Drug and medical device highlights 2020: Drugs for human use
On this page
- Drugs for human use: 2020 in brief
- Drugs for human use: What's new in 2020
- Drugs for human use: 2020 accomplishments
- Healthy clicks: Drugs for human use at glance
- Drugs for human use: Life-cycle
Drugs for human use: 2020 in brief
One of Health Canada's roles is to regulate drugs that can help Canadians maintain and improve their health. These include prescription and non-prescription ("over-the-counter") medicines, as well as vaccines.
The COVID-19 pandemic created an urgent need for access to safe, effective and high quality health products. Our response to the pandemic addressed critical issues related to drugs, as well as disinfectants and hand sanitizers, across product life-cycles.
Response to the COVID-19 pandemic
As part of the government's response to the pandemic, we introduced innovative and agile regulatory measures. These measures expedited the regulatory review of COVID-19 health products without compromising safety, efficacy and quality standards.
In 2020 we authorized 81 clinical trials related to drugs or vaccines for COVID-19. As a result of the pandemic, hundreds of clinical trials had to be amended in order to avoid contracting the infection through visits at trial sites. These amendments allowed for remote consent and visits, telemedicine, and different ways of dispensing and administering trial drugs. The pandemic also allowed for the decentralization of some clinical trials.
We approved two drugs to treat COVID-19, Veklury (remdesivir) and Bamlanivimab. We approved two vaccines, the Pfizer-BioNTech COVID-19 Vaccine and the COVID-19 Vaccine Moderna. As of December 31, 2020, two other vaccines and one other treatment were under review. We monitored the safety and effectiveness of health products related to COVID-19, and took action as needed. We also created the COVID-19 vaccines and treatments portal, which provides up-to-date information for consumers, health care professionals and researchers.
The regulation of hand sanitizers and disinfectants was an important part of our response to the COVID-19 pandemic. In 2020 we authorized over 4,400 new hand sanitizer products and approximately 200 new disinfectants.
For more information about our response to the COVID-19 pandemic, go to "Drugs for human use: 2020 accomplishments".
New drugs approved
In 2020 we approved 84 new drugs, including three new drugs (one treatment and two vaccines) that were authorized under the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19. These give patients more options for the treatment, prevention and diagnosis of various health conditions.
Forty of the new drugs we approved in 2020 contained medicinal ingredients that have never been approved for sale in Canada, what we call "new active substances". Forty-two percent of these were approved through an expedited pathway, including those that target specific health care needs. For example, Zolgensma is a one-time gene therapy for the treatment of spinal muscular atrophy. This is a rare disease and a leading genetic cause of infant mortality, for which few therapies exist. In addition, Givlaari was approved for the treatment of acute hepatic porphyria, a rare inherited disease.
It is also important to bring more choice and more affordable options to Canadians. We approved 138 new generic drugs and 16 new biosimilars in 2020. Generic drugs contain the same medicinal ingredients as the brand name drug, and are considered bioequivalent to the brand name drug. Biosimilars are biologic drugs that enter the market subsequent to a previously authorized drug in Canada with a demonstrated similarity to the previously authorized biologic drug. One of the new drugs (Sani-Cide EX3) and two of the generic drugs (clotrimazole and loperamide) we approved in 2020 were for non-prescription ("over-the-counter") use.
For a list and description of the new drugs we approved in 2020, go to "Approved in 2020: Drugs for human use".
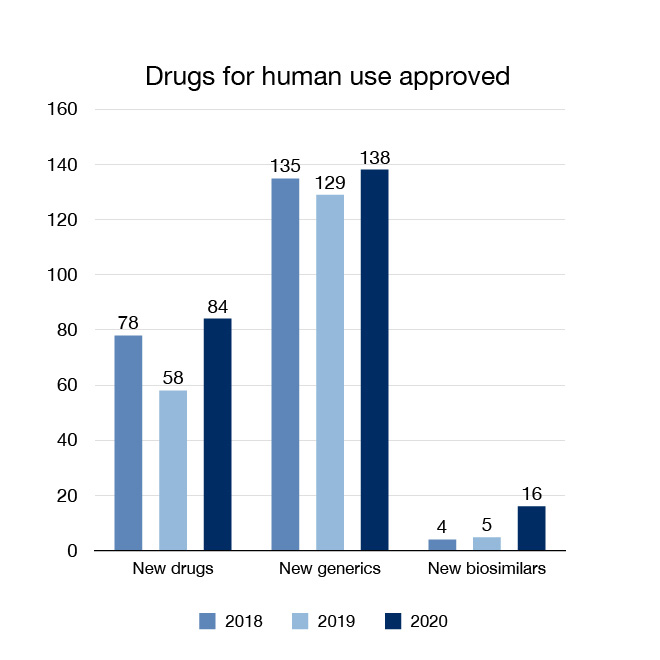
Figure 1: Drugs for human use approved: Text description
| Year | New drugs approved | New generic drugs approved | New biosimilars approved |
|---|---|---|---|
| 2018 | 78 | 135 | 4 |
| 2019 | 58 | 129 | 5 |
| 2020 | 84 | 138 | 16 |
Clinical trials and special access program
We review applications to allow companies and researchers to conduct clinical trials in Canada. New clinical trials mean Canadians may have access to more innovative treatment options. In 2020, 1,274 new clinical trial applications for drugs were approved.
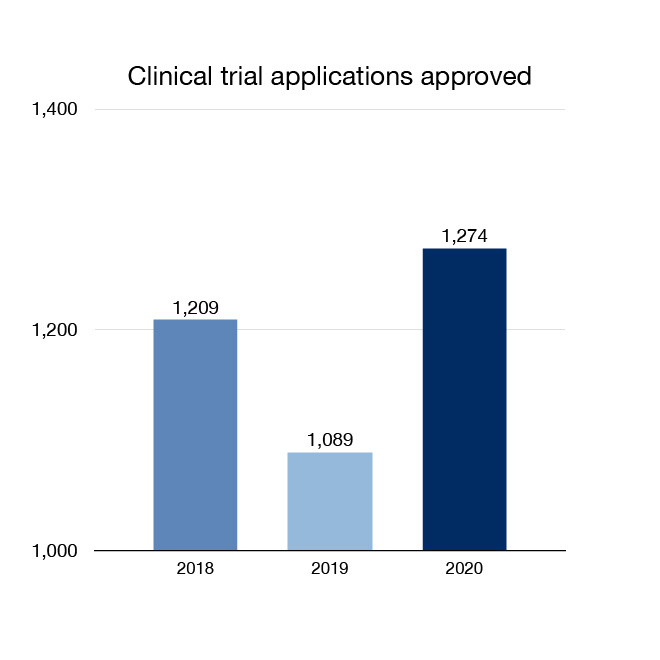
Figure 2: Clinical trial applications approved: Text description
| Year | Number of Clinical trial applications approved |
|---|---|
| 2018 | 1209 |
| 2019 | 1089 |
| 2020 | 1274 |
Through our Special Access Program, we give access to drugs that are not available for sale in Canada. We may grant access to doctors for emergency use or for the treatment of serious or life-threatening conditions. In 2020, 11,740 requests for special access to drugs were authorized. This included an array of drugs for infections, epilepsy, cancer and rare diseases. In 2020 etomidate, a drug that had been available through the program for more than 20 years, received market authorization and was transitioned from the Special Access Program to the market.
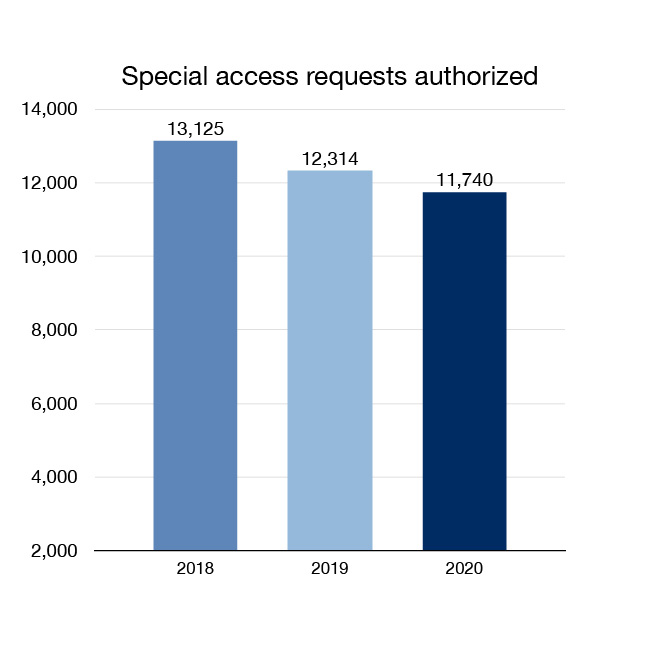
Figure 3: Special access requests authorized: Text description
| Year | Special access requests authorized |
|---|---|
| 2018 | 13,125 |
| 2019 | 12,314 |
| 2020 | 11,740 |
Surveillance
After we approve a drug for sale in Canada, we continue to monitor and evaluate reports of suspected adverse reactions.
In 2020 we received 603,705 reports of adverse reactions to drugs for human use and undertook 1,034 actions related to drugs. Adverse drug reactions come from domestic and international sources. Actions can include informing the public and health care professionals of safety information or recommending labelling changes. In cases where a serious risk is identified, we may remove a drug from the market.
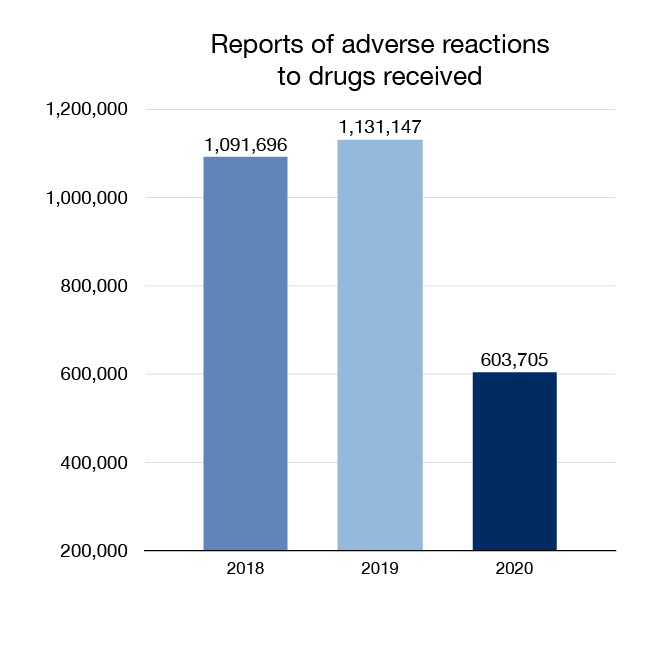
Figure 4: Reports of adverse reactions to drugs received: Text description
| Year | Reports of adverse reactions to drugs received |
|---|---|
| 2018 | 1,091,696 |
| 2019 | 1,131,147 |
| 2020 | 603,705 |
To learn how we addressed safety issues that arose for drugs in Canada in 2020, go to "Approved in 2020: Drugs for human use".
Transparency of decision making
In 2020 we continued to advance our openness and transparency efforts by expanding the amount of regulatory health and safety information that is made available to Canadians. We published 143 regulatory decision summaries and 57 summary basis of decision documents, which explain Health Canada's decisions for certain drugs seeking market authorization.
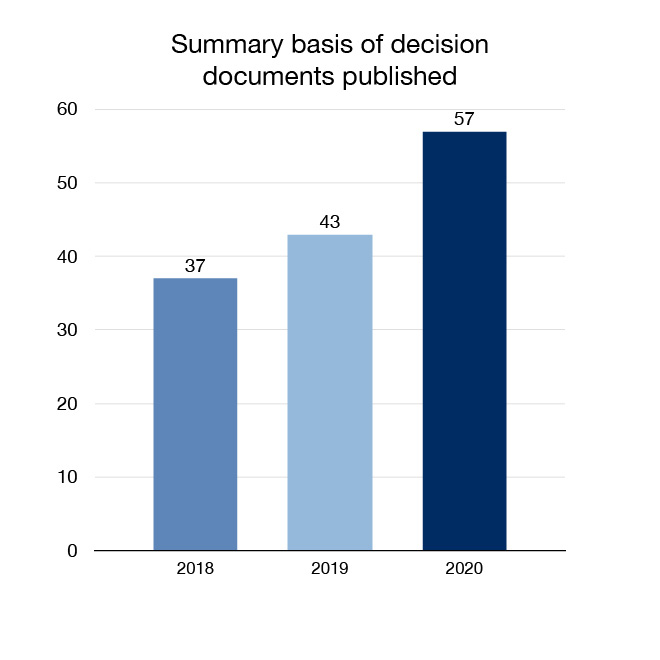
Figure 5: Summary basis of decision documents published: Text description
| Year | Summary basis of decision documents published |
|---|---|
| 2018 | 37 |
| 2019 | 43 |
| 2020 | 57 |
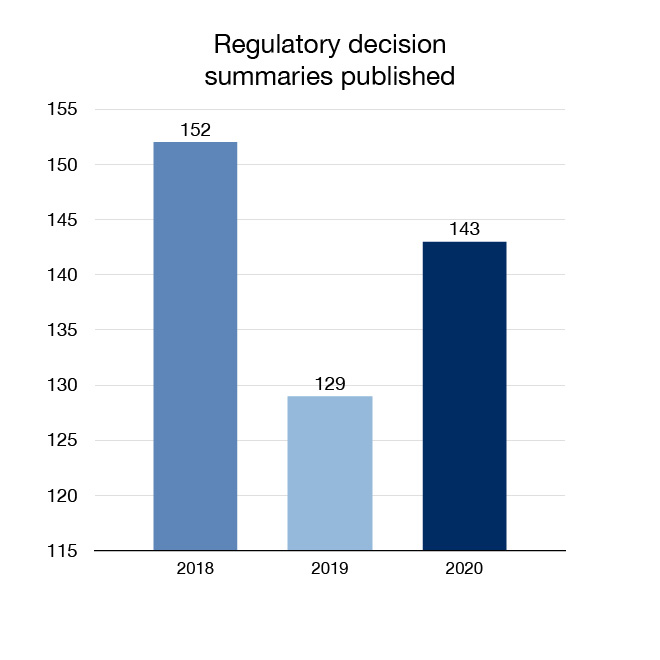
Figure 6: Regulatory decision summaries published: Text description
| Year | Regulatory decision summaries published |
|---|---|
| 2018 | 152 |
| 2019 | 129 |
| 2020 | 143 |
Through our "Clinical Information Portal", we published 2,185,079 pages of clinical information on 79 drugs. This clinical information is provided by companies when they seek approval to sell a drug in Canada, and is made publicly available after we decide to approve or reject the drug.
Health Canada also publishes summaries of its safety reviews, which describe Health Canada's decisions related to potential safety issues. In 2020 we published 19 such summaries for human drugs. These summaries complement other safety-related information to help Canadians make informed decisions about their medication choices.
This "Drugs for Human Use" chapter gives you more information about our work in 2020. For up-to-date information about our activities see the "Healthy clicks: Drugs for human use at a glance" section, and follow us on social media.

Director General,
Therapeutic Products

Director General,
Biologic and Radiopharmaceutical Drugs

Director General,
Natural and Non-Prescription Health Products

Director General,
Marketed Health Products
Drugs for human use: What's new in 2020
In 2020 Health Canada approved 84 new drugs, including 16 new biosimilars. More detail is available in the section "Approved in 2020: Drugs for human use".
Alimentary tract and metabolism
- Givlaari
- Ibsrela
- Mar-Trientine
- Rybelsus
- TrurapiFootnote *
- Uceris
Antiinfectives for systemic use
- Amoxicillin Sodium and Potassium Clavulanate for Injection
- Anthim
- Bamlanivimab
- Cabenuva / Vocabria
- COVID-19 Vaccine Moderna
- Menquadfi
- Pfizer BioNTech COVID-19 Vaccine
- Veklury
- Vocarvi
- Xenlata
- Xofluza
Antineoplastic and immunomodulating agents
- AmgevitaFootnote *
- AvsolaFootnote *
- Daurismo
- Enspryng
- Gleolan
- HulioFootnote *
- HyrimozFootnote *
- IdacioFootnote *
- Inqovi
- Inrebic
- KanjintiFootnote *
- Mayzent
- NivestymFootnote *
- Nubeqa
- NyvepriaFootnote *
- Odomzo
- Piqray
- Polivy
- Qinlock
- RiximyoFootnote *
- Rozlytrek (two submissions)
- RuxienceFootnote *
- Sarclisa
- Tukysa
- Verity-BCG
- Zeposia
- ZiextenzoFootnote *
Blood and blood forming organs
- Addnutriv
- Bivalirudin Injection
- Cablivi
- Essepna
- Inclunox, Inclunox HPFootnote *
- Noromby, Noromby HPFootnote *
- Reblozyl
- Redesca, Redesca HPFootnote *
- Regiocit
- Tavalisse
Cardiovascular system
- Corzyna
Dermatologicals
- Duobrii
- Teva-Betamethasone/ Calcipotriol
Genito urinary system and sex hormones
- Bijuva
- Imvexxy
- Nexplanon
Musculo-skeletal system
- Zolgensma
Nervous system
- Ajovy
- Dayvigo
- Firdapse
- Kynmobi
- Perseris
- Peyona
- Ruzurgi
- Spravato
- Suvexx
- Tomvi
- Vyndaqel
Respiratory system
- Atectura Breezhaler
- Enerzair Breezhaler
Sensory organs
- Beovu
- Luxturna
Systemic hormonal preparations, excluding sex hormones and insulins
- Increlex
- OsnuvoFootnote *
- Tirosint
Various
- Galliapharm
- Itulatek
- Sani-Cide EX3
- Sodium Pertechnetate 99mTc Injection
Drugs for human use: 2020 accomplishments
Responding to the COVID-19 pandemic
The COVID-19 pandemic has had a profound impact on the health and well-being of Canadians. It has created an unprecedented demand on Canada's health care system and has led to an urgent need for access to health products.
Health Canada's response to the pandemic addressed critical issues related to drugs for human use across product life-cycles, from clinical trials to authorizations of drugs to surveillance.

Director General,
COVID-19 Regulatory Response Team
Focus on…
The COVID-19 regulatory response team
The COVID-19 Regulatory Response Team was created to be the focal point for COVID-19 drugs and vaccines. The team supports the review areas in engaging with industry and other stakeholders. It also engages with partners across the Government of Canada to provide the regulatory perspective into the response to the COVID-19 pandemic.
"The dedication and hard work since the beginning of the pandemic has been incredible. I'm so proud of what we have all been able to do to support bringing tests, treatments, vaccines and other products to Canadians."
Clinical trials
In May 2020, the Minister of Health signed the Interim Order Respecting Clinical Trials for Medical Devices and Drugs related to COVID-19. This temporary measure facilitated a more efficient and flexible authorization process for clinical trials in Canada, allowing broader types of COVID-19 clinical trials to take place more efficiently. It offered greater patient access to potential COVID-19 drugs, while upholding strong patient safety requirements.
In addition, we expedited the authorization of trials for COVID-19 and published a Notice on the management of clinical trials during the pandemic. Applications were reviewed faster than usual, to speed up access without compromising patient safety. We worked with companies, academic research centres and investigators who had products in development, to provide guidance and help bring clinical trials to Canada.
In 2020 we authorized 81 clinical trials related to drugs or vaccines for COVID-19.
For more information, go to Drugs and vaccines for COVID-19: Clinical trials for drugs and vaccines.
Special Access Program
The Special Access Program is for health care professionals who are treating seriously ill patients where conventional therapies have failed, are unsuitable or are unavailable. The Special Access Program worked to help resolve critical shortages within the health care system during the pandemic. In addition, in 2020 we modernized the regulations for the program by improving processes used by health care providers and by reducing the administrative burden for requests. These changes will facilitate access to non-marketed drugs for Canadians, for COVID-19 and other medical emergencies.
For more information, go to Health Canada's special access programs: Overview.
Focus on…
Access to treatments during the pandemic
The COVID-19 pandemic caused border closures and restricted air transportation. As a result, some patients had difficulty accessing necessary medical treatments. The Special Access Program played a key role in preventing and resolving critical shortages within the health care system. For example, prior to the pandemic, the program considered requests for medicinal leeches used to treat venous congestion post surgery. However, because of COVID-19, importing leeches from France to the American distributor required extraordinary efforts by the Special Access Program. The program coordinated with various government departments such as Health Canada, Canada Border Services Agency and Global Affairs Canada, as well as regulators in the United States and France.
Ian MacKay
Manager,
Therapeutic Product
Drugs and vaccines
Drugs and vaccines will play an essential role in Canadians' ability to recover safely from the COVID-19 pandemic.
As part of the government's response to the pandemic, Health Canada introduced innovative and agile regulatory measures. These allowed us to expedite the regulatory review of COVID-19 drugs and vaccines, without compromising our high standards for safety, efficacy and quality.
For more information on the Interim Orders that were published to address the COVID-19 pandemic, go to "Message from the Chief Regulatory Officer".
In 2020 we approved two vaccines under the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19, the Pfizer-BioNTech COVID-19 Vaccine and the COVID-19 Vaccine Moderna. We also approved two drugs to treat COVID-19, Veklury and Bamlanivimab. As of December 31, 2020, two other vaccines and one other treatment were under review.
We will closely monitor the use of these products in the real world, evaluate potential safety and effectiveness issues and take action where there are identified problems.
Focus on…
Our science
In 2020 Dr. Sean Li published a paper entitled "The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity". The publication investigated which mutations in the coronavirus spike protein led to resistance against neutralising antibodies and the importance of glycosylation in viral infectivity. The spike protein is the main surface antigen and the target in vaccine development. This work contributes to the development of vaccines and therapeutic antibodies.
Dr. Sean Li
Research Scientist,
Biologic and Radiopharmaceutical Drug
Disinfectants and hand sanitizers
The COVID-19 pandemic created an urgent need for disinfectants and hand sanitizers. These products were an important part of Health Canada's response to the pandemic to prevent the spread of COVID-19. To increase supply and ensure Canadians have access to these products, our work included:
- expediting the review of disinfectants and hand sanitizers,
- introducing interim measures such as:
- allowing the importation of products that may not fully meet regulatory requirements, but do not compromise the safety of Canadians, to expedite access to disinfectants and hand sanitizers to address product shortages
- temporarily authorizing technical grade ethanol in the production of disinfectants and hand sanitizers to address an identified shortage of pharmaceutical-grade ethanol,
- providing guidance on obtaining a licence to sell alcohol- and non-alcohol-based sanitizers, on producing ethanol and isopropyl alcohol to make sure the alcohol used was safe, and on obtaining an authorization to manufacture and sell disinfectants,
- working with companies who entered the hand sanitizer market, such as distilleries, to help them navigate our regulations to manufacture and sell their new products, and
- working with the disinfectant industry to inform Canadians of products that can be used to help against the spread of COVID-19.
In 2020 we authorized over 4,400 hand sanitizer products and approximately 200 disinfectants. We also developed an online list of hard-surface disinfectants with evidence for use against COVID-19, which included over 500 products as of December 31, 2020.
For more information, go to Hard-surface disinfectants and hand sanitizers (COVID-19).
Surveillance of COVID-19 health products
We monitored the safety and effectiveness of health products related to COVID-19, and took action as needed to protect Canadians.
Our work on drugs and vaccines included:
- working with the Public Health Agency of Canada to continuously monitor COVID-19 vaccines on the market in Canada,
- taking proactive steps to identify adverse events from drugs and vaccines used for COVID-19 (products explicitly approved for use in COVID-19 as well as those that were being used "off-label", or outside of the approved use of the product),
- monitoring retailers and advertisements that were making false, misleading and illegal claims related to COVID-19,
- publishing risk communications about potential safety and effectiveness concerns, and
- working closely with international partners to monitor the safety and effectiveness of COVID-19 treatments in the real world.
Our work on disinfectants and hand sanitizers included:
- monitoring the safety of disinfectants and hand sanitizers on the market in Canada,
- monitoring retailers and advertisements that were making false, misleading and illegal claims related to COVID-19, and
- publishing risk communications on safety concerns associated with disinfectants and hand sanitizers.
Focus on…
Monitoring COVID-19-related drugs and vaccines
On December 9, 2020 – the day that Health Canada authorized the Pfizer BioNTech COVID-19 Vaccine – Health Canada learned of two reports of anaphylactoid reactions that had occurred following immunization on the first day of the United Kingdom's mass immunization campaign. Through our existing relationships with our partner regulators, we were able to gather additional information about these events, as well as the perspectives of other regulators who were also reviewing the vaccine in their jurisdictions. In addition, we reached out to stakeholders with expertise in allergies and immunology for advice in responding to this emerging information. On December 12, 2020, prior to the initiation of the mass immunization campaign in Canada, we issued an advisory. The advisory warned Canadians with allergies to any of the ingredients in the vaccine not to receive it, and recommended those with any serious allergies to speak with a health professional before receiving the vaccine. Health care professionals were also advised to follow guidance and recommendations for identifying and managing serious allergic reactions following immunization. This was aligned with advice from the Canadian Society for Allergists, the National Advisory Council on Immunization and Clinical Immunologists and Food Allergy Canada, among others.
Sophie Sommerer
Senior Executive Director,
Marketed Health Products
Drug shortages
Early in the pandemic, it was clear that COVID-19 was having an impact on the supply of critical health products in Canada. We worked with our partners within Health Canada to introduce regulatory measures that provided more tools to urgently address drug shortages related to COVID-19. We also worked with provinces and territories, companies and manufacturers, health care providers and patient groups to strengthen the drug supply chain. These collaborations helped us to identify, prevent and ease shortages for Canadians.
For more information, go to Addressing critical product shortages.
Communications and collaborations
Throughout the pandemic, Health Canada published a number of web pages to provide information to Canadians and regulatory partners. Our web pages provide information about the various health products we regulate, including clinical trials authorized, applications for drugs that have been submitted and approved, disinfectants and hand sanitizers that have been authorized, and more.
We also published web pages that provided critical information to the health product industry about our regulation of health products for COVID-19. We developed guidance to support industry in developing health products to help in the crisis.
Health Canada also published a number of risk communications to Canadians to provide important information about drugs for human use, disinfectants and hand sanitizers.
We worked closely with our domestic partners including provincial and territorial governments, health technology assessment organizations, health care providers and patient groups. We sought their perspectives on the priorities and challenges they were facing within the health care system as a result of the pandemic. Through webinars, we were able to share information with health care practitioners about important topics such as the regulatory approval of treatments for COVID-19.
We collaborated with regulators from other countries on issues related to clinical trials, drug review, risk assessments and potential drug shortages. This sharing of information and expertise helped ensure that health products are safe and effective, and are available quickly to Canadians. This is taking place as part of the European Medicines Agency's OPEN project and through the Access Consortium. We also worked closely with the International Coalition for Medicines Regulatory Authorities, the World Health Organization and the Pan American Health Organization's COVID-19 task group. These partnerships have helped us to align our approach with the global response to COVID-19.
Focus on…
The COVID-19 vaccines and treatments portal
Health Canada is committed to providing Canadians, health practitioners and researchers with timely and trusted information related to vaccines and treatments for COVID-19. To that end, we created the COVID-19 vaccines and treatments portal. The portal is an easy-to-navigate, mobile-friendly interface that provides information about each product in one place. It contains product information for consumers including what the product is used for, precautions and adverse events so that consumers can make informed decisions. For health practitioners and researchers, the portal provides the rationale for Health Canada's decision to approve the product, a copy of the Canadian label, guidance on how to administer the product, clinical information and how we are continuing to monitor its safety and effectiveness.
"Our objective was to provide a user-friendly, easily-navigated interface so that consumers, health practitioners and researchers can find timely and trusted regulatory information and have confidence in the products authorized for the prevention and treatment of COVID-19."
Shannon Laforce
Executive Director,
Resource Management and Operations
Focus on…
The COVID-19 health products industry website
At the start of the pandemic, Health Canada rapidly established a centralized website to inform health product stakeholders about regulatory requirements in the context of COVID-19. The website communicates priority information, guidance and advice to stakeholders on COVID-19-related health products, including disinfectants and hand sanitizers, as well as drugs and vaccines.
It includes easy-to-access information on the new temporary measures put in place to expedite review of these products. In this way it supports traditional stakeholders as well as those new to the sector who require additional guidance.
The website has been maintained and updated throughout the pandemic, receiving close to 10 million visits between its launch in March 2020 and the end of December. With an average of 230,000 visits per week it remains a useful resource for stakeholders.
"In March 2020 our teams were facing an incredible volume of COVID-19-related regulatory questions from stakeholders. Recognizing the urgent need to communicate with new and regular stakeholders, we established a website that would serve as a central hub of key information and help manage the volume of inquiries."
Elizabeth Toller
Executive Director,
Policy, Planning and International Affairs
We began 2021 in the midst of the second wave of the pandemic. We will continue this critical work to ensure Canadians continue to have access to COVID-19-related health products.
Regulatory innovation
The current pace of innovation is unprecedented. This has led to new health products that are increasingly complex and personalized. We need new regulatory approaches to better support access to these health technologies, while continuing to protect patient safety.
Health Canada continued to prioritize regulatory innovation in 2020. The COVID-19 pandemic affected the timelines of this work. However, it also provided an opportunity to test certain temporary agile measures. We will be using the lessons learned from our regulatory response to COVID-19 to inform our regulatory innovation work, including:
- modernizing clinical trial regulations to create an environment that supports more innovative trials,
- enabling access to advanced therapeutic products that do not fit our current system,
- agile licensing for drugs, using flexible tools to oversee products across their life-cycle, and
- updating how we communicate to Canadians about health product information.
For more information, go to Regulatory innovation for health products.
Building international partnerships
For many years, Health Canada has worked closely with regulators around the world on issues related to drugs. This cooperation continued in 2020, both on COVID-19-related files (see "Drugs for human use: 2020 accomplishments – Responding to the COVID-19 pandemic") and in our day-to-day work.
For example, we have built on the collaborative review initiatives between Health Canada and other regulators. These include the Access Consortium and Project Orbis. Through the Access Consortium, we work with partners to review new drugs together, to get them to market quickly and efficiently. Project Orbis is an initiative of the United States Food and Drug Administration Oncology Center of Excellence. Project Orbis brings together regulators from multiple countries to review cancer drugs at the same time so that patients can receive earlier access to needed treatments.
Through these key work-sharing initiatives, we authorized a number of new drugs and new uses for already-approved drugs in 2020. Two examples of new drugs include Nubeqa for the treatment of prostate cancer, and Xofluza for the treatment of influenza.
By sharing information and expertise with other regulators, we have also optimized our understanding of how drugs are being used after they are available for sale. This information is called "real world evidence" and is used by Health Canada and other regulators to make regulatory decisions about drugs.
We updated our agreements with various regulators including the European Union's European Medicines Agency, the United Kingdom's Medicines and Healthcare products Regulatory Agency and the Health Science Authority of Singapore. These agreements allow regulators to share information about drug submissions, and helped us further align our approaches with our global partners.
Focus on…
Collaborating internationally on product safety
In the summer of 2018 several medications were recalled in Canada and elsewhere in the world, due to a nitrosamine impurity. Long-term exposure to nitrosamine impurities at a level above what is considered safe may increase the risk of cancer. To better understand this global issue, we are collaborating and sharing information with other major regulators around the globe. Health Canada is also the chair of two international working groups on nitrosamines. The groups meet regularly to foster global alignment on new developments, technical and regulatory positions, and on risk management for nitrosamines. This work has been critical to providing unified expectations and guidelines for industry and minimizing the health risk to consumers.
Mandatory reporting of serious adverse drug reaction by hospitals
In 2020 Health Canada supported the implementation of new regulations for mandatory reporting by hospitals that came into force in December 2019. These regulations are expected to increase the quantity and improve the quality of reports of serious adverse drug reactions. Hospitals are now required to report to Health Canada all serious adverse drug reactions 30 days from being documented within the hospital.
Health Canada developed tools to support hospitals including a guidance document, education modules and promotional materials.
The number of adverse drug reaction reports submitted to Health Canada by hospitals for drugs increased by 336 percent in 2020. After the implementation of mandatory reporting, several data elements in the report form became mandatory to ensure that important details were provided to Health Canada. This additional information aims to expand the data used by Health Canada to monitor the safety and effectiveness of drugs for human use.
Addressing antimicrobial resistance
Antimicrobials, such as antibiotics and antifungals, are essential to modern health care. However, the widespread use of these products over past decades has resulted in increasing levels of antimicrobial resistance. Commonly used antimicrobials become less effective as the pathogens they target (bacteria, viruses, fungi and parasites) become resistant to them.
Antimicrobial resistance is a global challenge and a growing threat to public health, the health care system, economic prosperity and health security. When there are fewer effective antimicrobials available, it will be harder to protect Canadians from common infectious diseases. The COVID-19 pandemic provides a clear example of the challenges created by infections that are difficult to treat.
Health Canada continues to take important steps to encourage the development of new and innovative therapeutic products, to help combat antimicrobial resistance.
In 2020 we launched a consultation on updates to the Pathogens of Interest List, inviting feedback from a wide range of stakeholders. This list is an important tool that lets companies know which pathogens are in most urgent need of additional therapeutic options. The list is having an impact; this year we approved Xenleta for the treatment of community-acquired pneumonia. Xenleta was granted priority review status because it targets difficult-to-treat pathogens on our Pathogens of Interest List.
Also in 2020, Health Canada provided funding to four companies that were successful in the first phase of the Innovative Solutions Canada challenge that was launched in 2019. This funding supports the development of novel tools so that health care providers can detect or diagnose antibiotic-resistant bacteria in humans or animals. These tools will aid in decision making about possible treatments.
We continue to work closely with regulators from other countries, including through the International Coalition of Medicines Regulatory Authorities. This Coalition has called on global health leaders to develop incentives for research and development, the pharmaceutical industry to continue to invest in research and development, and the media to keep the issue of antimicrobial resistance on the front page.
To learn about antimicrobial resistance related to animals and veterinary drugs, go to "Drugs for veterinary use: 2020 accomplishments".
Focus on…
World antimicrobial awareness week, November 18-24, 2020
Every year Health Canada joins the World Health Organization, international health agencies and other national authorities to support World Antimicrobial Awareness Week. This global initiative aims to raise awareness about antimicrobial resistance and to encourage best practices to avoid the spread of drug-resistant infections in humans and animals. In 2020 we welcomed a panel of international speakers to discuss the challenges associated with antimicrobial research, development and access to therapies.
Drugs for use in children
Children are different from adults, in their physiology and their anatomy. Therefore, they can respond differently to health products. Medicines need to be studied in pediatric populations to ensure that they are safe and effective. However, these studies are complex and challenging. Currently, up to 80% of drugs prescribed to children in Canada are considered "off-label", which means outside of the approved use of the drug.
To address these challenges, in 2020 Health Canada established the Pediatric Drug Action Plan. The goal of the plan is to improve children's access to safe and effective health products.
Early work on the plan has begun, and activities include:
- Collaborating with regulators around the world, including the European Medicines Agency, the United States Food and Drug Administration, and the World Health Organization. These collaborations help to strengthen our pediatric expertise and align our activities.
- Working with the Goodman Pediatric Formulation Centre of the Centre hospitalier universitaire Sainte-Justine to improve access to pediatric formulations in Canada.
- Analyzing ways to encourage companies to test health products in pediatric populations, when appropriate, and submit pediatric data to Health Canada.
- Developing a Canadian pediatric data strategy to analyse the availability of health products for use in children, trends in pediatric trials and drug safety issues associated with pediatrics.
Non-prescription drugs
Health Canada is responsible for the review of non-prescription drugs, also known as "over-the-counter" products, to ensure that they are safe, effective and of high quality. These products include, but are not limited to:
- antiseptics,
- pain relievers,
- cold and cough medicines,
- sunscreens, and
- hard surface disinfectants.
The nature of our review of non-prescription drugs depends on a number of factors. These include the ingredients, the health claims and the evidence that is needed to support the safety, efficacy and quality of the product. Health Canada issues a Drug Identification Number (DIN) to every approved non-prescription drug. Canadians should look for this number on the product label, which indicates that the drug has met our requirements.
Presently, products that contain a phytocannabinoid produced by or found in the cannabis plant can only be prescribed by a health care practitioner for medicinal purposes. We are aware that some Canadians are interested in potential therapeutic uses of cannabis for purposes such as pain relief, without the need for practitioner oversight (that is, in "over-the-counter" products). In 2019 Health Canada consulted on the potential market for such health products. We published a summary report in 2020, which provides an overview of the comments received from Canadians.
As part of this consultation, we also established a Scientific Advisory Committee on Health Products Containing Cannabis. This committee will provide us with independent scientific and clinical advice about appropriate safety, efficacy and quality considerations for health products containing cannabis. Health Canada will use this information to inform its regulatory path forward on these products.
Focus on…
Our science
In 2020 our research scientists teamed up to evaluate the effects of excipients on the structure and dynamics of active ingredients in recombinant protein biologics. In biological drugs, the excipients are substances that protect or enhance stability and/or bioavailability of the active pharmaceutical ingredients (the substance(s) responsible for the pharmaceutical activity or prevention of disease). The study was titled "Effects of Excipients on the Structure and Dynamics of Filgrastim Monitored by Thermal Unfolding Studies by CD and NMR Spectroscopy". This works contributes to the development and regulation of formulations for biologics.
Dr. Yves Aubin
Research Scientist,
Biologic and Radiopharmaceutical Drugs
Grant Frahm
Research Chemist,
Biologic and Radiopharmaceutical Drugs
Dr. Houman Ghasriani
Research Chemist,
Biologic and Radiopharmaceutical Drugs
Dr. Michael Johnston
Research Scientist,
Biologic and Radiopharmaceutical Drugs
Healthy clicks: Drugs for human use at glance
To stay informed about our activities:
- Follow us on Facebook
- Follow us on Twitter
- Follow us on YouTube
- See the latest news from Health Canada on our website
- Find other Health-related information on the Government of Canada website
You can also find specific information about drugs by following the links below.
Drugs and vaccines for use in COVID-19
The COVID-19 vaccines and treatments portal provides information for consumers, health care professionals and researchers on vaccines and treatments authorized for COVID-19, as well as those currently under review.
COVID-19 vaccines and treatments portal
The List of authorized drugs shows the drugs that are currently authorized for use in relation to the COVID-19 pandemic.
Drug and vaccine authorizations for COVID-19: List of authorized drugs and expanded indications
The List of applications received shows the drugs that are currently being reviewed for use in relation to the COVID-19 pandemic.
Drug and vaccine authorizations for COVID-19: List of applications received
Health Canada publishes up-to-date information about the use of disinfectants, hand sanitizers, cleaners and soaps in relation to COVID-19.
COVID-19 Disinfectants, sanitizers, cleaners and soaps
The List of authorized clinical trials shows the COVID-19 drugs, including vaccines, which are currently being investigated in clinical trials authorized by Health Canada.
Drugs and vaccines for COVID-19: List of authorized clinical trials
New drugs approved
The Drug and Health Product Register provides information for consumers about drugs that are currently marketed in Canada.
Drug and Health Product Register
The Drug Product Database is a listing of all drugs approved for sale in Canada. In the database, many drugs are accompanied by their Product Monographs, which describe the conditions of use of the product.
Search for data about the tests and trials that were performed on drugs to evaluate their safety and efficacy.
Clinical information on drugs and health products
The Submissions Under Review Lists show the drugs that are currently being reviewed by Health Canada.
Submissions Under Review Lists
The Generic Submissions Under Review List shows the generic drugs that are currently being reviewed by Health Canada.
Generic Submissions Under Review List
The Notice of Compliance database lists the approvals (Notices of Compliance or NOCs) issued for new drugs.
Regulatory Decision Summaries include the purpose of a drug submission and the reasons for Health Canada's decision to approve or reject it.
Summary Basis of Decision documents give the detailed regulatory, safety, effectiveness and quality considerations that factored into Health Canada's decision to approve certain drug submissions.
Drug shortages
The Drug Shortages in Canada website gives information on actual and anticipated drug shortages as well as discontinuations.
Clinical trails for drugs
The Clinical Trials Database provides a listing of specific information relating to phase I, II and II clinical trials, conducted in patients, which have been approved for drugs in Canada.
Surveillance of drugs
Report an adverse drug reaction
You can report adverse drug reactions to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
The Recalls and Safety Alerts Database includes the recalls, advisories, safety alerts and other publications issued by Health Canada.
Recalls and Safety Alerts Database
Summary Safety Reviews summarize our completed reviews of potential safety issues for drugs.
New Safety and Effectiveness Reviews are tables listing reviews that are currently ongoing in Health Canada.
Health Product InfoWatch is a monthly publication intended primarily for health care professionals. The Health Product InfoWatch provides clinically relevant information about health products and their safety.
The summary tables of advertising complaints list the complaints about health product advertising that have been filed with Health Canada, and the action we have taken.
Health Product Advertising Complaints
The Canada Vigilance Adverse Reaction Online Database includes information about suspected adverse reactions to health products. These reports have been submitted by consumers and health professionals as well as drug manufacturers and distributors.
Drugs for human use: Life-cycle
As part of Health Canada's mission to help Canadians maintain and improve their health, we evaluate drugs before they reach the Canadian market and continue to monitor real world evidence while they are on the market. Health Canada is involved throughout the life-cycle of a drug for human use, from clinical trials to after the drug is being sold in Canada.
Clinical trials
Clinical trials are conducted by sponsors (manufacturers or researchers) to gather information on a drug's safety and effectiveness in humans. Sponsors of clinical trials submit their applications to conduct a clinical trial with a drug in Canada. Health Canada reviews these applications before the trial is conducted in Canada.
Special access program
Drugs that are not approved in Canada may be available through our Special Access Program. In this program, access is given to an individual health care practitioner who is treating a specific patient. Access may be granted for emergency use, or to patients with serious or life-threatening conditions when conventional therapies have failed, are unavailable or are unsuitable.
Drug submission and review
When a company decides that it would like to market a drug in Canada, it files a submission with Health Canada. A new drug submission contains detailed scientific information about the drug's safety, quality and efficacy.
Our scientists and medical officers perform a thorough review of the information submitted. Sometimes we also consult with advisory committees or external consultants. Reviewers evaluate the safety, efficacy and quality data to assess the benefits and potential risks of the drug. They also review the information that will be provided to health care practitioners and consumers about the drug.
Expedited review pathways
We have several review processes that can provide an expedited path for certain drugs, including those that target specific health care needs. That is, there are several review pathways that have shorter review targets for certain drugs. Products approved through expedited review pathways can be available to patients sooner.
Priority review: Drugs for serious, life-threatening, or severely debilitating diseases or conditions can be given a priority review status. Drug submissions that are granted priority review status are subject to an expedited review process.
Notice of Compliance with conditions: When a new drug is approved it is issued a Notice of Compliance (NOC). A Notice of Compliance may be issued with conditions (NOC/c) to a drug that showed promising clinical benefit, for serious, life-threatening, or severely debilitating diseases or conditions. The manufacturer must still demonstrate that the drug has an acceptable safety profile and is of high quality, and also commits to undertake additional studies to verify the clinical benefit of the drug. Submissions that are reviewed under this pathway are subject to an expedited review process.
Approval of drugs
After its review of a drug submission, Health Canada may conclude that the benefits of the product outweigh the potential risks and approve the drug for sale in Canada. When a new drug is approved, it is issued a Notice of Compliance (NOC) and a Drug Identification Number (DIN). This does not mean the drug will immediately be available to patients, as many other factors can influence that timeline.
Surveillance
It is not possible to know or predict all of the possible adverse reactions to a drug through clinical studies. After a product is approved and available for sale in Canada, we continue to monitor its use in the real world, that is, in the broader Canadian population that may be taking other medications. We evaluate potential safety and effectiveness issues, and take action when there are identified problems.
Collecting information
Health Canada collects safety information about a product after it is approved from a variety of sources. One source of information is suspected adverse reactions that are reported after products are approved for sale. Adverse reactions are undesirable effects potentially caused by drugs.
You can report adverse drug reactions to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
Another source of information are documents called Risk Management Plans that are submitted by manufacturers as part of their drug submissions. A Risk Management Plan includes information on a drug's safety profile and how its risks will be prevented or minimized. It also contains plans for studies and other activities to learn more about the safety and effectiveness of the drug.
Evaluating safety signals
Health Canada evaluates the data we collect to detect new safety signals, which we then investigate more closely. A "safety signal" can be defined as information on a new or known adverse event that may be associated with a drug. These investigations are called signal assessments and they may result in recommendations for actions to be taken by the company, by Health Canada, or both. These actions can include informing the public and health care professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a drug from the market.
Advertising complaints
Health Canada also regulates the advertising of drugs in Canada to ensure that companies are not making false claims about their products. We review advertising complaints to determine if a company is complying with our requirements, and we take appropriate action when non-compliance is identified. This may include requesting a company to stop disseminating non-compliant advertising and taking steps towards avoiding any future issues.
Footnotes
- Footnote 1
-
New biosimilar