Drug and medical device highlights 2020: Drugs for veterinary use
On this page
- Drugs for veterinary use: 2020 in brief
- Drugs for veterinary use: What's new in 2020
- Drugs for veterinary use: 2020 accomplishments
- Healthy clicks: Drugs for veterinary use at a glance
- Drugs for veterinary use: Life-cycle
Drugs for veterinary use: 2020 in brief
One of Health Canada's roles is to regulate drugs for veterinary use, which play an important role in protecting human and animal health. We evaluate and monitor the safety, quality and effectiveness of veterinary drugs. In doing so, we work to protect animals and Canada's food supply.
The COVID-19 pandemic created an urgent need for access to safe, effective and high quality health products, including veterinary drugs should the specific need to treat animals arise. Although COVID-19 is understood to be primarily a new human disease, its impacts on animal health may not be fully known at this time.
Response to the COVID-19 pandemic
As part of the government's response to the pandemic, we introduced innovative and agile regulatory measures, including for veterinary drugs. These measures expedited the regulatory review of COVID-19 health products, without compromising safety, efficacy and quality standards.
In 2020 we modernized the regulations for the Emergency Drug Release program to facilitate emergency access to unapproved drugs for veterinary use.
For more information about our response to the COVID-19 pandemic, go to "Drugs for veterinary use: 2020 accomplishments".
New health products approved
In 2020 we approved nine new drugs for companion or food-producing animals. This enabled access to innovative new products and therapies to help maintain and improve the health of animals.
We also approved 11 new generic drugs to provide additional options for more cost-effective prevention and treatment.
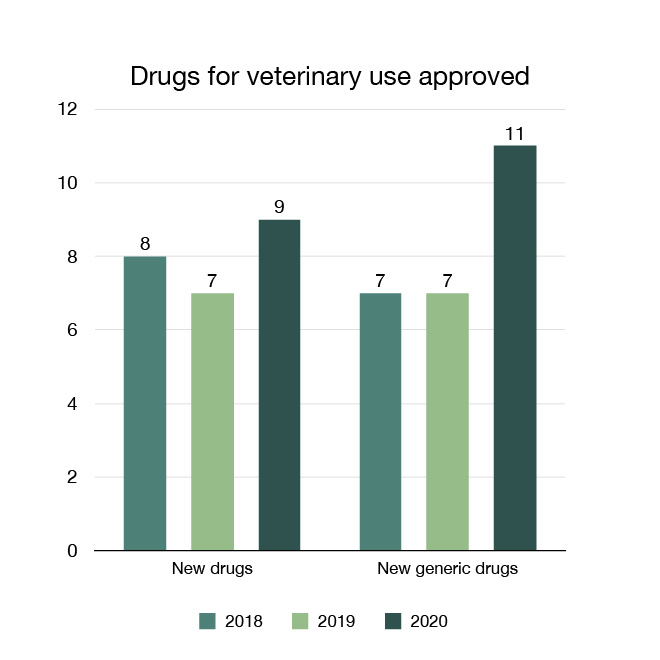
Figure 12: Drugs for veterinary use approved: Text description
| Year | New drugs approved | New generic drugs approved |
|---|---|---|
| 2018 | 8 | 7 |
| 2019 | 7 | 7 |
| 2020 | 9 | 11 |
For a list and description of the new drugs we approved in 2020, go to "Approved in 2020: Drugs for veterinary use".
This year, 761 veterinary health products were notified within our veterinary health product program. These offer more low-risk options to maintain or promote health and wellness in animals.
Clinical trials and Emergency Drug Release program
We review applications to allow companies and researchers to conduct studies on veterinary drugs in Canada. New veterinary drug trials (called investigational new drugs and experimental studies) mean Canadians may have access to new products in the future. In 2020, 94 Experimental Studies Certificates that support clinical trials or research activities were authorized.
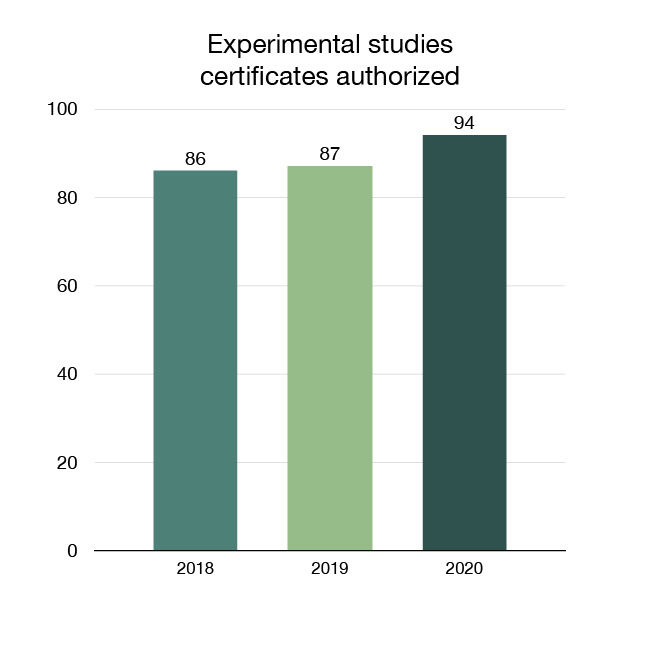
Figure 13: Experimental studies certificates authorized: Text description
| Year | Experimental studies certificates authorized |
|---|---|
| 2018 | 86 |
| 2019 | 87 |
| 2020 | 94 |
Through our Emergency Drug Release program, veterinarians can request authorization for drugs for veterinary use that are not available in Canada for emergency situations. In 2020, 310 requests under the Emergency Drug Release program were authorized.
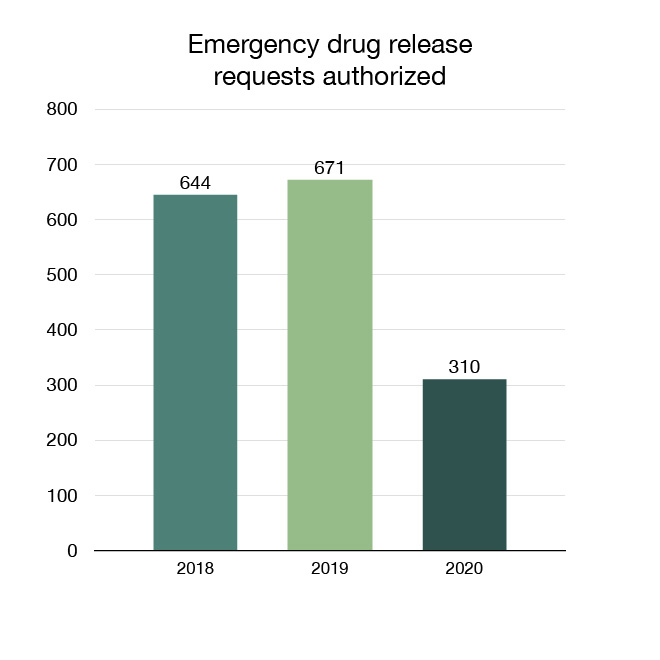
Figure 14: Emergency drug release requests authorized: Text description
| Year | Emergency drug release requests authorized |
|---|---|
| 2018 | 644 |
| 2019 | 671 |
| 2020 | 310 |
Surveillance

Director General,
Veterinary Drugs
After we approve a drug for sale in Canada, we continue to monitor and evaluate reports of suspected adverse veterinary drug reactions.
This "Drugs for Veterinary Use" chapter gives you more information about our work in 2020. For up-to-date information about our activities see the "Healthy clicks: Drugs for veterinary use at a glance" section, and follow us on social media.
Drugs for veterinary use: What's new in 2020
In 2020 Health Canada approved nine new drugs for veterinary use. More detail is available in the section "Approved in 2020: Drugs for veterinary use".
New drugs for veterinary use
- Amodip Flavoured Tablets
- Aservo Equihaler
- Bravecto One
- Comfortan
- Cosacthen
- Eradia
- Gonabreed
- Mirataz
- Zeleris
Drugs for veterinary use: 2020 accomplishments
Responding to the COVID-19 pandemic
COVID-19 is understood to be primarily a human disease and the virus is believed to spread mainly from person-to-person. However, COVID-19 is a new disease and its impacts on animal health may not be fully known at this time.
Emergency Drug Release program
Drugs that are not approved in Canada may be accessible through Health Canada's Emergency Drug Release (EDR) program. Veterinarians may request access to unapproved veterinary drugs to treat patients (an animal or group of animals) with serious or life-threatening conditions. Access to these drugs is only considered when conventional therapies have failed, are unsuitable or unavailable.
In 2020 we modernized the regulations for the Emergency Drug Release program to facilitate emergency access to unapproved drugs for veterinary use.
For more information, go to Health Canada's special access programs: Request a veterinary drug through EDR.
Focus on…
Emergency Drug Release program
"Reviewing applications received through the Emergency Drug Release program puts us in direct contact with veterinarians in clinical practice. We are fortunate to have updated regulations in place to help ensure that life-saving and welfare-improving drugs are made available to animals, especially when veterinarians are faced with treating emerging diseases in their patients. It is a rewarding experience to be part of the multifaceted collaborative effort to support animal health and welfare."
Shaunna Lucas
Information Officer,
Veterinary Drugs
Annie Tourangeau
Veterinary Information Analyst,
Veterinary Drugs
Julie Burke
Veterinary Information Analyst,
Veterinary Drugs
Drugs for veterinary use
As part of the government's response to the pandemic, Health Canada introduced innovative and agile regulatory measures. This will allow us to expedite the regulatory review of COVID-19 veterinary drugs, without compromising our high standards for safety, efficacy and quality if a manufacturer files an application in the future.
For more information on the Interim Orders that were published to address the COVID-19 pandemic, go to "Message from the Chief Regulatory Officer".
Drug shortages
Early in the pandemic, it was clear that COVID-19 was having an impact on the supply of critical health products in Canada. We worked with companies and manufacturers, as well as other regulators globally, to identify and respond to potential shortages. These helped us to identify, prevent and ease shortages for Canadians.
For more information, go to Addressing critical product shortages.
Communications and collaborations
Throughout the pandemic, Health Canada published a number of web pages to provide information to Canadians, regulatory partners and the health product industry about our response to the COVID-19 pandemic. Our websites provide information about the various health products we regulate, including applications for drugs that have been submitted and approved.
We worked closely with our domestic partners including manufacturers, practitioners and food producers. We sought their perspectives on the priorities and challenges they were facing as a result of the pandemic.
Addressing antimicrobial resistance related to animals
Antimicrobial resistance is a growing public health threat in Canada and worldwide. The overuse and misuses of antimicrobial drugs allow illness-causing germs like bacteria and fungi to evolve and become resistant to antimicrobials.
Antimicrobial use in animals can contribute to the development and spread of resistant bacteria in humans. A "One Health" approach acknowledges the interconnection between the health of humans, animals and their shared environment, and the need for collaborative efforts across sectors to improve health for all.
In 2020 Health Canada continued to focus on our next set of veterinary drug antimicrobial resistance initiatives on keeping animals healthy to reduce the use of antimicrobials, and to promote their responsible use when they are needed. This included:
- Publishing the post-market re-evaluation framework for antimicrobials important to human medicine to strengthen their responsible use in animals.
- Completing the analysis of the first year (2018) of veterinary antimicrobial sales data collected under the Veterinary Antimicrobial Sales Reporting program to support surveillance efforts. Analysis is underway for the data collected in the second year (2019) of the program.
- Launching a pilot project that expands access to veterinary health products that could be used in livestock feed, in partnership with the Canadian Food Inspection Agency. This project aims to facilitate increased access to general health and wellness products to improve animal health and reduce the reliance on conventional drugs, including antimicrobials.
These initiatives are in line with the Federal Action Plan on Antimicrobial Resistance and Use in Canada and the Pan-Canadian Framework for Action on Tackling Antimicrobial Resistance and Antimicrobial Use.
To learn about antimicrobial resistance related to humans, go to "Drugs for human use: 2020 accomplishments".
Focus on…
World Antimicrobial Awareness Week, November 18-24, 2020
Every year Health Canada joins the World Health Organization, international health agencies and other national authorities to support World Antimicrobial Awareness Week. This global initiative aims to raise awareness about antimicrobial resistance in animals and humans and to encourage best practices to avoid the spread of drug-resistant infections. In 2020 we welcomed a panel of international speakers to discuss the challenges associated with antimicrobial research, development and access to therapies.
Regulatory innovation
The current pace of innovation is unprecedented. This has led to new health products that are increasingly complex. We need new regulatory approaches to better support access to these health technologies, while continuing to protect patient safety.
Health Canada continued to prioritize regulatory innovation in 2020. The COVID-19 pandemic affected the timelines of this work. However, it also provided an opportunity to test certain temporary agile measures (for more information, go to "Message from the Chief Regulatory Officer"). We will be using the lessons learned from our regulatory response to COVID-19 to inform our regulatory innovation work, including these related to drugs for veterinary use:
- modernizing regulations for investigational studies, including experimental studies certificates,
- enabling access to advanced therapeutic products that do not fit our current system, and
- agile licensing for drugs, using flexible tools to oversee products across their life-cycle.
For more information, go to "Regulatory innovation for health products".
Collaborating internationally
For many years, Health Canada has worked closely with regulators around the world on issues related to drugs for veterinary use. This cooperation progressed significantly in 2020.
We continued our simultaneous reviews of veterinary drugs in partnership with the United States Food and Drug Administration's Center for Veterinary Drugs. This partnership offers manufacturers access to two major markets, expands the access to treatment options for animals in Canada, and helps Canadian food producers stay competitive globally.
In 2020 we published the Guidance on Veterinary Drug Joint Reviews with Australia and New Zealand, which outlines the joint review process for veterinary drugs. Also in 2020, we launched a process for simultaneous reviews with the United Kingdom. As of December 31, 2020, we had 1 joint review ongoing with Australia/New Zealand, and 10 simultaneous reviews ongoing with the United States.
Providing more choices to improve animal health and wellness
By keeping animals healthy, we can reduce the need to use drugs, including antimicrobials. Veterinary health products are low-risk products that can help maintain or promote the health and wellness of animals. They include ingredients such as vitamins, minerals and traditional medicines.
Presently, products that contain a phytocannabinoid produced by or found in the cannabis plant can only be prescribed by a health care practitioner for medicinal purposes. Health Canada is aware that some Canadians are interested in potential therapeutic uses of cannabis for purposes such as pain relief for animal use, without the need for practitioner oversight. In 2019 Health Canada consulted on the potential market for such health products. We published a summary report in 2020, which provides an overview of the comments received from Canadians.
As part of this consultation, we also established a Scientific Advisory Committee on Health Products Containing Cannabis, which includes a subcommittee to address issues related to health products containing cannabis for use in animals. This committee will provide us with independent scientific and clinical advice about appropriate safety, efficacy and quality considerations for health products containing cannabis, including for veterinary use. Health Canada will use this information to inform its regulatory path forward on these products.
Focus on…
Veterinary health products
Health Canada has in place a flexible and risk-appropriate framework for low risk health products for both food-producing and companion animals. This framework facilitates access to these veterinary health products. There are now over 2300 such products notified for sale in Canada. As the Notification Program for veterinary health products grows in popularity with Canadians and stakeholders, we continue to expand the type of ingredients and products included in the program. We do so without compromising the safety of animals or humans, including food safety. Our program also provides extensive and searchable information to Canadians.
"Our program provides information to Canadians about veterinary health products – the web-based application is user-friendly and information is easy to find and search."
Femma Van As
Drug Evaluator,
Veterinary Drugs
Consulting on changes to our regulatory approach
Many veterinary drugs are intended for use in food-producing animals such as cattle, chickens and pigs. Health Canada works to ensure the safety of food that comes from animals treated with veterinary drugs. We do this by setting standards such as Maximum Residue Limits for veterinary drugs in foods, and establishing withdrawal periods. In 2020 we consulted on a proposal to amend the list of maximum residue limits for veterinary drugs in foods.
We also consulted in 2020 on a proposed regulatory classification of products that contain acid based products intended for livestock species. Historically, these products may have been classified as either a veterinary drug, veterinary health product or livestock feed. This proposal will provide clarification about how these important products should be classified and subsequently regulated.
Healthy clicks: Drugs for veterinary use at a glance
To stay informed about our activities:
- Follow us on Facebook
- Follow us on Twitter
- Follow us on YouTube
- See the latest news from Health Canada on our website
- Find other Health-related information on the Government of Canada website
You can also find specific information about veterinary drugs by following the links below.
New drugs approved
The Drug Product Database is a listing of all drugs approved for sale in Canada. In the database, many drugs are accompanied by their veterinary labelling, which describe the conditions of use of the product.
The Notice of Compliance database lists the approvals (Notices of Compliance or NOCs) issued for new drugs.
Surveillance of veterinary drugs
Report a veterinary drug reaction.
Drugs for veterinary use: Life-cycle
Every year, we approve new veterinary drugs in Canada, giving more choices to help maintain and improve the health and well-being of animals. We work to protect human and animal health and the safety of Canada's food supply.
As part of this work, we evaluate veterinary drugs throughout the life-cycle, from research/studies to after the drug is sold in Canada.
Clinical trials
Experimental studies certificates and investigational new drug applications support the development of potential new veterinary drugs or new uses of already approved products. Sponsors of such research/studies (including manufacturers and researchers) submit their applications for Health Canada review before the trial is conducted in Canada.
Emergency drug release program
Drugs that are not approved in Canada may be available through our Emergency Drug Release program. Veterinarians may request access to veterinary drugs to treat patients (an animal or group of animals) with serious or life-threatening conditions. Access to these drugs is only considered when conventional therapies have failed, are unsuitable or are unavailable.
Drug submission and review
When a company decides that it would like to market a veterinary drug in Canada, it files a submission with Health Canada. A new drug submission contains detailed scientific information about the drug's safety, efficacy and quality.
Submissions for drugs are reviewed by our scientists to assess the potential benefits and risks to human and animal health. They also help to ensure veterinary drug labels have clear directions for use and warning statements.
Many veterinary drugs are intended for use in food-producing animals such as cattle, chickens and pigs. We work to ensure the safety of food that comes from animals treated with veterinary drugs. We do this by setting standards such as Maximum Residue Limits for veterinary drugs in foods, and establishing withdrawal periods.
Innovative reviews (international regulatory cooperation)
Through international regulatory cooperation, we conduct reviews of submissions for new veterinary drugs with certain partners. These include the United States Food and Drug Administration Center for Veterinary Medicines, the Australian Pesticides and Veterinary Medicines Authority, and the New Zealand Ministry of Primary Industries.
These collaborative reviews bring veterinary drugs to market in Canada at the same time as in other countries, which would otherwise not be possible. As a result, companion animal owners, veterinarians and producers have faster access to safe, effective and quality veterinary drugs.
Approval of drugs
After its review of a drug submission, Health Canada may conclude that the benefits of the product outweigh the potential risks and approve the veterinary drug for sale in Canada. When a new veterinary drug is approved, it is issued a Notice of Compliance (NOC) and a Drug Identification Number (DIN). This does not mean the drug will immediately be available in Canada, as many other factors can influence that timeline.
Surveillance
After Health Canada approves a veterinary drug, we continue to monitor its use in the real world. We evaluate potential safety and effectiveness issues, and take action when there are identified problems.
Collecting information
Health Canada collects information about a product after it is approved from a variety of sources.
One source of information is suspected adverse events that are reported after products are approved for sale. Adverse events are unwanted or harmful events that occur after administration of a veterinary drug. Included are:
- adverse reactions or events in animals,
- adverse reactions or events in humans who administered a veterinary drug to an animal, and
- events that result from a suspected lack of effect.
You can report a veterinary drug reaction to Health Canada.
Evaluating safety signals
Health Canada evaluates adverse event reports submitted by manufacturers and the public (including animal owners and veterinarians), to find out if they are related to the administered drug(s). We work with manufacturers and veterinarians to ensure that any important safety information is communicated.