Information Note: Human Health Reference Values for Pesticides in drinking water sources
Last updated: July 2025
On this page
- Introduction
- Development of Human Health Reference Values for pesticides
- Using Human Health Reference Values for pesticides
- Additional information
- Appendix 1: Table of Human Health Reference Values
Introduction
Health Canada is mandated under the Pest Control Products Act (PCPA) to evaluate pesticides on a pre-market basis, and to re-evaluate pesticides on a post-market cyclical basis, as well as when aspects of concern have been identified. A pesticide registration is approved, or continued registration is supported, only when a comprehensive scientific evaluation finds that when properly used, no harm to human health or the environment will result from exposure to, or directed use of a pesticide, including exposure from drinking water.
The outcome of a pre- or post-market evaluation allows Health Canada's Pest Management Regulatory Agency (PMRA) to establish or update existing conditions of use for all registered pesticides, which includes conditions designed to prevent or minimize contamination of drinking water sources that could otherwise pose unacceptable health risks. It also allows the PMRA to develop Human Health Reference Values (HHRVs) for pesticides in drinking water sources such as groundwater and surface watersFootnote 1.
HHRVs have been developed for a large set of pesticides approvedFootnote 2 for use in Canada that may potentially enter drinking water sources based on their chemical properties and registered uses. These pesticides may be detected following unexpected events, such as spills, legacy contaminations, over sprays, or the unexpected accumulation of a certain pesticide in the environment.
The HHRV data table (Appendix 1) presents a listing of these pesticide Human Health Reference Values, which are based on PMRA health risk assessments. They serve as benchmarks to determine if levels found in drinking water sources, such as groundwater and surface waters, warrant further investigation and can also be used to inform regulatory actions under the PCPA.
Development of Human Health Reference Values for pesticides
HHRVs are calculated using an internationally accepted, health protective approach, which considers the toxicity of the pesticide, typical body weight, drinking water intake estimates, and an allocation factor for long-term exposure. They are also protective of vulnerable subpopulations, such as children. HHRVs are based on modern risk assessments and are updated regularly through the pesticide pre- and post-market review processes. The same approach is described in the WHO's Guidelines for Drinking-Water Quality, and this approach is also used to calculate the USEPA's human health benchmarks for pesticides in drinking water (HHBP).
Acceptable Daily Intakes (ADI) and Acute Reference Doses (ARfD)
HHRVs are based on acceptable daily intakes (ADIs) and acute reference doses (ARfDs) for pesticides that are developed by PMRA as part of the pesticide regulatory process.
- Acceptable daily intake (ADI): the amount of a specific pesticide that will not cause harm when consumed through food and drinking water on a daily basis over a lifetime (long term exposure). It is expressed in units of milligrams per kilogram body weight per day.
- Acute reference dose (ARfD): the amount of a specific pesticide that will not cause harm when consumed through food and drinking water in a single day (short-term exposure). It is expressed in units of milligrams per kilogram body weight.
Acceptable daily intakes and acute reference doses take into consideration vulnerable populations such as children and females of reproductive age, and therefore, there may be different values for different subpopulations, depending on the toxicity profile of the pesticide. To be protective of all populations, the lowest ADI and ARfD for a given pesticide are used to calculate the HHRVs.
For details on specific ADIs and ARfDs, please refer to the individual pesticide registration decisions listed in Appendix 1. In cases where a published reference document is not listed, please contact the Pest Management Information Service.
Calculating Human Health Reference Values
HHRVs are calculated using the following formulas:
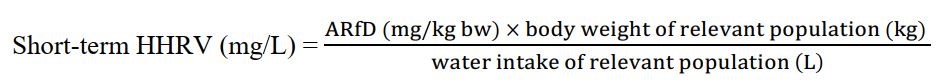
Equation 1 - Text Equivalent
This equation calculates the short-term HHRV. The ARfD in mg/kg bw for a pesticide is multiplied by the body weight estimated for the specific Canadian subpopulation in kg and then divided by the estimated drinking water intake for that subpopulation in L/day. The HHRV is expressed in mg/L.
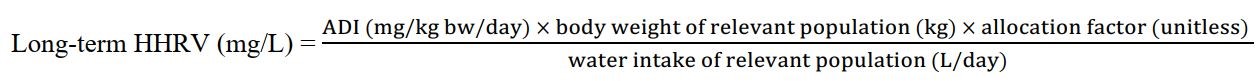
Equation 2 - Text Equivalent
This equation calculates the long-term HHRV. The ADI in mg/kg bw/day for a pesticide is multiplied by the body weight estimated for the specific Canadian subpopulation in kg and by an allocation factor of 20% which accounts for other sources of exposure then divided by the estimated drinking water intake for that subpopulation in L/day. The HHRV is expressed in mg/L.
Allocation factor
The allocation factor for the long-term HHRV is 20%. This is the same value used for the USEPA's HHBPs, and for the default allocation factor in the WHO's Guidelines for Drinking-water Quality. This is a generic allocation factor that does not account for a pesticide's specific use pattern. In essence, it assumes that the population may be exposed to the pesticide from other sources such as residues on food, and adjusts the long-term HHRV to be lower to account for these other potential exposures (20% of the total exposure from drinking water, and 80% from other sources).
An allocation factor is not included when developing the short-term HHRV. While over the long term it is reasonable to assume that the population has some exposure to the pesticide from other sources, the short-term case refers to a person being exposed to a pesticide in their drinking water for a single day as a result of an unexpected contamination. It is unlikely that this individual will also have been exposed significantly to the same pesticide on the same day from other sources, and therefore the short-term HHRV allocates 100% of the single-day exposure to drinking water.
Body weight and water intake
The typical body weight and water intake values used for calculating HHRVs are based on the same data used in PMRA's comprehensive regulatory risk assessments for pesticides. For the adult population, typical body weight is 80 kg and typical water intake is 2.5 L. For females aged 13-49 years, typical body weight is 69 kg and typical water intake is 2.5 L. For children, a normalized ratio of drinking water intake to body weight of 0.15 L/kg is used.
Using Human Health Reference Values for pesticides
A person is unlikely to experience adverse health effects from drinking water containing a pesticide at or below the long-term HHRV over a lifetime. Small exceedances above the long-term value for short periods of time are also not expected to cause harm.
Short-term HHRVs are also developed for certain pesticides as part of the pesticide regulatory review process. In the event of a single exposure to a pesticide, such as an accidental release to a body of water, a person drinking water containing the pesticide at or below the short-term HHRV is unlikely to experience health effects. Short-term HHRVs should be used when exposure is limited to one day. If a short-term HHRV is not available or the exposure occurred over more than one day, then the long-term HHRV should be used.
If a pesticide is detected in drinking water sources at levels that exceed the HHRV, additional investigation may be needed to determine the cause and extent of the contamination. If the levels are deemed unacceptable, regulatory action may be taken. This may result in new restrictions on the use of a pesticide to minimize or prevent future contamination.
Additional information
For more information on Health Canada's Pest Management Regulatory Agency's review process and opportunities to comment on pesticide assessments, please see Pesticides and Pest Management.
Appendix 1: Table of Human Health Reference Values
To view the documents listed in the "Reference Document" column, please visit the Government of Canada publications website and search for the reference document. In cases where a reference document is not listed, or for any other inquiries related to the Human Health Reference Values (HHRV) for Pesticides, please contact the Pest Management Information Service.
| Pesticide | Long-term HHRV (mg/L) | Short-term HHRV (mg/L) | Reference Document |
|---|---|---|---|
| 2,4-D | 0.11 | 1.7 | PACR2007-06 |
| 2,4-DB | 0.2 | 1.7 | PRVD2018-08 |
| 2-phenylphenol | 2.5 | – | PRVD2008-04 |
| 5,5-dimethylhydantoin | 2 | – | – |
| Abamectin | 0.003 | 0.011 | PRVD2023-01 |
| Acephate | 0.0077 | 0.011 | PRVD2016-01 |
| Acequinocyl | 0.15 | – | ERC2007-10 |
| Acetamiprid | 0.05 | 0.05 | PRD2010-02 |
| Acibenzolar-s-methyl | 0.017 | 0.018 | PRD2010-19 |
| Acifluorfen | 0.005 | 0.6 | PACR2006-02 |
| Ametoctradin | 54.3 | – | PRD2011-25 |
| Aminocyclopyrachlor | 7 | – | PRD2014-08 |
| Aminopyralid | 3 | – | REG2007-01 |
| Amitraz | 0.002 | 0.002 | ERC2013-04 |
| Atrazine | 0.03 | 0.1 | PSRD2023-01 |
| Aviglycine hydrochloride | 0.03 | – | PRVD2017-14 |
| Azamethiphos | 0.005 | 0.005 | PRD2016-25 |
| Azoxystrobin | 1 | 2 | PRVD2023-02 |
| Bensulide | 0.6 | 1 | – |
| Bentazon | 0.02 | 3 | – |
| Benzovindiflupyr | 0.3 | 0.7 | PRD2015-07 |
| Bicyclopyrone | 0.006 | 0.03 | PRD2015-02 |
| Bifenazate | 0.06 | – | REG2006-01 |
| BifenthrinFootnote * | 0.02 | 0.06 | PRD2017-11 |
| Bixafen | 5 | 0.1 | PRD2019-04 |
| Boscalid | 0.9 | – | REG 2004-02 |
| Broflanilide | 0.1 | – | PRD2020-06 |
| Bromacil | 0.6 | – | PACR 2004-22 |
| Bromoxynil | 0.02 | 0.5 | PSRD2019-01 |
| Buprofezin | 0.06 | 3 | PRD2016-07 |
| Butoxypolypropylene glycolFootnote * | 7.7 | 8 | PRVD2010-05 |
| Captan | 0.4 | 2 | PRVD2016-13 |
| Carbaryl | 0.07 | 0.073 | PRVD2009-14 |
| Carbathiin/carboxin | 0.05 | – | PRVD2008-25 |
| Carbendazim | 0.06 | 0.3 | PRVD2019-07 |
| Carfentrazone-ethyl | 0.6 | – | ERC2008-05 |
| Chlorantraniliprole | 10.1 | – | PRD2013-08 |
| Chlorfenapyr | 0.03 | 0.1 | PRD2013-01 |
| Chlorimuron-ethyl | 0.3 | 2 | PRVD2018-14 |
| Chlormequat chloride | 0.3 | 6 | PRVD2009-13 |
| Chloropicrin | 0.006 | 0.007 | PRVD2017-01 |
| Chlorothalonil | 0.096 | 3.9 | RVD2018-11 |
| Chlorpropham | 0.1 | 23 | PACR2007-04 |
| ChlorpyrifosFootnote * | 0.001 | 0.005 | PACR2003-03 |
| Chlorsulfuron | 0.1 | – | PRVD2007-09 |
| ChlorthalFootnote * | 0.06 | – | PRVD2008-18 |
| Clethodim | 1 | 7 | PRVD2016-11 |
| Clodinafop-propargyl | 0.02 | 0.2 | PRVD2018-16 |
| Clofentezine | 0.03 | – | PRVD2013-05 |
| Clomazone | 3 | – | – |
| Clopyralid | 0.96 | 5 | PRVD2010-17 |
| Cloquintocet-mexyl | 3 | – | – |
| Cloransulam-methyl | 0.3 | – | PRD2008-09 |
| Clothianidin | 0.3 | 1.7 | PRD2016-04 |
| CoumaphosFootnote * | 0.004 | 0.017 | – |
| Cyantraniliprole | 0.06 | – | PRD2016-13 |
| Cyazofamid | 2.1 | – | PRD2016-34 |
| Cyclaniliprole | 2 | – | PRD2023-11 |
| Cyflumetofen | 1 | 20 | PRD2014-10 |
| Cyfluthrin | 0.03 | 0.03 | RVD2018-35 |
| Cymoxanil | 0.02 | 0.1 | PRVD2021-04 |
| Cypermethrin | 0.1 | 0.1 | PRVD2016-18 |
| Cyprodinil | 0.2 | 5 | – |
| Cyprosulfamide | 2.5 | – | – |
| Cyromazine | 0.03 | 0.1 | PRVD2020-02 |
| Deltamethrin | 0.02 | 0.02 | PRVD2015-07 |
| Desmedipham | 0.2 | 0.7 | – |
| DiazinonFootnote * | 0.01 | 0.01 | – |
| Dicamba | 0.07 | 2 | PRVD2007-05 |
| Dichlobenil | 0.06 | 4.1 | – |
| Dichlorprop-p | 0.4 | 0.5 | PRD2013-15 |
| Dichlorvos | 0.0006 | 0.093 | PRVD2017-16 |
| Didecyl dimethyl ammonium chloride (DDAC) cluster | 0.06 | 0.3 | – |
| Difenoconazole | 0.06 | 2 | PRVD2021-06 |
| Diflubenzuron | 0.1 | – | PACR 2004-35 |
| Diflufenican | 1 | – | PRD2023-07 |
| Dimethenamid-p | 0.3 | 4 | – |
| Dimethoate | 0.01 | 0.087 | PRVD2011-12 |
| Dimethomorph | 1 | 5 | PRVD2019-03 |
| Diodofon | 0.01 | – | PRVD2010-04 |
| Diphenylamine | 0.16 | – | REV2017-25 |
| Diquat (present as dibromide) | 0.03 | 5 | PRVD2008-12 |
| Dithiopyr | 0.03 | – | PRVD2009-01 |
| Diuron | 0.02 | – | PACR2006-07 |
| Dodine | 0.1 | – | PRVD2008-11 |
| D-phenothrin | 0.4 | 7 | PRVD2015-05 |
| Endothall | 0.04 | – | REV2006-06 |
| EPTC/S-ethyl dipropylthiocarbamate | 0.05 | 1 | PRVD2007-03 |
| Ethaboxam | 0.35 | 7 | PRD2014-13 |
| Ethalfluralin | 0.3 | 6.9 | PRVD2011-16 |
| Ethametsulfuron-methyl | 1.7 | 6.9 | PRVD2008-05 |
| Ethephon | 0.03 | 1.1 | RVD2020-09 |
| Ethofumesate | 0.1 | 0.6 | – |
| Etoxazole | 0.2 | – | PRD2015-15 |
| Etridiazole | 0.03 | 4.1 | PACR2006-03 |
| Famoxadone | 0.009 | – | REG 2003-10 |
| Fenamidone | 0.3 | – | PRD2014-03 |
| Fenazaquin | 0.1 | 0.1 | PRD2022-11 |
| Fenbuconazole | 0.0819 | 3 | PRDD2005-03 |
| Fenbutatin oxide | 0.11 | – | RVD2007-06 |
| Fenhexamid | 1 | – | PRVD2020-01 |
| Fenoxaprop-p-ethyl | 0.03 | 3 | RVD2012-07 |
| Fenpropathrin | 0.06 | 0.1 | PRD2020-05 |
| Fenpyroximate | 0.04 | 0.2 | PRD2016-01 |
| Flazasulfuron | 0.06 | 3 | PRD2018-03 |
| Flonicamid | 0.3 | – | PRD2010-25 |
| Florasulam | 0.3 | 17 | PRVD2021-03 |
| Florpyrauxifen-benzyl | 10 | – | PRD2022-17 |
| Florylpicoxamid | 0.2 | – | PRD2022-14 |
| Fluazaindolizine | 1 | 8.7 | PRD2021-3 |
| Fluazifop-p-butyl | 0.03 | – | PRVD2011-11 and RVD2012-05 |
| Fluazinam | 0.024 | 0.087 | PRD2017-14 |
| Flucarbazone | 3 | 7 | PRVD2022-02 |
| Fludioxonil | 0.24 | – | PRVD2016-03 |
| Fluensulfone | 0.1 | 0.4 | PRD2017-02 |
| Flufenacet | 0.01 | 0.01 | PRVD2021-01 |
| Flumethrin | 0.01 | 0.01 | PRD2016-29 |
| Flumetsulam | 6 | – | PRVD2014-05 |
| Flumioxazin | 0.02 | 0.08 | ERC2010-05 |
| Fluopicolide | 0.43 | 6 | PRD2015-18 |
| Fluopyram | 0.077 | 3 | PRD2016-11 |
| Fluoxastrobin | 0.096 | – | PRD2012-07 |
| Flupyradifurone | 0.5 | 3 | PRD2018-07 |
| Fluroxypyr | 6 | – | PRVD2017-11 |
| Flutianil | 5 | – | PRD2021-09 |
| Flutriafol | 0.14 | 0.3 | PRD2014-16 |
| Fluxapyroxad | 0.13 | 8.33 | PRD2012-09 |
| Folpet | 0.2 | 0.7 | PRVD2018-05 |
| Fomesafen | 0.06 | 7 | PRVD2018-15 |
| Foramsulfuron | 54.3 | – | PRD2008-05 |
| Formetanate hydrochloride | 0.004 | 0.004 | PRVD2008-26 |
| Fosetyl-aluminum | 6 | – | PRVD2017-19 |
| Glufosinate-ammonium | 0.1 | – | – |
| Glyphosate | 2 | 7 | PRVD2015-01 |
| Halauxifen-methyl | 1 | – | PRD2014-12 |
| Halosulfuron-methyl | 0.4 | 6 | PRD2014-05 |
| Hexazinone | 0.6 | 3 | REV2017-11 |
| Imazamethabenz-methyl | 0.8 | – | PRVD2008-29 |
| Imazamox | 60 | – | RVD2016-04 |
| Imazapyr | 16 | – | PRD2020-17 |
| Imazethapyr | 3.6 | – | PRVD2010-02 |
| Imidacloprid | 0.4 | 0.5 | PRVD2016-20 |
| Indaziflam | 0.1 | 3 | PRD2017-09 |
| Inpyrfluxam | 0.4 | 2 | PRD2020-10 |
| Iodosulfuron-methyl-sodium | 0.47 | – | PRD2008-06 |
| Ipconazole | 0.1 | 0.91 | PRD2012-05 |
| Ipflufenoquin | 2 | 8.7 | PRD2022-18 |
| Iprodione | 0.09 | 1.8 | RVD2018-16 |
| Isofetamid | 0.3 | 8 | PRD2014-19 |
| Isoxaben | 0.3 | – | PRVD2014-04 |
| Isoxaflutole | 0.1 | – | PRVD2021-02 |
| Kasugamycin | 0.6 | – | PRD2012-30 |
| Kresoxim-methyl | 3 | – | PRVD2020-10 |
| Lambda-cyhalothrin | 0.002 | 0.006 | RVD2021-04 |
| Linuron | 0.016 | 0.345 | RVD2020-10 |
| Malathion | 0.2 | 1.5 | PRVD2010-18 |
| Maleic hydrazide | 1.6 | – | PRVD2008-24 |
| Mancozeb | 0.15 | 1 | RVD2020-12 |
| Mandestrobin | 2 | – | PRD2016-03 |
| Mandipropamid | 0.3 | – | PRD2016-20 |
| MCPA | 0.23 | 3 | – |
| MCPB | 0.11 | 0.47 | PRVD2011-06 |
| Mecoprop-p | 0.06 | 11.7 | – |
| Mefentrifluconazole | 0.3 | 10 | PRD2019-09 |
| Mesosulfuron-methyl | 9.92 | – | PRD2010-01 |
| Mesotrione | 0.006 | – | REG2005-02 |
| Metalaxyl | 0.48 | – | – |
| Metaldehyde | 0.06 | 0.5 | PRVD2008-15 |
| Metconazole | 0.01 | 0.06 | PRD2014-24 |
| Methomyl | 0.004 | 0.005 | PRVD2016-02 |
| Methoxyfenozide | 0.6 | – | REG 2004-08 |
| Metiram | 0.016 | 2 | PRVD2014-03 |
| Metrafenone | 1.6 | – | PRD2013-07 |
| Metribuzin | 0.053 | – | – |
| Metsulfuron-methyl | 0.533 | – | PRVD2008-08 |
| MGK-264 (N-octyl bicycloheptene dicarboximide) | 0.4 | 7 | PRVD2017-15 |
| Myclobutanil | 0.16 | 0.8 | PRVD2010-14 |
| Naled | 0.01 | 0.4 | PSRD2019-03 |
| N-alkyl dimethyl benzyl ammonium chloride (ADBAC) cluster | 2.8 | – | PRVD2008-23 |
| N-alkyl dimethylbenzylammonium saccharinate | 2.8 | – | PRVD2008-23 |
| Naphthalene acetates | 0.96 | 3 | PRVD2008-28 |
| Napropamide | 0.77 | – | PRVD2007-06 |
| Nicosulfuron | 9.02 | – | PRVD2008-01 |
| Novaluron | 0.3 | 0.3 | – |
| Oxamyl | 0.004 | 0.005 | – |
| Oxathiapiprolin | 30 | – | PRD2017-15 |
| Oxirane derivatives | 0.02 | 0.07 | PRVD2008-02 |
| Oxyfluorfen | 0.2 | – | PACR2005-03 |
| Paclobutrazol | 0.1 | 0.8 | PRD2023-10 |
| Pendimethalin | 0.6 | – | PRVD2007-07 |
| Penflufen | 0.3 | 3 | PRD2012-02 |
| Penthiopyrad | 0.6 | 8.33 | PRD2014-01 |
| Permethrin | 0.1 | 0.5 | PRVD2017-18 |
| Pethoxamid | 0.06 | 0.5 | PRD2019-12 |
| Phenmedipham | 0.21 | – | – |
| Phorate | 0.0007 | 0.00073 | – |
| Phosmet | 0.04 | 0.07 | PRVD2017-07 |
| Phosphine | 0.0723 | 0.12 | PACR2004-43 |
| Picarbutrazox | 0.1 | – | PRD2021-2 |
| Picloram | 1 | – | PRVD2007-04 |
| Picolinafen | 0.09 | – | PRDD2005-05 |
| Picoxystrobin | 0.29 | 4.5 | PRD2012-10 |
| Pinoxaden | 0.6 | 3 | REG2006-14 |
| Piperonyl butoxide | 0.2 | 10 | PRVD2020-09 |
| Potassium dimethyldithiocarbamate | 0.04 | 0.11 | – |
| Prohexadione-calcium | 1 | – | PRD2022-05 |
| Prometryn | 0.04 | 1 | – |
| Propamocarb hydrochloride | 0.77 | – | PRVD2015-03 |
| Propiconazole | 0.2 | 0.8 | PRVD2011-02 |
| Propoxur | 0.003 | 0.003 | RVD2014-01 |
| Propoxycarbazone-sodium | 5 | – | PRD2016-06 |
| Propyzamide | 0.17 | – | PRVD2008-20 |
| Prosulfuron | 0.34 | 0.7 | PRVD2015-02 |
| Prothioconazole | 0.01 | 0.06 | PRD2010-08 |
| Pydiflumetofen | 0.6 | 7 | PSRD2024-03 |
| PymetrozineFootnote * | 0.24 | 2.8 | PRDD 2002-03 |
| Pyraclostrobin | 0.11 | 0.47 | PRD2008-04 |
| Pyraflufen-ethyl | 1 | – | ERC2014-03 |
| Pyrasulfotole | 0.006 | 0.36 | ERC2007-11 |
| Pyraziflumid | 0.1 | 11 | PRD2022-04 |
| Pyrethrins | 0.06 | 0.5 | PRVD2020-08 |
| Pyridaben | 0.01 | 3 | PRVD2016-04 |
| Pyridate | 0.4 | 2 | PRD2021-4 |
| Pyrifluquinazon | 0.03 | 0.1 | PRD2022-15 |
| Pyrimethanil | 1.1 | 7 | PRD2013-10 |
| Pyriofenone | 0.6 | – | PRD2023-08 |
| Pyriproxyfen | 1 | – | PRVD2019-10 |
| Pyroxasulfone | 0.1 | 0.7 | PRD2012-20 |
| Pyroxsulam | 6 | – | ERC2010-04 |
| Quinclorac | 2 | 10 | PRVD2016-15 |
| Quintozene | 0.006 | – | PRVD2009-02 |
| Quizalofop-p-ethyl | 0.06 | – | PRVD2022-17 |
| Rimsulfuron | 0.61 | – | PRVD2008-06 |
| Rotenone | 0.003 | 0.41 | REV2008-01 |
| Saflufenacil | 0.094 | 0.47 | PRD2017-07 |
| Sedaxane | 0.6 | 2 | PRD2015-03 |
| Sethoxydim | 0.9 | 12 | PRVD2007-17 |
| S-methoprene | 0.4 | – | PRD2015-21 |
| S-metolachlor and R-enantiomer | 0.96 | 30 | PRVD2024-01 |
| Sodium chlorate | 0.2 | – | PRVD2008-09 |
| Sodium chlorite | 0.2 | – | PRVD2008-09 |
| Sodium dimethyldithiocarbamateFootnote * | 0.04 | 0.11 | – |
| Spinetoram | 0.2 | – | PRD2012-31 |
| Spinosad | 0.2 | 0.7 | – |
| Spirodiclofen | 0.09 | – | – |
| Spiromesifen | 0.04 | – | ERC2007-08 |
| Spirotetramat | 0.1 | 7 | PRD2008-07 |
| Spiroxamine | 0.1 | 0.7 | PRD2015-14 |
| Streptomycin | 0.3 | – | PRVD2008-16 |
| Sulfentrazone | 0.29 | 2.3 | ERC2010-08 |
| Sulfoxaflor | 0.03 | 0.2 | PRD2015-08 |
| Sulfuryl fluoride | 0.11 | 19.4 | PRD2016-30 |
| Tebuconazole | 0.02 | 0.08 | RVD2024-09 |
| Tebufenozide | 0.1 | 0.3 | RVD2021-01 |
| Tefluthrin | 0.003 | 0.007 | PRVD2010-01 |
| Tembotrione | 0.003 | 0.02 | – |
| TepraloxydimFootnote * | 0.1 | 3.6 | PRDD 2004-01 |
| Tetrachlorvinphos | 0.2 | 0.2 | PSRD2019-04 |
| Tetraconazole | 0.03 | 3 | PRD2012-29 |
| Tetraniliprole | 0.4 | – | PRD2019-14 |
| Thiacloprid | 0.03 | 0.07 | PRD2007-02 |
| Thiamethoxam | 0.03 | 0.7 | PRD2018-14 |
| Thiencarbazone-methyl | 7.49 | – | ERC2010-03 |
| Thifensulfuron-methyl | 0.08 | 15 | PRVD2008-03 |
| Thiophanate-methyl | 0.17 | 0.3 | PRVD2019-07 |
| Thiram | 0.01 | 0.01 | RVD2018-38 |
| Tiafenacil | 0.03 | – | PRD2022-01 |
| Tioxazafen | 0.3 | 5 | PRD2017-10 |
| Tolpyralate | 0.06 | 0.7 | PRD2017-13 |
| Topramezone | 0.0083 | 0.047 | REG2006-09 |
| Tralkoxydim | 0.03 | 1 | PRVD2009-08 |
| Triallate | 0.016 | 0.47 | PRVD2007-08 |
| Tribenuron-methyl | 0.042 | – | E95-04 |
| Triclopyr | 0.16 | – | – |
| Trifloxystrobin | 0.24 | – | PRD2009-02 |
| Trifludimoxazin | 0.5 | 0.5 | PRD2020-15 |
| Trifluralin | 0.15 | 30 | PRVD2008-22 |
| Triflusulfuron-methyl | 0.3 | 6 | PRVD2014-06 |
| Triforine | 0.1 | 10 | PRVD2019-02 |
| Trinexapac-ethyl | 0.2 | 0.8 | PRVD2022-01 |
| Triticonazole | 0.2 | 0.3 | PRVD2021-05 |
| Uniconazole-p | 0.1 | 0.3 | PRVD2019-09 |
| Ziram | 0.04 | 0.11 | RVD2018-39 |
| Zoxamide | 3 | – | PRVD2022-06 |
|
No longer approved for use in Canada, but these HHRVs are retained in the event of trace amount detection in drinking water sources. |
|||
Footnotes
- Footnote 1
-
Distinct from Guidelines for public water supplies sourced from water treatment facilities.
- Footnote 2
-
Nine pesticides with asterisks are no longer approved for use in Canada. These HHRVs are retained in the event of trace amount detection in drinking water sources.