Recommendations on the Duration of the Post-vaccination Observation Period for Influenza Vaccination during the COVID-19 Pandemic
An Advisory Committee Statement (ACS)
National Advisory Committee on Immunization (NACI)
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (hereafter referred to as PHAC) with ongoing and timely medical, scientific, and public health advice relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the consideration of programmatic factors in developing evidence-based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be considered by NACI include: economics, ethics, equity, feasibility, and acceptability. Over the coming years NACI will be refining methodological approaches to include these factors. Not all NACI Statements will require in-depth analyses of all programmatic factors. As NACI works towards full implementation of the expanded mandate, select Statements will include varying degrees of programmatic analyses for public health programs.
PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Download the alternative format
(PDF format, 41 pages)
Organization: Public Health Agency of Canada
Publication Date: 2020-10-15
Related topics
Table of contents
- Summary of the Information Contained in this NACI Statement
- I. Introduction
- II. Methods
- III. Onset of adverse events following immunization
- IV. Recommendations
- Tables and Figures
- List of Abbreviations
- Acknowledgments
- References
Summary of the Information Contained in this NACI Statement
The following highlights key information for immunization providers. Please refer to the remainder of this statement for details.
1. What
- It is important to continue routine immunization programs during the COVID-19 pandemic, especially for the upcoming influenza season, which will prevent influenza-related morbidity and mortality and contribute to reducing the burden on the Canadian health care system. This is particularly important in anticipation of a potential resurgence in COVID-19 in the fall or winter months.
- During the COVID-19 pandemic, large crowds at influenza immunization clinics could contribute to increased SARS-CoV-2 transmission risks if not managed appropriately; therefore, adjustments to regular immunization practices and clinic design may be required.
- In addition to other public health and infection prevention and control measures, reducing the post-vaccination observation period in post-vaccination observation areas provides an option to reduce crowding. Decreasing close interactions among vaccine recipients and between vaccine recipients and clinic staff may help to mitigate against potential SARS-CoV-2 transmission in clinic settings. Vaccine recipients who do not meet the criteria for a reduced post-vaccination observation period will also benefit from a less crowded post-vaccination waiting area. This intervention may also allow more individuals to be vaccinated in a given time period (increase vaccination setting "throughput").
2. Who
- Settings providing influenza vaccinations for the upcoming influenza season during the COVID-19 pandemic.
3. How
- NACI recommends that the current post-vaccination observation period, as specified in the Canadian Immunization Guide Reference a, should be maintained for influenza vaccination settings that can adhere to appropriate public health and infection prevention and control measures Reference b to reduce SARS-CoV-2 transmission, particularly physical distancing (Strong NACI recommendation).
- NACI concludes that there is fair evidence to maintain a 15-minute post-vaccination observation period during the COVID-19 pandemic for individuals with no known history of severe allergic reactions (including anaphylaxis) to any component of the influenza vaccine being considered for administration or any history of other immediate post-vaccination reactions (e.g., syncope with or without seizure) (Grade B Evidence).
- NACI recommends that a shorter post-vaccination observation period, between 5 to 15 minutes after influenza immunization, may be considered during the COVID-19 pandemic, but only during times when appropriate physical distancing in post-vaccination waiting areas cannot otherwise be maintained due to the volume of individuals seeking immunization, and only when specific conditions are metEndnotes * (Discretionary NACI recommendation).
- NACI concludes that there is insufficient evidence from retrospective, passive adverse event following immunization (AEFI) case series reports to support a reduced post-vaccination observation period. However, other factors may be taken into consideration to support a reduced post-vaccination observation period, if necessary for public health and infection prevention and control purposes (Grade I Evidence).
Endnotes *
- Endnotes *
-
A shorter observation period may be considered only if the vaccine recipient meets the following conditions Reference c:
- Past history of receipt of influenza vaccine and no known history of severe allergic reactions (including anaphylaxis) to any component of the influenza vaccine being considered for administration (note that novel technology vaccine recipients should be exempt from reduced post-observation period eligibility) Reference d.
- No history of other immediate post-vaccination reactions (e.g., syncope with or without seizure) after receipt of any vaccines.
- The vaccine recipient is accompanied by a parent/guardian (in the case of a child) or responsible adult who will act as a chaperone to monitor the vaccine recipient for a minimum of 15 minutes post-vaccination. In the case of two responsible adults, both can be vaccine recipients for the purposes of this criterion, if both agree to monitor the other post-vaccination.
- The vaccine recipient will not be operating a motorized vehicle or self-propelled or motorized wheeled transportation (e.g., bicycle, skateboard, rollerblades, scooter), or machinery for a minimum of 15 minutes after vaccination.
- The vaccine recipient and the parent/guardian or responsible adult chaperone are aware of when and how to seek post-vaccination advice and given instructions on what to do if assistance and medical services are required.
- The vaccine recipient and the parent/guardian/responsible adult agree to remain in the post-vaccination waiting area for the post-vaccination observation period and to notify staff if the recipient feels or looks at all unwell before leaving. They should be informed that an individual exhibiting any symptom suggestive of an evolving AEFI at the end of the shortened post-observation period necessitates a longer period of observation in the clinic.
4. Why
- A vaccination clinic setting that is able to maintain recommended public health and infection prevention and control measures, such as physical distancing in post-vaccination areas, is unlikely to achieve significant additional reductions in the risk of SARS-CoV-2 transmission by implementing a shorter post-vaccination observation period.
- A vaccination clinic setting that at times is unable to maintain recommended physical distancing in post-vaccination areas may consider implementing a shorter post-vaccination observation period to reduce the risk of SARS-CoV-2 transmission under specified conditions.
- Serious adverse events in the minutes following immunization that may require medical intervention are relatively rare.
I. Introduction
A cluster of cases of pneumonia of unknown origin was reported from Wuhan, Hubei Province, China in December 2019. These cases were determined to be due to a novel coronavirus (SARS-CoV-2) that causes a disease now referred to as coronavirus disease 2019 (COVID-19). The World Health Organization (WHO) characterized COVID-19 as a pandemic on March 11, 2020Footnote 1.
Canadian provinces and territories instituted a series of public health measures to limit SARS-CoV-2 transmission and to reduce the burden on the healthcare system, including the temporary deferral of non-essential medical services. Recognizing the need to continue routine immunization programs during the pandemic, the Public Health Agency of Canada (PHAC), with consultation from the National Advisory Committee on Immunization (NACI) and the Canadian Immunization Committee, released interim guidance on priority populations for immunization programs in Canada,Footnote 2 as well as guidance on strategies and modifications of usual immunization clinic processes for the upcoming influenza season to reduce the risk of SARS-CoV-2 transmission in a variety of immunization clinic settingsFootnote 3.
There are a variety of process modifications (administrative, environmental and engineering controls, and personal protective measures) that can be implemented in the vaccination clinic setting to prevent SARS-CoV-2 transmission, such as promoting physical distancing (at least two metres) among vaccine recipients, and staff and their co-workers. Another possible measure may be to reduce the post-vaccination observation period for rare, but serious AEFIs. Reducing the amount of time vaccine recipients spend in the vaccination clinic post-vaccination could reduce the opportunity for SARS-CoV-2 transmission from an unrecognized, infectious COVID-19 case. However, any consideration of a reduction in post-vaccination observation period must weigh the potential risk of delayed identification of an adverse event that may require immediate medical intervention against the potential benefits of reducing the risk of SARS-CoV-2 transmission and allowing more individuals to be vaccinated (increase vaccination clinic "throughput") in a given time period.
In Canada, it is recommended that vaccine recipients remain under observation for at least 15 minutes after vaccination, unless there is a specific concern about a possible vaccine allergy in which case a 30-minute post-vaccination observation period is considered a safer intervalFootnote 4. Guidelines for post-vaccination observation in other countries vary Footnote 5Footnote 6Footnote 7Footnote 8. In April 2020, the Australian Technical Advisory Group on Immunization (ATAGI) released a recommendation that the post-vaccination observation period could be reduced from 15 minutes to 5 minutes in immunization clinics where adequate physical distancing was not possible, as long as specific criteria were metFootnote 9. The ATAGI guidance was based on a targeted review of the literature and expert opinion.
Guidance objective
The objectives of this NACI statement are to review the evidence on the timing of onset of serious AEFIs (anaphylaxis, syncope with or without seizure). These data will be used to assess the potential impact of a reduction in the post-vaccination observation period on the risk of delayed identification of a serious adverse event that may require immediate medical intervention, balanced against the potential benefits of reducing SARS-CoV-2 transmission in influenza vaccination settings and allowing more individuals to be vaccinated in a given time period. The analysis will be used to provide guidance to provincial and territorial immunization programs and frontline clinicians on the duration of the post-vaccination observation period for influenza vaccination during the COVID-19 pandemic.
II. Methods
Rapid literature review
In preparation for influenza vaccination clinics in fall 2020, a rapid review of literature was conducted to determine the likelihood of missing serious AEFIs (anaphylaxis, syncope with or without seizure) with shorter post-vaccination observation periods. The rapid review's search strategy was developed in conjunction with a federal Reference Librarian and was used to search a single electronic database for studies on the timing of serious AEFIs in children and adults (all ages and populations).
Research question
What is the minimum observation period needed post-vaccination to observe serious AEFI?
- P (population):
- Individuals 6 months of age and older
- I (intervention):
- Observation period post-vaccination, any vaccine
- C (comparison):
- N/A
- O (outcomes):
- Time to onset of serious AEFI
The electronic database (EMBASE) was searched from inception to May 22, 2020 using the following search terms: seizure, syncope, anaphylaxis, adverse event, immunization, vaccination reaction, allergic reaction, and post-vaccination reaction. Searches were restricted to articles published in English and French. Two reviewers (NF, PDP) independently screened the titles and abstracts and full-text of eligible articles, but not in duplicate. The PRISMA Flow Diagram is shown in Appendix A. The complete search strategy is available upon request.
Studies were included if they met the following criteria:
- The study recorded the specific time of onset of at least a subset of AEFIs and reported events that occurred within 30 minutes of vaccination; and
- The study assessed the incidence of the following serious AEFIs:
- anaphylaxis, syncope with or without seizure.
Studies were excluded if they met one or more of the following criteria:
- The study was in a language other than English or French;
- The article was an editorial, opinion, conference abstract or news report;
- The minimum observation period for AEFIs was greater than 30 minutes; or
- The study did not report the time to onset of serious AEFIs.
The eligible studies that were identified were divided amongst three reviewers (NF, PDP, GC). The reviewers independently extracted data from their assigned studies into evidence tables and appraised the risk of bias of the studies. The data extraction and risk of bias assessments were not done in duplicate for any of the eligible studies.
The extracted data were used to summarize key study characteristics and to analyze the studies for risk of bias, using a modified Institute of Health Economics Tool (IHE)Footnote 10, as all included studies were case reports or case series. A narrative synthesis of the extracted data was created to summarize the outcomes of interest (number of AEFIs, severity, time to onset, and incidence, if reported). As not all of the identified articles evaluated outcomes by severity, the number of serious reports identified were compiled from reports that were explicitly classified as serious, as well as from inference from stated sequelae of individual cases that would meet usual serious outcome definitions (e.g., death, life-threatening illness, hospitalization, prolonged hospitalization, permanent disability).
Canadian Adverse Events Following Immunization Surveillance System
The Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) database was searched for AEFI reports of anaphylaxis, syncope, and afebrile seizure associated with a date of vaccine administration between 2014 and 2018. The Medical Dictionary for Regulatory Activities (MedDRA) terms used to code CAEFISS reports were used to search CAEFISS for reports for the three outcomes of interest. Reports of anaphylaxis had to meet the Brighton Collaboration definition for anaphylaxis Footnote 11 and the individual in the report managed as such. CAEFISS uses the World Health Organization definition of a serious AEFIFootnote 12: an event that is life threatening, results in hospitalization or a prolongation of hospitalization, persistent or significant disability or where the outcome is a birth defect or death. Reports that did not include a time to onset of symptoms were excluded.
The time to symptom onset for each AEFI was summarized by 5-minute intervals within the first 15 minutes post-vaccination, if available; and the time of onset that includes 25%, 50%, 75%, 95%, and 100% of all reports. These summary measures were analyzed by age groups: less than 2 years, 2 to less than 7 years, 7 to less than 18 years, 18 to less than 50 years, 50 to less than 65 years, 65 years and older, all ages, and by all reports versus serious AEFI.
Qualitative assessment of reducing the post-vaccination observation period
It is challenging to accurately quantify the impact of a reduction in the post-vaccination observation period on the risk for SARS-CoV-2 transmission in a vaccination setting. This is particularly the case because the clinic settings in Canada are diverse; community prevalence of SARS-CoV-2 varies between communities and fluctuates over time within communities; and because this measure would be one of several public health and infection prevention and control measures implemented to reduce the risk of transmission in these settings. Consequently, a qualitative assessment of this intervention was undertaken to weigh the potential risk of any potential delay in identifying serious AEFIs that may require immediate medical intervention against the potential benefit of reducing the risk of SARS-CoV-2 transmission and enhancing the ability to vaccinate more individuals during a given time period (increase vaccination clinic "throughput").
Recommendation Development
The draft statement was developed through the NACI Influenza Working Group and in consultation with the NACI Vaccine Safety Working Group. These three analyses, outlined above, were then used by NACI to draft recommendations on whether to reduce the duration of post-vaccination observation period in the context of the COVID-19 pandemic. NACI critically appraised the available evidence and approved the final recommendation on August 13, 2020.
III.Onset of adverse events following immunization
Twenty-two studies on time to onset of serious AEFI were identified through the rapid review. All studies were evaluated for risk of bias using a modified IHE tool and all were deemed to be at serious risk of bias, except two studies, which were deemed to be at unclear risk of biasFootnote 14Footnote 19. Both studies with unclear risk of bias described the incidence of anaphylaxis after vaccine administration using a restricted set of ICD-9 codes to search a health care database, a process deemed to be more sensitive at identifying anaphylaxis episodes than relying on reports submitted to passive AEFI reporting systems. McNeil et al.Footnote 19 used the same code set used by Bohlke et al.Footnote 14, but supplemented their search with allergy codes combined with epinephrine-dispensing codes. The majority of the included articles used data from passive surveillance systems, and had limitations such as underreporting, insufficient information on cases reported, lack of denominators and inconsistent case definitions. For measurement of whether the intervention of interest was described, articles were considered to be of low bias if they reported the vaccine name and manufacturer and whether this was the first or subsequent immunization dose, if relevant. A visual summary of the risk of bias assessments is provided in Figure 2.
The findings from the rapid review must be interpreted with caution in light of the findings of the risk of bias assessment for the articles identified in the rapid review and the inherent limitations of data obtained from retrospective case series studies that rely on passive AEFI surveillance systems. For example, the voluntary nature of reporting in most of these systems can lead to underreporting of AEFIs; the retrospective nature of these studies may make it difficult to obtain the necessary clinical data to verify outcomes and to determine that outcomes meet case definitions; the studies may also not have access to data on the sequelae for individuals in case reports, which may lead to an underestimation of the seriousness of some outcomes. There is often no access to denominator data (i.e., the number of vaccine doses distributed or ideally the number of doses administered) to estimate the incidence of outcomes. Also estimates of incidence based on relatively few case reports may be unreliable (i.e., relatively few additional case reports can significantly alter the calculated rate) and rates generated in heterogeneous populations may not be comparable.
III.1 Anaphylaxis
Rapid literature review
Time to symptom onset of anaphylaxis
There were 16 articles (12 retrospective case series Footnote 13 Footnote 14 Footnote 15 Footnote 16 Footnote 17 Footnote 18 Footnote 19 Footnote 20 Footnote 21 Footnote 22 Footnote 23 Footnote 24, 4 case reportsFootnote 25 Footnote 26 Footnote 27 Footnote 28) identified in the rapid review that provide data on the timing of reports of anaphylaxis following vaccine administration. The retrospective case series primarily use AEFI surveillance systems to examine reports of anaphylaxis in vaccine recipients. Across all of the identified articles, 981 reports of anaphylactic reactions were described, with 816 (83%) reports assessed against the Brighton Collaboration definition of anaphylaxis and the criteria for the three levels of diagnostic certainty (level 1: 413 reports; level 2: 372; level 3: 14; other/level not reported: 17). Of the 981 reports of anaphylaxis, 729 (74%) of the reports were classified as serious.
Overall, 873 (89%) of the anaphylaxis reports had some time to symptom onset recorded, of which the majority (492/873, 56%) occurred within 30 minutes of vaccination. The majority of the data (735/873, 84%) came from a single study using reports from the US Department of Health and Human Service's Vaccine Adverse Events Reporting System (VAERS) database, which categorized onset times into relatively broad time intervals (within 30 minutes, 30-119 minutes, 2-4 hours, 4-8 hours and 8-24 hours)Footnote 23.
There were 8 retrospective case series Footnote 13 Footnote 14 Footnote 15 Footnote 16 Footnote 17 Footnote 19 Footnote 22 Footnote 24 and 3 case reports Footnote 25 Footnote 26 Footnote 28 that identified 55 reports that provided descriptions of or actual times to anaphylaxis symptom onset in intervals within the first 15 minutes post-vaccination. Of these 55 reports, 35 (64%) occurred between 0 and 5 minutes post-vaccination, 4 (7%) "within minutes" or "within a few minutes," 9 (16%) between 5 and 10 minutes, 1 (2%) between 10 and 15 minutes, and 6 (11%) within 5 to 15 minutes.
Three of the retrospective case series studies Footnote 13 Footnote 20 Footnote 23 provided summary measures of the time to anaphylaxis symptom onset after vaccination. Baxter et al. evaluated the Australian Surveillance of Adverse Events following Vaccination in the Community (SAEFVIC) database for reports of anaphylaxis occurring within the first 60 minutes following immunization in preschool aged (<5 years) children. The study found all 12 identified anaphylaxis reports had a time of symptom onset of 0-40 minutes, with an average of 7.5 minutes and a median of 5 minutesFootnote 13. Su et al., using anaphylaxis case reports from VAERS from 1990-2016, found 828 case reports that occurred within 1 day post-vaccination. These reports had a median time of onset after vaccination of 20 minutes (ranging from <1 minute to 24 hours)Footnote 23. And finally Pahud et al., examining serious, non-fatal adverse events reported to VAERS following receipt of 2009 H1N1 vaccine in children (<18 years of age) from October 2009-January 2010 found 12 true allergic reactions (i.e., not just reports of anaphylaxis) occurred within 3 hours of vaccination with a median of 30 minutes (range: 5 minutes-3 hours)Footnote 20.
Estimates of anaphylaxis incidence based on literature
There were 6 retrospective case series Footnote 13 Footnote 14 Footnote 15 Footnote 16 Footnote 18 Footnote 23 that had access to the data on the number of vaccine doses either distributed or administered during the period of the study; these data were used to calculate either overall or vaccine-specific estimates of anaphylaxis incidence during the study period.
The incidence of anaphylaxis was fairly consistent across studies, although there was some variation. The variation could be due to the small number of anaphylaxis case reports and the different denominators used to calculate incidence (doses distributed or doses administered). McNeil et al. reported an overall incidence of anaphylaxis in children and adults of 1.31 per 1,000,000 doses administeredFootnote 18. Two other studies calculated the incidence of anaphylaxis in children <18 years of age; estimates ranged from 0.65 to 1.3 per 1,000,000 dosesFootnote 13 Footnote 14.
Four studies reported incidence estimates for specific vaccines that varied significantly by vaccine and by study (0.1 to 26 per 1,000,000 doses)Footnote 15 Footnote 16 Footnote 18 Footnote 23. The highest reported incidence was from a study based on anaphylaxis reports submitted to the New South Wales Health Immunization Unit for human papillomavirus (HPV) vaccineFootnote 15 and the lowest reported incidence was for influenza vaccine based on reports submitted to VAERSFootnote 23. Incidence of anaphylaxis estimates after administration of influenza vaccine was 0.1 to 1.8 per 1,000,000 doses (administered or distributed)Footnote 18 Footnote 23.
Canadian Adverse Events Following Immunization Surveillance System
Time to symptom onset of anaphylaxis
Of the 136 reports of anaphylaxis identified, 112 (82%) were classified as serious. Just over half of all the anaphylaxis reports occurred in individuals 7 to less than 18 years of age (n=30, 22%) and 18 to less than 50 years of age (n=46, 34%). There were fewer numbers of AEFI reports in each of the other age groups (range: 9-20 AEFI reports per age group).
For all ages combined, 25% of anaphylaxis reports had onset within 5 minutes of vaccination, increasing to 50% of reported cases by 15 minutes post-vaccination, with an overall median time to symptom onset of 15 minutes (range: 1 minute to 48 hours). The proportion of individuals with symptom onset within the first 15 minutes post-vaccination did not vary significantly by age grouping, with the exception of children less than 2 years of age (6 minutes vs 15 minutes for all ages) and individuals 50 to less than 65 years of age (45 minutes vs 15 minutes) (Table 1). When comparing the time to symptom onset for all anaphylaxis reports compared to reports classified as serious, similar trends were found. For all ages combined, 29% of case reports had onset of symptoms by 5 minutes post-vaccination, 46% by 10 minutes post-vaccination and 54% by 15 minutes post-vaccination. These proportions did not differ significantly by age grouping, with the exception of children less than 2 years of age (50% by 5 minutes, 67% by 15 minutes) and individuals 50 to less than 65 years of age (8% by 5 minutes, 31% by 15 minutes) (data not shown), reflecting the differences in median time to symptom onset in these age groups (Table 1).
| Age group (years) | Number of cases (%) | Percentage of case reports by time to symptom onset | |||||
|---|---|---|---|---|---|---|---|
| 25% of cases | 50% of cases | 75% of cases | 95% of cases | 100% of cases | Median (range) | ||
| Less than 2 | 15 (11%) | 3 minutes | 6 minutes | 40 minutes | 3 hours | 3 hours | 6 minutes (1 minute to 3 hours) |
| 2 to less than 7 | 20 (15%) | 5 minutes | 13 minutes | 32 minutes | 25 hours | 48 hours | 13 minutes (1 minute to 48 hours) |
| 7 to less than 18 | 30 (22%) | 5 minutes | 15 minutes | 30 minutes | 60 minutes | 2 hours | 15 minutes (1 minute to 2 hours) |
| 18 to less than 50 | 46 (34%) | 7 minutes | 15 minutes | 30 minutes | 6 hours | 19 hours | 15 minutes (1 minute to 19 hours) |
| 50 to less than 65 | 16 (12%) | 17 minutes | 45 minutes | 7 hours | 48 hours | 48 hours | 45 minutes (1 minute to 48 hours) |
| 65 and older | 9 (7%) | 6 minutes | 18 minutes | 25 minutes | 2 hours | 2 hours | 18 minutes (5 minutes to 2 hours) |
| All Ages | 136 | 5 minutes | 15 minutes | 31 minutes | 7 hours | 48 hours | 15 minutes (1 minute to 48 hours) |
III.2 Syncope and Seizure
As seizures may occur secondary to syncopal episodes, the two outcomes are considered together in this section.
Rapid literature review
Time to symptom onset of syncope or seizure
There were 8 articles (7 retrospective case series Footnote 19 Footnote 21 Footnote 29 Footnote 30 Footnote 31 Footnote 32 Footnote 33, 1 case reportFootnote 34) identified in the rapid review that provide data on the outcomes of either syncope or seizure following vaccine administration. The retrospective case series studies all utilized AEFI surveillance systems to evaluate reports of syncope or seizure in vaccine recipients. In the 8 articles that identified reports of syncope or seizure, there were 1,180 reports of syncope alone (i.e., not associated with any reported seizure activity), 199 reports of seizures associated with syncopal episodes ("syncopal seizures"), and 16 reports of seizures alone (either afebrile or febrile) for a total of 1,395 reports. Of the 1,395 reports, 103 (7%) were considered serious (97 syncope alone, 1 syncopal seizure, and 5 febrile seizures).
Of the 1,180 reported cases of syncope alone, 453 (38%) had some indication of time to symptom onset. The range of reported onset times varied from within 5 minutes to 2 days; however, 340 (75%) of the syncopal case reports with a recorded time to symptom onset occurred within the first 15 minutes post-vaccination. Twenty-four (5%) of these reports were from four studies Footnote 19 Footnote 21 Footnote 31 Footnote 32 using retrospective case series data: 15 (63%) cases had onset from 0-5 minutes post-vaccination and 1 (4%) case with onset within 10-15 minutes. The time of onset of the remaining 8 cases were not provided in the same 5-minute time intervals: 4 (17%) occurred within 10 minutes of vaccination and 4 (17%) within 15 minutes of vaccination.
Among the studies identified, a fifth studyFootnote 29 also using retrospective case series data provided the largest number of syncopal case reports with symptom onset within the first 15 minutes post-vaccination. This study by Braun et al evaluated reports of syncope occurring within 12 hours of immunization submitted to VAERS and injury reports for syncope-related falls from the US National Vaccine Injury Compensation Program in individuals of any age from 1990-October 1995. Of the 571 reports that had a recorded time to symptom onset (81.9% of total reports), 323 (63.2%) occurred within 5 minutes of vaccination, increasing to 416 (81.4%) within 10 minutes, and 454 (88.8%) within 15 minutes of vaccination. Of the 454 syncopal episodes, 153 were associated with "tonic or clonic movements" ("syncopal seizures"): 138 (30. 4%) within 15 minutes of vaccination and 15 (12.8%) greater than 15 minutes after vaccination. Of note, 67 (9.6%) cases reported subsequent hospitalization. Six individuals sustained head injuries after syncope-induced falls, which included skull fractures, cerebral contusions, cerebral hematomata and one case of major cerebral hemorrhage. Three of the injuries necessitated neurosurgery to alleviate cerebral swelling or bleeding and 2 individuals still had significant neurologic deficits up to 2 years after the incidents. All 6 of these head injuries occurred from syncopal events within 15 minutes after vaccination.
In the study by Sutherland et al., there were 10 secondary injuries recorded among the 26 serious syncopal reports: head injuries after syncopal falls (n=9), including one death of a 15-year-old boy due to intracranial hemorrhage, and a motor vehicle incident after a loss of consciousness while driving (n=1). Seven (70%) of the injuries occurred within 15 minutes of vaccinationFootnote 32.
A description of the time to symptom onset was available for 154 (77%) of the 199 reports of seizures associated with syncopal episodes ("syncopal seizures") and for all 16 (100%) reports of seizures alone. Two articles provided the data on syncopal seizure following vaccine administration Footnote 29 Footnote 31. Braun et al.Footnote 29 reported 153 syncopal reports with associated "tonic or clonic movements": 138 (90.2%) occurred within 15 minutes of vaccination and 15 (9.8%) occurred greater than 15 minutes after vaccination. The remaining case which was reported in the second study occurred at 0 minutes post-vaccination (i.e., immediately)Footnote 31. The 16 cases of seizure alone were identified in two studiesFootnote 30 Footnote 19. Crawford et al analyzed detailed clinical information on SAEFVIC case reports of syncope and seizures after quadrivalent HPV vaccination in females 12-26 years of age vaccinated as part of the Australian National HPV Vaccination Program from May 2007-April 2009.Footnote 30 The study identified 31 episodes of syncope with associated seizures, but the time to seizure onset was reported only for 3 reports of afebrile seizure, all in individuals with confirmed underlying epilepsy. There were no deaths reported in the study population, but there were 7 injuries reported: head injuries (n=5), mouth bleeding (n=1) and a T5/T6 vertebral fracture (n=1). The study by Milstien et al.Footnote 19 identified 13 reports that met the US Food and Drug Administration (FDA) case definition for a potentially vaccine-associated convulsion, four afebrile convulsions and nine febrile convulsions. The time of onset for the afebrile convulsions was 30 minutes, 2 hours, 24 hours and 2 days after vaccination.
Estimates of syncope and/or seizure incidence based on literature
There were 3 retrospective case series studies that had access to the data on the number of vaccine doses either distributed or administered during the period of the study, allowing for calculation of either overall or vaccine-specific syncope or seizure incidence during the study periodFootnote 30 Footnote 31 Footnote 32.
In an analysis of SAEFVIC case reports of syncope and seizures after quadrivalent HPV vaccination as part of the Australian National HPV Vaccination Program, Crawford et al. estimated an incidence of 7.8 and 2.6 per 100,000 doses of vaccine distributed for syncope and syncopal seizures respectivelyFootnote 30.Subelj et al. estimated the incidence of syncope and seizures associated with syncope to be 13.4 and 3.4 per 100,000 doses, respectively, based on 4 years of data from mandatory reports to the National Institute of Public Health of a school-based four-valent HPV (HPV4) vaccine program targeting girls 11-14 years of age in SloveniaFootnote 31. Sutherland et al evaluated reports of "syncope" or "syncope vasovagal" occurring in individuals ≥5 years of age on the same day as vaccination submitted to the VAERS surveillance system from January 1, 2005-July 31, 2007 and compared the rates of syncopal events to the rate of VAERS reports received from 2002-2004Footnote 32. The rates of syncopal events per 1,000,000 doses of vaccines distributed in the US during the respective study periods was increased in 2005-2006 (0.31-0.54) compared to 2002-2004 (0.28-0.35), as were the proportion of reports from females (77.5% vs 61.6%) and persons 11-18 years of age (62.0% vs 47.3%).
Canadian Adverse Events Following Immunization Surveillance System
Time to symptom onset of syncope or seizure
Of the 52 reports of syncope, 20 (38%) were classified as serious. Individuals 7 to less than 18 years of age (n=33, 65%) and 18 to less than 50 years of age (n=8, 16%) accounted for the majority of all 52 reports. There were very few AEFI reports in each of the remaining age groups (N is between 2 and 4 for each).
Age group (years) |
Number of cases (%) | Percentage of case reports by time to symptom onset | |||||
|---|---|---|---|---|---|---|---|
| 25% of cases | 50% of cases | 75% of cases | 95% of cases | 100% of cases | Median (range) |
||
| Less than 2 | 2 (4%) | 5 days | 12 days | 18 days | 18 days | 18 days | 12 days (5 to 18 days) |
| 2 to less than 7 | 2 (4%) | 4 minutes | 7 minutes | 10 minutes | 10 minutes | 10 minutes | 7 minutes (4 to 10 minutes) |
| 7 to less than 18 | 33 (65%) | 1 minute | 2 minutes | 6 minutes | 20 minutes | 35 minutes | 2 minutes (1 to 35 minutes) |
| 18 to less than 50 | 8 (16%) | 2 minutes | 63 minutes | 48 hours | 29 days | 29 days | 63 minutes (1 minute to 29 days) |
| 50 to less than 65 | 2 (4%) | 1 minute | 31 minutes | 60 minutes | 60 minutes | 60 minutes | 31 minutes (1 to 60 minutes) |
| 65 and older | 4 (8%) | 2 hours | 11 hours | 33 hours | 48 hours | 48 hours | 11 hours (1 minute to 48 hours) |
| All Ages | 52 | 1 minute | 3 minutes | 15 minutes | 5 days | 29 days | 3 minutes (1 minute to 29 days) |
For all ages combined, 25% of syncope reports had onset within 1 minute of vaccination, increasing to 50% of reported cases by 3 minutes post-vaccination and 75% within 15 minutes, with an overall median time to symptom onset of 3 minutes (range: 1 minute to 29 days) (Table 2). The proportions were similar for syncope reports classified as serious (data not shown).
There were 61 reports of afebrile seizure between 2014 and 2018, 50 (82%) of which were classified as serious. The majority (74%) of reports were in individuals less than 2 years of age. There were very few AEFI reports in the other age groups. For all ages combined, the median time to onset of afebrile seizure was 21 hours (range: 1 minute to 29 days). Only for the group of individuals 7 to less than 18 years were a significant proportion (25%) of afebrile seizures reported to have occurred within 2 minutes of vaccination, based on a small number of case reports (n=8).
III.3 Risk-benefit assessment
The rationale for potentially considering a reduction in the post-vaccination observation period in influenza vaccination settings during the COVID-19 pandemic is to reduce the duration of time vaccine recipients spend together with others in post-vaccination areas and to potentially reduce the number of contacts recipients encounter in vaccination settings. This could then reduce the opportunity for SARS-CoV-2 transmission from an unrecognized, infectious COVID-19 case and allow more individuals to be vaccinated (increase vaccination clinic "throughput") in a given time period. However, it is very challenging to quantify the impact of a reduction in the post-vaccination observation period on the risk for SARS-CoV-2 transmission, independent of other public health and infection prevention and control measures (engineering, environmental and administrative controls, and personal protective equipment) that are likely to be implemented in influenza vaccination settings during the COVID-19 pandemic. In addition, there will be differences in the risk of transmission in various settings due to variability in disease prevalence at different locations and at different points in time. Any consideration of a reduction in post-vaccination observation period would be a deviation from the current standard practice in Canadian provinces and territories. The current standard of a 15-minute post-vaccination observation period is intended to identify serious AEFIs that may require immediate medical intervention. This must be weighed against the potential benefits of reducing risk of SARS-CoV-2 transmission and allowing more individuals to be vaccinated (increase vaccination clinic "throughput") in a given time period.
Potential benefits of a reduced post-vaccination observation period
- Reduction in crowding within post-vaccination waiting areas of vaccination clinics will improve the likelihood of maintaining physical distancing among vaccine recipients and between vaccine recipients and clinic staff.
- Less waiting post-vaccination may encourage members of the public to attend vaccination clinics if there is perceived to be less risk of COVID-19 infection due to less crowding in the vaccination clinics areas and if there is less time overall required to get vaccinated. Perceptions of reduced risk of infection will be enhanced by the other infection prevention and control measures taken in the context of COVID-19 that will also reduce crowding within the vaccination clinic setting (e.g., scheduled vaccination appointments, arrival just in time for appointments, screening of clinic staff and attendees).
- A reduced number of individuals in the post-vaccination waiting areas would benefit vaccine recipients who do not meet the criteria for a reduced post-vaccination observation period, and may remain in the vaccination setting for the standard 15-minute observation period or a 30-minute observation period if there is a specific concern about possible vaccine allergy.
- A reduced post-vaccination period may allow more individuals to be vaccinated (increase vaccination clinic "throughput") in a given time period and also allow vaccine recipients to be in contact with fewer individuals.
Potential risks of a reduced post-vaccination observation period
- There would be a small but potentially increased risk of delayed identification of an adverse event that may require immediate medical intervention (e.g., anaphylaxis and syncope with or without seizure).
- Reducing the post-vaccination observation period may have only a relatively modest, independent impact in reducing the risk of SARS-CoV-2 transmission in the vaccination clinic setting during the COVID-19 pandemic when compared with the other public health and infection prevention and control measures (e.g., environmental and engineering controls, administrative processes, personal protective equipment). The overall risk will also depend upon factors such as local disease prevalence at the time of the immunization clinics.
- The reduction in the post-vaccination observation period may be perceived as an action being taken primarily to improve clinic efficiency rather than an important infection prevention measure.
IV. Recommendations
Following a thorough review of the evidence summarized above, NACI has made two recommendations for public health program decision-making. These recommendations are based on currently available scientific evidence and expert opinion and are relevant only during the COVID-19 pandemic, after which the current guidance for the post-vaccination observation period as identified in Part 2 of the Canadian Immunization Guide should be followedFootnote 4.
In considering these recommendations and for the purposes of publicly funded program implementation, provinces and territories may take into account economic factors and other local operational factors (e.g. current immunization programs, resources). Recognizing that there are differences in operational contexts across Canada, jurisdictions are advised to refer to the local epidemiology of COVID-19 and additional PHAC guidanceFootnote 3 to determine the relative merits of modifying the post-vaccination observation period. NACI will continue to monitor the scientific literature for developments related to post-vaccination observation period and will update recommendations as evidence evolves, if required.
Please note:
- A strong recommendation applies to most populations/individuals and should be followed unless a clear and compelling rationale for an alternative approach is present.
- A discretionary recommendation may be considered for some populations/individuals in some circumstances. Alternative approaches may be reasonable.
Refer to Table 3 for a more detailed explanation of strength of NACI recommendations and grade of the body of evidence.
IV.1 Recommendations for Public Health Program Level Decision-Making
- NACI recommends that the current post-vaccination observation period, as specified in the Canadian Immunization GuideEndnote a, should be maintained for influenza vaccination settings that can adhere to appropriate public health and infection prevention and control measures Endnote bto reduce SARS-CoV-2 transmission, particularly physical distancing (Strong NACI recommendation).
- NACI concludes that there is fair evidence to maintain a 15-minute post-vaccination observation period during the COVID-19 pandemic for individuals with no known history of severe allergic reactions (including anaphylaxis) to any component of the influenza vaccine being considered for administration or any history of other immediate post-vaccination reactions (e.g., syncope with or without seizure) (Grade B Evidence).
Evidence and Rationale
- A 15-minute post-vaccination observation period is the Canadian standard for individuals with no known history of prior severe allergic reaction or any other immediate post-vaccination reactions.
- A vaccination clinic setting that is able to maintain recommended public health and infection prevention and control measures, including physical distancing in post-vaccination areas, is unlikely to achieve significant additional reductions in the risk of SARS-CoV-2 transmission by implementing a shorter post-vaccination observation period.
- NACI recommends that a shorter post-vaccination observation period, between 5 to 15 minutes after influenza immunization, may be considered during the COVID-19 pandemic, but only during times when appropriate physical distancing in post-vaccination waiting areas cannot otherwise be maintained due to the volume of individuals seeking immunization, and only when specific conditions are met Endnotes * (Discretionary NACI recommendation).
- NACI concludes that there is insufficient evidence from retrospective, passive adverse event following immunization (AEFI) case series reports to support a reduced post-vaccination observation period. However, other factors may be taken into consideration to support a reduced post-vaccination observation period, if necessary for public health and infection prevention and control purposes (Grade I Evidence).
Endnotes *
- Endnotes *
-
A shorter observation period may be considered only if the vaccine recipient meets the following conditions Reference c:
- Past history of receipt of influenza vaccine and no known history of severe allergic reactions (including anaphylaxis) to any component of the influenza vaccine being considered for administration (note that novel technology vaccine recipients should be exempt from reduced post-observation period eligibility)Reference d.
- No history of other immediate post-vaccination reactions (e.g., syncope with or without seizure) after receipt of any vaccines.
- The vaccine recipient is accompanied by a parent/guardian (in the case of a child) or responsible adult who will act as chaperone to monitor the vaccine recipient for a minimum of 15 minutes post-vaccination. In the case of two responsible adults, both can be vaccine recipients for the purposes of this criterion, if both agree to monitor the other post-vaccination.
- The vaccine recipient will not be operating a motorized vehicle or self-propelled or motorized wheeled transportation (e.g., bicycle, skateboard, rollerblades, scooter), or machinery for a minimum of 15 minutes after vaccination.
- The vaccine recipient and the parent/guardian or responsible adult chaperone are aware of when and how to seek post-vaccination advice and given instructions on what to do if assistance and medical services are required.
- The vaccine recipient and the parent/guardian/responsible adult agree to remain in the post-vaccination waiting area for the reduced post-vaccination observation period and to notify staff if the recipient feels or looks at all unwell before leaving the clinic. They should be informed that an individual exhibiting any symptom suggestive of an evolving AEFI at the end of the shortened post-observation period necessitates a longer period of observation in the clinic.
Evidence and Rationale
- Public health and infection prevention and control measures (i.e., engineering, environmental and administrative controls, and personal protective equipment) should be implemented to reduce the risk for SARS-CoV-2 transmission.
- A consideration to reduce the post-vaccination observation period must weigh the potential risk of any delay in identifying serious adverse events that may require immediate medical intervention against the potential benefits of reducing contacts with others and the associated risk of SARS-CoV-2 transmission; and potentially allowing more individuals to be vaccinated in a given time period. It is recognized that several factors may influence decision-making and applicability of recommendations:
- The community levels of SARS-CoV-2 transmission at the time of vaccination clinics will influence the risk of SARS-CoV-2 transmission in the vaccination setting. This risk will vary by and within jurisdictions over time.
- Unanticipated or unprecedented increases in the number of individuals presenting for immunization in clinic settings at a given time could temporarily overwhelm the other public health and infection prevention measures in place.
- It is unknown how much a reduced post-vaccination observation period, independent of other public health and infection prevention and control measures, contributes to reducing the risk of SARS-CoV-2 transmission in the vaccination setting.
- The evidence from retrospective, passive AEFI case series reports is insufficient to support a reduced post-vaccination observation period.
- Overall estimates of anaphylaxis incidence using retrospective case series data from passive AEFI surveillance systems varied, but were consistent with estimates of anaphylaxis incidence for commonly administered vaccines (1 per 100,000 to 1 per 1,000,000 doses) cited by the World Allergy Organization in its International Consensus statement on allergic reactions to vaccinesFootnote 35.
- Estimates for the incidence of syncope using passive case series data ranged widely, but were consistently much higher than for anaphylaxis.
- The data from the rapid review indicate that a shortened post-vaccination observation period may still identify a majority of syncopal episodes, but not the majority of episodes of anaphylaxis.
- The study by Braun et alFootnote 29 that evaluated the largest number of reports of syncope found 63.2% occurred within 5 minutes of vaccination, increasing to 416 (81.4%) within 10 minutes, and 454 (88.8%) within 15 minutes of vaccination. CAEFISS data, for all ages combined, found 25% of syncope reports had onset within 1 minute of vaccination, increasing to 50% of reported cases by 3 minutes post-vaccination and 75% within 15 minutes, with an overall median time to symptom onset of 3 minutes (range: 1 minute to 29 days).
- A little over half of all anaphylaxis reports with a recorded time of symptom onset occurred within 30 minutes of vaccination, but the proportion of those reports occurring during the first 15 minutes post-vaccination was not reported. From the case series containing the largest number of anaphylaxis reports, the median time to anaphylaxis onset after vaccination was 20 minutes (range: from <1 minute-24 hours). CAEFISS reports, for all ages combined, found 25% of anaphylaxis reports had onset within 5 minutes of vaccination, increasing to 50% of reported cases by 15 minutes post-vaccination, with an overall median time to symptom onset of 15 minutes (range: 1 minute to 48 hours). Similar trends were found for anaphylaxis reports classified as serious.
- Use of a shortened post-vaccination observation period should be accompanied by safeguards, as outlined in the recommendation, to minimize situations that would place the vaccine recipient at risk for unintended injury should an adverse event occur after the shortened observation period, but within the standard 15-minute post-vaccination observation period.
Tables and figures
| STRENGTH OF NACI RECOMMENDATION | GRADE OF EVIDENCE |
|---|---|
| Based on factors not isolated to strength of evidence (e.g. public health need) | Based on assessment of the body of evidence |
Strong "should/should not be offered"
|
A - good evidence to recommend |
| B - fair evidence to recommend | |
| C - conflicting evidence, however other factors may influence decision-making | |
| D - fair evidence to recommend against | |
| E - good evidence to recommend against | |
| I - insufficient evidence (in quality or quantity), however other factors may influence decision-making | |
Discretionary "may be considered"
|
A - good evidence to recommend |
| B - fair evidence to recommend | |
| C - conflicting evidence, however other factors may influence decision-making | |
| D - fair evidence to recommend against | |
| E - good evidence to recommend against | |
| I - insufficient evidence (in quality or quantity), however other factors may influence decision-making |
| Level | Description |
|---|---|
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case-control analytic studies, preferably from more than one centre or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| Quality Rating | Description |
|---|---|
| Good | A study (including meta-analyses or systematic reviews) that meets all design- specific criteriaTable 5 tablenote * well. |
| Fair | A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterionTable 5 tablenote * but has no known "fatal flaw". |
| Poor | A study (including meta-analyses or systematic reviews) that has at least one design-specificTable 5 tablenote * "fatal flaw", or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
Table 5 Tablenotes
|
|
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
|---|---|---|---|---|---|---|
| Case Series | ||||||
Baxter et al., 2018 |
Any vaccine |
Case Series Australia SAEFVIC passive surveillance system July 2007 to June 2015 |
Children less than 5 years of age |
Anaphylaxis The incidence of anaphylaxis was found to be 0.13 per 100,000 doses of vaccine. The time interval between vaccine dose and onset of anaphylaxis ranged from 0-40 minutes (n=12), with an average of 7.5 minutes and a median of 5 minutes. Ten cases (83%) developed symptoms of anaphylaxis within 5 minutes, two cases (17%) had delayed symptom onset with one at 20 minutes and another at 40 minutes following vaccine administration. For the majority of cases (68%), more than one vaccine was given prior to the onset of anaphylaxis. |
III |
Poor |
Bohlke et al., 2003 |
Any vaccine |
Case Series US 1991 to 1997 |
Children 17 years of age or younger registered in one of 4 Health Maintenance Organizations participating in the US CDC Vaccine Safety Datalink project |
Anaphylaxis 5 cases of potential vaccine-associated anaphylaxis were identified after administration of 7,644,049 vaccine doses. Incidence of anaphylaxis was 0.65 cases per 1,000,000 doses (95% CI: 0.21-1.53). None of the episodes resulted in death. Among the 5 cases identified, one (20%) happened within 5-10 minutes of vaccine administration and the other 4 (80%) cases happen more than 1 hour following administration. Of the 5 cases, only one case of anaphylaxis reaction happened following a single vaccine administration (MMR vaccine). |
III |
Poor |
Braun et al., 1997 |
Any vaccine |
Case Series US VAERS passive surveillance system 1990 to 1995 |
All children and adults (no age restriction) |
Syncope A total of 697 syncopal episodes were reported that occurred within 12 hours of vaccine administration. Hospitalization was reported in 9.6% of cases. Of the 571 (73.3%) syncope events with known time, 511 (89.5%) occurred 1 hour or less after vaccination. Of these, 323 (63.2%) occurred within 5 minutes or less, 93 (18.2%) occurred within 10 minutes and 38 (7.4%) occurred within 15 minutes of vaccine administration. Six patients suffered skull fractures, cerebral bleeding, or cerebral contusion after falls; 3 of these patients required neurosurgery. All the falls occurred 15 minutes or less after vaccination, in or near the clinic or office. |
III |
Poor |
Brotherton et al., 2008 |
HPV4 vaccine |
Case Series Australia 2007 |
Female adults and children 12-26 years of age that received HPV4 vaccine through Australia National HPV Vaccination Program in either the school-based or primary care clinic setting |
Anaphylaxis 12 cases of suspected anaphylaxis were identified. Of the 11 cases that provided consent to be included in the study, 8 (73%) cases fulfilled the Brighton case definition for anaphylaxis (Level 1; n=1 and Level 2; n=7). All cases were reported to have an onset within 15 minutes of receiving vaccination; 4 (50%) cases were within 5 minutes and 3 (37.5%) cases occurred between 5-10 minutes of vaccine administration. |
III |
Poor |
Cheng et al., 2015 |
Any vaccine |
Case Series Australia SAEFVIC passive surveillance system May 2007 to May 2013 |
Children less than18 years of age |
Anaphylaxis 25 cases were identified that met Brighton Collaboration criteria for anaphylaxis. Of these cases, 9 (26%) met level 1 diagnosis certainty, 15 (60%) level 2 and one level 3 (4%). The majority of cases had rapid symptom onset with 13 (52%) cases happening within 5 minutes, 18 (72%) cases within the first 15 minutes and 20 (80%) cases during the first 30 minutes of vaccination. Overall, 20% (5/25) of cases had their first symptoms of anaphylaxis developed ≥30 minutes after immunization. |
III |
Poor |
Crawford et al., 2011 |
HPV4 vaccine |
Case Series |
Female adult and children 12-26 years of age who received HPV4 vaccine through Australia National HPV Vaccination Program |
Syncope and Seizure Overall incidence following HPV4 vaccination was 7.8 per 100,000 doses distributed for syncope and 2.6 per 100,000 for syncopal seizures. Of the 94 syncopal episodes, 67% (63/94) had syncope alone, and 33% (31/94) had associated seizure activity, of which 23% (7/31) had urinary incontinence. The HPV4 vaccine was given alone in 85% (82/97) of reports, with concomitant vaccines including: hepatitis B (6); diphtheria-tetanus-acellular pertussis (6); varicella (1); and varicella and hepatitis B vaccine (2). Three patients were identified with afebrile seizures without syncope and all had a confirmed underlying epilepsy disorder. One patient had a generalized seizure 4 hours after the HPV4 vaccine (dose 2). Another had an exacerbation of complex partial seizures 4 hours after the HPV4 vaccine (dose 2). Another patient experienced a generalized tonic-clonic seizure 2 days after receiving HPV4 vaccine (dose 1). This resulted in a wedge fracture of spinal vertebrae, which was treated conservatively. A generalized epilepsy disorder was confirmed, and anticonvulsant medication commenced. |
III |
Poor |
Johann-Liang et al., 2011 |
Any vaccine |
Case Series US Vaccine Injury Comensation Program Jan 2000 to Dec 2009 |
Adults and children of all ages |
Anaphylaxis 53 unique cases alleging "anaphylaxis or anaphylactic shock" were identified through the Vaccine Injury Compensation Program, accounting for 3% of the total of 1,819 non-autism claims for the study period. Of those, 9 (17%) were defined as anaphylaxis. One case occurred from minutes to hour, 2 cases were within 5 minutes, one case had onset at 15 minutes, 2 cases were between 20 and 30 minutes and 3 cases occurred more than one hour after vaccine administration (one at 1 hour, one at 2 hours and one at 2-3 hours). |
III |
Poor |
McNeil et al., 2016 |
Any vaccine |
Case Series US Jan 2009 to Dec 2011 |
Adults and children of all ages enrolled in health plans at one of 9 Vaccine Safety Datalink participating sites |
Anaphylaxis 76 cases of chart-confirmed anaphylaxis were identified that met Brighton Collaboration levels 1 and 2 criteria. Of these, 33 anaphylaxis cases [Brighton Collaboration level 1 (n=12; 36%) and level 2 (n=21; 64%)] were associated with vaccination and 43 were attributed to other causes. 29 cases (87.9%) of anaphylaxis had documented time to onset: 8 cases (24%) occurred within 30 minutes, 8 (24%) between 30 and 120 minutes, 10 (30%) from 2 to 4 hours and 3 cases (10%) occurred from 4 to 20 hours following vaccine administration. Only one case had specific reaction time which was an immediate reaction after multiple vaccine administration. |
III |
Poor |
Milstien et al., 1987 |
Hib vaccine |
Case Series US Apr 1985 to May 1986 |
Children 18 to 23 months of age at high risk of Hib and children 2 to 5 years of age who are not at high risk |
Anaphylaxis Two cases of anaphylactic-like reactions were reported. One case was in a 3-year-old boy who became pale and hypotensive and began to wheeze five minutes after vaccination. The other case was in a 4-year-old boy who became nauseated, pale, and bradycardia; circumoral cyanosis developed 20 minutes after vaccination. Both cases responded quickly to epinephrine and oxygen. Syncope Seven reports of syncope were identified, three that noted treatment with epinephrine and/or Benadryl. All but three episodes occurred within ten minutes of vaccination (others occurred at 30 minutes, 2 hours, and 24 hours). All episodes were in children 3 to 5 years of age. Seizure 13 patients whose reactions met the case definitions for seizures were identified: four afebrile convulsions and nine febrile convulsions. Five patients with febrile convulsions were hospitalized. All reactions occurred more than 2 hours after vaccine administration, except one afebrile convulsion that occurred within 30 minutes. The mean time to onset of febrile convulsions after receipt of the vaccine was 24 hours (median: 12 hours). Two of the children had a prior history of febrile convulsions, and an additional three had a history of febrile convulsions in a sibling or a parent. |
III |
Poor |
Pahud et al., 2013 |
2009 H1N1 monovalent influenza vaccine |
Case Series US VAERS passive surveillance system Oct 2009 to Jan 2010 |
Children less than 18 years of age |
Anaphylaxis 3928 reports and 214 (5.4%) were classified as having serious, nonfatal condition. 109 cases were referred for further review of which 99 cases had complete clinical information. Of the 99 cases, they found fifteen presumed allergic reactions. The reported diagnoses included anaphylaxis (n=5), hypersensitivity reaction (n=3), angioedema (n=3), hives (n=3) and allergic reaction (n=1). True anaphylaxis caused by a vaccine was present in 4 cases, urticaria or other skin manifestations of a true allergic reaction in 6 cases and allergic reaction to a vaccine with respiratory symptoms in 2 cases. Two cases were considered anxiety reactions and 1 was considered likely attributable to an independent viral infection. All anaphylaxis cases or true allergic reactions (n = 12) occurred within 3 hours of vaccination (median, 30 minutes; range, 5 minutes-3 hours). |
III |
Poor |
Patja et al., 2000 |
MMR vaccine |
Case Series Finland Passive surveillance system 1982 to 1996 |
Adults and children of all ages |
Anaphylaxis 30 suspected cases of anaphylaxis were identified, all of which appeared within 20 minutes of vaccination, except one case who developed symptoms several hours after vaccination. Seizure 52 vaccinees reported febrile seizures 12 hours to 15 days after vaccination. Apart from 3 children who were 3 to 6 years of age, all reports were in children less than 3 years of age. Undefined seizures were observed in four children 2 to 12 days post-vaccination. |
III |
Poor |
Schumacher et al., 2010 |
Any vaccine |
Case Series Switzerland Passive surveillance system 1991 to 2001 |
Adults and children of all ages |
Anaphylaxis There were 18 cases of non-fatal anaphylaxis reaction reported. Two occurred within minutes after immunization, five within 6 hours, four within 6-24 hours, and seven after an unknown time interval. Of the 7 (3.6%) serious AEFI that were assessed as very likely or certainly related to immunization, there were 5 cases of anaphylaxis reaction with time to symptom onset ranging from 1 minute to 1 hour post-vaccine administration. One case happened several minutes after DTP-based combination vaccine, one case after 1 minute after DTP-based combination vaccine, one case after 5 minutes of DTP-based combination, one case after 1h of DTP-based combination vaccine and one case following MMR vaccination without information on timing of onset. |
III |
Poor |
Su et al., 2019 |
Any vaccine |
Case Series US VAERS passive surveillance system Jan 1990 to Dec 2016 |
Adults and children of all ages |
Anaphylaxis 828 reports that either met the Brighton case definition or included a diagnosis of anaphylaxis by a physician described symptoms within 24 hours of receiving the vaccine. Of reports with time to onset of symptoms available, 77% described symptoms less than 2 hours after vaccination with a median time to onset after vaccination of 20 minutes (range; <1 minute to 24 hours). Moreover, 402 (49%) of the reports had time to onset of symptoms < 30 minutes after vaccination. Finally, the authors identified 8 reports of death. Of 7 reports with time to onset symptoms available, 3 (43%) cases happened within 5 minutes and 4 (57%) cases within 15 minutes following vaccination. The other report of death happened at 20 min, 258 min or on the same day of vaccine administration. |
III |
Poor |
Subelj et al., 2016 |
HPV4 vaccine |
Case Series Slovenia Sept 2009 to Aug 2013 |
Girls 11 to 14 years of age who received HPV4 vaccine as part of a school-based vaccination program |
Syncope Seizure 2 seizure events, accounting for 0.9% of all AEFIs reported, were identified. Incidence was 3.4 per 100,000 HPV4 vaccine doses distributed. One seizure was deemed serious and occurred immediately following vaccine administration. |
III |
Poor |
Sutherland et al., 2008 |
Any vaccine |
Case Series US VAERS passive surveillance system Jan 2002 to Dec 2004 and Jan 2005 to July 2007 |
Adults and children 5 years of age or older |
Syncope 26 (5.6%) of the 463 post-vaccination syncope reports during 2005-2007 were coded as serious, which was not substantially different from the 20 (9.9%) serious reports during 2002-2004. Among the 23 patients for whom times of vaccination and syncope onset were indicated, 12 (52.2%) occurred within 5 minutes of vaccination, and 16 (69.6%) occurred within 15 minutes. |
III |
Poor |
Woo et al., 2005 |
Any vaccine |
Case Series US VAERS passive surveillance system 2000 to 2005 |
Adults and children of all ages who experienced syncope/presyncope and accidental injury on the day of vaccination |
Syncope 107 reports of syncope/presyncope and unintentional injury on the day of vaccination were identified. 100 of the reported injuries occurred within 20 minutes of vaccination. There were three reports of serious head injury, including one that was deemed fatal due to vasovagal syncope. Following the third dose of hepatitis B vaccine, a 15-year-old boy with no antecedent of event or medical problems, experienced vasovagal syncope several minutes after vaccination. He fell backward and hit his head, momentarily lost consciousness, then complained of pain in the chest and arms. Then, he reportedly had convulsions and went into cardiopulmonary arrest. A 21- year-old man experienced intracerebral bleeding after falling backwards onto the floor 3 minutes after hepatitis B vaccine, tetanus toxoid, and diphtheria vaccine. He reportedly recovered without sequelae. An 18-year-old woman was in a fatal motor vehicle collision after she "passed out while driving her car" approximately 7 hours after meningococcal polysaccharide vaccine; the 7-hour interval between vaccination and the collision makes vaccine-related vasovagal syncope unlikely. |
III |
Poor |
Zafack et al., 2019 |
Any vaccine |
Case Series Passive surveillance system database Jan 1998 to Dec 2016 |
5,600 children and adults of all ages receiving additional doses of vaccine(s) previously temporally associated with an AEFI |
Anaphylaxis Of the 5,600 individuals identified who had experienced an AEFI, 18 patients had an anaphylaxis reaction, of which 14 (77.8%) had information regarding delay of onset following immunization. Among the 18 patients with reported anaphylaxis, 3 met the Brighton Collaboration level 1 of diagnostic certainty, 8 met level 2, 6 met level 3 and one did not have a description of signs and symptoms. Among the 14 patients with timing of symptom onset, 4 (28.6%) occurred by 5 minutes, 9 (64.3%) by 10 minutes and 10 (71.4%) patients had symptom onset within 30 minutes of vaccine administration. |
III |
Poor |
Case reports |
||||||
Poddighe et al., 2014 |
HPV2 vaccine |
Physician case report Funding: |
A 14-year old girl |
Syncope A 14-year-old girl reported an AE after the second dose of bivalent HPV vaccine. The patient experienced sudden onset of general malaise and other symptoms after the vaccine administration. She fainted around 60 minutes after intramuscular injection. The patient did not have any previous physical and/or psychiatric diseases or complaints. Upon further evaluation in the following days, the patient was diagnosed with a neuropsychiatric syndrome. |
III |
Poor |
Stone et al., 2019 |
MMR, Varicella, and DTaP/ IPV vaccine |
Physician case report Funding: |
A 5-year-old male with a history of alpha-gal allergy |
Anaphylaxis Five minutes after receiving the vaccines, 5 year-old developed shortness of breath, wheezing, disseminated urticaria, and angioedema of the face and oropharynx, prompting an emergency room visit where he received epinephrine, diphenhydramine, prednisone and famotidine with relief of symptoms within ten minutes. He had no history of egg, latex, dairy, or gelatin allergies and had uneventfully received all prior childhood vaccinations. |
III |
Poor |
Stone et al., 2017 |
Live-attenuated herpes zoster vaccine |
Physician case report Funding: |
A 71-year-old woman with documented history of allergy to red meat |
Anaphylaxis A 71-year-old women with documented allergies to red meat required emergency department treatment and epinephrine administration upon receipt of live attenuated herpes zoster virus vaccine containing the Oka VZV strain. The vaccine was administered in a local pharmacy and within minutes she had a sensation of mental clouding progressing to lightheadedness, wheezing, and throat tightness. She self-administered 50 mg diphenhydramine five minutes after symptom onset. Thirty minutes after her vaccination, she sought emergency care at which point she was documented to be dyspneic, flushed, with facial, oral, and uvular angioedema and bilateral conjunctival infections with stable vital signs and blood pressure, without documented wheezing on pulmonary examination. Her symptoms resolved within 20-30 minutes. She was later diagnosed with alpha-gal and the event was attributed to the presence of mammalian products within the vaccine. |
III |
Poor |
Turktas et al., 1999 |
Whole-cell DTP vaccine |
Physician case report Funding: |
A 6-month-old infant |
Anaphylaxis A 6-month-old infant experienced anaphylaxis following the third dose of the whole-cell DTP vaccine. The infant experienced drowsiness, followed by loss of consciousness, an urticarial rash throughout the body and swelling of the eyes. He was brought into a community hospital 20 minutes after vaccine administration and was then referred to the Department of Pediatrics within 2 hours of vaccination. During follow-up, the patient had mild monthly wheezing and cough episodes in winter, and perennial rhinitis throughout the year. |
III |
Poor |
Worm et al., 2000 |
Tick-borne encephalitis vaccine |
Case report Funding: |
A 29-year-old woman |
Anaphylaxis Patient developed a generalized urticaria, dyspnea and hypotension a few minutes after the third immunization. She immediately received antihistamines and steroids intravenously and the symptoms resolved completely within 1 hour. |
III |
Poor |
Abbreviations: AE: adverse events; AEFI: adverse event following immunization; DTP: Diphtheria, tetanus, pertussis; HPV: human papillomavirus; HPV4 vaccine: 4-valent human papillomavirus vaccine; MedDRA: Medical Dictionary for Regulatory Activities; MMR: Measles-mumps-rubella; SAEFVIC: Surveillance of Adverse Events following Vaccination in the Community; US: United States; VAERS: Vaccine Adverse Events Reporting System |
||||||
Appendix A: Rapid review PRISMA Flow Diagram
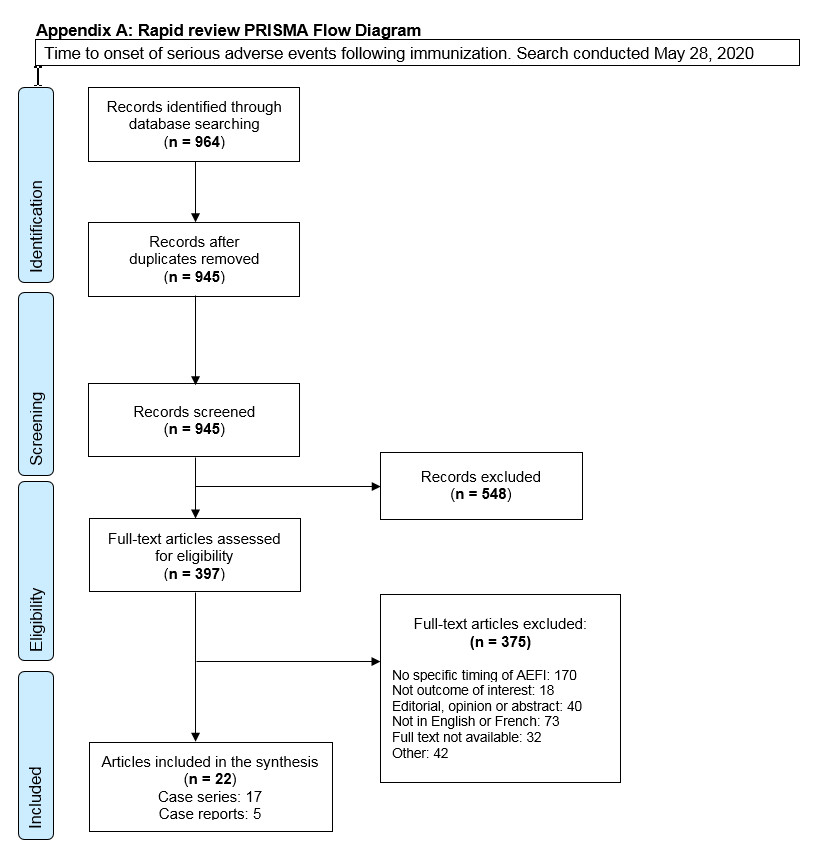
PRISMA flow diagram: Text description
The PRISMA flow diagram describes the process by which articles were selected for the literature review. The process is broken down into four stages: Identification, Screening, Eligibility and Included.
Stage 1: Identification
- 964 records were identified through the May 28, 2020 database search.
- 945 records remained after duplicates were removed.
Stage 2: Screening
- 945 records were then screened.
- Of these 945 records, 548 records were excluded.
Stage 3: Eligibility
- 397 full-text articles were assessed for eligibility.
- Of these 397 full-text articles, 375 were excluded. The exclusion breakdown is as follows: No specific timing of AEFI: 170; Not outcome of interest: 18
- Editorial, opinion or abstract: 40; Not in English or French: 73; Full text not available: 32; Other: 42
Stage 4: Included
- 22 articles were included in the final synthesis: 8 case series and 5 case reports.
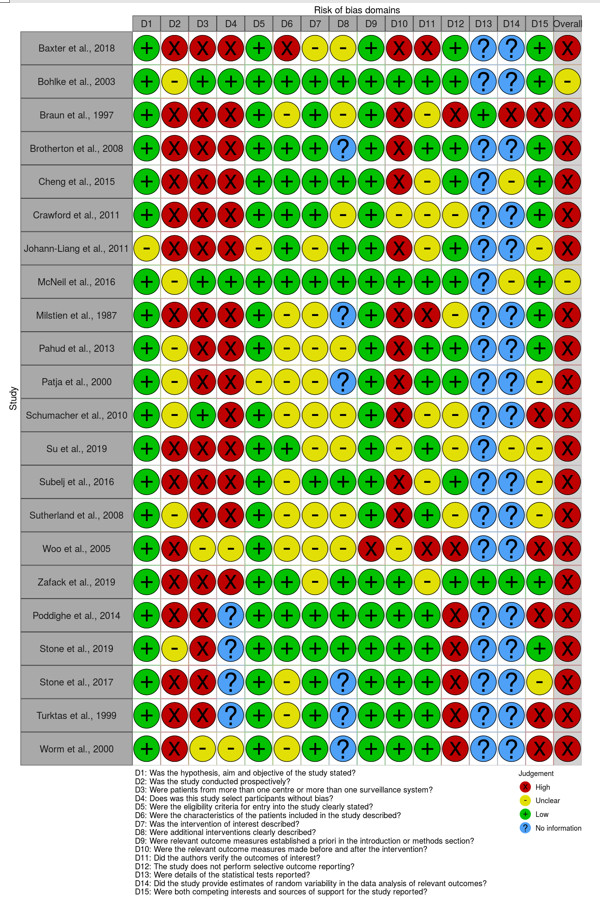
Risk of Bias Domains assessed (Modified IHE tool): Text description
| Study | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Domain 6 | Domain 7 | Domain 8 | Domain 9 | Domain 10 | Domain 11 | Domain 12 | Domain 13 | Domain 14 | Domain 15 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baxter et al., 2018 | Low | High | High | High | Low | High | Unclear | Unclear | Low | High | High | Low | No information | No information | Low | High |
| Bohlke et al, 2003 | Low | Unclear | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | No information | No information | Low | Unclear |
| Braun et al., 1997 | Low | High | High | High | Low | Unclear | Low | Unclear | Low | High | Unclear | High | Low | High | High | High |
| Brotherton et al., 2008 | Low | High | High | High | Low | Low | Low | No information | Low | High | Low | Low | No information | No information | Low | High |
| Cheng et al., 2015 | Low | High | High | High | Low | Low | Low | Low | Low | High | Unclear | Low | No information | Unclear | Low | High |
| Crawford et al., 2011 | Low | High | High | High | Low | Low | Low | Unclear | Low | Unclear | Unclear | Unclear | No information | No information | Low | High |
| Johann-Liang et al., 2011 | Unclear | High | High | High | Unclear | Low | Unclear | Low | Low | High | Unclear | Low | No information | No information | Unclear | High |
| McNeil et al., 2016 | Low | Unclear | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | No information | Unclear | Low | Unclear |
| Millstein et al., 1987 | Low | High | High | High | Low | Unclear | Unclear | No information | Low | High | High | Unclear | No information | No information | Low | High |
| Pahud et al., 2013 | Low | Unclear | High | High | Low | Unclear | Unclear | Unclear | Low | High | Low | Low | No information | No information | Low | High |
| Patja et al., 2000 | Low | Unclear | High | High | Unclear | Unclear | Unclear | No information | Low | High | Low | Low | No information | No information | Unclear | High |
| Schumacher et al., 2010 | Low | Unclear | Low | High | Low | Unclear | Unclear | Unclear | Low | High | Unclear | Unclear | No information | No information | High | High |
| Su et al., 2019 | Low | High | High | High | Low | Low | Unclear | Unclear | Low | Unclear | Low | Unclear | No information | Unclear | Unclear | High |
| Subelj et al., 2016 | Low | High | High | High | Low | Unclear | Low | Low | Low | High | Unclear | Low | No information | No information | Unclear | High |
| Sutherland et al., 2008 | Low | Unclear | High | High | Low | Unclear | Unclear | Unclear | Low | High | Low | Unclear | No information | No information | Unclear | High |
| Woo et al., 2005 | Low | High | Unclear | Unclear | Low | Unclear | Unclear | Unclear | High | Unclear | High | High | No information | No information | High | High |
| Zafack et al., 2019 | Low | High | High | High | Low | Low | Unclear | Low | Low | Low | Unclear | Low | Low | Low | Low | High |
| Poddighe et al., 2014 | Low | High | High | No information | Low | Low | Low | Low | Low | Low | Low | High | No information | No information | High | High |
| Stone et al., 2019 | Low | Unclear | High | No information | Low | Low | Low | Low | Low | Low | Low | High | No information | No information | Low | High |
| Stone et al., 2017 | Low | High | High | No information | Low | Unclear | Low | No information | Low | Low | Low | High | No information | No information | Unclear | High |
| Turktas et al., 1999 | Low | High | High | No information | Low | Unclear | Low | No information | Low | Low | Low | High | No information | No information | High | High |
| Worm et al. 2000 | Low | High | Unclear | Unclear | Low | Unclear | Low | No information | Low | Low | Low | High | No information | No information | High | High |
List of abbreviations
| Abbreviation | Term |
|---|---|
| AE | Adverse events |
| AEFI | Adverse event following immunization |
| ATAGI | Australian Technical Advisory Group on Immunisation |
| CAEFISS | Canadian Adverse Event Following Immunization Surveillance System |
| DTP | Diphtheria, tetanus, pertussis |
| FDA | United States Food and Drug Administration |
| IHE | Institute of Health Economics |
| HPV | Human papillomavirus |
| HPV4 vaccine | 4-valent human papillomavirus vaccine |
| MedDRA | Medical Dictionary for Regulatory Activities |
| MMR | Measles-mumps-rubella |
| NACI | National Advisory Committee on Immunization |
| PHAC | Public Health Agency of Canada |
| SAEFVIC | Surveillance of Adverse Events following Vaccination in the Community |
| US | United States |
| VAERS | Vaccine Adverse Events Reporting System |
Acknowledgments
This statement was prepared by: R Stirling, P Doyon-Plourde, N Forbes, K Young, and R Harrison, on behalf of the NACI Influenza Working Group and was approved by NACI.
NACI gratefully acknowledges the contribution of: G Crichlow, H Anyoti, A House, K Johnson, M Laplante, A Sinilaite, M Tunis, and E Westhaver
NACI Influenza Working Group
Members: R Harrison (Chair), N Dayneka, I Gemmill, K Klein, D Kumar, J Langley, J McElhaney, A McGeer, D Moore, S Smith, and B Warshawsky
Liaison representatives: L Grohskopf (Centers for Disease Control and Prevention [CDC], United States)
Ex-officio representatives: L Whitmore (Centre for Immunization and Respiratory Infectious Diseases [CIRID], PHAC), A Gartley (First Nations and Inuit Health Branch [FNIHB], Indigenous Services Canada [ISC]), and J Xiong (Biologic and Radiopharmaceutical Drugs Directorate [BRDD], Health Canada [HC]).
NACI
Members: C Quach (Chair), S Deeks (Vice-Chair), J Bettinger, N Dayneka, P De Wals, E Dubé, V Dubey, S Gantt, R Harrison, K Hildebrand, K Klein, J Papenburg, B Sander, S Smith and S Wilson.
Liaison representatives: LM Bucci (Canadian Public Health Association), E Castillo (Society of Obstetricians and Gynaecologists of Canada), A Cohn (CDC, United States), L Dupuis (Canadian Nurses Association), J Emili (College of Family Physicians of Canada), D Fell (Canadian Association for Immunization Research Evaluation), M Lavoie (Council of Chief Medical Officers of Health), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), and A Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada).
Ex-officio representatives: D Danoff (Marketed Health Products Directorate, HC), E Henry (CIRID, PHAC), M Lacroix (Public Health Ethics Consultative Group, PHAC), J Pennock (CIRID, PHAC), R Pless (BRDD, HC), G Poliquin (National Microbiology Laboratory, PHAC), V Beswick-Escanlar (National Defense and the Canadian Armed Forces), and T Wong (FNIHB, ISC).
References
References
- Reference a
-
Vaccine recipients should be kept under observation for at least 15 minutes after immunization; 30 minutes is a safer interval when there is a specific concern about possible vaccine allergy.
- Reference b
-
Appropriate public health and infection prevention and control measures are to be determined by the responsible public health authority in the jurisdiction of the vaccination clinic.
- Reference c
-
Adapted from the Australian Technical Advisory Group on Immunisation (ATAGI), "Statement on the duration of observation after vaccination in the context of minimizing risk of exposure to COVID-19 at health care facilities
- Reference d
-
Refer to the NACI Statement on Mammalian cell-culture based influenza vaccines
Footnotes
- Footnote 1
-
WHO. Coronavirus disease 2019 (COVID-19) Situation Report - 51. [online] Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.
- Footnote 2
-
PHAC. Interim guidance on continuity of immunization programs during the COVID-19 pandemic. Updated May 13, 2020. [online] Available at: /content/canadasite/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/interim-guidance-immunization-programs-during-covid-19-pandemic.html
- Footnote 3
-
PHAC. Guidance for influenza vaccine delivery in the presence of COVID-19. [online] Available at: /content/canadasite/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/guidance-influenza-vaccine-delivery-covid-19.html
- Footnote 4
-
PHAC. Canadian Immunization Guide: Part 2 - Vaccine Safety. [online] Available at: /content/canadasite/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-2-vaccine-safety/page-4-early-vaccine-reactions-including-anaphylaxis.html
- Footnote 5
-
US CDC. 2017. General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). [online] Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/adverse-reactions.html
- Footnote 6
-
The Vaccine Administration Task Force. 2001. United Kingdom Guidance on Best Practice in Vaccine Administration [online] Available at: http://www.wales.nhs.uk/sitesplus/documents/861/UK%20best%20practice%20guidance%20vacc%20admin%202001.pdf
- Footnote 7
-
New Zealand Ministry of Health. 2018. Immunisation Handbook 2017 (2nd edn). Wellington: Ministry of Health. [online] Available at: https://www.health.govt.nz/system/files/documents/publications/immunisation-handbook-2017-2nd-edition-mar18-v9_1.html
- Footnote 8
-
Australian Government Department of Health. 2019. Australian Immunisation Handbook. [online] Available at: https://immunisationhandbook.health.gov.au/vaccination-procedures/after-vaccination
- Footnote 9
-
Australian Technical Advisory Group on Immunisation (ATAGI). 2020. ATAGI Clinical Statement on Vaccination Observation Time. [online] Available at: https://www.health.gov.au/resources/publications/atagi-clinical-statement-on-vaccination-observation-time
- Footnote 10
-
Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. Journal of clinical epidemiology. 2016 Jan 1;69:199-207.footnote1text
- Footnote 11
-
Rüggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, de Souza Brito G, Heininger U, Imoukhuede B, Khamesipour A, Erlewyn-Lajeunesse M. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007 Aug 1;25(31):5675-84.
- Footnote 12
-
PHAC. Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) [online] Available at: /content/canadasite/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html
- Footnote 13
-
Baxter CM, Clothier HJ, Perrett KP. Potential immediate hypersensitivity reactions following immunization in preschool aged children in Victoria, Australia. Human Vaccines & Immunotherapeutics. 2018 Aug 3;14(8):2088-92.
- Footnote 14
-
Bohlke K, Davis RL, Marcy SM, Braun MM, DeStefano F, Black SB, Mullooly JP, Thompson RS. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003 Oct 1;112(4):815-20.
- Footnote 15
-
Brotherton JM, Gold MS, Kemp AS, McIntyre PB, Burgess MA, Campbell-Lloyd S. Anaphylaxis following quadrivalent human papillomavirus vaccination. Cmaj. 2008 Sep 9;179(6):525-33.
- Footnote 16
-
Cheng DR, Perrett KP, Choo S, Danchin M, Buttery JP, Crawford NW. Pediatric anaphylactic adverse events following immunization in Victoria, Australia from 2007 to 2013. Vaccine. 2015 Mar 24;33(13):1602-7.
- Footnote 17
-
Johann-Liang R, Josephs S, Dreskin SC. Analysis of anaphylaxis cases after vaccination: 10-year review from the National Vaccine Injury Compensation Program. Annals of Allergy, Asthma & Immunology. 2011 May 1;106(5):440-3.
- Footnote 18
-
McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, Hambidge SJ, Lee GM, Jackson LA, Irving SA, King JP. Risk of anaphylaxis after vaccination in children and adults. Journal of Allergy and Clinical Immunology. 2016 Mar 1;137(3):868-78.
- Footnote 19
-
Milstien JB, Gross TP, Kuritsky JN. Adverse reactions reported following receipt of Haemophilus influenzae type b vaccine: an analysis after 1 year of marketing. Pediatrics. 1987 Aug 1;80(2):270-4.
- Footnote 20
-
Pahud BA, Williams SE, Dekker CL, Halsey N, LaRussa P, Baxter RP, Klein NP, Marchant CD, Sparks RC, Jakob K, Aukes L. Clinical assessment of serious adverse events in children receiving 2009 H1N1 vaccination. The Pediatric infectious disease journal. 2013 Feb 1;32(2):163-8.
- Footnote 21
-
Patja A, Davidkin I, Kurki T, Kallio MJ, Valle M, Peltola H. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. The Pediatric infectious disease journal. 2000 Dec 1;19(12):1127-34.
- Footnote 22
-
Schumacher Z, Bourquin C, Heininger U. Surveillance for adverse events following immunization (AEFI) in Switzerland-1991-2001. Vaccine. 2010 May 28;28(24):4059-64.
- Footnote 23
-
Su JR, Moro PL, Ng CS, Lewis PW, Said MA, Cano MV. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. Journal of Allergy and Clinical Immunology. 2019 Apr 1;143(4):1465-73.
- Footnote 24
-
Zafack JG, Toth E, Landry M, Drolet JP, Top KA, De Serres G. Rate of Recurrence of Adverse Events Following Immunization: Results of 19 Years of Surveillance in Quebec, Canada. The Pediatric infectious disease journal. 2019 Apr 1;38(4):377-83.
- Footnote 25
-
Stone CA, Commins SP, Choudhary S, Vethody C, Heavrin JL, Wingerter J, Hemler JA, Babe K, Phillips EJ, Norton AE. Anaphylaxis after vaccination in a pediatric patient: further implicating alpha-gal allergy. The Journal of Allergy and Clinical Immunology: In Practice. 2019 Jan 1;7(1):322-4.
- Footnote 26
-
Stone CA, Hemler JA, Commins SP, Schuyler AJ, Phillips EJ, Peebles RS, Fahrenholz JM. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. Journal of Allergy and Clinical Immunology. 2017 May 1;139(5):1710-3./p>
- Footnote 27
-
Türktas I, Ergenekon E. Anaphylaxis following diphtheria-tetanus-pertussis vaccination-a reminder. European journal of pediatrics. 1999 Apr 1;158(5):434.
- Footnote 28
-
Worm M, Sterry W, Zuberbier T. Gelatin-induced urticaria and anaphylaxis after tick-borne encephalitis vaccine. Acta dermato-venereologica. 2000;80(3).
- Footnote 29
-
Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Archives of pediatrics & adolescent medicine. 1997 Mar 1;151(3):255-9.
- Footnote 30
-
Crawford NW, Clothier HJ, Elia S, Lazzaro T, Royle J, Buttery JP. Syncope and seizures following human papillomavirus vaccination: a retrospective case series. Medical journal of Australia. 2011 Jan;194(1):16-8.
- Footnote 31
-
Šubelj M, Učakar V, Kraigher A, Klavs I. Adverse events following school-based vaccination of girls with quadrivalent human papillomavirus vaccine in Slovenia, 2009 to 2013. Eurosurveillance. 2016 Apr 7;21(14):30187.
- Footnote 32
-
Sutherland A, Izurieta H, Ball R, Braun MM, Miller ER, KR Broder KR, Slade BA, Iskander JK, Kroger AT, Markowitz LE, Huang WT. Syncope after vaccination--United States, January 2005-July 2007. MMWR. Morbidity and mortality weekly report. 2008 May 2;57(17):457.
- Footnote 33
-
Woo EJ, Ball R, Braun MM. Fatal syncope-related fall after immunization. Archives of pediatrics & adolescent medicine. 2005 Nov 1;159(11):1083.
- Footnote 34
-
Poddighe D, Castelli L, Marseglia GL, Bruni P. A sudden onset of a pseudo-neurological syndrome after HPV-16/18 AS04-adjuvated vaccine: might it be an autoimmune/inflammatory syndrome induced by adjuvants (ASIA) presenting as a somatoform disorder?. Immunologic research. 2014 Dec 1;60(2-3):236-46.
- Footnote 35
-
Dreskin SC, Halsey NA, Kelso JM, Wood RA, Hummell DS, Edwards KM. International Consensus (ICON): Allergic reactions to vaccines. World Allergy Organ J. 2016; 9: 32.
- Footnote 36
-
McGuinness, LA, Higgins, JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2020; 1- 7. https://doi.org/10.1002/jrsm.1411
