Recommendations for public health programs on the use of pneumococcal vaccines in children, including the use of 15-valent and 20-valent conjugate vaccines
Download in PDF format
(1.57 MB, 65 pages)
Organization: Public Health Agency of Canada
Date published: 2024-03-11
Cat.: HP5-239/1-2024E-PDF
ISBN: 978-0-660-70388-6
Pub.: 230765
On this page
- Preamble
- Summary of information contained in this NACI statement
- Introduction
- Methods
- Vaccine
- Evidence summary
- Ethics, equity, feasibility and acceptability considerations
- Economics
- Recommendations
- Research and surveillance priorities
- List of abbreviations
- Acknowledgements
- Appendix A: Tables
- References
Preamble
The National Advisory Committee on Immunization (NACI) is an External Advisory Body that provides the Public Health Agency of Canada (PHAC) with independent, ongoing and timely medical, scientific, and public health advice in response to questions from PHAC relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the systematic consideration of programmatic factors in developing evidence-based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be systematically considered by NACI include economics, ethics, equity, feasibility and acceptability. Not all NACI statements will require in-depth analyses of all programmatic factors. While systematic consideration of programmatic factors will be conducted using evidence-informed tools to identify distinct issues that could impact decision-making for recommendation development, only distinct issues identified as being specific to the vaccine or vaccine-preventable disease will be included.
This statement contains NACI's independent advice and recommendations, which are based upon the best current available scientific knowledge. This document is being disseminated for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph. Recommendations for use and other information set out herein may differ from that set out in the product monographs of the Canadian manufacturers of the vaccines. Manufacturer(s) have sought approval of the vaccines and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Summary of information contained in this NACI statement
The following highlights key information for immunization providers. Please refer to the remainder of the statement for details.
What
The bacterium Streptococcus pneumoniae can lead to invasive pneumococcal disease (IPD), a serious communicable disease, and other infections such as community acquired pneumonia and acute otitis media. There are currently more than 100 known serotypes of S. pneumoniae. The majority of pneumococcal infections are caused by only a portion of these serotypes.
Health Canada has recently authorized two new pneumococcal conjugate (Pneu-C) vaccines for infants, children and adolescents 6 weeks through 17 years of age:
- Pneu-C-15 (15-valent) is authorized with an indication for prevention of IPD caused by 15 serotypes of S. pneumoniae.
- Pneu-C-20 (20-valent) is authorized with an indication for prevention of IPD caused by 20 serotypes of S. pneumoniae.
Who
IPD is most common in young children, older adults, and groups at increased risk due to a medical condition and/or environmental/living conditions (see Table 1). NACI continues to recommend that all routine infant immunization programs in Canada include a conjugate pneumococcal vaccine.
For routine immunization programs in children less than 5 years of age who are not at increased risk of IPD:
- NACI recommends that either Pneu-C-15 or Pneu-C-20 should be the current product of choice for children less than five years of age in routine immunization programs.
For children at increased risk of IPD due to a medical and/or environmental/living conditions (Table 1):
- NACI recommends that Pneu-C-20 should be used for children 2 months to less than 18 years of age who have conditions that result in increased risk of IPD. Children who have started their pneumococcal vaccine series with Pneu-C-13 or Pneu-C-15 should complete their series with Pneu-C-20.
- NACI recommends that children under 18 years of age who are at increased risk of IPD due to medical and/or environmental/living conditions and who have completed their recommended immunization schedule with Pneu-C-13 or Pneu-C-15, should receive one catch-up (additional) dose of Pneu-C-20.
- NACI recommends that Pneu-C-20 should be offered to children less than 18 years of age who received a hematopoietic stem cell transplant (HSCT) after consultation with their transplant specialist.
For additional information, including supporting evidence and rationale for these recommendations, please see Recommendations.
Table 1: Risk conditions resulting in increased risk of IPD
Medical risk conditions
- Chronic cerebrospinal fluid (CSF) leak
- Cochlear implants, including those who are to receive implants. The highest risk is in the weeks following implant. It is best to administer prior to implant but surgery should not be delayed to administer vaccine. Vaccine should be given as soon as possible.
- Chronic kidney disease, particularly those with nephrotic syndrome, on dialysis, or with renal transplant
- Chronic liver disease, including hepatic cirrhosis and biliary atresia
- Chronic neurologic condition that may impair clearance of oral secretions
- Functional or anatomic asplenia (including sickle cell disease and other hemoglobinopathies, congenital or acquired asplenia, or splenic dysfunction)
- Diabetes mellitus
- Chronic heart disease (including congenital heart disease and cyanotic heart disease)
- Chronic lung disease, including asthma requiring acute medical care in the preceding 12 months
- Congenital immunodeficiencies involving any part of the immune system, including B-lymphocyte (humoral) immunity, T-lymphocyte (cell) mediated immunity, complement system (e.g. properdin, or factor D deficiencies)
- Immunocompromising therapy, including use of long-term corticosteroids, chemotherapy, radiation therapy, and post-organ transplant therapy
- HIV infection
- Hematopoietic stem cell transplant (recipient)* see separate recommendation
- Malignant neoplasms, including leukemia and lymphoma
- Solid organ transplant
Environmental or living conditions for individuals
- Who live in communities or settings experiencing sustained high IPD rates
- Who are underhoused or experiencing houselessness
- Who are in residential care for children with complex medical needs
How
Pneu-C-15 and Pneu-C-20 are administered intramuscularly using a single-dose, prefilled syringe. A single dose of Pneu-C-15 and Pneu-C-20 is 0.5ml.
Pneu-C-15 and Pneu-C-20 are provided either using a 3-dose (2+1) schedule at 2 months, 4 months and 12 months of age, or a 4-dose (3+1) schedule at 2 months, 4 months and 6 months followed by a dose at 12 to 15 months of age. For more information on immunization schedules, including for children at increased risk of IPD and by immunization history, refer to the pneumococcal vaccines chapter of the Canadian Immunization Guide and Recommendations (Table 7).
Contraindications for Pneu-C-15 and Pneu-C-20 include hypersensitivity (e.g., anaphylaxis) to the vaccine or any of its components. Pneumococcal vaccines may be administered concurrently with other vaccines, except for a different formulation of pneumococcal vaccine (e.g., do not concurrently administer conjugate and polysaccharide pneumococcal vaccines).
Why
Pneumococcal disease can lead to long-lasting complications and can result in significant morbidity and mortality, especially for young children and other children at increased risk of IPD. The most effective way to prevent these infections is through immunization. Pneu-C-15 and Pneu-C-20 are designed to prevent infection from a larger number of serotypes than previous pneumococcal vaccines.
Introduction
Guidance objective
The need for updated National Advisory Committee on Immunization (NACI) guidance on the pediatric pneumococcal vaccine program arose from the authorization of new pediatric indications for two pneumococcal conjugate (Pneu-C) vaccines. On July 8, 2022, Health Canada authorized the use of a pneumococcal conjugate 15-valent vaccine (Pneu-C-15, Vaxneuvance®), for active immunization of children from 6 weeks through 17 years of age for the prevention of invasive pneumococcal disease (IPD) caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F. On July 21, 2023, Health Canada authorized the use of a pneumococcal conjugate 20-valent vaccine (Pneu-C-20, Prevnar 20®) for the prevention of IPD caused by S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F and 33F in children 6 weeks through 17 years of age.
Note: For purposes of this document, 'children' refers to infants, children and adolescents under 18 years of age.
On October 4th, 2022, NACI provided interim guidance on the use of Pneu-C-15 vaccine, recommending that it may be used interchangeably with Pneu-C-13 in children 6 weeks to 17 years of age. Following the approval of Pneu-C-20 for use in children, additional guidance was requested by Provinces and Territories on the use of these vaccines in routine pediatric immunization programs in Canada.
The primary objectives of this Statement are to:
- review the evidence on the potential benefits (immunogenicity), risks (safety) and modelled impact and cost-effectiveness of pediatric pneumococcal immunization programs using Pneu-C-15 or Pneu-C-20 on the reduction on the pneumococcal disease burden in Canada.
- provide recommendations for the use of Pneu-C-15 and Pneu-C-20 vaccines in routine pediatric programs in Canada and for children at increased risk of IPD.
Background on pneumococcal vaccines, immunization programs and recommendations for children in Canada
In Canada, routine immunization programs for IPD for infants include the use of Pneu-C vaccines provided either using a 3-dose (2+1) schedule at 2 months, 4 months and 12 months of age or a 4-dose (3+1) schedule at 2 months, 4 months and 6 months followed by a dose at 12 to 15 months of age. Infants at increased risk of IPD due to an underlying medical condition have previously been recommended to receive the 4-dose schedule of Pneu-C-13 vaccine, as well as one dose of Pneu-P-23 vaccine at 24 months of age, at least 8 weeks after Pneu-C-13 vaccine, to increase protection against additional S. pneumoniae serotypes not contained in Pneu-C-13. Children with certain medical conditions were also recommended an additional booster dose of Pneu-P-23 vaccine at least 5 years after any previous dose of Pneu-P-23 vaccine.
In 2021, it was estimated that 85.1% (95% confidence interval [CI]: 83.1% to 87.0%) of 2-year-old children in Canada had at least 3 doses of the pneumococcal vaccineFootnote 1. The current national vaccination coverage goal by 2025 is to have 95% of 2-year-old children immunized with at least 3 doses of the recommended pneumococcal vaccineFootnote 2.
Methods
In brief, the stages in the preparation of a NACI statement are:
- Knowledge synthesis: retrieval and summary of individual studies, and assessment of the risk of bias of included studies.
- Summary of vaccine-relevant evidence: benefits (examples would include: immunogenicity, efficacy and effectiveness profiles compared with existing vaccines) and potential harms (safety profile compared with existing vaccines), considering the certainty of the synthesized evidence and, where applicable, the magnitude of effects observed across the studies.
- Systematic assessment of program-relevant considerations related to ethics, equity, feasibility, and acceptability (EEFA).
- Economic evaluation, including a systematic review of economic evaluations and economic modelling.
- Use of all available evidence to inform recommendations.
NACI reviewed the available evidence on Pneu-C-15 and Pneu-C-20 immunogenicity and safety, pneumococcal disease burden, and cost-effectiveness and considerations for ethics, equity, feasibility, and acceptability during meetings held on June 12, July 7 and September 27, 2023. Following NACI discussion, additional evidence on the risk of IPD in children with risk factors was reviewed by the Committee on November 16, 2023. Recommendations on the use of pneumococcal vaccines in pediatric populations were approved on December 5, 2023.
Further information on NACI's process and procedures is available elsewhereFootnote 3. Comprehensive NACI recommendations for the use of pneumococcal vaccines in Canada are provided in the pneumococcal vaccines chapter in the Canadian Immunization Guide (CIG).
Burden of invasive pneumococcal disease
Since 2000, IPD has been nationally notifiable in Canada through the Canadian Notifiable Disease Surveillance System (CNDSS), with all provinces and territories reporting cases that meet the national case definition. For this Statement, the CNDSS line list data used to assess the burden of IPD among different pediatric age groups were available from ten provinces and from the International Circumpolar Surveillance (ICS) program for the three territories. In addition to the three territories, regions of Canada captured in the ICS system also include northern Labrador and northern Quebec. The incidence of IPD in these regions was compared to national IPD incidence using aggregate CNDSS data. All cases were presumed to meet the national case definition of IPD. More information about the CNDSS data is provided at Notifiable Diseases Online, including limitations to the CNDSS data.
The National Microbiology Laboratory (NML) collaborates with public health laboratories to conduct passive, laboratory-based surveillance of IPD in Canada and releases regular reportsFootnote 4. All IPD isolates from public health laboratories are serotyped by the NML, although specimen collection may be limited by variable regional standards, the preliminary nature of some data, and the availability of bacterial isolates for testing. Serotype data may also be biased toward overrepresentation of more virulent serotypes for which medical treatment is sought and clinical specimens are taken. Information on serotype distribution is provided for 80% to 98% of all IPD cases reported to CNDSS.
For the serotype reporting in this Statement, serotype 6C was included with Pneu-C-13 serotypes due to cross protection with 6A. Serotypes 15B and 15C were grouped together as 15B/C because of reported reversible switching between them in vivo during infection, making it difficult to precisely differentiate between the two types.
Additional information on complications of pneumococcal disease were collected through the pediatric hospital-based surveillance network IMPACT (Immunization Monitoring Program, ACTive) which covers 90% of tertiary care pediatric beds in Canada.
There are limited Canadian-specific data on pneumococcal disease in children with medical risk conditions. NACI reviewed information on pneumococcal disease in this population based on data from a recent review conducted to inform the United States (US) Advisory Committee on Immunization PracticesFootnote 5.
Literature review of Pneu-C-15 and Pneu-C-20
The overarching research question to support the evidence review is: What is the efficacy, effectiveness, immunogenicity, and safety of Pneu-C-15 and Pneu-C-20 compared to Pneu-C-13, when used to reduce the risk of IPD in children under 18 years of age?
Population: Children 6 weeks to under 18 years of age with and without additional risk factors for IPD (Table 1) who have never been vaccinated, have an incomplete vaccine series, or have completed their vaccine series (for catch-up/additional doses).
Intervention: Pneu-C-15 or Pneu-C-20 (alone or in a mixed series).
Comparator: Currently recommended age and risk factor-appropriate pneumococcal vaccine schedule.
Outcomes: Death due to S. pneumoniae serotypes included in the vaccine (i.e., vaccine serotype), IPD due to vaccine serotypes, acute otitis media (AOM) or pneumococcal community acquired pneumonia (CAP) due to vaccine serotypes, and serious adverse events following vaccination.
As efficacy/effectiveness and safety data were not published at the time of the initial evidence review, NACI evaluated immunogenicity and safety data accessible in the regulatory submission to Health Canada and/or were presented to NACI by the manufacturer.
Immunogenicity datapoints that were reviewed by NACI included opsonophagocytic (OPA) geometric mean titers (GMT), IgG geometric mean concentrations (GMC), and seroresponse rates (defined according to the World Health Organization standard of either a greater or equal to 4-fold increase in pre-vaccination GMC/GMT ratios or GMC values of greater or equal to 0.35 μg/mL)Footnote 6. The data that were extracted for analysis included information on the study design, population, intervention, comparator, and outcomes of interest. The risk of bias for each study was assessed using the Cochrane Risk of Bias Tool. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework was used to assess the certainty of evidence where possible, based on availability of outcome information. Immunogenicity outcomes (ratios/differences) with Pneu-C-15 or Pneu-C-20 compared to Pneu-C-13 were considered to be numerically lower or higher when all CI values in all studies were less than 1.00 or greater than 1.00, respectively. Serotypes for which these values could not be determined were either those in which CIs were null-inclusive (i.e., included 1.00) or where results were mixed across studies. GRADE data tables for analyzed studies are available in Appendix A.
Literature review of Pneu-C-15 and Pneu-C-20 cost-effectiveness
A systematic review of the cost-effectiveness of Pneu-C-15 and Pneu-C-20 vaccines for preventing pneumococcal disease in infants and children included published studies of economic evaluations conducted in populations aged less than 18 years, comparing currently used vaccines to prevent pneumococcal disease to Pneu-C-15 or Pneu-C-20 and measures of economic outcomes (incremental cost per quality-adjusted life year [QALY], cost per life year, etc.). A systematic literature search for English- and French-language studies was conducted in six electronic databases: Embase, Ovid Medline, International Pharmaceutical Abstracts, EBM Reviews, SCOPUS, and Econlit. The search was limited to records published between January 1, 2018, to March 7, 2023. All costs were adjusted to 2022 Canadian dollars and are reported as such. Additional details of the economic literature review are provided in a supplementary economic evidence summary.
NACI cost-utility analysis
A model-based cost-utility analysis was conducted from health system and societal perspectives. A multi-age static cohort model was used to compare the benefits (in QALYs) and costs (in 2022 Canadian dollars) associated with using Pneu-C-15 or Pneu-C-20 compared to Pneu-C-13 in previously unvaccinated infants that are eligible for routine pneumococcal vaccination. The analysis used a 10-year time horizon, with lifetime costs and consequences of pneumococcal disease discounted at 1.5% and all costs adjusted to 2022 Canadian dollars. Details of the cost-utility analysis are provided in a supplementary economic evidence summary.
Vaccine
There are currently five vaccines in Canada that are authorized for use in children less than 18 years of age:
- Pneu-P-23 (Pneumovax®23) is a sterile solution of 23 highly purified capsular polysaccharidesFootnote 7.
- Pneu-C-10 (Synflorix®) is a sterile suspension of saccharides of the capsular antigens of 10 serotypes of S. pneumoniae conjugated to the Non-Typeable Haemophilus influenza protein D, diphtheria or tetanus toxoidFootnote 8.
- Pneu-C-13 (Prevnar®13) is a sterile solution of polysaccharide capsular antigen of 13 serotypes of S. pneumoniae. The antigens are individually conjugated to a Corynebacterium diphtheriae (CRM197) protein carrierFootnote 9.
- Pneu-C-15 (Vaxneuvance®) is a sterile suspension of purified capsular polysaccharides from 15 serotypes of S. pneumoniae. The antigens are individually conjugated to diphtheria CRM197 protein carrierFootnote 10.
- Pneu-C-20 (Prevnar 20TM) is a sterile saccharide suspension of the capsular antigens of 20 serotypes of S. pneumoniae. The antigens are individually conjugated to diphtheria CRM197 proteinFootnote 11.
Additional information about the composition of these vaccines is available in Table 2.
| Vaccine name | PneuMOVAX®23 (Pneu-P-23) |
SYNFLORIX® (Pneu-C-10) |
PREVNAR®13 (Pneu-C-13) |
VAXNEUVANCE® (Pneu-C-15) |
PREVNAR 20TM (Pneu-C-20) |
|---|---|---|---|---|---|
| Manufacture | Merck | GSK | Pfizer | Merck | Pfizer |
| Date of initial authorization in Canada / pediatric age of authorization | December 23, 1983 / 2 years old to 17 years old |
December 11, 2008/ 6 weeks to 5 years old |
December 21, 2009/ 6 weeks to 17 years old |
July 8, 2022 / 6 weeks to 17 years old |
May 9, 2022 (adult) July 21, 2023 (children)/ 6 weeks to 17 years old |
| Type of vaccine | Polysaccharide | Conjugate | Conjugate | Conjugate | Conjugate |
| Vaccine composition | 25 mcg of capsular polysaccharides from each of S. pneumoniae serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F, sodium chloride 0.9 % w/w, phenol 0.25% w/w and water for injection. |
1 mcg of each saccharide for S. pneumoniae serotypes 1, 5, 6B, 7F, 9V, 14 and 23F, and 3 mcg of saccharide for serotype 4, 18C and 19F, Non-Typeable Haemophilus influenzae |
2.2 mcg of each saccharide for S. pneumoniae serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F and 23F, 4.4 mcg of saccharide for serotype 6B, 34 mcg CRM197 carrier protein, 4.25 mg sodium chloride, 100 mcg polysorbate 80, 295 mcg succinic acid and 125 mcg aluminum as aluminum phosphate adjuvant and water for injection |
32 mcg of total pneumococcal polysaccharide (2.0 mcg each of polysaccharide serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F and 4.0 mcg of polysaccharide serotype 6B) conjugated to 30 mcg of CRM197 carrier protein, 125 mcg of |
2.2 mcg of each of S. pneumoniae serotypes 1, 3, 4, 5, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F and 33F saccharides, 4.4 mcg of 6B saccharide, 51 mcg CRM197 carrier protein, 100 mcg polysorbate 80, 295 mcg succinic acid, 4.4 mg sodium chloride, and 125 mcg aluminum as aluminum phosphate adjuvant and water for injection |
Route of administration |
Intramuscular or subcutaneous injection |
Intramuscular injection |
Intramuscular injection |
Intramuscular injection |
Intramuscular injection |
Storage Requirements |
Multi-dose vial. |
Single-dose prefilled syringe. Refrigerate at 2°C to 8°C. Do not freeze. Store in original package. |
Single-dose prefilled syringe. Refrigerate at 2°C to 8°C. Do not freeze. Store in original package. |
Single-dose prefilled syringe. Refrigerate at 2°C to 8°C. Do not freeze. Protect from light. Administer as soon as possible after being removed from the refrigerator. |
Single-dose pre-filled syringe. Refrigerate at 2°C to 8°C. Do not freeze. Store horizontally in original package. |
A comparison of serotypes included in currently authorized vaccine formulations is provided in Table 3.
| Vaccine | Serotypes in penumonoccal vaccines | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 5 | 7F | 3 | 6A | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | 2 | 9N | 17F | 20 | |
| PNEU-C-10 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| PNEU-C-13 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N | N | N |
| PNEU-C-15 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | N | N | N | N | N |
| PNEU-C-20 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N |
| PNEU-C-23 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Abbreviations: Y: yes; N: no | ||||||||||||||||||||||||
Evidence summary
Burden of disease
IPD incidence rates by age group
Following the implementation of Pneu-C programs in Canada, between 2001 and 2021, there was an approximately 65% reduction in overall IPD incidence in children under five years of age. Although Pneu-C-7 vaccine had the largest impact on IPD incidence in the under-five age group, further decreases in this age group were observed with the switch to Pneu-C-13 in 2010. Since 2015, when the reported IPD incidence rate in children under five years of age reached 10.7 (95% CI: 9.3-12.2) cases per 100,000 population, the IPD in this age group has remained stable, with the exception being a decrease in the IPD incidence rate in 2020 (IR: 6.1, 95% CI: 5.0-7.3) during the first year of the COVID-19 pandemic. In contrast, since the introduction of Pneu-C programs, the IPD incidence in individuals 5 to 19 has remained low and relatively stable (Figure 1). Between 2014 and 2021, there have been, on average, 214 cases of IPD reported annually for children under five years of age (164 cases per year in the 1-to-4-year age group), and 108 cases per year in the 5- to 19-year-old age group.
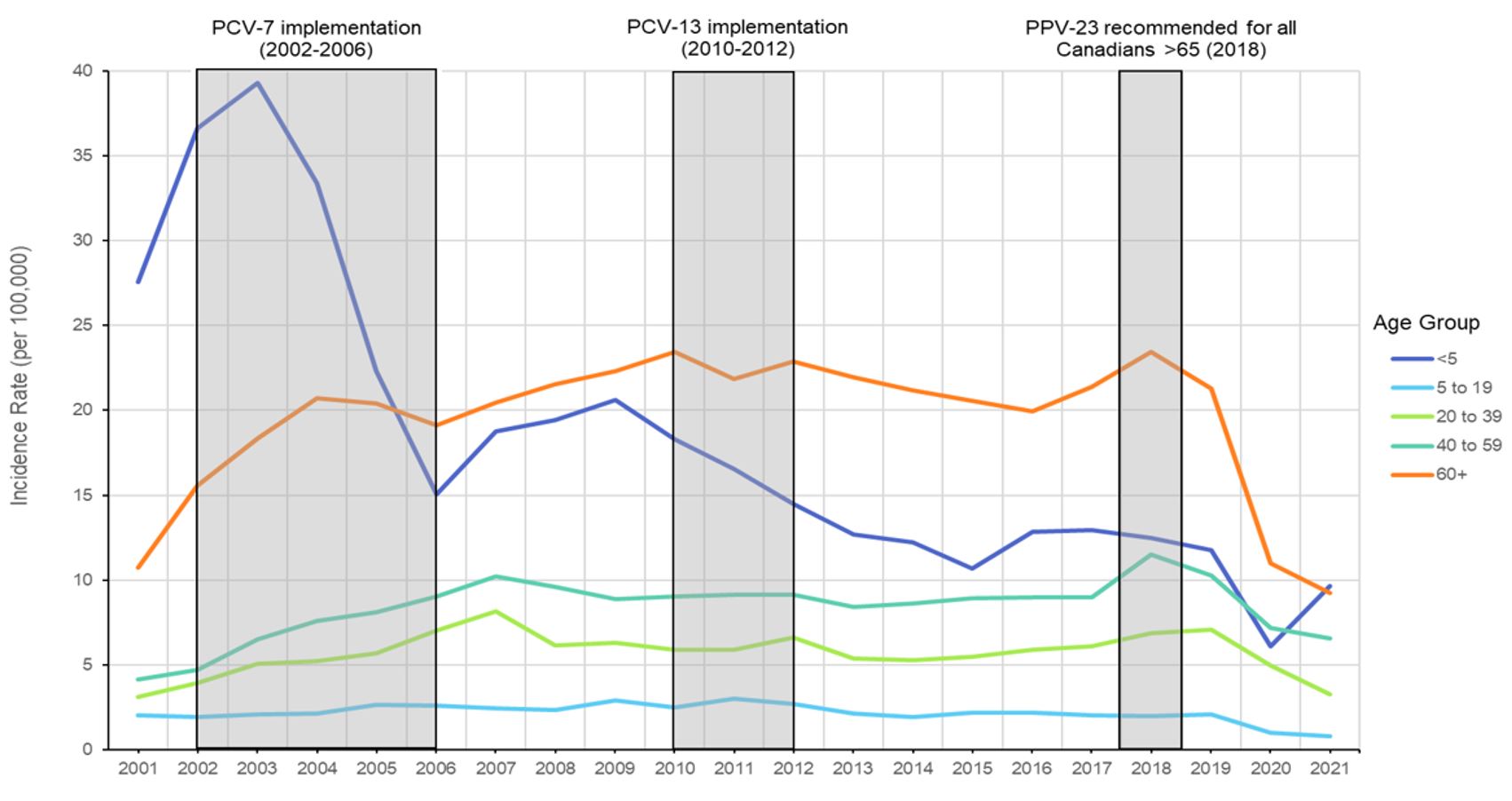
Figure 1: Text description
A line graph displaying the incidence rate of invasive pneumococcal disease each year from 2001 to 2021, for different age groups including less than 5 years of age, 5 to 19 years of age, 20 to 39 years of age, 40 to 59 years of age, and 60 years of age and older, and highlighting the dates of implementation of Pneu-C-7, Pneu-C-13, and Pneu-P-23 in Canada.
| Year | Less than 1 | 1 to 4 | 5 to 9 | 10 to 14 | 15 to 19 | 20 to 24 | 25 to 29 | 30 to 39 | 40 to 59 | 60 and over |
|---|---|---|---|---|---|---|---|---|---|---|
| 2001 | 37.8 | 25.2 | 3.6 | 1.1 | 1.5 | 1.6 | 1.8 | 2.5 | 4.1 | 10.8 |
| 2002 | 50.0 | 33.5 | 3.2 | 1.6 | 1.2 | 1.6 | 2.1 | 3.5 | 4.7 | 15.6 |
| 2003 | 54.5 | 35.6 | 4.4 | 1.2 | 1.0 | 1.4 | 3.0 | 4.7 | 6.5 | 18.3 |
| 2004 | 42.9 | 31.0 | 4.3 | 1.2 | 1.3 | 1.6 | 2.8 | 4.9 | 7.6 | 20.7 |
| 2005 | 25.9 | 21.4 | 5.3 | 1.8 | 1.3 | 2.6 | 3.2 | 5.0 | 8.1 | 20.4 |
| 2006 | 20.3 | 13.7 | 4.6 | 1.8 | 1.8 | 2.0 | 3.8 | 6.8 | 9.0 | 19.1 |
| 2007 | 30.3 | 15.8 | 3.4 | 1.9 | 2.1 | 3.4 | 4.5 | 7.1 | 10.2 | 20.5 |
| 2008 | 29.8 | 16.7 | 4.6 | 1.4 | 1.4 | 2.7 | 3.5 | 5.2 | 9.6 | 21.6 |
| 2009 | 27.8 | 18.7 | 5.8 | 1.6 | 1.8 | 2.4 | 3.5 | 5.5 | 8.9 | 22.3 |
| 2010 | 25.1 | 16.5 | 4.8 | 1.8 | 1.3 | 1.9 | 3.1 | 5.2 | 9.0 | 23.4 |
| 2011 | 20.7 | 15.5 | 5.3 | 2.5 | 1.7 | 1.4 | 3.1 | 5.4 | 9.2 | 21.8 |
| 2012 | 18.3 | 13.6 | 4.3 | 2.5 | 1.6 | 2.5 | 4.0 | 5.1 | 9.2 | 22.9 |
| 2013 | 18.7 | 11.2 | 3.8 | 1.5 | 1.3 | 1.5 | 2.8 | 4.6 | 8.4 | 21.9 |
| 2014 | 17.6 | 10.9 | 3.7 | 1.1 | 1.0 | 1.7 | 2.6 | 4.4 | 8.6 | 21.2 |
| 2015 | 14.4 | 9.8 | 3.9 | 1.2 | 1.5 | 1.9 | 2.8 | 4.5 | 8.9 | 20.6 |
| 2016 | 15.7 | 12.1 | 3.6 | 1.3 | 1.7 | 2.3 | 2.6 | 4.8 | 9.0 | 20.0 |
| 2017 | 15.6 | 12.3 | 3.5 | 1.2 | 1.4 | 1.5 | 2.8 | 5.3 | 9.0 | 21.4 |
| 2018 | 12.0 | 12.6 | 3.7 | 1.2 | 1.2 | 1.6 | 3.6 | 5.7 | 11.5 | 23.4 |
| 2019 | 13.4 | 11.3 | 3.8 | 1.2 | 1.3 | 2.1 | 2.8 | 6.0 | 10.3 | 21.3 |
| 2020 | 6.0 | 6.1 | 1.7 | 0.5 | 0.8 | 1.5 | 2.5 | 3.9 | 7.2 | 11.0 |
| 2021 | 10.3 | 9.5 | 1.5 | 0.4 | 0.6 | 2.0 | 2.5 | 4.2 | 6.6 | 9.3 |
Data Source: CNDSS
IPD incidence rates by geographic region
Differences in IPD incidence rates are also observed geographically. When compared to those reported for the rest of Canada, the incidence rates of IPD in Northern Canada have been higher across age groups, and particularly in children less than one year of age (incidence rate ratio of 5.9 [95% CI: 4.7-7.5]). Outside of Northern Canada, the incidence rates in 5- to 19-year-old age groups have consistently remained below 5 cases per 100,000 population between 2001 and 2021 (Table 4).
| Age group (years) | IR Northern Canada | IR Rest of Canada | IRR (95% CI) |
|---|---|---|---|
| Less than 1 | 133.0 | 22.6 | 5.9 (4.7-7.5) |
| 1-4 | 36.7 | 16.0 | 2.3 (1.8-2.9) |
| 5-9 | 10.9 | 3.9 | 2.8 (1.9-4.0) |
| 10-14 | 2.8 | 1.4 | 1.9 (0.9-4.1)Footnote b |
| 15-19 | 6.1 | 1.4 | 4.4 (2.6-7.3) |
|
|||
IPD reporting according to vaccine serotypes
Excluding a slight increase in 2022, the number of isolates caused by Pneu-C-7 vaccine serotypes has remained relatively stable following the introduction of Pneu-C-13 into routine pediatric programs despite the approximately 25% lower GMC IgG concentrations that were reported in the Pneu-C-7/Pneu-C-13 clinical trials for the shared antigens (although seroresponse rates were similar) [Figure 2]Footnote 9.
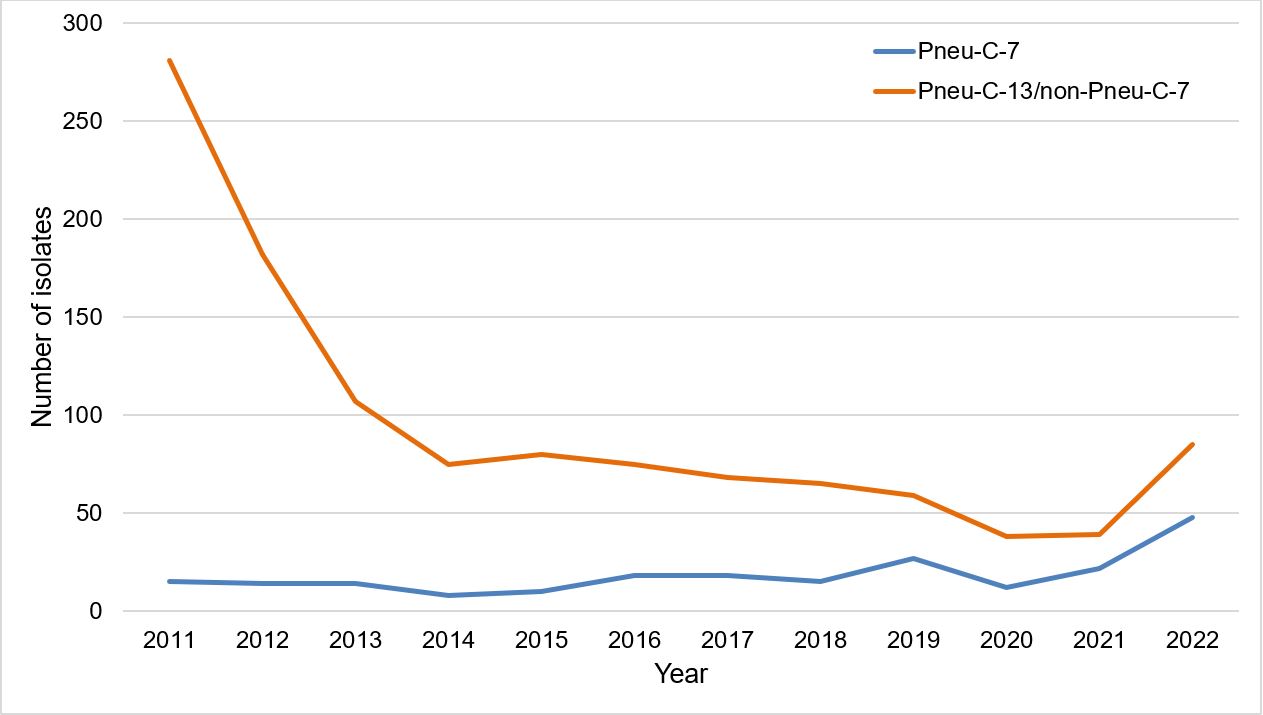
Figure 2: Text description
A line graph displaying the number of Streptococcus pneumoniae isolates collected each year from 2011 to 2022, for Pneu-C-7 serotypes and Pneu-C-13/non-Pneu-C-7 serotypes.
| Year | Number of isolates of: | |
|---|---|---|
| Pneu-C-7 serotypes | Pneu-C-13/non-Pneu-C-7 serotypes | |
| 2011 | 15 | 281 |
| 2012 | 14 | 182 |
| 2013 | 14 | 107 |
| 2014 | 8 | 75 |
| 2015 | 10 | 80 |
| 2016 | 18 | 75 |
| 2017 | 18 | 68 |
| 2018 | 15 | 65 |
| 2019 | 27 | 59 |
| 2020 | 12 | 38 |
| 2021 | 22 | 39 |
| 2022 | 48 | 85 |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); NML. Preliminary data for 2021 and 2022.
Following the introduction of Pneu-C-13 vaccine into routine pediatric immunization programs, there has been a generally decreasing trend in the proportion of pediatric IPD isolates due to vaccine serotypes, from approximately 58% in 2011 to 31% in 2022. However, the proportion of non-Pneu-C-13 vaccine serotypes has increased from 2011 to 2022. In children less than 18 years of age, the largest increases in vaccine serotypes were observed for Pneu-C-20/non-Pneu-C-15 serotypes (from 15.1% to 22.8%), followed by Pneu-C-15/non-Pneu-C-13 serotypes (from 8.3% to 14.9%) and Pneu-P-23/non-Pneu-C-20 serotypes (from 1.8% to 4.4%); the proportion of IPD isolates caused by non-vaccine type (NVT) serotypes increased from approximately 17% to 28%. Figure 3 provides information by age group.
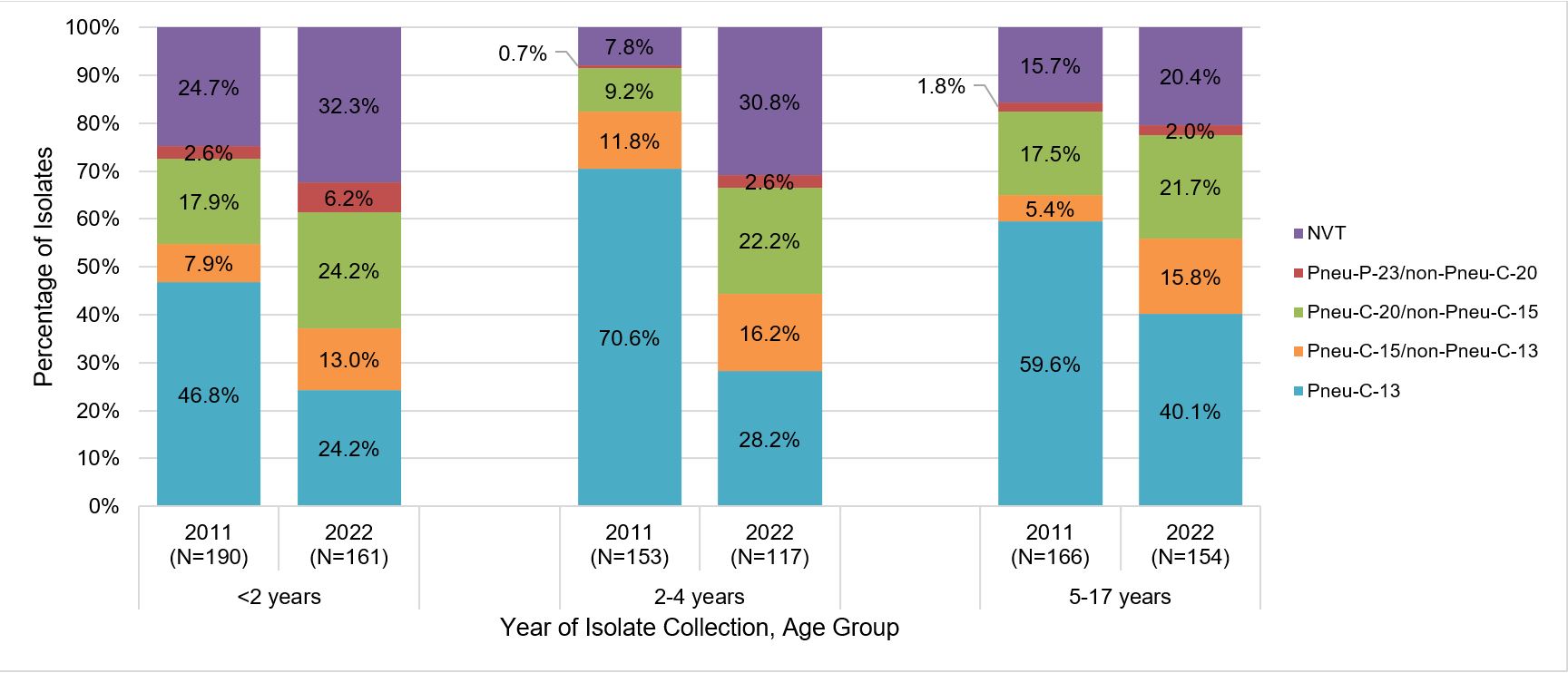
Figure 3: Text description
A stacked bar graph displaying the percentage of Streptococcus pneumoniae isolates collected from each vaccine category (PNEU-C-13, PNEU-C-15/non-PNEU-C-13, PNEU-C-20/non-PNEU-C-15, PNEU-P-23/non-PNEU-C-20 and other non-vaccine serotypes) in 2011 compared to 2022, for different pediatric age groups: <2 years, 2-4 years and 5-17 years of age.
| Age group | Year | Vaccine (%, N) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU-C-13 | PNEU-C-15/ non-PNEU-C-13 |
PNEU-C-20/ non-PNEU-C-15 |
PNEU-P-23/ non-PNEU-C-20 |
NVT | ||||||||
| Less than 2 years | 2011 | 46.8% | (89) | 7.9% | (15) | 17.9% | (34) | 2.6% | (5) | 24.7% | (47) | 190 |
| 2022 | 24.2% | (39) | 13.0% | (21) | 24.2% | (39) | 6.2% | (10) | 32.3% | (52) | 161 | |
| 2 to 4 years | 2011 | 70.6% | (108) | 11.8% | (18) | 9.2% | (14) | 0.7% | (1) | 7.8% | (12) | 153 |
| 2022 | 28.2% | (33) | 16.2% | (19) | 22.2% | (26) | 2.6% | (3) | 30.8% | (36) | 117 | |
| 5 to 17 years | 2011 | 59.6% | (99) | 5.4% | (9) | 17.5% | (29) | 1.8% | (3) | 15.7% | (26) | 166 |
| 2022 | 40.1% | (61) | 15.8% | (24) | 21.7% | (33) | 2.0% | (3) | 20.4% | (31) | 154 | |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); NML. Preliminary data for 2021 and 2022.
In children less than 2 years of age, apart from a slight increase in 19F and 19A serotype isolates, there was an overall decrease in the proportion of isolates caused by Pneu-C-13 serotypes from 2011 to 2014, after which the proportion stabilized (Figure 4).
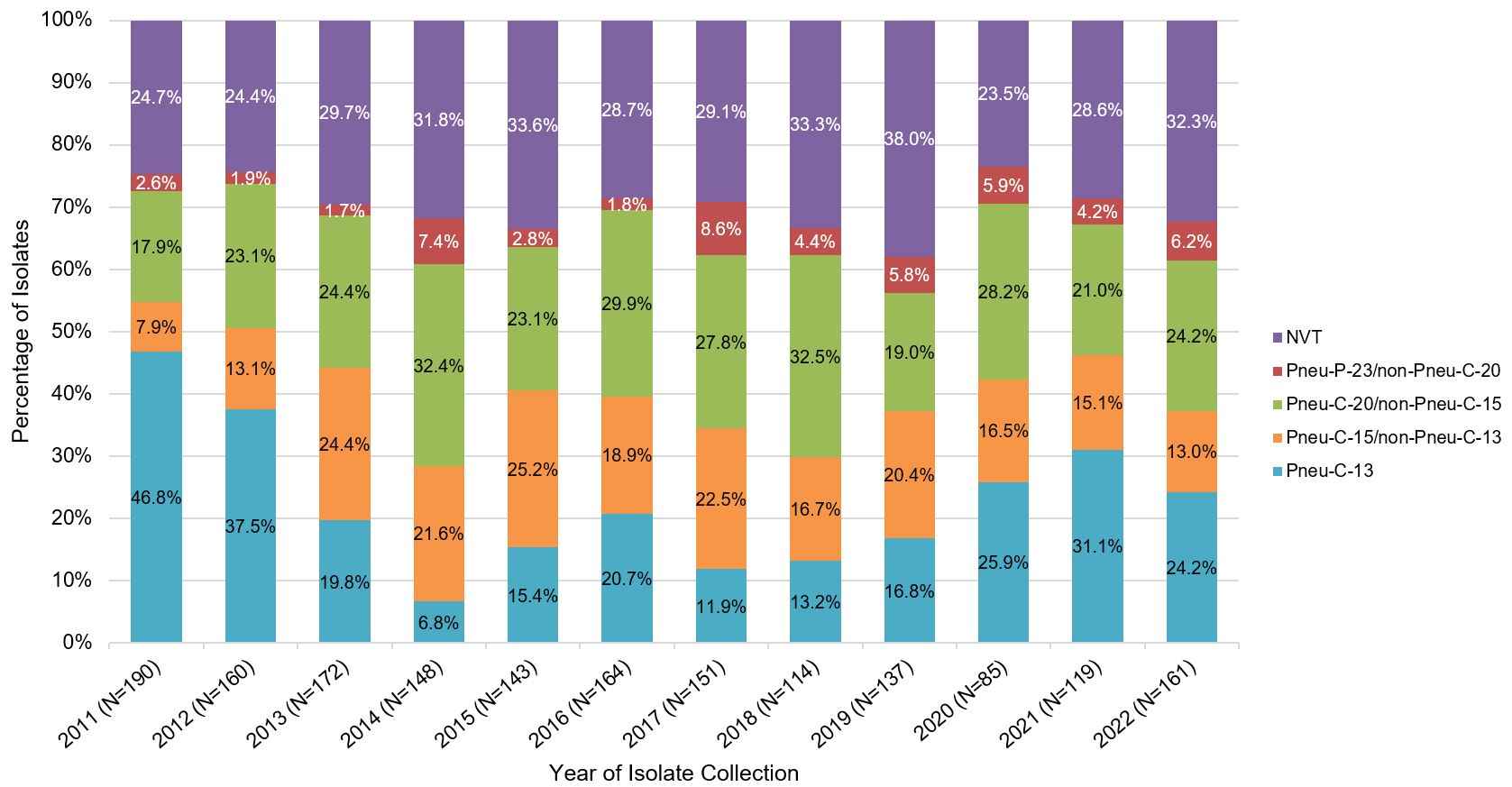
Figure 4: Text description
A stacked bar graph displaying the percentage of Streptococcus pneumoniae isolates collected from each vaccine category (PNEU-C-13, PNEU-C-15/non-PNEU-C-13, PNEU-C-20/non-PNEU-C-15, PNEU-P-23/non-PNEU-C-20 and other non-vaccine serotypes), for children <2 years of age in 2011 to 2022.
| Year | Vaccine (%, N) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU-C-13 | PNEU-C-15/ non-PNEU-C-13 |
PNEU-C-20/ non-PNEU-C-15 |
PNEU-P-23/ non-PNEU-C-20 |
NVT | |||||||
| 2011 | 46.8% | (89) | 7.9% | (15) | 17.9% | (34) | 2.6% | (5) | 24.7% | (47) | 190 |
| 2012 | 37.5% | (60) | 13.1% | (21) | 23.1% | (37) | 1.9% | (3) | 24.4% | (39) | 160 |
| 2013 | 19.8% | (34) | 24.4% | (42) | 24.4% | (42) | 1.7% | (3) | 29.7% | (51) | 172 |
| 2014 | 6.8% | (10) | 21.6% | (32) | 32.4% | (48) | 7.4% | (11) | 31.8% | (47) | 148 |
| 2015 | 15.4% | (22) | 25.2% | (36) | 23.1% | (33) | 2.8% | (4) | 33.6% | (48) | 143 |
| 2016 | 20.7% | (34) | 18.9% | (31) | 29.9% | (49) | 1.8% | (3) | 28.7% | (47) | 164 |
| 2017 | 11.9% | (18) | 22.5% | (34) | 27.8% | (42) | 8.6% | (13) | 29.1% | (44) | 151 |
| 2018 | 13.2% | (15) | 16.7% | (19) | 32.5% | (37) | 4.4% | (5) | 33.3% | (38) | 114 |
| 2019 | 16.8% | (23) | 20.4% | (28) | 19.0% | (26) | 5.8% | (8) | 38.0% | (52) | 137 |
| 2020 | 25.9% | (22) | 16.5% | (14) | 28.2% | (24) | 5.9% | (5) | 23.5% | (20) | 85 |
| 2021 | 31.1% | (37) | 15.1% | (18) | 21.0% | (25) | 4.2% | (5) | 28.6% | (34) | 119 |
| 2022 | 24.2% | (39) | 13.0% | (21) | 24.2% | (39) | 6.2% | (10) | 32.3% | (52) | 161 |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); NML. Preliminary data for 2021 and 2022.
In contrast, the proportion of Pneu-C-20 unique serotypes has increased in children less than 2 years of age, from 25.8% in 2011 to 37.3% in 2022. In 2022, the two additional serotypes contained in Pneu-C-15 would have potentially prevented an additional 13% of IPD cases in children less than 2 years of age, while the 7 additional serotypes contained in Pneu-C-20 would have potentially prevented approximately 37% of additional IPD cases in this age group (Figure 5).
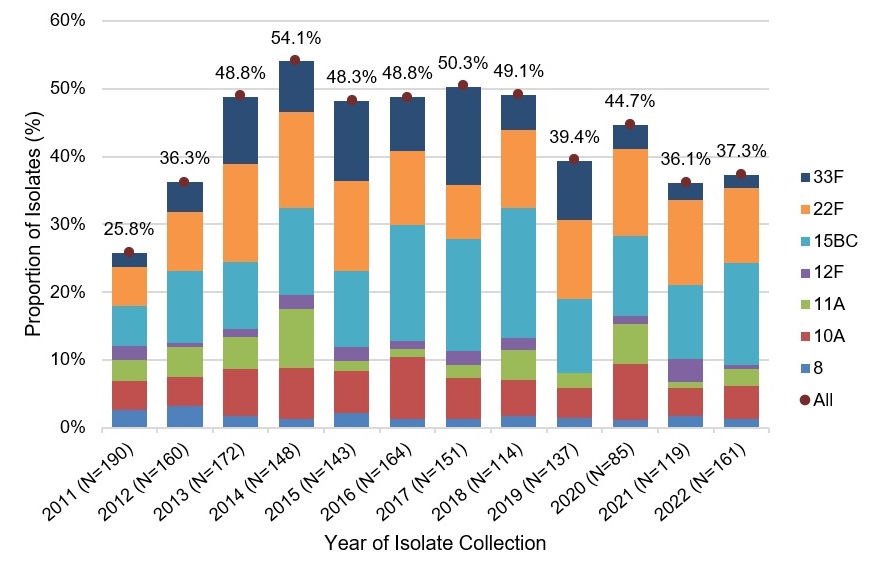
Figure 5: Text description
A stacked bar graph displaying the percentage of Streptococcus pneumoniae isolates collected of each Pneu-C-15/non-Pneu-C-13 (22F and 33F) and Pneu-C-20/non-Pneu-C-15 (8, 10A, 11A, 12F and 15B/C) serotype, including a total combined percentage of all seven serotypes, for children <2 years of age in 2011 to 2022.
| Year(n) | Serotype(%, n) | Combined total (%,n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU-C-15/non-PNEU-C-13 | PNEU-C-20/non-PNEU-C-15 | ||||||||||||||
| 22F | 33F | 8 | 10A | 11A | 12F | 15BC | |||||||||
| 2011 (190) | 5.8% | (11) | 2.1% | (4) | 2.6% | (5) | 4.2% | (8) | 3.2% | (6) | 2.1% | (4) | 5.8% | (11) | 25.8% (49) |
| 2012 (160) | 8.8% | (14) | 4.4% | (7) | 3.1% | (5) | 4.4% | (7) | 4.4% | (7) | 0.6% | (1) | 10.6% | (17) | 36.3% (58) |
| 2013 (172) | 14.5% | (25) | 9.9% | (17) | 1.7% | (3) | 7.0% | (12) | 4.7% | (8) | 1.2% | (2) | 9.9% | (17) | 48.8% (84) |
| 2014 (148) | 14.2% | (21) | 7.4% | (11) | 1.4% | (2) | 7.4% | (11) | 8.8% | (13) | 2.0% | (3) | 12.8% | (19) | 54.1% (80) |
| 2015 (143) | 13.3% | (19) | 11.9% | (17) | 2.1% | (3) | 6.3% | (9) | 1.4% | (2) | 2.1% | (3) | 11.2% | (16) | 48.3% (69) |
| 2016 (164) | 11.0% | (18) | 7.9% | (13) | 1.2% | (2) | 9.1% | (15) | 1.2% | (2) | 1.2% | (2) | 17.1% | (28) | 48.8% (80) |
| 2017 (151) | 7.9% | (12) | 14.6% | (22) | 1.3% | (2) | 6.0% | (9) | 2.0% | (3) | 2.0% | (3) | 16.6% | (25) | 50.3% (76) |
| 2018 (114) | 11.4% | (13) | 5.3% | (6) | 1.8% | (2) | 5.3% | (6) | 4.4% | (5) | 1.8% | (2) | 19.3% | (22) | 49.1% (56) |
| 2019 (137) | 11.7% | (16) | 8.8% | (12) | 1.5% | (2) | 4.4% | (6) | 2.2% | (3) | 0.0% | (0) | 10.9% | (15) | 39.4% (54) |
| 2020 (85) | 12.9% | (11) | 3.5% | (3) | 1.2% | (1) | 8.2% | (7) | 5.9% | (5) | 1.2% | (1) | 11.8% | (10) | 44.7% (38) |
| 2021 (119) | 12.6% | (15) | 2.5% | (3) | 1.7% | (2) | 4.2% | (5) | 0.8% | (1) | 3.4% | (4) | 10.9% | (13) | 36.1% (43) |
| 2022 (161) | 11.2% | (18) | 1.9% | (3) | 1.2% | (2) | 5.0% | (8) | 2.5% | (4) | 0.6% | (1) | 14.9% | (24) | 37.3% (60) |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); NML. Preliminary data for 2021 and 2022.
All/Combined total refers to the total combined percentage of all Pneu-C-15/non-Pneu-C-13 (22F and 33F) and Pneu-C-20/non-Pneu-C-15 serotypes (8, 10A, 11A, 12F and 15B/C).
In children 2 to 4 years of age, the proportion of isolates due to the Pneu-C-13 vaccine serotypes has decreased since 2011 from 70.6% to 28.2% in 2022. Similarly, to the under 2-year age group, a more rapid decrease was observed between 2011 to 2014/15, after which the proportion change stabilized. During the same period, an increase was observed in Pneu-C-15/non-Pneu-C-13 and Pneu-C-20/non-Pneu-15 serotypes (from 11.8% to 16.2%, and 9.2% to 22.2%, respectively) [Figure 6 ].
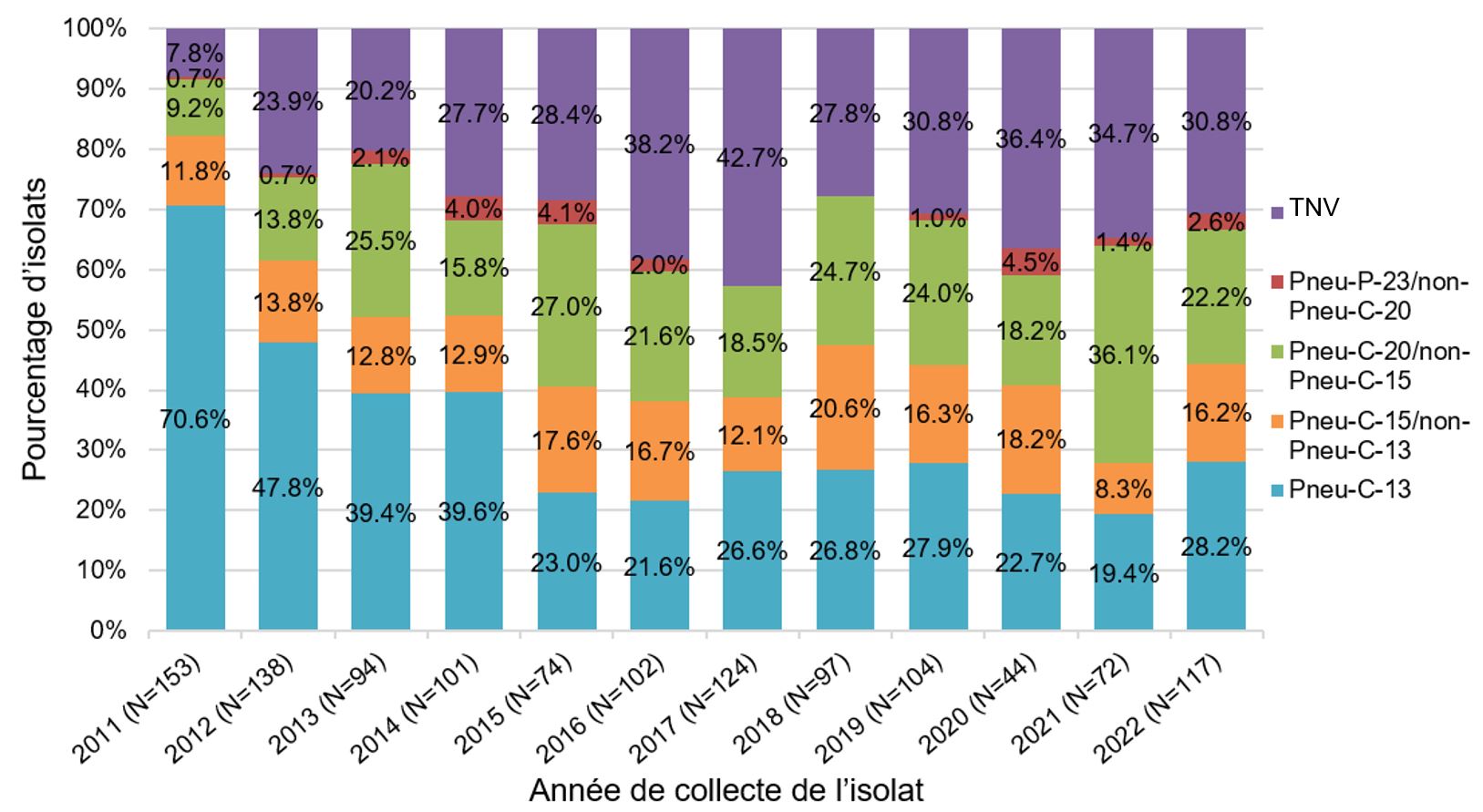
Figure 6: Text description
A stacked bar graph displaying the percentage of Streptococcus pneumoniae isolates collected from each vaccine category (PNEU-C-13, PNEU-C-15/non-PNEU-C-13, PNEU-C-20/non-PNEU-C-15, PNEU-P-23/non-PNEU-C-20 and other non-vaccine serotypes), for children 2 to 4 years of age in 2011 to 2022.
| Year | Vaccine (%, N) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU-C-13 | PNEU-C-15/ non-PNEU-C-13 |
PNEU-C-20/ non-PNEU-C-15 |
PNEU-P-23/ non-PNEU-C-20 |
NVT | |||||||
| 2011 | 70.6% | (108) | 11.8% | (18) | 9.2% | (14) | 0.7% | (1) | 7.8% | (12) | 153 |
| 2012 | 47.8% | (66) | 13.8% | (19) | 13.8% | (19) | 0.7% | (1) | 23.9% | (33) | 138 |
| 2013 | 39.4% | (37) | 12.8% | (12) | 25.5% | (24) | 2.1% | (2) | 20.2% | (19) | 94 |
| 2014 | 39.6% | (40) | 12.9% | (13) | 15.8% | (16) | 4.0% | (4) | 27.7% | (28) | 101 |
| 2015 | 23.0% | (17) | 17.6% | (13) | 27.0% | (20) | 4.1% | (3) | 28.4% | (21) | 74 |
| 2016 | 21.6% | (22) | 16.7% | (17) | 21.6% | (22) | 2.0% | (2) | 38.2% | (39) | 102 |
| 2017 | 26.6% | (33) | 12.1% | (15) | 18.5% | (23) | 0.0% | (0) | 42.7% | (53) | 124 |
| 2018 | 26.8% | (26) | 20.6% | (20) | 24.7% | (24) | 0.0% | (0) | 27.8% | (27) | 97 |
| 2019 | 27.9% | (29) | 16.3% | (17) | 24.0% | (25) | 1.0% | (1) | 30.8% | (32) | 104 |
| 2020 | 22.7% | (10) | 18.2% | (8) | 18.2% | (8) | 4.5% | (2) | 36.4% | (16) | 44 |
| 2021 | 19.4% | (14) | 8.3% | (6) | 36.1% | (26) | 1.4% | (1) | 34.7% | (25) | 72 |
| 2022 | 28.2% | (33) | 16.2% | (19) | 22.2% | (26) | 2.6% | (3) | 30.8% | (36) | 117 |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); NML. Preliminary data for 2021 and 2022.
In 2022, the two additional serotypes contained in Pneu-C-15 would have potentially prevented
approximately 16% of additional IPD cases in children 2 to 4 years of age, while the 7 additional serotypes contained in Pneu-C-20 would have potentially prevented approximately 39% of additional IPD cases in this age group (Figure 7).
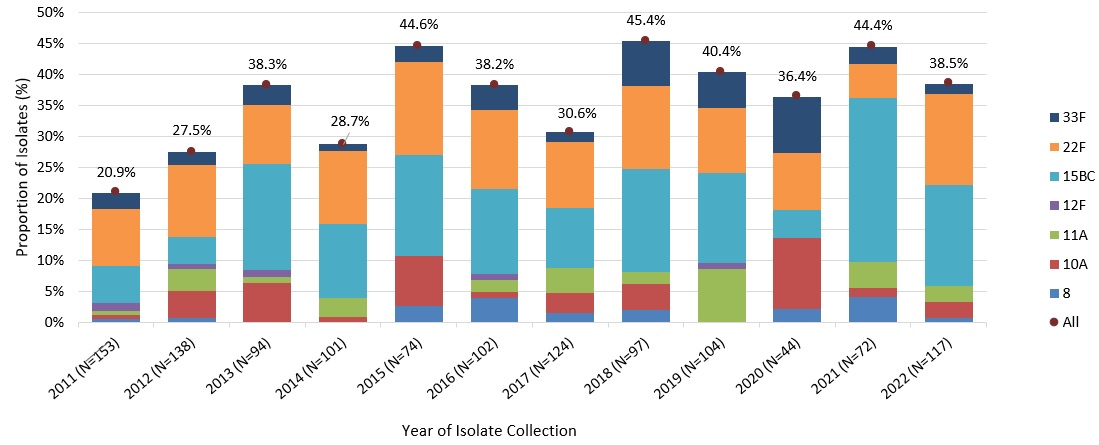
Figure 7: Text description
A stacked bar graph displaying the percentage of Streptococcus pneumoniae isolates collected of each Pneu-C-15/non-Pneu-C-13 (22F and 33F) and Pneu-C-20/non-Pneu-C-15 (8, 10A, 11A, 12F and 15B/C) serotype, including a total combined percentage of all seven serotypes, for children 2 to 4 years of age in 2011 to 2022.
| Year (n) | Serotype (%, N) | Combined total (%, N) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU-C-15/non-PNEU-C-13 | PNEU-C-20/non-PNEU-C-15 | ||||||||||||||
| 22F | 33F | 8 | 10A | 11A | 12F | 15BC | |||||||||
| 2011 (153) | 9.2% | (14) | 2.6% | (4) | 0.7% | (1) | 0.7% | (1) | 0.7% | (1) | 1.3% | (2) | 5.9% | (9) | 20.9% (32) |
| 2012 (138) | 11.6% | (16) | 2.2% | (3) | 0.7% | (1) | 4.3% | (6) | 3.6% | (5) | 0.7% | (1) | 4.3% | (6) | 27.5% (38) |
| 2013 (94) | 9.6% | (9) | 3.2% | (3) | 0.0% | (0) | 6.4% | (6) | 1.1% | (1) | 1.1% | (1) | 17.0% | (16) | 38.3% (36) |
| 2014 (101) | 11.9% | (12) | 1.0% | (1) | 0.0% | (0) | 1.0% | (1) | 3.0% | (3) | 0.0% | (0) | 11.9% | (12) | 28.7% (29) |
| 2015 (74) | 14.9% | (11) | 2.7% | (2) | 2.7% | (2) | 8.1% | (6) | 0.0% | (0) | 0.0% | (0) | 16.2% | (12) | 44.6% (33) |
| 2016 (102) | 12.7% | (13) | 3.9% | (4) | 3.9% | (4) | 1.0% | (1) | 2.0% | (2) | 1.0% | (1) | 13.7% | (14) | 38.2% (39) |
| 2017 (124) | 10.5% | (13) | 1.6% | (2) | 1.6% | (2) | 3.2% | (4) | 4.0% | (5) | 0.0% | (0) | 9.7% | (12) | 30.6% (38) |
| 2018 (97) | 13.4% | (13) | 7.2% | (7) | 2.1% | (2) | 4.1% | (4) | 2.1% | (2) | 0.0% | (0) | 16.5% | (16) | 45.4% (44) |
| 2019 (104) | 10.6% | (11) | 5.8% | (6) | 0.0% | (0) | 0.0% | (0) | 8.7% | (9) | 1.0% | (1) | 14.4% | (15) | 40.4% (42) |
| 2020 (44) | 9.1% | (4) | 9.1% | (4) | 2.3% | (1) | 11.4% | (5) | 0.0% | (0) | 0.0% | (0) | 4.5% | (2) | 36.4% (16) |
| 2021 (72) | 5.6% | (4) | 2.8% | (2) | 4.2% | (3) | 1.4% | (1) | 4.2% | (3) | 0.0% | (0) | 26.4% | (19) | 44.4% (32) |
| 2022 (117) | 14.5% | (17) | 1.7% | (2) | 0.9% | (1) | 2.6% | (3) | 2.6% | (3) | 0.0% | (0) | 16.2% | (19) | 38.5% (45) |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); NML. Preliminary data for 2021 and 2022.
All/Combined total refers to the total combined percentage of all Pneu-C-15/non-Pneu-C-13 (22F and 33F) and Pneu-C-20/non-Pneu-C-15 serotypes (8, 10A, 11A, 12F and 15B/C).
A similar trend has also been observed in the 5- to 17-year-old age group. In 2022, Pneu-C-15/non-Pneu-C-13 and Pneu-C-20/non-Pneu-15 serotypes contributed to approximately 16% and 22% of isolates, respectively (Figure 8).
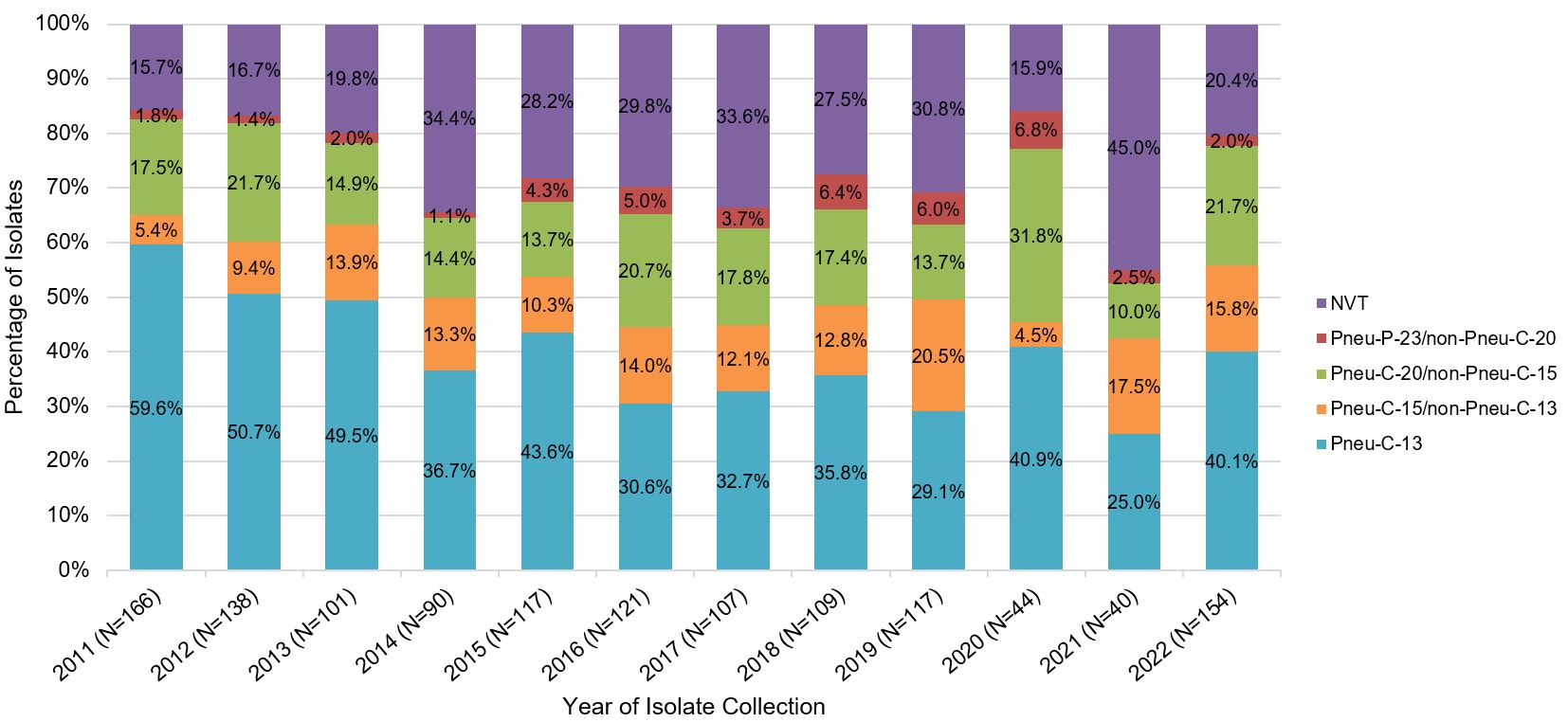
Figure 8: Text description
A stacked bar graph displaying the percentage of Streptococcus pneumoniae isolates collected from each vaccine category (PNEU-C-13, PNEU-C-15/non-PNEU-C-13, PNEU-C-20/non-PNEU-C-15, PNEU-P-23/non-PNEU-C-20 and other non-vaccine serotypes), for children 5 to 17 years of age in 2011 to 2022.
| Year | Vaccine (%, N) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU-C-13 | PNEU-C-15/ non-PNEU-C-13 |
PNEU-C-20/ non-PNEU-C-15 |
PNEU-P-23/ non-PNEU-C-20 |
NVT | |||||||
| 2011 | 59.6% | (99) | 5.4% | (9) | 17.5% | (29) | 1.8% | (3) | 15.7% | (26) | 166 |
| 2012 | 50.7% | (70) | 9.4% | (13) | 21.7% | (30) | 1.4% | (2) | 16.7% | (23) | 138 |
| 2013 | 49.5% | (50) | 13.9% | (14) | 14.9% | (15) | 2.0% | (2) | 19.8% | (20) | 101 |
| 2014 | 36.7% | (33) | 13.3% | (12) | 14.4% | (13) | 1.1% | (1) | 34.4% | (31) | 90 |
| 2015 | 43.6% | (51) | 10.3% | (12) | 13.7% | (16) | 4.3% | (5) | 28.2% | (33) | 117 |
| 2016 | 30.6% | (37) | 14.0% | (17) | 20.7% | (25) | 5.0% | (6) | 29.8% | (36) | 121 |
| 2017 | 32.7% | (35) | 12.1% | (13) | 17.8% | (19) | 3.7% | (4) | 33.6% | (36) | 107 |
| 2018 | 35.8% | (39) | 12.8% | (14) | 17.4% | (19) | 6.4% | (7) | 27.5% | (30) | 109 |
| 2019 | 29.1% | (34) | 20.5% | (24) | 13.7% | (16) | 6.0% | (7) | 30.8% | (36) | 117 |
| 2020 | 40.9% | (18) | 4.5% | (2) | 31.8% | (14) | 6.8% | (3) | 15.9% | (7) | 44 |
| 2021 | 25.0% | (10) | 17.5% | (7) | 10.0% | (4) | 2.5% | (1) | 45.0% | (18) | 40 |
| 2022 | 40.1% | (61) | 15.8% | (24) | 21.7% | (33) | 2.0% | (3) | 20.4% | (31) | 154 |
Data Source: National Laboratory Surveillance of IPD in Canada (eSTREP); National Microbiology Laboratory. Preliminary data for 2021 and 2022.
Between 2018 and 2022, the conjugate-vaccine serotypes that were the most common cause of IPD in children were ST22F, ST15B/C, ST19A and 3 which represented, on average, approximately 12%, 12%, 9% and 8% of all isolates collected per year, respectively (National Laboratory Surveillance of IPD in Canada (eSTREP); NML).
IPD incidence in children at increased risk of IPD due to underlying medical conditions
Data on the risk of pneumococcal disease in the pediatric population in the pneumococcal conjugate vaccine era is available from the US. Even with widespread use of pneumococcal conjugate vaccines, from the period 2007 to 2010, children with certain underlying medical conditions continued to demonstrate an increased burden of pneumococcal diseaseFootnote 12, including IPD, pneumococcal pneumonia and all-cause pneumonia. In 2018–2019, approximately 25% of IPD in children 5 to 18 years occurred in children with immunocompromising conditions, cochlear implants, or cerebrospinal fluid leaksFootnote 13.
Non-invasive pneumococcal disease burden
National surveillance data on AOM and CAP are not available in Canada, and disease burden estimates are based on provincial health administrative data from British Columbia (BC) and Ontario (ON). The estimated incidence rates for CAP following Pneu-C-13 vaccine programme implementation among children less than 5 years of age ranged from 2,500 per 100,000 (BC) to 5,000 per 100,000 (ON). Among children 5 to 17 years of age, the incidence of CAP ranged from 950 per 100,000 (BC) to 1,250 per 100,000 (ON)Footnote 14. The estimated incidence rates for AOM following Pneu-C-13 vaccine programme implementation among children less than 5 years of age ranged from 14,000 per 100,000 (BC) to 25,000 per 100,000 (ON). Among children 5 to 17 years of age, the incidence of AOM ranged from 6,200 per 100,000 (BC) to 7,200 per 100,000 (ON)Footnote 14. (Figure 9). Overall, following the implementation of the Pneu-C-13 vaccine program, the available data suggests limited changes to non-IPD burden, with a slight decrease in AOM incidence in younger childrenFootnote 14.
International studies show that the proportion of CAP cases attributable to S. pneumoniae was estimated to be 6% in infants under 1 year of age and 12% in children 1 to 15 years of ageFootnote 15Footnote 16. The proportion of AOM cases attributable to S. pneumoniae was estimated to be 17% in childrenFootnote 15Footnote 17. Given the reliance on administrative data and limited studies assessing the proportion of non-invasive CAP and AOM by etiology, there is considerable uncertainty about the proportion of non-invasive CAP and AOM due to S. pneumoniae as well as the relevant serotype distribution in children with these infections when they are caused by S. pneumoniae.
a. Community-acquired pneumonia
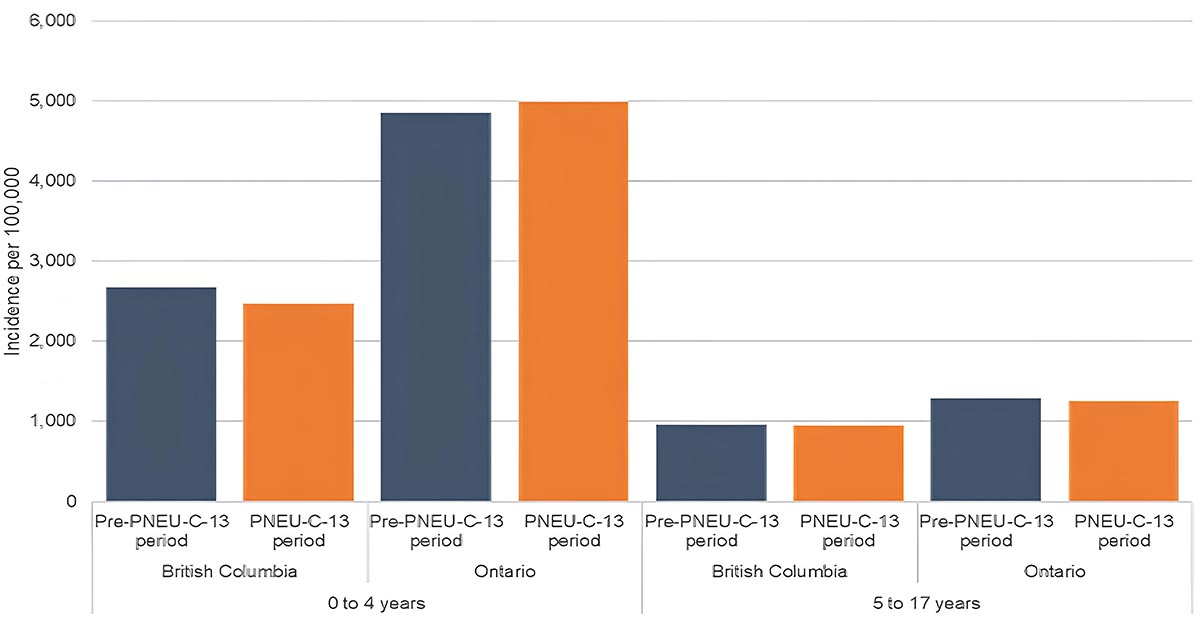
Figure 9a: Text description
Age group-specific incidence of CAP (a)
A bar graph displaying the incidence rate of Community Acquired Pneumonia (CAP) in British Columbia (on the far left), and in Ontario (on the left), pre-PNEU-C-13 in blue and following the introduction of PNEU-C-13 period in orange children 4 years old and younger and in British Columbia (on right) and in Ontario (on the far right) for pre PNEU-C-13 period in blue and following the introduction of PNEU-C-13 period for children 5 to 17 years old.
| Age group | Province | Period | Incidence per 100,000 population |
|---|---|---|---|
| 0 to 4 years | British Columbia | Pre-PNEU-C-13 period | 18,639.80 |
| 0 to 4 years | British Columbia | PNEU-C-13 period | 13,603.80 |
| 0 to 4 years | Ontario | Pre-PNEU-C-13 period | 28,937.90 |
| 0 to 4 years | Ontario | PNEU-C-13 period | 25,467.60 |
| 5 to 17 years | British Columbia | Pre-PNEU-C-13 period | 7,648.40 |
| 5 to 17 years | British Columbia | PNEU-C-13 period | 6,205.70 |
| 5 to 17 years | Ontario | Pre-PNEU-C-13 period | 7,998.60 |
| 5 to 17 years | Ontario | PNEU-C-13 period | 7,225.90 |
b. Acute otitis media
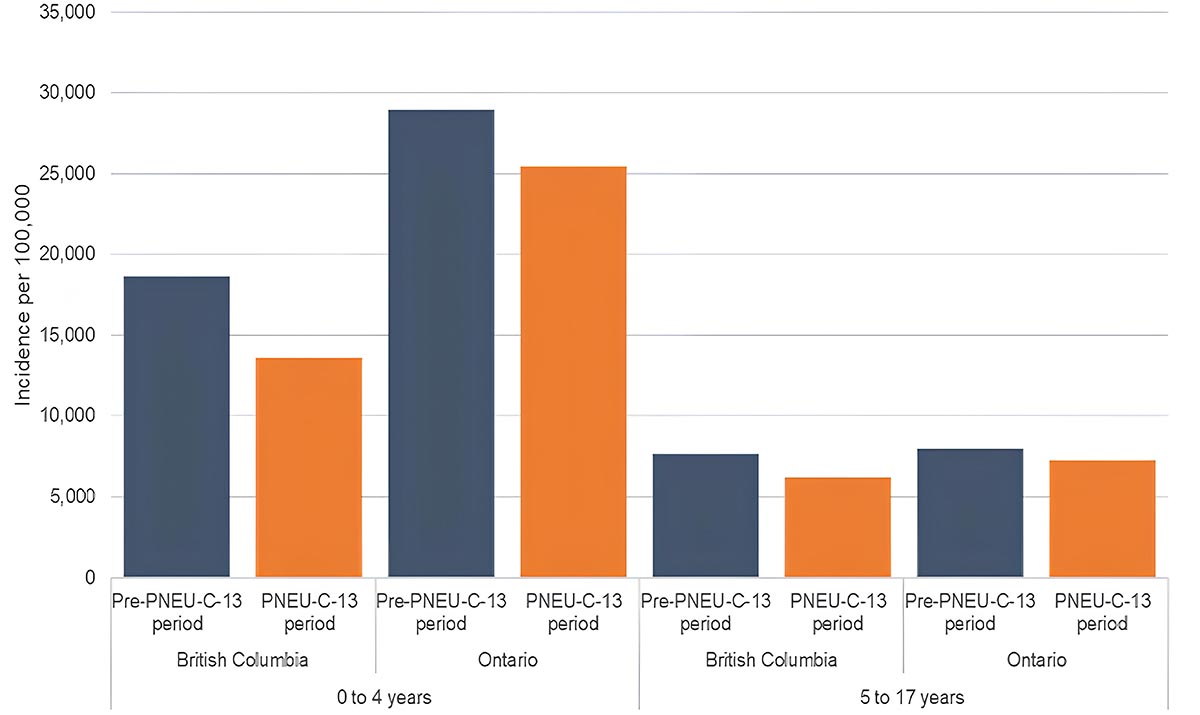
Figure 9b: Text description
Age group-specific incidence of AOM (b)
A bar graph displaying the incidence rate of Acute Otitis Media (AOM) in British Columbia (on the far left), and in Ontario (on the left), pre-PNEU-C-13 in blue and following the introduction of PNEU-C-13 period in orange children 4 years old and younger and in British Columbia (on right) and in Ontario (on the far right) for pre PNEU-C-13 period in blue and following the introduction of PNEU-C-13 period for children 5 to 17 years old.
| Age group | Province | Period | Incidence per 100,000 population |
|---|---|---|---|
| 0 to 4 years | British Columbia | Pre-PNEU-C-13 period | 2,671.60 |
| 0 to 4 years | British Columbia | PNEU-C-13 period | 2,464.10 |
| 0 to 4 years | Ontario | Pre-PNEU-C-13 period | 4,854.20 |
| 0 to 4 years | Ontario | PNEU-C-13 period | 4,991.10 |
| 5 to 17 years | British Columbia | Pre-PNEU-C-13 period | 957.60 |
| 5 to 17 years | British Columbia | PNEU-C-13 period | 945.20 |
| 5 to 17 years | Ontario | Pre-PNEU-C-13 period | 1,289.80 |
| 5 to 17 years | Ontario | PNEU-C-13 period | 1,249.00 |
Figures adapted from Nasreen S et al., 2022Footnote 14.
Immunogenicity, efficacy and safety of Pneu-C-15 and Pneu-C-20 in pediatric populations
There are currently no efficacy or effectiveness data available for Pneu-C-15 or Pneu-C-20 vaccines for any pediatric indication. NACI reviewed the evidence on safety and immunogenicity of Pneu-C-15 vaccine from eight Phase 2/3 clinical trials which included a range of pediatric populations, and which used different vaccine schedules. An additional trial, V114-022, provided information about immunogenicity and safety following the immunization of 14 hematopoietic stem cell transplant (HSCT) participants 3 to 17 years of age (with 8 receiving Pneu-C-15)Footnote 18. For the Pneu-C-20 vaccine, data on the safety and immunogenicity was available from five Phase 2/3 clinical trials. Summary of key clinical trial information that was reviewed by NACI is provided in Table 8.
Overall, a comparative analysis of data reported in Pneu-C-15 studies V114-008, V114-025, V114-027 and V114-029Footnote 19Footnote 20Footnote 21Footnote 22, and in Pneu-C-20 studies B7471003, B7471011, B747B1012, and B7471013Footnote 23Footnote 24Footnote 25Footnote 26 showed congruent immune response patterns following vaccination with using either a 2+1 or 3+1 immunization schedule in healthy vaccine-naïve infants (Table 9, Table 10, Table 11, Table 12, Table 13). In addition, there were no differences found in reported adverse events and the overall safety profiles of higher-valent vaccines when compared to Pneu-C-13 (Table 13, Table 14, Table 15).
Immunogenicity
NACI reviewed the available evidence on immunogenicity of Pneu-C-15 and Pneu-C-20 vaccines in the context of routine pediatric immunization programs as well as catch-up and re-immunization schedules. The infant schedules used in the studies included a two dose (provided at 2 and 4 months of age) or three dose (provided at 2, 4 and 6 months of age) priming series followed by an additional dose at 11 to 15 months of age. Studies that evaluated Pneu-C-15 immunogenicity in pre-term infants also used an alternative priming schedule in which the vaccine was provided at 2, 3 and 4 months of age as well as 11-15 months. Immunogenicity was measured one month following dose administration and evaluated post dose 2 (PD2), 3 (PD3) and 4 (PD4). In all infant studies, Pneu-C vaccines were provided concomitantly with other recommended pediatric vaccines.
Immunogenicity of Pneu-C-15 in pediatric populations
Immunogenicity following immunization with Pneu-C-15 was reported in seven Phase 2/3 clinical trials that included healthy children (five studies), children with sickle cell disease (one study) and children with HIV infection (one study) (Table 8).
In the pivotal double-blind clinical trial V114-025Footnote 20 that used a 2+1 vaccination schedule, immunogenicity was measured in healthy infants aged 2 to 15 months who were vaccinated with Pneu-C-13 (n=593) or Pneu-C-15 (n=591). When analyzed according to relative differences in antibody concentrations, lower GMC ratios were observed following dose 2 (PD2) and dose 3 (PD3) with Pneu-C-15 vaccination for the majority of serotypes (9/13 and 11/13, respectively). In Pneu-C-15 recipients, the lowest titres were reported for serotypes 1, 5 and 6A, PD3. Similarly, when assessed by OPA GMT, functional antibodies were lower for 11/13 serotypes PD3 in Pneu-C-15 recipients compared to Pneu-C-13 recipients. However, differences between these vaccines were less pronounced when immunogenicity was reported as seroresponse rate differences, with lower values measured for 3/13 and 6/13 shared serotypes PD2 and PD3, respectively. The patterns of IgG immune responses between doses in this study were generally comparable between the intervention groups, demonstrating significant antibody waning prior to the administration of the booster dose, as well as rapid boosting following the receipt of the booster dose, confirming the presence of immune memory. A summary of GRADE evidence is provided in Table 9.
In three clinical trials that used a 3+1 vaccination schedule (V114-008, V114-027, V114-029)Footnote 19Footnote 21Footnote 22, immunogenicity was assessed in close to 1,400 healthy infant Pneu-C-15 recipients. Lower antibody concentrations were consistently reported after Pneu-C-15 administration for 5/13 shared serotypes PD3 (synthesis of 2 trials) and PD4 (synthesis of 3 trials). Compared to Pneu-C-13, OPA GMT ratios were numerically lower for serotypes 4, 6A, 19A and 23F, PD3 as well as serotypes 1, 3, 5, 6B, 7F and 9V PD4 in Pneu-C-15 recipients (synthesis of 2 trials). There were notable uncertainties regarding the differences in seroresponse rates between groups PD3 and PD4. Similar to what was observed following a two-dose primary schedule, the pattern of antibody waning following the receipt of the primary series was comparable and significant PD3, while PD4 administration immune responses demonstrated a pattern that was conclusive with the establishment of immune memory. A summary of immunogenicity data reported in studies V114-008, V114-025, V114-027 and V114-029 and a summary of GRADE evidence is provided in Table 10.
Mixed schedules
The V114-027 trialFootnote 21 was the only trial that evaluated the immunogenicity of mixed schedules. In this study, 900 healthy participants 40 to 90 days of age were randomized to one of five groups (n=180 per group) to receive a complete four dose series with Pneu-C-13 or Pneu-C- 15, or a mixed regimen initiated with one, two or three doses of Pneu-C-13 and continued with Pneu-C-15.
When assessed by serotype-specific seroresponse rates, a mixed regimen consisting of two or three Pneu-C-15 doses elicited generally comparable immune responses PD3 to those observed after Pneu-C-13 only administration. Similar GMC ratios between a Pneu-C-13-only schedule and a mixed schedule using two doses of Pneu-C-15 were also observed PD3 and PD4. In a mixed schedule with three Pneu-C-15 doses, lower GMC ratios were observed for 4/13 shared serotypes both PD3 and PD4.
As seen with schedules that used only Pneu-C-13 or Pneu-C-15 vaccine, significant antibody waning was observed across all mixed intervention groups PD3, while the fourth dose led to a steep increase in antibody concentrations that were comparable or superior to those observed PD3.
Catch-up vaccination: vaccine-naïve and children with an incomplete Pneu-C series
In the double-blind V114-024 clinical trialFootnote 27, 602 healthy pneumococcal vaccine-naïve children or children who previously received a partial series of a licensed pneumococcal conjugate vaccine (Pneu-C-7, Pneu-C-10 or Pneu-C-13) were randomized to receive an age-appropriate schedule using one to three catch-up doses of either Pneu-C-13 or Pneu-C-15 vaccine. The study stratified children in five groups according to age (7 to 11 months, 12 to 23 months, greater or equal to 2 to less than 6 years, and greater or equal to 6 to 17 years), with all participants less than 2 years of age being Pneu-C vaccine-naïve. In all age groups, serotype-specific antibody concentrations and seroresponse rates at 30 days following the last dose were generally comparable between intervention groups for all common vaccine serotypes and significantly higher for the Pneu-C-15 unique serotypes.
Re-vaccination: children at increased risk of IPD with a completed Pneu-C-13 series
In the V114-023 trialFootnote 28, 99 children 5 to 17 years old with sickle cell disease were randomized in a 2:1 ratio to receive a single dose of either Pneu-C-15 or Pneu-C-13. When assessed by serotype-specific IgG GMCs at 30 days post Pneu-C vaccination, immune responses were generally similar between groups for the shared serotypes and higher for the Pneu-C-15 unique serotypes. Over 75% of Pneu-C-15 recipients achieved greater or equal to 4-fold rise in antibody concentrations for the two unique serotypes whereas only 42% and 58% of vaccine recipients achieved greater or equal to 4-fold rise OPA GMTs for the serotype 33F and 22F, respectively.
In the V114-030 studyFootnote 29, 400 children 6 to 17 years old with HIV were randomized in a 1:1 ratio to receive a single dose of either Pneu-C-15 or Pneu-C-13 vaccine followed by Pneu-P-23 at week 8 post immunization. At 30 days following the administration of Pneu-C-15, IgG GMC were numerically similar for all 13 shared and higher for the two unique serotypes. One month following Pneu-P-23 administration, antibody concentrations were generally similar between groups, although numerically lower for all serotypes compared to those observed one month prior, post Pneu-C administration.
Pre-term infants
An integrated analysis of over 350 pre-term infants who were enrolled in studies V114-025, V114-027, V114-029 and V114-031Footnote 20Footnote 21Footnote 22Footnote 30 demonstrated that, overall, immune responses following Pneu-C-15 vaccination were generally comparable to those observed in pre-term infants receiving Pneu-C-13 for the shared serotypes and consistent with those observed in term infants receiving four doses of Pneu-C-15 vaccine (including IgG GMC and OPA GMT). Over 85% and 96% of pre-term infants receiving Pneu-C-15 achieved seroprotective IgG concentrations of greater or equal to 0.35 μg/mL for each of the vaccine serotypes PD3 and PD4, respectively.
Immunogenicity of Pneu-C-20 in pediatric populations
A pivotal clinical study B7471012Footnote 25 measured immune responses in infants following a 2+1 immunization schedule. The observed IgG antibody concentrations PD2 and PD3 were lower for all common serotypes among Pneu-C-20 recipients compared to Pneu-C-13 recipients. While a lower seroresponse was reported for 9/13 shared serotypes PD2, seroresponse PD3 was only lower for ST3 while for the majority of other shared serotypes (12/13) it was uncertain due to the inclusion of null. The OPA GMTs were generally lower at all time points for the shared serotypes except for ST19A, PD3. A summary of the GRADE assessment is provided in Table 11.
Two clinical trials measured immune responses using a 3+1 infant schedule. In the pivotal B7471011 studyFootnote 24, antibody concentrations were lower for all shared serotypes PD3 and for 12/13 serotypes PD4 compared to Pneu-C-13 recipients. While, similarly, lower seroresponse rates were observed for the majority (8/13) of shared serotypes PD3, there were only 2 shared serotypes (ST1 and ST3) for which lower seroresponse rates were reported PD4. The OPA GMTs were also generally lower for the majority of shared serotypes PD3 and PD4. A very similar pattern of IgG GMC, seroresponse rates and OPA GMTs were also reported PD3 and PD4 in the second, smaller, trial titled B7471003Footnote 23. A summary of GRADE assessment is provided in Table 12.
In all infant studies there was an observed congruency in immune response patterns between intervention groups, independent of the schedule used. There was an observed antibody waning (both for total and functional antibodies) following the completion of the primary series and significant antibody boosting following the receipt of the additional dose. In all studies, one month after the additional dose, antibody levels surpassed those observed after the completion of the primary series, demonstrating the establishment of immune memory in all vaccine recipients.
Re-vaccination: children who completed a routine Pneu-C-13 series
In children 5 to 17 years of age, immunization elicited increases in OPA GMTs for all vaccine serotypes, with the pre/post increase in OPA GMTs for the 7 serotypes not contained in Pneu-C-13 ranging from 11.5 to 499-fold. Immunogenicity data from vaccine-experienced individuals was available from only one study (B7471014)Footnote 31. In this study, 425 children less than 5 years of age previously immunized with greater or equal to 3 doses of Pneu-C-13 and 406 children 5 to 17 years of age (regardless of prior pneumococcal vaccination status) were recruited to receive one dose of Pneu-C-20. Among study participants less than 5 years of age, increases in IgG concentrations were observed for all 20 vaccine contained serotypes with at least 83% achieving predefined IgG concentrations of the 7 additional serotypes, except for serotype 12F (40.0%).
Immunogenicity of routinely administered pediatric vaccines when administered concurrently with Pneu-C-15 or Pneu-C-20
Concurrent administration of Pneu-C-15 or Pneu-C-20 with other routinely administered pediatric vaccines was assessed in all infant clinical trials. In addition to pneumococcal vaccines, participants also received diphtheria, tetanus, pertussis, poliomyelitis (serotypes 1, 2 and 3), hepatitis A, hepatitis B, Haemophilus influenzae type b, measles, mumps, rubella, varicella, and rotavirus vaccines, either as monovalent or combination vaccines. Immune responses to all antigens provided concomitantly with Pneu-C-15 or Pneu-C-20 were similar to those observed in Pneu-C-13 recipients as assessed by the individual antigen-specific response rates (for the combination and monovalent vaccines) or GMT (rotavirus vaccine) at 30 days following the completion of the primary series and the receipt of the additional dose, in both the 2+1 and 3+1 immunization schedule.
Evidence on safety
NACI reviewed the available evidence on safety of Pneu-C-15 and Pneu-C-20 vaccines in the context of routine pediatric immunization programs and catch-up and re-immunization schedules. Evidence on safety was available from clinical trials involving children receiving one or more doses of Pneu-C-15 or Pneu-C-20 vaccine and, in most of the trials, one or more doses of Pneu-C-13 vaccine.
Evidence on safety of Pneu-C-15 in pediatric populations
Adverse events (AE) following immunization with Pneu-C-15 vaccine were reported in eight clinical trials. Overall, 5,399 children received one or more doses of Pneu-C-15 and 3,280 children received one or more doses of Pneu-C-13. The measured safety endpoints included the proportion of participants with solicited local and systemic AEs 1 to 14 days post-vaccination, maximum body temperature measurements 1 to 7 days post-vaccination and serious adverse events (SAEs), up to 6 months following vaccination.
A GRADE assessment of studies that reported safety results for three outcomes of interest (total SAEs, vaccine-related SAEs and death) using 2+1 and 3+1 schedules, concluded that in vaccine-naïve infants there was moderate to high certainty evidence of little to no difference between the Pneu-C-13 and Pneu-C-15 groups (and low to moderate certainty evidence of little to no difference for immunocompromised infants due to small sample size) [Table 13].
A GRADE assessment of studies that reported safety results of additional vaccine doses in vaccine-experienced children concluded that there was moderate certainty evidence of little to no difference between vaccines for all measured safety outcomes (Table 14).
A separate integrated analysis of the final safety database (data from studies V114-025, -027, -029 and -031 that had a similar study design and population) included data from 3,589 healthy infants who received at least one dose of Pneu-C-15 and 2,058 who received at least one dose of Pneu-C-13. Solicited AEs accounted for the majority of reported safety events and were mostly of short duration (3 days or less) and of mild to moderate intensity. The proportions of participants with local and systemic AEs (solicited and unsolicited) after each dose in the primary series, after the booster dose, and after any dose were similar in both intervention groups. In infants, the most frequently reported AEs after any dose of Pneu-C-15 were irritability (range: 47% to 55.1%), somnolence (22.8% to 40.7%), injection-site pain (19.1% to 27.1%), and decreased appetite and other injection-site reactions (less than 20%). In children 11 to 15 months of age, the most frequently reported AEs were irritability (45.7%), somnolence (21.8%), injection-site pain (21%), decreased appetite (19.4%) and other injection-site reactions (erythema, swelling, induration; all less than 22%).
For the majority of participants who received Pneu-C-15, study investigators reported maximum body temperature measurements of less than 38.0 °C, with a temperature distribution that was comparable between intervention groups. Of the participants with a maximum body temperature higher than 38.0 °C, no significant differences were observed between Pneu-C-13 and Pneu-C-15 vaccine recipients after any vaccine dose. SAEs were reported for 10% (n=358) of Pneu-C-15 recipients and 10.5% (n=217) of Pneu-C-13 recipients. While the majority of SAEs were deemed to be non-vaccine related, there were three vaccine-related SAEs reported in 2 participants in the Pneu-C-15 group and 1 participant in the Pneu-C-13 group (all were pyrexia requiring hospitalization). There were 4 deaths (2 in Pneu-C-13 and 2 in Pneu-C-15 recipients), of which none were considered to be related to either of the vaccines.
Pre-term infants
The safety of Pneu-C-15 was assessed in over 170 pre-term infants (gestational age less than 37 weeks) who were immunized using a four-dose schedule. The safety profile following vaccination with Pneu-C-15 was similar to that observed in term infants. The frequency of SAEs was similar between groups (14.9% for Pneu-C-15 and 14.4% for Pneu-C-13). There were no vaccine related SAEs or deaths reported among this small group of pre-term infants.
Evidence on safety of Pneu-C-20 in pediatric populations
Safety data following immunization with Pneu-C-20 vaccine was available from five clinical trials (B7471003, B7471011, B7471012, B7471013, and B7471014)Footnote 23Footnote 24Footnote 25Footnote 26Footnote 31. One (B7471014) was a single-arm trial (n=839) with vaccine-experienced children and was evaluated separatelyFootnote 31. Overall, from the four assessed trials (B7471003, B7471011, B7471012, B7471013)Footnote 23Footnote 24Footnote 25Footnote 26, 2,833 infants received one or more doses of Pneu-C-20 and 2,320 infants received one or more doses of Pneu-C-13. In addition, there were 831 children 15 months through 17 years of age who received at least 1 dose of Pneu-C-20 vaccine. The measured safety endpoints included the proportion of participants with solicited local and systemic AEs 1-7 days post-vaccination (including immediate AEs occurring within 30 minutes post-vaccination), AEs one month after each dose and SAEs up to 6 months following vaccination. A GRADE analysis of studies that reported safety results using 2+1 and 3+1 schedules concluded that there was low to moderate certainty evidence of little to no difference between vaccines for all measured safety outcomes (very low to low among the immunocompromised groups, Table 15).
A separate integrated safety analysis (data from studies B7471003, B7471011, B7471012 and B7471013 that had similar study designs and populations) included data from 2,812 healthy infants who received at least one dose of Pneu-C-20 and 2,299 who received at least one dose of Pneu-C-13Footnote 23Footnote 24Footnote 25Footnote 26. The rates of local reactions and systemic events among infants receiving Pneu-C-20 and Pneu-C-13 after any dose were similar, with the most commonly reported AEs in Pneu-C-20 recipients being irritability (range 59.1% to 71.3%), somnolence (37.0% to 66.5%), injection site pain (33.5% to 46.3%), decreased appetite (22.7% to 26.0%), and other injection site reactions (15.1% to 24.7%). In children 11 to 15 months of age, the most frequently reported AEs in the 3-dose study (B7471012)Footnote 25 and 4-dose studies (B7471003, B7471011, B7471013)Footnote 23Footnote 24Footnote 26 were irritability (58.5% in 3-dose study and 71% in 4-dose studies), somnolence (37.7% and 50.9%), injection-site pain (33.4% and 42.4%), decreased appetite (26.4% and 39.3%), and other injection site reactions (redness or swelling) (15.1% and 36.9%).
For the majority of participants who received Pneu-C-20, study investigators reported maximum body temperature measurements of less than 38.0 °C, with a temperature distribution that was comparable between vaccine groups. Of the participants with a maximum body temperature higher than 38.0°C, no significant differences were observed between Pneu-C-13 and Pneu-C-20 vaccine recipients after any vaccine dose. SAEs were reported for 4.8% of Pneu-C-20 recipients and 4.5% of Pneu-C-13 recipients. While the majority of SAEs were assessed as non-vaccine related, one SAE with onset 7 days after the first vaccine dose was assessed as possibly related to either Pneu-C-20 or one of the concomitant vaccines received. The study participant was hospitalized with fever, painful swelling in the right groin and a right inguinal hernia. Laboratory tests revealed elevated inflammatory markers (C-reactive protein and procalcitonin) and a negative blood culture. The study participant was treated with antibiotics in hospital and the event resolved. There were no deaths reported in any of the Pneu-C-20 pediatric trials.
Pre-term infants
The safety of Pneu-C-20 was assessed in 110 pre-term infants (gestational age greater or equal to 34 to less than 37 weeks) who were immunized with a four-dose schedule (at 2, 4, 6, and 12 to 15 months of age). The safety profile of Pneu-C-20 and Pneu-C-13 was similar to that observed in term infants, including both local reactions and systemic events after each dose. The frequency of AEs reported up to one month post dose 3 was 31.2% for Pneu-C-20 and 23.5% for Pneu-C-13. The frequency of AEs reported up to one month post dose 4 was similar between groups (14.3% for Pneu-C-20 and 17.2% for Pneu-C-13). The percentages of participants with SAEs from Dose 1 to 6 months after Dose 4 were also similar between the two groups (4.4% and 5.6% for Pneu-C-20 and Pneu-C-13 groups, respectively).
Ethics, equity, feasibility and acceptability considerations
NACI uses a published, peer-reviewed framework and evidence-informed tools to ensure that issues related to ethics, equity, feasibility, and acceptability (EEFA) are systematically assessed and integrated into its guidanceFootnote 32.
Infants and children under 5 years of age are at higher risk of IPD compared to children 5 years and age and older and benefit from a routine pneumococcal immunization program. Vaccination of the young pediatric population provides both direct protection and indirect protection to other vulnerable populations (e.g. older adults). Previous recommendations on the use of pneumococcal vaccines in children have also recognized that some groups require additional vaccine doses to provide optimal protection (i.e., the 3+1 vaccine schedule, additional dose with Pneu-P-23), particularly for individuals who may experience decreased immune response due to underlying medical conditions. The continued use of both age- and risk-based vaccine recommendations promotes equitable access to pneumococcal vaccination for those who are most in need of protection.
Children with medical risk factors have been identified as being at increased risk of IPD. Social, environmental, or living conditions can also increase the risk of severe illness and should be considered. The higher disease burden of IPD observed in Northern Canada can be attributed to a variety of intersecting factors, including environmental/living factors (e.g. crowding), the younger age distribution compared to the general Canadian population, and decreased access to health care. Therefore, living in communities or settings experiencing sustained high levels of IPD, or being subject to ongoing risk of IPD due to other environmental or living conditions (i.e., houselessness or residential care) should be identified as risk factors that result in increased risk of IPD. In the case of Indigenous communities, autonomous decisions should be made by Indigenous Peoples with the support of healthcare and public health partners in accordance with the United Nations Declaration on the Rights of Indigenous PeoplesFootnote 33.
The new higher-valent pneumococcal conjugate vaccines offer an opportunity to protect children against additional serotypes compared to Pneu-C-13, and further reduce the burden of IPD. Vaccine coverage of the pediatric pneumococcal vaccines is high in the Canadian population (with an estimated coverage of 85.1% in 2021), and with a routine vaccination program already in place, no change in vaccine uptake is anticipated as a result of the adoption of the higher-valent pneumococcal conjugate vaccines.
Conjugate vaccines induce formation of long-term memory cells, provide longer duration of protection, reduce mucosal carriage of bacteria, and have an anamnestic response, in a way that polysaccharide vaccines do not. Therefore, while Pneu-C-20 may not contain as many serotypes as Pneu-P-23, the advantages of a conjugate vaccine outweigh the slightly reduced breadth in serotype coverage. This was considered sufficient to eliminate the need for Pneu-P-23 and would contribute to simplifying the pediatric vaccine schedule, improving program feasibility.
Provinces and territories have previously transitioned from the use of Pneu-C-7 to Pneu-C-13 in pediatric pneumococcal vaccination programs and demonstrated the feasibility of adopting new vaccine products. There will be similar challenges including needing to update communications (e.g. protocols and training, public messaging), minimizing vaccine wastage, and implementing catch-up guidance for specific cohorts. A notable barrier expected in the transition to the use of higher-valent pneumococcal vaccines is the cost of the new vaccines, and there may be variability in how or when each jurisdiction is able to adopt any new recommendations on the use of the higher-valent vaccines. Some jurisdictions may opt to focus on offering the new products initially to certain populations at increased risk of IPD, while others may also factor in local serotype epidemiology in their choice of product. The use of a single product in both routine and high-risk programs for children and adults would reduce program complexity and reduce the chance for administering a different vaccine than intended.
Economics
A systematic review and de novo model-based economic evaluation were used as economic evidence to inform decision-making for the use of Pneu-C-15 and Pneu-C-20 in the pediatric population. Full details, including assumptions and limitations, are provided in a supplementary economic evidence summary.
Systematic review
A systematic search of the peer-reviewed and grey literature identified two model-based economic evaluations comparing Pneu-C-15 to Pneu-C-13 in pediatric populations. Both studies evaluated routine infant programs and did not evaluate vaccination strategies for groups at increased risk for IPD. No studies were identified that included Pneu-C-20 as of March 7, 2023. The studies were conducted in the United States and were cost-utility analyses that used a societal perspectiveFootnote 34Footnote 35. One of the studies was industry sponsoredFootnote 35. Both models were static and did not include transmission dynamics. Indirect protection effects were included as a percent reduction in IPD or all pneumococcal disease in individuals not receiving Pneu-C-15 vaccineFootnote 34Footnote 35. Both models employed a similar approach to model the risk of pneumococcal disease, including IPD, non-bacteremic pneumococcal pneumonia, pneumococcal AOM and long-term post-meningitis sequelae as health outcomes.
Despite differences in perspective used, model type, and time horizon, both studies concluded that Pneu-C-15 use was associated with lower costs and improved health outcomes, dominating Pneu-C-13; however, these analyses assumed that Pneu-C-15 and Pneu-C-13 were priced equivalently in the base caseFootnote 34Footnote 35. In sensitivity analyses, results were robust to alternate assumptions about vaccine effectiveness, coverage, and indirect effects, with Pneu-C-15 remaining the dominant strategy when Pneu-C-15 and Pneu-C-13 were priced equivalentlyFootnote 34Footnote 35. In a threshold analysis, Pneu-C-15 was shown to remain the dominant strategy up to a maximum price per dose that is 18% higher than Pneu-C-13Footnote 35. A catch-up campaign using Pneu-C-15 for all children aged 2 to 5 years who were previously fully vaccinated with Pneu-C-13 was unlikely to be cost-effective, with ICERs exceeding $3.5 million per QALY gainedFootnote 34.
Cost-utility analysis
A static cost-utility model developed by NACI evaluated the cost-effectiveness of the use of Pneu-C-15 and Pneu-C-20 compared to Pneu-C-13 in previously unvaccinated Canadian infants eligible for routine pneumococcal vaccination. The analysis did not include an evaluation of vaccination strategies for individuals at increased risk for IPD. Both Pneu-C-15 and Pneu-C-20 were projected to improve health outcomes compared to Pneu-C-13, with Pneu-C-20 averting twice as many IPD cases as Pneu-C-15 over the 10-year model time period (median estimate of 468 vs. 221 cases averted). In the base-case sequential analysis that compared all possible vaccination strategies, the ICERs for Pneu-C-15 and Pneu-C-20 were $58,800 (vs. Pneu-C-13) and $135,200 (vs. Pneu-C-15) per QALY gained, respectively, for the health system perspective.
For the societal perspective, sequential ICERs were $18,300 (vs. Pneu-C-13) and $93,400 (vs. Pneu-C-15) per QALY gained for Pneu-C-15 and Pneu-C-20, respectively. Results were highly sensitive to assumed vaccine price. Base case values were $71, $78, and $90 per dose of Pneu-C-13, Pneu-C-15, and Pneu-C-20, respectively; lower assumed vaccine prices for Pneu-C-15 and Pneu-C-20 increased their cost-effectiveness. The base-case analysis excluded indirect protection effects; if use of Pneu-C-15 or Pneu-C-20 in the pediatric population results in lower pneumococcal disease incidence in the rest of the population due to indirect protection effects, ICERs would be substantially reduced. Serotype replacement was not evaluated in the analysis. In a setting with higher pneumococcal disease burden and higher medical costs, use of Pneu-C-20 was a dominant strategy (less costly and more effective) compared to Pneu-C-13 and Pneu-C-15 for both the health system and societal perspectives. The results of the health economic modelling are subject to limitations associated with simplifying assumptions and data uncertainty, which are discussed in greater detail in a supplementary economic evidence summary.
Additional economic evidence
Two additional economic evaluations that included both Pneu-C-15 and Pneu-C-20 became available after completion of the systematic review and were also reviewed. One evaluation was a comparison of three cost-utility analyses (two industry funded) that was conducted in the United States (US)Footnote 36 and the second evaluation was a cost-utility analysis conducted in QuebecFootnote 37. The US analysis compared the use of Pneu-C-20 to Pneu-C-13 or Pneu-C-15, using a 3+1 schedule in children less than 2 years of age. The Quebec analysis compared the use of a 2+1 schedule of Pneu-C-15 or Pneu-C-20 to a schedule of 2 doses of Pneu-C-10 plus 1 dose of Pneu-C-13 (the current pneumococcal vaccine schedule used in Quebec). All models were static but differed in structure, analytic time frame, assumptions about indirect protection effects, vaccine price, and other parameters. Neither evaluation conducted a sequential analysis comparing all three vaccination schedules. A formal comparison across the models was not conducted.
Both evaluations showed that Pneu-C-20 was expected to improve health outcomes compared to other vaccination options. For the US analysis, which used a societal perspective, results varied across models, with ICERs for Pneu-C-20 ranging from dominant to $74,200 per QALY compared to Pneu-C-13, and dominant to $162,700 per QALY compared to Pneu-C-15. The largest ICERs were seen with the non-industry funded model. For the Quebec analysis, when compared to current standard of care, both Pneu-C-15 and Pneu-C-20 had ICERs above commonly used cost-effectiveness thresholds, using the health system perspective. When Pneu-C-20 was compared to Pneu-C-15, the ICER was below commonly used thresholds for the health system perspective, and Pneu-C-20 dominated Pneu-C-15 for the societal perspective. These additional economic evaluations demonstrate that the estimated cost-effectiveness of Pneu-C-15 and Pneu-C-20 are sensitive to model assumptions and input parameters.
Recommendations
Following the review of available evidence summarized above, NACI continues to recommend that all routine infant immunization programs in Canada include a conjugate pneumococcal vaccine. NACI further makes the following recommendations for public health program decision-making (see Table 6 for a more detailed explanation of strength of NACI recommendations and grade of the body of evidence). As previously indicated in this Statement, the reference to "children" includes infants, children, and adolescents.
Recommendations for routine immunization programs in children less than 5 years of age who are not at increased risk of IPD
- NACI recommends that either Pneu-C-15 or Pneu-C-20 should be the current product of choice for children less than five years of age for routine immunization programs. (Strong NACI recommendation)
- Pneu-C-15 or Pneu-C-20 should be provided to children using previously recommended routine schedules for Pneu-C-13 (please see the pneumococcal vaccines chapter of the Canadian Immunization Guide).
- Pneu-C-20 is the preferred product for children at increased risk of IPD (see Recommendations 2 through 4)
See the Management Options Table (Table 5) for a summary of the relative merits of each pneumococcal conjugate vaccine for use in routine programs, including EEFA considerations.
Summary of evidence, rationale, and additional considerations
- The vaccine effectiveness and duration of protection of Pneu-C-15 and Pneu-C-20 are currently unknown. Both Pneu-C-15 and Pneu-C-20 are immunogenic in pediatric populations and offer additional serotype coverage compared to Pneu-C-13. Immune responses are lower after Pneu-C-15 and Pneu-C-20 than Pneu-C-13 for several shared serotypes. It is unknown how this may impact vaccine effectiveness, duration of protection, carriage, and herd immunity. Ongoing and enhanced surveillance of pneumococcal disease in Canada is recommended.
- No safety signals following any of the Pneu-C vaccines have been identified to date (Table 13).
- The new higher-valent pneumococcal conjugate vaccines can protect children against additional serotypes compared to Pneu-C-13 and are expected to further reduce the burden of IPD. With five additional serotypes compared to Pneu-C-15, Pneu-C-20 is expected to have a larger impact on IPD. Inclusion of Pneu-C-20 in routine programs for all children may simplify immunization programs and provide the most indirect benefits to other populations.
- In the base case cost-effectiveness analysis performed by NACI, Pneu-C-15 is more likely to be a cost-effective option than Pneu-C-20. However, Pneu-C-15 or Pneu-C-20 may be cost-effective depending on the price per dose of each vaccine or whether the broader impacts of vaccination are considered with a societal perspective and/or inclusion of potential indirect effects.
- Pneu-C-13 only or a mixed Pneu-C-10/Pneu-C-13 schedule can be used if Pneu-C-15 or Pneu-C-20 are unavailable or inaccessible.
- Consistent with previous guidance, NACI recommends that all pneumococcal vaccines may be administered concomitantly with other vaccines, except for Pneu-P-23. No conjugate pneumococcal vaccine should be given concomitantly with Pneu-P-23.
Recommendations on the use of pneumococcal vaccines for children with medical conditions or environmental or living conditions that place them at increased risk of IPD
See Table 1 for medical risk factors and environmental or living conditions that result in increased risk of IPD in children.
- NACI recommends that Pneu-C-20 should be used for infants and children at increased risk of IPD who are initiating their pneumococcal vaccine series, and for series completion for those who have started their vaccine series with Pneu-C-13 or Pneu-C-15. (Strong NACI Recommendation)
- Infants at increased risk of IPD should be immunized with Pneu-C-20 vaccine using a 4-dose (3+1) schedule at 2 months, 4 months, 6 months and a dose at 12 to 15 months of age.
- Infants and children who have not initiated their immunization schedule by 7 months of age should be immunized according to the schedule provided in Table 7.
- Children two years of age and older who are at increased risk of IPD and have never received any pneumococcal vaccination, should receive one dose of Pneu-C-20.
- A vaccine series started with Pneu-C-13 or Pneu-C-15 should be completed with Pneu-C-20 according to the recommended schedule shown in Table 7.
- Children at increased risk of IPD who have completed a vaccine series appropriate for age that includes at least one dose of Pneu-C-20 do not require further doses at this time.
Summary of evidence, rationale, and additional considerations
- Pneu-C-20 was safe and immunogenic in studies with pediatric populations at high risk of IPD.
- The use of Pneu-C-20 in children at increased risk of IPD provides protection for a larger number of serotypes compared to other conjugate vaccines.
- Children at increased risk of IPD who have received at least one dose of Pneu-C-20, do not require Pneu-P-23.
- It is not known whether additional doses of Pneu-C-20 would confer added benefit, therefore additional doses are not required at this time. NACI will assess the evidence as it becomes available.
- A cost-effectiveness analysis for populations at increased risk of IPD was not conducted due to limited data for these populations.
Recommendations on catch-up doses for children with medical conditions or environmental or living conditions that place them at increased risk of IPD
- a) NACI recommends that children under 18 years of age who have medical risk factors and who have completed their recommended immunization schedule with Pneu-C-13 or Pneu-C-15, should receive one catch-up (additional) dose of Pneu-C-20. (Strong NACI recommendation)
- Those with medical risk factors who have previously received Pneu-P-23 should be given one dose of Pneu-C-20 at a one-year interval from the last dose of Pneu-P-23.
- b) NACI recommends that children under 18 years of age who have environmental or living conditions that result in increased risk for IPD, and who have completed their recommended immunization schedule with Pneu-C-13 or Pneu-C-15, should receive one catch-up (additional) dose of Pneu-C-20. (Strong NACI recommendation)
- In communities and settings experiencing sustained high IPD rates, an assessment of local epidemiologic trends should be used to inform groups for vaccination programs.
- In First Nations, Metis, or Inuit communities, autonomous decisions should be made by Indigenous Peoples with the support of healthcare and public health partners in accordance with the United Nations Declaration on the Rights of Indigenous Peoples.
Summary of evidence, rationale, and additional considerations
- A catch-up dose with Pneu-C-20 for children at increased risk of IPD (medical and/or environmental/ living conditions) who have completed their recommended immunization schedule will provide broader serotype protection, along with the immune advantages of conjugate vaccines.
- For children who are not at increased risk of IPD due to medical and/or environmental or living conditions and have completed a recommended immunization series with Pneu-C-13, a catch-up (additional) dose of Pneu-C-15 or Pneu-C-20 is not recommended.
- A cost-effectiveness analysis for catch-up programs was not conducted due to limited data for these populations.
Recommendations for recipients of hematopoietic stem cell transplant
- NACI recommends that pneumococcal conjugate vaccine Pneu-C-20 should be offered to children less than 18 years of age who received a hematopoietic stem cell transplant (HSCT) after consultation with their transplant specialist. (Strong NACI recommendation)
- A primary series of 3 doses of Pneu-C-20 starting 3 to 9 months after transplant should be administered at least 4 weeks apart, followed by an additional dose of Pneu-C-20 12 to 18 months post-transplant (6 to 12 months after the last dose of Pneu-C-20).
- The recommended timing of Pneu-C-20 for HSCT recipients should be determined in consultation with the recipient's transplant specialist.
Summary of evidence, rationale, and additional considerations
- Pneu-C-20 offers protection against additional serotypes for these individuals, along with the immune advantages of conjugate vaccines. Pneu-P-23 is no longer recommended in the vaccine series for this population.
| Vaccine | Factors for consideration | Decision points |
|---|---|---|
| Pneu-C-15 | Epidemiology In 2022, 13% (less than 2 years old) and 16% (2 to 4 and 5 to 17 years old) of isolates from IPD cases were due to the two unique serotypes (22F and 33F) contained in Pneu-C-15. Immunogenicity Safety Economic analysis |
Epidemiology
Immunogenicity
Effectiveness
Safety
Economics
Feasibility/Acceptability/Equity
|
| Pneu-C-20 | Epidemiology In 2022, 37% (less than 2 years old) and 38% (2 to 4 and 5 to 17 years old) of IPD cases were caused by the seven serotypes (8, 10A, 11A, 12F, 15B/C, 22F and 33F) contained in Pneu-C-20 and not in Pneu-C-13. Immunogenicity Safety Economic analysis |
|
| Pneu-C-13 | Epidemiology Feasibility |
| Strength of recommendation | Strong | Discretionary |
|---|---|---|
| Wording | "should/should not be offered" | "may/may not be offered" |
| Rationale | Known/anticipated advantages outweigh known/anticipated disadvantages ("should"), |
Known/anticipated advantages are closely balanced with known/anticipated disadvantages, OR uncertainty in the evidence of advantages and disadvantages exists |
| Implication | A strong recommendation applies to most populations/individuals and should be followed unless a clear and compelling rationale for an alternative approach is present. | A discretionary recommendation may be considered for some populations/individuals in some circumstances. Alternative approaches may be reasonable. |
| Age at presentation for immunization | Number of previously received Pneu-C doses | Recommended schedule for Pneu-C-20Footnote b |
|---|---|---|
| 2 months to less than 7 months | 0 dose | 3 doses + 1 dose at 12 to 15 months of age |
| 1 dose | 2 doses + 1 dose at 12 to 15 months of age | |
| 2 doses | 1 dose + 1 dose at 12 to 15 months of age | |
| 7 months to less than 12 months | 0 doses | 2 doses + 1 dose at 12-15 months of age |
| 1 dose | 1 dose at 7 to less than 12 months of age + 1 dose at 12 to 15 months of age | |
| 2 doses | 1 dose at 12 to 15 months of age | |
| 12 months to less than 24 months | 0 dose | 2 doses |
| 1 dose at less than 12 months of age | ||
| 2 or more doses at less than 12 months of age | 1 dose | |
| 0 or 1 dose at less than 12 months of age AND 1 dose at 12 months of age or older | ||
| 24 months to less than 60 months (5 years) |
0 doses of Pneu-C-20 | 1 dose |
| 5 years to less than 18 years | 0 doses of Pneu-C-20 | 1 dose only for children at high risk |
|
||
Research and surveillance priorities
- Determining the direct (vaccinated children) and indirect (unvaccinated children and adults) impact of Pneu-C-15 and Pneu-C-20 programs on the burden of disease (AOM, CAP, IPD) in individuals with and without risk factors for invasive disease.
- Determining the immunological correlates of protection against various disease outcomes (severity and disease manifestations).
- Determining the duration of immunity and need for additional doses for different Pneu-C-15 and Pneu-C-20 schedules including mixed schedule programs.
- Determining the impact of vaccination with Pneu-C-15 and Pneu-C-20 on carriage and serotype switching.
- Determining any rare and very rare adverse events that may not have been reported due to limited sample size in clinical trials.
- Enhancing ongoing pneumococcal disease surveillance including non-IPD pneumococcal diseases and S. pneumoniae carriage.
List of abbreviations
- AE
- Adverse event
- AEFI
- Adverse event following immunization
- AOM
- Acute otitis media
- CAP
- Community acquired pneumonia
- CI
- Confidence interval
- CIC
- Canadian Immunization Committee
- CIG
- Canadian Immunization Guide
- CIQ
- Quebec Immunization committee
- CNDSS
- Canadian Notifiable Disease Surveillance System
- CRM197
- Corynebacterium diphtheriae
- DALY
- Disability adjusted life years
- EEFA
- Ethics, equity, feasibility, acceptability
- eSTREP
- Enhanced Streptococcus Surveillance System
- EtD
- Evidence to Decision
- GMC
- Geometric mean concentration
- GMT
- Geometric mean titre
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluation
- HIV
- Human Immunodeficiency Virus
- HSCT
- Hematopoietic Stem Cell Transplant
- IC
- Immunocompromising conditions
- ICER
- Incremental cost-effective ratio
- ICS
- International Circumpolar Surveillance
- IgG
- Immunoglobulin G
- IMPACT
- Immunization Monitoring Program, ACTive
- IPD
- Invasive pneumococcal disease
- NACI
- National Advisory Committee on Immunization
- NML
- National Microbiology Laboratory
- NVT
- Non-vaccine type
- NWT
- Northwest Territories
- OPA
- Opsonophagocytic Activity
- OR
- Odds ratio
- pCAP
- Pneumococcal community-acquired pneumonia
- PD2
- Following dose 2
- PD3
- Following dose 3
- PD4
- Following dose 4
- PHAC
- Public Health Agency of Canada
- Pneu-C
- Pneumococcal conjugate vaccine
- Pneu-C-10
- 10-valent pneumococcal conjugate vaccine
- Pneu-C-13
- 13-valent pneumococcal conjugate vaccine
- Pneu-C-15
- 15-valent pneumococcal conjugate vaccine
- Pneu-C-20
- 20-valent pneumococcal conjugate vaccine
- Pneu-P
- Pneumococcal polysaccharide vaccine
- Pneu-P-23
- 23-valent pneumococcal polysaccharide vaccine
- PP
- Pneumococcal pneumonia
- QALY
- Quality adjusted life-years
- RD
- Risk Difference
- RoB
- Risk of bias
- RR
- Relative Risk
- SAE
- Serious adverse events
- SES
- Socioeconomic Status
- ST
- Serotype
- ST3
- Serotype 3
- US
- United States
- VE
- Vaccine Efficacy
Acknowledgements
This statement was prepared by: A Wierzbowski, M Salvadori, O Baclic, E Wong, A Tuite, N Islam, G Gebretekle, R Pless, A Howarth, M Hersi, A Stevens, K Young, and K Hildebrand on behalf of NACI.
NACI gratefully acknowledges the contribution of: C Tremblay, M Tunis, F Khan, R Yorke, J Montroy, A Haynes as well as A Golden, I Martin, K Franklin, C Primeau, M Laverty, G Metz and other members of the PHAC NML and VPD Surveillance Teams.
NACI Pneumococcal Working Group
NACI Pneumococcal Working Group Members: K Hildebrand (Chair), J Papenburg, P De Wals, N Brousseau, J Bettinger, D Fisman, J Kellner, S Rechner, G Tyrrell, A McGeer, S Nasreen, and M Kobayashi (Centers for Disease Control and Prevention, US).
Ex-officio representatives: M Knight (First Nations and Inuit Health Branch, ISC), G Coleman (Biologic and Radiopharmaceutical Drugs Directorate, HC), K Franklin (VPD Surveillance), C Primeau (VPD Surveillance), I Martin (National Microbiology Laboratory), A Golden (National Microbiology Laboratory) and G Metz (Vaccine Safety).
PHAC participants: E Wong, A Wierzbowski, R Pless, O Baclic, M Tunis, A Stevens, G Gebretekle, N Islam, J Montroy, A Tuite, F Crane, F Khan, M Hersi, A Simmons, and C Tremblay.
NACI
NACI members: S Deeks (Chair) R Harrison (Vice-Chair), M Andrew, J Bettinger, N Brousseau, H Decaluwe, P De Wals, E Dubé, V Dubey, K Hildebrand, K Klein, M O'Driscoll, J Papenburg, A Pham-Huy, B Sander, and S Wilson.
Liaison representatives: L Bill/ M Nowgesic (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), S Buchan (Canadian Association for Immunization Research, Evaluation and Education) E Castillo (Society of Obstetricians and Gynaecologists of Canada), J Comeau (Association of Medical Microbiology and Infectious Disease Control), J MacNeil (Center for Disease control and Prevention) M Osmack (Indigenous Physicians Association of Canada), J Potter (College of Family Physicians of Canada), M Lavoie (Council of Chief Medical Officers of Health), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), and A Ung (Canadian Pharmacists Association).
Ex-officio representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (Centre for Immunization Programs (CIP), PHAC), M Lacroix (Public Health Ethics Consultative Group, PHAC), C Pham (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), S Ogunnaike-Cooke (CIRID, PHAC), P Fandja (Marketed Health Products Directorate, HC), M Routledge (National Microbiology Laboratory, PHAC), and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
Appendix A: Tables
| Study | Comparisons | Study design | Participants |
|---|---|---|---|
V114-008: A Study to Evaluate the Safety, Tolerability, and Immunogenicity of Two Lots of V114 in Healthy InfantsFootnote 19 |
Pneu-C-15 Lot 1 4-dose series per group |
Phase 2, randomized to Pneu-C-15 Lot 1 (N=351), Pneu-C-15 Lot 2 (N=350), or Pneu-C-13 (N=350) |
Children aged 42 to 90 days, inclusively, in good health who are pneumococcal vaccine-naïve. Gender (total study): 49.8% female Authors state participant demographics were similar between groups. |
V114-023: A Study to Evaluate the Safety, Tolerability, and Immunogenicity of V114 in Children with Sickle Cell DiseaseFootnote 28 |
Pneu-C-15 vs |
Phase 3, randomized to single dose of Pneu-C-15 (N=70) or Pneu-C-13 (N=34) |
Children aged 5 to 17 years, who have sickle-cell disease and are pneumococcal vaccine-naïve (as defined in trial: did not receive vaccine within 3 years of study entry). Gender (total study): 45.6% female Clinical study report states participant demographics were similar between groups. |
V114-024: Safety and Immunogenicity of Catch-up Vaccination Regimens of V114Footnote 27 |
Pneu-C-15 vs Pneu-C-13 7 to 11 months of age: 3-dose series per group |
Phase 3, |
Children aged 7 to 11 months of age, or 12 to 23 months of age in good health who are pneumococcal vaccine-naïve. Children aged 2 to 17 years of age in good health who are either pneumococcal vaccine-naïve or vaccine-experienced. |
V114-025: Safety, Tolerability, and Immunogenicity of V114 in Healthy InfantsFootnote 20 |
Pneu-C-15 vs Pneu-C-13 Full-term infants (greater or equal to 37 weeks gestational age at birth): 3-dose series per group Pre-term infants (less than 37 weeks gestation age): 4-dose series per group |
Phase 3, randomized to Pneu-C-15 (N=591) or Pneu-C-13 (N=593) |
Children aged 42 to 90 days, inclusively, in good health who are pneumococcal vaccine-naïve. Gender (total study): 48.2% female Clinical study report states participant demographics were similar between groups. |
V114-027: A Study to Evaluate the Interchangeability of V114 and Prevnar 13™ in Healthy InfantsFootnote 21 |
Pneu-C-15 (4 doses) |
Phase 3, randomized to Pneu-C-15 (4 doses; N=180), or Total randomized: 900 |
Children aged 42 to 90 days, inclusively, in good health who are pneumococcal vaccine-naïve. |
V114-029: Safety, Tolerability, and Immunogenicity of V114 in Healthy InfantsFootnote 22 |
Pneu-C-15 vs Pneu-C-13 4-dose series per group |
Phase 3, randomized to Pneu-C-15 (n=860) or Pneu-C-13 (n=860) |
Children aged 42 to 90 days, inclusively, in good health who are pneumococcal vaccine-naïve. Clinical study report states participant demographics were similar between groups. |
V114-030: Safety and Immunogenicity of V114 in Children Infected with Human Immunodeficiency VirusFootnote 29 |
Pneu-C-15+ Pneu-P-23 vs Pneu-C-13 + Pneu-P-23 2-dose series per group (first dose=Pneu-C vaccine; second dose=Pneu-P-23 vaccine) |
Phase 3, randomized to Pneu-C-15+Pneu-P-23 (N=203) or Pneu-C-13+Pneu-P-23 (N=204) Total randomized = 407 |
Children aged 6 to 17 years, who have Human Immunodeficiency Virus and are vaccine-naïve, previously vaccinated with a less than 13-valent Pneu-C, partially vaccinated with Pneu-C-13, or has a history of previous Pneu-C-13 vaccination greater or equal to 3 years before Visit 2 (Day 1). Gender (total study): 47.9% female Clinical study report states participant demographics were similar between groups. |
V114-031: A Study to Evaluate the Safety and Tolerability of V114 and Prevnar 13™ in Healthy InfantsFootnote 30 |
Pneu-C-15 vs Pneu-C-13 |
Phase 3, randomized to Pneu-C-15 (n=1972) or Pneu-C-13 (n=437) |
Children aged 42 to 90 days, inclusively, in good health who are pneumococcal vaccine-naïve. Clinical study report states participant demographics were similar between groups. |
B7471003: Trial to Evaluate the Safety and Immunogenicity of a Multivalent Pneumococcal Vaccine in Healthy InfantsFootnote 23 |
Pneu-C-20 vs Pneu-C-13 4-dose series per group |
Phase 2, randomized to Pneu-C-20 (n=232) or Pneu-C-13 (n=228) Total randomized = 460 |
Children aged 42 to 98 days, inclusively, in good health who are pneumococcal vaccine-naïve. Gender (total study): 49.3% female Clinical study report states participant demographics were similar between groups. |
B7471011: 20-valent Pneumococcal Conjugate Vaccine Safety and Immunogenicity Study of a 4-Dose Series in Healthy InfantsFootnote 24 |
Pneu-C-20 vs Pneu-C-13 4-dose series per group |
Phase 3, randomized to Pneu-C-20 (n=1004) or Pneu-C-13 (n=993) Total randomized = 1997 |
Children aged 42 to 98 days, inclusively, in good health who are pneumococcal vaccine-naïve. Gender (total study): 48.5% female Clinical study report states participant demographics were similar between groups. |
B7471012: 20-valent Pneumococcal Conjugate Vaccine Safety and Immunogenicity Study of a 3-Dose Series in Healthy InfantsFootnote 25 |
Pneu-C-20 vs Pneu-C-13 3-dose series per group |
Phase 3, randomized to Pneu-C-20 (n=603) or Pneu-C-13 (n=604) Total randomized = 1207 |
Children aged 42 to 112 days, inclusively, in good health who are pneumococcal vaccine-naïve. Gender (total study): 49.3% female Clinical study report states participant demographics were similar between groups. |
B7471013: 20-valent Pneumococcal Conjugate Vaccine Safety Study in Healthy InfantsFootnote 26 |
Pneu-C-20 vs Pneu-C-13 4-dose series per group |
Phase 3, randomized to Pneu-C-20 (n=1006) or Pneu-C-13 (n=505) Total randomized = 1511 |
Children aged 42 to 98 days, inclusively, in good health who are pneumococcal vaccine-naïve. Gender (total study): 49.4% female Clinical study report states participant demographics were similar between groups. |
B7471014: Safety and Immunogenicity Study of 20vPnC in Healthy Children 15 Months Through 17 Years of AgeFootnote 31 |
Pneu-C-20 (before vs after vaccination) 1 dose per group |
Phase 3, greater or equal to 15 to less than 24 months of age: Pneu-C-20 (N=210) Total randomized = 839 |
Cohort 1 and 2: children aged greater or equal to 15 months to less than 5 years, in good health who previously received at least 3 doses of Pneu-C-13 with the last dose administered greater than 2 months before study enrollment. Excluded if previously received any investigational pneumococcal vaccine or with Pneu-P-23. Cohort 3 and 4: children aged greater or equal to 5 years to less than 18 years, in good health. Excluded if previously received any investigational pneumococcal vaccine or with Pneu-P-23. Gender (total study): Clinical study report states participant demographics were similar between groups. There were slightly more male participants in each age cohort, except in participants greater or equal to 2 to less than 5 years of age (Cohort 2, which had an approximately equal distribution). |
| Measured outcome | Healthy children |
|---|---|
GMT OPA ratios |
No data reported after dose 2. Lower for 11/13 shared serotypes following dose 3; higher for ST3 and ST14 following dose 3. Higher for both unique serotypes. |
GMC IgG ratios |
Lower for 9/13 shared serotypes 11/13 following dose 2 and dose 3, respectively higher for ST23F following dose 2, and for ST3 following dose 2 and dose 3; mixed or null-inclusive results for remaining serotypes. Higher for both unique serotypes following dose 2 and 3. |
% seroresponders: shared serotypes |
Lower for 3/13 shared serotypes 6/13 following dose 2 and dose 3, respectively; higher for ST23F following dose 2, and for ST3 following dose 2 and dose 3; null-inclusive results for remaining serotypes. |
% seroresponders: unique serotypes |
Higher for unique serotypes following dose 2 and dose 3. |
Vaccine-related serious adverse events |
Pneu-C-15, N= 0/587 |
Total serious adverse events |
Pneu-C-15, N= 57/587 |
Deaths |
No deaths were observed. |
| Measured outcome | Healthy children and immunocompetent, high-risk children | Immunocompromised children |
|---|---|---|
GMT OPA ratios |
Lower for 4/13 and 10/13 shared serotypes following dose 3 and 4, respectively; higher for ST3 following dose 3 but lower following dose 4; mixed results for remaining serotypes. Higher for both unique serotypes following dose 3 and 4. |
|
No GRADE assessment |
||
GMC IgG ratios |
Lower for 5/13 shared serotypes following dose 3 and dose 4; higher for ST3 following dose 3 and dose 4; mixed or null-inclusive results for remaining serotypes. Higher for both unique serotypes following dose 3 and 4. |
|
Moderate certainty of evidence (both doses) |
Low certainty of evidence (both doses) |
|
% seroresponders: shared serotypes |
Mixed or null-inclusive results for 12/13 shared serotypes following dose 3 and dose 4; higher for ST3 following dose 3 and dose 4. |
|
Moderate certainty of evidence (3rd dose), low certainty of evidence (4th dose) |
Low certainty of evidence (3rd dose), very low certainty of evidence (4th dose) |
|
% seroresponders: unique serotypes |
Higher for unique serotypes following dose 3 and dose 4. |
|
Moderate certainty of evidence (both doses) |
Low certainty of evidence (both doses) |
|
Vaccine-related serious adverse events at 6 months |
Pneu-C-15, N= 4/3699 |
|
High certainty of evidence |
Moderate certainty of evidence |
|
Total serious adverse events at 6 months |
Pneu-C-15, N= 338/3699 |
|
Moderate certainty of evidence |
Low certainty of evidence |
|
Deaths at 6 months |
Pneu-C-15, N= 3/3699 |
|
High certainty of evidence |
Moderate certainty of evidence |
|
| Measured outcome | Healthy children |
|---|---|
GMT OPA ratios |
Lower for 13/13 and 12/13 shared serotypes following dose 2 and dose 3, respectively; higher for ST19A following dose 3. Higher for all unique serotypes following dose 2 and dose 3. |
GMC IgG ratios |
Lower for 13/13 shared serotypes following both dose 2 and dose 3; higher for all unique serotypes following dose 2 and dose 3. |
% seroresponders: shared serotypes |
Lower for 9/13 and 1/13 (ST3) shared serotypes following dose 2 and dose 3, respectively; null-inclusive results for remaining serotypes. |
% seroresponders: unique serotypes |
Higher for all unique serotypes following dose 2 and dose 3. |
Vaccine-related adverse events |
Pneu-C-20, N= 1/601 |
Total serious adverse events |
Pneu-C-20, N= 34/601 |
Deaths |
No deaths were observed. |
| Measured outcome | Healthy children and immunocompetent, high-risk children |
Immunocompromised children |
|---|---|---|
| GMT OPA ratios | Lower for 8/13 and 12/13 shared serotypes following dose 3 and 4, respectively. Higher for both unique serotypes following dose 3 and 4. | |
| No GRADE assessment | ||
| GMC IgG ratios | Lower for 13/13 and 12/13 shared serotypes following dose 3 and dose 4, respectively; null-inclusive for ST14 following dose 4. Higher for both unique serotypes following dose 3 and 4. | |
| Moderate certainty of evidence (both doses) | Low certainty of evidence (both doses) | |
| % seroresponders: shared serotypes | Mixed or null-inclusive results for 8/13 and 2/13 shared serotypes following dose 3 and dose 4, respectively; null-inclusive results for remaining serotypes. | |
| Moderate certainty of evidence (dose 3), low certainty of evidence (dose 4) | Low certainty of evidence (dose 3), very low certainty of evidence (dose 4) | |
| % seroresponders: unique serotypes | Higher for unique serotypes following dose 3 and dose 4. | |
| Moderate certainty of evidence (both doses) | Low certainty of evidence (both doses) | |
| Vaccine-related serious adverse events at 6 months | No vaccine-related events observed. | |
| Moderate certainty of evidence | Low certainty of evidence | |
| Total serious adverse events at 6 months | Pneu-C-20, N= 101/2232 |
|
| Low certainty of evidence | Very low certainty of evidence | |
| Deaths at 6 months | No deaths were observed. | |
| Moderate certainty of evidence | Low certainty of evidence | |
| Outcome | Summary of findings | Comments/summary | ||||
|---|---|---|---|---|---|---|
| Number of studies, study design |
Pneu-C-15 | Pneu-C-13 | Impact – quantitative or narrative | Certainty of evidence | ||
| Up to 6 months after vaccine, per protocol analysis: 2+1 schedule | ||||||
| Vaccine-related SAE | Assessed with GRADE |
N=0/587 | N=1/591 | Relative effects: Peto OR: 0.14 (0.00 to 6.87) |
ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of vaccine-related SAEs |
| Total SAE | Assessed with GRADE: |
N=57/587 | N=70/591 | Relative effects: RR 0.82 (0.59 to 1.14) |
ModerateFootnote b | There is probably little to no difference between vaccines in the occurrence of SAEs |
| Death | Assessed with GRADE: |
N=0/587 | N=0/591 | No deaths were observed | ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of deaths |
| Up to 6 months after vaccine, per protocol analysis: 3+1 schedule | ||||||
| Vaccine-related SAE | Assessed with GRADE: |
N=4/3,699 | N=0/1,814 | Relative effects: Peto OR: 4.01 (0.41 to 39.22) |
High | There is little to no difference between vaccines in the occurrence of vaccine-related SAEs |
| ModerateFootnote d for immunocompromised persons (IC) | There is probably little to no difference between vaccines in the occurrence of vaccine-related SAEs | |||||
| Total SAE | Assessed with GRADE: |
N= 338/3,699 | N=162/1,814 | Relative effects: RR 1.03 (0.86 to 1.24) |
ModerateFootnote c | There is probably little to no difference between vaccines in the occurrence of SAEs |
| LowFootnote cFootnote d for IC | There may be little to no difference between vaccines in the occurrence of SAEs | |||||
| Death | Assessed with GRADE: |
N=3/3,699 | N=2/1,814 | Relative effects: Peto OR: 0.74 (0.11 to 5.15) |
High | There is little to no difference between vaccines in the occurrence of deaths |
| ModerateFootnote d for IC | There is probably little to no difference between vaccines in the occurrence of deaths | |||||
|
||||||
| Outcome | Summary of findings | Comments/summary | ||||
|---|---|---|---|---|---|---|
| Number of studies, study design | Pneu-C-15 | Pneu-C-13 | Impact – quantitative or narrative | Certainty of evidence | ||
| Up to 6 months after vaccine, per protocol analysis | ||||||
| Vaccine-related SAE | Assessed with GRADE: |
N=0/272 | N=0/238 | No vaccine-related events observed | ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of vaccine-related SAEs |
| Total SAE | Assessed with GRADE: |
N=14/272 | N=9/238 | Relative effects: RR 0.81 (0.38 to 1.72) |
ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of SAEs |
| Death | Assessed with GRADE: |
N=0/272 |
N=0/238 |
No deaths were observed |
ModerateFootnote a |
There is probably little to no difference between vaccines in the occurrence of deaths |
|
||||||
| Outcome | Summary of findings | Comments/summary | ||||
|---|---|---|---|---|---|---|
| Number of studies, study design | Pneu-C-20 | Pneu-C-13 | Impact – quantitative or narrative | Certainty of evidence | ||
| Up to 6 months after vaccine, per protocol analysis: 2+1 schedule | ||||||
| Vaccine-related SAE | Assessed with GRADE: |
N=1/601 | N=0/603 | Relative effects: Peto OR 7.41 (0.15 to 373.63) |
ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of vaccine-related SAEs |
| Total SAE | Assessed with GRADE: |
N=34/601 | N=40/603 | Relative effects: RR 0.85 (0.55 to 1.33) |
LowFootnote b | There may be little to no difference between vaccines in the occurrence of SAEs |
| Death | Assessed with GRADE: |
N=0/601 | N=0/603 | No deaths were observed | ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of deaths |
| Up to 6 months after vaccine, per protocol analysis: 3+1 schedule | ||||||
| Vaccine-related SAE | Assessed with GRADE: |
N=0/2,232 | N=0/1,717 | No vaccine-related events observed | ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of vaccine-related SAEs |
| Low for ICFootnote aFootnote c | There may be little to no difference between vaccines in the occurrence of vaccine-related SAEs | |||||
| Total SAE | Assessed with GRADE: |
N=101/2,232 | N=64/1,717 | Relative effects: RR 1.24 (0.71 to 2.15) |
LowFootnote dFootnote e | There may be little to no difference between vaccines in the occurrence of SAEs |
| Very low for ICFootnote cFootnote dFootnote e | There may be little to no difference between vaccines in the occurrence of SAEs, but the evidence is very uncertain | |||||
| Death | Assessed with GRADE: |
N=0/2,232 | N=0/1,717 | No deaths were observed | ModerateFootnote a | There is probably little to no difference between vaccines in the occurrence of deaths |
| Low for ICFootnote aFootnote c | There may be little to no difference between vaccines in the occurrence of deaths | |||||
|
||||||
| Immunogenicity measure | Findings Pneu-C-15 | Findings Pneu-C-20 |
|---|---|---|
| GMC IgG Ratios |
|
|
| % Seroresponders Shared serotypes |
|
|
| % Seroresponders Unique serotypes |
|
|
| GMT OPA RatiosFootnote a |
|
|
|
||
References
- Footnote 1
-
Statistic Canada. Childhood National Immunization Coverage Survey, 2021 [Internet]. Ottawa (ON): Government of Canada; 2023 Jun 12 [cited 2023 Jul 19]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/230612/dq230612b-eng.htm.
- Footnote 2
-
Public Health Agency of Canada (PHAC). Vaccination Coverage Goals and Vaccine Preventable Disease Reduction Targets by 2025 [Internet]. Ottawa (ON): Government of Canada; 2022 Aug 16 [cited 2023 Jun 26]. Available from: https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/national-immunization-strategy/vaccination-coverage-goals-vaccine-preventable-diseases-reduction-targets-2025.html.
- Footnote 3
-
National Advisory Committee on Immunization (NACI). National Advisory Committee on Immunization (NACI): Methods and process [Internet]. Ottawa (ON): Government of Canada; 2023 Apr 12 [cited 2023 Jun 26]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/methods-process.html.
- Footnote 4
-
Golden A, Griffith A, Demczuk W, Lefebvre B, McGeer A, Tyrrell G, et al. Invasive pneumococcal disease surveillance in Canada, 2020. Canada Communicable Disease Report. 2022 Sep;48(9):396-406. https://doi.org/10.14745/ccdr.v48i09a04.
- Footnote 5
-
ACIP Updates: Recommendations for Use of 20-Valent Pneumococcal Conjugate Vaccine in Children - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023 Sep 29;72(39):1072. https://doi.org/10.15585/mmwr.mm7239a5.
- Footnote 6
-
Expert Committee on Biological Standardization. Recommendations for the production and control of pneumococcal conjugate vaccines [Internet]. Geneva (CH): World Health Organization; 2009 [cited 2023 Jun 23]. Available from: https://www.who.int/docs/default-source/biologicals/vaccine-quality/48-pneumo-final-23april-2010.pdf?sfvrsn=6f700ed5_1.
- Footnote 7
-
Merck Canada Inc. Product Monograph: Pneumovax23 (pneumococcal vaccine, polyvalent, MSD Std.) [Internet]. Kirkland (QC): Merck Canada Inc.; 2016 Apr 15 [cited 2023 Jun 26]. Available from: https://pdf.hres.ca/dpd_pm/00035855.PDF.
- Footnote 8
-
GlaxoSmithKline Inc. Product Monograph: Synflorix Pneumococcal conjugate vaccine (Non-Typeable Haemophilus influenzae (NTHi) protein D, diphtheria or tetanus toxoid conjugates) adsorbed [Internet]. Mississauga (ON): GlaxoSmithKline Inc.; 2019 Nov 13 [cited 2023 Jun 26]. Available from: https://pdf.hres.ca/dpd_pm/00053908.PDF.
- Footnote 9
-
Pfizer Canada ULC. Product Monograph: Prevnar13 Pneumococcal 13-valent Conjugate Vaccine (Diphtheria CRM197 Protein) [Internet]. Kirkland (QC): Pfizer Canada ULC; 2019 Aug 08 [cited 2023 Jun 26]. Available from: https://pdf.hres.ca/dpd_pm/00052583.PDF.
- Footnote 10
-
Merck Canada Inc. Product Monograph: Vaxneuvance (Pneumococcal 15-valent Conjugate Vaccine [CRM197 Protein], adsorbed) [Internet]. Kirkland (QC): Merck Canada Inc.; 2022 Jul 08 [cited 2023 Jun 26]. Available from: https://pdf.hres.ca/dpd_pm/00066824.PDF.
- Footnote 11
-
Pfizer Canada ULC. Product Monograph: Prevnar20 Pneumococcal 20-valent Conjugate Vaccine (Diphtheria CRM197 Protein) [Internet]. Kirkland (QC): Pfizer Canada ULC; 2022 May 09 [cited 2023 Jun 26]. Available from: https://pdf.hres.ca/dpd_pm/00065729.PDF.
- Footnote 12
-
Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis. 2014 Sep 01;59(5):615-23. https://doi.org/10.1093/cid/ciu348.
- Footnote 13
-
National Center for Immunization and Respiratory Diseases. EtR framework for PCV20 use in children aged 2–18 years with certain underlying conditions that increase the risk of pneumococcal disease [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2023 Sep 12 [cited 2023 Nov 23]. Available from: https://www.cdc.gov/vaccines/acip/recs/grade/PCV20-child-risk-based-etr.html.
- Footnote 14
-
Nasreen S, Wang J, Sadarangani M, Kwong JC, Quach C, Crowcroft NS, et al. Estimating population-based incidence of community-acquired pneumonia and acute otitis media in children and adults in Ontario and British Columbia using health administrative data, 2005-2018: a Canadian Immunisation Research Network (CIRN) study. BMJ Open Respir Res. 2022 Jun;9(1):e001218. https://doi.org/10.1136/bmjresp-2022-001218.
- Footnote 15
-
King L. Pediatric outpatient ARI visits and antibiotic use attributable to serotypes in higher valency PCVs [slides presented at Advisory Committee on Immunization Practices (ACIP) meeting February 22, 2023] [Internet]. Atlanta (GA): Centers for Disease Control and Prevention (CDC); 2023 Feb 22 [cited 2023 Sep 12]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/Pneumococcal-03-King-508.pdf.
- Footnote 16
-
LeBlanc JJ, ElSherif M, Ye L, MacKinnon-Cameron D, Ambrose A, Hatchette TF, et al. Recalibrated estimates of non-bacteremic and bacteremic pneumococcal community acquired pneumonia in hospitalized Canadian adults from 2010 to 2017 with addition of an extended spectrum serotype-specific urine antigen detection assay. Vaccine. 2022 Apr 20;40(18):2635-46. https://doi.org/10.1016/j.vaccine.2022.02.081.
- Footnote 17
-
Kim SH, Jeon EJ, Hong SM, Bae CH, Lee HY, Park MK, et al. Bacterial Species and Antibiotic Sensitivity in Korean Patients Diagnosed with Acute Otitis Media and Otitis Media with Effusion. J Korean Med Sci. 2017 Apr;32(4):672-8. https://doi.org/10.3346/jkms.2017.32.4.672.
- Footnote 18
-
Merck Sharp & Dohme LLC. A Study to Evaluate the Safety, Tolerability, and Immunogenicity of V114 in Allogeneic Hematopoietic Stem Cell Transplant Recipients (V114-022/Pneu-STEM) [Internet]. Bethesda (MD): ClinicalTrials.gov; 2022 [cited 2023 Jun 26]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03565900.
- Footnote 19
-
Platt HL, Greenberg D, Tapiero B, Clifford RA, Klein NP, Hurley DC, et al. A Phase II Trial of Safety, Tolerability and Immunogenicity of V114, a 15-Valent Pneumococcal Conjugate Vaccine, Compared With 13-Valent Pneumococcal Conjugate Vaccine in Healthy Infants. Pediatr Infect Dis J. 2020 Aug;39(8):763-70. https://doi.org/10.1097/INF.0000000000002765.
- Footnote 20
-
Martinon-Torres F, Wysocki J, Szenborn L, Carmona-Martinez A, Poder A, Dagan R, et al. A Phase III, multicenter, randomized, double-blind, active comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of V114 compared with PCV13 in healthy infants (Pneu-PED-EU-1). Vaccine. 2023 May 16;41(21):3387-98. https://doi.org/10.1016/j.vaccine.2023.04.036.
- Footnote 21
-
Bili A, Dobson S, Quinones J, Phongsamart W, Oberdorfer P, Kosalaraksa P, et al. A phase 3, multicenter, randomized, double-blind study to evaluate the interchangeability of V114, a 15-valent pneumococcal conjugate vaccine, and PCV13 with respect to safety, tolerability, and immunogenicity in healthy infants (Pneu-DIRECTION). Vaccine. 2023 Jan 16;41(3):657-65. https://doi.org/10.1016/j.vaccine.2022.10.072.
- Footnote 22
-
Lupinacci R, Rupp R, Wittawatmongkol O, Jones J, Quinones J, Ulukol B, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (Pneu-PED). Vaccine. 2023 Jan 27;41(5):1142-52. https://doi.org/10.1016/j.vaccine.2022.12.054.
- Footnote 23
-
Senders S, Klein NP, Lamberth E, Thompson A, Drozd J, Trammel J, et al. Safety and Immunogenicity of a 20-valent Pneumococcal Conjugate Vaccine in Healthy Infants in the United States. Pediatr Infect Dis J. 2021 Oct 01;40(10):944-51. https://doi.org/10.1097/INF.0000000000003277.
- Footnote 24
-
Pfizer. 20-valent Pneumococcal Conjugate Vaccine Safety and Immunogenicity Study of a 4-Dose Series in Healthy Infants [Internet]. Bethesda (MD): ClinicalTrials.gov; 2022 Nov 03 [cited 2023 Jun 26]. Available from: https://clinicaltrials.gov/study/NCT04382326.
- Footnote 25
-
Pfizer. 20-valent Pneumococcal Conjugate Vaccine Safety and Immunogenicity Study of a 3-Dose Series in Healthy Infants [Internet]. Bethesda (MD): ClinicalTrials.gov; 2023 Jun 05 [cited 2023 Jun 26]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04546425.
- Footnote 26
-
Pfizer. 20-valent Pneumococcal Conjugate Vaccine Safety Study in Healthy Infants [Internet]. Bethesda (MD): ClinicalTrials.gov; 2023 Jun 13 [cited 2023 Jun 26]. Available from: https://clinicaltrials.gov/study/NCT04379713.
- Footnote 27
-
Banniettis N, Wysocki J, Szenborn L, Phongsamart W, Pitisuttithum P, Rämet M, et al. A phase III, multicenter, randomized, double-blind, active comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of catch-up vaccination regimens of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants, children, and adolescents (Pneu-PLAN). Vaccine. 2022 Oct 19;40(44):6315-25. https://doi.org/10.1016/j.vaccine.2022.09.003.
- Footnote 28
-
Quinn CT, Wiedmann RT, Jarovsky D, Lopez-Medina E, Rodriguez HM, Papa M, et al. Safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, in children with SCD: a V114-023 (Pneu-SICKLE) study. Blood Adv. 2023 Feb 14;7(3):414-21. https://doi.org/10.1182/bloodadvances.2022008037.
- Footnote 29
-
Wilck M, Barnabas S, Chokephaibulkit K, Violari A, Kosalaraksa P, Yesypenko S, et al. A phase 3 study of safety and immunogenicity of V114, a 15-valent PCV, followed by PPSV23, in children living with HIV. AIDS. 2023 Mar 20;37(8):1227-37. https://doi.org/10.1097/QAD.0000000000003551.
- Footnote 30
-
Merck Sharp & Dohme LLC. A Study to Evaluate the Safety and Tolerability of V114 and Prevnar 13™ in Healthy Infants (V114-031/Pneu-LINK) [Internet]. Bethesda (MD): ClinicalTrials.gov; 2022 [cited 2023 Jun 26]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03692871.
- Footnote 31
-
Pfizer. Safety and Immunogenicity Study of 20vPnC in Healthy Children 15 Months Through 17 Years of Age [Internet]. Bethesda (MD): ClinicalTrials.gov; 2023 Apr 26 [cited 2023 Jun 26]. Available from: https://clinicaltrials.gov/study/NCT04642079.
- Footnote 32
-
Ismail SJ, Hardy K, Tunis MC, Young K, Sicard N, Quach C. A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations. Vaccine. 2020 Aug 10;38(36):5861,5876. https://doi.org/10.1016/j.vaccine.2020.05.051.
- Footnote 33
-
Office of the High Commissioner for Human Rights. UN Declaration on the Rights of Indigenous Peoples [Internet]. Geneva (CH): United Nations; 2007 Sep 13 [cited 2023 Jun 23]. Available from: https://www.ohchr.org/en/indigenous-peoples/un-declaration-rights-indigenous-peoples.
- Footnote 34
-
Prasad N, Stoecker C, Xing W, Cho B, Leidner AJ, Kobayashi M. Public health impact and cost-effectiveness of 15-valent pneumococcal conjugate vaccine use among the pediatric population of the United States. Vaccine. 2023 May 02;41(18):2914-21. https://doi.org/10.1016/j.vaccine.2023.03.045.
- Footnote 35
-
Huang M, Hu T, Weaver J, Owusu-Edusei K, Elbasha E. Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population. Vaccines (Basel). 2023 Jan 06;11(1):135. https://doi.org/10.3390/vaccines11010135.
- Footnote 36
-
Ayabina D. Y. Summary of three economic analyses of the use of 20-valent pneumococcal conjugate vaccine (PCV20) in children in the United States [slides presented at Advisory Committee on Immunization Practices (ACIP) meeting June 22, 2023] [Internet]. Atlanta (GA): Centers for Disease Control and Prevention (CDC); 2023 Jun 22 [cited 2023 Nov 24]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-Pneumococcal-Ayabina-508.pdf.
- Footnote 37
-
De Wals P. Personal Communication. 2023.
