Archived: SARS-CoV-2 variant rapid risk assessment report: XBB.1.5
Download in PDF format
(616 KB, 10 pages)
Organization: Public Health Agency of Canada
Date published: 2023-01-27
Assessment completed: January 20, 2023
On this page
- Background
- Risk statement
- Risk assessment summary
- Proposed actions for public health authorities
- Appendix 1: Supporting information
- Appendix 2: Methods
- References
Background
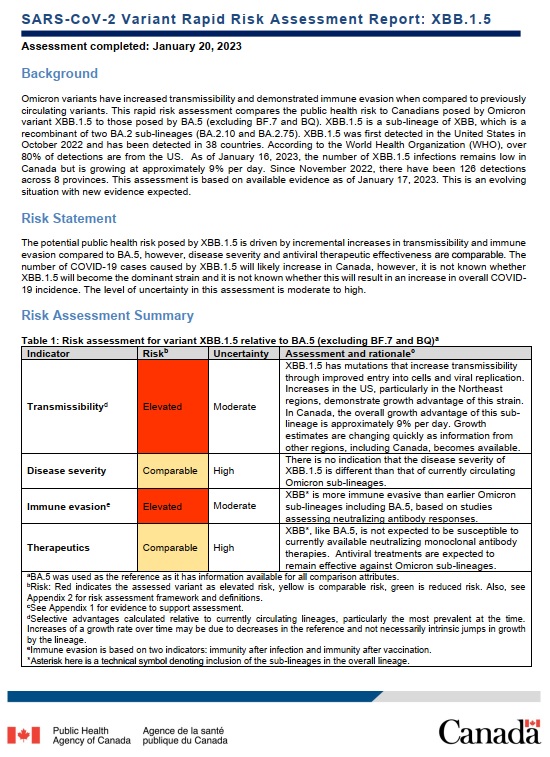
Omicron variants have increased transmissibility and demonstrated immune evasion when compared to previously circulating variants. This rapid risk assessment compares the public health risk to Canadians posed by Omicron variant XBB.1.5 to those posed by BA.5 (excluding BF.7 and BQ). XBB.1.5 is a sub-lineage of XBB, which is a recombinant of two BA.2 sub-lineages (BA.2.10 and BA.2.75). XBB.1.5 was first detected in the United States in October 2022 and has been detected in 38 countries. According to the World Health Organization (WHO), over 80% of detections are from the US. As of January 16, 2023, the number of XBB.1.5 infections remains low in Canada but is growing at approximately 9% per day. Since November 2022, there have been 126 detections across 8 provinces. This assessment is based on available evidence as of January 17, 2023. This is an evolving situation with new evidence expected.
Risk statement
The potential public health risk posed by XBB.1.5 is driven by incremental increases in transmissibility and immune evasion compared to BA.5, however, disease severity and antiviral therapeutic effectiveness are comparable. The number of COVID-19 cases caused by XBB.1.5 will likely increase in Canada, however, it is not known whether XBB.1.5 will become the dominant strain and it is not known whether this will result in an increase in overall COVID-19 incidence. The level of uncertainty in this assessment is moderate to high.
Risk assessment summary
| Indicator | RiskTable 1 Footnote b | Uncertainty | Assessment and rationaleTable 1 Footnote c |
|---|---|---|---|
| TransmissibilityTable 1 Footnote d | Elevated | Moderate | XBB.1.5 has mutations that increase transmissibility through improved entry into cells and viral replication. Increases in the US, particularly in the Northeast regions, demonstrate growth advantage of this strain. In Canada, the overall growth advantage of this sub-lineage is approximately 9% per day. Growth estimates are changing quickly as information from other regions, including Canada, becomes available. |
| Disease severity | Comparable | High | There is no indication that the disease severity of XBB.1.5 is different than that of currently circulating Omicron sub-lineages. |
| Immune evasionTable 1 Footnote e | Elevated | Moderate | XBBTable 1 Footnote * is more immune evasive than earlier Omicron sub-lineages including BA.5, based on studies assessing neutralizing antibody responses. |
| Therapeutics | Comparable | High | XBBTable 1 Footnote *, like BA.5, is not expected to be susceptible to currently available neutralizing monoclonal antibody therapies. Antiviral treatments are expected to remain effective against Omicron sub-lineages. |
|
|||
Proposed actions for public health authorities
These actions are for consideration by jurisdictions according to their local epidemiology, policies, resources, and priorities. Due to the current level of uncertainty associated with XBB.1.5, it is important that the public health response be proportionate to the risk.
Surveillance and reporting
- Continue to monitor for changes in XBB.1.5 epidemiology, particularly related to growth rate, severity of cases and immune evasion.
- Continue sequencing SARS-CoV-2 to understand circulating strains.
- Continue to share emerging evidence between local, provincial/territorial and federal levels to inform the public health response.
Risk communication
- Continue regular communication with Canadians and health professionals on the current COVID-19 situation and share associated guidance. When necessary, correct and counter mis- or disinformation.
Public health interventions
- Continue public health interventions based on local epidemiology. There are no changes to the federal guidance on individual public health measures, COVID-19 vaccination, infection prevention and control measures and the use of antivirals.
Appendix 1: Supporting information
| Indicator | Evidence |
|---|---|
Transmissibility |
International
Domestic
|
Disease severity |
International
Domestic
|
Immune evasionTable 2 Footnote a |
Neutralizing antibody responses
Cellular responses
There is no data on vaccine effectiveness against XBB.1.5 infection or severe outcomes as well as no data on protective effectiveness from a prior infection against XBB-related severe outcomes. |
Therapeutics |
Considerations
|
|
|
| Criteria | Considerations |
|---|---|
Health systems impact, including hospital capacity and infection prevention and control measures |
|
Border measures |
|
Surveillance capacity |
|
Public health measures |
|
Vaccination |
|
Appendix 2: Methods
For the variant risk assessment framework and criteria for estimating the level of uncertainty, consult:
References
- Footnote 1
-
Yue C, Song W, Wang L, et al. Enhanced transmissibility of XBB.1.5 is contributed by both strong ACE2 binding and antibody evasion. bioRxiv. 2023:2023.01.03.522427. doi:10.1101/2023.01.03.522427 Available at: https://www.biorxiv.org/content/10.1101/2023.01.03.522427v1
- Footnote 2
-
World Health Organization (WHO). XBB.1.5 Rapid risk assessment, 11 January 2023. Geneva: WHO; 2023. https://www.who.int/docs/default-source/coronaviruse/11jan2023_xbb15_rapid_risk_assessment.pdf
- Footnote 3
-
covSPECTRUM. Detect and analyze variants of SARS-CoV-2. https://cov-spectrum.org/
- Footnote 4
-
Centers for Disease Control and Prevention (CDC). COVID Data Tracker Weekly Review. Atlanta, GA: CDC. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
- Footnote 5
-
Public Health Ontario. SARS-CoV-2 Genomic Surveillance in Ontario, January 13, 2023. https://www.publichealthontario.ca/-/media/Documents/nCoV/epi/covid-19-sars-cov2-whole-genome-sequencing-epi-summary.pdf?rev=6fc38d12cd6a45dfb100fb85a5604a0f&sc_lang=en
- Footnote 6
-
European Centre for Disease Prevention and Control. Implications for the EU/EEA of the spread of the SARS-CoV-2 Omicron XBB.1.5 sub-lineage for the EU/EEA – 13 January 2023. ECDC: Stockholm; 2023. https://www.ecdc.europa.eu/en/publications-data/covid-19-threat-assessment-brief-implications-spread-omicron-xbb
- Footnote 7
-
World Health Organization (WHO). TAG-VE statement on Omicron sublineages BQ.1 and XBB. Geneva: WHO. https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sublineages-bq.1-and-xbb
- Footnote 8
-
Uriu K, Ito J, Zahradnik J, et al. Enhanced transmissibility, infectivity and immune resistance of the SARS-CoV-2 Omicron XBB.1.5 variant. bioRxiv. 2023:2023.01.16.524178. doi:10.1101/2023.01.16.524178. Available at: https://www.biorxiv.org/content/10.1101/2023.01.16.524178v1
- Footnote 9
-
Qu P, Faraone JN, Evans JP, et al. Extraordinary Evasion of Neutralizing Antibody Response by Omicron XBB.1.5, CH.1.1 and CA.3.1 Variants. bioRxiv. 2023:2023.01.16.524244. doi:10.1101/2023.01.16.524244. Available at: https://www.biorxiv.org/content/10.1101/2023.01.16.524244v1
- Footnote 10
-
Uraki R, Ito M, Furusawa Y, et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis. Jan 2023;23(1):30-32. doi:10.1016/S1473-3099(22)00816-7. Available at: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00816-7/fulltext
- Footnote 11
-
Akerman A, Milogiannakis V, Jean T, et al. Emergence and antibody evasion of BQ and BA.2.75 SARS-CoV-2 sublineages in the face of maturing antibody breadth at the population level. medRxiv. 2022:2022.12.06.22283000. doi:10.1101/2022.12.06.22283000. Available at: https://www.medrxiv.org/content/10.1101/2022.12.06.22283000v1
- Footnote 12
-
Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. bioRxiv. 2022:2022.11.23.517532. doi:10.1101/2022.11.23.517532. Available at: https://www.biorxiv.org/content/10.1101/2022.11.23.517532v1.full
- Footnote 13
-
Tamura T, Ito J, Uriu K, et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. bioRxiv. 2022:2022.12.27.521986. doi:10.1101/2022.12.27.521986. Available at: https://www.biorxiv.org/content/10.1101/2022.12.27.521986v1.full
- Footnote 14
-
Vikse EL, Fossum E, Erdal MS, Hungnes O, Bragstad K. Poor neutralizing antibody responses against SARS-CoV-2 Omicron BQ.1.1 and XBB in Norway in October 2022. bioRxiv. 2023:2023.01.05.522845. doi:10.1101/2023.01.05.522845. Available at: https://www.biorxiv.org/content/10.1101/2023.01.05.522845v1.full
- Footnote 15
-
Chalkias S, Whatley J, Eder F, et al. Safety and Immunogenicity of Omicron BA.4/BA.5 Bivalent Vaccine Against Covid-19. medRxiv. 2022:2022.12.11.22283166. doi:10.1101/2022.12.11.22283166. Available at: https://www.medrxiv.org/content/10.1101/2022.12.11.22283166v1
- Footnote 16
-
Zou J, Kurhade C, Patel S, et al. Improved Neutralization of Omicron BA.4/5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent BA.4/5 Vaccine. bioRxiv. 2022:2022.11.17.516898. doi:10.1101/2022.11.17.516898. Available at: https://www.biorxiv.org/content/10.1101/2022.11.17.516898v1
- Footnote 17
-
Kurhade C, Zou J, Xia H, et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1, and XBB.1 by 4 doses of parental mRNA vaccine or a BA.5-bivalent booster. bioRxiv. 2022:2022.10.31.514580. doi:10.1101/2022.10.31.514580. Available at: https://www.biorxiv.org/content/10.1101/2022.10.31.514580v2.full
- Footnote 18
-
Davis-Gardner ME, Lai L, Wali B, et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N Engl J Med. Jan 12 2023;388(2):183-185. doi:10.1056/NEJMc2214293 Available at: https://www.nejm.org/doi/full/10.1056/NEJMc2214293
- Footnote 19
-
Muik A, Lui BG, Diao H, et al. Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T-cell recognition epitopes. bioRxiv. 2022:2022.12.15.520569. doi:10.1101/2022.12.15.520569. Available at: https://www.biorxiv.org/content/10.1101/2022.12.15.520569v1.full.pdf
- Footnote 20
-
Tan CY, Chiew CJ, Pang D, et al. Protective Effectiveness of Natural SARS-CoV-2 Infection and Vaccines against Omicron BA. 4/BA. 5 and XBB Reinfection in Singapore: A National Cohort Study. Available at SSRN 4308740. 2022. Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4308740
- Footnote 21
-
Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. bioRxiv. 2022:2021.12.27.474275. doi:10.1101/2021.12.27.474275. Available at: https://www.biorxiv.org/content/10.1101/2021.12.27.474275v2
- Footnote 22
-
Imai M, Ito M, Kiso M, et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N Engl J Med. Jan 5 2023;388(1):89-91. doi:10.1056/NEJMc2214302. Available at: https://www.nejm.org/doi/full/10.1056/NEJMc2214302
- Footnote 23
-
Government of Canada. COVID-19 vaccination in Canada. https://health-infobase.canada.ca/covid-19/vaccination-coverage/