Approved in 2020: Medical devices
On this page
- Medical devices: Approved in 2020
- Health categories
- Important definitions
- New class IV medical devices approved in 2020
- Body fluid and tissue management devices
- Body tissue manipulation and reparation devices
- Cardiovascular devices
- Gastro-urological devices
- General hospital devices
- In vitro diagnostic medical devices
- Neurological devices
- Plastic surgery and cosmetic devices
- Radiological devices
- Various
Medical devices: Approved in 2020
There are different classes of medical devices, ranging from Class I to IV. Class I devices are considered low-risk devices, for example, a tongue depressor. Class IV devices present the greatest potential risk, for example, a pacemaker.
This section outlines the new Class IV medical devices approved for sale in Canada in 2020, and the safety updates issued.
Health categories
The medical devices listed have been divided into categories according to the Global Medical Device Nomenclature system for naming and grouping medical devices.
We have included the indication of each new medical device to give you some additional information. In addition, each new device has a hyperlink to the Decision summary (when available). These documents provide a brief overview of the rationale for our decision to approve the medical device.
The categories are:
- Blood fluid and tissue management devices - for example, blood separation systems.
- Body tissue manipulation and reparation devices - for example, bone grafts and dermal dressings.
- Cardiovascular devices - for example, cardiovascular catheters and pacemakers.
- Gastro-urological devices - for example, incontinence control systems.
- General hospital devices - for example, infusion pumps.
- In vitro diagnostic medical devices - for example, instrument/analyser and viral infection disease in vitro devices.
- Neurological devices - for example, neurological stimulation devices.
- Plastic surgery and cosmetic devices - for example, breast implants.
- Radiological devices – for example, ultrasound imagine systems.
- Various - applicable to medical devices generally.
Important definitions
Licence with conditions (identified with 'C' icon)
A medical device licence may be issued with conditions set out by Health Canada. For example, the manufacturer may be required to submit additional information on an on-going basis for the medical device to demonstrate that it continues to meet our regulatory requirements.
Medical device
Medical devices are products that are used for diagnostic and/or therapeutic purposes. Newly approved medical devices provide a broader range of products used to treat, manage, diagnose or prevent a disease or a physical condition.
Novel technology (identified with 'N' icon)
Medical devices with novel technology introduce a new apparatus, appliance, software or material with novel technology never before approved for sale in Canada.
Safety updates
Safety updates are designed to communicate information about potential health risks, so that patients and health care professionals can make informed decisions about their health.
For more information about the types of risk communications that can be found on the Government of Canada's website, go to "Healthy clicks: Medical devices at a glance".
You can report medical device incidents to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
New class IV medical devices approved in 2020
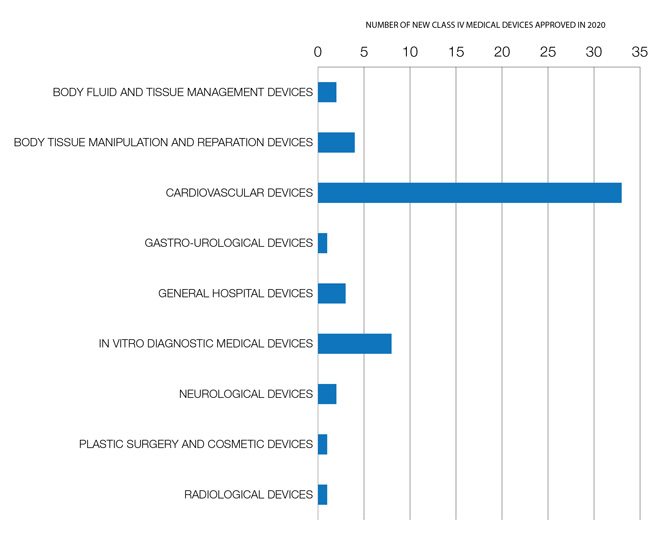
Figure 16: New Class IV medical devices approved in 2020: Text description
Number of new Class IV medical devices approved in 2019, according to the Global Medical Device Nomenclature (GMDN) system for naming and grouping medical devices.
| Health Category | Number of Class IV medical devices approved |
|---|---|
| Body fluid and tissue management devices | 2 |
| Body tissue manipulation and reparation devices | 4 |
| Cardiovascular devices | 33 |
| Gastro-urological devices | 1 |
| General hospital devices | 3 |
| In vitro diagnostic medical devices | 8 |
| Neurological devices | 2 |
| Plastic surgery and cosmetic devices | 1 |
| Radiological devices | 1 |
Body fluid and tissue management devices
For example, blood separation systems.
2 New medical devices
CliniMACS Prodigy T Cell Transduction (TCT) System
- Licence with conditions
- Indication
- The CliniMACS Prodigy T Cell Transduction (TCT) System is an automated cell processing for the generation of gene modified T cells from heterogeneous, hematological cell populations for clinical purposes.
Sonopet iQ Ultrasonic Aspirator System
Decision summary: Sonopet iQ Ultrasonic Aspirator System
- Indication
- The Sonopet iQ Ultrasonic Aspirator System is indicated for use in surgical procedures where fragmentation, emulsification, and aspiration of soft and hard tissue is desirable.
Body tissue manipulation and reparation devices
For example, bone grafts and dermal dressings.
4 New medical devices
Chondro-Gide Bilayer Collagen Membrane
Decision summary: Chondro-Gide Bilayer Collagen Membrane
- Indication
- The Chondro-Gide Bilayer Collagen Membrane is a bilayer collagen membrane of porcine origin to be used to cover cartilage defects treated with microfracturing techniques.
Myriad
- Indication
- Myriad is intended to cover, protect and provide a moist wound environment. The device may be fixed via sutures, staples or tacks to surround the tissue if desired.
Puracol Plus
Decision summary: Puracol Plus
- Indication
- The Puracol Plus is a highly porous collagen matrix for wound healing, it is a wound dressing.
Regeneten Bioinductive Implant System
- Licence with conditions
- Indication
- The Regeneten Bioinductive Implant is indicated for the management and protection of rotator cuff tendon injuries in which there has been no substantial loss of tendon tissue.
Cardiovascular devices
For example, cardiovascular catheters and pacemakers.
33 new medical devices
Agilis HisPro Steerable Catheter with Electrodes
- Indication
- The Agilis HisPro Steerable Catheter with Electrodes is a delivery tool indicated to provide transvenous access during a cardiac procedure or device implant (i.e. lead placement) to structures in the heart.
Athletis Over-the-Wire PTA Balloon Dilatation Catheter
- Indication
- The Athletis Percutaneous Transluminal Angioplasty (PTA) Balloon Dilatation Catheter is indicated for percutaneous transluminal angioplasty in the peripheral vasculature including upper extremity, renal, iliac, and infrainguinal vessels and the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. It is also indicated for post-dilatation of stents and stent grafts in the peripheral vasculature.
Attain Stability Quad MRI SureScan 4798
Decision summary: Attain Stability Quad MRI SureScan 4798
- Indication
- The Attain Stability Quad MRI SureScan 4798 is a left ventricular (LV) lead to be used in a Medtronic Cardiac Resynchronization Therapy (CRT) system. This device incorporates an active fixation side helix to assist the implanter in lead placement in the cardiac vein and to prevent dislodgement once implanted.
Cardiac Resynchronization Therapy Defibrillator (CRT-DS)
- Indication
- The Implantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy Defibrillator (CRT-D) devices are indicated for automated treatment of life-threatening ventricular arrhythmias. CRT-D devices are also indicated to treat symptoms in patients who have congestive heart failure with ventricular dyssynchrony.
Cobalt XT / Cobalt / Crome ICD and CRT-D MRI SureScan
Decision summary: Cobalt XT / Cobalt / Crome ICD and CRT-D MRI SureScan
- Indication
- The Cobalt XT/Cobalt/Crome Implantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy Defibrillator (CRT-D) magnetic resonance imaging (MRI) SureScan device is indicated for the automated treatment of patients who have experienced, or are at significant risk of developing, life-threatening ventricular arrhythmias through the delivery of antitachycardia pacing, cardioversion, and defibrillation therapies.
Diamondback 360 Coronary Orbital Atherectomy System
- Indication
- The Diamondback 360 Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease who are acceptable candidates for percutaneous transluminal coronary angioplasty or stenting due to de novo, severely calcified coronary artery lesions.
Eluvia Over-the-Wire Drug-Eluting Vascular Stent System
- Licence with conditions
- Indication
- The Eluvia Over-the-Wire Drug Eluting Vascular Stent System is a drug eluting vascular stent. It is intended to improve luminal diameter in the treatment of symptomatic de-novo or restenotic lesions in the native superficial femoral artery (SFA) and/or proximal popliteal artery with reference vessel diameters (RVD) ranging from 4.0-6.0 mm and total lesion lengths up to 190 mm.
EPstar Fixed Electropysiology Catheter / Epstar Electrophysiologt Cable
- Licence with conditions
- Indication
- The EPstar Fixed Electrophysiology Catheter is intended for electrogram recording and pacing during diagnostic electrophysiology studies.
Evolut PRO+ Transcatheter Aortic Valve
- Licence with conditions
Decision summary: Evolut PRO+ Transcatheter Aortic Valve
- Indication
- Evolut PRO+ System is a transcatheter aortic valve replacement system. This device replaces the existing aortic valve.
Implantable Cardioverter Defibrillator (ICDS)
- Licence with conditions
- Indication
- The Implantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy Defibrillator (CRT-D) devices are indicated for automated treatment of life-threatening ventricular arrhythmias. CRT-D devices are also indicated to treat symptoms in patients who have congestive heart failure with ventricular dyssynchrony.
IntellaNav ST
- Licence with conditions
- Indication
- The IntellaNav ST is a standard power temperature-controlled ablation catheter. When used with a compatible radiofrequency controller, is indicated for interruption of accessory atrioventricular (AV) conduction pathways associated with tachycardia, for treatment of AV nodal reentrant tachycardia and for creation of complete AV block in patients with a rapid ventricular response to an atrial arrhythmia.
IntellaNav StablePoint Ablation Catheter
- Licence with conditions
- Indication
- The IntellaNav StablePoint Catheter is indicated for use in patients who require catheter-based cardiac electrophysiological mapping (stimulating and recording) and, when used in conjunction with a radio frequency (RF) generator, for cardiac ablation.
Jade PTA Balloon Dilatation Catheter
- Licence with conditions
- Indication
- The Jade Percutaneous Transluminal Angioplasty (PTA) Balloon Dilatation Catheter is a rapid exchange balloon catheter for peripheral indications. It is used for percutaneous transluminal angioplasty in the peripheral vasculature, including iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae.
Konect Resilia Aortic Valved Conduit
- Licence with conditions
Decision summary: Konect Resilia Aortic Valved Conduit
- Indication
- The Konect Resilia Valved Graft Conduit is indicated for patients who require replacement of their native or prosthetic aortic valve and the associated repair or replacement of a damaged or diseased ascending aorta.
Lotus Edge Valve System
- Licence with conditions
- Indication
- The Lotus Edge Valve System is a pre-loaded, stent-mounted tissue valve prosthesis for transcatheter aortic valve replacement (TAVR). It is indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are at high or greater risk for open surgical therapy.
Micra AV MC1AVR1
- Licence with conditions
- Novel technology
- Indication
- The Micra AV is a leadless pacemaker delivering VDD therapy that is implanted directly into the right ventricle by a delivery catheter through the femoral vein. Atrial-Ventricular (AV) synchrony is provided through use of an on-board accelerometer that senses atrial contractions.
Penumbra LP Coil System
- Licence with conditions
- Indication
- The Penumbra LP Coil System is indicated for the embolization of intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenus fistulae, arterial and venous embolizations in the peripheral vasculature.
QDot Micro Navigation Catheter
- Licence with conditions
Decision summary: QDot Micro Navigation Catheter
- Indication
- The QDot Micro Navigation Catheter is a steerable, multi-electrode luminal catheter with a deflectable tip designed to facilitate electrophysiological mapping of the heart and to transmit radiofrequency (RF) energy to the catheter tip electrode for ablation purposes.
Ranger and Ranger SL Over-the-Wire Paclitaxel-Coated PTA Balloon Catheter
- Licence with conditions
- Indication
- The Ranger is a Drug Coated Balloon (DCB). The device is used in angioplasty procedures in the leg.
Reprocessed ViewFlex Xtra ICE Catheter
- Licence with conditions
- Indication
- The Reprocessed ViewFlex Xtra ICE catheter is indicated for use in adult and adolescent pediatric patients to visualize cardiac structures, blood flow and other devices within the heart.
Sapphire II NC Coronary Dilatation Catheter
- Licence with conditions
- Indication
- Sapphire II NC Coronary Dilatation Catheter is used for balloon dilatation of a stenotic portion of a coronary artery in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
Scoreflex NC Coronary Dilatation Catheter
- Licence with conditions
- Indication
- The Scoreflex NC is intended for balloon dilatation of a stenotic portion of a coronary artery in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
Scoreflex PTA Balloon Dilatation Catheter
- Licence with conditions
- Indication
- The Scoreflex Percutaneous Transluminal Angioplasty (PTA) Balloon Dilatation Catheter is indicated for percutaneous transluminal angioplasty in the peripheral vasculature, including iliac, femoral, ilio-femoral, popliteal, infra-popliteal, renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae.
Sentinel Cerebral Protection System
- Licence with conditions
- Indication
- The Sentinel Cerebral Protection System is indicated for use as an embolic protection device to capture and remove thrombus/debris while performing transcatheter aortic valve replacement procedures.
Smart Touch Programming System
- Licence with conditions
- Indication
- The Smart Touch Programming System is a programmer system for implanted devices (pacemakers, defibrillators, and cardio resynchronization therapy-defibrillator). The software interrogates and programs the devices. Additionally, it provides measurement, electrocardiogram (ECG) display and report printing functions.
Surpass Evolve Flow Diverter System
- Licence with conditions
- Indication
- The Surpass Evolve Flow Diverter System is a flow diverter comprised of a self-expandable braided implant preloaded on a delivery wire, housed inside an introducer sheath designed for endovascular treatment of intracranial aneurysms. The Surpass Evolve Flow Diverter System is indicated for use for the treatment of saccular or fusiform intracranial aneurysms arising from a parent vessel with a diameter between 2.0 mm and 5.0 mm.
Synergy Monorail Everolimus-Eluting Platinum Chromium Coronary Stent System
- Licence with conditions
- Indication
- The Synergy Everolimus-Eluting Platinum Chromium Coronary Stent System is a medical device that provides a mechanical structure for vascular lumen support (the stent component) and a pharmacological agent (everolimus) targeted towards reducing the injury response that leads to restenosis after stent implantation.
Synergy XD Monorail Everolimus-Eluting Platinum Chromium Coronary Stent System
- Licence with conditions
- Indication
- The Synergy XD Monorail Everolimus-eluting Platinum Chromium Coronary Stent System is used for improving luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease, stable angina, unstable angina, non-ST elevation MI or documented silent ischemia due to atherosclerotic lesions in native coronary arteries between 2.25 mm and 5.00 mm in diameter in lesions 44 mm or less in length.
TriClip System
- Licence with conditions
- Novel technology
- Indication
- The TriClip System is a transcatheter Tricuspid Valve Repair System (TVRS) intended for use in reconstruction of the tricuspid valve (TV) through tissue approximation.
Vega endocardial pacing lead
- Licence with conditions
- Indication
- The Vega pacing lead is a bipolar, endocardial, steroid eluting, silicone-insulated lead with an extendable/retractable active-fixation screw, intended for permanent pacing and sensing of either the right atrium or right ventricle.
Wattson temporary pacing guidewire
- Licence with conditions
- Indication
- The Wattson temporary pacing guidewire is a dual purpose guidewire designed for the delivery of devices (e.g. Transcatheter Aortic Valve Replacement) and temporary rapid pacing of the heart when used with an external pulse generator.
Xience ProA Everolimus Eluting Coronary Stent System
Decision summary: Xience ProA Everolimus Eluting Coronary Stent System
- Indication
- The Xience ProA Everolimus Eluting Coronary Stent System (Xience ProA) consists of a drug and polymer coated stent pre-mounted on a balloon catheter delivery system. The Xience ProA stent system is indicated for improving coronary artery luminal diameter in patients, and for treating de novo chronic total coronary occlusions.
Zoll X Series Advanced
Decision summary: Zoll X Series Advanced
- Indication
- The Zoll X Series Advanced devices are portable, lightweight defibrillators that combine defibrillation and external pacing with several monitoring capabilities. The X Series Advanced system is indicated for defibrillation on victims of cardiac arrest where there is apparent lack of circulation as indicated by unconsciousness, absence of breathing, or absence of pulse.
Gastro-urological devices
For example, incontinence control system.
1 new medical device
InterStim Micro SureScan MRI
- Licence with conditions
- Indication
- The InterStim system is an implantable programmable neuromodulation system that delivers electrical stimulation to the sacral nerve. Sacral neuromodulation therapy provided by the InterStim system is indicated for the management of the following chronic intractable (functional) disorders of the pelvis and lower urinary or intestinal tract: overactive bladder, fecal incontinence, and non-obstructive urinary retention.
Safety update
- Single incision mini-sling (SIMS) made from non-absorbable synthetic material (polypropylene):
General hospital devices
For example, infusion pumps.
3 new medical devices
EPstar Fixed Electrophysiology Catheter with Lumen / EPstar Electrophysiology Cable
- Licence with conditions
- Indication
- The EPstar Fixed Electrophysiology Catheter with Lumen/ EPstar Electrophysiology Cable can be used in the evaluation of a variety of cardiac arrhythmias from endocardial and intravascular sites.
Minimed 770G
- Licence with conditions
- Indication
- The MiniMed 770G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons two years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin, in the abdomen and buttock, or back of upper arm, depending on the person's age.
T:slim X2 Insulin Pump with Control-IQ Technology
- Licence with conditions
- Indication
- The t:slim X2 Insulin Pump with Control-IQ Technology is an insulin delivery system, intended to treat people with Type 1 diabetes, over the age of 6. It is intended for the subcutaneous delivery of insulin. The Control-IQ technology is intended for use with a compatible continuous glucose monitor (CGM) and the t:slim X2 insulin pump to automatically increase, decrease, and suspend delivery of basal insulin based on CGM readings and predicted glucose values.
Safety updates
Respirators:
- Advisory: Important safety information for certain respirator masks
- Information Update: Medical Device Respirator recalls
In vitro diagnostic medical devices
For example, instrument/analyser and viral infection disease in vitro devices.
8 new medical devices
Advia Centaur HBC Total 2 (HBcT2) (donor screening for transplantation)
- Licence with conditions
- Indication
- The Advia Centaur HBcT2 assay is an in vitro diagnostic device which measures total antibodies to the core antigen of hepatitis B virus in human serum and plasma. The presence of anti-HBc indicates previous or ongoing infection with hepatitis B virus.
Advia Centaur Quantitative HBsAg (QHBs)
- Licence with conditions
- Indication
- The Advia Centaur Quantitative HBsAg (QHBs) assay is for in vitro diagnostic use in the quantitative determination of hepatitis B surface antigen (HBsAg) in human serum and plasma (lithium heparin, sodium heparin, and dipotassium EDTA) that are confirmed positive for HBsAg using the Advia Centaur XP and Advia Centaur XPT systems.
Alinity s HIV Ag/Ab Combo Assay (donor screening and cadaveric testing)
- Licence with conditions
- Indication
- The Alinity s HIV Ag/Ab Combo Assay is intended for blood donor screening and it is run off the Alinity s platform.
Atellica IM Quantitative HBsAg (QHBs)
- Licence with conditions
- Indication
- The Atellica IM Quantitative HBsAg (QHBs) assay is for in vitro diagnostic use in the quantitative determination of hepatitis B surface antigen (HBsAg) in human serum and plasma (dipotassium EDTA, lithium heparin, and sodium heparin) that are confirmed positive for HBsAg using the Atellica IM Analyzer.
INSTI HIV Self Test
- Licence with conditions
- Indication
- The INSTI HIV Self Test is a single use in vitro self-test for the detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) and Type 2 (HIV-2) in whole blood. The test is intended for use as a self-test by users 18 years or older.
Liaison XL Murex HCV AB
- Licence with conditions
- Indication
- The Liaison XL Murex HCV AB is intended to be used as an aid in the diagnosis of hepatitis C virus (HCV) infection. The assay may also be used as an aid in the diagnosis of HCV infection in pediatric subjects and in pregnant women.
PK CMV-PA System Control Set
- Licence with conditions
Decision summaryPK CMV-PA System Control Set
- Indication
- The PK CMV-PA System Control Set is an in vitro diagnostic device. This application for a new medical device license for the PK CMV-PA System Control Set (PA2302) is to add the Beckman Coulter PK7400 Automated Microplate System as an intended use for this control set.
PK7400 TP HA reagent and controls
- Licence with conditions
- Indication
- PK7400 TP HA reagent is intended for the qualitative screening of blood donors for the detection of Treponema pallidum IgG and IgM antibodies to syphilis in human serum, EDTA plasma, or CPDA plasma.
Neurological devices
For example, neurological stimulation devices.
2 new medical devices
Percept PC
- Licence with conditions
- Indication
- Bilateral anterior thalamic nucleus (ATN) stimulation using the Medtronic DBS System for epilepsy is indicated as adjunctive therapy for reducing the frequency of seizures in adults diagnosed with epilepsy characterized by partial-onset seizures, with or without secondary generalization, that are refractory to antiepileptic medications.
Proclaim Implantable Pulse Generators
- Indication
- The Proclaim XR Implantable Pulse Generator (IPG) is a spinal cord stimulation device. This device generates weakly electric pulses and delivers them to the site of interest in the spinal cord for pain control.
Plastic surgery and cosmetic devices
For example, breast implants.
1 new medical device
Natrelle Inspira Cohesive Breast Impant
- Licence with conditions
- Indication
- Indications for use include breast augmentation, breast reconstruction, and revision for previous breast augmentation or reconstruction to correct or improve the result of the previous surgery.
Radiological devices
For example, ultrasound imaging systems.
1 new medical device
Acist HDi System
Decision summary: Acist HDi System
- Indication
- The Acist HDi System is intended to be used for the ultrasound examination of coronary and peripheral intravascular pathology. Intravascular ultrasound imaging is indicated in patients who are candidates for transluminal interventional procedures.
Various
Applicable to medical devices generally.
Safety update
- Some medical devices that use Bluetooth Low Energy (BLE) chips: