Pest Management Regulatory Agency Annual Report 2020–2021

Download the alternative format
(PDF format, 1,405 Kb, 42 pages)
- Organization: Health Canada
- Date published: February 2022
Pest Management Regulatory Agency
18 February 2022
Table of Contents
- Message from the Executive Director
- 2020–2021 PMRA performance highlights
- Impacts of the COVID-19 pandemic on PMRA operations
- About the Pest Management Regulatory Agency
- New pesticide registrations
- Regulation of pesticides on the market
- Health Canada's compliance and enforcement activities on pesticides
- Keeping pace with change
- International scientific and regulatory cooperation
- Regulatory updates
- Formulants and contaminants of health and environmental concern
- Amendments to certain spa chemicals
- Miscellaneous amendments to the Pest Control Products Regulations
- Targeted regulatory reviews
- Parliamentary review of the Pest Control Products Act
- Pest Control Products Regulations review
- Regulatory guidance for sanitizers
- Stakeholder relations and outreach communications
- Financial profile
- Appendix
Message from the Executive Director
I am pleased to present to you the Pest Management Regulatory Agency's (PMRA) Annual Report for 2020–2021.
In March 2020, the COVID-19 pandemic led to an abrupt and profound upheaval to the way we do our work. The sudden shift to an entirely virtual work environment presented a long list of technical, logistical and work/life challenges for employees and stakeholders alike, affecting program delivery in a number of ways (please see the section entitled "Impacts of the COVID-19 pandemic on PMRA operations").
Despite these challenges, as you will see in this report, the PMRA workforce pressed on to uphold our high standards of health and environmental protection in the regulation of pesticides, and offered time and expertise in the global fight against COVID-19. Despite some inevitable delays, Appendix Figure A1 shows that our core work of pesticide review, registration and re-evaluation continued and generally approached and even surpassed some timeline standards, which is no small feat under the circumstances.
Over the course of 2020–2021, PMRA completed foundational work on the multi-year program renewal project aiming to build a stronger, more transparent and more sustainable pesticide regulatory program. Information Technology and data strategies were initiated to support the integrated lifecycle approach, and respond to the Minister's mandate commitment to make science-based regulatory decisions in a timely manner. In August 2021, the Government of Canada announced that $42 million in new funding over the next three years will allow PMRA to build on this work, including by increasing the availability of independent data to support pesticide review decisions and improving the transparency of decision-making. The Government of Canada will also begin consulting on specific provisions of the Pest Control Products Act (2002) to consider, among other elements, ways to balance how pesticide review processes are initiated in Canada and increase transparency. These changes will further strengthen health and environmental protection and lead to improved quality and timeliness of scientific decisions.
PMRA continued to work with grower groups, provincial partners, Agriculture and Agri-Food Canada, Environment and Climate Change Canada, environmental groups and industry on various pesticide-related issues and initiatives such as drones and alternative testing methodologies, and an important new framework for data collection related to agricultural pesticides and environmental monitoring. Although the COVID-19 pandemic led to the suspension of some international activities, where possible, the PMRA continued working virtually with other countries and organizations on Joint Reviews and international agreements.
This report is a testament to the agility and dedication of PMRA's workforce through the ongoing challenges posed by the COVID-19 pandemic, and an assurance to Canadians that PMRA will continue to prioritize health and environmental protection through the changes ahead.
Peter Brander
Executive Director
Pest Management Regulatory Agency
2020–2021 PMRA performance highlights
- New active ingredients: 10
- New generic products (active ingredient and end-use): 101
- New minor uses: 477
- Emergency registrations: 12
- Joint reviews: 3
- Final re-evaluation decisions: 11
- Proposed re-evaluation decisions: 14
- Final special review decisions: 7
- Proposed special review decisions: 7
- Pesticide incident reports received: 1437
- Scientific studies received through incident reporting: 52
- Compliance verifications: 1213
- Enforcement actions taken: 1336
- Compliance promotion activities: 142
Impacts of the COVID-19 pandemic on PMRA operations
During the ongoing COVID-19 public health crisis, the PMRA continued to maintain operations while supporting the Government of Canada's public health efforts. PMRA deployed resources within the Health Portfolio in support of critical activities related to the pandemic, including employees volunteering in a variety of ways.
Initially, remote access to government network was limited, and in order for Health Canada to effectively respond to the COVID-19 pandemic, network access was prioritized for critical activities. In addition, COVID-19 related material was given priority access to publication.
The cumulative impact of these measures resulted in an increased backlog, delays with the registrations of new pesticide products and in the post-market decisions for existing pesticide products in Canada.
While in a 'business as possible' mode of operation, various interim measures were put in place to help maintain service delivery. These included measures to address a shortage of certain formulants and technical grade active ingredients used in the formulation of pesticide products, and new guidance for applying for an import certificate under the Grower Requested Own Use (GROU) Program.
On a case-by-case basis, PMRA lengthened public consultation periods on various documents to ensure stakeholders had sufficient time to review and comment on proposed decisions. However, in some cases these necessary extensions led to delayed completion of certain reviews.
In order to limit the impact on stakeholders and address some of their own challenges related to the pandemic, administrative changes and regulatory flexibilities were implemented. Health Canada worked with pesticide registrants to identify their most important applications so that resources could be focused on their highest priorities as opposed to the traditional sequential approach.
Flexibilities were incorporated to allow registrants to address ingredient shortages and meet the increased need for sanitizers. Requests were processed in an expedited manner for regulatory guidance and registration of sanitizers (or similar products) for use against the COVID-19 virus.
Health Canada liaised regularly with international regulatory partners to review novel approaches to the regulation of sanitizers, discuss response to regulatory issues and supply chain challenges, align communications where possible, and to share compliance and enforcement strategies.
PMRA will continue to adapt and respond to the challenges of the COVID-19 pandemic moving forward.
About the Pest Management Regulatory Agency
The Pest Management Regulatory Agency (PMRA) is the branch of Health Canada responsible for regulating pesticides under the authority of the Pest Control Products Act. PMRA's primary mandate is to prevent unacceptable risks to Canadians and the environment from the use of these products.
PMRA applies current, evidence-based scientific approaches to assess whether the health and environmental risks of pesticides proposed for registration are acceptable, and if the products have value.
This same approach is used to regularly and systematically review whether pesticides already on the Canadian market continue to meet modern scientific standards.
Health Canada's Regulatory Operations and Enforcement Branch collaborates with PMRA on promoting, monitoring and enforcing compliance with the Pest Control Products Act across Canada. Health Canada is committed to doing this in an open and transparent manner.
This work is carried out by a highly skilled workforce, the majority of whom are scientists, with additional expertise in areas such as regulatory and policy development, stakeholder engagement, international collaboration, and information management.
Vision
Canadians are confident that Canada's pesticide regulatory system protects their health and the environment.
Mission
To protect the health and environment of Canadians by using modern, evidence-based, scientific approaches to pesticide regulation, in an open and transparent manner.
What are pesticides?
In general, pesticides are toxic chemicals intentionally released into the environment to control pests on crops, in homes and workplaces, and in industrial processes. These can include personal insect repellents, wood preservatives, and pool sanitizers. Pesticides also include biologicals (derived from natural sources such as bacteria, fungi, viruses, plants, animals and minerals) and devices.
There are more than 600 registered active ingredients in more than 7600 registered pesticide products in Canada.
New pesticide registrations
Pesticides are regulated in Canada by Health Canada, reflecting the importance placed on human health and environmental protection in the regulation of these products. The Pest Control Products Act governs how pesticides are regulated based on scientific risk assessment and risk management, before and after they are registered for use.
Before a pesticide can be registered for sale in Canada, pesticide applicants are required to provide PMRA with extensive scientific data to show that their product does not pose unacceptable risks to health and the environment, and that the product has value. These data are reviewed by PMRA scientists to determine whether a product is acceptable for registration in Canada.
PMRA's science-based risk assessment includes the following:
- an examination of all sources and routes (oral, dermal or inhalation) of potential exposure to a given pesticide, including exposure through diet, from drinking water and from contact with treated areas like lawns and gardens
- an estimation of the amount of pesticides that people, including children, may come in contact with, both during and after a pesticide application
- a human health risk assessment with a particular focus on vulnerable populations, including pregnant women, infants, children, women, and seniors; this considers the potential for a pesticide to cause adverse health effects such as cancer, birth defects and endocrine effects, and allows registration only for those pesticides with exposures well below levels that cause adverse effects
- an environmental risk assessment that considers the fate (movement, persistence and transformation), toxicity, and risks to plants, birds, mammals, beneficial insects, and aquatic organisms
- a value assessment that considers the contribution of the product to pest management, as well as its health, safety and environmental benefits, and social and economic impact
For some currently registered pesticides, registrants may request changes to the use pattern. For these types of registrations, PMRA may also assess:
- additional environmental data, such as levels of pesticides detected through monitoring of pesticide concentrations in water across Canada or the United States
- any incident reports from Canada or other jurisdictions where the pesticide is already registered
- any other information needed to evaluate the health and environmental risks and the value of the pest control product
Various factors determine which studies are required to be submitted by applicants for registration, such as the nature of the product, the intended use, and the type of registration (for an overview of product submission types, see Appendix, Table A1). PMRA follows established service standards, or defined timelines, for these evaluations as outlined in the Management of Submissions Policy (Regulatory Directive DIR2017-01).
The PMRA continues to work to meet the review timelines across all submission categories, however, the additional challenges presented due to the COVID-19 pandemic did impact the review timelines for certain submission categories (Appendix, Figure A1).
The number and type of submissions reviewed by PMRA can vary significantly by year, as shown in Appendix, Figure A2.
New active ingredients and products registered in 2020–2021
In 2020–2021, 10 new active ingredients (the substance with the pesticidal effect) were registered for use in Canada, resulting in the registration of 13 new related end-use products (different formulations of products containing the active ingredient). Of the 10 new active ingredients, six were biopesticides (derived from natural sources such as bacteria, fungi, viruses, plants, animals and minerals) and four were conventional chemical pesticides. Please see Appendix, Table A2 for a full list of new active ingredients registered.
A total of 278 new end-use products were registered, containing new and existing registered active ingredients. Some examples of end-use products registered in 2020–2021 include:
- products to protect field food crops and greenhouse crops
- a product to control wireworm and rootworm in certain crops
- a biopesticide seed treatment to protect corn and soybean against parasitic nematodes
- biopesticides to protect greenhouse and field crops
- a non-conventional product to suppress varroa mites in honeybee hives
In the last decade, the total number of registered products increased from approximately 6000 to more than 7600, even though a number of products were removed from the market, either at the manufacturer's request or as a result of re-evaluation decisions.
PMRA met its performance targets on some pre-market evaluations (e.g., Category Cs) but missed performance on other categories of submissions due to an increasingly complex workload and COVID-19 impacts on PMRA operations. Reviews of some Category A applications were delayed due to the deployment of resources to assist with COVID-19 support as well as the prioritization of the publication of COVID-19 related materials, which delayed the publication of consultation and final decision documents.
PMRA also responded to a high number of requests for pre-submission consultations or Subject to Regulation enquiries, including those for application of pesticides and/or devices that were proposed to control, reduce, destroy or inactivate bacteria, viruses or other pathogens.
As PMRA adjusted to working in a virtual environment, digital processes were implemented and electronic tools employed to ensure seamless workflow. In addition, to further assist in improving the quality of information submitted with applications, PMRA published updated data-code (DACO) guidance as well as two videos to guide registrants on the pre-market registration process and on filling a product specification form: the Pest Management Regulatory Agency registration toolkit and the Pest Management Regulatory Agency Statement of Product Specification Form.
Joint reviews
Joint reviews are pesticide assessments conducted in cooperation with other jurisdictions. In the last two decades, Canada has progressed from developing pilot pesticide joint review approaches with the United States, to conducting joint reviews as a primary course of business for pre-market reviews. Registrants must apply to register their product in each participating jurisdiction at the same time for a joint review to be conducted.
In 2020–2021, of the 10 active ingredients registered, three were joint reviews. PMRA is continuing to pilot new joint review approaches with the United States Environmental Protection Agency to increase efficiencies of the review process. The pilot approaches have been shared with international partners with the aim of increasing international interest in joint reviews, potentially leading to more global joint reviews in the future.
Generic registrations
When a new pesticide is developed, the innovator invests substantial funds into the studies required to show that the product works as intended, has value, and poses no unacceptable health and environmental risks. The data supporting an innovation to Canada (i.e., a new active ingredient) receives exclusive use protection for a period of time, to prevent it from being used for the benefit of a competitor without the innovator's approval. Data subsequently used to amend or maintain a registration or register a new product are given compensable protection.
This practice allows the innovator the opportunity to recover their investment, but also encourages further innovation by allowing competition on the market after a period of time. Allowing timely introduction of equivalent products by generic manufacturers following the exclusive period can enhance market competition to the benefit of users, including growers. These regulations are important to innovators, generic companies and to growers.
In 2020–2021, the PMRA received 279 applications to register generic products. The number of generic applications received continues to remain high. There were 101 generic products (52 technical and 49 end-use products) registered in 2020–2021.
Minor uses
The term 'minor use' describes a potential use for a pest control product whose anticipated volume of sales is not sufficient to persuade a manufacturer to register and sell the product in Canada. The definition emphasizes that it is the projected sales of the pest control product that is minor and not necessarily the size of the crop. A minor use may be registered on a major crop because the use may be needed only occasionally or is limited to a small percentage of the total area of the crop.
To help resolve these pesticide access issues for Canadian growers, PMRA works with Agriculture and Agri-Food Canada's Pest Management Centre to support growers and grower associations in identifying priorities for new minor use registrations in Canada. PMRA also works directly with the provinces to assist in addressing regional minor use needs.
In 2020–2021, PMRA reviewed minor use submissions from Agriculture and Agri-Food Canada and the provinces, and made 100 regulatory decisions, of which twenty were joint reviews or workshares with the United States Environmental Protection Agency. Final label reviews resulted in the registration of 477 new minor uses.
Emergency registrations
A pest control product can be registered for up to one year for the emergency control of seriously detrimental pest infestations, for example, following the introduction of an invasive species. The product must have acceptable value and the human health and environmental risks must be acceptable. Emergency registrations are sponsored by the provincial ministry or federal agency that supports the management of the pest problem.
The number of emergency registration submissions that the PMRA receives can vary each year, depending on pest outbreaks, environmental conditions, and the availability of alternative products and control methods. In 2020–2021, the PMRA granted 12 emergency registrations.
Maximum residue limits (MRLs)
A maximum residue limit (MRL) is the maximum amount of residue that is expected to remain on food products when a pesticide is used according to label directions. These are set at levels well below the amount that could pose a health concern, and are established for each combination of pesticide and treated food crops.
Health Canada sets science-based MRLs to ensure the food Canadians eat is safe. As of December 2020, Canada had approximately 24,000 pesticide MRLs set (Figure 1). Typically, an MRL applies to the identified raw agricultural food commodity as well as to any processed food product derived from these raw commodities. If it is determined that an unacceptable risk exists based upon how the pesticide is intended to be used, the pesticide will not be permitted for sale or use in Canada.
The Canadian Food Inspection Agency (CFIA) is responsible for monitoring MRL compliance in foods in the Canadian marketplace. In two of their most recent reports from 2018–2019 surveys, the overall compliance rates for pesticide MRLs were 100% in products sampled for the Children's Food Project, and 99.3% of samples in CFIA's Pesticides and Metals in Selected Foods. The compliance rate from previous reports along with these recent surveys continues to indicate that the vast majority of food on the market meets Canadian pesticide standards.
Differences in MRLs between countries can lead to trade barriers. If an importing country's MRL for a given commodity is set lower than Canada's, this can lead to the importing country refusing entry to the Canadian commodity, despite the fact that the difference does not reflect a health risk.
International differences in MRLs can occur as a result of differences in both use patterns and data available to regulators at the time of MRL establishment, as well as other factors. Aligning MRLs globally has become increasingly important to reduce barriers to the movement of treated agricultural food products around the world. Domestic and international collaboration is critical in resolving these issues.
PMRA continued work with its international partners under the Canada-United States-Mexico Agreement, or CUSMA, the Organisation for Economic Co-operation and Development (OECD) and Codex Alimentarius Commission, on science policies relevant to establishing MRLs internationally.
PMRA is also undertaking an import MRL pilot project, which is based on a similar pilot project recently conducted by the United States Environmental Protection Agency's Office of Pesticide Programs. The objective of this project is to explore the feasibility of specifying import MRLs using only foreign country reviews, if available. Preference is given to reviews prepared by the Joint Food and Agriculture Organization/World Health Organization Meeting on Pesticide Residues (Joint Meeting on Pesticide Residues), in which Canada actively participates, along with the European Food Safety Association, the United States and other OECD countries such as Australia and New Zealand.
The absence of an MRL for a particular pesticide-crop combination in an export market (sometimes called a "missing MRL"), or MRL differences can also be a challenge for agricultural exporters. PMRA supports Agriculture and Agri-Food Canada in efforts to address this challenge.
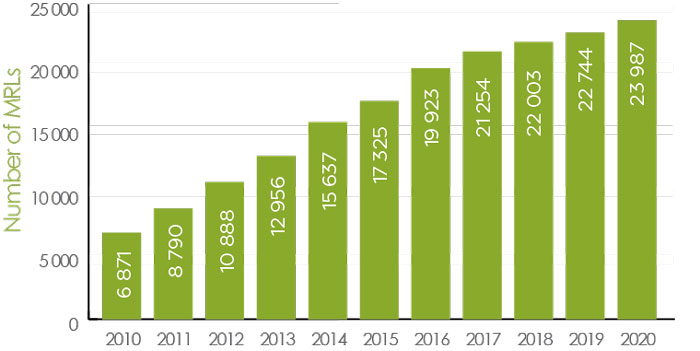
Figure 1 - Text Description
| Year | Number of MRLs |
|---|---|
| 2010 | 6871 |
| 2011 | 8790 |
| 2012 | 10888 |
| 2013 | 12956 |
| 2014 | 15637 |
| 2015 | 17325 |
| 2016 | 19923 |
| 2017 | 21254 |
| 2018 | 22003 |
| 2019 | 22744 |
| 2020 | 23987 |
Regulation of pesticides on the market
Once a pesticide has been registered, it becomes subject to a system of post-market risk management controls under the Pest Control Products Act. This includes re-evaluations and special reviews, compliance and enforcement activities, and reporting of health and environmental incidents.
Over the 10-year period between 2010 and 2020, the number of registered pest control products increased from approximately 6000 to over 7600. This represents an increase in post-market activity workload.
Re-evaluation and special review programs
Under the Pest Control Products Act, registered pesticides currently available on the market are subject to re-evaluations, which are initiated on a 15-year cycle based on the most recent major decision affecting the registration, including its initial registration. Pesticides registered after 1995 are referred to in the re-evaluation context as 'cyclical pesticides'.
Pesticides registered prior to 1995 are referred to as 'older pesticides', and when the re-evaluation program was established, there were 401 of these older pesticides.
As of March 31, 2021, 395 of the original 401 were completed. The re-evaluation of older pesticides was scheduled to be completed by the end of 2020; however, delays due to the COVID-19 pandemic affected the timing of some final decisions.
Extensions of consultation periods for certain post-market consultations were granted to provide adequate opportunities for stakeholders impacted by the COVID-19 pandemic to provide comments. These extensions further delayed completion of the remaining older pesticides. Furthermore, these remaining older pesticides are complex re-evaluations based on their large use patterns, and require large volumes of scientific data, and in some cases data that may be complex to generate.
Under the re-evaluation program, new methodologies, data, and scientific approaches are incorporated into the assessments to ensure that registered pesticides continue to meet modern standards for health and environmental protection, and have value.
Special reviews are another mechanism used under the Pest Control Products Act to determine the continued acceptability of registered pesticides. Unlike a re-evaluation, the intent of a special review is to address the specifically identified aspect(s) of concern, and may be triggered when:
- there are reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable; or
- an OECD member country prohibits all uses of an active ingredient for health or environmental reasons.
In 2019, the Pest Control Products Act was amended to clarify that an identified aspect(s) of concern that would otherwise prompt a new special review can also be addressed through an ongoing re-evaluation or special review, reducing the need to duplicate work that is already being done.
Five-year re-evaluation and special review work plan
As part of its commitment to improve transparency, PMRA is publishing interim updates to its five-year Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2020–2025 (Re-evaluation Note REV2020-01 and later). This work plan includes the target timelines to publish proposed and final decisions for ongoing re-evaluations and special reviews, as well as the list of anticipated re-evaluation initiations in the next five years.
In 2020–2021, PMRA made good progress on the re-evaluation of older pesticides, supported by additional temporary resources. Completing these remaining large and complex re-evaluations will continue to be a priority, acknowledging that workload continues to increase as new re-evaluations and special reviews are initiated every year. As of March 31, 2021, 130 re-evaluations and special reviews are underway with a requirement to initiate 49 new re-evaluations later in 2021. As PMRA focused its resources in completing older complex pesticide re-evaluations and special reviews, progress on the review of cyclicals re-evaluations was impacted.
Over the past five years, PMRA has completed an average of 27 final decisions per year for re-evaluations and special reviews. Though this is an improvement over previous years, workload continues to increase as new re-evaluations and special reviews are initiated. Based on the projected number of re-evaluation initiations for the next five years, along with the average number of final decisions made per year, work on hand will continue to grow. The Program Renewal section in this Annual Report describes how PMRA is working to address this challenge.
Outreach and stakeholder engagement in re-evaluation and special review programs
PMRA has increased outreach efforts with global regulators such as the United States Environmental Protection Agency, the Australian Pesticides and Veterinary Medicines Authority and the European Food Safety Authority, to build awareness and potential opportunities for post-market collaboration.
PMRA continued its work to provide stakeholders with an opportunity for improved collaboration during re-evaluations. The agriculture stakeholder engagement unit continues to promote understanding of PMRA's re-evaluation process and risk assessments. To this end, the unit has been giving presentations to stakeholders, responding to information requests and hosting stakeholder webinars related to specific re-evaluation decisions. In addition, work has begun on exploring options to obtain pesticide use information with a goal of collecting the information needed before a re-evaluation begins.
Pest control product sales information reporting
Since 2007, PMRA's Pest Control Product Sales Information Reporting Program has been collecting sales information, in the form of total quantity (by volume or mass), for all registered products available for sale. These data are reported by calendar year (January 1 to December 31). The purpose of the program is to collect sales data to be used by PMRA to better understand pesticide use in Canada.
Sales data are considered in risk assessments of pesticides, in policy decisions, in identifying trends in pesticide use, and in providing guidance for risk-reduction strategies.
For example, sales data are used in the re-evaluation of pesticides to help understand the presence and scale of use of the pesticide in the Canadian marketplace, as well as the potential impacts if changes are made to the registration status of the pesticide. Sales data are also used to inform the Pesticide Incident Reporting Program on the market share of particular pesticides to help identify potential risks that may require attention.
Note that due to differences between fiscal year and calendar year reporting, as well as the time required to collect and verify sales information, the most recent sales reports are not for the same year as the current annual report.
In 2020–2021, PMRA published the annual sales report for the 2018 calendar year.
Sales of pest control products in Canada have increased from 101.1 million kg of active ingredients (kg a.i.) in 2014 to 121.3 million kg a.i. in 2018 (Figure 2).
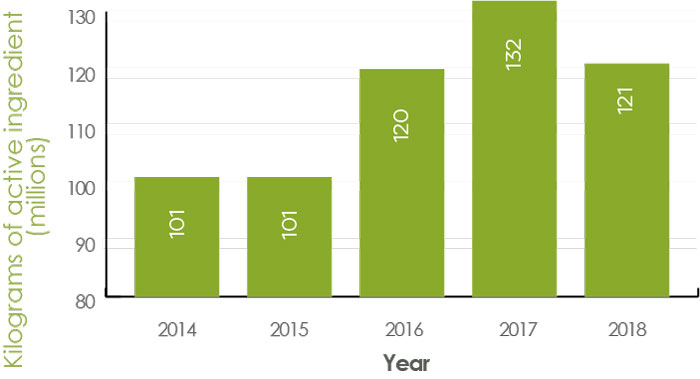
Figure 2 - Text Description
| Year | Kilograms of active ingredients |
|---|---|
| 2014 | 101.1 million |
| 2015 | 101.4 million |
| 2016 | 120.1 million |
| 2017 | 132.1 million |
| 2018 | 121.3 million |
In 2018, 71.1% of pesticide sales in Canada were agricultural sector products (Figure 3), whereas 24.3% were non-agricultural sector products, and 4.5% were domestic sector products.
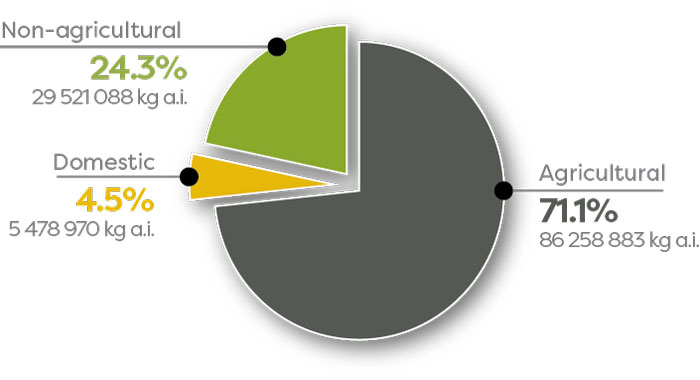
Figure 3 - Text Description
| Sector | Kilograms of active ingredients |
|---|---|
| Agricultural | 86 258 883 (71.1%) |
| Non-agricultural | 29 521 088 (24.3%) |
| Domestic | 5 478 970 (4.5%) |
Glyphosate remained the top active ingredient sold in Canada in 2018 (Table 1). Six of the top 10 active ingredients sold in 2018 had been among the top 10 selling active ingredients since 2014. These top 10 active ingredients accounted for 68.7% of all pesticides sold in Canada in 2018.
| Active ingredient | Product type |
|---|---|
| Glyphosate | Herbicide |
| Available chlorine, present as sodium hypochlorite | Antimicrobial |
| Creosote | Antimicrobial |
| Prothioconazole | Fungicide |
| Glufosinate ammonium | Herbicide |
| Bromoxynil | Herbicide |
| MCPA | Herbicide |
| Surfactant blend | Other |
| Borates | Insecticides/Fungicides/Antimicrobial |
| 2,4-D | Herbicide |
Incident reporting
A pesticide incident is a negative effect (adverse reaction) on humans, animals (pets or livestock) or the environment (plants or wildlife) that can result from exposure to a pesticide. Pesticide registrants are required by law to report all incidents related to their products to Health Canada. Canadians may also report pesticide incidents to registrants, or directly to Health Canada's PMRA via the Public Engagement Portal Voluntary Incident Reporting Form.
PMRA uses incident reports to identify and characterize potential risks to humans, domestic animals and the environment from the use of pesticides, which were not evident during the initial registration of a pesticide.
Incident report assessments are prioritized based on the type of incident. Serious adverse effects such as death or life-threatening effects are evaluated immediately and mitigation measures are put into place, if warranted. If a potential risk is identified, it is investigated and protective action may be taken, such as changes to how a pesticide may be manufactured, packaged, labelled, or used.
Incident reports also inform risk assessments for new registrations and re-evaluations. New scientific studies must also be submitted as an incident report to PMRA by registrants of a registered pesticide if the study demonstrates any new hazard, any risk that may be greater than the risk determined at the time of registration, or the presence of a previously undetected component or derivative of a pest control product.
Monitoring incidents for unanticipated effects is an ongoing process that includes re-assessing previous conclusions, as necessary. In cases where mitigation strategies have been adopted, PMRA also monitors incident reports to determine if the actions were effective in managing the identified risk.
In the 2020–2021 fiscal year, 1437 pesticide incident reports and 52 scientific studies were submitted to PMRA. Details of these reports can be found through the Pesticide Incident Reporting Database, by visiting Canada.ca/pesticides and selecting the link for the "Pesticide product information database".
Below is an overview of the incidents reported in 2020–2021:
- Domestic animal incidents were reported most frequently, followed by human and packaging failure incidents.
- The majority of reported domestic animal incidents involved spot-on pesticides used for flea, tick and mosquito control, and the reported health effects were mostly minor in nature.
- 1083 incidents occurred in Canada and 354 incidents relevant to Canadian products occurred in the United States.
- Overall, Canadian incidents involved approximately 180 different pesticide products.
- The majority of products in reported incidents were domestic class pesticides, followed by commercial class pesticides.
- Only a very small number of products classified as restricted class or manufacturing class were reported in incidents.
To report a pesticide incident, visit Canada.ca/pesticides and select the link for "Report a pesticide incident".
Health Canada's compliance and enforcement activities on pesticides
Health Canada's Pesticide Compliance Program is responsible for promoting, monitoring and enforcing compliance with the Pest Control Products Act and Regulations. The primary objective of this legislation is to prevent unacceptable risks to the health and safety of Canadians and the environment from the use of pest control products. Compliance and enforcement functions and accountability are managed by Health Canada's Regulatory Operations and Enforcement Branch.
The Pesticide Compliance Program has oversight on all parties regulated by the Pest Control Products Act, including pesticide registrants, manufacturers, importers, retailers, and users, and conducts a variety of compliance verification and compliance promotion activities within all sectors. Compliance verification includes carrying out inspections and collection of samples to assess compliance. When required, enforcement action is taken against regulated parties to address non-compliance with the Pest Control Products Act and Regulations.
Health Canada uses a range of enforcement tools including warning letters, compliance orders, notices of violation with warning or monetary penalty, prosecution, seizure, and partnering with the Canada Border Services Agency to refuse entry of unregistered pesticides into Canada.
Compliance promotion includes presentations, exhibits at trade shows, written articles, and the development and distribution of publications, such as fact sheets and information packages. These activities increase the reach of the Pesticide Compliance Program and support overall levels of compliance by providing important information to regulated parties to foster compliance with the Pest Control Products Act and Regulations.
The delivery of compliance activities is prioritized based on risk. Criteria used in the selection of priority areas for compliance activities includes potential risks to human health and the environment, compliance history, and outcomes of PMRA re-evaluation decisions. Considerations to assess risk include observations from the field, information from PMRA and provincial regulators, and data analysis.
2020–2021 Summary of compliance and enforcement activities
As a result of the public health restrictions associated to COVID-19, the program's focus shifted to off-site compliance verification and promotion activities where possible. A total of 609 compliance verifications were conducted as a result of both planned and reactive activities (such as complaints), as well as 604 admissibility recommendations (verifications of acceptability for importation) to the Canada Border Services Agency, for a total of 1213 compliance verifications.
A total of 1336 enforcement actions addressing single or multiple violations were issued to non-compliant parties, including:
- 796 warning letters
- 16 compliance orders
- refusal of entry into Canada for 500 importations containing unauthorized products, in partnership with the Canada Border Services Agency
- 12 seizures of non-compliant pest control products
- 12 administrative monetary penalties under the Agriculture and Agri-Food Administrative Monetary Penalties Act, for a total value of $100 000 in penalties
The most common violation types noted were importation of unregistered products (29%), sale of unregistered products (20%) and advertising of pest control products in a way that is contrary to the Pest Control Products Act (16%).
Furthermore, 142 compliance promotion activities were conducted, including mail outs and virtual events.
Keeping pace with change
Globalization, rapid technological advances, evolving science, economic pressures and various other challenges and opportunities require a pesticide regulatory system that is flexible and responsive to change. PMRA is continuously modernizing risk assessment and risk management approaches, refining business practices to help ensure the needs of all stakeholders are met, and responding to major scientific and environmental developments.
Program renewal
In 2018, PMRA launched a two-year project to explore options to create a more sustainable program delivery model and enhance health and environmental protection. A new program model for an integrated approach to pesticide reviews was developed. While current pre-and post-market processes tend to operate distinctly, PMRA's Integrated Approach proposes a more holistic approach that involves continuous risk evaluation throughout a pesticide's regulatory life cycle.
While initially the project centered on the re-evaluation program, as the analysis continued, it became apparent that PMRA needed to broaden its scope and embrace changes across the Agency to achieve sustainable change. In addition to advancing the new Integrated Approach, PMRA is pursuing broader, program-wide changes to provide the tools and processes required to move PMRA towards becoming a more agile, modern and efficient organization (Figure 4). To support this broader scope of change, the Office of Program Renewal (OPR) was established to lead PMRA through the program transformation.
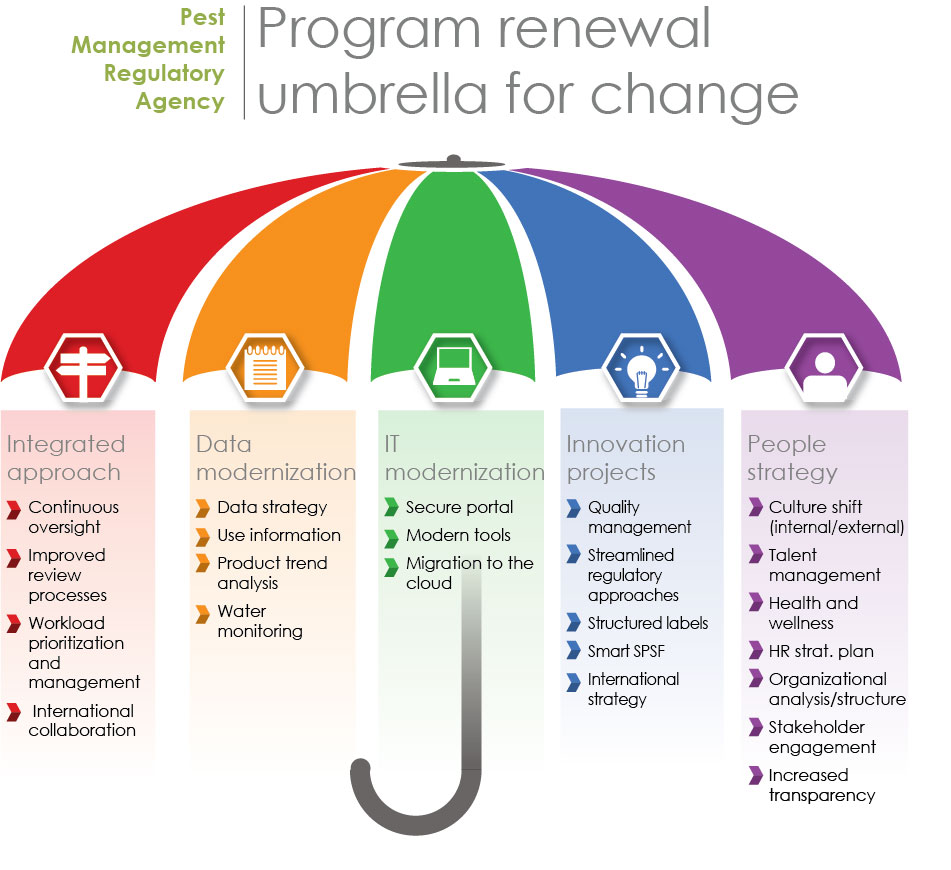
Figure 4 - Text Description
Pest Management Regulatory Agency - Program renewal umbrella for change
| Theme | Activities |
|---|---|
| Integrated approach |
|
| Data modernization |
|
| IT modernization |
|
| Innovation projects |
|
| People strategy |
|
PMRA has engaged with stakeholders from across Canada representing 141 associations and organizations through 24 sessions. Through these discussions, PMRA sought feedback from stakeholders on the Integrated Approach. This feedback helped further the development and design of the new regulatory model. The results of the external consultations on the Integrated Approach were published in the external What Was Heard report. The full report is available by request on the Pesticides section of the Canada.ca website.
In 2020–2021, PMRA began implementing new workload and management systems to focus resources on timely decision making for priority post-market reviews. Additionally, PMRA began developing new streamlined approaches to address lower priority pesticides that do not merit a comprehensive post-market evaluation, for example where existing assessments are up-to-date and no further risk management is required. The PMRA also advanced pilots on a new continuous lifecycle approach, which will allow PMRA to identify and address risks sooner by capturing key findings and data needs throughout the full regulatory lifecycle of an active ingredient.
As part of the broader scope of change under Program Renewal, the PMRA also began refining its data strategy to align with that of Health Canada. An evaluation of PMRA data requirements and its linkages to specific Program Renewal projects has been initiated. The refined data strategy will further support Program Renewal and inform both information management and information technology solutions.
In 2020–2021, the PMRA also conducted some additional preliminary stakeholder engagement on certain specific components of program renewal, with further detailed engagement planned for the fall of 2021. Currently, PMRA is examining how to incorporate data requirements and timing into a new regulatory request format that will align with the continuous lifecycle approach. The PMRA's goal will be to ensure that it obtains the right data at the right time from the right sources, in support of timely and informed decision-making.
Evaluating new technologies
In addition to assessing the potential health and environmental risks of chemical and biological pesticides, PMRA scientists monitor new developments such as machine learning, robotics and drones. While these technologies may have many benefits (for example, for precision agriculture), their potentially unique health and environmental risks must be identified and assessed carefully. PMRA is working with manufacturers, other regulatory authorities globally, and international organizations such as the OECD and the World Health Organization Chemical Risk Assessment Network, to understand and assess new technologies and equipment that support modern agricultural practices.
PMRA also seeks opportunities to reduce the need for animal testing wherever possible, while continuing to ensure scientifically robust approaches are in place for assessing risk. Integrated approaches to testing and assessment (IATA) or new approach methodologies (NAM) involve using data from existing laboratory animal studies, in vitro high-throughput screening assays, predictive models, mechanistic studies and other data in order to refine, reduce and in some cases even replace laboratory animal studies for human health and environmental assessment of pesticides.
PMRA collaborates with North American and OECD partners, and through other multi-stakeholder initiatives that are examining new approaches to current study types. This includes alternative approaches to acute studies, such as defined approaches for skin sensitization, the ongoing work on incorporating non-animal assays for the developmental neurotoxicity study, and availability of internationally developed tools such as Risk21.
PMRA's multi-stakeholder collaboration on dermal absorption was acknowledged in a peer-reviewed journal published in March 2021. This is another example of where the availability of robust data (triple-pack protocol) has allowed PMRA to transition to use of non-animal assays, when applicable.
The PMRA is also a member of the Health and Environmental Sciences Institute (HESI) Transforming the Evaluation of Agrochemicals (TEA) Committee, which is developing a fit-for-purpose framework for the safety evaluation (health and environment) for agrochemicals. The timing of the TEA project is also relevant to PMRA given the ongoing work related to program renewal, as noted above.
PMRA also continues to collaborate with a Department-wide community of experts on science-policy initiatives to explore further incorporation of race and gender considerations in exposure scenarios and risk assessments, as well as in the evaluation of incident report data.
Remotely piloted aircraft systems (drones) for pesticide application
PMRA continues to receive a number of inquiries related to the application of pesticides by remotely piloted aircraft systems (RPAS, or drones). Currently there are no registered uses of RPAS for pesticide application on Canadian labels. However, the PMRA has approved a limited number of Research Authorizations in support of data generation for RPAS regulatory applications. Parties interested in adding the use of RPAS are encouraged to work with registrants using the PMRA pre-submission process to determine the kinds of data necessary to assess this new application technology.
In 2020–2021, the PMRA continued to work closely with two international working groups to coordinate information sharing on the health and environmental safety of this new application method, in support of regulatory reviews:
- The OECD Working Group on Pesticides Drone Subgroup – The PMRA assisted with coordinating a critical review of available global research related to environmental exposure (e.g., spray deposition and drift), human health exposure (e.g., operator/bystander exposure and crop residues) and product efficacy (e.g., comparison to traditional application equipment). A final report is being prepared for the 2021 OECD Working Party on Pesticides.
- The North American Remotely Piloted Aerial Application Systems Working Group – PMRA continues to engage with the working group on developments in RPAS research.
Also, the PMRA participated in the 2020 Center of Excellence for Regulatory Science in Agriculture (CERSA) Workshop on Advances in Regulatory Risk Assessment of Pesticide Drift from Unmanned Application Systems (UAS) and Manned Aerial Application, hosted by the North Carolina State University. A final report can be found on the CERSA UAS/Spray Drift Virtual Workshop webpage (https://cersauas.wordpress.ncsu.edu/files/2021/05/CERSA-UAS-Spray-Drift-Workshop-Final-Report.pdf).
PMRA continues to move forward, in conjunction with international regulatory partners, on identifying regulatory data needs for this rapidly emerging spray technology.
Water monitoring
Canada has significant freshwater resources and the Government of Canada is committed to keeping our water safe, clean and well-managed. The PMRA works to minimize harmful pesticides from entering water in multiple ways such as restricting where a pesticide can be used, reducing application rates, requiring buffer zones to prevent pesticides in spray drift from entering water, or requiring vegetative filter strips to prevent pesticides running off fields into water during rainstorms.
Despite these measures, pesticides may sometimes enter water. In order to protect human health and the environment, the PMRA determines if there are any risks from pesticides that may be found in water. This includes potential risks to health from drinking water sources (groundwater and surface water) as well as risks to aquatic organisms from pesticides that may be present in lakes, rivers and streams.
For some pesticides, the Agency receives sufficient water monitoring data from other federal departments, provinces, municipalities, and research scientists, or other organizations to enable the PMRA's scientists to estimate exposure to pesticides that can then be incorporated into regulatory decisions. In the absence of such robust data, the Agency's scientists rely on mathematical models to estimate the amount of pesticides in water.
Over the past year, the Agency continued to refine water-related tools. This included updating a list of pesticides where water-monitoring data should be prioritized based on factors such as the likelihood of a pesticide to move to water bodies, ecotoxicity endpoints and the use of the pesticides. The re-evaluation schedule is also considered alongside the list of prioritized pesticides. The aquatic life reference values (ALRVs) were updated to include newly registered pesticides and changes resulting from re-evaluation. The ALRVs are available from the Agency on request. In addition, the Agency enhanced its water monitoring database of over 2 million data points by including new data and performing a quality control check of entries.
Vegetative filter strips
PMRA continued to examine the use of vegetative filter strips (VFSs) as a risk mitigation tool. A VFS is a permanent strip of dense, perennial vegetation situated on the downslope border of the treated area (such as an agricultural field, plantation or woodlot), along the edge of the water body into which the area drains. The vegetation within a VFS contains grasses, but may also contain other vegetation, such as shrubs and trees. The VFS reduces the velocity of water runoff to allow soil and pollutants, such as pesticides, to settle out before entering the water.
The PMRA is working to incorporate VFS computer modelling into environmental risk assessments using the Vegetative Filter Strip Modeling System (VFSMOD). Modelling will help identify products for which a mandatory 10-metre VFS will reduce environmental risks. Integration of the computer model VFSMOD into PMRA's water modelling framework may also allow exploration of the effectiveness of VFSs for more water soluble pesticides, without the need for field trials. As part of an international Working Group on Vegetative Filter Strips, the PMRA is working with partners within Canada and the Americas to standardize an approach to modelling VFSs.
Future work includes investigating ways to incorporate site-specific considerations (such as slope) to determine the most optimal width for a VFS.
International scientific and regulatory cooperation
Canada's internationally respected regulatory model has allowed Canada to form strong partnerships with other regulators, and to play a significant role in developing collaborative approaches to joint pesticide reviews, promoting international regulatory alignment, and addressing barriers to agricultural innovation and trade. These activities also involve bilateral information exchange to promote regulatory capacity building amongst other national and international pesticide regulatory authorities, thereby enhancing pesticide safety beyond our borders. In 2020-2021, technical meetings were held virtually.
Stockholm Convention
The Stockholm Convention is a legally binding international treaty with a focus on the elimination or restriction of the production and use of persistent organic pollutants (POPs). PMRA is the responsible federal authority for meeting the obligations and for ongoing participation at the Stockholm Convention as it pertains to pesticides.
PMRA collaborates with other federal partners by providing scientific experts to work with the Persistent Organic Pollutants Review Committee (POPRC) and the Conference of the Parties (COP) of the Stockholm Convention, and in the development of Canadian positions and submissions.
- At POPRC, PMRA actively participates in the review of the scientific justification for identifying substances as POPs and making recommendations on how these substances can be managed globally.
- At the COP meetings, PMRA provides experts to negotiate international decisions on restrictions working toward the elimination of each POP at the global level.
In 2020–2021, the POPRC adopted the risk profile for the insecticide methoxychlor (not a registered pesticide in Canada), and as a result of its long-range environmental transport and its potential for significant adverse human health and environmental effects, it was decided that global action is warranted, thereby advancing it to the evaluation stage.
Rotterdam Convention
The Rotterdam Convention promotes information exchange and informed consent in the international trade of chemicals, with the aim of protecting human health and the environment. The Convention is a multilateral treaty to promote shared responsibilities in relation to importation of hazardous chemicals.
The Convention calls on exporters of hazardous chemicals to use proper labelling, include directions on safe handling, and inform purchasers of any known restrictions or bans.
PMRA collaborates with other federal partners by providing scientific experts to work with the Chemical Review Committee (CRC) and the COP of the Rotterdam Convention, and in the development of Canadian positions and submissions.
For the CRC, PMRA actively reviews submissions to the Rotterdam Convention against established Convention criteria and participated in the Sixteenth Meeting of the CRC. At the COP meetings, PMRA provides experts to negotiate international decisions for each substance at the global level.
This year's meetings of the COPs for both the Rotterdam and Stockholm conventions were delayed due to the pandemic. They will be replaced by virtual meetings with limited agendas in 2021 and in-person meetings in 2022 to allow for negotiations on substantive items.
Organisation for Economic Co-operation and Development
PMRA is involved with several OECD initiatives, including various OECD task forces and expert group projects. PMRA routinely participates in meetings of both the OECD Working Party on Pesticides (WPP) as well as the OECD Working Group on Biocides. Both working groups function as vehicles for global cooperation, information exchange and alignment of approaches with respect to pesticides assessment.
PMRA also contributes input (via the Canadian Delegation) to the OECD Joint Meeting of the Chemicals and Biotechnology Committee (CBC) as required. For example, PMRA contributed to the development of the "OECD Possible Elements for an Updated Council Act and Best Practice Guide on Intellectual Property Rights Related to Chemical Safety Data" led by the CBC. After three years of participation in the ad hoc group, the draft revised Recommendation is expected to be endorsed by the CBC in spring / summer 2021 for final approval by the OECD Council. PMRA routinely provides experts to participate in the OECD WPP Expert Groups on Residue Chemistry, Pollinator Safety, Bio-pesticides, and Electronic Exchange of Pesticide Data.
Some examples of OECD WPP initiatives include:
- development of a common approach to regulating novel pest control products, such as RNAi-pesticides and new approach methods
- implementation of technical guidelines (for example, those that provide guidance on alternative approaches to animal testing)
- identification of residues, metabolites and degradation products
- development of a guidance document for regulating bacteriophages
- ongoing dialogue related to pollinator protection
- aligning risk assessment of new digital and mechanical technologies for applying pesticides such as innovative drone technology
PMRA also plays a lead role on the OECD WPP e-label project to identify commonalities in pesticide labels that would support development of e-label solutions. Furthermore, PMRA actively contributes to the Expert Group on Claims Development for Treated Articles.
In support of the OECD WPP's objectives, PMRA has led discussions with global manufacturers of pesticides regarding new chemistries to broaden collaboration and promote global joint reviews and alignment between international regulatory partners. PMRA has also initiated discussion with OECD partners on post-market review challenges and the potential benefit of having a greater collaboration in this area.
Codex
PMRA plays an active role in the World Health Organization/Food and Agriculture Organization Codex Committee on Pesticide Residues, which is responsible for setting international food standards. Codex participation enables PMRA to:
- enhance Canada's influence on Codex deliberations and outcomes
- promote the development of science-based standards that will result in fair practices in food trade (for example, establishment of MRLs)
- promote more effective work-planning by the committee (help ensure priorities include Canadian stakeholders' interests)
- promote the timely development of standards (for example, continue to explore opportunities for parallel reviews with the Joint Meeting on Pesticide Residues)
This year's meeting of the CCPR52 was delayed due to the pandemic until July 26–30, 2021.
Regulatory updates
In 2020–2021, PMRA continued to take steps to modernize its legislative framework.
Due to the ongoing public health crisis and associated reprioritization of activities, certain regulatory proposals were delayed from their original anticipated delivery dates.
Formulants and contaminants of health and environmental concern
In June 2020, PMRA published an Order that amended the List of Pest Control Product Formulants and Contaminants of Health or Environmental Concern to:
- remove those formulants and contaminants that either are no longer found in a pest control product registered in Canada or are no longer considered to adversely affect human health or the environment due to specific or approved use patterns; and
- add those formulants and contaminants that either are known to cause anaphylactic-type reactions or are designated as being of health or environmental concern under relevant domestic or international agreements.
The Order also clarified that the List only pertains to formulants and contaminants that are in pest control products registered in Canada.
Amendments to certain spa chemicals
In December 2020, PMRA amended the Pest Control Products Regulations to remove sodium bromide at a 35% concentration and potassium monopersulfate at a 32% concentration from the list of spa products in Schedule 2 of the Regulations that, subject to certain conditions, are exempt from registration. The amendment provided regulatory clarity and reflected the outcome of a past re-evaluation decision, which found that the health risks associated with the use of those chemicals in spa products were not acceptable.
Miscellaneous amendments to the Pest Control Products Regulations
In March 2021, the Pest Control Products Regulations were amended as part of Health Canada's miscellaneous amendments regulations process. The following changes addressed a number of minor issues in regulations identified through Health Canada's good regulatory stewardship practices:
- correcting a cross-reference to the Food and Drugs Act in subsection 24(1) of the Pest Control Products Regulations
- addressing a typographical error in the name of the standard referenced in the definition of "common chemical name"
- clarifying paragraph 64(b) of the Pest Control Products Regulations, which prescribes the conditions for import for research purposes
Targeted regulatory reviews
The Government of Canada announced in Budget 2018 that it would fund, over three years, "targeted reviews of regulatory requirements and practices that are bottlenecks to economic growth and innovation."
Agri-food and aquaculture sector
As part of this initiative, in 2018, PMRA participated in the targeted regulatory review of the agri-food and aquaculture sector. A central feature of the review was to invite input from businesses, Canadians, academia and other stakeholders, on ways to make regulations more agile, transparent, and responsive.
In 2020–2021, PMRA continued work on other regulatory modernization initiatives on the Roadmap, including those related to the post-market review process, labelling, data protection, and the authorization of pesticides not requiring registration.
Also in 2020–2021, as part of the implementation the Agri-food and Aquaculture Regulatory Review Roadmap, PMRA continued work on proposed statutory changes to:
- broaden the Minister's powers to make risk-based authorizations and exercise appropriate post-market oversight over authorized products; and,
- broaden the Minister's power to amend pest control product labels without an application in certain situations (for example, to clarify wording of an existing health or environmental protection requirement).
International standards, digitalization and technology neutrality, and clean technology
In 2019, the Government of Canada announced the next round of targeted regulatory reviews that included a review of international standards, digitalization and technology neutrality, and clean technology. In 2020–2021, PMRA participated in the process that would identify projects to be included in the roadmaps for these targeted regulatory reviews.
Parliamentary review of the Pest Control Products Act
The Pest Control Products Act must be reviewed every seven years by Parliamentary Committee.
The Act stood referred to Parliamentary Committee as of June 29, 2020, which signaled that the review process could be initiated. Due to other government priorities (related to COVID-19), the review process has not been initiated and a specific committee has not yet been identified.
In early 2020, the Pest Management Advisory Council indicated that the Pest Control Products Act is fit for purpose, with only minor amendments as outlined in the Agri-food and Aquaculture Roadmap being required.
Pest Control Products Regulations review
Prior to the launch of the TBS-led regulatory reviews, the departments and agencies responsible for regulating the agri-food and aquaculture sector, including PMRA, each had an ambitious regulatory modernization agenda that extended over several years.
In 2020–2021, PMRA continued its comprehensive review of the Pest Control Products Regulations, the first such review since they were established in 2006. The review is aimed at ensuring the regulations continue to meet program objectives (for example, of health and environmental protection) in an effective and efficient manner, while attempting to minimize regulatory burden on regulatory parties.
In 2020–2021, the review included public and industry engagement on certain aspects of the application and importation process for pest control products in Canada.
Regulatory guidance for sanitizers
Since the beginning of the pandemic, Health Canada's PMRA has faced a significant increase in requests for regulatory guidance from manufacturers, distributors and importers of sanitizers and similar type products (e.g., UV radiation-emitting devices, self-sanitizing coatings) who wish to bring their products to market in Canada. The PMRA has facilitated timely responses through regular collaboration between branches and international counterparts to ensure alignment of communications and consistency in regulatory requirements, where possible. By mid-year, the increased demand for regulatory guidance subsequently resulted in a five-fold increase in applications for registration for sanitizers and similar type products. The PMRA has communicated flexibilities to streamline applications for pest control products used to control or kill SARS-CoV-2 and requests for an expedited review are considered on a case-by-case basis, following confirmation that all required data and non-data elements have been submitted as part of the application to the PMRA.
Stakeholder relations and outreach communications
PMRA recognizes that the transparency and openness of its work is critical to strengthening trust in its regulatory decisions.
Outreach activities and public opinion research
Due to the pandemic having an impact on dissemination of outreach materials, both the production of new material and social media postings were temporarily reduced.
As one of the top active ingredients sold in Canada, a webpage on Glyphosate in Canada was published in August 2020, which summarizes information about the active, and provides links to further information, including its regulatory status in a number of other OECD countries.
Refinements to existing outreach materials have been underway, and work was begun on analysis of the results of the 2020 public opinion research study. This information assists us in our efforts on how best to inform Canadians about the regulatory system, which can include safe use information as well as product changes and recalls.
In July 2020, PMRA published a fact sheet on personal protective equipment (PPE) for anyone who works with pesticides with guidance information on how protect yourself when applying pesticides and includes tips for PPE use and care information.
Stakeholder engagement
In the interests of public safety due to the pandemic, the spring 2020 stakeholder information session was not held. Instead, previous attendees of this event received an email from the Executive Director on how the PMRA was managing operations as a result of COVID-19, with updates on pre- and post-market performance evaluation statistics.
On December 15, 2020, the PMRA hosted its first fully virtual session, which was well attended by 184 stakeholders from across the country. The webcast provided a diverse group of stakeholders with updates on pesticide regulation, as well as an opportunity to ask questions. Participant feedback following the event was positive and will help inform future similar events.
Due to the COVID-19 pandemic, the annual meetings of the Pest Management Advisory Council and of the Federal/Provincial/Territorial (FPT) Committee on Pest Management and Pesticides were delayed to April and June 2021, respectively. While some monthly FPT meetings had to be cancelled earlier in the pandemic, they resumed in virtual format later in the year.
Financial profile
| 2020-2021 funding and revenue (in millions of dollars) | Total |
|---|---|
| A-Base | 26.3 |
| Revenue – application fees $5.2 and annual charge $7.8 | 13.0 |
| Canadian Agricultural Partnership | 3.3 |
| Chemicals Management Plan | 4.9 |
| Departmental pressure funding | 2 |
| COVID-19 funding | 0.8 |
| Total PMRA fiscal year 2020–2021 | $50.3 |
- Financial profile includes employee benefit plan.
- A portion of revenues paid by regulated parties is allocated to support employee benefits plans (non-respendable revenue) and internal services. These amounts are not included in the $13 million reported above.
- Departmental pressure funding of $2 million and COVID-19 funding of $0.8 million was not included in PMRA main estimates. (Funding received as in-year funding.)
- PMRA received $3.3 million through the Canadian Agricultural Partnership initiative to support the registration of minor use products. As a result, newer, more environmentally sustainable, and more modern products have been made available to Canadian producers, which helps sustain Canada's competitive position globally.
- Through Canada's Chemicals Management Plan, PMRA received $5 million to re-evaluate older pesticides, improve risk management approaches through incident reporting and sales reporting regulations, and contribute to the development of scientific and regulatory approaches with other jurisdictions on high-priority issues. For more information, please consult the Chemicals Management Plan webpage.
Appendix
| Submission Category | Service Standard in Days |
|---|---|
| Category A New active ingredients or integrated system products, their related end-use products and manufacturing-use products; major new use of registered pest control products; maximum residue limits for an unregistered active ingredient; and user requested minor use registrations (URMUR). |
|
| Conventional chemicals and import MRLs for an unregistered active ingredient | 665 |
| Reduced risk, other biopesticides, non-conventionals, non-straight chain lepidopteran pheromone (NSCLP) | 555 |
| Microbials, and URMUR for all pesticide types (conventional chemical, reduced risk, microbial, other biopesticides, non-conventionals, NSCLP) | 470 |
| Straight chain lepidopteran pheromone (SCLP), including URMUR | 285 |
| Applications with atypical timelines (joint reviews, tailgaiters, renegotiated timelines, synchronized timelines, coordination with re-evaluation | Variable |
| Category B New pest control products containing registered active ingredients; an amendment to existing pest control products (for example, product chemistry, labelling); emergency registration; the addition of import MRLs for previously assessed active ingredients. |
|
| Conventional chemicals (including emergency use) and new import MRL for previously assessed active ingredient | 425 |
| Reduced risk, other biopesticides, non-conventionals, NSCLP (including emergency use) | 360 |
| Microbials and SCLP (including emergency use) | 240 |
| Streamlined applications (application rate changes, tank mixes, new pests, or changes to level of control) | 158 |
| Applications with atypical timelines (joint reviews, tailgaiters, renegotiated timelines, synchronized timelines, coordination with re-evaluation) | Variable |
| Category C Product registrations and amendments with no data requirements. These applications involve minor label or formulation reviews, such as product registration based on registered precedent products. |
|
| New/changes to product labels; addition of approved minor use; similar product | 240 |
| New/changes to technical grade active ingredient, integrated system product, manufacturing concentrate or end-use product chemistry; administrative changes; administrative re-instatement | 180 |
| Applications with atypical timelines (tailgaters, renegotiated/ synchronized timelines, coordination with re-evaluation) | Variable |
| Category D Submissions within particular programs. |
|
| Registration renewal | 255 |
| Registration/ amendment to registration of active ingredient to be used in pest control product manufactured for export only | 46 |
| Master copies | 42 |
| Private labels | 10 |
| Own use import equivalency and permitsTable 1 Footnote * | 70 (Equivalency) 30 (Permits) |
| Grower requested own use equivalency and permitsTable 1 Footnote * | TBD (Equivalency) 30 (Permits) |
| DiscontinuationsTable 1 Footnote * | 45 |
| Category E Authorizations and notifications for research in Canada. |
|
| Research authorization for new technical grade active ingredients | 159 |
| Research authorization for new uses of registered active ingredients | 69 |
| Research notification for research carried out in Canada | 30 |
| Category F Notification |
|
| Registration and amendments to registered pest control products via notification | 45 |
| Category L Submissions to register or amend products where the applicant wishes to use or rely upon data provided by another registrant. |
|
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (conventional chemical) | 425 |
| Equivalency and data compensation assessment of active ingredient, end-use product and manufacturing concentrate with no data (all product types) | 365 |
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (reduced risk, other biopesticide, non-conventional, NSCLP) | 360 |
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (microbial and SCLP) | 240 |
| Applications with atypical timelines (tailgaters, renegotiated/ synchronized timelines, coordination with re-evaluation) | Variable |
| Regulatory DecisionTable 1 Footnote * | 45 |
| Requests to extend the exclusive use protection period based upon minor usesTable 1 Footnote * | 240 |
| Category P Pre-submission Consultations |
|
| Pre-submission consultations excluding those for joint reviews and subject to registration inquiriesTable 1 Footnote * | 80 |
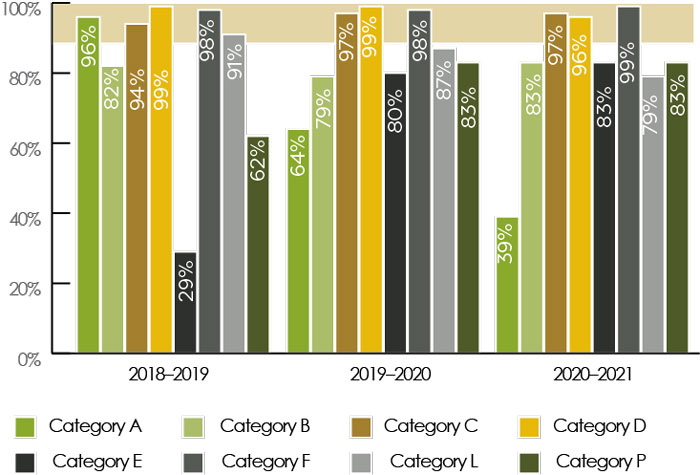
Figure A1 - Text Description
| Year | Performance against review timelines |
|---|---|
| 2018-2019 |
|
| 2019-2020 |
|
| 2020-2021 |
|
- Please see Appendix, Table A1 for a description of each registration category.
- Effective April 1, 2017, categories F, L and P were added to the Management of Submissions Policy.
- This figure shows the percentages of submissions by submission category that met the applicable review timelines outlined in the Management of Submissions Policy over the last three fiscal years.
- All categories of pre-market submissions have a performance standard of 90% against the established review timelines for the different submission categories.
- PMRA continued to meet its performance targets on some pre-market evaluations (C, D, F), while for some categories of submissions (A, B, E, L), due to an increasingly complex workload and COVID-19 impacts on PMRA operations, performance targets were not met.
| Number | New active Ingredient | End-use product(s) | Product type | Product category | Uses/sites |
|---|---|---|---|---|---|
| 1 | Bacillus amyloliquefaciens, strain PTA-4838 | AVEO EZ Nematicide | Nematicide | Biopesticide | Corn (field, sweet and pop) and soybean. |
| 2 | Bacillus amyloliquefaciens strain FZB42 | AmyProtec 42 | Fungicide | Biopesticide | Potato and garlic. |
| 3 | Broflanilide | Cimegra | Insecticide | Conventional Chemical | Corn (field, pop, sweet and seed) and potato. |
| Teraxxa F4 | Insecticide, Fungicide | Conventional Chemical | Barley, canary seed, annual canary grass (grown for human consumption), oats, rye, triticale, wheat (all types: winter, spring and durum). | ||
| Teraxxa | Insecticide | Conventional Chemical | Barley, buckwheat, pearl millet, proso millet, oats, rye, sorghum, triticale, canary seed, annual canarygrass (grown for human consumption), wheat (all types: winter, spring and durum). | ||
| 4 | Fenpropathrin | Danitol Insecticide | Insecticide | Conventional Chemical |
|
| 5 | Inpyrfluxam | Excalia Fungicide | Fungicide | Conventional Chemical | Apple, soybean, and sugar beet. |
| Zeltera Fungicide | Fungicide | Conventional Chemical |
|
||
| 6 | Lecanicillium muscarium strain 19.79 | Mycotal Biological Insecticide | Insecticide | Biopesticide | Greenhouse tomato |
| 7 | L-Menthol | Api Life VAR | Acaricide | Biopesticide | Honeybee colonies. |
| 8 | Racemic Camphor | ||||
| 9 | Stearic Acid And Related Fatty Acids | Rodents Away Odor Free | Animal Repellent | Biopesticide | Indoor spaces including modes of transport (e.g., homes and recreational vehicles) where house mice are present. |
| 10 | Trifludimoxazin | Vulcarus | Herbicide | Conventional Chemical | Barley, field corn, peas (dried field), soybeans, wheat (including durum, spring and winter) and chemfallow |
| Voraxor | Herbicide | Conventional Chemical | Barley, field corn, peas (dried field), lentils, soybeans, wheat (including durum, spring, and winter) and chemfallow |
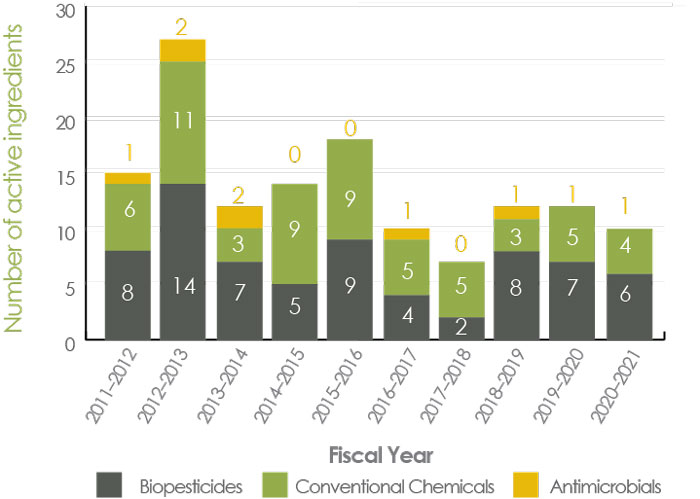
Figure A2 - Text Description
| Year | Number of new active ingredients |
|---|---|
| 2011-2012 |
|
| 2012-2013 |
|
| 2013-2014 |
|
| 2014-2015 |
|
| 2015-2016 |
|
| 2016-2017 |
|
| 2017-2018 |
|
| 2018-2019 |
|
| 2019-2020 |
|
| 2020-2021 |
|
- This figure provides the number of new active ingredients registered over the course of the last ten fiscal years. It represents active ingredients that have been registered for use in Canada and excludes any new active ingredients for which only a maximum residue limit on imported food was established.
| Active ingredient | Document number | Summary of decision or proposed decision |
|---|---|---|
| Re-evaluation decisions | ||
| Acephate | RVD2020-07 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health and environmental risk concerns. |
| Dichlorvos | RVD2020-08 | Acceptable for continued registration for some products. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. |
| Ethephon | RVD2020-09 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. |
| Linuron | RVD2020-10 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. |
| Phosmet | RVD2020-11 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. |
| Mancozeb | RVD2020-12 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses that were not supported by manufacturers. |
| Thiophanate-methyl | RVD2020-13 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. |
| Chlorpyrifos (Environment) | RVD2020-14 | Acceptable for continued registration for certain outdoor uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other outdoor uses due to environmental risk concerns. |
| Tebufenozide | RVD2021-01 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Fenhexamid | RVD2021-02 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Pyriproxyfen | RVD2021-03 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Special review decisions | ||
| Acephate | SRD2020-01 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health and environmental risk concerns. |
| Dichlorvos | SRD2020-02 | Acceptable for continued registration for some products. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. |
| Metaldehyde | SRD2020-03 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect the environment. |
| Tetrachlorvinphos | SRD2021-01 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health. Cancellation of other uses due to health risk concerns. |
| Linuron | SRD2021-02 | Acceptable for continued registration. |
| Clothianidin (aquatic invertebrates) | SRD2021-03 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to environmental risk concerns. |
| Thiamethoxam (aquatic invertebrates) | SRD2021-04 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Cancellation of other uses due to environmental risk concerns. |
| Proposed re-evaluation decisions | ||
| Sodium omadine (paints, coatings and related uses) | PRVD2020-03 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health. |
| Ziram (paints, coatings and related uses) | PRVD2020-04 | Proposed cancellation due to health risk concerns. |
| Folpet (paints, coatings and related uses) | PRVD2020-05 | Proposed for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health. Proposed cancellation of other uses due to health risk concerns. |
| Chlorothalonil (paints, coatings and related uses) | PRVD2020-06 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health. |
| Dazomet (paints, coatings and related uses) | PRVD2020-07 | Proposed continued registration for some uses. Mitigation includes new/revised label statements to further protect human health. Proposed cancellation of other uses due to health risk concerns. |
| Pyrethrins | PRVD2020-08 | Proposed for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Proposed cancellation of other uses due to health risk concerns and lack of data to conduct risk assessments. |
| Piperonyl butoxide | PRVD2020-09 | Proposed for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Proposed cancellation of other uses due to health risk concerns. |
| Kresoxim-methyl | PRVD2020-10 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| (S)-kinoprene | PRVD2020-11 | Proposed cancellation of all uses due to human health and environmental risk concerns. |
| Flufenacet | PRVD2021-01 | Proposed cancellation of all uses due to human health risk concerns. |
| Isoxaflutole | PRVD2021-02 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Florasulam | PRVD2021-03 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Cymoxanil | PRVD2021-04 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Triticonazole | PRVD2021-05 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. |
| Proposed special review decisions | ||
| Diodofon | PSRD2020-01 | Proposed continued registration for some uses. Mitigation includes new/revised label statements to further protect human health. Proposed cancellation of other uses due to health risk concerns. |
| Metaldehyde | PSRD2020-02 | Proposed for continued registration. New/revised label statements proposed for consistency between product labels. |
| Pentachlorophenol | PSRD2020-03 | Proposed cancellation of all uses due to environmental risk concerns. |
| Pymetrozine (subsection 17(1) of Pest Control Products Act) | PSRD2020-04 | Proposed continued registration for some uses. Mitigation includes new/revised label statements to further protect human health and the environment. Proposed cancellation of other uses due to health risk concerns. |
| Pymetrozine (subsection 17(2) of Pest Control Products Act) | PSRD2020-04 | Proposed continued registration for some uses. Mitigation includes new/revised label statements to further protect human health and the environment. Proposed cancellation of other uses due to health risk concerns. |
| Linuron | PSRD2020-05 | Proposed for continued registration. |
| Iprodione | PSRD2021-01 | Proposed for continued registration. |