PMRA Guidance Document, Management of Submissions Policy
Pest Management Regulatory Agency
4 February 2025
Document (Revision/Update) History
| Updated: | Update/Rationale: |
|---|---|
| February 2025 | Revision to Section 4.2.1 Science Evaluation to specify two opportunities to address recurring Review Stage deficiencies. |
| May 2021 | This version of the PMRA Guidance document is an update to and replaces the previously published Regulatory Directive DIR2017-01, Management of Submissions Policy. Update references from the User Fees Act to the Service Fees Act as well as some minor updates. |
| March 2017 | PMRA Published Regulatory Directive DIR2017-01, Management of Submissions Policy. This document was an update to the previously published Regulatory Directive DIR2013-01, Revised Management of Submissions Policy. |
| April 2013 | PMRA Published Regulatory Directive DIR2013-01, Revised Management of Submissions Policy. This version of the document was a result of a consultation process via Regulatory Proposal PRO2010-05, Revised Management of Submissions Policy and replaced the previous Regulatory Proposal PRO96-01, Management of Submissions Policy. |
Table of Contents
- 1.0 Purpose
- 2.0 Scope
- 3.0 Instructions for Submitting an Application
- 4.0 The Management of Submissions Policy Process
- Appendix I Service Standards (Review Timelines), Completeness Check Timelines and Public Consultation Timelines for Pest Control Product Applications
- Appendix II Submission Process Using Category A as an Example
1.0 Purpose
Pursuant to subsection 6(1) of the Pest Control Products Act, no person shall manufacture, possess, handle, store, transport, import, distribute or use a pest control product that is not registered under the Pest Control Products Act, except as otherwise authorized under the Act or unless specifically exempted by the Pest Control Products Regulations.
The purpose of this version of the Management of Submissions Policy (MOSP) is to update the previous policy as outlined in Regulatory Directive DIR2017-01, Management of Submissions Policy:
- To incorporate changes related to the Service Fees Act on performance reporting and fee remissions that will apply to submissions received on or after 1 April 2021 that are subject to cost recovery fees. These changes were previously presented for feedback at the 29 May 2019 PMRA Stakeholder Information Session.
- To remove references to the User Fees Act which was repealed on 22 June 2017 and replaced by the Service Fees Act.
- To incorporate changes related to the payment of fees that came into effect 1 January 2020.
- To change all references to "re-evaluations" to "post-market reviews".
2.0 Scope
This document pertains to all applications for:
- The registration or amendment of a pest control product,
- The specification of a maximum residue limit (MRL),
- The authorization or notification of research,
- The issuance of an equivalency certificate and authorisation of importation for own use,
- Pre-submission consultations,
- Extension of exclusive use protection, and
- Equivalency and data compensation assessments.
The same general process applies to all categories of submissions. However, some steps are not required for all categories. Depending on the purpose of the application and the type of information required, every submission subject to the MOSP is assigned to one of the eight following categories:
Category A
- New active ingredients or integrated system products, their related end-use products, and manufacturing-use products.
- Major new use of registered pest control products (defined as the addition of a new use-site category to the use pattern for a specific registered active ingredient).
- Specification of import MRLs for an unregistered active ingredient.
Category B
- New pest control products containing registered active ingredients.
- Amendment to existing pest control products (for example, product chemistry, labelling).
- Emergency registration.
- The addition of import MRLs for previously assessed active ingredients.
Category C
- Product registrations and amendments with no data requirements. These applications involve minor label or formulation reviews, such as product registration based on registered precedent products.
Category D
- Submissions within particular programs including:
- Import for Manufacture and Export Program (IMEP),
- Own Use Import (OUI),
- Grower Requested Own Use (GROU) Equivalency and import permits,
- Master Copies,
- Private Labels,
- Registration Renewal, and
- Discontinuations.
Category E
- Research authorisations for new active ingredients and new use(s) of registered active ingredients.
- Research notification for research carried out in Canada.
Category F
- Registration and amendments to registered pest control products via notification.
Category L
- Submissions to register or amend products including new sources of technical grade active ingredient, manufacturing concentrates and end use products where the applicant wishes to use or rely upon data provided by another registrant.
- Requests to extend the exclusive use protection period based upon minor uses.
Category P
- Pre-submission consultations.
3.0 Instructions for submitting an application
Various guidance documents and instructions on submitting information are available on the Pesticides section of the Canada.ca website to help applicants prepare a complete application package.
4.0 The Management of Submissions Policy process
The following sections provide a step-by-step description of the submission review process.
Under the MOSP, applications are typically reviewed in chronological order within each MOSP category subdivision. However, under certain circumstances timelines may be adjusted:
- If there is a critical need, an expedited reviewFootnote 1 may be considered. The intent of expedited reviews is to meet the urgent needs of users of pest control products, to facilitate risk reduction or to address a public health or environmental concern. For example, a formulation amendment to replace a formulant of concern, or products needed to mitigate a public health or environmental risk may be expedited.
- Related submissions may be grouped in order to follow the same review timeline. The grouping of related submissions occurs when one submission depends upon the success of the other. For example, an end use product cannot be registered before the technical grade active ingredient is registered and conversely, an active ingredient must have a use associated with it to be registered.
- PMRA Guidance Document, Organizing and Formatting a Complete Submission for Pest Control Products contains information on how the PMRA processes tailgater submissions which can result in atypical timelines for submissions. A tailgate submission is defined as a submission for a new or existing product for which a current submission is open, past screening and awaiting a regulatory decision. A tailgate submission cannot be reviewed until the previously submitted application has been accepted or proposed for registration. Tailgate submissions delay the processing of the original submission by causing the review to cease and refocus to encompass the amendment. The following options are given to the applicant of a tailgater submission: (a) withdraw the tailgate submission and resubmit when the precedent submission is approved; (b) maintain the tailgate submission but combine it with the precedent submission (adopt its category and status) and reset the timelines for the precedent submission to start at the time of the tailgate submission; or (c) screen and delay: that is, allow tailgate submission, perform preliminary screen and delay processing within the PMRA until the original submission is accepted for registration. During the delay status, any changes in scientific approach or new policies will apply to the submission in delay.
- PMRA sometimes receives submissions to expand, or change use patterns, or to make substantial amendments to the conditions of registration while a post-market review is underway. Thus, in order to reach consistent and timely regulatory decisions, PMRA coordinates the review of these pre-market submissions and the science review component of the post-market review. Consequently, PMRA applies any updated science findings to any subsequent (pre-market and post-market) decisions.
The MOSP completeness check timelines, service standards (review timelines) and public consultation timelines in calendar days are summarized in Appendix I, Tables 1 to 8 (for Categories A to F, L and P respectively).
Within each category there are further subdivisions (for example, submission subcategories, submission types, classes) that may have different completeness check timelines and/or service standards (review timelines) because they are conducted under specific programs (for example, Joint Review).
A submission flow diagram is depicted in Appendix II to illustrate the MOSP examination process for a standard Category A submission. Note: other categories will follow the same process using the performance timelines provided in Appendix I. However, some steps will not apply to every submission category and/or submission subdivision (for example, public consultation is not required for Category C submission decisions). Also, for some submission subdivisions the screening and review are combined, for example, master copy and private labels or in the case of notifications the completeness check and review are combined.
4.1 Completeness check (verification and screening)
A completeness check will be performed on all submissions to ensure a complete submission has been received before the review stage is started. The timelines for the completeness check are outlined in Appendix I. When the submission is received by the PMRA, the completeness check clock will start. The completeness check will generally consist of an initial verification step and a more detailed screening step.
4.1.1 Verification
For most submission categories there is a seven-calendar-day verification step during which submissions are verified to ensure that non-data elements have been provided. Non-data elements may include the covering letter, the appropriate application form, the Statement of Product Specification Form, the Fee Estimate Form, and the e-index. A submission found to be deficient at the verification step will result in an e-mail, outlining the deficiencies, being sent to the applicant and the submission being placed "on-hold". The applicant is given 14 calendar days to address the deficiencies. When a response is received from the applicant, a second verification period of a maximum of seven calendar days will apply and the completeness check clock will be reset to day 0. Lack of an adequate response will result in the submission being rejected.
Applicants are provided a submission number acknowledging receipt of the submission. This number should appear on all subsequent correspondence to the Agency relating to that submission.
4.1.2 Screening
For most submission categories there is a 30 calendar day screening step during which submissions are screened to ensure they meet the format, data and fee requirements of the PMRA before they are accepted for review.
Clarifications
The PMRA may request minor clarifications concerning submitted information by e-mail or facsimile (for example, clarification of the Statement of Product Specification Form). The applicant has 10 calendar days to respond to the clarification request; screening continues during this time.
If an adequate response to the request for clarification is not provided within the timeframe specified, a Notice of Deficiencies will be issued. A Notice of Deficiencies can also be sent to the applicant when significant deficiencies are identified during screening.
Note: Clarifications may also be sent out during the review stage–refer to Section 4.2.
Notice of deficiencies
During the screening stage, the PMRA will place a submission "on-hold" if deficiencies are identified in the application requirements, or if insufficient information has been submitted (for example, if required test data is missing). Note: deficiencies may also be identified during the review stage; refer to Section 4.2.
When a Notice of Deficiencies is issued at screening (at which time the completeness check clock stops), the applicant must respond to the Notice of Deficiencies within the timeframe specified in the notice, usually 45 calendar days, and provide all of the requested information as directed. (Note: When one submission in a group of related submissions is put "on-hold", all of the related submissions will also be put "on-hold"). When a response to the Notice of Deficiencies is received by the PMRA, the completeness check clock will be reset with 15 calendar days on the clock.
No reminders will be issued. If there is no response or if the response is incomplete or inadequate, the application will be denied in accordance with subsection 7(5) of the Pest Control Products Act, unless the applicant withdraws the application. Any submission that has been previously withdrawn by the applicant, or denied by the PMRA during a previous examination, may be re-submitted at a future date. It will be considered a new submission and assigned a new submission number.
4.2 Review stage
The review stage includes the following activities:
- science evaluation of the health and environmental risks and the value of the pest control product to determine if they are acceptable,
- the review of the bilingual product label, and
- the decision-making process.
The service standards (review timelines) outlined in Appendix I are in calendar days allotted to the PMRA to conduct all of the steps in the review stage. Note: If related submissions have different review timelines, the longer review timeline will usually apply to all of the submissions in the group.
The review stage clock will start as soon as the completeness check is completed.
Excluded from the review timeline is the 45 calendar day public consultation period for major regulatory decisions (for example, new active ingredients and major new uses) conducted via the publication of a Proposed Registration Decision.
4.2.1 Science evaluation
The PMRA may request clarifications of minor points on submitted data by e-mail or facsimile. The applicant has 10 calendar days to respond to the clarification request; the review continues during this time. If an adequate response to the request for clarification is not provided within the 10 calendar days, a Notice of Deficiencies will be issued, the submission will be placed "on-hold", and the review stage clock will stop. Note: As indicated previously, when one submission in a group of related submissions is placed "on-hold", all related submissions will also be put "on-hold".
If deficiencies are identified by a single science review stream at any time during the review stage, a Notice of Deficiencies will be sent to the applicant, the submission will be placed "on-hold", and the review stage clock will stop. The science review stream to which the deficiencies apply will stop that portion of the review; however, the remaining science review streams will continue to actively work on the submission during this time if this is possible, and determined to be efficient. The applicant is given a specified number of days (usually 90 calendar days) to fulfil the requirements outlined in the Notice of Deficiencies. There will be no reminders provided during the "on-hold" period. When the response is received within the required timeframe, the review stage clock will immediately restart and the affected science review stream will continue their review. If the response is inadequate, a second Notice of Deficiencies may be issued in which case the preceding process will repeat. If, following the second Notice of Deficiencies issued for a recurring deficiency, the response is inadequate, the application may be denied in accordance with subsection 7(5) of the Pest Control Products Act, unless the applicant withdraws the application. Lack of a response, or an incomplete response to any Notice of Deficiencies within the required time frame will result in the application being denied in accordance with subsection 7(5) of the Pest Control Products Act, unless the applicant withdraws the submission.
The PMRA will issue one consolidated Notice of Deficiencies to the extent possible; however, in the event that deficiencies are identified by a second science review stream during the course of the first "on-hold", a second Notice of Deficiencies will be sent to the applicant and the review stage clock will remain stopped. The applicant will be given a specified number of days (usually 90 calendar days) from the time of the second Notice of Deficiencies to fulfil the identified data requirements. This new timeline will serve as the timeline for receipt of all data. Upon receipt of a response to a Notice of Deficiencies the science review stream for whom the deficiencies have been addressed will resume review of the submission; however, the review stage clock will remain "stopped" if any Notices of Deficiencies remain outstanding, and the review will remain on-hold for any science review stream for whom a Notice of Deficiencies remains outstanding.
The review stage clock will not restart until such time that a response to all outstanding Notices of Deficiencies has been received by the PMRA.
If the applicant has questions on any Notice of Deficiencies, the applicant is encouraged to contact the administrative coordinator immediately.
4.2.2 Label review
To facilitate timely issuance of the approved product label, separate French and English labels must be submitted with the application. Provided the proposed product label is acceptable, the label review continues throughout the review process and the PMRA will make necessary label revisions to the proposed product label. Should extensive changes be required to the proposed product label the PMRA will request the applicant to make changes and provide an updated proposed product label during the review process. Where possible, translation of label revisions resulting from the science evaluation will be provided by the PMRA.
The PMRA will communicate to the applicant any changes resulting from the science evaluation before finalizing the product label. This will provide an opportunity for the applicant to clarify issues arising from the label revisions.
4.2.3 Decision
If there is sufficient scientific evidence to show that a product does not pose unacceptable health or environmental risks and that it has value, a decision to issue an authorisation (permit) or register the product will be made. The applicant will receive a decision letter and at the same time, if applicable, the PMRA will issue the approved bilingual label and the certificate of registration.
4.3 Public consultation
A bilingual consultation document (Proposed Registration Decision) is published for all major decisions (for example, new active ingredients and major new uses of registered pesticides) as defined under subsection 28(1) Pest Control Products Act.
The consultation period for a Proposed Registration Decision is 45 days from the date of publication. Any comments received during the consultation period are considered before a final decision is made, that is, issuance of the final decision letter, approved label and certificate of registration.
4.4 Renegotiation of review timelines
Review timelines as detailed in Appendix I may need to be renegotiated by the PMRA and the applicant for the purpose of synchronizing the reviews of related submissions, or to allow for the review of additional information required to make a regulatory decision.
4.5 Measures
Completeness check time
The time taken from initial receipt of an application (or from when a response to a verification deficiency is received) to the end of the first screening.
Review time
In general, the time after the completeness check is completed to when a final regulatory decision is made, excluding applicant time (when a submission is placed "on-hold" pending an applicant response to a Notice of Deficiencies) and excluding public consultation time.
Applicant time
The time when a submission is pending an applicant to respond to a Notice of Deficiencies, in other words, when the completeness check or review clocks are "on-hold".
Total time
From the date that an application is received to the date that the submission is registered, rejected, withdrawn, denied or completed.
Performance target
The PMRA's performance target is that 90% of submissions in all categories are to be processed within the applicable review timelines.
4.6 Service Fees Act reporting
For the purpose of reporting under the Service Fees Act, the PMRA will report performance against service standards (review timelines) on submissions subject to fees for each individual submission category and MOSP review timeline.
Fee remissions will be issued in accordance with the PMRA Remission Policy for Missed Service Standards (https://www.canada.ca/en/health-canada/services/funding/cost-recovery-service-fees/pest-management-regulatory-agency-remission-policy-missed-service-standards.html).
4.7 Dispute resolution
To minimize disputes, applicants are encouraged to familiarize themselves with the pesticide registration process and registration requirements and to request, when appropriate, a pre-submission consultation.
The PMRA will make every effort to manage and resolve disputes at the organizational level at which they take place.
Disputes regarding the screening of an application, including: screening deficiencies, data requirements, and screening timeline should be addressed to the screening officer assigned to the submission.
Disputes regarding the review of the submission, including: review deficiencies, data requirements, review timeline, labelling revisions and review decision should be addressed to the administrative coordinator assigned to the submission.
If mechanisms for early dispute resolution fail, applicants should contact the Chief Registrar's Office of the PMRA. For major regulatory decision proposals, there is an opportunity for the applicant (or any member of the public) to comment during the public consultation period. In addition, for any major regulatory decisions for which a public consultation under section 28(1) of the Pest Control Products Act was required before a regulatory decision was taken, the applicant (or any member of the public) has another opportunity to comment by filing a Notice of Objection requesting the reconsideration of the decision within 60 days after the decision is made public.
For additional information on the reconsideration of decision process, please consult the Pesticides section of the Canada.ca website (Request a Reconsideration of Decision) or contact the Health Canada PMRA's Pest Management Information Service.
Appendix I Service standards (review timelines), completeness check timelines and public consultation timelines for pest control product applications
(MOSP Performance Target = 90% of submissions to be processed within the applicable review timelines)
| Category Subdivision | Completeness Check Timeline in Calendar Days | Service Standard (Review Timelines in Calendar Days (Months)Footnote 1 | Public Consultation timeline in Calendar Days |
|---|---|---|---|
| Conventional chemical | 37 | 655 (22) | 45 |
| Import MRLFootnote 2 | 37 | 655 (22) | n/a |
| Reduced-riskFootnote 3, other biopesticides, non-conventionals, NSCLPFootnote 4 | 37 | 555 (18.5) | 45 |
| Microbials including URMURFootnote 6 | 37 | 470 (15.5) | 45 |
| URMURFootnote 6 for conventional chemical, Reduced-risk, other biopesticides, non-conventionals, NSCLPFootnote 4 | 37 | 470 (15.5) | 45 |
| Pheromones – SCLPFootnote 5 including URMURFootnote 6 | 37 | 285 (9.5) | 45 |
| Joint reviews | Negotiated | 45 | |
| Submissions with atypical timelines, for example, tailgater submissions, renegotiated timelines, synchronized timelines, coordination with post-market reviews | 37 or variable | Variable | 45 |
| Category Subdivision | Completeness Check Timeline in Calendar Days | Service Standard (Review Timelines) in Calendar Days (Months)Footnote 1 |
|---|---|---|
| Conventional chemical including emergency use (priority) | 37 | 425 (14) |
| New MRL for previously assessed active ingredientFootnote 2 | 37 | 425 (14) |
| Reduced-riskFootnote 3, other biopesticides, non-conventionals, NSCLPFootnote 4 including emergency use (priority) | 37 | 360 (12) |
| Microbials including emergency use (priority) | 37 | 240 (8) |
| Pheromones – SCLPFootnote 5 including emergency use (priority): | 37 | 240 (8) |
| Streamlined (application rate changes, tank mixes, new pests or changes to level of control) | 37 | 158 (5) |
| Joint review | Negotiated | Negotiated |
| Submissions with atypical timelines, for example, tailgater submissions, renegotiated timelines, synchronized timelines, coordination with post-market reviews | 37 or variable | Variable |
| Category SubdivisionFootnote 2 | Completeness Check Timeline in Calendar Days | Service Standard (Review Timelines) in Calendar Days (Months)Footnote 1 |
|---|---|---|
| New/Changes TGAI or ISP Product Chemistry (C.1) | 37 | 180 (6) |
| New/Changes to EP or MA Product Chemistry (C.2) | ||
| Administrative Changes (C.6.2) | ||
| Administrative Re-instatement (C.9) | ||
| New/Changes to Product Labels (C.3) | 37 | 240 (8) |
| Addition of approved minor use (C.6.3)Footnote 3 | ||
| Similar Product (C.7) | ||
| Upgrade to Initial or Master Product (C.8)Footnote 4 | See category F | See category F |
| Submissions with atypical timelines, for example, tailgater submissions, renegotiated timelines, synchronized timelines, coordination with post-market reviews | 37 or variable | Variable |
| Category Subdivision | Completeness Check Timeline in Calendar Days | Service Standard (Review Timelines) in Calendar DaysFootnote 1 |
|---|---|---|
| IMEP | 21 | 46 |
| OUI equivalency certificate | 21 | 70 |
| OUI permit | Not Applicable | 30 days total time |
| GROU equivalency certificate | To be determined | To be determined |
| GROU permit | Not Applicable | 30 days total time |
| Master copy | 7 verification | 42 screen and review |
| Private label | 7 verification | 10 screen and review |
| Registration renewal | Not Applicable | Number of days from the issuance of the renewal notice to March 15 of the following year |
| Discontinuation | 7 verification | 45 screen and review |
| Submissions with atypical timelines, for example, tailgater submissions, renegotiated timelines, synchronized timelines, coordination with post-market reviews | 37 or variable | Variable |
| Category Subdivision | Completeness Check Timeline in Calendar Days | Service Standard (Review Timelines) in Calendar DaysFootnote 1 |
|---|---|---|
| New technical grade active ingredient (food and non-food use) | 21 | 159 |
| New use | 21 | 69 |
| Notification of research | Not Applicable | 30 days total time |
| Category Subdivision | Service Standard (Review Timelines) in Calendar DaysFootnote 1 |
|---|---|
| All Notifications | 45 days total time |
| Category Subdivision | Completeness Check Timeline in Calendar Days | Service Standard (Review Timelines) in Calendar DaysFootnote 1 | ||
|---|---|---|---|---|
| Equivalency and data compensation assessment TGAI, EP and MA with no data | Not Applicable | 365 day total time | ||
| Equivalency and data compensation assessment EP and MA with partial data package | Conventional Chemical | 37 | 425 | |
| Reduced-riskFootnote 2, other biopesticides, non-conventionals, NSCLPFootnote 3 | 37 | 360 | ||
| Microbial and SCLPFootnote 4 | 37 | 240 | ||
| Regulatory Decision | Not Applicable | 45 days total time | ||
| Requests to extend the exclusive use protection period based upon minor uses | 37 | 240 | ||
| Submissions with atypical timelines, for example, tailgater submissions, renegotiated timelines, synchronized timelines, coordination with post-market reviews | 37 or variable | Variable | ||
| Category Subdivision | Service Standards (Review Timelines) in Calendar DaysFootnote 1 |
|---|---|
| Pre-submission ConsultationFootnote 2 | 80 days total time |
Appendix II Submission Process Using Category A as an Example
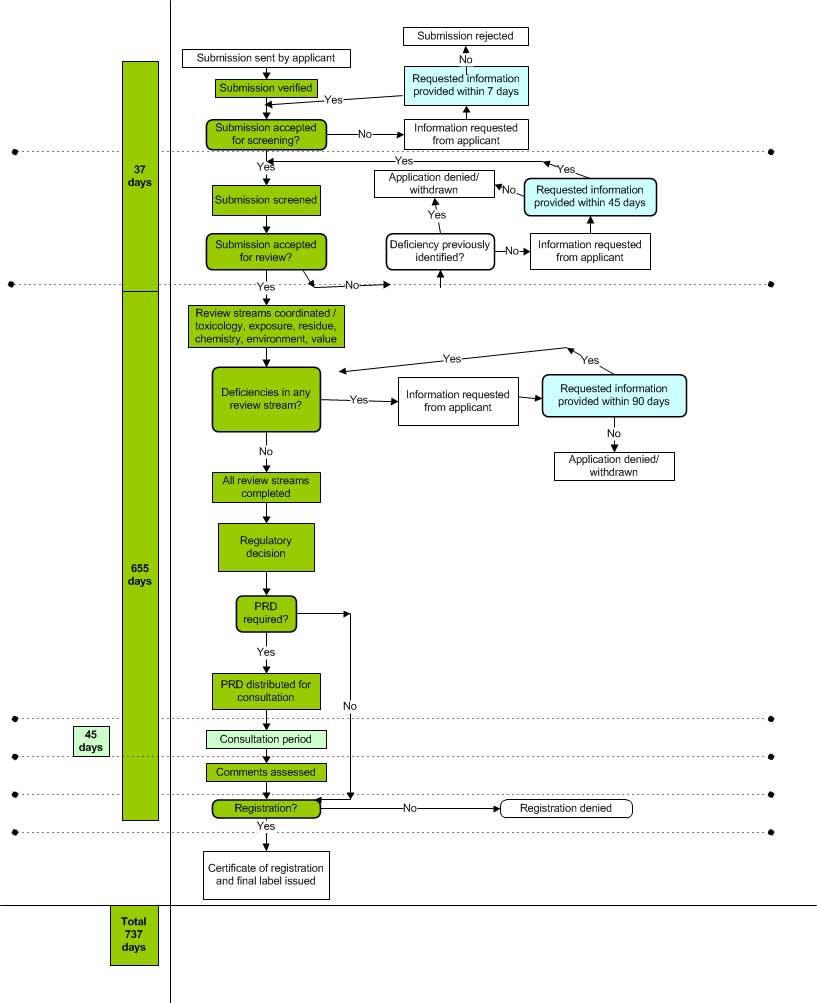
Text Description
The image is a flowchart that outlines the process for submissions using Category A as an example.
The applicant sends their application to the PMRA. The PMRA verifies the submission to ensure that non-data elements - including the covering letter, the appropriate application form, Statement of Product Specification Form (SPSF), fee form, fee and e-index - have been provided.
If the submission has met the verification criteria, the submission can be advanced to the screening level. If the submission has not met the verification criteria, the submission will go on hold. If the submission has not met the verification criteria, an email requesting the missing information is sent to the applicant. The applicant has 14 days to provide the missing information. If the applicant did not provide the missing information within 14 days, the submission is rejected.
At the screening level, the submission is screened to ensure the submission meets the format, data and fee requirements of the PMRA. If the submission has met the screening criteria, the submission can be advanced to the review stage. If the submission has not met the screening criteria, the submission will go on hold. If the deficiency was not sent to the applicant previously, a notice of screening deficiencies is sent to the applicant to request the missing information. The applicant has 45 days to provide the missing information. The application is denied or withdrawn when the applicant requests the submission be withdrawn, the submission is denied because the applicant did not provide the missing information within 45 days or the information provided was inadequate.
The PMRA has 37 days to conduct the verification and screening of the application.
During the review stage, the work of the different review streams (toxicology, exposure, residue, chemistry, environment, value) is coordinated.
If the submission is deficient, a notice of deficiencies is sent to the applicant requesting the missing information. The applicant must provide the requested information within 90 days. If the missing information was provided, the review resumes. The application is denied or withdrawn when the applicant has requested that the submission be withdrawn, the submission is denied because the applicant did not provide the missing information within 90 days or the information provided was inadequate.
If the submission is not deficient, all the review streams have completed their review and the PMRA makes a regulatory decision on the submission.
For some submissions, public consultation may be required via the publication of a Proposed Registration Decision. If a public consultation is required, a Proposed Registration Decision is prepared. The public has 45 days to comment on the Proposed Registration Decision. The PMRA assesses any comments made on the Proposed Registration Decision. The PMRA makes a final regulatory decision on the submission.
The PMRA has 655 days to conduct the review of the application. The review starts after screening is completed and ends when the final regulatory decision is made.
When making a decision, the PMRA denies the registration of the submission or the PMRA issues a certificate of registration and the final approved labels of the product.
The total number of days from when the submission is received from the applicant to when a final regulatory decision is made is 737 days. This is the number of days it should take the PMRA to examine the submission if there are no deficiencies in the submission.