Draft guidance on sampling and mitigation measures for controlling corrosion: Tables, figures and protocols
On this page
Tables
Principal factors influencing the corrosion and leaching of lead, copper, iron and cement
|
Factors |
Key effects |
|
Age of the pipes |
Leaching of lead, copper, iron and cement usually decreases with the aging of distribution materials. However, heavily tuberculate iron pipes are often a source of red water problems and associated with increased levels of lead at the tap. |
Stagnation time |
Lead and iron concentrations at the tap rapidly increase with water stagnation in the plumbing system, but reach fairly constant levels after 8 h or more. Copper levels rapidly increase with initial water stagnation, but can then decrease or continue to increase, depending on the oxidant levels. Long residence time may also increase water quality deterioration from cement-based materials. |
| pH | Lead, copper and iron levels at the tap usually decrease with increasing pH. Higher pH favours iron corrosion and a higher degree of tuberculation. Low pH favours leaching from cement. In turn, cement leaching increases pH. |
Alkalinity |
Lead and copper levels at the tap usually increase with low alkalinity. Copper levels can also increase with very high alkalinity. Low alkalinity will favour iron leaching. Low alkalinity will favour leaching from cement. In turn, cement leaching will increase alkalinity. |
| Temperature | No simple relationship exists between lead, copper and iron levels at the tap and temperature. |
Calcium |
Lead, copper and iron levels at the tap are not significantly influenced by calcium. Low calcium concentration in the drinking water will favour leaching from cement. In turn, cement leaching will increase calcium concentration in the drinking water. |
| Free chlorine | The presence of chlorine may yield stable lead IV scales. Free chlorine may increase copper corrosion rates at low pH. Free chlorine may decrease copper corrosion rates at high pH. Data also indicates that free chlorine may increase lead and iron corrosion rates. |
| Chloramines | Chloramines may dissolve lead scales formed under chlorinated water conditions. The presence of chloramines may yield unstable lead scales. Little information on the effect of chloramines on copper or iron was found. |
Chloride and sulphate |
Chloride alone has not been shown to conclusively influence lead levels at the tap. Chloride may reduce the rate of copper corrosion up to relatively high concentrations. High concentrations of chloride may cause copper pitting. Lead and copper levels at the tap may not be significantly influenced by sulphate. Sulphate may cause copper pitting. A CSMR greater than 0.58 may lead to higher lead levels at the tap. High levels of sulphate may induce the formation of cracks in cement pipes. |
| Natural organic matter (NOM) | The effects of NOM on levels of lead, copper and iron at the tap are not conclusively determined. NOM may decrease copper pitting and iron corrosion. NOM may increase lead, copper and iron solubility. |
Conditions favouring lead leaching and indicators of lead leaching in drinking water distribution and plumbing systems
At the treatment plant
|
Condition |
Comment |
|
When pH is less than 7.0 or greater than 9.5 |
Although pH is controlled at the treatment plant, it may vary within the distribution system. Low-pH water has been strongly correlated with higher lead levels at the tap. A pH exceeding 9.5 can lead to an increase in lead solubility. |
| When alkalinity is less than 30 mg/L | Although alkalinity is controlled at the treatment plant, it may vary within the distribution system. Low-alkalinity water has been correlated with higher lead levels at the tap. In addition, low-alkalinity water offers poor buffering capacity and can jeopardize pH stability. |
| Treatment change | Any change in treatment that will have a chemical, biological or physical impact on the distributed water should be carefully monitored in the distribution system. Lead corrosion and lead levels are easily influenced by small changes in the quality of the water distributed. Lead levels at the tap and within the distribution system should be closely monitored during a treatment change, especially a coagulant or disinfectant change. |
| Change from chlorine to chloramines | Changing the residual disinfectant treatment will have an impact on the electrochemical potential and the pH of the water. This, in turn, may destabilize corrosion by-products within the distribution and plumbing systems. Lead levels at the tap and within the distribution system should be closely monitored during a treatment change, especially a coagulant or disinfectant change. |
Within the distribution system
|
Condition |
Comment |
|
Lead-based fittings or in-line devices |
Lead in goosenecks/pigtails, valve parts or gaskets used in water treatment plants or distribution mains can release lead. |
Old unlined cast iron pipes |
Old unlined cast iron pipes are heavily corroded. The presence of tubercles reduces the diameter of the pipe and offers niches for micro-organisms to proliferate. The high surface-to-pipe ratio, long residence time and greater microbiological activity may change the water’s pH, alkalinity and chemical balance. These pipes may also be followed by lead service lines. Iron adsorbs lead and other metals and may increase their levels at the tap. |
| Dead ends | Dead ends provide a stagnation period where the contact time between the water and the pipe material is increased. This longer contact time favours microbiological and chemical activity. |
| Microbiological activity | Biofilms are present in distribution and plumbing systems. The presence of micro-organisms will influence the biochemical balance of the water and subsequently influence corrosion. |
| Nitrification | Nitrification could play a role in depressing pH and increasing lead dissolution, especially when chloramine is used as a secondary disinfectant. |
| Change in hydraulic flow | A sudden change in hydraulic flow may release solids previously attached as corrosion by-products. |
| Lead service lines | Lead service lines will continue to leach lead after many years of service. A strong correlation between the period of stagnation and lead release from lead service lines has been established. Partial lead service line replacement may result in temporary increases of lead levels due to filings or mechanical or hydraulic disturbances, which release solids previously attached as corrosion by-products. |
Within the plumbing system
|
Condition |
Comment |
|
Lead service lines |
Lead service lines will continue to leach lead after many years of service. A strong correlation between the period of stagnation and lead release from lead service lines has been established. Partial lead service line replacement may result in temporary increases of lead levels due to filings or mechanical or hydraulic disturbances, which release solids previously attached as corrosion by-products. |
| Brass fittings or in-line devices | Lead brass fittings and in-line devices, including water meters, may still contain lead and be released from these devices. Older devices may contain up to 8% lead in lead-based brass and be a source of lead for a period of time. Water meters are found in residential homes, but are typically the responsibility of the municipality. |
| Lead solder | Lead solders may be present in plumbing systems installed prior to 1990. These solders continue to be a source of lead at the tap. |
| Stagnation time | There is a strong correlation between the period of stagnation and lead release. The lead concentration will peak after 8 h. |
At the tap
|
Condition |
Comment |
|
Consumers’ complaints |
Consumers’ complaints provide a good source of information to determine where lead problems may occur. Complaints may arise from direct concern about lead concentration or indirect aesthetic concerns about the water. |
| Colour, turbidity or debris | The presence of colour, turbidity or debris at the consumer’s tap can be a good source of information with respect to corrosion. Although most often correlated with iron, it may also adsorb lead and other metals. |
| Lead levels | Lead levels remain the only truly reliable information to evaluate population exposure to lead from drinking water. |
Figures
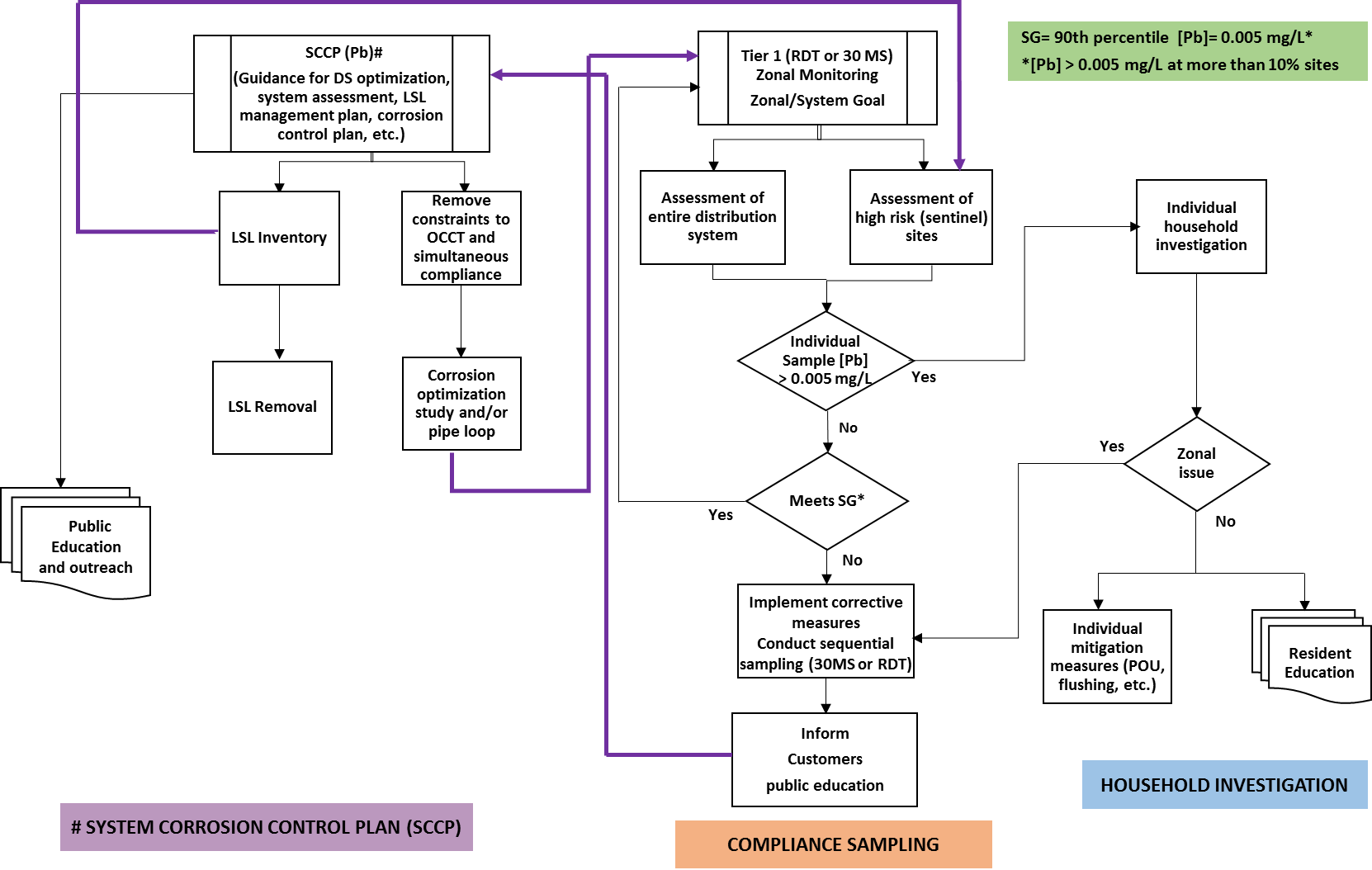
Long description for Framework for residential corrosion control program
This is a flowchart outlining a framework for a residential corrosion control program. The framework describes activities and steps to undertake to achieve a holistic approach to corrosion control and corrosion control treatment. It outlines the interconnection between the various elements of the System Corrosion Control Plan such as guidance for distribution system optimization followed by lead service line inventory and subsequent removal of the lead service line. It also includes, in parallel, the removal of constraints to optimal corrosion control and simultaneous compliance followed by corrosion optimization study and/or pipe loop studies, which then inform the protocols for zonal monitoring of residential dwellings. The lead service line inventory informs the selection and assessment of sentinel sites, which are used in both options of the residential dwellings protocols (RDT and 30 MS).
Protocols
Alternative monitoring protocol for non-residential and residential buildings (two-tier stagnation)
Sampling protocol
The objectives of this alternative sampling protocol and system goal for non‑residential and residential buildings, such as child care centres, schools and larger buildings, are to locate specific lead problems within the buildings and identify where and how to proceed with remedial actions. The intention is to minimize lead concentrations at the cold drinking water outlets (i.e., fittings/fixtures such as faucets and fountains) used for drinking and cooking and therefore protect occupants’ health from exposure to lead. The sampling protocols and system goal are based on an understanding of the variations in lead concentrations observed at outlets in a non-residential building resulting from sources of lead within the plumbing and water use patterns.
Stagnation periods will be influenced by such things as the frequency of use of the outlet, whether bottled water is distributed in the building, whether the building is occupied 24 or 8 h per day and the number of occupants. As such, establishing the source of the problem within a specific building becomes a critical tool in assessing which measures to take to reduce lead exposure. The locations of specific lead problems are determined by measuring lead levels at water fountains and cold drinking water outlets. When elevated concentrations of lead occur at an outlet, they can be from lead-containing material within the outlet itself (e.g., faucet, bubbler, water cooler), from the plumbing upstream of the outlet or from the water entering the building. A two-tier sampling approach is used to identify the source of the elevated lead concentration.
Since elevated concentrations of lead can be found in drinking water as a result of leaching from plumbing materials, including fittings and fixtures, within a building, this protocol should be followed by responsible authorities, such as building owners or managers, school boards and employers, as part of the overall management of the health and safety of the occupants of schools, child care centres and other non-residential buildings. This protocol may also be followed by utilities that want to include non-residential or residential buildings such as schools and multi-dwelling buildings in their corrosion control monitoring programs. The extent of sampling conducted by an individual responsible authority within a building may vary depending on the objective of the sampling and the authority conducting the sampling.
In some cases, responsible authorities may want to collect Tier 1 and Tier 2 samples at the same time to eliminate the need to return to the site. In this case, authorities should be aware that the confidence in some sample results will decrease, since flushing water through one outlet may compromise the flushed samples taken from other outlets that are located in close proximity.
Tier 1 sampling protocol
The objective of Tier 1 sampling is to identify specific cold drinking water outlets that have elevated levels of lead following periods of stagnation. Collection of a smaller sample volume helps to pinpoint whether the source of lead is from the specific outlet and to direct the appropriate corrective measures. Tier 1 sampling should be conducted at the locations identified in the sampling plan for the non-residential/residential building. In addition, a sample that is representative of the water that is entering the building (water main sample) should be collected at each monitoring event. Water main samples should be collected from a drinking water faucet in close proximity to the service line following a period of approximately 5 min of flushing (longer flushing may be necessary to ensure that the sample is representative of water that has been flowing in the main). All other samples in the building should be collected using the protocol described below.
A first-draw 250 mL sample is taken at the locations identified in the sampling plan after the water has been stagnant for a minimum of 8 h but generally not more than 24 h. To ensure that representative samples are collected, the aerator or screen on the outlet should not be removed prior to sampling. It is recommended that samples be separated into smaller volumes (e.g., 2 x 125 mL). This is a form of profile sampling that helps in the investigative phase if the analysis of the sample(s) indicates that lead is present. These smaller samples represent the water from the fitting (fountain or faucet) and a smaller section of plumbing and have the added benefit of being more effective at identifying the source of lead at an outlet. Collecting the Tier 2 samples provides the benefit of not having to return to the location to resample in order to identify the source of lead.
The use of wide-mouth sample bottles allows the sampler to fill the bottle at a medium to high (i.e., typical) flow rate, which provides a more accurate result. Sample bottles with a smaller opening will be difficult to fill at a typical flow rate and provide inaccurate results with respect to potential exposure and for investigative/remediation purposes.
If the lead concentration exceeds 0.005 mg/L (system goal) at any of the monitoring locations, it is recommended that the following measures be undertaken:
- Educate the occupants (e.g., teachers, day care providers, students) of the building and other interested parties (e.g., parents, occupational health and safety committees) on the sampling results and the interim measures that are being undertaken, as well as the plans for additional sampling.
- Conduct additional sampling at the outlets with lead concentrations that exceed 0.005 mg/L to determine the source of lead, as outlined in the Tier 2 protocol.
- Implement interim corrective measures immediately to reduce occupants’ exposure to lead in first-draw water. These measures may include any or a combination of the following:
- cleaning debris from the screens or aerators of the outlet
- flushing the plumbing system following periods of stagnation
- taking the outlet out of service
- using certified drinking water treatment devices
- supplying an alternative water supply
- Where a substantial amount of debris was removed from the aerator or screen, authorities may want to retest the water from these outlets following the same protocol. If results of the retesting show lead concentrations below 0.005 mg/L, authorities should investigate whether particulate lead may be contributing significantly to elevated lead levels and whether regular cleaning of the aerator or screen should be implemented as part of the maintenance or flushing program.
Tier 2 sampling protocol
Tier 2 sampling is used in combination with results from Tier 1 to determine the source of the lead in the plumbing within the building. Sampling after a short period of flushing (30 s) will determine the concentration of lead in the water that has been stagnant in the plumbing upstream of the outlet.
At those water fountains and cold drinking water outlets with lead concentrations that exceeded 0.005 mg/L for Tier 1, a second 250 mL flushed sample is taken after the water has been stagnant for a minimum of 8 h (but generally not more than 24 h) and then flushed for 30 s. It is recommended that samples be separated into smaller volumes (e.g., 2 x 125 mL). This is a form of profile sampling that helps in the investigative phase if the analysis of the sample(s) indicates that lead is present. These smaller samples represent the water from the fitting (fountain or faucet) and a smaller section of plumbing and have the added benefit of being more effective at identifying the source of lead at an outlet.
When the lead concentration in any of these second samples exceeds 0.005 mg/L (MAC), corrective measures should be undertaken immediately. Corrective measures can include interim measures, such as routine flushing of the outlet before the facility opens (a minimum of 5 min to obtain water from the water main), removing the outlet from service, using certified drinking water treatment devices or providing an alternative water supply, that are put in place until a permanent solution can be implemented. In addition, depending on the results of the Tier 1 and Tier 2 sampling, one or a combination of the following corrosion control measures should be initiated:
- Educate the occupants of the building (e.g., teachers, day care providers, students) and other interested parties (e.g., parents, occupational health and safety committees) on the sampling results and the interim and long-term corrective measures that are being undertaken.
- Compare the Tier 1 and Tier 2 sampling results to determine whether the source of the lead contamination is the fitting, fixture or internal plumbing. If the results of the Tier 1 and Tier 2 sampling both indicate lead contamination, conduct additional sampling from the interior plumbing within the building to further determine the sources of lead contamination.
- Additional measures to consider:
- Flush the outlets.
- Install certified drinking water treatment devices.
- Replace the outlets, fountains or pipes.
- Remove the outlets from service.
- Replace lead brass fittings or in-line components.
- Work collaboratively with the water supplier to ensure that the water delivered to the building is not aggressive.
- Distribute an alternative water supply.
Rationale: Alternative stagnation sampling protocol
As with residential monitoring programs, each component of a sampling protocol in non-residential settings, such as the stagnation time, the volume of water collected and the system goal, has important implications as to the usefulness of the data collected. Since the objectives of conducting sampling in non-residential buildings are different from those in residential settings, the volume of water collected is also different.
The Tier 1 and Tier 2 sampling protocols for non-residential sites are based on the collection of a 250 mL sample volume. Studies have demonstrated that to evaluate the amount of lead leaching from outlets such as kitchen faucets, more than 95% of the lead can be found in the first 200–250 mL of water from the faucet (Gardels and Sorg, 1989). Lead levels in non‑residential and large residential buildings have generally been found to decrease significantly following flushing of the outlet for 30 s. This suggests that the fountain or faucet and the connecting plumbing components can be major contributors to elevated lead concentrations at outlets in non-residential and institutional buildings (Bryant, 2004; Boyd et al., 2008a,b; McIlwain et al., 2016; Doré et al., 2018; Katner, et al., 2018; Miller-Schulze et al., 2019).
The collection of a larger volume of water, such as 1 L, would include a longer line of plumbing prior to the outlet. This plumbing may contain valves, tees and soldered joints that could contribute to the lead concentration in the 1 L sample; however, it would not be possible to identify which material was releasing the lead. In addition, it is suggested that collecting such a large volume from a drinking water fountain might dilute the initial high concentrations observed in the outlet. This is not desirable, since water collected from sections of plumbing farther from the outlet typically have lower lead concentrations (U.S. EPA, 2004). Therefore, the collection of a sample volume that is smaller (250 mL) than those typically used to assess corrosion (1 L and greater) in residential dwellings of 6 units or less, is considered important for sampling in non-residential buildings. A 250 mL sample volume is selected for sampling in non-residential buildings, as it represents water from the fitting (fountain or faucet) and a smaller section of plumbing and is therefore more effective at identifying the source of lead at an outlet, especially if this volume is broken down into smaller volumes (e.g., 2 x 125 mL) so as to obtain a profile of the plumbing (U.S. EPA, 1994, 2006). However, if additional volumes of water were collected following the initial 250 mL sample (i.e., 250 to 1 000 mL), the result from this larger volume may correspond to a lower concentration when calculated as a 1 L sample. This is due to the fact that the subsequent volumes would most likely contain lower concentrations of lead than that seen in the initial 250 mL sample and result in a dilution effect (U.S. EPA, 2004). However, studies have also shown an increase in lead concentration with increasing volume (McIlwain et al., 2016; Miller-Schulze et al., 2019).
Studies examining sources of lead at the tap have found lead solder and brass fittings to be significant sources of elevated lead concentrations following a period of stagnation (Lee et al., 1989; Singh and Mavinic, 1991; AwwaRF, 2004; U.S. EPA, 2007). Depending on the age and type of material, the concentrations of lead from brass fittings have been shown to increase significantly following stagnation periods of between 4 and 20 h (Lytle and Schock, 2000). As a result, the water use pattern in a building is an important factor in determining lead concentrations at the tap. Since water use patterns are often intermittent in buildings such as day care centres, schools, residential and office buildings, sampling following a period of stagnation will capture this type of scenario. The most conservative standing time prior to sampling is between 8 and 18 h, since it is most likely to result in the measurement of peak concentrations of lead. Therefore, first-flush samples should be collected following a minimum period of stagnation of 8 h, but not greater than 24 h, so that they are representative of the longer periods in which outlets are not used for drinking during most days of the week in a non-residential building.
Tier 1 sampling protocol
The Tier 1 sampling protocol has been used in non-residential and residential buildings for locating specific lead issues, determining how to proceed with remedial measures and demonstrating that remediation has been effective. Numerous studies have been published on extensive sampling programs for measuring lead concentrations at the tap, conducted in schools and other non-residential and residential buildings. These studies demonstrated that the collection of 250 mL samples following a period of stagnation of a minimum of 8 h, but generally not more than 24 h, is effective at identifying outlets with elevated lead concentrations (Gnaedinger, 1993; Murphy, 1993; Maas et al., 1994; Bryant, 2004; Boyd et al., 2008a,b). Using this sampling method, several studies were able to determine the source of lead within schools and develop a remediation plan (Boyd et al., 2008a,b; Deshommes et al., 2016; Doré et al., 2018).
Tier 2 sampling protocol
In order to help identify the source of lead at outlets that exceed the Tier 1 system goal, follow-up samples are taken of the water that has been stagnant in the upstream plumbing but not in the outlet itself. The results can then be compared to assess the sources of elevated lead and to determine the appropriate corrective measures. In order to be able to compare the results, a second 250 mL sample is collected following the same period of stagnation. To obtain water that has been stagnant in the plumbing prior to the outlet, a 250 mL sample is taken after a period of stagnation of a minimum of 8 h, but generally not more than 24 h, followed by a 30-s flush. Water fountains and cold water outlets exceeding the 30 s flushing was selected, since it should normally eliminate the water present in the outlet. If the lead concentration in the second 250 mL sample decreases below 0.005 mg/L (system goal), then it can be concluded that the water fountain, the cold drinking water outlet or the plumbing in the immediate vicinity is the source of the lead. If concentrations of lead above 0.005 mg/L (MAC) are found in the Tier 2 samples, then the lead sources may include the plumbing materials that are behind the wall, a combination of both the outlet and the interior plumbing or contributions of lead from the service connection.
The results of Tier 1 and Tier 2 sampling should be interpreted in the context of the plumbing profile so that an assessment of the lead contributions can be made and the appropriate interim and long-term corrective measures can be taken. Information on other sampling that can be conducted to help determine the source of lead if it has not been identified as well as detailed information on the interpretation of Tier 1 and Tier 2 sampling results can be obtained from other reference material (U.S. EPA, 2018).