Products Used in Preventive Conservation – Technical Bulletin 32
Jean Tétreault
CCI Technical Bulletins
Technical Bulletins are published at intervals by the Canadian Conservation Institute (CCI) in Ottawa as a means of disseminating information on current techniques and principles of conservation of use to curators and conservators of Canada’s cultural objects as well as collection care professionals worldwide. The author welcomes comments.
Abstract
This Technical Bulletin provides a critical review of products typically used for the display, storage and transportation of objects by explaining how certain products can affect objects and their preservation, and it proposes guidelines for minimizing damaging effects. Products used to treat objects are not covered here, nor are electronic features such as lighting and security devices.
Author
Jean Tétreault studied at Montréal University where he received a Master’s degree in Analytical Chemistry in 1989. That same year he joined CCI, where he currently works as a conservation scientist in the Preservation Services Division. His main research interests focus on pollutants, products used for display and storage, paper degradation and passive environmental controls in collections. Results of his research have been published in various peer-reviewed journals. He has given more than 100 seminars in Canada and Western Europe on preventive conservation issues such as lighting, environmental guidelines, and exhibit and storage materials. Jean is the author of the book Airborne Pollutants in Museums, Galleries, and Archives: Risk Assessment, Control Strategies, and Preservation Management, published by CCI in 2003. Since 1998, he has also been a board member of the Indoor Air Pollution Working Group, which holds conferences every two years.
Table of contents
- Abbreviations
- Principles for selecting products for preventive conservation
- List of products used in preventive conservation
- Adhesives and tapes
- Boards and panels
- Coatings, laminates and films
- Containers
- Cushioning and padding
- Fabrics
- Films, sheeting, sleeves and envelopes
- Gaskets
- Glazing and glazing films
- Lines, twill tape, strings and wires
- Sealants
- Tubing, tubes and rods
- Where to buy products for preventive conservation purposes
- Tests for products used in preventive conservation
- Examples of preventive conservation practices
- References and further readings
Disclaimer: The information provided here is based on the current understanding of the issues presented. The guidelines given in this Technical Bulletin will not necessarily provide complete protection in all situations or protection against every possible adverse effect caused by the use of products in museum contexts.
Abbreviations
- ABS
- acrylonitrile-butadiene styrene
- CAMEO
- Conservation and Art Material Encyclopedia Online
- CCI
- Canadian Conservation Institute
- cm
- centimetre
- CPVC
- chlorinated poly(vinyl chloride)
- EPDM
- ethylene-propylene-diene monomer
- EPS
- expanded polystyrene
- EVOH
- ethylene vinyl alcohol
- g
- gram
- HDPE
- high-density polyethylene
- HDO
- high-density overlay
- ISO
- International Organization for Standardization
- LDPE
- low-density polyethylene
- LLDPE
- linear low-density polyethylene
- MDO
- medium-density overlay
- mL
- milliliter
- mm
- millimetre
- PC
- polycarbonate
- PE
- polyethylene
- PET
- poly(ethylene terephthalate)
- PETG
- poly(ethylene terephthalate) glycol
- pH
- potential hydrogen
- PMMA
- poly(methyl methacrylate)
- PP
- polypropylene
- PS
- polystyrene
- PTFE
- poly(tetrafluoroethylene)
- PUR
- polyurethane
- PVC
- poly(vinyl chloride)
- PVDC
- poly(vinylidene chloride)
- RH
- relative humidity
- SBR
- styrene-butadiene rubbers
- SDS
- safety data sheet
- SPNHC
- The Society for the Preservation of Natural History Collections
- TVOC
- total volatile organic compounds
- µg
- microgram
- µm
- micrometre
- UV
- ultraviolet
- VOC
- volatile organic compound
- XLPE
- crosslinked polyethylene
- XPS
- extruded polystyrene
Principles for selecting products for preventive conservation
This section describes the principles that guide the selection of products typically used for display, storage and transportation. Safe use of products depends not only on their stability, but also on the context and way in which they are used.
Key terms related to products
Enclosure
A structure or covering that completely surrounds and encloses a limited volume of space and in which one or several museum objects may be contained. Examples include plastic bags, display cases, storage cabinets, boxes and transportation crates. Note that an enclosure is constructed or assembled using one or more products.
Material
A substance that makes up an object or a product, e.g. copper, oak and cotton are materials.
Object
In this context, as opposed to products, an object is an item that is collected by museums, archives or private individuals, because the item is judged by society, or some of its members, to be of historical, artistic, social or scientific importance. Objects can be composed of one or more materials and can be movable (such as archaeological and historic artifacts, paintings, books, archival documents, furniture, costumes, etc.) or immovable (such as architectural interiors, monuments and historic buildings).
Pollutant
In this context, the term “pollutant” is used to describe any compound originating from a product that reacts with objects or becomes strongly bonded to them. Pollutants reach objects as:
- airborne pollutants such as compounds in the form of gases, vapours, aerosols or particles (dust, smoke and spores)
- falling debris (loose material falling on objects)
- compounds transferred by contact (i.e. surface-to-surface transfer)
More general information about pollutants can be found on the CCI website under Agent of deterioration: pollutants.
Product
In this context, the term “product” describes a manufactured or processed substance composed of one or many materials, which can be used alone or assembled with other products into more complex finished items. For example, plywood is a product made of two materials (wood and adhesive) and can be used as is as a platform or can be assembled with other products to make display cases or packing crates.
The ideal product versus reality
Ideally, products used in museums should not contain pollutants and should have long-term chemical and physical stability. Modern glass meets these criteria fairly well but not completely. Metals such as steel plates might also be considered ideal products because of their durability and their absence of off-gassing. However, most uncoated metals will tarnish slowly with oxygen and water vapour in the environment and can eventually stain objects through contact (Figure 1).

© Government of Canada, Canadian Conservation Institute. CCI 120171-0002
Figure 1. Paper stained by corrosion of a steel paperclip.
Many plastics are far from perfect for museum and archival use. For example, numerous types of plastic sheets or panels are expected to embrittle or weaken to such an extent that within 30 to 50 years they are liable to tear or crack under stress. Some plastic sheets, such as certain types of polyethylene sheeting, become brittle after only five years. Consult Plastics – gifts of the 20th century and challenges of the 21st century.
Although some products have deficiencies, using only ideal products for all applications can be restrictive and expensive. Fortunately, for many applications, products do not need to be chemically and physically perfect, such as when the objects are non-reactive (or slightly reactive) or robust or are not located close to emissive products. Thus, many less-than-ideal products can still be considered because of their other benefits, such as low cost, availability, functionality or sustainability.
Many products can be used in the care of objects in museum collections with a low risk of damage to many types of objects. However, before choosing your materials, a proper risk assessment needs to be made.
Assessing the risks of damage
Understanding the nature of both the objects and the products that will occupy the same environment is important when assessing the risk of any potential interaction between them.
Assessing the vulnerability of the objects
In order to identify risks of damage from products to objects, it is useful to recognize which type of object is most susceptible to various pollutants or to degradation mechanisms and the types of damage that may occur. Tables 1 to 3 detail:
- damage that occurs to objects by airborne pollutants emitted by products (Table 1);
- damage that occurs to objects by direct contact with products (Table 2); and
- damage that occurs to the object by incorrect use of products (Table 3).
Use these tables to determine if your objects could be at risk when sharing the same environment or context with potentially harmful products.
| Objects and types of damageTable 1 note1 | Pollutants | Products that can release the pollutants |
|---|---|---|
|
Acetic acid |
|
|
Adipic acid |
|
|
Ammonia |
|
|
Benzoic acid |
|
|
Formic acid |
|
|
Hydrogen peroxide |
NB: Peroxides can oxidize aldehydes and form carboxylic acids such as acetic and formic acids. |
|
Hydrogen sulfide; Carbonyl sulfide |
|
|
Nitrogen oxides |
|
Table 1 note
- Table 1 note 1
-
Damage reported in ambient conditions, often in airtight enclosures. No accelerated methods were used. High relative humidity (RH) will increase the rate or the extent of damage in most cases
| Types of damage on objectsTable 2 note 1 | Objects at risk, with details of damage | Product and/or compounds within product causing damage |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
Products or compounds that risk sticking to objects include:
|
|
Various types of objects, including:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2 note
- Table 2 Footnote 1
-
Damage reported in ambient conditions. No accelerated methods were used.
| Types of physical damage to objects | Causes |
|---|---|
| Breakage or cracks on objects |
|
| Deformation of the object |
|
| Surface abrasion or grooves |
|
| Surface alteration and dust generation |
|
Assessing potential threats from products
In order to evaluate potential risks to objects from specific products, various information about the products should be consulted and assessed, as discussed below.
Chemical composition
Determine the product’s chemical composition and its formation process. This will help identify any potential short- or long-term source of pollutants (consult Tables 1 and 2).
If both the object and the product have a similar composition (e.g. a wooden sculpture on a wooden base), there will usually be no significant damage observed over time.
Physical properties
Determine the product’s physical properties that are useful for its function or can have an impact on the object (e.g. dimension, porosity, strength, flexibility, abrasiveness of the surface). If the product is used to physically support an object, it must provide adequate support and a sufficient area of contact to avoid deformations or stresses due to gravity or handling; the points of contact should not cut, scratch, dent or deform the object’s surface; and the product should be strong enough to support the weight. Consult Table 3 for details on various types of physical damage that can be caused by incorrect use of products.
Rigid, tough products are often padded where they come into contact with an object. For more details on these issues, consult the CCI resource Mount-making for Museum Objects (Barclay et al. 2002).
Changes to properties over time
The long-term qualities or properties of most products vary, especially those composed of organic materials. Because most products are not designed for the preservation of museum collections, their use in some cases can lead to unintended damage. For example, products used inside a new display case can initially emit high levels of pollutants causing corrosion on objects within a few weeks or months. However, these levels usually diminish over time. A change in a product’s properties over time is caused or influenced by four factors:
- the chemical properties of the product;
- the physical properties of the product;
- the environment to which the product is exposed (e.g. RH, temperature, light and oxygen); and
- the context of its use (physical forces exerted on the product, such as tensions, compressions, load-bearing stresses, etc. [consult Table 3]).
Because certain deleterious properties of a product may evolve to become significant or insignificant over time, the product may be used in proximity to objects during the time frame when it does not cause significant adverse effects, while avoiding it otherwise.
Volatile emissions
One property of products that is of concern in preventive conservation is the emission of pollutants over time. Emissions tend to follow typical patterns. Generally, solvent- or water-based products (e.g. paints, varnishes, coatings, adhesives, caulking) release more volatile compounds when fresh (recently applied) than after a few weeks. Depending on a product’s emission rate at different phases of its life cycle, it may be possible to use the product under certain conditions and not others.
There are two typical phases in a product’s life cycle: a formation phase characterized by high emissions, followed by a stable phase. In addition, there is sometimes a third phase in which rapid degradation after a period of time causes significant emissions. Figure 2 represents these phases based on products emitting acetic acid.
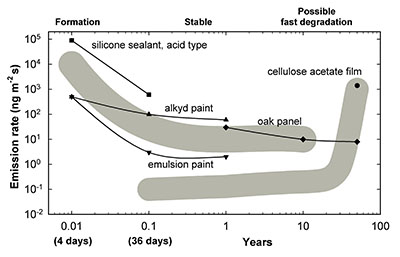
© Government of Canada, Canadian Conservation Institute. CCI 120171-0003
Figure 2. Emission of acetic acid from products over time. For acetoxy cure silicone sealants, alkyd paints and emulsion paints (acrylic, vinyl, vinyl-acrylic, vinyl ethylene, acrylic-styrene), emissions taper off after one month of airing out. Cellulose acetate film is initially stable when made, but after 40 years it starts off-gassing significantly.
Figure 2 – text version
| Years | Alkyd paint | Acetoxy cure silicone sealant | Oak panel | Cellulose acetate film | Emulsion paint | General trend of decreasing emission (grey band) | General trend of increasing emission (grey band) |
|---|---|---|---|---|---|---|---|
0.01 (4 days) |
500 |
90000 |
- | - | 500 |
10000 |
- |
0.1 (36 days) |
100 |
610 |
- | - | 3 |
30 |
0.1 |
1 |
60 |
- | 30 |
- | 2 |
10 |
0.1 |
10 |
- | - | 10 |
- | - | 10 |
1 |
50 |
- | - | 8 |
1400 |
- | - | 1000 |
100 |
- |
- |
- |
- |
- |
- |
- |
During the formation phase, liquid products, such as solvent- or water-based paints and adhesives, can generate significant amounts of volatile compounds when they are being applied and while still fresh. The same is true for products formed by chemical processes, such as room-temperature-vulcanizing (crosslinking) silicone, two-part urethane paints and two-part epoxy paints.
Many types of damage caused by high levels of volatile compounds reported in the conservation literature relate to objects that were stored or displayed in newly made enclosures. Corrosion or efflorescent compounds are usually noticed within the first three months of the object being placed in the inappropriate enclosure. Exposure to high concentrations of volatile compounds may not result in visible damages or mechanical changes in objects in the short term but can initiate chemical alterations that could affect them in the future.
After the initial period of curing or evaporation, typically three to four weeks, many products will enter a stable phase. In this phase, they typically release only a steady, low level of pollutants resulting from the ongoing processes of hydrolysis, oxidation or thermal degradation. Many products in this steady-state phase are generally safe to use in enclosures. Most coated wood and plastic products that release organic acids fall into this category. There are some exceptions to this rule: uncoated oak and cedar are affected by hydrolysis and release high amounts of organic acid vapours well into the stable phase. For example, a 50-year-old piece of oak will still have a noticeable smell of vinegar and can contribute to the corrosion of lead. Another example is the continual release of significant amounts of organic acids by oil-based paints that cure slowly over a long period of time (by oxidative polymerization). Formic acid released by these coatings can affect paper documents over the long term.
Although some products are not recommended for use during the early phase of their life cycle, it is quite the opposite for others. For example, some products—and objects—deteriorate after a period ranging from a few years to a few decades, and this deterioration causes an increase in the rate at which pollutants are emitted. Cellulose acetate sheets fall into this category as they suffer accelerating deterioration due to acid hydrolysis and release acetic acid. Low-cost polyurethane foam, often used as cushioning in transport packing crates, is also problematic at the end of its life cycle. As the foam degrades by photooxidation and hydrolysis, the foam progressively turns a yellowish-brown colour, which could stain an object by contact and can emit corrosive acids (it will also slowly loose its physical properties, as further discussed below).
Changes to physical properties
The physical properties of products tend to reflect their specific life-cycle phase. At the formation phase, organic products, such as adhesives and coatings, are typically fluid. At the stable phase, the products tend to maintain their desired properties. This stability is often extended by the use of additives such as antioxidants. However, cumulative chemical deterioration, fatigue or stress will eventually cause the physical properties of products to change. Some products or materials show chemical changes fairly quickly (e.g. within months or a couple of years); they are sometimes called “unstable” because of this. For example, acidic paper noticeably degrades at room temperature within a few decades, becoming yellower and much more brittle and fragile with age. As mentioned above, polyurethane foam will become brittle, no longer maintaining its original cushioning ability after only a few months (if receiving shocks or compressions) up to a few years. A polyurethane gasket is another example of a product with a limited lifespan of about 10–20 years, changing from rubbery to brittle (as shown in Figure 3) and thus losing its gasketing ability.

© Government of Canada, Canadian Conservation Institute. CCI 124703-0081
Figure 3. Some materials have a limited lifetime. For example, a 20-year-old urethane gasket, shown here, that was kept in the dark and in stable environmental conditions is now falling apart.
Assessing the context
The probability and extent of damage to an object by a product’s emissions also depends on their context. This context can be identified by three main parameters:
- contact. the proximity between a product and an object and whether or not they touch each other
- enclosure: the surface area of the emissive products in the enclosure compared to the volume within the enclosure and its airtightness
- length of exposure: the length of time a product and an object occupy the same location and can interact with each other
Table 4 summarizes the risks of pollutant damage on collections stored in different contexts: with the product and object in contact or not, and in different enclosure designs (in an enclosed, either partially or fully airtight space, or in an open space, such as a room).
| Enclosure | Non-contact | Contact |
|---|---|---|
| Room or large and well-ventilated space |
|
|
| Small enclosure not fully airtight (1 to 10 air exchanges per day) |
|
|
| Small enclosure fully airtight (leakage rate below 1 air exchange per day) |
|
|
Table 4 note
- Table 4 Footnote 1
-
The risk is high in small enclosures if the product is a freshly applied solvent-based product (e.g. adhesive and paint) or a freshly applied chemically cured product (e.g. some silicone sealants).
Contact
Physical damage can occur at contact points between the object and the product. The product may provide an inadequate contact surface, such as one that is narrow or sharp (resulting in unequal pressure points) or that is abrasive or too hard. A hard surface can damage a softer or weaker one. In the case of a hard or an abrasive surface, vibrations can cause damage if the product and object slide or chatter against each other.
Chemical reactions can also occur at contact points when a product contains harmful compounds that migrate to the surface and transfer to the object or react with it. The transfer of pollutants can be avoided by choosing a different product or by using a good gas barrier interleaf. A list of types of damage to objects caused by contact with a product is listed in Table 2. More information on interleaves can be found in Interleaves – discrete and efficient.
Undesirable transfer also happens when an object is in contact with a sticky product, such as freshly applied paint (less than four weeks old), dark-coloured paint softened because of the nature of colourant added (this can happen in the case of paint up to a few years old), leatherette or old flexible PVC. In time, parts of the object such as fragile coatings, laminations and inlays can become detached and remain stuck to the product. The reverse can also happen where solid compounds from the product can become stuck to the object, as shown in Figure 4.

© Government of Canada, Canadian Conservation Institute. CCI 124703-0082
Figure 4. An object made of acrylic was placed for a few weeks on a surface that had been painted a dark brown colour a year before. When the object was removed, fragments of the paint layer remained strongly adhered to the object.
Compounds can also transfer from objects to products depending on the nature of the object and this is particularly likely with dirty, acidic or fatty/oily objects. Compounds from such objects can stain their mount, their container or even their label, affecting the look of the display and its capacity to be reused, since stained products can stain or damage other objects with which they subsequently come into contact.
Finally, contact between dissimilar metals can cause galvanic corrosion. An electrochemical cell is created where the metals act as anodes and cathodes in the presence of an electrolyte (water with salt). The corrosion affects the anodic metal, i.e. the more reactive or less noble metal. This process is usually negligible in most indoor situations but can be observed in humid environments. For example, if a large copper object is in contact with an iron wire, the wire will corrode at the contact point when exposed to high humidity since iron is less noble than copper. The rust that developed on the iron will then stain the copper object. Metal-to-metal contact also risks causing physical abrasion of surfaces if some vibration occurs.
Enclosure
The different physical parameters of the enclosure will have an influence on the concentration of pollutants generated by a product used within it. The concentration of pollutants released by products can be expressed by a simple formula:
C = E A / V N
Where
C: concentration in the enclosure (μg/m3)
E: emission rate of the pollutant from the product (μg/m2h)
A: product surface area (m2)
V: volume of the enclosure (m3)
N: leakage rate of the enclosure (h-1)
From the formula, it can be seen that the most dangerous scenario is when a vulnerable object is placed in a well-sealed enclosure with a large surface area of an emissive product. At the other extreme, the risk of damage will be negligible when either a well-sealed enclosure does not contain emission products or a moderate amount of product-generated pollutants can be easily dissipated from the enclosure. In between, the risk of damage will vary between low and high.
For a more complete formula, one needs to consider extra parameters such as the concentration of pollutants outside the enclosure and the capacity of other products to sorb pollutants. More parameters can be added by considering the contributions of both space and time, such as the concentration gradient through the enclosure and the emission or sorption rate over time (which are also influenced by the RH and temperature).
In general, the use of enclosures such as small boxes and display or transportation cases is the best way to protect objects from outdoor pollutants, with the highest performance achieved when they are well-sealed, but this can expose them to pollutants released by products or other objects inside the enclosures. Table 5 describes in more detail how enclosures at three different levels of airtightness affect objects exposed to pollutants or to other agents of deterioration. Overall, tightly fitted enclosures are an excellent preventive conservation strategy on many fronts, provided that no off-gassing object or product is trapped inside the enclosure.
| Issue | Leaky enclosures (more than 10 air exchanges per day) |
Semi-airtight enclosures (1–10 air exchanges per day) |
Airtight enclosuresTable 5 note 1 (less than 1 air exchange per day) |
|---|---|---|---|
| Pollutants generated in the room or from the outside | Relies on the controls provided in the room. Dust deposition is a problem. |
Provides weak to good protection. | Provides the best protection against outdoor pollutants and pollutants related to human activities in the room. |
| Pollutants generated inside the enclosure from some of the objects or products | The leaks allow pollutant levels inside to remain low. | Levels inside will be low to medium. | Levels inside can reach maximum levels (equilibrium). This is usually a concern mainly for objects sensitive to carbonyls or sulfur compounds. Sorbents may help to reduce levels if the rate of emission is not too high. |
| Relative humidity(RH) | The RH will be the same as in the room. | It runs the risk of high localized RH if there is a cold enclosure wall. Moisture-buffering products will have low to moderate efficiency. |
Generally beneficial but there are risks of high RH or large RH fluctuations if the temperature fluctuates more than 5°C combined with no moisture-buffering surface or no product inside the enclosure to help buffer the RH change. There is a risk of high RH or mould if the stored objects are moist or if there is a cold enclosure wall. |
| Temperature | The temperature will be the same as in the room. | The temperature will be the same as in the room with a delay depending on the thermal insulation of the enclosure walls. Lamps inside enclosures increase the temperature (which will affect the RH). |
The temperature will be the same as in the room with a delay depending on the thermal insulation of the enclosure walls. Lamps inside enclosures increase the temperature (which will affect the RH). Keep the enclosure at least 10 cm (4 in.) away from an exterior (cold) wall or from the floor if the enclosure panel facing the wall or the bottom is not thermally isolated. |
| Insects | No protection from risk of infestation. | Little protection from risk of infestation. | Usually good protection from risk of infestation, although insects can perforate and go through products such as a thin plastic sheet. |
| Water leaks | Provides weak protection (depends on the design). | Provides weak to good protection (depends on the design). | Provides the best protection. |
| Consider leak-proof enclosure tops. Raise enclosures 10 cm (4 in.) above the floor. | |||
| Other agents of deterioration |
|
||
| Design and maintenance | Ventilation inside a leaky enclosure occurs by stack pressure, which allows dust infiltration. This can be counteracted using a dust screen or a positive-pressure filter system. Filters must be replaced periodically. | Can use a positive-pressure filter system. | Must use sealed seams and adequate gaskets. Ensure proper air circulation between objects and moisture and pollutant sorbents. Pollutant sorbents will provide additional long-term protection. Their periodic maintenance is required. Frequent need for access may limit airtightness. |
Table note
- Table 5 note 1
-
These require special attention to the assembly, in particular good-quality, carefully designed gaskets and sealants.
Length of exposure
It is not always possible to display or transport objects in pollutant-free enclosures. Sometimes enclosures may be made with potentially problematic products for different reasons such as design, the recycling of existing enclosures or simply due to financial and time constraints. A balance between optimum preservation and visitor access must be considered. For temporary use, some small imperfections in the set-up may be acceptable. However, for storage or permanent display, it is best to investigate the most appropriate products.
Instead of displaying an object in an exhibition case lacking an adequate long-term set-up, the object could be displayed for a limited time before being returned to better storage conditions. The same approach is used for exposure to light: an object can follow display–storage cycles where it is displayed only a few months per year and spends most of its time stored in an airtight archival box while other objects are displayed.
Risk management knowledge helps decision makers understand that they have some flexibility in selecting products for their specific contexts and needs. Some low-cost or easily accessible products that have a limited lifetime, such as 10 years or less, can still be used temporarily in some cases; for example, polyurethane foam can be used as inside padding within shipping crates for short-term loan programs. This flexibility can be useful for assigning priorities when resources are limited. However, there is a risk that a product suitable for a well-defined short-term purpose might be used beyond the planned time frame or is reused for another purpose without an assessment of the new context, leading to the possibility of the product causing damage to an object.
If the risks of using a product in a specific context cannot be assessed with confidence, it is best to err on the side of safety.
Monitoring
As in any management project, it is a wise practice to monitor whether products are being properly used to fulfil their initial purposes. Manufacturers do change the formulation and manufacturing processes of products over time, usually for the best. Unfortunately, from time to time, the change may affect properties that are a concern for museums; for example, the adhesive is more acidic or the lifetime of a product is shorter. A significant problem occurred in the 1990s when polyethylene foams degraded and crumbled after only a few years, as shown in Figure 5. No products are exempt from failures. This is true of foam as well as any other kind of product.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0005
Figure 5. In the 1990s, one company made polyethylene foam board with a different process or formulation, which caused the dramatic degradation of the foam after a few years. Faulty foams (on the right) had an acrid smell. Some museums encountered this problem and had to replace all of the defective foam. The company has since fixed the problem (on the left), but a museum professional still has to wonder if it can happen again.
List of products used in preventive conservation
This section provides advice about choosing the right product from the wide range available and about avoiding situations that present significant risks of damage to objects. The products are grouped alphabetically according to function and material. A better understanding of products is just one part of the overall decision-making process when choosing products for preventive conservation—one must also consider the nature of the object and its context (placement, environment and intended use). Some products that are recommended for general use may present risks under certain conditions. For example, a product that could be used safely in an open space where ventilation is adequate may release unsuitably high levels of pollutants in an enclosure. Caution and critical assessment are always needed.
Disclaimer: The information provided in this Bulletin is based on the current understanding of the issues presented. The guidelines will not necessarily provide complete protection in all situations or protection against every adverse effect possibly caused by the use of products in museum contexts.
Adhesives and tapes
Applying adhesives or tapes directly to objects is unsafe and not recommended. Over time, adhesive on an object can become brittle or viscous, suffer bond failure and cause staining (Figure 6) that can be difficult to remove. Adhesives should be used only for assembling storage and display fittings, such as mounts, backings, boxes, sleeves and cases, and should not be applied directly to objects for the purpose of mounting or labelling. It can be assumed that all tapes will fail to adhere one day; it is just a question of time. In storage, it is not uncommon to find examples of old misguided practices; for example, labels that were applied directly onto objects now becoming completely dissociated (unglued). This not only causes stains but also puts very important information concerning the object (e.g. the accession number) at high risk of being lost. Similarly in displays, when objects have been glued to the wall, this has not only caused stains but also high risk of physical damage, because adhesive failure often occurs over time and results in the objects crashing to the floor.

© Government of Canada, Canadian Conservation Institute. CCI 120279-0005
Figure 6. Adhesive tape has left a residue on the soapstone sculpture.
Starch paste and methyl cellulose are examples of adhesives acceptable for use on paper artwork because they are removable if necessary (by a trained conservator) and do not stain. Even so, its use for preservation purposes is confined to attaching supportive hinges, and it is best applied under conservation supervision.

© Government of Canada, Canadian Conservation Institute. CCI 120078-0083

© Government of Canada, Canadian Conservation Institute. CCI 120078-0223
Figures 7a and 7b. Stains on a historic scrapbook caused by tape adhesive: before treatment (left) and after treatment (right). The stains are greatly reduced, and areas of damage are reinforced.
Unless the tape adhesive is part of the artist’s intent, all tape on objects that can be safely removed from objects should be done sooner rather than later. Tape adhered onto fragile substrates such as paper and photographs typically requires the expertise of a conservator (Figures 7a and 7b). If any doubt arises regarding the safety of the removal process, a conservator should be consulted. With archival documents, all sticky notes (e.g. Post-it) should be removed from direct contact but kept and preserved properly. The same applies for all staples and paperclips (Figure 1).
Adhesives used in an open space do not present a significant off-gassing problem. However, adhesives used in an enclosure should be allowed to off-gas or dry out (cure or set) for three to four weeks, especially if there is a large surface area to be glued, before sensitive objects are placed in the enclosure.
One way to avoid the constraint of drying time is to use hot-melt glue (hot glue sticks). This type of thermoplastic adhesive can be made up of a large variety of raw materials such as ethylene vinyl acetate, polyethylene and polyurethanes. Colourless hot-melt glues are preferable because they do not contain rosin or sulfur-based compounds. Rosin, a natural resin containing sulfur, yellows within a short period of time, while sulfur compounds tend to tarnish silver and discolour many organic objects (consult Test #1: Lead acetate test for detecting sulfur compounds for instructions on how to detect sulfur compounds in materials).
Sealants such as silicone and latex acrylic caulking are sometimes used as adhesives, e.g. when assembling cases, etc. More information on these products appears under Sealants.
Modelling clays, putties, waxes or clear gels (such as museum gel) are sometimes used to stabilize small objects against small shocks and vibrations. They are applied underneath the object, then the object is pressed down to the horizontal surface. This unobtrusive method can be a short- to medium-term solution for waterproof and smooth surfaces such as glass, metal or coated metal. However, it is not suitable for many types of porous objects such as wood, fabrics, unglazed ceramics, stone or historic glass (which can be porous) because the clay or gel contains organic or silicone oil which will leave stains or residues at the contact point. Some of these materials also contain sulfur compounds. The oil may be removable on non-porous surfaces but not on a porous object. Also, as the gel ages, it will lose its adherence properties. Risk analysis would be required to determine whether these products provide more benefit than risk, such as the possibility of failure and stains on the objects. Otherwise, the alternative would be the use of padded mechanical fasteners.
Alternatives to adhesives used for assembling storage and display fittings include mechanical fasteners such as nails, screws and hook-and-loop fasteners, such as Velcro, TouchTape, Aplix and Dura-Grip. Velcro products that contain an adhesive are not recommended because failure of the adhesive is likely an issue in the future. This is why it is preferable to use the type of Velcro that can be mechanically fixed (i.e. by sewing). For more information on mechanical fasteners, consult CCI’s resource Mount-making for Museum Objects (Barclay et al. 2002).
Boards and panels
Boards and panels are used extensively in a museum context to build structures such as shelving, display cases and shipping crates and to make storage trays, boxes and other enclosures, as well as backing boards, mounts and supports. The most common types of boards and panels are those that are plastic-based, wood and wood-based products (such as plywood) and paper-based products (such as matboard). Other types include metal panels, which are ideal for applications requiring strength and durability, and composite panels, which are ideal when looking for lightweight panels that remain rigid without bowing, even when used in extra-large dimensions. These are described in more detail below.
Plastic boards and panels
Note that transparent glazing materials for display cases and framing are discussed in their own section, Glazing and glazing films.
Poly(methyl methacrylate) (PMMA and acrylic)
Known as Plexiglas, Perspex, ACRYLITE or simply acrylic, this transparent sheet is popular for making display mounts (Figure 8) as well as cases (consult Glazing and glazing films for more information on display cases). The extruded type of acrylic sheet, which offers superior forming capabilities as compared to the cell cast type, is recommended for mount making since bending, forming and finishing are usually required. (In contrast, cell cast acrylic is recommended for framing and display cases because of its superior optical clarity.) Acrylic sheets in general can be joined using methylene chloride (solvent-dissolving acrylic) or with acetoxy cure silicone sealants or an epoxy. The resulting joints are strong but not strong enough to be load-bearing for medium to heavy weights; bending the acrylic, rather than joining pieces at an angle, is a stronger and less time-consuming alternative. Note that coloured translucent and opaque acrylic sheets are also available, as well as those with a mirrored look. Scratch-resistant types are also sold.

© Government of Canada, Canadian Conservation Institute. CCI 69345-0023
Figure 8. Acrylic sheet is widely used to make custom mounts for the display of museum objects.
Polycarbonate
Known as Lexan or Makrolon (formerly Tuffak), polycarbonate resists shattering, which gives it a security advantage as an impact-proof material. Some polycarbonates are slightly less crystal clear than acrylic. Polycarbonate is not as easy to glue due to its resistance to solvents, and the resulting joints are not as strong as joints in acrylic. Unlike acrylics, sharp bends in thin sheets (up to 3 mm or 1/8 in.) can be done cold by hand. Scratch-resistant varieties are also available.
Poly(ethylene terephthalate) glycol (PETG)
PETG, or copolyester sheets (a common brand name is Vivak), is especially useful for lightweight applications when transparency and ease of forming are required.
Corrugated sheets
Corrugated polymer sheets, such as fluted plastic sheet, polyflute, Coroplast, Cor-X, Correx, Hi-Core, Corru-Lite (Corulite) and Diversi-Plast, are made of polypropylene and polyethylene, or both as a copolymer. They are commonly used in conservation as flat bases or platforms or to make backing boards, folders, trays (Figure 9) and boxes. The corrugated sheets are available in different thicknesses and many colours. Factors such as the thickness of the walls composing the flutes, the stress of folding, exposure to UV radiation and extent of handling can limit the lifetime of corrugated sheets to a few decades. Panels having polypropylene may have a longer lifetime than those made only of polyethylene. Some corrugated plastics are specially made to be biodegradable, such as the Hi-Core Oxo-Biodegradable. If used in institutions, they must always be well-identified and used only for short-term purposes. Ideally, biodegradable products should not be used for conservation to avoid unwanted results. Recycled corrugated sheets should also be used for short and medium terms only because a portion of the polymer has probably been partly broken down and oxidized in its previous life. Consult the section on Containers to see how corrugated sheets are used to make boxes.
Some large panels made of polycarbonate (such as Verolite) and acrylic are available and are used for building structures (twinwall or multiwall) such as greenhouses. In museums, they can be used to support large-sized flat objects.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0006
Figure 9. Objects stored in a custom-made corrugated plastic tray, with cotton twill tape securing objects to the tray.
High-density polyethylene (HDPE)
High-density polyethylene hard boards or panels are popular choices when strength and rigidity are needed, e.g. to make display case bases. The product can be cut into various intricate shapes, can be used to make replicas and comes in a variety of colours. Marine and outdoor quality boards (e.g. Marine Board, StarBoard, Seaboard) typically have better chemical stability (e.g. addition of UV stabilizers). This could be beneficial if enclosures made of this product are exposed to direct sunlight.
Poly(methyl methacrylate) and polycarbonate (PC)
Coloured translucent and opaque acrylic and polycarbonate sheets are available, as well as those with a mirrored look. Consult Plastic boards and panels for further information on these materials.
Foam boards
For polyethylene (PE) foam board and polystyrene (PS) insulation foam board, consult Foam planks, thick (>13 mm or 1/2 in.).
Poly(vinyl chloride) (PVC)
Some hard foam boards are made of rigid PVC foams. Gatorcel, Komacel and Sintra are common trade names for these boards. Rigid PVC foam boards are not recommended for use in contact with objects or in a fairly airtight enclosure for long-term purposes. This constraint is to minimize the impact of the possible release of low levels of hydrochloric acid during the slow degradation of the PVC.
Wood and wood products
Both solid wood or wood product panels are commonly used. Wood products or wood-based boards are composites made chiefly of wood and glue. They include plywood, waferboard (oriented strand board and flakeboard), particleboard (chipboard) and fibreboard (low, medium and high density). The usual adhesives used for wood products are urea formaldehyde and phenol formaldehyde. The main problem with wood and wood products is the acidic emission of the wood components and their capacity to stain during contact (Figures 10a and 10b). A non-porous interleaf (e.g. Melinex) between the wood and the object will avoid the problem of staining (consult Interleaves – discrete and efficient for details on using an interleaf).

© Government of Canada, Canadian Conservation Institute. CCI 120171-0007

© Government of Canada, Canadian Conservation Institute. CCI 120171-0008
Figures 10a and 10b. An example of a stain caused by contact with a knot in a piece of wood. The brownish stain on the paper (left) was caused by contact with the back wooden panel, which has a large knot (as seen on the right).
Lead, copper alloys and paper are commonly known to be among the most susceptible types of objects to acidic vapours and thus, if possible, should not be exposed to emissions from wood and wood products, especially within enclosure settings where the emissions can accumulate.
When choosing wood and wood products for museum uses, the species can matter. Oak and cedar are the most acidic wood species, and in general, they should not be used to make enclosures. Rather, choose a wood species that has a pH higher than 5. It should be well-aged and conditioned to the temperature and humidity of the room. Elm, maple, poplar, ash and aspen are some of the Canadian species that usually have a pH above 5. An extended list of wood species with their respective pH values and how it can be measured can be found in CCI Technical Bulletin 21 Coatings for Display and Storage in Museums (Tétreault 1999). The pH of wood products may be hard to predict because they are composite materials. The use of pH strips saturated in a glycerol and water solution can be used to estimate the level of acidity generated by a product or by an enclosure. Consult Test #2: pH test using glycerol to measure the acidity of volatile compounds for a description of this test.
The adhesive used in wood products is usually not the main issue. Wood products may have a smell of adhesive when new, but the main concern should be focused on the acid emission from the wood itself. It is important to realize that no wood can be referred to as being of “museum-quality.” Formaldehyde-free wood products are beneficial in terms of human health but not usually for objects.
In terms of sustainability, it is best to use local wood and wood products, if possible, and from a well-managed forest. In Canada and the United States, the Forest Stewardship Council can certify that the wood meets sustainability criteria.
Also, some wood products, such as medium density fibreboard, can be made of post-consumer recycled content which is good from a sustainability point of view. In terms of conservation, these materials should be dealt with in the same way as new wood products.
Be aware that wood products can be treated with fire-retardant compounds. Some of these compounds make wood products more corrosive. As a precautionary approach, avoid these types of specially treated wood products for use within airtight enclosures.
A common method to reduce emissions from wood and wood products is to seal them with a coating or to buy them already laminated (for information about Formica and melamine laminates and medium- and high-density overlaid plywood [MDO and HDO], consult Coatings, laminates and films). In general, when ambient RH is well-controlled, adequately coated wood and wood products should be acceptable for enclosing most objects, except those containing lead. A coating on wood products will protect them and limit the generation of airborne wood particles.
Paperboards
For simplicity, as the name states, paperboards include all boards made of wood pulp that are thicker than a single sheet of paper. The most common are matboards and corrugated cardboards, but many other types of paper-based boards are possible. These products are lightweight and generally used in conservation to make boxes, trays, window mats, mounts, folders, backing boards, etc.
The best practice recommends the use of buffered (acid-free) wood pulp-based products including paperboards for the storage and display of photographs and paper documents. The alkaline reserve from the calcium carbonate in the board will extend the lifetime of the mount or box itself compared to a regular acidic product. Although it is not conclusively proven that buffered boards can slow down the degradation of papers, at least they will not further contribute to their degradation.

© Government of Canada, Canadian Conservation Institute. CCI 124703-0054
Figure 11. Paper artwork stained by an acidic mat after many years of contact.
In practice, books and paper documents are sometimes stored in acidic boxes. This situation is tolerated if the content is acidic, such as papers made of wood-pulp fibres. Although acidic boxes are not ideal, at least the papers will be protected against outdoor gases and dust deposition and light. In terms of chemistry, the main cause of deterioration of acidic paper is the amount of water in the paper (acid-catalyzed hydrolysis). To reduce this deterioration, strategies can aim at preventing the paper from becoming more fragile and yellowing by limiting periods of high temperature and high RH.
Boards in contact with photographic documents should also pass the photographic activity test (ISO Standard 14523:1999).
Matboards
Matboard is a paperboard typically used by the picture framing field as well as in conservation for mounting and storing works of art on paper and photographs (folders, window mats) and, in glazed frames, for increasing the gap between the mounted artwork and the glass. Acidic matboards are known to damage paper artwork, as shown in Figure 11. Matboard recommended for conservation purposes, described by the manufacturers as “100% rag fibre,” is available either buffered (pH 7.5–8.5) or unbuffered (pH 7). Matboard made from highly purified wood pulp buffered to pH 8.5 is an acceptable, more economical alternative. Matboard that is buffered (i.e. has a pH slightly above 7.0) to protect paper from acidity is appropriate for most works on paper. Generally, the use of white or buffered four-ply matboard is recommended (Figure 12). If using coloured matboard, ensure that the dye is fast (consult Test #3: Testing for colourfastness).

© Government of Canada, Canadian Conservation Institute. CCI 85428-0012
Figure 12. Aquatint on paper depicting a large boat on water, in window mat. Entitled Commencement of the Action Between His Majesty’s Ship Shannon and the United States Frigate Chesapeake, this work of art belongs to the Art Gallery of Nova Scotia, accession number 2003.397.
Corrugated paperboards
The common corrugated cardboards used everywhere commercially for storage and packing of manufactured goods are acidic, but acid-free and buffered varieties are produced and marketed for the museum and archival field. Corrugated boards are available in a few different thicknesses (e.g. single or double corrugated layer). They are useful as lightweight platforms and trays or to make storage boxes and containers (Figures 13a and 13b).

© Government of Canada, Canadian Conservation Institute. CCI 126389-0039

© Government of Canada, Canadian Conservation Institute. CCI 126389-0037
Figures 13a and 13b. A twined basket with a silk lining, stored within a custom-made box constructed of acid-free corrugated paperboard. The basket rests on a cloth-covered, foam-padded movable base inside the box (left). The basket within its box with the lid on (right).
Metals
Metal sheets such as aluminum, steel or galvanized steel are also used for building display cases or storage cabinets. In indoor environments, many metals tend to slowly tarnish and may stain objects that they are in contact with. Contact between metallic objects and metallic products may cause galvanic corrosion in a highly humid environment (described under Contact). For this reason, the use of coated metallic units is recommended if contact with objects is expected in both the short and long term. In particular, powder-coated metal shelving is often used in museums, as it is sturdy, does not contain solvents nor corrode and eliminates the risk of staining. More details about the selection and use of coatings can be found under Coatings, laminates and films.
Composite panels
Composite panels are typically lightweight and usually quite rigid. They include foam core panels, honeycomb panels and metallic composites.
Foam core boards
Boards with foam centres (foam core boards or laminated foam boards such as Gatorplast, Gator board or Gatorfoam, Fome-Cor) are chiefly made of polystyrene foam laminated on each side with thin sheets usually made of plastic (e.g. a hard polystyrene sheet or a sheet of resin-impregnated paper). Foam boards are light and somewhat fragile. Those made of a thin layer of polystyrene adhered to the foam core can be used safely in terms of minimal risk of off-gassing. As for the resin-impregnated paper types, the most common products are acidic but some made for the museum and archival field are acid-free. The nature of the adhesive gluing the laminated layers is not critical since it is covered by the vapour-proof outer layers of plastic or buffered paper. No problems related to emissions or contact are expected with them. However, foam boards with a laminate made of acidic paper, thin cardboard or wood veneer, or PVC rigid board may need evaluation to ensure their compatibility with the objects that they may be in contact with. Products made of hard foam cores covered with metal sheets are discussed further below.
Honeycomb panels
Honeycomb panels are a very light and versatile alternative to other kinds of heavy sheet products such as wood panels. They can be made of paper and cardboard, aluminum, polyethylene, polycarbonate or other plastics. Their top surfaces are often laminated with paper, wood products, aluminum or plastic film. They can be used for flat mounts or for backing boards used in framing, as well as flat supports for paintings and textiles. They are less readily available in local hardware stores and tend to be expensive. Note that some honeycomb panels are finished with potentially problematic materials such as acidic paper, cardboard or wood veneer. Some honeycomb panels fall in the category of metallic composite panels, described below.
Metallic composite panels
Composite laminated panels with rigid metal skins on rigid or foam plastic cores, such as the aluminum composite products made by Dibond, Reynobond or Alucobond, are being successfully used to provide a metallic look when building display cases. They can also be called by the generic name of “foam sandwich panels.”
Sometimes, metal sheets are used on their own to build the structure of the display cases.
Coatings, laminates and films
Coatings are often applied on wood, metal and concrete surfaces for aesthetic purposes but also to protect the substrates and to block potentially harmful compounds that can be released by wood products. However, sometimes, there may be more harmful volatile compounds released by coatings than by the wood itself. It is important to follow guidelines for the selection of coatings and to adhere to their drying periods in order to avoid unsafe conditions. Where time is limited, consider using laminated wood products that prevent emissions, instead of other coatings that may need several weeks of airing out to off-gas naturally.
Coatings
Coatings such as paints, varnishes and stains are used for aesthetic purposes and to provide vapour barriers. Coatings formed by oxidative polymerization, such as oil-based paints, oil-based urethanes, alkyds, melamine and epoxy esters (one-component or one-part systems), should be avoided in museums because they release acids and peroxides that can harm objects, especially papers, metal objects (lead, copper and their alloys) and black and white photographs. Fortunately, with new volatile organic compound (VOC) regulations in Canada and the United States, these coatings are becoming less and less available. The coatings that are generally acceptable in archival and museum contexts are acrylic or acrylic-urethane emulsion paints (latex) and, for special applications, two-part epoxy and two-part urethane paints.
Drying period
Even with recommended coatings, there is an important factor to consider: the drying period. As shown in Figure 14, many volatile compounds are released when the coating is still relatively fresh; the emissions decrease as the paint dries or cures. As the figure shows, the emission rate tapers off (the curve flattens) after 20 to 30 days. This is why a drying period of four weeks is recommended. Planning this drying period ahead of time when schedules and timelines are being developed is important. However, there may be situations where a balance is required between reaching a low level of volatile compounds in the freshly painted enclosure (and consequently reaching a low risk of damage) and the need to install the object in that enclosure with only a short delay. For example, there may be a delay in one or more preceding steps during an exhibition project with a fixed deadline which can lead to pressure to cut down the drying period. Research done in 1997 at CCI did not conclude that low volatile organic compound paints can be safely used with a shorter drying time. Today, most paints that are on the market emit fewer volatile compounds than 10–20 years ago; unfortunately, no research had been done to support a reduction of the drying period while keeping the risk of damage low. A rough estimate of the acidity generated by painted enclosures can be done with pH strips saturated with a glycerol and water solution (consult Test #2: pH test using glycerol for measuring the acidity of volatile compounds).
The drying period for a painted surface in a room is less critical since normal room ventilation will prevent high levels of pollutants from accumulating. Volatile compounds released by the painted walls, floors or shelves should dissipate easily.
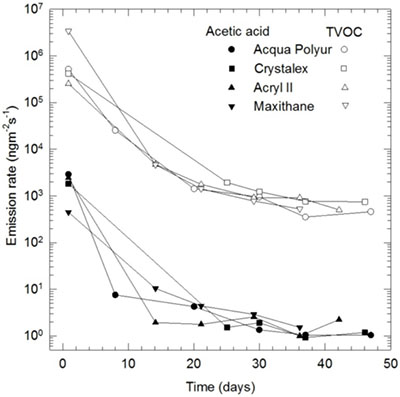
© Government of Canada, Canadian Conservation Institute. CCI 120171-0009
Figure 14. Emission of volatile compounds over time for four brands of acrylic and urethane varnishes. The emission rate of different emulsion varnishes reduces exponentially over time. The curves show that the volatile emission after two weeks is about 10 times less than after one week. After four weeks of off-gassing, the curves flatten off, so there is not much to be gained by airing out the painted surfaces further. (TVOC: total volatile organic compounds)
Figure 14 – text version
| Acqua Polyur | Crystalex | Acryl II | Maxithane | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (days) | Acetic acid (AA) | Total volatile organic compounds (TVOC) | Time (days) | AA | TVOC | Time (days) | AA | TVOC | Time (days) | AA | TVOC |
0.833 |
2912.112 |
519105.6 |
0.833 |
1820.592 |
419559.8 |
0.833 |
2452.32 |
254931.8 |
0.833 |
447.552 |
3413318 |
7.98 |
7.55 |
25555.6 |
25.06 |
1.525 |
1932.6 |
14.119 |
1.938 |
4750 |
14.119 |
10.39 |
4666.6 |
19.999 |
4.255 |
1425 |
29.96 |
1.91 |
1232.9 |
21.07 |
1.775 |
1767.45 |
21.07 |
4.39 |
1416.487 |
29.96 |
1.35 |
946.2 |
36.995 |
0.92 |
765.4 |
29.12 |
2.56 |
921.86 |
29.12 |
2.893 |
774.19 |
36.995 |
1.05 |
353 |
46.06 |
1.19 |
748.5 |
36.12 |
1 |
913.58 |
36.12 |
1.5186 |
525.92 |
46.97 |
1.04 |
458.47 |
- | - | - | 42.119 |
2.235 |
502.92 |
- | - | - |
A drying period of four days has been recommended for a painted surface in a room. In practice, if removing all objects from the room being painted is not feasible, an option may be to move the collection to one side of the room and paint the walls on the other side, while protecting the collection with plastic sheeting and providing as much ventilation as possible. In the context of a large enclosure such as a room, if a delay of four days for off-gassing is impossible to commit to, it is safer to avoid painting the room if acid-sensitive objects are present, such as lead, colour photographs, and paper, unless localized means of protection are provided such as tightly sealed boxes or display cases.
Selection of coatings based on substrate and purpose
Different types of coatings for different substrates and purposes are summarized below:
- Inside wooden enclosures: The common choice is a latex acrylic paint (emulsion). Two-part epoxy paint can be chosen if there is a requirement for good chemical resistance, and two-part urethane is recommended for good abrasion resistance. Allow a four-week drying period before inserting objects.
- Walls, ceilings, open wooden shelves and any exterior wooden surfaces of enclosures: Same recommendation for coatings as for inside wooden enclosures. Allow a four-day drying period.
- Wooden and concrete floors: Two-part epoxy paint can be chosen if there is a requirement for good chemical resistance, and two-part urethane is recommended for good abrasion resistance. Latex acrylic-urethane paint is also an option if a moderate abrasive-resistant coating is needed. Allow a four-day drying period.
- Metallic enclosures: Powder coatings are a good popular choice. Baked alkyd paints should be avoided due to the potential for off-gassing problems from incompletely baked paint. Small aluminum parts can be anodized and dyed. For both powder-coated and anodized metals, no drying time is needed.
- Metallic fixtures outside of enclosures: Powder coatings, anodized coatings and baked alkyd paint can be used in a large-sized well-ventilated room since the first two release no emissions and emissions are fairly low for the third. Even regular (unbaked) alkyd paint, which has higher emissions, can be used if the surface to be painted is very small compared to the dimensions of the room (emissions will become quickly diluted). No drying time is needed in either context. (Do not use these products inside an enclosure without making a proper risk assessment.)
- Coated surfaces in contact with objects: To avoid tackiness, choose a paint based on the type of substrate mentioned above. Allow a four-week drying period before contact between a white (or pale-coloured) painted surface and an object. Coloured paint, especially dark colours, will need a longer drying period (Figure 4). If the stated drying periods are hard to fulfill, the best option is to use an interleaf between the surface and the object. Consult Interleaves – discrete and efficient for more on the use of interleaves. As a precaution, it is recommended to always use an interleaf between objects and any painted surfaces. Surfaces with a coating cured by radiation, anodized or covered with powder coatings do not need a drying period prior to contact with an object.
For more information on coatings, consult “Sustainable Use of Coatings in Museums and Archives – Some Critical Observations” (Tétreault 2011).
Laminates
One way to avoid dealing with the prolonged airing-out period needed when using wet coatings is to use wooden panels already covered by a stable vapour barrier or laminate, or to apply a laminating film on the wood product that will seal in any off-gassing emissions. These options are described below.
Formaldehyde-based laminates
Panels surface-finished with a high-pressure laminate are an alternative (e.g. Formica, Arborite). They are often made of formaldehyde-based resins such as phenol formaldehyde and/or melamine formaldehyde, and they are not problematic (emissive or corrosive). High-pressure laminates are good vapour barriers and are available in different colours or patterns. Most of the time, these laminates are applied on particleboard, which have limited load capacity, but the laminates can also be applied on more robust types of wood products such as plywood.
Plywood panels can also be laminated with an overlay such as a high-density overlay (HDO) and a medium-density overlay (MDO). The laminate in this case is a kraft paper impregnated with phenol formaldehyde of high or medium density. These laminated surfaces are good gas and vapour barriers. These products are commercially available with the laminate surface on one or both sides. The high-density overlay surface has a slick or waxy surface and cannot be painted (in case this is necessary for display aesthetics), while the medium-density overlay can accept paint. The side with the overlay surface is placed towards the interior of packing crates. They can also be used for the base of display cases where the interior surface of the panels is not visible to visitors. Their core being plywood panels, these products can be cut and assembled easily and are quite sturdy. They can be found in regular hardware stores.
Films
Plastic-laminated aluminum foil
Another option for sealing wooden panels is to apply a plastic-laminated aluminum foil such as Marvelseal 360 or Valsem S27 (in France), as shown in Figure 15. This excellent vapour barrier has a shiny metallic appearance due to its aluminum foil core. It can be covered with fabric or coloured matboard to mask this appearance, as necessary. An in-house version of the film can be made with domestic aluminum foil and polyethylene sheets (consult CCI Note 1/9 Low-Cost Plastic/Aluminum Barrier Foil for details on this application). The aluminum can also be used to seal and block the transfer of potentially harmful products from different materials, such as papier-mâché, to objects, as shown in Figure 16.

© Government of Canada, Canadian Conservation Institute. CCI 69345-0024
Figure 15. A hot iron is applied to plastic-laminated aluminum foil to melt the polyethylene side of the foil onto the plywood panel. The plastic on the other side of the foil is nylon, which has a higher melting point. Use an interlayer when applying heat to avoid any risks of the foil sticking to the iron or hot spatula. In this case, the iron was covered with a sheet of beige-coloured Teflon, but just a sheet of paper would do.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0011
Figure 16. Mannequins made of papier-mâché and covered with plastic-laminated aluminum foil.
Containers
Small enclosures such as containers are extremely useful for storing museum objects because they can provide very good protection against physical forces and, depending on how they are made, can also protect against external agents such as pests, pollutants and large RH fluctuations in the room.
The ideal storage containers should be:
- airtight (protection against external pollutants and insects and to some degree against large RH fluctuations);
- waterproof (if not stored in a waterproof cabinet or not covered by a plastic sheet);
- made of non-emissive products;
- stable for many decades;
- strong enough to support the weight of objects they contain when lifted, plus other possible loads (such as the weight of one or two other boxes potentially placed on top);
- labelled to allow ease of identification during retrieval; and
- easy to handle (provided with sturdy handles and not too heavy).
Containers could also be insulated if they are located in an area where the temperature fluctuates. For example, if the box is placed near cold walls, it is at high risk of becoming cool and damp inside. Ideally though, the temperature should be controlled on a larger scale via the room and building features (adding more insulation in the walls or within enclosed cabinets). For more details, consult the CCI webpage Agent of deterioration: incorrect relative humidity.
It may even be good to have fire-resistant containers, although the products used to make them should not be impregnated by flame retardants since not all of the compounds that make up flame retardants are safe for objects and some could cause alterations to objects in a closed environment. It is better to have a good system of fire detection and suppression as a first line of defence.
It is also wise to avoid stacking many boxes on top of each other, as shown in Figure 17. Typically avoid stacking more than three boxes, and use shelving with sufficient shelves to store the objects both safely and in a compact, space-saving manner (Figure 18).

© ICCROM
Figure 17. Many containers are not designed to be excessively piled.

© Government of Canada, Canadian Conservation Institute. CCI 126753-0005
Figure 18. Example of paperboard boxes used in storage. Notice that shelves were adjusted so that no more than two or three boxes are stacked.
Boxes and small containers can be made using a wide variety of products. If they are custom-made, most commonly the raw materials or products for their walls (including top and base) consist of plastic or paper-based panels. Sturdier options are also possible, such as wood or wood products, or metal; these are discussed under Boards and panels. A wide variety of fastening devices and techniques can be used to connect the walls together to make the box. As well, other products discussed within this Technical Bulletin are usually integrated within the design, such as light board separators, foam padding, interleaf sheeting or fabric liners, cotton twill tape and paper or plastic labels. Choosing which product is most appropriate to make containers will depend on the characteristics (size, weight, fragility, etc.) of the object(s) being contained as well as on the context, environment and intended use.
Although making boxes out of boards and panels is usually more economical and offers more possibilities in terms of materials and designs (consult Boards and panels), there are many types of pre-made containers that are commercially available in many standard sizes and that can be used in a museum setting, as discussed below.
Clear small containers
Acrylic, polystyrene (Figure 19), polypropylene, polyethylene (e.g. many types of Tupperware containers, Lock & Lock food containers) and poly(ethylene terephthalate) (PET) are the most commonly used materials for small clear plastic boxes. Polyethylene containers, which are usually milky rather than fully transparent, may turn slightly yellow with time. Transparent plastics offer the advantage of allowing objects to be viewed without handling (note though that the wrapping or padding which usually covers the object, at least partially, is usually not transparent). Plastic containers will not be able to maintain an RH very different from the RH in the room over the long term: the water vapour in the air can slowly infiltrate the container by leakage at the lid joint or through the plastic walls (permeability).

© Government of Canada, Canadian Conservation Institute. CCI 124703-0030
Figure 19. Examples of small clear boxes made of polystyrene plastic.
Plastic bins with lids
Plastic storage bins are usually made of polyethylene and available at a good price. These containers are supplied in a range of colours, or they can be slightly opaque. Colours in plastic containers are not a problem as the colour does not transfer. Plastic boxes, such as Tupperware and Rubbermaid boxes (Figure 20), are usually supplied with fairly tight lids. High-density polyethylene should have a longer working life than low-density polyethylene. Look for a company that offers a 10-year warranty for its products.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0010
Figure 20. Polyethylene bins can be very useful for storing and handling objects.
Corrugated plastic containers
Custom-making a box from corrugated (or fluted) plastic sheets allows for the box to be tailor fitted to the object’s size and needs and also makes it possible for the box to be fully opened flat to allow easy retrieval of fragile objects (Figure 21).
Pre-made corrugated plastic die-cut boxes are available, sold either flat-packed or assembled (Figure 22). The boxes are made of polypropylene and polyethylene or both as a copolymer. The weakest point of the boxes is the stress at bent edges. A good option to relieve this stress, which is observed as a whitening of the plastic, is to gently warm the bends with a blowtorch. Information on making boxes and trays with corrugated plastic can be found in CCI Technical Bulletin 14 Working with Polyethylene Foam and Fluted Plastic Sheet (Schlichting 1994).

© Government of Canada, Canadian Conservation Institute. CCI 100548-0024
Figure 21. A collapsible, custom-fitted box made of a plastic corrugated sheet for a fragile ivory casket. The box opens up fully to allow easy retrieval of the object. The box is padded with foam lined with a non-woven fabric.

© Government of Canada, Canadian Conservation Institute. CCI 99201-0030
Figure 22. Die-cut assembled corrugated plastic box.
Paperboard containers
Pre-made acid-free laminated paperboard or corrugated cardboard boxes are available for the museum and archival field in several standard sizes (Figures 23 and 24). Some types of laminated board boxes are reinforced with metal edges for added strength and durability. The common and ubiquitous corrugated cardboard box is acidic and so has limited use in a museum setting (as discussed under Boards and panels).

© Government of Canada, Canadian Conservation Institute. CCI 124901-0001
Figure 23. Example of a die-cut acid-free corrugated cardboard box.

© Government of Canada, Canadian Conservation Institute. CCI 124840-0001
Figure 24. Example of a store-made laminated paperboard box with reinforced metal edges.
Cushioning and padding
Cushioning and padding materials include foams of different densities, battings, felts and cushioned wraps used to support or pad out objects, or to wrap objects. They are often used in a combination of layers of increasing densities (e.g. as a first layer of shock-absorbent material), to fill up the extra space inside a hard container such as a shipping crate or to bulk out the inside of objects with a soft cushioning material generally added to more fully pad and protect areas of the object that are in contact with the container (example in Figures 20 and 21). Ensure that the cushioning is adequate for the expected shocks and vibration. Information on how to use cushioning material within shipping crates can be found on the CCI website: Step 5: Use Cushioning Material Effectively in Six Steps to Safe Shipping.
Bubble wrap (air bubble packing)
Bubble wrap (air bubble pack) is a generic name given to a range of clear cellular cushioning sheets made of low-density polyethylene. The air cells can be as small as 6 mm (1/4 in.) in diameter to as large as 26 mm (1 in.). Bubble wraps are recommended mainly for wrapping fragile or lightweight objects for short periods of time because the cells tend to rupture and deflate with time. When bubble wrap is placed in direct contact with objects or held taut against surfaces for long periods, there is a risk that stains in the shape of the air cells can become imprinted on objects (Figure 25). This undesirable pattern can be quite noticeable, affecting the appearance of the object. In general, the recommended practice is to avoid direct contact between bubble wrap and objects. Because of these drawbacks, overall polyethylene foam sheeting is a preferable option. Otherwise, the use of an interleaf between the bubble wrap and the object is recommended to avoid potential staining.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0334
Figure 25. A silver-plated brass bowl became tarnished on areas in direct contact with bubble wrap.
Foams
Foams are commonly used for cushioning, padding and support. They are plastics whose density is reduced by the presence of many small cavities or cells dispersed throughout the mass, creating a cushioning effect. Foams are classified as open cell or closed cell (although there are semi-closed cells, as well). In closed-cell foams, each cell is completely enclosed by a thin wall of plastic; in open-cell foams, the individual cells are interconnected. Open-cell foams are recommended in situations where the foam will be compressed often or will be under steady, heavy pressure. Closed-cell foams can be a good option where less pressure will be applied and a good vapour barrier is needed; however, note that they tend to lose their cushioning ability if submitted to constant pressure or impacts (shock). Many types of foam are available with differing densities (hardness) and compressive strengths. Care should be taken to select a foam with the proper compressive strength and in using it over a sufficiently large area to prevent crushing under the load. Information on how much cushioning is required for shipping specific loads can be found on CCI’s web page PadCAD (a cushion design software) and in CCI Note 20/2 Foam Corner Pads.
Foam thickness is a primary consideration for effective padding and cushioning. For practical reasons, foams are presented below in three groups which differ by their form and/or thickness: planks, sheets and agglomerates. While some foams are available in a range of thicknesses, it is convenient to separate them into two categories: those less than or equal to 13 mm (1/2 in.) and those greater than this thickness.
Foam planks, thick (>13 mm or 1/2 in.)
Thick foams are quite useful as padding inside transportation crates and for making supports (Figures 13, 21 and 26).
Polyethylene
Often called “Ethafoam,” polyethylene foam is a common product made by different companies. They are typically available non-pigmented (whitish) or in a black colour. Ethafoam 220 is a popular and safe polyethylene foam plank for preservation. It is a medium density 35.2 kg/m3 (2.2 lb./ft.3), closed-cell foam available with thicknesses from 38 to 102 mm (1.5 to 4 in.). Ethafoam is also available in different densities. PolyPlank is a similar product, offering similar density and sizes. Stratocell is another thick polyethylene foam, which is made of multi-layered thin polyethylene foams. Stratocell foam can be produced in thicknesses from 25 to 203 mm (1 to 8 in.).
Waxy residues have been found on the skin of polyethylene foam planks, probably acting as a releasing agent, observable under optical magnification deep in the creases of the skin’s film. No damages have been reported. However, as a precaution, the skin should be cut off or covered. When the skin of the foam is cut, the new foam surface may be too abrasive for very delicate objects. A layer of quilt batting and a lining of soft fabric can be used to cover the foam (Figure 26). A soft padding layer is also often added in between (e.g. polyester batting).

© Government of Canada, Canadian Conservation Institute. CCI 120714-0002
Figure 26. Example of a storage mount carved out of polyethylene foam and lined with fabric.
Obtaining polyethylene foam planks that are partly made of recycled materials is possible. For long-term use of foams, such as for object storage mounts, it is best to use only new foam. Avoid using recycled foams since they may contain polyethylene that is already degraded, and this can affect the lifetime of the new recycled foam.
Polyethylene foam planks can be easily glued together with hot-melt glue or welded with a hot air gun, if greater thicknesses are needed (Figures 27a and 27b).
Consult Figure 5 for the fast degradation problem of PE foam in the 1990s.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0012

© Government of Canada, Canadian Conservation Institute. CCI 120171-0013
Figures 27a and 27b. Polyethylene foam planks glued together to make a period mannequin (left) and padded with polyester quilt batting and covered with jersey knit (right).
Polypropylene
Polypropylene foam, known as PropaFoam or PolyPro, has similar properties to polyethylene foam with the potential for a longer lifetime.
Polystyrene
Polystyrene foam is formed by either extrusion (XPS) or expansion (EPS). Both types are shown in Figure 28. Extruded plank polystyrene foam, such as Stylite and Styrofoam, has a uniform closed-cell structure, a smooth continuous skin and consistent product qualities. It is also easy to cut using a band saw. Expanded bead polystyrene foam is made from expanded polystyrene beads (pre-puffed beads), which are placed in a mold and heated so that the softened, swollen beads stick together. Polystyrene coffee cups are made this way. This type of foam is less expensive than the extruded type. However, it is less suitable because beads along a plank’s exposed edges tend to crumble apart during handling and cutting, and as well because this type of plank will structurally weaken with age as the beads inside eventually shrink a little bit and separate from each other.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0014
Figure 28. Extruded plank polystyrene boards (above) and expanded bead (below). Note the loose beads from the expanded board.
XPS foam boards have well-defined thermal insulation properties and are commonly available in Canada in white, blue and pink colours. A lot of static charge is created when cutting or just handling the EPS. They should not be used close to objects having powdery surfaces (e.g. pastels and charcoal).
Polyurethane
Polyurethane foam is available as an ether or ester type. The least stable polyurethane foam is the ether type, which tends to turn yellow within a year if exposed to air, and within a few years to a few decades it stains and becomes brittle. Ester-type polyurethane is somewhat more stable, but after a few decades it will become fully degraded. Degraded ester-type foam has been found to release adipic acid, which causes metal corrosion. Despite their low stability, both low-cost polyurethane foams provide a useful level of soft cushioning when transporting light and fragile objects, which would be difficult or expensive to reproduce with other types of foams or materials. However, they should be used only for short-term applications and should not be in direct contact with objects. An interleaf should be inserted between the foam and the object to prevent contact (as shown in Interleaves – discrete and efficient).
Foam sheeting, thin (≤13 mm or 1/2 in.)
Thin foams are useful for light padding on a shelf or within boxes or trays, or for wrapping objects. These products are available either in sheets or rolls.
Polyethylene
Polyethylene (PE) foam sheets, such as Cellu-Cushion, are available by the roll with a thickness ranging from 1.6 to 6 mm (1/16 to 1/4 in.) (Figure 29).
Crosslinked polyethylene
Crosslinked polyethylene (XLPE) foam, such as Volara and Plastazote, is available in different thicknesses (3 to 13 mm or 1/64 to 1/2 in.) and degrees of softness (density). They are used to provide soft cushioning for support and transportation—or as soft interleaves for lining drawers and shelves, as well as for padding inside boxes, trays and mounts (Figure 30).
Polypropylene
Polypropylene (PP) foam wrap, such as Microfoam, looks similar to thin sheet polyethylene foam but makes a squeaky sound when rubbed against itself. This foam is used in the same types of applications as those for polyethylene foam sheeting; for example, it is often used as a soft lining on storage shelves.

© Government of Canada, Canadian Conservation Institute. CCI 123327-0008
Figure 29. Example of thin polyethylene sheeting used to individually wrap piano legs in a box for travel.

© Government of Canada, Canadian Conservation Institute. CCI 122641-0005
Figure 30. Support for archaeological objects made of crosslinked polyethylene foam. Recesses were carved out of the foam, and a base was made out of a second sheet of foam plus an outer sheet of corrugated plastic board. Cotton twill tape is used to secure the larger pieces.
Foam beads, peanuts and strands
Extruded polystyrene (XPS) foam also comes in the form of various agglomerates that can serve to bulk out a package. The most common forms are tiny beads, peanuts (S-shaped), flakes (shell-shaped) and strands (spaghetti-shaped). The foam itself is stable. However, when moved, foam agglomerates tend to settle with time. Consult also the discussion on polystyrene foam, under Foam planks, thick (>13 mm or 1/2 in.).
Heat-activated molding felts (FOSSHAPE)
FOSSHAPE is a dense felt-like material made of polyester fibres. It can be heat-formed or heat-molded to take on certain shapes and, therefore, is especially suited to make interior structures for hats, shoes and costumes. It is lighter in weight than polyethylene foam when used in similar applications. It requires a form against which to be molded, e.g. a master mannequin shape. Heat-molding it can be less time-consuming than other options, such as having to carve out PE foam cylinders and assemble them to make a mannequin, for example.
Polyester quilt batting and fibrefill
Polyester quilt batting is a flat and thick lining material that is useful to provide very soft cushioning (Figure 31), e.g. for padding lightweight objects or internal foam supports, covering wooden or plastic panels to form padded flat supports or for padding coat hangers. It is composed of non-woven polyester fibres held together by the mechanical interlocking of fibres, by thermal bonding or by adhesive bond (with, for example, an acrylic adhesive). Interleaving (e.g. of cotton or non-woven fabric) between the batting and the object is recommended to prevent the fibres from snagging on surfaces.

© Government of Canada, Canadian Conservation Institute. CCI 124883-0021
Figure 31. Polyester quilt batting is wrapped over a polyethylene carved foam shape to provide a soft-padded surface for an internal shoe mount. The batting will be covered with jersey knit fabric.
Polyester fibrefill consists of the same type of polyester fibres with no additives, loosely clumped together in a large ball (as compared to the flat two-dimensional structure of the batting). It is enclosed within a non-woven fabric or plastic pouch and is used most commonly as inner mounts to provide volume inside objects that would otherwise collapse or lose their shape (such as moccasins, hats, baskets, etc.).
Fabrics
General considerations
Fabrics, including jersey knits, plain weaves, felts and velvets, as well as non-woven materials such as Tyvek, are commonly used as finishing materials, providing an aesthetically pleasing soft covering layer or interleaf and in some cases providing a certain amount of padding. Sheets of fabric are also used as dust covers (Figures 32a and 32b). Cotton, linen and polyester are safe products to use with objects.

© Government of Canada, Canadian Conservation Institute. CCI 73062-0049

© Government of Canada, Canadian Conservation Institute. CCI 73062-0065
Figures 32a and 32b. Egyptian revival armchair uncovered (left) and covered with a cotton cloth dust cover (right).
The use of wool in museums tends to be a concern because of the possible emission of sulfur gases. This might be a problem with large surface areas of wool, but damage in enclosures has not been demonstrated clearly. Only when wool is almost touching sensitive metals, such as silver, is there a cause for concern. The main source of hydrogen sulfide in collections remains visitors and external sources. Silk is safe to use because it is made of a protein called fibroin which does not contain sulfur.
Fabrics should be washed prior to their use. This will remove possible finishing products and oxidant residues if they have been bleached.
Dyes used in natural or synthetic fabrics for museum purposes should meet the following conditions. They should
- not contain sulfur-based compounds (consult Test #1: Lead acetate test for detecting sulfur compounds for a test to ensure that no sulfur compounds are present in a dyed fabric),
- be colourfast (consult Test #3: Testing for colourfastness for instructions on how to test colourfastness in a dyed fabric) and
- be stable under light (a criterion for a long-term exhibition).
Non-woven polyethylene (Tyvek)
Non-woven polyethylene (under the common trade name Tyvek) resembles a white plastic paper. It is available in a variety of thicknesses and strengths. In conservation, the water-impermeable Tyvek 1443R grade is often recommended, but breathable (punctured with tiny air holes) varieties are also made. Tyvek is thin and has good strength; it is often used for rolling textiles and can also be used as a dust cover in collection storage. The smooth side of the Tyvek should be placed in contact with objects. Tyvek has certain advantages: it does not shed fibres (lint) so does not contribute to dust in storage or within enclosures; as well, its slick surface is safe to use in contact with objects that have uneven surfaces or asperities which could otherwise catch within the fibres of woven fabrics (e.g. as a bag for inner mounts for woven baskets; Figure 33). Tyvek also has various other uses as described under the section Films, sheeting, sleeves and envelopes. Non-woven polypropylene does exist, but it is not yet used very often in museums and archives.
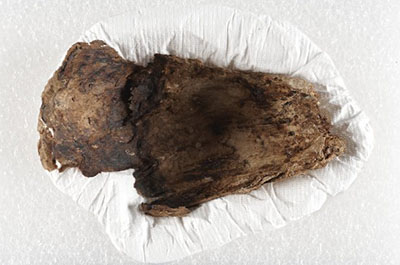
© Government of Canada, Canadian Conservation Institute. CCI 124970-0114
Figure 33. Top view of a fragile archaeological object made of muskox horn within its recessed storage mount. It is stored on a plank of PE foam, padded with a layer of polyester quilt batting and lined with Tyvek.
Films, sheeting, sleeves and envelopes
A variety of good-quality paper and card stocks or plastic-based films and sheets are available to make sleeves, folders and envelopes in order to store flat thin objects such as archival paper documents and photographic materials. Commercially manufactured sleeves and envelopes of many standard sizes are also available for the archival and museum fields (Figure 34). Sheets, sleeves and envelopes are discussed further below.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0015
Figure 34. A variety of sleeves and envelopes are commercially available.
Paper
Most paper today is made from wood pulp fibres. Depending on its composition and the fabrication process, paper can be acidic, neutral or alkaline. Papers made mechanically from wood pulps tend to be acidic, and for this reason, they tend to have a short shelf life. These papers are not recommended for long-term contact with objects sensitive to acid. Papers made from chemically processed wood are neutral or slightly alkaline because the lignin is removed. These acid-free papers will slowly become acidic, depending upon air quality and the acidity of the objects they touch. If a paper’s pH becomes too low (too acidic), the paper may need replacing. Stable papers (with or without lignin) have a small quantity of calcium carbonate added as a pH buffer. These papers are alkaline and more permanent than unbuffered papers. Usually, both neutral and alkaline papers are suitable for preventive conservation and can be used for wrapping many types of objects, such as paper documents, black and white photographs and black and white negatives. As well, it is recommended that two types of photographs be stored in neutral pH papers because they are potentially sensitive to high pH. These are cyanotypes (produced from 1842 until the 1950s) and dye-transfer prints (rare, produced between 1946 and 1993), which are stabilized at an acidic pH (Lavédrine 2009). Papers used to store photographic documents should also pass the photographic activity test (ISO Standard 14523:1999).
Archival quality sheets of paper and card stock are often used as interleaves or in a variety of other ways for storing archival papers and photographs (Figure 35). Sheets of acid-free buffered or unbuffered paper are useful as a light covering material over objects, as thin interleaves or as a wrapping layer. They can also be used crumbled up to pad out three-dimensional shapes (Figure 36).

© Government of Canada, Canadian Conservation Institute. CCI 99948-0010
Figure 35. Storage of cellulose acetate negatives. They are first placed in archival quality paper sleeves, then inserted into a paper envelope and stored in a box.

© Government of Canada, Canadian Conservation Institute. CCI 124883-0041
Figure 36. Storage of Georges Vanier’s boots. Internal padding was provided, then the boots were secured to foam blocks with cotton twill tape and padded out with crumbled acid-free non-buffered tissue paper.
Wrapping a rolled textile with two to three layers of acid-free or alkaline-buffered tissue paper provides an effective, low-cost barrier against dust and reactive outdoor pollutants, such as ozone and nitrogen dioxide. Over time, it is possible to monitor the quantity of pollutants trapped by the top layer compared to the layers below by observing the colour change or by measuring the pH change. Weak acids, such as formic and acetic acids, will penetrate the paper layers easily.
A semi-transparent paper called “glassine” is sometimes used as an envelope to store negatives and photographs. Glassine can be waxed or laminated. Under high humidity, the photographic emulsion can adhere to it. In addition, high humidity can cause glassine to deform, which can affect the wrapped object. In general, glassine should be avoided unless it is acid-free or buffered and is free of surface treatments such as wax, laminates or humectants (e.g. glycerol). Even though many users do not report problems with glassine, many archival institutes will avoid it.
Paper envelopes and sleeves
Archival quality paper sleeves and envelopes can be made in-house using acid-free paper or card stock, or they may also be purchased in standard sizes. Paper envelopes and sleeves are largely used for conservation and archival purposes and are relatively easy to get in specialized artist and/or archival supplies stores. Some can also be found in office supply stores as well as hardware stores. Contact of acidic papers with objects should be avoided. For storing photographic documents, archival institutions commonly select envelopes or sleeves that pass a test called the photographic activity test (ISO 14523:1999, Tétreault 1999). This accelerating aging test determines if any constituent of the product can potentially affect the colloidal silver particles. Some archival material suppliers are able to suggest products that have passed the test.
Clear plastic films
Poly(ethylene terephthalate) (PET) and polypropylene (PP) are the most suitable plastic sheets for general purposes, offering a clear and very stable sheet. Polyethylene (PE) has some merit, especially for its low cost and availability, but its lifetime is limited and the film is not as clear as PET and PP. Flexible poly(vinyl chloride) (PVC) is to be avoided for preventive conservation use. Alternative products to plastic films are some non-woven fabrics such as Tyvek (described under Fabrics).
Poly(ethylene terephthalate) (PET)
Melinex and Mylar are trade names for PET, also commonly called polyester. It is supplied in sheets of various thicknesses, is durable and has a crystal-clear transparency. Mylar D used to be recommended for long-term archival use, but it is no longer produced. Melinex 516 is the recommended substitute. In general, the use of other types of Mylar and Melinex are probably acceptable since no damage has been reported from their use in normal indoor conditions. In museums, PET films are commonly used as interleafing (Figure 37) and encapsulation (Figure 38) materials, as well as window materials for mats and boxes (Figure 39). PET film also has a very low permeability coefficient to hydrogen sulfide (Tétreault 2003 p. 135) and thus, if made into a tightly sealable bag, it can be useful to store silver, possibly in combination with a sorbent such as Pacific Silvercloth.

© Government of Canada, Canadian Conservation Institute. CCI 124883-0019
Figure 37. A polyester film sleeve in between the metal and the leather prevents green corrosion products from forming in areas of contact.

© Government of Canada, Canadian Conservation Institute. CCI 88702-0154
Figure 38. Melinex film-encapsulated leaves from the Catharine Parr Traill scrapbook.

© Government of Canada, Canadian Conservation Institute. CCI 124008-0027
Figure 39. A Melinex window on the top or (as shown here) on the side of a storage box permits viewing of the object inside without having to open the box.
Polypropylene (PP)
Polypropylene is more specifically termed “oriented polypropylene.” It is a clear sheet similar to PET and with equivalent performance characteristics. Due to its great stability, this plastic is often recognized as an archival product. Some special polypropylene films have a reduced-glare finish for increased visibility of documents.
Some polypropylene sheets are made to be biodegradable. They are typically less stable than regular plastic due to their modification to be vulnerable to microorganisms. They take months or a few years to degrade when buried in an anaerobic landfill. An alternative sustainable practice would be to reuse the regular polypropylene or to recycle it properly.
Polyethylene (PE)
PE, also called polythene, costs less than PET or polypropylene. It is also less transparent and less reliable. PE sheets are typically available in high and low density. High-density PE (HDPE) tends to have a greater lifetime than low-density PE (LDPE). Both densities are recyclable and are often used to cover objects or shelving against dust or potential water leakage from the ceiling or pipes. PE film is also commonly used as a waterproof wrap over boxes intended for shipment. In the form of bags, they are commonly used to store small items such as archaeological metals or organic fragments (Figure 40).
Bags made of PE sheeting are a cost-effective means of individually isolating objects during a pest or mould infestation, for example, as the film is fairly economical and can easily be cut to any size and heat-sealed. Some PE bags and sheets were found to embrittle and tear after only 5 to 10 years in a museum environment. Those early failures are more expected with LDPE, but all types of PE can be damaged prematurely if in contact with objects that contain oils, fats or solvents. For long-term storage of objects with a plastic sheet, it is wise to consider PET or polypropylene.

© Government of Canada, Canadian Conservation Institute. CCI 100426-0013
Figure 40. Archaeological wood fragments from Sannirajaq (Hall Beach), Nunavut, stored in zipper-type PE bags.
Another LDPE that is becoming more common is the linear low-density polyethylene (LLDPE), also called “stretch wrap.” This PE probably has a limited lifetime, especially when stretched, and provides low gas barrier protection. Some of the brand names for the LLDPE are Glad Cling Wrap, Handi-Wrap and Saran Premium Wrap. Consult Poly(vinylidene chloride) (PVDC) as another stretch wrap.
Nylon-6,6
Nylon-6,6 is a transparent film. The sheet is often used to cover large items such as paintings. If possible, select nylon film that does not contain plasticizers, additives or surface coatings.
Ethylene vinyl alcohol (EVOH)
The film, known by the brand name EVAL, provides a good oxygen barrier and is highly transparent. EVOH is often used as a layer in a composite plastic film or container to improve the overall performance of the product.
Poly(vinyl chloride) (PVC)
Commonly known simply as PVC, this plastic is usually supplied in the form of flexible sheets or tubing. Flexible PVC contains as much as 30% plasticizer. Over time, the plasticizer tends to degrade and migrate, causing the product to turn yellow, deform, lose its flexibility, become brittle and stain objects in contact with it (Figure 41; consult also Plastics – gifts of the 20th century and challenges of the 21st century for more information on the properties of plastics). PVC is suspected of off-gassing hydrochloric acid over time, but this has not been confirmed under normal museum conditions. Avoid direct contact of PVC with an object and avoid using it in an airtight enclosure for more than 10 years.

© Government of Canada, Canadian Conservation Institute. CCI 120210-0155
Figure 41. Detail of two PVC slide holders showing stains and warping, signs of their physical and chemical deterioration over time.
Poly(vinylidene chloride) (PVDC)
PVDC provides an efficient vapour or gas barrier. In particular, it is an excellent barrier film against water vapour (Tétreault 2003 Appendix 5), which explains its extensive use in the food industry to keep foods moist. Although PVDC contains chlorine, its molecular structure gives it greater stability than flexible PVC. In sheet form, PVDC may contain up to 10% plasticizer. Migration of this plasticizer has not been observed, but it may be safer to avoid using this product for medium- or long-term applications. The trade name Saran Wrap used to be the most common stretch wrap on the market, and it was originally made of PVDC. Since 2004, in Canada and the United States, Saran Wrap is made of a LLDPE (consult Polyethylene (PE) for details on this type of plastic).
Cellulose acetate
Cellulose acetate sheets, used to enclosed small thin objects such as textiles, papers or photographs, should not be used for more than a decade because it will eventually release a significant amount of acetic acid vapour. This so-called vinegar syndrome is most noticeable with collections of acetate negatives. A better choice is polypropylene sheets.
Multi-layer films
Multi-layer plastic films are typically specialty products intended to provide enhanced gas impermeability and strength. The film Escal (or Escal Neo) from Mitsubishi has been used in conservation because it provides a very good gas barrier and is fairly clear. The sheet, made of ceramic deposited in PET/PE, can be formed and sealed to make a bag that can be used to maintain an anoxic environment, i.e. an oxygen-free environment. This environment is ideal for the protection of any object that can be degraded by oxidation, such as natural rubber and salt-contaminated iron objects. Figure 42 shows an example of the use of a multi-layer sheet. Security films and UV-filtering films are often composed of multi-layer films, and they are discussed in the section on Glazing and glazing films.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0016
Figure 42. A natural rubber shoe sealed in a bag made of multi-layer film to protect against oxidation or any outdoor pollutants.
Non-transparent films
Plastic-laminated aluminum
Consult Coatings, laminates and films.
Non-woven polyethylene (Tyvek)
Tyvek in general is further described under Fabrics. In the archival field, Tyvek envelopes are used to store archival documents, photographic negatives or other small items. Tyvek 6060 or 1073 is recommended for paper-like labels; however, a sample should be tested before use to ensure that the ink does not soak through and transfer to the object below.
Gaskets
A gasket is a compressible, rubber-like material that is squeezed between two mating surfaces to form a static seal. A gasket is usually made from a material that yields so that it is able to deform and tightly fill the space it is designed for, including any slight irregularities. The materials should also remain springy or elastic if the gasket is joining an area that is occasionally opened and closed, so that they may rebound and fill up new gaps even after having been compressed. Gaskets can be made of rubber or rubber composites, as well as of plastic foams, flexible graphite and many other materials. Natural vulcanized rubber has great sealing qualities, but the vulcanization process that increases its elasticity and resistance also leads to some sulfur off-gassing. Thus, gaskets made with this type of rubber are generally inappropriate in a museum setting.
When selecting a gasket for use in preventive conservation, choose stable, non-emissive products such as:
- polyethylene foams of appropriate density—these can be sold as sheets, strips or rods (i.e. backer rods or insulation dowels, such as Climaloc and Magic FoamCord);
- crosslinked polyethylene foam;
- polypropylene foam;
- Viton (fluoropolymer elastomer);
- silicone (may leave an oily residue which can stain but is not volatile);
- neoprene foam (polychloroprene—some may stain if placed in direct contact with objects; no chlorine off-gassing has been reported);
- micro-cellular urethane foam (select those of high quality based on given specifications such as low volatile organic compound content or no tendency to fog); or
- Teflon [poly(tetrafluoroethylene) (PTFE), which is very stable but tends to stay deformed after compression. It is best if the compression is done once or no more than a few times; otherwise, an airtight seal will not be easily achieved].
Avoid the following products:
- low-cost common urethane foam (ether or ester type due to their short lifetime issues [consult Table 1 and Table 2])
- a gasket containing sulfur (consult Test #1: Lead acetate test for detecting sulfur compounds for instructions on how to identify if a gasket contains sulfur.) Vulcanized natural rubber will release sulfur compounds. For other types of synthetic rubbers, it depends. A dark- or bright-coloured gasket may contain sulfur, while a semi-transparent one should not. Some styrene-butadiene rubbers (SBR) and ethylene-propylene-diene monomers (EPDM) may contain sulfur compounds, but not all.
- soft PVC foam (it can degrade faster than rigid PVC)
To create airtight storage cabinets or display cases, the gasket chosen should be able to maintain its initial properties, such as compressibility, for 10 years or more. Ideally, the profile (shape) of the gasket should be rather standard, so that it can be easily replaced in the future. Airtightness performance of an enclosure can be optimized by choosing a good quality gasket material as well as by good joint design.
Glazing and glazing films
Glazing for display cases or frames can be made of glass or clear rigid plastic panels. Having a clear protective cover over objects on display shields them from many potential threats. In particular, it provides a degree of protection against opportunistic thieves and vandals, especially if the panels are physically fixed to the base of the cases. Cases and glazed frames can be further secured against theft or vandalism by the application of a security film that makes it difficult to smash and break through the glazing. As well, UV radiation which damages many types of objects can be filtered at the level of the glazing layer when display cases or glazed framing are used.
Glass
Modern glass is stable and provides an excellent barrier.
As opposed to plastics, glass is not permeable to water vapour, making it a more effective material for creating a microclimate and airtight seal within a display case or frame. As well, glass is fairly rigid (more than plastics). Display cases covering large surfaces are usually made of glass because glass does not flex or bow out as much as clear plastic glazing. For optimal transparency, it is better to choose low-iron glass because regular glass tends to be slightly green when it is thicker. Regular glass and tempered glass are both breakable, although tempered glass is stronger than the regular kind. Tempered glass also shatters into many small fragments when it is broken, which prevents major injuries. Tempered glass can sometimes be recognized by little marks on the edge, which are caused by the metal tongs used to support the glass during the heat process. Both types of glass panels can be reinforced with a security film. Some types of glass panels (e.g. Gross Glass and Tru Vue) incorporate UV-filtering compounds to minimize the amount of UV that reaches the object. Anti-reflective products that help reduce glare caused by spotlights or room lighting are also options.
Plastics
Although polycarbonate panels have better impact resistance, acrylic panels remain a more popular choice for glazing. For skylights, frame glazing and glass display cases, cell cast acrylic panels are usually chosen because they offer superior optical clarity (less distortion) compared to extruded acrylic panels. Compared to glass, acrylic panels are more resistant to impacts and significantly lighter. However, plastic panels allow slow permeation of oxygen and water vapour, and very large variations in RH can affect the dimensional stability of plastic sheets. As a result, glass is a better choice for obtaining very airtight enclosures.
Some plastic glazing panels are designed to provide enhanced UV filtering (e.g. ACRYLITE OP2 or OP3, LuciteLux Museum Grade and Tru Vue). Anti-reflective products that help reduce glare caused by spotlights or room lighting are also options. Note that acrylic panels are easily scratched unless scratch-resistant products are used. Consult also Plastic boards and panels for details on plastic materials used for display purposes.
Ultraviolet-filtering glazing and films
If UV radiation is not controlled at the source (i.e. from lamps or windows), glazing products that incorporate UV filtration can be used, as discussed above. Another option is to apply a thin plastic, UV-filtering film on normal glazing. These products should reduce UV to a low level. ISO Standard 18902:2007 for framing materials recommends a UV filter of at least 97% in the UV range of 300 to 380 nm. For information on UV filters, consult CCI Note 2/1 Ultraviolet Filters.
Security films
Security films applied on glass panels or windows help to impede access into the building or inside a display case. Security films are laminates (i.e. they are made of different layers of plastic laminated together). Instead of being able to break a window or display case in one hammer strike, it may take more than 10 strikes to get through the secured glazing. Some offer very good UV protection as well.
Lines, twill tape, strings and wires
Using monofilament or multifilament lines made of nylon or polyester for suspension places objects at some risk because the lines could, and often will, eventually break. As a general rule, the line used to support an object should have a breaking strength of at least 10 times the weight of the object (e.g. use at least a 5-kg-strength string for a 0.5 kg object), and the line should be changed after 10 years. To avoid strain that could lead to breakage, the line should not pass over sharp edges and corners, and all points of contact should be padded (using tubing, for example). Consult Suspending with proper padding for a recommendation on suspending an object using line.
Twill tapes are used for securing objects to supports or within boxes and shipping crates; for example, to immobilize objects within a padded support while they are being moved or handled (Figure 43). These products should be selected and treated in the same manner as for Fabrics, described above.
© Government of Canada, Canadian Conservation Institute. CCI 121831-0016
Figure 43. A swordfish bill before treatment at CCI. Cotton twill tape was used to secure it to the carved, recessed and padded polyethylene foam within its transportation crate.
Rubber bands should not be used as they will degrade within only a few months under stress and so are unreliable for securing items together; due to the presence of sulfur, they may also stain the objects. Strings and wires are generally not used directly in contact with objects but can be useful to assemble products together when making boxes and enclosures.
Sealants
A sealant, or caulk, is an unformed flexible product (elastomer) applied permanently in a joint to prevent the passage of air. They may be used when assembling airtight display cases and containers. Sealants are also used to glue products together. Most sealants available are composed of acrylic latex, silicone, butyl rubber or polyurethane. The shelf life of these products is usually a year, after which they may not cure properly. Their service lifetime is a maximum of 30 years, but they may fail earlier if they are thin and if there is any movement in the joints.
Many types of silicone sealant are available on the market; they come in clear or coloured varieties and some can be painted. The most common silicone is acetoxy cure silicone, which provides the best bond for a wide range of products. Unfortunately, this type of silicone releases a substantial amount of acetic acid during curing (easily recognized by its vinegar smell) and should not be used inside enclosures. A low-odour or neutral cure silicone (based on oxime or alkoxy), such as GE Silicone II, is a much better alternative as a sealant. After a few weeks, most of the solvents (acetone, methanol or ammonia) will have evaporated.
Like some low-odour silicones, acrylic latex sealants release ammonia, which dissipates easily within a few weeks in a ventilated area.
It is not advisable to apply any liquid sealant inside an enclosure containing objects and then to close it immediately.
Tubing, tubes and rods
This section covers cylindrical products of different sizes and flexibility. Tubing refers to cylinders having a rather small diameter, while tubes are flexible and rigid cylinders with a large diameter. Rods are defined as filled rigid cylinders.
Tubing
Small and flexible tubes are mainly used as padding and interleaf over monofilament thread or metal rod mounts. Examples of tubing include heat-shrinkable tubing (made of Teflon, polyethylene or polypropylene) on metal wire, or silicone and polyethylene (surgical) tubing over monofilament lines to provide padding at points of contact (consult Suspending with proper padding).
Polyethylene foam tubes and foam cylinders, sold typically as insulation to cover metal pipes, can also be used as padding and support in a variety of museum applications. Tubing made of flexible PVC, vulcanized rubber or any rubber containing sulfur compounds should not be used. Most rubber-like tubing could contain harmful products (sulfur or plasticizer or both), while most low-cost heat-shrink tubing sold in electronic shops are made of PVC and contain plasticizer. CCI Note 17/1 The Beilstein Test: Screening Organic and Polymeric Materials for the Presence of Chlorine, with Examples of Products Tested provides a means of testing to know whether chlorine is present, which could help to identify the PVC.
Rigid tubes and rods
Large and rigid tubes or rods are used primarily for display and for storing rolled flat textiles and similar objects. They can be made of different materials:
Tubes
- Acrylonitrile-butadiene styrene (ABS): a good choice.
- Polyethylene (PE): good but has a limited load capacity.
- Poly(vinyl chloride) (PVC), chlorinated poly(vinyl chloride) (CPVC) and poly(vinylidene chloride) (PVDC): as rigid tubes, they do not contain much plasticizer. However, it is not recommended they be in contact with an object over the long term due to concerns over the release of chloride.
- Regular (acidic) board (e.g. sonotubes, sold commercially as forms for pouring cement columns): to avoid transfer of acid compounds from the tube, interleaf should be placed between the tube and the object (Figure 44).
Rods
- Plexiglas: good with limited load capacity (Figure 45).
- Wood and metals: both need an interleaf to block acid compounds from the wood and to avoid staining the object in contact with the tarnished metal.
Consult CCI Note 13/3 Rolled Storage for Textiles for more details.
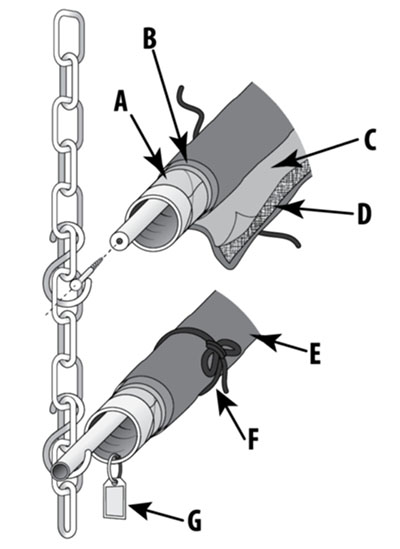
© Government of Canada, Canadian Conservation Institute. CCI 120171-0017
Figure 44. Suspension storage system. A. Mylar covering the cardboard tube; B. Acid-free tissue or prewashed cotton sheeting over Mylar; C. Interleaving of neutral-pH tissue or prewashed cotton sheeting; D. Textile with pile on outside; E. Prewashed cotton cover; F. Cotton twill tape; G. Identification tag.
Figure 2 – text version
- Mylar covering the cardboard tube;
- Acid-free tissue or prewashed cotton sheeting over Mylar;
- Interleaving of neutral-pH tissue or prewashed cotton sheeting;
- Textile with pile on outside;
- Prewashed cotton cover;
- Cotton twill tape;
- Identification tag.

© Government of Canada, Canadian Conservation Institute. CCI 120171-0001
Figure 45. A Chinese wedding gown supported by a Plexiglas rod.
Where to buy products for preventive conservation purposes
Many of the products described in this Bulletin can be found in hardware or office supply stores. However, there is no guarantee that the products found there are acceptable for museum applications. Caution is required. In terms of more specialized distributors of archival and museum products, The Society for the Preservation of Natural History Collections (SPNHC) website provides a list of specialized distributors with links to their online stores. These distributors supply products commonly used by the heritage community.
When products are stored in your institution, make sure that they remain well-labelled in order to avoid confusion about their nature. Large institutions tend to buy a lot of products that are safe for any purpose so that staff never has any doubts about their low level of risk to objects, and they can, therefore, use them freely. An example of how a large institution (the Musée du Quai Branly in Paris) has handled this issue is provided in Big move at the Musée du Quai Branly – keep it simple.
Tests for products used in preventive conservation
There are not many tests recognized by the conservation field to investigate whether products contain harmful compounds. The archival community uses the photographic activity test (ISO Standard 14523:1999) for products that are intended to enclose photographic documents. An accelerated corrosion test called the Oddy test (Green and Thickett 1995) is often used to detect the presence of volatile acids and other gaseous compounds that can harm metals and other sensitive materials. Both tests need an oven that can maintain a temperature of 60°C for four weeks.
In this section, three tests for detecting harmful compounds in products (sulfur compounds, volatile compounds and non-waterfast dyes) are presented. They can be done at a low cost and do not require specialized skills and tools.
- Test #1: Lead acetate test for detecting sulfur compounds
- Test #2: pH test using glycerol for measuring the acidity of volatile compounds
- Test #3: Testing for colourfastness
Further tests for detecting harmful compounds in products used in preventive conservation can be found in the following publications:
- Airborne Pollutants in Museums, Galleries, and Archives: Risk Assessment, Control Strategies, and Preservation Management (Tétreault 2003).
- Pollutants in the Museum Environment: Practical Strategies for Problem Solving in Design (Hatchfield 2002).
- CCI Note 17/1 The Beilstein Test: Screening Organic and Polymeric Materials for the Presence of Chlorine, with Examples of Products Tested.
Test #1: Lead acetate test for detecting sulfur compounds
Purpose of test
To determine the presence of sulfur. Products containing sulfur compounds are known to discolour, corrode and weaken some objects.
Before doing the test, review the safety data sheet (SDS) for the product to be tested and in particular the section on hazardous decomposition products to see if any sulfur compounds are released during combustion or degradation. If compounds such as sulfur dioxide (SO2), sulfur oxides (SOX), sulfuric acid (H2SO4), hydrogen sulfide (H2S) and carbonyl sulfide (COS) are listed, the presence of sulfur is confirmed and the test is no longer needed.
Materials and reagents to be used
- Lead acetate strips (lead acetate indicators, lead acetate test papers), which are available through distributors of chemical products and at some drugstores
- Hydrogen peroxide (3–10% solution), which is available at drugstores
- Water (tap water is suitable)
- Glass test tube (10 mL works best)
- Eye dropper
- Alcohol burner or propane torch
Sample preparation
Liquid samples, such as paints and adhesives, should be dried on aluminum foil for approximately one day; solid samples, such as gaskets or plastic films, need no preparation.
Procedure
Please note: This test can produce strong irritant odours and should be conducted under a fume hood or outside.
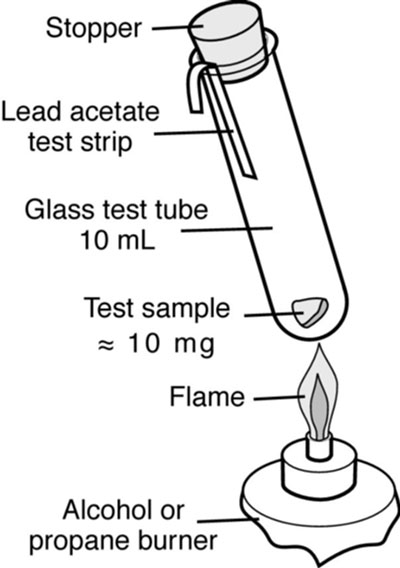
© Government of Canada, Canadian Conservation Institute. CCI 120171-0018
Figure 46. Diagram of lead acetate test set-up. A glass test tube with the sample at the bottom, and lead acetate test strip at the top (held by the stopper), is heated over a flame.
The procedure should be carried out as follows:
- Wet the lead acetate test strip with one or two drops of clean water.
- Put the wet test strip and a small (approximately 10 mg) sample of the film or product in a glass test tube as shown in Figure 46; the test strip and sample should not be in contact. Seal the tube.
- Use a flame to gently pyrolyze the sample (thermochemically decompose it in a low oxygen environment) in the glass test tube. Keep the flame under the sample and away from the test strip. This is to avoid yellowing/browning the test strip due to excessive heat. If dense smoke is generated, tilt the tube horizontally to make sure the smoke reaches the test strip. The test strip will turn brown in the presence of sulfur.
- After about 30 seconds of direct exposure to the smoke, remove the brown test strip from the glass test tube. Quickly reseal the tube to limit the amount of gas released.
- Add one drop of hydrogen peroxide onto the strip. The presence of sulfur compounds in the sample will be confirmed if the brown test strip turns white. If the test strip remains yellow/brown, there is no sulfur and the colour was simply due to the burning of the strip.
Test results
The product should not be used if the test strip turns yellow or brown after burning the sample AND the strip turns white after being in contact with one drop of peroxide.
Products that contain sulfur, such as vulcanized rubber, should not be placed in contact with objects and should not share the same enclosure, whether airtight or leaky.
Alternative test
Azide test (Daniel and Ward 1982)
Test #2: pH test using glycerol for measuring the acidity of volatile compounds (Tétreault 1992)
Purpose of test
To determine the acidity of volatile compounds from a specific product, enclosure or room. Significant amounts of volatile acid compounds emitted from a product or present in an enclosure or a room can potentially cause damage, such as corrosion of metals or acidification of papers.
Materials and reagents to be used
- pH strips covering a narrow range of 4 to 7. A common pH strip product is EMD ColorpHast pH Test Strips. Avoid using a universal pH indicator covering the range of pH from 1 to 14 because the range is too broad to allow good sensitivity in the detection of small changes in the pH.
- Glycerol (glycerine), which is available at drugstores
- Glass jar with screw-top lid (250 mL or smaller) for testing a product
- Eye dropper
Sample preparation
Liquid samples, such as paint and adhesives, should be dried on aluminium foil for at least a week; solid samples, such as a gasket, need no preparation.
Prepare a solution of 20 mL water and 80 mL glycerine.
Procedure
All pH strips used in this test need to be wetted with one or two drops of the water-glycerine solution. The initial wet pH should be at least 6. (During the test, volatile compounds will accumulate on the wet strip and the colour will change based on the concentration and nature of the acids absorbed.)
If testing a product: place at least 2 g of the product in a glass jar with two or three previously wetted pH strips, and seal the jar. As well, place two or three wetted pH strips in a similar sealed jar without any sample—this will serve as a control (blank). Wait 24 hours. It is useful to reproduce this test with one or two more samples, each in its own jar with wetted test strips.
If testing an enclosure: place two or three wetted pH strips in the enclosure and two or three strips (blank) outside of the enclosure, ideally in a clean room for 24 hours.
If testing a room: place pH strips in the room and outside (blank) in a clean environment for 24 hours.
Avoid any contact between the glycerol-wetted pH strip and the test sample or enclosure.
Test results
For a product, enclosure or room to be acceptable for acid-sensitive objects, no difference in the pH value should be observed between the pH strip in the jar (or in the enclosure) and the blank test strip.
Volatile compounds released by films formed by coalescence (e.g. latex emulsions) are usually alkaline due to the presence of ammonia, which should evaporate in a few days. Paint films should only be tested after airing for a one-week period to ensure that most of the early-release volatile compounds have dissipated. Otherwise, some volatile compounds (such as peroxide, which bleaches the pH strips) could interfere with the test.
Alternative tests
Alternative tests for the determination of the acidity of the air can be found in Tétreault (2003).
Test #3: Testing for colourfastness
Purpose of test
To determine if a fabric, paper or matboard (whether wet or dry) could transfer dye to objects.
Materials and reagents to be used
- 2 pieces of glass or hard plastic plates
- Absorbent material, such as blotting paper or a piece of prewashed white cotton cloth, approximately of the same size as the plates mentioned above
- Tap water
- A 1 kg weight (approximate), for example a book or a block of metal
Procedure
A sample of fabric is placed on a piece of glass or plastic and wetted with several drops of water. Absorbent blotting paper or prewashed white cotton are laid on the sample (as shown in Figure 47 in the case of a fabric sample), allowing the absorbent paper or cotton above it to be wetted by contact. A second piece of glass or hard plastic is placed on top. Place the weight above the stacked assembly to compress it for good contact. After 30 minutes, observe the results.
Test results
The coloured fabric, paper or matboard undergoing the test can be used if no colour transfer is observed on the wet absorbent material above (blotting paper or cotton cloth).
If the colour has not transferred to the absorbent paper or cotton, the fabric is colourfast. If the colour has transferred, the fabric should be washed once or twice, allowed to dry, and retested. If the colour still transfers, it should not be used in the vicinity of museum objects.
© Government of Canada, Canadian Conservation Institute. CCI 120171-0020
Figure 47. Diagram showing how to assemble the materials and sample to carry out a colourfast dye test.
Figure 47 – text version
A sample of fabric is placed on a plate. Absorbent material is laid on the sample. A second plate is placed on top. The weight is placed on top of the stacked assembly.Complementary test
White residues that may leach out of some fabrics, such as gelatin or other finishing compounds, cannot be observed easily on a white absorbent material (blotting paper or cotton cloth). To detect these compounds, wet the fabric on a glass plate with a few drops of clean water. Gently rub the fabric for 10 minutes against the glass plate, then remove it and allow the glass plate to dry. Inspect the dry plate for residues or stains. If these are observed, this confirms that the fabric contains compounds that should be removed prior to its use within collections: wash the fabric and redo the test to check that all residues have been removed, or select another prewashed fabric that passes this test.
Alternative test
Consult CCI Note 13/14 Testing for Colourfastness for an alternative version of this test.
Examples of preventive conservation practices
Plastics – gifts of the 20th century and challenges of the 21st century
Plastics are synthetic compounds made of long molecular chains called polymers. The characteristics of polymers can be modified by additives such as fillers, plasticizers, antioxidants and colourants. Plastic products appear throughout museums in various forms such as: adhesives, coatings, fabrics, foams, gaskets, panels, sealants, sheets and lines. They offer many new design and application possibilities, sometimes at a lower cost than traditional products such as metal, glass and wood. However, many plastic products have limited stability over time and may turn yellow (if initially uncoloured), become fragile and cause stains. Their chemical and physical stability depends on how they are made, their form, the additives present and their function. Examples of the most common problems found with plastics products are listed in Table 6.
| Plastic | Problems |
|---|---|
| Tape adhesives | Most will stain or lose strength and become brittle after a few weeks to a few decades. |
| Flexible PVC in tubing and sheets | The plasticizer that gives PVC its flexibility tends to migrate to the surface and stain after one or a few decades. Degraded plasticizers may contain benzoic acid, which can cause corrosion of copper and copper alloys in sealed enclosures. The risk of hydrochloric acid emission from flexible or rigid PVC under normal conditions has not been demonstrated clearly. Due to this theoretical possibility, however, as a precaution, rigid PVC foam boards (e.g. Sintra) are not recommended for use in contact with objects for long-term purposes (limit their use to five years maximum). |
| Low-cost ether- or ester-type polyurethane foams | The ester-type polyurethane (PUR) foam is more stable, but both types tend to become sticky and brittle after a few years, losing their cushioning ability. They will also stain through contact. Foam gaskets made of PUR foam used to tightly seal storage cabinets or other enclosures should be monitored more closely after five years and replaced at that time if degraded or ineffective. |
| Rubbers and molding materials that contain sulfur compounds | Sulfur compounds are used in the crosslinking process for creating many types of rubbers. These rubbers can stain other objects through contact or by off-gassing in enclosures. They are never transparent. Molding materials such as plasticine may also contain sulfur. Consult Test #1: Lead acetate test for detecting sulfur compounds for instructions on testing for sulfur compounds in products. |
| Rubber bands | Synthetic and natural rubber bands will fail (break) after a few months under light stress and after a few years at rest. They can stain through contact when old. Rubber bands used to attach accession number labels to objects will fail and become dissociated from the objects, leading to a high risk of losing critical information. |
| Rubber gaskets and tubes | Synthetic and natural rubbers will lose their initial chemical and physical properties after a few decades. Gaskets used to tightly seal enclosures should be monitored more closely after 10 years of use and replaced if degraded or ineffective. |
| Plastic lines and strings under tension | Overloaded lines (monofilaments) and strings can break prematurely. They can also cut into softer surfaces (such as wood, plaster, paints and finishes, other plastics, leather, basketry fibres, soapstone, some ceramics) which results in disfiguring grooves on the objects if there is no padding (e.g. with tubing). Consult Suspending with proper padding for recommendations on preventing harm to objects when using lines and strings. |
| Acetoxy cure silicone sealant | Common silicone sealants are often of the acetoxy type. Such products release a high amount of acetic acid vapour (which smells vinegary) when freshly applied. Objects that are sensitive to this acid (lead, copper, paper, cotton) can be affected if the objects are installed in enclosures freshly sealed with this type of sealant. |
Suspending with proper padding
The suspension of objects requires special care. Tiny filaments can cut into many types of materials that are technically softer, including wood and wood-based products (Figure 48), plaster, paints and finishes, other plastics, leather, basketry fibres, soapstone, some ceramics, etc. Padding using safe tubing is essential. Make sure to make non-slip knots. Consult section Lines, twill tape, strings and wires.
© Government of Canada, Canadian Conservation Institute. CCI 120171-0022
Figure 48. The string used to attach the wooden fork for its suspension is both thin and hard, a combination that often leads to it cutting into the suspended object due to gravitational force (the weight of the object) and vibrations, as shown on the left. The solution is to increase the contact area between the string and the object. Tubing covering the string increases the overall diameter, thereby spreading the pressure on a larger contact surface on the object, as seen on the right. In practice, it is best to use transparent wire and tubing to minimize visual interference.
Interleaves – discrete and efficient
Avoiding all transfer of compounds from a product to the object is important. If there is any risk of such transfer and the product cannot be easily substituted to eliminate this risk, place an interleaf between the object and the product, as illustrated in Figure 49. The interleaf must have good gas-barrier properties and be free of compounds that could be transferred through contact. Melinex 516 (formerly called Mylar type D), Hostaphan 43SM and Terphane 10.10 are transparent PET films that are recommended because of their high-stability and low-additive content. In practice, most PET films will be acceptable.
Another option is plastic-laminated aluminum film, such as Marvelseal 360. The aluminum layer gives the film excellent barrier properties. Thin sheets of polycarbonate or acrylic are also good interleaves. Note that neutral pH papers and polyethylene sheet can be used, but they have poor to moderate barrier properties that may limit their long-term use.
© Government of Canada, Canadian Conservation Institute. CCI 120171-0023
Figure 49. The vase is on a pedestal base that releases harmful compounds, some of which migrate into the object at the contact point (left). The introduction of an interleaf will block the migration (middle) but not the off-gassing (right). If there is efficient dissipation of vapour in the immediate environment, this off-gassing is acceptable. Otherwise, the base should be sealed or replaced. For aesthetic reasons, the interleaf should not be visible to the viewer.
Figure 49 – text version
A drawing of a vase on a pedestal with arrows depicting migration and off-gassing of harmful compounds from the pedestal (left); an interleaf has been inserted under the vase (middle); a vase on an interleaf with arrows depicting only off-gassing from the pedestal (right).Big move at the Musée du Quai Branly – keep it simple
In the first years of the 21st century, about 275,000 objects from two museums were moved to the new Musée du Quai Branly in Paris. A huge number of support and packaging products were needed for safe transfer of the collection. For simplicity, only a few products that were known to be safe for this purpose were used:
- Rigid products:
- Acid-free corrugated paper board (2.5 mm and 3.2 mm thick)
Acid-free honeycomb board (15 mm sheets)
Corrugated polypropylene board (3.3 mm and 4 mm sheets)
Polypropylene sheeting (1 mm and 2 mm sheets) - Foams:
- Polyethylene foam planks (2.5 cm, 5 cm and 10 cm thick)
Thin foam (rolls) (0.3 cm thick sheets) - Wrapping products:
- Acid-free tissue papers
Cotton twill tape (tested for colourfastness)
Non-woven polyethylene (Tyvek)
Polyethylene bubble wraps
Polyethylene bags - Barrier films:
- 50-µm poly(ethylene terephthalate) sheets (Melinex 516)
75-µm poly(ethylene terephthalate) sleeves - Adhesives:
- Hot-melt glues (colourless) used with hot-melt glue gun, not applied to objects
References and further readings
Not all products were reviewed in this Technical Bulletin. External sources may help you find information on other products, as well as further information on products covered here.
While several web sources on products used in preventive conservation exist, the following independent ones (i.e. not associated with any specific distributors or manufacturers) are free and particularly recommended:
- Centre de conservation Québec. Préserv’Art
- Préserv’Art is developed by the Centre de conservation du Québec and is a searchable database of products and equipment used in conservation. CCI has been involved in the revision of these files.
- Museum of Fine Arts, Boston. CAMEO (Conservation and Art Material Encyclopedia Online)
- CAMEO is a searchable encyclopedia developed by the Museum of Fine Arts in Boston. CAMEO contains information on historical and current products used in preserving and producing artistic, architectural and archaeological materials.
The following are recommended as useful references that provide information on products and on strategies to use them safely with objects:
- Hatchfield, P.B. Pollutants in the Museum Environment: Practical Strategies for Problem Solving in Design, Exhibition and Storage. London, UK: Archetype Publications, 2002.
- Raphael, T., N. Davis and K. Brookes. Exhibit Conservation Guidelines: Incorporating Conservation into Exhibit Planning, Design and Fabrication [CD-ROM]. Washington, D.C.: US National Park Service, 1999.
- Tétreault, J. Airborne Pollutants in Museums, Galleries, and Archives: Risk Assessment, Control Strategies, and Preservation Management. Ottawa, ON: Canadian Conservation Institute, 2003.
References used in this resource:
Barclay, R.L., A. Bergeron and C. Dignard. Mount-making for Museum Objects. 2nd ed. Illustrated by C. Schlichting. Ottawa, ON: Canadian Conservation Institute, 2002.
Brimblecombe, P., D. Shooter and A. Kaur. “Wool and Reduced Sulphur Gases in Museum Air.” Studies in Conservation 37 (1992). pp. 53–60.
Daniel, V.D., and S. Ward. “A Rapid Test for the Detection of Substances which will Tarnish Silver.” Studies in Conservation 27 (1982), pp. 58–60.
Green, L.R., and D. Thickett. “Testing Materials for Use in the Storage and Display of Antiquities — A Revised Methodology.” Studies in Conservation 40 (1995), pp. 145–152.
International Organization for Standardization (ISO). ISO 14523:1999, Photography — Processed Photographic Materials — Photographic Activity Test for Enclosure Materials. Geneva, Switzerland: ISO, 1999.
International Organization for Standardization (ISO). ISO 18902:2007, Imaging Materials — Processed Imaging Materials — Albums, Framing and Storage Materials. Geneva, Switzerland: ISO, 2007.
Lavédrine, B. Photographs of the Past: Process and Preservation. Los Angeles, CA: The Getty Conservation Institute, 2009.
Marcon, P. Foam Corner Pads. CCI Notes 20/2. Ottawa, ON: Canadian Conservation Institute, 2020.
Marcon, P., and T. Strang. PadCAD: cushion design software. Ottawa, ON: Canadian Conservation Institute, 1999.
Schlichting, C. Working with Polyethylene Foam and Fluted Plastic Sheet. Technical Bulletin 14. Ottawa, ON: Canadian Conservation Institute, 1994.
Tétreault, J. "La mesure de l'acidité des produits volatils." Journal of the International Institute for Conservation-Canadian Group 17 (1992) pp. 17–25.
Tétreault, J. Coatings for Display and Storage in Museums. Technical Bulletin 21. Ottawa, ON: Canadian Conservation Institute, 1999.
Tétreault, J. Low-Cost Plastic/Aluminum Barrier Foil. CCI Notes 1/9. Ottawa, ON: Canadian Conservation Institute, 2010.
Tétreault, J. “Sustainable Use of Coatings in Museums and Archives – Some Critical Observations.” e-Preservation Science 8 (2011).
© Government of Canada, Canadian Conservation Institute, 2017
Published by:
Canadian Conservation Institute
Department of Canadian Heritage
1030 Innes Road
Ottawa ON K1B 4S7
Canada
Cat. No.: CH57-3/1-32-2017E-PDF
ISSN 0706-4152
ISBN 978-0-660-04138-4