Guidelines for the Safety Assessment of Novel Foods
Food Directorate
Health Products and Food Branch
Health Canada
June, 2006
Updated: July 2022
Table of contents
- 1. Introduction
- 2. Notification procedure
- 3. Other regulatory considerations
- 4. Information requirements for safety assessment
- 4.1 Novel foods derived from plants
- 4.2 Novel foods derived from microorganisms
- Appendix 1: Health Canada Guidance on the Novelty Interpretation of Products of Plant Breeding
- Appendix 2: Health Canada Guidance on the Pre-Market Assessment of Foods Derived from Retransformants
This document replaces the Guidelines for the Safety Assessment of Novel Foods (Volumes I and II) originally published in September, 1994.
1. Introduction
1.1 Background
The globalization of the food supply, the demand for more food sources globally, and rapid advances in food science and technology have resulted in the introduction of foods not previously available in the Canadian marketplace. Novel whole foods and food ingredients may appear in Canada through the importation of new products, the introduction of a new species as a food source, the use of new processing techniques, or changes in the genetic make-up of the microorganisms, plants and animals from which foods are derived.
Advances in transportation technology and lower transportation costs have increased the variety of food and food products imported into Canada. Changing consumer food preferences driven by exposure to different cultural and ethnic traditions as well as nutritional and health concerns, have also resulted in the diversification of our food supply. In addition, the increasing global population continues to drive the introduction of new food sources worldwide. Foods that are not traditional in Canada may be widely consumed in other parts of the world. In some cases, adverse effects may be associated with their consumption or with the traditional methods needed to prepare the food prior to consumption. Foods derived from sources not previously used as human foods must be evaluated for safety as they may contain toxins, contaminants and anti-nutritional factors. Conclusions from these assessments permit appropriate risk management measures to be taken.
New techniques for food preservation and processing continue to be developed to extend the shelf life of foods and food products, to reduce fuel energy requirements for processing, and for many other purposes. As processing techniques can alter the characteristics of a food, including nutritional and any toxic characteristics, human health impacts of new processes must be considered.
Genetic modifications to change the agronomic, production, processing or nutritional characteristics of microorganisms, plants and animals may be achieved through traditional breeding techniques or modern gene technologies. The application of genetic modification through either traditional breeding or genetic engineering is not considered to increase or decrease the inherent risk associated with consuming the organism as a food. However, the wide variety of manipulations possible through genetic modification, and the potential for the introduction of toxic compounds, unexpected secondary effects and changes in the nutritional and toxic characteristics of the food product may give rise to safety concerns.
Federal responsibility for the regulations dealing with foods sold in Canada, including novel foods, is shared by Health Canada and the Canadian Food Inspection Agency (CFIA). Health Canada is responsible for establishing standards and policies governing the safety and nutritional quality of foods and developing labelling policies related to health and nutrition. The CFIA develops standards related to the packaging, labelling and advertising of foods, and handles all inspection and enforcement duties. The CFIA also has responsibility for the regulation of seeds, veterinary biologics, fertilizers and livestock feeds. More specifically, CFIA is responsible for the regulations and guidelines dealing with cultivating plants with novel traits and dealing with livestock feeds and for conducting the respective safety assessments, whereas Health Canada is responsible for the regulations and guidelines pertaining to novel foods and for conducting safety assessments of novel foods.
The mechanism by which Health Canada controls the sale of novel foods in Canada is the mandatory pre-market notification requirement as set out in Division 28 of Part B of the Food and Drug Regulations (see Figure 1). Manufacturers or importers are required under these regulations to submit information to Health Canada regarding the product in question so that a determination can be made with respect to the product's safety prior to sale.
The safety criteria for the assessment of novel foods outlined in the current document were derived from internationally established scientific principles and guidelines developed through the work of the Organization for Economic Cooperation and Development (OECD), Food and Agriculture Organisation (FAO), World Health Organisation (WHO) and the Codex Alimentarius Commission. These guidelines provide for both the rigour and the flexibility required to determine the need for notification and to conduct the safety assessment of the broad range of food products being developed. This flexibility is needed to allow novel foods and food products to be assessed on a case-by-case basis and to take into consideration future scientific advances.
1.2 Purpose
These Guidelines are intended to assist the petitioner in preparing a novel food notification and to ensure that the information is sufficient to for a safety assessment. Novel food safety assessments are conducted by the Food Directorate, Health Products and Food Branch of Health Canada. The Food Directorate is the federal health authority responsible for establishing policies, setting standards and providing advice and information on the safety and nutritional value of food.
These Guidelines are not intended to define explicitly all the data that might be required in the course of a safety assessment. Further data requirements may be identified on a case-by-case basis during the safety assessment process.
1.3 Scope
This document encompasses novel foods, whether whole foods, food products, or food ingredients, that are derived from plant or microbial sources. Safety assessment criteria for novel foods derived from animals are under development. Manufacturers or importers of novel foods derived from animal sources should consult with the Food Directorate to discuss what information is appropriate to the evaluation of the safety of a particular product.
The definition of 'novel food', and the definitions for 'genetically modify' and 'major change' are set out in B.28.001 of the Food and Drug Regulations. Please see Figure 1.
Figure 1. Division 28 of Part B of the Food and Drug Regulations
Novel Foods
Interpretation
B.28.001. The definitions in this section apply in this Division.
"genetically modify" means to change the heritable traits of a plant, animal or microorganism by means of intentional manipulation.(modifier génétiquement)
"major change" means, in respect of a food, a change in the food that, based on the manufacturer's experience or generally accepted nutritional or food science theory, places the modified food outside the accepted limits of natural variations for that food with regard to
- the composition, structure or nutritional quality of the food or its generally recognized physiological effects;
- the manner in which the food is metabolized in the body; or
- the microbiological safety, the chemical safety or the safe use of the food. ( changement majeur)
"novel food" means- a substance, including a microorganism, that does not have a history of safe use as a food;
- a food that has been manufactured, prepared, preserved or packaged by a process that
- has not been previously applied to that food, and
- causes the food to undergo a major change; and
- a food that is derived from a plant, animal or microorganism that has been genetically modified such that
- the plant, animal or microorganism exhibits characteristics that were not previously observed in that plant, animal or microorganism,
- the plant, animal or microorganism no longer exhibits characteristics that were previously observed in that plant, animal or microorganism, or
- one or more characteristics of the plant, animal or microorganism no longer fall within the anticipated range for that plant, animal or microorganism. ( aliment nouveau)
Pre-market notification
B.28.002. (1) No person shall sell or advertise for sale a novel food unless the manufacturer or importer of the novel food
- has notified the Director in writing of their intention to sell or advertise for sale the novel food; and
- has received a written notice from the Director under paragraph B.28.003(1)(a) or subsection B.28.003(2).
(2) A notification referred to in paragraph (1)(a) shall be signed by the manufacturer or importer, or a person authorized to sign on behalf of the manufacturer or importer, and shall include the following information:
- the common name under which the novel food will be sold;
- the name and address of the principal place of business of the manufacturer and, if the address is outside Canada, the name and address of the principal place of business of the importer;
- a description of the novel food, together with
- information respecting its development,
- details of the method by which it is manufactured, prepared, preserved, packaged and stored,
- details of the major change, if any,
- information respecting its intended use and directions for its preparation,
- information respecting its history of use as a food in a country other than Canada, if applicable, and
- information relied on to establish that the novel food is safe for consumption;
- information respecting the estimated levels of consumption by consumers of the novel food;
- the text of all labels to be used in connection with the novel food; and
- the name and title of the person who signed the notification and the date of signing.
B.28.003. (1) Within 45 days after receiving a notification referred to in paragraph
B.28.002(1)(a), the Director shall review the information included in the notification and
- if the information establishes that the novel food is safe for consumption, notify the manufacturer or importer in writing that the information is sufficient; or
- if additional information of a scientific nature is necessary in order to assess the safety of the novel food, request in writing that the manufacturer or importer submit that information.
(2) Within 90 days after receiving the additional information requested under paragraph (1)(b) the Director shall assess it and, if it establishes that the novel food is safe for consumption, notify the manufacturer or importer in writing that the information is sufficient.
2. Notification procedure
2.1 Submission of a novel food notification
Division 28 of Part B of the Food and Drug Regulations requires a manufacturer or importer to notify Food Directorate in writing of their intention to sell or advertise for sale a novel food. It should be noted that in accordance with subsection B.28.002(1) no person shall sell or advertise for sale a novel food unless the manufacturer or importer of the novel food has:
- notified Food Directorate of their intention to sell or advertise for sale the novel food; and
- received a letter of no objection to the sale of the novel food in Canada as stated in B.28.002(1)(b)
It is recommended that manufacturers or importers consult with the Food Directorate if they are not sure if a product is a novel food or not, or if they would like to discuss data requirements specific for a novel food. This will help to determine if a notification is needed and, if so, how to use these Guidelines to develop a safety assessment data package.
For information on requesting a pre-submission consultation with the Food Directorate, please see the Pre-submission consultation guidance: Pre-market mandatory submissions for the Food Directorate.
The notification must include the information set out in subsection B.28.002 (2), as shown in Figure 1 and repeated below:
A notification referred to in paragraph (1)(a) shall be signed by the manufacturer or importer, or a person authorized to sign on behalf of the manufacturer or importer, and shall include the following information:
- the common name under which the novel food will be sold;
- the name and address of the principal place of business of the manufacturer and, if the address is outside Canada, the name and address of the principal place of business of the importer;
- a description of the novel food, together with
- information respecting its development,
- details of the method by which it is manufactured, prepared, preserved, packaged and stored,
- details of the major change, if any,
- information respecting its intended use and directions for its preparation,
- information respecting its history of use as a food in a country other than Canada, if applicable, and
- information relied on to establish that the novel food is safe for consumption;
- information respecting the estimated levels of consumption by consumers of the novel food;
- the text of all labels to be used in connection with the novel food; and
- the name and title of the person who signed the notification and the date of signing.
The notification should be sent electronically through the Application Form for Pre-Market Submissions to the Food Directorate (Online Application Form). It is recommended that the notification should provide the information in the order of paragraphs (a) to (f) of subsection B.28.002 (2). Upon receipt of the notification, a case number is assigned and the data package is verified within 10 calendar days. Upon successful verification of the notification, a letter of acknowledgement with the case number is sent to the petitioner. This number should be used in all subsequent correspondence.
Section B.28.003 provides for two stages of notification and review. The first is a period of 45 days, for reviewing notifications to determine if the information provided establishes that the food is safe and to advise proponents if additional information is needed to review the product properly. The notification will be reviewed and the petitioner will be informed in writing that either there is no objection to the sale of the novel food for consumption or that additional scientific data must be submitted in order to assess the safety of the novel food. If additional information is necessary, a period of 90 days, after receiving the information requested, is provided under subsection B.28.003 (2) to assess the information (see section 2.2 below).
2.2 Submission of a safety assessment data package
If the information provided in the notification for a novel food is not considered adequate to determine the novel food's safety, additional data supporting the safety of the food will be required. The type of information required to conduct the safety assessment of a novel food will depend on a number of factors such as the nature of the food, processing methods and the intended use. The approaches used to assess the safety of novel foods are outlined in these Guidelines. However, the types of studies considered appropriate to demonstrate the safety of a novel food change with scientific knowledge and development. These Guidelines are expected to be used in conjunction with information available in the scientific literature and from research and development conducted by the manufacturer.
Since novel foods represent a diverse range of products, not all types of data outlined in this document will be appropriate for a specific submission. Petitioners should consider the novel characteristics of their particular product when addressing the criteria in these Guidelines. Consultation with the Food Directorate is encouraged during the development phase of a product to determine the specific data necessary to demonstrate the safety of the product. Information sufficient to establish that a novel food is safe for consumption may include experimental data as well as sound scientific rationales.
To enhance the efficiency of the review process, petitioners should prepare their safety assessment data packages according to the following headings, as applicable:
- History of use
- Dietary exposure
- Detail of novel process
- History of organism(s)
- Characterization of derived line/strain
- Genetic modification considerations
- Nutritional considerations
- Toxicology considerations
- Allergenicity considerations
- Chemical considerations
- Microbiological considerations
The Guidelines should be consulted to determine what should be included under each heading. Details concerning the process and time lines of the review and notification to petitioners of the results are given in Section 2.5.
2.3 When to apply
Written notification should be provided as far in advance as possible of the period when the manufacturer intends to market the product. Food Directorate is obligated to respond regarding the safety of the novel food or whether further information is required for assessment within 45 days of receiving the notification.
2.4 How to submit
A Novel Foods Section has been established in the Food Directorate to coordinate the safety evaluation of novel foods intended for human consumption in Canada. Notifications and safety assessment data packages should be sent electronically through the Online Application Form.
Additional requested information (for example, responses to information requests or deficiencies) should be sent electronically through the Transport Form for Submitting Additional Documents to Health Canada (Transport Form).
2.5 Standard operating procedure
As the coordinating office, the Novel Foods Section in the Food Directorate is responsible for communicating with petitioners, receiving novel foods notifications and submissions of additional information requested for evaluation, and initiating the review process outlined in Figure 2. This provides for a single window approach to submission reviews. The Novel Foods Section distributes the submission material to relevant Food Directorate bureaux, namely the Bureau of Chemical Safety for evaluation of chemical and toxicological considerations, the Bureau of Nutritional Sciences for nutritional considerations, and the Bureau of Microbial Hazards for microbial and molecular biological aspects of the files. In some cases, where the CFIA is not conducting an environmental review under the Feeds Act or Seeds Act, the Environmental Assessment Unit, Healthy Environments and Consumer Safety Branch of Health Canada, will by asked by the Food Directorate to conduct an environmental assessment of the novel food pursuant to the Canadian Environmental Protection Act, 1999 (CEPA) (see section 3.1 of these Guidelines for more information).
During the evaluation, a thorough analysis is conducted of the data submitted and of the protocols used to acquire the data to ensure the validity of the results. If an evaluator determines that the data is not sufficient or the submission is unclear or incomplete, additional information, clarification or testing will be requested in order to assess the safety of the novel food. In addition, evaluators take into consideration data published in Canada or internationally that is relevant to the product in question.
Under the Regulations, a novel food notification must include specific information described in subsection B.28.002(2) including details of any major change in the food and information respecting its intended use and directions for its preparation, as well as the text of all labels to be used in connection with the novel food. Petitioners wishing to promote an attribute of a novel food on a label or in advertising should be aware that such representations or claims need to be addressed independently of the novel food safety assessment. Although subsection B.28.002 (2) requires manufacturers to advise Food Directorate at the time of making a novel food notification of the intended use of the product and the details of the major change, if any, as well as to provide the text of all labels to be used in connection with the novel food, this does not constitute a request for a review and approval of a claim. At present, the Food and Drug Regulations permit only certain health and nutrition claims for foods, i.e. those referred to in section B.01.311 ("biological role claims"), tables following section B.01.513 (nutrient content claims), and section B.01.603 (health claims).
If a petitioner has an interest in any type of claim, it is recommended that this interest be identified before or at the same time as the novel food notification. Petitioners should be aware that health claims not currently listed in the table to section B.01.603 would require provision of appropriate data for review and amendment to the Regulations to provide for their use. This interest in a claim will be routed to the appropriate Bureau for consideration. Petitioners should also consult the sections on intended nutritional effects under Nutritional Considerations in the part of the Guidelines that addresses their type of novel food (see Figure 3) for further important information about regulatory issues concerning health claims and novel foods.
The intent of B.28.003 is to provide for two stages of notification and review: a period of 45 days for reviewing notifications and an additional 90 days if further information is requested to assess the novel food. The 45 days period allows the Food Directorate to review the information submitted and determine if it establishes that the food is safe for consumption. During this period, the Directorate may advise proponents that additional information is needed to review the product properly. If the proponent is advised that additional data is needed, then the Food Directorate has to conclude a review of all of the available data submitted within 90 days from receipt of that package, contingent upon having a complete data set that includes all the information needed to arrive at a conclusion.
At the completion of the safety assessment, if and only if there are no outstanding concerns regarding any aspect of the safety assessment and it is determined that there are no health risks associated with the consumption of the novel food product in question, a document proposing that the food be permitted for sale is drafted. This proposal, which contains a summary of the scientific reviews conducted by the relevant bureaux of the Food Directorate is presented to the Food Rulings Committee for consideration. This Committee is chaired by the Director General of the Food Directorate and consists of Food Directorate senior management and representatives from the Canadian Food Inspection Agency. If the food rulings proposal is found acceptable by the Committee, the petitioner is notified in writing that, based on the evaluation of the submitted data, Health Canada has no objection to the sale of the novel food product as human food in Canada as specified in the notification.
In the event that the scientific reviews conducted by the bureaux conclude that the product cannot be considered safe for consumption as a food, this determination would also be brought to the Food Rulings Committee. Also, where companies are not able to provide data required to reach a conclusion about the safety of a product, they may choose to withdraw the submission.
In light of widespread interest in novel foods and, in particular, those produced by the techniques of biotechnology, the Food Directorate is of the view that mechanisms to inform the public about such new products are needed. One such approach entails the use of a novel food decision document. In order to maintain a transparent approach, yet respect the confidential business information contained in a submission, a general summary document is drafted which describes the product and summarizes the information collected to demonstrate its safety. This summary is prepared according to the appropriate sections of these Guidelines (i.e. Development and Production, Product Information, Dietary Exposure, Nutritional Data, and Toxicology Data). The manufacturer or importer is given an opportunity to review this document and provide comments. The novel food decision document is subsequently made available on the Novel Foods page of the Health Canada website for all products for which Health Canada has issued a letter of no objection to the use as food in Canada.
Figure 2. Processing a novel food notification and requests for additional information in the Food Directorate.Footnote 1, Footnote 2
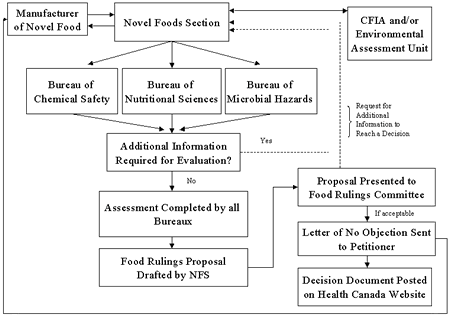
Figure 2 - Text Equivalent
As the coordinating office, the Novel Foods Section is responsible for communicating with petitioners and receiving novel food notifications and submission material and initiating the review process.
The Novel Foods Section distributes the submission material to the relevant Food Directorate bureaux, namely the Bureau of Chemical Safety, the Bureau of Nutritional Sciences, and the Bureau of Microbial Hazards for their respective reviews.
Evaluators will review the novel food notification submission material and determine whether or not the product is considered novel under Section B.28.001 of the Novel Foods Regulations. If the product is considered novel, a safety assessment is then conducted as outlined in the Guidelines for the Safety Assessment of Novel Foods. Evaluators may make requests for additional information if relevant data has not been included in the original notification/submission material.
An environmental assessment is also conducted for a novel food product. If the novel food product is derived from a genetically modified plant, an environmental assessment is conducted by the Canadian Food Inspection Agency. For all other novel foods, an environmental assessment is conducted by the Environmental Assessment Unit of Health Canada if the novel food is a new substance.
Upon completion of the safety assessment, if and only if all members of the evaluation team agree there are no health risks associated with the consumption of the novel food product in question, a proposal is drafted and presented to the Food Rulings Committee. If the proposal is accepted by the Committee, the petitioner is notified in writing by the Director General that, based on the evaluation of the submitted material, Health Canada has no objection to the sale of the novel food product as human food in Canada as specified in the letter. Health Canada will also post a summary of the safety assessment and the final decision that is made by the Committee on the Health Canada website.
3. Other regulatory considerations
3.1 Environmental impact
Health Canada is responsible for the safety assessment of novel foods under the Food and Drugs Act and Regulations. However, the Food and Drugs Act and Regulations do not currently provide for the assessment of the environmental impact or for assessment of certain indirect human health aspects that the manufacture or import of a regulated product, such as a novel food, may have.
Health Canada is in the process of developing a regulatory framework for the environmental assessment of products regulated under the Food and Drugs Act and Regulations. Until this framework is developed, information on potential environmental and indirect human health impact of a novel food is required pursuant to the New Substances Notification Regulations under CEPA.
It is the responsibility of the petitioner to ensure that a novel food meets all applicable regulatory requirements, including those required under CEPA. A guidance document on current New Substances Notification requirements for products regulated under the Food and Drugs Act is available on Health Canada's website or upon request at 1-888-492-1104. Once an environmental assessment is deemed necessary, the petitioner is then responsible for preparing the information package addressing CEPA requirements, which will be reviewed by the Environmental Assessment Unit of the Healthy Environments and Consumer Safety Branch (HECSB) (see Figure 2).
Substances which are on the Domestic Substances List (DSL) or substances that are already regulated under other federal statutes listed in CEPA Schedule III, such as the Seeds Act and the Feeds Act, are exempted from the notification regulation under CEPA. For instance, if a novel food is derived from a plant for which an application has been submitted to the CFIA for unconfined environmental release or for use as animal feed, this should be stated in the novel food notification to Health Canada because those Acts already have CEPA equivalent environmental assessment requirements.
3.2 Plants with novel traits and novel feeds
The CFIA is responsible for the regulation of plants with novel traits (PNTs) to be cultivated in Canada. Under the Seeds Act, a new variety of a cultivated species that possesses a novel trait (i.e., a novel characteristic) would be subject to Regulatory Directive Dir94-08 (Assessment Criteria for Determinating Environmental Safety of Plants with Novel Traits). More information on the regulations of plants with novel traits is available through the CFIA's Plant Biosafety Office 613-225-2342).
The Feed Section of the CFIA administers a national livestock feed program, under the authority of the Feeds Act and Regulations, to verify that livestock feeds, including novel feeds, manufactured or sold in Canada are safe, efficacious and labelled properly. Novel feeds consist of organisms or parts of products thereof that have not been evaluated and approved for use as livestock feed in Canada. Novel feeds may be from plant sources, including PNTs, that could be used as feed must be assessed by the Feed Section prior to their use as a livestock feed. More information on the regulation of novel feeds from plant sources is available. Please refer to the Guidelines for the Assessment of Novel Feeds: Plant Sources for data requirements for a novel feed submission.
Livestock feed is an outlet for by-products and residual material of the food processing industry. By-products of foods derived from novel microorganisms must be assessed by the Feed Section prior to their incorporation into livestock feed. The draft Guidelines for the Safety Assessment of Novel Feeds: Microbial Products can be obtained by contacting the Feed Section of the CFIA.
Before introducing a new plant to the Canadian market it is necessary to consider whether it would be classified as novel under the regulatory provisions of the Feeds Act, the Food and Drugs Act, and the Seeds Act. To increase harmonization and reduce unnecessary delays and conflicting decisions, the CFIA and Health Canada have developed a formalized process to coordinate the determination of novelty for new plant varieties or foods or feeds derived from these plants as defined under the regulatory provisions of these Acts.
When a petitioner contacts the Feed Section (CFIA), Novel Foods Section (Health Canada), and/or the Plant Biosafety Office (CFIA) for an opinion on the novelty of a plant and its feed and food products, a meeting will be organized among all three groups to review the case in order to analyse the factors that contribute to its status and provide guidance on the appropriate regulatory oversight. Where a plant variety has been determined to be a Plant with a Novel Trait (PNT), the feed and food products derived from it are most often classified as novel. On the other hand, in some cases, a plant variety may not be found to be a PNT but the characteristics of the feed and food products would lead the Feeds Section and Novel Foods Section to conclude that they are novel. In other cases, a plant could be considered a PNT by the Plant Biosafety Office if it is new to the Canadian environment, but the feed and food products would not be considered novel since they have a previous history of safe use in the marketplace. In order to respond to a request for an opinion on novelty from a petitioner, additional information may be required in order for regulatory authorities to reach a decision.
3.3 Coordination of regulatory decisions for novel foods and novel feeds derived from plants with novel traits
Health Canada and the CFIA conduct interdepartmental consultations in order to coordinate the granting of their respective approvals to minimize the potential for unapproved food products to enter the Canadian marketplace. This approach will continue through a formalized process which will ensure the decisions related to plants with novel traits and their use as food and feed are made in a harmonized fashion.
Where products are intended for exclusive use as food, feed or molecular farming (use of plants to produce industrial or therapeutic products), consultations among regulatory authorities will be required to assess any potential risks associated with release of the product in a commodity stream for which it is not intended. For these products, an identity preservation system or alternative will be essential to minimize the likelihood of such an event.
Please note that once the safety assessments have been completed, the applicant is notified in writing by Health Canada and the CFIA (separate letters) on their respective decisions.
3.4 New information
If the Food Directorate concludes that the novel food is safe for consumption, it will be permitted to enter the marketplace in the same manner as traditional food products and thereafter is subject to the same regulatory requirements applicable to all foods in Canada. It remains the responsibility of a company to ensure that its products are in compliance with all applicable statutory and regulatory requirements.
At the current pace of technological advancement, it is expected that new information on previously approved products will be identified on occasion. Any post-market information obtained, which has potential health and safety implications, should be forwarded to Health Canada for consideration in order to ensure the continued safety and integrity of all novel foods available in the Canadian marketplace. The sale of a food that poses a hazard to the health of the consumer would contravene the provisions of the Food and Drugs Act.
3.5 Post-market monitoring
Novel foods are not permitted for sale if there is any evidence to suggest that they are not safe for consumption. Post-market monitoring may be an appropriate risk management measure in specific circumstances. Following the safety assessment, the need and utility for post-market monitoring should be considered, on a case-by-case basis, during risk assessment and its practicability should be considered during risk management.
4. Information requirements for safety assessment
The starting point for the safety assessment of novel foods is the evaluation of these foods relative to conventional counterparts that have a history of safe use. This approach takes both intended and unintended effects into account. The intention is to identify new or altered hazards relative to the conventional counterpart. If a new or altered hazard, nutritional or other food safety concern is identified, it would be assessed to determine its relevance to human health. Where no conventional counterpart exists for comparison, the safety of a novel food must be evaluated from data derived directly from historical experience or experimental studies with the food. The role that the food will play in the diet can be used as a background in considering nutritional implications of the product.
The safety assessment of novel foods follows a stepwise process of addressing relevant factors that include:
- History of use
- Dietary exposure
- Detail of novel process (if applicable)
- History of organism(s)
- Characterization of derived line/strain (if applicable)
- Genetic modification considerations (if applicable)
- Nutritional considerations
- Toxicology considerations
- Allergenicity considerations
- Chemical considerations
Given the great variety of potential novel foods and the many reasons why a food can be classified as novel, the amount of information necessary for the safety assessment will also vary widely from one case to another. Therefore, in order to provide guidance for petitioners, this document will highlight the types of information likely to be required for specific types of novel foods. Not all information described may be relevant in every case. The explanations and interpretations in this document are subject to change as additional knowledge and experience are gained in evaluating data and information supplied in novel food submissions.
Experiments intended to generate data to demonstrate the safety of a novel food should be designed and conducted in accordance with sound scientific concepts and principles, as well as, where applicable, Good Laboratory Practice. Primary data should be made available to regulatory authorities upon request. Data should be obtained using sound scientific methods and analysed using appropriate statistical techniques, as applicable. The sensitivity of all analytical methods should be documented and references to analytical methods made available.
The decision tree in Figure 3 is intended to help petitioners to consider whether their product is indeed a food, whether it might be something other than a novel food and, if it is a novel food, to determine which sections of the Guidelines are most appropriate for the various novel food categories (genetic modification, novel process, and history of safe use) and whether plant, microorganism or animal (the latter under development). Petitioners are encouraged to consult with the Novel Foods Section to clarify which information requirements should be addressed for a particular novel food product prior to making a notification or submission.
Figure 3. Decision tree for guidance on regulations or safety assessment guidelines for novel foods or similar products.

Figure 3 - Text Equivalent
When a petitioner has a product that they think is regulated under the Food and Drugs Act, they can answer the following series of questions to determine what regulations (if any) their product may be subject to.
The first question to answer is: Is this product a food or food ingredient as defined in the Food and Drugs Act? If the answer is no, a novel food notification is not required under part B of the Food and Drugs Regulations. However, a petitioner should then consider whether their product is a drug, for which a petitioner should refer to Part C of the Food and Drugs Regulations entitled "Drugs". If the product is not a drug, a petitioner should then consider whether their product is a natural health product and therefore subject to the Natural Health Products Regulations.
If the answer to the first question is yes, the second question to answer is: Is this food or food ingredient a food additive as defined in B.01.001 of the Food and Drugs Regulations? If the answer is yes, a petitioner should refer to B.16.002 (a) of the Food and Drugs Regulations which addresses food additives.
If the answer is no, the third question to answer is: Is this food or food ingredient intended to be a dietary fibre source? If the answer is yes, a petitioner should refer to the novel fibre guidelines.
If the answer is no, the fourth question to answer is: Is this food or food ingredient intended to fortify foods with vitamins, minerals, or amino acids? If the answer is yes, a petitioner should refer to Part D of the Food and Drugs Regulations entitled "Vitamins, Minerals and Amino Acids".
If the answer is no, the fifth question to answer is: Is this a food or food ingredient produced by, and/or containing an organism (to the species level) not previously used in food in Canada? If the answer is yes, a petitioner should refer to the Guidelines for the Safety Assessment of Novel Foods, particularly sections 4.1.1 and 4.2.1 which discuss plants and microorganisms with no history of safe use, respectively.
If the answer is no, the sixth question to answer is: Is the source organism from which the food or food ingredient is derived genetically modified as defined in B.28.001 of the Food and Drugs Regulations? If the answer is yes, a petitioner should refer to the Guidelines for the Safety Assessment of Novel Foods, particularly sections 4.1.3 and 4.2.3 which discuss genetically modified plants and microorganisms, respectively.
If the answer is no, the seventh question to answer is: Is the food or food ingredient the result of a process not previously used on that food or food ingredient and has this process in a major change to the food or food ingredient? For the complete definition of a "major change", please refer to B.28.001 of the Food and Drugs Regulations. If the answer is yes, a petitioner should refer to the Guidelines for the Safety Assessment of Novel Foods, particularly sections 4.1.2 and 4.2.2 which discuss the novel processing of plants and microorganisms, respectively.
Lastly, if a petitioner has identified their product as a food or food ingredient as defined in the Food and Drugs Act, but has answered "no" to the other six questions, a novel food notification is not required for the sale of that food or food ingredient in Canada.
4.1 Novel foods derived from plants
Plants may be consumed as food or used to produce materials which are used in food or food processing. Novel foods can be derived from plants with no history of safe use as a food source in Canada, manufactured by new processes applied to plant materials, or produced by plants that have been genetically modified by a variety of techniques.
It is recommended that the following information be included for assessing the acceptability of plant-derived foods that are novel for one or more of the above reasons. Note that not all information requirements outlined below may be applicable to all cases.
4.1.1 Substance with no history of safe use
- 4.1.1.1 History of use
- 4.1.1.2 Dietary exposure
- 4.1.1.3 Nutritional considerations
- 4.1.1.4 Toxicology considerations
- 4.1.1.5 Allergenicity considerations
- 4.1.1.6 Chemical considerations
4.1.2 Novel process
- 4.1.2.1 Detail of novel process
- 4.1.2.2 Dietary Exposure
- 4.1.2.3 History of organism
- 4.1.2.4 Nutritional considerations
- 4.1.2.5 Toxicology considerations
- 4.1.2.6 Allergenicity considerations
- 4.1.2.7 Chemical considerations
4.1.3 Genetic modification
- 4.1.3.1 Characterization of derived line
- 4.1.3.2 Genetic modification considerations
- 4.1.3.3 History of organism (Host and Donor(s))
- 4.1.3.4 Dietary exposure
- 4.1.3.5 Nutritional considerations
- 4.1.3.6 Toxicology considerations
- 4.1.3.7 Allergenicity considerations
- 4.1.3.8 Chemical considerations
4.1.1 Substance with no history of safe use
The safety assessment of novel foods in this category follows a stepwise process of addressing relevant factors that include:
- 4.1.1.1 History of use
- 4.1.1.2 Dietary exposure
- 4.1.1.3 Nutritional considerations
- 4.1.1.4 Toxicology considerations
- 4.1.1.5 Allergenicity considerations
- 4.1.1.6 Chemical considerations
4.1.1.1 History of use
A substance may be considered to have a history of safe use as a food if it has been an ongoing part of the diet for a number of generations in a large, genetically diverse human population where it has been used in ways and at levels that are similar to those expected or intended in Canada. The fact that a product has had a history of use according to the above definition in a jurisdiction with a similar food safety system would increase the level of confidence in the evidence presented. The following information would be needed to support a claim that a product has a history of safe use:
- Historical evidence indicating ongoing, frequent consumption by a cross-section of the population where it has been used over several generations. This evidence may be derived from various sources including, but not limited to, scientific publications and patents, non-scientific publications and books, cookbooks, books on the history of food culture, and/or affidavits from two or more independent, reputable authorities that include well-documented accounts of the way the food is used and how they know it has the history it does. Limited usage or short term exposure would not be adequate to demonstrate a history of safe use.
- A declaration of any possible adverse effects linked to the food documented in its country of origin and/or a country where there is a high degree of consumption.
- A description of the standard methods of commercial and/or domestic processing and preparation for consumption.
- A description of how the food is cultivated or (if from wild sources) harvested.
- Amounts of the food that people are likely to consume in Canada, including typical serving sizes and expected frequency of consumption, at both average and extremely high consumption levels.
- Analysis of the composition of the food based on randomly selected, statistically valid samples. This analysis should include proximate data as well as amino acid profile, fatty acid profile, mineral and trace mineral composition and vitamin composition, as well as any nutrients, antinutrients and bioactive phytochemicals known to be of particular interest in the product. The analysis should pay special attention to the presence of compounds in the food which may have implications for the health of any groups of the Canadian population (e.g. possible toxicants or allergens or unusually high levels of nutrients in the food source or final food product).
- Metabolism and/or gastrointestinal effects in humans.
The submission should include reliable, high quality information and reference sources. Anecdotal evidence will be given less weight than scientifically derived data. Information on the history of human exposure will be particularly important where there are traditional handling or cooking requirements for a food that is novel. This information will need to be made available to consumers in a consistent manner.
4.1.1.2 Dietary exposure
The role of the dietary exposure assessment for substances with no history of use as foods intended for use as food is to estimate:
- how much of the food is likely to be consumed and at what frequency and what role it is likely to play in the diet (e.g. a significant protein source, a condiment, etc.);
- the potential impact of that food on the dietary intake of nutrients by combining the results of the findings in (a) with information on the nutrient composition of the food; and
- if there are any anti-nutrients, toxins, contaminants or novel substances determined to occur in the food, the potential exposure to those substances.
The introduction of foods with no history of safe use may give rise to nutritional, toxicological or allergen issues that may impact upon food safety, and, therefore, estimation of exposure to components of the food of significance to health should be considered. For such foods, dietary exposure assessment has special challenges since the food is not simply an existing food with changes made to it; there is no pre-existing experience of its role in the food supply upon which to base exposure determinations. The approach needed to predict potential consumption patterns would likely involve using intakes of products of similar nutritional composition that play a similar role in the diet and that are currently routinely consumed in the diets of Canadians. The impact of palatability of the food and how it will be promoted will need to be estimated. An exposure simulation could then be conducted based on current dietary intake databases, preferably using data from Canadian subjects, in which the novel food has been incorporated by substituting it for a food or foods it might be expected to replace in the diet. These intake estimates may then be used to calculate the potential dietary exposure to specific components of the novel food that will be the subject of the safety assessment.
The exposure assessment results should describe the estimated changes in the dietary intake distribution of microconstituents using measures such as average, variability and percentiles (upper and lower) of intake. A differential impact in subgroups of the population (e.g. children, infants, elderly, ethnic groups, susceptible populations) should be evaluated as well as the impact on the population as a whole.
Although introduction of a completely novel food could change dietary patterns and therefore have a broader impact on nutrition and on exposure to other components in the diet than can be directly attributed to the food itself, these types of changes may not be possible to predict and the need for and feasibility of such projections or of post-market follow up would be assessed on a case by case basis. Periodic nutrition surveys of the population may be best placed for identifying dietary trends that may have resulted from newly introduced foods, foods having increased availability or popularity, as well as the many other factors that change diets in Canada and that could have either beneficial or adverse health impacts (See Section 3.4).
4.1.1.3 Nutritional considerations
General observations
The introduction of a novel food into the Canadian food supply requires a determination of nutritional quality of the food and the implications of its nutritional characteristics for the population as a whole and/or for specific subgroups. Population subgroups may be more vulnerable for different reasons: e.g. young children, pregnant and lactating women, those with particular metabolic characteristics, adolescents and others who may consume large amounts of food, or the elderly who consume small amounts of food. A nutrition evaluation which includes assessment of intended and unintended nutritional effects is needed in order to ensure that the nutritional status of consumers is not likely to be jeopardized by:
- substitution of foods and food ingredients of significant nutritive value with less nutritious varieties of the same or similar foods
- excessive intakes of nutrients or other bioactive substances as a result of unusually high levels in the novel food, or
- new or increased levels of anti-nutrients that could adversely affect the nutritional value of the food or the diet.
What is nutritional quality?
Nutritional quality as applied to food is related to the presence of essential nutrients and energy-yielding substances (in appropriate quantity and quality) and to other aspects of food traditionally considered as part of the science of nutrition. These other aspects include the nutritional roles of non-essential amino acids, specific types of fatty acids and carbohydrates, dietary fibre, cholesterol, lipotropic substances, other components of specific foods (e.g. human milk), nutrient bioavailability and nutrient interactions with other nutrients, with food additives and with natural toxicants. They also include nutrient excesses and the effects (both positive and negative) of food processing on the nutrients and on the organoleptic properties of the food. More recently, a wide range of "bioactive" substances found principally in plants are being shown to have a possible role to play in improving or protecting human health. These roles are also included in the broad definition of nutritional quality.
Foods with no history of safe use
The main concern with respect to a food with no history of safe use would be to verify that the consumption of the food would not have an adverse effect on the nutritional health of the consumer. Information on nutritional composition and quality is primarily needed to determine how the food could be used in the diet, to establish basic composition information for the food for use in food composition databases, and to permit the validation of nutrient content claims and quantity declarations.
Guidelines for producing data for nutritional evaluation
- Function of the data to be submitted
- The information provided for a food with no history of safe use should be of sufficient quantity and quality to determine its role in the diet and to characterize the average nutritional composition of the food.
- Any studies conducted to evaluate nutritional quality should be performed using the food as it is expected to be consumed by humans.
- Where published data on nutrient composition of the novel food are inadequate, analytical data may need to be obtained by the petitioner. In this case, a clear hypothesis should be formulated with an appropriate study design for obtaining data on nutritional composition that:
- Considers all major sources of potential variation in nutritional quality, e.g. geographic area, season, soil type and fertility, amount of sunlight, temperature, crop management, etc, in designing the study, to ensure these factors are controlled.
- Subjects the novel plant during cultivation to the conditions expected for it in commercial production.
- Locates test plots in several locations where the plant is expected to be grown or collected. Ideally, the conditions under which the plant is grown for collecting data should aim at representing different geographical locations where the plant may be grown as well as different years, rather than relying on data from many replicates at a single field location for only one year. Ensure sampling is conducted at the appropriate stage of maturity for the respective crop.
- Establishes a sampling plan prior to the commencement of the study based on a power calculation using at least one or a few components to indicate the number of samples required. This could be based on the most important analytes and/or the analytes which are expected to be more variable and require more samples. Details about the planned power and size of the chosen design for the proposed statistical test and hypothesis testing should be provided.
- Ensures that the appropriate analyses are performed on all the parts of the plant that may be used as food in Canada. The compositional data should be provided for the raw food, in other words, the edible part of the plant in its unprocessed state as well as for the food prepared for human consumption by recommended and/or expected means to examine the effects, where applicable, of processing, storage and cooking.
- Provides the criteria used for selecting the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed in Nutrient Composition section below.
- Ensures that analyses for each nutritive or non-nutritive component are conducted for all samples by a single laboratory using internationally approved and validated analytical methods and following consistent and appropriate sample storage and preparation procedures throughout. The study samples are analysed within an acceptable time frame from date of collection.
- Uses appropriate and consistent statistical methods chosen in advance based on the study design to analyse and report the results.
-
Nutrient composition
In the context of the above study guidelines, the following components of novel foods should be analysed. Where not all are analysed, the petitioner should provide the criteria used to select the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed below.
- proximate composition (i.e. ash, moisture, protein, fat, fibre, carbohydrate)
- content of true protein, non-protein nitrogenous material (e.g. nucleic acids and aminoglycosides) and amino acid profile - unusual amino acids should be determined if their presence is suspected (e.g. d-amino acids from bacterial proteins)
- complete fatty acid profile expressed as % total fatty acids, total nonsaponifiable components, and total sterols - the list of fatty acids should be grouped as monounsaturated, polyunsaturated and saturated
- composition of the carbohydrate fraction (e.g. sugars, starches, chitin, tannins, non-starch polysaccharides and lignin)
- composition of micronutrients (i.e. significant vitamin and mineral analysis)
- presence of naturally occurring or adventitious anti-nutritional factors (e.g. phytates, trypsin inhibitors, etc.)
- predictable secondary metabolites, physiologically active (bioactive) substances, other detected substances
-
Nutrient bioavailability/Presence of anti-nutrients
In situations where the food with no history of safe use may be a significant component of the Canadian diet, and/or a major supplier of nutrients, animal studies should be conducted to assess nutritional adequacy. This pertains in particular to the evaluation of protein quality, the possibility of unknown anti-nutrients, and questions of nutrient bioavailability.
Information should be provided, if applicable, describing the processing conditions that would be used in the production of the novel food, and the effects of the processing on nutrient levels and nutrient bioavailability.
- Information to include in the submission:
- the name of the plant including Latin and common names.
- a complete description of the experimental design, experimental conditions, and how sources of variation for nutrient levels were controlled.
- a complete description of sample collection and sample preparation.
- a citation and/or description of the analytical and statistical methods which were used to obtain data for the nutritive and non-nutritive components.
- nutrient and related data expressed as mean ± standard deviation, and as a range.
- results of statistical analyses.
- raw data for all components analysed from all locations used to grow the plant.
- published data if available.
- intended use of the organism as food in Canada, i.e. ingredient type(s), possible end products, level of use if different from current products which it would replace, known patterns of use and consumption of the food and its derivatives.
- any foreseeable unintended uses.
- Decision-making process
- All aspects of nutritional quality will be evaluated based on modern nutritional principles, standards and guidelines aimed at meeting human nutritional needs. The bases of evaluation include: nutrient intake recommendations, the role of the food in the diet of the population and the role of diet and nutrition in reducing the risk of developing diet-related disease and health promotion.
- The first phase of nutritional evaluation will be based on the nutrient composition data and an assessment of both intended and unintended effects on nutrient composition. If there is a finding of unusual or unanticipated components or levels of nutrients or nutritive substances, the food may need to be subjected to further analysis.
- A novel food with no history of safe use is not required to meet specific criteria of nutritional quality. The main concern is to document the composition of the food in order to evaluate claims and to determine its potential role in the diet.
4.1.1.4 Toxicology considerations
Toxicological testing is required for substances of unknown safety that may be introduced to the food supply. For foods that have no history of safe use, it may be difficult to identify individual components which are novel in the context of human consumption in the absence of a traditional counterpart.
Where it is not possible to identify novel components of the food, a case-by-case approach should be used to determine the appropriate toxicological tests to be carried out on the food. The history of the organism from which the food is derived as a source of toxins or antinutrients and a chemical analysis of its components will be considerations in determining requirements for toxicological testing. Depending on these determinations, conventional studies of toxicity, including chronic toxicity, developmental toxicity, genotoxicity or carcinogenicity, may need to be performed on the final food product or its components as appropriate.
It should be noted that the conduct of studies with whole foods presents some challenges due to the potential for inducing nutritional imbalances when the food is incorporated into the diet at high concentrations. In addition, toxicology studies on novel foods are used to reach a conclusion as to whether the food is safe to consume under expected consumption patterns, rather than to derive a quantitative limit such as an acceptable daily intake in the manner used for simple chemicals like food additives.
4.1.1.5 Allergenicity considerations
The primary consideration in allergenicity assessment of a novel food is the prevention of unexpected and/or unavoidable exposure of susceptible individuals to food allergens. For foods with no history of safe use, the potential exists that one or more component proteins would have the capacity to cross-react with known food allergens or lead to the development of de novo hypersensitivity. It should be noted, however, that the vast majority of proteins consumed in the diet are not allergenic.
At present, there is no definitive test that can be relied upon to measure directly the allergenic potential of an individual protein or of a whole food. Because existing strategies for the assessment of the allergenic potential of proteins were developed for the evaluation of individual, well-defined proteins (Section 4.1.3.7), they are not easily applied to the entire protein component of a whole food. The protein component of foods with no history of safe use will not be characterized to the extent necessary to apply these assessment strategies.
A preliminary strategy for assessing the allergenic potential of foods with no history of safe use would be to investigate whether plants from the same taxonomic family that are commonly part of the food supply are implicated in the induction of allergic response. The association of a particular family of plants with allergic response might not necessarily preclude the introduction of the novel food from a related species into the marketplace, but risk management measures such as post-market surveillance and labelling where identification of the food item is not obvious will need to be considered. Proteins from an allergenic source should not be added to foods where identity preservation cannot be guaranteed.
4.1.1.6 Chemical considerations
The identification and levels of chemical contaminants must be reported in a food with no history of safe use. Potential levels and types of contaminants would be specific to the novel food type. It would therefore be necessary to determine the levels and ranges of contaminants which may be present in the food. If possible, a comparison of the levels of chemical contaminants in the novel food with those typically found in similar food products should be made. Examples of potential chemical contaminants are inorganic contaminants including metals (e.g. arsenic, cadmium, mercury and lead), organic contaminants including persistent organic pollutants or "POPs" (e.g. DDT, aldrin, dieldrin, etc.), and natural contaminants including mycotoxins (e.g. aflatoxins, vomitoxin, ochratoxin, zearalenone, etc.).
Any food additives present in the final food (e.g. anticaking agents, carrier solvents, solid diluents, colours, preservatives) or processing aids used during the course of manufacture of the food (e.g. precipitation aids, filtering agents, etc,) should be identified and their levels indicated.
In the case of novel foods intended for use as ingredients in other foods, specifications of identity and purity should be provided, along with a sample label and Directions for Use.
4.1.2 Novel process
Some processes applied to foods or food ingredients may result in the generation of foods which would be considered novel in relation to traditional counterparts. The application of new processes which cause a food to undergo a major change would trigger the requirement to notify Health Canada. A major change is defined in Division 28 of the Regulations as a change in a food that, based on the manufacturer's experience or generally accepted nutritional or food science theory, places the food outside the accepted limits of natural variations for that food with regard to: the composition, structure, nutritional quality of the food or its generally recognized physiological effects; the manner in which the food is metabolized in the body; or the microbiological safety, the chemical safety or the safe use of the food. Examples of novel processes include: new heat processing techniques; new packaging technologies; and the use of ultraviolet light for reducing the microbial load of a product.
The safety assessment of novel foods in this category follows a stepwise process of addressing relevant factors that include:
- 4.1.2.1 Details of novel process
- 4.1.2.2 Dietary Exposure
- 4.1.2.3 History of organism
- 4.1.2.4 Nutritional considerations
- 4.1.2.5 Toxicology considerations
- 4.1.2.6 Allergenicity considerations
- 4.1.2.7 Chemical considerations
4.1.2.1 Details of novel process
While the focus of the safety assessment is on the food product, consideration of the process or preparation of the product can guide the safety assessment. Any novel processing or preparation techniques used to produce a novel food should be described in sufficient detail since such processing or preparation techniques may result in potential microbiological, toxicological, allergenic, or nutritional concerns.
4.1.2.2 Dietary exposure
The role of the dietary exposure assessment for novel foods resulting from the application of a novel process, is to estimate:
- how much of the food is likely to be consumed and at what frequency and what role it is likely to play in the diet, if different from the role of the unprocessed food or the food processed by conventional means;
- the potential impact of that food on the dietary intake of nutrients by combining the results of the findings in (a) with information on changes, if any, in the nutrient composition of the food due to the novel process;
- if there are any modifications in the level or nature of bioactive substances, anti-nutrients, contaminants or toxins, or if there are novel substances produced in the food, the potential exposure to those substances.
In cases where the nutrient composition of food has been altered, either intentionally or unintentionally, through the novel process, the magnitude of that change should be assessed against the expected nutritional value of the unprocessed food and/or against the changes that result from conventional processes used on the same food, and also to determine if it would have a significant impact on overall dietary nutrient intakes for consumers. A decision to do a full exposure assessment might depend on how big a change has taken place and how much of the food supply might be affected as a result. An exposure assessment could be done by intake modelling using current dietary intake databases, preferably using data from Canadian subjects, in which the novel food with its modified nutrient composition has been inserted in place of the food that is processed for the same purpose by conventional means. For example, if the novel process is meant to replace heat pasteurization, then the heat-pasteurized food in the database would be the food to substitute.
The results should describe the estimated changes in the intake distribution of nutrients using measures such as the average, the variability and percentiles (upper and lower) of intake. A differential impact in subgroups of the population (e.g. children, infants, elderly, ethnic groups, susceptible populations) should be evaluated as well as the impact on the population as a whole.
Where there is an intentional nutritional or health-related modification, the impact of how it will be promoted also needs to be considered, to the extent possible, since this could change the current level of use of the non-novel counterpart.
Where there are changes in the levels of bioactive substances, anti-nutrients, contaminants or toxins or if novel substances are generated as reaction by-products in the food as a result of the process, intake estimates for the food would then be used to calculate the potential dietary exposure to these specific components of the novel food that will be the subject of the safety assessment.
Novel processes applied to foods to reduce spoilage due to microbial activity can also increase the availability of foods previously consumed relatively rarely in Canada, for example, fruits growing in tropical regions. The increased availability of such foods may give rise to nutritional, toxicological or allergenic issues that may impact upon food safety and, therefore, estimation of exposure to food components of significance to health should be considered. Estimating the potential for such increases to occur and their impact should be considered if there are reasonable grounds upon which to make quantifiable predictions. Although increases of this type could change dietary patterns and therefore have an impact on nutrition and on exposure to other components in the diet, these types of changes may not be possible to predict. The need for and feasibility of projections of future intakes or of post-market follow up should be assessed on a case by case basis. Periodic nutrition surveys of the population may be best placed for identifying dietary trends that may have resulted from newly introduced foods, foods having increased availability or popularity, as well as the many other factors that change diets in Canada and that could have either beneficial or adverse health impacts (See Section 3.4).
4.1.2.3 History of organism(s)
The history of an organism can provide information that is important to the assessment of a novel food. There may be a history of toxin production by certain strains, species or genera and it would be important in such cases to examine the particular variety of the organism being used for the potential to produce such toxins, both under the conditions used in normal manufacturing and also under extreme conditions.
4.1.2.4 Nutritional considerations
I Unintended nutritional effects
General Observations
The introduction of a novel food into the Canadian food supply requires a determination of nutritional quality of the food and the implications of its nutritional characteristics for the population as a whole and/or for specific subgroups. Population subgroups may be more vulnerable for different reasons: e.g. young children, pregnant and lactating women, those with particular metabolic characteristics, adolescents and others who may consume large amounts of food, or the elderly who consume small amounts of food. A nutrition evaluation which includes assessment of intended and unintended nutritional effects is needed in order to ensure that the nutritional status of consumers is not likely to be jeopardized by:
- substitution of foods and food ingredients of significant nutritive value with less nutritious varieties of the same or similar foods
- excessive intakes of nutrients or other bioactive substances as a result of unusually high levels in the novel food, or
- new or increased levels of anti-nutrients that could adversely affect the nutritional value of the food or the diet.
What is nutritional quality?
Nutritional quality as applied to food is related to the presence of essential nutrients and energy-yielding substances (in appropriate quantity and quality) and to other aspects of food traditionally considered as part of the science of nutrition. These aspects include the nutritional effects of non-essential amino acids, specific types of fatty acids and carbohydrates, dietary fibre, cholesterol, lipotropic substances, other components of specific foods (e.g. human milk), nutrient bioavailability and nutrient interactions with other nutrients, with food additives and with natural toxicants. They also include nutrient excesses and the effects (both positive and negative) of food processing on the nutrients and on the organoleptic properties of the food. More recently, "bioactive"substances found principally in plants are being shown to have a possible role to play in improving or protecting human health. These substances are also included in the broad definition of nutritional quality.
Application of novel process to plant foods
The development of novel foods or novel food ingredients through application of a novel process, could result in unintended changes in the composition of the food product which could in turn have an impact on the nutritional value of the food and the nutritional status of the persons consuming it.
Unintended nutritional effects can occur whether the novel process is intended for nutritional or microbiological or other reasons. Evaluation of an intended effect on the nutritional quality of a food is discussed in Part II of this section.
An important step in the safety and nutritional assessment of this type of novel food is a comparison of its composition with its appropriate counterpart(s). To determine whether there are any differences in the nutritional quality of the novel food compared to its appropriate counterpart(s), the major constituents of the food must be analysed, i.e. macronutrients and their component parts, as well as individual micronutrients and other bioactive substances selected based on valid criteria. If any nutrients are excluded from the analyses, this should be justified by an acceptable rationale. Also, circumstances may warrant an evaluation of the nutritional "performance" of the new food in its ready-to-eat form, thus either raw or when further processed by traditional/conventional methods used to make the product ready-to-eat. The purpose would be to provide an opportunity to identify major changes that may not have been detected by compositional analysis, but which could affect, for example, the stability or bioavailability of nutrients in the food or the susceptibility of anti-nutrients to processing that normally destroys them. A performance test could involve re-analysis of a substance following cooking or it could require animal testing for bioavailability.
Guidelines for producing data for nutritional evaluation
- Function of the data to be submitted
- The information provided for a novel food should be of sufficient quantity and quality to allow an assessment of whether any significant unintended effect on the nutritional quality of the food has occurred as a result of the application of the novel process on the food, relative to the food processed using current commercial processes. It should also allow an assessment of the nutritional significance of any change that is detected.
- Data should be provided for the food in its final product state (i.e. processed using novel method). Data may also be required for the food prepared for human consumption by conventional means. This would examine the effects of further processing, storage and cooking. For example, in cases where cooking the food normally destroys anti-nutrients, data would be collected on the levels of anti-nutrients after cooking.
- Data on the novel food should be compared, at a minimum, to data on two appropriate counterparts, the unprocessed food and the food processed by a currently used equivalent process (see section b, below). It is suggested that the study design include a representation of the various cultivars that are commercially available in the Canadian market; these cultivars should all be subjected to the test and control processes. This would permit assessment with respect to the normal variation expected between cultivars. Literature data (if available) may also be valid for assessing the nutritional relevance of any unintended effect.
- Where published data on nutrient composition of the novel food are inadequate, analytical data may need to be obtained by the petitioner. In this case, a clear hypothesis should be formulated with an appropriate study design for obtaining data on nutritional composition that:
- Considers all potential sources of variation in nutritional quality, e.g. conditions of application (dose, duration, temperature), surface area or volume of plant food, cultivar, consistency of nutrient levels in the starting material, etc, in designing the study, to ensure these factors are controlled.
- Includes in the same study the novel food that is the subject of the notification as well as the appropriate counterparts, i.e. the same food in its pre-processed raw state, and the same food subject to a currently used equivalent process. A currently used equivalent process would be a non-novel process that is currently used commercially to achieve the same effect as the novel process (if applicable). In the absence of a currently used equivalent process, the counterpart would be simply the same food in its pre-processed raw state.
- Applies the novel process (test), and currently used equivalent process (control) to a selection of the commercial cultivars available in the current market.
- Establishes a sampling plan prior to the commencement of the study based on a power calculation using at least one or a few components to indicate the number of samples required. This could be based on the most important analytes and/or the analytes which are expected to be more variable and require more samples. Details about the planned power and size of the chosen design for the proposed statistical tests and hypothesis testing should be provided.
- Ensures processing is conducted at the appropriate stage of maturity for the plant food, and that sampling is conducted at the appropriate stage of processing for the plant food (i.e. final product).
- Ensures that the appropriate analyses are performed on all the parts of the plant that may be used as food in Canada.
- Provides the criteria used for selecting the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed in c. Nutrient Composition below.
- Ensures samples are analysed within an acceptable time frame from date of collection.
- Ensures that analyses for each nutritive or non-nutritive component are conducted for all samples by a single laboratory using internationally approved and validated analytical methods and following consistent and appropriate sample storage and preparation procedures throughout.
- Uses appropriate and consistent statistical methods chosen in advance, based on the study design, to compare levels of each nutrient in the novel food versus its controls.
-
Nutrient composition
In the context of the above study guidelines, the following components of foods should be analysed. Where not all are analysed, the petitioner should provide the criteria used to select the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed below.
- proximate composition (i.e. ash, moisture, protein, fat, fibre, carbohydrate)
- content of true protein, non-protein nitrogenous material (e.g. nucleic acids and aminoglycosides), amino acid profile - unusual amino acids should be determined if their presence is suspected (e.g. d-amino acids from bacterial proteins)
- complete fatty acid profile expressed as % total fatty acids, total nonsaponifiable component, and total sterols. The list of fatty acids should be grouped as monounsaturated, polyunsaturated and saturated
- composition of the carbohydrate fraction (e.g. sugars, starches, chitin, tannins, non-starch polysaccharides and lignin)
- composition of micronutrients, i.e. significant vitamin and mineral analyses
- presence of naturally occurring or adventitious anti-nutritional factors e.g. phytates, trypsin inhibitors, etc.
- predictable secondary metabolites, physiologically active (bioactive) substances, other detected substances
-
Nutritional "performance" of novel plant food
Consideration should be given to the possible need for the following types of information regarding the novel food:
- Response of known anti-nutrients to processes normally expected to neutralize their activity, measured using compositional analysis.
- Storage stability with regard to nutrient degradation.
- Performance of product in relation to the intended benefit (other than direct health benefits) e.g. improved stability of an oil to heating after fatty acid profile modification.
-
Nutrient bioavailability/Presence of new or altered anti-nutrients
In situations where the novel food may become a significant component of the Canadian diet, and/or a significant supplier of nutrients, animal studies may be needed in assessing nutritional adequacy to determine if there have been changes in the bioavailability of nutrients or if the composition is not comparable to conventional foods.
Information should be provided, if applicable, describing the conditions used in the further processing of the novel food and its derivatives, and the potential effects of the processing on nutrient levels and nutrient bioavailability.
- Information to include in the submission:
- a full description of the novel process, the purpose of the process, and the food (s) on which it could be applied, and the food (s) on which it will be applied (for the purpose of the submission).
- the foods on which the test and control processes were applied in the study, and the names and source (i.e. where purchased and grown) of all commercial cultivars which were represented in the study).
- a complete description of the experimental design, experimental conditions, and how sources of variation for nutrient levels were controlled.
- a complete description of sample collection and sample preparation.
- a citation and/or description of the analytical and statistical methods used to obtain data for the nutritive and non-nutritive components.
- nutrient and related data for test, control, and commercial cultivars (expressed as mean ± standard deviation, and as a range).
- results of statistical analyses.
- raw data for all components analysed.
- published data if available.
- intended use of the plant as food in Canada, i.e. ingredient type(s), possible end products, level of use if different from current products which it would replace, known patterns of use and consumption of the food and its derivatives.
- any foreseeable unintended uses.
- Decision-making process
- Appropriate modern statistical methods should be applied to the data to permit a demonstration that no significant change has resulted from the intervention. According to CodexFootnote 3, "the statistical significance of any observed differences should be assessed in the context of the range of natural variations for that parameter to determine its biological significance". If the composition of the novel food is judged not to be nutritionally equivalent to that of its counterparts, i.e. significant differences (statistical and biological) exist in the nutrient data, additional nutritional data may be required on a case-by-case basis.
- All aspects of nutritional quality will be evaluated based on modern nutritional principles, standards and guidelines aimed at meeting human nutritional needs. The bases of evaluation include: nutrient intake recommendations, the role of the food in the diet of the population and the role of diet and nutrition in reducing the risk of developing a diet-related disease and health promotion.
- Detection of a major change due to an unintended nutritional effect may not preclude the marketing of the product. However, such changes may require limits on the use of the food in food products or a requirement for labelling that goes beyond basic provisions. See also Part II with respect to safety assessment of high levels of nutrients or bioactive substances.
- The first phase of nutritional evaluation will be based on the nutrient composition data and an assessment of both intended and unintended effects on nutrient composition. If there is a finding of unusual or unanticipated components or levels of nutrients or nutritive substances, the food may need to be subjected to further analysis and assessment.
- The safety of a major increase in the level of a nutrient or other bioactive component would need to be assessed in a similar way to the safety assessment of an intended nutritional change. For details on this see Part II below.
II Intended nutritional modifications
The term "intended nutritional modification" is taken to include any change or introduced characteristic intended to improve the nutritional quality or health-related profile of the food, including but not limited to essential nutrients, beneficial bioactive phytochemicals, quantities and nature of the energy-yielding substances, improved nutrient bioavailability, and reduction in anti-nutrient levels.
Evaluation of an intended nutritional change requires steps that are similar to those used in either the addition of a vitamin or mineral nutrient to a food or the evaluation of foods with health claims or both. For instance, such a change would trigger questions concerning the intended target group, what level of the targeted nutrient or other substance is expected in the food, what is the expected change in level of exposure to the targeted nutrient or other substance across all age and sex groups and at the upper and lower extremes of intake of the food, and the safety of this level of exposure.
Addition of vitamins and mineral nutrients to foods by direct addition will continue to be regulated under Part D of the Food and Drug Regulations and pertinent parts of Part B of the Regulations. However, foods with intentional increased nutrient content as a result of other types of processes including use of special fertilizers to enhance nutrient content in a vegetable or grain would be considered as novel foods. The assessment of the proposed new levels of vitamins or minerals will take into consideration the regulatory limits of addition of vitamins and minerals.
A novel food with an introduced health or nutritional benefit would likely fall into the unofficial category of "functional food". B.28.002 (2) requires manufacturers to advise Health Canada at the time of making a novel food notification of the intended use and the details of the major change, if any, as well as to provide the text of all labels to be used in connection with the novel food. The manufacturer or importer is expected therefore, to advise Health Canada of the nature of the intended health or nutritional benefit and any desired health claims that are being considered for these products. Since there are different types of health claims, including biological role claims for nutrients and diet-related disease risk reduction claims, the manufacturer or importer are encouraged not to refrain from disclosing desired claims since the regulatory implications may vary.
Manufacturers and importers should note that the assessment of any health or nutritional claims is a separate step from the safety evaluation although information about the intended use may provide important information for the safety evaluation and affect such key considerations as dietary exposure. The assessment of the claim is most likely to be done after the decision regarding the product's safety and appropriate use as food.
In some cases, these products would be evaluated in accordance with the criteria being laid out for the product-specific authorization of health claims for foods. At this time, however, regulations for product-specific authorization of health claims for foods have not been promulgated. For information, prospective petitioners should refer to the proposed regulatory framework for product-specific health claims which was published in November, 2001, and the Interim Guidance Document on Standards of Evidence which was published in February, 2002.
These include attention to the evidence in support of the claim, as well as to product safety and product quality considerations.
Product safety of this type of novel food is intended to be controlled through application of the novel food regulations. The safety evaluation of a food manufactured using a novel process for the purpose of having an intended nutritional modification should be the same as for other novel foods. With regard to the safety and nutritional evaluation of the intended nutritional modification itself, data requirements are described below. It is important to note that if a modification to the food is such that it will not be safe for ad libitum consumption, a regulatory amendment may be required to specify the levels of use that are permitted for the food or ingredient. This would extend the approval period for the product considerably.
Product quality assurance refers to ensuring the consistency of the level of biologically active substances in the novel food in delivering the claimed benefits, and to conformance with acceptable procedures in all aspects of product testing. Details about quality assurance are discussed in the Interim Guidance Document on Standards of Evidence, mentioned below.
As part of quality assurance, it is important to ascertain to what extent the intended nutritional effect of a novel process remains stable with storage, further processing, and cooking.
The review of unintended nutritional effects in a food manufactured using a novel process for the purpose of having an intended nutritional effect would follow the same steps as for other novel foods.
Nutritional evaluation of expected or unexpected increased levels of a nutrient or bioactive substance
- Increased levels of a nutrient or other intrinsic bioactive substance in a food need to be evaluated for safety.
- Data needed for this include:
- the level of the targeted nutrient or other substance expected in the food;
- intended target group, if applicable, or which group(s) is or are most likely to have high intakes of the food;
- expected level of exposure to the substance through consumption of the food by the target group, by vulnerable sub-groups and at the upper and lower extremes of intake of the food across all age and sex groups using recent Canadian food consumption data where possible;
- how the expected level of exposure to the targeted nutrient or other substance differs from the current levels of exposure from all sources;
- any potential use of the product as a replacement of existing foods; and
- data in support of the safety of the expected level of exposure.
- acceptability of new levels of a vitamin or mineral will be evaluated on the basis of the levels permitted for direct addition under the Food and Drug Regulations.
4.1.2.5 Toxicology considerations
Toxicological testing is required for substances of unknown safety that are introduced to the food supply. The application of novel processes to foods may result in the generation of novel substances in the resulting food be they intentional or unintentional. Because of the potential wide variety of products generated by the application of novel processes, a determination of the appropriate toxicological testing should be conducted on a case-by-case basis.
Identification of any novel substances generated in the food subjected to a novel process is assisted by the use of a comparator such as unprocessed food or conventionally processed food. Chemical analysis may provide information on any new substances that have been formed. In addition, information on the nature, duration and intensity of treatment and the chemical composition of the food may be useful in predicting the types of alterations to the food components. Depending on these determinations, conventional studies of toxicity, including assays of metabolism, toxicokinetics, chronic toxicity/carcinogenicity, impact on reproductive function, and teratogenicity, may need to be performed on the final food product or its components as appropriate.
Intentional alteration of the composition of foods by the addition of food components at levels that fall outside the accepted limits for natural variations (e.g. "functional" foods) may result in exposures for which there is no history of safe use. Substances that have been traditionally consumed in foods but which have been added to foods at levels outside their normal range will result in consumption of higher amounts of the substance than from a traditional diet. In such cases, the novel aspect of the food is the extent of exposure to the substance, rather than the substance itself, and toxicological testing of the enhanced component will be required to establish an upper limit of tolerability to the substance. The types of studies conducted should be guided by a knowledge of the role of the component in human physiology. Evidence from animal and in vitro studies as indicated in the previous paragraph would be required to determine safety. Studies in experimental animals may be of limited usefulness if the commonly used animal model (e.g. the rat) differs markedly from humans in the metabolic pathways and chronic conditions that are the basis of the intended functional effect, and it may be necessary to place greater reliance on human response to increased intakes of such food components. Epidemiologic studies may be available for substances that are normally components of foods, and these can provide important information on long-term effects.
4.1.2.6 Allergenicity considerations
The primary consideration in allergenicity assessment of a novel food is the prevention of unexpected and unavoidable exposure of sensitized individuals to food allergens. In cases where the application of a novel process to a food results in the generation of a novel protein or an alteration of the protein content of a food containing allergenic proteins, a consideration of the allergenic potential of the novel food would be required.
Novel proteins
At present, there is no definitive test that can be relied upon to measure directly the allergenic potential of an individual protein or of a whole food. If the application of a novel process to a food results in the generation of a novel protein that can be isolated and characterized, the assessment strategy that has been developed for foods which are the products of recombinant DNA technology and described in section 4.1.3.7 can be used to assess its potential allergenicity. This strategy involves a weight of evidence approach that relies on the assessment of amino acid sequence homology to known food allergens, and a consideration of the similarity of its properties, in particular, resistance to digestion in the mammalian gastrointestinal tract, to those of known food allergens.
Alteration of endogenous allergen content
If the application of a novel process to a food that contains allergenic proteins results in altered protein content of that food, the potential for increase in the allergenic content should be assessed. While the health impacts of such increases is uncertain, this result would be considered undesirable. Techniques used for assessing the potential for effects on endogenous allergen expression are: the quantitative comparison of protein composition of the edible portion of the modified organism or, where sera from sufficient numbers of individuals with allergies to the food are available, the comparative immunoreactivity to the edible portion of the modified organism can be determined using immunoblotting techniques.
4.1.2.7 Chemical considerations
The identification and levels of chemical contaminants must be reported. Contaminants could be naturally present in the food before application of the novel process or could be introduced as a result of application of the novel process. It would be necessary to provide a comparison of the levels of chemical contaminants in the novel food with those levels typically found in the food product prepared by accepted traditional processes. Examples of potential chemical contaminants naturally present in the food are inorganic contaminants including metals (e.g. arsenic, cadmium, mercury and lead), organic contaminants including persistent organic pollutants or "POPs" (e.g. DDT, aldrin, dieldrin, etc.), and natural contaminants including mycotoxins (e.g. aflatoxins, vomitoxin, ochratoxin, zearalenone, etc.). Examples of substances that might be introduced as a result of application of the novel process would include extraction solvent residues, manufacturing aids, machinery lubricants, etc.
Any food additives present in the final food (e.g. anticaking agents, carrier solvents, solid diluents, colours, preservatives) or processing aids used during the course of manufacture of the food (e.g. precipitation aids, filtering agents, etc.) should be identified and their levels indicated.
In the case of foods, prepared by a novel process and intended for use as ingredients in other foods, specifications of identity and purity should be provided, along with a sample label and Directions for Use.
4.1.3 Genetic modification
Plants may be consumed as food or used to produce materials which are used in food or food processing. The variety of ways by which plants can be modified, and the degree of modification that can be produced, preclude standardization of the means to assess safety. The methods and extent of genetic modification, in part, determine both the type and quantity of information required to make an assessment.
The point in the development of the new variety at which data are generated is central to the assessment of safety. It is expected that for many "novel plants," the final product will be the result of repeated backcrosses between the initially-modified plant and the host variety. Some data generated in the initial stages would be accepted for an assessment of the final product. This would specifically relate to information on the method of modification, the stability of the transformed plant and molecular biology. The detailed data on the chemical and toxicological characterization should be generated with genetically stable, converted lines which are representative of the final food product.
It is important to note that not all information requirements outlined below may be appropriate to all cases. Applicants are encouraged to consult the Food Directorate early in product development in order to reach agreement on what information is appropriate to the evaluation of the safety of the product. The following information is recommended for assessing the acceptability of genetically modified plants and their products intended for use in or as a food. Once a genetically modified plant is determined to be acceptable, further variety development using traditional breeding techniques would not result in varieties requiring notification unless another major change occurs in the plant.
Wherever possible, transformation markers which generate safety concerns should not be present in the final food product. If selectable markers are present in the final food, they will be evaluated for safety.
The safety assessment of novel foods in this category follows a stepwise process of addressing relevant factors that include:
- 4.1.3.1 Characterization of derived line
- 4.1.3.2 Genetic modification considerations
- 4.1.3.3 History of organism
- 4.1.3.4 Dietary exposure
- 4.1.3.5 Nutritional considerations
- 4.1.3.6 Toxicology considerations
- 4.1.3.7 Allergenicity considerations
- 4.1.3.8 Chemical considerations
4.1.3.1 Characterization of derived line
Where a plant has been modified, whether by conventional breeding, selection and mutagenesis techniques or by recombinant nucleic acid technology, the relationship of the derived variety with the parent varieties should be characterised. The approach of the safety assessment is based on the principle that the safety of novel products is assessed relative to a conventional counterpart having a history of safe use, taking into account both intended and unintended effects. Any significant differences between the novel and the conventional variety are then assessed for potential adverse health effects. Of particular interest to the safety assessment is whether the modification could inadvertently develop or increase the toxicity or allergenicity potential of a new variety or reduce it's nutritional quality.
4.1.3.2 Genetic modification considerations
Genetic modification by traditional techniques
Many non-recombinant nucleic acid modification procedures are relatively undefined and poorly characterized in terms of insertion, deletion or rearrangement of genetic material, and the procedures are generally used for transfer of multi-genic characteristics. Strain selection or conventional breeding techniques can influence the toxin-producing capacity of an organism and may also influence desirable nutritional factors such as vitamin levels or the proportions of unsaturated fatty acids.
It is understood that specific information on the genetic differences between a novel organism such as a plant derived by mutagenesis or traditional breeding methods may not be available. The breeder may have knowledge of the characteristic selected and the source of that trait which should be provided if available. Agronomic characterization in addition to a consideration of key nutrients (macro and micro nutrients), anti-nutrients, and toxicants will be required to demonstrate the safety of a novel food derived from mutagenesis or traditional breeding techniques. The number of key nutrients, toxicants, and anti-nutrients required for analysis and assessment will be determined on a case-by-case basis and are associated with the organism under consideration. The nutrients and toxicants considered significant for the purposes of establishing the safety of a new food also depends on the potential intake of the food in Canada (dietary exposure considerations).
It is recognized that major food crops have an extensive history of safe use and that the introduction of new varieties of existing crop plants has only rarely resulted in adverse effects in humans. Novel food varieties obtained by outbreeding traditional crop varieties with wild types or exotics could potentially cause nutritional or toxicological concerns. In crosses where parental varieties are well known, toxins may be known and standards of toxin levels may be established. However, where crosses involve wild plants or wild relatives of crop plants, more extensive analysis for toxins in the edible portions of the plant and feeding studies may be necessary. It should be noted that the extent of backcrossing should be fully described as the process can remove a large percentage of the donor parents genetic material from the progeny selected for food use.
Traditionally developed plants require a multi-disciplinary assessment since details of the modifications may be largely unknown. As experience in the safety assessment of novel foods develops, it may be possible to identify data requirements for particular groups of products more clearly, or to preclude certain products from further detailed evaluation.
Conventional breeding may result in a food crop that requires a pre-market notification if selected characteristics fall well outside the agronomic, nutritional and compositional range for that species. A food derived from a crop species could also be considered novel if it exhibits major variations in composition in comparison to its unmodified counterpart.
Plants derived from mutagenesis and traditional breeding may result in a food crop that requires a pre-market notification if selected characteristics fall well outside the agronomic, nutritional and compositional range for that species. For example, a food derived from a food crop could be considered novel if it exhibits major variations in composition in comparison to its traditional counterpart. The goal of plant breeding is to change the heritable characteristics of an organism to introduce new characteristics or improve upon existing characteristics. Health Canada does not wish to review all new plant varieties being developed, only those that are truly novel and could pose a safety risk.
Genetic modification by modern techniques
In cases where a plant has been modified using modern genetic techniques, such as recombinant nucleic acid technology, the safety assessment will consider detailed characterization data of a novel organism at the molecular level. The following requirements are taken from safety assessment guidelines endorsed by the Codex Alimentarius Commission in 2003Footnote 4. In addition to the requirements of previous sections, the following areas should be addressed for these types of products:
i) Description of the genetic modification(s)
Details of all methods and manipulations involved in the modification of an organism must be provided to allow for the identification of all genetic material potentially inserted, deleted, mutated, or rearranged in the host genome. This will provide the necessary information for the analysis of the data supporting the characterization of the modified organism.
The description of the modification process should include:
- information on the method(s) of modification used, e.g. Agrobacterium-mediated transformation or direct transformation by methods such as particle bombardment, electroporation, etc.;
- description and characterization of all genetic material potentially delivered, if applicable, including the source, identity and expected function in the organism; and
- details of manipulations or modifications to introduced, intermediate and recipient genetic material (e.g. change that affects the amino acid sequence of expression product).
Information should be provided on DNA added, inserted, deleted, or modified, including:
- the characterization of all the genetic components including marker genes, regulatory and other elements affecting the function of the DNA;
- the size and identity;
- the location and orientation of the sequence in the final vector/construct; and
- function in the organism.
A summary diagram, outlining the key features of the final construct should be provided. Depending on the nature of the genetic modification, restriction maps and sequence data of the introduced or modified genetic material and adjacent regions, may be required.
ii) Characterization of the genetic modification(s)
In order to provide clear understanding of the impact on the composition and safety of foods derived from genetically modified organisms, a comprehensive molecular and biochemical characterization of the organism should be carried out.
Information should be provided on the DNA insertions into the genome; this should include:
- the characterization and description of all inserted genetic materials;
- the number of insertion sites;
- data to demonstrate if complete or partial copies have inserted into the genome;
- data to demonstrate whether the arrangement of the genetic material used for insertion has been conserved or whether significant rearrangements have occurred upon integration;
- the organization of the inserted genetic material at each insertion site including copy number and, where appropriate, provide sequence data of the inserted material and of the surrounding regions (sequencing information may be informative in some cases, i.e., to fully characterize a partial or rearranged DNA insert).
- identify any potential chimeric open reading frames created by the insertion(s) with contiguous plant genomic DNA if the inserted genetic material is truncated;
- in the case of modifications that involve deletions, rearrangements or site-specific, in vitro mutagenesis, sequence data of the region before and after modification should be provided.
Information should be provided on any expressed substances in the modified organism; this should include:
- the gene product (e.g. a protein or an untranslated RNA);
- the gene product's function;
- the phenotypic description of the new characteristic(s);
- the level and site of expression of the gene product(s), and the levels of its metabolites;
- to demonstrate whether deliberate modifications made to the amino acid sequence of the expressed protein result in changes in its post-translational modification or affect sites critical for its structure or function;
- where genetic manipulations are directed to altered regulation of endogenous genes, the characteristics and level of gene expression should be compared with that of the unmodified host;
- to indicate whether there is any evidence to suggest that one or several endogenous genes in the host plant has been affected by the modification process;
- to confirm the identity and expression pattern of any new fusion proteins;
- to demonstrate the intended effect of the modification has been achieved and that all expressed characteristics are expressed and inherited in a manner that is stable through several generations consistent with laws of inheritance. It may be necessary to examine the inheritance of the DNA itself or the expression of the corresponding RNA if the phenotypic characteristics cannot be measured directly; and
- to demonstrate that the newly expressed characteristic(s) are expressed as expected in the appropriate tissues in a manner and at levels that are consistent with the associated regulatory sequences driving the expression of the corresponding gene.
Conventionally bred hybrids derived from the crossing of previously approved genetically modified plant lines
Consistent with the definition of "novel food" in Division 28 of the Food and Drug Regulations, the progeny derived from the conventional breeding of approved genetically modified plants (one or both parents are genetically modified) would not be classified as a novel food unless some form of novelty was introduced into such progeny as a result of the cross, hence triggering the requirement for pre-market notification under Division 28. For example, notification may be required for modifications observed in the progeny that result in a change of existing characteristics of the plant that places those characteristics outside of the accepted range, or, that introduce new characteristics not previously observed in that plant (e.g. a major change has occurred in the expression levels of characteristics when stacked). In addition, the use of a wild species (interspecific cross) not having a history of safe use in the food supply in the development of a new plant line may also require notification to Health Canada.
4.1.3.3 History of organism(s)
The history of both donor and host organisms can provide information that is important to the assessment of a novel food. There may be a history of toxin production by certain strains, species or genera and it would be important in such cases to examine the particular organism(s) being used in the development of the novel food for the potential to produce such toxins, both under the conditions used in normal manufacturing and also under extreme conditions.
4.1.3.4 Dietary exposure
The role of the dietary exposure assessment for novel foods produced through genetic modification, is to estimate:
- how much of the food is likely to be consumed at what frequency and what role it is likely to play in the diet, if different from the role of the unmodified food;
- the potential impact of that food on the dietary intake of nutrients, by combining the results of the findings in (a) with information on changes, if any, in the nutrient composition of the food due to the genetic modification;
- if there are any modifications in the level or nature of bioactive substances, anti-nutrients or toxins;
- the potential exposure to gene expression products produced as a result of the genetic modification.
In cases where the nutrient composition of a food has been altered, either intentionally or unintentionally, through genetic modification, the magnitude of that change should be assessed against the expected nutritional value of the unchanged food, and also to determine if it would have a significant impact on overall dietary nutrient intakes for consumers. A decision to do a full exposure assessment might depend on how big a change has taken place and how much of the food supply might be affected as a result. An exposure assessment could be done by intake modelling using current dietary intake databases, preferably using data for Canadian subjects, in which the novel food with its modified nutrient composition has been inserted in place of the unmodified food.
The results should describe estimated changes in the intake distribution of nutrients using measures such as average, variability and percentiles (upper and lower) of intake. A differential impact in subgroups of the population (e.g. children, infants, elderly, ethnic groups, susceptible populations) should be evaluated as well as the impact on the population as a whole.
Where there is an intentional nutritional or health-related modification, the impact of how it will be promoted also needs to be considered to the extent possible, since this could change the current level of use of the non-novel counterpart.
Where there are changes in the levels of bioactive substances, anti-nutrients, contaminants or toxins or if there are novel substances produced in the food, these intake estimates would then be used to calculate the potential dietary exposure to these specific components of the novel food that will be the subject of the safety assessment.
In the case of commodity crops that undergo genetic modification to alter agronomic characteristics, dietary exposure to food or food ingredients derived from the crop is unlikely to be altered. However, if these genetic modifications result in changes in the levels of bioactive substances, anti-nutrients, contaminants or toxins or a novel protein or novel metabolites in the food products derived from them, the dietary exposure to these substances should be determined using dietary modelling as described above and considered together with the toxicological data as part of the risk assessment. The effects of typical food processing procedures on the novel component(s) should be considered in deriving the exposure estimate. In the case of substances covered by existing safety data (e.g. permitted agricultural chemicals), documentation of the anticipated increase in exposure to these substances should be provided.
Genetic modification of crops to alter agronomic characteristics such as disease resistance can also increase the availability of foods previously consumed relatively rarely in Canada. The increased availability of such foods may have nutritional, toxicological or allergenic consequences related to increased consumption of individual food components of significance to health. Estimating the potential for such increases and their impact should be considered if there are reasonable grounds upon which to make quantifiable predictions. Although increases of this type could change dietary patterns and therefore have an impact on nutrition and on exposure to other components in the diet, these types of changes may not be possible to predict. The need for and feasibility of projections of future intakes or of post-market follow up should be assessed on a case by case basis. Periodic nutrition surveys of the population may be best placed for identifying dietary trends that may have resulted from newly introduced foods, foods having increased availability or popularity, as well as the many other factors that change diets in Canada and that could have either beneficial or adverse health impacts (See Section 3.4).
4.1.3.5 Nutritional considerations
I Unintended nutritional effects
General observations
The introduction of a novel food into the Canadian food supply requires a determination of nutritional quality of the food and the implications of its nutritional characteristics for the population as a whole and/or for specific subgroups. Population subgroups may be more vulnerable for different reasons: e.g. young children, pregnant and lactating women, those with particular metabolic characteristics, adolescents and others who may consume large amounts of food, or the elderly who consume small amounts of food. A nutrition evaluation which includes assessment of intended and unintended nutritional effects is needed in order to ensure that the nutritional status of consumers is not likely to be jeopardized by:
- substitution of foods and food ingredients of significant nutritive value with less nutritious varieties of the same or similar foods
- excessive intakes of nutrients or other bioactive substances as a result of unusually high levels in the novel food, or
- new or increased levels of anti-nutrients that could adversely affect the nutritional value of the food or the diet.
What is nutritional quality?
Nutritional quality as applied to food is related to the presence of essential nutrients and energy-yielding substances (in appropriate quantity and quality) and to other aspects of food traditionally considered as part of the science of nutrition. These aspects include the nutritional effects of non-essential amino acids, specific types of fatty acids and carbohydrates, dietary fibre, cholesterol, lipotropic substances, other components of specific foods (e.g. human milk), nutrient bioavailability and nutrient interactions with other nutrients, with food additives and with natural toxicants. They also include nutrient excesses and the effects (both positive and negative) of food processing on the nutrients and on the organoleptic properties of the food. More recently, "bioactive" substances found principally in plants are being shown to have a possible role to play in improving or protecting human health. These substances are also included in the broad definition of nutritional quality.
Foods from genetically modified plants
The development of novel foods or novel food ingredients through genetic modification, whether by traditional breeding, mutagenesis or recombinant DNA techniques, could result in unintended changes in the composition of the food product which could in turn have an impact on the nutritional value of the food and the nutritional status of the persons consuming it. As more complex or layered genetic modifications are attempted through recombinant DNA techniques, for instance to introduce both improved nutritional characteristics and agronomic characteristics into the same organism, these could increase the potential for unintended effects compared to simpler modifications. By the same token, other methods of genetic modification could also introduce multiple changes.
Unintended nutritional effects can occur whether the intended modification is nutritional or agronomic or something else. Evaluation of a modification intended to affect the nutritional quality of a food is discussed in Part II of this section.
An important step in the safety and nutritional assessment of the modified food is a comparison of its composition with its appropriate counterpart. To determine whether there are any significant differences, the major constituents of the food must be analysed, i.e. macronutrients and their component parts, as well as individual micronutrients and other bioactive substances selected based on valid criteria. If any nutrients are excluded from the analyses, this should be justified by an acceptable rationale. Also, circumstances may warrant an evaluation of the nutritional "performance" of the new food in its ready-to-eat form, thus either raw or when processed by traditional/conventional methods used to make the product ready-to-eat. The purpose would be to provide an opportunity to identify major changes that may not have been detected by compositional analysis, but which could affect, for example, the stability or bioavailability of nutrients in the food or the susceptibility of anti-nutrients to processing that normally destroys them. A performance test could involve re-analysis of a substance following cooking or it could require animal testing for bioavailability or some other nutritional factor.
Guidelines for producing data for nutritional evaluation
- Function of the data to be submitted
- The information provided for a novel food should be of sufficient quantity and quality to allow an assessment of whether any significant unintended genetic modification affecting the nutritional quality of the food has occurred as a result of the introduction of the novel characteristic. It should also allow an assessment of the nutritional significance of any change that is detected.
- Data should be provided for the raw food, in other words, the edible part of the plant in its unprocessed state. Data may also be required for the food prepared for human consumption by conventional means to examine the effects, where applicable, of processing, storage and cooking to look, for example, at the effectiveness of cooking to destroy anti-nutrients in cases where anti-nutrients normally destroyed by cooking are present.
- Data on the novel food should be compared, at a minimum, to data on the near isogenic, non-modified parent variety, the most appropriate counterpart, if available, or else a closely related non-modified cultivar. Since one or more significant differences could arise, the study design should include crops of the same species from a range of standard cultivars that are in commercial production for the same purposes and grown in the same geographical areas as those typically found on the Canadian market. This would permit assessment with respect to normal variation. Literature data (if available) may also be valid for assessing the nutritional relevance of any unintended effect.
- A clear hypothesis should be formulated with an appropriate study design for obtaining data on nutritional quality that:
- Considers all sources of potential variation in nutritional composition, e.g. geographic area, season, soil type and fertility, amount of sunlight, temperature, crop management, etc, in designing the study, to ensure these factors are controlled.
- Subjects the modified plant to the conditions expected for it in commercial production, i.e. a plant which is made tolerant to environmental or other stresses (insects, salt, drought, herbicides, etc.) should be grown under those conditions for the purposes of data collection. The control plants should likewise be grown under conditions appropriate for them.
- Includes in the same study the novel food that is the subject of the notification as well as the appropriate counterpart, i.e. the near isogenic parent cultivar, and a selection of the commercial cultivars available in the current market. In the absence of a near isogenic parent cultivar, the most closely related non-modified cultivar may be chosen.
- Locates the test plots in several locations which are representative of the major growing areas for the organism. Ideally, the conditions under which the organisms are grown for collecting data should aim at representing different geographical locations where the plant is normally grown as well as different years, rather than relying on data from many replicates at a single field location for only one year.
- Establishes a sampling plan prior to the commencement of the study based on power calculation using at least one or a few components to indicate the number of samples required. This could be based on the most important analytes and/or the analytes which are expected to be more variable and require more samples. Details about the planned power and size of the chosen design for the proposed statistical tests and hypothesis testing should be provided.
- Ensures sampling is conducted at the appropriate stage of maturity for the respective crop.
- Ensures that the appropriate analyses are performed on all the parts of the plant that may be used as food in Canada. For example, if the intended uses of a novel corn include the oil and the meal, samples of both corn oil and cornmeal should be analysed for the appropriate nutrients.
- Provides the criteria used for selecting the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed in the Nutrient Composition section below.
- Ensures samples are analysed within an acceptable time frame from date of collection.
- Ensures that analyses for each nutritive or non-nutritive component are conducted for all samples by a single laboratory using internationally approved and validated analytical methods and following consistent and appropriate sample storage and preparation procedures throughout.
- Uses appropriate and consistent statistical methods chosen in advance, based on the study design to compare levels of each nutrient in the novel food versus its controls.
-
Nutrient composition
In the context of the above study guidelines, the following is a generic list of the components of novel foods that should be analysed. Specifics for individual crops and their products will vary. Consensus documents on compositional considerations for new varieties of various crops, developed by the Organization for Economic Cooperation and Development (OECD)'s Task Force for the Safety of Novel Foods and FeedsFootnote 5, are available and may be consulted for further guidance with respect to particular crops. These documents suggest which key nutrients, anti-nutrients and toxicants associated with the crop should be analysed and provide data on the typical composition of the crops and their main products. Since these are guidelines, it is recommended that Health Canada be consulted with regard to which components should be included in an assessment. Where not all nutrients below are analysed, the petitioner should provide the criteria used to select the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed below:
- proximate composition (i.e. ash, moisture, protein, fat, fibre, carbohydrate)
- content of true protein, non-protein nitrogenous material (e.g. nucleic acids and aminoglycosides), amino acid profile - unusual amino acids should be determined if their presence is suspected (e.g. d-amino acids from bacterial proteins)
- complete fatty acid profile expressed as % total fatty acids, total nonsaponifiable component, and total sterols. The list of fatty acids should be grouped as monounsaturated, polyunsaturated and saturated
- composition of the carbohydrate fraction (e.g. sugars, starches, chitin, tannins, non-starch polysaccharides and lignin)
- composition of micronutrients, i.e. significant vitamin and mineral analyses
- presence of naturally occurring or adventitious anti-nutritional factors e.g. phytates, trypsin inhibitors, etc.
- predictable secondary metabolites, physiologically active (bioactive) substances, other detected substances
-
Nutritional "performance" of modified plant
Consideration should be given to the possible need for the following types of information regarding the modified plant:
- Response of known anti-nutrients to processes normally expected to neutralize their activity measured using compositional analysis.
- Storage stability with regard to nutrient degradation.
- Performance of product in relation to the intended benefit (other than direct health benefits), e.g. improved stability of an oil to heating after fatty acid profile modification.
-
Nutrient bioavailability/Presence of new or altered anti-nutrients
In situations where the food from a genetically modified source may become a significant component of the Canadian diet, and/or a significant supplier of nutrients, animal studies may be needed in assessing nutritional adequacy to determine if there have been changes in the bioavailability of nutrients or if the composition is not comparable to conventional foods.
Information should be provided, if applicable, describing the processing conditions used in the production of the novel food and its derivatives, and the potential effects of the processing on nutrient levels and nutrient bioavailability.
- Information to include in the submission:
- the names of all the cultivars which were represented in the study.
- a complete description of the experimental design, experimental conditions, and how sources of variation for nutrient levels were controlled.
- a complete description of sample collection and sample preparation.
- a citation and/or description of the analytical and statistical methods used to obtain data for the nutritive and non-nutritive components.
- nutrient and related data for test, control, and commercial cultivars (expressed as mean ± standard deviation, and as a range).
- results of statistical analyses.
- raw data for all components analysed from all locations used to grow the plant.
- published data if available.
- intended use of the plant as food in Canada, i.e. ingredient type(s), possible end products, level of use if different from current products which it would replace, known patterns of use and consumption of the food and its derivatives.
- any foreseeable unintended uses.
- Decision-making process
- Appropriate modern statistical methods should be applied to the data to permit a demonstration that no significant change has resulted from the intervention. According to CodexFootnote 6 "the statistical significance of any observed differences should be assessed in the context of the range of natural variations for that parameter to determine its biological significance". If the composition of the novel food is judged not to be nutritionally equivalent to that of its counterparts, i.e. significant differences (statistical and biological) exist in the nutrient data, additional nutritional data may be required on a case-by-case basis.
- All aspects of nutritional quality will be evaluated based on modern nutritional principles, standards and guidelines aimed at meeting human nutritional needs. The bases of evaluation include: nutrient intake recommendations, the role of the food in the diet of the population and the role of diet and nutrition in reducing the risk of developing a diet-related disease and health promotion.
- Detection of a major change due to an unintended nutritional effect may not preclude the marketing of the product. However, such changes may require limits on the use of the food in food products or a requirement for labelling that goes beyond basic provisions. See also Part II with respect to safety assessment of high levels of nutrients or bioactive substances.
- The first phase of nutritional evaluation will be based on the nutrient composition data and an assessment of both intended and unintended effects on nutrient composition. If there is a finding of unusual or unanticipated components or levels of nutrients or nutritive substances, the food may need to be subjected to further analysis and assessment.
- The safety of a major increase in the level of a nutrient or other bioactive component would need to be assessed in a similar way to the safety assessment of an intended nutritional change. For details on this see Part II below.
II Intended nutritional modifications
The term "intended nutritional modification" is taken to include any change or introduced characteristic intended to improve the nutritional quality or health-related profile of the food, including but not limited to essential nutrients, beneficial bioactive phytochemicals, quantities and nature of the energy-yielding substances, improved nutrient bioavailability, and reduction in anti-nutrient levels.
Evaluation of an intended nutritional change requires steps that are similar to those used in either the addition of a vitamin or mineral nutrient to a food or the evaluation of foods with health claims or both. For instance, such a change would trigger questions concerning the intended target group, what level of the targeted nutrient or other substance is expected in the food, what is the expected change in level of exposure to the targeted nutrient or other substance across all age and sex groups and at the upper and lower extremes of intake of the food, and the safety of this level of exposure.
A novel food with an introduced health or nutritional benefit would likely fall into the unofficial category of "functional food". It is expected that manufacturers will be interested in making health claims for these products. These products would therefore be evaluated in accordance with the criteria being laid out for foods with product-specific health claims. These include attention to the evidence in support of the claim, as well as to product safety and product quality considerations.
Product safety of this type of novel food is intended to be controlled through application of the novel food regulations. The safety evaluation of a novel food genetically modified to have an intended nutritional modification should be the same as for other genetically modified foods. With regard to the safety and nutritional evaluation of the intended nutritional modification, itself, data requirements are described below.
Product quality assurance refers to ensuring the consistency of the level of biologically active substances in the novel food in delivering the claimed benefits, and to conformance with acceptable procedures in all aspects of product testing. Details about quality assurance are discussed in the Interim Guidance Document on Standards of Evidence, mentioned below.
At this time, regulations for product-specific health claims have not yet been promulgated. Prospective petitioners should refer to the proposed regulatory framework for product-specific health claims which was published in November, 2001, and the Interim Guidance Document on Standards of Evidence which was published in February, 2002.
Adding a substance through genetic modification differs from adding one through applying it to or mixing it with the food after it is harvested. The decision to proceed with or cease the addition would take place at different stages of production. This could have an effect on the ability to manage the presence of the "added" substance or characteristic in the food supply if it was later decided that there was a need to control it. Given this potential need, such products should be subject to post-market surveillance to ensure the ability to monitor and control the products. To promote a product that has been altered with the intention of benefiting the consumer, manufacturers themselves would have a requirement for post-market surveillance, in any case, and therefore this should not add any significant additional burden.
It is important to ascertain to what extent the modified nutrient (if the intent was to deliberately modify the level of a nutrient) is bioavailable and remains stable with cultivation, time, processing, storage and cooking.
The review of unintended nutritional effects in a food modified to have an intended nutritional effect would follow the same steps as for other novel foods.
Nutritional evaluation of expected or unexpected increased levels of a nutrient or bioactive substance
- Increased levels of a nutrient or other intrinsic bioactive substance in a food need to be evaluated for safety.
- Data needed for this include:
- the level of the targeted nutrient or other substance expected in the food
- intended target group, if applicable, or which group(s) is or are most likely to have high intakes of the food
- expected level of exposure to the substance through consumption of the food by the target group, by vulnerable sub-groups and at the upper and lower extremes of intake of the food across all age and sex groups using recent Canadian food consumption data where possible
- how the expected level of exposure to the targeted nutrient or other substance differs from the current levels of exposure from all sources
- any potential use of the product as a replacement of existing foods
- data in support of the safety of the expected level of exposure
4.1.3.6 Toxicology considerations
Toxicological testing is required for substances of unknown safety that are introduced to the food supply. Novel substances may be introduced to the food supply through recombinant DNA technology or other genetic modification processes. Introduction of novel substances may be intentional or unintentional.
Genetic modification techniques can result in the production of novel substances by the organism or the intentional or unintentional modification of substances already produced by the organism or their expression.
Novel substances
In vitro nucleic acid techniques enable the introduction of DNA which can result in the synthesis of new substances in plants. These include the protein expression product and other substances which may be generated as a result of enzymic activity of the protein expression product. The new substances can be conventional components of plant foods such as proteins, fats, carbohydrates, or vitamins that are novel in the context of that recombinant DNA plant.
The introduced characteristic should be shown to be unrelated to any characteristics of donor organisms that could be harmful to human health. Information should be provided to ensure that genes coding for known toxins or anti-nutrients present in the donor organisms are not transferred to recombinant DNA plants that do not normally express those toxic or anti-nutritious characteristics. This assurance is particularly important in cases where a recombinant DNA plant is processed differently from a donor plant, since traditional processing techniques associated with the donor organisms may deactivate anti-nutrients or toxicants.
Toxicology studies are not considered necessary where the substance or a closely related substance has been consumed safely in food at equivalent intakes or where the new substance is not present in the food. Otherwise, the use of conventional toxicology studies on the new substance will be necessary. This may require the isolation of the new substance from the recombinant DNA plant, or the production of the substance from an alternative source, in which case, the material should be shown to be biochemically and functionally equivalent to that produced in the recombinant DNA plant.
For proteins, the assessment of potential toxicity should focus on amino acid sequence similarity between the protein and known protein toxins and anti-nutrients (e.g. protease inhibitors, lectins) as well as stability to heat or processing and to degradation in appropriate/representative gastric and intestinal model systems. Since proteins that are enzymes have never been shown to be direct-acting carcinogens, mutagens, teratogens or reproductive toxicants (Pariza and Foster 1983) it is generally not necessary to test proteins for these toxicological endpoints when exposure occurs by the oral route. Protein toxins act through acute mechanisms after the administration of a single dose at doses in the nanogram to milligram per kilogram body weight. Therefore, acute oral toxicity studies using gram per kilogram body weight doses of the novel protein are appropriate for assessing the potential toxicity of proteins. A negative result using doses in the gram/kg body weight range together with evidence that the protein is digested to small peptides and amino acids would provide assurance that the protein is not a toxin and is digested to nutrients as are the vast majority of dietary proteins.
Different types of in vivo or in vitro studies would be needed to assess the toxicity of introduced substances other than proteins. The types of studies are determined on a case-by-case basis and depend on the original source of the introduced substances and their function. Such studies may include assays of metabolism, toxicokinetics, chronic toxicity/carcinogenicity, impact on reproductive function, and teratogenicity.
Unintended effects
Techniques used in the genetic modification of plants or microorganisms have the potential to induce unintended effects on the genome of the modified organism that could be manifested as an alteration in the levels of toxicants or antinutrients normally produced by the organism. The intended genetic alteration may also influence the behaviour of the organism with respect to accumulation of contaminants, pesticides, or other substances from the environment that were not anticipated.
Compositional analysis is the method currently used for detection of unintended changes to the genome that result in accumulation of toxic substances either of endogenous or exogenous origin. Because of the influence of environmental stress on production of endogenous components such as toxins and anti-nutrients, data should be collected from a number of different test sites. New, more sensitive technologies that allow the determination of alterations to expression of the organisms' genome are presently under development.
4.1.3.7 Allergenicity considerations
The primary consideration in allergenicity assessment of a novel food is the prevention of unexpected and unavoidable exposure of sensitized individuals to food allergens. This includes the assessment of the potential for foods containing novel proteins to cross-react with known food allergens or to lead to the development of de novo hypersensitivity. In addition, the potential of increasing the allergenic potential of foods already containing allergens as an unintended result of genetic modification should be assessed. The following requirements are based on the Codex guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants.
Section 1 - Introduction
All newly expressed proteins in recombinant-DNA plants that could be present in the final food and are novel in the context of that food, need to be assessed for their potential to cause allergic reactions. This should include consideration of whether a newly expressed protein is one to which certain individuals may already be sensitive as well as whether a protein new to the food supply is likely to induce allergic reactions in some individuals.
At present, there is no definitive test that can be relied upon to measure directly the allergenic potential of a newly expressed protein in humans. Based upon the [best], currently-available scientific information, the recommended approach used takes into account the preponderance of evidence derived from several types of information and data in an integrated, stepwise, case-by-case manner.
Section 2 - Assessment strategyFootnote 7
The initial steps in assessing possible allergenicity of any newly expressed proteins involve determination of: the allergenicity of the source of the introduced protein; any similarity between the amino acid sequence of the protein and that of known allergens; and certain physicochemical properties, including but not limited to, its susceptibility to enzymatic degradation.
Genes derived from known allergenic sources should be assumed to encode an allergen unless scientific evidence demonstrates otherwise.
Determination of amino acid sequence homology and physicochemical characteristics will require the isolation of the newly expressed protein from the recombinant-DNA organism, or the synthesis of production of the substance from an alternative source, in which case the material should be shown to be functionally and biochemically equivalent to that produced in the recombinant-DNA organism.
Food proteins that are not allergens and that are altered by mutagenesis techniques need only be assessed for the likelihood that the mutagenized protein is a de novo allergen.
The absolute exposure to the newly expressed protein and the effects of relevant food processing will contribute toward an overall conclusion about the potential for human health risk. In this regard, the nature of the food product intended for consumption should be taken into consideration in determining the types of processing that would be applied and its effects on the presence of the protein in the final food product.
Section 3 - Initial assessment
Section 3.1 - Source of the protein
As part of the data supporting the safety of foods derived from recombinant-DNA organisms, information should describe any reports of allergenicity associated with the donor organism. Allergenic sources of genes would be defined as those organisms for which reasonable evidence of IgE-mediated oral, respiratory or contact allergy is available. Specific tools and relevant data that permit confirmation of allergenic potential are available for proteins from some allergenic sources. These include: the availability of sera for screening purposes; documented type, severity and frequency of allergic reactions; and structural characteristics and amino acid sequence (when available) of known allergenic proteins from that source.
Section 3.2 - Amino acid sequence homology
Amino acid sequence homology comparisons should be used to assess the extent to which a newly expressed protein is similar in structure to known allergens in order to determine whether that protein has allergenic or cross-reactivity potential. Overall structural similarities can be predicted using sequence homology searches that compare the structure of newly expressed proteins with all known allergens should be conducted using various algorithms such as FASTA or BLASTP. Strategies such as stepwise contiguous identical amino acid segment searches may also be performed for the purpose of identifying sequences that may represent linear epitopes. The size of the contiguous amino acid search should be based on a scientifically justified rationale in order to minimize the potential for false negative or false positive resultsFootnote 8. Validated search and evaluation procedures should be used in order to produce biologically meaningful results. IgE cross-reactivity between the newly expressed protein and a known allergen should be considered a possibility when there is more than 35% identity in a segment of 80 or more amino acids (FAO/WHO 2001).
Sequence homology searches have certain limitations. In particular, comparisons are limited to the sequences of known allergens in publicly available databases and the scientific literature. There are also limitations in the ability of such comparisons to detect non-contiguous IgE-binding epitopes.
A negative sequence homology result indicates that a newly expressed protein is not a known allergen and is unlikely to be cross-reactive to known allergens. A result indicating absence of significant sequence homology should be considered along with the other data outlined under this strategy in assessing the allergenic potential of newly expressed proteins. This does not preclude further studies where considered necessary (see also section 6). A positive sequence homology result indicates that the newly expressed protein has a high probability of being allergenic. If the product is to be considered further, it should be assessed using serum from individuals sensitized to the identified allergenic source (see section on Specific Serum Screening).
Section 3.3 - Pepsin resistance
Resistance to pepsin digestion has been observed in several food allergens; thus, a correlation exists between resistance to digestion by pepsin, and allergenic potentialFootnote 9. The resistance of a protein to degradation in the presence of pepsin under appropriate conditions indicates that further analysis should be conducted to determine the likelihood of the newly expressed protein being allergenic. The establishment of a consistent and well-validated pepsin degradation protocol may enhance the utility of this method.
Although the pepsin resistance protocol is strongly recommended, it is recognized that other enzyme susceptibility protocols exist. Alternative protocols may be used where adequate justification is provided.
Section 4 - Specific serum screening
For those proteins that originate from a source known to be allergenic, or have sequence homology with a known allergen, testing in immunological assays is required. Sera from individuals with a clinically validated allergy to the source of the protein can be used to test IgE-binding of the protein in in vitro assays. A critical issue for testing will be the availability of human sera from sufficient numbers of individualsFootnote 10. In addition, the quality of the sera and the assay procedure need to be standardized to produce a valid test result.
In the case of a newly expressed protein derived from a known allergenic source, a negative result in in vitro immunoassays may not be considered sufficient, but should prompt additional testing, such as the possible use of skin test and ex vivo protocols.
The identification of a newly expressed protein as an allergen through immunological assays suggests that further development for commercialization of the product be discouraged, unless adequate risk management and risk communication measures could be assured throughout marketing and distribution of the product, since segregation and identity preservation of the new source of this allergen may be difficult or impossible to enforce.
Section 5 - Areas requiring further development
The endpoint of the assessment of the data discussed above is a conclusion as to the likelihood of the protein being a food allergen. The techniques of targeted serum screening (i.e. the assessment of binding to IgE in sera of individuals with clinically-validated allergic responses to broadly-related categories of foods) and the use of animal models, once developed and validated, could enhance the weight of evidence used to derive this conclusion. To allow serum screening, steps should be taken to organize an international serum bank. As scientific knowledge and technology evolves, other methods, such as examination of newly expressed proteins for T-cell epitopes and structural motifs associated with allergens, might also be useful.
Unintended effects on endogenous allergens
Genetic modification techniques have the potential to produce unintended effects on the genome that could lead to an increase in the expression of endogenous allergens. While the potential for health impacts of such increases is uncertain, they are in any case considered undesirable. Techniques used for assessing the potential for effects on endogenous allergen expression are the quantitative comparison of protein composition of the edible portion of the modified organism or, where sera from sufficient numbers of individuals with allergies to the food are available, the comparative immunoreactivity to the edible portion of the modified organism can be determined using immunoblotting techniques.
4.1.3.8 Chemical considerations
The identification and levels of chemical contaminants must be reported. Potential levels and types of contaminants would, of course, be specific to the food to be modified and, also, the type of process employed to achieve the genetic modification. In this regard, contaminants could be naturally present in the food before genetic modification or contaminants may be introduced or their levels altered in food derived from the modified crop compared with the unmodified parental strain under the same growing conditions as a result of the application of the genetic modification. To address the latter case, it would be necessary to provide a comparison of the levels of chemical contaminants in the genetically modified food with those levels typically found in the original food product. Examples of potential chemical contaminants naturally present in the food are inorganic contaminants including metals (e.g. arsenic, cadmium, mercury and lead), organic contaminants including persistent organic pollutants or "POPs" (e.g. DDT, aldrin, dieldrin, etc.), and natural contaminants including mycotoxins (e.g. aflatoxins, vomitoxin, ochratoxin, zearalenone, etc.).
Any food additives present in the final food (e.g. anticaking agents, carrier solvents, solid diluents, colours, preservatives) or processing aids used during the course of manufacture of the food (e.g. precipitation aids, filtering agents, etc.) should be identified and their levels indicated.
In the case of novel foods intended for use as ingredients in other foods, specifications of identity and purity should be provided, along with a sample label and Directions for Use.
4.2 Novel foods derived from microorganisms
Microorganisms have been an important component of food for millennia. They may be consumed as inocula in fermented milk, meat or vegetable products or their metabolites may be used in food and in food processing. More recently, microorganisms have also been consumed directly as food in the form of single cell protein. Novel foods or ingredients can be derived from microorganisms not traditionally used as a food source in Canada, manufactured by new processes involving microorganisms, or produced by microorganisms that have been genetically modified by a variety of techniques.
It is recommended that the following information be included for assessing the acceptability of novel microorganisms and their products that are intended for use in or as a food. It is important to note that not all information requirements outlined below may be appropriate to all cases.
4.2.1 Substance with no history of safe use
- 4.2.1.1 History of use
- 4.2.1.2 Dietary exposure
- 4.2.1.3 Nutritional considerations
- 4.2.1.4 Toxicology considerations
- 4.2.1.5 Allergenicity considerations
- 4.2.1.6 Chemical considerations
4.2.2 Novel process
- 4.2.2.1 Detail of novel process
- 4.2.2.2 Dietary Exposure
- 4.2.2.3 History of organism
- 4.2.2.4 Nutritional considerations
- 4.2.2.5 Toxicology considerations
- 4.2.2.6 Allergenicity considerations
- 4.2.2.7 Chemical considerations
4.2.3 Genetic modification
- 4.2.3.1 Characterization of derived strain
- 4.2.3.2 Genetic modification considerations
- 4.2.3.3 History of organism (Host and Donor(s))
- 4.2.3.4 Dietary exposure
- 4.2.3.5 Nutritional considerations
- 4.2.3.6 Toxicology considerations
- 4.2.3.7 Allergenicity considerations
- 4.2.3.8 Chemical considerations
4.2.1 Substance with no safe history of use
The safety assessment of novel foods in this category follows a stepwise process of addressing relevant factors that include:
- 4.2.1.1 History of use
- 4.2.1.2 Dietary exposure
- 4.2.1.3 Nutritional considerations
- 4.2.1.4 Toxicology considerations
- 4.2.1.5 Allergenicity considerations
- 4.2.1.6 Chemical considerations
- 4.2.1.7 Microbiological considerations
4.2.1.1 History of use
A substance may be considered to have a history of safe use as a food if it has been an on-going part of the diet for a number of generations in a large, genetically diverse human population where it has been used in ways and at levels that are similar to those expected or intended in Canada. The fact that a product has had a history of use according to the above definition in a jurisdiction with a similar food safety system would increase the level of confidence in the evidence presented. The following information would be needed to support a claim that a product has a history of safe use:
- Historical evidence indicating ongoing, frequent consumption by a cross-section of the population where it has been used over several generations. This evidence may be derived from various sources including, but not limited to, scientific publications and patents, non-scientific publications and books, cookbooks, books on the history of food culture, and/or affidavits from two or more independent, reputable authorities that include well-documented accounts of the way the food is used and how they know it has the history it does. Limited usage or short term exposure would not be adequate to demonstrate a history of safe use.
- A declaration of any possible adverse effects linked to the food documented in its country of origin and/or a country where there is a high degree of consumption.
- A description of the standard methods of commercial and/or domestic processing and preparation for consumption.
- A description of how the food is produced.
- Amounts of the food that people are likely to consume in Canada, including typical serving sizes and expected frequency of consumption, at both average and extreme high consumption levels.
- Analysis of the composition of the food based on randomly selected, statistically valid samples. This analysis should include proximate data as well as amino acid profile, fatty acid profile, mineral and trace mineral composition and vitamin composition, as well as any nutrients, antinutrients and bioactive phytochemicals known to be of particular interest in the product. The analysis should pay special attention to the presence of compounds in the food which may have implications for the health of any groups of the Canadian population (e.g. possible toxicants or allergens or unusually high levels of nutrients in the food source or final food product).
- Metabolism and/or gastrointestinal effects in humans.
The submission should include reliable, high quality information and reference sources. Anecdotal evidence will be given less weight than scientifically derived data. Information on the history of human exposure will be particularly important where there are traditional handling or cooking requirements for a food that is novel. This information will need to be made available to consumers in a consistent manner.
4.2.1.2 Dietary exposure
The role of the dietary exposure assessment for substances with no history of use as foods intended for use as food is to estimate:
- how much of the food is likely to be consumed and at what frequency and what role it is likely to play in the diet (e.g. a significant protein source, a condiment, etc.);
- the potential impact of that food on the dietary intake of nutrients by combining the results of the findings in (a) with information on the nutrient composition of the food; and
- if there are any anti-nutrients, toxins, contaminants or novel substances determined to occur in the food, the potential exposure to those substances.
The introduction of foods with no history of safe use may give rise to nutritional, toxicological or allergen issues that may impact upon food safety, and, therefore, estimation of exposure to components of the food of significance to health should be considered. For such foods, dietary exposure assessment has special challenges since the food is not simply an existing food with changes made to it; there is no pre-existing experience of its role in the food supply upon which to base exposure determinations. The approach needed to predict potential consumption patterns would likely involve using intakes of products of similar nutritional composition that play a similar role in the diet and that are currently routinely consumed in the diets of Canadians. The impact of palatability of the food and how it will be promoted will need to be estimated. An exposure simulation could then be conducted based on current dietary intake databases, preferably using data from Canadian subjects, in which the novel food has been incorporated by substituting it for a food or foods it might be expected to replace in the diet. These intake estimates may then be used to calculate the potential dietary exposure to specific components of the novel food that will be the subject of the safety assessment.
The exposure assessment results should describe the estimated changes in the dietary intake distribution of microconstituents using measures such as average, variability and percentiles (upper and lower) of intake. A differential impact in subgroups of the population (e.g. children, infants, elderly, ethnic groups, susceptible populations) should be evaluated as well as the impact on the population as a whole.
Although introduction of a completely novel food could change dietary patterns and therefore have a broader impact on nutrition and on exposure to other components in the diet than can be directly attributed to the food itself, these types of changes may not be possible to predict and the need for and feasibility of such projections or of post-market follow up would be assessed on a case by case basis. Periodic nutrition surveys of the population may be best placed for identifying dietary trends that may have resulted from newly introduced foods, foods having increased availability or popularity, as well as the many other factors that change diets in Canada and that could have either beneficial or adverse health impacts (See Section 3.4).
4.2.1.3 Nutritional considerations
General observations
The introduction of a novel food into the Canadian food supply requires a determination of nutritional quality of the food and the implications of its nutritional characteristics for the population as a whole and/or for specific subgroups. Population subgroups may be more vulnerable for different reasons: e.g. young children, pregnant and lactating women, those with particular metabolic characteristics, adolescents and others who may consume large amounts of food, or the elderly who consume small amounts of food. A nutrition evaluation which includes assessment of intended and unintended nutritional effects is needed in order to ensure that the nutritional status of consumers is not likely to be jeopardized by:
- substitution of foods and food ingredients of significant nutritive value with less nutritious varieties of the same or similar foods
- excessive intakes of nutrients or other bioactive substances as a result of unusually high levels in the novel food, or
- new or increased levels of anti-nutrients that could adversely affect the nutritional value of the food or the diet.
What is nutritional quality?
Nutritional quality as applied to food is related to the presence of essential nutrients and energy-yielding substances (in appropriate quantity and quality) and to other aspects of food traditionally considered as part of the science of nutrition. These aspects include the nutritional roles of non-essential amino acids, specific types of fatty acids and carbohydrates, dietary fibre, cholesterol, lipotropic substances, other components of specific foods (e.g. human milk), nutrient bioavailability and nutrient interactions with other nutrients, with food additives and with natural toxicants. They also include nutrient excesses and the effects (both positive and negative) of food processing on the nutrients and on the organoleptic properties of the food. More recently, "bioactive" substances found principally in plants are being shown to have a possible role to play in improving or protecting human health. These roles are also included in the broad definition of nutritional quality.
Foods with no history of safe use
The main concern with respect to a food with no history of safe use would be to verify that the consumption of the food would not have an adverse effect on the nutritional health of the consumer. Information on nutritional composition and quality is primarily needed to determine how the food could be used in the diet, to establish basic composition information for the food for use in food composition databases, and to permit the validation of nutrient content claims and quantity declarations.
Guidelines for producing data for nutritional evaluation
- Function of the data to be submitted
- The information provided for a food with no history of safe use should be of sufficient quantity and quality to determine its role in the diet and to characterize the average nutritional composition of the food.
- Any studies conducted used to evaluate nutritional quality should have been performed using the food as it is expected to be consumed by humans.
- Where published data on nutrient composition of the novel food are inadequate, analytical data may need to be obtained by the petitioner. In this case, a clear hypothesis should be formulated with an appropriate study design for obtaining data on nutritional quality that:
- Considers all major sources of potential variation in nutritional composition, e.g. composition of the growing medium, fermentation conditions (temperature, pH, stage of growth), etc., in designing the experimental design and sampling methodologies.
- Subjects the novel microorganism or food containing it to the conditions expected for it in commercial production.
- Establishes a sampling plan prior to the commencement of the study based on a power calculation using at least one or a few components to indicate the umber of samples required. This could be based on the most important analytes and/or the analytes which are expected to be more variable and require more samples. Details about the planned power and size of the chosen design for the proposed statistical tests and hypothesis testing should be provided.
- Ensures sampling is conducted at the appropriate stage of production.
- Ensures that the appropriate analyses are performed on all products containing the microorganism that are expected to be used as food in Canada.
- Provides the criteria used for selecting of the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed in the Nutrient Composition section below.
- Ensures samples are analysed within an acceptable time frame from date of collection.
- Ensures that analyses for each nutritive or non-nutritive component are conducted for all samples by a single laboratory using internationally approved and validated analytical methods and following consistent and appropriate sample storage and preparation procedures throughout.
- Uses appropriate and consistent statistical methods chosen in advance based on the study design to analyse and report the results.
-
Nutrient composition
In the context of the above study guidelines, the following components of novel foods should be analysed. Where not all are analysed, the petitioner should provide the criteria used to select the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed below.
- proximate composition i.e. ash, moisture, protein, fat, fibre, carbohydrate
- content of true protein, non-protein nitrogenous material (e.g. nucleic acids and aminoglycosides), amino acid profile, -- unusual amino acids should be determined if their presence is suspected (e.g. d-amino acids from bacterial proteins)
- complete fatty acid profile expressed as % total fatty acids, total nonsaponifiable component, and total sterols. The list of fatty acids should be grouped as monounsaturated, polyunsaturated and saturated
- composition of the carbohydrate fraction e.g. sugars, starches, chitin, tannins, non-starch polysaccharides and lignin
- composition of micronutrients, i.e. significant vitamin and mineral analyses
- presence of naturally occurring or adventitious anti-nutritional factors e.g. phytates, trypsin inhibitors, etc.
- predictable secondary metabolites, physiologically active (bioactive) substances, other detected substances.
-
Nutrient bioavailability/Presence of anti-nutrients
In situations where the novel microorganism or food containing it may become a significant component of the Canadian diet, and/or a significant supplier of nutrients, animal studies should be conducted to assess nutritional adequacy. This pertains in particular to the evaluation of protein quality, the possibility of unknown anti-nutrients, and nutrient bioavailability.
Information should be provided, if applicable, describing the processing conditions that would be used in the production of the novel food, and the effects of the processing on nutrient levels and nutrient bioavailability.
- Information to include in the submission:
- the name of the microorganism including Latin and common names.
- a complete description of the experimental design, experimental conditions, and how sources of variation for nutrient levels were controlled.
- a complete description of sample collection and sample preparation.
- a citation and/ or description of the analytical and statistical methods which were used to obtain data for the nutritive and non-nutritive components.
- nutrient and related data expressed as mean ± standard deviation, and as a range.
- results of statistical analyses.
- raw data for all components analysed.
- published data if available.
- intended use(s) of the microorganism as food in Canada, i.e. as food itself or as an ingredient that might modify a food through culture, possible end products, level of use if different from current products which it would replace, known patterns of use and consumption of the food and its derivatives.
- any foreseeable unintended uses.
- Decision-making process
- All aspects of nutritional quality will be evaluated based on modern nutritional principles, standards and guidelines aimed at meeting human nutritional needs. The bases of evaluation include: nutrient intake recommendations, the role of the food in the diet of the population and the role of diet and nutrition in reducing the risk of developing a diet-related disease and health promotion.
- The first phase of nutritional evaluation will be based on the nutrient composition data and an assessment of both intended and unintended effects on nutrient composition. If there is a finding of unusual or unanticipated components or levels of nutrients or nutritive substances, the food may need to be subjected to further analysis.
- A novel food with no history of safe use is not required to meet specific criteria of nutritional quality. The main concern is to document the composition of the food in order to evaluate claims and to determine its potential role in the diet.
4.2.1.4 Toxicology considerations
Toxicological testing is required for substances of unknown safety that may be introduced to the food supply. For foods that have no history of safe use, it may be difficult to identify individual components which are novel in the context of human consumption in the absence of a traditional counterpart.
Where it is not possible to identify novel components of the food, a case-by-case approach should be used to determine the appropriate toxicological tests to be carried out on the food. The history of the organism from which the food is derived as a source of toxins or antinutrients and a chemical analysis of its components will be considerations in determining requirements for toxicological testing. Depending on these determinations, conventional studies of toxicity, including chronic toxicity, developmental toxicity, genotoxicity or carcinogenicity, may need to be performed on the final food product or its components as appropriate.
It should be noted that the conduct of studies with whole foods presents some challenges due to the potential for inducing nutritional imbalances when the food is incorporated into the diet at high concentrations. In addition, toxicology studies on novel foods are used to reach a conclusion as to whether the food is safe to consume under expected consumption patterns, rather than to derive a quantitative limit such as an acceptable daily intake in the manner used for simple chemicals like food additives.
4.2.1.5 Allergenicity considerations
The primary consideration in allergenicity assessment of a novel food is the prevention of unexpected and/or unavoidable exposure of susceptible individuals to food allergens. For foods with no history of safe use, the potential exists that one or more component proteins would have the capacity to cross-react with known food allergens or lead to the development of de novo hypersensitivity. It should be noted, however, that the vast majority of proteins consumed in the diet are not allergenic.
At present, there is no definitive test that can be relied upon to measure directly the allergenic potential of an individual protein or of a whole food. Because existing strategies for the assessment of the allergenic potential of proteins were developed for the evaluation of individual, well-defined proteins (Section 4.1.3.7), they are not easily applied to the entire protein component of a whole food. The protein component of foods with no history of safe use will not be characterized to the extent necessary to apply these assessment strategies.
A preliminary strategy for assessing the allergenic potential of foods with no history of safe use would be to investigate whether microorganisms from the same taxonomic family that are commonly part of the food supply are implicated in the induction of allergic response. The association of a particular family of microorganisms with allergic response might not necessarily preclude the introduction of the novel food from a related species into the marketplace, but risk management measures such as post-market surveillance and labelling where identification of the food item is not obvious will need to be considered. Proteins from an allergenic source should not be added to foods where identity preservation cannot be guaranteed.
4.2.1.6 Chemical considerations
A complete chemical characterization of potentially-toxic microconstituents of the novel food should be presented. The identification and levels of inadvertently-introduced chemical contaminants must also be reported. Potential contamination by residues of inorganic, organic or natural contaminants could occur, for instance, by chemicals used in or produced by fermentation, extraction or purification procedures used to produce the desired food product from microorganisms.
Any food additives present in the final food (e.g. anticaking agents, carrier solvents, solid diluents, colours, preservatives) or processing aids used during the course of manufacture of the food (e.g. precipitation aids, fermentation co-factors, filtering agents, etc.) should be identified and their levels indicated.
In the case of novel foods intended for use as ingredients in other foods, specifications of identity and purity should be provided, along with a sample label and Directions for Use.
4.2.1.7 Microbiological considerations
For novel microorganisms, petitioners should address the following criteria:
-
Strain identification
The accurate identification of a strain will provide important information for the safety assessment of microorganisms and/or their products. A microbial strain should have an appropriate taxonomic designation following international codes of nomenclature and standard taxonomic references. The taxonomic designation should be provided to a level that distinguishes the microorganism from pathogenic species of the same genus. In the event the identification is not conclusive, additional data may be required to address the safety of a microorganism.
In general, the methods used to identify an organism should be consistent with methods currently used in microbial classification. A taxonomic designation should be accompanied by a list of the tests used to arrive at the designation, with the results and any other information used to make the designation. A brief description of the type of tests used, or references, should be provided.
-
Pathogenicity
The potential of a viable microorganism in a food product to have adverse effects on human health must be considered. Adverse effects would include, but not be limited to, infection, disease, adverse immunologic reactions and toxicosis. While information from a review of the scientific literature is sufficient to satisfy this information requirement to address these points, petitioners should search various sources for information on the human health effects of a microorganism (databases, regulatory authorities, etc.). The search should provide information that would give a complete and thorough overview of any known involvement of a microorganism in an adverse health effect or the lack of any documented adverse health effects caused by a microorganism. In some cases, further testing may be required to address the pathogenic potential of an organism.
-
Antimicrobial production
Information should be provided on the production of antimicrobial compounds by the notified microorganism or its close relatives. These include classical antibiotics and other antimicrobials such as bacteriocins. The significance of these compounds in relation to clinically important antimicrobials will be considered.
-
Antimicrobial resistance
The presence of antimicrobial resistance factors, especially resistance to clinically important antibiotics, must be addressed if the microorganism is present in the final food product. Data on the susceptibility of the microorganism to various antibiotics must be provided if the microorganism can survive or colonize the gastrointestinal tract (e.g. a new starter culture/probiotic strain used in yoghourt).
-
Production/Specifications
Microbial specifications for assuring microbial safety and data demonstrating compliance with these specifications should be provided for a number of production batches. The identification and levels of microbial contaminants must be reported. A food grade fermentation would be expected to yield a pure culture without microbiological contamination prior to down stream processing. Certificates of analysis for appropriate indicator organisms should be provided to demonstrate microbial safety. Documentation on the quality control of the manufacturing process should be provided, including a description of the manufacturing process and control measures that are applied to ensure quality and prevent microbial contamination.
4.2.2 Novel process
Some processes applied to foods or food ingredients may result in the generation of foods which would be considered novel in relation to traditional counterparts. The application of new processes which cause a food to undergo a major change would trigger the requirement to notify Health Canada under the Novel Foods Regulation. A major change is defined in Division 28 of the Regulations as a change in a food that, based on the manufacturer's experience or generally accepted nutritional or food science theory, places the food outside the accepted limits of natural variations for that food with regard to; the composition, structure or nutritional quality of the food or its generally recognized physiological effects; the manner in which the food is metabolized in the body; or the microbiological safety, the chemical safety or the safe use of the food. Examples of novel processes include: new heat processing techniques; new packaging technologies; the use of ultraviolet light for reducing the microbial load of a product.
The safety assessment of novel foods in this category follows a stepwise process of addressing relevant factors that include:
- 4.2.2.1 Details of novel process
- 4.2.2.2 Dietary exposure
- 4.2.2.3 History of organism
- 4.2.2.4 Nutritional considerations
- 4.2.2.5 Toxicology considerations
- 4.2.2.6 Allergenicity considerations
- 4.2.2.7 Chemical considerations
- 4.2.2.1 Details of novel process
4.2.2.1 Details of novel process
While the focus of the safety assessment is on the food product, consideration of the process or preparation of the product can guide the safety assessment. Any novel processing or preparation techniques used to produce a novel food should be described in sufficient detail since such processing or preparation may result in potential microbiological, toxicological, allergenicity, or nutritional concerns.
4.2.2.2 Dietary exposure
The role of the dietary exposure assessment for novel foods resulting from the application of a novel process, is to estimate:
- how much of the food is likely to be consumed and at what frequency and what role it is likely to play in the diet, if different from the role of the unprocessed food or the food processed by conventional means;
- the potential impact of that food on the dietary intake of nutrients by combining the results of the findings in (a) with information on changes, if any, in the nutrient composition of the food due to the novel process;
- if there are any modifications in the level or nature of bioactive substances, anti-nutrients, contaminants or toxins, or if there are novel substances produced in the food, the potential exposure to those substances.
In cases where the nutrient composition of food has been altered, either intentionally or unintentionally, through the novel process, the magnitude of that change should be assessed against the expected nutritional value of the unprocessed food and/or against the changes that result from conventional processes used on the same food, and also to determine if it would have a significant impact on overall dietary nutrient intakes for consumers. A decision to do a full exposure assessment might depend on how big a change has taken place and how much of the food supply might be affected as a result. An exposure assessment could be done by intake modelling using current dietary intake databases, preferably using data from Canadian subjects, in which the novel food with its modified nutrient composition has been inserted in place of the food that is processed for the same purpose by conventional means. For example, if the novel process is meant to replace heat pasteurization, then the heat-pasteurized food in the database would be the food to substitute.
The results should describe the estimated changes in the intake distribution of nutrients using measures such as the average, the variability and percentiles (upper and lower) of intake. A differential impact in subgroups of the population (e.g. children, infants, elderly, ethnic groups, susceptible populations) should be evaluated as well as the impact on the population as a whole.
Where there is an intentional nutritional or health-related modification, the impact of how it will be promoted also needs to be considered, to the extent possible, since this could change the current level of use of the non-novel counterpart.
Where there are changes in the levels of bioactive substances, anti-nutrients, contaminants or toxins or if novel substances are generated as reaction by-products in the food as a result of the process, intake estimates for the food would then be used to calculate the potential dietary exposure to these specific components of the novel food that will be the subject of the safety assessment.
Novel processes applied to foods to reduce spoilage due to microbial activity can also increase the availability of foods previously consumed relatively rarely in Canada, for example, fruits growing in tropical regions. The increased availability of such foods may give rise to nutritional, toxicological or allergenic issues that may impact on food safety and, therefore, estimation of exposure to food components of significance to health should be considered. Estimating the potential for such increases to occur and their impact should be considered if there are reasonable grounds upon which to make quantifiable predictions. Although increases of this type could change dietary patterns and therefore have an impact on nutrition and on exposure to other components in the diet, these types of changes may not be possible to predict. The need and feasibility of projecting future intakes or post-market follow up should be assessed on a case by case basis. Periodic nutrition surveys of the population may be best placed for identifying dietary trends that may have resulted from newly introduced foods, foods having increased availability or popularity, as well as the many other factors that change diets in Canada and that could have either beneficial or adverse health impacts (See Section 3.4).
4.2.2.3 History of organism(s)
The history of an organism can provide information that is important to the assessment of a novel food. There may be a history of toxin production by certain strains, species or genera and it would be important in such cases to examine the particular strain of the organism being used for the potential to produce such toxins, both under the conditions used in normal manufacturing and also under extreme conditions.
4.2.2.4 Nutritional considerations
I Unintended nutritional effects
General observations
The introduction of a novel food into the Canadian food supply requires a determination of nutritional quality of the food and the potential implications of its nutritional quality characteristics for the population as a whole and/or for specific subgroups. Population subgroups may be more vulnerable for different reasons: e.g. young children, pregnant and lactating women, those with particular metabolic characteristics, adolescents and others who may consume large amounts, or the elderly who consume small amounts. A nutrition evaluation which includes assessment of intended and unintended nutritional effects is needed in order to ensure that the nutritional status of consumers is not likely to be jeopardized by:
- substitution of foods and food ingredients of significant nutritive value with less nutritious varieties of the same or similar foods
- excessive nutrient intakes as a result of unusually high levels of a given nutrient, or
- new or increased levels of anti-nutrients that could adversely affect the nutritional value of the food or the diet.
What is nutritional quality?
Nutritional quality as applied to food is related to the presence of essential nutrients and energy-yielding substances (in appropriate quantity and quality) and to other aspects of food traditionally considered as part of the science of nutrition. These aspects include the nutritional effects of non-essential amino acids, specific types of fatty acids and carbohydrates, dietary fibre, cholesterol, lipotropic substances, other components of specific foods (e.g. human milk), nutrient bioavailability and nutrient interactions with other nutrients, with food additives and with natural toxicants. They also include nutrient excesses and the effects (both positive and negative) of food processing on the nutrients and on the organoleptic properties of the food. More recently, "bioactive" substances found principally in plants are being shown to have a possible role to play in improving or protecting human health. These intrinsic bioactive substances are also included in the broad definition of nutritional quality.
Application of novel process to microorganisms
Microorganisms constitute a minor component of foods in the Canadian diet. The use of single cell protein is rare. Therefore, it is very unlikely that a change in the microorganisms that are currently in foods would have a direct impact on the nutritional quality of foods and diets. There are two ways, however, that a microorganism in a food could have an impact on the nutritional quality of the food or diet and in turn on the health of the consumer. One way is that microorganisms can have a significant indirect impact on the nutritional quality of foods that they are in. For example, the use of yeast to leaven bread reduces the phytate content which makes the minerals more available for intestinal absorption. The yeast also produces B vitamins in sufficient quantities to significantly affect the content of some of the B vitamins, for example folate, in bread. The other way that a microorganism in a food can have an impact on health is potentially as a "probiotic". Probiotics are thought to be able to populate or alter the population of bacteria in the large intestine and as a result have various beneficial effects on the health of the intestine and the individual.
The development of novel forms of microorganisms through application of a novel process could result in intended or unintended changes in the composition of the food product. This could in turn have an impact on the nutritional value of the food and the nutritional status of the persons consuming it.
Unintended nutritional effects can occur whether the novel process applied to the microorganism is intended for nutritional or functional or other reasons. Evaluation of a microorganism, which was produced using a novel process, intended to affect the nutritional quality of the microorganism or the food of which it is part is discussed in Part II of this section. Thus, discussion of probiotic aspects of microorganisms is limited to that part.
An important step in the safety and nutritional assessment of this type of novel food is a comparison of its composition with its appropriate counterpart. In the case of a novel microorganism (i.e. the microorganism which was produced using a novel process), this could apply to the microorganism itself in the event that it constitutes a significant portion of the food mass but it is more likely to apply to the food containing the novel microorganism. To determine whether there are any differences in the nutritional quality of the food containing the novel microorganism compared to its appropriate counterpart, the microorganism should first be subject to laboratory testing of the metabolic products of the microorganism in controlled media. Once into the food production trial phase, the major constituents of the food containing the microorganism must be analysed, i.e. macronutrients and their component parts, as well as individual micronutrients selected based on validated criteria. If any nutrients (in the list below) are excluded from the analyses, this should be justified by an acceptable rationale.
Also, circumstances may warrant an evaluation of the nutritional "performance" of the new food in its ready-to-eat form, thus either raw or when processed by traditional/conventional methods used to make the product ready-to-eat. The purpose would be to provide an opportunity to identify major changes that may not have been detected by compositional analysis, but which could affect, for example, the stability or bioavailability of nutrients in the food or the susceptibility of anti-nutrients to further processing that normally destroys them. A performance test could involve re-analysis of a substance following cooking or it could require animal testing for satisfactory growth and nutrient bioavailability.
Guidelines for producing data for nutritional evaluation
- Function of the data to be submitted
- The information provided for a novel food comprising or derived from a microorganism should be of sufficient quantity and quality to allow an assessment of whether any significant unintended effect on the nutritional quality of the food has occurred as a result of the introduction of the application of the novel process on the food. It should also allow an assessment of the nutritional significance of any change that is detected.
- Data should be provided for the novel food comprising or derived from a microorganism, before further processing. Data may also be required for the food prepared for human consumption by conventional means to examine the effects, where applicable, of further processing, storage and cooking, for example, to look at the effectiveness of cooking to destroy anti-nutrients in cases where anti-nutrients normally destroyed by cooking are present.
- Data on the novel food comprising or derived from a microorganism should be compared, at a minimum, to data on the most appropriate counterpart (see section b, below). Literature data (if available) may also be valid for assessing the nutritional relevance of any unintended effect.
- Where published data on nutrient composition of the novel food are inadequate, analytical data may need to be obtained by the petitioner. In this case, a clear hypothesis should be formulated with an appropriate study design for obtaining data on nutritional quality that:
- Considers all major sources of potential variation in nutritional quality (e.g. composition of the growing medium, production conditions, processing conditions, etc.) in designing the study, to ensure these factors are controlled.
- Subjects the novel food comprising or derived form a microorganism to the conditions expected for it in commercial production.
- Includes in the same study the novel process that is the subject of the notification as well as the appropriate counterpart, i.e. the food comprising or derived from a microorganism, where the microorganism component was prepared using an equivalent commercial process (i.e. a process which is not novel, and which is currently used to achieve the same or similar effect)
- Establishes a sampling plan prior to the commencement of the study based on a power calculation using at least one or a few components to indicate the number of samples required. This could be based on the most important analytes and/or the analytes which are expected to be more variable and require more samples Details about the planned power and size of the chosen design for the proposed statistical tests and hypothesis testing should be provided.
- Ensures that the appropriate analyses are performed on all products containing the microorganism that are expected to be used as food in Canada.
- Provides the criteria used for selection of the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed in the following section entitled "Nutrient Composition".
- Ensures samples are analysed within an acceptable time frame from date of collection.
- Ensures that analyses for each nutritive or non-nutritive component are conducted for all samples by a single laboratory using internationally approved and validated analytical methods and following consistent and appropriate sample storage and preparation procedures throughout.
- Uses appropriate and consistent statistical methods chosen in advance based on the study design to compare levels of each nutrient in the novel food versus its controls.
-
Nutrient composition
In the context of the above study guidelines, the following components of foods should be analysed. Where not all are analysed, the petitioner should provide the criteria used to select the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed below.
- proximate composition i.e. ash, moisture, protein, fat, fibre, carbohydrate
- content of true protein, non-protein nitrogenous material (e.g. nucleic acids and aminoglycosides), amino acid profile, -- unusual amino acids should be determined if their presence is suspected (e.g. d-amino acids from bacterial proteins)
- complete fatty acid profile expressed as % total fatty acids, total nonsaponifiable component, and total sterols. The list of fatty acids should be grouped as monounsaturated, polyunsaturated and saturated.
- composition of the carbohydrate fraction e.g. sugars, starches, chitin, tannins, non-starch polysaccharides and lignin
- composition of micronutrients, i.e. significant vitamin and mineral analyses
- presence of naturally occurring or adventitious anti-nutritional factors e.g. phytates, trypsin inhibitors, etc.
- predictable secondary metabolites, physiologically active (bioactive) substances, other detected substances
-
Nutritional "performance" of novel microorganism
Consideration should be given to the possible need for the following types of information regarding the novel microorganisms or the foods containing them:
- Response of known anti-nutrients to processes normally expected to neutralize their activity measured using compositional analysis.
- Storage stability with regard to nutrient degradation.
- Performance of product in relation to the intended benefit (other than direct health benefits) e.g. improved stability of an oil to heating after fatty acid profile modification.
-
Nutrient bioavailability/Presence of new or altered anti-nutrients
In situations where the novel food may become a significant component of the Canadian diet, and/or a significant supplier of nutrients, animal studies may be needed in assessing nutritional adequacy to determine if there have been changes in the bioavailability of nutrients or if the composition is not comparable to conventional foods.
Information should be provided, if applicable, describing the conditions used in the further processing of the novel food and its derivatives, and the potential effects of the processing on nutrient levels and nutrient bioavailability.
- Information to include in the submission:
- a full description of the novel process, the purpose of the process, and the microorganism(s) on which it could be applied, and the microorganism(s) on which it will be applied (for the purpose of the submission).
- the microorganism(s) on which the test and control processes were applied in the study, and the names and sources of all the strains which were represented in the study.
- a complete description of the experimental design, experimental conditions, and how sources of variation for nutrient levels were controlled.
- a complete description of sample collection and sample preparation.
- a citation and/ or description of the analytical and statistical methods which were used to obtain data for the nutritive and non-nutritive components.
- nutrient and related data for test, control, and commercial strains (expressed as mean ± standard deviation, and as a range).
- results of statistical analyses.
- raw data for all components analysed.
- published data if available.
- intended use(s) of the novel food comprising or derived from a microorganism as food in Canada, i.e. as food itself or as an ingredient that might modify a food through culture, possible end products, level of use if different from current products which it would replace, known patterns of use and consumption of the food and its derivatives.
- any foreseeable unintended uses.
- Decision-making process
- Appropriate modern statistical methods should be applied to the data to permit a demonstration that no significant change has resulted from the intervention. According to CodexFootnote 11 "the statistical significance of any observed differences should be assessed in the context of the range of natural variations for that parameter to determine its biological significance". If the composition of the novel food is judged not to be nutritionally equivalent to that of its counterparts, i.e. if significant differences (statistical and biological) exist in the nutrient data, additional nutritional data may be required on a case-by-case basis.
- All aspects of nutritional quality will be evaluated based on modern nutritional principles, standards and guidelines aimed at meeting human nutritional needs. The bases of evaluation include: nutrient intake recommendations, the role of the food in the diet of the population and the role of diet and nutrition in reducing the risk of developing a diet-related disease and health promotion.
- Detection of a major change due to an unintended nutritional effect may not preclude the marketing of the product. However, such changes may require limits on the use of the food in food products or a requirement for labelling that goes beyond basic provisions.
- The first phase of nutritional evaluation will be based on the nutrient composition data and an assessment of both intended and unintended effects on nutrient composition. If there is a finding of unusual or unanticipated components or levels of nutrients or nutritive substances, the food may need to be subjected to further analysis and assessment.
- The safety of a major increase in the level of a nutrient or other bioactive component would need to be assessed in a similar way to the safety assessment of an intended nutritional change. For details on this see Part II, below.
II Intended nutritional modifications
The term "intended nutritional modification" is taken to include any change or introduced characteristic intended to improve the nutritional quality or health-related profile of the food, including but not limited to both essential nutrients and beneficial phytochemicals, quantities and nature of the energy-yielding substances, improved nutrient bioavailability, improved probiotic function and reduction in anti-nutrient levels.
Evaluation of an intended nutritional change requires steps that are similar to those used in either the addition of a vitamin or mineral nutrient to a food or the evaluation of foods with health claims or both. For instance, such a change would trigger questions concerning the intended target group, what level of the targeted nutrient or other bioactive substance is expected in the food, what is the expected change in level of exposure to the targeted nutrient or other bioactive substance across all age and sex groups and at the upper and lower extremes of intake of the food, and the safety of this level of exposure.
A novel food with an introduced health or nutritional benefit would likely fall into the unofficial category of "functional food". It is expected that manufacturers will be interested in making health claims for these products. These products would therefore be evaluated in accordance with the criteria being laid out for foods with product-specific health claims. These include attention to the evidence in support of the claim, as well as to product safety and product quality considerations.
Product safety of this type of novel food is intended to be controlled through application of the novel food regulations. The safety evaluation of a microorganism or of a food containing a microorganism, where the microorganism was subjected to a novel process, which resulted in the food having an intended nutritional modification (i.e. novel food), should cover the same aspects as for other novel foods. With regard to the safety and nutritional evaluation of the intended nutritional modification, itself, data requirements are described below.
At this time, regulations for product-specific health claims have not yet been promulgated. Prospective petitioners should refer to the proposed regulatory framework for product-specific health claims which was published in November, 2001, and the Interim Guidance Document on Standards of Evidence which was published in February, 2002.
It is important to ascertain to what extent the intended nutritional effect of a novel process remains stable with cultivation, time, further processing, storage and cooking.
The review of unintended nutritional effects in a novel microorganism or a food containing a novel microorganism, i.e. where a novel process was applied on the microorganism for the purpose of having an intended nutritional effect would follow the same steps as for other novel foods.
Nutritional evaluation of expected or unexpected increased levels of a nutrient or bioactive substance
- Increased levels of a nutrient or other bioactive substance (including a microorganism) in a food need to be evaluated for safety.
- Data needed for this include:
- the level of the targeted nutrient or other bioactive substance expected in the food;
- intended target group, if applicable, or which group(s) is or are likely to consume the most of the food;
- expected level of exposure to the substance through consumption of the food by the target group, by vulnerable sub-groups, and at the upper and lower extremes of intake of the food and across all age and sex groups using recent Canadian food consumption data where possible;
- how the expected level of dietary exposure to the targeted nutrient or other substance differs from the current levels of exposure from all sources;
- data in support of the safety of the expected level of exposure.
4.2.2.5 Toxicology considerations
Toxicological testing is required for substances of unknown safety that are introduced to the food supply. The application of novel processes to foods may result in the generation of novel substances in the resulting food be intentional or unintentional. Because of the potential wide variety of products generated by the application novel processes as determination of the appropriate toxicological testing should be conducted on a case-by-case basis.
Identification of any novel substances generated in the food subjected to a novel process is assisted by the use of a comparator such as unprocessed food or conventionally processed food. Chemical analysis may provide information on any new substances that have been formed. In addition, information on the nature, duration and intensity of treatment and the chemical composition of the food may be useful in predicting the types of alterations to the food components. Depending on these determinations, conventional studies of toxicity, including assays of metabolism, toxicokinetics, chronic toxicity/carcinogenicity, impact on reproductive function, and teratogenicity, may need to be performed on the final food product or its components as appropriate.
Intentional alteration of the composition of foods by the addition of food components at levels that fall outside the accepted limits for natural variations (e.g. "functional" foods) may result in exposures for which there is no history of safe use. Substances that have been traditionally consumed in foods but which have been added to foods at levels outside their normal range will result in consumption of higher amounts of the substance than from a traditional diet. In such cases, the novel aspect of the food is the extent of exposure to the substance, rather than the substance itself, and toxicological testing of the enhanced component will be required to establish an upper limit of tolerability to the substance. The types of studies conducted should be guided by a knowledge of the role of the component in human physiology. Evidence from animal and in vitro studies as indicated in the previous paragraph would be required to determine safety. Studies in experimental animals may be of limited usefulness if the commonly used animal model (i.e. the rat) differs markedly from humans in the metabolic pathways and chronic conditions that are the basis of the intended functional effect, and it may be necessary to place greater reliance on human response to increased intakes of such food components. Epidemiologic studies may be available for substances that are normally components of foods, and these can provide important information on long-term effects.
4.2.2.6 Allergenicity considerations
The primary consideration in allergenicity assessment of a novel food is the prevention of unexpected and unavoidable exposure of sensitized individuals to food allergens. In cases where the application of a novel process to a food results in the generation of a novel protein or an alteration of the protein content of a food containing allergenic proteins, a consideration of the allergenic potential of the novel food would be required.
Novel proteins
At present, there is no definitive test that can be relied upon to measure directly the allergenic potential of an individual protein or of a whole food. If the application of a novel process to a food results in the generation of a novel protein that can be isolated and characterized, the assessment strategy that has been developed for foods which are the products of recombinant DNA technology and described in section 4.1.3.7 can be used to assess its potential allergenicity. This strategy involves a weight of evidence approach that relies on the assessment of amino acid sequence homology to known food allergens, and a consideration of the similarity of its properties, in particular, resistance to digestion in the mammalian gastrointestinal tract, to those of known food allergens.
Alteration of endogenous allergen content
If the application of a novel process to a food that contains allergenic proteins results in altered protein content of that food, the potential for increase in the allergenic content should be assessed. While the health impacts of such increases is uncertain, this result would be considered undesirable. Techniques used for assessing the potential for effects on endogenous allergen expression are: the quantitative comparison of protein composition of the edible portion of the modified organism or, where sera from sufficient numbers of individuals with allergies to the food are available, the comparative immunoreactivity to the edible portion of the modified organism can be determined using immunoblotting techniques.
4.2.2.7 Chemical considerations
A complete chemical characterization of potentially-toxic microconstituents of the novel food should be presented. The identification and levels of inadvertently-introduced chemical contaminants must also be reported. Potential contamination by residues of inorganic, organic or natural contaminants could occur, for instance, by chemicals used in or produced by fermentation, extraction or purification procedures used to produce the desired food product from microorganisms. In this regard, it would be necessary to provide a comparison of the levels of chemical contaminants in the novel food with those levels typically found in the food product prepared by accepted traditional processes.
Any food additives present in the final food (e.g. anticaking agents, carrier solvents, solid diluents, colours, preservatives) or processing aids used during the course of manufacture of the food (e.g. precipitation aids, fermentation co-factors, filtering agents, etc.) should be identified and their levels indicated.
In the case of novel foods intended for use as ingredients in other foods, specifications of identity and purity should be provided, along with a sample label and Directions for Use.
4.2.3 Genetic modification
Microorganisms referred to in this section are those developed by recombinant nucleic acid technology and other methods of DNA introduction, such as protoplast fusion in eukaryotic cells, ballistic microinjection, and electroporation. Microorganisms developed by deletion, rearrangement or suppression of native DNA should also be considered. In addition, those microorganisms that have undergone genetic modification by traditional selection techniques (spontaneous mutation, selective pressures) and intentionally induced mutagenesis (i.e. through the application of techniques such as chemical treatment and ultra-violet irradiation) resulting in alteration of the phenotype or composition, may also be included.
The data to be submitted are to include, but are not necessarily limited to, those outlined here. Of special concern may be modified microorganisms where a parent or vector originates from a species known to produce toxic compounds. Wherever possible, transformation markers which generate safety concerns should not be present in the final food product. The acceptability of such markers however, will be evaluated on a case-by-case basis.
The safety assessment of novel foods in this category follows a stepwise process of addressing relevant factors that include:
- 4.2.3.1 Characterization of derived strain
- 4.2.3.2 Genetic modification considerations
- 4.2.3.3 History of organism (Host and Donor(s))
- 4.2.3.4 Dietary exposure
- 4.2.3.5 Nutritional considerations
- 4.2.3.6 Toxicology considerations
- 4.2.3.7 Allergenicity considerations
- 4.2.3.8 Chemical considerations
4.2.3.1 Characterization of derived strain
Where a microorganism has been modified, whether by selection and mutagenesis techniques or by recombinant nucleic acid technology, the relationship of the derived strain with the parent organism(s) should be characterised. The approach of the safety assessment is based on the principle that the safety of novel products is assessed relative to a conventional counterpart having a history of safe use, taking into account both intended and unintended effects. Any significant differences between the novel and the conventional strain are then assessed for potential adverse health effects. Of particular interest to the safety assessment is whether the modification could inadvertently develop or increase the pathogenicity, toxicity, or allergenicity potential of an organism.
4.2.3.2 Genetic modification considerations
Genetic modification by traditional techniques
Many non-recombinant nucleic acid modification procedures are relatively undefined and poorly characterized in terms of insertion, deletion or rearrangement of genetic material. Strain selection and mutagenesis techniques can influence the toxin-producing capacity of an organism and may also influence the expression of antimicrobial compounds or other substances not present in food.
For microorganisms derived through classical mutagenesis and selection techniques, information should be provided to fully characterize the novel strain that enables a comparison with the parent organism(s). This characterization will include details of the methods used to modify the organism and a phenotypic and genotypic comparison of the parents and donors, as appropriate. New or altered characteristics and characteristics acquired and expressed should be described. A comparison of the biological activity, growth and physiological characteristics of the novel microorganism to the parent apart from the intended modification should be performed. In all cases, the degree of exposure to the modified microorganism or its products will be an important factor in determining the extent of the data required for the safety assessment (dietary exposure considerations).
Traditionally modified microorganisms require a multi-disciplinary assessment since details of the modifications may be largely unknown. As experience in the safety assessment of novel foods develops, it may be possible to more clearly identify data requirements for particular groups of products or to preclude certain products from further detailed evaluation.
Genetic modification by modern techniques
In cases where a microorganism has been modified using modern genetic techniques, such as recombinant nucleic acid technology, the safety assessment will consider detailed characterization data of a novel food at the molecular level. The following requirements are taken from safety assessment guidelines endorsed by the Codex Alimentarius Commission in 2003Footnote 12. In addition to the requirements of previous sections, the following areas should be addressed for these types of products:
I) Description of the genetic modification(s)
Details of all methods and manipulations involved in the modification of an organism must be provided to allow for the identification of all genetic material potentially inserted, deleted, mutated, or rearranged in the host genome. This will provide the necessary information for the analysis of the data supporting the characterization of the modified organism.
The description of the modification process should include:
- information on the method(s) of modification used, e.g. conjugation, electroporation, etc.;
- description and characterization of all genetic material potentially delivered, if applicable, including the source, identity, expected function in the organism, and copy number for plasmids; and
- details of manipulations or modifications to introduced, intermediate and recipient genetic material.
Information should be provided on DNA added, inserted, deleted, or modified, including:
- the characterization of all the genetic components including marker genes, vector genes, regulatory and other elements affecting the function of the DNA;
- the size and identity;
- the location and orientation of the sequence in the final vector/construct; and
- function in the organism.
A summary diagram, outlining the key features of the final construct, should be provided. Depending on the nature of the genetic modification, restriction maps and sequence data of the introduced or modified genetic material and adjacent regions, may be required.
ii) Characterization of the genetic modification(s)
In order to provide clear understanding of the impact on the composition and safety of foods derived from genetically modified microorganisms, a comprehensive molecular and biochemical characterization of the organism should be carried out.
Information should be provided on the DNA insertions into the genome; this should include:
- the characterization and description of all inserted, deleted, or otherwise modified genetic materials;
- the number of insertion sites;
- data to demonstrate if complete or partial copies have inserted into the genome;
- data to demonstrate whether the arrangement of the genetic material used for insertion has been conserved or whether significant rearrangements have occurred upon integration;
- the organization of the inserted genetic material at each insertion site including copy number and sequence data of the inserted material and, where appropriate, of surrounding region;
- identification of any open reading frames within the inserted DNA or created by the insertions with contiguous DNA in the chromosome or in a plasmid, including those that could result in fusion proteins; and
- in the case of modifications that involve deletions, rearrangements or site-specific, in vitro mutagenesis, sequence data of the region before and after modification should be provided.
Information should be provided on any expressed substances in the modified organism; this should include:
- the gene product (e.g. a protein or an untranslated RNA);
- the gene product's function;
- the phenotypic description of the new characteristic(s);
- the level and site of expression of the gene product(s), and the levels of its metabolites;
- to demonstrate whether deliberate modifications made to the amino acid sequence of the expressed protein result in changes in its post-translational modification or affect sites critical for its structure or function;
- where genetic manipulations are directed to altered regulation of endogenous genes, the characteristics and level of gene expression should be compared with that of the unmodified host;
- to indicate whether there is any evidence to suggest that one or several endogenous genes in the host microorganism has been affected by the modification process;
- to confirm the identity and expression pattern of any new fusion proteins;
- to demonstrate the intended effect of the modification has been achieved and that all expressed characteristics are expressed and inherited in a manner that is stable through several generations consistent with laws of inheritance. It may be necessary to examine the inheritance of the DNA itself or the expression of the corresponding RNA if the phenotypic characteristics cannot be measured directly; and
- to demonstrate that the newly expressed characteristic(s) are expressed as expected in the appropriate cellular location or is secreted in a manner and at levels that are consistent with the associated regulatory sequences driving the expression of the corresponding gene.
iii) Special considerations if a viable recombinant-DNA microorganism is present in the food
There is a recognition that horizontal gene transfer can be an issue if the modified microorganism is viable and present in the food. Therefore, special considerations should be given for the potential of the microorganism to colonize the gut and to transfer genetic material to other gut flora. Due to these concerns, the following requirements should be addressed:
Assessment of viability and residence of microorganisms in the human gastrointestinal tract:
- In some foods produced using recombinant-DNA microorganisms, ingestion of these microorganisms and their residence may have an impact on the human intestinal tract. The need for further testing of such microorganisms should be based on the presence of their conventional counterpart in foods, and the nature of the intended and unintended effects of genetic modifications. If processing of the final food product eliminates viable microorganisms (by heat treatment in baking bread, for example), or if accumulations of end products toxic to the microorganism (such as alcohol or acids) eliminate viability, then viability and residence of microorganisms in the alimentary system need no examination.
- For applications in which recombinant-DNA microorganisms used in production remain viable in the final food product, (for example, organisms in some dairy products), it may be desirable to demonstrate the viability (or residence time) of the microorganism alone and within the respective food matrix in the digestive tract and the impact on the intestinal microflora in appropriate systems. The nature of intended and unintended effects of genetic modification and the degree of differences from the conventional counterpart will determine the extent of such testing.
Antibiotic resistance and gene transfer:
- In general, traditional strains of microorganisms developed for food processing uses have not been assessed for antibiotic resistance. Many microorganisms used in food production possess intrinsic resistance to specific antibiotics. Such properties need not exclude such strains from consideration as recipients in constructing recombinant-DNA microorganisms. However, strains in which antibiotic resistance is encoded by transmissible genetic elements should not be used where such strains or these genetic elements are present in the final food. Any indication of the presence of plasmids, transposons, and integrons containing such resistance genes should be specifically addressed.
- Alternative technologies, demonstrated to be safe, that do not rely on antibiotic resistance marker genes in viable microorganisms present in foods should be used for selection purposes in recombinant-DNA microorganisms. In general, use of antibiotic resistance markers for constructing intermediate strains should pose no significant hazards that would exclude the use of the ultimate strains in food production, provided that the antibiotic resistance marker genes have been removed from the final construct.
- Transfer of plasmids and genes between the resident intestinal microflora and ingested recombinant-DNA microorganisms may occur. The possibility and consequences of gene transfer from recombinant- DNA microorganisms and food products produced by recombinant-DNA microorganisms to gut microorganisms or human cells should also be considered. Transferred DNA would be unlikely to be maintained in the absence of selective pressure. Nevertheless, the possibility of such events cannot be completely discounted.
- In order to minimize the possibility of gene transfer, the following steps should be considered:
- chromosomal integration of the inserted genetic material may be preferable to localization on a plasmid;
- where the recombinant-DNA microorganism will remain viable in the gastrointestinal tract, genes should be avoided in the genetic construct that could provide a selective advantage to recipient organisms to which the genetic material is unintentionally transferred; and
- sequences that mediate integration into other genomes should be avoided in constructing the introduced genetic material.
4.2.3.3 History of organism(s)
The history of both donor and host organisms can provide information that is important to the assessment of a novel food. There may be a history of toxin production by certain strains, species or genera and it would be important in such cases to examine the particular strain of the organism being used for the potential to produce such toxins, both under the conditions used in normal manufacturing and also under extreme conditions.
The following detailed information should be provided:
- taxonomic designation of the microorganism to the species level and where applicable, to include subspecies and strains, accompanied by technical data substantiating this designation;
- other names (synonyms, common usage, strain numbers, culture collection accession number) associated with the microorganism;
- origin (environmental/clinical/food isolate, culture collection) of the microorganism;
- strain development and enhancement history of the microorganism;
- pathogenicity of genus and species;
- evidence pertaining to the potential for production of any toxic compounds and antibiotics; and
- history of extended safe use, particularly in foods, of the subject microorganism and closely related strains.
4.2.3.4 Dietary exposure
In conducting dietary exposure assessments for novel foods produced through genetic modification, the primary issues to be addressed as part of the safety assessment are: the potential for alteration of nutrient content of the food, and the potential for introduction of novel substances to the food supply.
In cases where the nutrient composition of foods has been altered, either intentionally or through genetic modification, changes to nutrient intake should be determined for the food itself and in the context of the food as a source of the nutrient in the total diet. Variation of dietary patterns in subgroups the population (e.g. children, infants, elderly, ethnic groups) as well as the potential for change in use and/or exposure to the food compared with the related, traditional food product should be taken into consideration.
For foods produced from genetically-modified microorganisms, that result in the introduction of a novel protein or novel metabolites to the food supply, their content should be determined and considered together with the toxicological data as part of the safety assessment. The effects of typical food processing procedures on the novel component(s) should be considered in deriving the exposure estimate.
4.2.3.5 Nutritional considerations
I Unintended nutritional effects
General Observations
The introduction of a novel food into the Canadian food supply requires a determination of nutritional quality of the food and the implications of its nutritional characteristics for the population as a whole and/or for specific subgroups. Population subgroups may be more vulnerable for different reasons: e.g. young children, pregnant and lactating women, those with particular metabolic characteristics, adolescents and others who may consume large amounts of food, or the elderly who consume small amounts of food. A nutrition evaluation which includes assessment of intended and unintended nutritional effects is needed in order to ensure that the nutritional status of consumers is not likely to be jeopardized by:
- substitution of foods and food ingredients of significant nutritive value with less nutritious varieties of the same or similar foods;
- excessive intakes of nutrients or other bioactive substances as a result of unusually high levels in the novel food; or
- new or increased levels of anti-nutrients that could adversely affect the nutritional value of the food or the diet.
What is nutritional quality?
Nutritional quality as applied to food is related to the presence of essential nutrients and energy-yielding substances (in appropriate quantity and quality) and to other aspects of food traditionally considered as part of the science of nutrition. These aspects include the nutritional effects of non-essential amino acids, specific types of fatty acids and carbohydrates, dietary fibre, cholesterol, lipotropic substances, other components of specific foods (e.g. human milk), nutrient bioavailability and nutrient interactions with other nutrients, with food additives and with natural toxicants. They also include nutrient excesses and the effects (both positive and negative) of food processing on the nutrients and on the organoleptic properties of the food. More recently, "bioactive" substances found principally in plants are being shown to have a possible role to play in improving or protecting human health. These substances are also included in the broad definition of nutritional quality.
Genetically modified microorganisms
Microorganisms constitute a quantitatively minor component of foods in the Canadian diet. The use of single cell protein as a food ingredient is rare. For most foods containing microorganisms, a change in the microorganisms would be unlikely to have a significant direct impact on the nutritional quality of foods and diets. There are two other ways, however, that a microorganism in a food could have an impact on the nutritional quality of the food or diet and in turn on the health of the consumer.
One way is that microorganisms can have a significant indirect impact on the nutritional quality of foods that they are in. For example, the use of yeast to leaven bread reduces the phytate content which makes the minerals more available for intestinal absorption. The yeast also produces B vitamins in sufficient quantities to significantly affect the content of some of the B vitamins, for example folate, in bread. The other way that a microorganism in a food can have an impact on health is potentially as a "probiotic". Probiotics are thought to be able to populate or alter the population of bacteria in the large intestine and as a result have various beneficial effects on the health of the intestine and the individual.
Therefore, the development of novel forms of microorganisms that are used in food through genetic modification, whether by traditional selection methods, mutagenesis or recombinant DNA techniques, could result in intended or unintended changes in the composition of the food product which could in turn have an impact on the nutritional value of the food and the nutritional status of the persons consuming it. As more complex or layered genetic modifications are attempted through rDNA techniques, for instance to introduce both improved nutritional characteristics and functional characteristics into the same organism, these could increase the potential for unintended effects compared to simpler modifications. By the same token, other methods of genetic modification could also introduce multiple changes.
Unintended nutritional effects can occur whether the intended modification of the microorganism is nutritional or functional or something else. Evaluation of a modification of a microorganism intended to affect the nutritional quality of the microorganism or the food of which it is part is discussed in Part II of this section. Thus, discussion of probiotic aspects of microorganisms is limited to that part.
An important step in the safety and nutritional assessment of the modified food is a comparison of its composition with its appropriate counterpart. In the case of a modified microorganism, this could apply to the microorganism itself in the event that it constitutes a significant portion of the food mass but it is more likely to apply to the food containing the modified microorganism. To determine whether there are any differences in the nutritional quality of the food containing the modified microorganism compared to its appropriate counterpart, the microorganism should first be subject to laboratory testing of the metabolic products of the microorganism in controlled media. Once into the food production trial phase, the major constituents of the food containing the microorganism must be analysed, i.e. macronutrients and their component parts, as well as individual micronutrients selected based on validated criteria. If any nutrients (in the list below) are excluded from the analyses, this should be justified by an acceptable rationale.
Also, circumstances may warrant an evaluation of the nutritional "performance" of the new food in its ready-to-eat form, thus either raw or when processed by traditional/conventional methods used to make the product ready-to-eat. The purpose would be to provide an opportunity to identify major changes that may not have been detected by compositional analysis, but which could affect, for example, the stability or bioavailability of nutrients in the food or the susceptibility of anti-nutrients to processing that normally destroys them. A performance test could involve re-analysis of a substance following cooking or it could require animal testing for satisfactory growth and nutrient bioavailability.
Guidelines for producing data for nutritional evaluation
- Function of the data to be submitted
- The information provided for a novel microorganism or for a food containing one should be of sufficient quantity and quality to allow an assessment of whether any significant unintended genetic modification affecting the nutritional quality of the food has occurred as a result of the introduction of the novel characteristic. It should also allow an assessment of the nutritional significance of any change that is detected.
- Data should be provided for the novel microorganism or for the food containing one, before further processing. Data may also be required for the food prepared for human consumption by conventional means to examine the effects, where applicable, of processing, storage and cooking, for example, to look at the effectiveness of cooking to destroy anti-nutrients in cases where anti-nutrients normally destroyed by cooking are present.
- Data on the novel food should be compared, at a minimum, to data on the near isogenic, non-transgenic parent strain, i.e. the most appropriate counterpart, if available, or else a closely related non-transgenic strain. Since one or more significant differences could arise, the study design should include strains of the same species from a range of standard strains that are used in commercial production for the same purposes and, possibly, at a variety of production plants in Canada. This would permit assessment with respect to normal variation. Literature data (if available) may also be valid for assessing the nutritional relevance of any unintended effect.
- A clear hypothesis should be formulated with an appropriate study design for obtaining data on nutrient composition that:
- Considers all major sources of potential variation in nutritional quality (e.g. composition of the growing medium, incubation conditions, etc.) in designing the study, to ensure these factors are controlled.
- Subjects the modified microorganism or food containing it to the conditions expected for it in commercial production.
- Includes in the same study the novel microorganism that is the subject of the notification as well as the appropriate counterpart, i.e. the near isogenic, non-transgenic parent strain, if available, and a selection of the commercial strains available in the current market. In the absence of a near isogenic parent strain, the most closely related non-transgenic strain may be chosen.
- Establishes a sampling plan prior to the commencement of the study based on a power calculation using at least one or a few components to indicate the number of samples required. This could be based on the most important analytes and/or the analytes which are expected to be more variable and require more samples. Details about the planned power and size of the chosen design for the proposed statistical tests and hypothesis testing should be provided.
- Ensures sampling is conducted at the appropriate stage of incubation.
- Ensures that the appropriate analyses are performed on all products containing the microorganism that are expected to be used as food in Canada.
- Provides the criteria used for selecting of the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed in c. Nutrient Composition below.
- Ensures that analyses for each nutritive or non-nutritive component are conducted for all samples by a single laboratory using internationally approved and validated analytical methods and following consistent and appropriate sample storage and preparation procedures throughout.
- Ensures samples are analysed within an acceptable time frame from date of collection.
- Uses appropriate and consistent statistical methods chosen in advance based on the study design to compare levels of each nutrient in the novel food versus its controls.
- Nutrient composition
In the context of the above study guidelines, the following components of foods should be analysed. Where not all are analysed, the petitioner should provide the criteria used to select the nutrients analysed and the rationale for the exclusion from analysis of any nutrients and other substances listed below.
- proximate composition i.e. ash, moisture, protein, fat, fibre, carbohydrate
- content of true protein, non-protein nitrogenous material (e.g. nucleic acids and aminoglycosides), amino acid profile, -- unusual amino acids should be determined if their presence is suspected (e.g. d-amino acids from bacterial proteins)
- complete fatty acid profile expressed as % total fatty acids, total nonsaponifiable component and total sterols. The list of fatty acids should be grouped as monounsaturated, polyunsaturated and saturated.
- composition of the carbohydrate fraction e.g. sugars, starches, chitin, tannins, non-starch polysaccharides and lignin
- composition of micronutrients, i.e. significant vitamin and mineral analyses
- presence of naturally occurring or adventitious anti-nutritional factors e.g. phytates, trypsin inhibitors, etc.
- predictable secondary metabolites, physiologically active (or bioactive) substances, other detected substances
-
Nutritional "performance" of a modified microorganism
Consideration should be given to the possible need for the following types of information regarding the modified microorganism or the foods containing them:
Response of known anti-nutrients to processes normally expected to neutralize their activity measured using compositional analysis.
Storage stability with regard to nutrient degradation.
Performance of product in relation to the intended benefit (other than direct health benefits) e.g. improved stability of an oil to heating after fatty acid profile modification.
-
Nutrient bioavailability/Presence of new or altered anti-nutrients
In situations where the food from a genetically modified source may become a significant component of the Canadian diet, and/or a significant supplier of nutrients, animal studies may be needed in assessing nutritional adequacy to determine if there have been changes in the bioavailability of nutrients or if the composition is not comparable to conventional foods.
Information should be provided, if applicable, describing the processing conditions used in the production of a food, and the potential effects of the processing on nutrient levels and nutrient bioavailability.
- Information to include in the submission:
- the names of all the strains which were represented in the study.
- a complete description of the experimental design, experimental conditions, and how sources of variation for nutrient levels were controlled.
- a complete description of sample collection and sample preparation.
- a citation and/ or description of the analytical and statistical methods which were used to obtain data for the nutritive and non-nutritive components.
- nutrient and related data for test, control, and commercial strains (expressed as mean ± standard deviation, and as a range).
- results of statistical analyses.
- raw data for all components analysed from all test sites.
- published data if available.
- intended use(s) of the microorganism as food in Canada, i.e. as food itself or as an ingredient that might modify a food through culture, possible end products, level of use if different from current products which it would replace, known patterns of use and consumption of the food and its derivatives.
- any foreseeable unintended uses.
- Decision-making process
- Appropriate modern statistical methods should be applied to the data to permit a demonstration that no significant change has resulted from the intervention. According to CodexFootnote 13 "the statistical significance of any observed differences should be assessed in the context of the range of natural variations for that parameter to determine its biological significance". If the composition of the novel food is judged not to be nutritionally equivalent to that of its counterparts, i.e. if significant differences (statistical and biological) exist in the nutrient data, additional nutritional data may be required on a case-by-case basis.
- All aspects of nutritional quality will be evaluated based on modern nutritional principles, standards and guidelines aimed at meeting human nutritional needs. The bases of evaluation include: nutrient intake recommendations, the role of the food in the diet of the population and the role of diet and nutrition in reducing the risk of developing a diet-related disease and health promotion.
- Detection of a major change due to an unintended nutritional effect may not preclude the marketing of the product. However, such changes may require limits on the use of the food in food products or a requirement for labelling that goes beyond basic provisions. See also Part II with respect to safety assessment of high levels of nutrients or bioactive substances.
- The first phase of nutritional evaluation will be based on the nutrient composition data and an assessment of both intended and unintended effects on nutrient composition. If there is a finding of unusual or unanticipated components or levels of nutrients or bioactive substances, the food may need to be subjected to further analysis and assessment.
- The safety of a major increase in the level of a nutrient or other bioactive component would need to be assessed in a similar way to the safety assessment of an intended nutritional change. For details on this see Part II, below.
II Intended nutritional modifications
The term "intended nutritional modification" is taken to include any change or introduced characteristic intended to improve the nutritional quality or health-related profile of the food, including but not limited to essential nutrients, beneficial bioactive phytochemicals, quantities and nature of the energy-yielding substances, improved nutrient bioavailability, and reduction in anti-nutrient levels.
Evaluation of an intended nutritional change requires steps that are similar to those used in either the addition of a vitamin or mineral nutrient to a food or the evaluation of foods with health claims or both. For instance, such a change would trigger questions concerning the intended target group, what level of the targeted nutrient or other bioactive substance is expected in the food, what is the expected change in level of exposure to the targeted nutrient or other bioactive substance across all age and sex groups and at the upper and lower extremes of intake of the food, and the safety of this level of exposure.
A novel food with an introduced health or nutritional benefit would likely fall into the unofficial category of "functional food". It is expected that manufacturers will be interested in making health claims for these products. These products would therefore be evaluated in accordance with the criteria being laid out for foods with product-specific health claims. These include attention to the evidence in support of the claim, as well as to product safety and product quality considerations.
Product safety of this type of novel food is intended to be controlled through application of the novel food regulations. The safety evaluation of a novel food genetically modified to have an intended nutritional modification should be the same as for other genetically modified foods. With regard to the safety and nutritional evaluation of the intended nutritional modification itself, data requirements are described below.
At this time, regulations for product-specific health claims have not yet been promulgated. Prospective petitioners should refer to the proposed regulatory framework for product-specific health claims which was published in November, 2001, and the Interim Guidance Document on Standards of Evidence which was published in February, 2002.
Adding a substance through genetic modification differs from adding one through applying it to or mixing it with the food after it is harvested. The decision to proceed with or cease the addition would take place at different stages of production. This could have an effect on the ability to manage the presence of the "added" substance or characteristic in the food supply if there were later considered to be a need to control it. Given this potential need, such products should be subject to post-market surveillance to ensure the ability to monitor and control the products. To promote a product that has been altered with the intention of benefiting the consumer, manufacturers themselves would have a requirement for post-market surveillance, in any case, and therefore this should not add a significant additional burden.
It is important to ascertain to what extent the nutrient or other targeted substance whose levels have been changed (if the intent was to deliberately modify the level of a nutrient) is bioavailable and remains stable with cultivation, time, processing, storage and cooking.
The review of unintended nutritional effects in a food modified to have an intended nutritional effect would follow the same steps as for other novel foods.
Nutritional Evaluation of expected or unexpected increased levels of a nutrient or bioactive substance
- Increased levels of a nutrient or other bioactive substance (including a microorganism) in a food need to be evaluated for safety.
- Data needed for this include:
- the level of the targeted nutrient or other bioactive substance expected in the food;
- expected level of exposure to the targeted nutrient or other bioactive substance through consumption of the food at the upper and lower extremes of intake of the food and across all age and sex groups using recent Canadian food consumption data where possible;
- intended target group, if applicable, or which group(s) is or are likely to consume the most of the food;
- how the expected level of dietary exposure to the targeted nutrient or other bioactive substance differs from the current levels in the diet; and
- data in support of the safety of the expected level of exposure
4.2.3.6 Toxicology considerations
Toxicological testing is required for substances of unknown safety that are introduced to the food supply. Novel substances may be introduced to the food supply through recombinant DNA technology, or may be generated by the application of novel processes to foods or [other DNA modification processes]. Introduction of novel substances may be intentional or unintentional.
Genetic modification techniques can result in the production of novel substances by the organism or the intentional or unintentional modification of substances already produced by the organism or their expression.
Novel Substances
In vitro nucleic acid techniques enable the introduction of DNA which can result in the synthesis of new substances in microorganisms. These include the protein expression product and other substances which may be generated as a result of enzymic activity of the protein expression product. The new substances can be conventional components of genetically modified microorganisms.
The introduced characteristic should be shown to be unrelated to any characteristics of donor organisms that could be harmful to human health. Information should be provided to ensure that genes coding for known toxins present in the donor organisms are not transferred to recombinant DNA organisms.
Toxicology studies are not considered necessary where the substance or a closely related substance has been consumed safely in food at equivalent intakes or where the new substance is not present in the food. Otherwise, the use of conventional toxicology studies on the new substance will be necessary. This will require the isolation of the new substance from the recombinant DNA microorganism.
For proteins, the assessment of potential toxicity should focus on amino acid sequence similarity between the protein and known protein toxins and anti-nutrients (e.g. protease inhibitors, lectins) as well as stability to heat or processing and to degradation in appropriate/representative gastric and intestinal model systems. Since proteins that are enzymes have never been shown to be direct-acting carcinogens, mutagens, teratogens or reproductive toxicants (Pariza and Foster 1983) it is generally not necessary to test proteins for these toxicological endpoints when exposure occurs by the oral route. Protein toxins act through acute mechanisms after the administration of a single dose at doses in the nanogram to milligram per kilogram body weight (bw). Therefore, acute oral toxicity studies using gram/kg bw doses of the novel protein are appropriate for assessing the potential toxicity of proteins. A negative result using doses in the gram/kg bw range together with evidence that the protein is digested to small peptides and amino acids would provide assurance that the protein is not a toxin and is digested to nutrients as are the vast majority of dietary proteins.
Different types of in vivo or in vitro studies would be needed to assess the toxicity of introduced substances other than proteins. The types of studies are determined on a case-by-case basis and depend on the original source of the introduced substances and their function. Such studies may include assays of metabolism, toxicokinetics, chronic toxicity/carcinogenicity, impact on reproductive function, and teratogenicity.
Unintended effects
Techniques used in the genetic modification of microorganisms have the potential to induce unintended effects on the genome of the modified organism that could be manifested as an alteration in the levels of toxicants normally produced by the organism. The intended genetic alteration may also influence the behaviour of the organism with respect to accumulation of contaminants, metabolites, or other substances that were not anticipated.
Compositional analysis is the method currently used for detection of unintended changes to the genome that result in accumulation of toxic substances either of endogenous or exogenous origin. Because of the influence of environmental stress on production of endogenous components such as toxins, data should be collected from a number of different test sites. New, more sensitive technologies that allow the determination of alterations to expression of the organisms' genome are presently under development.
4.2.3.7 Allergenicity considerations
The primary considerations in allergenicity assessment of a novel food are the prevention of unexpected and unavoidable exposure of sensitized individuals to food allergens. This includes the assessment of the potential for foods containing novel proteins to cross-react with known food allergens or to lead to the development of de novo hypersensitivity. In addition, the possibility of increasing the allergenic potential of foods already containing allergens as a result of genetic modification should be assessed.
Section 1 - Introduction
All newly expressed proteins in recombinant-DNA microorganisms that could be present in the final food and are novel in the context of that food, need to be assessed for their potential to cause allergic reactions. This should include consideration of whether a newly expressed protein is one to which certain individuals may already be sensitive as well as whether a protein new to the food supply is likely to induce allergic reactions in some individuals.
At present, there is no definitive test that can be relied upon to measure directly the allergenic potential of a newly expressed protein in humans. Based upon the best, currently-available scientific information, the recommended approach takes into account the preponderance of evidence derived from several types of information and data in an integrated, stepwise, case-by-case manner.
Section 2 - Assessment strategyFootnote 14
The initial steps in assessing possible allergenicity of any newly expressed proteins involve determination of: the allergenicity of the source of the introduced protein; any similarity between the amino acid sequence of the protein and that of known allergens; and certain physicochemical properties, including but not limited to, its susceptibility to enzymatic degradation.
Genes derived from known allergenic sources should be assumed to encode an allergen unless scientific evidence demonstrates otherwise.
Determination of amino acid sequence homology and physicochemical characteristics will require the isolation of the newly expressed protein from the recombinant-DNA organism, or the production of the substance from an alternative source, in which case the material should be shown to be functionally and biochemically equivalent to that produced in the recombinant-DNA organism.
Food proteins that are not allergens and that are altered by mutagenesis techniques need only be assessed for the likelihood that the mutagenized protein is a de novo allergen.
The absolute exposure to the newly expressed protein and the effects of relevant food processing will contribute toward an overall conclusion about the potential for human health risk. In this regard, the nature of the food product intended for consumption should be taken into consideration in determining the types of processing that would be applied and its effects on the presence of the protein in the final food product.
Section 3 - Initial assessment
Section 3.1 - Source of the protein
As part of the data supporting the safety of foods derived from recombinant-DNA organisms, information should describe any reports of allergenicity associated with the donor organism. Allergenic sources of genes would be defined as those organisms for which reasonable evidence of IgE-mediated oral, respiratory or contact allergy is available. Specific tools and relevant data that permit confirmation of allergenic potential are available for proteins from some allergenic sources. These include: the availability of sera for screening purposes; documented type, severity and frequency of allergic reactions; and structural characteristics and amino acid sequence (when available) of known allergenic proteins from that source.
Section 3.2 - Amino acid sequence homology
Amino acid sequence homology comparisons should be used to assess the extent to which a newly expressed protein is similar in structure to known allergens in order to determine whether that protein has allergenic or cross-reactivity potential. Overall structural similarities can be predicted using sequence homology searches that compare the structure of newly expressed proteins with all known allergens should be conducted using various algorithms such as FASTA or BLASTP. Strategies such as stepwise contiguous identical amino acid segment searches may also be performed for the purpose of identifying sequences that may represent linear epitopes. The size of the contiguous amino acid search should be based on a scientifically justified rationale in order to minimize the potential for false negative or false positive resultsFootnote 15. Validated search and evaluation procedures should be used in order to produce biologically meaningful results.
IgE cross-reactivity between the newly expressed protein and a known allergen should be considered a possibility when there is more than 35% identity in a segment of 80 or more amino acids (FAO/WHO 2001).
Sequence homology searches have certain limitations. In particular, comparisons are limited to the sequences of known allergens in publicly available databases and the scientific literature. There are also limitations in the ability of such comparisons to detect non-contiguous IgE-binding epitopes.
A negative sequence homology result indicates that a newly expressed protein is not a known allergen and is unlikely to be cross-reactive to known allergens. A result indicating absence of significant sequence homology should be considered along with the other data outlined under this strategy in assessing the allergenic potential of newly expressed proteins. This does not preclude further studies where considered necessary (see also section 6). A positive sequence homology result indicates that the newly expressed protein has a high probability of being allergenic. If the product is to be considered further, it should be assessed using serum from individuals sensitized to the identified allergenic source (see section on Specific Serum Screening).
Section 3.3 - Pepsin resistance
Resistance to pepsin digestion has been observed in several food allergens; thus, a correlation exists between resistance to digestion by pepsin, and allergenic potentialFootnote 16. The resistance of a protein to degradation in the presence of pepsin under appropriate conditions indicates that further analysis should be conducted to determine the likelihood of the newly expressed protein being allergenic. The establishment of a consistent and well-validated pepsin degradation protocol may enhance the utility of this method.
Although the pepsin resistance protocol is strongly recommended, it is recognized that other enzyme susceptibility protocols exist. Alternative protocols may be used where adequate justification is provided.
Section 4 - Specific serum screening
For those proteins that originate from a source known to be allergenic, or have sequence homology with a known allergen, testing in immunological assays is required. Sera from individuals with a clinically validated allergy to the source of the protein can be used to test IgE-binding of the protein in in vitro assays. A critical issue for testing will be the availability of human sera from sufficient numbers of individualsFootnote 17. In addition, the quality of the sera and the assay procedure need to be standardized to produce a valid test result.
In the case of a newly expressed protein derived from a known allergenic source, a negative result in in vitro immunoassays may not be considered sufficient, but should prompt additional testing, such as the possible use of skin test and ex vivo protocols.
The identification of a newly expressed protein as an allergen through immunological assays suggests that further development for commercialization of the product be discouraged, unless adequate risk management and risk communication measures could be assured throughout marketing and distribution of the product, since segregation and identity preservation of the new source of this allergen may be difficult or impossible to enforce.
Section 5 - Areas requiring further development
The endpoint of the assessment of the data discussed above is a conclusion as to the likelihood of the protein being a food allergen. The techniques of targeted serum screening (i.e. the assessment of binding to IgE in sera of individuals with clinically-validated allergic responses to broadly-related categories of foods) and the use of animal models, once developed and validated, could enhance the weight of evidence used to derive this conclusion. To allow serum screening, steps should be taken to organize an international serum bank. As scientific knowledge and technology evolves, other methods, such as examination of newly expressed proteins for T-cell epitopes and structural motifs associated with allergens, might also be useful.
Unintended effects on endogenous allergens
Genetic modification techniques have the potential to produce unintended effects on the genome that could lead to an increase in the expression of endogenous allergens. While the potential for health impacts of such increases is uncertain, they are in any case considered undesirable. Techniques used for assessing the potential for effects on endogenous allergen expression are the quantitative comparison of protein composition of the edible portion of the modified organism or, where sera from sufficient numbers of individuals with allergies to the food are available, the comparative immunoreactivity to the edible portion of the modified organism can be determined using immunoblotting techniques.
4.2.3.8 Chemical considerations
A complete chemical characterization of potentially-toxic microconstituents of the novel food should be presented. The identification and levels of inadvertently-introduced chemical contaminants must also be reported. Potential contamination by residues of inorganic, organic or natural contaminants could occur, for instance, by chemicals used in or produced by fermentation, extraction or purification procedures used to produce the desired food product from microorganisms. In this regard, it would be necessary to provide a comparison of the levels of chemical contaminants in the novel food with those levels typically found in the non-modified food product.
Any food additives present in the final food (e.g. anticaking agents, carrier solvents, solid diluents, colours, preservatives) or processing aids used during the course of manufacture of the food (e.g. precipitation aids, fermentation co-factors, filtering agents, etc.) should be identified and their levels indicated.
In the case of novel foods intended for use as ingredients in other foods, specifications of identity and purity should be provided, along with a sample label and Directions for Use.
Appendix 1: Health Canada Guidance on the Novelty Interpretation of Products of Plant Breeding
1. Introduction
Canada's Novel Food Regulations (Division 28, Part B, of the Food and Drug Regulations, or FDRs) were published in 1999. These regulations require a manufacturer to file a pre-market notification and for the regulator to determine that the information in that notification establishes that the food is safe before it can be sold. The scope of these requirements is delineated by the definition "novel food" in these regulations. When a food is "novel" the regulations require pre-market notification and regulatory approval before sale.
To aid in the interpretation of the Novel Food Regulations, Health Canada published the Guidelines for the Safety Assessment of Novel Foods. These guidelines describe how regulators conduct novel food pre-market safety assessments and the information that must be contained in a notification. This guidance document was last updated in 2006.
Since that time, technological advancements have created new tools of genetic modification by which new plant varieties can be developed (e.g., gene editing technologies). Consequently, developersFootnote 18 expressed the need for the Novel Food Regulations to be clearer, more predictable, and more transparent regarding products of plant breeding, including those developed using these new tools of modification.
This new guidance takes account of the health and safety objectives of the regulations, making use of the regulator's experience as this field of science has evolved since the regulations were published. This new guidance interprets the Novel Food Regulations in a manner that takes account of the current scientific knowledge and the underlying scheme and the purpose of these rules (i.e., to require pre-market assessment of novel foods that may pose a potential risk to the consumer) in a way that reflects a greater understanding of plant breeding technologies, and the benefits of aligning with our international regulatory partners.
2. Background
2.1 Development of the Novel Food Regulations
In 1999, new regulations were made in part to address uncertainty in the safety of plants developed using biotechnology (i.e., recombinant DNA [rDNA] technologies). However, unlike most jurisdictions which developed legislation based on a specific process of genetic modification, Canada's Novel Food Regulations were written in a manner that could be interpreted to generally define many foods derived from genetically modified plants - including certain conventionally-bred products - as "novel", requiring pre-market notification and safety assessment prior to sale. To date, these rules have been interpreted in a way that has not taken account of whether the breeding methods used to develop the plant are well characterized as safe in determining whether these pre-market notification and assessment requirements apply to a food.
2.2 Plant breeding practices that support food safety
Plant developers rigorously analyze and understand their new plant varieties in relation to food safety during the breeding process prior to commercialization, and throughout the commercial lifespan of the plant variety including one developed using biotechnology. In-depth product characterization limits the risk that plants can have unintended food safety effects related to an intentionally developed characteristic. These characterizations also provide assurance that any consequences of new characteristics are well understood in relation to food safety. These principles of characterization have always been a part of plant breeding practices regardless of the methods used to develop a new plant variety. Through time, technological advancements have given developers new analytical tools to more precisely characterize their products. This guidance does not change the responsibility of developers to analyze or characterize their product, nor their responsibility to ensure that their products are safe for food use.
The following considerations represent Health Canada's understanding of well established, universally applied plant developer practices and competencies relating to the full characterization of a new product of plant breeding, regardless of what methods were used in its development and selection.
- Plant developers are experts in their plant variety and the plant species in relation to its use in food, and related food safety. This means that:
- Developers understand the typical range of important analytes that make up the nutrients, anti-nutrients, allergens, and toxins of the plant variety they are developing or have developed;
- Developers understand the intended characteristic(s) in their new plant variety and how it can influence the exposure of Canadians to nutrients, anti-nutrients, allergens, and toxins of the plant-derived food; and
- Developers are aware of expected end uses of their products and how these end uses influence the exposure of Canadians to nutrients, anti-nutrients, allergens, and toxins of the plant-derived food.
- It is well established that plant developers are current in their knowledge of how the intended characteristic(s) may affect the expression of other characteristics, and how this may affect food safety. For example, how changes in a metabolic pathway could influence nutrient bioavailability, or lead to the accumulation of other metabolites that may have allergenic and/or toxic and/or anti-nutrient properties.
- It is well established that plant developers consider whether there are possible risks to food safety linked to the new characteristics that they have or plan to introduce in the plant. In this sense, plant developers are considering risk hypotheses involving altering the levels of nutrients, anti-nutrients, allergens, and toxins.
- It is well established that throughout the plant development process, any plants with characteristics that can negatively affect food safety are noted and discarded.
- It is well established that following commercialization, plant developers carefully observe their plant varieties for characteristics that can negatively affect food safety in relation to the plant variety they are working with. Any plants observed to possess characteristics that can negatively affect food safety are noted and discarded.
A combination of developer knowledge, scientific rationale, and data generated during plant variety development, as well as during post-commercialization monitoring (which is consistently of high quality and of sufficient rigor) adequately supports that these products are safe and as such, do not fit within the meaning of "novel food" as defined in section B.28.001(c) of the FDRs. Health Canada recognizes that the components of a plant subject to analysis are highly specific and targeted. As such, the determination of what analyses to perform is based on multiple considerations specific to the plant.
3. Foods derived from products of plant breeding that are not novel foods
Taking account of these conclusions, it is Health Canada's position that the following five categories of foods would not add to our body of knowledge about their safety, if assessed individually as novel foods in accordance with sections B.28.002-B.28.003 of the FDRs because their safety is already consistently well characterized:
- Foods derived from plants with genetic modifications that do not alter an endogenous protein in a way that introduces or increases similarity with a known allergen or toxin relevant to human health;
- Foods derived from plants with genetic modifications that do not increase levels of a known endogenous allergen, a known endogenous toxin, or a known endogenous anti-nutrient beyond the documented ranges observed for these analytes in the plant species;
- Foods derived from plants with genetic modifications that do not have an impact on key nutritional composition and/or metabolism;
- Foods derived from plants with genetic modifications that do not intentionally change the food use of the plant; and
- Foods derived from plants with genetic modifications that do not result in the presence of foreign DNAFootnote 19 in the final plant productFootnote 20.
The Department interprets the Novel Food Regulations to classify foods derived from plants that meet all five (5) of these categories as ones whose characteristics do not meet the threshold of novelty required for them to meet the definition "novel food" set out in section B.28.001(c) of the FDRs. The definition, interpreted narrowly in this way takes account of the precautionary safety objectives that underlie these pre-market safety assessment regulations.
4. Impacts on food safety and nutrition
While most characteristics introduced or altered in plants are known to not affect food safety, there are certain characteristics that would pose a potential safety concern and would be considered "novel food" and require a pre-market assessment.
Characteristics that are considered by Health Canada to pose potential safety or nutritional concerns and that merit a food derived from a plant being classified as "novel" are outlined below:
4.1 Impacts on allergens, toxins, and anti-nutrients
The expression of an altered endogenous protein that now demonstrates significantFootnote 21 similarity with a known allergen or toxin relevant to human health; increased expression of an endogenous allergen or toxin; or an increased expression of an endogenous anti-nutrient is considered to be a trigger for a food being "novel", requiring a pre-market notification and safety assessment prior to sale.
4.2 Impacts on key nutritional composition and metabolism
For the purpose of this guidance, Health Canada will focus on nutrients indicated by the Dietary Reference Intake values as nutrients associated with adequacy and/or those with established adverse health effects (referred to as key nutrientsFootnote 22 within this guidance). Changes in one of these key nutrients may occur due to the direct manipulation of that nutrient or its metabolism, or indirectly by manipulation of other nutrients or metabolic pathways. When a characteristic is known to influence a metabolic pathway related to nutritional composition, the key nutrients relevant to that plant should be measured, and compared with their documented rangesFootnote 23.
When a key nutrient relevant to that plant is at a level outside the documented range, the potential impact on the dietary intakes of Canadians for this key nutrient should be considered. If consumption of this plant as a food is likely to result in an overall higher or lower dietary intake of that key nutrient by Canadians, the product requires pre-market notification and assessment as a novel food.
Furthermore, developers should consider the bioavailability of nutrients or how the food is metabolized in the body. If a characteristic of a plant results in a key nutrient relevant to that plant becoming more or less available upon consumption of the plant-derived food, and is likely to result in an overall higher or lower dietary intake of that key nutrient by Canadians, the product requires pre-market notification and assessment as a novel food.
4.3 Intentional changes in the food use of the plant
In some cases, new plant varieties may exhibit altered or new characteristic(s) that change the intended usage of the plant-derived foods. The developer may introduce or alter a characteristic such that consumers will now eat a part of the plant not previously consumed as food, or a plant with no previous use as food is modified to now be consumed as food. Similarly, one or more components of the edible tissues may be altered such that consumers are intended to consume greater or lesser amounts of the plant-derived foods such that it may impact the health and safety of the consumerFootnote 24. Plant varieties that have altered or new characteristics that result in any of the above-mentioned changes in food use continue to be considered "novel food" that require pre-market notification and assessment prior to sale.
4.4 Food products derived from plants that contain foreign DNA in the final plant product
Foods derived from plants that have been genetically modified such that they contain foreign DNA in the final plant product require pre-market notification and assessment as novel foods.
Of specific importance for gene-edited plants, the DNA encoding gene editing machinery (e.g., CRISPR Cas protein(s) and associated guide RNAs) is considered to be foreign DNA if present in the final product. Most plant developers will remove this DNA through subsequent plant breeding; however, in the event that a gene-edited plant still contains the DNA encoding this machinery within its genome, foods derived from such a gene-edited plant require pre-market notification and assessment as novel foods.
5. List of conventional methods of plant breeding
Techniques for overcoming reproductive barriers
Techniques for chromosome and genome manipulation
Other plant-breeding techniques
* Detailed descriptions and examples of the listed techniques can be found in van de Wiel et al., 2010. Traditional plant breeding methods. Wageningen UR Plant Breeding, Wageningen. Report 338.
** This table represents a list of methods which were, at the minimum, researched as potential tools for plant breeding. The majority of these methods were adopted by plant developers and are used in current breeding programs.
Appendix 2: Health Canada Guidance on the Pre-Market Assessment of Foods Derived from Retransformants
1. Introduction
Since the Novel Food Regulations were enacted in 1999, Health Canada has evaluated the safety of foods derived from over 140 genetically modifiedFootnote 25 (GM) plant varieties. In all cases, foods derived from these GM plant varieties were found to be safe for food use. As a result, there are some characteristics that Health Canada has assessed multiple times and have continually been determined not to pose a food safety concern. This extensive experience has allowed regulators to develop a substantial degree of familiarity with these characteristics.
In addition, Canadian regulators have reviewed the scientific literature regarding the types of genetic changes that can occur as a result of inserting DNA into a plant's genome (collectively referred to as insertional effects) (Schnell et al., 2015). This review concluded that the unintended genetic changes that may result from the insertion of DNA into a plant's genome are no different from those that can occur through conventional plant breeding or as a result of plant-environment interactions. These unintended effects are accounted for in the practices used by plant developersFootnote 26. Developers produce thousands of plants containing the same inserted DNA and, through analysis, select the ideal plant (i.e., no unintended effects observed).
Furthermore, developers often then breed multiple generations of the ideal plant to ensure that, with the exception of the inserted DNA, the plant's genome is as similar to its unmodified counterpart as possible. Consequently, the potential for unintended effects of interest are those related to what is encoded and expressed from the inserted DNA itself. In cases where a new plant variety has been developed through the insertion of a DNA sequence identical to another previously assessed GM plant, the potential unintended effects that could occur are either controlled through breeding practices or have been considered as part of the assessment of the previous GM plant.
On this basis, Health Canada has developed a tiered approach to the information requirements for the pre-market assessment of novel foods derived from GM plants that have been transformed with the identical DNA sequence as a previously assessed GM plant, referred to as a retransformant. While the specific genetic changes in the genome of the retransformant do not need to be identical to the genetic changes in the previously assessed GM plant, they must result in the same mechanism(s) of actionFootnote 27 and characteristic(s) as that plant in order to be considered a retransformant. The information requirements are tiered in a way that takes into account prior knowledge regarding the level of risk associated with these plant-derived food products while maintaining the safety of Canadians. Furthermore, the pre-market safety assessment of retransformants will be conducted under an expedited service standard. The following sections describe the information requirements and the service standard for the pre-market safety assessment of retransformants.
Health Canada will continue to conduct pre-market safety assessments with a regular service standard (i.e., 410 calendar days) for those products that are novel and unlike any previously assessed novel food.
2. Pre-market safety assessment with an expedited service standard of novel foods derived from retransformants
Those products that undergo the pre-market assessment process with an expedited service standard will be published on the List of Completed Assessments for Novel Foods and the subject food products that were notified to Health Canada will only be permitted for sale when a written confirmation is provided by Health Canada.
2.1 Information requirements for novel foods derived from retransformants
The information requirements for the pre-market safety assessment of a retransformant are as follows:
- A summary description of the product demonstrating it meets the definition of a retransformant. This would include a description of the product's characteristic(s) and the mechanism(s) of action by which it is achieved.
- Data supporting a complete molecular characterization of the retransformant as described in Section 4.1.3.2 of Health Canada's Guidelines for the Safety Assessment of Novel Foods (2006). These data can include but are not limited to:
- A map of the intended insert compared with the intended insert of the previously assessed GM plant
- Sequencing data demonstrating the full characterization of the insert
- A rationale or data addressing why the mechanism of action(s) between the retransformant and the previously assessed GM plant are identical
- A rationale or data addressing why the characteristic(s) between the retransformant and the previously assessed GM plant are identical
The purpose of this data is to demonstrate that the retransformant possesses a genetic change which results in the same characteristic(s) achieved through the same mechanism(s) of action as a previously assessed GM plant.
- A scientific rationale which supports that the nutritional composition of the retransformant is equivalent to the previously assessed GM plant.
- A scientific rationale which supports that the data pertaining to the toxicological and allergenic safety of the previously assessed GM plant is applicable to the retransformant.
- If the characteristic(s) of the retransformant involves an intentional change in the uptake of chemical compounds by the plant, a chemical analysis of the product is required.
- If applicable, address any potential differences in the estimated dietary exposure between the retransformant and the previously assessed GM plant and their relevance to human health.
2.2 Service standard for the pre-market assessment of novel foods derived from retransformants
The pre-market safety assessment of novel foods derived from retransformants will be completed within 120 calendar days of receipt of the complete submission package by Health Canada.
During the pre-market safety assessment with an expedited service standard, if additional clarification and/or information is required by Health Canada, a request will be made to the petitioner of the submission. An additional 45 calendar days will be added to the service standard to accommodate time for the petitioner to respond to the request. Should the information provided by the petitioner be insufficient to complete the assessment or the time to respond exceed the 45 days, the submission will be closed without prejudice to resubmit.
2.3 Outcome
Like all novel foods that undergo a pre-market safety assessment, the positive outcome for the pre-market safety assessment of a retransformant with an expedited service standard will include the issuance of a Letter of No Objection to the petitioner. Furthermore, technical and summary documents that describe the assessed plant will be published as part of Health Canada's list of Completed safety assessments of novel foods including genetically modified (GM) foods.
If Health Canada determines that a product does not meet the definition of a retransformant, this will be communicated to the petitioner as early in the process as possible and the submission will be closed. A new submission for the pre-market assessment of the product with a regular service standard (i.e., 410 calendar days) can be submitted without prejudice.
3. Reference
SCHNELL, J., STEELE, M., BEAN, J., NEUSPIEL, M., GIRARD, C., DORMANN, N., PEARSON, C., SAVOIE, A., BOURBONNIÈRE, L., and MACDONALD, P., 2015. A comparative analysis of insertional effects in genetically engineered plants: considerations for pre-market assessments. Transgenic research, 24(1), pp. 1-17.
Footnotes
- Footnote 1
-
Prior to entering this process, manufacturers or importers may request a pre-notification consultation to help them prepare a complete package.
- Footnote 2
-
CFIA = Canadian Food Inspection Agency. Environmental Assessment Unit is part of the Healthy Environments and Consumer Health Branch of Health Canada.
- Footnote 3
-
"Codex Alimentarius Commission", Joint FAO/WHO Food Standard Programme; Codex Ad Hoc Intergovernmental Task Force on Foods Derived from Biotechnology", 3rd Session: Yokohama, Japan 4-8 March 2002: Consideration of Proposed Draft Guideline for the Conduct of Food Safety Assessment of Recombinant-DNA Microorganisms in Food At Step 4"; page 13.
- Footnote 4
-
Codex Alimentarius. Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants.
- Footnote 5
- Footnote 6
-
"Codex Alimentarius Commission", Joint FAO/WHO Food Standard Programme; Codex Ad Hoc Intergovernmental Task Force on Foods Derived from Biotechnology", 3rd Session: Yokohama, Japan 4-8 March 2002: Consideration of Proposed Draft Guideline for the Conduct of Food Safety Assessment of Recombinant-DNA Microorganisms in Food At Step 4"; page 13.
- Footnote 7
-
This assessment strategy is not applicable for assessing whether newly expressed proteins are capable of inducing gluten-sensitive or other enteropathies. In addition, the strategy is not applicable to the evaluation of foods where gene products are down regulated for hypoallergenic purposes.
- Footnote 8
-
It is recognized that the 2001 FAO/WHO consultation suggested moving from 8 to 6 identical amino acid segment searches. The smaller the peptide sequence used in the stepwise comparison, the greater the likelihood of identifying false positives; inversely, the larger the peptide sequence used, the greater the likelihood of false negatives, thereby reducing the utility of the comparison.
- Footnote 9
-
The method outlined in the U.S. Pharmacopoeia (1995) was used in the establishment of the correlation (Astwood et al. 1996).
- Footnote 10
-
According to the Joint Report of the FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology (22-25 January 2001, Rome, Italy) a minimum of 8 relevant sera is required to achieve a 99% certainty that the new protein is not an allergen in the case of a major allergen. Similarly, a minimum of 24 relevant sera is required to achieve the same level of certainty in the case of a minor allergen. It is recognized that these quantities of sera may not be available for testing purposes.
- Footnote 11
-
"Codex Alimentarius Commission", Joint FAO/WHO Food Standard Programme; Codex Ad Hoc Intergovernmental Task Force on Foods Derived from Biotechnology", 3rd Session: Yokohama, Japan 4-8 March 2002: Consideration of Proposed Draft Guideline for the Conduct of Food Safety Assessment of Recombinant-DNA Microorganisms in Food At Step 4"; page 13.
- Footnote 12
-
Codex Alimentarius. Guideline for the Conduct of Food Safety Assessment of Foods Produced using Recombinant-DNA Microorganisms.
- Footnote 13
-
"Codex Alimentarius Commission", Joint FAO/WHO Food Standard Programme; Codex Ad Hoc Intergovernmental Task Force on Foods Derived from Biotechnology", 3rd Session: Yokohama, Japan 4-8 March 2002: Consideration of Proposed Draft Guideline for the Conduct of Food Safety Assessment of Recombinant-DNA Microorganisms in Food At Step 4"; page 13.
- Footnote 14
-
This assessment strategy is not applicable for assessing whether newly expressed proteins are capable of inducing gluten-sensitive or other enteropathies. In addition, the strategy is not applicable to the evaluation of foods where gene products are down regulated for hypoallergenic purposes.
- Footnote 15
-
It is recognized that the 2001 FAO/WHO consultation suggested moving from 8 to 6 identical amino acid segment searches. The smaller the peptide sequence used in the stepwise comparison, the greater the likelihood of identifying false positives; inversely, the larger the peptide sequence used, the greater the likelihood of false negatives, thereby reducing the utility of the comparison.
- Footnote 16
-
The method outlined in the U.S. Pharmacopoeia (1995) was used in the establishment of the correlation (Astwood et al. 1996).
- Footnote 17
-
According to the Joint Report of the FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology (22-25 January 2001, Rome, Italy) a minimum of 8 relevant sera is required to achieve a 99% certainty that the new protein is not an allergen in the case of a major allergen. Similarly, a minimum of 24 relevant sera is required to achieve the same level of certainty in the case of a minor allergen. It is recognized that these quantities of sera may not be available for testing purposes.
- Footnote 18
-
For the purpose of this guidance, the term 'developer' is used as an umbrella term that represents all parties involved in the development of a new plant variety from concept to commercialization.
- Footnote 19
-
For the purposes of this guidance, the term 'foreign DNA' refers to DNA that is originally sourced from genetic sources outside the plant species and cannot be introduced into that plant species using conventional methods of plant breeding as defined in 5. List of conventional methods of plant breeding.
- Footnote 20
-
The final plant product refers to the plant variety intended for commercialization and food use.
- Footnote 21
-
Significant similarity is typically >35 % overall sequence identity between amino acid sequences, or 100 % sequence identity over 8 consecutive amino acid residues with the sequence of a known allergen.
- Footnote 22
-
Key nutrients are considered any nutrients listed in the Dietary Reference Intakes (DRI) that have a nutrient-based reference value associated with adequacy (i.e., an Estimated Average Requirement [EAR] and Recommended Dietary Allowance [RDA] value or an Adequate Intake [AI] value) and/or those with established adverse health effects (i.e., a Tolerable Upper Intake Level [UL] value). All four reference values are directly associated with consumer health.
- Footnote 23
-
For the purpose of this guidance, documented ranges refers to the ranges documented in the Organisation for Economic Co-operation and Development (OECD) Consensus Documents on Compositional Considerations for New Varieties and other crop composition databases (e.g., the Agriculture and Food Systems Institute Crop Composition Database), the published scientific literature, and from commercialized varieties.
- Footnote 24
-
For example, a developer may intentionally reduce the allergenicity of a plant with the intent that its edible tissues could now be consumed by sensitive individuals.
- Footnote 25
-
As defined in Section B.28.001 of the FDRs, "genetically modify" means to change the heritable traits of a plant, animal or microorganism by means of intentional manipulation.
- Footnote 26
-
For the purpose of this guidance, the term 'developer' is used as an umbrella term that represents all parties involved in the development of a new plant variety from concept to commercialisation.
- Footnote 27
-
Within this guidance, 'mechanism of action' is defined as the specific biochemical reaction(s) and/or interaction(s) through which a characteristic functions.