Health Product InfoWatch – June 2019

Download the alternative format
(PDF format, 629 KB, 8 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Organization: Health Product InfoWatch Editorial Team
ISSN: 2368-8025
Cat.: H167-1E-PDF
Pub.: 190000
Contents
Health products mentioned in this issue
Pharmaceuticals and Biologics
Actemra (tocilizumab)
Extraneal peritoneal dialysis solution
Gentian violet-containing, non-prescription
pms-Methotrexate (methotrexate)
Medical Devices
Alaris Infusion Sets
Biocell breast implants
Cellex Photopheresis System
Gentian violet-containing, medical devices
Paclitaxel-coated balloons and paclitaxel-eluting stents
Natural Health Products
Health products manufactured by Konsar Future Nutrition Inc.Other
Foreign health products
Unauthorized cell therapy treatments
Unauthorized health products
Announcement
Health Canada warns Canadians of potential cancer risk associated with non-prescription gentian violet drug products
Health Canada conducted 2 health risk assessments on gentian violet-containing products and the risk of cancer. One focused on a non-prescription drug product (Gentiane Violet Liquid Topical) and the other on medical devices (Hydrofera antibacterial foam dressings). Health Canada's assessment of the non-prescription drug product concluded that there is a risk of cancer, due to oral exposure. The assessment of the medical devices containing gentian violet did not find this risk.
Health Canada's risk assessment of the non-prescription drug product concluded that there is evidence in the scientific literature, based on animal studies, that there is a potential for gentian violet to cause cancer. There was only one non-prescription drug product containing gentian violet marketed in Canada, Gentiane Violet Liquid Topical. The manufacturer voluntarily discontinued the sale of the product in Canada in May 2019, and the product licence has been cancelled.
Health Canada's risk assessment of licensed medical devices containing gentian violet (Hydrofera antibacterial foam dressings), based on the limited exposure to gentian violet, did not find a risk of cancer. However, no evidence to support the safety of the devices for pregnant and nursing women was provided to Health Canada by the manufacturer. As a precaution, Health Canada worked with the manufacturer to strengthen the device safety information to better inform users.
Summary Safety Review
Information Update
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls, as well as summaries of completed safety reviews published in May 2019 by Health Canada.
Actemra (tocilizumab)
Serious drug-induced liver injury, in some cases resulting in acute liver failure requiring a transplant, has been reported in patients treated with Actemra. Healthcare professionals are advised not to recommend Actemra in patients with active hepatic disease or hepatic impairment, and refer to the approved Canadian product monograph for guidance on the recommended dose adjustments (reduction, interruption or discontinuation) in patients with liver enzyme elevations. Patients are advised to contact a healthcare professional if they experience signs of liver injury. Health Canada is working with the manufacturer to include this new safety information in the Canadian product monograph.
Health Professional Risk Communication - Actemra (tocilizumab)
Alaris Infusion Sets
Becton Dickinson (BD) has recalled additional lots of the Alaris Pump Infusion Sets due to a manufacturing defect that can cause unintended delivery which can result in over-infusion. The April 28, 2019 list of affected products has been expanded to include all model codes and lot numbers with an expiration date between 05/2019 and 08/2020. BD has also confirmed that the affected lots were distributed beginning in August 2018, and not November 2018, as previously indicated.
Update - Health Professional Risk Communication - Alaris Infusion Sets
Biocell breast implants
Health Canada's safety review evaluated the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). This safety review found that there was an increased risk of BIA-ALCL with the use of highly textured (macro-textured) implants, in comparison with those having less textured or smooth implants. Biocell breast implants made by Allergan are the only macro-textured implants currently available in Canada. Health Canada has suspended the licences held by Allergan for their Biocell textured breast implants. Health Canada will work with all breast implant manufacturers to strengthen the Instructions for Use of all breast implants regarding the risk of BIA-ALCL. Health Canada has also communicated this information to Canadians.
Information Update - Biocell breast implants
Summary Safety Review - Biocell breast implants
Cellex Photopheresis System
This safety review evaluated the risk of venous thromboembolism and pulmonary embolism associated with Cellex Photopheresis System (CPS). Health Canada's review concluded that the majority of thromboembolic events occurred after the use of CPS for treatment of graft versus host disease (GvHD), which is not an intended use of CPS. Health Canada has requested the manufacturer of CPS to update the Instructions for Use to include a warning that treatment of patients with GvHD is not an authorized use in Canada.
Summary Safety Review - Cellex Photopheresis System
Extraneal peritoneal dialysis solution
Two lots of Extraneal peritoneal dialysis solution were recalled as a precautionary measure because of high levels of sodium hydroxide. Use of the affected product in patients could cause chemical peritonitis. The lots affected by this recall were distributed by Baxter between March 14, 2019 and March 20, 2019. Further to the original communication, Baxter Corporation has issued an update indicating that one of the recalled lots (Extraneal 2L/2L Twinbag, product code JB9912, lot number W9B28T0), may have also been distributed on March 21, 2019, in addition to March 14 to 20, 2019.
Update - Extraneal peritoneal dialysis solution
Foreign health products
These foreign health products have been found by regulators in the United States, Singapore and Australia to contain undeclared drug ingredients, which may pose serious health risks. These products are not authorized for sale in Canada and have not been found in the Canadian marketplace, but it is possible they may have been brought into the country by travellers or purchased over the Internet.
Health products manufactured by Konsar Future Nutrition Inc.
All products manufactured by Konsar Future Nutrition Inc. may pose serious health risks. Health Canada inspected the manufacturing site (located in Dollard-des-Ormeaux, Quebec) and found serious unsanitary conditions and quality control issues. As a result, Health Canada has suspended the company's site licence and all 101 of its product licences.
Advisory - Health products manufactured by Konsar Future Nutrition Inc.
Paclitaxel-coated balloons and paclitaxel-eluting stents
A meta-analysis published in the Journal of the American Heart Association has identified a possible increased risk of death after the use of paclitaxel-coated balloons (PCB) and paclitaxel-eluting stents (PES) to treat peripheral arterial disease (PAD). Healthcare professionals are advised to continue surveillance of patients who have been treated for PAD with PCB and PES according to current standards of care, determine whether the benefits of using these devices outweigh the risks, and follow the additional recommendations provided in the communication. Health Canada has also requested that manufacturers update the labelling of paclitaxel-containing devices to include details about this potential risk.
Health Professional Risk Communication - Paclitaxel-coated balloons and paclitaxel-eluting stents
Unauthorized cell therapy treatments
Health Canada advised Canadians that some clinics and healthcare professionals are offering unauthorized cell therapies, such as stem cell therapy, suggesting that these treatments are safe, and are making unproven health claims to patients. Unauthorized treatments have not been proven to be safe, or effective, and may cause life-threatening or life-altering risks, such as serious infections. Health Canada published a position paper to clarify the regulatory status of these products.
Information Update - Unauthorized cell therapy treatments
Unauthorized health products
Health Canada advised Canadians about various unauthorized health products being sold at retail locations across Canada or online that may pose serious health risks.
Advisory - Multiple unauthorized health products at Sunrise Lee Chinese Herbs Centre in Calgary, Alberta
Advisory - Unauthorized "popper"
Advisory - Unauthorized products (Part 1)
Advisory - Unauthorized products (Part 2)
New health product safety information
The following topics have been selected to raise awareness and, in some cases, to stimulate reporting of similar adverse reactions.
Vaccine safety biannual summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility for monitoring the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
Provincial and territorial public health authorities report adverse events following immunization (AEFIs) from publicly-funded vaccine programs to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC to monitor the safety of immunization programs.
Report for July 1, 2018 to December 31, 2018
Key Messages:
- No new safety signals (potential safety issues) were identified during this period.
- From July 1, 2018 to December 31, 2018, the Canada Vigilance Program received 592 reportsFootnote a of adverse events following immunization for which vaccines were a suspected cause.
This biannual vaccine safety summary includes reports of adverse events following immunization received by the Canada Vigilance Program between July 1, 2018 and December 31, 2018. To access reports published by the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), please visit the CAEFISS Web site.
- From July 1, 2018 to December 31, 2018, the Canada Vigilance Program received 592 reportsFootnote a of adverse events following immunization for which vaccines were a suspected cause.
- The largest proportion of the reports was from spontaneous reporting (Figure 1). The source of the reports is presented in Figure 2 (Note: 4 reports with unknown reporter type were not included in the graph).
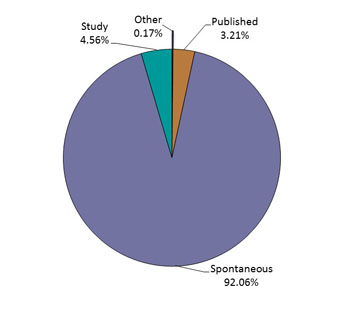
Figure 1: Total number of reports received by report type - Text description
The figure shows the percentage of immunization reports received from July 1 to December 31, 2018, by type of report.
| Report Type | Percentage (%) |
|---|---|
| Other | 0.17 |
| Published | 3.21 |
| Spontaneous | 92.06 |
| Study | 4.56 |
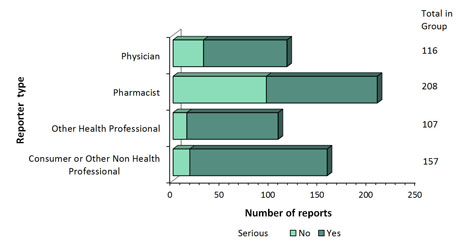
Figure 2: Total number of reports received by type of originating reporter - Text description
The figure shows the total number of reports received from July 1 to December 31, 2018, by type of originating reporter and severity.
| Originating reporter | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Consumer Or Other Non Health Professional | 17 | 140 |
| Other Health Professional | 14 | 93 |
| Pharmacist | 95 | 113 |
| Physician | 31 | 85 |
- Most of the reports involved females (Figure 3), and the most common age group was adults from 19 to 64 years of age (Figure 4).
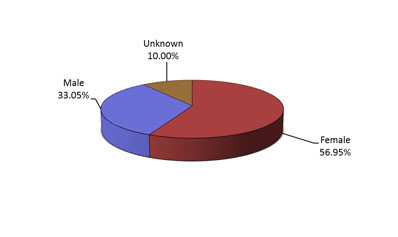
Figure 3: Total number of reports received by gender - Text Description
The figure shows the percentage of immunization reports received from July 1 to December 31, 2018, by gender.| Sex | Percentage (%) |
|---|---|
| Female | 56.95 |
| Male | 33.05 |
| Unknown | 10.00 |
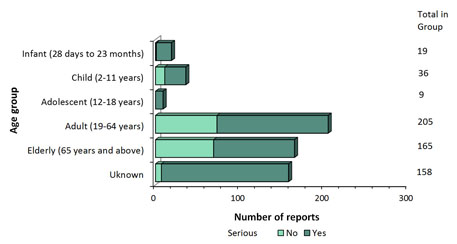
Figure 4: Total number of reports received by age group - Text Description
The figure shows the total number of reports received from July 1 to December 31, 2018, by age group and severity.
| Age | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Adolescent (12-18 years) | 0 | 9 |
| Adult (19-64 years) | 73 | 132 |
| Child (2-11 years) | 11 | 25 |
| Elderly (65 years and above) | 69 | 96 |
| Infant (28 days to 23 months) | 1 | 18 |
| Unknown | 7 | 151 |
- There were 431 (73%) serious reports. Most of these involved patients with underlying medical conditions and the serious adverse events were unlikely related to the vaccination.
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (309 reports; 48%), followed by influenza vaccines (136 reports; 21%) and pneumococcal vaccines (62 reports; 10%).
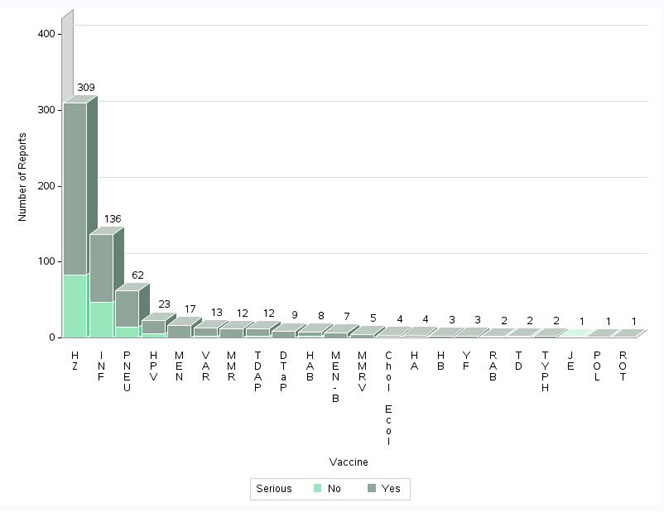
Figure 5: Total number of reports received by vaccine - Text Description
The figure shows the total number of reports received from July 1 to December 31, 2018, by vaccine type and severity.
| Vaccine Type | Number of non-serious reports | Number of serious reports | |
|---|---|---|---|
| Cholera and travellers' diarrhea | Chol Ecol | 1 | 3 |
| Diphtheria, tetanus, acellular pertussis | DTaP | 0 | 9 |
| Hepatitis A | HA | 1 | 3 |
| Hepatitis A, B | HAB | 2 | 6 |
| Hepatitis B | HB | 0 | 3 |
| Human papillomavirus | HPV | 6 | 17 |
| Herpes zoster | HZ | 83 | 226 |
| Influenza | INF | 47 | 89 |
| Japanese encephalitis | JE | 1 | 0 |
| Meningococcal | MEN | 0 | 17 |
| Meningococcal B | MEN-B | 0 | 7 |
| Measles, mumps, rubella | MMR | 0 | 12 |
| Measles, mumps, rubella, varicella | MMRV | 0 | 5 |
| Pneumococcal | PNEU | 15 | 47 |
| Poliomyelitis | POL | 0 | 1 |
| Rabies | RAB | 1 | 1 |
| Rotavirus | ROT | 0 | 1 |
| Tetanus, diphtheria (reduced) | TD | 1 | 1 |
| Tetanus, diphtheria (reduced), acellular pertussis | TDAP | 2 | 10 |
| Typhoid | TYPH | 0 | 2 |
| Varicella | VAR | 2 | 11 |
| Yellow Fever | YF | 0 | 3 |
- The majority of reports for herpes zoster vaccines (309 reports) were for Shingrix (290 reports). Of these 290 reports for Shingrix, 84 were described as social/digital media cases retrieved by the Market Authorization Holder (MAH). None of the social/digital media reports were assessable for the relatedness to the vaccine, due to missing information.
- There were 10 reports with an outcome of death. Five were male, 3 were female and 2 were of unknown gender. Age was not provided in 5 of the reports. In the remaining 5 reports, 3 were elderly, 1 was an adult, and 1 was an infant. The reported vaccines were: Pediacel (1), Prevnar 13 (1), Zostavax (1), Gardasil 9 (1), influenza vaccines (2), Shingrix (2), and Gardasil (2). The 2 Gardasil reports are possibly from a case that was assessed in 2015Footnote b. The information provided from these reports was not sufficient to adequately assess the causal association with the vaccine.
- The most frequently reported adverse events (serious and non-serious) included vaccination failure, herpes zoster, pyrexia, fatigue and pain. These were mostly reported for Shingrix. The adverse events of vaccination failure and herpes zoster were from social/digital media extracted by the MAH. None of the social/digital media reports were assessable for the relatedness to the vaccine, because of 2 or more of the following missing information: doses received (first or second dose or both); time to onset; pre-existing and concurrent conditions prior to vaccination; medical history; concomitant treatments; corrective treatment; and medical confirmation/test/results.
- No new safety signals (potential safety issues) were identified during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with the Public Health Agency of Canada (PHAC), will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Product monograph updates
The following safety labelling updates, which were recently made to the Canadian product monograph, have been selected for your awareness. A complete list of safety labelling updates is available on Health Canada's Product Monograph Brand Safety Updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
pms-Methotrexate (methotrexate)
The following information has been included in the Contraindications, Warnings and Precautions and Consumer Information sections of the Canadian product monograph for pms-Methotrexate. Health Canada is working with the manufacturers to harmonize and update the Canadian product monographs for other methotrexate products marketed in Canada.
Key messages for healthcare professionals:1
- Methotrexate is contraindicated in patients with severe renal impairment including end stage renal disease with or without dialysis.
- Pulmonary alveolar haemorrhage has been reported with methotrexate. This event may also be associated with vasculitis and other comorbidities. Prompt investigation should be considered when pulmonary alveolar haemorrhage is suspected.
References
- Reference 1
-
pms-Methotrexate (methotrexate) [product monograph]. Montreal (QC): Pharmascience Inc.; 2019.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffectTM Canada
- Recalls and Safety Alerts Database
- New Safety Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Register
- Drug Shortages Canada
- Annual trends for adverse reaction case reports and medical device problem incidents
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at HC.infowatch-infovigilance.SC@canada.ca
Health Canada
Marketed Health Products Directorate
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Fax: 613-952-7738
Copyright
©2019 Her Majesty the Queen in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.
Footnotes
- Footnote a
-
Glossary of Fields in the Canada Vigilance Adverse Reaction Online Database
- Footnote b
-
Summary Safety Review for Gardasil in 2015 is available at the following link: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-gardasil-quadrivalent-human-papillomavirus-types-6-11-16-18.html