Original quantitative research – Encouraging older adults with pre-frailty and frailty to “MoveStrong”: an analysis of secondary outcomes for a pilot randomized controlled trial

HPCDP Journal Home
Published by: The Public Health Agency of Canada
Date published: June 2022
ISSN: 2368-738X
Submit a manuscript
About HPCDP
Browse
Previous | Table of Contents | Next
Isabel B. Rodrigues, PhDAuthor reference footnote 1; Justin B. Wagler, BScAuthor reference footnote 1; Heather Keller, PhDAuthor reference footnote 1Author reference footnote 2; Lehana Thabane, PhDAuthor reference footnote 3; Zachary J. Weston, MScAuthor reference footnote 4Author reference footnote 5; Sharon E. Straus, MSc, MDAuthor reference footnote 6Author reference footnote 7; Alexandra Papaioannou, MSc, MDAuthor reference footnote 3Author reference footnote 8; Marina Mourtzakis, PhDAuthor reference footnote 1; Jamie Milligan, MDAuthor reference footnote 8; Wanrudee Isaranuwatchai, PhDAuthor reference footnote 6Author reference footnote 9; Desmond Loong, MScAuthor reference footnote 6Author reference footnote 9; Ravi Jain, MScAuthor reference footnote 10; Larry Funnell, BScAuthor reference footnote 10; Angela M. Cheung, MD, PhDAuthor reference footnote 11; Sheila Brien, RN, diploma of public healthAuthor reference footnote 10; Maureen C. Ashe, PhDAuthor reference footnote 12Author reference footnote 13; Lora M. Giangregorio, PhDAuthor reference footnote 1Author reference footnote 2
https://doi.org/10.24095/hpcdp.42.6.02
This article has been peer reviewed.
Author references
Correspondence
Lora M. Giangregorio, Department of Kinesiology and Health Sciences, University of Waterloo, 200 University Ave W., Waterloo, ON N2L 3G1; Tel: 519-888-4567 ext. 46357; email: lora.giangregorio@uwaterloo.ca
Suggested citation
Rodrigues IB, Wagler JB, Keller H, Thabane L, Weston ZJ, Straus SE, Papaioannou A, Mourtzakis M, Milligan J, Isaranuwatchai W, Loong D, Jain R, Funnell L, Cheung AM, Brien S, Ashe MC, Giangregorio LM. Encouraging older adults with pre-frailty and frailty to “MoveStrong”: an analysis of secondary outcomes for a pilot randomized controlled trial. Health Promot Chronic Dis Prev Can. 2022;42(6):238-251. https://doi.org/10.24095/hpcdp.42.6.02
Abstract
Background: This 8-week pilot stepped-wedge randomized controlled trial evaluated the MoveStrong program for teaching adults who have frailty/pre-frailty about balance and functional strength training and sufficient protein intake to prevent falls and improve mobility.
Methods: We recruited individuals aged 60 years and over, with a FRAIL scale score of 1 or higher and at least one chronic condition, who were not currently strength training. The program included 16 exercise physiologist-led hour-long group sessions and two dietitian-led hour-long nutrition sessions. We analyzed secondary outcomes—weight, gait speed, grip strength, physical capacity (fatigue levels), sit-to-stand functioning, dynamic balance health-related quality of life (HRQoL), physical activity levels and protein intake—using a paired t test and a generalized estimating equation (GEE).
Results: Of 44 participants (mean [SD] age 79 [9.82] years), 35 were pre-frail and 9 were frail. At follow-up, participants had significantly improved grip strength (1.63 kg, 95% CI: 0.62 to 2.63); sit-to-stand functioning (2 sit-to-stands, 95% CI: 1 to 3); and dynamic balance (1.68 s, 95% CI: 0.47 to 2.89). There were no significant improvements in gait speed, HRQoL index scores, self-rated health, physical activity levels (aerobic activity and strength training) or protein intake. GEE analysis revealed an interaction between exposure to MoveStrong and gait speed, sit-to-stand functioning, dynamic balance and HRQoL index scores. The total cost to administer the program and purchase equipment was CAD 14 700, equivalent to CAD 377 per participant.
Conclusion: Exploratory analyses suggest MoveStrong exercises may improve gait speed, sit-to-stand functioning, dynamic balance and HRQoL index scores in older individuals who are frail and pre-frail.
Keywords: FRAIL score, exercise, nutrition, complex intervention, RCT, protein intake, balance, functional strength
Highlights
- The MoveStrong program teaches older adults who are pre-frail and frail about balance and functional strength training and sufficient protein intake.
- The program may improve grip strength, sit-to-stand functioning and dynamic balance.
- The program may be associated with improvements in other outcomes, such as health-related quality of life and gait speed.
Introduction
The Canadian 24-Hour Movement Guidelines for adults aged 65 years and older recommend muscle-strengthening and balance-challenging activities at least twice a week.Footnote 1Footnote 2 There is moderate- to high-certainty evidence that functional strength and balance training are crucial for promoting functional independence and mobility and reducing the risk of falls in older adults.Footnote 3Footnote 4Footnote 5Footnote 6
As many as 88% of Canadian adults 65 years and older are not meeting these exercise guidelines.Footnote 7 Furthermore, inadequate nutrition and low protein intake is common among older adults. Initiating exercise when protein intake is insufficient may cause weight loss and limit gains in muscle strength.Footnote 8 The PROT-AGE group recommends individuals 65 years and older consume at least 1.0 to 1.2 grams of protein per kilogram of body weight per day to maintain or regain lean body mass and muscle function.Footnote 9 However, Wijnhoven et al.Footnote 10 found that almost 50% of adults aged 55 years and older consumed less than 1.0 g/kg/d. Lower protein intake is associated with a higher prevalence of frailty.Footnote 11
A major knowledge gap exists in promoting and sustaining programs to increase the uptake of balance and functional strength training as well as protein intake among older adults, particularly individuals who are pre-frail or frail.
Previous complex interventionsFootnote 12 evaluating the implementation of specific types of exercises under real-world conditions for older adults include home-based exercise programs such as the Otago Exercise ProgramFootnote 13Footnote 14 and the Lifestyle-integrated Functional Exercise (LiFE) programFootnote 15, or facility-based exercise programs such as Mi-LiFE, which is a group-based version of the LiFE programFootnote 16. The goal of these three programs is to promote the uptake of balance and functional strength training to prevent falls and manage chronic diseases in older adults.
A meta-analysis found the Otago Exercise Program reduced the number of falls and fall-related injuries (incidence rate ratio [IRR] = 0.65, 95% confidence interval [CI]: 0.57 to 0.75; and IRR = 0.65, 95% CI: 0.53 to 0.81, respectively) compared with the control group.Footnote 17 Similarly, Clemson and colleaguesFootnote 15 found that teaching older adults how to integrate functional strength and balance exercises into daily life activities (the LiFE program) was associated with a reduced fall rate (IRR = 0.69, 95% CI: 0.48 to 0.99) and improvements in static and dynamic balance and sit-to-stand functioning, compared with controls. Yet, there is less evidence on how to effectively implement strength and balance training programs into community-based programs, especially for older adults who are pre-frail or frail.Footnote 16 In addition, it is still unclear which type of program or combination of programs promotes long-term participation in physical activity and encourages older adults to exercise at a frequency and intensity to confer gains.
The aim of this pilot study was to evaluate the feasibility of implementing a balance and functional strength training program, with attention to protein intake, under real-world settings. Our research team collaborated with several stakeholders to create MoveStrong, a program to teach balance and functional strength training with attention to protein intake to older adults who are pre-frail or frail. In a previous manuscript, we describe the feasibility of implementation, the adverse events, program fidelity and the participants’ and providers’ experience with the MoveStrong program.Footnote 18
The aim of this paper is to report on the effects of the MoveStrong program on secondary outcomes such as frailty indicators (i.e. body weight, physical capacity, sit-to-stand functioning, dynamic balance, grip strength and gait speed), health-related quality of life (HRQoL), physical activity levels and protein intake at baseline and follow-up. We also report health care resource utilization and costs at 6 months prior to starting the intervention and at follow-up.
Methods
We conducted this study in accordance with the extension of the CONSORT 2010 reporting guidelines for stepped-wedge cluster randomized trialsFootnote 19 and pilot and feasibility trials.Footnote 20 We also used the TIDieR (Template for Intervention Description and Replication) checklist to promote full and accurate description of the intervention.Footnote 18Footnote 21
Trial design
The study design was an 8-week pilot, assessor-blinded, multisite, closed cohort stepped-wedge randomized controlled trial (RCT). Each site was exposed to the intervention but not at the same time. Before the program began, all sites were randomized to start at different time points, each 3 weeks apart. At regular 3-week intervals (the “steps”), one site crosses from the control group to the intervention group (Figure 1).Footnote 22 This process continues until all sites have been exposed to the MoveStrong program.
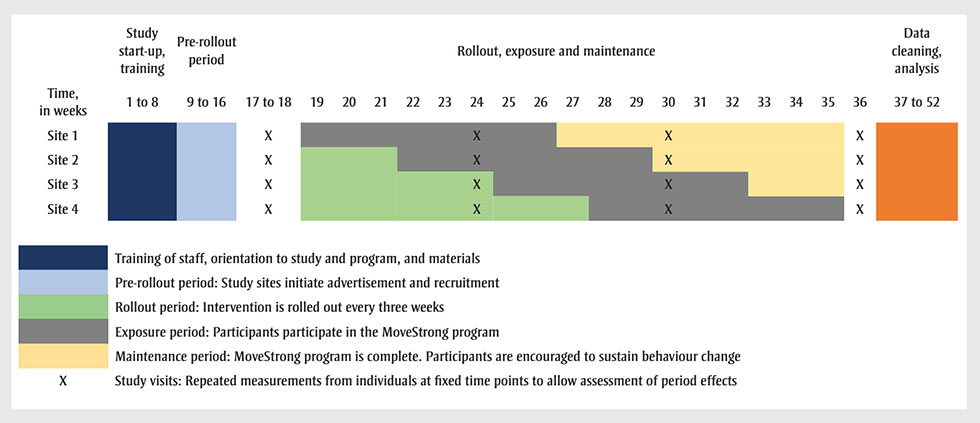
Figure 1 - Text description
| Site | Study start-up, training | Pre-rollout period | Rollout, exposure and maintenance | Data cleaning, analysis | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time, in weeks |
1 to 8 | 9 to 16 | 17 to 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 to 52 |
| Site 1 | Footnote a | Footnote b | Footnote x | Footnote c | Footnote c | Footnote c | Footnote c | Footnote c | Footnote x | Footnote c | Footnote c | Footnote d | Footnote d | Footnote d | Footnote x | Footnote d | Footnote d | Footnote d | Footnote d | Footnote d | Footnote x | Footnote e |
| Site 2 | Footnote a | Footnote b | Footnote x | Footnote f | Footnote f | Footnote f | Footnote c | Footnote c | Footnote x | Footnote c | Footnote c | Footnote c | Footnote c | Footnote c | Footnote x | Footnote d | Footnote d | Footnote d | Footnote d | Footnote d | Footnote x | Footnote e |
| Site 3 | Footnote a | Footnote b | Footnote x | Footnote f | Footnote f | Footnote f | Footnote f | Footnote f | Footnote x | Footnote c | Footnote c | Footnote c | Footnote c | Footnote c | Footnote x | Footnote c | Footnote c | Footnote d | Footnote d | Footnote d | Footnote x | Footnote e |
| Site 4 | Footnote a | Footnote b | Footnote x | Footnote f | Footnote f | Footnote f | Footnote f | Footnote f | Footnote x | Footnote f | Footnote f | Footnote f | Footnote c | Footnote c | Footnote x | Footnote c | Footnote c | Footnote c | Footnote c | Footnote c | Footnote x | Footnote e |
|
||||||||||||||||||||||
Abbreviation: RCT, randomized controlled trial.
We selected the stepped-wedge design because all participants eventually receive the intervention—and hence the benefits of progressive resistance training.Footnote 22 In addition, in a parallel design, participants allocated to the no-exercise control group are more likely to drop out and participant blinding is not possible in exercise trials. The stepped-wedge RCT is also preferred over the traditional parallel RCT when sites are substantially heterogenous (e.g. rural vs. urban populations, community dwelling vs. residential) and the intra-cluster correlation may be high.Footnote 19 Lastly, this design allowed us to determine the feasibility of using a stepped-wedge design for a larger pragmatic trial.
Study setting
We evaluated the program in areas that typically represent real-world practice, and we selected three distinct settings in Ontario: retirement homes/assisted living facilities, community centres and a family health team. We chose one rural site (Sudbury) and three urban sites (Cambridge, Guelph, Kitchener–Waterloo) to ensure diversity in city population, structure and health service. There are differences between urban and rural populations in terms of health-seeking behaviours, health status and health service use, cost and outcomes. In general, rural residents have access to fewer health services and providers than urban residents.Footnote 23
The MoveStrong program was implemented and delivered at a kinesiologist-led clinic partnered with Arbour Trails (retirement home/assisted living and independent living facility, Guelph, site 1); Kinnect to Wellness (physical fitness centre, Sudbury, site 2); the Village of Winston Park (retirement home/assisted living and independent living facility, Kitchener, site 3); and a YMCA that operated at two locations (Cambridge and Kitchener–Waterloo; site 4).
To deliver the exercise program, we contracted exercise physiologists already working at the sites or teaching exercise in the community who had at least one year of experience delivering exercise to older adults. This allowed us to assess the feasibility of real-world implementation rather than have it delivered in a research setting. We also contracted two registered dietitians to deliver education sessions at the northern and southern Ontario sites.
Participants
We included participants if they (1) spoke English or attended with a translator; (2) were aged 60 years or older; (3) had a FRAIL (Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight) scale score of 1 or higher (i.e. a score of 0 indicates robustness, of 1 or 2 indicates pre-frailty and of 3 to 5 indicates frailty)Footnote 24; and (4) had at least one of the following chronic conditions diagnosed by a physician: diabetes, obesity, cancer (other than minor skin cancer), chronic lung disease, cardiovascular disease, congestive heart failure, hypertension, osteoporosis, arthritis, stroke or kidney disease.
We encouraged participants to attend with a caregiver/friend for social or physical support; the caregiver/friend could also complete the screening and assessment process to determine if they were eligible to enrol in the study.
We excluded individuals who (1) were currently doing a similar resistance exercise two or more times per week; (2) were receiving palliative care; (3) could not perform basic activities of daily living; (4) had severe cognitive impairment (e.g. were unable to follow two-step commands or could not explain the research study to the research assistant); (5) planned to be away for more than 1 week during the trial; or (6) had absolute exercise contraindications. We determined absolute exercise contraindications using the American College of Sports Medicine guidelines.Footnote 25 We did not exclude individuals who were participating in regular aerobic physical activity.
Recruitment and randomization
We recruited participants from local primary care practices, retirement homes/assisted living facilities and via advertisement in the local community (e.g. physiotherapy clinics, libraries and churches) using face-to-face techniques, traditional and social media (Facebook and Twitter), posters, flyers and brochures. We set up recruitment booths at the two retirement home/assisted living facility sites. Because of the delay between recruitment and randomization, we decided a priori that participants who dropped out prior to randomization could be replaced up until the start of the intervention.
A biostatistician, independent of the study, created a computer-generated randomization sequence to randomize sites to start the program at one of four start times, each 3 weeks apart. A co-investigator (MCA) kept the randomization sequence concealed, communicating it to all sites after randomization. Each site was assigned to receive the intervention at calendar weeks 19, 22, 25 or 28 (see Figure 1); participants who received the intervention during later weeks were asked to continue their usual activities until the start of the program.
Intervention
Exercise program
The MoveStrong exercise program includes functional strength training movements for older adults of varying abilities, using minimal equipment. Each exercise was informed by the GLA:D program for arthritisFootnote 26, BoneFitFootnote 27 and meta-analyses on resistance exercise and fall prevention for older adultsFootnote 6Footnote 28Footnote 29Footnote 30Footnote 31. We sought input from representatives from the YMCAs of Cambridge and Kitchener–Waterloo, Community Support Connections, and Osteoporosis Canada, as well as patient advocates. To promote personal relevance, the exercises are aligned with functional movements such as lunging/stepping, reaching, squatting, pulling, lifting and carrying, and pushing.
Participants were prescribed one exercise from each category: stepping (e.g. foot stomps, heel drops); step-up or leg extension (e.g. stationary lunge, seated leg extension, step-up); reach (e.g. resisted thoracic extension, back to wall shoulder flexion, shoulder press); squat (e.g. squat, sit-to-stand); pull (e.g. elastic band row, pull apart, bent-over dumbbell row); hinge with or without carry (e.g. seated back extension, glute bridge, wall tap hip hinge, weighted hinge, hinge plus weighted carry); and push (e.g. resisted chest press, wall push-up, counter/table push-up).
Each site received a standardized toolkit with materials for participant workbooks and a trainer manual. The trainer manual provided guidance on how to deliver the workshop, select and progress exercises, adapt exercises for common impairments, cueing tips and discussion topics. The research team met with the exercise physiologists at each site for one to two hours to demonstrate how to deliver the MoveStrong program and to review the manual. Each exercise physiologist was advised to use informal assessments of multiple repetitions maximum and a repetition in reserve strategy to guide exercise selection and progression. We instructed the exercise physiologist to increase the difficulty of the movement if participants could perform more than eight repetitions.
Exercise physiologists could decide how to deliver the program in their setting—as an exercise class or by allowing participants to work through the program on their own or in stations.
Each participant received a one-to-one session with an exercise physiologist (not blinded to site allocation) who selected a starting level and variations for each functional movement, intensity, and number of repetitions and sets. The participant workbooks included pictures and instructions for each exercise so that the participants could practise and exercise at home or elsewhere; each participant received their workbooks during the one-on-one session with the exercise physiologist. Participants attended physiologist-led group exercise sessions (1 exercise physiologist to ≤6 participants) twice a week for 8 weeks. Program components included a warm-up (5 minutes), the exercise program (50 minutes) and cool-down (5 minutes), during which the exercise physiologist led a group discussion on when and where participants could practise the exercise(s), at home or in a setting of choice.
During the first 2 weeks, the focus was on form rather than on intensity. Exercise difficulty, resistance or volume (up to 3 sets, up to 8 repetitions) was increased over time, with a target intensity of a maximum of eight repetitions. We did not formally assess one-repetition maximum.
Nutrition education
The nutrition program included two components: a nutrition education booklet; and two dietitian-led hour-long group seminars to answer questions and discuss topics related to protein intake. The dietitians were not blinded to allocation. The booklet and seminars reviewed the cost of preparing high-protein foods; how and why to spread protein intake throughout the day; how much protein was in the participant’s usual diet and how much was recommended; low-cost options to add protein to meals; easy-to-consume protein-rich snacks with minimal preparation; high quality protein supplements (e.g. rapidly digested, high leucine-content foods, such as whey); and how to prioritize high-protein choices in retirement home/assisted living facility restaurants. During each seminar, the dietitian provided samples of protein-rich snacks. Seminars were held during weeks 2 and 5 to allow time to review material, revisit topics and address questions.
We recommended 1.2 grams of protein per kilogram of body weight per day and 20 to 30 grams of protein per meal.Footnote 8Footnote 32 As protein intake may be influenced by living conditions (e.g. living in a retirement home/assisted living facility vs. independent living), the dietitian reviewed methods on how to select high-protein options from the retirement home restaurant menu. For example, residents learned how to estimate the amount of protein in common foods listed on the menu (e.g. 85 grams salmon has 19 grams of protein, or 1 cup of 2% milk has 8 grams of protein).
Outcomes
Frailty indicators
The Fried Frailty Index guided the selection of frailty indicators. The indicators included change in body weight, gait speed, physical capacity, physical activity (fatigue) levels and handgrip strength.Footnote 33 We measured body weight using a calibrated scale at baseline (study visit 1) and follow-up (study visit 4).
We assessed gait speed using the 10-metre walk testFootnote 34; physical capacity (i.e. fatigue levels) using two questions from the Center for Epidemiologic Studies Depression Scale (“I felt that everything I did was an effort” and “I could not get going”)Footnote 35; and physical activity levels with the physical activity screenFootnote 36. The physical activity screen assesses moderate- to vigorous-intensity aerobic physical activity in minutes per week and strength training in days per week. We did not include the MoveStrong exercise program sessions in our calculation for strength training.
Grip strength of the non-dominant hand was measured in kilograms using a digital Jamar Hand Dynamometer.Footnote 37Footnote 38 Other predictor variables of frailtyFootnote 39 included sit-to-stand functioning, assessed with the 30-second chair–stand testFootnote 40, and dynamic balance, assessed with the four-square step testFootnote 41. All frailty indicators, except body weight, were measured at baseline (study visit 1), study visit 2, study visit 3 and follow-up (study visit 4).
Health-related quality of life
We assessed HRQoL using the EuroQol Group 5 Dimension 5 Level (EQ-5D-5L) questionnaire.Footnote 42 The first part of the questionnaire comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and each dimension has five levels (no problems, slight problems, moderate problems, severe problems, extreme problems). Scores range from 0.9489 (highest reported quality of life) to 0.2041 (lowest reported quality of life).Footnote 43 The second part of the questionnaire records the participant’s self-rated health on a vertical visual analog scale, where the endpoints are labelled “The best health you can imagine” (score of 100) to “The worst health you can imagine” (score of 0).
Protein intake
We used the 2018 Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool to conduct interviewer-administered diet recalls. We collected three-day food records (two weekdays and one weekend) to capture an accurate description of each participant’s typical daily diet. The ASA24 Dietary Assessment Tool is a free web-based instrument that enables highly standardized multipass recall to obtain detailed information about dietary intake using multiple probes and reminders to enhance recall.Footnote 44 The tool generates a “total calorie consumption” across all meals and snacks consumed in a single day and automatically codes carbohydrate, fat, protein and alcohol intake.Footnote 44
Health care resource utilization and costs
We used a health care resource utilization and costs questionnaire to assess direct and indirect costs of health service utilization developed in consultation with two health economists (WI and DL). We collected data on intervention costs and resource use to assess the feasibility of data collection methods for a larger trial. The health care resource utilization and costs questionnaire consists of six direct health care service categories: (1) primary care visits; (2) emergency department or specialist visits; (3) hospital days; (4) other health care provider visits (e.g. nurse, physiotherapist, occupational therapist); (5) adverse events such as falls and fractures; and (6) lab services.
The questionnaire also asks about participants’ out-of-pocket costs, such as over-the-counter medications, supplements or devices, the use of homecare, complementary therapy (e.g. massage therapist, naturopath) and transportation costs. The total cost per person was calculated by multiplying the number of units of service (quantity) by the unit cost (price). We reported costs using the 2020 Canadian dollar (CAD).
We obtained costs for implementing the program from financial records. Because the costs associated with developing the program were incurred before the trial, these are not included. We also did not include the costs of evaluating the program or of recruiting the exercise physiologist, because in many instances, the relevant organization had existing staff that could deliver the program. We also did not put a value on the time participants spent exercising or attending the nutrition sessions as we assumed these activities were done in their leisure time.
Sample size
We selected a recruitment rate of 10 participants at each site because of the proposed class ratio of one instructor to five participants. Having 10 participants allowed us to determine the feasibility of delivering two nutrition sessions and two groups of exercise sessions at each site.Footnote 18 We allowed sites to over-recruit by one or two people.
Data safety monitoring committee
A physiotherapist, a physician and a biostatistician, not involved in the trial, reviewed potential adverse events after three sites completed the program and provided guidance for a future trial. There were no interim analyses and there were no guidelines on stopping the pilot trial.
Statistical analyses
Demographic, health care resource utilization and cost data were reported using means and standard deviations or as 95% confidence intervals for continuous data, and as a count and percentage for categorical outcomes. We conducted a paired t test (α = 0.05) on secondary outcomes at baseline and follow-up using imputed data. We used multiple imputation procedures to impute the missing data values (fully conditional specification method, number of imputations = 5, maximum iterations = 25). We used baseline data for sites 1 and 2 at weeks 17 to 18 and for sites 3 and 4 at week 24. Follow-up data for sites 1 and 2 were at week 30 and for sites 3 and 4 at week 36 (see Figure 1). To model the interaction between exposure to the MoveStrong program and site on secondary outcomes we applied a generalized estimating equation (GEE). In our protocol, we originally planned to do linear regression, but revised our analysis plan to better account for clustering by site.Footnote 45 We had planned to do a subgroup analysis with and without caregiver or friend participation but not enough caregivers/friends participated.
For protein intake at baseline, we only collected baseline measures for 40 individuals.
We calculated health care resource utilization by multiplying unit costs from the 2015 Common Billing Codes for family physicians and the 2020 Ministry of Health Ontario Health Insurance Plan Laboratories and Genetics Branch to each resource to calculate direct medical costs. We estimated specialist visits at CAD 300.00 and allied health professional visits at CAD 61.25Footnote 46; if data were missing, we assumed the value to have no associated costs and did not include it.
Some participants did not consent to measuring their body weight; we used the average body weight for their sex to estimate their protein and energy (kcal/kg/day) intakes.
Significance (p values) was reported to three decimal places, with statistical significance defined as p < 0.05. No correction (e.g. Bonferroni correction) for multiple testing was made because of the exploratory nature of the analyses.
All analyses were performed using SPSS Statistics for Windows version 27 (IBM Corp., Armonk, NY, US).
Ethics
We obtained ethics approval from University of Waterloo Ethics committee (#31752).
Results
We screened 75 individuals for eligibility and enrolled 44 participants prior to randomization (Table 1, Figure 2); only 39 individuals started the intervention. One participant attended with a caregiver, but the caregiver did not enrol in the program.
Mean (SD) age was 79 (9.82) years); 35 participants were pre-frail, and 9, frail.
| Characteristics | Site 1: Arbour TrailsFootnote a (n = 9) |
Site 2: Kinnect to WellnessFootnote b (n = 15) |
Site 3: Village of Winston ParkFootnote c (n = 9) |
Site 4: YMCAFootnote d (n = 11) |
|---|---|---|---|---|
| Mean age (SD), years | 78 (11.50) | 81 (5.39) | 84 (8.80) | 72 (7.71) |
| Mean height (SD), cm | 161 (10.89) n = 7 |
156 (26.18) | 160 (7.63) n = 7 |
161 (7.71) |
| Mean weight (SD), kg | 72 (19.17) n = 7 |
73 (12.44) | 65 (7.64) n = 8 |
67 (12.80) |
| Body mass index (SD) | 24.96 (3.52) n = 7 |
29.17 (4.27) | 24.99 (4.12) n = 7 |
25.65 (4.56) |
| Female sex, n (%) | 7 (78) | 10 (67) | 7 (78) | 10 (91) |
| Ethnicity, n (%) | ||||
| White | 8 (89) | 15 (100) | 8 (89) | 9 (82) |
| South Asian | 0 (0) | 0 (0) | 1 (11) | 2 (18) |
| Middle Eastern | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| Marital status, n (%) | ||||
| Married | 2 (22) | 7 (47) | 4 (44) | 7 (64) |
| Widowed | 4 (44) | 6 (40) | 5 (56) | 2 (18) |
| Single/separated/divorced | 3 (33) | 2 (13) | 0 (0) | 2 (18) |
| Highest level of education, n (%) | ||||
| Middle school | 0 (0) | 5 (33) | 0 (0) | 1 (9) |
| High school | 0 (0) | 8 (53) | 4 (44) | 3 (27) |
| Higher education (college or university) | 9 (100) | 2 (13) | 5 (56) | 7 (64) |
| Employment, n (%) | ||||
| Retired (not working) | 6 (67) | 15 (100) | 9 (100) | 11 (100) |
| Medical leave | 2 (22) | 0 (0) | 0 (0) | 0 (0) |
| Part-time (<40 h/wk) | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| Annual income, CAD | ||||
| <40 000 | 3 (33) | 7 (47) | 3 (33) | 4 (36) |
| 40 000–60 000 | 1 (11) | 5 (33) | 0 (0) | 3 (27) |
| >60 000 | 3 (33) | 0 (0) | 2 (22) | 0 (0) |
| Prefer not to say | 2 (22) | 3 (20) | 4 (44) | 4 (36) |
| Place of residence, n (%) | ||||
| Retirement home, alone | 5 (56) | 1 (7) | 5 (56) | 0 (0) |
| Retirement home, with someone | 0 (0) | 0 (0) | 2 (22) | 0 (0) |
| In the community, alone | 2 (22) | 4 (27) | 1 (11) | 4 (36) |
| In the community, with someone | 2 (22) | 10 (67) | 1 (11) | 7 (64) |
| Visits from friends and family, n (%) | ||||
| Daily | 3 (33) | 9 (60) | 2 (22) | 1 (9) |
| Weekly | 3 (33) | 5 (33) | 7 (78) | 9 (82) |
| Monthly | 2 (22) | 1 (7) | 0 (0) | 1 (9) |
| Yearly | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| Use of homecare in the last 6 months, n (%) | 1 (11) | 1 (7) | 1 (11) | 1 (11) |
| Mean FRAIL scale score (SD) | 2.00 (0.50) | 2.07 (0.96) | 2.11 (0.60) | 1.36 (0.67) |
| FRAIL scale, n (%) | ||||
| Time feeling tired during the past 4 weeks | 5 (56) | 6 (40) | 5 (56) | 7 (64) |
| Difficulty walking up 10 steps without resting | 4 (44) | 7 (47) | 4 (44) | 2 (18) |
| Difficulty walking several hundred yards | 5 (56) | 12 (80) | 8 (89) | 2 (18) |
| ≥5 physician-diagnosed chronic diseases | 3 (33) | 2 (13) | 1 (11) | 0 (0) |
| Weight change >5% in the last 6 months | 3 (33) | 4 (27) | 1 (11) | 4 (36) |
| ≥2 components on the FRAIL scale | 8 (89) | 10 (67) | 8 (89) | 3 (27) |
| ≥3 components on the FRAIL scale | 1 (11) | 5 (33) | 2 (22) | 1 (9) |
| Comorbidities, n (%) | ||||
| Cardiovascular diseases | 4 (44) | 6 (40) | 5 (56) | 2 (18) |
| Hypertension | 8 (89) | 11 (73) | 6 (67) | 4 (36) |
| Respiratory illnesses | 3 (33) | 5 (33) | 2 (22) | 1 (9) |
| Bone disease (osteoporosis) | 4 (44) | 8 (53) | 5 (56) | 6 (55) |
| Joint disease | 5 (56) | 15 (100) | 6 (67) | 5 (45) |
| Type 2 diabetes | 3 (33) | 6 (40) | 2 (22) | 4 (36) |
| Low back pain | 5 (56) | 13 (87) | 4 (44) | 5 (45) |
| Falls and fractures in the last 6 months, n (%) | ||||
| Individuals who fell | 1 (11) | 4 (27) | 1 (11) | 0 (0) |
| Individuals who sustained a fragility fracture | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Use of assistive devices, n (%) | ||||
| Walker | 2 (22) | 0 (0) | 1 (11) | 1 (9) |
| Wheelchair | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| Physical activity screen, n (%) | ||||
| Achieved ≥75 min/wk of vigorous-intensity aerobic physical activity or ≥150 min/wk of moderate-intensity aerobic physical activity | 2 (22) | 1 (7) | 0 (0) | 7 (64) |
Abbreviations: CAD, Canadian dollar; FRAIL, Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight; h, hours; min, minute; SD, standard deviation; wk, week.
|
||||
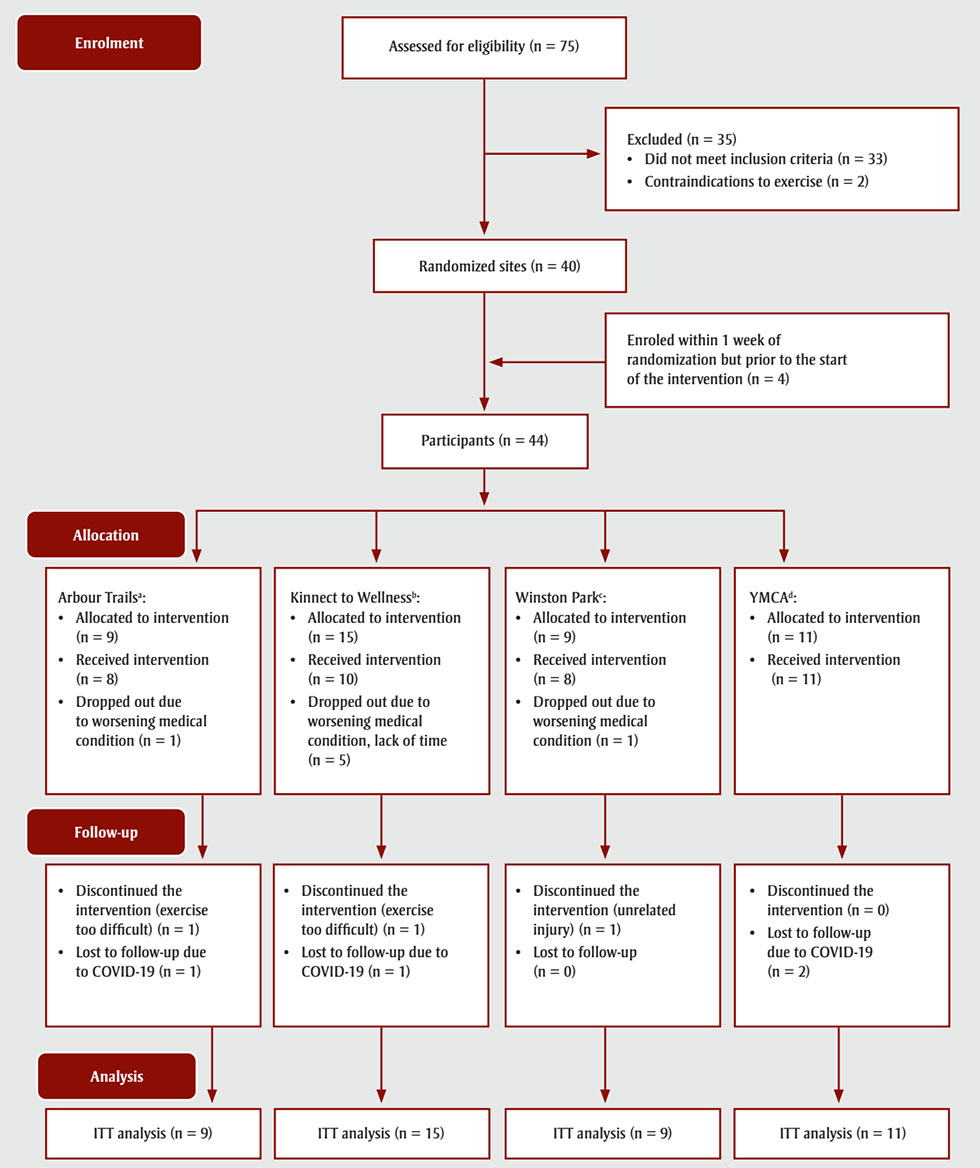
Figure 2 - Text description
This figure depicts the CONSORT flow diagram reporting participant enrolment, allocation, follow-up and analysis in the MoveStrong pilot randomized controlled trial.
In the enrolment phase, n = 75 participants were assessed for eligibility. Of those, n = 35 were excluded because they did not meet inclusion criteria (n = 33) or due to contraindications to exercise (n = 2), resulting in n = 40 participants at randomized sites. An additional 4 participants enroled within 1 week of randomization but prior to the start of the intervention, bringing the total up to 44.
As part of the allocation phase, participants were assigned to a site as follows:
Arbour Trails:
- Allocated to intervention (n = 9)
- Received intervention (n = 8)
- Dropped out due to worsening medical condition (n = 1)
Kinnect to Wellness:
- Allocation to intervention (n = 15)
- Received intervention (n = 10)
- Dropped out due to worsening medical condition, lack of time (n = 5)
Winston Park:
- Allocation to intervention (n = 9)
- Received intervention (n = 8)
- Dropped out due to worsening medical condition (n = 1)
YMCA:
- Allocation to intervention (n = 11)
- Received intervention (n = 11)
The follow-up phase revealed the following about participants from each site:
Arbour Trails:
- Discontinued the intervention (exercise too difficult) (n = 1)
- Lost to follow-up due to COVID-19 (n = 1)
Kinnect to Wellness:
- Discontinued the intervention (exercise too difficult) (n = 1)
- Lost to follow-up due to COVID-19 (n = 1)
Winston Park:
- Discontinued the intervention (unrelated injury) (n = 1)
- Lost to follow-up (n = 0)
YMCA:
- Discontinued the intervention (n = 0)
- Lost to follow-up due to COVID-19 (n = 2)
The analysis phase saw an intention-to-treat analysis as follows:
- Arbour Trails (n = 9)
- Kinnect to Wellness (n = 15)
- Winston Park (n = 9)
- YMCA (n = 11)
Abbreviations: ITT, intention-to-treat; RCT, randomized controlled trial.
a Arbour Trails retirement home/assisted
living and independent living facility, Guelph, Ontario.
b Kinnect to Wellness physical fitness centre,
Sudbury, Ontario.
c Village of Winston Park retirement
home/assisted living and independent living facility, Kitchener, Ontario.
d YMCA operating in two locations, Cambridge,
Ontario, and Kitchener–Waterloo, Ontario.
Frailty indicators
Intention-to-treat analyses revealed a significant difference from baseline to follow-up for grip strength, sit-to-stand functioning and dynamic balance (Table 2). There were no significant differences in body weight, gait speed, physical capacity (fatigue) or physical activity levels at baseline to follow-up.
| Secondary outcomes | Mean values (95% CI) |
Mean change score (95% CI) Paired t test (baseline vs. follow-up) |
||
|---|---|---|---|---|
| Baseline | During the MoveStrong program | Follow-up | ||
| Frailty indicators | ||||
| Body weight, kg | 69.79 (65.92 to 73.66) |
69.80 (65.93 to 73.65) |
69.62 (65.73 to 73.52) |
0.17 (−0.34 to 0.68) |
| Gait speed (10-m walk test), m/s | 1.06 (0.95 to 1.18) |
1.06 (0.95 to 1.16) |
1.12 (1.00 to 1.24) |
0.60 (0.00 to 0.12) |
| Physical capacity (“I felt that everything I did was an effort” on the CES-D) | 0.70 (0.45 to 0.96) |
0.86 (0.53 to 1.20) |
1.00 (0.67 to 1.33) |
−0.30 (−0.65 to 0.06) |
| Physical capacity (“I could not get going” on CES-D) | 0.73 (0.48 to 0.98) |
0.82 (0.49 to 1.15) |
1.00 (0.65 to 1.35) |
−0.27 (−0.63 to 0.08) |
| Grip strength (non-dominant hand), kg | 20.45 (17.95 to 22.95) |
21.82 (18.96 to 24.69) |
22.07 (19.44 to 24.71) |
1.63 (0.62 to 2.63)Footnote * |
| Physical activity screen – aerobic activity, min/wk | 100.00 (49.59 to 150.41) |
150.20 (111.37 to 189.04) |
118.64 (84.22 to 153.05) |
31.25 (−8.50 to 71.00) |
| Physical activity screen – strength training, d/wk | 0.41 (0.03 to 0.79) |
2.18 (1.57 to 2.79) |
1.70 (1.09 to 2.32) |
−1.30 (−2.03 to 0.06) |
| Sit-to-stand functioning (30-s chair–stand test), n | 9.18 (7.73 to 10.63) |
9.70 (8.23 to 11.18) |
11.32 (9.60 to 13.04) |
2.14 (1.07 to 3.20)Footnote * |
| Dynamic balance (FSST), s | 14.86 (13.09 to 16.62) |
14.10 (12.06 to 16.15) |
13.17 (11.49 to 14.87) |
1.68 (0.47 to 2.89)Footnote * |
| HRQoL | ||||
| EQ-5D-5L index score | 0.79 (0.75 to 0.83) |
0.83 (0.80 to 0.85) |
0.82 (0.78 to 0.85) |
−0.02 (−0.06 to 0.01) |
| Self-rated health on the visual analog scale | 71.01 (65.16 to 76.87) |
75.42 (71.30 to 79.54) |
77.10 (72.35 to 81.85) |
−6.09 (−12.43 to 0.26) |
| Protein intake – ASA24 Dietary Assessment Tool | ||||
| Protein, g/d | 69.46 (69.46 to 22.29) |
N/A | 70.88 (54.80 to 77.00) |
1.65 (−4.44 to 7.73) |
| Protein, g/kg/d | 1.01 (0.91 to 1.11) |
N/A | 1.00 (0.91 to 1.09) |
0.01 (−0.07 to 0.10) |
| % Energy from protein | 16.76 (15.80 to 17.70) |
N/A | 17.83 (16.60 to 19.00) |
0.92 (−0.37 to 2.20) |
| Energy, kcal/kg/d | 23.81 (21.40 to 26.30) |
N/A | 22.52 (20.20 to 24.80) |
−0.64 (−1.69 to 0.40) |
Abbreviations: ASA24, Automated Self-Administered 24-Hour [dietary assessment tool]; CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; d, days; EQ-5D-5L, EuroQol Group 5 Dimension 5 Level; FSST, four-square step test; HRQoL, health-related quality of life; kcal, kilocalories; min, minute; N/A, not applicable; wk, week.
|
||||
The GEE analysis (linear response, factor = exposure to MoveStrong by site, covariates = site, within-subject variable = study visit, maximum likelihood estimate, Wald chi-square) suggests a significant interaction for exposure to MoveStrong on the following variables: gait speed (10-metre walk test), sit-to-stand functioning (30-second chair–stand test), dynamic balance (four-square step test) and HRQoL according to the EQ-5D-5L index score (Table 3).
| Secondary outcomes | Estimate of difference-adjusted clustering within a site | 95% CI | p value |
|---|---|---|---|
| Frailty indicators | |||
| Body weight | −2.94 | −6.77 to 0.90 | 0.13 |
| Gait speed (10-m walk test) | 0.15 | 0.06 to 0.24 | <0.05 |
| Physical capacity (“I felt that everything I did was an effort” on the CES-D) | −0.19 | −0.66 to 0.28 | 0.43 |
| Physical capacity (“I could not get going” on CES-D) | −0.277 | −0.71 to 0.15 | 0.21 |
| Grip strength (non-dominant hand) | 1.59 | −0.69 to 3.88 | 0.17 |
| Physical activity screen – aerobic activity | −0.11 | −23.16 to 22.94 | 0.99 |
| Physical activity screen – strength training | −0.11 | −0.42 to 0.21 | 0.51 |
| Sit-to-stand functioning (30-s chair–stand test) | 2.78 | 1.56 to 3.97 | <0.05 |
| Dynamic balance (FSST) | −1.61 | −3.14 to −0.08 | <0.05 |
| HRQoL | |||
| EQ-5D-5L index score | 0.03 | 0.01 to 0.06 | <0.05 |
| Self-rated health on the visual analog scale of the EQ-5D-5L | 2.29 | −1.18 to 5.76 | 0.19 |
| Protein intake (ASA24 Dietary Assessment Tool) | |||
| Protein (g/kg/d) | 1.05 | 0.89 to 1.22 | 0.06 |
| Protein (g/d) | 77.90 | 72.78 to 83.03 | 0.08 |
| Energy | 26.24 | 20.65 to 31.83 | 0.13 |
Abbreviations: ASA24, Automated Self-Administered 24-Hour [dietary assessment tool]; CES-D, Center for Epidemiologic Studies Depression Scale; d, day; EQ-5D-5L, EuroQol Group 5 Dimension 5 Level; FSST, four-square step test; HRQoL, health-related quality of life; RCT, randomized controlled trial. |
|||
GEE analysis indicated there were no interactions for body weight, grip strength, physical activity levels or protein intake. We conducted a similar GEE analysis for physical capacity using an ordinal response and found no interaction for exposure to the MoveStrong program and physical capacity (fatigue levels).
Health-related quality of life
Intention-to-treat analysis revealed no significant difference from baseline to follow-up on the EQ-5D-5L index score and on the self-rated health score using the visual analog scale of the EQ-5D-5L (Table 2); however, GEE analysis indicates there may be an interaction for exposure to the MoveStrong program on EQ-5D-5L index scores (Table 3).
Protein intake
Intention-to-treat analyses of average protein (g/d and g/kg/d) and energy (kcal/kg/d) intake revealed no significant differences from baseline to follow-up (Table 2). The GEE analysis revealed no significant interaction between exposure to the MoveStrong program on energy intake in kcal/kg/d or protein intake in g/d (Table 3).
We found that participants do not consume an equal amount of protein at each meal; the highest amount of protein was consumed at dinner (baseline: 32.60 [13.07] g, n = 33; follow-up: 30.71 [8.55] g, n = 33), and it was also the only meal where the average protein intake was within the recommended range of 20 to 30 g/meal.
After attending the nutrition sessions, participants reported consuming new protein-rich foods (i.e. foods they did not report eating at baseline) including meat (fish, chicken, turkey, pork, beef); dairy (milk, yogurt, cheese); plant-based (whole wheat, rice, quinoa); and others (eggs, seeds, nuts, protein powder). The average protein intake at baseline was 69.46 g/d (95% CI: 69.46 to 22.29; n = 39) or 1.01 g/kg/d (95% CI: 0.91 to 1.11; n = 33) and was above the recommended dietary allowance (RDA; 0.8 g/kg/d). However, 14 participants (35%) had a protein intake below the RDA, while 27 participants (67%) consumed less than our target of 1.2 g/kg/d.
At baseline, the average percentage of energy from protein was within the Acceptable Macronutrient Distribution Range (AMDR) of 10% to 35%.
The average energy intake at baseline was 23.81 kcal/kg/d (95% CI: 21.40 to 26.30; n = 39), which was less than the RDA (30 kcal/kg/d). Of the 40 participants, 28 (70%) had an average energy intake less than the RDA and 20 (50%) consumed less than 21 kcal/kg/d.
Resource use
The total cost to administer the program and purchase equipment at all four sites was CAD 14 700, or CAD 377 per participant. The total direct medical cost during the study was CAD 22 430, while the total indirect medical cost was CAD 21 610. Six weeks prior to starting the intervention, participants reported a direct medical cost of CAD 6148 over six weeks, and of CAD 7389 over six weeks at follow-up. Six weeks prior to starting the intervention, participants reported an indirect medical cost of CAD 6464 over six weeks; after the intervention, this was CAD 5916 over six weeks. The main cost drivers were identified to be physician visits, test procedures and transportation.
Discussion
The main challenge in evaluating complex interventions is in the number of components that act both independently and interdependently.Footnote 12Footnote 47For this reason, Campbell and colleaguesFootnote 12 suggest evaluating complex interventions in several phases. This pilot study is considered part of phase IIFootnote 12 and involves testing the feasibility of delivering the intervention and piloting outcomes for a larger trial.
We piloted several secondary outcomes and found an interaction between participating in the MoveStrong program and gait speed (10-metre walk test), sit-to-stand functioning (30-second chair–stand test), dynamic balance (four-square step test) and HRQoL (EQ-5D-5L index score). We found no interaction between participating in the MoveStrong program and body weight, grip strength, physical capacity (i.e. fatigue levels), self-rated health on the visual analog scale of the EQ-5D-5L and protein intake. Future trials on balance and functional strength training among older adults with frailty or pre-frailty should consider the responsiveness of frailty indicators when selecting study outcomes, such as those reported in our pilot study.
The MoveStrong exercises aim to mimic activities performed in real-life situations. Maintaining adequate strength and balance using functional movements intuitively makes sense for improving physical function and preventing falls because specificity is important in exercise prescription. The efficacy of balance in combination with functional training as types of exercise that can mediate fall risk and mobility impairments has been highlighted in several systematic reviews.Footnote 3Footnote 6Footnote 29Footnote 48 We found that participating in the MoveStrong program may improve activities that involve grip strength, sit-to-stand functioning and dynamic balance.
We also saw improvements in outcomes that were directly related to movements in our exercise program. For example, the 30-second sit-to-stand is a feasible outcome to measure sit-to-stand functioning in the lower limbs, and daily activities that use these muscles include getting up from a chair. Program participants completed two additional sit-to-stands by the end of the study; an increase of two or more repetitions for the 30-second sit-to-stand represents the minimum clinically important difference.Footnote 49
Foot clearance is an important function in everyday life, and the ability to do this in different directions is essential when reacting to stimuli in the real world (i.e. navigating a busy street or walking on an uneven pavement).Footnote 50 The four-square step test incorporates rapid stepping while changing direction; however, we found this test was difficult for older adults categorized as frail, with six participants (≥3 on the FRAIL scale) unable to complete the test. Future studies should consider adding another test of dynamic balance and a static balance test feasible for older adults who are frail. If a research study includes older adults with either pre-frailty or frailty, at least two tests to measure balance should be considered, such as the Berg Balance Scale as well as the four-square step test.
Lastly, we did not see an improvement in gait speed using the 10-metre walk test; however, the average gait speed at baseline was average for adults over 75 years old (mean gait speed 1.06 m/s, 95% CI: 0.95 to 1.18); high functioning gait speed is greater than 1.1 m/s.Footnote 51 In addition, three of the four sites did not have the 14-metre cleared pathway required to conduct this test, so we performed several 10-metre walk tests in the hallway where other residents were walking, which could have interfered with our results. Future trials should consider specificity and target population in program design and outcome selection and ensure that they select outcomes that are feasible and responsive in the target population.
The interactions between the MoveStrong program and HRQoL were significant, but the interactions between the program and physical capacity levels (i.e. fatigue levels) were not. Several systematic reviews suggest that exercise may make little difference to HRQoL in older adults.Footnote 2Footnote 3Footnote 5Footnote 52 However, many exercise studies in older adults may be exhibiting healthy responder bias and ceiling effect. Most participants who enrol in exercise trials may already have high HRQoL scores at baseline so there would be little room for improvement; however, the individuals in our study had multiple chronic conditions and were pre-frail or frail. Although, the mean change EQ-5D-5L score was not significant in our study (−0.02 points, 95% CI: −0.06 to 0.01), the minimum clinically important difference for this scale is 0.18 (95% CI: 0.03 to 0.54; 18 studies).Footnote 53 It is possible that a longer study may show a more meaningful change.
We also found no significant interaction between exercise exposure and site on protein intake. Protein intake is significantly associated with eating occasion among older Canadians in long-term care, with the greatest intake at dinner.Footnote 54 In the current study, dinner was the only meal where the average amount of protein consumed was 20 to 30 g. There is evidence that higher protein intake and a more even distribution of daily protein intake across meals are associated with greater muscle mass and strength.Footnote 55Footnote 56
In terms of energy, the average intake was less than the RDA (i.e. 30 kcal/kg/d), but above 21 kcal/kg/d; a daily energy intake of less than 21 kcal/kg/d is associated with frailty.Footnote 57 Our current intervention mainly focussed on increasing protein intake while maintaining energy intake; however, it may be important for future interventions to also emphasize maintaining or increasing energy intake to meet the RDA and to avoid a level that may be associated with frailty.
In Canada, the total health care costs of physical inactivity have been estimated at CAD 6.8 billion.Footnote 58 The total cost of implementing and delivering our program was CAD 14 700, or CAD 377 per participant, which is similar to that of other strength and balance training interventions.Footnote 59Footnote 60Footnote 61 A 2016 study found the cost of implementing a community-based version of the Otago Exercise Program to be USD 585 per client, inclusive of administrative costs.Footnote 62 Assuming an average exchange rate of CAD 1 to USD 0.7553 in 2016, with an inflation of 1.74% per year, USD 585 would be equivalent to CAD 830 per client in 2020, which is substantially more than our cost of CAD 377 per participant. Our program was designed to use as little equipment as possible to help reduce costs. A larger multisite trial is now needed to determine the cost-effectiveness of implementing the MoveStrong program at a larger scale.
We could not perform a subgroup analysis by sex/gender, living arrangement or frailty level because of the sample size, the small number of male participants or of frail individuals at each site, and the potential of conflating differences between sites with differences in living arrangement. If subgroup analyses are not performed under the correct circumstances or if several subgroup analyses are performed, the likelihood of false negative and false positive significance tests increases rapidly.Footnote 63Footnote 64A subgroup analysis by sex/gender, living arrangements (i.e. retirement/assisted living vs. community dwelling) and frailty level should be considered in future larger trials.
Strengths and limitations
Our study had several strengths. Our research team is represented by a collaborative group including implementation scientists, health care providers, health economists and patient partners. Involving knowledge users and individuals who can use the research evidence to inform policy and practice decisions is an important shift from solely scientist-driven research to collaborative problem-based research. We also recruited a diverse group of participants from across Ontario, which increases the generalizability through relevance, applicability and impact of the research results. Lastly, our program operationalized specific models and frameworks in the Knowledge to Action cycleFootnote 65 to pilot the feasibility of the MoveStrong program in practice.
We acknowledge some limitations in our study. Some individuals had trouble completing the balance assessment. To impute the missing data we used multiple imputation, which could have led to a Type II error. In addition, data collection during the last assessment was abruptly stopped due to the COVID-19 pandemic, and we were not able to collect the performance-based outcomes for eight participants.
Protein intake was based on three days. Although three days is a commonly used timeframe to assess changes in food intake, it may not have been a sufficient to demonstrate significant clinical and statistical change. Furthermore, the capacity of participants to recall food consumption may be affected by their not preparing their own meals. To mitigate the challenge of recalling dietary intake, we asked for the menus from the retirement homes to ensure some reliability in data collection.
Lastly, statistical analysis of stepped-wedged trials is complex, and we opted to use a GEE analysis. One of the limitations of using GEE with few clusters is the risk of Type I error.
Conclusion
Participating in the MoveStrong program may improve grip strength, sit-to-stand functioning and dynamic balance. We did not see improvements in gait speed, physical capacity, HRQoL or protein intake. There may be an interaction between exposure to the MoveStrong program and gait speed, sit-to-stand functioning, dynamic balance and HRQoL index scores. Future trials on balance and functional strength training in older adults with pre-frailty and frailty should consider specificity of the exercises and the potential for ceiling or floor effects of certain outcomes.
Acknowledgements
The authors would like to acknowledge the assistance of several individuals. We would like to thank all the exercise physiologists, Jessica Bodson, Katarina Bubulj, Katelyn Corke, Bridget Misener, Eliza Reid and Ellen Wang, and dietitians, Kathy Lepp and Nicole Selman, who helped to deliver the program. We would also like to thank Denise Maki and Tina Treitz for allowing us to use their location, Kinnect to Wellness, in Sudbury, Ontario, to deliver the program, and Dave Courtemanche, Sarah Crichton, Nathalie Chisholm and Meghan Peters at the Family Health Teams in Sudbury, Ontario, for helping with recruiting participants and assessing outcomes. In addition, we would like to thank Jennifer Bucino, Josie d'Avernas, Andrea Grantham, Nathan Honsberger, Michael Lewiecki, Shaen Gingrich, Alex Steinke and Cindy Wei for their ongoing support. Lastly, we would like to thank Dr. Sayem Borhan, at St. Joseph’s Healthcare in Hamilton, Ontario, for providing us with the randomization sequence for our study.
The Data and Safety Monitoring Board (DSMB) chair was represented by Dr. Stephanie Kaiser from the Division of Endocrinology and Metabolism at Dalhousie University, Halifax, Nova Scotia. The DSMB clinical investigator was Dr. Christine Friedenreich, a senior director of Cancer Epidemiology and Prevention Research, Alberta Health Services, Calgary, Alberta, and the DSMB biostatistician was Dr. Eleanor Pullenayegum from the Dalla Lana School of Public Health at the University of Toronto, Toronto, Ontario.
Funding for this project was received from the Canadian Institutes of Health Research catalyst grant: SPOR Innovative Clinical Trials (grant number SCT-162968). The funding sponsors did not provide a role, other than financial support. IBR was supported by the Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (CGS-D).
Conflicts of interest
The authors have no conflicts of interest to declare.
Authors’ contributions and statement
LMG, LT, HK, MA, SB, AC, LF, RJ, DL, WI, JM, MM, AP, SES, ZW: Conceptualization, methodology, writing – review and editing
IBR: Project administration, writing-original draft
IBR, JW: Data curation, formal analysis
LT, HK, LMG: Resources, software, validation of formal analysis
LGM: Supervision, funding acquisition
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
Registration
This trial was registered in ClinicalTrials.gov under identifier NCT04037436.
Protocol
The original protocol was published online in ClinicalTrials.gov.
