PMRA Guidance Document, Approach to Special Reviews of Pesticides
Pest Management Regulatory Agency
2 February 2021
(PDF Version) (343 KB, 21 pages)
Document (Revision/Update) History
- Updated:
- February 2, 2021
- Update/Rationale:
- This document replaces Regulatory Directive DIR2014-01, Approach to Special Reviews providing additional information on special review triggers and the processes, taking into consideration the 2019 amendments of the Pest Control Products Act related to the special reviews.
Table of Contents
- 1.0 Introduction
- 2.0 Purpose
- 3.0 General information about the post-market review processes for pesticides
- 4.0 Special review triggers and initiation requirements
- 5.0 Special review process
- Appendix I Special review triggers and the 2019 amendments of the Pest Control Products Act related to special review requirements
- Appendix II Approach for addressing the aspect(s) of concern
1.0 Introduction
In Canada, pest control products, or pesticides, are regulated by Health Canada’s Pest Management Regulatory Agency (PMRA) on behalf of the Minister of Health and under the authority of the Pest Control Products Act. The Pest Control Products Act prescribes both the pre-market and post-market assessment of pesticides to determine the acceptability or continued acceptability of human health and environmental risks, and, value of a pesticide in Canada. Special review is one of the post-market review mechanisms provided for under the Pest Control Products Act.
2.0 Purpose
The purpose of this document is to describe the special review process for registered pesticides in Canada. In May 2014, the PMRA published a regulatory directive (DIR2014-01 Approach to Special Reviews) outlining the requirements for the special review of pesticides as set out in the Pest Control Products Act as well as the PMRA’s approach to special reviews. Subsequently, the 2019 amendments of the Pest Control Products Act give discretion to the Minister of Health as it relates to the initiation of a special review of a pesticide. This document replaces DIR2014-01, providing additional information on special review triggers and the processes, taking into consideration the 2019 amendments of the Pest Control Products Act related to special reviews (Section 4.0; Appendix I).
3.0 General information about the post-market review processes for pesticides
Under the Pest Control Products Act, pesticides are registered if the risks to human health and the environment are determined to be acceptable considering the conditions of use of the pesticide, and if the pesticide has value.
Following the registration of a pesticide, there are two post-market review processes available to determine the continued acceptability of registered pesticides according to acceptable standards: re-evaluation and special review. These processes differ in two aspects, namely, the triggers for the review, and the scope of the review. According to subsection 16(1) of the Pest Control Products Act, the PMRA may initiate a re-evaluation of a registered pesticide if there has been a change in the information required or the procedures used by the PMRA to determine that the pesticide meets health, environment, and value standards. In addition, subsection 16(2) of the Pest Control Products Act requires the PMRA to initiate re-evaluations of a registered pesticide on a 15-year cycle, based on the most recent major decision affecting the registration, including its initial registration. The scope of a re-evaluation is broad and addresses all relevant aspects of a pesticide.
Unlike a re-evaluation, the intent of a special review is to address specifically the identified aspect(s) of concern, and a special review is triggered only under certain circumstances, as described in section 17 of the Pest Control Products Act (see Section 4.0). The scope of a special review is narrower than a re-evaluation and it only evaluates the aspect(s) of concern that triggered the special review. The identified aspect(s) of concern that would otherwise prompt a new special review can also be addressed through an ongoing re-evaluation or special review (See Section 4.0).
For both re-evaluation and special review decisions, the PMRA applies internationally accepted science-based risk assessment and risk management approaches. The re-evaluation or special review assessment may result in a range of outcomes, including cancellation of a pesticide, or changes to the conditions of registration, including additional risk reduction measures.
Following the assessments, the PMRA publishes the proposed re-evaluation and special review decisions for public consultation before making a final decision. Documents associated with re-evaluations and special reviews, including decision documents, notices related to initiations and notices requiring additional information during the evaluations (for example, test data), are available to the public in the PMRA’s Public Registry on Canada.ca.
4.0 Special review triggers and initiation requirements
Section 17 of the Pest Control Products Act describes the triggers for initiating a special review, as well as the conditions under which initiation of a new special review is not required even when the triggers are met under subsections 17(1), 17(2), or 17(3).
The triggers for a special review of a registered pesticide outlined in the Pest Control Products Act are:
- Under subsection 17(1), if the Minister has reasonable grounds to believe that the health or environmental risks of a registered pest control product are, or its value is unacceptable.
- Under subsection 17(2) when an Organisation for Economic Co-operation and Development (OECD) member country prohibits all uses of a pesticide for health or environmental reasons.
- Under subsection 17(3), if the Minister has reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable based on information submitted by federal or provincial government departments or agencies.
- In addition, under section 14 of the Pest Control Products Act, if the Minister has reasonable grounds to believe that the health or environmental risks of the pest control product are, or its value is, unacceptable as per subsection 17 (1) based on the information reported under the additional information (section 12 of the Pest Control Products Act) or mandatory reporting (section 13 of the Pest Control Products Act) provisions of the Pest Control Products Act.
The 2019 amendments of the Pest Control Products Act give discretion to the Minister of Health as it relates to the initiation of a special review of a pesticide under subsection 17(1), 17(2) and 17(3). The amendments allow the PMRA to address the identified aspect(s) of concern through an existing post-market review, instead of initiating a new special review (see Section 5.1 for details), as follows:
- Subsection 17(7) allows the PMRA to expand the scope of an existing re-evaluation or special review before the final decision is made public under subsection 28(5), to include any aspect(s) of concern of a pesticide that would otherwise prompt a new special review under subsection 17(1), 17(2), or 17(3).
- Under subsection 17.1(1), the PMRA can decide not to initiate a new special review under subsection 17(1), 17(2) or 17(3), if the aspect of concern that would otherwise prompt a new special review is already being addressed in an ongoing re-evaluation or another special review.
- As per subsection 17.1 (2), the PMRA can decide not to initiate a new special review under subsection 17(2), if the aspect(s) of concern was previously addressed in a final, published re-evaluation or special review decision, and there is no additional information in relation to the risk of the product that would provide reasonable grounds to believe that the risks are unacceptable.
These amendments reduce the duplicative reviews of similar information under different post market reviews (re-evaluation or special review), while ensuring that the aspect(s) of concern has been assessed and adequately addressed. Based on the above considerations, when the special review triggers are met, the PMRA determines the best approach for addressing the aspect(s) of concern of the registered pesticide during the preliminary analysis phase (see Section 5.1 and Appendix II for details).
A special review may be triggered based on additional information that the PMRA becomes aware of (for example, through monitoring of international decisions). In addition, any person may request a special review through a request made to the Minister in the form and manner prescribed.Footnote 1 The reasons for requesting a special review must be relevant to registered Canadian uses and, if based on subsections 17(1) or 17(3), may include scientific and other relevant information relating to health or environmental risks or to the value of the product. If an OECD member country ban as described in subsection 17(2) is the reason for the special review request, the requestor should provide information concerning the applicable decision (for example, the OECD member country decision, a news item about the decision, etc.) that prohibits all uses of an active ingredient for health or environmental reasons.
5.0 Special review process
The PMRA uses a systematic approach for special reviews as described below. The depth of the review and the length of time required to conduct it are dependent on the number and complexity of the aspect(s) of concern associated with a given pesticide, the registrant’s requirements to conduct new studies, as well as the amount of information that requires assessment and/or that is received during consultation. Based on the above factors, following initiation, the average timeline for completion of a special review varies from two to four years. At any point during the special review, section 20 of the Pest Control Products Act allows the PMRA to cancel or amend the registration of the registered pesticide, if there are reasonable grounds to believe that it is necessary to manage a situation that endangers human health or the environment, taking into account the precautionary principle as outlined in the Pest Control Products Act.
Step 1: Preliminary Analysis
Step 2: Special Review Announcement
Step 3: Science Review of the Aspect(s) of Concern
Step 4: Public Consultation
Step 5: Final Special Review Decision
5.1 Step 1 – Preliminary analysis
The PMRA will carry out a preliminary analysis of the additional information and any relevant information in its possession relating to the aspect(s) of concern to determine whether the criteria under subsection 17(1), 17(2), or 17(3) are met. If the criteria are met, the PMRA will determine the approach for addressing the aspect(s) of concern; either through an existing post-market review or by initiating a new special review. Preliminary analysis will also determine information requirements for the special review.
5.1.1 Criteria for initiation of special reviews
a) Criteria to determine if there are reasonable grounds to believe that the risk or value of a pesticide is unacceptable (subsection 17(1) and 17(3)): As part of the preliminary analysis of information considered under sections 12, 13, subsection 17(1), 17(3), or 17(4) of the Pest Control Products Act, the PMRA determines whether the information indicates reasonable grounds to believe that the human health or environmental risks of the pesticide are, or its value is, unacceptable.
In general, the PMRA has reasonable grounds to believe that the risks are, or value of a pesticide is, unacceptable, when the additional information indicates a serious possibility of an increase in risk or a lack of value, or identifies a new risk, that
- may result in changes to the existing risk assessment conclusions, and
- anticipates changes will be needed to the existing conditions of registration to mitigate the risk or bring the value to an acceptable level.
If the above conditions are met, the PMRA considers that the criteria under subsections 17(1) or 17(3) are met. To determine this, as part of the preliminary analysis step, the PMRA compares the additional information with the existing assessment(s) and registered uses of the pesticide, including its conditions of use that mitigate the risks or maintain value. Conditions of use can include how the pesticide may be used and any existing restrictions (for example, application rate and method, protective equipment required, and buffer zones for sensitive areas).
Information that could alter the outcome of an existing risk assessment includes scientific results that indicate a greater hazard and potential for a lower toxicology reference value, a new risk (previously unidentified), or higher exposure levels than those previously considered by the PMRA. Consideration is given to the nature and degree of risk, or if there is change in value. If the potential change in hazard or risk is small, it is unlikely the additional information considered would alter the conclusions of the existing risk assessment and consequently the conditions of registration. In such situations, the reasonable grounds threshold to initiate a special review under subsection 17(1) or 17(3) would not be met. In addition, if the identified risk can be mitigated through the existing conditions of use (for example the label directions already include adequate personal protective equipment to minimize human health exposure or buffer zones to minimize exposure to non-target organisms), and no additional risk mitigation is anticipated, the PMRA considers that the criteria under subsection 17(1) or 17 (3) would not be met, and a special review would not be initiated.
Additional consideration for section 13 prescribed information (incident reports): Incident reports submitted to the PMRA under the Pest Control Products Incident Reporting Regulations can include a variety of information, including scientific studies and reported cases of adverse effects (reported incidents) involving the use of a pesticide. The PMRA conducts an in-depth review of all serious reported incidents as they are received to determine if there were unanticipated effects from the use of registered pesticides. Scientific studies are submitted to the PMRA if there is a new health or environmental hazard, increased health or environmental risk, or the presence of a component or derivative that has not been previously detected. The data are subjected to a preliminary analysis to determine if the criteria under subsection 17(1) have been met.
There are additional considerations when determining if the criteria under subsection 17(1) have been met for the cases of adverse effects reported under the Pest Control Products Incident Reporting Regulations. This information is considered critical for detecting potential risks, including those that are not evident at the time of the initial registration of a pesticide. However, there may be limitations with the submitted information that must be taken into account when reviewing these types of incident reports.
For example, the information is often unsubstantiated and incomplete, and the adverse effects reported may also be caused by non-pesticide related factors. Reporting of a particular effect does not necessarily mean that it was associated with the pesticide.
During the preliminary analysis of reported incidents, the following considerations are used to determine if there are increased health or environmental risks that could change the existing risk assessment:
- if there is an indication of harm based on the severity of the reported effect(s), the type of organism(s) affected, and/or the number of people, animals or other organisms affected;
- if there is strong supporting evidence that the pesticide could be associated with the reported effect;
- if it is likely that the incident could occur again, under the current conditions of use; and,
- if the risk has not already been addressed with the current conditions of use (for example, through existing risk mitigation measures on the label).
If the above criteria are met for the reported incidents, the next step would be to determine if changes to the conditions of registration of the pesticide (including cancellation) would likely be required in order to address the risk, as discussed above in Section 5.1.1a).
Conversely, some incidents occur, for example, because label directions were not followed. These types of incidents would not generally meet the criteria to initiate a special review because the risk was already addressed by the condition of use outlined on the product label. This situation would more likely be addressed through compliance actions, education, or improvement of the pesticide label.
Information that does not meet the criteria under subsection 17(1) or (3): If the information submitted under sections 12, 13, or subsections 17(3) or 17(4), does not meet the reasonable grounds test under subsection 17(1) or 17(3), it can still be used by the PMRA to inform regulatory assessments. For example, it can be used to help clarify or improve pesticide labels, develop education and training materials, and modify product packaging. The submitted information may also lead to monitoring and compliance activities.
b) Criteria under subsection 17(2) related to OECD-member country prohibitions: The PMRA gathers information regarding OECD member country prohibitions of pesticides through participation in international working group supporting activities of the OECD and the Rotterdam Convention, as well as from publicly available databases.
In determining the criteria under subsection 17(2), a preliminary analysis of the OECD member country’s decision regarding the prohibition of the pesticide is necessary to confirm that it meets the following conditions:
- the decision is from a member country of the OECD;
- all uses of the pesticide have been prohibited; and
- the prohibition is based on health or environmental reasons.
In determining the above conditions, the PMRA considers relevant information from other regulatory authorities and other sources as appropriate. For example, the PMRA may contact other international regulatory organizations to confirm the regulatory status and/or reasons for prohibition. Information from other sources, such as registrants, may also be considered. If the above criteria are not met, a special review will not be initiated under subsection 17(2). However, the PMRA may consider the information under subsection 17(1).
Following initiation of a special review of a pesticide under subsection 17(2), the regulatory status of that pesticide in the OECD member country may change, and the initial prohibition may be lifted. In such situations, the PMRA would terminate the special review under subsection 17(2), and would determine whether criteria to initiate a special review under subsection 17(1) has been met.
5.1.2 Identification of aspect(s) of concern:
As part of the preliminary analysis, the PMRA would identify the aspect(s) of concern that warrants further investigation.
5.1.3 Determination of the approach for addressing the aspect(s) of concern:
When the criteria under subsection 17(1), (2) or (3) are met for a pesticide, the PMRA addresses the aspect(s) of concern either:
- by initiating a new special review, or
- through an ongoing post-market review (re-evaluation or special review) of the implicated pesticide.
If criteria under subsection 17(1), (2) or (3) are met, the PMRA verifies whether there is any ongoing re-evaluation or special review of the implicated pesticide that includes the identified aspect(s) of concern and related information, or whether the scope of the ongoing post-market review can be expanded to include the aspect(s) of concern (and related information). The scope of the ongoing review would be expanded to address the new aspect(s) of concern only if it does not have a major impact on the timely completion of the ongoing review.
When the identified aspect(s) of concern can be addressed, and the accompanying additional information can be considered through an ongoing post-market review of the implicated pesticide in a timely manner, a new special review would not be initiated, and the aspect(s) of concern would be addressed in accordance with subsection 17(8) or section 17.1.
Additional consideration when the criteria under subsection 17(2) are met: There are additional considerations in determining the approach for addressing the aspect(s) of concern, when the criteria under subsection 17(2) are met. A new special review would not be initiated, even if the criteria under subsection 17(2) are met, when the following conditions are met:
- the aspect(s) of concern was previously addressed in a published, final re-evaluation or special review decision; and,
- there is no additional information from the OECD member country decision that provides reasonable grounds to believe that the health or environmental risks are unacceptable.
5.1.4 Duty to make decisions public:
The PMRA will publish the following decisions, and the reasons for those decisions in the Public Registry of the PMRA. They would also be communicated to the registrants, and, where applicable, the requestor of the special review.
- When the scope of a post-market review is expanded to address the aspect(s) that would otherwise prompt a new special review.
- When a new special review is not initiated, as the aspect(s) of concern was addressed previously in a final, published post-market decision, or
- When a new special review is not initiated because an ongoing post-market review already addresses the concern(s).
Figure 1 and Appendix II provide additional information regarding the approach for addressing the aspect(s) of concern.
5.1.5 Information requirements:
As part of the preliminary analysis, the PMRA would identify any additional information that may be required from registrants to address the aspect(s) of concern, either as part of a new special review or through an existing post-market review.
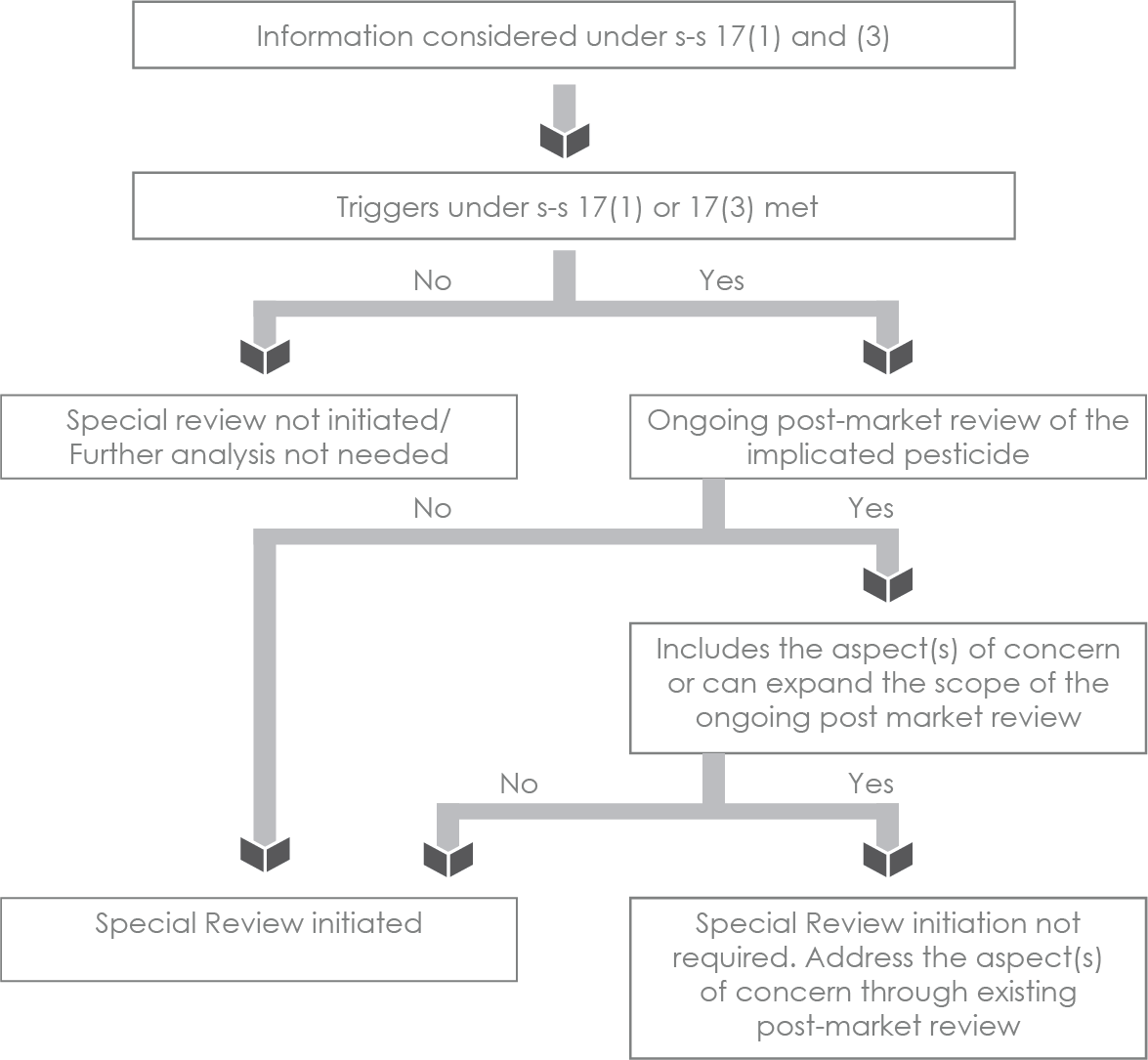
Figure 1a - Text description
The steps included in Figure 1a are:
- Determine whether triggers under s-s 17(1) or 17(3) are met, based on the information considered.
- If the triggers are not met, special review is not initiated and further analysis is not needed.
- If the triggers under s-s 17(1) or 17(3) are met, determine whether there is any ongoing post-market review of the implicated pesticide:
- If there is no ongoing post-market review, initiate a special review.
- If there is an ongoing post-market review of the implicated pesticide, determine whether it includes the aspect(s) of concern or the scope of the ongoing post-market review can be expanded:
- If the ongoing post-market review doesn’t include the aspect(s) of concern or the scope of the on-going post-market review cannot be expanded to include the aspect(s) of concern), a special review is initiated.
- If the ongoing post-market review includes the aspect(s) of concern or the scope of the on-going post-market review can be expanded to include the aspect(s) of concern, a special review initiation is not required. Address the aspect(s) of concern through existing post-market review.
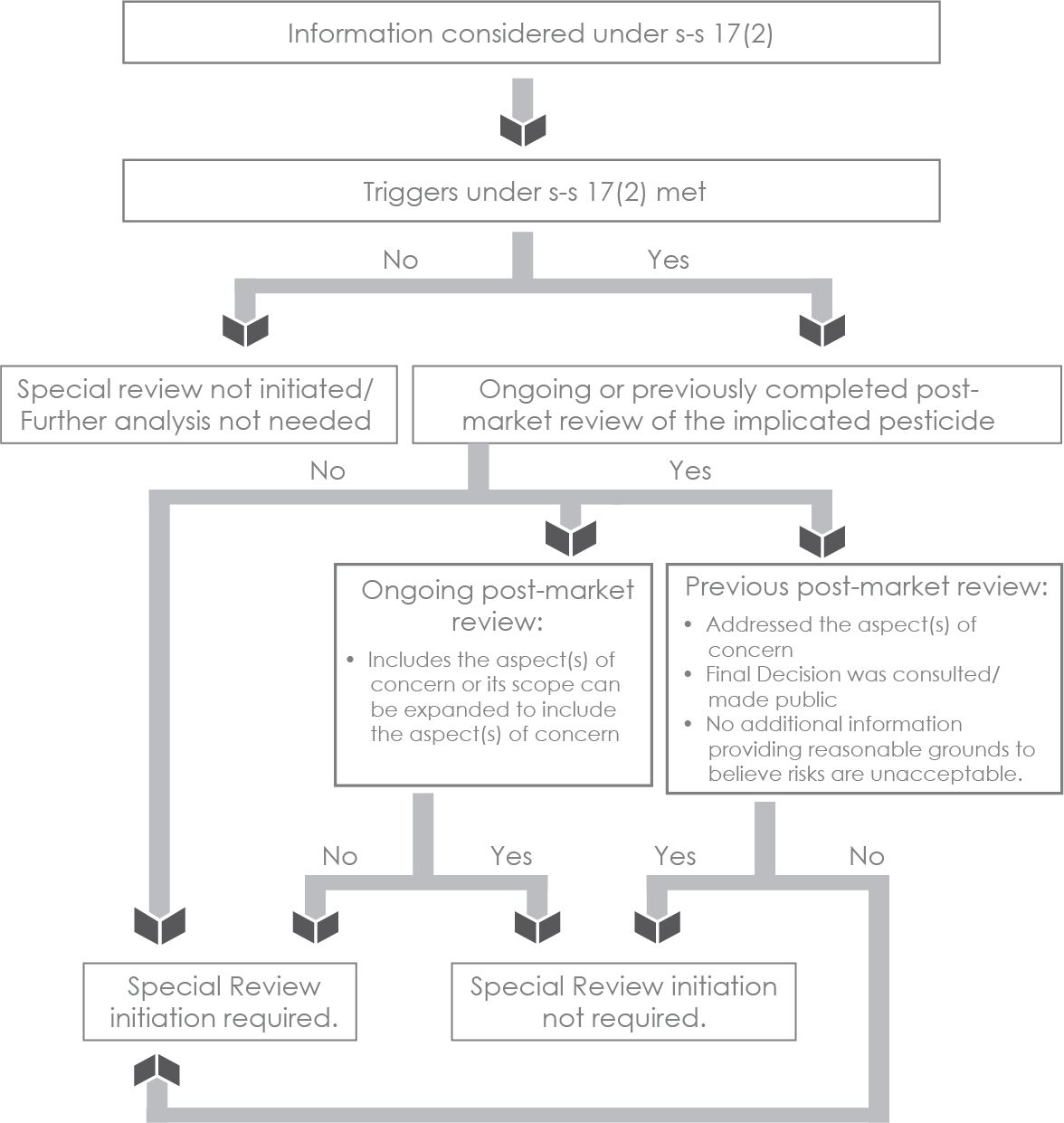
Figure 1b - Text description
The steps included in Figure 1b are:
- Determine whether triggers under s-s 17(2) are met, based on the information considered.
- If the triggers are not met, special review is not initiated and further analysis is not needed.
- If the triggers under s-s 17(2) are met, determine whether there is any ongoing or previously completed post-market review of the implicated pesticide.
- If there is no ongoing post-market review, or previously completed post-market review of the implicated pesticide, initiate a special review.
- Ongoing post-market review: If there is an ongoing post-market review of the implicated pesticide, determine whether it includes the aspect(s) of concern or the scope of the ongoing post-market review can be expanded:
- If the ongoing post-market review doesn’t include the aspect(s) of concern or the scope of the on-going post-market review cannot be expanded to include the aspect(s) of concern), a special review is initiated.
- If the ongoing post-market review includes the aspect(s) of concern or the scope of the on-going post-market review can be expanded to include the aspect(s) of concern, a special review initiation is not required. Address the aspect(s) of concern through existing post-market review.
Previous post-market review: If there is a previous post-market review, and the following conditions are met, special review initiation is not required. If the following conditions are not met, special review initiation is required.
- Addressed the aspect(s) of concern.
- Final decision was consulted/made public.
- No additional information providing reasonable grounds to believe risks are unacceptable.
5.2 Step 2 – Announcement of special review initiation (where applicable)
When a new special review is required under subsection 17(1), 17(2) or 17(3) of the Pest Control Products Act, registrants will be formally notified of the initiation, and an announcement will be published in the PMRA’s Public Registry. If there is a requestor, they will also be informed of the decision to initiate a special review.
If necessary, the PMRA will require the registrant to provide information relating to the aspect(s) of concern in accordance with subsection 18(1) of the Pest Control Products Act. The PMRA may also engage other stakeholders, such as product user associations, for information to clarify the current agricultural production practices and pesticide use. After a special review is initiated, the PMRA will request other relevant federal/provincial government departments and agencies to provide available information relevant to the aspect(s) of concern under subsection 18(2) of the Pest Control Products Act.
5.3 Step 3 – Science review of the aspect(s) of concern
The identified aspect(s) of concern will be evaluated, as required by subsection 18(4) of the Pest Control Products Act, either as part of a new special review or through an ongoing re-evaluation or special review. To assess the aspect(s) of concern, the PMRA considers the information that prompted the special review and other relevant information currently available, including existing assessments, information from international regulatory organizations, and information gathered from other federal/provincial government departments and agencies.
The PMRA uses the same internationally accepted science-based approaches for the assessment of the aspect(s) of concern as it does for all other scientific assessments (for example, new product registrations, re-evaluations). This step includes both risk (or value, if applicable) assessment and risk management to address the concerns identified.
In accordance with section 19 of the Pest Control Products Act, before the evaluations are completed, the PMRA provides registrants with an opportunity to make representations on any additional scientific data or information not submitted by the registrant that the PMRA is using in its review. The PMRA will assess the registrants’ comments in order to complete the evaluations.
5.4 Step 4 – Public consultation
Once the assessments are completed (Step 3), the PMRA prepares the proposed special review decision for public consultation. The consultation document contains a summary of the science evaluations and outlines the proposed special review decision. The consultation document is published on the Government of Canada website for a 45 day public consultation period.
When the aspect(s) of concern is addressed in an existing post-market review, the outcome of the assessment of that aspect(s) of concern will be included in the public consultation document for the existing post-market review. In cases where the aspect(s) of concern is addressed by expanding the scope of an existing re-evaluation or special review following the publication of its consultation statement under subsection 28(2) of the Pest Control Products Act, the PMRA will publish a new or amended consultation document that takes into account the aspect(s) of concern.
5.5 Step 5 – Final special review decision
After considering any comments and information received during the consultation period (Step 4), the PMRA will publish a final decision. A final decision includes: 1) a summary of the comments received, 2) PMRA’s response to those comments, 3) the final regulatory decision, including information regarding any required changes to the conditions of registration such as amended use directions on the label, and 4) implementation timelines. This information is also communicated to the registrants of the implicated products and, where applicable, the requestor of the special review.
Following the publication of a final regulatory decision, any person may file a Notice of Objection. The Notice of Objection must be filed within 60 days of the decision date. For more information regarding the basis of objection (which must be based on scientific grounds), please refer to the the Pesticides section of the Canada.ca website (Request a Reconsideration of Decision) or contact the PMRA’s Pest Management Information Service.
Appendix I Special review triggers and the 2019 amendments of the Pest Control Products Act related to special review requirements
1.0 Special review triggers
Subsection 17(1): The Minister shall initiate a special review of the registration of a pest control product if the Minister has reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable.
Subsection 17(2): Without limiting the generality of subsection (1), when a member country of the Organisation for Economic Co-operation and Development prohibits all uses of an active ingredient for health or environmental reasons, the Minister shall initiate a special review of registered pest control products containing that active ingredient.
Subsection 17(3): Without limiting the generality of subsection (1), the Minister shall initiate a special review of the registration of a pest control product if a federal or provincial government department or agency has provided information to the Minister that relates to the health or environmental risks or the value of the product and if, after considering the information provided, the Minister has reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable.
Section 14: After considering any information reported under section 12 or 13, the Minister shall determine whether a special review of the registration of the pest control product should be initiated.
Subsection 12(1) The Minister may, by delivering a notice in writing, require a registrant
- to compile information, conduct tests and monitor experience with the pest control product for the purpose of obtaining additional information with respect to its effects on human health and safety or the environment or with respect to its value; and
- to report the additional information to the Minister within the time and in the form specified in the notice.
Subsection 12(2) A requirement under subsection 12(1) is a condition of registration.
Section 13: An applicant for registration of a pest control product, a person who makes an application under subsection 10(2) or a registrant shall report any prescribed information that relates to the health or environmental risks or the value of the pest control product to the Minister within the prescribed time and in the form and manner directed by the Minister.
2.0 The 2019 amendments of the Pest Control Products Act related to special review requirements
Scope of special review:
Subsection 17(6): For the purposes of this section, the Minister shall initiate a special review only in relation to the aspect of the pest control product that prompted the special review.
Addition of aspect:
Subsection 17(7): If the Minister has initiated a re-evaluation of, or a special review in relation to, a pest control product, the Minister may, at any time before the decision statement is made public under subsection 28(5), expand the scope of the re-evaluation or special review to include any aspect of the product that would otherwise prompt a new special review under subsection (1), (2) or (3).
New or amended consultation statement:
Subsection 17(8): If the Minister expands the scope of a re-evaluation or special review under subsection (7) after the consultation statement relating to the re-evaluation or special review has been made public under subsection 28(2), the Minister shall make public a new or amended consultation statement under that subsection that takes into account the aspect referred to in subsection (7).
Discretion of Minister – aspect already covered:
Subsection 17.1(1): Despite section 17, the Minister may decide not to initiate a special review in relation to a pest control product if a re-evaluation of, or a special review in relation to, the product has already been initiated that includes the aspect of the product that would otherwise prompt a special review.
Discretion of Minister – previous decision statement:
Subsection 17.1(2): Despite subsection 17(2), the Minister may decide not to initiate a special review of a registered pest control product under that subsection if
- the Minister made public under subsection 28(5) a decision statement respecting a re-evaluation of, or a special review in relation to, that product;
- the aspect of the product that would otherwise prompt a special review was addressed by the re-evaluation or special review referred to in paragraph (a); and
- the Minister determines that there is no additional information in relation to the health or environmental risks of the product that provides the Minister with reasonable grounds to believe that those risks are unacceptable.
Duty to make decisions public:
Subsection 17.2: The Minister shall make public each of the following decisions and the reasons for it:
- a decision made under subsection 17(7) to expand the scope of a re-evaluation or special review to include an aspect that would otherwise prompt a new special review under subsection 17(2);
- a decision made under subsection 17.1(1) or (2) not to initiate a special review in relation to an aspect that would otherwise prompt such a review under sub section 17(2).
Appendix II Approach for addressing the aspect(s) of concern
| Special Review | Conditions |
|---|---|
| A new special review initiation is required under subsection 17(1) or 17(3): | When the preliminary analysis indicates there are reasonable grounds to believe that the health or environmental risks or value of a pesticide are unacceptable, and if,
|
| A new special review initiation is required under subsection 17(2): | When the preliminary analysis indicates that an OECD member country prohibits all uses of a pesticide for health or environmental reasons, and if,
|
| Special Review | Conditions |
|---|---|
| A new special review initiation is not required under subsection 17(1) or 17 (3) |
|
| A new special review initiation is not required under subsection 17(2) |
|
Footnotes
- Footnote 1
-
The Special Review Request Form is available on the Pesticides section of Canada.ca or may be requested through the Pest Management Information Service.