Agent of Deterioration: Pollutants
Jean Tétreault
Table of Contents
Pollutants are grouped into a range of compounds that can have chemical reactions with any component of an object. Pollutants can be gases, aerosols, liquids or solids of either anthropogenic or natural origin, and they are substances that are known to have adverse effects (negative consequences) on objects. Deposits of solid particles are considered pollutants, and while they may not necessarily cause damage, they are recognized as altering the aesthetic aspects of the objects. In some cases, fine particles deposited on an object's surface can be strongly bonded.
In a museum, there are three modes of action for pollutants to reach objects and cause deterioration. In the first mode, the pollutants are airborne; in the second, the pollutants are transferred between two materials at points of contact; as for the third, it is intrinsic, in that the pollutant already exists, as part of the materials composing the object, or is formed during chemical reactions on or within it. The latter is called also called a secondary pollutant. Each of these modes of action will be discussed further below. Table 1 shows some common pollutants, their nature and their effects.
| Pollutants | Nature | Effects |
|---|---|---|
| Airborne pollutants | Atmospheric sources: ozone, hydrogen sulfide, carbonyl sulfide, sulfur dioxide, nitrogen dioxide, and particles (e.g. soot, salts). From emissive products, objects and people: sulfur-based gases, organic acids (e.g. carboxylic acids), particles (e.g. lints, danders). |
Acidification of papers, corrosion of metals, discoloration of colorants, efflorescence of calcium-based objects with RH (e.g. seashells), loss of strength for textiles. Dust: disfiguration of objects; attractant for pests, scratching of soft surfaces by friction. |
| Pollutants transferred by contact | Plasticizer from flexible PVC (polyvinyl chloride), sulfur compounds from natural rubber, staining materials from wood (especially knots), viscous compounds from old polyurethane foams, paper clips on papers, adhesives on objects from previous presentation, oily materials from leather, acids from some mineral specimens, fatty acids from people or from greasy objects such as skin/leather. Impregnation of salts during burial or immersion in seawater. Impregnation of residue of cleaning agents. Impregnation of salt from brick or stone floors or foundation. | Discoloration or corrosion of surface of the object in contact with harmful material from products or objects. |
| Intrinsic pollutants | Composite objects having compounds harmful for the other parts of the object, such as alum or iron gall ink in papers, 'original' adhesive tape on papers, corrosion of copper in contact with leather (e.g. tanned leather object having copper parts), composite objects made of sulfur- based compounds and metals. Secondary pollutants such as acetic acid and nitrogen oxide compounds from the hydrolysis of cellulose acetate and cellulose nitrate respectively. |
Deterioration of the objects: acidification, discoloration or stain on objects. Secondary pollutant may speed up the degradation processes caused by oxygen, water vapour or other pollutants. |
Airborne Pollutants
Airborne pollutants are either of anthropogenic or natural origin, and are carried by the air. Traditionally, they are mostly associated with industrial and urban activities. Within buildings, some pollutants can be generated by construction products. Staff and visitors are also a potential source of indoor airborne pollutants.
It is uncertain how many airborne compounds in any environment are actually harmful to objects, but it is possible to focus on those that are known to be the most harmful. Seven compounds have been identified as key airborne pollutants:
- acetic acid
- hydrogen sulfide
- nitrogen dioxide
- ozone
- sulfur dioxide
- fine particles
- water vapour
These key pollutants are common, and their reactivity is equal to or greater than the other pollutants of the same chemical group. Thus, strategies to control the seven key pollutants will generally also control the other airborne pollutants.
Acetic acid
This carboxylic acid (CH3COOH) is mainly generated indoors when inappropriate products are used, and may cause problems in airtight enclosures. Typically, an enclosure constructed using poorly chosen wood and paints will cause problems. Lead is the most sensitive material to acetic acid (Figure 1 and Vignette 1) and it is often found corroded where acidic woods or inadequate paints are chosen to build display or storage cabinets.
Hydrogen sulfide
Hydrogen sulfide (H2S), a reduced-sulfur gas with a characteristic "rotten egg" odour, is a key pollutant due to its great ability to tarnish silver (Figure 2) and copper within a very short time, even outside of urban areas. The darkening of lead white pigment in paintings is also caused by the presence of reduced-sulfur gases. The main anthropogenic sources of hydrogen sulfide are the pulp-and-paper and petroleum industries. Outside the urban environment, hydrogen sulfide is emitted by oceans, volcanic and geothermal activities, marshes, and vegetation. Inside buildings, staff and visitors are often the major source of hydrogen sulfide.
Nitrogen dioxide
The most common compound of the nitrogen oxide group (NOX), nitrogen dioxide (NO2) is responsible for the reddish brown colour above cities, especially during periods of photochemical smog. NO2 is formed rapidly in the atmosphere by the action of ozone on nitric oxide (NO), which is the major nitrogen oxide emitted by combustion in vehicles (about 50% of emissions), power plants, and industrial activities. In the atmosphere, a fraction of nitrogen dioxide can be further oxidized to its acid form: nitric acid (HNO3). Both nitric acid and nitrogen dioxide cause artists' colorants to fade and can contribute to the degradation of paper and vegetable-tanned leather. It is also believed that the nitrogen oxide absorbed by objects becomes oxidized to nitric acid, the latter being responsible for most of the ensuing deterioration.
Ozone
Ozone (O3) is a strong oxidant that is normally present in the stratosphere and protects us against intense, harmful ultraviolet radiation. At ground level, it is formed during photochemical smog. Photochemical smog is the result of multiple chemical reactions between nitrogen oxides and hydrocarbons, and their oxygenated derivatives in the presence of sunlight. In Canada, the Windsor–Quebec City corridor has the highest levels of ozone. This is due to the high population density and the industrialization of the corridor, and to the dominant southwest winds that carry ozone precursors, especially from the area immediately south of the Great Lakes. The transport of ozone precursors in the atmosphere results in high levels of ozone in remote areas where there are no major human activities. Inside buildings, the main sources of ozone are electrostatic precipitators in the heating, ventilation, and air-conditioning (HVAC) system, electronic air cleaners (ozone generators), and photocopiers. Ozone has the capacity to attack materials by breaking apart any double bonds between carbon atoms. The degradation of vulcanized natural rubbers under stress and the fading of artists' colorants are the most studied phenomena.
Sulfur dioxide
In the United States and in Europe, power plants based on coal and oil combustion are the major sources of sulfur dioxide (SO2), followed by industrial processes and transportation. Only small quantities of sulfur dioxide come from gasoline-fuelled motor vehicle exhaust. In Canada, industrial activity, specifically metal smelting, is the main source of sulfur dioxide. Inside enclosures materials such as proteinaceous substances, sulfur-vulcanized rubbers, oxidizing sulfides in geological specimens, and some dyes are sources of sulfur compounds. sulfur dioxide causes corrosion of copper, fading of some artists' colorants and weakening of vegetable-tanned leathers (Figure 3). Both sulfur dioxide and nitrogen dioxide have been largely associated with the acidification of papers where acid rain and urban pollution are an acute problem.
Fine particles
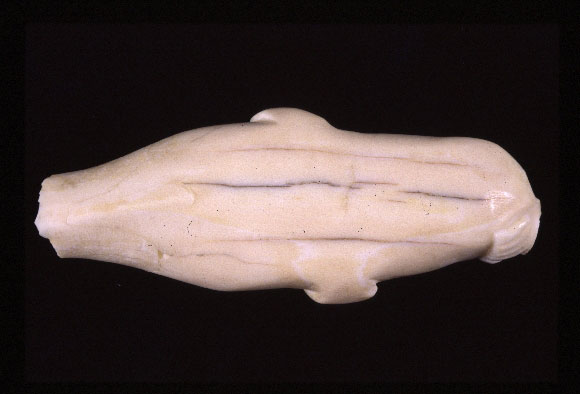
It is common to characterize particulate matter (dust) in terms of aerodynamic diameter, which is defined as the diameter of a sphere with a unit density having an aerodynamic behaviour equivalent to that of the particle in question. The aerodynamic diameter is important, because it determines its behaviour and control. For the control of pollutants, the fine particle (PM2.5: suspended particle matter having an aerodynamic diameter equal to or less than 2.5 µm) and the coarse particle (PM10: aerodynamic diameter between 2.5 and 10 µm) are commonly used as indicators. Due to the small size of PM2.5 , it is the most challenging particle size to control. Sulphate and nitrate compounds, organic carbon, crustal materials, and salts are the major harmful outdoor compounds forming fine particulate matter (PM2.5 ). Any attempt to control the level of PM2.5 must consider a priori the control of levels of PM10 and some super coarse particles (>10 µm), which still contain some potentially reactive compounds, such as combustion residues, human danders, and microbiological specimens. Fine particles are particularly damaging, because they discolour or soil surfaces. Soiling changes the visual appearance of objects. The more fragile, porous, or altered the surfaces, the more difficult they are to clean. Any control strategy designed to maintain low levels of particles is beneficial to objects, since cleaning fragile or porous objects can be difficult and time consuming. Object cleaning is a delicate process that requires trained conservators. The ivory sculpture (Figure 4) is a good example of an object that is challenging to clean. The deposit of hygroscopic, oily, or metallic particles on a surface can initiate or accelerate deterioration, as well as the formation of harmful compounds such as acids. With the exception of particles generated by cooking activities in a museum's cafeteria or the burning of combustibles (candles and lamps), most indoor-generated particles are composed of soil, and fibres from carpet and cloth. Fibres are not generally considered to have direct adverse effects on collections, except for of magnetic media such as audio and video tapes, where abrasive dusts are an issue during handling and playing. Dust accumulation can also provide an attractive foraging place for insects, and can initiate mould. From a wider viewpoint, another adverse consequence is the impact of the perception by visitors, including potential donors, that there is a basic lack of care for the collection.
Water vapour
Water (H2O) is included as a key airborne pollutant, even though there are well-established guidelines for relative humidity levels for museums in order to prevent physical deterioration caused by incorrect levels (too dry or too humid), or by excessive fluctuations (see section on Incorrect relative humidity). The action of water vapour as a pollutant relates to both physical and chemical damage. Through hydrolysis, water vapour can directly damage cellulose-based materials such as book and paper artworks, which are usually an important part of collections. Materials that are sensitive to the hydrolysis action of water vapour include cellulose acetate and cellulose nitrate (Figure 8 and Figure 9), especially in the form of thin sheets or rolled films, papers, and magnetic tapes. Water vapour also greatly influences the deterioration processes caused by other pollutants.
Oxygen
Oxygen (O2) is naturally part of the atmosphere. Without necessarily being the initiator, oxygen is involved in many deterioration processes of organic materials, such as some colorants, polymers, cellulose-based objects, and skins. The deterioration is caused by the oxidation of a compound after being photo-excited by UV or visible radiation. The resulting oxidation leads to physical changes, such as brittleness and cracking, as well as chemical changes, such as yellowing and fading. In the presence of moisture, metals such as iron will rust. Up to now, low oxygen or anaerobic environments have been mainly used for disinfestation and for long-term storage of individual objects in airtight bags. There are few case studies of the use of low-oxygen environments for large enclosures. This is partly due to problems related to airtightness, maintenance costs, and access. Because of the limited use of low-oxygen environments and the unnecessary need to include oxygen in basic monitoring campaigns, oxygen has not been classified as a key pollutant.
Mosaic of photographs showing typical damage caused by pollutants.








Control Strategies for Airborne Pollutants
Guidelines on levels of airborne pollutants
Airborne pollutants around collections are undesirable. Although it can be very expensive both in money and time to maintain a very low level of pollutants, the concept of degree of preservation can help in determining whether the preservation of the collection against pollutants is suitable in terms of the mandate and resources of the museum. This is based mainly on the concept of dose (the concentration of the pollutant multiplied by the duration of exposure) at which the first signs of deterioration caused by a pollutant are measurable in a material. In the jargon of risk management, this dose is called the "lowest observed adverse effect dose" (LOAED). In accordance with the principle of reciprocity, for a given dose of a pollutant on an object, it is possible to calculate the exposure time required before signs of damage appear. For example, basic fuchsin (a green dye) first begins to turn a lighter colour at a dose of 10 micrograms of sulfur dioxide per cubic metre year (10 µg m-3 yr). Therefore, if we are willing to accept a slight color change after 10 years, the average concentration of sulfur dioxide must be reduced to less than 1 µg m-3 (10 µg m-3yr / 10 years).
Table 2 shows the maximum allowable concentration for each key airborne pollutant to provide minimal risk to most materials for the indicated exposure periods. These maximum levels of pollutants have been grouped in three degrees, or targets, of preservation: 1, 10, and 100 years. The 100-year target provides the greatest preservation, and means that most objects should not show adverse effects for 100 years when exposed to the maximum level of pollutants allowed in the respective column. For most objects, it is probably unrealistic and unreliable to specify pollutant concentrations in the room lower than those allowed by the preservation target for 100 years. Intermediate targets, such as 50 or 80 years, can also be adopted. It is important to stress that Table 2 gives degree of preservation based on medium sensitivity objects that include the majority of the collection. High risk materials, shown in Table 3 are excluded because it can be too expensive to provide a global control strategy intended to preserve all objects, including the high risk ones. It is much more cost effective to provide a higher level of preservation to high risk objects, and a global approach to the overall collection. It is very important to identify the highly sensitive objects in the collection so that extra attention can be focussed on them.
| Key airborne pollutants | Maximum average concentration for indicated preservation targets, µg m-3 (ppb) Table 2 note 1 | Reference average concentration range, µg m-3 | |||
|---|---|---|---|---|---|
| 1 yr | 10 yrs | 100 yrs | Clean low troposphere | Urban area | |
| Acetic acid | 1000 (400) | 100 | 100 | 0.3–5 | 0.5–20 Table 2 note 2 |
| Hydrogen sulfide | 1 (0.71) | 0.1 | 0.01 | 0.01–1 | 0.02–1 |
| Nitrogen dioxide | 10 (5.2) | 1 | 0.1 | 0.2–20 | 3–200 |
| Ozone | 10 (5.0) | 1 | 0.1 | 2–200 | 20–300 |
| Sulfur dioxide | 10 (3.8) | 1 | 0.1 | 0.1–30 | 6–100 |
| Fine particles (PM2.5) | 10 | 1 | 0.1 | 1–30 | 1–100 |
| Water vapour | keep below 60% RH Table 2 note 3 | N/A | |||
Table 2 notes
- Table 2 Footnote 1
-
Preservation target is the length of time (in years) for which the objects can be exposed to the indicated level of pollutants with minimal risk of deterioration. These targets are based on the LOAED of most objects (exclude high risk objects; consult Table 3) and assume that average RH is kept between 50 and 60%, temperature ranges between 20 and 30°C, and the collection is kept clean (if not, the maximum levels of key airborne pollutants for each class of targets may need to be readjusted). These values are not applicable to high risk materials – ppb means parts per billion.
- Table 2 Footnote 2
-
Acetic acid levels can be emitted to levels as high as 10,000 µg m-3 in enclosures made with inappropriate materials, such as fresh acid-cured silicone.
- Table 2 Footnote 3
-
For permanent collections where the RH has not been kept between 50 and 60%, maintain the historical conditions.
| Objects and potential damage Table 3 Footnote 1 | Most harmful airborne pollutants and their sources |
|---|---|
| Sensitivity colorants such alizarin crimson, basic fuchsin, curcumin and pararosaniline base: they tend to fade. | Typically outdoor pollutants such as nitrogen dioxide, Sulfur dioxide and ozone. |
Lead: in enclosure, in presence of carboxylic acid-emissive products, lead tends to corrode by forming a white powder on its surface. |
Mainly acetic and formic acid from wood, especially oak and cedar and/or in presence of oil based or alkyd paints in enclosure. |
Natural rubber (vulcanized or not): especially under stress, the rubber tends to crack and become brittle. Synthetic rubbers are sensitive, but to a lesser extent. |
Ozone from the low atmosphere. |
Silver: well known to tarnish easily in ambient conditions in a year. Frequent cleaning may cause loss of information and can be a serious issue if the layer of silver is thin. |
Reduced sulfur gases such as Hydrogen sulfide, carbon disulphide and carbonyl sulphide from natural and anthropogenic sources. Inside museums, visitors can be the main source of Hydrogen sulfide. Some products may contain sulfur-based compounds. |
Magnetic tapes (audio, video, data): presence of dust will contaminate equipment and lead to read errors for digital tapes or signal losses known as dropout for analog tapes, and scratching of the tape surfaces. |
Particles from outdoors and from indoors (danders and lints from staff and visitors, and human activities such as renovation and cooking). |
Objects that are difficult to clean. This includes objects with powdery pigments or surfaces such as some painted ethnographic objects or feathers, some butterfly wings; physically fragile objects such as insect collections and filamentous mineral specimens; objects in which fine particles could become lodged in microcracks or interstices (e.g. ivories or painted objects with cracks); objects with sticky surfaces such as certain deteriorated plastics and some polyethylene glycol treated wooden waterlogged objects; objects that cannot be easily cleaned by vacuum cleaning, immersion baths, or poultices; and objects with numerous small components that would be difficult and time-consuming to clean well. |
Fine particles from outside, mainly caused by the combustion associated with urban transportation. |
Moisture-sensitive objects (sensitive to hydrolysis) such as cellulose acetate and cellulose nitrate, colour photographic prints, photographic gelatin, magnetic recording tapes, many types of papers, natural varnishes, flexible (plasticized) PVC. Damages depend of the nature of the material, but can include discoloration, acidification, tensile strength loss and deformation. |
Water vapour from visitors, water-based paints, and adhesives, wet cleaning activities and outdoor environment. |
Objects containing water soluble salts that are known to react with acetic and formic acids, including ceramics, seashells, minerals. They are prone to develop long filament crystals or snowy flakes (efflorescence). |
Water vapour, acetic acid, formic acid and objects with internal salt or acetate compounds. |
Table 3 notes
- Table 3 note 1
-
Consider control of strategies associated with a higher degree of preservation or consult Tétreault (2003).
The maximum concentration of key airborne pollutants allowed in Table 2 for the preservation targets of 1 and 10 years can be considered effective and achievable for preservation purposes. Many objects will not show the first signs of deterioration until after the designated exposure period. In the rooms of most historic buildings, a target of around 10 years is, in fact, the best that can be achieved. However, achieving pollutant levels with a low preservation target in a room can contribute to a better degree of preservation in an enclosure if there are no significant emissive sources of pollutants inside.
The ease with which a specific degree of preservation in a room is achieved depends on the LOAED value of the pollutant to the majority of the collection. This is exactly the same value as given in the one-year target in Table 2 (for NO2; 10 µg m-3 × 1 yr = 10 µg m-3 yr-1 ). The higher the LOAED value and the lower the outdoor concentration, the easier it is to achieve a high degree of preservation. If there is an internal source of a pollutant, such as a high number of visitors emitting hydrogen sulfide, a high degree of preservation can be more difficult to achieve. It all depends upon the actual facilities, and the resources available for improving air quality.
Systematic approach
Control strategies are coordinated measures intended to reduce one or more airborne pollutants to a certain level, thereby limiting the risk or the rate of deterioration of objects exposed to them. These measures can be derived from specifications, which are accurate descriptions of technical requirements for the performance of building features, portable fittings, and procedures. Table 4 summarizes the various control strategies to prevent adverse effects of airborne pollutants. It shows the possibilities for control at different stages. Avoid, block, dilute, and filter/sorb are strategies to reduce the levels of pollutants in the ambient air. Reduce reaction is a strategy for minimizing the adverse effects of pollutants on objects. This strategy consists, firstly, in the reduction of environmental factors such as radiations and compounds that are involved in the reaction without being the main pollutant. Secondly, the strategy consists in the neutralisation of the sorbed pollutants in the objects. The mass deacidification of books is based on this strategy. Reducing exposure is a strategy for controlling deterioration by limiting the exposure of objects to the harmful environment. Whenever feasible, avoiding sources of pollutants is the best option. However, there are unfortunately few options available to avoid outdoor pollutants, the most realistic being the blocking strategy. For indoor-generated pollutants, the avoid strategy (i.e. avoiding exposure by selecting safe products) is the most efficient choice for enclosures. If this cannot be done, the block, the dilution or the filter/sorb strategies will provide a partial reduction in pollutants. It is important to note that control strategies do not have to be uniformly applied throughout the museum. They can be tailored to the value and need for preservation of the objects, as well as to individual rooms and enclosures.
Control strategies for the control of airborne pollutants (Tétreault )
AVOID outdoor sources
- Select proper locations for new buildings based on surrounding sources of airborne pollutants such as pollution-emitting industries and dominant winds.
- Minimize the generation of pollutants, e.g. pave the parking lot, limit traffic in the immediate vicinity of the building.
AVOID sources in rooms/building
- Minimize dust- and gas-generating activities close to the collection or in the same ventilation zone.
- Limit the number of visitors per room, depending on the ventilation capacity.
- Carefully select and use products based on their chemical components.
AVOID sources in enclosures
- Carefully select and use products based on their chemical components.
BLOCK infiltration of pollutants in rooms/building
- Improve airtightness of the building membrane, or of some rooms; add vestibules for the main doors and open windows wisely.
- Select proper location for the air intake of the HVAC system system, provide different positive pressure zones with a minimum air intake ratio. Insert efficient gas and particle filters. Change filters periodically as recommended by the manufacturer, or based on the results of assessment done by an air quality firm.
BLOCK infiltration of pollutants in enclosures
- Use airtight enclosures or use air-filtered positive pressure systems. Change filters periodically.
- Wrap objects with sorbent tissues, such as acid-free tissues or cotton fabrics.
BLOCK emission of pollutants from products in rooms/enclosures
- Apply a barrier film on the surface of emissive wood products
BLOCK transfer (deposition or sorption) of pollutants on objects
- Apply a barrier film on the surface of objects (limited option).
DILUTE, FILTER, or SORB pollutants in rooms/building
- Consider wise distribution of collections in the different air quality zones of the building (including the possibility of using an enclosure).
- Increase the distance between the source and objects.
- Use local exhaust for the most polluting activities (cooking, workshop, chemical storage).
- Use portable fans to push the air out of the rooms/building (short-term high emission such as fresh painted walls or floors).
- Filter the recirculating air of the HVAC system system or use a portable filter unit. Change filters periodically.
- Remove dust accumulation in the building and on portable fitting surfaces periodically. Minimize resuspension of dust.
DILUTE or SORB pollutants in enclosures
- Consider stack pressure design of the enclosure if the environment of the room is well controlled, or use air-filtered positive pressure systems.
- Dilute (flush) air with a non-reactive gas such as argon, helium, or nitrogen.
- Use passive or active sorbent methods (can include gas, particles, water vapour, or oxygen sorbent). Change the sorbents periodically.
REDUCE REACTIONS on objects
- Decrease the level of RH, the temperature, or the ultraviolet, visible, and infrared radiation (where appropriate).
- Neutralize pollutants absorbed into the objects (e.g. alkaline compounds in papers inhibit acid deterioration).
REDUCE EXPOSURE TIME
- Limit exposure of objects to the inappropriate environment.
MONITOR the collection
- Inspect for signs of deterioration on objects and on the enclosure and building products periodically.
MONITOR pollutants in rooms and in enclosures
- Do appropriate in situ monitoring of pollutants.
MONITOR performance of control features
- Verify efficiency of the gas and dust filter systems periodically.
- Measure the leakage rate of the building and enclosures
RESPOND to the detection of pollutants or damage on objects
- Protect objects from the harmful environment, or monitor closely.
- Remove dust, efflorescence compounds or active corrosion compounds on objects.
- Re-evaluate the avoid, block, and reduce strategies; consider cost-benefit analysis.
Monitoring is an important element of the control strategy. However, methods for monitoring air quality in museums have not yet been standardized and are not extensively used, partly due to their high cost. This reinforces the function of the Avoid and Block strategies. When resources are available or when there is a specific important problem, Airborne Pollutants (Tétreault ) proposes an investigative approach. Detailed lists of commercial dosimeters can also be found in Grzywacz ( ). In the absence of monitoring equipment, visual inspection of the collection can be easily done. In a routine inspection, look for signs of new corrosion on metals, especially for those in new display cases or new storage cabinets. Off-gassing from fresh products in the enclosure may cause corrosion observable in only a few weeks or months. If the RH is maintained at high levels, or if there is high fluctuation, check for efflorescence, such as the formation of white powder or crystals on ceramic or shell object (Figure 6). The other signs of deterioration, such as discoloration, cracks and embrittlement, usually appear gradually, and the perception of change is difficult to assess without reference documentation. Accumulation of dust can also be monitored by placing clean glass plates in different locations. After a few months of exposure, a primary analysis of the nature of dust with an optical microscope can help to identify the main source in the specific location. The control strategies can be revised if the rate of deposit is unacceptable.
All the strategies above are for prevention mode. When a problem is noticed, it is necessary to respond by going into active mode. All objects at risk must be in a safe environment, or they should be removed to reduce exposure time. Close monitoring is necessary and, if it is considered that the compounds on objects resulting from the presence of pollutants may still be active, the damaged objects should be dealt with by qualified professionals. Once the cause has been investigated, the control strategies in place should be re-evaluated to prevent the same risk in the future.
Control strategies for different degrees of preservation
Certain typical sets of control strategies are grouped to fulfill three progressive degrees of preservation: basic, intermediate and advanced. Table 5 shows three different degrees of preservation for the majority of a collection. They are based mainly on making improvements in the selection of construction products, airtightness of enclosures and filtration of the fresh air intake. The degrees of preservation are not absolute, and can be adjusted as required.
| Degree of Preservation | Control Strategies |
|---|---|
Basic |
|
Intermediate |
|
Advanced |
|
Table 5 note
- Table 5 note 1
-
If not in conflict with RH required for overall collection. The % value is based on an average of a few weeks. For more information, see section on Incorrect RH.
- Table 5 note 2
-
Technical Bulletin 32 Products Used in Preventive Conservation.
- Table 5 note 3
- Table 5 note 4
- Table 5 note 5
-
Consult section on Pests.
Control strategies may vary according to the preservation needs of specific objects or collections. The highly sensitive objects (mentioned above) should be identified to allow an appropriate degree of preservation. They will probably need tighter control strategies to achieve the same degree of preservation as for the rest of the collection.
Basic preservation
The basic level insures minimum preservation by proposing control strategies that minimise the risk of damage by airborne pollutants for a period of one to three years. The highest risks with airborne pollutants are usually from new products such as building or retrofitting display cases without proper selection of products, or without sufficient curing time before the installation of objects. Oak and similar woods, and oxidative polymerization coatings, such as alkyd and oil based paints, are the most common problem products that cause corrosion on metals and efflorescence on calcium-based objects. High peaks of RH (above about 70%) also initiate and accelerate corrosion.
Since the cleaning of objects can be difficult and time consuming, it is better to prevent dust deposit. In storage, fabric or plastic sheets should cover large-scale objects, and objects difficult to clean should be enclosed, both in storage as well as in exhibit areas. Conservation advice may be needed in removing particles on fragile surfaces.
Intermediate preservation
After fulfilling the basic level of preservation, museum staff may consider a greater level of preservation. The control strategies described in intermediate preservation allow a minimum risk of damage for a period of 10 to 30 years for medium sensitivity objects. The main emphasis is on reducing the infiltration of airborne pollutants into the building and into enclosures, as well as better selection of low emission products inside enclosures. The minimal particle filter efficiency for the HVAC system is Class B Table 6 which is based on the performance of MERV 14 for the final stage filter.
| Class of speci- fication |
First-stage particle filter, minimum efficiency based on | Gas filter | Final-stage particle filter, minimum efficiency based on | Return air Table Footnote 3 |
||||
|---|---|---|---|---|---|---|---|---|
| ASHRAE 52.2 (MERV Table 6 note 1 ) | Dust spot efficiency Table 6 note 2 | EN 779 Table 6 note 2 | ASHRAE 52.2 (MERV) | Dust spot effi- ciency |
EN 779 | |||
| AA | >12 | >70% | >F6 | 1 or 2 stages | >16 | >99% | >H10 | filtered |
| A | 11 | 60–65% | F6 | 1 stage | 15 | >95% | F9 | filtered |
| B | 10 | 50–55% | F5 | 1 stage (preferable) | 14 | 90–95% | F8 | filtered |
| C | 9 | 40–45% | F5 | None | 13 | 80–85% | F7 | filtered (pre- ferable) |
| D | 8 | 30–35% | G4 | None | 12 | 70–75% | F6 | un- filtered |
Table 6 notes
- Table 6 note 1
-
MERV: Minimum efficiency reporting value from ANSI/ASHRAE Standard 52.2. This standard replaces the two previous American and European standard tests (Note Table 6 note 2 ). MERV covers a range from 1 to 20, where 20 is the highest filtration control. A MERV of 17 and above is designed for the needs of an ultra pure environment, such as storage of radioactive materials and surgery rooms.
- Table 6 note 2
-
Performance of the filter based on the atmospheric dust spot test ASHRAE 52.1 and EN 779 from the European Committee for Standardisation (included for reference).
- Table 6 note 3
-
Return air is filtered when it is recirculated through the filter system.
Advanced preservation
When resources are available, or when the value of objects justify it, advanced preservation can be achieved by tighter control strategies. A high barrier membrane is used for the building, and careful selection of non-emissive products for airtight enclosures is practiced. Sorbents in active or passive mode can be used in enclosures (Vignette 2). Due to the tight control required, access to visitors can be limited in storage areas as well as, to a certain level and in certain situations, the exhibition areas. The goal of advanced preservation is to minimize the risk of damage for 100 years or more. In some cases, a dry and/or low oxygen environment can be chosen for display or storage of highly sensitive or high value objects to insure this level of preservation.
Pollutants Transferred by Contact
A pollutant on the surface of an object or product can be transferred by contact with another surface, usually causing discoloration or staining. The rate of staining depends on the quantity and mobility of the pollutant in the material and the porosity or the reactivity of the surface receiving the pollutant. To a certain extent, all objects are vulnerable to staining from contact with inappropriate products. Such stains can be hard to remove from porous or very fragile objects. The most typical products that tend to transfer stains by contact over time are unsealed woods (especially where there are knots), acidic paper or cardboard (Vignette 3), flexible PVC, sulfur-based plastic, soft polyurethane foam, most adhesive tape, some liquid adhesives, salt contaminated surfaces (such as contaminated bricks or stone floors), metals under humid conditions (stain organic materials and can cause galvanic corrosion on metal objects), and oily materials such as skin and leather, but also the fatty acids from staff and visitors. Cleaning products can be harmful if incorrectly used, for example, general building cleaning supplies can splash onto objects on exhibit and in storage, or compounds associated with cleaning and polishing objects can cause problems if used inappropriately or in too large quantities. Sometimes the residue left by polishing compounds is not readily visible.
Control Strategies
Strategies to minimize the risk of staining by contact mainly concern avoiding inappropriate products/objects, blocking pollutant migration at contact points, or reducing the length of contact. Table 7 shows common strategies to reach three different degrees of preservation for contact with harmful products. The basic degree of preservation against damage caused by contact for the first years; intermediate and advance levels will prevent damage for a few decades and for a few centuries respectively.
| Degree of Preservation | Control Strategy |
|---|---|
Basic |
|
Intermediate |
|
Advanced |
|
Intrinsic Pollutants
An intrinsic pollutant is one that is already in the object as part of its original content, or has been added during processing or treatment. Inherent deterioration is a term sometimes used to refer to the action of intrinsic pollutants in the object. Intrinsic pollutants are either unstable or they can react with other compounds in the object to cause damage. New compounds formed inside objects during a degradation process can also be considered as intrinsic pollutants or, more precisely, secondary intrinsic pollutants. A good example is acetic acid released by cellulose acetate film undergoing hydrolysis. The acetic acid formed within the film accelerates its degradation. In paper artworks, alum (used as internal sizing) tends to speed up the degradation of the paper. Objects treated with oily materials, such as leather dressings, can result in corrosion of attached copper parts (Figure 7).
Plasticizer in flexible PVC objects is also a common problem, but it is not really associated with an intrinsic pollutant. Somehow the plasticizer is unstable in the object and it tends to migrate to the surface of the object. The flexible PVC will become sticky and will deform while the plasticizer is leaving. Certain colorants in the plastic can be dissolved and migrate with the plasticizer to stain other parts of the object with which it is in contact. Reducing the temperature will help to slow down the migration.
Control strategies
If intrinsic pollutants are a feature of unstable artists' materials or manufactured products used in artworks, there is little that can be done to avoid them, except education. Blocking the pollutant within the object is often a solution. Deacidification of books in archives and libraries is a typical strategy, blocking the action of acidic compounds by adding alkaline compounds. Otherwise, the main strategies are limited to reducing the reaction by keeping objects cool and dry. Thus, the basic degree of preservation will consist of avoiding excesses of relative humidity, temperature and light.
Objects in the collection having intrinsic pollutants should be identified and special control strategies initiated before any serious and irreversible damage can occur. Some materials from objects composed of sulfur- based compounds, polyurethane foams, flexible PVC, wool (Figure 10), inadequate glue or cellulose acetate can cause damage to the object itself by off gassing or contact.


References
Grzywacz, C. M. Monitoring for Gaseous Pollutants in Museum Environments. Los Angeles: Getty Publication, .
Key Readings
Hatchfield, P. B. Pollutants in the Museum Environment: Practical Strategies for Problem Solving in Design, Exhibition and Storage. London: Archetype Publications, .
Tétreault, J. Airborne Pollutants in Museums, Galleries and Archives: Risk Assessment, Control Strategies and Preservation Management, Ottawa: Canadian Conservation Institute. .
Tétreault, J. "Guidelines for Pollutant Concentrations in Museums". CCI Newsletter 31 ( ) p. 3-5.
Thomson, G. Museum Environment, second edition. London: Butterworths, .
Glossary
Enclosure
A collection of materials that surrounds a limited space (e.g. a plastic bag, display case, storage cabinet, or transportation box).
HVAC system
A heating, ventilation, and air-conditioning system that includes any interior surface of the facility's air distribution system for conditioned spaces and/or occupied zones.
Material
A substance that composes an object or a product, e.g. copper, oak, cotton are materials. See also Object and Product.
Object
An item judged by society, or by some of its members, to be of historical, artistic, social, or scientific importance. Objects can be composed of one or more materials, and can be movable (such as works of art, artifacts, books, archival-related items, and items of natural, historical, or archaeological origin) or immovable (such as architectural interiors or structures of historical or artistic interest).
Product
A manufactured or processed substance made up of one or more materials used in the care and housing of collections or objects. For example, plywood is a product made of two materials: wood and adhesive. Specifications for selecting products use a variety of terms such as inert, stable, safe (no or low risk), or suitable. These terms are not standardized, but "inert" almost always refers to a very high level of stability that is not usually mandatory in conservation. Otherwise, common recommended products such as acid free papers will have to be banned in conservation.
Vignettes
Vignette 1. Powdery White Corrosion in Storage
Problem: White, powdery corrosion was noticed on a medal. Examination showed that all the affected items were made of lead. This is the result of using products in the display or storage environment that emit carboxylic acids. Lead is particularly prone to corrosion from these acidic compounds.
Solution: Identify the source of pollution and deal with it. In this case, the oak paneling used in construction of the storage enclosures proved to be a source of acetic acid. Sealing the wood with acrylic latex paint with at least four weeks drying and ensuring basic control of the relative humidity can mitigate the problem. However, due to the sensitivity of lead to acetic acid and the probability of having high levels of acetic acid released from products, it is advisable to avoid using such acidic products as wood, acidic paper, fresh paint or fresh emulsion glue in displays of lead objects.

Courtesy of the Musée du séminaire de Sherbrooke.
Vignette 2. Use of Sorbents

Photo courtesy of Purafil, Inc.
Sorbents (or scavengers) can extract certain compounds present in the ambient air and retain them by an affinity, or reaction, process. A sorbent may work through absorption (interactions taking place largely within the pores of solids) or adsorption (interactions taking place on solid surfaces). The processes involved can also be divided into chemisorption (chemical bonding with the substrate) and physisorption (physical attraction, such as weak electrostatic forces). Desorption processes can also occur (assuming the sorbed gases have not already reacted) if the sorbent is close to equilibrium (between ambient pollutant's level and what the sorbent can absorb) and if the level of pollutants decreases in the ambient air. For good performance, the sorbent must be replaced or regenerated before it approaches the desorption process.
As a simple rule of thumb, in the case of a well-sealed enclosure (assuming one air exchange a day) without important emissive materials (objects and products), 500 grams of activated carbon per cubic meter can, for a period of one year, reduce the level of pollutants inside the enclosure by a factor of 10, as compared to the level in the room. After that, the carbon will need to be replaced. If the enclosure is more leaky, if there are substantial internal pollutants, or if the lifetime of the sorbent must be extended, more sorbent will be needed. In practice, the use of sorbents is recommended mainly when measures are already in place to minimize internal sources, and to maintain high level of airtightness of the enclosures.
For more details on the use of sorbents, see CCI Note 15/1 Care of Objects Made from Rubber and Plastic , or consult Tétreault (2003).
Vignette 3. Stain of paper artwork

This artwork on paper has been stained by the migrating component of an acidic tape (passe-partout) by contact over more than a decade. The stain is mostly irreversible. A buffered acid-free board should had been used instead. For long-term preservation, the use of acidic papers and cardboards, as well as metal staples or paperclips, rubber bands and adhesive tape should be avoided.
Vignette 4. Video

The use of commercial paints in museums
This video provides answers to the commonly received questions: what paint should be used to avoid any damage to museum collections and how long should a painted surface be left to dry before objects can be exposed to it? This video was created by the Canadian Conservation Institute.
Related resources
Time-lapse Video of Deterioration: Two Iron Keys, Incorrect Relative Humidity and Pollutants

Time-lapse Video of Deterioration: Two Iron Keys, Incorrect Relative Humidity and Pollutants
This video was created by the Canadian Conservation Institute.
Thanks to the Centro Nacional de Conservación y Restauración in Chile, the Canadian Conservation Institute’s web resource Agents of deterioration, translated into Spanish by ICCROM, is now available free of charge. Intended for curators and conservators, the resource identifies 10 primary threats specific to heritage environments.