A literature review for the Canadian National HIV Surveillance Program

 Download this article as a PDF (235 KB)
Download this article as a PDF (235 KB)Published by: The Public Health Agency of Canada
Issue: Volume 51-5, May 2025: Travel Health
Date published: May 2025
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 51-5, May 2025: Travel Health
Literature Review
Proposed approaches for public health surveillance: A literature review for the Canadian National HIV Surveillance Program
Anita Robert1, Wes Martin1, Leigh Jonah1, Dana Paquette2, Joseph Cox3, Laura H Thompson1
Affiliations
1 Centre for Communicable Disease and Infection Control, Public Health Agency of Canada, Ottawa, ON
2 Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON
3 School of Population and Global Health, Department of Global and Public Health, McGill University, Montréal, QC
Correspondence
Suggested citation
Robert A, Martin W, Jonah L, Paquette D, Cox J, Thompson LH. Proposed approaches for public health surveillance: A literature review for the Canadian National HIV Surveillance Program. Can Commun Dis Rep 2025;51(5):191–211. https://doi.org/10.14745/ccdr.v51i05a06
Keywords: HIV, surveillance, diagnosis, review literature as topic, public health practice
Abstract
Background: The National HIV Surveillance Program, managed by the Public Health Agency of Canada, is a passive surveillance system that collects de-identified data on HIV cases in Canada. Regular review of this surveillance system is required to maintain its accuracy, effectiveness and relevance in the face of a changing HIV epidemic. The National HIV Surveillance Program is undergoing a comprehensive review and renewal process with the aim of identifying and implementing potential improvements to meet the information needs of communities, service providers, researchers, provinces and territories and the federal government more effectively.
Methods: A non-systematic literature review was conducted in June to July 2023, with 3,521 articles found and 105 included.
Objective: This literature review aimed to identify proposed approaches for public health surveillance, with an emphasis on HIV surveillance and identify key findings relating to the following themes: surveillance system infrastructure, data collection, ethical considerations and stakeholder relationships.
Results: Key findings from the literature review pertained to standardization and centralization of data collection; collection of demographics, disease staging, social determinants of health and other data elements; and linking surveillance systems to other data sources or other surveillance systems. Additional findings concerned legislative and policy review, privacy strategies, informed consent, ethical surveillance system design, stakeholder consultation at all stages, knowledge translation and ensuring adequate resourcing.
Conclusion: In future work, lessons resulting from the literature review will be combined with evidence from other components of the overall review of Canada’s HIV surveillance system. Together, this information will be further assessed and prioritized for possible implementation after consultation with data providers and communities.
Introduction
In 2022, there were 1,833 newly diagnosed HIV cases in Canada Footnote 1. Given the persistence of HIV and other sexually transmitted and blood-borne infections (STBBIs) in Canada, the federal government highlighted surveillance systems as a key evidence source to inform programs and policies in its goal to reduce the burden of STBBIs. It committed to strengthening these systems in the Government of Canada's STBBI Action Plan (2024–2030) Footnote 2Footnote 3. Therefore, high-quality HIV surveillance data remains critical to Canada’s STBBI response.
Public health surveillance systems should be updated as proposed surveillance approaches, or the epidemiology of the condition of interest evolves. The National HIV Surveillance Program compiles data on HIV diagnoses in Canada from various sources: line-listed datasets and case report forms on individual HIV diagnoses reported in the provinces and territories (PT), aggregate data tables on perinatal exposure to HIV from the Canadian Perinatal HIV Surveillance Program, aggregate data tables on positive HIV tests during immigration medical exams provided by Immigration, Refugees and Citizenship Canada (IRCC) and HIV-related mortality data from Statistics Canada. In response to annual data requests sent to the PTs, line-listed datasets and case report forms with data on HIV diagnoses are received, data is cleaned and reformatted to match national HIV surveillance variable formats, PT-specific data is validated, national surveillance data is compiled and reports and infographics are prepared. Additional information on the operation of the surveillance system has been described elsewhere Footnote 4. The review and renewal process initiated in 2021 represents the first comprehensive review of the National HIV Surveillance Program. This review and renewal process was undertaken to improve the surveillance system for harmonization of methods related to data reporting and collection across jurisdictions and to better meet community, program and policy engagement and evidence needs. As the epidemiology of HIV has evolved over time, the review and renewal ensure that the National HIV Surveillance Program remains relevant and allows for better alignment of the surveillance system with the goals of the Government of Canada's STBBI Action Plan (2024–2030) Footnote 2Footnote 3. The National HIV Surveillance Program is undergoing a review and renewal process to identify areas for improvement and implement needed changes to provide better quality data to data users. Along with technical assessments and stakeholder and other key informant consultations, a literature review was undertaken to identify proposed approaches in public health surveillance, emphasizing HIV surveillance systems, to advance HIV surveillance in Canada. This literature review was conducted as an information gathering exercise to identify proposed approaches implemented by other surveillance systems that will be discussed with data providers and community stakeholders for potential implementation during the renewal process. This article reports the findings of the review grouped across the following themes: surveillance system structure and methodology, data collection, ethical considerations and stakeholder consultation.
Methods
A general, non-systematic literature review was performed in June to July 2023 to examine proposed approaches in local, provincial and national public health surveillance systems within Canada and globally. Follow-up for newly diagnosed HIV cases in Canada is completed locally, with required reporting to provincial/territorial health authorities and subsequent reporting to the national HIV surveillance program. The National HIV Surveillance Program reports on national trends in new HIV diagnoses stratified by various epidemiological factors. The following topics, determined to be relevant to the National HIV Surveillance Program, were examined: surveillance system processes and methodologies; data reporting methods; data infrastructure; considerations for collection, analysis and use of sociodemographic and epidemiological data; community engagement; ethical considerations; inter-surveillance system linkage; and program and policy engagement.
Distinct search strategies were developed for PubMed, Embase and Google Scholar to address each topic. PubMed and Embase were selected, as their combined use results in a high coverage rate of publications for research Footnote 5 and this search was supplemented by Google Scholar due to the large volume of publications included Footnote 6. Various search terms were developed for each aspect of the given topic. Search terms used for “surveillance” included: surveillance, monitoring, disease surveillance, passive surveillance, etc. The topics were divided equally across two reviewers responsible for search strategy development, database searches, article screening and assessment for inclusion, article detail extraction and analysis. Given that this was a non-systematic literature review and given the volume of articles identified for screening, each article was only reviewed by one of the two reviewers and no procedure existed for discordant assessments or disagreements between reviewers. Data extraction was completed by entering data into structured evidence tables on citation information, inclusion/exclusion decision, rationale for decision regarding inclusion, notes and the main message for the National HIV Surveillance Program.
Overall, 3,521 articles were identified for screening (Figure 1). Articles were assessed for inclusion based on criteria that included relevance, recency and geographic location. By including articles published on or after January 1, 2010, the authors were able to obtain information on improvements to public health surveillance in response to various events over time (i.e., swine flu pandemic, Ebola, COVID-19 pandemic, etc.) and including articles by study location allowed for the extraction of relevant information from jurisdictions with similar public health contexts. Although this literature review was completed as part of the review of the National HIV Surveillance Program, articles related to other conditions were included, as key findings from these articles can also be applied to HIV surveillance. Occasionally, articles with geographic locations outside of the listed regions or published before 2010 were included if reviewers deemed them particularly relevant for Canadian HIV surveillance. Included articles not meeting one or more inclusion criteria are detailed in the Appendix, Table A1. Such articles held lessons for the renewal of the National HIV Surveillance Program and met the other relevant criteria for inclusion: contains information that can be relevant to surveillance systems in Canada, related to passive surveillance systems, contains information that is otherwise relevant to surveillance system modernization and available in either English or French. Articles were not restricted by study design. While there was no specific separate search for grey literature, the intention in the use of Google Scholar as a searched database was to capture relevant grey literature Thus, 3,416 articles were excluded across all topics.
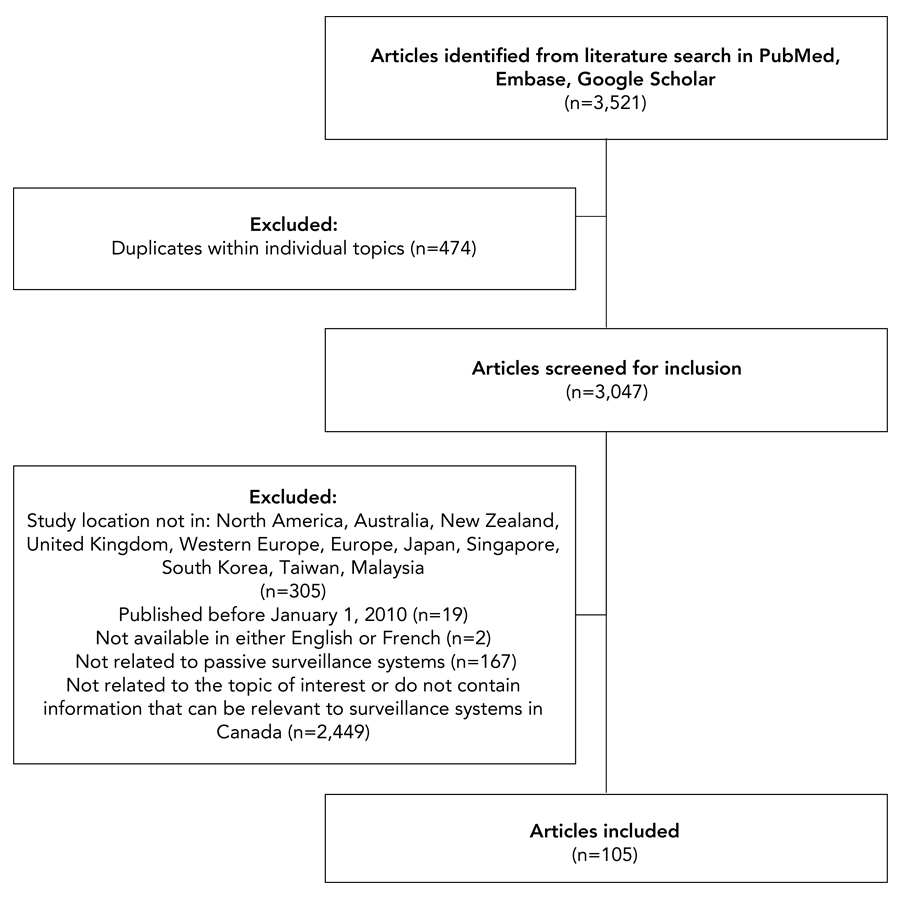
Figure 1: Descriptive text
This figure shows inclusion and exclusion of articles screened during the literature review. Overall, 3,521 articles were identified from literature searches in PubMed, Embase and Google Scholar. There were 474 articles excluded, as these were articles appearing multiple times within individual topics, leaving 3,047 articles being screened for inclusion. Of these, 305 were excluded, as the study location was not in: North America, Australia, New Zealand, United Kingdom, Western Europe, Europe, Japan, Singapore, South Korea, Taiwan, or Malaysia; 19 articles excluded, as these were published before January 1, 2010; 2 articles were excluded, as these were not available in either English or French; 167 articles were excluded, as these were not related to passive surveillance systems; and 2,499 articles were excluded, as these were not related to the topic of interest or do not contain information that can be relevant to surveillance systems in Canada. Overall, 105 articles were included in this literature review.
- Figure 1 Footnote a
-
As literature searches were conducted separately for each topic, the number of duplicates only includes articles identified as duplicated within a particular topic. As articles could be included in multiple themes, the number of duplicates does not include articles appearing under multiple themes
Practices were deemed to be “proposed approaches” if their implementation could improve data collection and surveillance system methodology and infrastructure, ensure ethical public health surveillance and improve stakeholder engagement for the National HIV Surveillance Program. Proposed approaches are potential actions for the National HIV Surveillance Program supported by the literature. Although each proposed approach is evidence-based, these have not been vetted for risk or bias. Practices and processes from included articles were grouped into key findings for the National HIV Surveillance Program’s renewal. Individual articles could support multiple findings. Thematic analysis was performed using an open-coding system to derive themes through an inductive process, where the main message or key finding for the National HIV Surveillance Program from each included article was derived. Individual main messages were inductively grouped based on similarity to each other into key findings. Key findings were grouped into the four larger themes and subthemes based on surveillance system development, operation and evaluation. The process was conducted manually and no formal qualitative analysis procedure or software was used. The results of this review are separated into “Novel findings,” representing potentially new proposed approaches and processes for the National HIV Surveillance Program and “Long standing public health practices,” representing practices or processes that have been consistently used in public health surveillance. “Novel findings” were related to modernization and updating surveillance systems and were similar to findings identified from previously completed steps of this review process. “Long standing public health practices” were most often practices for ongoing surveillance system operation and were already in place at other surveillance systems both in Canada and in other jurisdictions.
Results
Key findings
Literature searches were conducted separately for each topic and 3,521 articles were identified across all sources. Article exclusions for duplicates and other reasons are outlined in Figure 1. Overall, 105 articles were included. One third (n=33) were published during or post-COVID-19 pandemic (2020 onwards). The geographic distribution of articles shows that half came from North America (n=52) and 17.1% (n=18) compiled information about multiple countries. Key findings were derived using an inductive approach, where articles included were analyzed to identify any practices or processes potentially applicable to the National HIV Surveillance Program. Post-literature review, the findings were grouped into four key themes: surveillance system structure and methodology, data collection, ethical considerations and stakeholder engagement.
Novel findings
Novel findings were identified related to surveillance system structure and methodology, data collection, ethical considerations and stakeholder consultation. (Table 1). Most of the findings were supported by multiple articles, with additional details found in Table 1.
| Surveillance system stage where relevant | Novel findings | Articles that support findings | Examples of novel findings |
|---|---|---|---|
| Surveillance system structure and methodology | |||
| Surveillance system development | 1. Enhance timeliness of data with real-time or close to real-time surveillance for key metrics and data linkage between different surveillance systems (i.e., within the same level, or local surveillance systems to the national surveillance system) to ensure relevance of reporting and ability to respond quickly to transmission clusters (n=8). | Beyrer (2021) Footnote 7, Ghosh (2012) Footnote 8, Mao (2010) Footnote 9, McGill (2023) Footnote 10, Moore (2012) Footnote 11, Morse (2012) Footnote 12, Price (2021) Footnote 13, Sullivan (2022) Footnote 14 |
Mao (2010) Footnote 9: Description of the creation of the Chinese national, web-based HIV/AIDS information system. This system provides real-time data on HIV testing, prevention and treatment from provinces. Price (2021) Footnote 13: This article discusses the construction of local surveillance systems in the United Kingdom for COVID by linking data from laboratory information management systems with electronic medical records. Data about specimens collected from laboratory information management systems is linked to information about patient admissions and discharges from electronic medical records. This system allows for the real-time monitoring of hospital onset COVID infections. |
| Surveillance system operation | 2. Use web-based application to assist with data collection (n=3). | Cohen (2014) Footnote 15, Hood (2017) Footnote 16, Mao (2010) Footnote 9 |
Cohen (2014) Footnote 15: Review of the status of the United States (US) National HIV Surveillance System in 2013. This surveillance system uses specialized web application developed by the US Centers for Disease Control and Prevention (CDC) to receive monthly de-identified data submissions from HIV surveillance programs. Hood (2017) Footnote 16: A study using HIV epidemiological data and field service data from King County, Washington to assess the impact of misclassification of prior diagnoses on new diagnoses and partners services on linkage to care. Cases in the Laboratory Tracking Database, a database containing electronic laboratory data submissions, are matched to those in the Enhanced HIV/AIDS Reporting System (eHARS), a web-based HIV surveillance data repository, prior to submission. |
| Data collection | |||
| Surveillance system development | 3. Supplemental information obtained through passive surveillance systems with multiple internal or external data sources aside from other existing surveillance systems such as: laboratory data, survey data, clinical data or electronic health records, healthcare system data, testing data and population data (n=28). | Brandwagt (2019) Footnote 17, Buchacz (2015) Footnote 18, Chapman (2011) Footnote 19, Cohen (2014) Footnote 15, Ghosh (2012) Footnote 8, Goller (2010) Footnote 20, Hall (2021) Footnote 21, Hood (2017) Footnote 16, Jeong (2022) Footnote 22, Jones (2011) Footnote 23, Jung (2018) Footnote 24, Kliewer (2010) Footnote 25, Kraut (2023) Footnote 26, Leal (2010) Footnote 27, Moore (2012) Footnote 11, Morse (2012) Footnote 12, Newton (2012) Footnote 28, Paulukonis (2014) Footnote 29, Pierce (2017) Footnote 30, Price (2021) Footnote 13, Schmidt (2019) Footnote 31, Shaw (2011) Footnote 32, Sullivan (2022) Footnote 14, Tanaka (2021) Footnote 33, Weston (2018) Footnote 34, WHO (2021) Footnote 35, Wijayasri (2016) Footnote 36, Willis (2019) Footnote 37 |
Brandwagt (2019) Footnote 17: Discusses the evaluation of the national invasive meningococcal disease surveillance system in the Netherlands, created by linking data from clinical datasets, laboratory datasets and serogroup/subtype data from the national reference laboratory. Data from clinical and laboratory sources are merged with data on serotype and subtype information obtained from further testing at the national reference laboratory. Hall (2021) Footnote 21: This article discusses the creation of the HOSTED (Household Transmission Evaluation Database) surveillance system for household COVID19 transmission, developed by merging data from the Second Generation Surveillance System at Public Health England, National Health Service Personal Demographics Service data. The National Health Service Personal Demographics Service dataset has information on the home addresses of people registered with a general physician; unique households are indexed using a Unique Property Reference Number. Data linkage with the Second Generation Surveillance System for COVID occurs in a secure data access environment, generating a pseudonymized dataset for analysis. |
| 4. Include: race and/or ethnicity data, CD4 count and/or HIV staging, gender, sexual orientation data, residence data, socioeconomic data elements (i.e., income, poverty level, educational), data elements related to migration (i.e., country of infection or country of origin, immigration) and additional variables (i.e., insurance, coverage, incarceration status, occupation, homeless, pre-exposure prophylaxis [PrEP], reason for test) (n=12). | Dickson (2015) Footnote 38, Ford (2012) Footnote 39, Newton (2012) Footnote 28, Rice (2017) Footnote 40, Rossi (2017) Footnote 41, Schmidt (2019) Footnote 31, Sullivan (2022) Footnote 14, Cohen (2014) Footnote 15, Croxford (2022) Footnote 42, Beltran (2011) Footnote 43, Beyrer (2021) Footnote 7, WHO (2021) Footnote 35 |
Dickson (2015) Footnote 38: Overview of the epidemiology of HIV and AIDS in Aotearoa New Zealand since the inception of the surveillance system. This surveillance system collects data on age at diagnosis, gender, ethnicity, usual residence, likely means of infection, place of infection and initial CD4 cell count. Ford (2012) Footnote 39: Identifies critical data and indicators related to HIV care and access to services, looks at the impact of the US National HIV/AIDS Strategy and looks at the data systems that capture key data. This report recommended collection of clinical care indicators, mental health, substance use, housing indicators, food insecurity, demographic and other social determinant of health data (i.e., income, reimbursement for medical services, etc.). |
|
| Ethical considerations | |||
| Surveillance system development | 5. Design surveillance system and associated infrastructure considering ethical principles including equity and accessibility (n=5). | Aiello (2020) Footnote 44, Coltart (2018) Footnote 45, Molldrem (2020) Footnote 46, Moran-Gilad (2017) Footnote 47, Samuel (2018) Footnote 48 |
Aiello (2020) Footnote 44: Outlines principles related to public health surveillance systems involving the internet and social media. Public health surveillance systems must be designed considering the following ethical principles: non-maleficence, beneficence, respect for autonomy, equity and efficiency. Coltart (2018) Footnote 45: A review article that examined ethical issues in global HIV phylogenetic research. Risk-benefit assessments, with a special focus on key populations likely to be identified, should be conducted prior to designing, conducting and reporting phylogenetic studies. The rights and interests of individuals participating in these studies and the populations they represent need to be protected with clinically relevant results returned as soon as possible. Awareness regarding the social, legal, human rights and other aspects of society are needed with special focus on the effect of this research on criminalization and violence toward impacted populations (i.e., key populations). Risk mitigation strategies must be implemented to ensure that data cannot be re-linked for harmful purposes, training on privacy and confidentiality and including measures for ongoing monitoring and redress in cases of data misuse. True informed consent from participants is needed. Community engagement during the design, conduct and analysis of research is needed to ensure that projects are relevant to communities affected. Communication should be clear and understandable with special focus on sensitizing groups, such as communities, law enforcement and public health, to phylogenetic research and its implications. Equitable data sharing with governance structures to ensure accountability to communities is also required. |
| 6. Automate processes for data privacy and enhanced security with data anonymized, pseudonymized or using alternative identifiers in the event of data linkage (n=16). | Blais (2014) Footnote 49, Boes (2020) Footnote 50, Bublitz (2019) Footnote 51, Campo (2020) Footnote 52, Cauchi (2022) Footnote 53, Fan (2010) Footnote 54, Jeong (2022) Footnote 22, Johnson (2018) Footnote 55, McKerr (2015) Footnote 56, Ndeikoundam Ngangro (2022) Footnote 57, Polemis (2020) Footnote 58, Rivière (2018) Footnote 59, Sáez-López (2019) Footnote 60, Severi (2023) Footnote 61, Strobel (2019) Footnote 62, Trifirò (2014) Footnote 63 |
Boes (2020) Footnote 50: Discusses the review of the national hepatitis B surveillance system in Germany. Physicians and laboratories report hepatitis B diagnoses to the local public health authorities, which report to state health authorities. Data is pseudonymized at reporting to state health authorities, which then submit data to the Robert Koch Institute. McKerr (2015) Footnote 56: An evaluation of Taiwan’s national dengue surveillance system as part of the National Disease Surveillance System (NDSS). This system has an active component amongst travellers at ports of entry and a passive component amongst patients presenting for care at their healthcare providers, laboratory testing, notification to local health departments and subsequently the NDSS. Data is anonymized and extracted from the NDSS. |
|
| Stakeholder consultation | |||
| Surveillance system operation | 7. Community engagement and community-led monitoring throughout surveillance cycle (n=4). | Lee (2009) Footnote 64, Sullivan (2022) Footnote 14, Sweeney (2013) Footnote 65, WHO (2021) Footnote 35 |
Lee (2009) Footnote 64: Commentary that proposes a US national privacy protection standard for public health data. Prior to the use or release of potentially identifiable data, affected community groups should informed and provided the opportunity to provide input in decision making processes. Sweeney (2013) Footnote 65: Review of ethical considerations for using HIV surveillance data to facilitate HIV medical care. Prior to piloting a new program, community-based organizations were engaged in the development of strategies for the use and scale-up of HIV surveillance data. |
| 8. Consult with stakeholders on the development of specific information products with reporting in a sensitive/respectful manner that does not allow for the direct identification of affected individuals (n=8). | Burkom (2021) Footnote 66, Cauchi (2022) Footnote 53, Goldstick (2021) Footnote 67, Hargrove (2018) Footnote 68, Marshall (2017) Footnote 69, Marzano (2023) Footnote 70, Severi (2023) Footnote 61, Simonsen (2016) Footnote 71 |
Marshall (2017) Footnote 69: Examines the development of the Prevent Overdose Rhode Island (PORI) website. This website aimed to use public health surveillance data to provide information on drug overdoses to stakeholders and help direct those at risk to treatment. Prior to launching the web application, community and other stakeholders were sent email surveys. Over a hundred comments were received regarding how to improve the website. The next phase of evaluation will involve focus groups and user testing sessions to target content for audience. Marzano (2023) Footnote 70: Reviews a real-time suicide surveillance system in Great Britain. Police forces submit forms with information on demographics, life events, mental health problems, previous police contact and circumstances surrounding death (i.e., time, date, location and method) to the British Transport Police. Involvement of police forces in the surveillance system has been controversial, due to previous history of criminalizing suicides. Further, reporting should be conducted in a respectful and anonymized manner. |
|
| 9. Provide training to public health stakeholders on the surveillance system (i.e., data collection) and knowledge mobilization initiatives to affected communities, public health professionals, clinicians and other stakeholders to improve public health surveillance of the condition of interest and demonstrate the public health value of the surveillance system (n=7). | Contoli (2016) Footnote 72, Crain (2016) Footnote 73, Hennenfent (2017) Footnote 74, Magee (2011) Footnote 75, Paul (2019) Footnote 76, Rivière (2018) Footnote 59, Smith (2011) Footnote 77 |
Contoli (2016) Footnote 72: Discusses the implementation of PASSI d’Argento in Italy, a behavioural surveillance system monitoring health related behaviours in senior citizens. This surveillance system includes a web-based community of practice that offers workshops, communication tools and a forum for general discussions among stakeholders. Magee (2011) Footnote 75: Describes the creation of a training program for the National Tuberculosis Surveillance System, which receives data from all 50 states, the District of Columbia, New York City, Puerto Rico and other US jurisdictions in the Pacific and the Caribbean. The US CDC developed a training course for the National Tuberculosis Surveillance System. This course had a self study and a facilitator led version. Training materials included visual, auditory, reading/writing and movement aspects with self-study modules to be used in the completion of the training course and to serve as reference material to take back to the participants’ jobs. Study questions and case studies were included, along with notes, comments and diagrams. Field testing with quantitative and qualitative data from field tests with experts were analyzed and pre-test and post-tests were designed and conducted. Trainers were also trained using the Teach-Back system. As the training program was successful, this was used as a model for similar trainings in other surveillance systems and jurisdictions. |
|
| 10. Provide a media toolkit and other knowledge translation tools for community-led social and behavioural change communication (n=1). | Sullivan (2022) Footnote 14 | Sullivan (2022) Footnote 14: Describes the data visualization tool America’s HIV Epidemic Analysis Dashboard (AHEAD). A toolkit for various users (i.e., jurisdictions, community-based organizations, advocacy organizations and federal agencies) was also available. |
|
| 11. Consult stakeholders during the development of and ongoing operation of surveillance system infrastructure and implement any feasible changes suggested (n=5). | Dubiniecki (2022) Footnote 78, Marshall (2017) Footnote 69, Toutant (2011) Footnote 79, Yang (2020) Footnote 80, Zhang (2017) Footnote 81 |
Dubiniecki (2022) Footnote 78: Discusses the development and implementation of the Canadian Armed Forces Surveillance and Outbreak Management System (CAF SOMS). This system was developed by the National Contact Tracing Team and the Health Informatics Team and included the use of four distinct forms: case details, contagion elicitation, contact notification and contact follow-up. The database was presented to members of the National Contact Tracing Team for review. Perceptions of the system were positive and certain suggestions were acted on immediately. Other feasible suggestions that could not implemented immediately were prioritized for future implementation. Implemented changes received positive feedback. Toutant (2011) Footnote 79: Examines the development of SUPREME, a web application to examine the effect of extreme meteorological events on public health. This web application assists public health surveillance by combining data from various datasets including, health, meteorological, demographic, air quality and geospatial data. The article explains how feedback from stakeholder surveys and meetings were used to identify information needs, provincial capabilities and develop specifications for the web application. Based on the given specifications, the web application, SUPREME, was developed. |
|
Abbreviations: AHEAD, America’s HIV Epidemic Analysis Dashboard; CAF SOMS, Canadian Armed Forces Surveillance and Outbreak Management System; CDC, Centers for Disease Control and Prevention; HOSTED, Household Transmission Evaluation Database; NDSS, National Disease Surveillance System; PORI, Prevent Overdose Rhode Island; US, United States; WHO, World Health Organization |
|||
Novel findings regarding surveillance system and methodology addressed real- or near real-time data collection with surveillance system linkage and use of web-based applications in data collection. Web applications have been used in data submission to central agencies Footnote 15 and to receive data Footnote 16. Timeliness of data has been improved through real-time submissions to central agencies by jurisdictions Footnote 9 and institutions Footnote 13.
Findings for data collection indicate the need to supplement information from passive surveillance systems with other data sources and collect data on demographic traits, disease staging (e.g., CD4) and the social determinants of health. Disease subtype from laboratory data can supplement routine surveillance Footnote 17 and new systems developed by including population databases Footnote 21. While data on age, gender, ethnicity, residence, exposure and place of infection is routinely collected for HIV Footnote 38, clinical care indicators and social determinants of health data are needed Footnote 39.
Findings on ethical considerations included ethical, equitable and accessible surveillance system design and automated processes for data privacy alongside the ethical principles of non-maleficence, beneficence, respect for autonomy, equity and efficiency Footnote 44. Risk-benefit assessments, awareness, risk mitigation, community engagement and equitable data sharing Footnote 45 are needed in surveillance system design. Automated processes including pseudonymization Footnote 50 at data submission and anonymization at data extraction Footnote 56 enhance data privacy.
Stakeholder engagement is encouraged throughout the surveillance cycle and surveillance information product development. Training, knowledge mobilization and demonstration of the public health value of surveillance data, use of various knowledge translation tools and implementing changes recommended by stakeholders to the extent feasible are significant parts of surveillance system operation. Implementation included community engagement in decision and strategy making Footnote 64Footnote 65, as well as surveillance information product development Footnote 69Footnote 70; provision of resources, workshops Footnote 72 and training courses Footnote 75; audience specific knowledge translation toolkits Footnote 14; and implementation of community suggested changes in the development of technical tools Footnote 78Footnote 79.
Long standing public health practices
Long standing public health practices were identified applying to surveillance system structure and methodology, data collection, ethical considerations and stakeholder consultation. (Table 2). Most practices were supported by multiple articles with additional details found in Table 2.
| Surveillance system stage where relevant | Practice | Articles that support practices | Examples of practices |
|---|---|---|---|
| Surveillance system structure and methodology | |||
| Surveillance system development | 1. Delineate clear roles and responsibilities (n=3). | Gulaid (2012) Footnote 82, Kodan (2021) Footnote 83, O’Brien (2012) Footnote 84 |
Kodan (2021) Footnote 83: Describes the process of implementing a maternal death surveillance system in Suriname as experienced by healthcare providers, committee members and public health experts. A working group oversaw the clear delineation of roles and responsibilities by specifying tasks. O’Brien (2012) Footnote 84: Compares surveillance of transfusion-transmissible infections across the blood donation systems of five high income countries. Canada’s and France’s surveillance systems had differentiated responsibilities across programs and surveillance systems. |
| 2. Pilot test new data collection applications or systems in collaboration with other programs during surveillance system development (n=4). | Brady (2020) Footnote 85, Karp (2017) Footnote 86, Mao (2010) Footnote 9, Sweeney (2013) Footnote 65 |
Brady (2020) Footnote 85: Discusses the pilot testing of a voluntary community-based testing surveillance system for HIV in Ireland, with testing data submitted by clinics to the Health Protection Surveillance Centre. A steering group, including government and non-government organizations, was developed to oversee the development of the system including the development of a minimum dataset. Organizations were surveyed to develop database protocols and data collection tools. Karp (2017) Footnote 86: Examines the National Antimicrobial Resistance Monitoring System (NARMS) in the US. This surveillance system receives data on retail meat from the US Food and Drug Administration (FDA) and food animals from the United States Department of Agriculture (USDA) in addition to data on human illness from the US Centers for Disease Control and Prevention (which receives data from state and local public health units). Data from the surveillance system is used as part of risk assessment in drug approvals the creation of risk management strategies using data on resistance trends, types and pathogen prevalence. Pilot studies were conducted on farms to assess data and sample collection processes and lessons learned were used in the development of antimicrobial resistance in food animal surveillance programs. |
|
| Surveillance system operation | 3. Centralize and standardize data collection with standardized questionnaires and forms (n=7). | Kebisek (2021) Footnote 87, Lee (2009) Footnote 64, Mao (2010) Footnote 9, Mohammed (2016) Footnote 88, O’Brien (2012) Footnote 84, Weston (2018) Footnote 34, Zhang (2012) Footnote 89 |
Kebisek (2021) Footnote 87: Describes the process of conducting near real-time COVID-19 surveillance among the US Army population during the first year of the pandemic through the integration of several Department of Defense surveillance systems. Mao (2010) Footnote 9: Describes the creation of the Chinese national, web-based HIV/AIDS information system. Data collection on HIV testing, prevention and treatment is standardized across provinces. |
| Surveillance system evaluation | 4. Conduct periodic evaluations of surveillance system (n=5). | Cohen (2014) Footnote 15, Lee (2009) Footnote 64, Petty-Saphon (2019) Footnote 90, Rossi (2017) Footnote 41, Trepka (2016) Footnote 91 |
Cohen (2014) Footnote 15: Reviews the status of the US National HIV Surveillance System in 2013. The surveillance system will continue to evolve in response to the need for high-quality data and the findings from this evaluation. Rossi (2017) Footnote 41: Reviews the factors that influence the accuracy of infectious disease monitoring in migrants in Europe. The improvement of existing surveillance systems was recommended after this review. |
| Data collection | |||
| Surveillance system operation | 5. Resolve duplicates and data quality issues prior to upload into database (n=3). | Chapman (2011) Footnote 19, Jung (2018) Footnote 24, Strobel (2019) Footnote 62 |
Chapman (2011) Footnote 19: Examines the development of the Virginia Vital Events and Screening Tracking System (VVESTS), which is created by merging the Virginia Congenital Anomalies Reporting and Education System (VaCARES) (passive surveillance system) and the Virginia Early Hearing Detection and Intervention Program (VEHDIP). As duplicate records are a challenge, these records are assessed by querying for combination of variables. Strobel (2019) Footnote 62: Examines the evaluation of the Intellectual Disability Exploring Answers (IDEA) surveillance system in Western Australia, which was created by merging data from the Department of Education Western Australia and the Disability Services Commission (DSC). The surveillance system can be linked to administrative data as necessary. Data quality is assessed by cross-checking data across different systems and conducting cross-tabulations as needed. |
| 6. Manually verify data linkages (n=2). | Chapman (2011) Footnote 19, Leal (2010) Footnote 27 |
Chapman (2011) Footnote 19: Examines the development of the VVESTS, which is created by merging the VaCARES (passive surveillance system) and the VEHDIP. Data linkages are manually reviewed for accuracy. Leal (2010) Footnote 27: Examines the creation of a surveillance system for bloodstream infection in Calgary, which was created by merging regional laboratory data with data from hospital administrative databases. Regional laboratory data was merged with hospital administrative databases. Medical records were manually reviewed to verify data linkages. |
|
| Ethical considerations | |||
| Surveillance system development | 7. Review relevant legislations specific to the condition under surveillance (i.e., criminalization), their implications, privacy policies/legislations, other relevant legislations and obtaining the needed approvals to design surveillance systems in compliance with these laws and policies (n=13). | Abecasis (2018) Footnote 92, Blais (2014) Footnote 49, Bublitz (2019) Footnote 51, Campo (2020) Footnote 52, Condell (2016) Footnote 93, Fritsch (2019) Footnote 94, Ghosh (2012) Footnote 8, Hoppe (2022) Footnote 95, Leitner (2018) Footnote 96, Marzano (2023) Footnote 70, Ndeikoundam Ngangro (2022) Footnote 57, Scaduto (2010) Footnote 97, Trifirò (2014) Footnote 63 |
Blais (2014) Footnote 49: Discusses the evaluation of the Québec Integrated Chronic Disease Surveillance System (QICDSS). This surveillance system was created by linking several databases: the health insurance registry, hospitalization database, vital statistics death database, physician claims database and pharmaceutical services databases. The QICDSS creation process was evaluated by government bodies with legal ownership of databases, the public health ethics committee and Commission d’accès à l’information du Québec. Ghosh (2012) Footnote 8: Reviews the National Tuberculosis Surveillance System in the US, created by linking epidemiological data submitted by state and territorial public health departments with laboratory data from the National Tuberculosis Genotyping Service. Compliant with federal legislation, identifying information is not stored and user credentialing is also maintained. |
| Surveillance system operation | 8. Create data sharing and use agreements with surveillance partners with variables and additional sources limited to purposes of surveillance system for privacy reasons (n=3). | Blais (2014) Footnote 49, Ghosh (2012) Footnote 8, Strobel (2019) Footnote 62 |
Blais (2014) Footnote 49: Discusses the evaluation of the QICDSS. This surveillance system was created by linking several databases: the health insurance registry, hospitalization database, vital statistics death database, physician claims database and pharmaceutical services databases. Only relevant variables were used in the creation of the QICDSS. Strobel (2019) Footnote 62: Examines the evaluation of the IDEA surveillance system in Western Australia, which was created by merging data from the Department of Education Western Australia and the DSC. Variable collection is limited for privacy reasons. Data sharing agreements governing data provision from data sources are present. |
| 9. Restrict data access and sharing on need-to-know basis for data integrity and privacy with access monitored using logs (n=4). | Blais (2014) Footnote 49, Ghosh (2012) Footnote 8, Marzano (2023) Footnote 70, Ndeikoundam Ngangro (2022) Footnote 57 |
Blais (2014) Footnote 49: Discusses the evaluation of the QICDSS. This surveillance system was created by linking several databases: the health insurance registry, hospitalization database, vital statistics death database, physician claims database and pharmaceutical services databases. Data access is logged and limited to the team working within the surveillance system. Ndeikoundam Ngangro (2022) Footnote 57: Discusses the feasibility of an automated surveillance system for sexually transmitted infections in France. Sexually transmitted infections clinics submit data to Santé Publique France through a web portal. Data access is limited to authorized personnel. |
|
| 10. Seek ethics approvals for specific projects outside of routine surveillance (i.e., manuscripts) (n=2). | Fan (2010) Footnote 54, Sáez-López (2019) Footnote 60 |
Fan (2010) Footnote 54: Describes the development of the Alberta Real Time Syndromic Surveillance Network (ARTSSN), a real-time surveillance system obtaining data from several different databases simultaneously: Health Link calls, emergency department visits, school absenteeism, laboratory reports and online forms. Research uses of the data must be approved with the process simplified due to the pseudonymization of data. Sáez-López (2019) Footnote 60: Discusses the evaluation of Portuguese influenza surveillance system, which has a sentinel and non-sentinel component. The non-sentinel component operates by having laboratories associated with the Portuguese Laboratory Network for the Diagnosis of influenza infection. Approval for specific projects is granted by the Health Ethic Committee of National Institute of Health Doutor Ricardo Jorge. |
|
| 11. Obtain informed consent from individuals consulted as part of surveillance system evaluation (n=3). | Johnson (2018) Footnote 55, McKerr (2015) Footnote 56, Rivière (2018) Footnote 59 |
McKerr (2015) Footnote 56: This article is an evaluation of Taiwan’s national dengue surveillance system as part of the National Disease Surveillance System (NDSS). This system has an active component amongst travellers at ports of entry and a passive component amongst patients presenting for care at their healthcare providers, laboratory testing, notification to local health departments and subsequently the NDSS. Verbal informed consent was obtained from those interviewed. Rivière (2018) Footnote 59: Summarizes an evaluation of the surveillance of bovine tuberculosis in France. Passive surveillance is conducted on specimens killed by hunters and dead or dying animals with data submitted to local veterinary services and the National Hunting and Wildlife Office. There is also an active surveillance component on living specimens with data submitted to the local veterinary services. Verbal informed consent was obtained prior to interviews. |
|
| Stakeholder consultation | |||
| Surveillance system development | 12. Build stakeholder relationships with each other and with surveillance system based on ongoing communication with stakeholders using tools such as communication plans including multiple methods of communication (n=16). | Asburry (2019) Footnote 98, Burkom (2021) Footnote 66, Cauchi (2022) Footnote 53, Contoli (2016) Footnote 72, Crain (2016) Footnote 73, Dawson (2016) Footnote 99, European Food Safety Authority (2023) Footnote 100, Huot (2019) Footnote 101, Johnson (2018) Footnote 55, McGill (2023) Footnote 10, Rivière (2018) Footnote 59, Schönfeld (2018) Footnote 102, Schwartz (2022) Footnote 103, Smith (2011) Footnote 77, Strobel (2019) Footnote 62, Takla (2012) Footnote 104 |
Smith (2011) Footnote 77: Examines the implementation of a surveillance system model for child maltreatment developed by the US CDC. Numerous data sources are used, such as: death certificates, homicide files, medical examiner records, child protective services records, child welfare registries and Child Death Review Team (CDRT) reports. Respondents indicated that personal collaborative relationships between stakeholders were essential. However, frequent staff turn over and low meeting attendance were identified as hindrances in developing these relationships. Schwartz (2022) Footnote 103: Outlines the development of a COVID19 surveillance system in Memphis, US. The surveillance system combined regional laboratory COVID19 testing and epidemiological data with electronic medical records, social determinant and environmental data. The surveillance system included standard operating procedures for team meetings and community outreach in addition to a formal community outreach leadership team. |
| Surveillance system operation | 13. Ensure that the surveillance system is sustainable, ongoing and well resourced with the ability support the participation of all stakeholders in the surveillance system (n=15). | Asburry (2019) Footnote 98, Crain (2016) Footnote 73, Dawson (2016) Footnote 99, Ehlman (2021) Footnote 105, Fedorowicz (2010) Footnote 106, Hennenfent (2017) Footnote 74, Jermacane (2019) Footnote 107, Johnson (2018) Footnote 55, McGill (2023) Footnote 10, Moore (2012) Footnote 11, Paul (2019) Footnote 76, Schönfeld (2018) Footnote 102, Smith (2011) Footnote 77, Wijayasri (2016) Footnote 36, Wong (2022) Footnote 108 |
Ehlman (2021) Footnote 105: Discusses the evaluation of the National Electronic Injury Surveillance System. Coders in emergency departments complete data entry in the system, sending data to the Consumer Product Safety Commission, which submits data to the US CDC. Issues identified with the surveillance system include multiple approvals, updates and training required when the system is updated, data completion, issues with laptops and staff turnovers and the need to replace sites if any drop out of surveillance system. Moore (2012) Footnote 11: Describes the evaluation of the US Department of Defense Serum Repository and Defense Medical Surveillance System, linking laboratory data with demographic data to create a surveillance system for the health of the military. Recommendations included: setting and communicating priorities for resources, strengthening and resourcing organizational oversight, distinct chain of command for receiving guidance and resource from policymakers in addition to adequate staffing to meet user needs. |
| Surveillance system evaluation | 14. Stratify consultations based on different groups and include the following in surveillance system evaluation: surveys, focus groups and/or meetings and interviews (n=15). | Ehlman (2021) Footnote 105, Fedorowicz (2010) Footnote 106, Halliday (2013) Footnote 109, Hennenfent (2017) Footnote 74, Jermacane (2019) Footnote 107, Johnson (2018) Footnote 55, Kunze (2022) Footnote 110, Paul (2019) Footnote 76, Pratt (2020) Footnote 111, Rivière (2018) Footnote 59, Schönfeld (2018) Footnote 102, Smith (2011) Footnote 77, Takla (2012) Footnote 104, Yang (2020) Footnote 80, Zhang (2017) Footnote 81 |
Halliday (2013) Footnote 109: Describes the surveillance system evaluation of the Australasian Maternity Outcomes Surveillance System. In this surveillance system, monthly data requests are emailed to maternity unit data collectors who can either submit data or submit a nil report through web-based reporting. Data collected would then be accessed by study investigators for research and dissemination. In addition to conducting an anonymous online survey of stakeholders, documentation from advisory and project meetings in addition to official correspondence were reviewed to identify previous concerns, questions, comments and feedback. Takla (2012) Footnote 104: Discusses an event-specific surveillance system developed for the FIFA women’s world cup in Germany in 2011. The local district where the world cup was held would report to the Robert Koch Institute (national health authority) through state health authorities, with infectious disease notifications being provided daily. The national health authority would provide feedback and summary reports to the ministry of health, district and state health authorities twice weekly in addition to phone conferences with key stakeholders as needed. Results from the evaluation were stratified across district vs. state health authorities. |
Abbreviations: ARTSSN, Alberta Real Time Syndromic Surveillance Network; CDC, Centers for Disease Control and Prevention; CDRT, Child Death Review Team; DSC, Disability Services Commission; FDA, Food and Drug Administration; IDEA, Intellectual Disability Exploring Answers; NARMS, National Antimicrobial Resistance Monitoring System; NDSS, National Disease Surveillance System; QICDSS, Québec Integrated Chronic Disease Surveillance System; US, United States; USDA, United States Department of Agriculture; VaCARES, Virginia Congenital Anomalies Reporting and Education System; VEHDIP, Virginia Early Hearing Detection and Intervention Program; VVESTS, Virginia Vital Events and Screening Tracking System |
|||
Surveillance system structure and methodology should include clearly defined roles and responsibilities and pilot testing during surveillance system development. Questionnaires were centralized and standardized during operation. Periodic evaluation was also conducted. Measures to enhance surveillance system structure included: working groups specifying tasks Footnote 83 and program-specific responsibilities Footnote 84, pilot testing of surveillance systems in data collection settings Footnote 85Footnote 86, integration Footnote 87 and standardization across jurisdictions Footnote 9 and periodic evaluations to understand factors affecting accuracy Footnote 41 and ensure the relevance of these factors, as high-quality data is needed Footnote 15.
Data collection during operation should include resolving duplicates and data quality issues prior to upload onto databases and manually verifying data linkages. Structured queries Footnote 19Footnote 64 and cross-tabulations Footnote 53Footnote 62 and manual verification between data sources Footnote 19Footnote 64 or against raw data, such as medical records Footnote 27, also ensure high data quality.
Ethical considerations include legislative and policy review and approval during development. Data sharing agreements, data access monitoring with need-to-know restriction and non-routine project-specific ethics approval were present during ongoing operation. Informed consent from stakeholders was obtained during evaluation. Ethics committee evaluation Footnote 49, data protection for legislative compliance Footnote 8, minimum data collection for privacy Footnote 49, data sharing agreements governing data provision Footnote 62, maintaining data access logs Footnote 49 with data access limited to authorized individuals Footnote 57, simplified oversight processes for extra projects Footnote 60 accounting for existing data protection Footnote 54 and verbal informed consent during evaluation interviews Footnote 56Footnote 59 all ensure ethical public health surveillance.
During surveillance system development, inter- and intra-stakeholder relationships should be built and facilitated using various tools. Dedicated resources should be provided for ongoing operation. Consultations during periodic evaluations should be stratified by stakeholder group. Consistent staff Footnote 77, formal outreach processes Footnote 103, dedicated resources Footnote 105, senior guidance and oversight Footnote 11 and consultations stratified by jurisdictional level during evaluation Footnote 104 supplemented by existing communications Footnote 109 improves stakeholder engagement.
Discussion
Several proposed approaches with respect to areas for improvement, such as surveillance system infrastructure and methodology, data collection, ethical public health surveillance and stakeholder engagement were identified through this literature review. As the use of evidence-informed policy and programs is a guiding principle of the Government of Canada’s sexually transmitted and blood-borne infections (STBBI) action plan 2024–2030, surveillance data has been emphasized as a guide for developing and implementing programs and interventions Footnote 2Footnote 3. Thus, it is important to periodically review and modernize surveillance systems to ensure their effectiveness. Key attributes of surveillance systems have been defined as follows by the United States Centers for Disease Control and Prevention (CDC), as part of its guidelines on public health surveillance system evaluation to develop effective surveillance systems: simplicity, flexibility, data quality, acceptability, sensitivity, predictive value positive, representativeness, timeliness and stability Footnote 112. Findings from the current review show that effective surveillance systems are characterized as standardized across jurisdictions, timely, developed and tested in conjunction with stakeholders and linked to other data sources. Data collection may benefit from including demographic information, disease staging and social determinant of health indicators, supplemented by other data sources as needed and include data quality and duplicate checks. Ethical surveillance system design considers equity and accessibility, privacy issues, legislation and policy review and informed consent. Effective stakeholder engagement is meaningful and conducted at all stages of public health surveillance, integrating various methods and programs.
The positive impact of modernizing national HIV surveillance systems was noted previously in the United States, since it updated its National HIV Surveillance System in 2013 with enhancements, such as improvements in data collection on sexual orientation, gender identity, social determinants of health and improved data deduplication procedures to enhance data quality Footnote 113. This update has resulted in an increased capacity to rapidly respond to HIV clusters and support ongoing viral suppression through “data-to-care” activities Footnote 113. Although the National HIV Surveillance Program in Canada presents information on the epidemiology of HIV on a national level, the program does not have a centralized database with consistent data standards and relies on data received from thirteen PTs. This affects the timeliness of surveillance data, impacting the relevance of data used to inform evidence-based policies and programs for HIV prevention and treatment. As the data for the Canadian National HIV Surveillance Program is provided by the PTs, data linkages to additional data sources for other social determinants of health data is not available. As such, proposed approaches included incorporating a centralized database, data quality procedures and additional data sources and social determinant of health data collection as part of the National HIV Surveillance Program renewal. Furthermore, it is important to update the National HIV Surveillance Program to better address the burden of HIV in Canada. It is anticipated that the potential renewal of the program in Canada will result in improved data collection and quality, providing key evidence needed to meet the federal government’s commitments under its STBBI action plan Footnote 2Footnote 3, improved public health outcomes and improved outcomes for those diagnosed and living with HIV.
The proposed improvements to surveillance system infrastructure and methodology may improve the simplicity, timeliness and stability of the National HIV Surveillance Program as data standardization across jurisdictions simplifies data submission, which in turn could improve timeliness and stabilizes data submission processes. Proposed approaches for ethical surveillance system design may result in improved ethical surveillance practices and subsequently increase the acceptability of the surveillance system. Improving stakeholder engagement using the proposed approaches could improve the acceptability as stakeholders may be more likely to engage with the surveillance program.
Although numerous proposed approaches addressed various areas of improvement, not all findings from this literature review may be applicable to national HIV surveillance in Canada. For instance, a real-time HIV surveillance system Footnote 35 may not be needed at the national level, as provincial, territorial and local public health units would be primarily addressing HIV outbreaks or clusters, as opposed to the National HIV Surveillance Program and the national program serves to provide a national overview of HIV epidemiology, instead of localized data.
Findings from this review were consistent with findings from consultations with data providers and community organizations conducted as part of the National HIV Surveillance Program’s review and renewal process and the recommendations in the Pan-Canadian Health Data Strategy: Toward a world-class health data system Footnote 114. While the need for meaningful community engagement and involvement has been acknowledged previously Footnote 115Footnote 116, the program’s consultations and the Pan-Canadian Health Data Strategy (PCHDS) Footnote 114 emphasized the importance of community consultations regarding data collection, interpretation and storage. Regarding data infrastructure and quality, the program’s consultations and the PCHDS Footnote 114 suggest developing consistent data standards, with the PCHDS advocating for timely data access, health data literacy promotion and data-driven social and technological innovation Footnote 114. Furthermore, the program’s consultations recommended the collection of information on sex, gender, sexual orientation, race and/or ethnicity and disease staging (e.g., CD4 cell count). The PCHDS proposed prioritizing the following related to data governance: ethical data use, First Nations, Inuit and Metis data sovereignty, oversight, federal leadership and measures to address security and privacy concerns Footnote 114. The findings of this review are consistent with the Public Health Agency of Canada’s vision for what public health surveillance should look like by 2030, with enhanced surveillance workflows, high-quality data, improved data sharing and linkage, community partnerships and public engagement Footnote 117. The National HIV Surveillance Program’s ongoing collaboration with data providers and community members to update data management, improve data collection on key variables and stakeholder engagement represents meaningful efforts to advance HIV surveillance in Canada.
This literature review provides a comprehensive overview of current and future proposed approaches in public health surveillance relevant to Canada’s national HIV surveillance. It contains evidence gathered from diverse sources over a broad timeframe, encompassing technological advances and surveillance system enhancements in response to public health events. As the epidemiology of diseases change over time, surveillance systems are updated to ensure relevance, resulting in the development and incorporation of new public health practices. The wide timeframe allows for the aggregation of practices gained from experience dealing with various public health events (e.g., COVID-19 pandemic) and technological updates, such as databases incorporating automated data linkage. Including information from a wide range of jurisdictions, with similar surveillance systems or public health contexts, allowed for a wider range of public health practices to be incorporated in this literature review for future discussion. In addition, the inclusion of information on other surveillance systems, instead of solely focusing on HIV surveillance systems, allowed for the inclusion of various practices. These include linked national and state tuberculosis surveillance systems in the United States Footnote 8 and additional methods for stakeholder collaboration and communication through resources tailored to stakeholders in a surveillance system examining health related behaviours in senior citizens in Italy Footnote 72. With the potential incorporation of these practices, linking the National HIV Surveillance Program to other surveillance systems or data sources could improve data collection on HIV and other relevant indicators. Resources tailored to stakeholders could improve the relevance of surveillance information products such as surveillance reports and infographics, as well as improve stakeholder engagement with the national program. Incorporating grey literature allowed novel and innovative practices found in emerging research to be included. Information can often be found in evidence reviews or reports in addition to published journal articles, such as a World Health Organization report on achieving key goals related to HIV, hepatitis and sexually transmitted infections, outlining various advances, such as case-based surveillance systems enhanced with additional data for improved care Footnote 35.
Limitations
This review was limited to information available online (i.e., additional information may be found in internal surveillance protocols and standard operating procedures). There may be additional technical details from these internal documents that may guide the development and operation of data management systems and other surveillance system tools that may not be accessible for review. While attempts were made to include articles primarily focused on passive surveillance systems, decisions regarding inclusion were sometimes difficult. For instance, articles regarding more recent surveillance systems often included components involving active or sentinel surveillance that supplemented passive surveillance. Additionally, this review was not a systematic review. Therefore, quality of evidence and risk of bias were not assessed. As quality of evidence was not considered and risk of bias was not assessed, evidence presented here could not be weighted based on these factors, rendering all sources as equal. This allows for over emphasis on lower quality and biased sources and under emphasis on higher quality sources with less bias. Despite these limitations, the results of this review provide evidence for improving surveillance practices as the National HIV Surveillance Program seeks to modernize surveillance practices and processes.
In addition to identifying possible directions for improvement of Canada’s national HIV surveillance program, this literature review summarizes public health surveillance practices for HIV and other conditions. The potential for applying public health surveillance practices for other conditions to HIV surveillance has been highlighted in this review and it serves as a valuable reference compiling information on proposed approaches to improving surveillance system infrastructure and methodology, data collection, ethical public health surveillance and stakeholder engagement in a single place. As such, this review may be relevant to other surveillance systems looking to review and renew their own systems.
Conclusion
In conclusion, as part of a comprehensive review of Canada’s HIV surveillance program, several current and future proposed approaches for surveillance systems were identified. Promising potential innovations include updates to data collection infrastructure, inclusion of new data elements (e.g., CD4 cell count), improving collection of existing data elements (i.e., sex and gender, race and/or ethnicity, HIV exposure information), developing a data governance framework and ongoing engagement with data providers and communities. Next steps include examining ways to validate and operationalize these key findings in collaboration with data providers and affected communities.
Authors' statement
- AR — Methodology, investigation, writing–original draft
- WM — Methodology, investigation, writing–original draft
- LJ — Conceptualization, writing–review & editing, supervision
- DP — Writing–review & editing
- JC — Writing–review & editing
- LHT — Conceptualization, writing–review & editing, supervision
Competing interests
None.
ORCID numbers
- Wes Martin — 0009-0005-2238-2104
- Joseph Cox — 0000-0002-7041-1556
- Laura H Thompson — 0000-0003-3792-9772
Acknowledgements
None.
Funding
This work was supported by the Public Health Agency of Canada.
References
- Footnote 1
-
Public Health Agency of Canada. HIV in Canada: 2022 surveillance highlights. Ottawa, ON: PHAC; 2023. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/hiv-2022-surveillance-highlights.html
- Footnote 2
-
Public Health Agency of Canada. Reducing the health impact of sexually transmitted and blood-borne infections in Canada by 2030: A pan-Canadian STBBI framework for action. Ottawa, ON: PHAC; 2018. https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/reports-publications/sexually-transmitted-blood-borne-infections-action-framework.html
- Footnote 3
-
Public Health Agency of Canada. Government of Canada’s sexually transmitted and blood-borne infections (STBBI) action plan 2024–2030. Ottawa, ON: PHAC; 2024. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/sexually-transmitted-blood-borne-infections-action-plan-2024-2030-brief.html
- Footnote 4
-
Public Health Agency of Canada. HIV in Canada, Surveillance Report to December 31, 2022. Ottawa, ON: PHAC; 2024. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/hiv-canada-surveillance-report-december-31-2022.html
- Footnote 5
-
Frandsen TF, Eriksen MB, Hammer DM, Christensen JB, Wallin JA. Using Embase as a supplement to PubMed in Cochrane reviews differed across fields. J Clin Epidemiol 2021;133:24–31. https://doi.org/10.1016/j.jclinepi.2020.12.022
- Footnote 6
-
Gusenbauer M, Haddaway NR. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res Synth Methods 2020;11(2):181–217. https://doi.org/10.1002/jrsm.1378
- Footnote 7
-
Beyrer C, Adimora AA, Hodder SL, Hopkins E, Millett G, Mon SH, Sullivan PS, Walensky RP, Pozniak A, Warren M, Richman B, Copeland R, Mayer KH. Call to action: how can the US Ending the HIV Epidemic initiative succeed? Lancet 2021;397(10279):1151–6. https://doi.org/10.1016/S0140-6736(21)00390-1
- Footnote 8
-
Ghosh S, Moonan PK, Cowan L, Grant J, Kammerer S, Navin TR. Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol 2012;12(4):782–8. https://doi.org/10.1016/j.meegid.2011.10.013
- Footnote 9
-
Mao Y, Wu Z, Poundstone K, Wang C, Qin Q, Ma Y, Ma W. Development of a unified web-based national HIV/AIDS information system in China. Int J Epidemiol 2010;39 Suppl 2(Suppl 2):ii79–89. https://doi.org/10.1093/ije/dyq213
- Footnote 10
-
McGill E, Coulby C, Dam D, Bellos A, McCormick R, Patterson K. Canadian COVID-19 Outbreak Surveillance System: implementation of national surveillance during a global pandemic. Can J Public Health 2023;114(3):358–67. https://doi.org/10.17269/s41997-023-00766-5
- Footnote 11
-
Moore M, Eiseman E, Fisher G, Olmsted SS, Sama PR, Zambrano JA. Harnessing Full Value from the DoD Serum Repository and the Defense Medical Surveillance System. Rand Health Q 2012;2(2):3.
- Footnote 12
-
Morse SS. Public health surveillance and infectious disease detection. Biosecur Bioterror 2012;10(1):6–16. https://doi.org/10.1089/bsp.2011.0088
- Footnote 13
-
Price JR, Mookerjee S, Dyakova E, Myall A, Leung W, Weiße AY, Shersing Y, Brannigan ET, Galletly T, Muir D, Randell P, Davies F, Bolt F, Barahona M, Otter JA, Holmes AH. Development and Delivery of a Real-time Hospital-onset COVID-19 Surveillance System Using Network Analysis. Clin Infect Dis 2021;72(1):82–9. https://doi.org/10.1093/cid/ciaa892
- Footnote 14
-
Sullivan PS, Woodyatt CR, Kouzouian O, Parrish KJ, Taussig J, Conlan C, Phillips H. America’s HIV Epidemic Analysis Dashboard: Protocol for a Data Resource to Support Ending the HIV Epidemic in the United States. JMIR Public Health Surveill 2022;8(2):e33522. https://doi.org/10.2196/33522
- Footnote 15
-
Cohen SM, Gray KM, Ocfemia MC, Johnson AS, Hall HI. The status of the National HIV Surveillance System, United States, 2013. Public Health Rep 2014;129(4):335–41. https://doi.org/10.1177/003335491412900408
- Footnote 16
-
Hood JE, Katz DA, Bennett AB, Buskin SE, Dombrowski JC, Hawes SE, Golden MR. Integrating HIV Surveillance and Field Services: Data Quality and Care Continuum in King County, Washington, 2010–2015. Am J Public Health 2017;107(12):1938–43. https://doi.org/10.2105/AJPH.2017.304069
- Footnote 17
-
Brandwagt DA, van der Ende A, Ruijs WL, de Melker HE, Knol MJ. Evaluation of the surveillance system for invasive meningococcal disease (IMD) in the Netherlands, 2004-2016. BMC Infect Dis 2019;19(1):860. https://doi.org/10.1186/s12879-019-4513-2
- Footnote 18
-
Buchacz K, Frazier EL, Hall HI, Hart R, Huang P, Franklin D, Hu X, Palella FJ, Chmiel JS, Novak RM, Wood K, Yangco B, Armon C, Brooks JT, Skarbinski J. A Matter of Perspective: Comparison of the Characteristics of Persons with HIV Infection in the United States from the HIV Outpatient Study, Medical Monitoring Project, and National HIV Surveillance System. Open AIDS J 2015;9:123–33. https://doi.org/10.2174/1874613601509010123
- Footnote 19
-
Chapman DA, Ford N, Tlusty S, Bodurtha JN. Evolution of an integrated public health surveillance system. J Registry Manag 2011;38(1):15–23.
- Footnote 20
-
Goller JL, Guy RJ, Gold J, Lim MS, El-Hayek C, Stoove MA, Bergeri I, Fairley CK, Leslie DE, Clift P, White B, Hellard ME. Establishing a linked sentinel surveillance system for blood-borne viruses and sexually transmissible infections: methods, system attributes and early findings. Sex Health 2010;7(4):425–33. https://doi.org/10.1071/SH09116
- Footnote 21
-
Hall JA, Harris RJ, Zaidi A, Woodhall SC, Dabrera G, Dunbar JK. HOSTED-England’s Household Transmission Evaluation Dataset: preliminary findings from a novel passive surveillance system of COVID-19. Int J Epidemiol 2021;50(3):743–52. https://doi.org/10.1093/ije/dyab057
- Footnote 22
-
Jeong D, Kang HY, Kim J, Lee H, Yoo BN, Kim HS, Choi H. Cohort Profile: Korean Tuberculosis and Post-Tuberculosis Cohort Constructed by Linking the Korean National Tuberculosis Surveillance System and National Health Information Database. J Prev Med Public Health 2022;55(3):253–62. https://doi.org/10.3961/jpmph.21.635
- Footnote 23
-
Jones J, Gastellu-Etchegorry M, Stenz FK, Baudon C, Bloem SJ, Bondonneau M. Epidemiology, surveillance and control of infectious diseases in the European overseas countries and territories, 2011. Eurosurveillance 2011;16(29):19923. https://researchportal.ukhsa.gov.uk/en/publications/epidemiology-surveillance-and-control-of-infectious-diseases-in-t
- Footnote 24
-
Jung JK, Feinstein SG, Palma Lazgare L, Macleod JS, Arrandale VH, McLeod CB, Peter A, Demers PA. Examining lung cancer risks across different industries and occupations in Ontario, Canada: the establishment of the Occupational Disease Surveillance System. Occup Environ Med 2018;75(8):545–52. https://doi.org/10.1136/oemed-2017-104926
- Footnote 25
-
Kliewer EV, Demers AA, Brisson M, Severini A, Lotocki R, Elias B, Hammond G, Wurtak G; Manitoba HPV Research Group. The Manitoba human papillomavirus vaccine surveillance and evaluation system. Health Rep 2010;21(2):37–42. https://www150.statcan.gc.ca/n1/en/pub/82-003-x/2010002/article/11153-eng.pdf?st=lHi899S7
- Footnote 26
-
Kraut A, Peters CE, Rydz E, Walld R. Acute myocardial infarctions identified in the Manitoba Occupational Disease Surveillance System: A linkage of worker’s compensation and provincial health data. Am J Ind Med 2023;66(8):679–86. https://doi.org/10.1002/ajim.23505
- Footnote 27
-
Leal J, Gregson DB, Ross T, Flemons WW, Church DL, Laupland KB. Development of a novel electronic surveillance system for monitoring of bloodstream infections. Infect Control Hosp Epidemiol 2010;31(7):740–7. https://doi.org/10.1086/653207
- Footnote 28
-
Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clin Infect Dis 2012;54 Suppl 5(0 5):S391-5. https://doi.org/10.1093/cid/cis243
- Footnote 29
-
Paulukonis ST, Harris WT, Coates TD, Neumayr L, Treadwell M, Vichinsky E, Feuchtbaum LB. Population based surveillance in sickle cell disease: methods, findings and implications from the California registry and surveillance system in hemoglobinopathies project (RuSH). Pediatr Blood Cancer 2014;61(12):2271–6. https://doi.org/10.1002/pbc.25208
- Footnote 30
-
Pierce AB, El-Hayek C, McCarthy D, Armishaw J, Watson K, Wilkinson A, Price B, Wright EJ, Hoy JF, Stoové MA. Comparing non-occupational post-exposure prophylaxis drug regimens for HIV: insights from a linked HIV surveillance system. Sex Health 2017;14(2):179–87. https://doi.org/10.1071/SH16132
- Footnote 31
-
Schmidt R, Carson PJ, Jansen RJ. Resurgence of Syphilis in the United States: An Assessment of Contributing Factors. Infect Dis (Auckl) 2019;12:1178633719883282. https://doi.org/10.1177/1178633719883282
- Footnote 32
-
Shaw K, Coleman D, O’Sullivan M, Stephens N. Public health policies and management strategies for genital Chlamydia trachomatis infection. Risk Manag Healthc Policy 2011;4:57–65. https://doi.org/10.2147/RMHP.S12710
- Footnote 33
-
Tanaka T, Oshima K, Kawano K, Tashiro M, Tanaka A, Fujita A, Tsukamoto M, Yasuoka A, Teruya K, Izumikawa K. Nationwide surveillance of AIDS-defining illnesses among HIV patients in Japan from 1995 to 2017. PLoS One 2021;16(8):e0256452. https://doi.org/10.1371/journal.pone.0256452
- Footnote 34
-
Weston EJ, Kirkcaldy RD, Stenger M, Llata E, Hoots B, Torrone EA. Narrative Review: Assessment of Neisseria gonorrhoeae Infections Among Men Who Have Sex With Men in National and Sentinel Surveillance Systems in the United States. Sex Transm Dis 2018;45(4):243–9. https://doi.org/10.1097/OLQ.0000000000000740
- Footnote 35
-
World Health Organisation. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva, CH: WHO; 2021. https://iris.who.int/bitstream/handle/10665/341412/9789240027077-eng.pdf?sequence=1
- Footnote 36
-
Wijayasri S, Li YA, Squires SG, Martin I, Demczuk W, Mukhi S. Evaluation of the Enhanced Invasive Pneumococcal Disease Surveillance System (eIPDSS) Pilot Project. Can Commun Dis Rep 2016;42(4):83–8. https://doi.org/10.14745/ccdr.v42i04a02
- Footnote 37
-
Willis SJ, Cocoros NM, Randall LM, Ochoa AM, Haney G, Hsu KK, DeMaria A Jr, Klompas M. Electronic Health Record Use in Public Health Infectious Disease Surveillance, USA, 2018–2019. Curr Infect Dis Rep 2019;21(10):32–5. https://doi.org/10.1007/s11908-019-0694-5
- Footnote 38
-
Dickson N, Lee B, Foster T, Saxton PJ. The first 30 years of HIV in New Zealand: review of the epidemiology. N Z Med J 2015;128(1426):31–48. https://nzmj.org.nz/media/pages/journal/vol-128-no-1426/the-first-30-years-of-hiv-in-new-zealand-review-of-the-epidemiology/39686ba75e-1696475830/the-first-30-years-of-hiv-in-new-zealand-review-of-the-epidemiology.pdf
- Footnote 39
-
Ford MA, Spicer CM, editors. Monitoring HIV Care in the United States: Indicators and Data Systems. National Academies Press 2012. https://www.ncbi.nlm.nih.gov/books/NBK201380/
- Footnote 40
-
Rice BD, Yin Z, Brown AE, Croxford S, Conti S, De Angelis D, Delpech VC. Monitoring of the HIV Epidemic Using Routinely Collected Data: the Case of the United Kingdom. AIDS Behav 2017;21 Suppl 1:83–90. https://doi.org/10.1007/s10461-016-1604-6
- Footnote 41
-
Giorgi Rossi P, Riccardo F, Pezzarossi A, Ballotari P, Dente MG, Napoli C, Chiarenza A, Velasco Munoz C, Noori T, Declich S. Factors Influencing the Accuracy of Infectious Disease Reporting in Migrants: A Scoping Review. Int J Environ Res Public Health 2017;14(7):720. https://doi.org/10.3390/ijerph14070720
- Footnote 42
-
Croxford S, Stengaard AR, Brännström J, Combs L, Dedes N, Girardi E, Grabar S, Kirk O, Kuchukhidze G, Lazarus JV, Noori T, Pharris A, Raben D, Rockstroh JK, Simões D, Sullivan AK, Van Beckhoven D, Delpech VC; EuroTEST HIV Late Diagnosis Definition Working Group. Late diagnosis of HIV: an updated consensus definition. HIV Med 2022;23(11):1202–8. https://doi.org/10.1111/hiv.13425
- Footnote 43
-
Beltran VM, Harrison KM, Hall HI, Dean HD. Collection of social determinant of health measures in U.S. national surveillance systems for HIV, viral hepatitis, STDs, and TB. Public Health Rep 2011;126 Suppl 3(Suppl 3):41–53. https://doi.org/10.1177/00333549111260S309
- Footnote 44
-
Aiello AE, Renson A, Zivich PN. Social Media- and Internet-Based Disease Surveillance for Public Health. Annu Rev Public Health 2020;41(1):101–18. https://doi.org/10.1146/annurev-publhealth-040119-094402
- Footnote 45
-
Coltart CE, Hoppe A, Parker M, Dawson L, Amon JJ, Simwinga M, Geller G, Henderson G, Laeyendecker O, Tucker JD, Eba P, Novitsky V, Vandamme AM, Seeley J, Dallabetta G, Harling G, Grabowski MK, Godfrey-Faussett P, Fraser C, Cohen MS, Pillay D; Ethics in HIV Phylogenetics Working Group. Ethical considerations in global HIV phylogenetic research. Lancet HIV 2018;5(11):e656–66. https://doi.org/10.1016/S2352-3018(18)30134-6
- Footnote 46
-
Molldrem S, Smith AK. Reassessing the Ethics of Molecular HIV Surveillance in the Era of Cluster Detection and Response: Toward HIV Data Justice. Am J Bioeth 2020;20(10):10–23. https://doi.org/10.1080/15265161.2020.1806373
- Footnote 47
-
Moran-Gilad J. Whole genome sequencing (WGS) for food-borne pathogen surveillance and control - taking the pulse. Euro Surveill 2017;22(23):30547. https://doi.org/10.2807/1560-7917.ES.2017.22.23.30547
- Footnote 48
-
Samuel GN, Farsides B. Public trust and ‘ethics review’ as a commodity: the case of Genomics England Limited and the UK’s 100,000 genomes project. Med Health Care Philos 2018;21(2):159–68. https://doi.org/10.1007/s11019-017-9810-1
- Footnote 49
-
Blais C, Jean S, Sirois C, Rochette L, Plante C, Larocque I, Doucet M, Ruel G, Simard M, Gamache P, Hamel D, St-Laurent D, Emond V. Quebec Integrated Chronic Disease Surveillance System (QICDSS), an innovative approach. Chronic Dis Inj Can 2014;34(4):226–35. https://doi.org/10.24095/hpcdp.34.4.06
- Footnote 50
-
Boes L, Houareau C, Altmann D, An der Heiden M, Bremer V, Diercke M, Dudareva S, Neumeyer-Gromen A, Zimmermann R. Evaluation of the German surveillance system for hepatitis B regarding timeliness, data quality, and simplicity, from 2005 to 2014. Public Health 2020;180:141–8. https://doi.org/10.1016/j.puhe.2019.11.012
- Footnote 51
-
Bublitz FM, Oetomo A, Sahu KS, Kuang A, Fadrique LX, Velmovitsky PE, Nobrega RM, Morita PP. Disruptive Technologies for Environment and Health Research: An Overview of Artificial Intelligence, Blockchain, and Internet of Things. Int J Environ Res Public Health 2019;16(20):3847. https://doi.org/10.3390/ijerph16203847
- Footnote 52
-
Campo G, Cegolon L, De Merich D, Fedeli U, Pellicci M, Heymann WC, Pavanello S, Guglielmi A, Mastrangelo G. The Italian National Surveillance System for Occupational Injuries: Conceptual Framework and Fatal Outcomes, 2002–2016. Int J Environ Res Public Health 2020;17(20):7631. https://doi.org/10.3390/ijerph17207631
- Footnote 53
-
Cauchi JP, Borg ML, Džiugytė A, Attard J, Melillo T, Zahra G, Barbara C, Spiteri M, Drago A, Zammit L, Debono J, Souness J, Agius S, Young S, Dimech A, Chetcuti I, Camenzuli M, Borg I, Calleja N, Tabone L, Gauci C, Vassallo P, Baruch J. Digitalizing and Upgrading Severe Acute Respiratory Infections Surveillance in Malta: system Development. JMIR Public Health Surveill 2022;8(12):e37669. https://doi.org/10.2196/37669
- Footnote 54
-
Fan S, Blair C, Brown A, Gabos S, Honish L, Hughes T, Jaipaul J, Johnson M, Lo E, Lubchenko A, Mashinter L, Meurer DP, Nardelli V, Predy G, Shewchuk L, Sosin D, Wicentowich B, Talbot J. A multi-function public health surveillance system and the lessons learned in its development: the Alberta Real Time Syndromic Surveillance Net. Can J Public Health 2010;101(6):454–8. https://doi.org/10.1007/BF03403963
- Footnote 55
-
Johnson I, Hansen A, Bi P. The challenges of implementing an integrated One Health surveillance system in Australia. Zoonoses Public Health 2018;65(1):e229–36. https://doi.org/10.1111/zph.12433
- Footnote 56
-
McKerr C, Lo YC, Edeghere O, Bracebridge S. Evaluation of the national Notifiable Diseases Surveillance System for dengue fever in Taiwan, 2010–2012. PLoS Negl Trop Dis 2015;9(3):e0003639. https://doi.org/10.1371/journal.pntd.0003639
- Footnote 57
-
Ndeikoundam Ngangro N, Pioche C, Vaux S, Viriot D, Durand J, Berat B, Hamdaoui M, Lot F. An Automated Surveillance System (SurCeGGID) for the French Sexually Transmitted Infection Clinics: Epidemiological Monitoring Study. JMIR Form Res 2022;6(10):e31136. https://doi.org/10.2196/31136
- Footnote 58
-
Polemis M, Tryfinopoulou K, Giakkoupi P, Vatopoulos A; WHONET-Greece study group. Eight-year trends in the relative isolation frequency and antimicrobial susceptibility among bloodstream isolates from Greek hospitals: data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance - WHONET-Greece, 2010 to 2017. Euro Surveill 2020;25(34):1900516. https://doi.org/10.2807/1560-7917.ES.2020.25.34.1900516
- Footnote 59
-
Rivière J, Le Strat Y, Hendrikx P, Dufour B. Perceptions and acceptability of some stakeholders about the bovine tuberculosis surveillance system for wildlife (Sylvatub) in France. PLoS One 2018;13(3):e0194447. https://doi.org/10.1371/journal.pone.0194447
- Footnote 60
-
Sáez-López E, Pechirra P, Costa I, Cristóvão P, Conde P, Machado A, Rodrigues AP, Guiomar R. Performance of surveillance case definitions for respiratory syncytial virus infections through the sentinel influenza surveillance system, Portugal, 2010 to 2018. Euro Surveill 2019;24(45):1900140. https://doi.org/10.2807/1560-7917.ES.2019.24.45.1900140
- Footnote 61
-
Severi E, Tavoschi L, Carrillo-Santisteve P, Westrell T, Marrone G, Giesecke J, Lopalco P. Hepatitis A notifications in the EU/EEA, 2010–2019: what can we learn from case reporting to the European Surveillance System? Euro surveillance 2023;28(19):11. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2023.28.19.2200575?crawler=true
- Footnote 62
-
Strobel NA, Bourke J, Leonard H, Richardson A, Edmond KM, McAullay D. Assessing the quality, efficiency and usefulness of the Western Australian population-based Intellectual Disability Exploring Answers (IDEA) surveillance system: a surveillance system evaluation. BMJ Open 2019;9(10):e026003. https://doi.org/10.1136/bmjopen-2018-026003
- Footnote 63
-
Trifirò G, Coloma PM, Rijnbeek PR, Romio S, Mosseveld B, Weibel D, Bonhoeffer J, Schuemie M, van der Lei J, Sturkenboom M. Combining multiple healthcare databases for postmarketing drug and vaccine safety surveillance: why and how? J Intern Med 2014;275(6):551–61. https://doi.org/10.1111/joim.12159
- Footnote 64
-
Lee LM, Gostin LO. Ethical collection, storage, and use of public health data: a proposal for a national privacy protection. JAMA 2009;302(1):82–4. https://doi.org/10.1001/jama.2009.958
- Footnote 65
-
Sweeney P, Gardner LI, Buchacz K, Garland PM, Mugavero MJ, Bosshart JT, Shouse RL, Bertolli J. Shifting the paradigm: using HIV surveillance data as a foundation for improving HIV care and preventing HIV infection. Milbank Q 2013;91(3):558–603. https://doi.org/10.1111/milq.12018
- Footnote 66
-
Burkom H, Loschen W, Wojcik R, Holtry R, Punjabi M, Siwek M, Lewis S. Electronic Surveillance System for the Early Notification of Community-Based Epidemics (ESSENCE): Overview, Components, and Public Health Applications. JMIR Public Health Surveill 2021;7(6):e26303. https://doi.org/10.2196/26303
- Footnote 67
-
Goldstick J, Ballesteros A, Flannagan C, Roche J, Schmidt C, Cunningham RM. Michigan system for opioid overdose surveillance. Inj Prev 2021;27(5):500–5. https://doi.org/10.1136/injuryprev-2020-043882
- Footnote 68
-
Hargrove SL, Bunn TL, Slavova S, Quesinberry D, Corey T, Ralston W, Singleton MD, Ingram V. Establishment of a comprehensive drug overdose fatality surveillance system in Kentucky to inform drug overdose prevention policies, interventions and best practices. Inj Prev 2018;24(1):60–7. https://doi.org/10.1136/injuryprev-2016-042308
- Footnote 69
-
Marshall BD, Yedinak JL, Goyer J, Green TC, Koziol JA, Alexander-Scott N. Development of a Statewide, Publicly Accessible Drug Overdose Surveillance and Information System. Am J Public Health 2017;107(11):1760–3. https://doi.org/10.2105/AJPH.2017.304007
- Footnote 70
-
Marzano L, Norman H, Sohal B, Hawton K, Mann R. Police-led real-time surveillance system for suspected suicides in Great Britain. BMJ Ment Health 2023;26(1):e300643. https://doi.org/10.1136/bmjment-2022-300643
- Footnote 71
-
Simonsen L, Gog JR, Olson D, Viboud C. Infectious Disease Surveillance in the Big Data Era: Towards Faster and Locally Relevant Systems. J Infect Dis 2016;214 suppl_4:S380–5. https://doi.org/10.1093/infdis/jiw376
- Footnote 72
-
Contoli B, Carrieri P, Masocco M, Penna L, Perra A; PDA Study Group. PASSI d’Argento (Silver Steps): the main features of the new nationwide surveillance system for the ageing Italian population, Italy 2013–2014. Ann Ist Super Sanita 2016;52(4):536–42. https://doi.org/10.4415/ANN_16_04_13
- Footnote 73
-
Crain J, McFaull S, Thompson W, Skinner R, Do MT, Fréchette M, Mukhi S. Status report - The Canadian Hospitals Injury Reporting and Prevention Program: a dynamic and innovative injury surveillance system. Health Promot Chronic Dis Prev Can 2016;36(6):112–7. https://doi.org/10.24095/hpcdp.36.6.02
- Footnote 74
-
Hennenfent A, DelVento V, Davies-Cole J, Johnson-Clarke F. Expanding veterinary biosurveillance in Washington, DC: the creation and utilization of an electronic-based online veterinary surveillance system. Prev Vet Med 2017;138:70–8. https://doi.org/10.1016/j.prevetmed.2017.01.009
- Footnote 75
-
Magee E, Tryon C, Forbes A, Heath B, Manangan L. The National Tuberculosis Surveillance System training program to ensure accuracy of tuberculosis data. J Public Health Manag Pract 2011;17(5):427–30. https://doi.org/10.1097/PHH.0b013e31820f8e43
- Footnote 76
-
Paul JA, Chateau J, Green C, Warda L, Heaman M, Katz A. Evaluating the Manitoba Infant Feeding Database. Canadian Journal of Public Health = Revue Canadienne de Santé Publique. 2019;110(5):649–56. Paul JA, Chateau J, Green C, Warda L, Heaman M, Katz A, Perchuk C, Larocque L, Lee JB, Nickel NC. Evaluating the Manitoba Infant Feeding Database: a Canadian infant feeding surveillance system. Can J Public Health 2019;110(5):649–56. https://doi.org/10.17269/s41997-019-00211-6
- Footnote 77
-
Smith LR, Gibbs D, Wetterhall S, Schnitzer PG, Farris T, Crosby AE, Leeb RT. Public health efforts to build a surveillance system for child maltreatment mortality: lessons learned for stakeholder engagement. J Public Health Manag Pract 2011;17(6):542–9. https://doi.org/10.1097/PHH.0b013e3182126b6b
- Footnote 78
-
Dubiniecki C, Gottschall S, Praught J. Development and formative evaluation of the Canadian Armed Forces Surveillance and Outbreak Management System (CAF SOMS): applications for COVID-19 and beyond. Health Promot Chronic Dis Prev Can 2022;42(3):96–9. https://doi.org/10.24095/hpcdp.42.3.02
- Footnote 79
-
Toutant S, Gosselin P, Bélanger D, Bustinza R, Rivest S. An open source web application for the surveillance and prevention of the impacts on public health of extreme meteorological events: the SUPREME system. Int J Health Geogr 2011;10(1):39. https://doi.org/10.1186/1476-072X-10-39
- Footnote 80
-
Yang L, Weston C, Cude C, Kincl L. Evaluating Oregon’s occupational public health surveillance system based on the CDC updated guidelines. Am J Ind Med 2020;63(8):713–25. https://doi.org/10.1002/ajim.23139
- Footnote 81
-
Zhang J, Ho K. Designing a Surveillance System in Canada to Detect Adverse Interactions Between Traditional Chinese Medicine and Western Medicine: issues and Considerations. Stud Health Technol Inform 2017;234:412–7. https://doi.org/10.3233/978-1-61499-742-9-412
- Footnote 82
-
Ackerman Gulaid L, Kiragu K. Lessons learnt from promising practices in community engagement for the elimination of new HIV infections in children by 2015 and keeping their mothers alive: summary of a desk review. J Int AIDS Soc 2012;15 Suppl 2(Suppl 2):17390. https://doi.org/10.7448/IAS.15.4.17390
- Footnote 83
-
Kodan LR, Verschueren KJ, Boerstra G, Gajadien I, Mohamed RS, Olmtak LD, Mohan SR, Bloemenkamp KW. From Passive Surveillance to Response: Suriname’s Efforts to Implement Maternal Death Surveillance and Response. Glob Health Sci Pract 2021;9(2):379–89. https://doi.org/10.9745/GHSP-D-20-00594
- Footnote 84
-
O’Brien SF, Zou S, Laperche S, Brant LJ, Seed CR, Kleinman SH. Surveillance of transfusion-transmissible infections comparison of systems in five developed countries. Transfus Med Rev 2012;26(1):38–57. https://doi.org/10.1016/j.tmrv.2011.07.001
- Footnote 85
-
Brady M, Shanley A, Hurley C, O’Donnell K, O’Tuathail M, Fitzgerald M, Flynn C, Carson R, Igoe D. Establishment of a national surveillance system to monitor community HIV testing, Ireland, 2018. Ir J Med Sci 2020;189(4):1507–14. https://doi.org/10.1007/s11845-020-02217-3
- Footnote 86
-
Karp BE, Tate H, Plumblee JR, Dessai U, Whichard JM, Thacker EL, Hale KR, Wilson W, Friedman CR, Griffin PM, McDermott PF. National Antimicrobial Resistance Monitoring System: Two Decades of Advancing Public Health Through Integrated Surveillance of Antimicrobial Resistance. Foodborne Pathog Dis 2017;14(10):545–57. https://doi.org/10.1089/fpd.2017.2283
- Footnote 87
-
Kebisek J, Maule A, Smith J, Allman M, Marquez A, McCabe A, Mafotsing Fopoussi A, Gibson K, Steelman R, Superior M, Ambrose J. Integration of Multiple Surveillance Systems to Track COVID-19 in the U.S. Army Population. Mil Med 2021;usab501. https://doi.org/10.1093/milmed/usab501
- Footnote 88
-
Mohammed H, Hughes G, Fenton KA. Surveillance systems for sexually transmitted infections: a global review. Curr Opin Infect Dis 2016;29(1):64–9. https://doi.org/10.1097/QCO.0000000000000235
- Footnote 89
-
Zhang L, Chow EP, Zhang J, Jing J, Wilson DP. Describing the Chinese HIV surveillance system and the influences of political structures and social stigma. Open AIDS J 2012;6:163–8. https://doi.org/10.2174/1874613601206010163
- Footnote 90
-
Petty-Saphon N, Brady M, Cullen G, Cooney F, Downes P, Doyle S, Holder P, Lyons F, Igoe D. A proposal for changes to the European Union syphilis surveillance case definition using evidence from evaluations in Ireland. Euro Surveill 2019;24(45):1900311. https://doi.org/10.2807/1560-7917.ES.2019.24.45.1900311
- Footnote 91
-
Trepka MJ, Sheehan DM, Fennie KP, Niyonsenga T, Lieb S, Maddox LM. Completeness of HIV reporting on death certificates for Floridians reported with HIV infection, 2000–2011. AIDS Care 2016;28(1):98–103. https://doi.org/10.1080/09540121.2015.1069786
- Footnote 92
-
Abecasis AB, Pingarilho M, Vandamme AM. Phylogenetic analysis as a forensic tool in HIV transmission investigations. AIDS 2018;32(5):543–54. https://doi.org/10.1097/QAD.0000000000001728
- Footnote 93
-
Condell O, Gubbels S, Nielsen J, Espenhain L, Frimodt-Møller N, Engberg J, Møller JK, Ellermann-Eriksen S, Schønheyder HC, Voldstedlund M, Mølbak K, Kristensen B. Corrigendum to ‘Automated surveillance system for hospital-acquired urinary tract infections in Denmark’ [Journal of Hospital Infection 93 (2016) 290–296]. J Hosp Infect 2016;94(4):410. https://doi.org/10.1016/j.jhin.2016.09.001
- Footnote 94
-
Fritsch A, Schweiger B, Biere B. Influenza C virus in pre-school children with respiratory infections: retrospective analysis of data from the national influenza surveillance system in Germany, 2012 to 2014. Euro Surveill 2019;24(10):1800174. https://doi.org/10.2807/1560-7917.ES.2019.24.10.1800174
- Footnote 95
-
Hoppe T, McClelland A, Pass K. Beyond criminalization: reconsidering HIV criminalization in an era of reform. Curr Opin HIV AIDS 2022;17(2):100–5. https://doi.org/10.1097/COH.0000000000000715
- Footnote 96
-
Leitner T, Romero-Severson E. Phylogenetic patterns recover known HIV epidemiological relationships and reveal common transmission of multiple variants. Nat Microbiol 2018;3(9):983–8. https://doi.org/10.1038/s41564-018-0204-9
- Footnote 97
-
Scaduto DI, Brown JM, Haaland WC, Zwickl DJ, Hillis DM, Metzker ML. Source identification in two criminal cases using phylogenetic analysis of HIV-1 DNA sequences. Proc Natl Acad Sci USA 2010;107(50):21242–7. https://doi.org/10.1073/pnas.1015673107
- Footnote 98
-
Asburry A, Blatt M. Improving Childhood Lead Poisoning Surveillance and Data Management in Arizona Through an Evaluation of the Transition to a New Surveillance System. J Public Health Manag Pract. 2019;25 Suppl 1, Lead Poisoning Prevention:S58–S62. https://doi.org/10.1097/PHH.0000000000000873
- Footnote 99
-
Dawson G, Gilmour R, Tobin S, Travaglia J. Strengthening public health systems: assessing the attributes of the NSW influenza surveillance system. Public Health Res Pract 2016;26(2):2621621. https://doi.org/10.17061/phrp2621621
- Footnote 100
-
Berezowski J, De Balogh K, Dórea FC, Ruegg S, Broglia A, Zancanaro G, Gervelmeyer A; European Food Safety Authority (EFSA). Coordinated surveillance system under the One Health approach for cross-border pathogens that threaten the Union - options for sustainable surveillance strategies for priority pathogens. EFSA J 2023;21(3):e07882. https://doi.org/10.2903/j.efsa.2023.7882
- Footnote 101
-
Huot C, Paradis A, Hammond-Collins K, Bélair MA, Villeneuve J, Brousseau N, Goupil-Sormany I, Riffon J. A public health enhanced surveillance system for a mass gathering event. Can Commun Dis Rep 2019;45(7/8):212–24. https://doi.org/10.14745/ccdr.v45i78a05
- Footnote 102
-
Schönfeld V, Diercke M, Gilsdorf A, Eckmanns T, Walter J. Evaluation of the statutory surveillance system for invasive MRSA infections in Germany, 2016–2017. BMC Public Health 2018;18(1):1063. https://doi.org/10.1186/s12889-018-5971-y
- Footnote 103
-
Schwartz DL, Stewart A, Harris L, Ozdenerol E, Thomas F, Johnson KC, Davis R, Shaban-Nejad A. The Memphis Pandemic Health Informatics System (MEMPHI-SYS)-Creating a Metropolitan COVID-19 Data Registry Linked Directly to Community Testing to Enhance Population Health Surveillance. Disaster Med Public Health Prep 2022;17:e326. https://doi.org/10.1017/dmp.2022.284
- Footnote 104
-
Takla A, Velasco E, Benzler J. The FIFA Women’s World Cup in Germany 2011--a practical example for tailoring an event-specific enhanced infectious disease surveillance system. BMC Public Health 2012;12(1):576. https://doi.org/10.1186/1471-2458-12-576
- Footnote 105
-
Ehlman DC, Haileyesus T, Lee R, Ballesteros MF, Yard E. Evaluation of the National Electronic Injury Surveillance System - All injury program’s self-directed violence data, United States, 2018. J Safety Res 2021;76:327–31. https://doi.org/10.1016/j.jsr.2020.12.002
- Footnote 106
-
Fedorowicz J, Gogan JL. Reinvention of interorganizational systems: A case analysis of the diffusion of a bio-terror surveillance system. Inf Syst Front 2010;12(1):81–95. https://doi.org/10.1007/s10796-009-9167-y
- Footnote 107
-
Jermacane D, Coope CM, Ironmonger D, Cleary P, Muller-Pebody B, Hope R, Hopkins S, Puleston R, Freeman R, Hopkins KL, Johnson AP, Woodford N, Oliver I. An evaluation of the electronic reporting system for the enhanced surveillance of carbapenemase-producing Gram-negative bacteria in England. J Hosp Infect 2019;102(1):17–24. https://doi.org/10.1016/j.jhin.2019.01.005
- Footnote 108
-
Wong BL, Maaß L, Vodden A, van Kessel R, Sorbello S, Buttigieg S, Odone A; European Public Health Association (EUPHA) Digital Health Section. The dawn of digital public health in Europe: implications for public health policy and practice. Lancet Reg Health Eur 2022;14:100316. https://doi.org/10.1016/j.lanepe.2022.100316
- Footnote 109
-
Halliday LE, Peek MJ, Ellwood DA, Homer C, Knight M, McLintock C, Jackson-Pulver L, Sullivan EA. The Australasian Maternity Outcomes Surveillance System: an evaluation of stakeholder engagement, usefulness, simplicity, acceptability, data quality and stability. Aust N Z J Obstet Gynaecol 2013;53(2):152–7. https://doi.org/10.1111/ajo.12020
- Footnote 110
-
Kunze M, Banović P, Bogovič P, Briciu V, Čivljak R, Dobler G, Hristea A, Kerlik J, Kuivanen S, Kynčl J, Lebech AM, Lindquist L, Paradowska-Stankiewicz I, Roglić S, Smíšková D, Strle F, Vapalahti O, Vranješ N, Vynograd N, Zajkowska JM, Pilz A, Palmborg A, Erber W. Recommendations to Improve Tick-Borne Encephalitis Surveillance and Vaccine Uptake in Europe. Microorganisms 2022;10(7):1283. https://doi.org/10.3390/microorganisms10071283
- Footnote 111
-
Pratt RH, Manangan LP, Cummings CN, Langer AJ. Noncountable Tuberculosis Case Reporting, National Tuberculosis Surveillance System, United States, 2010-2014. Public Health Rep 2020;135(1):18–24. https://doi.org/10.1177/0033354919884302
- Footnote 112
-
German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN; Guidelines Working Group Centers for Disease Control and Prevention (CDC). Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep 2001;50 RR-13:1–35.
- Footnote 113
-
Satcher Johnson A, Peruski A, Oster AM, Balaji A, Siddiqi AE, Sweeney P, Hernandez AL. Enhancements to the National HIV Surveillance System, United States, 2013–2023. Public Health Rep 2024;139(6):654–61. https://doi.org/10.1177/00333549241253092
- Footnote 114
-
Public Health Agency of Canada. Pan-Canadian Health Data Strategy. Toward a world-class health data system. Ottawa, ON: PHAC; 2022. https://www.canada.ca/content/dam/phac-aspc/documents/corporate/mandate/about-agency/external-advisory-bodies/list/pan-canadian-health-data-strategy-reports-summaries/expert-advisory-group-report-03-toward-world-class-health-data-system/expert-advisory-group-report-03-toward-world-class-health-data-system.pdf
- Footnote 115
-
Lin CY, Loyola-Sanchez A, Hurd K, Ferucci ED, Crane L, Healy B, Barnabe C. Characterization of indigenous community engagement in arthritis studies conducted in Canada, United States of America, Australia and New Zealand. Semin Arthritis Rheum 2019;49(1):145–55. https://doi.org/10.1016/j.semarthrit.2018.11.009
- Footnote 116
-
Tan YR, Agrawal A, Matsoso MP, Katz R, Davis SL, Winkler AS, Huber A, Joshi A, El-Mohandes A, Mellado B, Mubaira CA, Canlas FC, Asiki G, Khosa H, Lazarus JV, Choisy M, Recamonde-Mendoza M, Keiser O, Okwen P, English R, Stinckwich S, Kiwuwa-Muyingo S, Kutadza T, Sethi T, Mathaha T, Nguyen VK, Gill A, Yap P. A call for citizen science in pandemic preparedness and response: beyond data collection. BMJ Glob Health 2022;7(6):e009389. https://doi.org/10.1136/bmjgh-2022-009389
- Footnote 117
-
Public Health Agency of Canada. Best Brains Exchange what we heard report: A vision for the future of public health surveillance in Canada by 2030. Ottawa, ON: PHAC; 2024. https://www.canada.ca/en/public-health/services/publications/science-research-data/best-brains-exchange-what-we-heard-report-vision-future-surveillance-2030.html
Appendix
| Article | Inclusion criteria not met | Rationale for inclusion |
|---|---|---|
| Kodan (2021) Footnote 83 | Study location in: North America, Australia, New Zealand, United Kingdom, Western Europe, Europe, Japan, Singapore, South Korea, Taiwan, Malaysia This study was conducted in Suriname. |
This article was included as it outlined the development and operation of a working group overseeing the assignment of tasks and clearly delineating roles and responsibilities. This is relevant during stakeholder collaboration during a potential renewal. |
| Mao (2010) Footnote 9 | Study location in: North America, Australia, New Zealand, United Kingdom, Western Europe, Europe, Japan, Singapore, South Korea, Taiwan, Malaysia This study was conducted in China. |
This article was directly related to HIV surveillance and detailed the development of a national web-based HIV/AIDS information system based on real-time data. This is relevant to surveillance structure methodology and infrastructure during a potential renewal. |
| Zhang (2012) Footnote 89 | Study location in: North America, Australia, New Zealand, United Kingdom, Western Europe, Europe, Japan, Singapore, South Korea, Taiwan, Malaysia This study was conducted in China. |
This article had discussed the effect of social stigma and political structures on HIV surveillance in China and highlighted the importance of centralized data collection. This is relevant to data collection during a potential renewal. |
| Lee (2009) Footnote 64 | Published on or after January 1, 2010 This article was published in 2009. |
This article was a commentary in the United States proposing a national privacy protection standard for public health data and emphasized the importance of community groups providing input in decision making, standardized data collection and periodic surveillance system evaluation. This is relevant during stakeholder collaboration during a potential renewal. |
Additional supplementary information on search strategies and included articles is available upon request. Please contact hass@phac-aspc.gc.ca
