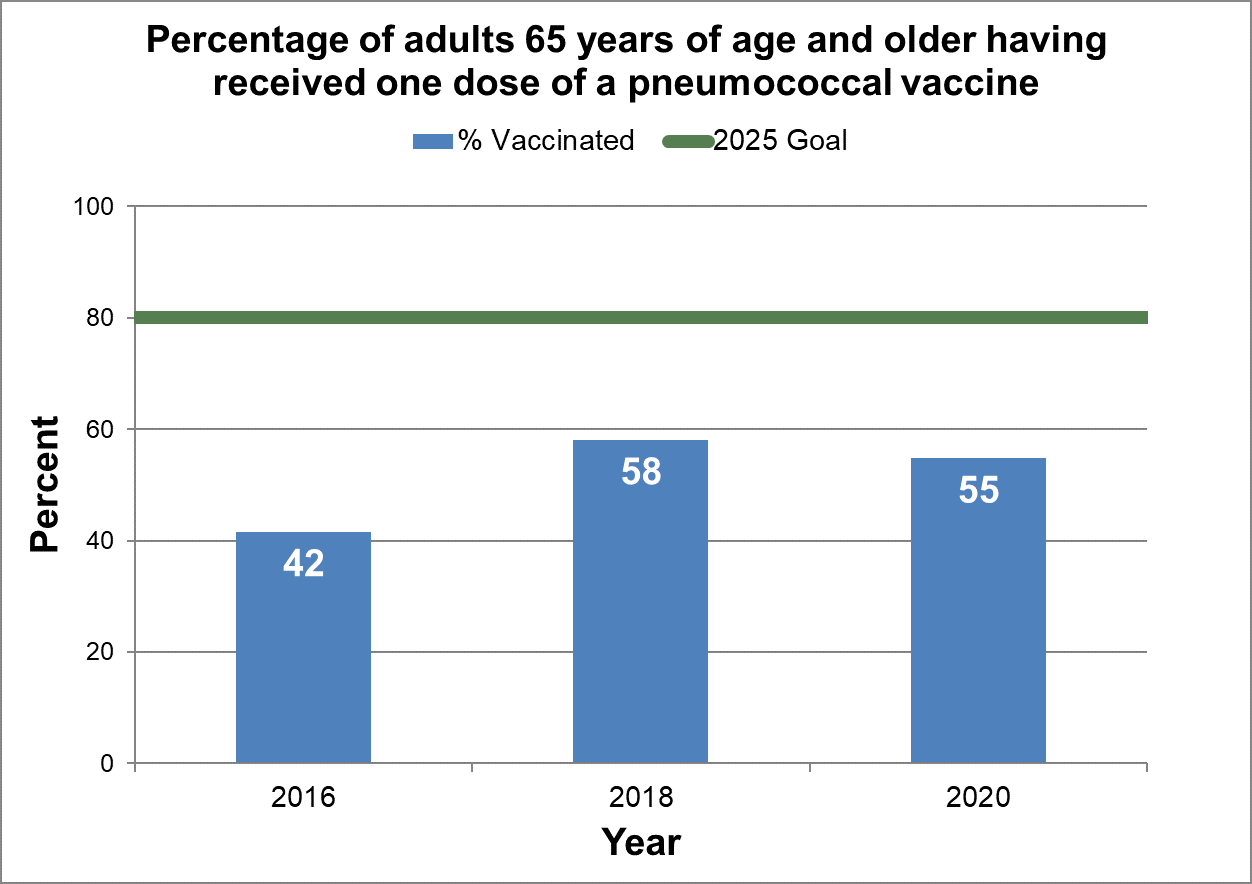Vaccination Coverage Goals and Vaccine Preventable Disease Reduction Targets by 2025
As part of the National Immunization Strategy objectives for 2016-2021, vaccination coverage goals and vaccine preventable disease reduction targets were set based on international standards and best practices. The goals and targets are consistent with Canada's commitment to the World Health Organization's (WHO) disease elimination targets and Global Vaccine Action Plan, while reflecting the Canadian context.
Table of Contents
Vaccination Coverage Goals by 2025
Vaccination coverage goals were developed for infant, childhood, adolescent and adult vaccines that are publicly funded in all provinces and territories (PT). Progress toward the national vaccination coverage goals will be reported based on the data collected using national coverage surveys, such as the childhood National Immunization Coverage Survey (cNICS), the adult National Immunization Coverage Survey (aNICS) and the Seasonal Influenza Vaccination Coverage Survey. Vaccine coverage monitoring at the national level takes into account variations in PT vaccination programs.
Infants and Children
To ensure children are protected through routine vaccination, a high vaccination coverage goal of 95% has been established for all childhood vaccines by two and seven years of age.
This level of vaccination coverage is based on the level of population protection required for measles, the most easily-spread vaccine preventable disease.
Achieve 95% vaccination for the first three doses of the pertussis vaccine for infants
The majority of hospitalizations due to pertussis occur in infants under one year of age. The first three doses of the vaccine are highly effective in preventing death. Specific vaccination goals have been set for infants under one year of age.
First dose of pertussis vaccine by three months of age
- Baseline:
- In 2015, 87% of two-year-old children in Canada had their first dose of pertussis vaccine by three months of age.
- Target:
- 95%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
Figure 1 - Percentage of two-year-old children having received their first dose of pertussis vaccine by three months of age
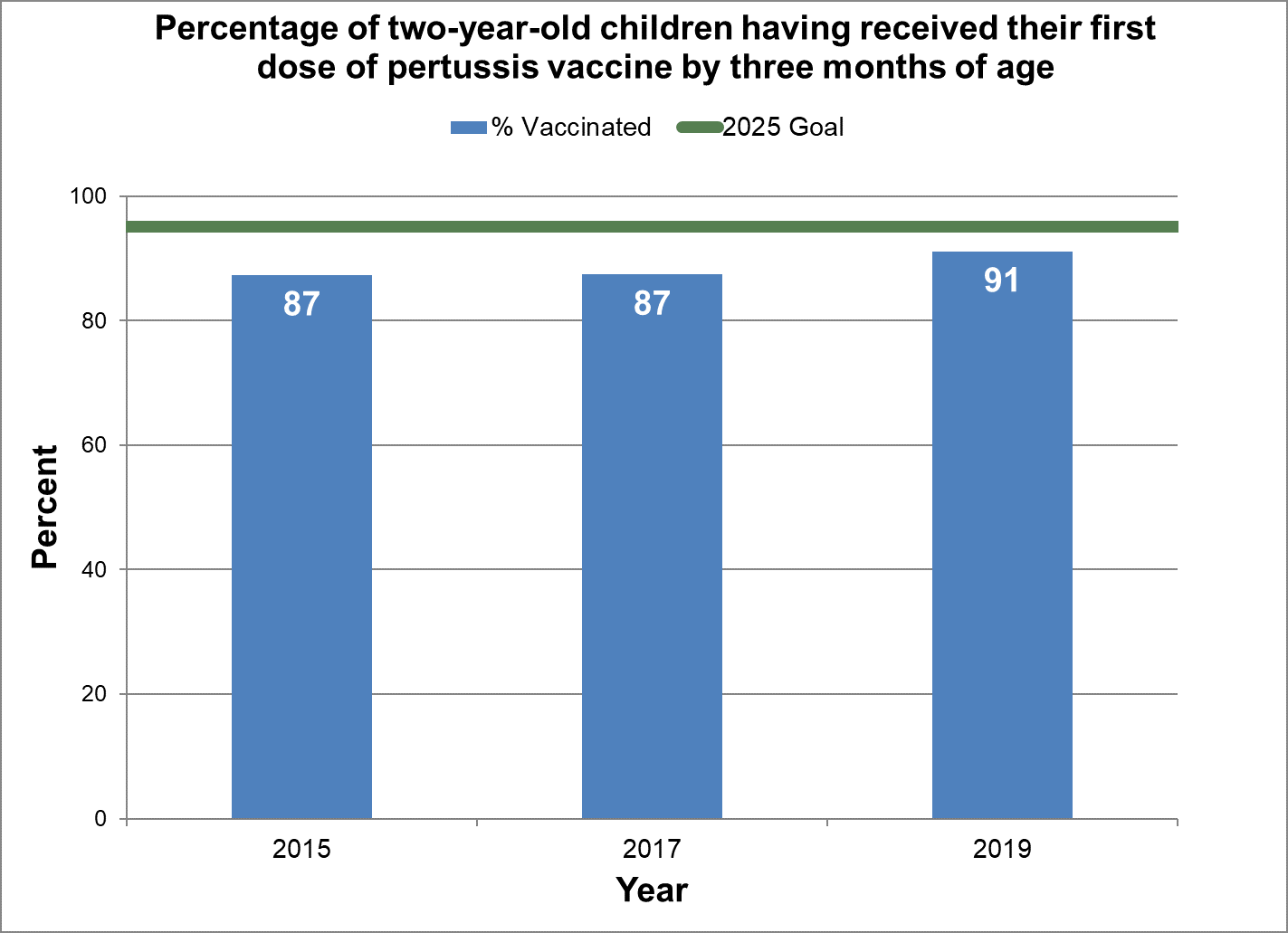
Text description: Figure 1
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 87.3 | 2015 | 95 |
| 87.4 | 2017 | 95 |
| 91.1 | 2019 | 95 |
Three doses of pertussis vaccine by seven months of age
- Baseline:
- In 2015, 72% of two-year-old children in Canada had three doses of pertussis vaccine by seven months of age.
- Target:
- 95%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
Figure 2 - Percentage of two-year-old children having received three doses of pertussis vaccine by seven months of age
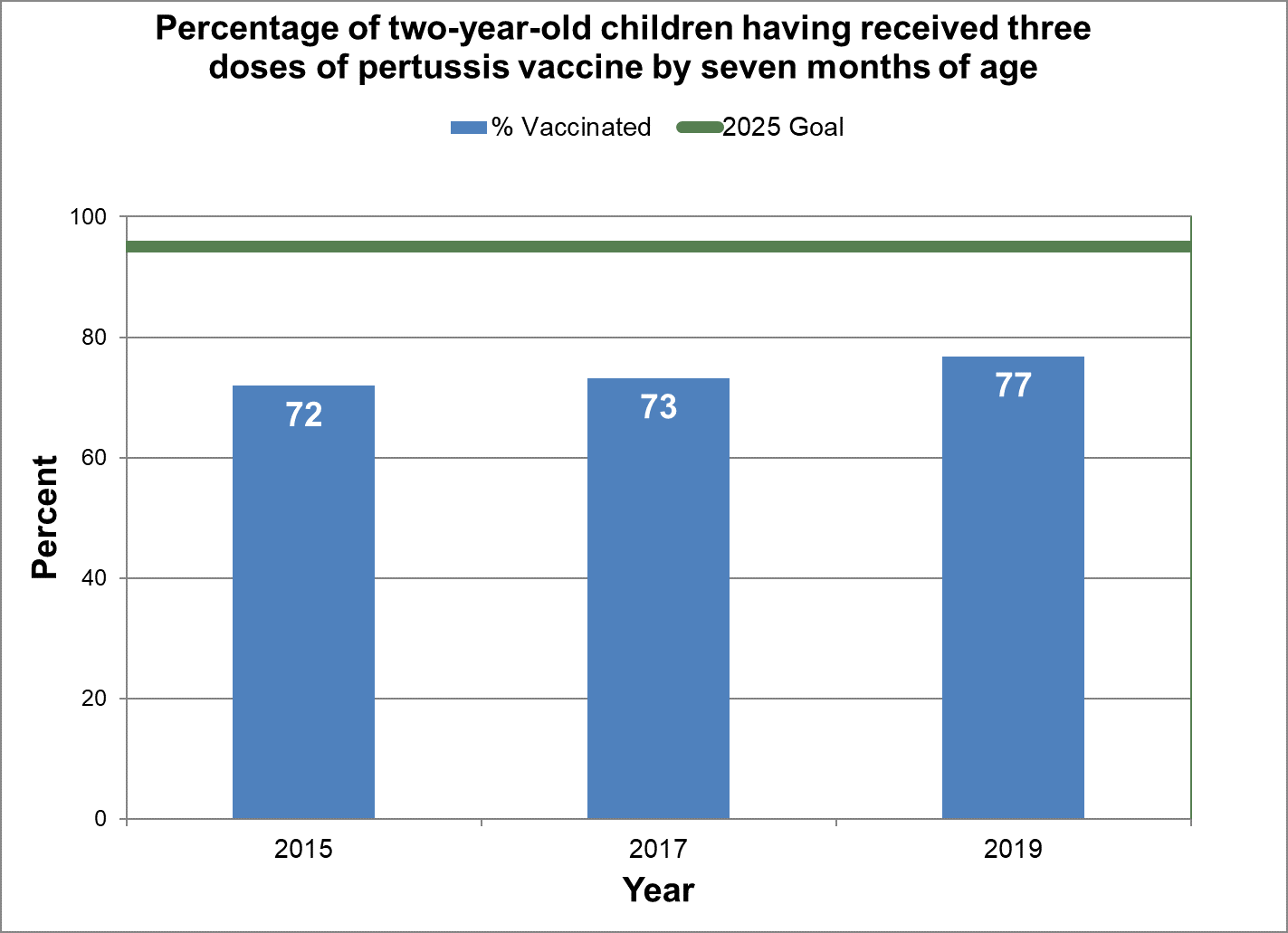
Text description: Figure 2
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 71.9 | 2015 | 95 |
| 73.2 | 2017 | 95 |
| 76.8 | 2019 | 95 |
Three doses of pertussis vaccine by 12 months
- Baseline:
- In 2015, 87% of two-year-old children in Canada had three doses of pertussis vaccine by 12 months of age.
- Target:
- 95%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
Figure 3 - Percentage of two-year-old children having received three doses of pertussis vaccine by 12 months of age
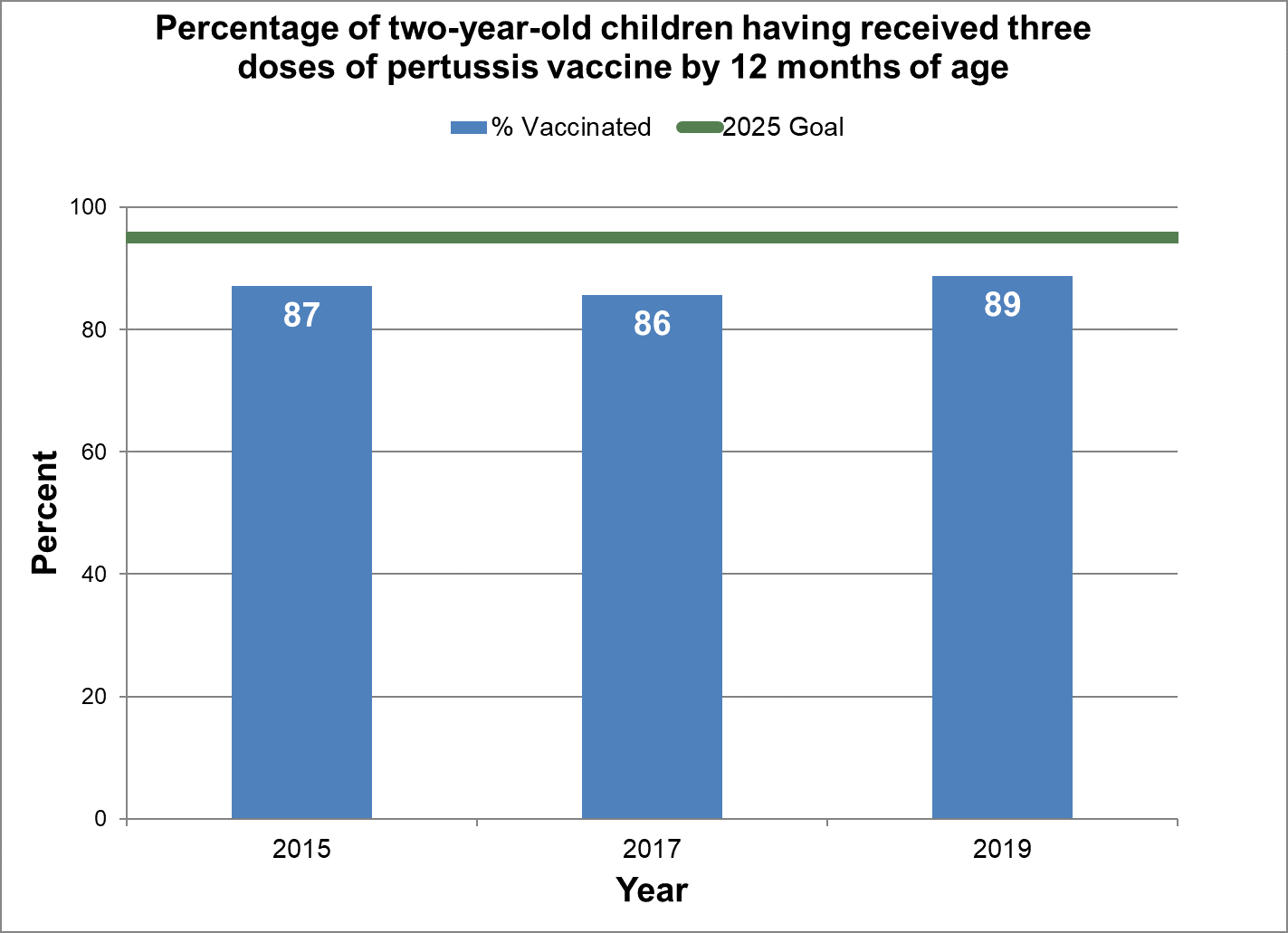
Text description: Figure 3
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 87.1 | 2015 | 95 |
| 85.7 | 2017 | 95 |
| 88.8 | 2019 | 95 |
Achieve 95% vaccination coverage by two years of age for the following childhood vaccines:
Four doses of diphtheria, tetanus, pertussis and Haemophilus influenzae type B (Hib) vaccine
- Baseline:
- In 2015, 77% of two-year-old children had four doses of diphtheria, tetanus, pertussis and Hib vaccine by their second birthday.
- Target:
- 95%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
Figure 4 - Percentage of children having received four doses of diphtheria, tetanus, pertussis and Haemophilus influenzae type B (Hib) vaccine by their second birthday
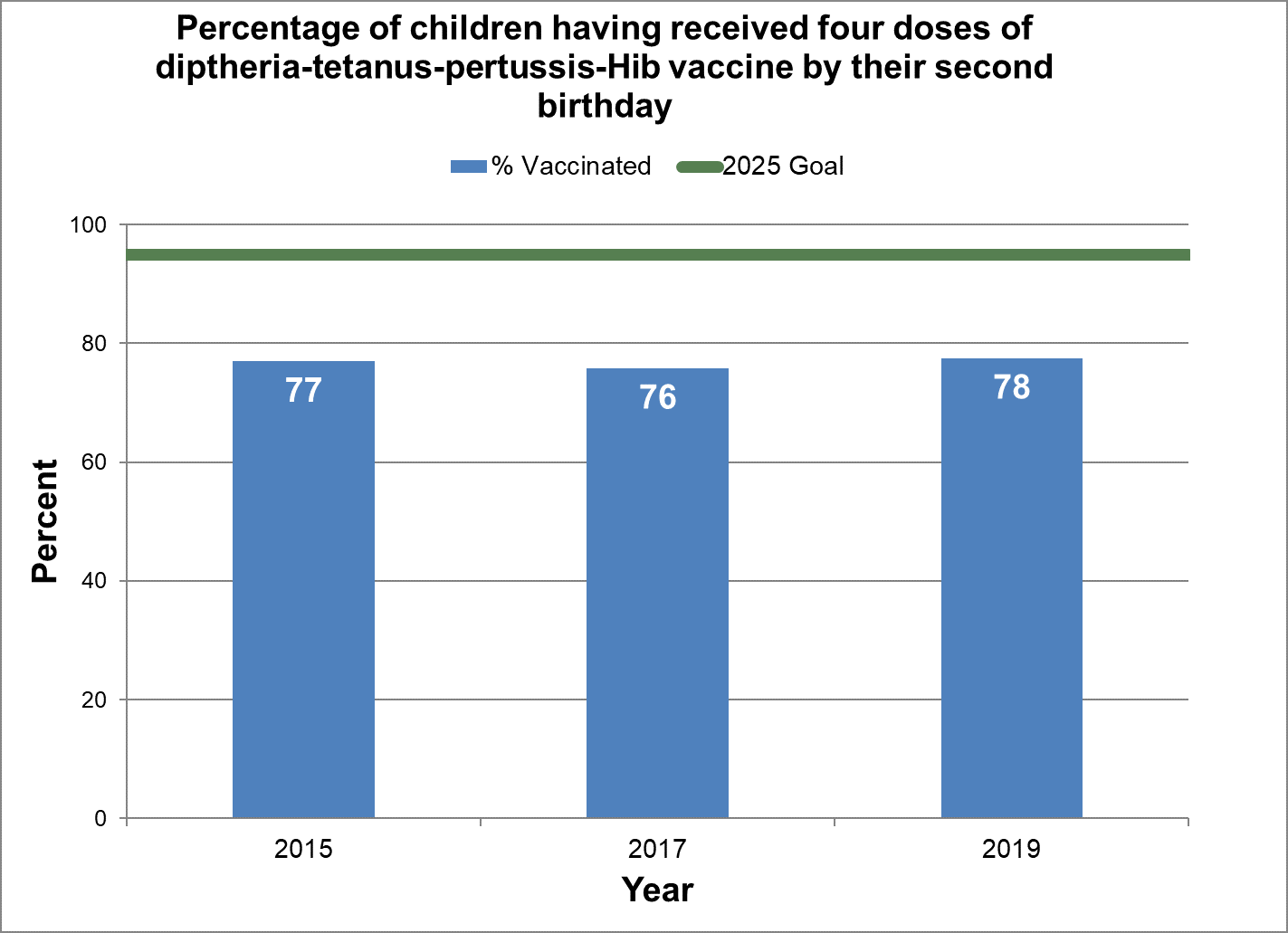
Text description: Figure 4
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 76.9 | 2015 | 95 |
| 75.8 | 2017 | 95 |
| 77.5 | 2019 | 95 |
Three doses of polio vaccine
- Baseline:
- In 2015, 91% of two-year-old children in Canada had three doses of polio vaccine by their second birthday.
- Target:
- 95%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
Figure 5 - Percentage of children having received three doses of polio-containing vaccine by their second birthday
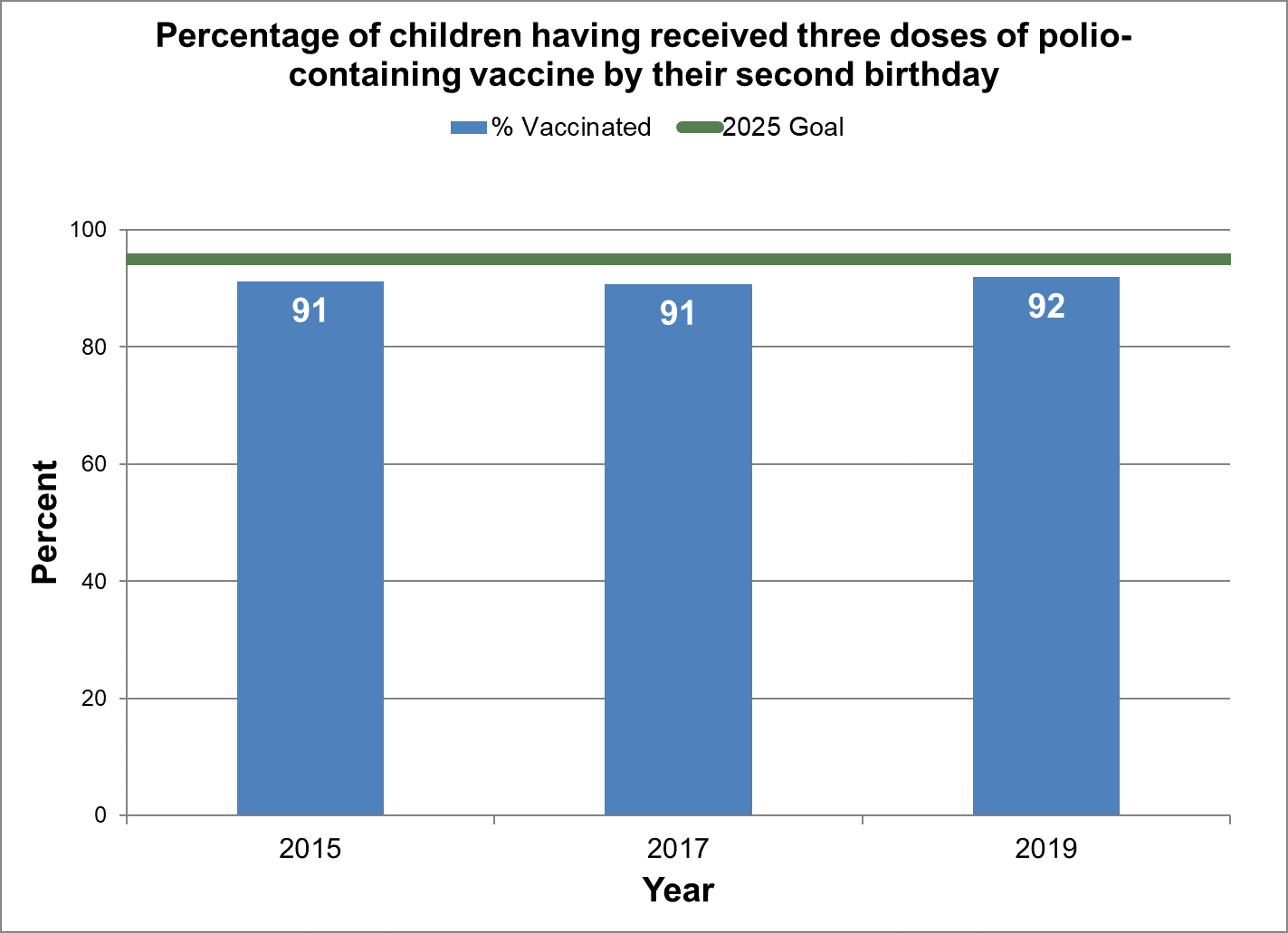
Text description: Figure 5
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 91.2 | 2015 | 95 |
| 90.7 | 2017 | 95 |
| 91.9 | 2019 | 95 |
Three doses of hepatitis B vaccine among children targeted in publicly-funded programs
- Baseline:
- In 2015, 69% of two-year-old children in Canada had three doses of hepatitis B vaccine by their second birthday
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
-
Coverage estimates were measured in jurisdictions where a 3-dose program for infants was in place (British Columbia, Québec, New Brunswick, Prince Edward Island, Yukon, Northwest Territories, and Nunavut). In other jurisdictions, the vaccine is given at school. Children were considered vaccinated if they received the number of doses recommended by the child’s province/territory of residence.
High vaccination coverage is required to support the WHO goal of eliminating viral hepatitis by 2030.
Figure 6 - Percentage of children having received three doses of hepatitis B vaccine by their second birthday
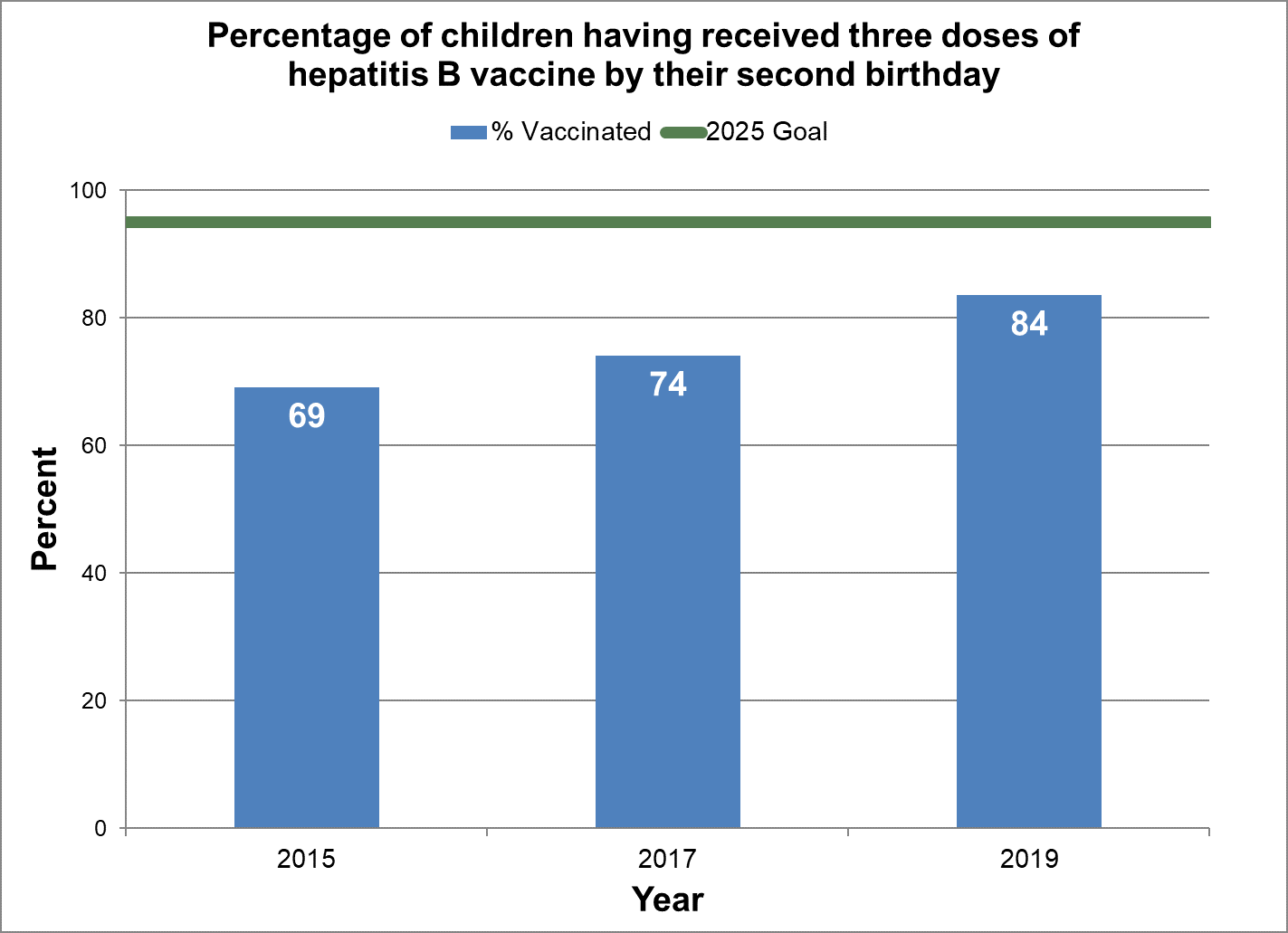
Text description: Figure 6
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 69.2 | 2015 | 95 |
| 74.1 | 2017 | 95 |
| 83.6 | 2019 | 95 |
One dose of measles, mumps, and rubella vaccine
- Baseline:
- In 2015, 89% of two-year-old children in Canada had their first dose of measles, mumps and rubella vaccine by their second birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
-
The estimates for mumps coverage are used to report on uptake of the measles, mumps and rubella (MMR) vaccine. Measles is not used because foreign-born children in Canada may have received the monovalent measles vaccine in another country and coverage would by consequence be higher.
Figure 7 - Percentage of children having received one dose of measles, mumps and rubella vaccine by their second birthday
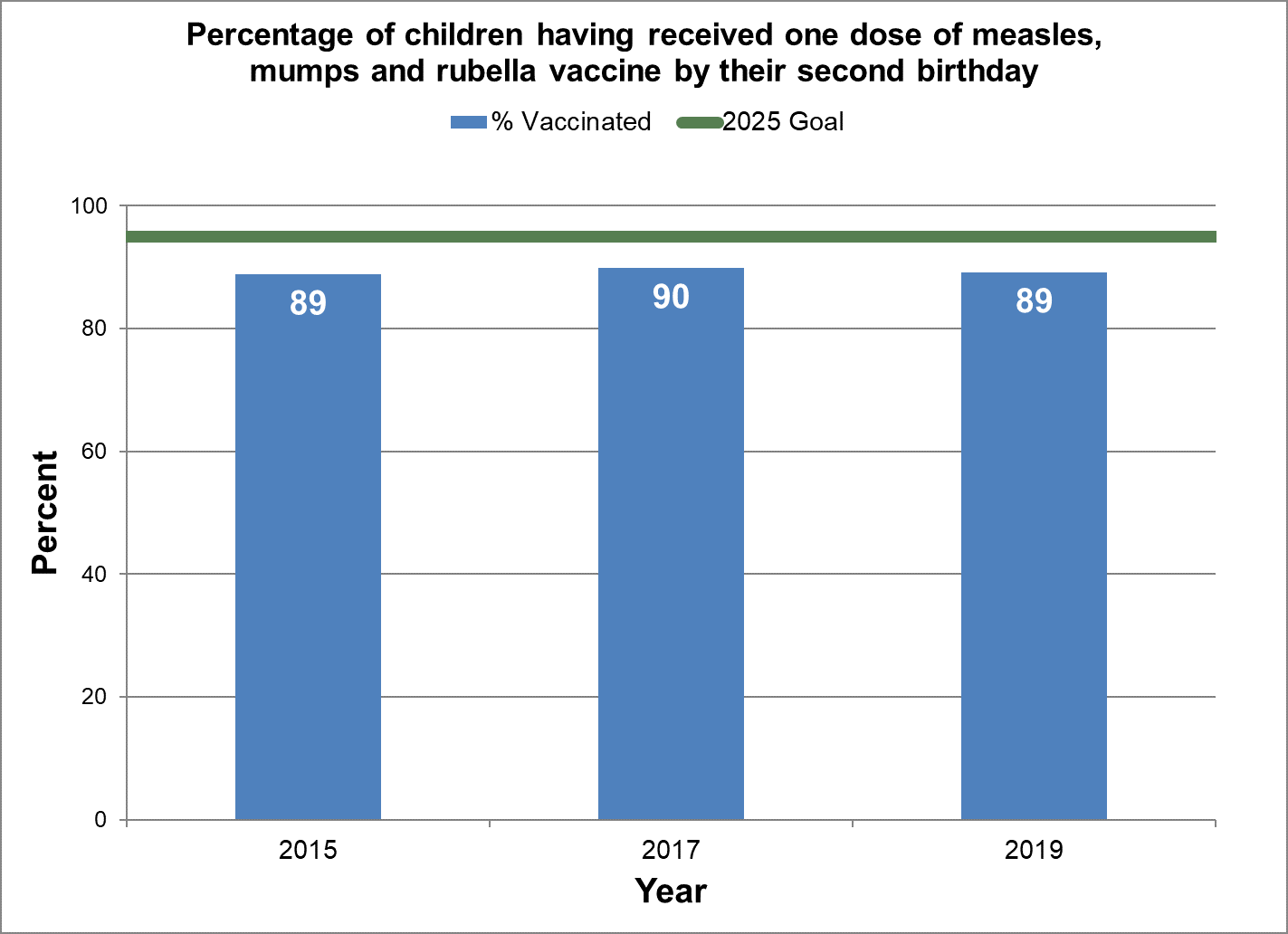
Text description: Figure 7
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 88.9 | 2015 | 95 |
| 89.9 | 2017 | 95 |
| 89.2 | 2019 | 95 |
One dose of varicella vaccine
- Baseline:
- In 2017, at least 83% of two-year-old children in Canada had their first dose of varicella vaccine by their second birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
- Due to an issue with data collection, varicella vaccination was underreported in previous cNICS cycles (see cNICS 2015 for more information). This issue was corrected from cNICS 2017 onwards.
Figure 8 - Percentage of children having received one dose of varicella-containing vaccine by their second birthday

Text description: Figure 8
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 82.9 | 2017 | 95 |
| 82.7 | 2019 | 95 |
Three or four doses of pneumococcal vaccine
- Baseline:
- In 2015, 80% of two-year-old children in Canada had three or four doses of pneumococcal vaccine by their second birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
- Coverage estimated by a four-dose program in Northwest Territories and Nunavut; 3-dose program in all provinces and in Yukon.
Figure 9 - Percentage of children having received three or four doses of pneumococcal vaccine by their second birthday
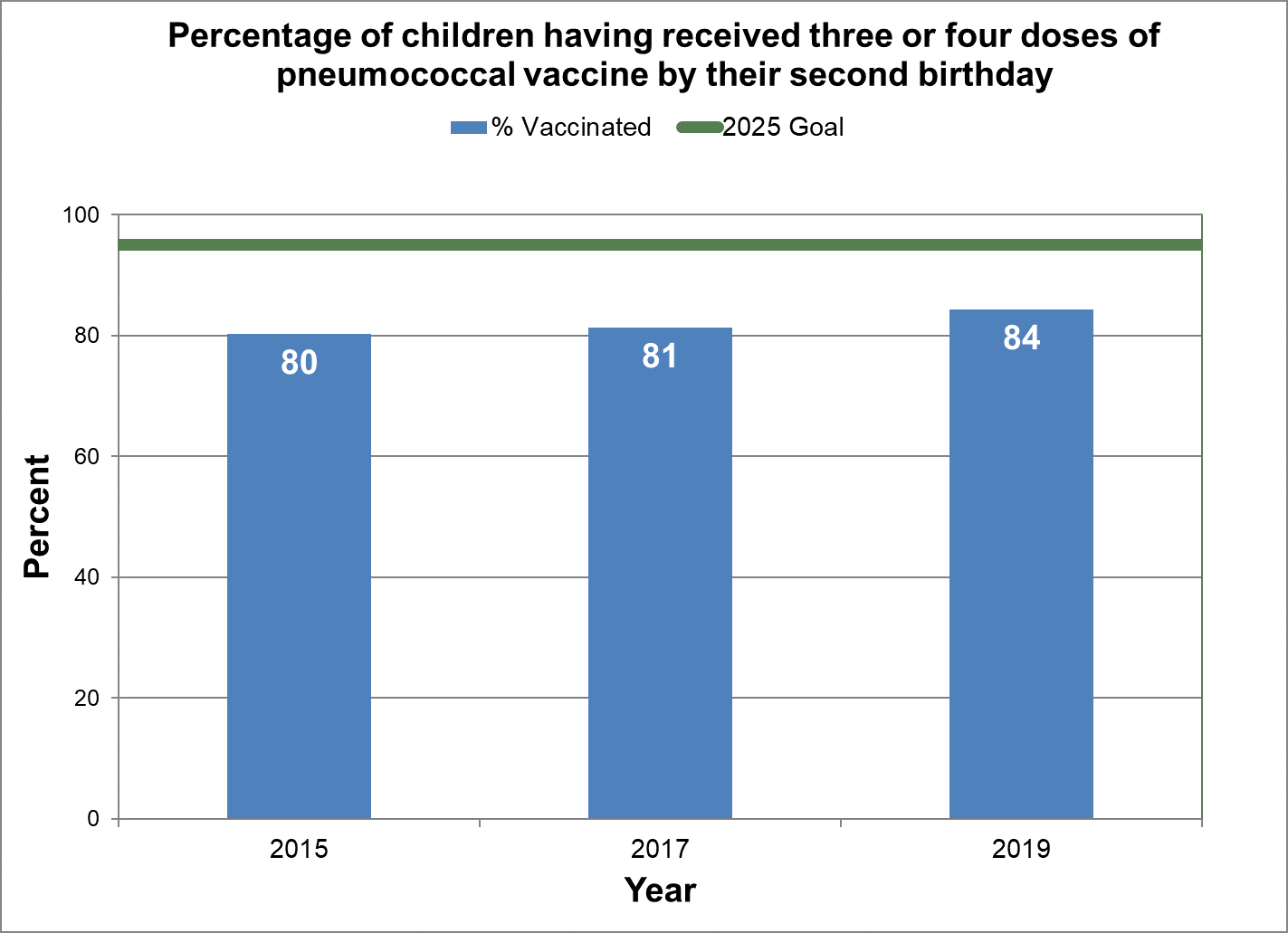
Text description: Figure 9
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 80.3 | 2015 | 95 |
| 81.4 | 2017 | 95 |
| 84.4 | 2019 | 95 |
One dose of meningococcal C vaccine
- Baseline:
- In 2015, 88% of two-year-old children in Canada had at least one dose of meningococcal C vaccine by their second birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
- Some of the provinces and territories (British Columbia, Alberta, Yukon and Northwest Territories) offer 2 doses of meningococcal C vaccine to children under 2 years of age.
Figure 10 - Percentage of children having received at least one dose of meningococcal C vaccine by their second birthday

Text description: Figure 10
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 87.8 | 2015 | 95 |
| 87.6 | 2017 | 95 |
| 91.1 | 2019 | 95 |
Achieve 95% vaccination coverage by seven years of age for the following childhood vaccines:
Five doses of diphtheria, tetanus and pertussis vaccine
- Baseline:
- In 2015, 75% of seven-year-old children in Canada had five doses of diphtheria, tetanus and pertussis vaccine by their seventh birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
- Outbreaks of pertussis occur during school-age; therefore, an additional dose before school entry is important.
Figure 11 - Percentage of children having received five doses of diphtheria-tetanus-pertussis vaccine by their seventh birthday
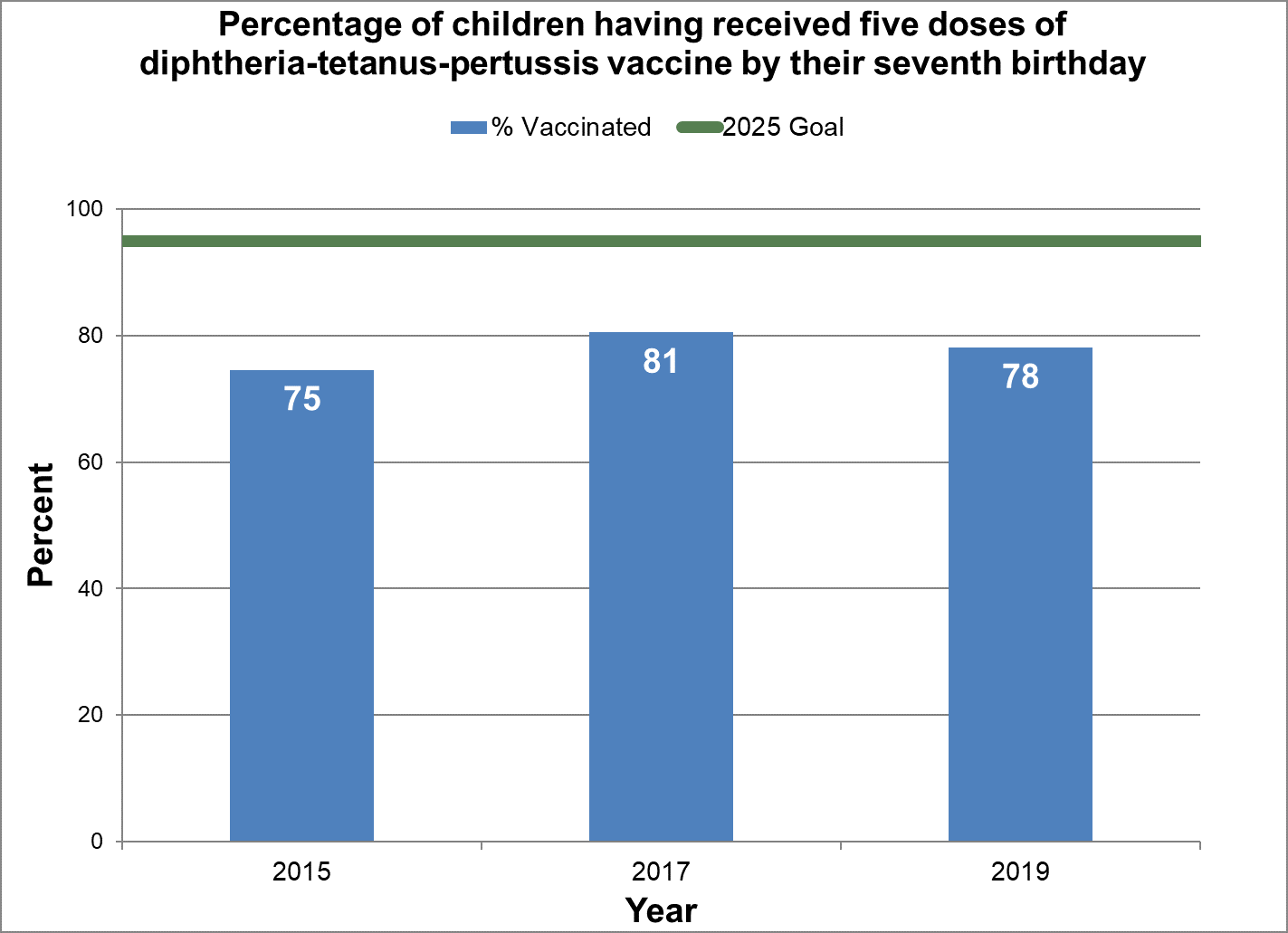
Text description: Figure 11
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 74.6 | 2015 | 95 |
| 80.5 | 2017 | 95 |
| 78.1 | 2019 | 95 |
Four doses of polio vaccine
- Baseline:
- In 2015, 90% of seven-year-old children in Canada had four doses of polio-containing vaccine by their seventh birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
Figure 12 - Percentage of children having received four doses of polio-containing vaccine by their seventh birthday
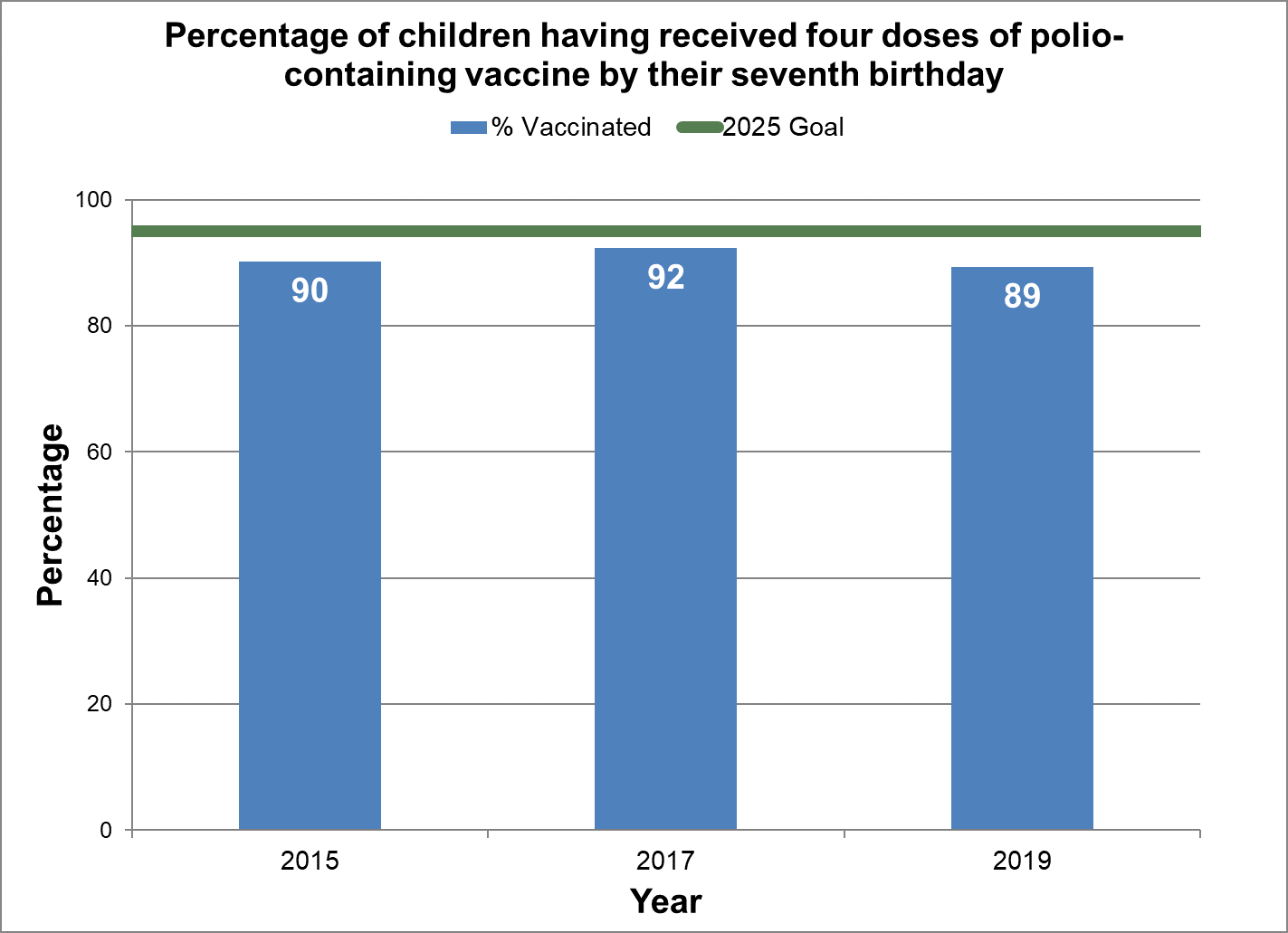
Text description: Figure 12
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 90.2 | 2015 | 95 |
| 92.3 | 2017 | 95 |
| 89.3 | 2019 | 95 |
Two doses of measles, mumps, and rubella vaccine
- Baseline:
- In 2015, 86% of seven-year-old children in Canada had two doses of measles, mumps, and rubella vaccine by their seventh birthday.
- Target:
- 95%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
- The estimates for mumps coverage are used to report on uptake of the measles, mumps and rubella (MMR) vaccine. Measles is not used because foreign-born children in Canada may have received the monovalent measles vaccine in another country and coverage would by consequence be higher.
Figure 13 - Percentage of children having received two doses of measles, mumps and rubella vaccine by their seventh birthday
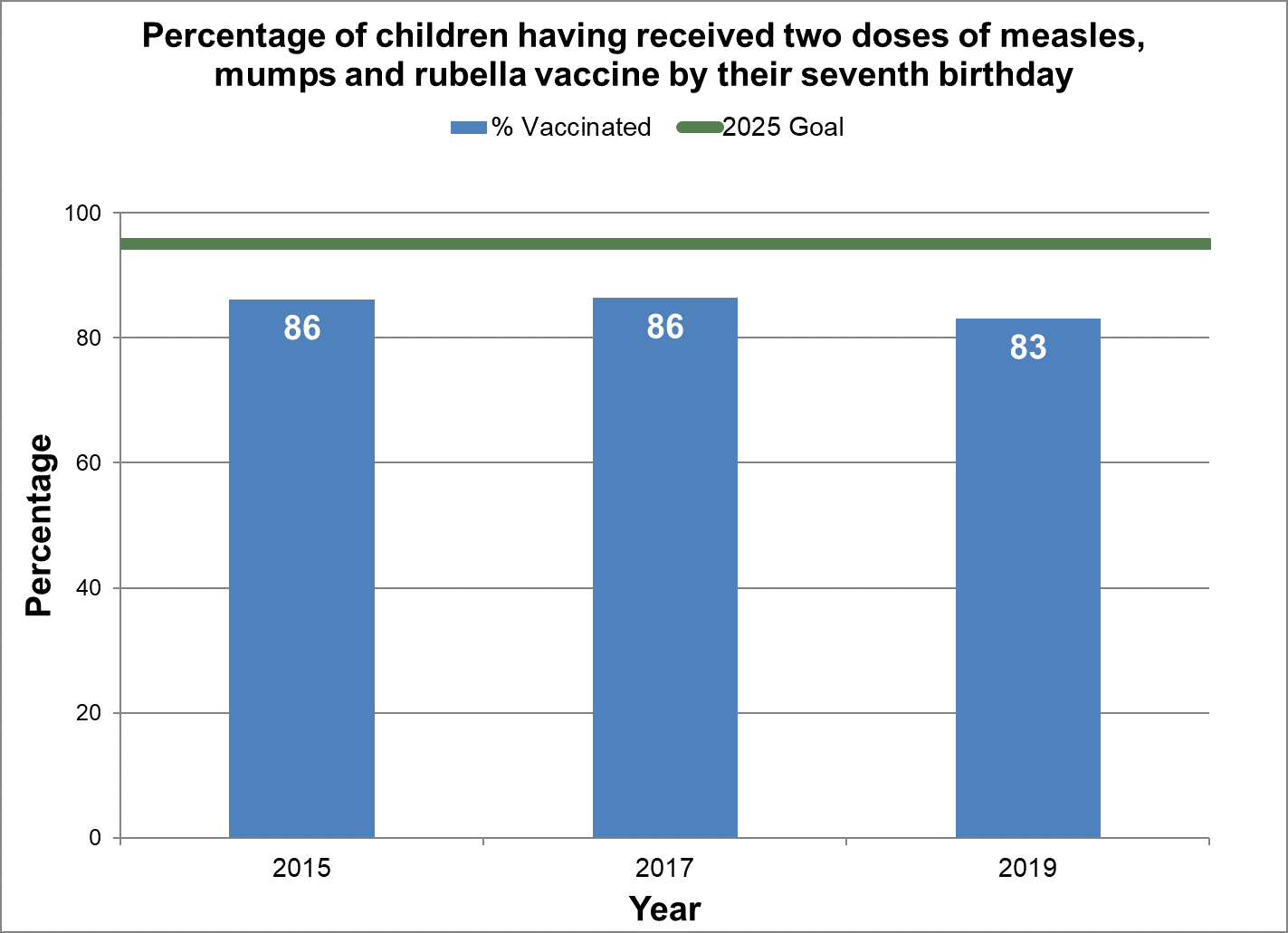
Text description: Figure 13
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 86.2 | 2015 | 95 |
| 86.4 | 2017 | 95 |
| 83.1 | 2019 | 95 |
Adolescents
The vaccine coverage goal of 90% for all adolescent vaccines is based on disease reduction targets, program impact, disease characteristics, achievability, and jurisdictional variation in vaccination programs as well as quality and effectiveness of the vaccine.
Achieve 90% vaccination coverage by 17 years of age for the following adolescent vaccines:
One dose of meningococcal vaccine
- Baseline:
- In 2019, 89% of 17-year-old adolescents in Canada had at least one dose of meningococcal vaccine.
- Target:
- 90%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2019.
- Notes:
- Meningococcal vaccine in adolescents was measured starting in the 2019 childhood National Immunization Coverage Survey (cNICS). The type of meningococcal vaccine administered varies by provincial/territorial program.
Figure 14 - Percentage of 17-year-old adolescents having received at least one dose of meningococcal vaccine
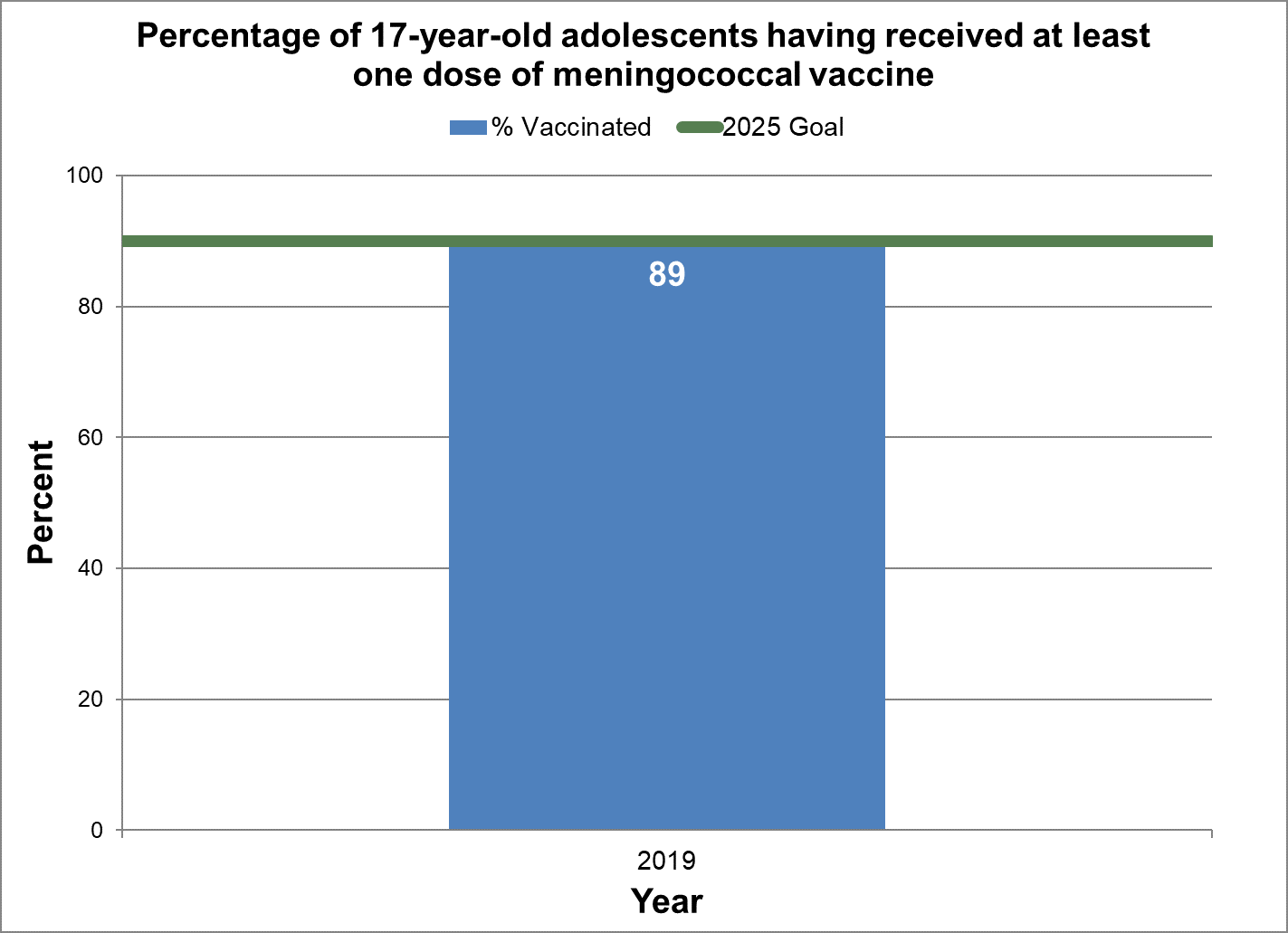
Text description: Figure 14
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 89.3 | 2019 | 90 |
One or more doses of hepatitis B vaccine among adolescents
- Baseline:
- In 2015, 88% of 17-year-old adolescents in Canada had at least one dose of hepatitis B vaccine.
- Target:
- 90%
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
-
cNICS reports by age 14 if parent recalled child ever having received a dose. High vaccination coverage is required to support the WHO goal of eliminating viral hepatitis by 2030.
Figure 15 - Percentage of 17-year-old adolescents having received at least one dose of hepatitis B vaccine
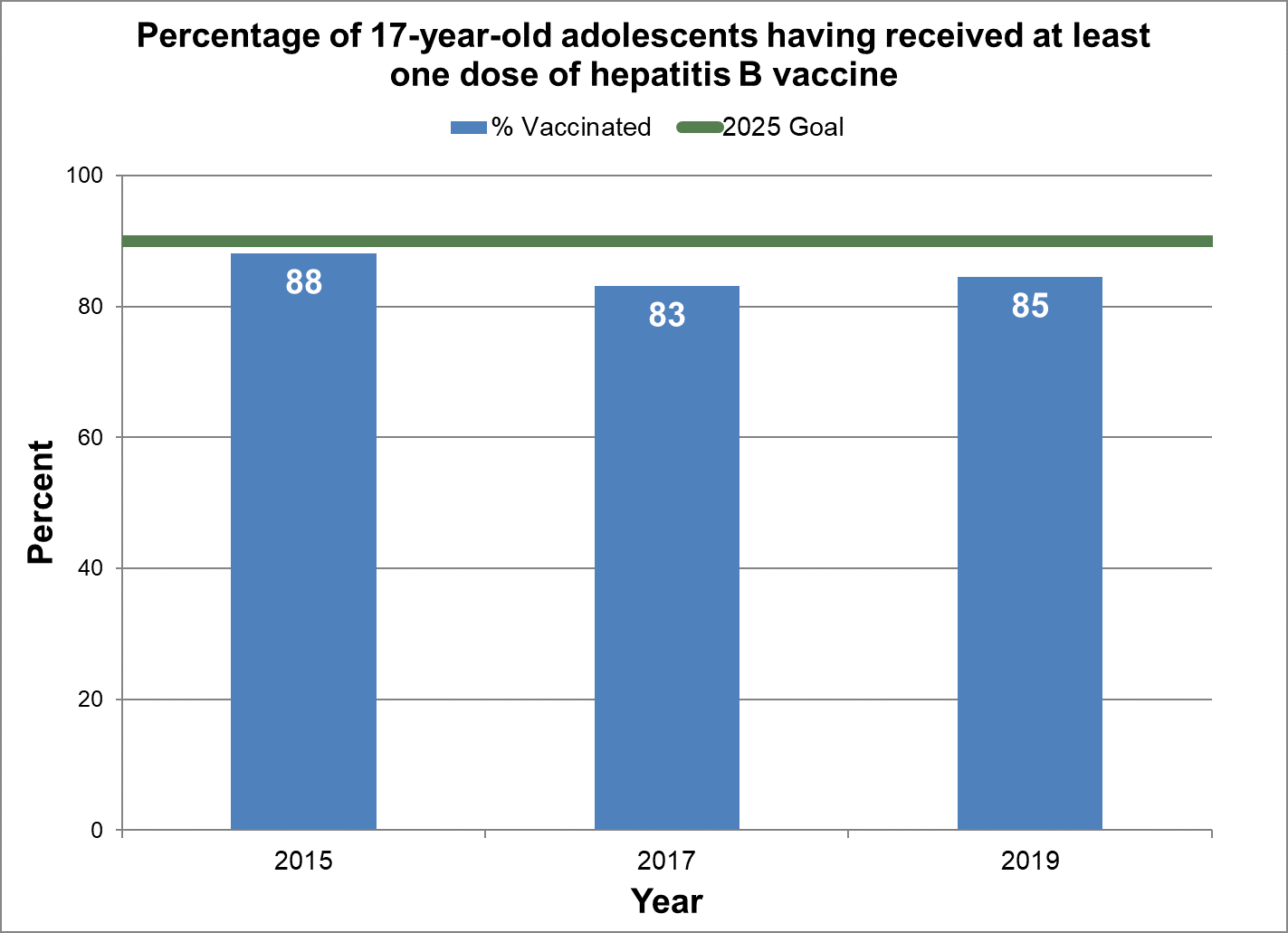
Text description: Figure 15
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 88.1 | 2015 | 90 |
| 83.1 | 2017 | 90 |
| 84.5 | 2019 | 90 |
Two or more doses of human papillomavirus vaccine (HPV)
Girls
- Baseline:
- In 2015, 75% of 14-year-old girls in Canada had at least one dose of HPV vaccine.
- Target:
- 90%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2015, 2017 and 2019.
- Notes:
- In the cNICS, only the estimate for receiving at least one dose of HPV vaccine was measured to minimize recall bias. cNICS reports by age 14 if parent recalled child ever having received a dose. The age group measured over the years has changed (13–14 years in 2015, 14 years in 2017 and 2019), therefore caution is advised when making comparisons over time.
Figure 16 - Percentage of 14-year-old girls having received at least one dose of HPV vaccine
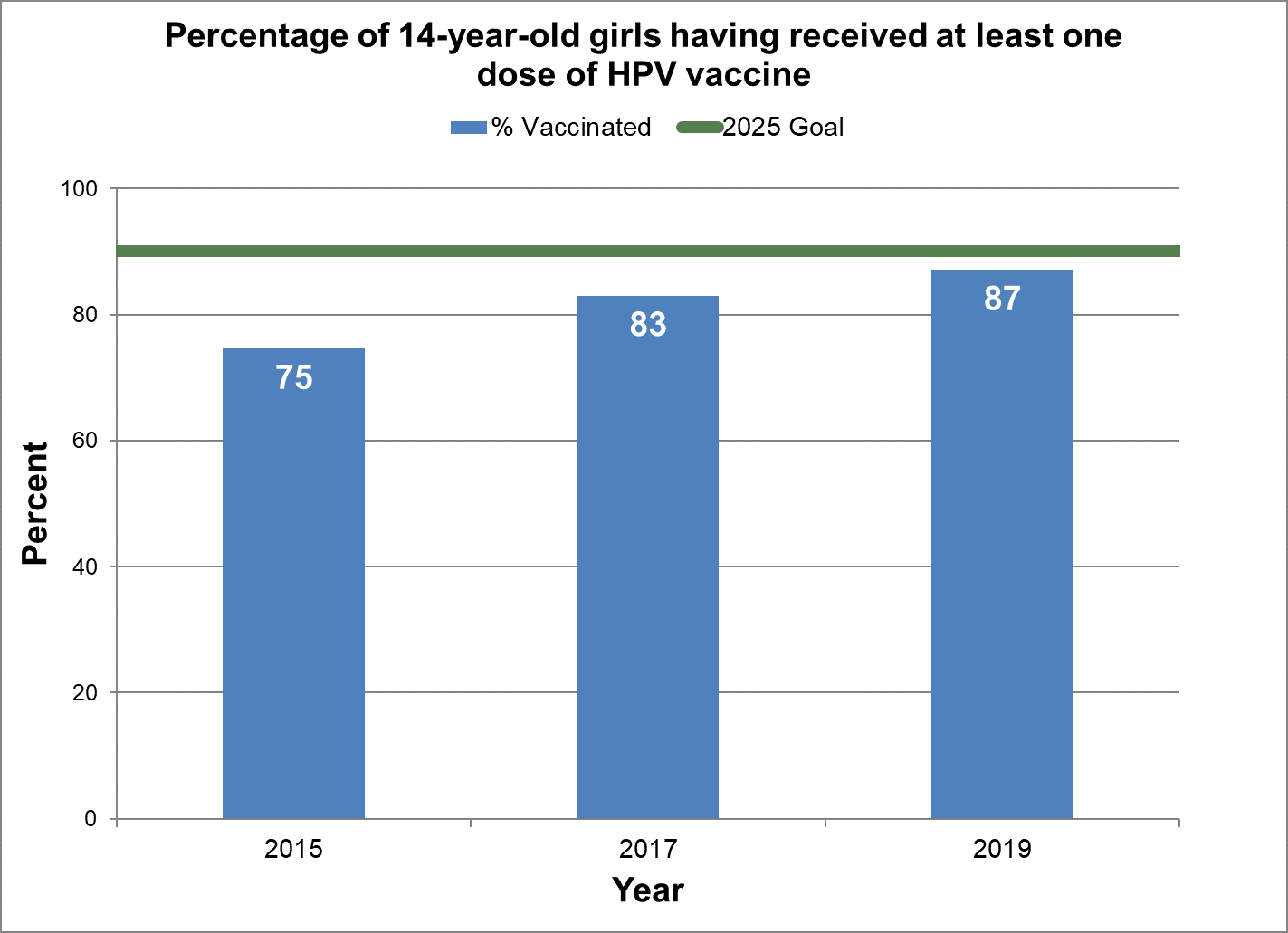
Text description: Figure 16
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 74.6 | 2015 | 90 |
| 83.0 | 2017 | 90 |
| 87.1 | 2019 | 90 |
Boys
- Baseline:
- In 2019, 73% of 14-year-old boys in Canada had at least one dose of HPV vaccine.
- Target:
- 90%
- Data sources:
- Childhood National Immunization Coverage Survey (cNICS) 2019.
- Notes:
- In 2017, HPV vaccination coverage was only measured among females, as the male vaccination program was not implemented consistently across provinces and territories, nor had there been enough time elapsed for those with programs at the time of data collection.
Figure 17 - Percentage of 14-year-old boys having received at least one dose of HPV vaccine
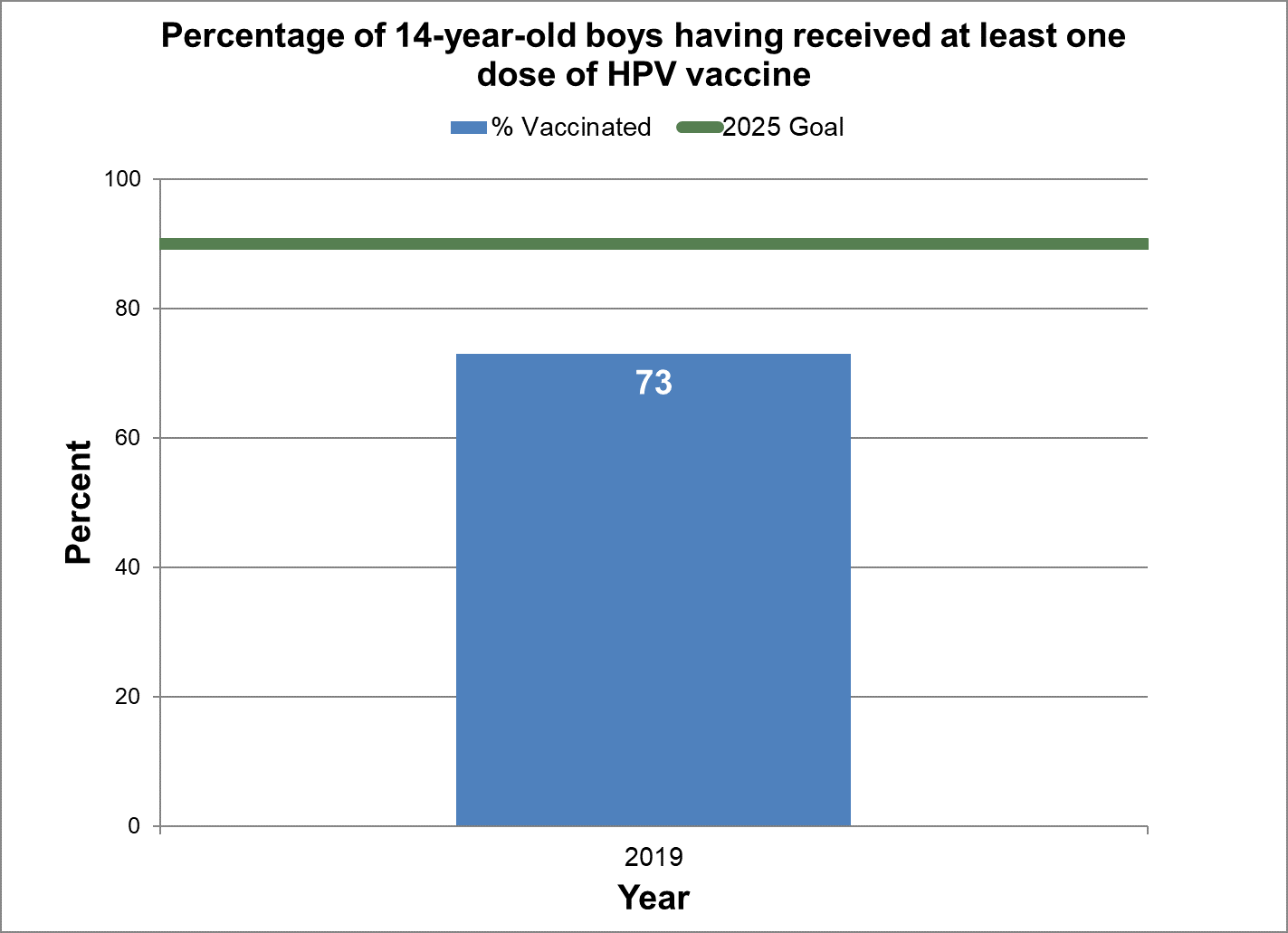
Text description: Figure 17
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 73.0 | 2019 | 90 |
One dose of tetanus-diphtheria-pertussis booster vaccine (Tdap)
- Baseline:
- In 2017, 89% of adolescents in Canada received one or more doses of diphtheria, tetanus, acellular pertussis (Tdap) booster by their 17th birthday.
- Target:
- 90%, which was met in 2019.
- Data sources:
-
Childhood National Immunization Coverage Survey (cNICS) 2017 and 2019.
- Notes:
-
Until cNICS 2015, the survey determined the percentage of children having received 6 doses of diphtheria, pertussis, and tetanus antigens by their 17th birthday. Starting in 2017, cNICS determined the percentage of adolescents having received one dose of Tdap vaccine between their 11th and 17th birthday.
Figure 18 - Percentage of adolescents having received one dose of Tdap vaccine between their 11th and 17th birthday
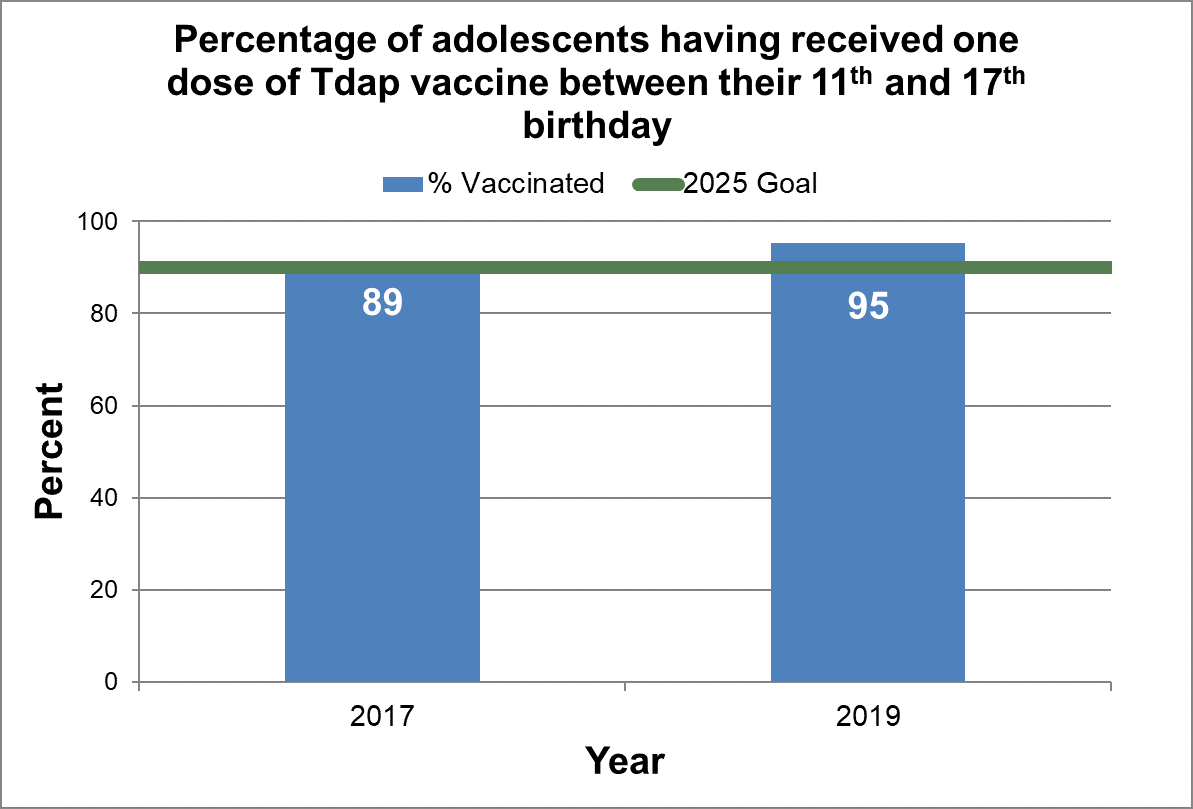
Text description: Figure 18
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 89.3 | 2017 | 90 |
| 95.3 | 2019 | 90 |
Adults
The following vaccination coverage goals for adults were established because of the importance of protecting Canadians at high risk for infection and medical complications.
Increase vaccination coverage for the following adult vaccines:
Achieve 80% vaccination coverage (one dose) of a pneumococcal vaccine among adults 65 years of age and older.
- Baseline:
- In 2016, 42% of seniors (aged 65 years or older) in Canada had been vaccinated with one dose of a pneumococcal vaccine.
- Target:
- 80%
- Data sources:
2016 adult National Immunization Coverage Survey;
Figure 19 - Percentage of adults 65 years of age and older having received one dose of a pneumococcal vaccine
Achieve 90% coverage (one dose) of hepatitis B vaccine among healthcare professionals.
- Baseline:
- Not available
- Target:
- 90%
- Data sources:
- To be determined
Seasonal Influenza Vaccination Coverage Goals
A vaccination coverage goal of 80% with the seasonal influenza vaccine has been established based on the importance of protecting Canadians at high risk for influenza-related complications or hospitalizations. This goal also reflects the importance of the influenza vaccine as a component of comprehensive infection control practices to protect healthcare professionals and patients from getting influenza.
Increase vaccination coverage for the seasonal influenza vaccine for the following groups:
Achieve 80% vaccination coverage among adults aged 65 years and older.
- Baseline:
- For the 2015/2016 influenza season, 65% of adults aged 65 years and older have been vaccinated.
- Target:
- 80%
- Data sources:
- National influenza immunization coverage survey 2015/16, 2016/17, 2017/18, 2018/19, 2019/20 and 2021/21
Figure 20 - Percentage of adults aged 65 years and older vaccinated with seasonal influenza vaccine
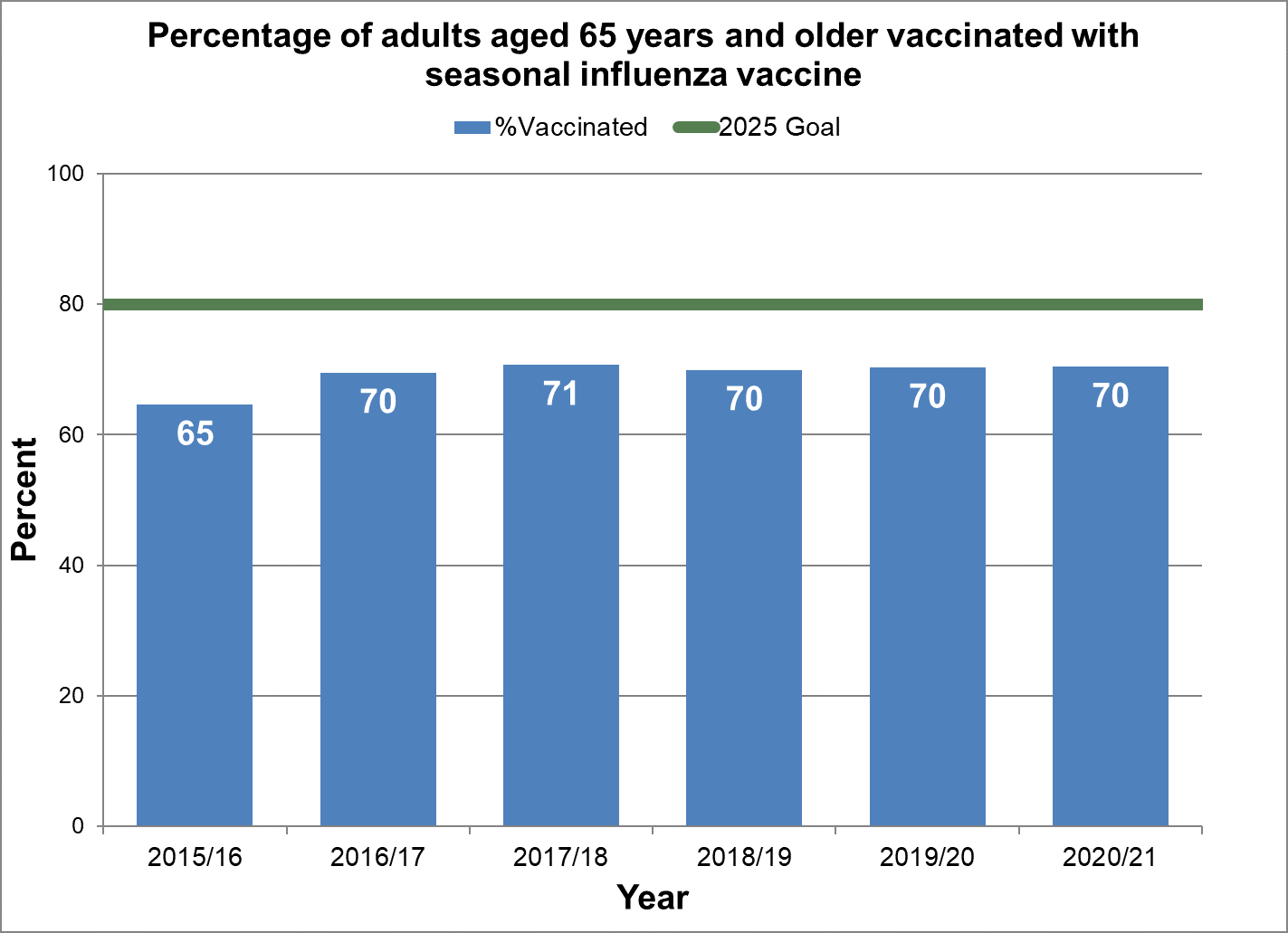
Text description: Figure 20
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 64.6 | 2015/16 | 80 |
| 69.5 | 2016/17 | 80 |
| 70.7 | 2017/18 | 80 |
| 69.9 | 2018/19 | 80 |
| 70.3 | 2019/20 | 80 |
| 70.4 | 2020/21 | 80 |
Achieve 80% vaccination coverage among adults aged 18-64 years with chronic medical conditions.
- Baseline:
- For the 2015/2016 influenza season, 37% of adults aged 18 to 64 years with a chronic medical condition have been vaccinated.
- Target:
- 80%
- Data sources:
- National influenza immunization coverage survey 2015/16, 2016/17, 2017/18, 2018/19, 2019/20 and 2021/21
- Notes:
- Chronic medical conditions are based on the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza for 2017-2018.
Figure 21 - Percentage of adults aged 18 to 64 years with chronic medical condition(s) vaccinated with seasonal influenza vaccine
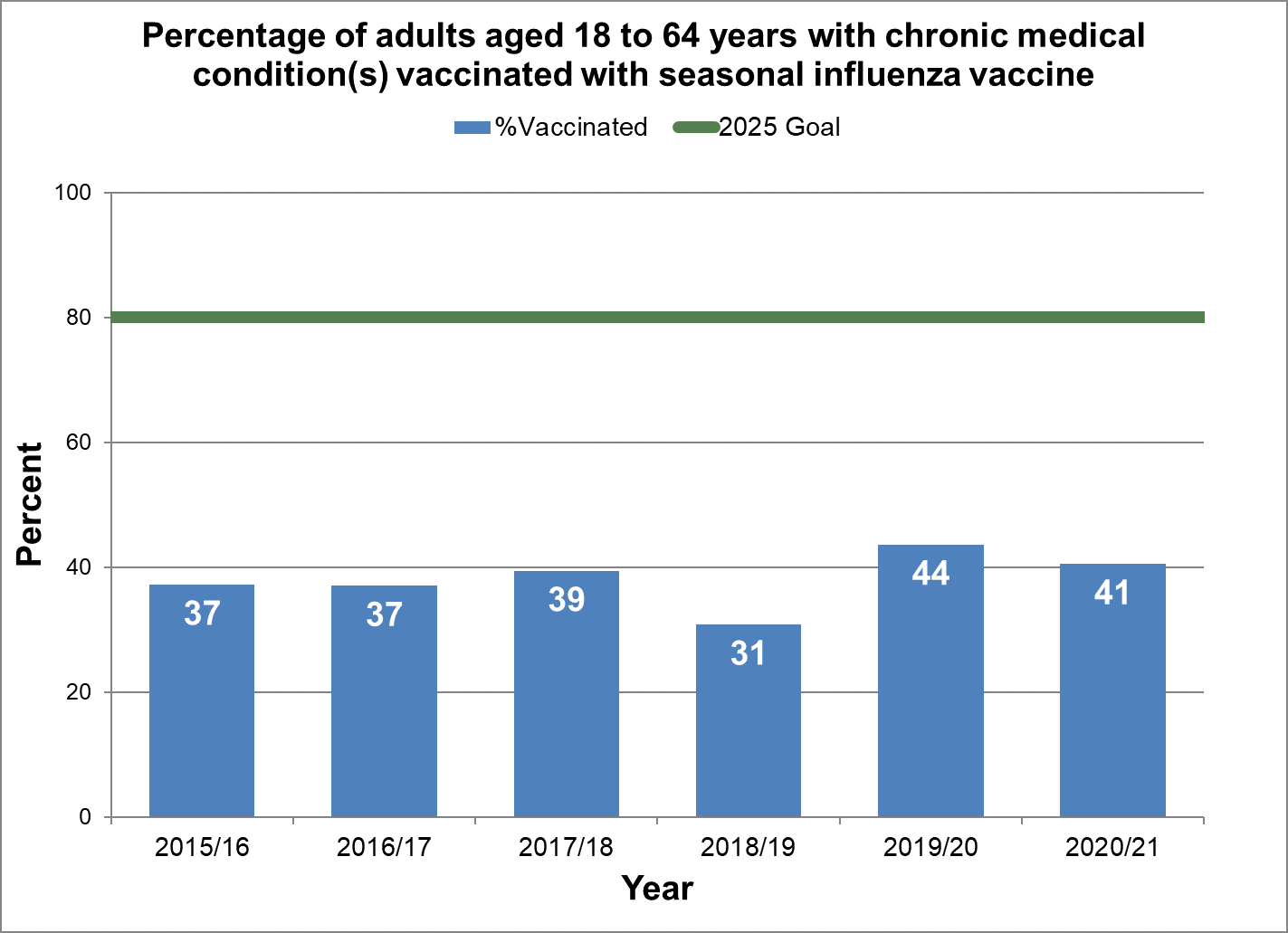
Text description: Figure 21
| % vaccinated | Year | 2025 goal of percent vaccinated |
|---|---|---|
| 37.2 | 2015/16 | 80 |
| 37.0 | 2016/17 | 80 |
| 39.4 | 2017/18 | 80 |
| 30.8 | 2018/19 | 80 |
| 43.6 | 2019/20 | 80 |
| 40.5 | 2020/21 | 80 |
Achieve 80% vaccination coverage among health care professionals.
- Baseline:
- Not Available
- Target:
- 80%
- Data sources:
- To be determined
Vaccine Preventable Disease Reduction Targets by 2025
Diseases under elimination: Maintain elimination status
Maintain zero cases of polio in Canada.
- 2015 Baseline (range 2011-2015):
- 0 cases
- 2017 (range 2016-2017):
- 0 cases
- 2019 (range 2018-2019):
- 0 cases
- Target:
- 0 cases
- Rationale:
- Polio is expected to be globally eradicated before 2025.
- Data sources:
-
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
- The last Canadian acquired case of wild polio was in 1996 (asymptomatic).
Maintain the elimination of endemic measles in Canada.
- 2015 Baseline (range 2011-2015):
- 0 endemic cases
- 2017 (range 2016-2017):
- 0 endemic cases
- 2019 (range 2018-2019):
- 0 endemic cases
- Target:
- 0 endemic cases
- Rationale:
-
Endemic measles has been eliminated from Canada since 1998 and in the Region of the Americas since 2016.
The World Health Assembly has endorsed the Global Vaccine Action Plan with the objective to eliminate measles in 5 WHO Regions by 2020.
- Data sources:
-
Canadian Measles and Rubella Surveillance System (CMRSS)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
Measles elimination is defined as an interruption of endemic measles virus transmission for a period ≥12 months, in the presence of high-quality surveillance.
Canada may continue to detect occasional imported cases, but with a vaccination coverage goal of 95% by 2025, community (herd) immunity will help to protect against second and subsequent generation cases.
Maintain the elimination of endemic rubella in Canada.
- 2015 Baseline (range 2011-2015):
- 0 endemic cases
- 2017 (range 2016-2017):
- 0 endemic cases
- 2019 (range 2018-2019):
- 0 endemic cases
- Target:
- 0 endemic cases
- Rationale:
- Endemic rubella has been eliminated in Canada since 2005 and the Region of the Americas since 2015.
- Data sources:
-
Canadian Measles and Rubella Surveillance System (CMRSS)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
- Canada may continue to detect occasional imported cases, but with a vaccination coverage goal of 95% by 2025, community (herd) immunity will help to protect against second and subsequent generation of cases.
Maintain zero cases of Congenital Rubella Syndrome (CRS)/Congenital Rubella Infection (CRI) in Canada.
- 2015 Baseline (range 2011-2015):
- 0 cases
- 2017 (range 2016-2017):
- 0 cases
- 2019 (range 2018-2019):
- 0 cases
- Target:
- 0 cases
- Rationale:
-
There have been no cases of endemically acquired CRS/CRI in Canada since 2000.
Endemic rubella has been eliminated in Canada since 2005 and the Region of the Americas since 2015.
- Data sources:
-
Canadian Measles and Rubella Surveillance System (CMRSS)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
Endemic diseases with low-level incidence: Maintain low levels
Achieve zero annual cases of respiratory diphtheria resulting from exposure in Canada.
- 2015 Baseline (range 2011-2015):
- Not Available
- 2017 (range 2016-2017):
- Not Available
- 2019 (range 2018-2019):
- Not Available
- Target:
- 0 cases
- Data sources:
-
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
- An average of 1 case per year (range 0-2) of diphtheria was reported from 2011 to 2015, while an average of 6 cases per year (range 1-10) were reported from 2016 to 2017 and an average of 6 cases per year (range 3-9) were reported from 2018 to 2019. In order to measure this target, the national case definition for diphtheria requires revision to distinguish between respiratory and cutaneous diphtheria and to address geographic location of exposure.
Maintain less than five cases of tetanus annually in Canada.
- 2015 Baseline (range 2011-2015):
- 4 cases (2-6)
- 2017 (range 2016-2017):
- 4 cases (4-5)
- 2019 (range 2018-2019):
- 4 cases (1-4)
- Target:
- Less than 5 cases
- Rationale:
-
Clostridium tetani spores are ubiquitous and are impossible to eliminate from the environment.
A threshold of less than 5 cases is sufficiently low to demonstrate success of the vaccination program.
- Data sources:
-
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
As tetanus is not a communicable disease, community (herd) immunity is not applicable.
Data are presented as the number of cases in the reporting year, followed by the range of cases over the years specified.
Maintain zero cases of maternal/neonatal tetanus in Canada.
- 2015 Baseline (range 2011-2015):
- Not Available
- 2017 (range 2016-2017):
- Not Available
- 2019 (range 2018-2019):
- Not Available
- Target:
- 0 cases
- Rationale:
- Remain consistent with WHO goal of eliminating maternal and neonatal tetanus.
- Data sources:
-
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
- Maternal and neonatal tetanus was eliminated in the Regions of the Americas in 2017. Due to universally available, high quality healthcare, maternal/neonatal tetanus is unexpected in Canada. Work is currently underway to estimate maternal/neonatal tetanus case counts based on diagnostic codes from Canadian hospital databases and consultations with provincial and territorial partners.
Maintain less than five cases of preventable Haemophilus influenzae type B (Hib) annually in children less than five years of age.
- 2015 Baseline (range 2011-2015):
- 0 cases (0-1)
- 2017 (range 2016-2017):
- 1 case (0-1)
- 2019 (range 2018-2019):
- 1 case (1-2)
- Target:
- Less than 5 cases annually of preventable Hib in children less than 5 years of age.
- Rationale:
- The threshold is sufficiently low to demonstrate success of the vaccination program and was set to address modifiable outcomes by targeting cases among un/partially-vaccinated children.
- Data sources:
-
Immunization Monitoring Program ACTive (IMPACT)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
Data from the Canadian Immunization Monitoring Program, ACTive (IMPACT) is obtained from 12 tertiary-care paediatric hospitals across Canada, representing 90% of all tertiary care paediatric beds in Canada; therefore, cases may be under-represented.
Preventable Hib cases refer to those who were either vaccine eligible but did not receive the vaccine or, are under-vaccinated for their age-appropriate vaccination schedule. Vaccine failures are not considered to be preventable.
Data are presented as the number of cases in the reporting year, followed by the range of cases over the years specified.
Maintain an average of less than 100 mumps cases annually in Canada (based on a five-year rolling average).
- 2015 Baseline (range 2011-2015):
- 103 cases (103-617)
- 2017 (range 2016-2017):
- 565 cases (122-565)
- 2019 (range 2018-2019):
- 734 cases (707-734)
- Target:
- Five year average of less than 100 cases annually
- Rationale:
- Given the cyclical nature of mumps outbreaks, the target number of cases is a five year average, rather than annual target.
- Data sources:
-
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
The intended target should reflect the need for continued control of mumps and recognizes the cyclical nature of mumps outbreaks.
Data are presented as the five-year rolling average in the reported year, followed by the range of the five-year rolling average over the years specified.
Maintain less than five cases of invasive meningococcal disease (IMD) serogroup C in children less than 18 years of age.
- 2015 Baseline (range 2011-2015):
- 0 cases (0-3)
- 2017 (range 2016-2017):
- 2 cases (0-2)
- 2019 (range 2018-2019):
- 3 cases (2-3)
- Target:
- Less than 5 cases in children under 18 years.
- Rationale:
- The low incidences in baseline years (2011-2015) are indicative of an effective vaccine delivered through mature vaccination programs in the provinces and territories. This level of success should be sustained.
- Data sources:
-
Enhanced Invasive Meningococcal Disease Surveillance System (eIMDSS)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
Data are presented as the number of cases in the reporting year, followed by the range of cases over the years specified.
Endemic disease with moderate levels of incidence: Reduce levels
Reduce incidence of invasive pneumococcal disease (IPD) in adults aged 65 years and older.
- 2015 Baseline (range 2011-2015):
- 23.6 cases per 100 000 population (23.6-26.0)
- 2017 (range 2016-2017):
- 24.0 cases per 100 000 population (22.7-24.0)
- 2019 (range 2018-2019):
- 23.2 cases per 100 000 population (23.2-25.8)
- Target:
- A 5% reduction in the overall incidence of IPD among ≥65 years of age.
- Rationale:
-
Due to the serotypes associated with adult IPD, it is estimated that 61% of isolates from patients aged 65 and older were potentially preventable by vaccination (serotypes contained in pneumococcal conjugate vaccine 13 and pneumococcal polysaccharide vaccine 23).
Despite the pneumococcal polysaccharide vaccine (PPV23) being funded in all provinces and territories, and the National Advisory Committee on Immunization recommending one dose for ages ≥65 years, the vaccine coverage is only 42%.
- Data sources:
-
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
- Data are presented as the incidence rate in the reporting year, followed by the incidence rate range over the years specified.
Maintain less than 50 hospitalizations annually for varicella in vaccine-eligible children less than 18 years of age.
- 2015 Baseline (range 2011-2015):
- 31 hospitalizations (12-31) – among non-immunized, vaccine-eligible children.
- 2017 (range 2016-2017):
- 9 hospitalizations (9-15) – among non-immunized, vaccine-eligible children.
- 2019 (range 2018-2019):
- 5 hospitalizations (5-5) – among non-immunized, vaccine-eligible children.
- Target:
- Less than 50 hospitalizations annually.
- Rationale:
- The varicella vaccine program was initiated in 2000 and was fully implemented across Canada in 2007. Compared to pre-vaccine era, an 85% reduction in varicella-related pediatric hospitalizations was seen since 2008. Since then, varicella-related hospitalizations have remained stable.
- Data sources:
-
Immunization Monitoring Program ACTive (IMPACT)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
Data from the Canadian Immunization Monitoring Program, ACTive (IMPACT) is obtained from 12 tertiary-care paediatric hospitals across Canada, representing 90% of all tertiary care paediatric beds in Canada; therefore, cases may be under-represented.
Data are presented as the number of hospitalizations in the reporting year, followed by the range of hospitalizations over the years specified.
Maintain an average of less than three deaths annually that are due to pertussis in infants less than six months of age (based on a three-year rolling average).
- 2015 Baseline (range 2011-2015):
- 1 death (1-2)
- 2017 (range 2016-2017):
- <1 death (<1-1)
- 2019 (range 2018-2019):
- 1 death (<1-1)
- Target:
- Less than 3 deaths in infants less than six months of age.
- Rationale:
-
The highest incidence of pertussis is in infants less than one year of age, and most deaths are in infants less than six months of age.
Due to the cyclical nature of pertussis incidence, targets should be based on a rolling average.
The goal of pertussis vaccination programs is to reduce hospitalizations and mortality in the under one age group. To align with this goal, the disease reduction target focuses on mitigating severe outcomes, including death, which are most likely to occur in infants less than six months.
- Data sources:
-
Immunization Monitoring Program ACTive (IMPACT)
Vaccine Preventable Disease Surveillance Report – 2015, 2017 and 2019
- Notes:
-
Data from the Canadian Immunization Monitoring Program, ACTive (IMPACT) is obtained from 12 tertiary-care paediatric hospitals across Canada, representing 90% of all tertiary care paediatric beds in Canada; therefore, cases may be under-represented.
Data are presented as the three-year rolling average in the reporting year, followed by the range of the three-year rolling average over the years specified.
Page details
- Date modified: