Community Pharmacy Inspection Program Annual Report, Fiscal Year 2018–2019
Background
Health Canada helps protect Canadians by authorizing the legitimate use of controlled substances and precursor chemicals using a risk-based assessment and evaluation process, and by performing compliance and monitoring activities such as inspections to minimize the risk of diversion.
The Office of Controlled Substances (OCS) of the Controlled Substances and Cannabis Branch regulates the distribution and sale of precursors and controlled substances. This includes administering legislation, regulations, policies and operational activities that support the monitoring of controlled substances such as oxycodone, morphine, fentanyl, and heroin and precursor chemicals used to make these and other drugs. This administration allows for oversight of legal controlled substances, and can therefore reduce the risk of substances moving from legitimate channels to illegal markets.
The Controlled Substances Program of the Regulatory Operations and Enforcement Branch (ROEB-CSP) carries out the operational functions that ensure controlled substances are appropriately managed and monitored. Its compliance and enforcement mandate includes inspecting community pharmacies to ensure compliance with the Controlled Drugs and Substances Act (CDSA) and its associated regulations. OCS and ROEB-CSP operate under the authority of the CDSA and its associated regulations including the Benzodiazepines and Other Targeted Substances Regulations, the Narcotic Control Regulations, the Precursor Control Regulations and Parts G and J of the Food and Drug Regulations.
Health Canada conducts compliance and monitoring activities such as inspections to ensure regulated parties (pharmacists, researchers, licensed dealers, etc.) are compliant with the CDSA and its regulations. The CDSA provides a framework to control substances that can alter mental processes and that may produce harm to health and to society when diverted or problematically used. Its purpose is to protect public health and maintain public safety by balancing the legitimate need for access to these substances for medical, scientific and industrial purposes with the need to minimize the risk of their diversion to the illegal market.
Health Canada works with partners including the Royal Canadian Mounted Police, local law enforcement, the Canada Border Services Agency, and provincial and territorial professional licensing authorities such as the colleges of pharmacists, the colleges of physicians and their professional associations. The program is designed to identify and minimize potential situations in which controlled substances could be diverted to illegal markets, and to ensure that researchers, health care practitioners and patients are engaged in the proper storage, use and disposal of these substances. In addition, Health Canada may refer suspicious activities or share other information upon request with the relevant provincial or territorial regulatory authorities and/or law enforcement agencies.
Through these and other activities, Health Canada is committed to minimizing the diversion and problematic use of controlled substances.
Problematic prescription drug use
As frontline health care service providers, pharmacists are important partners in supporting public health initiatives to prevent problematic use of prescription drugs. By conducting pharmacy inspections, and collecting, analyzing and reporting on the inspection data, Health Canada supports its mandate to maintain and improve the health of Canadians.
What we do – Community Pharmacy Inspection Program
The objectives of the Community Pharmacy Inspection Program are to: promote compliance among pharmacists; strengthen cooperation and communication between Health Canada, pharmacists and their provincial authorities and associations; and facilitate national information sharing to address problematic prescription drug use including problematic opioid use.
Community Pharmacy Inspections Program Annual Report, Fiscal Year 2018–2019
This report provides a summary of findings from the inspections of selected community pharmacies for the fiscal year 2018–19 (April 1, 2018 – March 31, 2019); 275 community pharmacy inspections were completed during this fiscal year.
During a pharmacy inspection, Health Canada inspectors examine relevant information such as purchase records, prescription records, loss and theft reports, destruction protocols, inventory reconciliation, and security measures. Inspectors are looking for discrepancies between records and actual inventory, gaps in security measures, and overall practices in the management of controlled substances. Observations, which are linked to regulatory requirements, are recorded in an inspection report, which is provided to the pharmacist to promote and encourage compliance, and to undertake corrective action. In cases where significant discrepancies are observed, Health Canada may undertake further compliance action such as a targeted inspection at the pharmacy at a later date, and/or sharing the findings of the inspection with the relevant provincial or territorial regulatory authority for follow-up.
The awareness of pharmacists regarding the requirements of the CDSA and its regulations may reduce the incidence of these types of observations.
Record keeping requirements
Under the CDSA and its regulations, pharmacists are required to keep records of all transactions, such as refills, prescription transfers, and orders received in respect of controlled substances for a period of at least two years. Inspectors verify records kept by pharmacists to ensure their accuracy.
Security requirements
Security requirements under the CDSA are also verified during an inspection. These requirements are in place to help pharmacies reduce the risk of loss or theft of products containing controlled substances. Inspectors check security practices such as alarm systems, access control, and the submission of loss and theft reports to Health Canada.
Provide, sale and return requirements
Pharmacists have specific requirements as per the regulations under the CDSA that they must adhere to when performing activities such as the provision, sale or return of controlled substances. For example, pharmacists must comply with the CDSA and its regulations when returning unserviceable stock to a licensed dealer, when providing controlled substances to another pharmacy for emergency purposes, and when verifying the identity of the practitioner who issued the order or prescription.
Other
The regulations under the CDSA contain provisions that do not fit into the categories listed above. These provisions, such as the improper advertising of controlled substances, are captured in this category.
Risk-based approach
Health Canada developed and implemented a risk-based approach to pharmacy inspections that characterizes and describes observations as critical, major or minor based on the risk of diversion. The purpose of this approach is to ensure national uniformity in the classification of observations and to assist in assigning inspection ratings as compliant or non- compliant.
When classifying an observation as critical, major or minor, inspectors consider the scope (number of occurrences and quantity of product) and severity (potential for diversion) of the deficiency or deviation from the requirements of the CDSA or its regulations. In addition, the previous compliance history, implemented corrective actions and the diligence of the pharmacist to correct previous observations (i.e. willingness to comply and ensuring corrective actions have been implemented) can be taken into consideration.
Critical observations
- Observations classified as critical represent the most serious contravention of the CDSA or its regulations.
- An observation is considered critical if it involves the possibility of deliberate fraud, misrepresentation, or falsification of information to circumvent the CDSA or its regulations, or if it clearly increases the immediate risk of diversion, prevents the detection of diversion, or prevents the pharmacy from taking action to address the risk of diversion.
- An example of a critical observation would be if the inventory reconciliation from the inspection revealed large, unexplainable inventory variances.
Major observations
- Observations classified as major represent a serious contravention of the CDSA or its regulations.
- An observation is considered major if it may increase the risk of diversion, impairs the detection of diversion, or impairs the pharmacy from taking action to address the potential risk for diversion.
- An example of a major observation would be if a pharmacy did not report the loss or theft of controlled substances to Health Canada.
Minor observations
- A minor observation represents a deficiency or deviation from the CDSA or its regulations.
- A minor observation may be issued if the pharmacist is generally able to prevent, detect and take actions to address potential risks for diversion, but improvements could be made.
- An example of a minor observation would be if some prescriptions were filed in the regular prescription file and not in the narcotics and controlled drugs file, as required.
Non-compliant inspection rating
- An inspection rating of non-compliant will be assigned to a pharmacy if one or more critical observations are identified, or if the inspection identifies that the pharmacist has failed to take all reasonable steps to protect all controlled substances on their premises or under their control, demonstrated by a combination of several major observations.
- When a non-compliant rating has been assigned to an inspection, Health Canada initiates compliance and/or enforcement actions, such as a referral to the provincial or territorial professional licensing authority or, when there is reason to believe that diversion is actively occurring at a pharmacy, a referral to law enforcement. Health Canada can also consider restricting a pharmacist’s privileges to order narcotics. Lastly, a follow-up “targeted” inspection may take place at the non-compliant pharmacy at a later date to verify that corrective actions have been implemented.
Inspection results
Over the course of the fiscal year 2018–19, community pharmacy inspections were conducted using a risk-based approach; the following is a summary of the results. The inspection results will help inform future compliance promotion activities for community pharmacies and will assist Health Canada in developing guidance for pharmacists on how to comply with the CDSA and its regulations. The risk-based approach was designed to be flexible and accommodate for changes, as needed.
It should be noted that due to variations in provincial and territorial regulations, there are differences in the frequency of some observations from province-to-province. For example, some provinces have explicit and strict requirements regarding the inventory of controlled substances, whereas other provinces are silent on inventory requirements. Health Canada works with pharmacy regulatory authorities to ensure consistency in the application of the CDSA across Canada. During the fiscal year 2018–19, a total of 275 community pharmacy inspections were conducted across Canada, with a total of 1276 observations noted during these inspections: 43 observations were critical, 902 were major, and 331 were minor. Of these inspections, 63 (23%) were rated as non-compliant and most were referred to the relevant provincial regulatory authorities for further review or action, as needed.
Figure 1 – Number of community pharmacy inspections in Canada and their compliance ratings
The map of Canada shows the number of community pharmacy inspections conducted in each province: 38 inspections were conducted in British Columbia, 34 in Alberta, 10 in Saskatchewan, 14 in Manitoba, 112 in Ontario, 44 in Quebec, 6 in New Brunswick, 8 in Nova Scotia, 4 in Prince Edward Island and 5 in Newfoundland. The pie chart shows that 77% of inspections were rated as compliant, while 23% were rated as non-compliant.
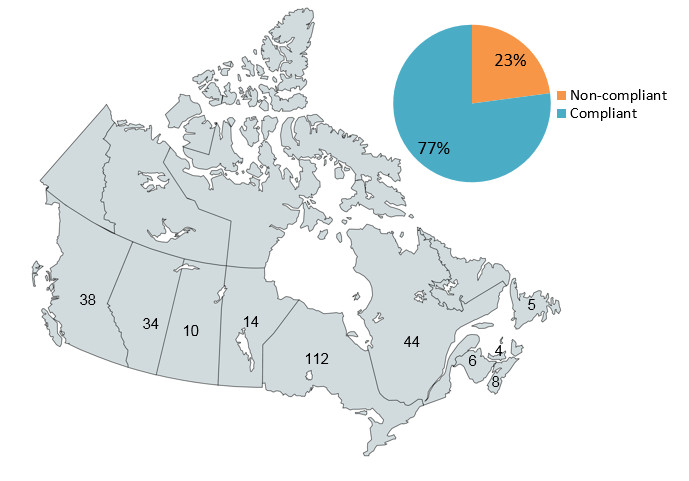
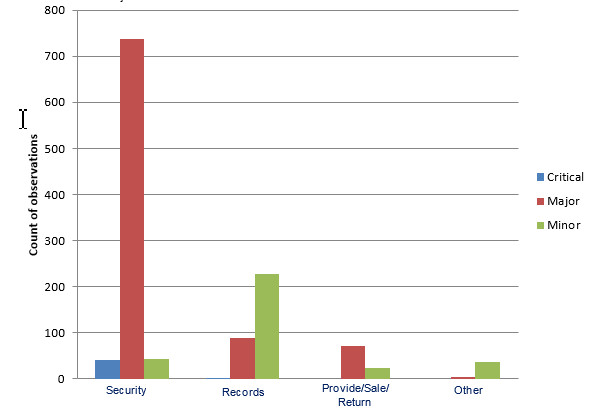
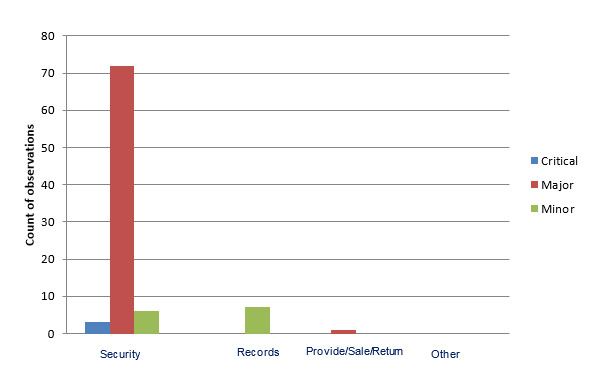
Figure 2 – Observation types and their associated risk classification noted during community pharmacy inspections
The bar graph shows observations noted during community pharmacy inspections in the categories of security, records, provide/sale/return and other. In the category of security, 41 observations were critical, 738 were major and 44 were minor. In the category of records, 2 observations were critical, 88 were major, and 227 were minor. In the category of provide/sale/return, 72 observations were major and 23 were minor. In the category of other, 4 observations were major and 37 were minor.
Targeted inspections
In addition to the 275 community pharmacies inspected under the Problematic Prescription Drug Use (PPDU) initiative, inspectors also conducted targeted inspections of community pharmacies, when there was intelligence to suggest that certain pharmacies may not be in compliance. During the fiscal year 2018–19, a total of 139 targeted inspections of community pharmacies occurred across Canada with a total of 525 observations noted: 19 observations were critical, 394 were major and 112 were minor. Of these inspections, 42 (30%) were rated as non-compliant and most were referred to the relevant provincial regulatory authorities for further review or action, as needed.
Number of targeted pharmacy inspections in Canada
Figure 3 – Number of targeted pharmacy inspections in Canada
The map of Canada shows the number of targeted community pharmacy inspections conducted per province: 22 targeted inspections were conducted in British Columbia, 12 in Alberta, 6 in Saskatchewan, 5 in Manitoba, 55 in Ontario, 16 in Quebec, 6 in New Brunswick, 14 in Nova Scotia, 2 in Prince Edward Island and 1 in Newfoundland.
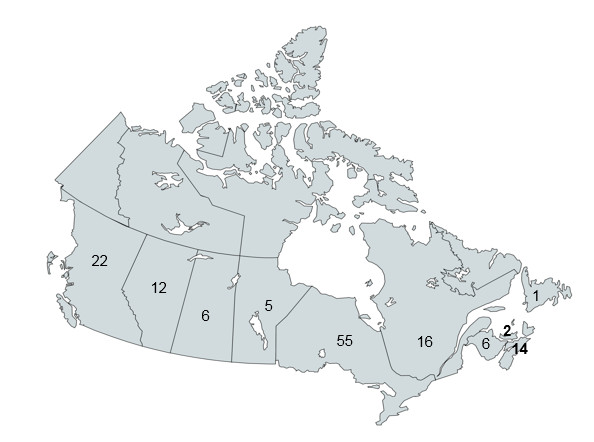
Figure 4 – Observation types and their associated risk classification noted during targeted inspections of community pharmacies
The bar graph shows observations noted during targeted inspections of community pharmacies in the categories of security, records, provide/sale/return and other. In the category of security, 17 observations were critical, 336 were major and 22 were minor. In the category of records, 1 observation was critical, 28 were major, and 65 were minor. In the category of provide/sale/return, 1 observation was critical, 29 were major and 4 were minor. In the category of other, 1 observation was major and 21 were minor.

Pharmacy inspection blitz
In addition to the community and targeted pharmacy inspections, Health Canada may conduct inspection blitzes across Canada. A blitz is defined as multiple targeted inspections conducted in specific area(s) with a focused and coordinated approach over a specified period of time to assess compliance with the CDSA and its Regulations and/or mitigate a suspected, discovered or referred issue(s). Pharmacy inspection blitzes also provide an opportunity for Health Canada to share information with pharmacists regarding legislative and regulatory requirements in respect of controlled substances.
During the fiscal year 2018–19, Health Canada conducted an inspection blitz of 24 community pharmacies in Hamilton, Ontario. A total of 89 observations noted: 3 observations were critical, 73 were major, and 13 were minor. Of these inspections, 6 (25%) were rated as non-compliant and most were referred to the relevant provincial regulatory authority for further review or action, as needed.
Blitz inspection results 2018-19, Hamilton ON
Figure 5 – Blitz inspection results 2018-19, Hamilton ON
The pie chart shows that 75% of the community pharmacy inspections conducted during the 2018-19 Hamilton blitz were rated as compliant, while 25% were rated as non-compliant.
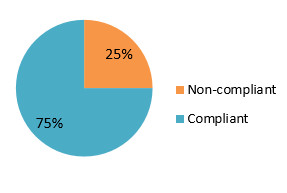
Figure 6 – Observation types and their associated risk classification noted during the Hamilton Blitz 2018-19
The bar graph shows observations noted during targeted inspections of community pharmacies conducted during the 2018-19 Hamilton, ON blitz in the categories of security, records, provide/sale/return and other. In the category of security, 3 observations were critical, 72 were major and 6 were minor. In the category of records, 7 observations were minor. In the category of provide/sale/return, 1 observation was major.
Conclusion
In addition to conducting inspections to promote compliance with the CDSA and its regulations, the Community Pharmacy Inspection Program provides the opportunity for Health Canada to identify common issues that may lead to an increased risk of diversion of controlled substances. These issues are addressed through the development of national guidance documents. The following documents are available on Health Canada’s website:
- Guidance Document: Reporting Loss or Theft of Controlled Substances and Precursors (CS-GD-005)
- Guidance Document: Handling and Destruction of Post-consumer Returns Containing Controlled Substances (CS-GD-021)
- Guidance Document for Pharmacists, Practitioners and Persons in Charge of Hospitals: Handling and Destruction of Unserviceable Stock Containing Narcotics, Controlled Drugs or Targeted Substances
The most common observations cited within a pharmacy inspection report during this past year were consistent with previous years. Health Canada continues to explore ways to promote compliance with the CDSA and its regulations including active engagement with national and provincial organizations, such as pharmacy colleges, that are partners in reducing risks associated with the diversion of controlled substances. Informed through the inspection of pharmacies, the risk-based approach to inspections has been adapted to address issues related to potential diversion.
Increasing security measures within a pharmacy and the education of pharmacists on requirements during an inspection may reduce the incidence of security-related observations.
Forward planning
The ongoing data being collected from this program will inform the future pharmacy inspection approach. Using a risk-based approach and other business intelligence, Health Canada will shift toward more targeted inspections. Therefore, future reporting may differ from the data presented for fiscal year 2018–19.