Risk management of chemical substances
On this page
- Implementation of risk management under the Plan of Priorities under the Canadian Environmental Protection Act, 1999 (CEPA)
- Overview of risk management
- Managing the risks of chemical substances under the CEPA
- Risk management instruments
- Objectives
- Risk management decision making – how to choose the best instrument(s) to control the risk
- Engaging in risk management
- Managing risks in a global context
- Compliance with risk management instruments
- Measuring our risk management performance
- Making information available
- Related resources
Implementation of risk management under the Plan of Priorities under the Canadian Environmental Protection Act, 1999 (CEPA)
CEPA was modernized in June 2023 and now requires a Plan of Priorities (the Plan). The Plan, published on July 19, 2025, specifies activities or initiatives in relation to controlling or otherwise managing the risks to the environment or to human health posed by substances.
Risk management activities and initiatives will be reported in the CEPA annual report as deliverables on the ongoing risk management priority put forth in the Plan. More information on the Plan and other delivery activities are available on the Plan of Priorities under CEPA web page.
Upcoming risk management
The following is a rolling list of upcoming risk management actions for substances assessed under the Chemicals Management Plan (CMP) as meeting section 64 criteria under CEPA. This list provides information on proposed or final actions expected for publication in the near term (that is, within the next 2 years). The list includes actions to be taken under CEPA as well as actions that may be taken under other federal acts, such as the Canada Consumer Product Safety Act (CCPSA), Food and Drugs Act (FDA), Fisheries Act, etc.
This list will be updated periodically to include newly identified actions. The last update was: October 4, 2024.
This list does not include risk management actions that have been taken outside of the CMP program, such as actions to address greenhouse gases.
Upcoming actions anticipated for publication in the 2024-2026 period:
Planned draft risk management actions:
- Proposed Coal Mining Effluent Regulations to include selenium and its compounds
- Proposed Code of Practice for benzophenone to reduce the concentrations of benzophenone in certain paints, to reduce the concentrations of benzophenone in certain paints, stains and coating products
- Proposed Release Guidelines for chemicals used in the rubber product manufacturing sector to manage releases of 1,4-Benzenediamine, N,N'-mixed phenyl and tolyl derivatives (BENPAT) and tetramethyl-thioperoxydicarbonic diamide (TMTD) to the environment
- Proposed amendments to the Pulp and Paper Effluent Regulations to modernize environmental protection measures, scope of the regulations, administrative improvements and compliance and administrative requirements
- Proposed amendments to the Chromium Electroplating, Chromium Anodizing and Reverse Etching Regulations to further reduce emissions of hexavalent chromium (HVC) compounds from regulated operations, harmonize requirements with other jurisdictions, and update and clarify regulatory requirements
- Proposal to list polyhexamethylene biguanide (PHMB) as a restricted or prohibited ingredient in cosmetics
- Notice of Intent to apply the significant new activity (SNAc) provisions to PHMB
- Regulatory measures to restrict Di(2-ethylhexyl) phthalate (DEHP) and products containing DEHP to prevent releases to the environment
- Developing and implementing risk management measures to prevent and control emissions of coal tars and their distillates from coal tar refining facilities
- Developing and implementing risk management measures to prevent and control releases of dinoseb from styrene production facilities
Planned final risk management actions:
- Restricting or prohibiting the use of talc, chlorocresol, malachite green, solvent violet 13 and benzophenone as an ingredient in cosmetics
- Publication of the final Prohibition of Certain Toxic Substances Regulations, 2024, which would further restrict the manufacture, use, sale and import of perfluorooctane sulfonate, its salts and its precursors (PFOS), perfluorooctanoic acid, its salts and its precursors (PFOA), and long-chain perfluorocarboxylic acids, their salts and their precursors (LC-PFCAs), and 2 flame retardants [hexabromocyclododecane (HBCD), polybrominated diphenyl ethers (PBDEs)], and products containing them, with time-limits for most remaining exemptions. It would also prohibit additional flame retardants, Dechlorane plus (DP) and decabromodiphenyl ethane (DBDPE), with time-limited exemptions
- Publication of the final Order Amending Schedule 3 to the Canadian Environmental Protection Act, 1999, which would add PFOA, LC-PFCAs, HBCD, PBDEs, DP, DBDPE and ferbam to the Export Control List in Schedule 3 to CEPA and amend the descriptions of certain substances already listed
- Order to apply the significant new activity (SNAc) provisions to TMTD
- Amendment to the Formaldehyde Emissions from Composite Wood Products Regulations to reduce administrative burden on industry
- Finalizing federal environmental quality guidelines - benzene, toluene, ethylbenzene, xylene (BTEX)
- Publication of the final Certain Products Containing Toxic Substances Regulations, which would restrict the manufacture, import, sale and offer for sale of certain sealant products containing coal tars and their distillates and polycyclic aromatic hydrocarbons (PAH)
- Amendments to the PCB Regulations to provide flexibility for the use and storage of polychlorinated biphenyls (PCB)-containing equipment under unique circumstances that were not foreseen when the Regulations came into force.
Substance specific web pages provide the most current status of the actions listed above. Information on planned or anticipated regulatory changes are included in the in the Environment and Climate Change Canada Forward Regulatory Plan and the Health Canada Forward Regulatory Plan.
Risk management in place
A list of proposed and final risk management actions that have already been published is available in the CMP risk management actions table.
Overview of risk management
The Government of Canada is committed to protecting people in Canada and the environment from chemical substances that could be harmful. The CMP is a Government of Canada initiative aimed at reducing the risks posed by chemical substances to people in Canada and the environment.
With proper use, storage and disposal, most chemical substances pose minimal risk. However, some chemical substances manufactured or used under certain conditions can lead to exposure that has harmful short- or long-term effects. Risk management of chemical substances involves preventing or controlling the conditions that may cause harm, such as the use of a chemical substance, the way it is made, how it is disposed of, and/or the amount or concentration that is released to the environment.
Risks to the environment or human health are determined through the risk assessment process. Once it has been determined that a chemical substance poses a risk, risk managers identify how best to manage the risk to help protect people in Canada and the environment. To do this, risk managers must understand how the chemical substance is created, used, who uses it and how it reaches the environment or people. Risk management instruments are then identified, developed and put into action to help prevent, reduce or eliminate that risk.
The performance measurement evaluation process helps to evaluate the effectiveness of risk management. If a risk management instrument has been in place for some time and the government is not satisfied that the risk has been sufficiently prevented or reduced, it can take further action.
The government is committed to implementing pollution prevention as a national goal and as the priority approach to environmental protection. Pollution prevention is about preventing the creation of waste at the source rather than cleaning it up after it has been produced. Minimizing or avoiding the creation of pollutants and wastes can be more effective in protecting the environment and less costly than treating them or cleaning them up after they have been created.
Managing the risks of chemical substances under the CEPA
CEPA defines "toxic" substances in section 64 as those that enter or may enter the environment at levels or conditions that:
- have or may have a harmful effect on the environment
- are or could be dangerous to the environment that life depends on; or
- are or could be dangerous to human life or health
Chemical substances which meet 1 or more of the criteria in section 64 generally require implementation of risk management actions in order to help prevent and control risks and protect human health and the environment. Before the government can take action on these chemical substances under CEPA, they have to be added to Schedule 1. In special cases, Schedule 1 chemical substances are included on the Non-Statutory List.
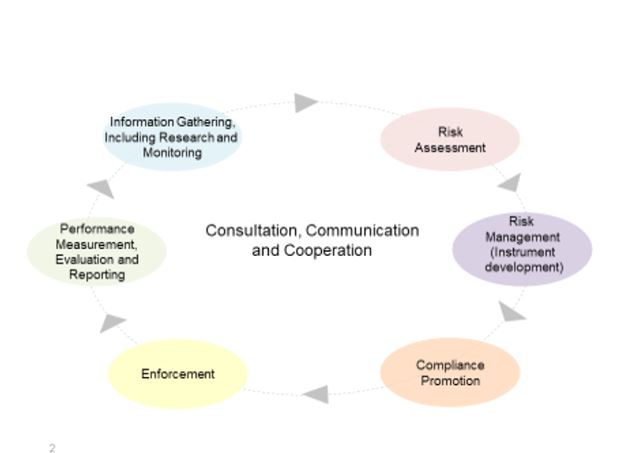
Figure 1 - Text description
The figure depicts Canada's chemicals management cycle, as it is known, made up of several-integrated components: a hub of information exchange through consultation, communication and cooperation in the middle that relates to the other 6 components.
These 6 interconnected components link in a circle:
- information gathering, including research and monitoring
- risk assessment
- risk management (instrument development)
- compliance promotion
- enforcement
- performance measurement, evaluation and reporting
Risk management instruments
Preventing or controlling risks is done by selecting and applying instruments that are most likely to achieve environmental and/or human health objectives. A variety of voluntary and mandatory instruments are used to manage risks posed by chemical substances. This range of options allows risk managers to use flexible, adaptable and phased approaches when dealing with harmful chemical substances in an integrated global market.
Information on actions taken to address risks from substances concluded to be harmful to the environment or human health as per section 64 of CEPA is available in the CMP risk management actions table.
Under CEPA, instruments are chosen based on a number of environmental, health, economic and social considerations, while risk managers also consider existing federal laws and programs, laws in provinces and territories, and sometimes those made in other countries.
Some examples of risk management instruments are:
- Regulations: these are enforceable laws that can restrict the use or release of a chemical substance, set limits on the concentrations allowed under various conditions, or prevent the use of chemical substances in certain products
- Pollution prevention planning notices: these require companies to prepare and implement a pollution prevention plan in order to minimize or avoid the creation of pollution or waste
- Release guidelines or codes of practice: these recommend limits and best practices to manage the use, release or disposal of a chemical substance
- Significant New Activity provisions: these require any major changes in the way a chemical substance is used be reported so that the government can decide whether to control the new use
- Ministerial conditions for new substances: these restrict the manner in which a new substance may be imported or manufactured
These risk management tools may be used to control various aspects of the life cycle of a Schedule 1 substance from the design and development stage to its manufacture, use, handling, storage, import, export, transport and ultimate disposal. For many chemical substances, CEPA also requires that risk management tools be developed and applied within strict timelines.
The Government of Canada also has access to risk management tools outside of CEPA that can address Schedule 1 or other chemical substances. Actions can be taken to develop instruments under other acts, such as the CCPSA and the FDA. When making risk management decisions, consideration is given to which act is best placed to manage the identified risks. Some examples of non-CEPA tools are to commit participating sectors or companies to specific challenges or performance levels:
- Additions to the Cosmetic Ingredient Hotlist: an administrative tool that sets out a list of substances that Health Canada describes as restricted or prohibited in cosmetics
- Various actions under the FDA: to address food, drug and health products
- Environmental performance agreements: these occur between one or more levels of government and a company or an industry sector
- Recall notices, regulations, packaging and labelling requirements under the CCPSA
Objectives
Before making decisions on which risk management instruments are the most appropriate to manage the risks, the objectives that should be achieved in order to protect people in Canada and the environment are established.
Environmental and human health objectives are statements that describe what should be done to address environmental and human health concerns related to a chemical substance or group of chemical substances. These can be qualitative (for example, reduce exposures to the general population) or quantitative (for example, achieve a level of 5 micrograms/L in surface water). Once human health or environmental objectives are decided upon, risk management objectives are established.
Risk management objectives are goals or targets that describe the expected results. These results would be achieved through the implementation of risk management instruments. Risk management objectives may be described in quantitative terms such as a performance standard, release limit, product content limit, percent reduction or more qualitative terms such as "lowest achievable levels". Risk management objectives are developed to contribute to the achievement of, or progress towards, an environmental or human health objective.
Risk management decision making - how to choose the best instrument(s) to control the risk
To identify the best-suited risk management instruments (mandatory or voluntary), risk managers follow a consistent and systematic approach. Through the instrument choice process, risk managers identify which instrument or mix of instruments are best suited to help achieve the risk management objectives on a sustained basis. The process takes into consideration the effectiveness and efficiency of the different risk management instruments, available information on the chemical substance and its sources of risk, and process guidance such as the Government of Canada's Cabinet Directive on Regulation.
Engaging in risk management
Consulting with interested and implicated stakeholders is an important element for the development and design of risk management instruments. Stakeholders, partners and members of the public are first engaged when a chemical substance is assessed and considered to be harmful to human health or the environment (that is, a Schedule 1 substance). When a toxic conclusion is proposed, a risk management scope is published at the same time as the draft assessment. The risk management scope outlines the Government of Canada's early thinking on risk management. If the final assessment maintains the toxic conclusion, the risk management approach is published and outlines in more detail the Government of Canada's plan for risk management. Both the draft assessment, the risk management scope, and the risk management approach provide an opportunity for stakeholders, partners and members of the public to provide feedback and information to inform the path forward. Consultations continue throughout risk management tool development, implementation, and publication. Stakeholder input on information gaps, technical considerations and socio-economic impacts can help inform the decisions made to manage risks for a Schedule 1 substance. Information on consultation opportunities is available on the CEPA Registry.
Managing risks in a global context
Sometimes taking action in Canada isn't enough to manage risks that originate outside our borders. Some chemical substances used in other countries can travel long distances through the air or water, and end up in the Canadian environment, such as mercury and persistent organic pollutants. Canada is an active participant in international efforts toward the sound management of substances and waste. Canada's participation in global agreements on substances and waste aims to protect the health of people in Canada and the environment, influence global priorities, and provide Canadian expertise based on our own domestic substances management experience.
View more information on the Compendium of Canada's Engagement in International Environmental Agreements.
Compliance with risk management instruments
Compliance means meeting specific requirements set in a risk management instrument and is secured through 2 types of activity: promotion and enforcement.
Promoting compliance contributes to the awareness and understanding of regulated communities of their obligations to comply with risk management instruments. Promotion includes products (factsheets, brochures, posters, guides, videos, social media messages, etc.) and activities (presentations at information sessions, tradeshows, workshops, meetings, responses to inquiries, etc.). Increasing the voluntary compliance of regulatees reduces the number of enforcement actions needed.
Environment and Climate Change Canada's Canadian Environmental Protection Act: compliance and enforcement policy will be applied when enforcement staff suspects non-compliance with a risk management instrument. This policy sets out the range of possible responses to alleged violations, including warnings, directions in case of release, environmental protection compliance orders, ticketing, ministerial orders, injunctions, prosecution and environmental protection alternative measures (which are an alternative to a court prosecution after the laying of charges for a CEPA violation). In addition, the policy explains when the Department of Environment and Climate Change will resort to civil suits by the Crown for cost recovery.
Measuring our risk management performance
Performance measurement evaluates the ongoing relevance, success and effectiveness of the actions taken to manage risks from Schedule 1 substances (that is, have human health and environmental objectives been met?).
Substance-based performance measurement and evaluation considers the overall performance of final risk management instruments applied to a chemical substance and relevant data or indicators of exposure to the environment or human health.
Instrument-based performance measurement evaluates the effectiveness of an individual instrument in meeting the specific risk management objectives that were set out when the risk management tool was designed. The results of performance measurement will help determine if additional risk management or assessment is needed.
View more information on the performance measurement for toxic substances is available.
Making information available
The Government of Canada works with stakeholders, such as the general public and industry, in addition to health and environmental organizations and Indigenous partners to help make sure decisions made on risk management are understood, and that the process used is transparent.
Information on actions the Government of Canada takes to manage risks is made available through the Canada Gazette and other government websites, such as the CEPA Registry. Information on the CMP is also available.
Stakeholders, partners and interested parties are informed of publications planned for the upcoming year through quarterly risk management mailouts. Stakeholders, partners and members of the public who are interested in being notified of CMP publications are invited to subscribe for the latest news on the CMP. Stakeholders, partners and members of the public who would like to receive CMP Publication Plans by email, inclusive of risk management Publication Plans on a quarterly basis, can contact: substances@ec.gc.ca.
Related resources
- Implementing the modernized Canadian Environmental Protection Act, 1999
A plain language summary of the amendments included in the June 2023 modernization of CEPA. - Plan of Priorities
A fully transparent, multi-year, integrated approach for the assessment of substances already in commerce in Canada, which identifies the activities and initiatives that support chemicals management. - The Substances Search tool
The tool provides the legislative status and program priorities for substances, such as chemicals, polymers and biotechnology organisms. - The toxic substances management process
An information source about the approach taken to develop management tools for substances found toxic under CEPA 1999 - Toxic Substances Management Policy
The federal Toxic Substances Management Policy puts forward a preventive and precautionary approach to deal with substances that enter the environment and could harm the environment or human health - Sources of toxic substances
A list of toxic substances managed under CEPA 1999 that are organized according to their sources - Environmental protection alternative measures (EPAMs)
A look at the Environmental Protection Alternative Measures that provide an alternative to legal prosecution in cases of CEPA 1999 violations - National Pollutant Release Inventory (NPRI)
A collection of information about releases and transfers of key pollutants across Canada - Pollution prevention planning
An information resource for pollution prevention planning under CEPA 1999, and access to the Pollution prevention resource finder - Summary of flame retardant assessments and management conducted under the Canadian Environmental Protection Act, 1999
A resource which provides a summary of risk assessment and management of flame-retardant substances assessed under the Government of Canada's Chemicals Management Plan (CMP).