Biological test method for measuring survival of springtails exposed to contaminants in soil: chapter 5
- Specific Procedures for Testing Field-collected Soil or Similar Particulate Material
- 5.1 Sample Collection
- 5.2 Sample Labelling, Transport, Storage and Analyses
- 5.3 Preparing Sample for Testing
- 5.4 Special Considerations for the Collection, Handling and Preparation of Soil from Canada’s Ecozones
- 5.5 Test Observations and Measurements
- 5.6 Test Endpoints and Calculations
Section 5
Specific Procedures for Testing Field-collected Soil or Similar Particulate Material
This section provides specific instructions for preparing and testing samples of field-collected (site) soil or similar particulate material, in addition to the procedures discussed in Section 4.
Detailed guidance for the collection, handling, transport, storage and preparation of field- collected soil for biological testing is given in Environment Canada’s Guidance Document on the Sampling and Preparation of Contaminated Soil for Use in Biological Testing (EC, 2012). General procedures are outlined therein for the preparation of collecting soil samples, including: developing study objectives; identifying the study area; collecting background data; conducting site surveys, soil surveys, and ecological land classifications; selecting sampling strategies and locations; determining the size and number of samples to collect; establishing proper quality assurance and quality control (QA/QC) procedures; considerations for environment, health and safety; and developing sampling plans. Guidance is also provided for soil collection, including: selecting sampling devices; collecting soil samples by horizon or by depth; handling soil samples on-site; selecting sample containers; and transporting samples. Procedures for personnel receiving, preparing (i.e., drying, wetting, sieving, grinding, homogenizing, reconstituting and characterizing), and storing soil samples for biological testing at the laboratory are also described in EC (2012). Additional procedures and considerations are included that are specific to the nature of the contaminants (i.e., soils contaminated with volatile or unstable contaminants), biological testing requirements and study objectives. Specific guidance is provided for sampling, handling, transporting, storing, and preparing soil from boreal forest, taiga, and tundra ecozones, as well as organic and wetland soils. Environment Canada’s soil collection guidance document (EC, 2012) should be consulted, and the guidance therein followed (in addition to the guidance provided here), when collecting samples of field-collected soil and preparing them for toxicity tests with springtails using the biological test method described herein.
5.1 Sample Collection
Environment Canada (2012) provides substantial guidance on field-sampling design and appropriate techniques for sample collection. The guidance provided therein assumes that some data on the characterization of the chemical and soil properties of the land under investigation are already available. Field surveys of soil toxicity using biological tests with springtails and/or other suitable, soil-associated test organisms (e.g., EC, 2004a, 2005a, 2013a) are frequently part of more comprehensive land assessments and remediation (Stephenson et al., 2008; EC, 2012). Such assessments often include a battery of toxicity tests to evaluate the toxicity of soil using more than one test type and test species in conjunction with tests for bioaccumulation of contaminants, chemical analyses, biological surveys of epifaunal and/or infaunal organisms, and perhaps the compilation of geological and hydrographic data. This integrated approach can provide more accurate information of the riskassociated with soil contamination in ecological risk assessments and contaminated land management (EC, 2012). Statistical correlation in these assessments can be improved and costs reduced if the samples are taken concurrently for these tests, analyses and data acquisitions. Samples of soil to be used in the biological test method described herein (Section 4) might be collected quarterly, semi-annually or annually from a number of contaminated or potentially contaminated sites for monitoring and compliance purposes. Samples of soil might also be collected on one or more occasions during field surveys of sites for spatial (i.e., horizontal or vertical) or temporal definition of soil quality. Increasingly, biological (toxicity) testing is being used in all levels (i.e., Tiers) of risk assessment. Depending on the specific objectives of the assessment and the conditions at a contaminated site, site-specific toxicity data can be used in a number of ways including:
- to screen soil at a site to locate highly toxic or sublethally toxic areas;
- to identify site soil (determine concentration of contaminant in a site soil) that has a toxic impact;
- to evaluate contaminated soil for lethal or sublethal toxic effects;
- to identify soil characteristics that modify bioavailability;
- to derive (in part) site-specific standards and/or remedial objectives;
- to identify the efficacy of bioremediation technologies and/or site remediation; and
- for long-term monitoring of a remediated site (EC, 2012).
Further guidance on the application of biological testing in contaminated soil assessment is provided in EC (2012).
Environment Canada (2012) provides extensive guidance on defining study objectives and developing a study plan that incorporates biological testing into contaminated land assessments and management. A study plan provides specific guidance for the methods and strategies for sample collection and the procedures required to ensure that all data quality objectives (DQOs) are met. Information incorporated into a study plan includes: identification of DQOs; definition of the study area; background data collection; selection and location of sampling; selection of sampling strategies; QA/QC; and considerations for environment, health and safety. The sampling strategy (i.e., the process by which the type, location and collection method of samples is determined) is driven primarily by the study objectives and secondarily by the site characteristics, and is discussed in detail in EC (2012).
The number of locations to be sampled at a study site and the number of replicate samples per location will be specific to each study. The number of samples to collect depends upon the study objectives, the data quality objectives, the desired level of certainty and site-specific considerations. The number of sample replicates required further depends on the experimental design of biological tests, and in most cases, logistical and budgetary constraints (e.g., time and cost). Various types of samples (i.e., point, composite and bulk) may be collected depending on the study objectives.
The majority of samples collected for biological testing are unconsolidated samples in which particles become loosened and separated in the sampling process. Consolidated samples are those collected such that the soil particles and pore structure remain unaltered (i.e., cores). Guidance on the collection of consolidated samples for biological testing is provided in EC (2012) and briefly discussed in Section 4.1 herein; however, this biological test method document and the guidance provided herein applies primarily to the use of unconsolidated soil samples.
Specific procedures for the collection, handling, and preparation of soils contaminated with volatile or unstable compounds are described in EC (2012), and include modifications to procedures for sample collection, transport, storage, preparation and contaminant analyses.
All of the procedures described therein should be applied in order to minimize the loss of contaminants when sampling and handling the soils in the field, transporting soils to the toxicity laboratory, and any further loss of these contaminants in the laboratory prior to testing (i.e., during sample storage, handling or preparation). Environment Canada’s soil sampling guidance document (2012) also addresses issues related to QA/QC.
For certain monitoring and regulatory purposes, multiple replicate samples of soil (i.e., five field replicates or separate samples from different point or bulksamples taken at the same location) should be taken at each sampling location, including one or more reference location(s). These replicate samplesFootnote87 provide information about the variability of the toxicity/bioavailability of the contaminants at the location and allow for statistical comparisons of soil toxicity among more than one location (EC, 2005b). Each of these “true replicate” samples of soil can be tested for its toxicity to springtails as a single replicate (i.e., using only one test vessel per replicate sample) or as multiple replicates (i.e., using more than one test vessel per replicate sample; see Section 5.6.1). The use of power analysis (see Section 5.6.2) with endpoint data obtained in previous tests of the same type, performed with previous samples from the same or similar sites, will assist in determining the number of field and/or laboratory replicates that need to be tested. Also, some of the statistical tests have requirements for a minimum number of replicates. For certain other purposes (e.g., preliminary study or extensive surveys of the spatial distribution of toxicity), the survey design might include only one replicate sample (i.e., field replicate) from each location, in which case the sample (including reference and/or control soils) must be homogenized and split between five replicate test vessels (i.e., laboratory replicates).Footnote88 The latter approach precludes any determination of mean toxicity at a given sampling location, and completely prevents any conclusion on whether a sampling location is different from the control or reference, or from another location. It does, however, allow a statistical comparison of the toxicity of that particular sample with the reference or control, or with one or more samples from other locations, using appropriate statistical tests (see Section 5.6.1). It is important to realize that any conclusion(s) about differences, which arise from testing single field samples lacking field replication, must not be extended to make any conclusion(s) about the sampling locations.
Regardless of the study objectives, one or more sites should be sampled for reference (presumably clean) soilduring each field collection (see Section 3.5).Footnote89 Sites for collecting reference soil should be sought where the geochemical properties of the soil are similar to soil characteristics encountered at the test sites. Some of the most critical soil physicochemical properties that should be matched between the reference and contaminated soils include: particle size distribution, total organic carbon content (%), organic matter content (%), pH and conductivity. In addition, other properties to match might include CEC, total inorganic carbon, redox potential, and water-holding capacity (EC, 2012). Matching of total organic carbon content (%) or organic matter content (%) might not be warranted in cases where pollution(e.g., from or within sewage or industrial sludge) is responsible for the high organic carbon content of test soils. Preliminary surveys to assess the toxicity and geochemical properties of soil within the region(s) of concern and at neighbouring sites are useful for selecting appropriate sites at which to collect reference soil. Further guidance on obtaining reference soils for biological testing and procedures to be followed when a site- specific reference soil cannot be located is provided in EC (2012).
Samples of municipal or industrial sludge (e.g., sewage sludge, dewatered mine tailings, or biosolids from an industrial clarifier or settling pond) might be collected for the assessment of their toxic effect(s) on springtails, and for geochemical and contaminant analyses. Other particulate wastes being considered for disposal to land might also be collected for toxicity and physicochemical evaluation. Environment Canada (2012) provides guidance on additional considerations unique to waste pile sampling.
A sampling plan is an important component of the study plan. The sampling plan is a written description of the detailed procedures to follow when collecting samples, handling and preparing samples on-site (if required), packaging, labelling, storing (if necessary), and transporting samples. Prior to extracting soil samples, it is important to obtain a thorough field description of the soil to be sampled. In addition, soils should be described at a detailed site-specific level. In Canada, soils are classified using the Canadian System of Soil Classification (CSSC). Soils collected for biological testing should be classified to the subgroup level according to the CSSC, following the guidance provided in EC (2012). Appendix E in EC (2012) provides detailed information on the CSSC and the basic components of soil taxonomic identification.
Procedures used for sample collection (i.e., point, bulk or composite) will depend on the study objectives and the nature of the soil or other particulate material being collected. A shovel, auger or soil corer (preferably stainless steel) is frequently used for collecting soil samples. Shovels, scoops or trowels are among the most commonly used tools in soil sampling when large volumes of soil are needed; however, care must be exercised to ensure that a representative and unbiased sample is collected (e.g., a constant depth or soil horizon must be removed). More precise sampling devices include soil corers, ring samplers, cutting frames or soil cylinders, but they are less convenient for extracting large soil sample volumes. If soil samples are collected at a depth, an auger can be a more efficient and less labour-intensive tool for soil collection. Descriptions of the more commonly used soil collection devices and the procedures that should be followed for collecting soils are provided in EC (2012).
Most Canadian forest or non-agronomic, ecozone soils are highly stratified into soil horizons. The structure and chemistry of soil horizons are often very different, and this can result in different bioavailability and toxicity of contaminants to soil organisms. The top layer (A horizon) is the most commonly sampled horizon for biological testing. This horizon contains the most organic matter and most of the biological activity in mineral soils. Depending on the study objectives, the forest litter (L layer), fulvic/humic (FH horizon) (e.g., at a forested site) or surficial organic layer (O horizon) of mineral soils (e.g., at a tundra site) might also be collected when present. Subsurface B horizons and less commonly C horizons might also be sampled. Soils from the boreal or taiga ecozones sampled for the assessment of effect(s) on springtails, described in this test method document, must be collected as separate soil horizons, where possible. Collection of soil samples according to depth is recommended for soils without distinct soil horizons (e.g., where the surface soil horizons have been mixed or disturbed due to human activity). To sample soil by horizon, the soil profile must first be classified, as described earlier and in EC (2012). Care should be taken when sampling soil horizons that dilution of the soil contamination does not occur. This is particularly important in cases where the vertical contamination extends only partially through a soil horizon. In this situation, the horizon can be sampled only to a certain depth, or collected as two different samples at two sampling depths (EC, 2012).
Guidance on the collection of soil samples for toxicity testing is provided in detail in EC (2012). The first step is to establish the boundaries of the sample location. The surface of the location where each sample is to be collected should then be cleared of debris such as twigs, leaves, stones, thatch and litter (unless the L layer is being collected as part of the study design). If the location is an area of grass or other herbaceous plant material, the plants should be cut to ground level and removed before the sample is collected. Removal of the vegetation should be done such that removal of soil particles with the roots is minimal. Dense root masses (e.g., grasses) should be removed and then shaken vigorously to remove soil particles adhering to the roots. The soil sample to be collected for toxicity evaluation and chemistry should be collected from one or more depths that represent the layer(s) of concern (e.g., a surficial layer of soil, or one or more deeper layers of soil or subsoil if there are concerns about historical deposition of contaminants). Soils exhibiting distinct horizons (e.g., undisturbed forest soils) must be sequentially collected in separate horizons as a soil pit is excavated (EC, 2012).
The minimum volume or mass of soil required for testing depends upon the study objectives, site conditions and the test to be conducted. For a given test, the amount of soil required can vary and depends on the experimental design of the toxicity test (e.g., single concentration test versus multi-concentration test), as well as the physical characteristics of the soil (e.g., bulk density, moisture content, amount of debris in the soil), the nature of the chemical analyses to be performed, and the distribution of the contaminants in the soil (e.g., vertical distribution). The required volume of soil per sample should be calculated before commencing a sampling program. This calculation should take into account the quantity of soil required to prepare laboratory replicates for soil toxicity tests, as well as that required for particle size characterization, total organic carboncontent (%), organic matter content (%), moisture content (%) and specific chemical analyses. Soil collection volume recommendations for specific biological tests are provided in EC (2012). To obtain the required sample volume, it is frequently necessary to combine subsample retrieved using the sampling device. Guidance provided in EC (2012) for compositing subsamples in the field should be followed. The same collection procedure should be used at all field sites sampled. For samples collected as distinct soil horizons, each horizon must be placed and stored in separate containers unless the soil profile has been disturbed through attempts to remediate the site.
The preparation of soil samples might begin in the field before the samples are shipped to a testing laboratory. This might include hand- sorting (to remove debris and/or organisms), air- drying, sieving and homogenization of soil samples. All of these procedures are described in detail in EC (2012).
5.2 Sample Labelling, Transport, Storage and Analyses
Containers for transport and storage of samples of field-collected soil or similar particulate material must be made of nontoxic, inert material. The choice of container for transporting and storing samples depends on the sample volume, the potential end uses of the sample, and the type and nature of the soil contamination. The containers must be clean and sealable and should be practical for handling and able to support the weight of the sample (EC, 2012). Thick (e.g., 4 mil) plastic bags are routinely used for sample transport and storage. If plastic bags are used, it is recommended that each be placed into a second clean, opaque sample container (e.g., a cooler or a plastic pail with a lid) to prevent tearing and to support the weight of the sample and to maintain darkened conditions during sample transport (ASTM, 2004). Plastic containers or liners should not be used if there is concern about the plastic affecting the characteristics of the soil (e.g., compounds from plastic leaching into the soil). For soils contaminated with volatile compounds, containers should be airtight and pressure resistant. Containers recommended for the transport and storage of soils are listed in Appendix H of EC (2012).
Following sample addition, the air space in each container used for sample transport and storage should be minimized (e.g., by collapsing and taping a filled or partially filled plastic bag). Immediately after filling, each sample container must be sealed, and labelled or coded. Labelling and accompanying records made at this time must include at least a code or description that identifies sample type (e.g., point, bulk, composite), sample date and time, sample site, precise location of sampling, sample condition, sample identification number (including replicate number, where applicable), and sample volume. The label information should also include the name and signature of sampler(s). Persons collecting samples of soil should also keep field records that describe details of:
- the nature, appearance and volume of each sample;
- the sampling procedure and apparatus;
- any procedure used to composite or subsample bulk or point samples in the field;
- any sample preparation (e.g., sieving, drying) carried out in the field;
- the number of replicate samples taken at each sampling location;
- the sampling schedule;
- the types and numbers of containers used for transporting samples;
- any field measurements (e.g., temperature, pH, soil moisture content, bulk density) of the soil at the collection site;
- soil horizon characterization;
- any in-situ field testing (e.g., litterbug, earthworm exposure, bait lamina) performed;
- procedures and conditions for cooling and transporting the samples;
- observations of environmental conditions at the time of sampling (e.g., raining);
- observations and any field sampling of soil fauna and flora at the collection site;
- sample storage duration and conditions prior to arrival at the laboratory; and
- information on sample transportation.
Additional recommendations for site observations and field measurements are provided in Table 10 of EC (2012).
Soil samples should be kept cool during transport and storage and should not freeze or become overheated. As necessary, gel packs, regular ice or other means of refrigeration should be used to assure that the temperature of the sample(s) remains cool (e.g., 7 ± 3°C) during transit. It is recommended that samples be kept in darkness (i.e., held in light-tight, opaque transfer containers such as coolers or plastic pails with lids) during transport, especially if they might contain PAHs or other chemicals or chemical products that could be photoactivated or otherwise altered due to exposure to sunlight. All samples must be shipped with appropriate documentation, including chain-of-custody forms as well as any specific regulatory documentation for transport of contaminated material (see EC [2012] for further guidance on sample transport).
The date the sample(s) is (are) received at the laboratory must be recorded. Sample temperature and moisture content upon receipt at the laboratory must also be measured and recorded. In addition, each sample of field-collected test soil or each separately collected soil horizon should be inspected and the following qualitative descriptions made and recorded: colour, texture, informal description of moisture content, presence of standing water, presence of indigenous invertebrates, fungi or plant material, and any strong odours (EC, 2012). Samples to be stored for future use must be held in airtight containers. If volatile contaminants are in the soil or of particular concern, any air “headspace” in the storage container should be purged with nitrogen gas before being capped tightly. Samples should not freeze or partially freeze during transport or storage (unless they are frozen when collected), and must not be allowed to dehydrate. If, however, one or more samples are saturated with excess water upon arrival at the laboratory (e.g., sampling occurred during a significant rainfall event), the sample(s) may be transferred to plastic sheeting for a brief period (e.g., one or more hours) to enable the excess water to run off or evaporate. Thereafter, the sample(s) should be returned to the transport container(s) or transferred to one or more airtight containers for storage.
It is recommended that samples be stored in darkness at 4 ± 2°C. These storage conditions must be applied in instances where PAHs or other light-sensitive contaminants are present, or if the samples are known to contain unstable volatiles of concern. It is recommended that samples of soil or similar particulate material be tested as soon as possible after collection. The effects of storage time and temperature on soil properties and toxicity depend on the contaminants and soil characteristics. The soil toxicity test(s) should begin within two weeks of sampling, and preferably within one week. The test must begin within six weeks, unless it is known that the soil contaminants are aged and/or weathered, and therefore considered stable. Further considerations for the storage of contaminated soil are provided in EC (2012), and the guidance therein should be followed.
In the laboratory, each sample of field-collected soil or distinct soil horizon should be thoroughly mixed (Section 5.3), and representative subsamples collected for physicochemical characterization. Each sample or soil horizon to be tested (including all associated samples of negative control soil and reference soil) must be characterized by analyzing subsample for at least the following:
- particle size distribution (% sand, % silt and % clay),
- total organic carbon content (%),Footnote90
- organic matter content (%),Footnote90
- pH,
- conductivity,
- moisture content (%),
- water-holding capacity (WHC), and
- cation exchange capacity (CEC).
Additionally, the following analyses should be performed:
- major cations and anions (Na+, K+, Mg2+, Ca2+, Al3+, S2-, S2-, Cl-).
- nitrogen as total N, nitrate (NO3-), nitrite (NO2-) and ammonium (NH4+),
- phosphorus as total and/or bioavailable,
- potassium as total and/or bioavailable, and
- C:N ratio.
Other analyses could include:
- bulk density,
- total inorganic carbon,
- total volatile solids,
- biochemical oxygen demand,
- chemical oxygen demand,
- redox potential,
- soluble salts,
- sodium adsorption ratio,
- contaminants of concern, and
- characteristics of the contamination (e.g., odour, staining, debris, presence of fuel or solvent).
In order to confirm that the reference soils are not contaminated, the following screening analyses are recommended:
- organophosphorous insecticide suite,
- organochlorine insecticide suite,
- herbicides suite,
- metals suite,
- petroleum hydrocarbons (including PAHs), and
- other site- or area-specific contaminants of concern.
Unless indicated otherwise, identical chemical, physical and toxicological analyses should be performed with subsample representative of each replicate sample of field-collected soil or soil horizon (including reference soil) taken for a particular survey of soil quality, together with one or more subsamples of negative control soil.
5.3 Preparing Sample for Testing
Field-collected soil or similar particulate waste material must not be sieved with water, as this would remove contaminants present in the interstitial water or loosely sorbed to particulate material. Large gravel or stones, debris, indigenous macroinvertebrates, or plant material should normally be removed using forceps or a gloved hand. If a sample contains a large quantity of undesirable coarse debris (e.g., plant material, wood chips, glass, plastic, large gravel) or large macroinvertebrates, these may be removed by pressing the soil through a coarse sieve (e.g., mesh size of 4 to 10 mm; EC, 2012). Dry sieving might also be desirable to ensure that the sample structure (i.e., aggregation, organic matter or clay distribution) is amenable for testing. Soils should not be sieved in the laboratory if they were sieved in the field, or if they have the crumbly texture that is optimal for testing (i.e., 3 to 5 mm clumps). Soil samples comprised of moist clayey subsurface soils are very cohesive and often cannot be directly sieved or homogenized. These soils should first be broken up manually and then dried prior to sieving and homogenization, as described in EC (2012). In general, grinding of soil samples should be avoided when possible, but may be necessary with some soils (i.e., clayey soils) or if greater homogeneity of a sample is desired than Field-collected soil or similar particulate waste material must not be sieved with water, as this would remove contaminants present in the interstitial water or loosely sorbed to particulate material. Large gravel or stones, debris, indigenous macroinvertebrates, or plant material should normally be removed using forceps or a gloved hand. If a sample contains a large quantity of undesirable coarse debris (e.g., plant material, wood chips, glass, plastic, large gravel) or large macroinvertebrates, these may be removed by pressing the soil through a coarse sieve (e.g., mesh size of 4 to 10 mm; EC, 2012). Dry sieving might also be desirable to ensure that the sample structure (i.e., aggregation, organic matter or clay distribution) is amenable for testing. Soils should not be sieved in the laboratory if they were sieved in the field, or if they have the crumbly texture that is optimal for testing (i.e., 3 to 5 mm clumps). Soil samples comprised of moist clayey subsurface soils are very cohesive and often cannot be directly sieved or homogenized. These soils should first be broken up manually and then dried prior to sieving and homogenization, as described in EC (2012). In general, grinding of soil samples should be avoided when possible, but may be necessary with some soils (i.e., clayey soils) or if greater homogeneity of a sample is desired than can be achieved by sieving. As with soil sampling and storage procedures, any soil preparation procedures should be documented and must be reported.
Reconstitution of soil sample constituents might be required prior to testing if the soil contained standing water that was decanted during preparation, or if portions of the sample were removed during preparation (e.g., thatch, plant root or other organic material) but need testing along with the soil (EC, 2012). Soil horizons are collected as separate components of a soil sample and must be tested independently as separate soil samples. If the contaminants of concern have only been confirmed in one soil horizon (e.g., upper organic horizon) based on previous analyses and/or toxicity testing, then, depending on the study objectives, a decision must be made whether to conduct toxicity testing on this horizon alone or in the additional soil horizons collected from the sampling location.
Unless research or special study objectives dictate otherwise, each sample or horizon of field-collected unconsolidated test material should be homogenized in the laboratory before use (USEPA, 1989).Footnote91 Any moisture that separates from a sample during its transport and/or storage must be remixed into it, if possible. Mixing can affect the concentration and bioavailability of contaminants in the soil, and sample homogenization might not be desirable for all purposes. To prepare a homogeneous sample, transfer the pre-calculated amounts of test and/or reference soil to a clean, rigid mixing container (e.g., a large stainless steel or plastic bowl) or, for larger volumes of soil, to clean plastic sheets spread out on the floor. The sample should be mixed manually (using a gloved hand or a nontoxic device such as a stainless steel spoon) or mechanically (e.g., using a domestic hand-held mixer with beaters at low speed or a hand-held wire egg beater) until its texture and colour are homogeneous. A number of methods used to homogenize soil samples (e.g., folding, mixing, coning) are described in detail in EC (2012). While mixing, care should be taken to ensure that the impact of mixing on soil structure is minimal and that the structure is not destroyed entirely. As soon as the texture and colour of the sample appears to be homogeneous, mixing should be discontinued.
For each sample or soil horizon included in a test, mixing conditions including duration and temperature must be as similar as possible. If there is concern about the effectiveness of sample mixing, subsamples of the soil should be taken after mixing and analyzed separately to determine the homogeneity of particle sizes, chemical(s) of interest, etc.
As indicated in Section 3.6, one or more samples or horizons of field-collected test soil might either be tested at a single concentration only (typically, 100%), or evaluated for toxicity in a multi-concentration test whereby a series of concentrations are prepared by mixing measured quantities with either negative control soil or reference soil. When performing a multi- concentration test, the following series of concentrations of test soil (mixed in negative control soil or reference soil), which spans the range of 100% to 1% test soil using eight concentrations, might prove suitable: 100%, 80%, 50%, 30%, 15%, 7.5%, 3% and 1%. Guidance on other concentration series that might prove as or more suitable is found in Section 6.2, along with that for preparing test mixtures that might apply equally when performing a multi-concentration test with one or more samples of field-collected soil. Refer to Section 4.1 for additional guidance when selecting test concentrations. In each instance, the test must include a treatment comprised solely of negative control soil (see Section 3.3).
As indicated in Section 4.1 for soils collected as distinct horizons, each horizon must be tested separately in independent definitive tests. For a multi-concentration test, the test soil horizon is mixed with the same horizon of negative control or reference soil at the various test concentration (0%, 6.25%, 12.5%, 25%, etc.). In some cases, it may not be possible to collect the same horizons of negative control soil and test soil. For example, preliminary remedial action may have already been taken at the test site, resulting in disturbed or mixed natural soil horizons. In these scenarios, the test soil can be tested as a mixed soil where test concentrations are prepared by mixing suitable weights of test soil into the available horizon(s) of negative control soils at the appropriate test concentrations. The study objectives must take into account the soil profile of the reference soil and the location and/or mobility of the contaminants in the test soil. The goal is to match equivalent horizons in reference and contaminated soil, if possible.
Soil structure is an important factor that influences the survival and reproduction of springtails, and moisture content plays an important role in the determination of soil structure. A qualitative procedure, informally known as a “squeeze test,” is useful when determining if the optimal moisture content of a sample of test soil has been achieved. Investigators might find it useful to apply this procedure when adjusting the moisture content of each sample of test soil to a particular percentage of the sample’s water-holding capacity (see following paragraphs), in preparation for a toxicity test. To perform this test, a small, representative subsample of the test soil (e.g., a “pinch” of soil) is randomly taken using a gloved hand, and gently compressed between the thumb and forefinger. If a small quantity of water can be squeezed from the soil with gentle pressure, then the soil’s moisture content is acceptable. If, however, no water appears, the soil is likely too dry. Conversely, if a substantial amount of water can be squeezed from the subsample of soil, it is likely too wet. The squeeze test can also be applied as a test proceeds,Footnote92 or test vessels can be weighed to determine water loss (see Section 4.6).
The moisture content of a given sample of field- collected test soil should be standardized during its preparation by determining its water-holding capacity (WHC) and then hydrating the soil to an optimal moisture content based on a percentage of this value. The optimal percentage of the WHC for each sample of field-collected soil must be determined prior to sample preparation and test initiation. In order to do so, the moisture content of each homogenized sample (i.e., each sample of test soil, including the negative control soil) must be determined (Sections 4.1 and 4.6). Thereafter, the WHC of each sample must be determined using a recognized standard procedure (see following three paragraphs). A subsample of each soil sample is then hydrated to a homogeneous, crumbly consistency with clumps approximately 3 to 5 mm in diameter.Footnote93
The moisture content, WHC and optimal percentage of the WHC of each soil horizon must be determined separately. Soil horizons with higher organic matter content can be expected to have higher WHC than mineral horizons, so will require greater amounts of water to hydrate to a moist, crumbly texture. Based on the initial moisture content of the sample, the WHC of the sample, and the amount of water added to achieve the desired soil consistency, the sample’s optimal moisture content can be calculated and expressed as a percentage of the WHC for each soil.Footnote94 Once this target (or optimal) percentage of the WHC has been determined, the moisture content of each sample of test soil (including the negative control soil) can be standardized to the selected (sample-specific) moisture content. Test water (i.e., de-ionized or distilled waterFootnote95) should be added to each sample with a moisture content that is less than the pre-determined optimal percentage of its WHC, until this moisture content is achievedFootnote96 (AquaTerra Environmental Ltd., 1998). If a sample is too wet, it should be spread as a thin layer on a clean sheet of plastic (e.g., a new plastic garbage bag or vapour-barrier plastic) or a clean, non-reactive (e.g., stainless steel or plastic) tray, and allowed to air-dry by evaporation at ambient (~20°C) room temperature;Footnote97 rehydration to the pre-determined optimal percentage of its WHC might be necessary. Upon completion of adjustment of a sample’s moisture content to the desired percentage of its WHC, the moisture content (%) of the hydrated soil must be determined and the percent WHC and percent moisture content recorded and reported.
The WHC (and the percent WHC that is optimal for biological testing) of a particular soil is generally unique to each soil type and/or horizon, and is ultimately the result of the interaction of many variables associated with soil structure (e.g., micro/macro-aggregation, pore space, bulk density, texture organic matter content). There are a number of methods that can be used to determine WHC; however, most of these methods require measurements to be made on an intact soil sample (e.g., soil core) where characteristics (structural aggregations, pore space, bulk density, texture, and organic matter content) are preserved during collection. The USEPA (1989) has described an appropriate method for toxicity testing using unconsolidated materials (such as samples of field-collected soils that have been dried, sieved, and homogenized, or samples of soil formulated in the laboratory from constituents).Footnote98 This method is outlined here.
For this method, ~130 g (wet wt)Footnote99 of sample is placed in an aluminum pan or petri dish (15 x 1 cm), and dried at 105°C until a constant weight is achieved (this usually takes a minimum of 24 h). The soil is then cooled for a minimum of 20 min. in a dessicator. Thereafter, 100 g of the oven-dried soil is placed into a 250-mL glass beaker with 100 mL of distilled or de-ionized water. The resulting slurry is mixed thoroughly with a glass stir rod. A folded filter paper (e.g., 185-mm diameter Fisherbrand™ P8 coarse porosity, qualitative creped filter paper; catalogue number 09-790-12G) is placed into a glass funnel (with a top inside diameter of 100 mm and a stem length of 95 mm). The folded filter paper should be level with the top of the glass funnel. Using a pipette, up to 9 mL of distilled or de-ionized water is slowly added to the filter paper to wet the entire surface. The funnel and hydrated filter paper are then weighed. To obtain the initial weight for the mass of the funnel plus hydrated filter paper plus dried soil (see “I” in Equation 1), the weight of the dried soil (100 g) is added to the weight of the funnel and the wet filter paper.
The funnel is then placed into a 500-mL Erlenmeyer flask, and the soil slurry is slowly poured onto the hydrated filter paper held in the funnel. Any soil remaining on the beaker and stir rod is rinsed into the funnel with the least amount of water necessary to ensure that all of the solid material has been washed onto the filter. The funnel is then tightly covered with aluminum foil and allowed to drain for three hours at room temperature. After three hours, the funnel containing the hydrated filter paper and wet soil is weighed. This weighing represents the final weight for the mass of the funnel plus hydrated filter paper plus (wet) soil (see “F” in Equation 1).
The water-holding capacity for the subsample of soil in the funnel, expressed as percentage of soil dry mass, is then calculated using the following equation:
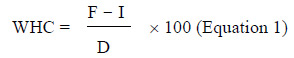
Equation 1
Equation 1 - The water-holding capacity for a subsample of soil (expressed as a percentage of the dry soils mass) is determined by subtracting the mass of the: funnel, hydrated filter paper, and the dried soil sample from the mass of the: funnel, hydrated filter paper, and wet soil sample. The result is then divided by the dry mass of the soil and multiplied by 100.
where:
WHC = water-holding capacity (%)
F = mass of funnel + hydrated filter paper + wet mass of soil
I = mass of funnel + hydrated filter paper + dry mass of soil
D = 100 g (i.e., dry mass of soil)
The WHC of each sample of test soil should be determined in triplicate, using three subsamples. The percentage of water (i.e., Pw) that is added to a sample of field-collected soil to achieve the desired hydration (i.e., the optimal percentage of the WHC) can be calculated as follows:Footnote100
PW = [WHC x (PWHC/100)] - MC (Equation 2)
where:
PW = percentage of water to add to the soil (%)
WHC = water-holding capacity (%)
MCi = initial moisture content of the soil
The volume of water (i.e., Vw) that should be added to a sample of field-collected soil to achieve the desired hydration (i.e., the optimal percentage of the sample’s water-holding capacity) can be calculated as follows (see footnote Footnote100):
VW = (PW x M)/100 (Equation 3)
where:
VW = volume of water to add to the soil (mL)
PW = percentage of water to add to the soil (%)
M = total mass of soil required for test (expressed as dry weight)Footnote101
Environment Canada (2012) describes various procedures that might be used for manipulating soil samples to render them testable to meet study objectives or DQOs when the conditions do not occur within the sample as collected. Detailed procedures for soil manipulations are described, and include: washing, aging/ weathering, adjusting soil pH, conditioning, adjusting soil fertility and reducing indigenous soil microorganisms (EC, 2012). In general, samples of field collected soil must not be adjusted or manipulated, except for research- oriented toxicity tests intended to determine the influence of a particular soil manipulation on sample toxicity. Soil horizons with high organic levels (e.g., LFH horizons), however, might require a double freeze/thaw cycle in order to remove indigenous invertebrates before testing (EC, 2012).Footnote102 Studies intending to investigate the effect of a soil manipulation (e.g., pH adjustment) on sample toxicity should involve two side-by-side tests, whereby one or more sets of treatments are adjusted, and one or more duplicate sets of treatments are not. Detailed, proper documentation of any soil manipulation procedures carried out must be made and reported.
Immediately following sample hydration (or dehydration) and mixing, subsamples of test material required for the toxicity test and for physicochemical analyses must be removed and placed into labelled test vessels (see Section 4.1), and into the labelled containers required for the storage of subsample for subsequent physicochemical analyses. Any remaining portions of the homogenized sample that might be required for additional toxicity tests using springtails or other test organisms (e.g., according to EC 2004a, 2005a, 2013a) should also be transferred to labelled containers at this time. Subsamples to be stored for future toxicity testing should be held in sealed containers with minimal air space, in darkness at 4 ± 2°C (Section 5.2) until tested. These storage conditions must be applied for subsamples collected for physicochemical analysis. Just before it is analyzed or used in the toxicity test, each subsample must be brought to room temperature and thoroughly remixed to ensure that it is homogeneous.
5.4 Special Considerations for the Collection, Handling and Preparation of Soil from Canada’s Ecozones
Specific guidance for sampling, handling, transporting, storing, and preparing soil from various Canadian ecozones is provided in EC (2012).
Previously published Environment Canada soil toxicity test methods (EC 2004a, 2005a) and the first edition of this test method document (EC, 2007c) were developed for the assessment of soils with neutral to near-neutral soil pH and organic matter content ranging from approximately 3% to 12%. These soils are generally characteristic of the Ah horizons of agricultural soils in Canada and soils from deciduous mixed forest eco-regions in the south- eastern part of the country (i.e., prairies and mixed-wood plains ecozones). There are many other soil types in Canada with widespread distributions that have properties falling outside the ranges considered typical by EC’s previously published standard methods, and therefore require special procedures for sampling, handling, transport, storage and preparation. These soils include: boreal forest soils, taiga soils, stony/shallow soils, organic soils, cryosolic soils and wetland soils, and are relevant for use with the revised test methodologies described in this second edition test method document. Given that these soils cover most of Canada’s land mass and that anthropogenic activities in these regions (e.g., mining, forestry, oil and gas production) have created or have the potential to create contaminated lands, specific guidance for sampling, handling, transporting, storing and preparing soils from these various ecozones is provided in EC (2012). Guidance is also provided on the variability of the soils within each of the described ecosystems and special considerations for selecting the appropriate test species when testing soils from these various ecosystems (EC, 2012).
5.5 Test Observations and Measurements
A qualitative description of each field-collected test material should be made at the time that the test is being set up. This might include observations of sample colour, texture, and homogeneity, and the presence of plants or macroinvertebrates. Any changes in the appearance of the test material observed during the test or upon its termination should be noted and reported.
Section 4.6 provides guidance and requirements for the observations and measurements to be made during or at the end of each test. These observations and measurements apply and must be made when performing the soil toxicity tests described herein using one or more samples of field-collected (site) soil.
Depending on the test objectives and experimental design, additional test vessels might be set up at the beginning of the test (Section 4.1) to monitor soil chemistry. These would be destructively sampled during and at the end of the test. Test organisms might or might not be added to these extra test vessels, depending on the study’s objectives. Measurements of chemical concentrations in the soil within these vessels may be made by removing aliquots of the soil for the appropriate analyses (see Section 5.2).
5.6 Test Endpoints and Calculations
The common theme for interpreting the results of tests with one or more samples of field- collected test soil, is a comparison of the biological effects for the test (site) soil(s) with the effects found in a reference soil. The reference sample should be used for comparative purposes whenever possible or appropriate, because this provides a site-specific evaluation of toxicity (EC, 1997a, b, 2001, 2004a, 2005a). Sometimes the reference soil might be unsuitable for comparison because of toxicity or atypical physicochemical characteristics. In such cases, it would be necessary to compare the test soils with the negative control soil. Results for the negative control soil will assist in distinguishing contaminant effects from non-contaminant effects caused by soil physicochemical properties such as particle size, total organic carboncontent (%) and organic matter content (%). Regardless of whether the reference soil or negative control soil is used for the statistical comparisons, the results from negative control soil must be used to judge the validity and acceptability of the test (see Section 4.4).
The biological endpoints for this method are survival (a quantal measurement) and reproductive success (a quantative measurement) at the end of the test. Because of the different nature of the measurements involved, different statistical approaches are needed, and these approaches are further refined to reflect the objectives and design of the experiment. This section provides statistical guidance for data from single-concentration tests (i.e., soil samples from multiple sampling locations tested at full strength only). The simplest testing scenario involves the comparison of one test sampling location with one reference sampling location, whereas more complex designs might include a comparison of several sampling locations with a reference sampling location, or with each other. Only summary guidance is provided here for analysing the mortality and reproduction endpoints as more extensive statistical guidance is available elsewhere (EC, 2005b). Standard statistical procedures are generally all that is needed for analyzing the results. Section 3 in EC (2005b) should be consulted for guidance when comparing the findings for single- concentration tests from multiple locations, using parametric or non-parametric tests. As always, the advice of a statistician familiar with toxicology should be sought for the design and analysis of tests.
Guidance in Section 6 (including that in Section 6.2 for performing range-finding tests, and that in Sections 6.4 and 4.8 for calculating test endpoints) should be followed if a multi- concentration test is performed using one or more samples of field-collected soil diluted with negative control soil or clean reference soil. Section 9 in EC (2005b) should be consulted when comparing such point estimates of toxicity for multiple samples of field-collected soil.
5.6.1 Variations in Design and Analysis
Environment Canada (EC, 2005b) provides guidance on the statistical analysis of quantal data in various test designs that examine multiple sampling locations. Choice of a specific statistical test depends on:
- the type of comparison that is sought (e.g., complete series of pairwise comparisons between all sampling locations, or compare the response from each sampling location only with that of the reference site);
- if a chemical and/or biological response gradient is expected;Footnote103 and
- the level and type (laboratory or field) of replication.
Environment Canada has also provided detailed statistical guidance on the analysis of quantitative measurements (EC 2005b),Footnote104 which can be readily applied to measurements of Collembola reproduction (i.e., number of surviving progeny at the end of the test) in a multiple sampling location scenario. If test results at a single test sampling location are to be compared with test results at a reference sampling location, a t-testFootnote105 is normally the appropriate statistical test (Section 3.2 in EC 2005b). In situations where more than one test sampling location (treatment) is under study, and the investigator wishes to compare multiple sampling locations with the reference, or compare sampling locations with each other, a variety of ANOVA and multiple comparison tests (and non-parametric equivalents) exist (Section 3.3 in EC, 2005b). Choice of a specific test depends on the three conditions described above for quantal tests, in addition to assumptions of normality and homoscedasticity being met.
A very preliminary survey might have only one sample of test soil (i.e., contaminated or potentially contaminated site soil) and one sample of reference soil, without replication. Simple inspection of the results might provide guidance for designing more extensive studies. A preliminary evaluation might conceivably be conducted with samples from many locations, but without either field replicates or laboratory (within-sample) replicates. The objective might be to identify a reduced number of sampling locations deserving of more detailed and further study. Opportunities for statistical analysis would be limited (EC, 2005b).
A more usual survey of soils would involve the collection of replicate samples from several places by the same procedures, and their comparison with replicate samples of a single reference soil and/or negative control soil. There are several pathways for analysis, depending on the type and quality of data. In these multi- location surveys, the type of replication would influence the interpretation of results (i.e., field replicates or laboratory replicates, or both). If both replicate samples (i.e., field replicates) and replicate vessels (i.e., laboratory replicates) have been tested, a statistician should be consulted for analysis options. If only laboratory replicates and no field replicates were tested, it is difficult to make statistically robust conclusions regarding differences between sampling sites (see also Section 5.1). The laboratory replicates would only show any differences in the samples that were greater than the baseline variability in the within-laboratory procedures for setting up and running the test. Sample variability due to location would not really be assessed in the statistical analysis, except that it would contribute to any difference in test results associated with sampling location.
If it were desired to compare the test results for the replicate samples from each sampling location with those for the reference soil, a number of tests are recommended, depending on whether the samples show a gradient and depending on whether there are an even or uneven number of replicates (see Section 3 in EC, 2005b).
In a multi-location survey, an investigator might wish to know which of the samples from various sampling locations showed results that differed statistically from the others, as well as knowing which ones were different from the reference and/or negative control sample(s). Such a situation might involve sampling from a number of locations at progressively greater distances from a point source of contamination, in which instance the investigator might want to know which sampling locations provided samples that had significantly higher toxicity than others, and thus which locations were particularly deserving of cleanup. Sections 3.1, 3.3 and 7.5 in EC, 2005b provide further details, alternate tests, and non-parametric options and the guidance therein should be followed.
5.6.2 Power Analysis
A critical feature of toxicity test design is the potential for declaring false positives (i.e., calling a clean site contaminated; Type I error) or false negatives (i.e., calling a contaminated site clean; Type II error). Scientists are usually cautious in choosing the level of significance (α) for tolerating false positive results (Type I error), and usually set it at p = 0.05 or 0.01. Commonly, scientists following a specified test design will never consider the relationship between power, variability and effect size, leaving the Type II error completely unspecified. There are several factors that influence statistical power, including:
- variability of replicate samples representing the same treatment;
- α (i.e., the probability of making a Type I error);
- effect size (ES), (i.e., the magnitude of the true effect for which you are testing); and
- n (i.e., the number of samples or replicates used in a test, and in some cases, the allocation of those replicatesFootnote106).
Environment Canada’s guidance document on statistical methods for environmental toxicity tests (EC, 2005b) provides further information and guidance on errors of Types I and II.
Power analysis can be used a priori to determine the magnitude of the Type II error and the probability of false negative results. It can also be used to ascertain the appropriate number of field and laboratory replicates for subsequent surveys involving this test, or to assist in the selection of future sampling sites. It is always prudent to include as many replicates in the test design as is economically and logistically warranted (see Section 5.1); power analysis will assist in this determination. Guidance on power analysis is provided in EC (2005b).
In research-based science, power analysis is most useful as part of a preliminary test design (Hoenig and Heisey, 2001; Lenth, 2007; Newman, 2008). Here, a preliminary experiment is run to determine the approximate standard deviation (variability), and to troubleshoot the execution of the experiment in general. Other factors in power analysis, such as effect size and number of replicates, can then be considered along with the standard deviation so that the final test design is optimized (e.g., number of replicates needed to detect a certain effect size is determined).
In the development of standardized test methods, the purpose of employing power analysis remains the optimization of test design (or at least estimating the power of the current test design).Footnote107 However, instead of a single estimate for variability and effect size, there would typically be a much richer data set to consider. For example, test method experts could collect a number of estimates of variability, across different laboratories and different contaminant scenarios (Thursby et al., 1997; Van der Hoeven, 1998; Denton et al., 2011). Standardized tests are often used in monitoring or regulatory programs, which may specify the expected effect size (e.g., 25%) to be detected (AE, 2007).
A limited power analysis was performed for two collembolan species (F. candida and P. minuta) used in this test method. Using only the reproduction endpoint, estimates of variability were collected from routine test data (F. candida, n = 47) or method validation data (P. minuta, n = 95). The performance of this test was evaluated by investigating the relationships between number of replicates, power, variability and effect size. Specifically, the detectable effect size was calculated to be 45-50%.Footnote108,Footnote109 Labs should establish expectations of power and effect size for each project or test. If smaller effect sizes are necessary or desirable, contact the Methods Development and Application Unit (methods@ec.gc.ca) or consult a statistician to discuss alternate test designs.