Consultation on further strengthening protection of health and the environment: Targeted review of the Pest Control Products Act - What we heard
Table of contents
- Executive summary
- Introduction
- About the consultations
- What we heard – Key takeaways
- Modernized business processes
- Improved transparency
- Real world data
- Additional stakeholder comments
- Conclusion and next steps
- Appendices
Executive summary
Protecting the health of people living in Canada and the environment from the risks of pesticides is the purpose of Canada's pesticide regulatory system. The system ensures access to pest control products that can be used safely and effectively for agricultural, industrial and other purposes, and consumer products, such as insect repellents.
Pesticides help manage pests, pathogens, and invasive species and when used properly are valuable products that people living and working in Canada rely on every day.
Health Canada's Pest Management Regulatory Agency (PMRA) is the regulatory authority in Canada responsible for the regulation of pest control products.
PMRA established a transformation process following the Government of Canada’s August 4, 2021 announcement, which highlighted that Health Canada will begin a review of specific provisions of the Pest Control Products Act (PCPA) to strengthen PMRA’s oversight and protection of human health and the environment, and to increase transparency in the regulation of pesticides. This commitment for a targeted legislative review aligns with the Minister of Health’s Mandate Letter.
In Spring 2022, PMRA launched consultations with a broad range of partners and stakeholders on a targeted review of the PCPA through a discussion document published on Canada.ca. A total of 121 written submissions were received. Additionally, over 40 meetings were held with partners and stakeholders, including meetings of the Transformation Steering Committee (TSC), technical working groups (TWG), public information sessions, and meetings on an ad-hoc basis with stakeholders.
Throughout these consultations, we heard from a variety of groups – pesticide manufacturer associations, agricultural and non-agricultural pesticide users, non-governmental organizations (NGOs), academia, Indigenous organizations, the provinces and territories, foreign jurisdictions, including the United States, Australia and the European Union, and the general public.
This intensive consultation phase of the transformation process is now complete. However, PMRA continues to welcome feedback from all stakeholders and partners about our transformation work at the following e-mail address: pmra.info-arla@hc-sc.gc.ca.
This report provides an overview of what we heard through the consultation. As such, it is intended to reflect the views of respondents as they were expressed and does not necessarily reflect the views of the Government of Canada, the existing legislative framework in the PCPA or the operational practices of PMRA.
Targeted amendments to the Pest Control Products Act (PCPA)
The primary goal of the consultations was to gather stakeholder views on whether possible amendments to the PCPA are required to achieve the objectives of transformation. Pesticide manufacturer associations and pesticide users viewed the Act as “fit for purpose” and indicated that much of the transformation agenda could be achieved through existing policy and regulatory channels.
Provincial and territorial partners did not have a firm position on whether the PCPA should be updated.
In contrast, NGOs were supportive of updating the PCPA to strengthen legislative requirements and called for a broader review of the statute, including expanding risk assessments and data requirements (such as requiring registrants to immediately submit any information provided to other Organization for Economic Co-operation and Development (OECD) regulators), bolstering renewal and re-evaluation requirements, requiring assessment of cumulative risks to the environment and to species at risk, and making specific amendments to the maximum residue limits (MRLs) process.
We also heard support for the incorporation of Indigenous knowledge and science into the assessment process, and when considering the impacts of pesticide use on boreal forests versus agricultural land. Other comments expressed that any bans on pesticide use by a First Nation, province, or territory should trigger a review by PMRA.
Modernized business processes
Stakeholders and partners showed a high level of support for increased efficiencies and business process modernization, while maintaining a science and evidence-based approach.
Regarding the continuous oversight and proportional effort approaches, pesticide manufacturer associations, pesticide user groups and NGOs agreed that PMRA should devote increased resources to modernizing business processes and clearing its re-evaluation backlog. Pesticide user groups noted that consultation should not delay the addition of new minor uses on labels. Also, that it is important that PMRA work with pesticide users when there are cancellations to identify other products that may be used instead. NGOs called for PMRA to immediately remove cancelled pesticides from the market and require new and updated data when a pesticide registration is being renewed.
Pesticide manufacturer associations expressed that any changes to business processes should not be made hastily to avoid any “unintended consequences” and were supportive of a stepwise approach that utilized pilot initiatives before full implementation.
Maximum Residue Limits (MRLs)
Among user groups and pesticide manufacturers, there was agreement that the current process for establishing MRLs is effective and does not require adjustment. These groups emphasized the importance of the MRL process continuing to be based on sound science, with an appropriate risk assessment approach. They added that PMRA should improve communication to help the public better understand how MRLs are set and avoid misinterpretation. Within the MRL Technical Working Group (TWG), there was support for a petition process, whereby a notice would be published online when an applicant requests a new MRL or a change to an MRL. NGOs had several recommendations for improving the current MRL process and pointed to the precautionary principle enshrined in the PCPA and recommended that MRLs take into account the most recent scientific data available and give priority to impacts on health and not commercial interests. It was also suggested that MRLs be a condition of product registration and that any request to increase an MRL should only be allowed at the time of registration, re-evaluation, or under a special review, while also quickly taking action on any proposed decrease to an MRL. Other comments received suggested that PMRA include Indigenous traditional knowledge into risk assessments and when setting MRLs.
Proposed authorization pathway
Related to PMRA’s proposed authorization pathway for pesticides of low or well-characterized risk, pesticide user groups were supportive, as this would increase access to certain products. However, some users were concerned that the current scope of the proposal is too narrow and would not provide them with appropriate access to pesticides with lower risks. Others called for clear, strict, and narrow criteria in the PCPA to prevent higher-risk pesticides from following this pathway and bypassing registration.
There were a range of views among pesticide manufacturer associations, ranging from neutral to supportive, however there was no desire to update the PCPA, and they added that this proposal should be consulted on more fully, outside of the transformation process.
NGO stakeholders were generally not supportive of the proposal, with concerns that the proposed pathway could be made available to higher-risk products.
Improved transparency
Overall, there was consensus from stakeholders and partners that PMRA needs to communicate more clearly, openly, and in plain language, and that this applies to regulatory decisions, the review process, re-evaluations, science communications, and public consultations. Stakeholders and partners also agreed that better organization of PMRA’s information on the web is necessary - specifically searches for PMRA decisions on re-evaluations, special reviews, and available publications.
Several stakeholders emphasized that access to this data and information would be significantly improved if PMRA was able to create a one-stop portal or online platform which would include all PMRA notices, published documents, documents associated with regulatory decisions, and the data and information that informed regulatory decisions such as initial applications, amendments, and post-market reviews.
Access to data
Insufficient access to data was seen by NGOs and academics as a barrier to further improving the trust that Canadians have in their pesticide regulatory system. Several pesticide manufacturer organizations noted that while Canada’s pesticide regulatory system is already very transparent, a lack of timely access to data and information, and a lack of understanding about PMRA decisions reduces Canadians’ overall confidence in the system.
Several ideas and areas of concern were discussed by stakeholders including the methods that PMRA could use to release more data and information and the types and extent to which information should be released. These also included consideration of confidential business information (CBI) and confidential test data (CTD), and current barriers to accessing data, where there were clear differences of views between stakeholder groups.
Real-world data
Pesticide user groups, NGOs, and pesticide manufacturer associations all expressed support for the increased use of use of real-world data, if it reflects actual use patterns in Canada and is held to the same high level of scrutiny as all data currently received by PMRA.
Some user groups supported the use of out-of-country data for agronomic conditions similar to regions of Canada, but not for data sets where climatic or agricultural conditions are different. It was noted that pesticide use data would need to be collected in sufficient detail to be truly useful in meeting PMRA’s mandate for health and safety of people and the environment.
Pesticide users and manufacturer associations commented that PMRA could use real-world data instead of using overly conservative models in decision-making, or refine risk assessments when a conservative model shows that a risk may be unacceptable. In contrast, some NGOs noted that PMRA should recognize that real-world data is often incomplete and should put clear policies in place so that modelling is not automatically regarded as “overly conservative.”
A concern was raised from some agricultural user associations that complete monitoring of water and soil is important but should not outweigh innovation and access to improved pesticides. Others remarked that PMRA should not use real-world data to justify lower thresholds for pesticide use. Pesticide manufacturers identified that "chemical-specific" use information should only be requested in response to an identified risk of concern, due to burden for growers and registrants to provide this information.
Other comments raised
Costs
Several stakeholders expressed concerns related to the cost of undertaking some of the proposed transformation initiatives, particularly those aiming to improve transparency. For example, some pesticide manufacturer associations and user groups raised concerns that transformation efforts would take resources from PMRA’s core business and add to existing capacity challenges, and that the costs of any measures taken, particularly those related to transparency, should be balanced with the benefits of those measures.
Continued engagement
Stakeholders and partners wish to continue to be engaged throughout the transformation process and the implementation of new measures.
Science advisory committee
There were mixed views on the concept of an external Science Advisory Committee (SAC). Some stakeholders and partners expressed concerns with whether it was needed, and others noted concerns over how it would be used, how its membership would be selected, and transparency around its objectives and establishment process.
Introduction
On August 4, 2021, the Government of Canada announced a series of measures to further strengthen human and environmental health and safety from risks posed by pesticides, which align with commitments in the Minister of Health’s Mandate Letter. To support the delivery of these commitments, the Government invested $50 million over three years in the Pest Management Regulatory Agency (PMRA) and Agriculture and Agri-Food Canada (AAFC), supported by Environment and Climate Change Canada (ECCC).
In response to this announcement, Health Canada established a Transformation Agenda, in close collaboration with AAFC and ECCC. This Agenda is built on four pillars of action: improved transparency, increased use of real-world data and independent advice, strengthened human health and environmental protection through modernized pesticide business processes, and a targeted review of the Pest Control Products Act (PCPA). PMRA created a Transformation Task Force to deliver on the Agenda while ensuring that resources dedicated to PMRA’s core functions are not compromised, and that PMRA’s day-to-day business remains fully operational. Additional information on the pesticide regulatory framework in Canada can be found in Appendix A.
A crucial part of delivering on the ambitious Transformation Agenda and informing the targeted review of the PCPA is gathering input from interested Canadians, stakeholder groups and partner organizations. On March 21, 2022, Health Canada published a Discussion Document, DIS2022-01, Further strengthening protection of health and the environment: Targeted review of the Pest Control Products Act where it identified and posed seven questions to help inform whether targeted legislative changes to the PCPA would be needed to achieve the transformation objectives. On June 2, 2022, an additional eighth question was posted on the transformation web page for additional input. In response to a consensus request from stakeholders, and to give more time for feedback on the additional question, Health Canada extended the consultation period from May 20, 2022, to June 30, 2022.
This “What We Heard” report synthesizes key messages received through this consultation process.
About the consultations
Health Canada took a phased approach to the consultations to make sure we received the broadest input possible on key issues. An overview of the engagement undertaken by PMRA within the scope of the targeted review of the Pest Control Products Act can be found in Appendix B.
Over 40 engagement opportunities were held virtually, including:
- The Transformation Steering Committee (TSC): Intended to set the context for the broader Transformation Agenda and identify issues for further discussion in technical working groups. Four sessions were held to solicit feedback from pesticide manufacturer organizations, agricultural and non-agricultural user associations, non-governmental organizations (NGOs), Indigenous organizations, and academia.
- Technical working groups (TWGs): Intended to provide technical input on the design and implementation of transformation proposals. Stakeholders participated in 19 sessions across five technical working groups that were aligned with the Transformation Agenda pillars: modernized business processes, improved transparency, water-monitoring, pesticide use as well as a TWG dedicated to maximum residue limits (MRLs).
- Webinars: Three information webinars, oriented towards the agricultural sector, PMRA stakeholders and the public, were held to provide information and address any questions regarding the Transformation Agenda and targeted review of the Pest Control Products Act.
- Provincial/territorial (P/Ts) and international sessions: Over 14 bilateral engagement sessions with provincial/territorial partners, and bilateral sessions with foreign regulatory partners were held to seek views on the Transformation Agenda and the consultation questions.
- Indigenous engagement: PMRA consulted directly with a national Indigenous organization via the steering committee and held a further follow-up meeting with the organization, as well as with Indigenous communities on specific pesticide-related issues. PMRA is continuing to work with our Indigenous partners to consult on key issues and to ensure that engagement is ongoing.
- Written submissions: A total of 121 written submissions were received from a broad range of stakeholders and partners. See the chart below that shows the number of written submissions from each stakeholder/partner group.
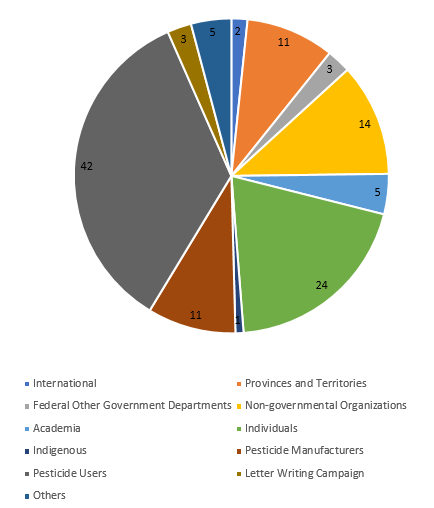
Figure 1 - Text description
Pie chart depicts the number of written submissions related to the PCPA targeted review that PMRA received from different stakeholders: international stakeholders sent 2 written submissions; federal government departments submitted 3; academia submitted 5; Indigenous groups submitted 1; pesticide users submitted 42; others submitted 5; provinces and territories submitted 11; non-government organizations submitted 14; individuals submitted 24; pesticide manufacturers submitted 11; letter writing campaign submitted 3.
PMRA placed significant importance on the contributions of its stakeholders and partners. These included academia, associations of pesticide manufacturers, agricultural and non-agricultural users and associations, individuals, non-governmental organizations (NGOs), provinces and territories (PTs), and Indigenous groups throughout this consultation process.
To ensure greater transparency and openness, Health Canada outlined a new approach to transparency related to engagement with stakeholders on its Transformation Agenda. A calendar of meetings and engagement activities is available publicly, which highlights the various engagements held between Health Canada officials and stakeholders concerning PMRA’s transformation initiatives. Documents which have been distributed at these meetings are available upon request.
The consultation process on the targeted review of the PCPA sought input on eight questions, in support of three transformation objectives:
- Objective 1 – Further strengthening human health and the environment through modernized business processes governing pesticide reviews
- What barriers, if any, exist in the Pest Control Products Act to implementing continuous oversight?
- Are there any changes you would like to see in how MRLs are established?
- Do you have views on Health Canada's proposal on ministerial authorizations and recall and how it should be implemented? This question was added to the consultations on June 2. Health Canada’s proposal to broaden authorization powers in the PCPA to allow the Minister to authorize products of low or well-characterized risks was originally part of the Annual Regulatory Modernization Bill (Bill S-6). The proposal was removed and made part of the review of the PCPA to ensure a more aligned approach, in support of PMRA’s Transformation Agenda.
- Objective 2 – Improved transparency
- Would introducing plain language summaries of our pesticide decisions, as well as more plain language information on how we conduct our science, improve transparency?
- What information would you most need to access, why, and how could that information be best made available to you?
- What barriers exist in the Pest Control Products Act to increasing access to information, considering our obligations to protect confidential business information (CBI) and our international commitments?
- How can PMRA improve the approach to consultation with the public on regulatory decisions?
- Objective 3 – Increased use of real-world data and independent advice in the pesticide regulatory process
- Are there any issues PMRA should consider in terms of accessing, sharing and releasing comprehensive water monitoring and pesticide use data?
What we heard – Key takeaways
Shared perspectives
There was an appreciation for the level of engagement to date. There was also appreciation for the flexibility demonstrated by PMRA to extend the consultation period to allow for meaningful feedback and to accommodate users, while recognizing that the volume of engagement sessions created capacity challenges for many. Throughout the engagement process, there was strong interest for ongoing stakeholder and partner engagement as Health Canada continues to implement its Transformation Agenda.
There was support for improved communication and greater, timelier access to information to increase stakeholder understanding of the regulatory process and decisions.
There was also consensus amongst participants for maintaining the current robust science-based decision-making approach to pesticides and support for the overall concept of continuous oversight and proportional effort approaches. Strong support was also received for the increased use of real-world data to better inform regulatory decisions.
Areas of divergence
Regarding the need to amend the PCPA, pesticide manufacturers and user groups felt that current legislative frameworks within the PCPA are “fit for purpose” and sufficient to enable implementation of the proposed transformation proposals, at least in the short-term, and that these should be implemented through policy and/or regulations. NGOs called for a broad review of the PCPA, and several comments and suggestions were received in support of amendments to the legislation.
Another area of difference among stakeholders was around confidential business information CBI and confidential test data (CTD). Pesticide manufacturer organizations were strongly opposed to any revisions in the definitions and handling of CBI/CTD. NGOs supported restrictions on CTD to be as limited as possible with a clear test of harms to commercial interests in the case of CBI. Provincial governments shared the views of pesticide manufacturers, with user groups expressing support for improved transparency while keeping effective protections on confidential information.
There were also differences between user groups, pesticide manufacturers, and NGOs regarding MRLs. Pesticide manufacturers/user groups supported the current systems for MRLs (with potentially increased alignment with major trading partners and Codex to avoid trade disruption) and the immediate lift of the current MRL pause. NGOs had several suggestions on the MRL process, preferring a model closer to that of the European Union and a rigorous assessment process for any increase to an MRL, while quickly addressing any potential decrease to an MRL.
More detailed summaries of these comments are captured below, organized by the pillars of transformation: Modernized business processes, improved transparency, and real-world data. Additionally, several comments were received on broader issues, which are captured as additional stakeholder comments.
Modernized business processes
Objectives
The primary objective of the Pest Control Products Act is to prevent unacceptable risks to individuals and the environment from the use of pest control products. To strengthen protection of human health and the environment, PMRA is proposing to modernize its review processes. This includes:
- moving from a reactive, point-in-time re-evaluation of pesticides that are on the Canadian market every 15 years to a continuous oversight approach that proactively identifies emerging risks and allows for better informed and more timely decision-making when new risks are identified. This approach includes proactively identifying and reviewing new information that could reveal the need for further investigation.
- introducing a risk-based approach to allow PMRA to focus more resources on pesticides with higher potential risks. This approach involves determining the relative risk profile of a pesticide and the extent of oversight needed to confirm that risks are acceptable, and then reviewing that relative-risk profile throughout the pesticide’s lifecycle.
- considering amendments to the PCPA to create a new authorization pathway that would give the Minister of Health the power to authorize pest control products that are of low or well-characterized risk. This proposal would also expand the current authorization of power under section 41 of the PCPA and provide for a recall power that is applicable to any pest control product that endangers human health or safety or the environment.
The consultation process sought input on three questions relating to this objective:
- What barriers, if any, exist in the Pest Control Products Act to implementing continuous oversight?
- Are there any changes you would like to see in how maximum residue limits (MRLs) are established?
- Do you have any views on Health Canada’s proposal on ministerial authorizations and recall and how it should be implemented?
What we heard
Continuous Oversight
What barriers, if any, exist in the Pest Control Products Act to implementing continuous oversight?
We heard broad support for the concept of a continuous oversight approach across stakeholders and stakeholder groups, though the views on implementation varied. There were concerns that the process should not be rushed to ensure that it is done “right” with standards based on rigorous and peer-reviewed science and does not compromise the integrity of the pest control product review process. The importance of transparency in the continuous oversight process was a common position across stakeholder groups.
There was common recognition from several stakeholders of the backlog of re-evaluations that PMRA faces and the need to appropriately resource this function in addition to bringing about improvements in process efficiency through a continuous oversight approach.
Pesticide manufacturer associations and user groups were supportive of implementing continuous oversight under the current Act, and in general opposed making any legislative changes, noting that the PCPA must not be too prescriptive, which would reduce the organizational agility of PMRA and reduce its capacity to respond. They noted that the changes proposed under continuous oversight could largely be achieved through regulatory and/or policy actions. In addition, several manufacturer associations indicated that while there is support for the modernization of business practices, the current regulatory system is effective in protecting human health and the environment.
Pesticide manufacturer associations indicated a desire to see a phased implementation to demonstrate the effectiveness of continuous oversight. They requested that PMRA take a “step-wise” approach and provide further details on continuous oversight to avoid any negative impacts or unintended consequences on registrants or to discourage innovation. Comments from pesticide manufacturer associations also noted that any proposed changes must continue to be driven by science.
While most user groups were supportive of a continuous oversight approach, some concerns included the risk that a continuous oversight approach could result in Canada falling out of harmonization with similar jurisdictions, such as the United States, whereas the established cyclical approach of re-evaluation of each pesticide every fifteen (15) years allows for planning. Concerns were also raised that continuous oversight should have clear review triggers to ensure reviews are warranted, with some questions shared regarding what constitutes emerging risks and who evaluates this.
There were a variety of stakeholder views on how to approach the 15-year requirement for re-evaluation that currently exists in the PCPA, ranging from support for its removal, to maintaining it as a “backstop” approach in addition to continuous oversight. Pesticide manufacturer associations were supportive of resetting the 15-year “clock” as continuous oversight pulse checks occurred, and by prioritizing re-evaluations based on new or updated science rather than the 15-year trigger alone.
Comments received from the NGO community, were supportive of bolstering the requirement to mandate that reviews are completed every 15 years, as opposed to being initiated every 15 years. Other comments from NGOs and individuals indicated that the “lifecycle” approach of continuous oversight should support, but not replace, cyclical post-market reviews or renewals. Also, there should be periodic post-market reviews and they should incorporate the current five-year renewal period into the PCPA. Some user groups provided similar comments, supporting moving away from the 15-year approach. Some NGOs also pointed out the current lack of incentives for registrants to provide data when required, and that disincentives for not complying with continuous oversight milestones should be incorporated in the Act.
Other user groups raised concerns that clear, narrow criteria would need to be established to limit access to inappropriate loopholes for higher-risk products, and that environmental conditions should be a trigger for a review.
Pesticide manufacturer associations suggested that PMRA initiate re-evaluations two to three years before the official start date and consider alternative approaches to re-evaluation (such as the use of emerging tools and third-party reviews).
Most NGOs were also supportive of the continuous oversight approach but emphasized that this should be adopted in addition to, not replacing, existing requirements under the PCPA, and should not be treated as a “cost-cutting measure.” They also commented that the objective of modernized business processes should not be to increase the rate at which pesticides are approved, but rather to place a clear focus on the health of Canadians and the environment. Another suggestion was that PMRA conduct systematic literature reviews, instead of having to rely on third parties and registrants for the current scientific literature.
Additional comments
There were comments from environmental and health-focused NGOs on expanding risk assessment practices to capture a wider range of risks, including cumulative health impacts, occupational risks, and impacts on environmental justice and climate change. These groups also strongly urged that hormone disruption/endocrine disruption be considered by PMRA as an adverse effect. We also heard from NGOs and individuals that environmental and health risks should be assessed at the same time, not as separate assessments, and the entire product should be assessed, not just the active ingredient(s).
Several NGOs re-iterated the centrality of human and ecological health in the pesticide review process, and that this focus should guide any amendments to the PCPA, not economic considerations. Environmental and health-focused NGOs were generally in favour of amendments to the PCPA and provided several recommendations for broader amendments. These recommendations included amending the PCPA to:
- require the Ministers of Agriculture, Health and Environment to develop a plan to reduce pesticide use and risk by 50% by 2030, and align federal pesticide regulation with Canada’s commitment to halt and reverse nature loss;
- expand requirements for assessing risks to vulnerable populations, including vulnerable workers and Indigenous populations;
- require assessment of cumulative risks to the environment and human health;
- require assessment of risk to species at risk and their habitats;
- require comparative assessments, with the goal of safer substitution;
- prohibit registration of cosmetic (lawn and garden) pesticides, except for products of minimum risk;
- limit ministerial discretion for any streamlined processes designed to facilitate access to minimum risk pesticides, and provide for public consultation/initiation of reviews;
- regulate treated seeds under the PCPA;
- make MRLs a condition of registration;
- codify a requirement that the scientifically based approach include consideration of up-to-date science and methodologies and a thorough review of all science on the pest control product that could identify and inform potential risks arising;
- establish in the PCPA a national system for reporting pesticide use and environmental monitoring; and
- recognize the human right to a healthy environment.
Some pesticide manufacturer associations also expressed several priorities relating to modernized business processes, including:
- a continuation of a science/risk-based approach;
- fulsome and transparent understanding of the value and resources (PMRA and stakeholder) of initiatives both individually and as part of the larger agenda;
- increased and more meaningful engagement with stakeholders early in the re-evaluation process and throughout the pesticide lifecycle;
- earlier identification, consultation and discussion with industry on areas of interest or data requirements, with consideration given to improving the transparency and predictability of processes, data requirements and decisions for all stakeholders;
- targeted and prioritized re-evaluations based on active ingredients in need of an updated review and/or new/changing science; and
- continued registrant and stakeholder engagement on integrated pesticide program development to ensure it meets its purpose and can be successfully implemented without undue regulatory burden or disrupting access to innovation.
Environmental and health-focused NGOs raised concerns specifically about occupational exposures to pest control products as an important aspect to be considered in the aggregate risk assessments, and to apply International Labour Organization standards when assessing occupational risks. Further comments were received on the use of personal protective equipment (PPE) as a mitigation measure against occupational risk and residential exposure, noting that this should be the last line of defence and that heavy reliance on PPE to justify otherwise dangerous uses is not appropriate.
NGOs also expressed support for more information to be proactively required by registrants, and to require information provided to other Organisation for Economic Co-operation and Development (OECD) regulators (including adverse risk information and updated science). NGOs also recommended taking proactive and timely steps to remove out-of-date labels from the market and improving the recall process – including recalling products where there is uncertainty as to the risk posed by the product.
There was also support from pesticide manufacturer associations, user groups, and some academics about moving towards a more harmonized approach with international partners, particularly the United States, while NGOs also noted their support for a more harmonized approach with the European Union.
User groups raised the implications of a product’s registration being cancelled, and the importance of notifying and working with users to understand the impacts of the cancellation and the identification of potential alternative products. Some user groups expressed concerns regarding what would constitute emerging risks, and how those risks would be identified. The predictability of re-evaluation was also identified as a priority.
NGOs were also supportive of continuous oversight and streamlining de-registration cancellation for high-risk active ingredients. They also agreed with requirements for registrants to update scientific information and meet the acceptable risk thresholds for each five-year renewal, and increased transparency of the renewal process and related decisions. Some environmental protection stakeholders expressed support for the elimination of the Policy on Cancellations and Amendments Following Re-Evaluation and Special Review.
Consultation process
There was support among pesticide manufacturer associations and user groups for identifying opportunities to streamline the re-evaluation process, as well as more meaningful and earlier engagement with stakeholders to improve confidence in the process. There was a strong desire among this group to see PMRA continue close collaboration with stakeholders on proposals to modernize business processes, and encouraged PMRA to share further details on specific initiatives.
Environmental NGOs indicated that PMRA should consult the wider public and not just NGOs, summarize/respond to all salient points raised, and publish this information online. The recognition of the consultation burden on stakeholders was also raised by NGOs, and there were calls for outlining reasonable engagement plans.
Maximum Residue Limits (MRLs)
Are there any changes you would like to see in how maximum residue limits (MRLs) are established?
Among user groups and pesticide manufacturer associations, there was agreement that the current process of establishing MRLs is effective in the protection of health, both from the perspective of domestic and import MRLs, and does not require adjustment. However, these stakeholders also identified a lack of clarity in PMRA’s communication of the MRL decision-making process as a major challenge. They encouraged PMRA to take action to improve risk communication and transparency to help the public better understand how MRLs are set and to avoid misinterpretation. In this vein, there was support for improved transparency and communication in the setting of MRLs among these stakeholder groups.
Another comment common from both pesticide manufacturer associations and user groups was the importance that the establishment of MRLs continue to be based on sound science and an appropriate risk assessment approach.
There were further suggestions from user groups and some pesticide manufacturers to improve the alignment with Codex standards when considering new MRLs or changes to existing MRLs, and the importance for Canada to continue to advocate for MRL harmonization at the international level. Several user groups expressed their support for Canada to use Codex MRLs in situations where there is no existing Canadian MRL. If that is not possible, they suggested aligning with countries that have the highest and most successful import/export of that product, such as the United States to avoid trade disruptions.
There was a strong view expressed within the MRL TWG that Canada should continue to use its sovereign right to establish Canadian MRLs, rather than using the Codex standard. Furthermore, it was recognized that a pesticide’s risk cup tends to be smaller in Canada due to legislative requirements to consider additional margins of safety and exposures. For that reason, it may not be appropriate to automatically adopt MRLs in Canada at the expense of domestic crop protection uses.
NGOs were not supportive of referring to Codex MRLs, and strongly stated that PMRA should continue to make its own decisions. We heard from NGOs that any changes to MRLs should not be done with trade considerations in mind, and that PMRA has an ongoing duty to ensure that MRLs meet acceptable risk standards based on up-to-date science. It was suggested by NGOs that the European Union model should be considered by PMRA if any changes to MRLs are to be made.
NGOs pointed to Section 20(2) of the PCPA, which outlines the precautionary principle, and recommended that the setting of MRLs should consider the most recent data on toxicology and give priority to the protection of health and not to commercial interests.
NGO stakeholders suggested amendments to the PCPA relating to MRLs, including requiring PMRA to publish annual comprehensive reports on MRL compliance, clarifying estimates of pesticide exposure in food by using Canadian food consumption data, and setting it at the time of registration. There were also comments received to amend the PCPA to require a continuous obligation to ensure that MRLs pose acceptable risks. It was also suggested by NGOs that if another country has a lower MRL for a certain food, Canada should match it.
There were also comments from NGOs that supported making MRLs a condition of registration. They also suggested that the establishment of MRLs for a pest control product should be part of the decision on the registration/renewal or authorization of a product. Additionally, any request to increase MRLs should only be allowed at the time of registration, re-evaluation, or during a special review.
Feedback received from Indigenous groups on MRLs were specific to glyphosate. They raised that MRLs should be established in specific recognition of the unique issues that arise from using the product in forestry, such as its cumulative effects and considering the differences between boreal forest environments and agricultural land. They added that MRLs should also be established for medicines on which First Nations rely.
Indigenous stakeholders also raised that bans by other OECD regulators on “all uses” of a pesticide will trigger a review by PMRA, but that bans by First Nations or provinces/territories does not trigger a review. They suggested this be changed. It was also noted that MRLs should be reviewed in response to a partial ban as well, not just “all uses.”
Potential process improvements considered
The members of the Technical Working Group (TWG) on MRLs were asked to look at the processes for establishing MRLs to think about whether improvements are needed. These included: having a separate category of “import tolerances;” incorporating international / foreign standards into MRLs; implementing a petition process for import MRLs; and extending the continuous oversight approach to MRLs.
Among user groups and pesticide manufacturer associations, there were comments supporting the development of a clear, transparent process that will inform applicants on how Canada establishes MRLs for imports.
Within the MRL TWG, there was a general preference for a process that resembles the US EPA petition process for pesticide import tolerances under the Federal Food Drug and Cosmetic Act as amended by the Food Quality Protection Act (1996). There was also support for improved communication materials and transparency to support proposals and MRL decisions.
Members of the MRL TWG were also asked to consider the idea of creating two categories for pesticide residues on food. The process for establishing MRLs for registered products and uses in Canada could continue using the same or similar approach to what is currently used. A separate import tolerance could be created for pesticides not used in Canada, crops not grown in Canada, or where higher residues for a particular chemical/commodity combination is warranted due to different growing conditions and or pest pressures from those in Canada. There was general agreement that a single harmonized global standard would be preferred, rather than creating a second MRL category for imports into Canada. NGOs commented that the PCPA should be precise when distinguishing import tolerances and domestic MRLs, and that the assessments/import tolerances should occur at the time of import.
Participants in the TWG reflected on the connection between MRLs and continuous oversight processes. In these discussions, PMRA indicated that while some MRLs may change as a result of updating a dietary risk assessment, all MRLs for that pesticide are reviewed and must continue to protect human health. It was felt by TWG participants that PMRA could be more transparent about when MRLs have been reviewed as part of a dietary risk assessment, rather than only referencing when the MRL was first established (e.g., in the public MRL database).
Ministerial authorizations and recall
Do you have any views on Health Canada’s proposal on ministerial authorizations and recall and how it should be implemented?
While some industry stakeholders and user groups were supportive of the adoption of a risk-based approach for the authorization pathway, there was some desire that these proposed changes be consulted on more broadly and approached separately from PMRA’s current transformation process. Other industry stakeholders did not believe that the PCPA should be amended to implement this new proposed pathway, but that the issue should be addressed through regulatory amendments.
NGO stakeholders were not supportive of the proposal, asserting that authorizations of pesticides are essential decisions that require full legislative thought and processes for the purposes of due process, transparency, and to ensure compliance with the purposes of the PCPA. They added that the proposed pathway could be made available for higher-risk products.
Other issues raised
MRL pause
On the MRL pause, there was significant agreement among user groups, pesticide manufacturers, and some provincial government stakeholders that the current embargo on future MRL increases should be removed immediately, and noted that the lack of clear communication from the federal government on a proposed increase to glyphosate MRLs led to misunderstanding and unfounded public concerns regarding health and safety. There were additional comments that MRLs should not be considered by the Science Advisory Committee that is part of PMRA transformation.
NGOs indicated their support for stringent requirements for any request to increase MRLs, suggesting that these should include a requirement to demonstrate the need to increase MRLs, regardless of whether pests are becoming resistant or a lack of non-chemical options.
There were additional comments received from NGOs that called for any requests to decrease MRL to happen more quickly. Additionally, any proposed MRL increase must undergo a rigorous re-assessment, and include considering possible alternatives, show that the increase is essential due to changes in circumstances (for example, climate), and give evidence that the increased MRL and associated increase in pesticide use will never exceed the acceptable daily intake through various routes of exposure.
Improved transparency
Objectives
As a regulator, PMRA is committed to greater transparency and openness to further strengthen trust in its regulatory decisions on pesticides. This is consistent with regulators around the world who are coming to understand that maintaining confidence and trust in their decisions requires enhanced public transparency.
The PCPA currently includes a number of provisions related to transparency and access to data and information that is considered in the decision-making process. As part of these consultations, PMRA considered options to improve transparency, and ensure that useful information and data are shared in a timely and usable manner and in a way that can be clearly understood.
As part of efforts to improve transparency, PMRA has proposed measures that aim to:
- revisit what types of information and data are currently available for review with an aim to improve access;
- better explain how data is considered and factored into the decision-making process; and
- ensure regulatory documents are written in clear, concise and plain language.
The consultation process sought input on four questions relating to this objective:
- Would introducing plain language summaries of our pesticide decisions, as well as more plain language information on how we conduct our science, improve transparency?
- What information would you most need to access, why, and how could that information be best made available to you?
- What barriers exist in the Pest Control Products Act to increasing access to information, considering our obligations to protect confidential business information (CBI) and our international commitments?
- How can PMRA improve the approach to consultation with the public on regulatory decisions?
What we heard
Plain language initiatives
Would introducing plain language summaries of our pesticide decisions, as well as more plain language information on how we conduct our science, improve transparency?
The goal of “plain language” is to ensure that a broad audience, including those without a technical education in the field, can understand the content presented. Stakeholders were divided over whether plain language summaries of pesticide decisions would be beneficial for overall transparency. Those stakeholders who were supportive of plain language initiatives included several agricultural user associations, some pesticide manufacturer organizations and individuals, who maintained that summaries written in plain language would be beneficial to both stakeholders and the broader public in understanding PMRA decisions.
Some stakeholders, such as pesticide manufacturer organizations and individuals who were in opposition to plain language summaries explained that other types of plain language materials which better explain how PMRA came to a decision, as well as explanations of PMRA procedures and science, would be more beneficial to increasing overall transparency, to better defend the scientific integrity and decision-making of PMRA. Provincial partners were supportive of plain language documents that explain rationales for PMRA decisions, and the supporting scientific methodologies and literature. A concern was raised that plain language would erode the use of precise, scientific terminology that is necessary for a full understanding of PMRA decisions. A question was also raised regarding the potential for the introduction of biases that would diminish the impartiality of the current summaries, and how this could be controlled.
Some user associations noted that the resources needed to keep up this plain language initiative would be overly burdensome to the overall business operations of PMRA. Other stakeholders, such as pesticide manufacturers, cautioned that PMRA should ensure that its resources are directed primarily to its core business.
Access to data
What information would you most need to access, why, and how could that information be best made available to you?
Insufficient access to data was reflected upon by stakeholders such as NGOs and academia as a barrier to further improving the trust that Canadians have in their pesticide regulatory system. Conversely, several pesticide manufacturer organizations noted that while Canada’s pesticide regulatory system is already very transparent, a lack of timely access to data and information, and a lack of understanding regarding PMRA decisions interferes with Canadians’ overall confidence in the system.
Overall, several areas of concern were discussed by stakeholders including the methods that PMRA could adopt to release more data and information in a timely manner, the types and extent to which information should be released, including considerations of confidential business information (CBI) and confidential test data (CTD), and current barriers to accessing data, including possible improvements that could be made in PMRA’s search tools.
Manufacturer associations indicated that PMRA should undertake early engagement with registrants to better position them to be aware of data requirements in the re-evaluation process, and expressed concern that in the past 24 months there has been a growing frequency of new requirements without proper notification and consultation.
Reading room and alternative approaches
PMRA currently makes use of a Reading Room approach to facilitate the inspection of CTD, whereby individuals are required to come to Ottawa to view information at PMRA offices. In the context of the COVID-19 pandemic, virtual measures were adopted to reduce barriers in accessing CTD, while maintaining the security and confidentiality, such as the use of portable data storage devices or USB sticks.
There were significant levels of support for improved transparency across stakeholder groups with respect to improving access to data. Several stakeholders, including agricultural user organizations, NGOs, and provincial governments, noted the challenges of the current process and the difficulties of requiring interested parties to physically travel to a Reading Room located in Ottawa to access test data. There was discussion that previous use of the Reading Room model is not a good way to estimate future possible use of a virtual access model given these geographic barriers. However, other stakeholders raised that further consideration should be given to what data people need access to and why before changing the approach.
Some provincial partners and individuals explored the possibility of a virtual access model, which would provide remote, digital access to technical data and information from the Reading Room. To address the potential travel requirement of the Reading Room, these portals could be accessible through select public offices across Canada. Some agricultural user stakeholders also noted that the information available in the reading room, or its virtual equivalent, should be expanded to include information that is publicly available in other jurisdictions.
Stakeholders supported the current digital approach (i.e., secure USB) to facilitate greater access to CTD, with appropriate protections in place for privacy and CBI or other proprietary information. There were further suggestions that a contract process for data access could be used versus a more open approach. The former approach would include having those individuals who require access to CTD to agree upon specific conditions of use defined within a contract, as opposed to the latter, open approach where access may be more difficult to control. A cost-recovery or user pay model was also suggested for larger data requests, though other stakeholders raised that a fee requirement would create unequal access. There were also suggestions that, given the cost to issue documents, PMRA should prioritise access based on need and demand.
There were concerns from pesticide manufacturer associations, who indicated that PMRA should take a cautious approach, beginning with limited pilots, and to continue the notification of registrants when confidential test data is accessed. Others expressed concerns that virtual access to CTD could facilitate unfair commercial use of the data, and as such would need to be consulted with data owners before any decisions are made. They also noted that resource implications for both PMRA and registrants should be considered in the case of a virtual access approach. They further noted that following the model of the European Food Safety Authority (EFSA) (which entails an “open-by-default” approach to releasing data) would not be preferable as it lacks sufficient security for data in their view.
NGOs were generally in favour of improved access and requiring a clear test for harm to commercial interests to qualify as confidential business information/test data protections and preferred a more open regime. They questioned how confidentiality is measured since such data relates to the protection of the environment and health, stating that disclosure to the public should outweigh the importance of commercial interests. Pesticide manufacturers and user groups noted that any changes to the determination of what data is available and how it is made available requires significant discussions with the data owners, and must ensure that the rights of data owners are protected to avoid any potential impact on future pesticide innovation in Canada.
Types of data and information
Regarding the types of data and information that were identified as being necessary for release, there was broad support across agricultural and non-agricultural users, NGOs and industry stakeholders for an increased availability of access to relevant documents earlier in the re-evaluation process, such as data evaluation reports (DERs), raw data, including CTD, data lists and risk assessment documents.
Several stakeholder groups, including pesticide manufacturers and user groups provided comments noting that the majority of the public is interested in simple summaries, whereas other stakeholders, such as those in academia for research and teaching may require more detailed, technical documents. There were also suggestions that PMRA should consider using a risk impact indicator in communications, such as the Cornell Environmental Impact Quotient (EIQ) as an example. Some stakeholders raised that information or data on non-target species should also be shared.
Across stakeholder groups, but primarily NGOs and academia, there was broad support for providing Canadians access to data that supports PMRA risk assessments. They thought it would help enable independent research and replicability of results, which would improve public trust in the regulatory process. Provincial authorities also require access to test data to enable a more in-depth research and analysis in support of provincial regulatory activities.
Researchers, academics and NGOs raised the importance of publishing pesticide sales and use data, organized across various categories. Those categories might include use per active ingredient and use classified by crops or geographic zone in an accessible format for Canadians.
Several stakeholders, particularly among pesticide manufacturer associations and user groups, indicated that improved access to test data must go hand-in-hand with clear improvements in how test data is presented: This includes better contextualization, the use of plain language, and other guidance tools, because test data is in a raw format. Science evaluation documents are currently written for one audience and need to be improved to address the different needs of different audiences. There were concerns that access to the test data which has not been appropriately contextualized could lead to misinterpretation and misunderstanding that has the potential to erode public confidence in PMRA.
There were also concerns raised by several stakeholders about the limited communication regarding PMRA’s roles and the regulatory process. There were suggestions that access to the risk assessment documentation may be more useful than the raw test data to explain why certain studies were used, or not used. Further, some noted the need for PMRA to explain to the general public how data was considered in making decisions. Other stakeholders raised that in addition to the public, registrants also need to better understand reasons for PMRA decisions.
Current barriers to access
There was a broad level of support across agricultural user associations, NGOs, individuals and pesticide manufacturer associations for improving the IT infrastructure and user-friendliness of search tools available on the PMRA website. Improvements could be made to locating PMRA decisions for re-evaluations and special reviews, as well as accessing available publications. Several stakeholders emphasized that access to this data and information would be significantly improved if PMRA were able to create a one-stop portal or online platform which would consolidate all PMRA notices, published documents, documents associated with regulatory decisions, and data and information on which regulatory decisions i.e., initial applications, amendments, and post-market reviews are based.
There were also comments received from several stakeholders regarding the PMRA website’s user interface. Stakeholders described it as “very challenging” to get information that is needed and that the website is more inaccessible than before. It was noted that the current process to request and access documents is laborious and slow. Some stakeholders indicated that the time for processing public registry process requests to have document(s) emailed is highly variable from one hour to two weeks, and that more immediate access might create more uptake from stakeholders. There were also suggestions that knowing what is available to request would be useful to stakeholders. The Canadian Food Inspection Agency (CFIA) database was cited as an example of a good approach.
There were suggestions that the public registry should be searchable by either use site or active ingredient. They also stated that there is a critical need for stakeholders to sign up for notifications and to be notified when labels change.
Barriers in the PCPA related to CBI/CTD
What barriers exist in the Pest Control Products Act to increasing access to information, considering our obligations to protect CBI and our international commitments?
On reviewing the definitions of confidential business information and test data (CBI/CTD), there were mixed views among stakeholders on whether changes would be needed to increase access. Pesticide manufacturer stakeholders expressed significant reservations about any potential changes to the definition of CBI/CTD under the PCPA. Meanwhile, other stakeholder groups, such as agricultural users, were more willing to consider changes, though most could see the potential challenges this would cause.
Some NGO stakeholders stated that the party that seeks confidentiality of information must be able to demonstrate that disclosure of such data would pose a real and substantive risk to their commercial interests. Other NGO and individual stakeholders raised that the definitions and confidentialities of such data should be reconsidered under the PCPA. Some pesticide manufacturer associations indicated that they have no issues with changes as long as commercially sensitive information continues to be protected the same way as it is today.
Some NGOs commented that the current definitions for CBI and CTD in the Act are quite narrow and suggested that the PMRA approach to disclosure is over-reaching. They also suggested that the words "risks or value of a pesticide" should be eliminated from the CTD definition and that the CTD definition be tightened up to revise "information" to "data" and that these changes be made in the Act, not regulations.
A number of stakeholders, including pesticide manufacturer organizations, user groups, and provincial governments, noted that protections must be in place to maintain the confidentiality of the test data. They felt that improved transparency must not come at the price of compromised or weakened protections for confidential commercial data. There was a recognition that the definition of CBI can be “operationally challenging”, but this could be addressed through other means such as the Annual Regulatory Modernization Bill (ARMB) process, rather than opening up the PCPA to change the definition. There were also comments that any changes to CBI/CTD definitions should not be a disincentive to bring new products to Canada.
Some stakeholders also raised possible resource implications that PMRA could face if they develop enhanced access to data tools and guidance. They explained that this should not impact the regulatory processes and obligations of the Agency. Some stakeholders suggested that with any new transparency requirements, PMRA should be mindful of the regulatory burden on registrants.
Consultation process on regulatory decisions
How can PMRA improve the approach to consultation with the public on regulatory decisions?
The consultation process and continuous oversight were also key areas that stakeholders responded to. Some NGO stakeholders indicated support for broader consultations with the general public, and to build a more collaborative relationship between PMRA and stakeholders. There was also support from pesticide manufacturer organizations for continued collaboration and engagement with pesticide industry stakeholders, and suggestions that PMRA adopt an approach similar to the United States Environmental Protection Agency (EPA) where all non-CBI information is located in an accessible “docket.”
Some stakeholders cautioned against additional consultations on use expansion submissions because this would place a large burden on many organizations to follow and provide input. It was noted by some stakeholders that there is flexibility in PCPA already to consult at any time and that no change to the Act is required for clarification purposes. Some agricultural user associations noted that certain groups engage, via various means such as petitions and emails, with individuals that are not directly affected by the pesticide regulatory framework to try to influence regulatory decisions. Similarly, there were comments received that PMRA should work with other jurisdictions to address cut-and-paste submissions, such as letter writing campaigns, that are not useful to the consultation process.
Some NGOs suggested that consultations should be required for matters that are in the public interest, i.e., that have the potential to pose risks to the environment or health of Canadians, including for increases to MRLs, and proposed a number of improvements to the current consultation process.
Timing of consultations
Some pesticide manufacturer organizations suggested that PMRA use consultation opportunities beyond legislative requirements. There was significant support across agricultural user associations and pesticide manufacturer stakeholders for earlier consultation and engagement with stakeholders during the re-evaluation process. The pesticide manufacturer organizations noted that earlier opportunities for engagement would allow registrants to understand potential regulatory concerns, provide supplemental data where appropriate and explore mitigation measures.
Conversely, agricultural users noted that in cases where a pest control product or label use is at risk of being cancelled, earlier engagement could help to lessen possible unforeseen consequences for users. Some pesticide manufacturer organizations proposed an additional step in the re-evaluation process which includes publishing a “draft risk assessment” for public consultation prior to the publication of proposed mitigation requirements and decisions.
Supporting information and improved communication
A wide array of stakeholders across agricultural user associations, NGOs, and industry agreed that more improved information on processes, timelines and PMRA decisions should be made available in a timely manner. This supporting information should be easily accessible and also provide a level of context and clarity on the decisions being made.
Several stakeholders also proposed additional information that should be provided. Indigenous partners asked that PMRA publish rationales in cases where a product banned in the provinces and territories is allowed for use as a result of PMRA’s assessment. NGO stakeholders proposed that PMRA also provide a better understanding of the qualitative and quantitative data that supports the decisions made, such as including use information corresponding to “No Observable Adverse Effect Levels” (NOAELs).
Some stakeholders suggested possible changes to PMRA decision templates, such as to include information on data requirements and uses, and how PMRA evaluates the value of studies it considers. Other suggestions included revising the templates so that the legal basis for the consultation is defined, e.g., type of consultation, whether it’s a re-evaluation or special review, the specific sections of the Pest Control Products Act that are involved, and possible policy considerations.
Real-world data
PMRA currently has only limited access to real-world data in areas such as water monitoring, pesticide use and crop production practices. More comprehensive and robust data is required to identify potential risks and to make timely and informed regulatory decisions. Access to such data would expand the evidence base for regulatory measures and would help to increase transparency and public trust in PMRA’s decisions. PMRA has committed to the development of a framework, which will inform the development of a national monitoring program. The pilot program will inform the development of the framework. This work has begun with a pilot program starting in spring, 2022, and an all-encompassing pesticide use data program for agriculture and non-agriculture sectors to identify and gather crop production and pesticide use data.
The consultation process sought input on one question relating to this objective:
- Are there any issues PMRA should consider in terms of accessing, sharing and releasing comprehensive water monitoring and pesticide use data?
A summary of what PMRA heard related to real-world data and independent scientific advice is outlined below. Additional discussions were held in the Water Monitoring Technical Working Groups, which will be captured in a separate report.
Support for use of real-world data
Agricultural and non-agricultural pesticide users, non-governmental organizations (NGOs), other federal government departments, industry and individuals all support the use of real-world data, provided that it reflects actual use patterns in Canada and is held to the same high level of scrutiny as all data received by PMRA.
Some pesticide users support the use of out-of-country data for agronomic conditions that are similar to regions of Canada, but not for data sets where climatic or agricultural conditions are different from those in Canada. Some pesticide users also noted that a sufficient amount of data would need to be collected in order to significantly influence PMRA’s decisions over time. It was noted that pesticide use data would need to be collected with enough detail to be truly useful in meeting PMRA’s mandate for the health and safety of people and the environment. NGOs indicated that real-world data is lacking and advised that PMRA should play a key role in addressing this gap. Some NGOs also recommended that the data be supplied directly to the public, rather than requiring the public to request it from PMRA.
Many stakeholders and partners identified uses for real-world data, including to:
- show either the impact, or lack thereof, on water and the environment from pesticide use;
- measure the impact of grower-led stewardship initiatives to block potential pathways into surface water;
- assist with the timeliness and rigour of evaluation and re-evaluation;
- help identify areas of risk; and,
- help researchers understand where Canadians’ water supplies have higher or lower concentrations of pesticides, and what Canadians’ overall cumulative pesticide exposures may be.
Some stakeholders and partners noted that it would be important to collect data from different regions to understand regional differences and identify geographical areas of concern.
Pesticide users and industry commented that PMRA could use real-world data instead of using overly conservative models in decision-making, or to refine risk assessments when a conservative model indicates a risk may be unacceptable. In contrast, some NGOs noted that PMRA should recognize that real-world data is often incomplete and should put clear policies in place so that modelling is not automatically regarded as “overly conservative”.
A concern was raised from some agricultural user associations that all-encompassing monitoring of water and soil is important but should not outweigh innovation and access to improved pesticides. Others remarked that PMRA should not use real-world data to justify lower thresholds for pesticide use.
Pesticide manufacturers identified that "chemical-specific" use information should only be requested in response to an identified risk of concern, due to burden for growers and registrants to provide this information. Concerns were raised that pre-emptive requests for active use information without an identified risk of concern would result in stakeholder fatigue and may increase hesitance in participation in data surveys. Concerns were also raised from manufacturers regarding the sharing of collected data without important contexts, which could result in misinterpretation and a diminished trust in the regulatory process.
Considerations for real-world data collection
Scientific rigour of real-world data
Agricultural and non-agricultural users, NGOs, pesticide manufacturers, individuals and academics all agree that it is very important that real-world data be subject to the same very high data quality standards as any other data used by PMRA. These high standards are fundamental to maintaining confidence and trust in PMRA’s work.
Stakeholders and partners noted that data must be credible, repeatable and relevant to the Canadian context. As one stakeholder indicated, the data sought by PMRA should be precisely defined, field-scaled, and properly compared among relevantly similar regional zones and environments, and that such precision is required to reduce the potential for any type of bias.
Pesticide users noted that monitoring must be sufficiently sensitive and fine-grained to ensure it can support the PMRA’s mandate to protect environmental and human health. Also, full data and test protocol details must be included for PMRA to assess the data and understand its context and any potential limitations. Pesticide users also noted the difference between data and information. They stated that it would undermine PMRA’s credibility if equal weight was given to opinions or anecdotal stories submitted by non-scientific sources and that letters and internet postings should not be considered "data."
Resources considerations
Many pesticide users indicated that there should be no burden placed on pesticide users due to PMRA’s need to collect real-world data. PMRA needs to appropriately resource its data collection needs from the outset. Some pesticide users specified that past instances where farm or commodity organizations generated water monitoring data to support PMRA’s decision-making were exceptional and cannot become the norm.
Some pesticide users suggested that PMRA should give incentives to farmers to collect accurate data. For example, it can develop educational materials on best practices and data collection tools.
Confidentiality and purpose
Pesticide users and pesticide manufacturer organizations identified concerns regarding data collection and confidentiality and noted that PMRA must ensure that data collection is sensitive to privacy concerns. These stakeholders recommended that data collection be anonymous and voluntary, and that PMRA not use this data for compliance purposes. Furthermore, they suggested that only aggregate data be released, and that careful attention be paid to the release of data that was collected on private land or that has commercial sensitivity.
Transparency related to real-world data
Pesticide users and NGOs agreed that PMRA needs to communicate the purpose and benefits of any data collection, in addition to what data is needed, when and how it will be used, and who will use it. These stakeholders also recommended that clear criteria and methods be established and communicated for removing and including data, including outliers. They further recommended that set processes be used to identify when new information requirements are triggered and to select priority areas for data collection.
Sharing real-world data
Pesticide users, NGOs, individuals and Indigenous groups recommended that water monitoring and pesticide use data be published at least annually, in a timely manner, and should be easy to access and publicly available by default, without having to make a request. Furthermore, they noted that this data should be made available directly, without any summarizing or filtering from PMRA, so that Canadians can understand the basis on which decisions about their health and wellness are being made.
NGOs noted that data sharing between stakeholders needs to happen more openly and transparently, and that data sharing has been a strong point of disagreement, particularly between industry and some members of the academic community. They further note that disputes over the data that met PMRA’s standards, can erode confidence in the process. Therefore, they recommended that PMRA provide greater clarity on how and when various data sets are used in the regulatory system to help maintain transparency and public trust. Some NGOs recommended that sales data for all approved pesticides should be proactively published on PMRA’s website with sales per pesticide expressed in total summed-up sales, not released only on request and not expressed as wide ranges (for example, 50,000 kg, >100,000 kg, >500,000 kg, and >1,000,000 kg per year).
Members of the academic community recommended using use data rather than sales data for greater transparency. They also recommended that PMRA increase the types of data required from the manufacturers for occupational exposure assessments and decrease the amount of optional data needed.
Provinces and territories indicated they would like to use water monitoring data to inform on the frequency of detection, to compare measured concentrations to defined threshold values, to analyze data for trends and to answer specific questions.
Understanding and misunderstanding data
Many pesticide users and manufacturers expressed concern that data could be misunderstood if shared publicly. They recommended that shared data be presented in context, for example, by specifying whether a detection of a pesticide is of concern or not and should include details on regional application where relevant.
Some pesticide users noted that caution needs to be applied to how water monitoring data is interpreted, for example a short-term conclusion may miss a long-term set of conditions, some of which may be out of the control of a particular pesticide user. It was also noted from pesticide manufacturers that it will be important for PMRA to qualify any data released publicly that the presence of a compound in the water does not automatically lead to the conclusion that there is an unacceptable risk to human health or the environment.
Indigenous organizations, industry, NGOs, academics, and pesticide users agree that the needs of different target audiences must be considered when sharing data, and that both raw data and some plain language explanations should be shared to help ensure the data is accurately understood. Many of these stakeholders and partners understand that producing materials for different target audience requires time and resources.
Using real-world data to monitor beyond active ingredients
Pesticide users noted that monitoring and use data should include the impacts of non-active ingredient components of pesticides, such as the mode of application. Individuals also noted that real-world data monitoring should be based on commercial formulations, including pesticide adjuvants, and not just active ingredients.
Members of the academic community recommended that real-world data could help inform risk assessments, noting that many pesticides banned elsewhere in the world due to their toxicity are authorized in Canada. These stakeholders also recommended that real-world data monitoring be based on complete formulas of the pesticides rather than just active ingredients.
Other issues raised
Pesticide users and NGOs stated that when real-world data shows regional differences in pesticide use or potential risks, other kinds of assessments could be used to inform the use of more suitable, alternative substances, technologies or approaches, and that communications and tools could be developed to help pesticide users adopt alternatives.
Independent science
Pesticide users, NGOs, individuals, members of the academic community and Indigenous partners are all supportive of independent advice and agree that decisions should be based on unbiased science.
These stakeholders noted that PMRA should not rely on monitoring data, research, studies or advice provided by companies, their advocacy organizations or scientists with financial or professional ties to the industry. They expressed that any reliance on third parties for science presents the risk of real or perceived bias in decision-making, which does not align with the guiding principles of the PCPA. These concerns were echoed particularly with regard to the Science Advisory Committee (SAC), with several user groups and NGOs expressing concerns over the adequate representation of SAC members.
These stakeholders also noted that PMRA should develop its own capacity to use independent scientific data. This includes improving capacity and using technology to conduct systematic literature reviews, considering available data from international sources, and improving direct access to data. They also strongly recommended that PMRA enable independent scientific research on pesticide-related risks in Canada, and share results broadly, noting that this would support pesticide registration, reviews, MRLs, renewal applications, and risk identification.
NGOs recommended separating management and decision functions from scientific recommendation functions, as well as introducing a more stringent overall conflict of interest policy for any expert involved in risk assessment. They also suggested that PMRA enforce and expand mandatory reporting of new scientific information, in combination with a timely review of such information. Finally, they recommended that pesticides be re-evaluated based only on studies that are independent of industry.
Other monitoring including air wildlife and biomonitoring
Pesticide users, NGOs, individuals and other federal government departments recommended that real-world data use go beyond water and pesticide use monitoring and should also include monitoring of pesticide levels in the air, soil, food, wildlife, and humans. Related to pesticides in the air, measurement should include the impacts of pesticide drift on neighboring farms and surrounding wildlife. Regarding monitoring pesticides in food, individuals recommended that PMRA update sources of information for food consumption to better reflect what Canadians eat, not what Americans eat. Regarding biomonitoring, stakeholders recommended a focus on farmers in particular, due to their potentially high levels of occupational exposure. Pesticide users further recommended biomonitoring that considers the use of personal protective equipment, and the use of passive dosimetry studies. NGOs suggested that PMRA incorporate more robust and consistent analysis on persistence and bioaccumulation in their monitoring. Indigenous partners expressed that PMRA should monitor pesticide use in forests, in particular.
Consultation and engagement
Indigenous partners expressed that PMRA should enhance consultation with First Nations for on-the-ground data that can be gathered about pesticide impacts on an ongoing basis.
Pesticide users encouraged continued collaboration with a broad range of stakeholders and stated that PMRA should keep engaging with Canadian producers and using existing work done by producer-government initiatives such as Agriculture and Agri-Food Canada’s Agricultural Species at Risk Core Planning Team; also, where applicable, use the Sustainability Sector Engagement Table. They also recommended that PMRA’s technical working groups on water monitoring and pesticide use data leave the door open for more members to be added, since recruitment took place largely during the spring planting season, when fewer agricultural producers were available.
Additional stakeholder comments
Context
In addition to the questions presented through the Discussion Document, several issues important to the transformation of PMRA were raised through written submissions and discussions in the Steering Committee and Technical Working Groups. These issues do not address any one transformation pillar but may relate to the broader pesticide regulatory system in Canada.
Stakeholders broadly agreed that the commitments of the Transformation Agenda should not affect the resources, capacities and operations of PMRA. Some stakeholders also raised concerns regarding food security, impacts on biodiversity and wildlife, scientific advisory work, and broader improvements to the regulatory process and management of pesticide use.
What we heard
On improving the regulatory process and broader management of pesticide use
Several stakeholders across NGOs, industry associations, and agricultural user associations criticized the Government of Canada for a lack of communication when it comes to identifying and correcting misunderstandings that Canadians may have on the decisions and science-based risk assessments of PMRA. These stakeholders raised that providing a robust defense of PMRA’s decisions would help to increase the trust that Canadians have in their regulatory authorities.
Some agricultural user associations emphasized the importance of considering economic factors for farmers when conducting pesticide reviews. Other user associations repeated that a balanced framework of health, the environment and the economy must be considered, all of which should be supported by science.
Some NGOs encouraged more interdepartmental data sharing, such as consolidating PMRA data with monitoring results from other agencies like the Canadian Food Inspection Agency (CFIA).
Some pesticide users noted that Canada should follow the lead of international partners making firm commitments to reducing pesticide use.
On biodiversity
Some NGO stakeholders raised that risk assessments should be required in order to fully examine the impact of pesticides on Canada’s at-risk species and to conserve and protect our biodiversity.
Other comments received from NGOs stated that in its current form, the Act cannot efficiently regulate products which are meant to conserve or enhance natural resources or the quality of the environment. These stakeholders suggested that the creation of a new product class within the Act would better support innovation and the marketing of environmental products, such as water remediation products.
On food security
Some non-agricultural user associations maintained that PMRA should consider the valuable role that certain pesticides and preservatives have played in ensuring food security during emergencies such as natural disasters, shortages and global pandemics.
Considering the broader, global role that Canada plays in ensuring food security, some industry stakeholders emphasized that changes set out in the Transformation Agenda should not place burdens on the Canadian agricultural sector.
On scientific advice and evidence-based decision-making
Select NGOs requested that PMRA release all independent scientific advice to Canadians, whether from the Science Advisory Committee or from established review panels.
Regarding the resources needed to support evidence-based decision-making, some NGOs recommended that PMRA improve their technological capacities to be able to review independent research systematically and consistently. Other NGOs noted that PMRA scientists must be equipped with current resources to understand recent and wide-spread scientific literature. Also, by using technology, PMRA could have more ‘direct access’ to scientific literature, which the stakeholders noted is more important for evidence-based decision-making than advice from an external body, such as the advisory committee.
Conclusion and next steps
Health Canada appreciates the views and comments received during the consultation period on the targeted review of the Pest Control Products Act. Invaluable input was provided to help Health Canada determine whether legislative changes are required to modernize and strengthen the Act to ensure it supports transparency, use of independent scientific evidence and input to the decision-making process. The input received will inform Health Canada’s work to ensure that Canadians are protected from risks associated with the use of pesticides and to better protect human health, wildlife and the environment.
Through these consultations, several comments were received that went beyond the targeted review of the PCPA, and implicated other pieces of legislation dedicated to the protection of human and ecological health such as the Species at Risk Act (SARA) and the Canadian Environmental Protection Act (CEPA). We have taken note of these comments and will work with our relevant federal partners to ensure they are captured.
Health Canada is grateful to everyone who took the time to share their feedback, either during engagement sessions and/or through written submissions. By generously offering their time and passion for the protection of human health and the environment, including wildlife, from risks posed by pesticides, Canadians provided valuable input on how to strengthen the current review process for the future.
The input received through this consultation will inform next steps of the review of the PCPA, as well as Health Canada’s transformation efforts to improve its oversight, as well as the protection of human health and the environment.
PMRA will continue to engage interested stakeholders and partners including Indigenous organizations as it continues to develop and implement its proposed initiatives on business process modernization, improved transparency and increased use of real-world data.
Appendices
Appendix A: Background information
The Government of Canada is committed to strengthening the protection of human health and the environment, including wildlife. Part of this commitment is the regulation of pest control products, otherwise known as pesticides, to ensure that they can be used safely and effectively for agricultural, industrial, and consumer purposes. Pest control products of acceptable risk and value can contribute significantly to food security, and to conserving biodiversity and natural resources. When used properly, these are valuable products that people living and working in Canada rely on every day.
The Canadian pesticide regulatory framework is the result of working together with federal, provincial and territorial governments, and the involvement of municipal governments where authority is granted by their governing jurisdictions. These efforts are supported by the contributions of a breadth of stakeholders, and various legislative instruments, such as the Pest Control Products Act. Several Acts beyond the Pest Control Products Act also contribute to the broader legislative framework of pesticide regulation in Canada, and include responsibilities for Health Canada and its federal partners, Agriculture and Agri-Food Canada (AAFC), Environment and Climate Change Canada (ECCC), and the Department of Fishers and Oceans Canada (DFO). Acts such as the Species at Risk Act, the Migratory Birds Convention Act and the Canadian Environmental Protection Act contribute to the broader protection of human health and the environment, including wildlife.
Provincial governments are also responsible for the transportation, sale, use, storage and disposal of pesticides, as well as training, certification and licencing. Provincial and territorial jurisdictions may also grant authorities to municipalities to enact by-laws which specify further conditions on the use of pesticides.
Under the authority of Health Canada and the Pest Control Products Act, the Pest Management Regulatory Agency (PMRA) acts as the regulatory authority responsible for the federal regulation of pesticides in Canada. Acting on behalf of the Minister of Health, PMRA’s primary mandate is to prevent unacceptable risks to Canadians and the environment from the use of pest control products. The Agency fulfills this mandate through its core responsibilities within the Canadian pesticide regulatory framework, which includes the registration of new pesticides and post-market monitoring and review, science-based health, environment and value assessments, and compliance and enforcement. Health Canada’s Regulatory Operations and Enforcement Branch (ROEB) collaborates with PMRA on promoting, monitoring, and enforcing compliance with the Pest Control Products Act across Canada.
The current Pest Control Products Act, which came into force on June 28, 2006, guides the registration, use, storage and importation of products which are used for the control of pests. It is the legislative framework which governs the regulation of pesticides based on scientific risk assessment and risk management, before and after they are registered for use. Prior to the registration of a pesticide in Canada, PMRA applies current, evidence-based, scientific approaches to assess whether the health and environmental risks of the proposed pesticides are acceptable, and to determine whether the products have value. The value of a pest control product is determined by evidence of its benefits and efficacy in its proposed uses. In conducting pesticide approvals, Health Canada considers data and information provided by manufacturers; published scientific reports; federal, provincial, and territorial governments; other regulatory agencies; and Canadians. Once a pesticide has been registered, it becomes subject to a system of post-market risk management controls under the Pest Control Products Act. This includes re-evaluations and special reviews, compliance and enforcement activities, and the reporting of health and environmental incidents.
Under the current legislation, PMRA is required to re-evaluate each registered pesticide every 15 years after the most recent major decision. With more than 7600 registered pesticide products in Canada, the current pesticide re-evaluation program is experiencing an increase in post-market activity workload, making it more difficult to meet this legislated requirement with current resource levels. Despite the current Act providing robust protection of human health and the environment, a review of some of its terms is needed to ensure that the pesticide approval process meets the expectations of Canadians in the areas of transparency and sustainability.
In recent years, PMRA has worked with key partners and stakeholders to address these concerns. Between 2018 and 2020, PMRA launched a two-year project to explore pathways that would lead to a more sustainable program delivery model and improve health and environmental protection. While the project initially focused on the re-evaluation program, it became apparent that changes across the broader pesticide program were needed to achieve the desired goals.
Building on these efforts, in August of 2021, the Government announced a series of measures to further strengthen the protection of human and environmental health and safety. These measures would become the guiding pillars of the Transformation Agenda advanced by PMRA in response to this new mandate. Aimed at improving several aspects of the pest control product framework in Canada, these measures included:
- further strengthening human health and environmental protection through modernized business processes for the review of pesticides
- improving transparency and public access to information and data across the regulatory pesticide processes
- increasing the use of comprehensive, real-world data on water monitoring, crop production and pesticide use, as well as independent scientific advice
- conducting a targeted review of the Pest Control Products Act to support the implementation of transformation initiatives, where necessary
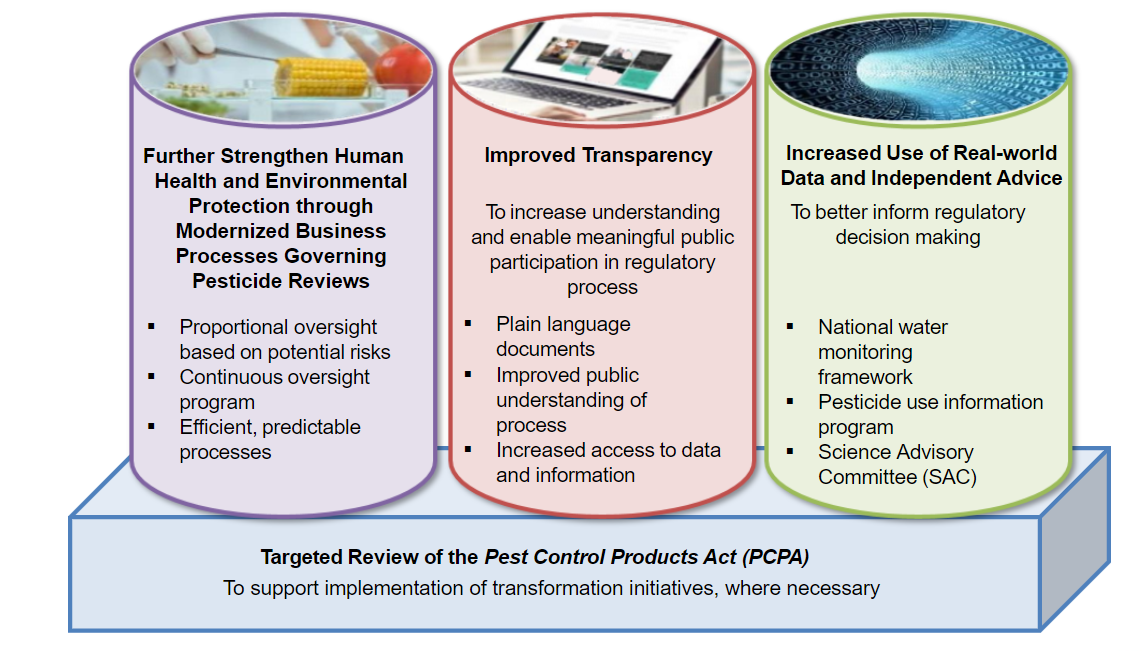
Figure 2 - Text description
Image depicting the three different pillars of the Transformation Agenda, with the targeted review of the PCPA supporting them all.
The first pillar on the far left includes the following title: Further Strengthen Human Health and Environmental Protection through Modernized Business Processes Governing Pesticide Reviews. Three bullets below this title are: proportional oversight based on potential risks; continuous oversight program; efficient predictable processes.
The second pillar, in the middle, includes the following title: Improved Transparency. This title is followed by the following text: To increase understanding and enable meaningful public participation in the regulatory process. Three bullets below this are: plain language documents; improved public understanding of process; increased access to data and information.
The third pillar on the far right includes the following title: Increased use of real-world data and independent advice. To better inform regulatory decision-making. Three bullets below this are: national water monitoring framework; pesticide use information program; Science Advisory Committee (SAC).
PMRA launched a broad engagement process to seek views from stakeholders and partners on whether these measures would require targeted legislative changes to the Pest Control Products Act. This targeted review is different from the broader, seven-year legislative review which requires an appropriate Standing Committee of Parliament to examine the administration and operation of the Act as needed. The scope of the targeted review is focused on potential changes that would support greater transparency and balance how pesticide review processes are started.
Appendix B: Summary of engagement on the targeted review of the Pest Control Products Act: An overview of the amount of engagement activities that have happened during the Pest Control Products Act review consultation process
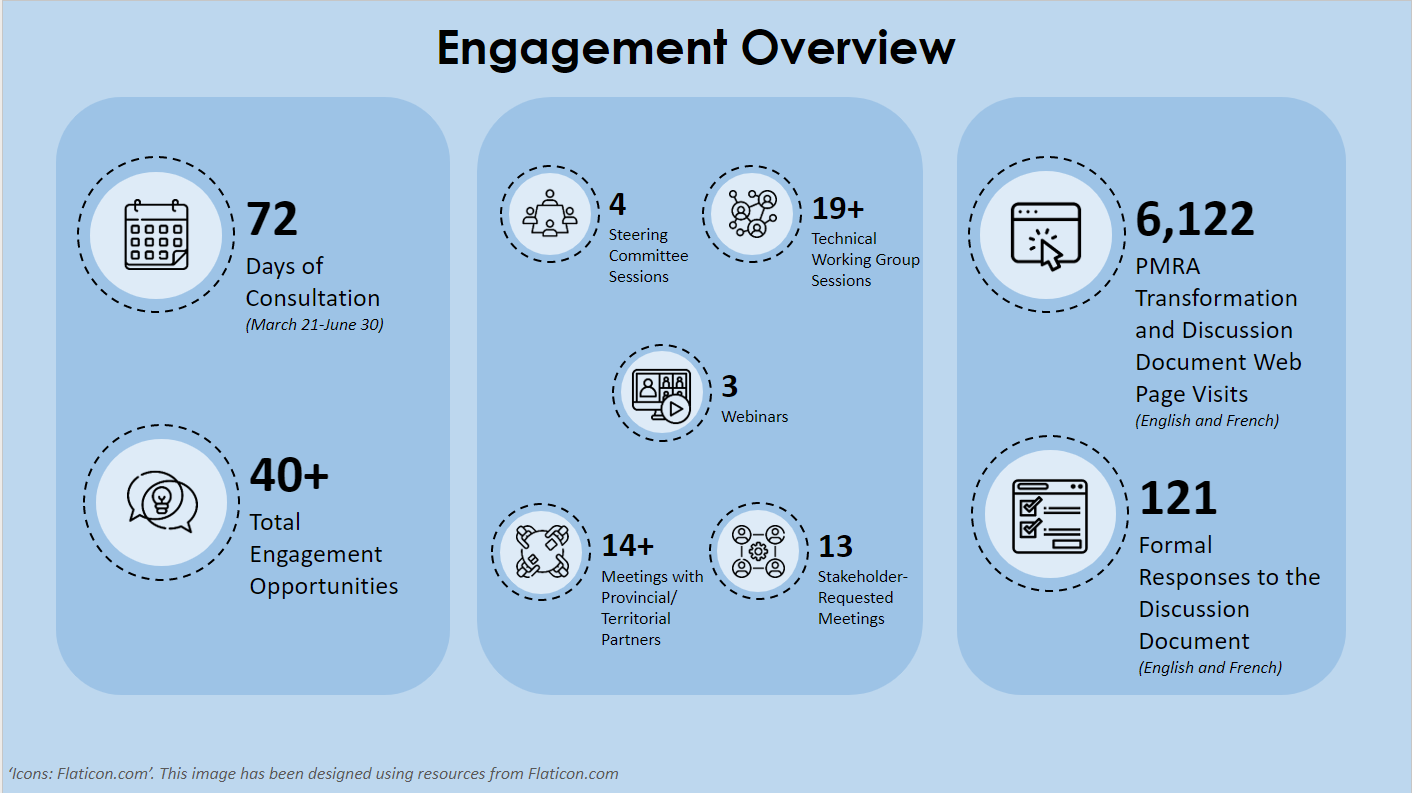
Figure 3 - Text description
Three blue boxes are depicted horizontally with images and text inside each box.
First box includes:
- An image of a calendar with the text “72 days of consultation (March 21- June 30)”
- An image of a lightbulb with the text, “40 plus total engagement opportunities.”
Second box includes:
- An image of people in a meeting with the text, “4 steering committee sessions”
- An image people connected together with the text, “19 plus technical working groups”
- An image of a computer screen with people on the screen with the text, “3 webinars.”
- An aerial image of people at a meeting with the text, “14 plus meetings with provincial/territorial partners.”
- An image with people connected together with the text, “13 stakeholder requested meetings”
Third box includes:
- An image of a web page being clicked on and the text, “6,122 transformation and discussion document web page visits”
- An image of document with checkmarks on it and the text, “121 formal responses to the discussion document in English and French”