Notification diagram for WTO and non-WTO importing countries under Canada's Access to Medicines Regime
The following diagram illustrates what information should be provided to which authority in the notification process. More complete details are available in Notification Requirements for Schedule 4, WTO and Non-WTO Countries.
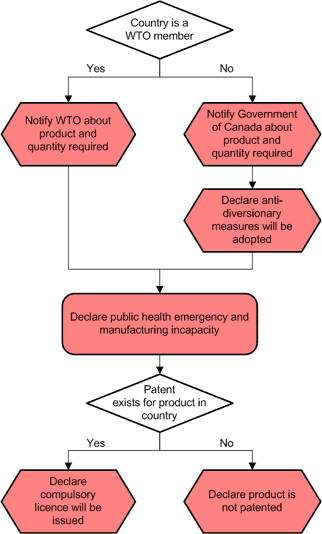
Notification Process Diagram - Schedule 4 World Trade Organization (WTO) and Non-WTO Importing countries - Text Description
Country is a World Trade Organization (WTO) member? If yes, notify WTO about product and quantity required. If no, notify Government of Canada about product and quantity required, declare anti-diversionary measures will be adopted. Declare public health emergency and manufacturing incapacity. Patent exists for product in country? If yes, declare compulsory licence will be issued. If no, declare product is not patented.