Ultraviolet radiation
Ultraviolet (UV) radiation comes from natural sources (like the sun), and artificial sources (like black lights, welding equipment, lasers, and tanning equipment).
On this page:
- Sunlight and radiation
- Electromagnetic radiation spectrum
- Types of UV radiation
- Factors that affect ultraviolet levels
- The ozone layer
- How the Government of Canada protects you
- More information
Sunlight and radiation
The sun is a star roughly 150 million kilometres away from the earth. Without the sun, life on earth would not exist - our planet would be frozen and dark.
A nuclear reaction at the sun's core creates massive amounts of radiation, or energy. This energy (also known as the electromagnetic spectrum) includes radiowaves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
Depending on its wavelength, different types of solar radiation do different things:
- Infrared radiation makes us feel the warmth of the sun.
- Visible light allows us to see the world around us.
- Ultraviolet radiation affects our health.
Electromagnetic radiation spectrum
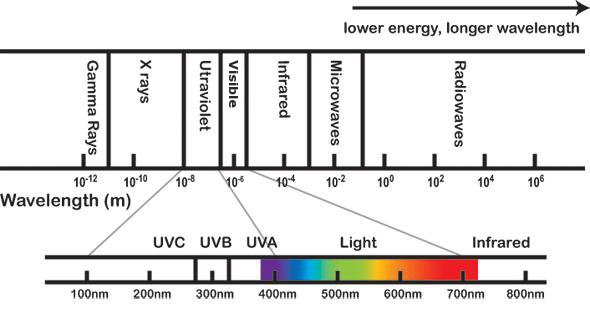
Electromagnetic radiation spectrum - description
This is an image of the different types of electromagnetic radiation. It is displayed as a line graph where the forms of electromagnetic radiation are plotted along a continuum in descending order of size and range.
The electromagnetic rays are displayed in two layers. The top layer shows a general range for all the types of electromagnetic radiation. The bottom layer shows a detailed range of Ultraviolet radiation Visible light, and Infrared radiation.
The top layer of the image showing electromagnetic radiation displays the rays in order of highest energy and shortest wavelength, through to lowest energy and longest wavelength.
From left to right are shown:
- Gamma Rays (wavelength range from 10 to the power of -12 metres, to 10 to the power of -11 metres)
- X rays (wavelength range from 10 to the power of -11 metres to 10 to the power of -8 metres)
- Ultraviolet light (wavelength range from 10 to the power of -8 metres to 10 to the power of -7 metres)
- Visible light (wavelength range from 10 to the power of -7 metres to 10 to the power of -5 metres)
- Infrared light (wavelength range from 10 to the power of -5 metres to 10 to the power of -3 metres)
- Microwaves (wavelength range from 10 to the power of -3 metres to 10 to the power of -1 metres), and
- Radiowaves (wavelength range from 10 to the power of -1 metres to 10 to the power of 6 metres and beyond).
The highest energy and shortest wavelength electromagnetic radiation is the Gamma Ray. The lowest energy and longest wavelength electromagnetic radiation is the Radiowave.
The second layer of the image displays detailed information about the range of Ultraviolet radiation, Visible light, and Infrared radiation. They are displayed along a continuum beginning with the types of Ultraviolet light, continuing with Visible light, and ending with Infrared radiation.
Ultraviolet radiation is shown to have a range of types. The types of radiation are displayed from left to right in descending order as UVC rays, UVB rays and UVA rays.
- UVC is displayed as having a range from 100 nanometres to 275 nanometres.
- UVB is displayed as having a range from 275 nanometres to 325 nanometres.
- UVA is displayed as having a range from 325 nanometres to 375 nanometres.
Along the same continuum in the image, Visible light is displayed after the Ultraviolet radiation waves. Visible light is displayed as having a range from 375 nanometres to 725 nanometres.
After the display of the range of visible light, Infrared radiation is placed last along the continuum. Infrared radiation displayed as having a range from 725 nanometres to beyond 825 nanometres.
Types of UV radiation
Ultraviolet radiation is invisible energy in the wavelength range from 100 to 400 nanometers (nm). A nanometer is one billionth of a meter. UV radiation has a shorter wavelength and is more energetic than visible light. Depending on its wavelength, it can get through the ozone layer and affect our health in different ways. The shorter the wavelength, the more harmful the UV radiation. However, shorter wavelength UV radiation is less able to penetrate the skin.
UV radiation is divided into three wavelength ranges:
- UVA is long-range UV radiation between 320 and 400nm. Although not as energetic as UVB, UVA can penetrate deep into our skin (dermis). This can cause immediate tanning and premature skin aging, and play a role in the development of certain skin cancers. UVA is not readily absorbed by the ozone layer - about 95% gets through.
- UVB is short-wave UV radiation between 280 and 320nm. It can just penetrate the outer protective layer of the skin and is responsible for delayed tanning, sunburns and most skin cancers. A large amount of UVB is absorbed by the ozone layer - only 5% reaches our planet's surface.
- UVC, with wavelengths between 100 and 280nm, is very energetic. It is very dangerous to all forms of life (even with short exposures). However, UVC radiation is filtered out by the ozone layer, and never reaches earth. It is created artificially to kill bacteria.
Factors that affect ultraviolet levels
The level (intensity) of UV radiation reaching our earth's surface depends on a number of factors:
- Time of day - UV radiation from the sun reaches its peak at solar noon, which is between 12 and 1 p.m. across Canada. At this time, the sun's rays have the least distance to travel through the atmosphere, increasing the intensity of UV. In general the UV Index in Canada can be 3 or higher from 11 a.m. to 3 p.m.
- Season - During the year, the sun's angle varies, which causes the intensity of UV rays to vary. UV intensity is highest during the spring and summer months. But the sun can still have an effect on your skin and eyes in the fall and winter, especially when UV is reflected back by large surfaces of fresh snow.
- Ozone layer thickness - A decrease in thickness of our ozone layer results in an increase in UV intensity. This effect is greatest in the spring and can be traced back to greenhouse gases. Also, pollutants like the chlorine from chlorofluorocarbons destroy ozone molecules at a faster rate.
- Weather conditions - Cloud cover can greatly affect the amount of UV radiation that reaches the earth's surface. Clouds that are dark and full of water can absorb up to 80% of the radiation. High thin clouds do not absorb much UV radiation. On the other hand, scattered clouds can actually increase the amount of UV radiation at the surface of the earth because of reflection. The intensity of UV radiation also depends on which pressure system is influencing the weather. A high-pressure area causes a thinner ozone layer, while a low-pressure area causes a thicker ozone layer.
- Surface reflections - Fresh white snow reflects up to 85% of UV radiation. Other bright surfaces (like sand, concrete, and water) reflect less. If you are skiing on a spring day at the end of March, for example, the reported UV index may only be 4, but because of reflection from the snow, you may experience a UV index of 7.
- Altitude - UV radiation increases with altitude (height above sea level) because there is less atmosphere to absorb the damaging rays. The UV index measured in Edmonton will be less than that measured at the top of a mountain in Jasper. At an altitude of around 2,000 metres, the amount of UV radiation can be up to 30% higher than at sea level.
- Latitude - UV is strongest at the equator where the UV index can reach about 12. In Canada, the UV index is highest in southern Ontario and is lowest at the North Pole.
The ozone layer
The earth's ozone layer protects us from the sun's harmful UV rays. But over time, the release of certain chemicals into the environment has damaged the ozone layer. As the ozone layer thins, more UV reaches the earth's surface.
Many countries around the world, including Canada, have recognized this problem and have taken steps to protect the ozone layer from more damage. Efforts have focused mainly on controlling the production and use of chemicals known to damage the ozone layer. These chemicals are used mainly in refrigeration and air-conditioning.
In 1987, Canada was one of 24 nations to sign the Montreal Protocol on Substances that Deplete the Ozone Layer. This was the first international agreement to limit the use of ozone-depleting chemicals. Since that time, more than 70 countries have signed the Montreal Protocol.
Canada has phased out the production of all ozone-depleting chemicals, and has taken steps to control their use through regulations that are part of the Canadian Environmental Protection Act.
How the Government of Canada protects you
Health Canada's Consumer and Clinical Radiation Protection Bureau assesses, monitors, and helps reduce health and safety risks from radiation emitting devices and other sources of radiation, including tanning equipment.
The Public Health Agency of Canada monitors cancer in Canada and develops programs to reduce cancer risks. Health Canada promotes public awareness about sun safety and the harmful effects of UV radiation.