Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal

Download the alternative format
(PDF format, 1.66 MB, 62 pages)
Organization:
Public Health Agency of Canada
Type: Guidance document
Date published: March 2017
March 2017
Table of Contents
- Preface
- Abbreviations and Acronyms
- Chapter 1 – Introduction
- Chapter 2 – Notification Reports
- Chapter 3 – Next Steps
- Chapter 4 – Glossary
- Appendix A – Exposure Reporting
- Appendix B – Investigating and Analyzing Incidents
Preface
In Canada, most facilities where human pathogens are handled and stored are regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR). The HPTA and HPTR set out reporting and notification requirements for licence holders, biological safety officers, and persons conducting controlled activities authorized under a Pathogen and Toxin Licence. The Canadian Biosafety Standard (CBS) sets out additional operational practice requirements for notification and reporting, detailing the specific timelines, reporting forms, and reporting processes. The Notification and Reporting under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal guideline (Guideline) serves as a resource for stakeholders, providing more in depth information and guidance on how to complete and submit a notification and subsequent follow-up report.
This Guideline was developed by the PHAC as part of an ongoing series of biosafety and biosecurity themed guidance documents. It is a continuously evolving document and subject to ongoing improvement. The PHAC welcomes comments, clarifications, and suggestions for incorporation into the future versions of the Guideline. To this end, please send information with references (where applicable) for continual improvement of this Guideline to:
PHAC e-mail: pathogens.pathogenes@phac-aspc.gc.ca
Abbreviations and Acronyms
- BSO
- Biological Safety Officer
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- HPTA
- Human Pathogens and Toxins Act
- HPTA Security Clearance
- Human Pathogens and Toxins Act Security Clearance
- HPTR
- Human Pathogens and Toxins Regulations
- LAI
- Laboratory Acquired Infection/Intoxication
- PHAC
- Public Health Agency of Canada
- RG
- Risk group (i.e., RG1, RG2, RG3, and RG4)
- SOP
- Standard operating procedure
- SSBA
- Security Sensitive Biological Agent
Chapter 1 – Introduction
The words in bold type are defined in the glossary found in Chapter 4.
In Canada, all facilities where human pathogens or toxins are handled or stored are regulated under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR).Footnote 1,Footnote 2 The Public Health Agency of Canada (PHAC) has the ultimate authority to implement provisions of the HPTA and HPTR.
The HPTR came into force on December 1, 2015. At the same time, the remaining sections of the HPTA also came into force and the Human Pathogens Importation Regulations were repealed. The Canadian Biosafety Standard (CBS) sets out additional operational practice requirements for notification and reporting, detailing the specific timelines, reporting forms, and reporting processes.Footnote 3 The Canadian Biosafety Handbook (CBH) provides additional guidance that supports the HPTA, the HPTR and the CBS.Footnote 4 The Notification and Reporting under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal guideline (Guideline) provides more in depth information and guidance on the notification, investigation, and reporting requirements set forth in the HPTA, HPTR, and CBS.
Section 16 of the HPTA specifies that no information provided under Sections 12 to 15 of the Act, by a licence holder or a person conducting activities under the authority of a Pathogen and Toxin Licence issued by the PHAC, may be used or received against that person in any criminal proceedings that are subsequently instituted against them, other than with respect to a contravention of Section 17 of the Act.
This guideline also contains a comprehensive glossary of definitions for technical terms, located in Chapter 4; words defined in the glossary appear in bold type upon first use. The appendices provide additional information related to notification and reporting, including guidance on incident investigation and step by step instructions for the completion and submission of exposure notification and exposure follow-up reports using the Biosecurity Portal.
1.1 Scope
This Guideline provides comprehensive guidance on how to complete and submit a notification report and, where exposure or laboratory acquired infections/intoxications (LAIs) is concerned, a subsequent follow-up report to the PHAC in accordance with requirements in the HPTA, HPTR and CBS.
Situations which require mandatory notification, as specified in the HPTA and HPTR, can generally be grouped into four categories: laboratory incidents, changes requiring issuance of a new licence, changes that may require an amendment or revocation of an existing Human Pathogens and Toxins Act Security Clearance (HPTA Security Clearance), and other events requiring notification.
This document provides detailed guidance on notification and reporting procedures pertaining to laboratory incidents and other events requiring notification.
Laboratory incidents include:
- Exposures and LAIs [HPTA 13];
- Inadvertent possession, production, or release [HPTA 12(1),(2), and HPTR 9(1)(c)(ii)]; and
- Missing, stolen, or lost biological agent (i.e., pathogen or toxin) [HPTA 14], including a security sensitive biological agent (SSBA) not received within 24 hours of an expected date and time [HPTR 9(1)(c)(iii)].
Other events requiring notification include:
- Changes that could affect biocontainment [HPTR 6(1)];
- Exemptions from a risk group – risk group reduction [HPTR 26(4)]; and
- Prohibiting an HPTA Security Clearance holder from accessing a part of the facility where SSBAs are handled or stored [HPTA 32].
1.2 Obligation to inform the Minister
In practice, the obligation to inform the Minister under the HPTA and/or the HPTR means providing information to the PHAC via the submission of a notification and/or follow-up report, as stipulated for the type of event and circumstances described below. Additional communication and reporting may be required on a case-by-case basis, where necessary.
The obligation to inform the PHAC of events is linked to either the licence holder or the biological safety officer (BSO). It is expected that licence holders may delegate their reporting role to the BSO; however, licence holders remain ultimately responsible for certain reporting under the HPTA.
1.3 Notification and Reporting Process
1.3.1 Biosecurity Portal
The PHAC online Biosecurity Portal (the Portal) facilitates notification and/or detailed reporting in accordance with the HPTA, HPTR, and the respective operational practice requirements specified in the CBS. Whenever possible, the laboratory incident and other reports specified in Section 1.1 should be reported through the Portal's reporting module.
1.3.1.1 Tips for using the Biosecurity Portal
| Tip | Description |
|---|---|
| Save early and often! | At each data entry page, always save the information that you have entered before navigating to another page. Not saving could result in the loss of the information that you have entered. |
| For the exposure follow-up report, the mandatory biological agent fields do not appear until the affected person record is saved. If these biological agent fields are not completed the report cannot be submitted. The system will indicate that fields in the Administrative Information section of the report have not been completed. If all visible mandatory fields on this page have been completed, open each affected person(s) record on this page and ensure that the biological agent fields have been completed. | |
| Answer all questions displayed on the form whenever the information is relevant and available | Complete all fields if the information is available whether the field is marked mandatory (*) or not. |
| Do not include sensitive personal information. | Do not provide names or any sensitive personal information about any affected persons in the report. Do not include names or any sensitive personal information about any holder of a HPTA Security Clearance who has been prohibited access to a facility. This information will be collected after the initial notification report is submitted, when the reporter is contacted by a representative from the Centre for Biosecurity. |
| Submit only one report type for a single incident | If your notification involves an exposure or infection/intoxication of one or more individuals in the laboratory/facility setting, report the incident as an exposure/LAI. If the incident also involves another incident type, clearly indicate this (e.g., inadvertent possession, missing pathogen) within the exposure report notes. |
| Electronic completion and submission of an exposure notification report | Some information will be prepopulated when completing a report based on the user profile of the individual making the report (e.g., licence number and reporter name). |
| Description of the event | The notification report includes a text box for submitting a description of the event. Provide sufficient details of the event so that representatives from PHAC can assess the situation and act accordingly. |
| If you have any questions about reporting or need assistance, contact the PHAC at pathogens.pathogenes@phac-aspc.gc.ca or 1-613-957-1779. | |
1.3.2 Other Methods of Reporting
In the event the Biosecurity Portal is unavailable (or for voluntary reporting) licence holder representatives and BSOs are asked to contact the PHAC for assistance:
- By emailing pathogens.pathogenes@phac-aspc.gc.ca
- By calling 1-613-957-1779
Chapter 2 – Notification Reports
Note: If your notification involves an exposure or infection/intoxication of one or more individuals in the laboratory/facility setting, report the incident as an exposure or LAI. If the incident also involves another incident type, clearly indicate this (e.g., inadvertent possession, missing pathogen) within the exposure report "Additional notes" Section A.2.6 at the end of the report. There is no need to submit more than one report type for a single incident as indicated in subsection 1.3.1.1.
2.1 Laboratory Incidents
2.1.1 Exposures and Laboratory Acquired Infections/Intoxications
A licence holder must inform the PHAC without delay if they have reason to believe an incident involving a human pathogen or toxin in the licence holder's possession has or may have caused disease in an individual. Notification without delay provides a timely alert (i.e., as soon as reasonably possible) and permits time for immediate response and control measures to be taken.
The CBS sets out the minimum operational practice requirements for licence holders to meet the HPTA reporting obligations, detailing the specific timelines, reporting forms, and reporting processes.
In particular, incidents involving exposure to a human pathogen or toxin or an LAI require submission of the following as per the CBS:
- an exposure notification report to be submitted without delay, (i.e., as soon as reasonably possible); followed by
- an exposure follow-up report, documenting the results of the investigation as known, to be submitted within the prescribed timelines.
See Appendix A for detailed guidance on how to complete the exposure notification report using the Portal.
2.1.1.1 Exposure Assessment
The determination of whether or not an incident involves exposure of one or more individuals is based on a facility's assessment of the incident, which should consider all relevant facts and circumstances to ultimately assess whether or not an inhalation, ingestion, inoculation, or absorption has, or may have, occurred.
Figure 2-1 illustrates a decision chart to assist in the exposure assessment.
Figure 2-1: Decision Chart to Assist in the Assessment of an Incident to Determine if an Exposure has Occurred and if Notification of the Public Health Agency of Canada (PHAC) is Required.Footnote 4 Figure 2-1 note *
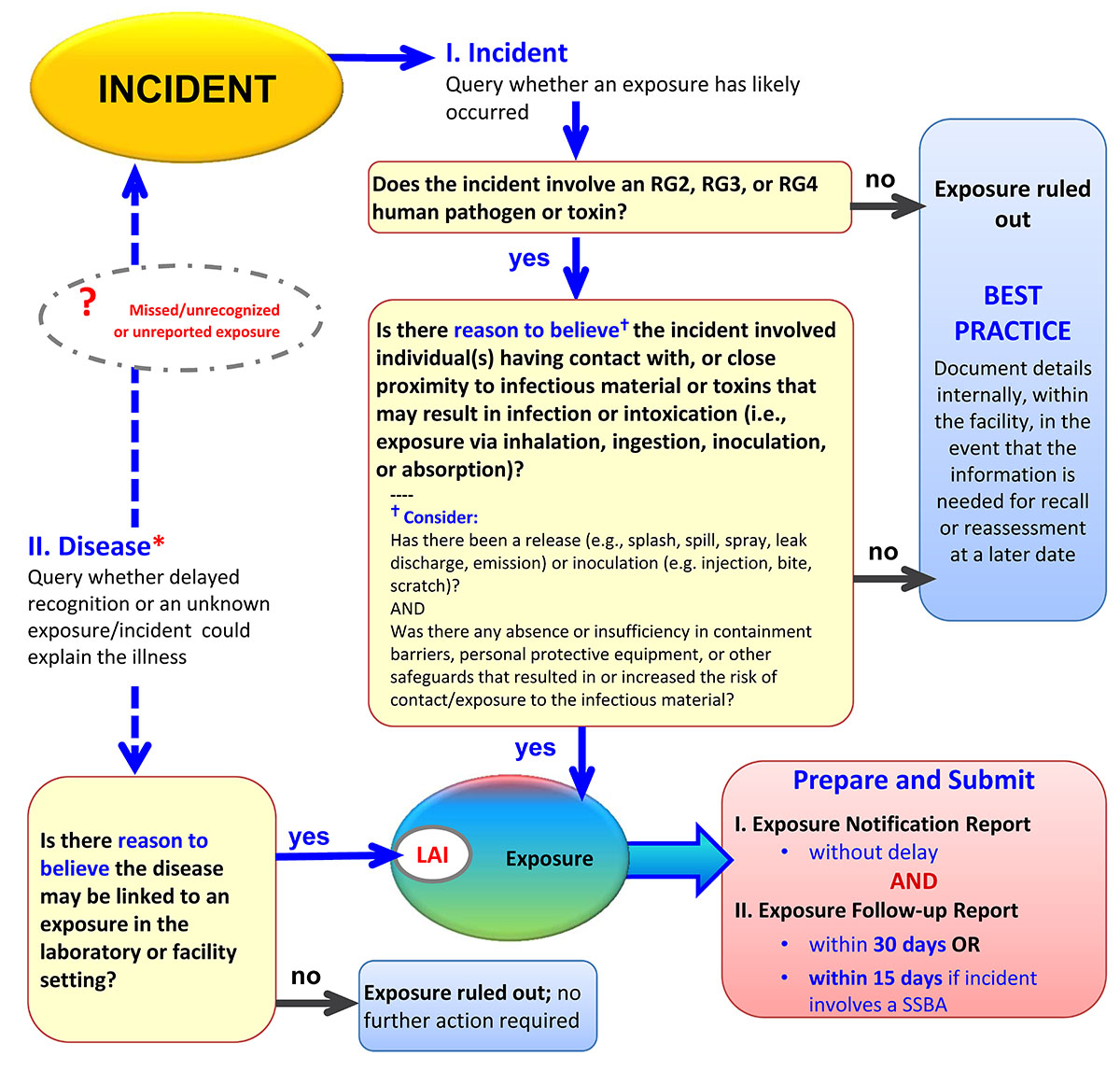
Figure 2-1 - Text description
Figure 2-1: Decision chart to assist in the assessment of an incident to determine if an exposure has occurred and if notification of the Public Health Agency of Canada (PHAC) is required.
If an incident involved an RG2, RG3, or RG4 pathogen or toxin, and there is reason to believe that the incident involved one or more individuals' contact with, or close proximity to, infectious material or toxins that may result in infection or intoxication then an exposure occurred. Contact or close proximity is defined as possible exposure via inhalation, ingestion, inoculation, or absorption. If an exposure has occurred, an exposure notification report is to be prepared and submitted without delay, and an exposure follow-up report submitted within 30 days, or in the case of an SSBA, within 15 days.
If the incident did not involve an RG2, RG3, or RG4 human pathogen or toxin, or if there is no reason to believe that contact or close proximity with pathogens or toxins may have resulted in infection or intoxication, then best practice dictates that the incident be documented internally within the facility in the event that the information is needed for recall or reassessment at a later date.
If a disease is detected and there is reason to believe that it may be linked to an exposure in the containment zone setting, then it is classified as an LAI and a previously missed or unreported exposure. In such a case, an exposure notification report and follow-up report are to be prepared and submitted as described above. If there is no reason to believe that the disease may be linked to the containment zone, then exposure is ruled out and there is no further action required.
In most cases, a disease state will include recognition of an illness, syndrome, or known disease. However, some facilities may employ medical surveillance practices that could identify a seroconversion, which may provide an additional source of information for recognition of infection or disease states.
Even if exposure/LAI is ruled out, documenting the details of the incident/disease and investigation findings within the facility's records is required in accordance with the re-examination at a later time, if necessary.
Additional guidance on assessing whether an exposure requiring notification of the PHAC has occurred and on conducting an incident investigation is available in the Appendix B of this document and Chapter 18 of the CBH.
2.1.2 Inadvertent Possession, Production or Release
Incidents involving inadvertent release, inadvertent production, or inadvertent possession of a human pathogen or toxin require a notification to the PHAC and will be followed up on a case by case basis where additional information may be required.
If a licence holder has reason to believe that a human pathogen or toxin has been released inadvertently from the facility in the course of an activity that is otherwise authorized by the licence, the licence holder shall, without delay, inform the Minister of the release [HPTA 12(1)].
The notification report provides the PHAC with a description of the event, which should include the following information:
- Licence number;
- Licence holder's name;
- Reporter's name;
- Name of the human pathogen or toxin;
- Quantity of the human pathogen or toxin;
- Date and time of the incident;
- Location of the incident; and
- Any other details of the incident, including information that supports the conclusion that a human pathogen or toxin has been released or produced.
2.1.3 Missing, Stolen or Lost Human Pathogen or Toxin, including when a Security Sensitive Biological Agent (SSBA) is not received within 24 hours of the expected date and time of receipt
Incidents involving a missing, stolen, or lost human pathogen or toxin, including when an SSBA is not received within 24 hours of the expected date and time of receipt, require a notification to the PHAC and will be followed up on a case-by-case basis where additional information may be required.
The notification report provides the PHAC with a description of the incident, including the following information:
- Licence number;
- Licence holder's name;
- Reporter's name;
- Name of the human pathogen or toxin;
- Quantity of human pathogen or toxin;
- Date and time of the incident;
- Location; and
- Any other details of the incident, including an up to date account of efforts to locate the human pathogen or toxin.
For reports involving incidents where an SSBA has not been received within 24 hours of the expected receipt, the report should also contain the following information:
- Name of company or organization sending/receiving the SSBA;
- Licence number of company or organization sending/receiving the SSBA (if known);
- Name of courier company and all persons who came into possession of the package during shipment; and
- Expected date and time of arrival.
2.2 Other events requiring notification
2.2.1 Changes affecting containment
Before making any changes to the physical structure of a facility, to any equipment, or to any standard operating procedures (SOPs) that could affect containment, a licence holder must notify the PHAC of their intent to make such a change.
The notification report provides the PHAC with a description of the proposed changes to a facility, equipment, or SOP that could affect containment, and should include the following information:
- Licence number;
- Licence Holder's name;
- Reporter's name;
- Location where the proposed changes will take place;
- Description of the proposed changes; and
- Proposed start date of work (for change to the physical structure of a facility).
2.2.2 Prohibiting a holder of an HPTA Security Clearance from accessing a part of the facility
When a licence holder decides to prohibit the holder of a HPTA Security Clearance from having access to a part of the facility to which the licence applies, they must notify the PHAC without delay.
The notification report provides the PHAC with information regarding the reason that the holder of a HPTA Security Clearance was prohibited from having access to the facility.
Although a notification report can be submitted using the Portal, please contact the Centre of Biosecurity at pathogens.pathogenes@phac-aspc.gc.ca or at 1-613-957-1779 BEFORE submitting a notification report. If the report is being submitted through the Portal, please do not include sensitive personal information.
2.2.3 Exemption from a Risk Group – Risk Group Reduction
A licence holder must notify the PHAC without delay when a Risk Group 2 (RG2), RG3, or RG4, human pathogen has been modified to the extent that it no longer meets the respective risk group definition, thereby potentially resulting in a risk group change. For example, if a an RG3 biological agent was modified such that it was no longer "likely to cause serious disease in a human", then it may no longer meet the definition of a RG3.
The notification report should provide the PHAC with a description of the modification and include:
- Licence number;
- Licence Holder's name;
- Reporter's name;
- Name of the human pathogen or toxin;
- Brief description of the modification; and
- Proposed change to risk group (i.e. RG3 to RG2).
This is followed by a pathogen risk assessment as described in Section 3.3.2.
Chapter 3 – Next Steps
3.1 Laboratory Incident Investigation
In accordance with the requirements specified in the CBS (Matrix 4.9), any incident involving pathogens, toxins, other regulated infectious material, infected animals, or failure of containment systems or control systems must be investigated and documented in order to determine the root cause(s).
While some guidance has been provided in Appendix B (Investigation and Analyzing Incidents), additional guidance on how to perform an investigation and root cause analysis is also available in the CBH and in a guideline available from the PHAC.
3.2 Laboratory Incidents
For incidents other than exposures/LAIs, the PHAC may request the facility to complete an Incident Investigation and Reporting Form to be submitted within a specified timeframe.
3.2.1 Exposure Follow-up Report
All exposure/LAI incidents require an exposure follow-up report documenting the results of the investigation, as known at the time the submission is due. Although the investigation may not be fully complete, the exposure follow-up report must be completed and submitted, with as much detail as is known and available, within the following timelines set out in Matrix 4.9 of the CBS:
- 15 days of the submission of an exposure notification report involving a an SSBA; or
- 30 days of the submission of an exposure notification report involving a human pathogen or toxin other than an SSBA.
See Appendix A for detailed guidance on how to complete the exposure follow-up report using the Portal.
3.3 Other events requiring notification
3.3.1 Prohibiting a holder of an HPTA Security Clearance from accessing a part of the facility
If a notification report is submitted through the Portal, a representative from the Centre for Biosecurity will contact the reporter.
3.3.2 Exemption from a Risk Group – Risk Group Reduction
Once the notification report has been submitted, regulated parties must conduct a pathogen risk assessment on the modified human pathogen or toxin and submit it to the PHAC for validation. If there is evidence to support the reduction in risk group, an amendment to the licence may be necessary. Please contact the PHAC at pathogens.pathogenes@phac-aspc.gc.ca for additional information and guidance on how to perform a pathogen risk assessment. A guideline on pathogen risk assessments is also available from the PHAC.
Chapter 4 – Glossary
It is important to note that while some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS, or the CBH; therefore, some definitions may not be applicable to facilities that fall outside of the scope of the CBS.
- Biological agent
- In the case of this guideline and the Biosecurity Portal, a human pathogen or toxin.
- Biological safety officer (BSO)
- An individual designated for overseeing the facility's biosafety and biosecurity practices.
- Biosafety
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to infectious material and toxins, or their accidental release.
- Containment
- The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material. The term "biocontainment" is also used in this context.
- Containment system
- Dedicated equipment that functions to provide and maintain containment. This includes, but is not limited to, primary containment devices (e.g., biological safety cabinets); heating, ventilation, and air conditioning (HVAC) and control systems; and decontamination systems (e.g., autoclaves).
- Disease
- A disorder of structure or function in a living human or animal, or one of its parts, resulting from infection or intoxication. It is typically manifested by distinguishing signs and symptoms.
- Exposure
- Contact with, or close proximity to, infectious material or toxins that may result in infection or intoxication, respectively. Routes of exposure include inhalation, ingestion, inoculation, and absorption.
- Facility
(plural: facilities) - Structures or buildings, or defined areas within structures or buildings, where infectious material or toxins are handled or stored. This could include individual research and diagnostic laboratories, large scale production areas, or animal housing zones. A facility could also be a suite or building containing more than one of these areas.
- Human Pathogens and Toxins Act Security Clearance (HPTA Security Clearance)
- An authorization following verification of an individual's background and reliability status issued by the Public Health Agency of Canada under Section 34 of the Human Pathogens and Toxins Act.
- Incident
- An event or occurrence with the potential of causing injury, harm, infection, intoxication, disease, or damage. Incidents can involve infectious material, infected animals, or toxins, including a spill, exposure, release of infectious material or toxins, animal escape, personnel injury or illness, missing infectious material or toxins, unauthorized entry into the containment zone, power failure, fire, explosion, flood, or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- Infectious material
- Any isolate of a pathogen or any biological material that contains human or animal pathogens and, therefore, poses a risk to human or animal health.
- Intoxication
- A substance-induced disorder or disease resulting in a symptomatic or asymptomatic condition or other physiological change resulting from an exposure (i.e., ingestion, inhalation, inoculation, or absorption) to a toxin produced by or isolated from a microorganism. This includes a similar response to that resulting from exposure to a synthetically produced microbial toxin.
- Laboratory
- An area within a facility, or the facility itself, where biological material is handled for scientific or medical purposes.
- Laboratory acquired infection/ intoxication (LAI)
- Infection or intoxication resulting from exposure to infectious material, infected animals, or toxins being handled or stored in the containment zone.
- Licence
- An authorization to conduct one or more controlled activities with human pathogens or toxins issued by the Public Health Agency of Canada under Section 18 of the Human Pathogens and Toxins Act.
- Pathogen
- A microorganism, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2 to 4 and in Part 2 of Schedule 5 of the Human Pathogens and Toxins Act but these are not exhaustive lists.
- Release
- The discharge of infectious material or toxins from a containment system.
- Risk group (RG)
- The classification of biological material based on its inherent characteristics, including pathogenicity, virulence, risk of spread, and availability of effective prophylactic or therapeutic treatments, that describes the risk to the health of individuals and the public as well as the health of animals and the animal population.
- Security sensitive biological agents (SSBAs)
- The subset of human pathogens and toxins that have been determined to pose an increased biosecurity risk due to their potential for use as a biological weapon. SSBAs are identified as prescribed human pathogens and toxins by Section 10 of the Human Pathogens and Toxins Regulations. This means all Risk Group 3 and Risk Group 4 human pathogens that are in the List of Human and Animal Pathogens for Export Control, published by the Australia Group, as amended from time to time, with the exception of Duvenhage virus, Rabies virus and all other members of the Lyssavirus genus, Vesicular stomatitis virus, and Lymphocytic Choriomeningitis Virus; as well as all toxins listed in Schedule 1 of the Human Pathogens and Toxins Act that are listed on the List of Human and Animal Pathogens for Export Control when in a quantity greater than that specified in Section 10(2) of the Human Pathogens and Toxins Regulations.
- Standard operating procedure (SOP)
- A document that standardizes safe work practices and procedures for activities with infectious material and toxins in a containment zone, as determined by a local risk assessment.
- Toxin
- A poisonous substance that is produced or derived from a microorganism and can lead to adverse health effects in humans or animals. Human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the Human Pathogens and Toxins Act.
Appendix A – Exposure Reporting
A.1 Exposure Notification Report
The following information is provided to assist licence holder representatives and BSOs in completing and submitting a notification report using the Portal.
If the Portal is unavailable (or for voluntary reporting) the PHAC must still be notified of the incident and this can be done via email to:
pathogens.pathogenes@phac-aspc.gc.ca
Please refer to the Biosecurity Portal User Guide for guidance on how to access the Portal and reporting module.
All fields should be completed if and when the information is available; fields marked with an asterisk (*) are mandatory to complete prior to saving or submitting the exposure notification report.
Tip: remember to save early and often!
- At the end of each data entry page, always save the information that you have entered so far in your report.
- Some key additional fields do not appear until the report is saved (i.e., biological agents fields on affected person page); as a result, after saving each page the reporter should scroll through the page again to ensure all fields have been completed.
- Not saving could result in the loss of the information that you have entered.
Privacy reminder: Do not include any personal name(s) or other personally identifying information on affected individuals anywhere in the report.
A.1.1 Administrative Information
- Exposure Incident Identification Number
- Prepopulated by the system once a new report has been created.
- Select the incident type that best describes the incident being reported
- Exposure,
- Laboratory Acquired Infection – Confirmed, or
- Laboratory Acquired Infection – Suspected.
Select "exposure" if an incident has involved individual(s) contact with or close proximity to infectious material or toxins that may result in infection or intoxication, respectively. Select "Laboratory acquired infection suspected" or "Laboratory acquired infection – confirmed" if an illness or infection/disease state (including seroconversion) is likely or confirmed as linked to a prior laboratory exposure incident.
Note: In the exposure follow-up report this field can also be used to rule out a previous notification of an exposure incident. If, at a later date, exposure is ruled out by the investigation (i.e., the investigation determined that either no pathogen or toxin was present or that there was no possibility of close contact that could have resulted in infection or intoxication of the person(s) in close proximity during the incident), the option "Ruled Out" can be selected from this field in the exposure follow-up report and no further information is required.
- * Licence Number associated with this incident
- A drop down list of all licence numbers associated with the reporter's profile is provided. Choose the licence number associated with the incident.
- Licence Holder
- Automatically populated by the system once the licence number has been selected.
- * Indicate the containment level of the laboratory associated with the incident
- Containment level 1,
- Containment level 2,
- Containment level 3, or
- Containment level 4.
A.1.2 Occurrence Information
- * Are/did any of the individuals exposed or infected during the incident travelling outside the province/territory during the potential incubation or infectious period?
- Yes – Travel to another Canadian jurisdiction,
- Yes – International travel,
- No, or
- Unknown.
- * Indicate if travel has/will take place during the known incubation (exposed individual) or infectious (LAI suspected or confirmed) period of the respective biological agent of infection.
Note: This field/question will only appear if "Yes" answered to the previous question.- Yes – travel during incubation period,
- Yes – travel during infectious period,
- No,
- Unknown, or
- Not applicable.
- * Incident Date known?
- Yes/No.
- * Incident Date
Note: This field/question will only appear if "Yes" answered to the previous question.- Select date the incident occurred using calendar icon.
- * Date first reported to internal authorities
- This may or may not be the same day that the incident occurred.
- Internal authorities means the licence holder representative or BSO.
- Select date the incident was first reported using calendar icon.
- * Location of Incident
- Indicate the location where the incident occurred
- Open text box – provide as much information as needed to identify the specific location within the facility where the incident took place. This may include the facility name, building name and address, room number, and, if known, the specific bench, biological safety cabinet (BSC), or other work area where the incident occurred.
- For example: Fairbridge building, room #101, bench C.
- * Select the occurrence type(s) that best characterise the incident (if more than one type is significant, check all that apply)
- Spill,
- Loss of containment,
- Sharps-related (needlestick/sharps injury),
- Animal-related (bites/scratches),
- Insect-related,
- PPE-related (inadequate or failure),
- Equipment-related,
- Procedure-related,
- Unknown, or
- Other.
- * Provide a brief description of what occurred during the incident
Note: This field/question will only appear if "Other" selected for the previous question.- Open text box – provide the sequence of events leading up to the incident as it is known at the time of notification (this can be revised during the completion of the exposure follow-up report). Indicate any equipment, tools, or other materials or item that may have contributed to the incident.
- *Indicate the main activity that best describes the work being undertaken during the incident
- Cell culture,
- Microbiology,
- Microscopy,
- Molecular investigations,
- Serology/hematology,
- Animal care,
- In vivo animal research,
- Autopsy/necropsy,
- Education/training,
- Maintenance (e.g., equipment upkeep or repairs, routine or general cleaning),
- Unknown, or
- Other.
- * If main activity is "Other", describe
Note: This field/question will only appear if "Other" selected for the previous question.- Open text box – provide a description work being undertaken.
- * Is/are the biological agent(s) involved in the incident known?
- Yes/No.
- * Biological Agents
Note: This section only appears if "Yes" selected for the previous question.
Note: Necessary to complete section for submission but not to save. - Specify the name(s) of the biological agent(s) involved in the incident
- The Portal contains information on a number of biological agents.
- Search for the biological agent(s) involved in the incident by starting to type in the name of the agent. Select the correct biological agent(s) from the list.
- More than one biological agent can be added.
- If the biological agent is not found leave this field blank and enter the information under "Other Biological Agents".
- Other Biological Agents (10 maximum. Use semicolons [;] to add multiple at a time)
- Only enter information in this field if one or more of the biological agents involved in the incident cannot be found in the search field of the Portal.
- Provide the name of the biological agent.
- Specify the specific strain, sub-type, and other identifiers of the biological agent, if known/applicable
- Open text box.
- *Was decontamination/disinfection performed using processes and methods in accordance with applicable standards and guidelines?
Note: This is the decontamination/disinfection of materials and surfaces.- Yes, provide further details,
- No, decontamination/disinfection was not required,
- No, other reason (explain below why not done or not done per standards and guidelines), or
- Unknown (explain below).
- * Additional Details
Note: If "No, decontamination/disinfection was not required" selected this field does not appear.- Open text box – provide the additional details required based on the answer selected for the previous question.
A.1.3 Submission Information
- Reporter's Name (first, last)
- Prepopulated by the system based on the user profile.
- * Reporter's role in the incident (select the option that best describes your role/participation during the incident)
- Directly involved in the incident;
- Witnessed the incident, not directly involved; or
- Not involved/did not witness, informed following the incident.
A.2 Exposure Follow-Up Report
The following information is provided to assist licence holder representatives and BSOs in completing and submitting an exposure follow-up report using the Portal. While only one exposure notification report can be submitted for an incident, multiple exposure follow-up reports can be submitted for the same incident as the investigation progresses.
If the Portal is unavailable an exposure follow-up report must still be submitted to the PHAC's email address pathogens.pathogenes@phac-aspc.gc.ca. Contact the PHAC to obtain a copy of the Incident Investigation and Reporting Form to facilitate submission of the required information.
Please refer to the Biosecurity Portal User Guide for guidance on how to access the Portal and reporting module.
Note: For exposure follow-up report fields that are identical to those in the exposure notification report, the information previously submitted will be prepopulated to fill these fields.
Reporters only need to review and revise the information in these fields if, as a result of the findings of incident investigation, the information previously submitted has now changed.
All fields should be completed if and where the information is available; fields marked with an asterisk (*) are mandatory to complete prior to saving or submitting the exposure follow-up report.
A.2.1 Administrative Information
- Exposure Incident Identification Number
- Prepopulated by the system once a new exposure follow-up report has been created.
- Select the incident type that best describes the incident being reported
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required, including ruling out an exposure.
- Ruled out – if a previous notification of exposure has been subsequently ruled out by the investigation (i.e. the investigation confirmed that no pathogen or toxin was present and/or that there was no possibility of close contact that could have resulted in infection or intoxication of person(s) in close proximity during the incident), then this menu option can be selected to rule out exposure.
- * Licence Number associated with this incident
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- Licence Holder
- Prepopulated from the exposure notification report.
- * Indicate the containment level of the laboratory associated with the incident
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Indicate the total number of individuals affected (exposed + infected) during the incident
- Numeric field – enter a value between 0 and 999.
- This number does not include number of individuals affected due to secondary transmission due to person-to-person spread. This is counted elsewhere in the exposure follow-up report.
- If number greater than 0, add an affected person record, otherwise save and go to the next page.
- Save the page once this section is completed and before adding affected persons. If the page is not saved the information entered in this field will disappear.
A.2.1.1 Affected Persons
For each affected person identified in the above question add an affected person record and complete the required fields.
Note: Due to the design limitations of the current iteration of the Portal, each affected person record must be saved before adding a new affected person record or returning to the administrative information page. By saving the record, additional fields related to the biological agent involved in the incident will appear and must be completed before the overall report can be submitted to the PHAC. If these additional fields have not been completed for each affected person, the system will not allow you to submit the report and an error message will be generated, indicating that information is missing in the Administrative Information section.
Privacy reminder: Do not include any personal name(s) or other personally identifying information on affected individuals anywhere in the report.
- Name
- This is NOT a personal name; this is locked system field.
- This field is auto-populated by the system with a unique identifier when an affected person record is saved.
- * Indicate the exposure versus disease status for this affected individual
- Exposure,
- Laboratory Acquired Infection – Confirmed,
- Laboratory Acquired Infection – Suspected,
- Ruled Out – if a previous notification of exposure for this person has been subsequently ruled out by the investigation (i.e., the investigation confirmed that no pathogen or toxin was present and/or that there was no possibility of close contact that could have resulted in infection or intoxication of this person in close proximity during the incident), then this menu option can be selected, or
- Seroconversion.
- * Indicate the primary (actual or potential) route of exposure
- Inhalation,
- Ingestion,
- Inoculation/Injection – needle/sharps,
- Inoculation/Injection – bite/scratch,
- Absorption via contact with mucous membrane,
- Absorption via contact with skin,
- Unknown, or
- Other, specify in additional details below.
- Explain further if the route of exposure is unknown OR provide any additional details on the known or suspected route of exposure
- Open text box – provide additional information that can better explain or provide detail to the exposure route, such as condition of the skin or other predisposing factors.
- Has the affected person experienced onset of illness?
- Yes – acute illness presentation,
- Yes – chronic illness presentation,
- No, or
- Unknown.
- Is Onset Date known?
Note: This field/question will only appear if "Yes" selected for the previous question.- Yes/No.
- * Date of first onset of symptoms for the affected individual
Note: This field/question will only appear if "Yes" selected for the previous question.- Select the date of first onset of symptoms from the calendar icon.
- * Select the disposition that best describes the affected individual's illness outcome at the time of this report
Note: This field/question will only appear if "Yes" selected for the question "Has the affected person experienced onset of illness?".- Recovered fully – no sequelae,
- Recovered – long term sequelae/disability,
- Ongoing chronic illness (non-infectious),
- Ongoing chronic illness (infectious),
- Ongoing carrier state (infectious),
- Not recovered,
- Death, or
- Unknown.
- * Indicate the affected individual's recovery time
Note: This field/question will only appear if "Recovered fully – no sequelae" or "Recovered – long term sequelae/disability" selected for the previous question.- 0-7 days,
- 8-14 days,
- > 14 days, or
- Unknown.
- * Indicate all the immediate and/or early post-exposure interventions that were administered within 0-7 days of the known/suspected exposure incident? (Check all that apply)
- First-aid administered immediately after exposure;
- Occupational health consultation within 0-7 days of the exposure;
- Medical consultation within 0-7 days of the exposure;
- Post-exposure prophylaxis (PEP) within 0-7 days of the exposure;
- Not applicable; or
- Other.
- * Indicate all the later post-exposure interventions that were administered more than 7 days after the known/suspected exposure incident? (Check all that apply)
- Occupational health consultation >7 days of the exposure;
- Medical consultation >7 days of the exposure;
- Post-exposure prophylaxis (PEP) >7 days of the exposure;
- Drug treatment (e.g., antibiotic, antiviral, antifungal) > 7 days after the exposure;
- Not applicable; or
- Other.
- Describe the post-exposure interventions administered, including where available, the name of PEP and/or treatment drugs and regimens (if any interventions were required or recommended but NOT administered, explain)
- Open text box – provide a description.
A.2.1.1.1 Laboratory Experience and Role(s)
- What is the affected person's laboratory experience in years?
- Numeric field – enter a value between 0 and 100.
- * What is the affected person's highest completed level of education?
- Grade School,
- High School,
- Technical/trades college diploma,
- Bachelor's degree,
- Master's degree,
- MD/PhD,
- Postdoctoral fellowship,
- Unknown, or
- Other.
- * Indicate the affected person's regular role in the laboratory/facility
- Technician/technologist,
- Supervisor/Manager,
- Animal handler,
- Researcher,
- Student,
- Unknown, or
- Other.
- * If other role is selected, specify
Note: This field/question will only appear if "Other" selected for the previous question.- Open text box – provide a description of the individual's role in the laboratory/facility.
A.2.1.1.2 Biological Agents
NOTE: Save the Affected Person record and complete the additional Biological Agent fields that have appeared on the page (under the Exposure/Disease status field). The Biological Agent section must be completed and saved before the overall report can be submitted to the PHAC.
- Specify the name(s) of the biological agent(s) involved in the incident
- The Portal contains information on a number of biological agents.
- Search for the biological agent(s) involved in the incident by starting to type in the name of the agent. Select the correct biological agent(s) from the list.
- More than one biological agent can be selected.
- If the biological agent is not found leave this field blank and enter the information under "Other Biological Agents".
- Other Biological Agents (10 maximum. Use semicolons [;] to add multiple at a time)
- Only enter information in this field if the/one of the biological agents involved in the incident cannot be found in the search field of the Portal.
- Provide the name of the biological agent.
- Specify the specific strain, sub-type, and other identifiers of the biological agent, if known/applicable
- Open text box – provide any additional information (e.g., strain, subtype, serogroup, drug sensitivity), as known, for the biological agent involved in the incident.
A.2.2 Occurrence Information
- * Are/did any of the individuals exposed or infected during the incident travelling outside the province/territory during the potential incubation or infectious period?
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Indicate if travel has/will take place during the known incubation (exposed individual) or infectious (LAI suspected or confirmed) period of the respective biological agent of infection
Note: This field/question will only appear if "Yes" answered for the previous question- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Indicate if any secondary transmission (person-to-person spread) occurred following the incident
- Yes,
- No, or
- Unknown.
- * Indicate the total number of secondary cases resulting from the incident in laboratory contacts (this is the total number of secondary cases in laboratory contacts due to person-to-person spread from all primary exposed lab cases)
Note: This field/question will only appear if "Yes" selected for the previous question.- Number of secondary cases that resulted from transmission that occurred in the laboratory facility.
- Numeric field-enter a value between 0 and 999.
- * Indicate the total number of secondary cases resulting from the incident in family or other community contacts (this is the total number of secondary cases in family/community contacts due to person-to-person spread from all primary exposed lab cases).
Note: This field/question will only appear if "Yes" selected for the question "Indicate if any secondary transmission (person-to-person spread) occurred following the incident".- Number of secondary cases (individuals affected) that resulted from transmission that occurred in the home or community setting.
- Numeric field-enter a value between 0 and 999.
- * Incident date known?
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- *Incident date
Note: This field/question will only appear if "Yes" answered to the previous question.- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Date the incident was first reported to internal authority(ies)
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Indicate the location where the incident occurred
- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
- * Select the occurrence type(s) that best characterise the incident (if more than one type is significant, check all that apply)
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Provide a brief description of what occurred during the incident
Note: This field/question will only appear if "Other" selected for the previous question.- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
- * Indicate the main activity that best describes the work being undertaken during the incident
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * If main activity is "Other", describe
Note: This field/question will only appear if "Other" selected for the previous question.- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
- * Is/are the biological agent(s) involved in the incident known?
- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Biological Agents
Note: this section only appears if "Yes" selected for the previous question.- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- Specify the name(s) of the biological agent(s) involved in the incident
- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
- Other Biological Agents (10 maximum. Use semicolons [;] to add multiple at a time)
- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
- Specify the specific strain, sub-type, and other identifiers of the biological agent, if known/applicable
- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
- * Was decontamination/disinfection performed using processes and methods in accordance with applicable standards and guidelines?
Note: This is the decontamination/disinfection of materials and surfaces.- Prepopulated from the exposure notification report.
- Confirm or revise this selection, as required.
- * Additional Details
- Prepopulated from the exposure notification report.
- Confirm or revise this information, as required.
A.2.3 Risk Rating
- * Indicate, on scale of 1-5, the actual severity of the incident, where 1 represents least severe and 5 represents most severe.
- 1 – Negligible. The incident presented a minimal risk for disease in the individual/other staff (e.g., no post-exposure prophylaxis or treatment is indicated) AND there is no risk to public health.
- 2 – Minor. The incident presented a low risk for disease in the individual/other staff (e.g., illness requiring only minor medical intervention or exposure warranting only individual health monitoring) AND/OR there is a low risk to public health.
- 3 – Moderate. The incident presented a moderate risk of disease in the individual/other staff (e.g., illness requiring post exposure prophylaxis or treatment, associated with mild to moderate illness, is readily treatable or vaccine preventable) AND/OR there is a moderate risk to public health (e.g., limited spread among close contacts, no deaths, and no widespread transmission in the community).
- 4 – Major. The incident presented a high risk of severe disease/death in the individual/other staff AND/OR there is a significant risk to public health (e.g., community spread/outbreak/fatalities).
- 5 – Catastrophic. The incident resulted in a high risk of disease in the individual/other staff AND a high risk of severe public health impact (e.g., severe epidemic/high mortality). For example, an incident involving multiple exposed laboratory personnel (or multiple LAIs with missed/unknown exposure incident) with a human pathogen or toxin causing severe morbidity and mortality or irreversible health effects AND involving community spread with a risk of serious public health impact (e.g., high morbidity, mortality, introduction of emerging/non-indigenous disease, and re-introduction of eliminated or eradicated disease).
- Unknown.
- * Indicate, on a scale of 1-5, the likelihood of a recurrence of this type of incident at the severity indicated above, where 1 represents least likely and 5 represents most likely.
- 1 – Rare. This type of incident will probably never happen/recur.
- 2 – Unlikely. This type of incident is not expected to happen/recur but it is possible.
- 3 – Possible. This type of incident may happen or recur occasionally.
- 4 – Likely. This type of incident will probably happen/recur, but it is not a persistent problem/circumstance.
- 5 – Almost Certain. This type of incident will undoubtedly happen/recur and is a persistent problem/circumstance.
- Unknown.
- Risk Rating (automatically calculated)
- Populated by system.
- * Was the actual severity less than the potential severity (i.e., was there a potential for the incident to have been more severe)?
- Yes,
- No, or
- Unknown.
- * If the actual severity was less than the potential severity, indicate what type of safeguards prevented a more severe outcome. (Check all that apply).
Note: This field/question will only appear if "Yes" selected for the previous question. - This section is intended to highlight the facility's biosafety/biosecurity program elements that helped to reduce the risk/exposure to the hazard. Select all that apply in relation to what safeguards were in place at the time of the incident that prevented a more severe outcome. While this field is mandatory and must be completed by all reporters, the text fields that allow further description of these safeguards/controls are not mandatory; nevertheless, these should be completed wherever possible. This additional information may be used by the PHAC for the development of standards or guidance for laboratories across the country, many of whom may be experiencing similar incidents but may not have such safeguards/controls in place. This additional information, therefore, may result in, or contribute to, improved biosafety in laboratories across Canada.
- Engineering Controls,
- Administrative Controls, or
- Individual Controls (Human action/individual last line of defence).
- Engineering Controls (Check all that apply)
Note: This field/question will only appear if "Engineering Controls" selected for the question "If the actual severity was less than the potential severity, indicate what type of safeguards prevented a more severe outcome".- Automation or computerization, e.g., use of devices or systems removed people from error prone or high risk activities.
- Design of facilities or equipment, e.g., use of design features such as ventilation, biosafety cabinets, engineered sharps, sharps containers, sealed biological waste containers, sealable centrifuge cups, reduced error, exposure/extent of hazard.
- Forcing function and constraints, e.g., physical/design barriers prevented errors or reduced the amount, potency, or extent of exposure/contact with the hazard.
- Administrative Controls (Check all that apply)
Note: This field/question will only appear if "Administrative Controls" selected for the question "If the actual severity was less than the potential severity, indicate what type of safeguards prevented a more severe outcome".- Standardization/simplification of tools and/or processes, e.g., use of standardized equipment and measures reduced errors and/or extent, severity or duration of the hazard/exposure.
- Standards/SOPs, policies, rules, electronic procedures, drop-down menus etc., (e.g., availability and required/reinforced use of guidance such as Biosafety Manuals, Pathogen Safety Data Sheets, laboratory notebooks reduced error/hazard).
- Reminders, checklists, double checks, e.g., pop up reminders, verification sign-offs, and checklist actions reduced errors or extent of exposure/contact with the hazard.
- Individual Controls (Human action/individual last line of defence – Check all that apply)
Note: This field/question will only appear if "Individual Controls" selected for the question "If the actual severity was less than the potential severity, indicate what type of safeguards prevented a more severe outcome".- Human observation (astute staff, regular monitoring, early and appropriate response), e.g., individual awareness, strict adherence to procedures/rules, and other administrative controls prevented errors or reduced contact/exposure to the hazard.
- Personal Protective Equipment, e.g., appropriate use of individual physical barriers such as lab coat, gloves, eye protection, face shield prevented or reduced contact/exposure to the hazard.
A.2.4 Investigation and Root Cause Analysis
- * Indicate investigation team members (provide first and last name and role of each member of the investigation team)
- Open text box – provide the names of all team members and their role (e.g., facilitator, scribe, lead, subject matter expert) in the investigation.
- * Indicate the current status of the investigation
Note: The CBS reporting requirements mean that the investigation may not have been fully completed by the reporting deadline. Identify the status of the investigation at the time of reporting.- Not started,
- In process, or
- Complete.
- * Explain why the investigation has not begun OR, if the investigation has begun, describe investigational activities to date
Note: This field/question will only appear if "Not started" or "In process" selected for the previous question.- Open text box – provide the information requested.
A.2.4.1 Root Causes
Note: This section will only appear if "Complete" selected for the question "Indicate the current status of the investigation".
- * Have root causes been established for the incident based on the investigation to date?
Note: This field/question will only appear if "Complete" selected for question "Indicate the current status of the investigation".- Root causes established, analysis complete;
- Some root causes established, investigation/analysis ongoing;
- Root causes not yet established, investigation/analysis in process; or
- Unable to establish root causes, investigation terminated.
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, explain further why root causes cannot be established
Note: This field/question will only appear if "Root causes not yet established, investigation/analysis in process" or "Unable to establish root causes, investigation terminated" selected for previous question.- Open text box – provide the information requested.
A.2.4.1.1 Standards, Policies, and Procedures
- * Were there standards, policies, procedures, or other expected practice documents that guided the work/activities (these may include SOPs, requirements, written guides, instructions, rules, and checklists)?
- Yes – there were standards, policies, procedures, or other documents that guided work/activities and they did play a role in this incident;
- No – there were standards, policies, procedures, or other documents that guided work/activities in place but they did NOT play a role in this incident;
- Unknown; or
- Not applicable – there were no standards, policies, procedures, or other documents that guided work/activities in place and there was no need for them.
- The standards, policies, procedures, or other expected practice documents that guided the work were: (Check all that apply)
Note: This field/question will only appear if "Yes" selected for previous question.- Known but not followed;
- Not followed because they were not known by the user(s);
- Not followed correctly (e.g., followed as written, but may have been confusing, not detailed enough, or unclear);
- Followed but not correct for the task/activity (e.g., contained wrong information or were inadequate to address the situation); or
- Not in place but should have been in place (e.g., the nature of the hazard warrants written direction).
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, provide more detail or explanation below
Note: This field/question will only appear if "Yes" selected for the question "Were there standards, policies, procedures or other expected practice documents that guided the work/activities (these may include SOPs, requirements, written guides, instructions, rules, and checklists)?".- Open text box – provide the information requested.
A.2.4.1.2 Training
- * Was there a training issue related to the incident?
- Yes – there was a training issue related to this incident;
- No – there was adequate training in place and it did NOT play a role in this incident;
- Unknown; or
- Not applicable – there was no training needed for the work/activity.
- Training (Check all that apply)
Note: This field/question will only appear if "Yes" selected for previous question.- There was no training for the task related to the incident;
- Training was inappropriate or insufficient to support adequate understanding;
- Appropriate and sufficient training was available, but not completed; or
- Staff was/were not qualified or not proficient in performing the task related to the incident.
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, provide more detail or explanation below
Note: This field/question will only appear if "Yes" selected for the question "Was there a training issue related to the incident?".- Open text box – provide the information requested.
A.2.4.1.3 Communications
- * Was there any communications factors directly related to the incident?
- Yes – there was a communication issue related to this incident;
- No – there was adequate communication that took place and it did NOT play a role in this incident;
- Unknown; or
- Not applicable – there was no communication required for the incident.
- Communications (Check all that apply)
Note: This field/question will only appear if "Yes" selected for previous question.- There was no method or system of communication;
- No communication occurred but should have; or
- Communication occurred but was unclear, ambiguous, misunderstood, incorrect, or not detailed enough.
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, provide more detail or explanation below
Note: This field/question will only appear if "Yes" selected for the question "Was there any communications factors directly related to the incident?".- Open text box – provide the information requested.
A.2.4.1.4 Management Oversight
- * Were there problems with management and/or oversight directly related to the incident?
- Yes – there was a management system and/or oversight issue related to this incident;
- No – the management system and/or oversight was adequate AND did not play a role in this incident;
- Unknown; or
- Not applicable – there was no management system and/or oversight required for the incident.
- Management Oversight (Check all that apply)
Note: This field/question will only appear if "Yes" selected for previous question.
Note: "Enforcement" below refers to facility-driven enforcement.- No supervision of the work related to the incident as/when there should have been;
- Improvement needed in regards to supervision of the work related to the incident;
- No auditing, evaluation, or enforcement in regards to the use of standards, policies or procedures or other documents;
- Improvement needed on auditing, evaluation, or enforcement in regards to the use of standards, policies, procedure, and similar documents;
- Training lacks auditing, evaluation, or enforcement;
- Training needs improvement in regards to auditing, evaluation, or enforcement;
- Preparation needs improvement (e.g., walk-through, job planning, pre-work briefing for activities related to the incident);
- Human factors need improvement (e.g., recognition of fatigue, impairment, work load, state of mind, and team selection);
- Risk assessment conducted prior to work was not done;
- Risk assessment conducted prior to work needs improvement; or
- Worker selection needs improvement.
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, provide more detail or explanation below
Note: This field/question will only appear if "Yes" selected for the question "Were there problems with management and/or oversight directly related to the incident?".- Open text box – provide the information requested.
A.2.4.1.5 Equipment
- * Were there equipment factors that directly related to the incident?
- Yes – there was an equipment issue related to this incident;
- No – the equipment was adequate and functioning as designed and did not play a role in the incident;
- Unknown; or
- Not applicable – there was no equipment as part of the incident.
- Equipment (Check all that apply)
Note: This field/question will only appear if "Yes" selected for previous question.- Equipment design needs improvement (e.g., design does not meet specifications or specifications inadequate);
- Equipment was not properly maintained (e.g., not maintained to manufacturer or facility standards);
- Equipment maintenance needs improvement (e.g., maintenance meets specifications but equipment still failed);
- Equipment used was not fit for purpose (e.g., equipment is being used beyond its intended/recommended use);
- Quality control was not done (e.g., calibration, validation or testing was not done as/when it should have been); or
- Quality control needs improvement (e.g., calibration, validation, testing done to accepted standards but still failed).
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, provide more detail or explanation below
Note: This field/question will only appear if "Yes" selected for the question "Were there equipment factors that directly related to the incident?".- Open text box – provide the information requested.
A.2.4.1.6 Human Interaction
- * Did any human interactions or human factors related to work demands or the work environment directly relate to the incident?
- Yes – there was human interaction and/or human factors issue related to the incident;
- No – the human interaction and/or human factors were adequate/functioning as intended and did not play a role in the incident;
- Unknown; or
- Not applicable.
- Human Interaction (Check all that apply)
Note: This field/question will only appear if "Yes" selected for previous question.- The labelling, placement, operation, displays, or other functions of tools/equipment in the work environment;
- Environmental factors within the work area (e.g., temperature, obstructions, clutter, distractions and peripheral noise, surfaces, lighting, location, or materials); or
- Workload constraints, pressures, or other demands (e.g., constraints/demands interfere with staff capability to manage tasks).
- If an RG3 or RG4 biological agent or an SSBA was involved in the incident, provide more detail or explanation below
Note: This field/question will only appear if "Yes" selected for the question "Did any human interactions or human factors related to work demands or the work environment directly relate to the incident?".- Open text box – provide the information requested.
A.2.4.1.7 Other Factors
- * Were there any other factors related to the incident?
- Yes/No.
- * Specify other factors
Note: This field/question will only appear if "Yes" selected for previous question.- Open text box – provide any information regarding other factors that may be related to the incident.
A.2.5 Corrective Actions
- Indicate whether corrective actions are planned or have been taken in relation to this incident.
- Yes/No.
- Indicate the categories of corrective actions that apply for this incident (check all that apply).
Note: This field/question will only appear if "Yes" selected for previous question.- Procedures, protocols, and SOPs;
- Standards and policies;
- Training;
- Communication;
- Management system and/or oversight;
- Equipment factors;
- Human interaction or human factors; or
- Other.
- Describe each corrective action implemented or planned AND indicate the expected completion date in brackets at the end of each corrective action.
Example: "Reinforce training on proper hand washing procedures among all staff and students working in the containment zone (January 31st, 2016)".
Note: This field/question will only appear if "Yes" selected for the question "Indicate whether corrective actions are planned or have been taken in relation to this incident".- Open text box – provide the information requested.
A.2.6 Outcome
- * Has management been informed of this incident?
- Yes,
- No, or
- Unknown.
- * Explain further
Note: This field/question will only appear if "No" or "Unknown"' selected for previous question.- Open text box – provide an explanation of why management has not been informed or why the reporter does not know if management has been informed.
- * Have there been previous occurrence(s) (i.e. one or more previous similar incidents) at your location in the past?
- Yes,
- No, or
- Unknown.
- * If "Yes", indicate the disposition of corrective actions for the previous occurrence(s) that best describes why this and/or similar incidents was not prevented. Previous corrective action(s):
Note: This field/question will only appear if "Yes" selected for the previous question.- Were appropriate but were not implemented;
- Were implemented but were not appropriate or not sufficient to address the root causes and prevent recurrence; or
- Other, explain below.
- * Describe corrective actions taken to address the previous occurrences AND/OR explain why corrective actions were NOT specified and/or taken
Note: This field/question will only appear if "Yes" selected for the question "Have there been previous occurrence(s) (i.e. one or more previous similar incidents) at your location in the past?".- Open text box – provide the information requested.
- * Based on the current incident investigation and root causes, what components of your biosafety program management system could be improved to reduce the likelihood of future occurrences? (Check all that apply)
- Procedures, protocols and SOPs,
- Standards and policies,
- Training,
- Communication,
- Management system and/or oversight,
- Equipment factors,
- Human interaction or human factors,
- Unknown, or
- Other.
- If the incident involved an RG3 or RG4 biological agent or an SSBA, explain or provide details on biosafety program improvements
- Open text box – provide further information about improvements to the biosafety program.
- Additional Notes (provide any further details to describe the main essence of the incident or additional comments on investigation findings)
- Open text box – provide further information regarding the incident or investigation that has not been captured elsewhere.
A.2.7 Submission
- Reporter's Name (first, last)
- Populated by system based on user profile.
- Reporter's role in the incident (select the option that best describes your role/participation during the event)
- Directly involved in the incident;
- Witnessed the incident; not directly involved; or
- Not involved/did not witness; informed following the incident.
Appendix B – Investigating and Analyzing Incidents
B.1 Investigating Incidents
This section describes the general principles and key steps involved in conducting an incident investigation and root cause analysis. From gathering thorough and credible information to conducting a comprehensive and systematic analysis of the evidence, the ultimate aim is to prevent a recurrence of the incident. Root cause analysis is a standardised method of deconstructing what happened leading up to and during an incident to explore the factors that contributed to the incident (casual factors) and uncover the initiating causes (root causes) that underpin each of these contributing factors. Through this process, causal pathways are defined and connect each causal factor back to its corresponding root cause(s), thus enabling a thorough and objective definition of problems and gaps in the system. On this foundation, unbiased interventions that target the root problems and issues at the base of each causal pathway can be planned and implemented (corrective action plan) to prevent a recurrence of the incident.
B.1.1 General Principles
- Organizations will have site specific policies and procedures for incident management for: reporting, investigating, analyzing, correcting, and preventing underlying root causes.
- Experience in performing investigations and root cause analysis is a valuable asset; additionally, formal methods, training, and tools for investigations and root cause analysis will reduce variability between users.
- Investigations and analyses may be performed in numerous ways, using various methods and tools; however, they should always be both thorough and credible in leading investigators to root causes and corrective and preventive actions.
- A thorough investigation is:
- Systematic and focuses on processes/systems, not individuals;
- An exploration of what happened and why, and should include a systematic "who, what, where, when, and why" based query;
- An inquiry into all of the causal factors and associated root causes that may have contributed to the incident (including a review of the human factors, equipment, systems, and processes in place); and
- A determination of corrective and preventive actions that lead to improvements in processes or systems that address root causes.
- A credible investigation:
- Includes participation by the internal authority, such as the BSO or other designate for the licence holder;
- Includes individuals that are most closely involved in the processes and systems under review;
- Includes relevant/appropriate subject matter experts with in-depth knowledge of processes/systems under review, when needed;
- Must be logical, consistent, and complete (i.e., it is conducted in a clear and systematic manner, does not contradict itself, is not biased, explains all findings, and does not leave obvious questions unanswered); and,
- Has an action plan that identifies changes that can be implemented to eliminate or significantly reduce the risk of recurrence of the root causes.
B.1.2 Conducting an Investigation and Follow-up Actions
This section provides a high-level overview of the key steps involved in an investigation process. This is intended as guidance only and is provided as an aid for collecting, organizing, and analyzing the facts surrounding an incident.
Additional guidance on incident reporting and investigation can be found in Chapter 18 of the CBH. For specific requirements and timelines for exposure notification and reporting refer to matrix 4.9 of the CBS and corresponding explanatory notes.
Figure B-1 illustrates the general steps involved in performing an incident investigation. Although the time required to conduct each step of the investigation will depend on the type, complexity, and severity of the incident, some approximate timelines are provided below. For the most part, steps will proceed in a sequential manner, although some elements of each step may overlap in time. For example, collection of information (beginning of Step 2) may begin before or at the same time that a notification (end of Step 1) is being prepared and submitted to internal (within the institution) or external (to the PHAC and/or other regulators) authorities.
Figure B-1: Incident Investigation, Steps, and Approximate Timelines
| Step | Timeline |
|---|---|
| Step 1 0-72 hours |
|
| Step 2 0-10 days |
|
| Step 3 0-3 weeks |
|
| Step 4 0-1 month |
|
| Step 5 3-12 months |
|
B.1.3 Initial Response
Although outside the scope of this guidance document, which aims to provide guidance for incident notification and reporting to the PHAC, the initial response to an incident (prior to notification/reporting at the latter end of this step) is critical for assessing the incident and responding to hazards. These immediate response actions can include providing first aid and/or other emergency services to the affected individuals, and controlling and/or containing the hazard to prevent/mitigate further harm. In addition, assessing available information, checking equipment status, documenting the names of persons involved in the event, and securing and preserving initial evidence should be part of the initial response once personnel have been treated and hazards have been controlled. Likewise, the formation of an investigation team with a defined scope, objectives, and roles should occur very early in the investigation process. This investigation team will likely consist of the BSO and others, depending on the type of incident.
At the latter end of the initial response step, an incident assessment, including exposure assessment, should be conducted to characterise the incident for immediate reporting to internal authorities and determine whether the incident requires immediate notification to external authorities (i.e., PHAC).
Notification to the PHAC without delay (i.e., as soon as reasonably possible) permits time for immediate response and control measures to be taken, while still providing a timely alert to the PHAC.
B.1.4 Collection of Evidence and Information
The amount and type of evidence/information gathered to describe and analyze a given incident will vary depending on the severity, complexity, extent, and/or uniqueness of the incident. The evidence collected must be sufficient to thoroughly document what happened and describe who, when, where, and how the event unfolded. For incidents that may not need to be formally reported (e.g., an incident involving a human pathogen or toxin where no known exposure, release, inadvertent possession, or other reportable incident is thought to have occurred), the incident should be investigated (as a near miss) and be fully documented should there be a need to reassess the evidence in the future. For example, if an incident was earlier ruled out as an exposure but later an individual who was in close proximity develops an associated illness, within a relevant timeframe, the investigation details should be reviewed to explore the possibility of an unrecognized exposure linked to the incident.
Initial information gathered may include (but is not limited to):
- Photos, sketches, or video recordings that can provide images of physical evidence;
- Details and observations of those involved in the occurrence. In more serious scenarios there may be a need to obtain a written (witness) statement from those involved as soon as possible so that the information they recall is still fresh in their memory;
- Physical evidence – tools, equipment, and samples/specimens; personal protective equipment worn and available;
- Environmental conditions (e.g., lighting, ventilation, testing conditions);
- Details of the other tasks going on in the lab and personnel; and
- Documents (e.g., policies, SOPs, training, competency records, maintenance logs, test results, training materials, standards of work, electronic records, audit/observation data, previous incident reports).
B.1.4.1 Incident timeline
A timeline is a simple yet effective way to identify gaps and missing information. Any associated information or conditions about the actions that contributed to the incident should be included in the timeline. It is difficult to determine if details are relevant until after the timeline is built.
B.1.4.2 Causal factors
The causal factors are errors or failures that, if corrected or eliminated, could have (i) prevented the incident from occurring or (ii) mitigated its results. For most incidents, there are multiple causal factors, any one of which could have changed the outcome of the incident. It is a good practice to take time with all those involved in the incident and any subject matter experts assisting with the investigation to come to an agreement on causal factors.
B.1.5 Analysis and Identification of Root Causes
As part of the incident investigation, the root cause(s) for each identified causal factor should be established. Root causes are the most basic, underlying reason a problem or causal factor exists. Most root cause analyses start by identifying general problems then continued questioning until specific, underlying system weaknesses, failures, or gaps have been defined that could have prevented the causal factor from existing.
- Root causes often point to a missing or poorly functioning part of a system and are something the organization has control to correct or fix.
- For each causal factor, there may be more than one root cause that allowed the causal factor to exist and contribute to the incident.
- There are many elements of a work system that should be considered when performing a thorough investigation.
- The elements of the system may vary based on the tool used to determine root causes; however, the system review should be thorough and systematic and include elements of a well-designed work system such as:
- Mechanisms that establish the ways in which work is performed, such as procedures, policies, requirements, standards, guidance, and training;
- Oversight of work through supervision activities, management systems, audits, evaluations, and tests;
- Human factors, such as competence, fitness for work, communication, team dynamics, cognitive demands, input for decision making factors, tool function, and ergonomics;
- The work environment, which includes the physical environmental conditions; and
- Equipment and system management, operation, and maintenance.
B.1.6 Corrective and Preventive Actions
Corrective and preventive actions eliminate or mitigate the root causes of an exposure/incident, or reduce the likelihood or consequence of a similar occurrence happening again.
- The actions should be based on a hierarchy of controls, starting with engineering controls that eliminate the hazard at the most effective end of the hierarchy, followed by controls measures that minimize the hazard in combination with engineering controls that reduce the magnitude or likelihood of events.
- Administrative controls, such as SOPs, checklists, training, signage, supervision, and coaching, can improve performance but tend to provide less reliable controls and should be used to provide "defence in depth" (i.e., multiple and overlapping layers of protection that are used in managing significant hazards).
- Corrective actions can address multiple root causes, and should be developed and documented in a way that ensures these actions are specific, measurable, achievable, reasonable, and timely.
- Corrective and preventive controls can include both immediate, short term, and long term actions.
- Each corrective and preventive action should identify individual(s) responsible for developing and implementing the action.
B.1.7 Evaluation and Continual Improvement
- Corrective actions should be monitored to ensure they are fully implemented in a timely manner.
- Each corrective and preventive action should identify individual(s) responsible for monitoring and evaluation.
- It is important to review the effectiveness of preventive and corrective actions to ensure that the identified root cause(s) is/are being addressed/controlled over time and that the corrective action is sustainable.
- If there are repeat occurrences with the same root causes, a more thorough and comprehensive review may need to take place or a more robust recommendation may need to be developed.