Guidance on obtaining electronic certificates of pharmaceutical product and good manufacturing practices (GUI-0024)
Date approved: March 27, 2023
Effective date: March 27, 2023
Replaces: Guidance document on the application for a certificate of a pharmaceutical product and good manufacturing practice certificate (GUI-0024)
Disclaimer
This document does not constitute legislation. In the event of any inconsistency or conflict between the legislation and this document, the legislation takes precedence. This document is an administrative document that is intended to facilitate compliance by the regulated party with the legislation and the applicable administrative policies.
On this page
- Purpose and scope
- Background
- Definitions
- Certificate applications
- Certificate application policies
- Contact us
Purpose and scope
Purpose
This guidance document outlines the policies and procedure for applying for and obtaining electronic certificates for:
- a pharmaceutical product
- good manufacturing practices (GMP)
Scope
This document covers electronic certificates issued:
- for human drugs (pharmaceutical, biological and radiopharmaceutical) and for veterinary drugs (food-producing animals and non-food producing animals)
- known as a certificate of a pharmaceutical product (CPP)
- to attest to the GMP compliance of a manufacturing site as per the drug establishment licence (DEL)
- known as a GMP certificate
Health Canada issues these certificates once we have confirmed compliance to all GMP requirements in accordance with the Food and Drugs Act and its Regulations.
Background
Access to quality pharmaceuticals is important and can be a major public health challenge in light of growing import and export activities.
The World Health Organization (WHO) develops standards and guidelines to promote quality assurance for pharmaceuticals. These standards and guidelines are endorsed and supported through numerous World Health Assembly (WHA) resolutions. The WHA has also adopted many resolutions to develop international standards, recommendations and instruments. These resolutions are designed to assure the quality of medicines, whether they are produced and traded nationally or internationally.
These WHA resolutions also provide the basis for the WHO's certification scheme (established in 1969) on the quality of pharmaceutical products moving in international commerce. The certification scheme is a voluntary international agreement. It's designed to enable countries with limited drug regulatory capacity to obtain assurance from exporting countries that the pharmaceutical products they plan to import are safe, effective and of good quality.
The most recent version of the certification scheme is outlined in the 55th report of the WHO Expert Committee on Specifications for Pharmaceutical Preparations. It includes revisions such as the establishment of standard time frames for decision-making and updates on legalization procedures.
Health Canada adopted the WHO Certification Scheme on May 1, 1996. Since then, we have been issuing CPPs as a service to the industry when required by an importing country.
We also issue GMP certificates, which are not included in the scope of the WHO's certification scheme. Health Canada voluntarily issues GMP certificates to enable and facilitate export activities for Canadian companies. Similar to CPPs, GMP certificates also provide assurance to countries with limited regulatory capacity that sites performing licensable activities are in compliance with Canadian GMP standards.
Health Canada's Regulatory Operations and Enforcement Branch's (ROEB) Compliance Directorate issues CPP and GMP certificates.
Definitions
Application package: Includes the application form(s), fee form and letter of authorization (if necessary).
Certificate of a pharmaceutical product (CPP): Indicates the status of the pharmaceutical, biological, radiopharmaceutical or veterinary product listed and the GMP status of the fabricator of the product. This certificate is issued electronically and in the format recommended by the WHO.
Drug: Any substance or mixture of substances manufactured, sold or represented for use in:
- the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals
- restoring, correcting or modifying organic functions in human beings or animals or
- disinfection in premises in which food is manufactured, prepared or kept
(Section 2 of the Food and Drugs Act)
Drug identification number (DIN): An 8-digit numerical code that Health Canada assigns to each drug product marketed under the Food and Drugs Act and Regulations. A DIN uniquely identifies the following product characteristics:
- manufacturer
- brand name
- medicinal ingredients
- strength of medicinal ingredients
- pharmaceutical form
- route of administration
Good manufacturing practices (GMP) certificate: Issued to attest to the GMP compliance of a manufacturing site as per the DEL. This certificate is issued electronically.
Certificate applications
Types of certificates
A CPP indicates 2 things:
- the status of the pharmaceutical product listed on the certificate and
- the GMP status of the sites conducting licensable activities of the pharmaceutical product in the exporting country
A GMP certificate attests to the GMP compliance of the sites conducting licensable activities as per the DEL. Health Canada issues electronic GMP certificates in a similar format to a CPP.
Requirements needed to request an electronic CPP
Health Canada issues an electronic CPP in the format recommended by the WHO to an applicant when the following requirements are met:
- the pharmaceutical product has a valid drug identification number (DIN) with a status of marketed, approved or dormant or, in the case of radiopharmaceuticals, a valid notice of compliance (NOC) and a valid date of notification
- the fabricator and packager/labeler are GMP compliant
- the applicant is located in Canada
If an applicant is applying on behalf of the DIN owner, the applicant must provide a valid letter of authorization for issuing the CPP from the DIN owner of the pharmaceutical product. The letter of authorization must include the DIN, the DIN owner's information and name of the company (applicant) being authorized. Letters should be dated within the current calendar year.
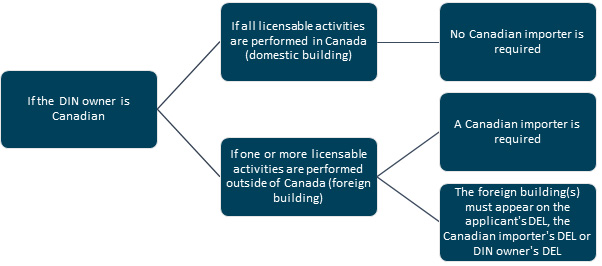
Diagram 1: Text description
[Situation 1: If the DIN owner is Canadian and if all licensable activities are performed in Canada (domestic building), then no Canadian importer is required.
Situation 2: If the DIN owner is Canadian and if 1 or more licensable activities are performed outside of Canada (foreign building), then:
- a Canadian importer is required and
- the foreign building(s) must appear on the applicant's DEL, the Canadian importer's DEL or the DIN owner's DEL]
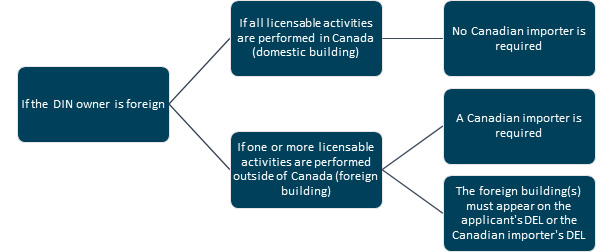
Diagram 2: Text description
[Situation 1: If the DIN owner is foreign and if all licensable activities are performed in Canada (domestic building), then no Canadian importer is required.
Situation 2: If the DIN owner is foreign and if 1 or more licensable activities are performed outside of Canada (foreign building), then:
- a Canadian importer is required and
- the foreign building(s) must appear on the applicant's DEL or the Canadian importer's DEL]
In both scenarios, if any foreign building conducting licensable activities is not listed on the DEL in question and is requested to be listed on the certificate, a request for an amendment must be submitted.
For more information on submitting an amendment application:
- refer to Guidance on Drug Establishment Licences (GUI-0002) or
- email the DEL unit at del.questions-leppp@hc-sc.gc.ca
The CPP can only be issued when the requested foreign building appears on the appropriate DEL.
Certificate application policies
There are 5 steps involved in the application process.
Step 1: Applicant submits application (English or French)
- If the application is for a pharmaceutical product (CPP application), fill out 1 FRM-0454 for each product (1 application per DIN), unless the batching section of the application form is being used.
- information on the CPP application form should match that of the Drug Product Database
- information on the CPP application form should match that of the DEL
- If the application is for a certificate that is not specific to a pharmaceutical product and the sites conducting licensable activities are GMP-compliant (GMP application), fill out a FRM-0455.
- 1 application form for each country of consignment, unless the batching section of the application form is being used
- Submit a completed FRM-0456 (fee form for an electronic certificate of a pharmaceutical product and good manufacturing practices certificate) with a completed FRM-0454 or FRM-0455.
- submit 1 single fee form per application package
- fee applies to total number of certificates requested (the original plus each supplemental copy) plus applicable tax (PST, GST or HST) based on your current address (province)
Note: The certificate fees increase on April 1 of every year. Applications submitted after 11:59 pm on the last business day in March are charged the new fee amount.
Please refer to the following forms:
Do not include the following with your application:
- requests for stamping and affidavits
- copies of product information
- including product labels, product monographs and lists of excipients
- payment or payment information
Do include the following items in your application package:
- fee form
- application form(s)
- letter of authorization (if necessary)
Email the application package to the CPP group at cpp_questions@hc-sc.gc.ca.
If you plan to submit more than 30 applications at one time, please contact the CPP (cpp_questions@hc-sc.gc.ca) group before you submit your applications.
Step 2: Health Canada confirms receipt
The CPP group will confirm receipt of a correctly completed application package. If any part of the application package (application forms, fee form or letter of authorization) is completed incorrectly before we send the acknowledgement notice to the applicant, the whole package will be rejected. The applicant may fix the deficiencies and resubmit the application package.
We'll reject an application for a number of reasons, such as the following:
- the country of consignment indicated in the application has a mutual recognition agreement with Canada
- the forms are not signed, dated, unlocked, in PDF format or legible
Step 3: Health Canada invoices
Once we confirm receipt of a complete application package, the applicant will be sent an invoice indicating the amount due from CRIU (Cost Recovery Invoicing Unit).
The fee for a CPP and GMP certificate are the same, as well as for any supplemental copies requested.
For current fees, consult the CPP chart in either of the following:
- Drug establishment licence (for human use) and associated fees or
- Establishment licence (drugs for veterinary use only) and associated fees
Step 4: Applicant makes payment to Health Canada
Applicant uses the invoice received by CRIU to submit payment to Health Canada. Do not submit payment before an invoice is issued to you by email.
You may pay by:
- cheque
- money order
- wire transfer
- direct deposit
- directly online through a Canadian financial institution
- credit card (Visa, MasterCard or American Express only)
- existing credit on your account or any Health Canada account
Find more instruction on How to Pay Your Establishment License Fees. Do not submit any credit card information by email.
Make your payment in Canadian funds. We will credit any overpayment to your company's customer account. Any payment being charged to a company's customer account must be accompanied by the appropriate account number.
For questions about payments or customer accounts, please contact our accounts receivable department by email at ar-cr@hc-sc.gc.ca or by telephone at 1-800-815-0506.
The CPP group reserves the right to withhold services for non-payment or partial payment.
Step 5: Health Canada issues certificate
Health Canada will review and aim to issue electronic CPP and GMP certificates within 25 business days from when we receive a complete application package. The service standard for processing the application begins when we receive a complete application package.
We will only issue a certificate when we have received and confirmed payment. If the CPP group finds deficiencies or incomplete or incorrect information after we send the acknowledgement notice to the applicant, we will contact the applicant with a clarification request. When a clarification request is sent regarding an application, reply directly to the clarification email with changes or the revised application form.
After 2 revisions or follow-up attempts, if the applicant is still unable to address the deficiencies or does not respond, we will reject the application. No refunds will be made.
We will reject an application for a number of reasons, such as:
- the pharmaceutical product DIN does not have a valid status
- the pharmaceutical product that is fabricated or packaged/labelled in a building outside of Canada does not comply with GMP requirements and is not listed on a Canadian DEL
- consult the guidance document How to demonstrate foreign building compliance with drug good manufacturing practices (GUI-0080)
- the foreign building is removed from the DEL foreign building annex because the Canadian importer has not submitted GMP evidence by the "new evidence required by" date or Health Canada considers the evidence is unacceptable or incomplete
The electronic CPP or GMP certificate is valid for a period of 1 year from the date it was issued.
Certificates will be issued with the official country name as per the World Health Organization (WHO) countries list.
Electronic certificates are signed with an electronic signature that uses a certificate-based digital ID to authenticate the signer's identity. Health Canada includes a letter of authorized e-signatories with the certificate. The letter validates the identity of the signatory on the certificate. It also gives assurance that the electronic e-signature on the certificate is authorized, valid and secure.
If a regulatory authority of the importing country has questions or concerns about a certificate's authenticity, integrity or validity, they may contact the CPP group at cpp_questions@hc-sc.gc.ca. They must include a copy of the electronic certificate that was provided to the regulatory authority in the email. We will reply to such requests as soon as possible.
Health Canada will notify WHO and importing authorities immediately if we find out that:
- a product exported under the WHO Certification Scheme has any new serious hazards associated with it or
- the Scheme has been criminally abused
- export of false or forged certificates
- falsely labelled, deceitful, counterfeited or substandard pharmaceutical product
If the Drug Establishment Licence Unit issues a certificate with errors, please submit a request for reissuance to CPP Questions at cpp_questions@hc-sc.gc.ca.
Requests for reissuance can be made up to the expiry date on the certificate without charge.
Contact us
Health Canada has set up generic email accounts to better assist you:
- For questions on CPP and GMP certificate applications and the WHO certification scheme: cpp_questions@hc-sc.gc.ca
- For questions about customer accounts and payment for CPP and GMP certificates: ar-cr@hc-sc.gc.ca
- For questions on DELs: del.questions-leppp@hc-sc.gc.ca
