Industry Guide to the Consumer Chemicals and Containers Regulations, 2001 Third Edition

Download the alternative format
(PDF format, 542 KB, 37 pages)
Organization: Health Canada
Published: 2001
Updated: 2023-10-27
Related Topics
Revised in 2023 (Previously titled: Guide to Canadian Consumer Chemical Product Assessment, Second Edition)
Health Canada’s mandate is to help protect the health and safety of Canadians. Health Canada’s Consumer Product Safety Program is responsible for the administration and enforcement of the Canada Consumer Product Safety Act (CCPSA) and its associated regulations.
The information in this guide provides an overview of the Consumer Chemicals and Containers Regulations, 2001 (CCCR, 2001) set out under the CCPSA. The information in this guide is not intended to substitute for, supersede or limit the requirements under the CCPSA or the CCCR, 2001. This guide is prepared for convenience of reference only and as such, has no official sanction. In case of any discrepancy, the CCPSA or the CCCR, 2001 legislation will prevail.
The CCPSA addresses dangers to human health or safety posed by consumer products in Canada. Any person who manufactures, imports, advertises, sells or tests a consumer product must comply with all applicable requirements of the CCPSA and its regulations. For example, the Act prohibits the manufacture, importation, advertisement or sale of any consumer product that poses a “danger to human health or safety” (paragraphs 7(a) and 8(a)). It also sets out requirements for preparing and maintaining documents and for mandatory incident reporting.
Appendix C provides information on how to: obtain a copy of the CCPSA and other references and contact your nearest Health Canada Consumer Product Safety Office.
This guide may be updated from time to time without notice. For the most recent version of the guide, consult the Consumer Product Safety Reports page.
1.0 Introduction
The Industry Guide to the Consumer Chemicals and Containers Regulations, 2001 (CCCR, 2001) presents key aspects of the assessment of consumer products to determine if they are chemical products or containers, as defined in the CCCR, 2001, and the applicable regulatory requirements.
This guide does not discuss all the requirements under the CCPSA or the CCCR, 2001.
The flow diagram below illustrates several of the key decision points and actions to be taken when assessing compliance with the CCCR, 2001. Information on these steps is presented in this guide.
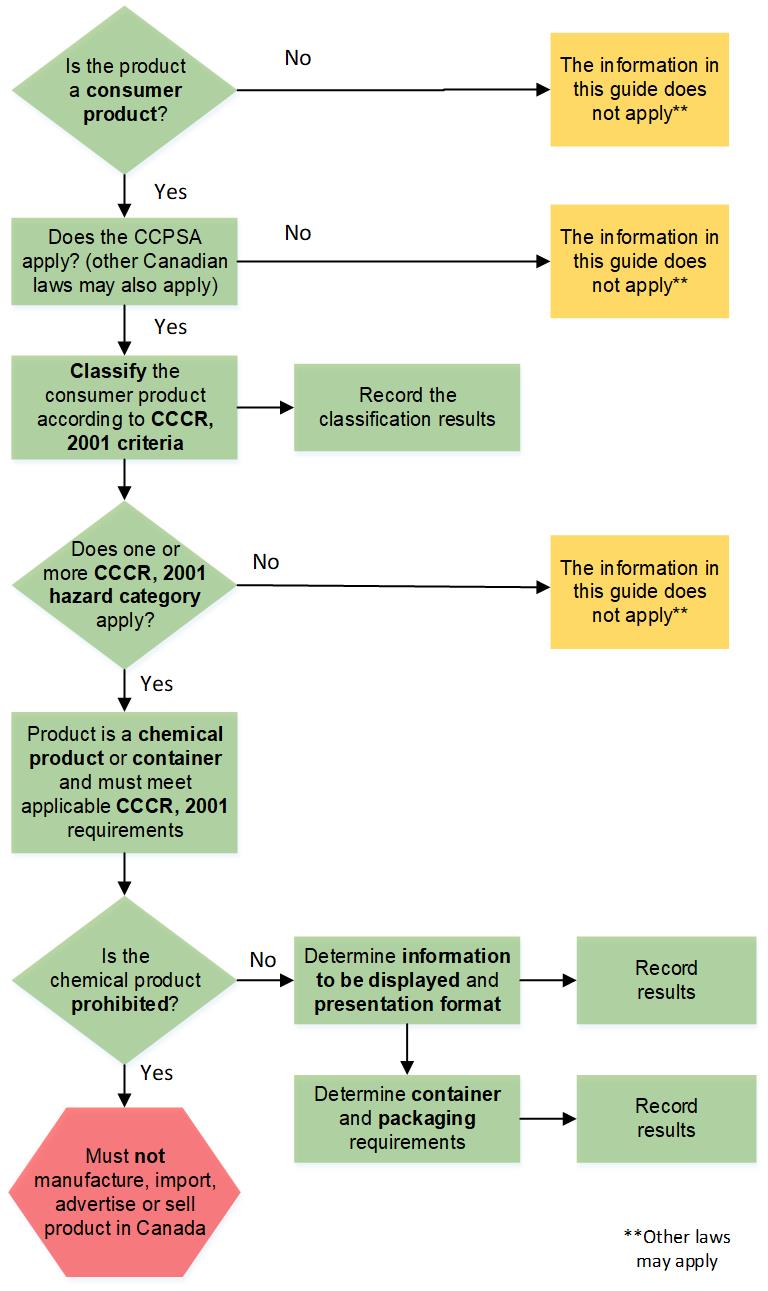
Figure 1: Flow diagram description - Text Equivalent
A flow diagram illustrating several of the key decision points and actions to be taken when assessing the applicability and compliance with the CCCR, 2001. Information on these steps are presented in the guide. The illustration does not discuss all requirements of the CCPSA or the CCCR, 2001. The process is presented as follows:
- “Is the product a consumer product?”
Yes: Down arrow guiding to step 2
No: Side arrow to a box indicating “The information in this guide does not apply**” - “Does the CCPSA apply? (other Canadian laws may also apply)”
Yes: Down arrow guiding to step 3
No: Side arrow to a box indicating “The information in this guide does not apply**” - “Classify the consumer product according to the CCCR, 2001 criteria”
A side arrow to box indicating “Record the results”; a down arrow guiding to step 4
- “Does one or more CCCR, 2001 hazard category apply?”
Yes: Down arrow guiding to step 5
No: Side arrow to a box indicating “The information in this guide does not apply**” - “Product is a chemical product or container and must meet applicable CCCR, 2001 requirements”
Down arrow guiding to step 6
- “Is the product prohibited?”
Yes: Down arrow to red hexagon indicating “Must not manufacture, import, advertise, or sell product in Canada”
No: onside arrow guiding to step 7 and 8 - “Determine information to be displayed and presentation format”
Side arrow to box indicating “Record results”; a down arrow to step 8
- “Determine container and packaging requirements”
Side arrow to box indicating “Record results”
Text indicating that ** means “Other laws may apply”
2.0 What Consumer Products Are Affected By These Regulations?
2.1 What is a Consumer Product?
For a chemical product to be subject to the requirements set out in the CCCR, 2001, it must first be classified as a consumer product that is subject to the CCPSA. To understand the scope of products that the CCPSA applies to, it is important to consider the definition of a “consumer product” as well as the range of consumer products that are excluded from the application of the Act.
Definition of Consumer Product (CCPSA, s. 2)
The term consumer product is defined in the CCPSA as:
“a product, including its components, parts or accessories, that may reasonably be expected to be obtained by an individual to be used for non-commercial purposes, including for domestic, recreational and sports purposes, and includes its packaging.”
Hazardous Products
The definition of consumer product in the CCPSA does not include products used exclusively for commercial or workplace purposes. As such, chemicals used exclusively in the workplace (including hazardous products as defined in the Hazardous Products Act) are not within the scope of the CCPSA or the CCCR, 2001.
Workers are likely familiar with reference to the Workplace Hazardous Materials Information System (WHMIS), which is Canada's national hazard communication standard. Hazardous products are governed by the requirements under the Hazardous Products Act (HPA) and its associated Hazardous Products Regulations. A statement such as “For Industrial Use Only”, on a chemical product subject to the CCPSA, does not remove the applicability of the requirements of the CCCR, 2001.
2.2 Excluded Products
Exclusions listed in the CCPSA (CCPSA, s. 4, Schedule 1)
Section 4 and Schedule 1 to the CCPSA sets out a list of consumer products to which the CCPSA does not apply. Below are examples of consumer products regulated under other Canadian legislation (see italics), and which the CCPSA and its associated regulations do not apply to:
- tobacco products: Tobacco and Vaping Products Act
- natural health products: Food and Drugs Act
- cosmetics: Food and Drugs Act
- drugs: Food and Drugs Act
- explosives: Explosives Act
- food: Food and Drugs Act
- medical devices: Food and Drugs Act
- nuclear substances: Nuclear Safety and Control Act
- pest control products: Pest Control Products Act
- cannabis products: Cannabis Act
The above list of exclusions is not intended be exhaustive. Please refer to section 4 and Schedule 1 to the CCPSA for complete information.
A product that is represented for use as a disinfectant or a sanitizer is not subject to the CCPSA or the CCCR, 2001 because drugs and pest control products are specifically excluded from the scope of the CCPSA.
While some products for animals, including animal grooming products, may be subject to the CCPSA and the CCCR, 2001, the following types of products are excluded from the CCPSA and CCCR, 2001:
- Products for use on animals that make a therapeutic claim may be classified as veterinary drugs and subject to the Food and Drug Regulations.
- Products that make pest control claims (e.g., flea and tick removal) may be classified as pest control products and subject to the Pest Control Products Regulations or the Food and Drugs Act, depending on the application.
Listed Exclusions in the CCCR, 2001 “Chemical Product” Definition (CCCR, 2001, ss. 1(1))
The term “chemical product” is defined in the CCCR, 2001 as:
“a product used by a consumer that has the properties of one or more of the following:
- (a) a toxic product;
- (b) a corrosive product;
- (c) a flammable product; or
- (d) a quick skin-bonding adhesive.
It does not include any of the following:
- (e) a product described in any of paragraphs (a) to (d) if it is not possible for a user to be exposed to the product or to any of its hazardous ingredients during reasonably foreseeable use;
- (f) a portable petroleum container that conforms with CSA B306 or CSA B376;
- (g) a lighter;
- (h) a fire extinguisher that conforms with ULC-S503, ULC-S504, ULC-S507 or ULC-S512; or
- (i) a container of fuel, such as gasoline, ethanol or propane, if the container is permanently attached to an internal combustion engine, a gas turbine or an appliance that uses the fuel.”
The definition of chemical product in the CCCR, 2001 excludes a product if a user cannot be exposed to it or any of its hazardous ingredients during reasonably foreseeable use. For example, in general, it is not possible for a person to be exposed to the refrigerant chemical, or its hazardous ingredients, in their refrigerator during reasonably foreseeable use. Where no exposure is possible, the product is excluded from the definition of chemical product. Note that reasonably foreseeable use includes foreseeable misuseFootnote 1.
The definition of chemical product in the CCCR, 2001 also specifically excludes certain products that must meet other stringent safety and performance standards in Canada. For example, lighters are required to conform to the Lighters Regulations under the CCPSA, and provincially mandated fire codes may require portable petroleum containers and fire extinguishers to meet CSA or ULC standards.
3.0 Product Classification
Once it has been established that a product is a consumer product subject to the CCPSA, the next step is to determine if the product is a chemical product or container within the meaning of the CCCR, 2001. A chemical product has hazardous properties described by any of the classification criteria set out in the CCCR, 2001. The classification criteria are based on a scientific assessment of the acute health and physical hazards that a consumer product may pose during foreseeable use (including foreseeable misuse). The classification criteria do not include effects that occur over longer-term or repeated exposures, such as cancer, reproductive effects or skin sensitization.
For a consumer product to be considered a chemical product within the meaning of the CCCR, 2001, it must meet the classification criteria of either a toxic product, a corrosive product, a flammable product or a quick skin-bonding adhesive. In addition, a consumer product that meets the classification criteria of a pressurized container is considered a container, within the meaning of the CCCR, 2001. These terms, and many other terms used in this guide, are defined in subsection 1(1) of the CCCR, 2001.
Below are the hazard categories addressed in the five parts of the CCCR, 2001 (note that Parts 1 to 3 are further broken down into sub-categories based on the severity of the hazard):
- Part 1: toxic products;
- Part 2: corrosive products;
- Part 3: flammable products;
- Part 4: quick skin-bonding adhesives; and
- Part 5: pressurized containers.
A chemical product may fall within more than one hazard category, based on its inherent hazards. For example, a toxic product packaged in an aerosol container would fall under both the “toxic” and “pressurized container” categories.
The classification results dictate:
- if any prohibitions apply;
- if there are specific container requirements that apply, such as a child-resistant container; and
- the information that must appear on the display surface of a chemical product, and if applicable, its packaging.
Definition of Container (CCCR, 2001, ss. 1(1))
The term “container” is defined in the CCCR, 2001 as:
- “a Category 5 pressurized container that is or is likely to be used by a consumer, including an empty container, as described in Part 5;
- an empty container that is destined for use by a consumer to store or dispense a chemical product; or
- any other container that is or is likely to be used by a consumer to store or dispense a chemical product.”
The definition of container in the CCCR, 2001 is intentionally broad. The definition does not exclude a container based on how long it may store a chemical product, whether it is designed to be decorative or if its function is to store a chemical product as it is evaporated or burned.
Some examples of products that fall within the definition of container include:
-
the fuel reservoir of certain fuel burning products, that require the use of a chemical product to function, such as decorative fuel burning products (including certain firepots, oil lamps and garden torch products) and food warming products (including certain chafing dishes, fondue pots and camping burners); and
-
certain product types where the chemical product is dispensed through evaporation, such as certain reed diffuser type products.
Responsible Person (CCCR, 2001, s. 4, 5)
The CCCR, 2001 define the term “responsible person” and use this term to assign obligations to specific participants in the supply chain. A manufacturer or importer of a chemical product or container in Canada is the responsible person and has the obligation to determine:
- the hazard categories that apply to the chemical product and, if applicable, its sub-categories;
- the type of container that is required; and
- the information that must appear on the display surface of a chemical product and, if applicable, its packaging.
The responsible person is required to prepare and maintain the documents relating to the above determinations. These documents must be kept for a period of at least three years after the day on which the chemical product or container is manufactured in Canada or the day on which it is imported.
An individual who is solely a retailer is not the responsible person, as defined in the CCCR, 2001.
Use of Consultants
The responsible person may choose to use the services of a third-party consultant to help with the classification process or to provide specific expert scientific advice on a component of the classification process. While the consultant will be the service provider in these instances, the manufacturer or importer remains the responsible person.
The responsible person should obtain all completed copies of work conducted by their consultants (if used) and keep the documents on file to provide them to a Health Canada inspector when requested.
Record of Results (CCCR, 2001, s. 5)
The responsible person must provide the documents generated by the classification process when requested by a Health Canada inspector. There is a time limit of 15 calendar days to provide the requested information to an inspector.
Checklists to aid in product classification and record keeping are given in Appendix A.
3.1 Hazard Categories and Sub-categories
Part 1 – Toxic Products (CCCR, 2001, s. 33, 34)
This hazard category includes the sub-categories: “very toxic”, “toxic” and “harmful”. The criteria describe chemical products that are hazardous because of the immediacy of their harmful effect following exposure and because exposure may lead to death. The distinction between sub-categories is the quantity of the chemical product that is required to produce a harmful or fatal effect. It should be noted that the manufacturing, importation, advertising, or sale of a “very toxic” chemical product is prohibited, and no exclusions exist.
Part 2 - Corrosive Products (CCCR, 2001, s. 41, 42)
This hazard category includes the sub-categories: “very corrosive”, “corrosive” and “irritant”. The criteria describe chemical products that are hazardous because they can cause a chemical burn or eye damage. The distinction between sub-categories is the degree of injury and whether permanent damage results. The manufacturing, importation, advertising, or sale of a “very corrosive” chemical product is prohibited but there are certain conditions that allow for these activities.
Part 3 - Flammable Products (CCCR, 2001, s. 48, 49)
This hazard category includes the sub-categories: “very flammable”, “flammable”, “combustible” and “spontaneously combustible”. The criteria describe chemical products that are hazardous because they can catch fire. The distinction between sub-categories is the ease of ignition or length of flame projection. The manufacturing, importation, advertising, or sale of a “very flammable” chemical product is prohibited but there are certain conditions that allow for these activities.
Part 4 - Quick Skin-bonding Adhesives (CCCR, 2001, s. 55)
The criteria for classifying a quick skin-bonding adhesive are that it has properties similar to an alkyl cyanoacrylate adhesive and that it is capable of bonding skin with skin instantly or nearly instantly.
Part 5 - Pressurized Containers (CCCR, 2001, s. 58)
The criteria for pressurized containers describe chemical products that are hazardous because of the internal pressure within the container. If punctured or heated, a pressurized container can rupture, resulting in flying debris or the release of dangerous contents.
4.0 Is the Chemical Product Prohibited?
4.1 Dangerous Chemical Products Are Prohibited (CCCR, 2001, s. 38, 45, 53)
Chemical products classified as “very toxic”, “very corrosive” or “very flammable” under the CCCR, 2001 are prohibited from manufacture, importation, advertising and sale in Canada. These chemical products are too dangerous to be available to the public as consumer products.
Conditions for Dangerous Chemical Products (CCCR, 2001, s. 45, 46, 53, 54)
There are conditions for manufacturing, importing, advertising or selling “very flammable” and “very corrosive” chemical products, including:
- Glass Etchants: “Very corrosive” glass etchants that contain fluoride are not prohibited, since there is no effective substitute for the frosting of glass, a technique used by artisans. To reduce the risk associated with using such products, the form of the glass etchant must be a paste or a gel. These forms promote a controlled application and reduce the possibility of splashing. As well, these products must be packaged in child-resistant containers and must display the required information for “very corrosive” products.
- Fuels: “Very flammable” fuels are not prohibited if the container of fuel is separate or detachable from specific product types that use the fuel and if that container displays the required information for “very flammable” products.
- Spray Containers: Spray containers that exhibit a flashback are classified as “very flammable” and are prohibited where they:
- have a flame projection of 100 cm or more; or
- contain a liquid with a flash point of less than -18°C.
However, spray containers that exhibit a flashback only are not prohibited if they display the required information of a “very flammable” product.
4.2 Canada Consumer Product Safety Act Prohibitions (CCPSA, Schedule 2)
All products listed in Schedule 2 to the CCPSA are prohibited. These include aerosol containers pressurized with vinyl chloride, sneezing powders, microscopy oils containing polychlorinated biphenyls (PCB’s), cutting oils and cutting fluids that may produce nitrosamines and urea formaldehyde foam insulation.
4.3 Prohibitions or Restrictions Under Other Canadian Legislation
Various chemical products are prohibited or restricted under other Canadian legislation, some examples include the Canadian Environmental Protection Act, 1999 and the Criminal Code.
A guide to several Government of Canada regulatory programs is given in Appendix B, however, it is not intended to be exhaustive. Regulated parties are advised to consult with relevant federal, provincial, territorial, municipal and other legislation for information on specific requirements.
Canadian Environmental Protection Act, 1999 (CEPA)
CEPA is an Act respecting pollution prevention and the protection of the environment and human health in order to contribute to sustainable development. Under this Act, the use of chlorofluorocarbon propellants in pressurized containers is prohibited by the Ozone Depleting Substances Regulations. In addition, some chemicals that have been declared toxic under paragraphs 64 (a) to (c) of CEPA are prohibited under the Prohibition of Certain Toxic Substances Regulations. For example, certain polychlorinated terphenyls and polybrominated biphenyls are prohibited. Other substances are restricted and must meet concentration limits, such as certain requirements in the 2-Butoxyethanol Regulations. In addition, many new regulations are being proposed or completed under CEPA and these may have an impact on the use of certain chemicals in consumer products. For more information about current, proposed, and repealed regulations under CEPA, please visit: https://pollution-waste.canada.ca/environmental-protection-registry/regulations.
The Criminal Code
Certain chemical products are prohibited under the Criminal Code (for example, tear gas and Mace). For more information, please refer to the Criminal Code and its associated regulations.
5.0 Information that Must be Displayed (CCCR, 2001, s. 15)
The required information that must be displayed according to the CCCR, 2001 depends on the hazard classification of the chemical product, and can include:
- hazard symbols;
- signal words;
- hazard statements;
- instructions for safe use; and
- first aid statements.
This required information must be on:
- the display surface of the container(s);
- the display surface of empty containers that the consumer may purchase to dispense and use a chemical product; and
- any outer packaging (such as kits or display cards).
Empty containers must be labelled and packaged for their presumed contents. The required information does not need to be displayed on shipping containers or package liners used only during transportation of the chemical product prior to sale to a consumer.
Small Containers (CCCR, 2001, s. 25)
Small containers, as defined in subsection 25(2) of the CCCR, 2001, are required to display limited information. Only the hazard symbols and signal words need to be displayed. When a small container is packaged in a larger outer package, the outer package must display all the required information unless the required information is visible through the outer package. This labelling exemption for small containers does not apply to chemical products that are “Quick Skin Bonding Adhesives”, which have their own rules for labelling under subsection 56(2) of the CCCR, 2001.
Kits and Display Cards (CCCR, 2001, s. 16)
There are special rules for displaying the required information on outer packaging such as kits and display cards.
A kit is a package that contains one or more chemical products. If the packaging is a multipack of one chemical product and the outer packaging is not transparent, then the outer packaging must display all the required information. But if the kit contains two or more chemical products that have different information requirements, then only the limited warnings for kits must be displayed on the outer packaging. Individual containers within a kit must display the required information.
Display cards and blister cards are considered to be outer packaging and must be labelled accordingly. However, if the required information is displayed on the container such that it is visible to the consumer through this outer packaging, then the display card or blister card is not required to display the information. If some of the required information on an inner container is obscured, this information needs to be displayed on the card, which may be on the back of the card.
5.1 Required Information (CCCR, 2001, s. 17, 39, 46, 54, 56, 59)
The required information is determined from the classification results in each of Parts 1 to 5 of the CCCR, 2001. The required information must be displayed in both official languages, that is, in English and French.
The wording for the hazard statements is mandatory unless specifically indicated otherwise. The hazard statements are generic so that a consistent message is provided to the public, and a consistent set of rules is in place for all regulated parties.
Did you know?
The CCCR, 2001 hazard symbols, signal words, hazard statements, and instructions for safe use were developed through a consensus process, with the active collaboration of interested stakeholders, including medical professionals, public health organizations, the chemical industry, consumer groups, senior groups, academia, technical experts and various federal government departments.
Additional Information (CCCR, 2001, s. 15)
Information in addition to what is required by the CCCR, 2001 may be displayed on a container as long as it does not disclaim or contradict the required information. The CCCR, 2001 set out the minimum requirements. The responsible person can add, and is encouraged to add, information considered necessary to fully inform Canadians of hazards associated with the use of the chemical product.
However, the responsible person is discouraged from deliberately over-stating the hazards posed by a chemical product. If unwarranted warnings are displayed on a chemical product, it may lead to unnecessary or inappropriate first aid treatment upon exposure to the product. The practice of over-stating hazards may minimize the perception of risk for other more dangerous products, which may lead to potential injuries from a lack of concern or precaution by a user.
Ingredient Disclosure (CCCR, 2001, s. 31, 32)
Hazardous ingredients, as defined in the CCCR, 2001, that are present at a concentration of 1% or more must be listed in the first aid statement. The hazardous ingredients must be listed in descending order of proportion. That is, the first listed ingredient is the one that is present in the highest percentage in the product.
An ingredient may be identified by its chemical name using the nomenclature system developed by the International Union of Pure and Applied Chemistry (IUPAC), or the Chemical Abstracts Service (CAS) rules of nomenclature, or any name that clearly gives the precise chemical identity. For complex mixtures, such as pine oil, the generic name may be used. The use of trade names, CAS numbers or other codes alone is not acceptable. In addition, it is strongly discouraged to use the words “may contain” and other phrases that create uncertainty regarding the composition and, consequently, the hazards associated with the use of a chemical product.
Is There a “Trade Secret” Exemption for Hazardous Ingredient Disclosure?
There is no “trade secret” exemption from hazardous ingredient disclosure for chemical products for the following reasons:
- identifying the exact percentage of ingredients present is not required;
- listing all ingredients is not required, only hazardous ingredients are listed; and
- listing ingredients that are present at a concentration below 1% is not required.
Record of Results (CCCR, 2001, s. 4, 5)
The responsible person must prepare and maintain a record of the steps taken to determine the information that is required to be displayed on their chemical product. The responsible person must provide these records when requested by a Health Canada inspector. There is a time limit of 15 calendar days to provide the requested information to a Health Canada inspector.
5.2 Presentation Format (CCCR, 2001, s. 17, 18, 22)
The presentation of the required information is intended to capture the attention of the user of the chemical product and to make it easy for the user to understand the safety information.
The required information must be clear and legible. There must be a distinct colour contrast between the information and the background: the colour contrast must be equivalent to at least a 70% screen of black on white. The information must remain legible throughout the lifetime of the product, and not fade, run, rub off or peel off under normal use. The required diameter of the hazard symbol and the print size increases with the area of the main display panel to promote information legibility under actual use conditions.
Colour
To allow for flexibility in product design, there is no specification for the colour of the required information. Readability is promoted through the requirements for print type, format, size and contrast. However, label designers should take into consideration that the combination of certain colours may create legibility challenges for people who have difficulty distinguishing between certain colours.
Main Display Panel (CCCR, 2001, ss.1(1), s. 25, 26)
The CCCR, 2001 defines the “main display panel” as:
“the part of the display surface that is displayed or visible under normal conditions of sale to the consumer. It includes
The safety information required by the CCCR, 2001 that must be displayed on the main display panel includes:
- in the case of a rectangular container, the largest side of the display surface;
- in the case of a cylindrical container, the larger of
- the area of the top, or
- 40% of the area obtained by multiplying the circumference of the container by the height of the display surface;
- in the case of a bag, the largest side of the bag; and
- in the case of any other container, the largest surface of the container that is not less than 40% of the display surface.”
- a hazard symbol(s);
- a signal word (for example, “DANGER”); and
- a primary hazard statement(s).
This information must always be displayed below the common name of the product. In this way, the main display panel provides a consistent and familiar location for this required safety information. The prominent placement of these elements on the main display panel alerts users and significantly increases their likelihood of reading the more detailed safety information on the rest of the chemical product container.
The hazard symbol also helps warn people with a limited ability to read English or French, including young children. The signal word reinforces the message given by the hazard symbol and indicates the degree of hazard. The primary hazard statement provides a brief description of the main hazard associated with exposure to the chemical product.
The order of the safety information required on the main display panel must be the hazard symbol at the top, followed by the signal word and then the primary hazard statement.
In certain cases, there may be multiple hazard categories that apply to a chemical product. When more than one hazard symbol is required, the hazard symbols must be placed in a row parallel to the base of the container, with the signal word displayed below, and the primary hazard statements displayed under the signal word (see example below). The signal word corresponding to the greatest hazard must be displayed. An exemption to the vertical format is provided for certain short-wide containers (CCCR, 2001 ss. 26(3)).
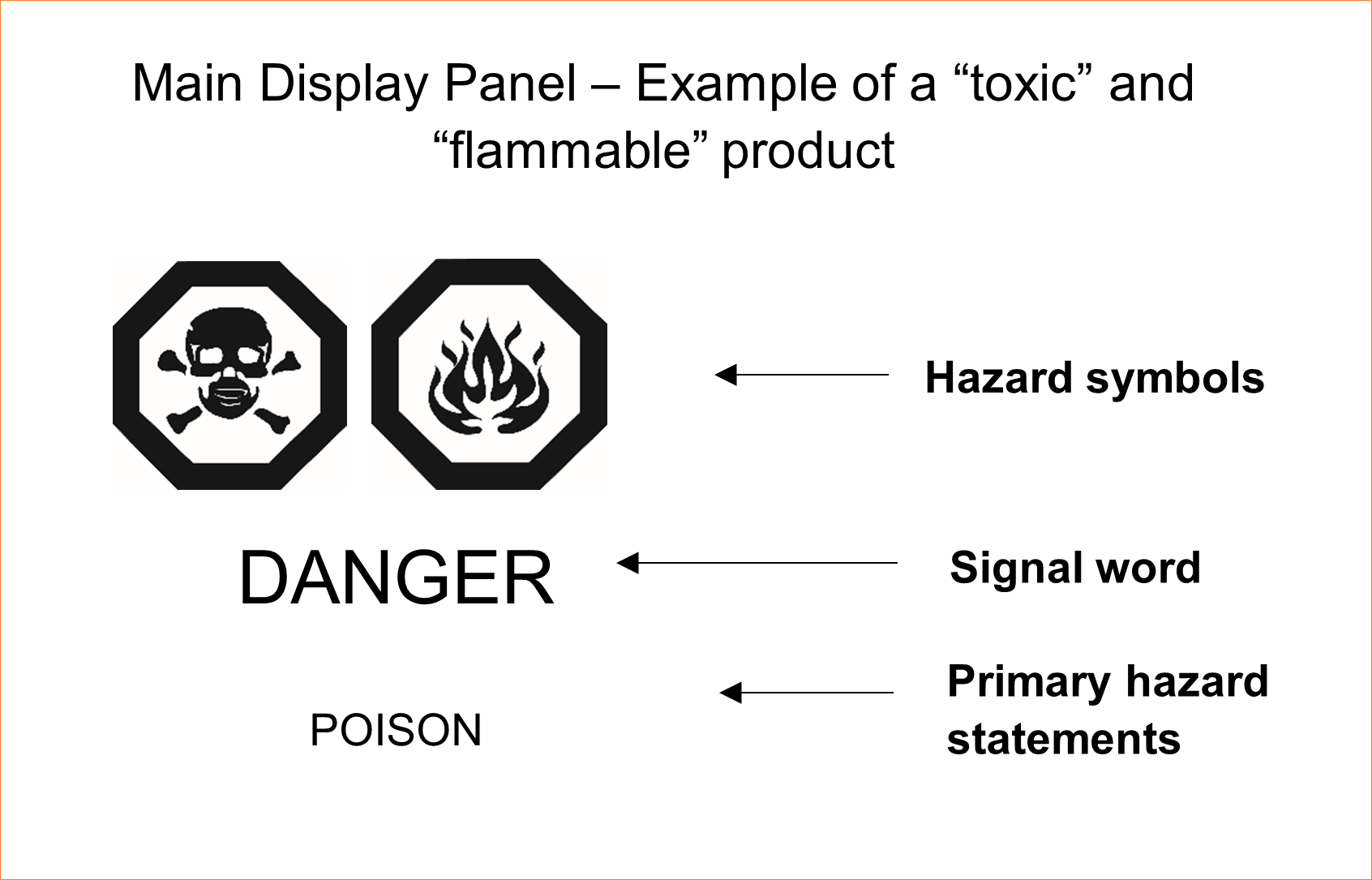
Figure 2: Main display panel - example - Text Equivalent
The illustration depicts information required by the CCCR, 2001 for the main display panel of a “toxic” and “flammable” product. The required information includes “toxic” and “flammable” hazard symbols, a “DANGER” signal word, and primary hazard statements that describe the nature of the hazards.
The "toxic" hazard symbol is a black line image of a skull and crossbones on a white background. The skull and crossbones are centred within a thick black lined hexagon. The "flammable" hazard symbol is a black line image of flames in a teardrop shape, emanating from a centre point at the base of the flames. The flames image is on a white background. Note that the sizing of the information to be displayed depends on the area of the main display panel.
Bordered Information on Any Part of the Display Surface (CCCR, 2001, ss.1(1), s. 25, 29, 30)
Since the space available on the main display panel limits the amount of safety information that can be displayed, the other required information including the specific hazard statements, positive and negative instructions, and the first aid statement must be displayed on any other part of the display surface of the chemical product.
The specific hazard statements, positive and negative instructions, and the first aid statement must be left-justified and placed within a border. The border enables users to easily distinguish the safety-related information required by the CCCR, 2001 from the other information on the display surface of the chemical product. For example, the first aid statement would be readily found during the stressful conditions of an emergency situation, when the reader may not have the time or may be too upset to search for the more detailed safety information.
The border must meet legibility and durability requirements, including the 70% contrast requirement. It may be made by using a difference in colour or shading of the background, or a series of dots or hatched lines or another graphic device, but it must be different from any other border on the label.
Label Example
Below is an illustration is an example of a CCCR, 2001 label for a chemical product that is classified as toxic (oral route), and that is not classified as corrosive, flammable, a quick-skin bonding adhesive, or a pressurized container.
Note that additional information such as product identity, product net quantity, and the dealer’s name and principal place of business are not requirements of the CCCR, 2001 and this information is not shown on the example. The content in italics is instructions to the responsible person who needs to insert the applicable details for their chemical product.

Figure 3: Label example - Text Equivalent
The left side of the figure depicting an example label shows the required information on the main display panel, including a skull and cross-bones hazard symbol, a “DANGER” signal word, and a “POISON” primary hazard statement, centred parallel to and near the base of the main display panel. The hazard symbol appears at the top, followed by the signal word and then the primary hazard statement.
The right side of the figure illustrates the information that must be displayed inside a border, including the specific hazard statement, negative and positive instructions, and the first aid statement. This information appears left justified and there are separate boxes for information in English and French.
Can Information Not Required be Placed in the Border?
The border is used to make the safety information required by the CCCR, 2001 stand out from other product information. It is appropriate to allow additional safety-related information within the CCCR, 2001 border. However, the presence of non-safety related information within the border conflicts with the objective of the bordered area and is not recommended.
6.0 Container and Packaging Requirements
6.1 Containers Must Not Leak (CCCR, 2001, s.7, Sch. 3)
A container of a liquid chemical product must not leak when sold or when used for the number of openings and closings normally required for the container’s size and contents. Although some products require containers that are designed to release vapours to avoid the build-up of internal pressures within the container, the liquid product must not be able to leak out.
The leakage test is done on the product, as it would be filled at the time of sale. The use of a placebo is not a satisfactory substitute for the actual chemical product.
Empty Containers
The leakage requirement also applies to empty containers that are destined to store and dispense a chemical product. The empty container must be filled with the intended product and tested in the same manner as a container that is full at the time of sale.
6.2 Child-resistant Containers (CCCR, 2001, s. 9 - 14)
A child-resistant container creates a barrier between a chemical product that may harm a child and a naturally curious child. A container that is child-resistant does not mean that it is child-proof. In general, a child-resistant container is required for chemical products classified as quick skin-bonding adhesives or those classified in the following sub-categories:
- “toxic”;
- “very corrosive”; and
- “corrosive”.
Containers are considered child-resistant within the meaning of the CCCR, 2001 when they are constructed so that they can be opened only with the use of a tool OR through acceptable results from the application of one of the recognized child test protocols.
Use of a Tool (CCCR, 2001, s. 9)
Containers that require a tool to be opened, such as paint cans that require a screwdriver to be opened, are considered child-resistant by design as long as a tool is not supplied with the container.
Child Test Protocols (CCCR, 2001, s. 9)
A container’s design is considered child-resistant where it satisfies the requirements of an acceptable test protocol as described in section 9 and summarised below. Children are used as the test subjects in the child test protocols. Children are not exposed to chemical products during these tests, as the containers are filled with a placebo that does not harm a child. In general, the test protocols require that at least 80% of those children being tested be unable to open the container during a 10-minute test. This requirement means that some children may still be able to open a container in the specified time.
At present, there are no universal mechanical tests to assess whether a package is child-resistant. Children will explore a variety of different ways to open a container. For example, if their fingers won’t work, their teeth might. Using a recognized child test protocol allows manufacturers to design packages that meet the child-resistant requirement without limiting technical innovation. However, once a container design has passed a child test protocol, mechanical testing may be appropriate for verification of continued product compliance to the specifications established as effective.
Child-Resistant Container Performance (CCCR, 2001, s. 10)
A child-resistant container must maintain its child-resistant characteristics for as long as it holds the chemical product for the number of openings and closings normally required for the container’s size and contents. While these characteristics must persist during shipping, during storage on the retail shelf prior to sale as well as after the product is sold, the re-testing of filled containers according to a child-test protocol is not required. Simple mechanical tests are available to verify the continued functioning of filled child-resistant containers once the design prototype is deemed child-resistant according to a child test protocol.
Record of Results (CCCR, 2001, s. 12)
To verify that the container and closure system will perform as originally designed, the specifications must be available for inspection. The required documents, as outlined in the CCCR, 2001, must be retained by the responsible person. Retailers are not required to keep these documents.
These documents must be kept for at least three years after the day on which the child-resistant container is manufactured in Canada or the day on which it is imported (to permit sufficient time for compliance actions to commence, where necessary, before records are destroyed). The responsible person must provide the documents when requested by a Health Canada inspector. There is a time limit of 15 calendar days to provide the requested information to a Health Canada inspector.
7.0 Importation Provisions (CCCR, 2001, s. 3)
An exception is set out in the CCCR, 2001 for the importation of non-compliant chemical products or containers. The importation exception applies only in three specific scenarios:
- the imported chemical product or container is brought into compliance prior to its sale to consumers;
- an importer sells the chemical product or container to a manufacturer who will bring it into compliance; and
- an importer may temporarily (that is, for a reasonable transition period) bring a non-compliant chemical product or container into Canada to export it to another country.
Bringing a chemical product or container into compliance with the CCCR, 2001 may involve re-formulation, re-packaging into a child-resistant container, or the application of over-labels with the required safety information. Both the chemical product and container are included in the importation exception to allow an importer to bring in empty containers from other countries that are to be filled and packaged in Canada.
7.1 Credible Evidence
The importer must provide credible evidence to support the importation of non-compliant chemical products or containers. Health Canada inspectors may request from the importer any or all of the following items as credible evidence:
- classification records to confirm the product’s ingredients and applicable labelling and packaging requirements;
- a purchase order or similar evidence that a printing company has been hired to produce a compliant label;
- a copy of the draft label or mock-up of the label demonstrating compliance to the CCCR, 2001;
- the contact information for the packager and labeller;
- the location of the production line, warehouse or similar facility where the chemical product or container will be brought into compliance; and
- the work plan or schedule for completion of the necessary work.
Appendix A: Checklists for Classification and Record Keeping
Please refer to the PDF version of the guide for Appendix A: Checklists for Classification and Record Keeping.
Health Canada recommends that the responsible person, as defined in the CCCR, 2001, completes the checklist for their records. The checklist has been provided as a template to assist the responsible person in collating the data described in section 4 of the CCCR, 2001. This data will help the responsible person determine the appropriate hazard categories and, if applicable, sub-categories that apply to the chemical product or container in question. The use of the checklist is not mandatory.
The checklist, along with the supporting documentation for the classification results, should be provided to Health Canada upon request to verify compliance to the CCCR, 2001.
The CCCR, 2001 Reference Manual may be consulted for additional clarification of regulatory requirements. A link to the CCCR, 2001 Reference Manual is found in Appendix C.
NOTE: The use of this checklist does not guarantee compliance with the regulations.
Appendix B: Examples of Government of Canada Regulatory Programs by Product Type
| Product type | Responsible Authority |
|---|---|
| chemicals; retail market | Health Canada, Consumer and Hazardous Products Safety Directorate |
| chemicals; intended for workplace | Health Canada, Consumer and Hazardous Products Safety Directorate |
| cosmetics | Health Canada, Consumer and Hazardous Products Safety Directorate |
| drugs, non-therapeutic | Health Canada, Office of Controlled Substances |
| drugs, therapeutic | Health Canada, Health Products and Food Branch |
| drugs, veterinary | Health Canada, Health Products and Food Branch |
| food | Health Canada, Health Products and Food Branch |
| medical devices | Health Canada, Health Products and Food Branch |
| natural health products | Health Canada, Health Products and Food Branch |
| pesticides | Health Canada, Pest Management Regulatory Agency |
| radioactive substances | Health Canada, Radiation Protection Bureau |
| tobacco and vaping | Health Canada, Tobacco Control Directorate |
| hazardous waste, disposal | Environment and Climate Change Canada |
| hazardous waste, transportation | Environment and Climate Change Canada |
| explosives | Natural Resources Canada, Explosives Regulatory Division |
| fertilizers | Agriculture and Agri-Food Canada |
| nuclear substances | Canadian Nuclear Safety Commission |
| transportation, of dangerous goods | Transport Canada, Transportation of Dangerous Goods Directorate |
| transportation, via pipeline | National Energy Board |
Appendix C: Information Resources
Health Canada Regional Product Safety Offices
For further information, visit the resources below or contact a Health Canada Consumer Product Safety Office via email (ccpsa-lcspc@hc-sc.gc.ca) or telephone (1-866-662-0666)
Online Resources
- Canada Consumer Product Safety Act (CCPSA) and its associated regulations
- Consumer Chemicals and Containers Regulations, 2001
- Reference Manual for the Consumer Chemicals and Containers Regulations, 2001
- Consumer Packaging and Labelling Act
- Canada Consumer Product Safety Act Quick Reference Guide
- Guidance on Mandatory Incident Reporting under the Canada Consumer Product Safety Act
- Industry Guidance - "Danger to Human Health or Safety" Posed by Consumer Products
- Information for Regulated Parties on the Enforcement Approach for the General Prohibitions under the Canada Consumer Product Safety Act
Footnotes
- Footnote 1
-
For more information on reasonably foreseeable use, refer to Health Canada’s Industry Guidance - "Danger to Human Health or Safety" Posed by Consumer Products