Drug and medical device highlights 2020: Medical devices
On this page
- Medical devices: 2020 in brief
- Medical devices: What's new in 2020
- Medical devices: 2020 accomplishments
- Healthy clicks: Medical devices at a glance
- Medical devices: Life-cycle
Medical devices: 2020 in brief
One of Health Canada's roles is to regulate medical devices that can help Canadians maintain and improve their health. Medical devices are used in the treatment, diagnosis or prevention of diseases or abnormal physical conditions.
In Canada, medical devices are categorized into four groups based on the level of risk associated with their use. These groups are called "Classes" and range from I to IV. Class I devices are considered low-risk devices – for example, a wheelchair. Class IV devices present the greatest potential risk – for example, a defibrillator.
In 2020 we launched a new Medical Devices Directorate, which is responsible for regulating medical devices throughout their life-cycle. This new structure is helping us adapt to the rapid growth and change in the medical device industry, and has allowed us to grow our capacity to regulate this industry.
The COVID-19 pandemic created an urgent need for access to safe, effective and high quality medical devices. Our response to the pandemic addressed critical issues related to medical devices across product life-cycles.
Response to the COVID-19 pandemic
As part of the government's response to the pandemic, we introduced innovative and agile regulatory measures. These measures expedited the regulatory review of COVID-19 medical devices without compromising safety, effectiveness and quality standards.
In 2020 we authorized 545 COVID-19 medical devices and 18 clinical trials for medical devices related to COVID-19.
For more information about our response to the COVID-19 pandemic, go to "Medical devices: 2020 accomplishments".
New medical devices approved
In 2020 we approved 332 new medical devices in the highest risk categories (Classes III and IV). These devices provide patients and health care professionals with new and innovative options for the treatment, prevention and diagnosis of various health conditions. For example, we approved the INSTI Rapid HIV test, the first home-based self test for HIV approved in Canada.
For a list and description of the 55 new Class IV (highest-risk) medical devices we approved in 2020, go to "Approved in 2020: Medical devices".
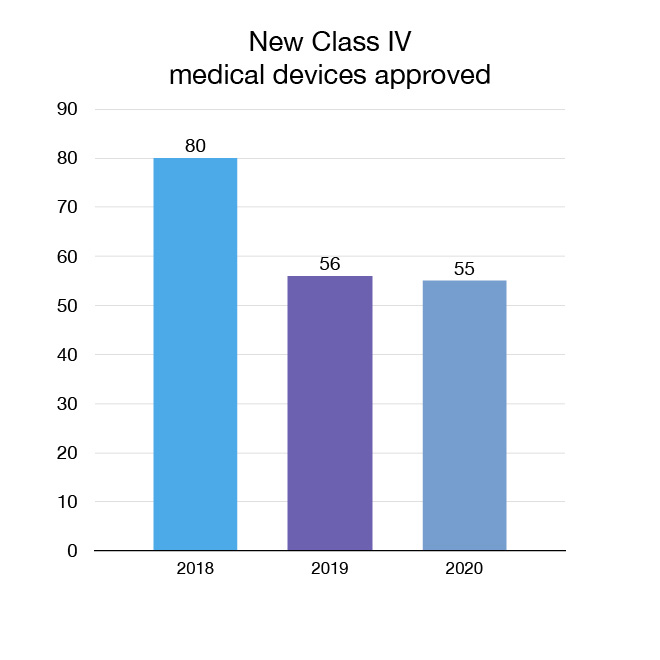
Figure 7: New Class IV medical devices approved: Text description
| Year | New Class IV medical devices approved |
|---|---|
| 2018 | 80 |
| 2019 | 56 |
| 2020 | 55 |
Investigational testing (clinical trials) and special access program
We review applications to allow companies to conduct investigational testing (clinical trials) on medical devices in Canada. New trials mean Canadians may have access to more innovative choices. In 2020, 122 new investigational testing applications for medical devices were approved.
We authorized investigational testing for leadless pacemakers as part of a global study, and a point-of-care HIV and syphilis antibody test for use in vulnerable populations and remote communities. We also authorized investigational testing for medical devices that used artificial intelligence for the early detection of COVID-19 in the lung, and for touch-free vital sign assessment.
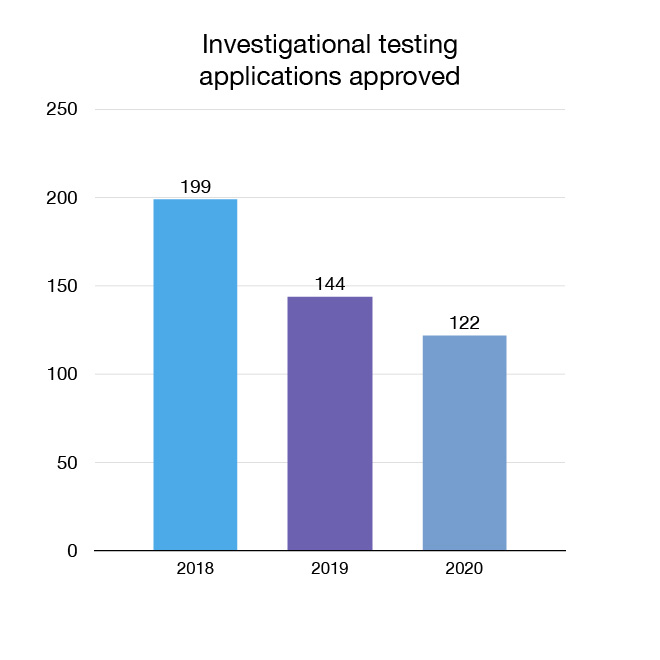
Figure 8: Investigational testing applications approved: Text description
| Year | Investigational testing applications approved |
|---|---|
| 2018 | 199 |
| 2019 | 144 |
| 2020 | 122 |
Through our Special Access Program, we grant access to health care professionals to medical devices that have not been approved for sale in Canada for emergency use or when alternatives are unsuitable or unavailable. In 2020, 2,693 requests for special access to medical devices were authorized. These included hand-held scanners to facilitate screening of COVID-19 patients and ventilators that were in short supply early in the pandemic. The program also facilitated the supply of medical devices that were in critical need after delays were created by the pandemic (for example, in transport).
In 2020 we also authorized:
- cardiovascular devices that allow for less traumatic procedures,
- devices treating complex recurrent aneurysms for compassionate use,
- devices related to organ transplant for young patients, and
- MRI-compatible infant incubators.
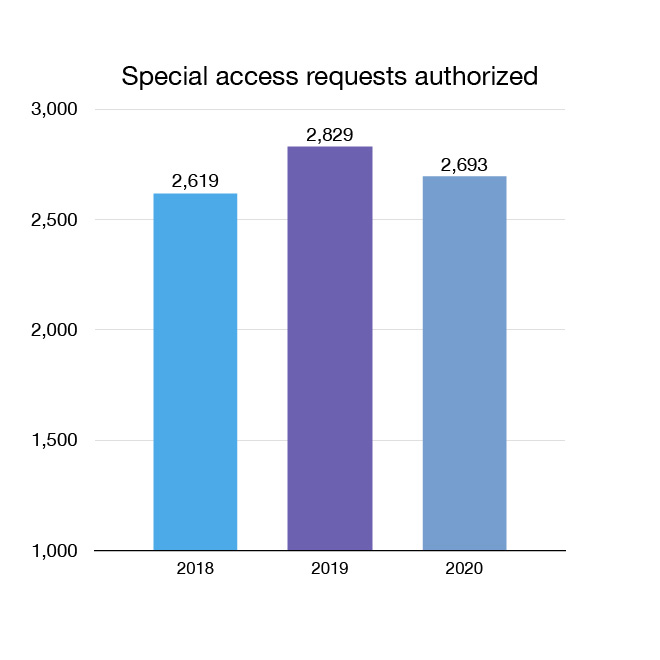
Figure 9: Special access requests authorized: Text description
| Year | Special access requests authorized |
|---|---|
| 2018 | 2,619 |
| 2019 | 2,829 |
| 2020 | 2,693 |
Surveillance
After we approve a medical device for sale in Canada, we continue to monitor and evaluate reports of suspected incidents involving that medical device.
In 2020 we received 39,304 reports of medical device incidents and undertook 2 actions related to medical devices. Incident reports come from domestic and international sources. Actions can include informing the public and health care professionals of safety information or recommending labelling changes, or affirming our current understanding. For example, following a recent review of dental amalgam, we reported that the most current data still showed no clear link between dental amalgam and harms from mercury. In cases where a serious risk is identified, we may remove a medical device from the market.
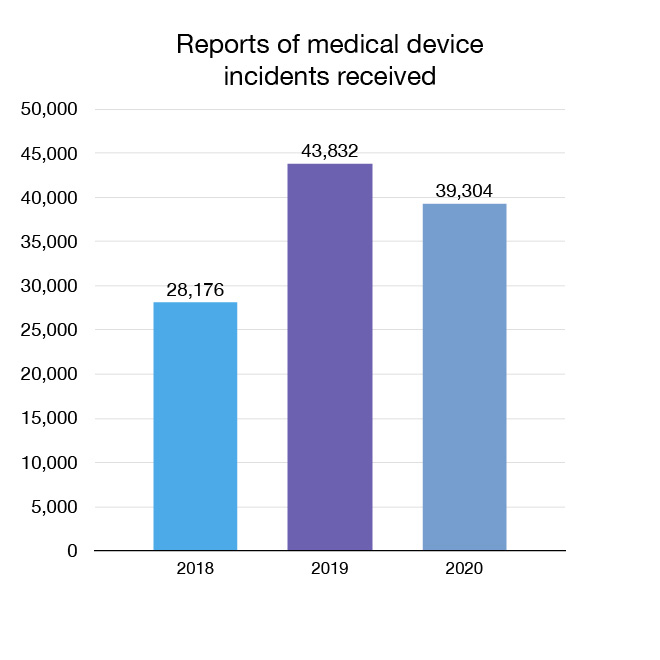
Figure 10: Reports of medical device incidents received: Text description
| Year | Reports of medical device incidents received |
|---|---|
| 2018 | 28,176 |
| 2019 | 43,832 |
| 2020 | 39,304 |
To learn how we addressed safety issues that arose for medical devices in Canada in 2020, go to "Approved in 2020: Medical devices".
Transparency of decision making
In 2020 we continued to advance our openness and transparency efforts by expanding the amount of regulatory health and safety information that is made available to Canadians. We published 387 regulatory decision summaries and 1 summary basis of decision document, which explain Health Canada's decisions for certain medical devices seeking market authorization.
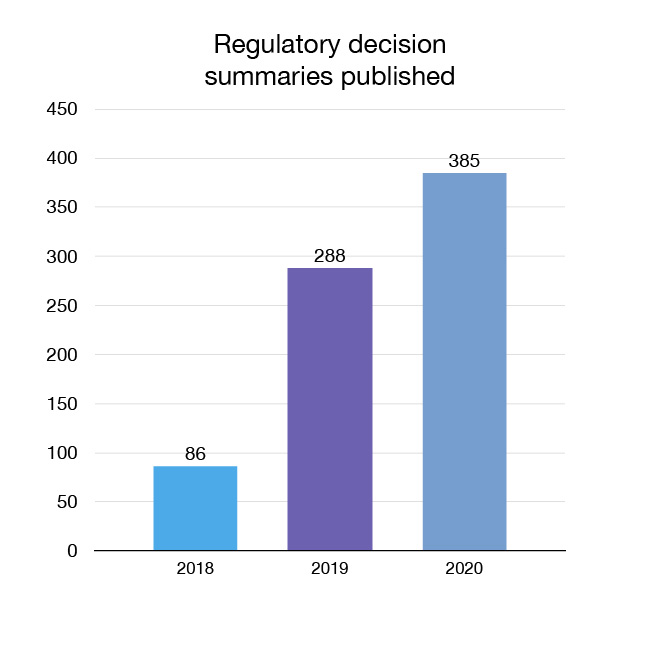
Figure 11: Regulatory decision summaries published: Text description
| Year | Regulatory decision summaries published |
|---|---|
| 2018 | 86 |
| 2019 | 288 |
| 2020 | 385 |
Through our "Clinical Information Portal", we published 1,859 pages of clinical information on 8 medical devices. This clinical information is provided by companies when they seek approval to sell a medical device in Canada, and is made publicly available on request after we decide to approve or reject the product.
Health Canada also publishes summaries of its safety reviews, which describe Health Canada's decisions related to potential safety issues. In 2020 we published one such summary for medical devices. These summaries complement other safety-related information to help Canadians make informed decisions about their medical device choices.
This "Medical Devices" chapter gives you more information about our work in 2020. For up-to-date information about our activities see the "Healthy clicks: Medical devices at a glance" section, and follow us on social media.

Director General,
Medical Devices

Director General,
Marketed Health Products
Medical devices: What's new in 2020
In 2020 we approved 55 new Class IV (highest-risk) medical devices. More detail is available in the section "Approved in 2020: Medical devices".
Body fluid and tissue management devices
- CliniMACS Prodigy T Cell Transduction (TCT) System
- Sonopet iQ Ultrasonic Aspirator System
Body tissue manipulation and reparation devices
- Chondro-Gide Bilayer Collagen Membrane
- Myriad
- Puracol Plus
- Regeneten Bioinductive Implant System
Cardiovascular devices
- Agilis HisPro Steerable Catheter With Electrodes
- Athletis Over-The-Wire PTA Balloon Dilatation Catheter
- Attain Stability Quad MRI SureScan 4798
- Cardiac Resynchronization Therapy Defibrillator (CRT-DS)
- Cobalt XT / Cobalt / Crome ICD and CRT-D MRI SureScan
- Diamondback 360 Coronary Orbital Atherectomy System
- Eluvia Over-the-Wire Drug- Eluting Vascular Stent System
- EPstar Fixed Electrophysiology Catheter
- Evolut PRO+ Transcatheter Aortic Valve
- Implantable Cardioverter Defibrillator (ICDS)
- IntellaNav ST
- IntellaNav StablePoint Ablation Catheter
- Jade PTA Balloon Dilatation Catheter
- Konect Resilia Aortic Valved Conduit
- Lotus Edge Valve System
- Micra AV MC1AVR1
- Penumbra LP Coil System
- QDot Micro Navigation Catheter
- Ranger and Ranger SL Over-the-Wire Paclitaxel-Coated PTA Balloon Catheter
- Reprocessed ViewFlex Xtra ICE Catheter
- Sapphire II NC Coronary Dilatation Catheter
- Scoreflex NC Coronary Dilatation Catheter
- Scoreflex PTA Balloon Dilatation Catheter
- Sentinel Cerebral Protection System
- Smart Touch Programming System
- Surpass Evolve Flow Diverter System
- Synergy Monorail Everolimus-Eluting Platinum Chromium Coronary Stent System
- Synergy XD Monorail Everolimus-eluting Platinum Chromium Coronary Stent System
- TriClip System
- Vega Endocardial Pacing Lead
- Wattson Temporary Pacing Guidewire
- Xience Pro A Everolimus Eluting Coronary Stent System
- Zoll X Series Advanced
Gastro-urological devices
- InterStim Micro SureScan MRI
General hospital devices
- EPstar Fixed Electrophysiology Catheter with Lumen / EPstar Electrophysiology Cable
- MiniMed 770G
- t:slim X2 Insulin Pump with Control-IQ Technology
In vitro diagnostic medical devices
- ADVIA Centaur HBC Total 2 (HBcT2) (Donor Screening for Transplantation)
- ADVIA Centaur Quantitative HBsAg (QHBs)
- Alinity s HIV Ag/Ab Combo Assay (Donor Screening and Cadaveric Testing)
- Atellica IM Quantitative HBsAg (QHBs)
- INSTI HIV Self Test
- Liaison XL Murex HCV AB
- PK CMV-PA System Control Set
- PK7400 TP HA Reagent and Controls
Neurological devices
- Percept PC
- Proclaim Implantable Pulse Generators
Plastic surgery and cosmetic devices
- Natrelle Inspira Cohesive Breast Implant
Radiological devices
- Acist HDi System
Medical devices: 2020 accomplishments
Responding to the COVID-19 pandemic
The COVID-19 pandemic has had a profound impact on the health and well-being of Canadians in 2020. It has created an unprecedented demand on Canada's health care system and has led to an urgent need for access to health products.
Health Canada's response to the pandemic addressed critical issues related to medical devices across product life-cycles, from investigational testing (clinical trials) to authorizations of medical devices and surveillance.
Investigational testing (clinical trials)
In May 2020, the Minister of Health signed the Interim Order Respecting Clinical Trials for Medical Devices and Drugs Related to COVID-19. This temporary measure facilitated a more efficient authorization process for investigational testing (clinical trials) in Canada, expanding the range of sponsors who may submit an investigational testing application. It offered greater flexibility in terms of administrative requirements, without compromising patient safety or the validity of trial results.
In addition, we expedited the authorization of trials for COVID-19. Applications were reviewed faster than usual, to speed up access without compromising patient safety. We worked with companies, academic research centres and investigators who had products in development, to provide guidance and help bring clinical trials to Canada.
In 2020 we authorized 18 clinical trials for medical devices relating to COVID-19.
For more information, go to Medical devices for COVID-19: Conducting a clinical trial.
Special Access Program
The Special Access Program is for health care professionals who are treating seriously ill patients where conventional therapies have failed, are unsuitable or are unavailable. In 2020 the pandemic created supply chain issues, resulting in shortages of needed medical devices. When these were not available in Canada, the program facilitated rapid access to medical devices that could respond to the urgent needs of health care professionals and their patients.
For more information, go to Health Canada's special access programs: Overview.
Focus on…
Access to medical devices during the pandemic
As a result of the pandemic, Canadians experienced shortages of much-needed medical devices due to manufacturing and shipping delays. Through the Special Access Program's emergency access mandate, the team worked directly with health care professionals and foreign manufacturers to obtain information on alternative devices. Early in 2020 this included wireless handheld ultrasound scanners that were used to diagnose respiratory failure in patients suspected to have COVID-19. As the pandemic progressed, ventilators and respiratory accessories for ventilators were also needed. Health Canada determined the safety and effectiveness of these new devices and worked closely with health care professionals and the manufacturers to ensure rapid access and delivery.
"Members of the medical devices Special Access Program Team include one supervisor, two administrative clerks and one student. Our team worked diligently with the health care practitioners and foreign manufacturers by phone and email throughout the work week, as well as the weekend with our 24/7 emergency phone line, to provide guidance and obtain sufficient information for our evaluation bureau."
Leo Periard
Clerk,
Medical Devices
Marina Whyte
Clerk,
Medical Devices
Peggy Seely
Supervisor,
Medical Devices
Erica Pierre-Pierre
Student,
Medical Devices
Medical devices
Medical devices play an important role in diagnosing, treating, mitigating or preventing COVID-19. Typical medical devices used in relation to COVID-19 include masks, N95 respirators, gloves, gowns, ventilators and testing devices.
As part of the government's response to the pandemic, Health Canada introduced innovative and agile regulatory measures. These allowed us to expedite the regulatory review of COVID-19 medical devices, without compromising our high standards for safety, effectiveness and quality. They also allowed certain medical devices that may not fully meet regulatory requirements to be imported and sold in Canada.
For more information on the Interim Orders that were published to address the COVID-19 pandemic, go to "Message from the Chief Regulatory Officer".
In 2020 we approved 545 medical devices for use in relation to COVID-19. For lists of authorized medical devices, go to Authorized medical devices for uses related to COVID-19: Overview.
We will closely monitor the use of these products in the real world, evaluate potential safety and effectiveness issues and take action where there are identified problems.
Focus on…
Encouraging 3D printing of medical devices
In March 2020, the demand for certain medical devices, including personal protective equipment (PPE), exceeded the available supply in Canada. Research centres, academic institutions and other industry sectors became interested in 3D printing personal protective equipment to assist with the shortage. We reached out urgently to our contacts in industry, hospitals, universities, colleges and industrial manufacturing facilities. A national network of 3D printing experts was quickly created that included over 80 organizations across Canada.
Through the network, we shared technical and regulatory information to help ensure that 3D-printed personal protective equipment were safe, effective, and of high quality. The network allowed open and transparent communication among regulators, physicians and non-traditional medical device manufacturers. Based on the information provided in this form, we published guidance for industry on 3D printing of personal protective equipment in response to COVID-19. This guidance helped ensure that safe, effective, and high quality personal protective equipment were produced for Canadian health care workers.
"It was incredible not only to see Canadians willing to help out during a time of crisis, but to see the passion and dedication from all those who stepped up to help raise the bar for the safety and quality of 3D-printed personal protective equipment for our Canadian health care workers."
Marc Lamoureux
Manager,
Medical Devices
Focus on…
N95 Respirators
Single use N95 respirators are a type of personal protective equipment (PPE) that protects health care workers from airborne particles. They are considered essential when working around airborne pathogens like SARS-CoV-2. As the COVID-19 pandemic unfolded, the shortage of N95 respirators in Canada reached a critical point where it threatened the ability of health care workers to deliver care to COVID-19 patients without risking their own health.
N95 respirators are normally disposed after a single use. To help address the shortage of N95 respirators, Health Canada collaborated extensively with stakeholders. We worked swiftly to establish the regulatory requirements for reprocessing of N95 respirators. We invited industry to provide innovative solutions for the reprocessing of N95 respirators and provided regulatory guidance as needed. Interim regulatory measures were also put in place to expedite the approval of medical devices that met regulatory requirements for the safe and effective reprocessing of N95 respirators.
While reprocessing single use devices is not a new concept, the reprocessing of N95 masks was an entirely new way of doing things. We conducted outreach to health authorities and health care providers to reassure them that the medical devices approved for the reprocessing of N95 respirators met regulatory standards, and would provide an option when new N95 respirators were not available.
"In the early days of the pandemic, health care professionals were faced with a dire situation as N95 respirators were in shortage. I was incredibly touched by the way we all came together and worked tirelessly to address this critical issue."
Evelyn Soo
Director,
Therapeutic Products
Surveillance of medical devices related to COVID-19
We monitored the safety and effectiveness of medical devices related to COVID-19, and took action as needed to protect Canadians.
Our work included:
- taking proactive steps to identify incidents related to medical devices used for COVID-19 (products explicitly approved for use in COVID-19 as well as those that were being used "off-label", or outside of the approved use of the product),
- monitoring retailers and advertisements that were making false, misleading and illegal claims related to COVID-19,
- publishing risk communications about potential safety and effectiveness concerns, and
- working closely with international partners to monitor the safety and effectiveness of medical devices related to COVID-19 in the real world.
Medical device shortages
Early in the pandemic, it was clear that COVID-19 was having an impact on the supply of critical medical devices in Canada. We introduced regulatory measures that provided more tools to urgently address medical device shortages related to COVID-19. We also worked with provinces and territories, companies and manufacturers, health care providers and patient groups to strengthen access to medical devices in Canada. These measures helped us to identify, prevent and ease shortages for Canadians.
For more information, go to Addressing critical product shortages.
Communications and collaborations
Throughout the pandemic, Health Canada published a number of web pages to provide information to Canadians and regulatory partners. Our web pages provide information about the various health products we regulate, including clinical trials authorized, medical devices authorized, testing devices that are under evaluation and more.
We also published web pages that provided critical information to the medical device industry about our regulation of products for COVID-19. We developed guidance to support industry in developing medical devices to help in the crisis.
We also published a number of risk communications to Canadians to provide important information about medical devices relating to COVID-19.
We worked closely with our domestic partners including provincial and territorial governments, health care providers and patient groups. We sought their perspectives on the priorities and challenges they were facing within the health care system as a result of the pandemic. Through webinars, we were able to share information with health care practitioners about important topics such as the reprocessing of N95 respirators.
We collaborated with regulators from other countries on issues related to investigational testing (clinical trials), medical device review, risk assessments and potential medical device shortages. This work helped ensure that medical devices are safe and effective, and are available quickly to Canadians. We also worked closely with the World Health Organization and the Pan American Health Organization's COVID-19 task group. These partnerships have helped us to align our approach with the global response to COVID-19.
Focus on…
The COVID-19 health product industry
At the start of the pandemic, Health Canada rapidly established a centralized website to inform health product stakeholders about regulatory requirements in the context of COVID-19. The website communicates priority information, guidance and advice to stakeholders on COVID-19-related health products, including medical devices (such as personal protective equipment and ventilators).
It includes easy-to-access information on the new temporary measures put in place to expedite review of these products. In this way it supports traditional stakeholders as well as those new to the sector who require additional guidance.
The website has been maintained and updated throughout the pandemic, receiving close to 10 million visits between its launch in March 2020 and the end of December. With an average of 230,000 visits per week it remains a useful resource for stakeholders.
"In March 2020 our teams were facing an incredible volume of COVID-19-related regulatory questions from stakeholders. Recognizing the urgent need to communicate with new and regular stakeholders, we established a website that would serve as a central hub of key information. The resulting website helped to reduce the volume of inquiries and enabled our staff to focus their efforts on core activities critical to the COVID-19 response."
Elizabeth Toller
Executive Director,
Policy, Planning and International Affairs
We began 2021 in the midst of the second wave of the pandemic. We will continue this critical work to ensure Canadians continue to have access to COVID-19-related medical devices.
Regulatory innovation
The current pace of innovation is unprecedented. This has led to new health products that are increasingly complex and personalized. We need new regulatory approaches to better support access to these health technologies, while continuing to protect patient safety.
Health Canada continued to prioritize regulatory innovation in 2020. The COVID-19 pandemic affected the timelines of this work. However, it also provided an opportunity to test certain temporary agile measures. We will be using the lessons learned from our regulatory response to COVID-19 to inform our regulatory innovation work, including these related to medical devices:
- modernizing investigational testing (clinical trials) regulations to create an environment that supports more innovative trials,
- enabling access to advanced therapeutic products that do not fit our current system,
- agile licensing for medical devices, using flexible tools to oversee products across their life-cycle, and
- updating how we communicate to Canadians about health product information.
For more information, go to Regulatory innovation for health products.
Building international partnerships
For many years, Health Canada has worked closely with regulators around the world on issues related to medical devices. This cooperation continued in 2020, both on COVID-19-related files (see "Medical devices: 2020 accomplishments – Responding to the COVID-19 pandemic") and in our day-to-day work.
We continued to build on our work with the International Medical Devices Regulators Forum. This group of medical device regulators works to harmonize the regulation of medical devices. This cooperation can make innovative medical devices available to patients more quickly around the world.
In 2020 we co-chaired an International Medical Devices Regulators Forum working group on cybersecurity, which published Principles and Practices for Medical Device Cybersecurity. We also participated in a new working group on artificial intelligence medical devices.
Under the Regulatory Co-operation Council, Health Canada continued to work with the United States Food and Drug Administration, through the International Medical Devices Regulators Forum, to build a Medical Device Single Review Program. This program will improve patient access to medical devices, support innovation and strengthen the development of standards. Health Canada and the Food and Drug Administration collaborated on the review of the Affinity NT Oxygenator with Balance Biosurface medical device, which was approved in both countries.
Medical devices action plan
Health Canada's Medical Devices Action Plan was launched in 2018 with the aim of strengthening the regulatory system for medical devices. Through the Action Plan, we aim to continuously improve the safety, effectiveness and quality of devices in Canada. In 2020 we remained focussed on the three components of the Medical Devices Action Plan.
Improving how medical devices get on the Canadian market
We continued to seek advice from the scientific, medical and patient communities about current and emerging issues. We held meetings of the Scientific Advisory Committees on Medical Devices Used in the Cardiovascular System and Health Products for Women. These committees provide us with ongoing advice and recommendations on regulatory issues for medical devices.
Strengthening monitoring and follow-up of medical devices
To improve our surveillance of medical devices once they are on the market, we published regulations relating to the post-market surveillance of medical devices. These regulations brought certain provisions of Vanessa's Law into effect for medical devices and introduced additional measures to gather safety information. They strengthen our ability to collect post-market information, and take appropriate action when a serious safety issue is identified.
Providing more information to Canadians
In 2020 we continued to advance our openness and transparency efforts by expanding the amount of regulatory health and safety information that is made available to Canadians. For more information about the clinical information portal and regulatory decision summaries, go to "Medical Devices: 2020 in brief".
Mandatory reporting of medical device incidents by hospitals
In 2020 Health Canada supported the implementation of new regulations for mandatory reporting by hospitals that came into force in December 2019. These regulations will increase the quantity and improve the quality of reports of medical device incidents. Hospitals are now required to report to Health Canada all medical device incidents within 30 days of being documented within the hospital.
Health Canada developed tools to support hospitals including a guidance document, education modules and promotional materials.
The number of medical device incident reports submitted to Health Canada by hospitals for medical devices increased by 620 percent in 2020. After the implementation of mandatory reporting, several data elements in the report form became mandatory, to ensure that important details were provided to Health Canada. This additional information aims to expand the data used by Health Canada to monitor the safety and effectiveness of medical devices.
Healthy clicks: Medical devices at a glance
To stay informed about our activities:
- Follow us on Facebook
- Follow us on Twitter
- Follow us on YouTube
- See the latest news from Health Canada on our website
- Find other Health-related information on the Government of Canada website
You can also find specific information about medical devices by following the links below.
Medical devices for use in COVID-19
The Lists of medical devices show the various medical devices that have been authorized for use in relation to the COVID-19 pandemic.
Authorized medical devices for uses related to COVID-19: Overview
Health Canada publishes up-to-date information about personal protective equipment in relation to COVID-19.
COVID-19 personal protective equipment (PPE)
The List of authorized clinical trials shows the COVID-19 medical devices that are currently being investigated in clinical trials authorized by Health Canada.
Conducting a clinical trial for COVID-19 medical devices: List of authorized clinical trials
New medical devices approved
The Medical Devices Active Licences Listing (MDALL) is a listing of all approvals (licences) issued for medical devices.
Medical Devices Active Licences
Search for data about the tests and trials that were performed on medical devices to evaluate their safety and efficacy.
Clinical information on drugs and health products
Regulatory Decision Summaries include the purpose of an application for a medical device licence and the reasons for Health Canada's decision to approve or reject it.
Summary Basis of Decision documents give the detailed regulatory, safety, effectiveness and quality considerations that factored into Health Canada's decision to approve certain medical devices.
Surveillance of medical devices
Report a medical device incident
Canada Vigilance Program
You can report medical device incidents to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
The Recalls and Safety Alerts Database includes the recalls, advisories, safety alerts and other publications issued by Health Canada.
Recalls and Safety Alerts Database
Summary Safety Reviews summarize our completed reviews of potential safety issues for medical devices.
New Safety and Effectiveness Reviews are tables listing reviews that are currently ongoing in Health Canada.
Health Product InfoWatch is a monthly publication intended primarily for health care professionals. The Health Product InfoWatch provides clinically relevant information about health products and their safety.
The summary tables of advertising complaints list the complaints about health product advertising that have been filed with Health Canada, and the action we have taken.
Health Product Advertising Complaints
The searchable extract of medical device incidents, complaints and recalls includes suspected medical device incidents reported to Health Canada. These incidents have been submitted by consumers and health professionals as well as medical device manufacturers and importers.
Medical devices: Life-cycle
As part of Health Canada's mission to help Canadians maintain and improve their health, we evaluate medical devices before and after they reach the Canadian market. Health Canada is involved throughout the life-cycle of a medical device, from investigational testing (clinical trials) to after the device is being sold in Canada.
Investigational testing (clinical trials)
Clinical trials are conducted by sponsors (manufacturers or importers) to gather information on a medical device's safety and effectiveness in humans. Sponsors of investigational tests submit their applications to conduct investigational testing with a medical device in Canada. Health Canada reviews these applications before the testing is conducted in Canada.
Special access program
Medical devices that are not approved in Canada may be available through our Special Access Program. In this program, access is given to an individual health care practitioner who is treating a specific patient. Access may be granted for emergency use, or when conventional therapies have failed, are unavailable or are unsuitable.
Medical device application and review
In Canada, medical devices are categorized in four groups based on their risk of use. These groups are called "Classes" and range from I to IV. Class I devices are considered low-risk devices – for example, a wheelchair. Class IV devices present the greatest potential risk – for example, a defibrillator.
When a company decides that it would like to market a Class II, III or IV medical device in Canada, it files an application to Health Canada for a new medical device licence. The application contains scientific information about the medical device's safety, effectiveness and quality. Class I devices do not require a medical device licence, but are monitored through establishment licences.
Applications for higher-risk medical devices are reviewed by our scientists and engineers. Sometimes we also consult with advisory committees or external consultants. Reviewers evaluate the safety, effectiveness and quality data submitted to assess the potential benefits and risks of the medical devices. They also review the information that will be provided to health care practitioners and consumers about the medical device.
Expedited review pathway: Priority review
The priority review pathway provides an expedited path to a final decision for certain medical devices, including those that target specific health care needs. Medical devices for serious, life-threatening, or severely debilitating diseases or conditions can be given a priority review status. Products approved through expedited review pathways can be available to patients sooner because they have shorter review targets.
Approval of medical devices
After its review of a medical device application, Health Canada may conclude that the benefits of the product outweigh the potential risks and approve the device for sale in Canada. When a new medical device is approved, it is issued a medical device licence. This does not mean the medical device will immediately be available to patients, as many other factors can influence that timeline.
Surveillance
It is not possible to know or predict all of the possible problems related to a medical device through clinical studies. After a product is approved and available for sale in Canada, we continue to monitor its use in the real world, that is, in the broader Canadian population. We evaluate potential safety and effectiveness issues and take action when there are identified problems.
Collecting information
Health Canada collects safety information about a product after it is approved from a variety of sources. One source of information is suspected medical device problems that are reported after products are approved for sale. These are undesirable effects potentially caused by medical devices.
You can report medical device incidents to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
Evaluating safety signals
Health Canada evaluates the data we collect to detect new safety signals, which we then investigate more closely. A "safety signal" can be defined as information on a new or known adverse event that may be associated with a medical device. These investigations are called signal assessments and they may result in recommendations for actions to be taken by the company, by Health Canada, or both. These actions can include informing the public and health care professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a medical device from the market.
Advertising complaints
Health Canada also regulates the advertising of medical devices in Canada to ensure that companies are not making false claims about their products. We review advertising complaints to determine if a company is complying with our requirements, and we take appropriate action when non-compliance is identified.