Statement on the prevention of respiratory syncytial virus disease in infants

Download in PDF format
(996 KB, 68 pages)
Organization: Public Health Agency of Canada
Date published: 2024-05-17
Cat.: HP40-355/1-2024E-PDF
ISSN: 978-0-660-74275-5
Pub.: 240619
Notice to reader
The following sentence, found in the Cost utility analysis, contained errors and has been corrected.
From: Year-round RSVpreF plus nirsevimab for infants at high-risk could be the optimal strategy if the price of nirsevimab was less than approximately $110 to $190 and the price of RSVpreF was greater than approximately $60 to $125 at a cost-effectiveness threshold of $50,000 per QALY.
Corrected to: Year-round RSVpreF plus nirsevimab for infants at high-risk could be the optimal strategy if the price of nirsevimab was greater than approximately $110 to $190 and the price of RSVpreF was less than approximately $60 to $125 at a cost-effectiveness threshold of $50,000 per QALY.
On this page
- Preamble
- Summary of information contained in this NACI statement
- Introduction
- Methods
- Epidemiology
- Medically attended RSV infection in infants and young children
- Hospitalization associated with RSV infection in infants and young children
- Intensive care unit admission associated with RSV infection in infants and young children
- Death associated with RSV infection in infants and young children
- RSV burden of disease in pregnant women and pregnant people
- RSV burden in northern and remote settings
- Immunization products
- Efficacy
- Efficacy of nirsevimab and RSVpreF vaccine against infant death due to RSV
- Efficacy of nirsevimab and RSVpreF vaccine against infant RSV tract infection with ICU admission
- Efficacy of nirsevimab and RSVpreF vaccine against respiratory tract infection with hospitalization
- Efficacy of nirsevimab and RSVpreF vaccine against medically attended RSV tract infection
- Immunization product administration and schedule
- Storage requirements
- Concurrent administration with other vaccines
- Immunization product safety
- Contraindications and precautions
- Immunization of specific populations
- Economics
- Ethics, equity, feasibility and acceptability considerations
- Recommendations
- Research priorities
- Surveillance issues
- Figure
- Tables
- List of abbreviations
- Acknowledgements
- References
Preamble
The National Advisory Committee on Immunization (NACI) is an External Advisory Body that provides the Public Health Agency of Canada (PHAC) with independent, ongoing and timely medical, scientific, and public health advice in response to questions from PHAC relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the systematic consideration of programmatic factors in developing evidence based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be systematically considered by NACI include: economics, ethics, equity, feasibility, and acceptability. Not all NACI statements will require in-depth analyses of all programmatic factors. While systematic consideration of programmatic factors will be conducted using evidence-informed tools to identify distinct issues that could impact decision-making for recommendation development, only distinct issues identified as being specific to the vaccine or vaccine-preventable disease will be included.
This statement contains NACI's independent advice and recommendations, which are based upon the best current available scientific knowledge. This document is being disseminated for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph. Recommendations for use and other information set out herein may differ from that set out in the product monographs of the Canadian manufacturers of the vaccines. Manufacturer(s) have sought approval of the vaccines and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Summary of information contained in this NACI statement
The following highlights key information for immunization providers. Please refer to the remainder of the statement for details.
What
Respiratory syncytial virus (RSV) is one of the most common respiratory viruses in infants and young children, infecting almost all children by the age of 2 years old. RSV can cause serious respiratory disease in infants, young children and older adults. RSV has a seasonal pattern of activity where infections are usually more common in the winter with variation in the timing and magnitude of the peak.
This statement focuses on the protection of infants and children from RSV disease. Health Canada has recently authorized two immunization products, both based on the pre-fusion stabilized F protein from RSV (RSVpreF), to protect infants from RSV using passive immunity:
- Nirsevimab (BEYFORTUSTM, Sanofi) is a monoclonal antibody authorized with an indication to directly protect all infants in their first RSV season and children who remain vulnerable to severe RSV disease in their second RSV season.
- RSVpreF (ABRYSVOTM, Pfizer) is a vaccine authorized with an indication to protect infants in their first RSV season through the passive transfer of maternal antibodies to the fetus by active immunization of a pregnant woman or pregnant person.
Who
Severe RSV disease is most common in young infants in their first months of life. Although the risk of severe RSV disease is higher in infants with certain medical conditions including prematurity (List 1), term infants account for the highest number of infants with severe RSV disease. Infants with certain medical conditions (List 1) remain at risk for severe RSV disease during their second RSV season.
The limited data currently available indicate that pregnancy does not appear to increase the risk of severe RSV disease in pregnant women or pregnant people. Immunization of this group though leads to maternal antibodies that cross the placenta and provide passive protection against severe RSV disease for the infant after birth. Immunization in pregnancy can therefore be used to protect the infant.
Considering the significant burden of disease in all infants from RSV and the impacts of RSV on the Canadian health system, NACI recommends building towards a universal RSV immunization program for all infants. Currently, nirsevimab is preferred over RSVpreF. Program introduction could occur in stages depending on access to supply, cost-effectiveness, and affordability of available options.
NACI recommends RSV immunization programs use nirsevimab to prevent severe RSV disease. Programs can build and expand over time depending on access to supply, cost-effectiveness, and affordability of available options. Nirsevimab should be prioritized for infants in the following way:
Priority 1:
- Infants entering, or born during, their first RSV season who are at increased risk of severe RSV disease, including those who are born at less than 37 weeks gestational age (wGA) (List 1).
- Infants entering their second RSV season and at ongoing increased risk of severe RSV disease (List 1).
- Infants entering, or born during, their first RSV season whose transportation for severe RSV disease treatment is complex, and/or whose risk of severe RSV disease intersects with established social and structural health determinants such as those experienced by some Indigenous communities across First Nations, Métis and Inuit populations.
Priority 2:
- If nirsevimab is priced in a manner to make such programs cost effective, NACI recommends nirsevimab be considered for any infant less than 8 months of age entering, or born during, their first RSV season through universal immunization programs to prevent severe RSV disease.
NACI recommends RSVpreF may be considered as an individual decision by a pregnant woman or pregnant person together with information from their pregnancy care provider, in advance of, or during, the RSV season, to prevent severe RSV disease in their infant. At the present time, NACI does not recommend an immunization program for RSVpreF. More data and information are expected to emerge over time and NACI will reconsider this recommendation in the future.
For administration of RSVpreF, consideration should be given to gestational timing and the start of the RSV season. For example, RSVpreF could be administrated starting in September to protect infants expected to be born during the RSV season in November, provided that gestational age is 32 weeks or greater at time of vaccination. For additional information, including supporting evidence and rationale for these recommendations, please see Recommendations.
List 1: Definition of infants at increased risk of severe RSV disease
Infants at increased risk of severe RSV disease during their first RSV season:
- All premature infants (i.e., born less than 37 wGA)
- Chronic lung disease, including bronchopulmonary dysplasia, requiring ongoing assisted ventilation, oxygen therapy or chronic medical therapy in the 6 months prior to the start of the RSV season
- Cystic fibrosis with respiratory involvement and/or growth delay
- Haemodynamically significant chronic cardiac disease
- Severe immunodeficiency
- Severe congenital airway anomalies impairing clearing of respiratory secretions
- Neuromuscular disease impairing clearing of respiratory secretions
- Down syndrome
Infants at ongoing risk of severe RSV disease during their second RSV season:
- All those listed above, except for infants born at less than 37 wGA and infants with Down syndrome who do not have another medical condition on the list.
How
Nirsevimab is administered intramuscularly using single-dose, prefilled syringes. For neonates and infants entering or in their first RSV season and weighing less than 5 kg, a single 0.5 mL dose (50 mg/0.5 mL) should be administered. For neonates and infants entering or in their first RSV season and weighing 5kg or more, a single 1 mL dose (100 mg/1 mL) should be administered. For children who remain vulnerable to severe RSV disease entering their second RSV season, the product monograph advises administration of 200 mg (2 x 100 mg/1 mL), divided between two injection sites.
RSVpreF is administered intramuscularly using single dose vials of lyophilized powder that are reconstituted with sterile water (diluent) in a prefilled syringe. A single 0.5 mL dose of RSVpreF is authorized for administration to pregnant women and pregnant people from 32 through 36 weeks of gestation to protect infants after birth until 6 months of age.
RSV exhibits a seasonal infection cycle that is somewhat variable by region. Prior to the COVID-19 pandemic, the RSV season in most of Canada was typically November to April. Jurisdictions are encouraged to define their RSV season and administer nirsevimab based on local epidemiology.
Why
RSV accounts for a significant burden of disease in infants and young children. RSV disease can have serious complications for infants, including hospitalization and intensive care unit (ICU) admission, as well as significant impact on caregivers and families. Nirsevimab and RSVpreF can help protect an infant from RSV disease by giving the infant antibodies, either via direct injection or transplacental transfer. Furthermore, reducing severe outcomes from RSV in infants at the population level may help to protect health system capacity. The prioritization of certain populations, such as infants with medical risk factors, infants who would require complex transportation for severe RSV disease, and equity-seeking groups such as some Indigenous communities, is cost-effective and may promote health equity.
Introduction
Guidance objective
The need for updated NACI guidance on the pediatric RSV vaccine program arose from the authorizations of two new products with pediatric indications. On April 19, 2023, Health Canada authorized the use of nirsevimab (BEYFORTUS, Sanofi) a novel monoclonal antibody for passive immunization of infants in their first RSV season and children under 24 months of age for the prevention of RSV lower respiratory tract disease. On December 21, 2023, Health Canada authorized the use of RSVpreF (ABRYSVO, Pfizer), a novel unadjuvanted subunit protein vaccine for passive immunization of infants via vaccination of pregnant women and pregnant people from 32 through 36 weeks of gestation.
This statement aligns with the earlier guidance provided by Canada's Drug and Health Technology Agency (CADTH) in July 2023 regarding the use of nirsevimab for RSV prevention in neonates and infants with certain high-risk conditions for the 2023-2024 respiratory virus seasonFootnote 1. A statement on June 2022 from NACI updated recommendations for the use of palivizumab (SYNAGIS®, AstraZeneca), a previous generation of monoclonal antibody for passive protection of infants with certain high-risk conditions (PDF). Nirsevimab differs from palivizumab in several ways, including the dosing interval. Palivizumab requires monthly dosing whereas one dose of nirsevimab may be protective for an entire RSV season.
The primary objectives of this statement are to:
- review the evidence on the potential benefits (efficacy), potential harms (safety) and cost-effectiveness of RSV immunization programs in Canada
- describe the ethics, equity, feasibility, and acceptability considerations for RSV immunization programs
- provide recommendations for the use of nirsevimab and RSVpreF immunizing products in Canada, including identifying groups that may be at increased risk of severe RSV disease and therefore would benefit the most from these products.
Background on RSV immunization programs in Canada
In Canada, palivizumab (SYNAGIS) has been authorized since May 2002 and available only as part of a narrowly targeted program for the highest risk infants with certain high-risk conditions, often administered outside the traditional public health immunization programs. The introduction of nirsevimab (BEYFORTUS) and RSVpreF (ABRYSVO) to the RSV product environment in Canada provides the opportunity to identify populations that may benefit from RSV prevention other than those where palivizumab is already used.
For the 2023-2024 season, CADTH developed guidance for the use of nirsevimab in high-risk populations. This NACI guidance for the prevention of RSV in infants is intended to replace this advice and provide guidance for physicians and program developers for immunizing products available to infants and pregnant women and pregnant people to protect their infants through transplacental transmission of antibodies. This NACI advice is relevant to all currently available products to prevent RSV, including nirsevimab, palivizumab and RSVpreFFootnote 1.
NACI will separately publish recommendations for the use of vaccines to prevent severe RSV disease in adults.
A note on language
NACI recognizes that not all people giving birth or breastfeeding will identify as women or mothers. The writing in this statement uses a gender additive approach where the term 'woman' is used alongside gender-neutral language. This is intended to demonstrate a commitment to redress the historic exclusion of trans and non-binary people, whilst avoiding the risk of marginalising or erasing the experience of women within the healthcare environment. However, in line with best practice, it is recognized that when discussing or caring for individuals in a one-on-one capacity language and documentation should reflect the gender identity of the individual.
In addition, much of the research available currently refers only to "women" when discussing pregnancy. When citing research, NACI refers to the language used in the study. In these cases, "woman" refers to someone who was assigned female at birth and "maternal" is used to identify the person who is pregnant or postpartum. For the purposes of this statement, the terms "woman", "women", and "maternal" should be considered to also apply to those individuals who do not specifically identify as female gender but are the parent gestating the fetus or breastfeeding or chestfeeding the infant.
Finally, NACI acknowledges the dynamic nature of language. It is likely that language deemed to be suitable or affirming in one context may not translate across others, and over the coming years will likely change and evolve with respect to appropriate representations.
Methods
In brief, the broad stages in the preparation of this NACI advisory committee statement were:
- analysis of burden of disease of RSV in children less than 24 months of age and in pregnant women and pregnant people
- retrieval and summary of individual studies of RSV prophylaxis, evidence synthesis including meta-analysis when appropriate, assessment of the quality of the evidence by the NACI Secretariat – summarized in the Summary of findings Table 4, Table 5, Table 6, Table 7.
- synthesis of the body of evidence of benefits and harms of nirsevimab and RSVpreF, considering the quality of the synthesized evidence and magnitude of effects observed across the studies
- use of a published, peer-reviewed framework and evidence-informed tools to ensure that issues related to ethics, equity, feasibility, and acceptability (EEFA) are systematically assessed and integrated into the guidance
- use of two systematic reviews and a de novo model-based economic evaluation of RSVpreF and nirsevimab for prevention of RSV-related outcomes in Canadian infants to generate economic evidence and
- translation of evidence into a recommendation
Further information on NACI's evidence-based methods is available elsewhere.
A framework has been developed to facilitate systematic consideration of programmatic factors (now included in NACI's mandate which includes ethics, equity, feasibility, acceptability and economics) in developing clear, evidence-based recommendations for timely, transparent decision-makingFootnote 2. This framework provides a clear outline with accompanying evidence informed tools to consider relevant aspects of each programmatic factor that may have an impact on the implementation of NACI recommendations. These tools have been completed by the PHAC NACI Secretariat and integrated into the statement.
For this advisory committee statement, NACI reviewed the key questions for the literature review as proposed by the RSV Working Group, including such considerations as the burden of illness to be prevented and the target population(s), safety, immunogenicity, efficacy, effectiveness, economic evaluation of the immunization product(s), immunization schedules, and other aspects of the overall immunization strategy. The knowledge synthesis was performed by the NACI Secretariat and supervised by the RSV Working Group. Where appropriate, results of individual trials were pooled in a meta-analysis using random effects model, except when calculating Peto odds ratios where fixed effect models were used, in RevMan by the NACI SecretariatFootnote 3.
Following critical appraisal of individual studies, summary tables with ratings of the certainty of the evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology were preparedFootnote 4Footnote 5Footnote 6. An assessment using the Evidence to Decision (EtD) framework was prepared for each question, and proposed recommendations for vaccine use were developed. The NACI Vaccine Safety Working Group reviewed and discussed the evidence on the safety of RSVpreF vaccine in pregnant women and pregnant people on October 26, 2023Footnote 7. NACI reviewed the available evidence as of January 28, 2024. The description of relevant considerations, rationale for specific decisions, and knowledge gaps are described.
The policy questions addressed in this statement are:
- What is the best use of nirsevimab to protect all infants entering their first RSV season from severe clinical outcomes due to RSV?
- What is the best use of nirsevimab to protect high-risk infants entering their first RSV season from severe clinical outcomes due to RSV?
- What is the best use of nirsevimab to protect high-risk infants entering their second RSV season from severe clinical outcomes due to RSV?
- What is the best use of RSVpreF in pregnant women and pregnant people to protect their infants from severe clinical outcomes due to RSV?
Epidemiology
RSV is an enveloped RNA virus classified within the Paramyxoviridae family. There are two subgroups based on differences in the G surface protein. The F surface protein has more limited variability between RSV A and B subgroups. Humans are the only source of infection and transmission occurs from direct or indirect exposure to respiratory secretions containing the virusFootnote 8. RSV is one of the most common respiratory viruses in infants and young children, infecting almost all children by the age of 2 years oldFootnote 9. Globally, in children aged 0 to 60 months, RSV is responsible for 31% of pneumonia cases and causes 33 million acute lower respiratory tract infections (LRTIs), 3.6 million acute LRTI hospitalizations, and 101,400 deaths per yearFootnote 10. The most common clinical presentations of RSV in young children requiring hospitalization are bronchiolitis (an acute LRTI associated with tachypnea, cough, and wheezing), and pneumonia. Primary infection does not confer complete protective immunity against re-infections, which recur throughout life. Reinfections tend to be less severe, except for in older adultsFootnote 8.
A recent rapid review highlighted RSV's impact on infants and young children, informing the 2022 NACI statement on palivizumab in infantsFootnote 11. This rapid review identified burden of illness in infants at risk for severe outcomes and complications with RSV, including prematurity, Down syndrome, chronic lung disease, congenital heart disease, congenital airway disorders, and immunocompromiseFootnote 11. More recently, a rapid review has been published regarding burden of disease in healthy infants, young children, and pregnant women and pregnant peopleFootnote 12. A detailed publication of these results is available in a Canada Communicable Disease Report (CCDR) and a summary of this rapid review is presented below.
RSV seasonality in Canada is typically November to April with peak incidence of cases in January and/or February. However, some differences were noted during the COVID-19 pandemic, and seasonality can vary by jurisdictionFootnote 13Footnote 14Footnote 15Footnote 16.
Medically attended RSV infection in infants and young children
There is a high incidence of medically attended RSV infections in healthy infants and young children, with RSV accounting for 10 to 20% of medically attended respiratory tract infections in infantsFootnote 12. In one United States (US) study, the burden of medically attended RSV was higher than that of medically attended influenzaFootnote 17. The risk of medically attended RSV appears to be higher in infants with comorbidities compared to healthy term infants entering their first RSV seasonFootnote 18. However, the majority of infants and young children who require medical office or emergency department visits associated with RSV have no underlying comorbiditiesFootnote 19. For infants with comorbidities entering their second RSV season there are not enough data to inform baseline risk of medically attended RSV infectionsFootnote 12.
Hospitalization associated with RSV infection in infants and young children
The range of annual hospitalizations for RSV-associated respiratory infections varies between seasons and studies but rates consistently decrease with increasing chronologic ageFootnote 12. The range of annual hospitalization rates for RSV-associated acute respiratory infections has varied in studies of healthy infants and young children from 5 to 28 per 1,000 in infants less than 6 months, 3 to 13 per 1,000 in infants 6 to 12 months, and 2.5 to 5 per 1,000 in children 1 to 5 years of ageFootnote 12. Compared with influenza, RSV causes up to 16 times more hospitalizations in young childrenFootnote 17Footnote 20Footnote 21Footnote 22. For infants with comorbidities entering their first or second RSV season, rates of RSV-associated hospitalizations are higher than for infants and young children without comorbiditiesFootnote 11Footnote 12. While the risk of hospitalization is higher in the presence of comorbidities, the majority of infants and young children hospitalized with RSV have no underlying comorbiditiesFootnote 12Footnote 22.
Intensive care unit admission associated with RSV infection in infants and young children
Approximately 5% of healthy infants and young children hospitalized with RSV require ICU admissionFootnote 12. In one Canadian study, ICU admission was more common with RSV than with influenzaFootnote 23. Among infants and young children hospitalized with RSV during their first or second RSV season, comorbidities are associated with greater risk of ICU admissionFootnote 11Footnote 12.
Death associated with RSV infection in infants and young children
There are very limited data on the burden of death in healthy infants and young children associated with RSV although available literature suggests the risk is very low (6.9 per 1 million live births) in high income countries including CanadaFootnote 12Footnote 24. For infants with comorbidities entering their first or second RSV season, there is a higher risk of mortality compared to healthy infantsFootnote 11Footnote 12. However, a greater absolute number of term children die due to RSV compared to infants with high-risk conditionsFootnote 12Footnote 24.
RSV burden of disease in pregnant women and pregnant people
Overall, there has been limited study of the impact of RSV disease on pregnant women and pregnant people themselves or their pregnancy outcomes. RSV burden of disease appears to be similar in pregnant versus non-pregnant women and non-pregnant people of equivalent age. Of pregnant women and pregnant people with respiratory symptoms, 10% to 13% have confirmed RSVFootnote 12Footnote 25. In one study there were higher hospitalization rates among pregnant women and pregnant people compared to non-pregnant adults with RSV, but the limited available literature demonstrates a wide range of RSV-associated hospitalization rates among pregnant women and pregnant peopleFootnote 12Footnote 26. There are not enough data to inform risk of ICU admission or death in pregnant women and pregnant people associated with RSV, nor preterm birth related to RSV infection in pregnant women and pregnant peopleFootnote 12.
RSV burden in northern and remote settings
While data are limited, both Canadian surveillance data and primary literature demonstrates a higher burden of RSV admissions in northern and remote settings compared to the rest of Canada. Canadian administrative hospitalization data (from September 2014 to August 2023) generally show higher rates of hospitalizations for the territories compared to the rest of Canada in most RSV seasons among infants less than 1 year of age and in the 0 to 4 year old age groups (with much higher rates in the less than 1 year old compared to the 0 to 4 year old group). Among the territories, higher rates were much more pronounced in the Northwest Territories in most seasonsFootnote 27. Primary surveillance data from the Yukon over five respiratory seasons (2018-2023) demonstrated a total of 73 RSV infections in children less than 24 months of age, and of these, 27 were severe cases. The number of RSV infections reported during this time period were higher than the number of influenza or COVID-19 infections (no denominator); burden was higher for RSV than for COVID-19 or influenzaFootnote 28. Studies have demonstrated RSV hospitalization rates of 5.0% for all infants less than 1 year of age (7.3% after adjustment for under detection) in Nunavik and 16.6% in Baffin Island compared to approximately 2% in other Canadian populationsFootnote 29Footnote 30. A recent study from Nunavut demonstrated a yearly RSV hospitalization incidence rate of 37.8 per 1,000 infants; among RSV hospitalizations, 41.1% were infants 0 to 2 months of ageFootnote 31.
In addition, there is a greater cost of RSV care to the healthcare system in these remote locations due to the need for air transportation of infants to regional hospitals or to tertiary care settings.
Immunization products
Preparation(s) authorized for use in Canada
Characteristics of the RSV immunizing agents currently authorized for use in Canada to prevent RSV disease in infants are summarized in Table 1.
| SYNAGIS® (palivizumab)Footnote 32 | BEYFORTUSTM (nirsevimab)Footnote 33 | ABRYSVOTM (RSVpreF)Footnote 34 | |
|---|---|---|---|
| Manufacturer | AstraZeneca | Sanofi | Pfizer |
| Date of authorization in Canada | May 15, 2002 | April 19, 2023 | December 21, 2023 |
| Type of immunization product | Monoclonal antibody | Monoclonal antibody | Stabilized subunit vaccine |
| Composition | Palivizumab (100 mg/mL), chloride, glycine, histidine, and water for injection | Nirsevimab (100mg/mL), L-arginine hydrochloride, L-histidine, L-histidine hydrochloride, polysorbate 80, sucrose, water for injection | Lyophilized powder containing 120 mcg of RSV stabilized prefusion F protein (60 mcg each of subgroup A and subgroup B antigens), 22.5 mg mannitol, 0.08 mg polysorbate 80, 1.1 mg sodium chloride, 11.3 mg sucrose, 0.11 mg tromethamine, 1.04 mg trometamol hydrochloride reconstituted with sterile water (diluent) |
| Schedule | Maximum 5-dose schedule (every 28 to 30 days during anticipated periods of RSV risk) | 1 dose schedule | 1 dose schedule |
| Route of administration | Intramuscular injection | Intramuscular injection | Intramuscular injection |
| Indications |
Authorized for the prevention of serious lower respiratory disease caused by RSV in pediatric patients up to 24 months at high risk of RSV disease, which includes infants with:
|
Authorized for the prevention of RSV lower respiratory tract disease in:
|
Authorized for active immunization of pregnant women and pregnant people from 32 through 36 weeks of gestation for the prevention of lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in infants from birth through 6 months of age. |
| Contraindications | Infants with known hypersensitivity to palivizumab injection or to any of its excipients and in patients with known hypersensitivity to other humanized monoclonal antibodies. | Infants with a history of severe hypersensitivity reactions, including anaphylaxis, to this drug or to any ingredients in the formulation, including any non-medicinal ingredient, or component of the container. | Individuals who are hypersensitive to the active substance or to any component of the vaccine |
| Precautions | Not indicated for adult use. | Not indicated for adult use. |
|
| Storage Requirements | Single use vials. Store in a refrigerator between 2°C and 8°C in its original container. Do not freeze. | Single dose pre-filled syringe. Store in a refrigerator between 2°C and 8°C. Keep the pre-filled syringe in the outer carton to protect from the light. Do not freeze, shake, or expose to heat. May be kept at room temperature (20°C – 25°C) for a maximum of 8 hours after removal from the refrigerator. | Single dose vials and pre-filled syringe with diluent. Store the unreconstituted vaccine in a refrigerator between 2°C and 8°C. Do not freeze. After reconstitution, ABRYSVO should be administered immediately (within 4 hours). Reconstituted vaccine should be stored between 15°C and 30°C. |
For complete prescribing information for BEYFORTUS, ABRYSVO and SYNAGIS, consult the product leaflet or information contained within Health Canada's authorized product monographs available through the Drug Product Database (DPD).
Efficacy
Among infants entering their first RSV season, the evidence suggests that nirsevimab results in a reduction in ICU admission associated with RSV, hospitalization associated with RSV, and medically attended RSV respiratory tract infection (RTI). In infants considered at high risk entering their first and second RSV season, the evidence suggests nirsevimab likely results in a reduction in ICU admission associated with RSV, hospitalization associated with RSV, and medically attended RSV RTI compared to palivizumab. However, there was limited evidence on the effect of nirsevimab against death due to RSV in infants.
Among infants entering their first RSV season, the evidence suggests RSVpreF vaccine administered to pregnant women and pregnant people results in a reduction in ICU admission associated with RSV, hospitalization associated with RSV, and medically attended RSV RTI in their infants. However, there was limited evidence available on the effect of RSVpreF vaccine against death due to RSV in infants.
Evidence on the efficacy of nirsevimab, a long-acting monoclonal antibody, in infants and young children to protect from severe clinical outcomes due to RSV were derived from 3 randomized controlled trials (RCTs). One phase IIb RCTFootnote 35 was conducted among healthy preterm infants born from 290/7 through 346/7 wGA entering their first full RSV season who received nirsevimab (n=570) or a placebo (n=290); of note, given that the weight-banded dosage was introduced after this trial, only infants weighing less than 5kg were included in the evidence synthesis if they received the dose of nirsevimab that was ultimately approvedFootnote 36. One phase III RCT (MELODY) was conducted among healthy late-preterm and term infants born on or after 35 wGA entering their first full RSV season who received nirsevimab (n=2,009) or a placebo (n=1,003)Footnote 37. One phase II/III RCT (MEDLEY) was conducted among infants (n=925) entering their first RSV season who were eligible to receive palivizumab, which included infants enrolled in the preterm cohort or the congenital heart disease (CHD) or chronic lung disease (CLD) of prematurity cohortFootnote 38. Infants enrolled into the preterm cohort included infants born on or before 350/7 wGA who did not have CHD or CLD of prematurity who received nirsevimab (n=407) or palivizumab (n=208). Infants enrolled in the CHD-CLD cohort included infants who had uncorrected, partially corrected or medically treated CHD or CLD requiring therapeutic intervention within the previous six months who received nirsevimab (n=209) or palivizumab (n=101). Infants in the CHD-CLD cohort were also followed in their second season; those who received nirsevimab in their first RSV season were given nirsevimab in their second RSV season (n=180), while those who received palivizumab in their first RSV season were randomized to nirsevimab (n=40) or palivizumab (n=42) for their second RSV seasonFootnote 39.
Data on the efficacy of nirsevimab are only available up to 150 days post-dose, and the effect of waning beyond this point is unclear. Available data suggest that a single dose of nirsevimab provided a consistent level of protection over 150 days and based on pharmacokinetic data, nirsevimab is expected to be effective up to 8 months following administration, but data are not yet availableFootnote 35Footnote 37Footnote 40. More information is needed on the efficacy of nirsevimab to prevent RSV disease in infants beyond 150 days.
Moreover, a recent phase IIIb open-label trial (HARMONIE) was conducted among healthy infants who were 12 months of age or younger, were born at a gestational age of 290/7 weeks or more, and were entering their first RSV season (n=8,058)Footnote 41. This study compared the use of nirsevimab (n=4,037) to the standard of care (no intervention, n=4,021). The results from this study were not available at the time of the GRADE analysis and therefore not included in these analysis but are described in this statement.
Evidence on the efficacy of RSVpreF vaccine, an unadjuvanted bivalent prefusion F protein vaccine administered during pregnancy for the prevention of severe clinical outcomes due to RSV in infants, were derived from two RCTs. One phase IIb study was conducted among healthy pregnant women from 18 through 49 years of age during their late second and third trimester of gestation (240/7 to 360/7 wGA) who received RSVpreF (n=327) or placebo (n=79)Footnote 42. At birth, their infants were enrolled into the corresponding group (n=325 infants born to pregnant participants who received RSVpreF and n=78 infants born to pregnant participants who received placebo). One phase III RCT (MATISSE) was conducted among healthy pregnant women 49 years of age and younger at the same gestational age range who received RSVpreF (n=3695) or placebo (n=3676)Footnote 43. At birth, their infants were enrolled into the corresponding group (n=3570 infants born to pregnant participants who received RSVpreF and n=3558 infants born to pregnant participants who received placebo). No efficacy data was available for prevention of RSV in the pregnant women themselves in these two RCTs. Therefore, the efficacy of RSVpreF vaccine in pregnant women and pregnant people was not evaluated and remains unknown at this time.
Data on the efficacy of RSVpreF vaccine among infants from the phase III RCT (MATISSE) was available up to 180 days after birth and interim estimates were conducted at 90, 120, and 150 days after birth. Although the confidence intervals (CIs) were large and overlapped, suggesting that estimates might not be statistically different, RSVpreF vaccine efficacy (VE) in infants decreases with increasing time after birth with the highest efficacy estimate observed at 90 days and the lowest at 180 days after birth. The clinical significance of the difference observed in VE between 90 and 180 days after birth is unknown, but RSVpreF VE may not exceed 6 months of age in infants due to waning of the effects of levels of passively transferred antibodies in neonates.
Efficacy of nirsevimab and RSVpreF vaccine against infant death due to RSV
There is limited evidence on the efficacy of nirsevimab and RSVpreF vaccine for the prevention of death due to RSV infection among infants.
There were no deaths due to RSV in the two RCTs evaluating the efficacy of nirsevimab in infants entering their first RSV season (n=3,872; 2,579 in the nirsevimab group and 1,293 in the placebo group) (Table 4), and the other RCT among high-risk infants (as defined in the study, see definition above) entering their first (n=918; 614 in the nirsevimab group and 304 in the palivizumab group) (Table 5) and second (n=262; 220 in the nirsevimab group and 42 in the palivizumab group) (Table 6) RSV seasonsFootnote 35Footnote 37Footnote 38Footnote 39. Moreover, no deaths were reported in the additional phase IIIb trial among infants entering their first RSV seasonFootnote 41.
In the RCTs evaluating the efficacy of RSVpreF vaccine, one death due to RSV occurred in a healthy infant born at 401/7 wGA in the placebo group (n=3,665) and none occurred in the RSVpreF group (n=3,675) (Table 7)Footnote 42Footnote 43.
Of note, both nirsevimab and RSVpreF clinical trials were not powered to assess this endpoint, consequently efficacy assessments for death due to RSV in infants were downgraded for imprecision.
Efficacy of nirsevimab and RSVpreF vaccine against infant RSV tract infection with ICU admission
The available evidence suggests that nirsevimab and RSVpreF vaccine likely reduce the risk of ICU admission due to RSV infection in infants. However, the evidence is limited due to the small number of events reported in each trial.
A meta-analysis of 2 RCTs demonstrated a beneficial protective effect of nirsevimab against RSV RTI requiring ICU admission when compared to a placebo (pooled efficacy of 90%; 95% CI: 54% to 98%) in infants entering their first RSV season which was deemed at moderate certainty (Table 4)Footnote 35Footnote 37Footnote 44. No ICU admission occurred among high-risk infants entering their first (n=925; 616 in the nirsevimab group and 309 in the palivizumab group) or second (n=262; 220 in the nirsevimab group and 42 in the palivizumab group) RSV seasonFootnote 38Footnote 39. The evidence for nirsevimab preventing ICU admission due to RSV in high-risk infants in their first RSV season (Table 5) and second RSV season (Table 6) was deemed at moderate certainty due to imprecision.
One RCT evaluating the efficacy of RSVpreF vaccine against RSV RTI with ICU admission in infant participants reported a VE of 43% (95% CI: -125% to 88%) which was deemed at low certainty due to imprecision (Table 7)Footnote 43Footnote 45. No data were provided on RSV RTI with ICU admission in the RSVpreF vaccine phase IIb RCTFootnote 42.
Efficacy of nirsevimab and RSVpreF vaccine against respiratory tract infection with hospitalization
The available evidence suggests that nirsevimab and RSVpreF vaccine reduce the risk of hospitalization due to RSV infection in infants.
A meta-analysis of RCTs demonstrated a beneficial protective effect of nirsevimab against RSV RTI with hospitalization in infants entering their first RSV season when compared to a placebo (pooled efficacy of 81%; 95% CI: 64% to 90%)Footnote 35Footnote 37Footnote 46. The evidence for nirsevimab was deemed at moderate certainty due to imprecision (Table 4). The phase IIIb study found similar results with an efficacy of 83.2% (95% CI: 67.8% to 92.0%) against hospitalization for RSV RTI for nirsevimab compared to the standard of care (no intervention) in healthy infants entering their first RSV seasonFootnote 41. One RCT reported an efficacy of 53% (95% CI: -279% to 94%) for nirsevimab compared to palivizumab in high-risk infants entering their first RSV seasonFootnote 38. The evidence was deemed at low certainty due to imprecision (Table 5). No RSV RTI with hospitalization occurred among high-risk infants entering their second RSV season (Table 6)Footnote 39Footnote 47.
One RCT evaluating the efficacy of RSVpreF vaccine against RSV RTI with hospitalization demonstrated a beneficial protective effect in infants entering their first RSV season when compared to placebo (VE of 57%; 99.17% CI: 10% to 81%)Footnote 43. The evidence was deemed at moderate certainty due to imprecision (Table 7). No data were provided on RSV RTI with hospitalization in the RSVpreF vaccine phase IIb RCTFootnote 42.
Efficacy of nirsevimab and RSVpreF vaccine against medically attended RSV tract infection
The available evidence suggests that nirsevimab and RSVpreF vaccine reduce the risk of medically attended RSV infection in infants.
A meta-analysis of two RCTs demonstrated a beneficial protective effect of nirsevimab against medically attended RSV RTI in infants entering their first RSV season when compared to a placebo (efficacy of 80%; 95% CI: 70 to 87%)Footnote 35Footnote 37Footnote 48. The evidence for nirsevimab efficacy was deemed at moderate certainty due to imprecision (Table 4). One RCT reported an efficacy of 33% (95% CI: -197 to 85%) for nirsevimab compared to palivizumab in high-risk infants entering their first RSV seasonFootnote 38. The evidence for nirsevimab compared to palivizumab efficacy was deemed at moderate certainty due to imprecision (Table 5). No medically attended RSV RTI occurred among high-risk infants entering their second RSV season in either the nirsevimab or the palivizumab group (Table 6)Footnote 39.
No data were provided on medically attended RSV RTI for infants in the RSVpreF vaccine phase IIb RCTFootnote 42. The Phase III RCT evaluating the efficacy of RSVpreF vaccine against medically attended RSV RTI demonstrated a beneficial protective effect in infants entering their first RSV season when compared to placebo (VE of 51%: 97.58% CI: 29 to 67%)Footnote 43. The evidence was deemed at high certainty (Table 7).
Immunization product administration and schedule
Nirsevimab
Nirsevimab is supplied in 50 mg and 100 mg single-dose, prefilled syringes. For neonates and infants entering or during their first RSV season and weighing less than 5 kg, a 0.5 mL dose (50 mg/0.5 mL) should be administered intramuscularly. For neonates and infants entering or during their first RSV season and weighing 5kg or more, a 1 mL dose (100 mg/1 mL) should be administered intramuscularly. For children who remain vulnerable to severe RSV disease entering their second RSV season, the product monograph advises a single dose of 200 mg (2 x 100 mg/1 mL) should be administered intramuscularly using two different injection sites. However, if the child weighs less than 10 kg entering their second RSV season, consideration can be given to administering a single dose of 100 mg at clinical discretion. Reimmunization is indicated for individuals undergoing cardiac surgery with cardiopulmonary bypass as soon as the individual is stable after surgery. Although not indicated in the product monograph, reimmunization can also be considered at conclusion of extracorporeal membrane oxygenationFootnote 49Footnote 50. If within 90 days after receiving the first dose of nirsevimab, the additional dose during the first RSV season should be 50 mg or 100 mg according to body weight, or 200 mg in the second RSV season (consideration can be given to administering 100 mg if the child weighs less than 10 kg in the second season). If more than 90 days since the first dose, the additional dose should be a single dose of 50 mg regardless of body weight during the first RSV season, or 100 mg during the second RSV season, to cover the remainder of the RSV season. Please see the product monograph for more detailsFootnote 33.
RSVpreF
RSVpreF is supplied as a single dose vial of lyophilized powder that is reconstituted with sterile water (diluent) in a prefilled syringe. A 0.5 mL dose of RSVpreF should be administered intramuscularly. The standard schedule for pregnant women and pregnant people from 32 through 36 weeks of gestation is one dose. Please see the product monograph for more detailsFootnote 34. If a dose of vaccine is inadvertently administered between 24 and 32 weeks of gestation, this should be considered a valid dose and an additional should not be administered. There are no data on the safety or efficacy of RSVpreF before 24 or after 36 weeks of gestation.
The need for revaccination of pregnant women and pregnant people with RSVpreF in subsequent pregnancies has not been established. No data are available on the efficacy or safety of additional doses of RSVpreF administered during subsequent pregnancies. NACI will update this information as needed as more evidence becomes available.
Storage requirements
Nirsevimab should be refrigerated at 2°C to 8°C. Do not freeze, shake, or expose to heat. Protect the syringe from light. After removal from the refrigerator, nirsevimab may be kept at room temperature (20°C to 25°C) for a maximum of 8 hours or discardedFootnote 33.
RSVpreF should be refrigerated at 2°C to 8°C. Do not freeze; discard if the vaccine has been frozen. Protect the vaccine from light. After reconstitution, RSVpreF should be stored between 15°C and 30°C and administered within 4 hoursFootnote 34.
Concurrent administration with other vaccines
Nirsevimab
Nirsevimab can be administered on the same day, or at any time before or after, routine childhood vaccines. Given that the monoclonal antibody targets a specific antigen, nirsevimab would not be expected to interfere with immunizations for protection from other infectionsFootnote 51. Although there are limited data available, when nirsevimab was given to healthy preterm and term infants with 7 prespecified vaccine groups (tuberculosis vaccine; influenza vaccine; measles/mumps/rubella/varicella vaccine; rotavirus vaccine; polyvalent diphtheria, pertussis, tetanus [DPT]-containing vaccine; pneumococcal vaccine; and Hepatitis B vaccine) on the same day, ±7 days, or ±14 days, the safety and reactogenicity profile was similar to the vaccines given aloneFootnote 33Footnote 52.
RSVpreF
RSVpreF is a recombinant protein subunit vaccine and is not live. Concurrent administration of RSVpreF to pregnant women and pregnant people with other recommended vaccines can be considered according to basic vaccine principles outlining that, in general, non-live vaccines may be administered concurrently with, or at any time before or after, other vaccinesFootnote 53.
Concurrent administration of RSVpreF with the tetanus, diphtheria, and acellular pertussis vaccine (Tdap) in healthy, non-pregnant women 18 to 49 years of age has been shown to be safe and immunogenicity data demonstrated non-inferiority was met for the tetanus and diphtheria components of the Tdap vaccine, as well as the RSV-A and RSV-B components of the RSVpreF vaccine. For the pertussis component, at one month after vaccination the ratios of anti-pertussis toxin (PT), anti-filamentous hemagglutinin (FHA), and anti-pertactin (PRN) antibody geometric mean concentrations (GMCs) for the combined RSVpreF and Tdap groups relative to the corresponding GMCs for the placebo and Tdap group were 0.80 (95% CI: 0.64 to 1.00), 0.59 (95% CI: 0.50 to 0.70), and 0.60 (95% CI: 0.48 to 0.76), respectivelyFootnote 54. Thus, compared with the criterion of 0.67, non-inferiority was not established for the pertussis component of the Tdap vaccine. For the RSV-A and RSV-B components, the geometric mean ratios (GMRs) for the combined RSVpreF and Tdap groups relative to the corresponding GMRs for the placebo and Tdap group were 0.97 (95% CI: 0.84 to 1.13) and 0.96 (95% CI: 0.81 to 1.08)Footnote 54. Thus, compared with both the criterion of 0.5 for the primary objective and 0.67 for the secondary objective, non-inferiority was established for both components.
When concurrently administered with the quadrivalent seasonal inactivated influenza vaccine (SIIV) in healthy participants aged 18 to 49 years, the RSVpreF vaccine was safe and well-tolerated but SIIV immune responses trended lower across all strainsFootnote 55Footnote 56Footnote 57. The GMRs at 1 month after SIIV for concurrent (RSVpreF+SIIV) to sequential (SIIV alone 1 month after RSVpreF) were 0.55 (95% CI: 0.32 to 0.93) for A/Michigan/45/2015 (H1N1) pdm09-like, 0.71 (95% CI: 0.40 to 1.27) for A/Singapore/INFIMH-16-0019/2016 A(H3N2)-like, 0.48 (95% CI: 0.30 to 0.78) for B/Colorado/06/2017-like (Victoria lineage), and 0.80 (95% CI: 0.49 to 1.29) for B/Phuket/3073/2013-like (Yamagata lineage)Footnote 55. Although there are limited data, influenza and RSV vaccines can be administered concurrently. No data are available on concurrent administration of RSVpreF with vaccines other than Tdap and influenza.
Immunization product safety
Nirsevimab
Overall, the evidence suggests that among infants entering their first RSV season, nirsevimab is not likely to increase the risk of severe systemic and local adverse events (AEs) compared to placebo. In infants considered at high risk entering their first and second RSV season, nirsevimab is not likely to increase the risk of severe systemic and local AEs when compared to palivizumab. Moreover, no meaningful differences in serious AEs were observed when nirsevimab is compared to placebo or to palivizumab. Details are presented below.
Evidence on the safety of nirsevimab in infants and young children to protect from severe clinical outcomes due to RSV were derived from the three RCTs previously describedFootnote 35Footnote 37Footnote 38. Results from the additional phase IIIb study were not included in the GRADE analyses but are presented in the statementFootnote 41.
RSVpreF
Among pregnant women and pregnant people, RSVpreF vaccine may not result in an increase in severe systemic AEs but may increase the risk of severe local AEs compared to placebo. With respect to any potential effects on the fetus, when RSVpreF is administered in pregnancy, receipt does not result in an increase in severe systemic AEs compared to placebo among infants entering their first RSV season. When RSVpreF is administered in pregnancy, the frequency of serious AEs was similar in pregnant women as well as in infant participants across the RSVpreF and placebo recipients. However, an imbalance was observed in preterm birth between RSVpreF and placebo recipients. It is unclear whether there is a causal relation with the vaccine as the currently available data are inconclusive. Limiting vaccine administration to the Health Canada approved dosing interval from 32 through 36 weeks of gestation will mitigate potential risk of preterm birth. NACI continues to monitor the RSVpreF vaccine safety data as it emerges and will update its recommendation if needed. Details are presented below.
Evidence on the safety of RSVpreF vaccine were derived from the 2 RCTs previously describedFootnote 42Footnote 43.
Reactogenicity
Among infants entering their first RSV season, the proportion of participants reporting at least one AE were comparable between nirsevimab and placebo groups, with most events being mild or moderateFootnote 58. Comparing nirsevimab and palivizumab, the percentage of participants reporting at least one AEs was well balanced between the groups, with the majority of AEs being mild or moderateFootnote 59. Overall, no meaningful differences in severe systemic and local AEs were observed when comparing nirsevimab to a placebo or to palivizumabFootnote 58Footnote 59.
When RSVpreF was administered during pregnancy, the proportion of pregnant and infant participants reporting at least one AEs were similar across the RSVpreF and placebo groupsFootnote 60. Most AEs reported were mild or moderate for both pregnant and infant participants across the RSVpreF and placebo groupsFootnote 60. Overall, no meaningful differences in severe systemic AEs were observed between the RSVpreF and placebo groups among pregnant participants and their infantFootnote 61Footnote 62.However, RSVpreF vaccine results in more severe local AEs compared to placebo among pregnant women and pregnant peopleFootnote 63.
Severe systemic adverse events following immunization
The available evidence suggests that nirsevimab does not increase the risk of severe systemic AEs in infants and that RSVpreF does not increase the risk of severe systemic AEs in pregnant women and pregnant people or their infants.
A meta-analysis of 2 RCTs did not demonstrate a higher risk of severe systemic AEs with nirsevimab (8.1%, n=208/2,570) in infants entering their first RSV season when compared to a placebo (11.1%, n=143/1,284) (pooled risk ratio [RR] of 0.73; 95% CI: 0.59 to 0.89)Footnote 35Footnote 37. Of note, AEs did include infections related to RSV, including RSV bronchiolitis, RSV pneumonia, and RSV bronchitis. The evidence on the safety of nirsevimab for infants entering their first RSV season was deemed to be at low certainty due to imprecision (Table 4). In the phase IIIb RCT comparing nirsevimab to the standard of care (no intervention), there were 94 severe systemic AEs (1.2%, n=48/4015 [nirsevimab] and 1.1%, n=46/4020 [standard of care])Footnote 41. Again, AEs did include infections related to RSV, including RSV bronchiolitis and RSV infectionFootnote 41.
One RCT reported non-significant increase in severe systemic AEs with nirsevimab when compared to palivizumab in high-risk infants entering their first (13.7% in the nirsevimab group, n=84/614; and 12.8% in the palivizumab group, n=39/304; RR of 1.07; 95% CI: 0.75 to 1.52) or second (10.9% in the nirsevimab group, n=24/220; and 2.4% in the palivizumab group, n=1/42; RR of 4.58; 95% CI, 0.64 to 32.95) RSV seasonFootnote 38Footnote 39. The evidence for nirsevimab compared to palivizumab for high-risk infants entering their first (Table 5) or second (Table 6) RSV season were deemed at low certainty due to imprecision.
Meta-analyses of 2 RCTs did not demonstrate a significant increase in severe systemic AEs with RSVpreF vaccine compared to placebo in pregnant women (2.3% in the RSVpreF group, n=85/3,777; and 2.3% in the placebo group, n=87/3,756; pooled RR of 0.97; 95% CI: 0.72 to 1.31)Footnote 42Footnote 43Footnote 61Footnote 62. In addition, although there was no direct exposure to the product, there were no increased risk of severe systemic AEs in their infants (18.1% in the RSVpreF group, n=666/3,682; and 18.0% in the placebo group, n=661/3,674; pooled RR of 1.01; 95% CI: 0.91 to 1.11)Footnote 64. The evidence for severe AEs related to RSVpreF was deemed at low certainty for pregnant women and pregnant people and their infants (Table 7).
Severe local adverse events following immunization
The available evidence suggests that nirsevimab does not increase the risk of severe local AEs in infants compared to palivizumab for high risk infants and compared to placebo for infants not at increased risk. However, evidence suggests that RSVpreF vaccine results in an increase in severe local AEs compared to placebo in pregnant women and pregnant people.
Across the 3 nirsevimab RCTs and the phase IIIb study, only one severe local AE was reported; this AE was among high-risk infants entering their first RSV seasonFootnote 35Footnote 37Footnote 38Footnote 39. A non-significant increase in severe local AEs was observed with nirsevimab (0.2%, n=1/614) compared to palivizumab (0.0%, n=0/304) (odds ratio of 4.46; 95% CI: 0.07 to 287.06)Footnote 38. No severe local AEs were reported in infants entering their first RSV season (2,579 in the nirsevimab group, and 1,293 in the placebo group) (Table 4), and high-risk infants entering their second RSV season (220 in the nirsevimab group, and 42 in the palivizumab group) (Table 6)Footnote 35Footnote 37Footnote 39. The certainty of evidence for high-risk infants entering their first RSV season was deemed to be moderate due to imprecision for nirsevimab compared to placebo and to palivizumab (Table 5).
There were no severe local adverse reactions reported among the pregnant participants in the RSVpreF phase IIb RCTFootnote 42. A meta-analysis of the phase IIb and phase III RCTs demonstrated an increase in severe local AEs with RSVpreF (0.3%; n=11/3,777) compared to placebo (0.0%; n=0/3,756) in pregnant participants (pooled odds ratio [OR] of 7.36; 95% CI: 2.26 to 24.02)Footnote 42Footnote 43Footnote 63. The certainty of evidence was deemed to be high (Table 7). Among pregnant participants with severe local AEs (n=11) who received RSVpreF vaccine, 6 (0.2%) had severe redness, 4 (0.1%) had severe swelling, and 4 (0.1%) had severe pain at injection site.
Serious adverse events
Among infants entering their first RSV season, there were no differences observed in the frequency of serious AEs between nirsevimab and placebo recipientsFootnote 58. Comparing nirsevimab and palivizumab, no meaningful differences in serious AEs were observed among high-risk infants entering their first and second RSV seasonsFootnote 59.
When RSVpreF is administered in pregnancy, the frequency of serious AEs was similar in the pregnant women as well as in infant participants across the RSVpreF and placebo groupsFootnote 65. However, an imbalance in preterm births was observed among the RSVpreF and placebo recipientsFootnote 65.
Preterm birth
There is uncertainty around an imbalance in preterm births observed in the phase III MATISSE trialFootnote 66. Available data were insufficient to definitively exclude a causal relationship between preterm birth and RSVpreF vaccination. At this time, limiting vaccine administration to the Health Canada approved dosing interval of 32 through 36 weeks of gestation reduces a potential risk of preterm birth. NACI continues to carefully monitor the evidence on the safety of RSVpreF vaccine in pregnant women and pregnant people and will update guidance accordingly.
Two RCTs from Pfizer reported preterm birth events that occurred in the RSVpreF vaccine group (5.6%, n=207/3,683) and the placebo group (4.7%, n=172/3,675)Footnote 42Footnote 43Footnote 66. A meta-analysis of these 2 RCTs did not demonstrate a significant increase in preterm birth with RSVpreF vaccine compared to placebo (pooled RR of 1.20; 95% CI: 0.98 to 1.46) and the evidence was deemed at low certainty due to imprecision (Table 7)Footnote 42Footnote 43Footnote 66. Of note, reported rates (including the vaccine group) were lower than the expected background rate of preterm births in all the participating countries, including Canada which is approximately 8%Footnote 65Footnote 67Footnote 68. Also, a numerical imbalance in preterm births was not observed in high-income countries (5.1% [RSVpreF] vs 5.1% [placebo]) similar to CanadaFootnote 65. When restricting the analysis to participants who received the intervention in the Health Canada approved dosing interval from 32 through 36 weeks of gestation, preterm birth events occurred in 4.2% (68/1,628) of the RSVpreF vaccine group and 3.7% (59/1,604) in the placebo groupFootnote 69.
It is noteworthy that in 2020, GSK initiated a phase III, double-blind, randomized, placebo-controlled study in 24 countries to assess the safety and efficacy of a single dose of their RSVPreF3 vaccine candidate, containing 120 µg of the RSVPreF3 antigen, administered to pregnant women 18 to 49 years of age in the late second or third trimester of pregnancy to prevent RSV-associated lower RTI in infants (RSV MAT-009)Footnote 70Footnote 71. In February 2022, the trial was halted to investigate an imbalance in the proportion of preterm birth (birth at less than 37 completed weeks of gestation) in the vaccine group compared to the placebo group.
In GSK's phase III trial, they found a significant increased risk of preterm birth (RR of 1.38; 95% CI: 1.08 to 1.75) including 238 events among 3,496 people in the RSVPreF3 groups (6.8%) compared to 86 events among 1,739 people in the placebo group (4.9%)Footnote 70Footnote 71. However, the imbalance in preterm births was observed more with low and middle-income countries (RR of 1.57; 95% CI: 1.17 to 2.10) than high-income countries (RR of 1.04; 95% CI: 0.68 to 1.58). The overall incidence of preterm birth in GSK trial is low in both groups and is below the background rates observed for the majority of the participating countries, including CanadaFootnote 70Footnote 71.
Contraindications and precautions
Nirsevimab and RSVpreF are contraindicated in individuals with a known hypersensitivity or history of a severe allergic reaction (e.g., anaphylaxis) to any component of the products.
Immunocompromised individuals may have a diminished immune response to RSVpreF. There are limited data on the use of RSVpreF in pregnant women and pregnant people less than 24 weeks of gestation, pregnant women and pregnant people less than 18 years of age, and pregnant women and pregnant people with underlying medical conditions, including conditions that put them at risk for premature delivery.
There have been documented administration errors in the United States, where some new RSV vaccines have been administered to the wrong populations including young children and pregnant peopleFootnote 72. Given the increasingly complex product environment for RSV vaccines and immunizing agents in Canada, it will be important for programs to take steps to minimize potential administration errors.
Immunization of specific populations
Immunization of immunocompromised persons
In general, immunocompromised people are more susceptible to vaccine-preventable infections and may have more severe infectionsFootnote 73. The effectiveness of vaccines in immunocompromised people is determined by the type of immunodeficiency and degree of immunosuppression. Each immunocompromised person is different and presents unique considerations regarding immunization. The relative degree of immunodeficiency is variable depending on the underlying condition, the progression of disease, and use of immunosuppressive agents.
There are no data on the use of RSVpreF in pregnant women and pregnant people who are immunocompromised. Immunocompromised individuals may have a diminished immune response to the vaccineFootnote 74.
Nirsevimab is authorized for the prevention of RSV lower respiratory tract disease in immunocompromised children less than 24 months of age. The efficacy and safety of nirsevimab have been evaluated in clinical trials in immunocompromised infants and children less than 24 monthsFootnote 33.
Immunization in pregnancy and breastfeeding
There is no evidence to suggest that RSVpreF would be present in human milk, but there is theoretical evidence, based on other vaccines studies, that there would likely be a modest transfer of protective antibodies via breast milk if the breastfeeding individual received vaccine in pregnancy or during breast feedingFootnote 75. There are limited or no available data to assess the effects of immunization with RSVpreF of breastfeeding women and breastfeeding people to protect the infant from RSV disease.
Nirsevimab is not indicated for use in adults. There is no evidence to suggest that the transfer of antibodies in human milk affects the efficacy of monoclonal antibodies in breastfed infants.
Immunization of individuals previously infected with RSV
All pregnant women and pregnant people are likely to have been previously infected with RSV. RSVpreF may be administered regardless of past RSV infection. A previous RSV infection is not a contraindication to administration of nirsevimab. However, nirsevimab is not typically necessary or recommended for an infant who has a current or previous confirmed RSV infection in the current RSV season. The additional benefit of nirsevimab after an infant has recovered from RSV infection is unknown but is expected to be low, as the risk of rehospitalization in the same RSV season is very lowFootnote 49Footnote 76. Consideration may be given in the case of severely immunocompromised infants who may still benefit as they may not mount an immune response to the RSV infection.
Economics
Systematic reviews and a de novo model-based economic evaluation were conducted by the NACI secretariat to support decision-making for the use of nirsevimab and RSVpreF for prevention of RSV in infants.
In summary, the model-based economic analyses showed that an all-infant program for nirsevimab and an RSVpreF for all pregnant women and pregnant people were not cost-effective at commonly used cost-effectiveness thresholds, even with modelled longer durations of protection. However, programs that limit nirsevimab use to those at increased medical risk due to RSV (defined as prematurity less than or equal to 366/7 wGA in the economic analysis) or living in settings with a higher RSV hospitalization rate and healthcare costs, were considered cost-effective at a cost-effectiveness threshold of $50,000 per quality-adjusted life year (QALY) gained. RSVpreF for all pregnant women and pregnant people combined with a high-risk program for nirsevimab may be cost-effective in settings with higher RSV hospitalization rates and healthcare costs.
The systematic review showed heterogeneity in the study findings. For instance, dominant results (i.e., immunization programs being less costly and more effective) were seen in Nunavik, Canada, especially during moderate or severe RSV seasons; whereas in many other settings programs using nirsevimab alone, RSVpreF alone, or both in combination generated incremental cost-effectiveness ratios (ICERs) that far exceeded commonly used cost-effectiveness thresholds.
Below are a descriptions of the systematic reviews and model-based economic evaluation, and additional details are provided in the Statement on the prevention of RSV disease in infants: Supplementary systematic review of economic evidence and a preprint publication, respectively.
Taken altogether, the systematic reviews of economic evaluations and a de novo cost-utility analysis detailed below, show that the cost-effectiveness of the immunization programs is impacted by how the program is implemented, such as offering seasonally versus year-round, offering with a nirsevimab catch-up dose versus none, or offering to the entire cohort versus subpopulations (i.e., high-risk groups).
Systematic reviews of RSVpreF and nirsevimab cost-effectiveness
Systematic reviews of economic evaluations of RSVpreF vaccine and nirsevimab products for preventing RSV-related outcomes in infants were conducted by CADTHFootnote 77Footnote 78. An additional literature review of the grey literature supplemented the systematic reviews of the peer-reviewed literature. The reviews included economic evaluations that compared RSVpreF or nirsevimab to any comparator (e.g., placebo, no intervention, alternative RSV prevention interventions [e.g., short-acting monoclonal antibodies such as palivizumab]). Outcomes included measures of cost-effectiveness (e.g., incremental cost per QALY, net monetary benefit, net health benefit). All costs were adjusted to 2023 Canadian dollars and are reported as such below. In terms of sources of funding, two studies reported industry fundingFootnote 79Footnote 80, and one study reported private/public funding with no involvement from the fundersFootnote 81. In terms of study quality, all studies met more than 50% of the Joanna Briggs Institute Critical Appraisal Checklist criteriaFootnote 82.
Of the 11 included studies, 2 were conducted in a Canadian setting. One of the studies was reflective of the Canadian Arctic region conducted from the health system perspective (Nunavik)Footnote 83, and one study was reflective of the Canadian south conducted from both the health system and societal perspectivesFootnote 84. In the Nunavik setting during mild RSV seasons (i.e., 30 to 50% of households had individuals infected with RSV), pregnancy vaccine (not specific to RSVpreF) had an ICER above $200,000 per QALYFootnote 83. During moderate or severe RSV seasons (i.e., more than 50% of households had individuals infected with RSV), a pregnancy vaccine program (not specific to RSVpreF) was dominant (i.e., less costly and more effective) compared to no interventionFootnote 83. Nirsevimab programs for infants at high-risk, with or without a pregnancy vaccine program, were dominant compared to no intervention, regardless of the severity of the RSV seasonFootnote 83. In many other settings, programs using nirsevimab alone, a pregnancy vaccine alone, or in combination generated ICERs that generally exceeded commonly used cost-effectiveness thresholdsFootnote 80Footnote 83Footnote 85Footnote 86Footnote 87Footnote 88Footnote 89Footnote 90. This is regardless of whether programs were seasonal versus year-round or offered with a nirsevimab catch-up dose to infants born outside of the RSV season versus none. There was variability in study findings due to the wide variation in model inputs. For instance, many of the peer-reviewed studies were conducted when product prices and clinical trial data for RSVpreF were not yet available.
Some studies conducted threshold analyses on product price. That is, they determined at what price an intervention would be considered cost-effective under specific cost-effectiveness thresholdsFootnote 80Footnote 81Footnote 84Footnote 85Footnote 86Footnote 91. Overall, studies found low product prices were required. For instance, the study in the Canadian south estimated that RSVpreF needed to be below $160 per dose for a pregnant woman, that nirsevimab needed to be below $215 per dose for an all-infant program, and that nirsevimab needed to be below $300 to $615 for various high-risk infant programs (prices were dependent on the eligibility criteria of prematurity, less than or equal to 32 wGA or less than or equal to 36 wGA, and presence of chronic lung disease or congenital heart disease)Footnote 84.
Cost-utility analysis
A model-based cost-utility analysis was conducted to assess the cost-effectiveness of the use of RSVpreF vaccine in pregnant women and pregnant people and nirsevimab in infants for the prevention of RSV-related outcomes in Canadian infants, compared to the standard of care (i.e., palivizumab for high-risk infants). A static cohort model was used to compare the discounted health outcomes (in QALYs) and costs (in 2023 Canadian dollars) of the interventions implemented over a one-year time period that included one RSV season, from both the health system and societal perspectives. Three possible program implementations were modelled for nirsevimab: (1) a dose at birth administered year-round, (2) a dose at birth for infants born from November to May (i.e., seasonal), and (3) a seasonal program with catch-up doses administered at the start of the RSV season (i.e., November) for infants born outside of the RSV season (i.e., from June to October). RSVpreF was assumed to be administered year-round. Two options for program eligibility were considered for nirsevimab: (1) all infants entering their first RSV season or (2) only infants at moderate-risk (330/7 to 366/7 wGA) or high-risk (born before 33 wGA), based on palivizumab eligibility entering their first RSV season. A program of year-round RSVpreF offered to all pregnant women and pregnant people plus nirsevimab offered year-round to infants at high-risk (assuming no protection from RSVpreF) was also evaluated. The model did not include infants entering their second RSV season. Canadian list prices were used in the base case for nirsevimab and RSVpreF: $952 and $230 per dose, respectively. Scenario and sensitivity analyses were performed to examine the impact of uncertainty related to input parameters and assumptions on the results. Details of the cost-utility analysis are provided in a preprint publication of the studyFootnote 92.
All RSVpreF and nirsevimab programs under consideration were projected to improve health outcomes, with nirsevimab for all-infants strategies averting more cases of RSV than year-round RSVpreF over the model time period. A year-round RSVpreF program had lower intervention costs than the all-infant nirsevimab strategies (due to lower costs per dose and lower assumed coverage), but its lower effectiveness and uptake also resulted in higher RSV-related costs. ICERs from the societal perspective were generally higher than from the health system perspective owing to the significant productivity loss incurred by caregivers during the administration of the vaccine or monoclonal antibodies ($141 per visit) but this did not qualitatively change the conclusions. Results are summarized for the health system perspective, with results for the societal perspective provided in the preprint publicationFootnote 92.
In the base case analysis, none of the modelled nirsevimab programs for all infants were expected to be cost-effective when compared sequentially against each other and standard of care, with ICERs well above commonly used thresholds (Figure 1). Across analyses, seasonal nirsevimab for all-infants with catch-up was more effective than a year-round program as it protected infants when protection was most needed (i.e., during the RSV season) as opposed conferring protection out of season. However, the ICER for seasonal nirsevimab program for all infants with catch-up was $512,265 per QALY compared to a program of year-round RSVpreF with nirsevimab for high-risk infants.
Programs prioritizing infants at moderate and high-risk had lower ICERs than nirsevimab for all infant programs. All base case and scenario analyses found that a seasonal nirsevimab program for infants at moderate and high risk with a catch-up program was cost-effective (under commonly used thresholds) compared sequentially to the standard of care (palivizumab programs) and other nirsevimab strategies considered for this population, with an ICER of $27,891 per QALY in the base case.
In all base case and scenario analyses, year-round RSVpreF for all pregnant women and pregnant people was dominated by other program options (i.e., other options had lower expected costs and higher QALYs compared to year-round RSVpreF). A strategy of combined use of RSVpreF for all pregnant women and pregnant people plus nirsevimab for infants at high-risk was not considered cost-effective at an ICER of $204,621 per QALY compared to seasonal nirsevimab for infants at moderate and high risk, with catch-up in the base case.
In settings with higher RSV hospitalization rates and health care costs due to complex transport requirements for receiving care, seasonal nirsevimab for all infants with a catch-up program was cost-effective compared to year-round RSVpreF with nirsevimab for infants at high-risk ($5,768 per QALY) and dominated all other strategies.
All results were sensitive to assumed nirsevimab price, and a two-way sensitivity analysis indicated that a seasonal nirsevimab for all infants with catch-up program is the optimal strategy if the price of nirsevimab was less than approximately $110 to $190 (depending on the price of RSVpreF vaccine) at a cost-effectiveness threshold of $50,000 per QALY.
Year-round RSVpreF plus nirsevimab for infants at high-risk could be the optimal strategy if the price of nirsevimab was greater than approximately $110 to $190 and the price of RSVpreF was less than approximately $60 to $125 at a cost-effectiveness threshold of $50,000 per QALY. The per dose prices are presented in ranges as cost-effectiveness of each intervention depends on the price of the alternative intervention.
The assumption of a longer duration of protection (10 months) for nirsevimab resulted in lower ICERs for all-infant nirsevimab programs, but a strategy of nirsevimab for all infants remained above commonly used cost-effectiveness thresholds, i.e., is not considered cost-effective.
The results of the health economic modelling are subject to limitations associated with simplifying assumptions and data uncertaintyFootnote 92. The prophylactic effect of the interventions was limited to medically attended LRTI (i.e., it did not included protection against upper respiratory tract infections and asymptomatic LRTI) and the study used a static model that did not account the impact of interventions on disease dynamics. There are possible program configurations that were not considered in the economic evaluation. Of note, a program of seasonal use of RSVpreF for all pregnant women and pregnant people plus nirsevimab for high-risk infants was not evaluated but would be expected to have lower ICERs than a year-round program.
Ethics, equity, feasibility and acceptability considerations
NACI uses a published, peer-reviewed framework and evidence-informed tools to ensure that issues related to ethics, equity, feasibility, and acceptability (EEFA) are systematically assessed and integrated into its guidanceFootnote 2.
Ethics
NACI consulted with the Public Health Ethics Consultative Group (PHECG) and also evaluated the following ethical considerations when making its recommendations: promoting well-being and minimizing risk of harm; maintaining trust; respect for people and fostering autonomy; and promoting justice and equity. In developing these recommendations, no significant ethical issues were identified by NACI or PHECG other than the equity considerations discussed below.
Equity
The nirsevimab recommendation was considered where costs of medical transportation and treatment are higher due to remoteness, limited availability of medical services, or other intersecting factors (e.g., racialization) that limit access to care. Complex transport settings include situations where transport distance may be very long (e.g., ground ambulance transport over several hours), but also may include shorter distances that require air or other complex transport or complex strategies. Complex medical transport for RSV care can have a disproportionate impact on infant health and can create community disruption in these settings.
NACI evaluated equity considerations when interpreting the epidemiological, clinical, and economic evidence summarized above, including intersecting factors leading to higher incidence of severe RSV disease, longer RSV season, and decreased access to health care for some populations. Equity considerations were used as a lens to identify trends in the data that are useful for recommendation synthesis, particularly where gaps in the data exist. Biases in available data were acknowledged, for example those due to systemic limitations in available data for racialized groups. Recommendations were synthesized based on equity-informed trends in available data extended to similar contexts where gaps in the data exist. NACI's recommendations aim to address the severe health inequities that exist and prioritize an intervention for people who have historically been and continue to be marginalizedFootnote 93. For example, the nirsevimab recommendation was considered in communities where rates of circulating RSV disease and RSV hospitalization are higher due to intersecting factors including social determinants of healthFootnote 30Footnote 31. In particular, Indigenous peoples experience a high burden of illness due to social, environmental, and economic factors, rooted in the history of colonization and systemic racismFootnote 93Footnote 94. By providing age-based and risk-based recommendations as well as inclusion of settings of higher disease burden, inequity may be reduced.
NACI consulted with Indigenous Services Canada (ISC)'s Vaccine Preventable Disease Working Group (VPD WG) to better understand if and how Indigenous peoples should be referenced and prioritized in this statement. The wording of the recommendations with regard to Indigenous groups comes directly from VPD WG members' feedback. In consultation with the ISC VPD WG, NACI recommends that the implementation of RSV immunization programs should seek to minimize inequities as much as possible. Implementation should be prioritized for groups who can benefit the most and be culturally safe given documented barriers to feasibility and acceptability of similarly broad palivizumab programs in similar settingsFootnote 95. Consideration should also be made for diverse contexts of equity-seeking communities, for example Indigenous groups across various settings (e.g., urban, rural, on-reserve, off-reserve). Furthermore, autonomous decisions should be made by Indigenous peoples with the support of healthcare and public health partners in accordance with the United Nations Declaration on the Rights of Indigenous PeoplesFootnote 96.
With respect to RSVpreF, pregnant women and pregnant people continue to experience paternalism during medical decision-makingFootnote 97. Therefore, the autonomy of pregnant women and pregnant people in making informed decisions, based on clear information communicated in a way that is easily understood, regarding accepting vaccines for themselves to protect their infants must be prioritized.
Feasibility
NACI consulted with the Canadian Immunization Committee (CIC) regarding feasibility of implementing programs for administration of nirsevimab and RSVpreF. There were no distinct, significant feasibility issues identified by either NACI or CIC. In general, CIC advised that new immunization programs that are similar to current programs (e.g., administering monoclonal antibodies to infants based on certain risk factors such as prematurity or medical conditions) are likely to be feasible. A program of seasonal administration of nirsevimab with "catch-up" at the start of the RSV season of infants born before the RSV season may implicate at least two different settings for administration (e.g., hospital and vaccination clinic or primary care provider office). Please see the Management options table (Table 2) for more considerations.
Given the available products, an immunization program to protect infants against severe RSV disease may encompass interventions for two patient populations (i.e., pregnant women and pregnant people, and their infants) by different disciplines of care providers. Clinicians may find themselves considering immunization products outside their usual patient population; for example, a prenatal care provider may be asked questions about nirsevimab for a pregnant woman or pregnant person's infant in the context of RSVpreF decision-making. At the program level, advice must be aligned for care providers of pregnant women/people and their infants so that a coordinated and unified program is implemented. At the patient level, information about publicly available immunization programs should be shared with care providers of pregnant women and pregnant people to facilitate individual decision-making. interventions should be coordinated between birthing parent/infant dyads and communicated between their care providers (including immunization status).
Acceptability
Nirsevimab is the first monoclonal antibody authorized for all infants and the injection can be given at birth. Because there is no similar immunization intervention authorized for all infants, acceptability of nirsevimab may be imperfectly informed by a few considerations. Firstly, data are lacking on the uptake of palivizumab but some clinicians report anecdotally that palivizumab uptake is high, although historically palivizumab has only been offered to infants and young children at the highest risk of severe RSV disease. Secondly, the uptake of routine childhood immunizations in Canada is relatively high, though important differences in uptake exist within and between racialized and non-racialized groupsFootnote 98. Moreover, many Canadian parents or guardians of 2-year-old children report that they believe childhood vaccines are safe (97%) and effective (98%), and 92% of parents or guardians "trust" or "really trust" medical doctors as a source of information on immunizationFootnote 98, highlighting the importance of a provider recommendation. There is a lack of data on the uptake of hepatitis B vaccine when indicated and offered at birth in Canada, but a study in the US showed uptake of 91.6%Footnote 99. Finally, the COVID-19 Snapshot Monitoring Study (COSMO) in Canada Wave 2.8 survey of parents reported that 50% of parents with children under 18 said they would be likely to get their children a vaccine for RSV if one became available, and 36% indicated they would be unlikely to do soFootnote 100. In Galicia, Spain, a universal nirsevimab program started in September 2023 saw an uptake of 100% (348/348) among high-risk infants; 81.4% (6,231/7,294) among infants 0 to 6 months born before the RSV season; and 93.0% (4,491/4,829) among infants born since the start of the program as of 28 January 2024Footnote 101.
A few small studies may help inform the acceptability of RSVpreF by pregnant women and pregnant people and providers. A survey in Ireland of awareness and acceptability of RSV and RSV vaccines amongst pregnant women and pregnant people found that 57% would accept an RSV vaccine if one was made availableFootnote 102. A survey in England found 81% of prenatal providers reported they would support (i.e., "definitely" or "likely") an RSV vaccine if routinely recommendedFootnote 103. Similar studies have been undertaken in Canada and 59% of respondents who were recently pregnant, pregnant, or planning pregnancy indicated that they would get an RSV vaccine to protect their infantFootnote 104. This appears to mirror other trends in pregnancy such as the uptake of pertussis vaccine and influenza vaccine where 65% and 53% of pregnant women and pregnant people respectively are vaccinatedFootnote 105. However, acceptability of an additional vaccine in pregnancy is unknown, and the imbalance in preterm births between the vaccine and placebo arm in the MATISSE trial may affect uptake. Furthermore, the acceptability of administering a monoclonal antibody to infants compared to a vaccination in pregnancy to protect the infant is unknown at this time. The availability of both nirsevimab and RSVpreF could also impact the uptake of RSVpreF. In the United States as of January 27, 2024, the overall coverage of RSVpreF among pregnant women and pregnant people at greater than or equal to 32 weeks of gestation was 16.2%, though this was in the context of challenges to the rollout of the vaccineFootnote 106.
Recommendations
Following the review of available evidence summarized above (and in the Management options table below), NACI makes the following recommendations for public health level and individual level decision-making.
Please note:
- A strong recommendation applies to most populations/individuals and should be followed unless a clear and compelling rationale for an alternative approach is present.
- A discretionary recommendation may be considered for some populations/individuals in some circumstances. Alternative approaches may be reasonable.
Please see Table 8 for a more detailed explanation of GRADE certainty of evidence for NACI recommendations.
NACI will continue to carefully monitor the scientific developments related to passive immunizing products for RSV and will update recommendations as evidence evolves.
Recommendation 1. Considering the significant burden of disease in all infants from RSV and the impacts of RSV on the Canadian health system, NACI recommends building towards a universal RSV immunization program for all infants. Program introduction could occur in stages depending on access to supply, cost-effectiveness, and affordability of available options. (Strong Recommendation)
Considerations:
- Nirsevimab is preferred over palivizumab. In contexts where there is limited or no availability of nirsevimab, palivizumab should be used according to the NACI 2022 recommendations.
- Jurisdictions are encouraged to define the RSV season and administer nirsevimab based on local epidemiology (prior to the COVID-19 pandemic, the RSV season was typically November to April).
- Nirsevimab is preferred over RSVpreF. If it is anticipated that nirsevimab will be administered to a healthy infant, then RSVpreF in pregnancy may not provide added benefit for the healthy infant.
- For the following infants whose gestational parent received RSVpreF, nirsevimab should still be administered:
- Infants who meet medical criteria for increased risk of severe RSV disease as per Recommendation 2 (List 1).
or
- Infants who are born less than 2 weeks after administration of RSVpreF.
Summary of evidence and rationale:
- Nirsevimab is preferred over palivizumab due to the balance of effects including the extended half-life and comparable, or potentially better, protective efficacy (when nirsevimab was compared to palivizumab, relative VE was 53% (-279% to 94%) at 150 days for RSV RTI with hospitalization). In addition, the increase in predicted half-life may mean once-per-season dosing of nirsevimab compared to monthly dosing for palivizumab.
- At this time, nirsevimab is preferred over RSVpreF given nirsevimab's higher efficacy and possible longer duration of protection. An imbalance in preterm births was also observed in the RSVpreF trial, although this potential risk is thought to be mitigated by Health Canada's authorized timing of administration (i.e., 32 to 36 wGA). If it is anticipated that nirsevimab will not be administered to an infant, RSVpreF may be considered.
- RSV previously had an established seasonal pattern of activity; however, the patterns have variability due to environmental or social factors and have shifted since the COVID-19 pandemic. Given the once-per-season dosing of nirsevimab and RSVpreF, flexibility for local decision-making on timing of administration is important.
Recommendation 2. NACI recommends RSV immunization programs use nirsevimab to prevent severe RSV disease. Programs can build and expand over time depending on access to supply, cost-effectiveness, and affordability of available options. (Strong recommendation)
Nirsevimab should be prioritized for infants in the following way:
Priority 1:
- Entering, or born during, their first RSV season who are at increased risk of severe RSV disease, including those who are born at less than 37 weeks gestational age (List 1).
- Entering their second RSV season and at ongoing increased risk of severe RSV disease (List 1).
- Entering, or born during, their first RSV season whose transportation for severe RSV disease treatment is complex, and/or whose risk of severe RSV disease intersects with established social and structural health determinants such as those experienced by some Indigenous communities across First Nations, Métis and Inuit populations.
Priority 2:
- If nirsevimab is priced in a manner to make such programs cost effective, NACI recommends nirsevimab be considered for any infant less than 8 months of age entering, or born during, their first RSV season through universal immunization programs to prevent severe RSV disease.
Considerations:
Eligibility:
- Infants at increased risk of severe RSV disease during their first RSV season include:
- All premature infants (i.e., born at less than 37 wGA)
- Chronic lung disease, including bronchopulmonary dysplasia, requiring ongoing assisted ventilation, oxygen therapy or chronic medical therapy in the 6 months prior to the start of the RSV season
- Cystic fibrosis with respiratory involvement and/or growth delay
- Haemodynamically significant chronic cardiac disease
- Severe immunodeficiency
- Severe congenital airway anomalies impairing clearing of respiratory secretions
- Neuromuscular disease impairing clearing of respiratory secretions
- Down syndrome
- Infants at ongoing risk of severe RSV disease during their second RSV season include all those listed above, except for infants born at less than 37 wGA and infants with Down syndrome (without another medical condition on the list above).
- For severe immunodeficiency, the list of immunocompromising conditions developed for COVID-19 may be usedFootnote 107. The following criteria apply for HIV: CD4 less than 750 cells/µL if age less than 1 year or CD4 less than 500 if age 1 to 2 years.
- Jurisdictions are encouraged to define the RSV season and administer nirsevimab based on local epidemiology (prior to the COVID-19 pandemic, the RSV season was typically November to April).
- Infants entering or in their first RSV season are usually below 8 months of age and infants entering or in their second RSV season are usually between 8 and 19 months of age.
Administration of nirsevimab:
- Nirsevimab should not be used as treatment and is intended only as a prophylactic to prevent severe RSV disease.
- Nirsevimab is not necessary or required for an infant who has a current confirmed RSV infection or a previous confirmed RSV infection in the current RSV season. The additional benefit of nirsevimab after an infant has recovered from RSV infection is unknown but expected to be low. Consideration may be given in the case of severely immunocompromised infants who may still benefit as they may not mount an immune response to the RSV infection.
- When supply of nirsevimab is limited, supply should be prioritized for infants/children at increased or ongoing increased risk of severe RSV disease receiving nirsevimab through public health programs.
Factors that intersect with social determinants of health:
- Indigenous peoples experience a high burden of illness due to social, environmental, and economic factors, rooted in the history of colonization and systemic racism (i.e., structural inequity); this recommendation for nirsevimab aims to address the severe health inequities that exist and prioritize an intervention for people who have historically been and continue to be marginalized.
- In First Nations, Métis, or Inuit communities, autonomous decisions should be made by Indigenous peoples with the support of healthcare and public health partners in accordance with the United Nations Declaration on the Rights of Indigenous Peoples ActFootnote 96.
- Consideration should be made for diverse contexts of equity-implicated communities. One example where diverse contexts may apply is Indigenous groups across various settings (e.g., urban, rural, on-reserve, off-reserve).
- Jurisdictions are encouraged to implement culturally-safe immunization programs. There have been documented barriers to the feasibility and acceptability of palivizumab programs for some populations. Nirsevimab is the best available clinical option for RSV prevention in infants at this time, therefore it is being recommended for populations where it will have the greatest potential to reduce health inequities. For infants without identified medical risk factors for severe RSV disease (listed in List 1) and whose gestational parent received RSVpreF at least 2 weeks before birth, administration of nirsevimab is expected to provide minimal additional benefit and therefore is not recommended.
Summary of evidence and rationale:
- The criteria for infants at increased and ongoing increased risk of severe RSV disease during their first and second RSV seasons respectively was established based on evidence reviews conducted for the NACI statement on palivizumab as well as expert opinion. Given the predicted reduced cost of nirsevimab compared to palivizumab as well as the increased feasibility of administration (i.e., 1 dose vs. 4 or 5 doses), NACI expanded the criteria for infants recommended to be made eligible for nirsevimab programs.
- The review of available evidence from clinical trials demonstrated that nirsevimab is safe and effective in preventing severe RSV disease during an infant's first RSV season.
- A model-based economic analysis showed:
- At list price, an all-infant program for nirsevimab was not cost-effective at commonly used cost-effectiveness thresholds, even when longer durations of protection were modelled.
- Nirsevimab use in those at increased risk of severe RSV disease (defined as birth at less than 37 wGA in the economic analysis) was expected to be cost-effective.
- Programs that extend nirsevimab use to those living in settings with higher RSV hospitalization rates and healthcare costs were also expected to be cost-effective.
- The economic analysis showed that administration of nirsevimab to all infants born during the RSV season, with immunization at the start of the RSV season for those infants born before it, could be an optimal strategy at a cost-effectiveness threshold of $50,000 per QALY if the price of nirsevimab was less than approximately $110 to $190 per dose.
- When interpreting the epidemiological trends to inform the recommendations, equity considerations include acknowledgement that available evidence for some populations is limited and may be biased, for example due to systemic limitations in available data for racialized groups.
- Complex medical transport for RSV care can have a disproportionate impact on infant health and can create community disruption. Complex transport settings include situations where transport distance may be very long (e.g., ground ambulance transport over several hours), but also may include shorter distances that require air or other complex transport or other complex strategies.
Recommendation 3. NACI recommends RSVpreF may be considered as an individual decision by a pregnant woman or pregnant person together with information from their pregnancy care provider, in advance of, or during, the RSV season, to prevent severe RSV disease in their infant. At the present time, NACI does not recommend an immunization program for RSVpreF. More data and information are expected to emerge over time and NACI will reconsider this recommendation in the future. (Discretionary recommendation)
Considerations:
- Pregnant women and pregnant people continue to experience paternalism during medical decision-making. Therefore, the autonomy of pregnant women and pregnant people in making informed decisions regarding accepting vaccines for themselves to protect their infants must be prioritized.
- Use of RSVpreF may not be required if a universal nirsevimab program is implemented.
- Jurisdictions are encouraged to define the RSV season and provide information for care providers of pregnant women and pregnant people (in addition to care providers of infants) about their publicly available immunization programs to facilitate informed individual level decision making.
- For administration of RSVpreF, consideration should be given to gestational timing and the start of the RSV season, allowing for the development of a humoral immune response and passive antibody transfer (e.g., at least two weeks). For example, RSVpreF could be administered starting in September to protect infants expected to be born during the RSV season in November, if gestational age is at least 32 weeks at the time of administration.
- No data are available on either the efficacy or safety of additional doses of RSVpreF given during subsequent pregnancies. NACI may revisit this topic as more evidence becomes available.
- RSVpreF may be administered regardless of past RSV infection.
Summary of evidence and rationale:
- The review of available evidence from clinical trials demonstrated that RSVpreF is effective at preventing severe RSV disease in an infant during the first months of life (VE of 57% (27 to 75%) at 180 days months for RSV RTI with hospitalization). The working group considered this alongside the available data for nirsevimab (VE of 81%; 95% CI: 64 to 91%) at 150 days for RSV RTI with hospitalization).
- An imbalance in preterm births in the manufacturer's trial was observed. Available data are insufficient to definitively exclude a causal relationship between preterm birth and RSVpreF vaccination. Consequently, at this time, limiting vaccine administration to the Health Canada approved dosing interval of 32 through 36 weeks of gestation reduces the potential risk of preterm birth. NACI will continue to carefully monitor the evidence on the safety of RSVpreF vaccine in pregnant women and people and will update guidance accordingly.
- The available efficacy (including duration of protection) and safety data for RSVpreF and nirsevimab underpin NACI's preferential recommendation for nirsevimab. This may be revisited as additional evidence emerges.
- The model-based economic evaluation showed that:
- Across all analyses, a year-round RSVpreF program was dominated by other programs (i.e., year-round RSVpreF had higher expected costs and lower QALYs compared to other programs).
- A year-round RSVpreF program in combination with nirsevimab for infants at high-risk was not cost-effective with an ICER of $204,621 per QALY compared to seasonal nirsevimab for infants at moderate and high risk, with catch-up in the base case.
- Year-round RSVpreF plus nirsevimab for infants at high-risk could be an optimal strategy if the price of nirsevimab was greater than approximately $110 to $190 and the price of RSVpreF was less than approximately $60 to $125 at a cost-effectiveness threshold of $50,000 per QALY.
Management options table
Various options for the vaccine and immunization product type, schedule, age cohort and risk group are available. The decision on which option is preferable will depend on the considerations listed below.
| Product | Considerations | Decision points |
|---|---|---|
| Nirsevimab |
Epidemiology: Severe RSV disease is most frequently observed in young infants (below6 months of age) in their first RSV season. Preterm infants are at higher risk but term infants make up the majority of severe RSV cases. Some infants are at higher risk for severe disease due to certain medical conditions (see recommendations above). A minority of infants are at risk for severe RSV disease in their second RSV season. Indigenous peoples experience a high burden of illness due to social, environmental, and economic factors, rooted in the history of colonization and systemic racism. RSV seasonality: RSV exhibits a seasonal infection cycle that may vary depending on environmental or other factors. Efficacy and duration of protection: The protective efficacy of nirsevimab takes effect immediately. Efficacy is high in the first months of life when infants are most at risk for RSV. Nirsevimab was shown to be efficacious through the first 5 months and may provide full season protection. Safety: Available data stem from clinical trials only. Safety of nirsevimab continues to be monitored and no signals were observed in clinical trials. Economics: Economic analysis indicated that administration of nirsevimab to infants at higher or ongoing risk may be cost-effective. An all-infant universal program for nirsevimab was not shown to be cost-effective at current list prices. Ethics, equity, feasibility and acceptability: A broader nirsevimab recommendation may mean nirsevimab may be administered to more infants than current palivizumab programs. This program expansion may be feasible to implement using established systems and programs. The nirsevimab recommendation was considered where costs of medical treatment and transportation are higher due to remoteness, limited availability of medical services or other intersecting factors (e.g., racialization) that limit access to care. Recommendations for Indigenous peoples aim to address the severe health inequities that exist and prioritize an intervention for people who have historically been and continue to be marginalized. |
Epidemiology: Although all infants may benefit from protection offered by nirsevimab and RSVpreF during their first months of life during the RSV season, some infants are at higher risk for severe disease (see recommendations above) and therefore may be prioritized in the context of immunization programs. RSV seasonality: The timing of administration should consider the wait time to protection and the start of the RSV season. Efficacy and duration of protection: From clinical trial data for each product, the efficacy reported for nirsevimab is higher than that reported for RSVpreF. Based on currently available data, the protection offered by RSVpreF to infants appears to wane earlier and faster than those reported for nirsevimab. Safety: The imbalance with respect to the number of pre-term births observed in the RSVpreF clinical trial may be a consideration. Economics: Nirsevimab is unlikely to be cost-effective for all infants at current list prices. A program for RSVpreF alone or a program combining RSVpreF and nirsevimab for infants at high risk is unlikely to be cost-effective at current list prices. A program for nirsevimab for all infants or a program that combines RSVpreF and nirsevimab for infants at high risk may be cost-effective in populations with complex transportation for medical care and higher rates of hospitalization. Ethics, equity, feasibility and acceptability: Nirsevimab programs may be similar to current palivizumab programs and therefore may be more feasible and acceptable. RSVpreF would require a new program for pregnant women and pregnant people to be established, and therefore may be less acceptable and feasible particularly where timing of administration may not align with influenza or pertussis immunization. |
| RSVpreF |
Epidemiology: See above RSV seasonality: See above Efficacy and duration of protection: The protective efficacy of RSVpreF takes some time to develop (at least 2 weeks between administration and birth needed). Efficacy is high in the first months of life when infants are most at risk for RSV. RSVpreF was shown to be somewhat efficacious the first 5 months after administration though protection is not expected to last more than 4 to 5 months. Safety: Available data stem from clinical trials only. The safety of RSVpreF is being monitored, particularly in the context of an imbalance in preterm births observed in the clinical trial. Economics: Economic analysis indicated that administration of RSVpreF to all pregnant women and pregnant people is not cost effective unless in combination with a high risk nirsevimab program, except in the context of complex transportation for medical care and other risk factors. Feasibility and acceptability: Immunization of pregnant women and pregnant people may be less acceptable than other vaccine strategies, particularly in the context of the imbalance with respect to the number of pre-term births observed in the RSVpreF clinical trial. Historically, vaccine programs in pregnancy (e.g., Tdap, influenza) have lower uptake compared to the general population in Canada (65% and 53%, respectively in 2021). |
| Season | Population protected (population receiving the intervention, if different) |
Recommendation |
|---|---|---|
| First | Infant at increased risk | Should be offered nirsevimab - Priority 1Footnote a |
| Second | Infant/child at ongoing increased risk | |
| First | Infant requiring complex transport and/or risk intersects with social/structural health determinants (e.g. Indigenous Peoples) | |
| First | All infants | Should be offered nirsevimab - Priority 2Footnote a |
| First | All infants (pregnant women and pregnant people) |
Individual level: Consider RSVpreF factoring in any potentially available program options |
|
Immunization programs: At the present time, universal RSVpreF program not recommended |
||
|
||
Research priorities
Research to address the following outstanding issues is encouraged:
- Safety and effectiveness of nirsevimab and RSVpreF outside of the clinical trial settings
- Safety and effectiveness of RSVpreF at gestational ages earlier than authorization, i.e., before 32 wGA, in preventing severe RSV disease in infants identified to be at increased risk in utero
- Safety and effectiveness of RSVpreF in preventing RSV disease in pregnant women and pregnant people
- Safety and efficacy or effectiveness of concurrent administration of RSVpreF with other vaccines for pregnant women and pregnant people
- Duration of antibody response to initial RSVpreF dose in pregnancy – does antibody persist into a subsequent pregnancy?
- Safety and efficacy or effectiveness of RSVpreF in subsequent pregnancies, if previously administered
- Additional research to explore whether the imbalance in preterm births observed in the RSVpreF trial constitutes a safety signal
- Safety and efficacy or effectiveness of administration of RSVpreF to gestational parent and nirsevimab to infant to protect the same infant
- Long-term impact of delaying RSV disease for cohorts of children through passive immunity acquired from the administration of monoclonal antibodies or RSVpreF
- Long-term health impact of nirsevimab or RSVpreF
- Durability of protection of nirsevimab
- Impact, if any, of polyclonal antibody response elicited by RSVpreF as compared to monoclonal antibody response elicited by nirsevimab
- Safety and efficacy or effectiveness of concurrent administration of nirsevimab with childhood vaccines and/or other monoclonal antibodies
- Impacts on equity due to nirsevimab and RSVpreF immunization programs or lack thereof
- Acceptability and uptake of nirsevimab and RSVpreF
Surveillance issues
Ongoing and systematic data collection, analysis, interpretation and timely dissemination is fundamental to planning, implementation, evaluation, and evidence-based decision-making. RSV is currently not a reportable disease in Canada. To support such efforts, NACI encourages ongoing surveillance and continued expansion of surveillance details in the epidemiology of RSV in Canada. This includes surveillance of selection pressure on changes to the viral evolution of RSV virus due to potential selection pressures related to the introduction of a novel monoclonal antibody and RSV vaccines.
The Respiratory Virus Detection Surveillance System (RVDSS), Canada's national RSV surveillance system, monitors the spread of RSV by province/territory. Robust enhanced surveillance data on infants, children and pregnant women and pregnant people including health status, and granularity by age group, ethnicity, and RSV-related complications (e.g., hospitalization and ICU admission) is limited. In addition, the impact of RSV on infants based on underlying health status, sex and other potential confounders is not well documented. Therefore, initiatives are needed to collect data on RSV infection (e.g., non-medically attended-RSV, medically attended-RSV, hospitalization, ICU admission, and death incidence) in pregnant women and pregnant people, their infants, and children to determine the burden of RSV infections in Canada.
Figure
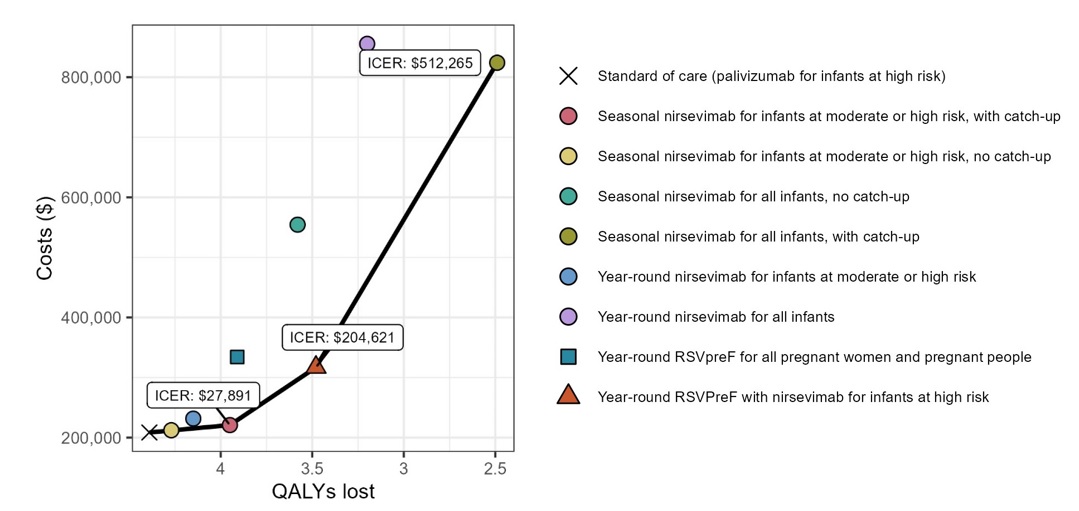
Figure 1: Text description
| Strategy | Costs ($) | Effect (QALYs lost) | Sequential ICERs ($ per QALY) |
|---|---|---|---|
| Standard of care (palivizumab for infants at high risk) | 208,527 | 4.39 | - |
| Seasonal nirsevimab for infants at moderate or high risk, no catch-up | 212,251 | 4.27 | Dominated |
| Seasonal nirsevimab for infants at moderate or high risk, with catch-up | 220,799 | 3.95 | 27,891 |
| Year-round nirsevimab for infants at moderate or high risk | 231,567 | 4.15 | Dominated |
| Year-round RSVPreF plus nirsevimab for infants at high risk | 316,971 | 3.48 | 204,621 |
| Year-round RSVPreF for all pregnant women and pregnant people | 334,187 | 3.91 | Dominated |
| Seasonal nirsevimab for all infants, no catch-up | 554,487 | 3.58 | Dominated |
| Seasonal nirsevimab for all infants, with catch-up | 824,113 | 2.49 | 512,265 |
| Year-round nirsevimab for all infants | 855,494 | 3.20 | Dominated |
Note: The solid line connects strategies that are not dominated and the labels indicate the sequential incremental cost-effectiveness ratios (ICERs) for each of these strategies (i.e., ICERs comparing a less costly strategy compared with the next most costly strategy). Note that seasonal nirsevimab for infants at moderate and high-risk, without catch-up of infants born outside the RSV season, is dominated despite appearing to be on the efficiency frontier due to the scale of the graph.
Tables
| Outcome | No. of studies (study design) | No. of events/No. of participants | Effect | Certainty | ||
|---|---|---|---|---|---|---|
| Nirsevimab | Placebo | Relative effect (95% CI) | Absolute effect (95% CI) | |||
| Death due to RSV (follow up: 150 days) | 2 (RCTS) | 0/2579 (0.0%) | 0/1293 (0.0%) |
Not estimable |
ModerateFootnote a | |
| RSV RTI with ICU admission (follow up: 150 days) | 2 (RCTS) | 1/2579 (0.0%) | 6/1293 (0.5%) |
OR 0.10 (0.02 to 0.46) Efficacy 90% (54% to 98%) |
4 fewer per 1,000 (from 5 fewer to 3 fewer) | ModerateFootnote a |
| RSV RTI with hospitalization (follow up: 150 days) | 2 (RCTS) | 12/2579 (0.5%) | 33/1293 (2.6%) |
RR 0.19 (0.10 to 0.36) Efficacy 81% (64% to 90%) |
21 fewer per 1,000 (from 23 fewer to 16 fewer) | ModerateFootnote a |
| Medically attended RSV RTI (follow up: 150 days) | 2 (RCTS) | 31/2579 (1.2%) | 80/1293 (6.2%) |
RR 0.20 (0.13 to 0.30) Efficacy 80% (70% to 87%) |
49 fewer per 1,000 (from 54 fewer to 43 fewer) | ModerateFootnote a |
| Severe systemic AEs (follow up: 150 days) | 2 (RCTS) | 208/2570 (8.1%) | 143/1284 (11.1%) | RR 0.73 (0.59 to 0.89) | 30 fewer per 1,000 (from 46 fewer to 12 fewer) | ModerateFootnote b |
| Severe local AEs (follow up: 150 days) | 2 (RCTS) | 0/2579 (0.0%) | 0/1293 (0.0%) |
Not estimable |
ModerateFootnote a | |
|
||||||
| Outcome | No. of studies (study design) |
No. of events/No. of participants |
Effect |
Certainty | ||
|---|---|---|---|---|---|---|
| Nirsevimab | Palivizumab | Relative effect (95% CI) | Absolute effect (95% CI) | |||
| Death due to RSV (follow up: 360 days) | 1 (RCT) | 0/614 (0.0%) | 0/304 (0.0%) |
Not estimable |
ModerateFootnote a | |
| RSV RTI with ICU admission (follow up: 150 days) | 1 (RCT) | 0/616 (0.0%) | 0/309 (0.0%) |
Not estimable |
ModerateFootnote a | |
| RSV RTI with hospitalization (follow up: 150 days) | 1 (RCT) | 2/616 (0.3%) | 2/309 (0.6%) |
OR 0.47 (0.06 to 3.79) Efficacy 53% (-279 to 94%) |
3 fewer per 1,000 (from 6 fewer to 18 more) | LowFootnote b |
| Medically attended RSV RTI (follow up: 150 days) | 1 (RCT) | 4/616 (0.6%) | 3/309 (1.0%) |
RR 0.67 (0.15 to 2.97) Efficacy 33% (-197 to 85%) |
3 fewer per 1,000 (from 8 fewer to 19 more) | ModerateFootnote c |
| Severe systemic AEs (follow up: 360 days) | 1 (RCT) | 84/614 (13.7%) | 39/304 (12.8%) | RR 1.07 (0.75 to 1.52) | 9 more per 1,000 (from 32 fewer to 67 more) | LowFootnote b |
| Severe local AEs (follow up: 360 days) | 1 (RCT) | 1/614 (0.2%) | 0/304 (0.0%) | Peto OR 4.46 (0.07 to 287.06) | 2 more per 1,000 (from 4 fewer to 8 more)Footnote d | ModerateFootnote a |
|
||||||
| Outcome | No. of studies (study design) |
No. of events/No. of participants |
Effect |
Certainty | ||
|---|---|---|---|---|---|---|
| Nirsevimab | Palivizumab | Relative effect (95% CI) | Absolute effect (95% CI) | |||
| Death due to RSV (follow up: 360 days) | 1 (RCT) | 0/220 (0.0%) | 0/42 (0.0%) |
Not estimable |
ModerateFootnote a | |
| RSV RTI with ICU admission (follow up: 150 days) | 1 (RCT) | 0/220 (0.0%) | 0/42 (0.0%) |
Not estimable |
ModerateFootnote a | |
| RSV RTI with hospitalization (follow up: 150 days) | 1 (RCT) | 0/220 (0.0%) | 0/42 (0.0%) |
Not estimable |
ModerateFootnote a | |
| Medically attended RSV RTI (follow up: 150 days) | 1 (RCT) | 0/220 (0.0%) | 0/42 (0.0%) |
Not estimable |
ModerateFootnote a | |
| Severe systemic AEs (follow up: 360 days) | 1 (RCT) | 24/220 (10.9%) | 1/42 (2.4%) | RR 4.58 (0.64 to 32.95) | 85 more per 1,000 (from 9 fewer to 761 more) | LowFootnote b |
| Severe local AEs (follow up: 360 days) | 1 (RCT) | 0/220 (0.0%) | 0/42 (0.0%) |
Not estimable |
ModerateFootnote a | |
|
||||||
| Outcome | No. of studies (study design) |
No. of events/No. of participants |
Effect |
Certainty | ||
|---|---|---|---|---|---|---|
| RSVpreF | Placebo | Relative effect (95% CI) | Absolute effect (95% CI) | |||
| Death due to RSV (follow up: 180 days) | 2 (RCTs) | 0/3675 (0.0%) | 1/3665 (0.0%) |
OR 0.13 (0.00 to 6.80) VE 87% (-580 to 100%) |
0 fewer per 1,000 (from 0 fewer to 2 more) | LowFootnote a |
| RSV RTI with ICU admission (follow up: 180 days) | 1 (RCT) | 4/3495 (0.1%) | 7/3480 (0.2%) |
RR 0.57 (0.12 to 2.25) VE 43% (-125 to 88%) |
1 fewer per 1,000 (from 2 fewer to 3 more) | LowFootnote a |
| RSV RTI with hospitalization (follow up: 180 days) | 1 (RCT) | 19/3495 (0.5%) | 44/3480 (1.3%) |
RR 0.43 (99.17% CI, 0.19 to 0.90) VE 57% (99.17% CI, 10 to 81%) |
7 fewer per 1,000 (99.17% CI from 10 fewer to 1 fewer) | ModerateFootnote b |
| Medically attended RSV RTI (follow up: 180 days) | 1 (RCT) | 57/3495 (1.6%) | 117/3480 (3.4%) |
RR 0.49 (97.58% CI, 0.33 to 0.71) VE 51% (97.58% CI, 29 to 67%) |
17 fewer per 1,000 (97.58% CI from 22 fewer to 10 fewer) | High |
| Preterm Birth | 2 (RCTs) | 207/3683 (5.6%) | 172/3675 (4.7%) | RR 1.20 (0.98 to 1.46) | 9 more per 1,000 (from 1 fewer to 22 more) | LowFootnote a |
| Severe systemic AEs in pregnant participants (follow up: 7 days) | 2 (RCTs) | 85/3777 (2.3%) | 87/3756 (2.3%) | RR 0.97 (0.72 to 1.31) | 1 fewer per 1,000 (from 6 fewer to 7 more) | LowFootnote a |
| Severe systemic AEs in infant participants | 2 (RCTs) | 666/3682 (18.1%) | 661/3674 (18.0%) | RR 1.01 (0.91 to 1.11) | 2 more per 1,000 (from 16 fewer to 20 more) | LowFootnote a |
| Severe local AEs in pregnant participants (follow up: 7 days) | 2 (RCTs) | 11/3777 (0.3%) | 0/3756 (0.0%) | Peto OR 7.36 (2.26 to 24.02) | 3 more per 1,000 (from 1 more to 5 more)Footnote c | High |
|
||||||
| GRADE certainty of evidence rating | Description |
|---|---|
| High | Very confident that the true effect lies close to that of the effect estimate. |
| Moderate | Moderately confident: the true effect is likely to be close to the effect estimate, but there is a possibility that it is substantially different. |
| Low | Limited confidence in the effect estimate: the true effect may be substantially different from the effect estimate. |
| Very Low | Very little confidence in the effect estimate: true effect likely to be substantially different from the effect estimate. |
List of abbreviations
- AE
- Adverse event
- AEs
- Adverse events
- CCDR
- Canada Communicable Disease Report
- CHD
- Congenital Heart Disease
- CI
- Confidence Interval
- CIC
- Canadian Immunization Committee
- CLD
- Chronic Lung Disease
- COSMO
- COVID-19 Snapshot Monitoring Study
- DPD
- Drug Product Database
- DPT
- Diphtheria, Pertussis, and Tetanus
- EEFA
- Equitability, feasibility, and acceptability
- FHA
- Filamentous hemagglutinin
- G and F surface protein
- Glycoprotein and Fusion surface protein
- GMCs
- Geometric mean concentrations
- GSK
- GlaxoSmithKline
- GRADE
- Grading of recommendations assessment, development, and evaluation
- HARMONIE trial
- Hospitalized RSV monoclonal antibody prevention
- ICER
- Incremental cost-effectiveness ratio
- ICU
- Intensive Care Unit
- ISC
- Indigenous Services Canada
- LRTI
- Lower respiratory tract infection
- MATISSE trial
- MATernal Immunization Study for Safety and Efficacy trial
- MEDLEY trial
- Monoclonal Antibody with an Extended Half-life Against Respiratory Syncytial Virus, in High-risk Children
- MELODY trial
- Monoclonal Antibody with an Extended Half-life Against Respiratory Syncytial Virus, in Healthy Late Preterm and Term Infants
- N
- Number of Participants
- NACI
- National Advisory Committee on Immunization
- OR
- Odds ratio
- PHAC
- Public Health Agency of Canada
- Phase IIb RCT
- Phase 2b Randomized Controlled Trials
- Phase III RCT
- Phase 3 Randomized controlled Trials
- PHECG
- Public Health Ethics Consultative Group
- PRN
- Pertactin
- PT
- Pertussis toxin
- QALY
- Quality-adjusted life year
- RCT
- Randomized Control Trial
- RNA
- Ribonucleic Acid
- RSV
- Respiratory Syncytial Virus
- RSV MAT-009
- Respiratory Syncytial Virus Monoclonal Antibody Trial 009
- RSVpreF
- Respiratory Syncytial Virus prefusion F protein
- RR
- Risk ratio
- RTI
- Respiratory Tract Infection
- RVDSS
- Respiratory Virus Detection Surveillance System
- SIIV
- Seasonal inactivated influenza vaccine
- Tdap
- Tetanus, Diphtheria, and Pertussis
- VPD
- Vaccine Preventable Disease
- VPD WG
- Vaccine Preventable Disease Working Group
- VE
- Vaccine Efficacy
- wGA
- Weeks Gestational Age
Acknowledgements
This statement was prepared by: A Killikelly, W Siu, P Doyon-Plourde, P Davis, E Abrams, G Gebretekle, M Yeung, A Tuite, and N Brousseau on behalf of NACI RSV Working Group and approved by NACI.
NACI gratefully acknowledges the contribution of: F Crane, A Cernat, S Cortes-Kaplan, A Howarth, C Jensen, S Lim, P Luth, A Roselli, M Rudd, A Simmons, A Stevens, M Salvadori, M Tunis, K Wilkinson, R Ximenes, R Yorke, K Young, L Zhao, the Vaccine Safety Working Group, the Public Health Ethics Consultative Group, the Indigenous Services Canada Vaccine Preventable Disease Working Group and the Yukon Communicable Disease Control, Health and Social Services.
NACI RSV Working Group
NACI RSV Working Group Members: N Brousseau (Chair), M Andrew, A Britton (Centers for Disease Control and Prevention, US), T Bogler, S Buchan, K Campbell, M Cao, E Castillio, K Fleming-Dutra (Centers for Disease Control and Prevention, US), J Jones (Centers for Disease Control and Prevention, US), S McNeil, M Melgar, D Money, D Moore, J Papenburg, V Poliquin, E Rafferty, J Robinson, and F Schwarz.
Ex-officio representatives: S Buckrell (Centre for Immunization and Respiratory Infectious Diseases, PHAC), M Cao (Biologic and Radiopharmaceutical Drugs Directorate, HC), L Lee (Centre for Immunization and Respiratory Infectious Diseases, PHAC), and F Schwarz (Biologic and Radiopharmaceutical Drugs Directorate, HC).
PHAC participants: F Crane, S Cortes-Kaplan, A Howarth, C Jensen, S Lim, A Roselli, M Rudd, A Simmons, A Stevens, M Salvadori, M Tunis, K Wilkinson, R Ximenes, R Yorke, K Young, and L Zhao.
NACI
NACI Members: R Harrison (Chair), V Dubey (Vice Chair), M Andrew, J Bettinger, N Brousseau, A Buchan, H Decaluwe, P De Wals, E Dubé, K Hildebrand, K Klein, M O'Driscoll, J Papenburg, A Pham-Huy, B Sander and S Wilson.
Former NACI Members: S Deeks (Chair)
Liaison Representatives: L Bill (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), S Buchan (Canadian Association for Immunization Research and Evaluation), E Castillo (Society of Obstetricians and Gynaecologists of Canada), J Comeau (Association of Medical Microbiology and Infectious Disease Canada), M Lavoie (Council of Chief Medical Officers of Health), J MacNeil (Centers for Disease Control and Prevention, US), M McIntyre (Canadian Nurses Association), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), M Nowgesic (Canadian Indigenous Nurses Association), M Osmack (Indigenous Physicians Association of Canada), J Potter (College of Family Physicians of Canada), and A Ung (Canadian Pharmacists Association).
Ex-Officio Representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (Centre for Immunization Programs (CIP), PHAC), P Fandja (Marketed Health Products Directorate, HC), M Lacroix (Public Health Ethics Consultative Group, PHAC), C Pham (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), S Ogunnaike-Cooke (Centre for Immunization Surveillance, PHAC), M Patel (Vaccine Supply and Assurance, PHAC), M Routledge (National Microbiology Laboratory, PHAC), M Su (COVID-19 Epidemiology and Surveillance, PHAC), and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
References
- Footnote 1
-
Canada's Drug and Health Technology Agency, (CADTH). Nirsevimab (Beyfortus) for respiratory syncytial virus prevention in neonates and infants [Internet]. CADTH; 2023 Jul 26 [cited 2023 Dec 18]. Available from: https://www.cadth.ca/nirsevimab-beyfortus-respiratory-syncytial-virus-prevention-neonates-and-infants.
- Footnote 2
-
Ismail SJ, Hardy K, Tunis MC, Young K, Sicard N, Quach C. A framework for the systematic consideration of ethics, equity, feasibility, and acceptability in vaccine program recommendations. Vaccine. 2020 Aug 10;38(36):5861-76. https://doi.org/10.1016/j.vaccine.2020.05.051.
- Footnote 3
-
Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020.
- Footnote 4
-
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. 2011 Apr 1;64(4):383-94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
- Footnote 5
-
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011 Apr 1;64(4):401-6. https://doi.org/10.1016/j.jclinepi.2010.07.015.
- Footnote 6
-
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015 Jan 1;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
- Footnote 7
-
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE evidence to decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ (Online). 2016 Jun 28;353:i2016. https://doi.org/10.1136/bmj.i2016.
- Footnote 8
-
American Academy of Pediatrics Committee on Infectious Diseases. Red book (2018): Report of the Committee on Infectious Diseases, 31st Edition. Itasca (IL): American Academy of Pediatrics; 2018 May 01.
- Footnote 9
-
Zylbersztejn A, Pembrey L, Goldstein H, Berbers G, Schepp R, van der Klis F, et al. Respiratory syncytial virus in young children: community cohort study integrating serological surveys, questionnaire and electronic health records, Born in Bradford cohort, England, 2008 to 2013. Eurosurveillance. 2021 Feb 11;26(6):20. https://doi.org/10.2807/1560-7917.ES.2021.26.6.2000023.
- Footnote 10
-
Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022 May 28;399(10340):2047-64. https://doi.org/10.1016/S0140-6736(22)00478-0.
- Footnote 11
-
Wingert A, Pillay J, Moore DL, Guitard S, Vandermeer B, Dyson MP, et al. Burden of illness in infants and young children hospitalized for respiratory syncytial virus: A rapid review. Can Commun Dis Rep. 2021 Sep 10;47(9):381-96. https://doi.org/10.14745/ccdr.v47i09a05.
- Footnote 12
-
Abrams EM, Doyon-Plourde P, Davis P, Brousseau N, Irwin A, Siu W, et al. Burden of disease of respiratory syncytial virus in infants, young children and pregnant women and people. Canada Communicable Disease Report. 2024 Feb;50(1/2):1-15. https://doi.org/10.14745/ccdr.v50i12a01.
- Footnote 13
-
Groves HE, Piché-Renaud P, Peci A, Farrar DS, Buckrell S, Bancej C, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Regional Health - Americas. 2021 Sept 01;1:100015. https://doi.org/10.1016/j.lana.2021.100015.
- Footnote 14
-
Bourdeau M, Vadlamudi NK, Bastien N, Embree J, Halperin SA, Jadavji T, et al. Pediatric RSV-associated hospitalizations before and during the COVID-19 pandemic. JAMA Network Open. 2023 Oct 04;6(10):e2336863. https://doi.org/10.1001/jamanetworkopen.2023.36863.
- Footnote 15
-
Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nature Reviews. Microbiology. 2023 Mar 1;21(3):195-210. https://doi.org/10.1038/s41579-022-00807-9.
- Footnote 16
-
Viñeta Paramo M, Ngo LPL, Abu-Raya B, Reicherz F, Xu RY, Bone JN, et al. Respiratory syncytial virus epidemiology and clinical severity before and during the COVID-19 pandemic in British Columbia, Canada: A retrospective observational study. Lancet Regional Health - Americas. 2023 Sep 1;25:100582. https://doi.org/10.1016/j.lana.2023.100582.
- Footnote 17
-
Bourgeois FT, Valim C, McAdam AJ, Mandl KD. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics (Evanston). 2009 Dec 1;124(6):e1072-80. https://doi.org/10.1542/peds.2008-3074.
- Footnote 18
-
Ambrose CS, Anderson EJ, Simões EAF, Wu X, Elhefni H, Park CL, et al. Respiratory syncytial virus disease in preterm infants in the US born at 32–35 weeks gestation not receiving immunoprophylaxis. The Pediatric Infectious Disease Journal. 2014 Jun;33(6):576-82. https://doi.org/10.1097/INF.0000000000000219.
- Footnote 19
-
Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. The New England Journal of Medicine. 2009 Feb 5;360(6):588-98. https://doi.org/10.1056/NEJMoa0804877.
- Footnote 20
-
Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P, Steiner C, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993—2008. Clinical Infectious Diseases. 2012 May 15;54(10):1427-36. https://doi.org/10.1093/cid/cis211.
- Footnote 21
-
Fleming DM, Pannell RS, Elliot AJ, Cross KW. Respiratory illness associated with influenza and respiratory syncytial virus infection. Archives of Disease in Childhood. 2005 Jul 1;90(7):741-6. https://doi.org/10.1136/adc.2004.063461.
- Footnote 22
-
Bont L, Checchia PA, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther. 2016 Sep 1;5(3):271-98. https://doi.org/10.1007/s40121-016-0123-0.
- Footnote 23
-
Amini R, Gilca R, Boucher FD, Charest H, De Serres G. Respiratory syncytial virus contributes to more severe respiratory morbidity than influenza in children <;2 years during seasonal influenza peaks. Infection. 2019 Aug 1;47(4):595-601. https://doi.org/10.1007/s15010-019-01287-5.
- Footnote 24
-
Reichert H, Suh M, Jiang X, Movva N, Bylsma LC, Fryzek JP, et al. Mortality associated with respiratory syncytial virus, bronchiolitis, and influenza among infants in the United States: a birth cohort study from 1999 to 2018. The Journal of Infectious Diseases. 2022 Aug 15;226(Supplement_2):S246-54. https://doi.org/10.1093/infdis/jiac127.
- Footnote 25
-
Hause AM, Avadhanula V, Maccato ML, Pinell PM, Bond N, Santarcangelo P, et al. Clinical characteristics and outcomes of respiratory syncytial virus infection in pregnant women. Vaccine. 2019 Jun 6;37(26):3464-71. https://doi.org/10.1016/j.vaccine.2019.04.098.
- Footnote 26
-
Nowalk MP, D'Agostino H, Dauer K, Stiegler M, Zimmerman RK, Balasubramani GK. Estimating the burden of adult hospitalized RSV infection including special populations. Vaccine. 2022 Jun 3;40(31):4121-7. https://doi.org/10.1016/j.vaccine.2022.05.077.
- Footnote 27
-
Canadian Institute for Health Information. Discharge Abstract Database (DAD) metadata. Data cut-off Sept 2014 - August 2023 [Internet]. Canadian Institute for Health Information [cited 2024 Mar 7]. Available from: https://www.cihi.ca/en/discharge-abstract-database-dad-metadata.
- Footnote 28
-
Yukon Communicable Disease Control, Health and Social Services. Data cut-off May 30, 2023. [Internet]. Whitehorse (YT): Government of Yukon; 2023 [cited 2023 May 30].
- Footnote 29
-
Gilca R, Billard M, Zafack J, Papenburg J, Boucher FD, Charest H, et al. Effectiveness of palivizumab immunoprophylaxis to prevent respiratory syncytial virus hospitalizations in healthy full-term <6-month-old infants from the circumpolar region of Nunavik, Quebec, Canada. Preventive Medicine Reports. 2020 Dec 1;20:101180. https://doi.org/10.1016/j.pmedr.2020.101180.
- Footnote 30
-
Banerji A, Lanctôt KL, Paes BA, Masoud ST, Tam DY, Macdonald WA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. The Pediatric Infectious Disease Journal. 2009 Aug;28(8):702-6. https://doi.org/10.1097/INF.0b013e31819df78e.
- Footnote 31
-
Kinshella MW, Allen J, Pawa J, Papenburg J, Jetty R, Dwilow R, et al. Hospital admissions for acute respiratory tract infections among infants from Nunavut and the burden of respiratory syncytial virus: A 10-year review in regional and tertiary hospitals. medRxiv. 2024 Feb 23:2024.02.21.24303174. https://doi.org/10.1101/2024.02.21.24303174.
- Footnote 32
-
AstraZeneca Canada. SYNAGIS®(palivizumab) Product Monograph [Internet]. Ontario: AstraZeneca Canada Inc.; 2022 Feb 24 [cited 2024 Mar 22]. Available from: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/synagis-product-monograph-en.pdf.
- Footnote 33
-
Sanofi Pasteur. BEYFORTUS®(nirsevimab) Product Monograph [Internet]. Ontario: Sanofi Pasteur Limited; 2024 Mar 14 [cited 2024 Mar 22]. Available from: https://www.sanofi.com/assets/countries/canada/docs/products/vaccines/beyfortus-en.pdf.
- Footnote 34
-
Pfizer Canada. ABRYSVO™ (respiratory syncytial virus stabilized prefusion F subunit vaccine) product monograph [Internet]. Quebec: Pfizer Canada; 2023 Dec 21 [cited 2024 Mar 15]. Available from: https://pdf.hres.ca/dpd_pm/00073900.PDF.
- Footnote 35
-
Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. The New England Journal of Medicine. 2020 Jul 30;383(5):415-25. https://doi.org/10.1056/NEJMoa1913556.
- Footnote 36
-
AstraZeneca. BEYFORTUS (Nirsevimab) for the prevention of RSV lower respiratory tract disease in infants and children [briefing document for Antimicrobial Drugs Advisory Committee Meeting June 8, 2023]. FDA; 2023 May 17. Table 8, Analysis Populations – Trial 04 and Study Trial 03; p. 51. Available from: https://www.fda.gov/media/169228/download.
- Footnote 37
-
Muller WJ, Madhi SA, Seoane Nuñez B, Baca Cots M, Bosheva M, Dagan R, et al. Nirsevimab for prevention of RSV in term and late-preterm infants. The New England Journal of Medicine. 2023 Apr 20;388(16):1533-4. https://doi.org/10.1056/NEJMc2214773.
- Footnote 38
-
Domachowske J, Madhi SA, Simões EAF, Atanasova V, Cabañas F, Furuno K, et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. The New England Journal of Medicine. 2022 Mar 3;386(9):892-4. https://doi.org/10.1056/NEJMc2112186.
- Footnote 39
-
Domachowske JB, Chang Y, Atanasova V, Caba Amp X F As F, Furuno K, Nguyen KA, et al. Safety of re-dosing nirsevimab prior to RSV season 2 in children with heart or lung disease. Journal of the Pediatric Infectious Diseases Society. 2023 Aug 31;12(8):477-80. https://doi.org/10.1093/jpids/piad052.
- Footnote 40
-
Simões EAF, Madhi SA, Muller WJ, Atanasova V, Bosheva M, Cabañas F, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. The Lancet Child & Adolescent Health. 2023 Mar 1;7(3):180-9. https://doi.org/10.1016/S2352-4642(22)00321-2
- Footnote 41
-
Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. The New England Journal of Medicine. 2023 Dec 28;389(26):2425-35. https://doi.org/10.1056/NEJMoa2309189.
- Footnote 42
-
Simões EAF, Center KJ, Tita ATN, Swanson KA, Radley D, Houghton J, et al. Prefusion F protein–based respiratory syncytial virus immunization in pregnancy. The New England Journal of Medicine. 2022 Apr 28;386(17):1615-26. https://doi.org/10.1056/NEJMoa2106062.
- Footnote 43
-
Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. The New England Journal of Medicine. 2023 Apr 20;388(16):1451-64. https://doi.org/10.1056/NEJMoa2216480.
- Footnote 44
-
AstraZeneca. BEYFORTUS (Nirsevimab) for the prevention of RSV lower respiratory tract disease in infants and children [briefing document for Antimicrobial Drugs Advisory Committee Meeting June 8, 2023]. FDA; 2023 May 17. Table 16, Healthcare Resource Utilization for MA RSV LRTI Through 150 Days Post Dose; p. 63. Available from: https://www.fda.gov/media/169228/download.
- Footnote 45
-
Advisory Committee on Immunization Practices (ACIP). Grading of recommendations, assessment, development, and evaluation (GRADE): Pfizer maternal RSV vaccine [Internet]. CDC; 2023 Sep 28 [cited 2023 Dec 28]. Available from: https://www.cdc.gov/vaccines/acip/recs/grade/pfizer-RSVpreF-pregnant-people.html#table-3c.
- Footnote 46
-
AstraZeneca. BEYFORTUS (Nirsevimab) for the prevention of RSV lower respiratory tract disease in infants and children [briefing document for Antimicrobial Drugs Advisory Committee Meeting June 8, 2023]. FDA; 2023 May 17. Table 13, Summary of efficacy against MA RSV LRTI with hospitalization through 150 days post dose; p. 38. Available from: https://www.fda.gov/media/169228/download.
- Footnote 47
-
AstraZeneca. A study to evaluate the safety of MEDI8897 for the prevention of medically attended respiratory syncytial virus (RSV) lower respiratory track infection (LRTI) in high-risk children [Internet]. ClinicalTrials.gov; 2023 Sept 21 [cited 2024 Mar 15]. Available from: https://clinicaltrials.gov/study/NCT03959488?term=MEDLEY&rank=4&tab=results.
- Footnote 48
-
Advisory Committee on Immunization Practices (ACIP). Grading of recommendations, assessment, development, and evaluation (GRADE): Nirsevimab, season 1 [Internet]. CDC; 2023 Aug 24 [cited 2023 Dec 28]. Available from: https://www.cdc.gov/vaccines/acip/recs/grade/nirsevimab-season1-rsv-infants-children.html#table-3a.
- Footnote 49
-
Brady MT, Byington CL, Davies HD, Edwards KM, Jackson MA, Maldonado YA, et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics (Evanston). 2014 Aug 1;134(2):e620-38. https://doi.org/10.1542/peds.2014-1666.
- Footnote 50
-
Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532,540. 18 ref. https://doi.org/10.1067/S0022-3476(03)00454-2.
- Footnote 51
-
Esposito S, Abu-Raya B, Bonanni P, Cahn-Sellem F, Flanagan KL, Martinon Torres F, et al. Coadministration of anti-viral monoclonal antibodies with routine pediatric vaccines and implications for nirsevimab use: A white paper. Frontiers in Immunology. 2021 Aug 11;12:708939. https://doi.org/10.3389/fimmu.2021.708939.
- Footnote 52
-
AstraZeneca. BEYFORTUS (Nirsevimab) for the prevention of RSV lower respiratory tract disease in infants and children [briefing document for Antimicrobial Drugs Advisory Committee Meeting June 8, 2023]. AstraZeneca; 2023 May 17. Available from: https://www.fda.gov/media/169228/download.
- Footnote 53
-
Public Health Agency of Canada (PHAC). Timing of vaccine administration: Canadian Immunization Guide [Internet]. Ottawa (ON): Government of Canada; 2017 May [cited 2023 Dec 18]. Available from: /en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-10-timing-vaccine-administration.html#p1c9a4.
- Footnote 54
-
Peterson JT, Zareba AM, Fitz-Patrick D, Essink BJ, Scott DA, Swanson KA, et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F vaccine when co-administered with a tetanus, diphtheria, and acellular pertussis vaccine. J Infect Dis. 2022 Jun 15;225(12):2077-86. https://doi.org/10.1093/infdis/jiab505.
- Footnote 55
-
Falsey AR, Walsh EE, Scott DA, Gurtman A, Zareba A, Jansen KU, et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J Infect Dis. 2022;225(12):2056-66.
- Footnote 56
-
Walsh EE, Falsey AR, Scott DA, Gurtman A, Zareba AM, Jansen KU, et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis. 2022;225(8):1357-66. https://doi.org/10.1093/infdis/jiab612.
- Footnote 57
-
Pfizer. A study to describe the safety and immunogenicity of a RSV vaccine in healthy adults [Internet]. ClinicalTrials.gov; 2023 Mar 3 [cited 2024 Mar 15]. Available from: https://clinicaltrials.gov/study/NCT03529773?term=NCT03529773&rank=1&tab=results.
- Footnote 58
-
AstraZeneca. BEYFORTUS (Nirsevimab) for the prevention of RSV lower respiratory tract disease in infants and children [briefing document for Antimicrobial Drugs Advisory Committee Meeting June 8, 2023]. FDA; 2023 May 17. Table 23, Overall summary of treatment-emergent adverse events through at least 150 days post dose – proposed-dose safety pool; p. 83. Available from: https://www.fda.gov/media/169228/download.
- Footnote 59
-
AstraZeneca. BEYFORTUS (Nirsevimab) for the prevention of RSV lower respiratory tract disease in infants and children [briefing document for Antimicrobial Drugs Advisory Committee Meeting June 8, 2023]. FDA; 2023 May 17. Table 25, Overall summary of treatment-emergent adverse events through 360 days post first dose in RSV season 1 – trial 05 and Table 26, Overall summary of treatment-emergent adverse events through at least 150 days post first dose in season 2 – trial 05; p. 87-89. Available from: https://www.fda.gov/media/169228/download.
- Footnote 60
-
Pfizer. Respiratory syncytial virus stabilized bivalent prefusion F subunit vaccine (RSVPREF / ABRYSVO) [briefing document from Vaccines and Related Biological Products Advisory Committee Meeting May 18, 2023]. FDA; 2023 May 18. Table 7, Number (%) of participants reporting adverse events by category within 1 month after vaccination - maternal participants - safety population and Table 10, Number (%) of participants reporting adverse events by category within 1 month after birth - infant participants - safety population; p.50-51 & 56-57. Available from: https://www.fda.gov/media/168186/download.
- Footnote 61
-
Pfizer. A phase 2B placebo-controlled, randomized study of a respiratory syncytial virus (RSV) vaccine in pregnant women [Internet]. ClinicalTrials.gov; 2023 Oct 18 [cited 2024 Mar 15]. Available from: https://clinicaltrials.gov/study/NCT04032093?term=NCT04032093&rank=1&tab=results.
- Footnote 62
-
Pfizer. Respiratory syncytial virus stabilized bivalent prefusion F subunit vaccine (RSVPREF / ABRYSVO) [briefing document from Vaccines and Related Biological Products Advisory Committee Meeting May 18, 2023]. FDA; 2023 May 18. Table 22, Systemic events, by maximum severity within 7 days before and within 7 days after vaccination - maternal participants - safety population; p.85-87. Available from: https://www.fda.gov/media/168186/download.
- Footnote 63
-
Pfizer. Respiratory syncytial virus vaccine (proposed trade name: Abrysvo) [briefing document from Vaccines and Related Biological Products Advisory Committee Meeting May 18, 2023]. FDA; 2023 May 18. Table 11, Local reactions, by maximum severity, within 7 days after vaccination, maternal participants, safety population; p. 30. Available from: https://www.fda.gov/media/168185/download.
- Footnote 64
-
Pfizer. Respiratory syncytial virus stabilized bivalent prefusion F subunit vaccine (RSVPREF / ABRYSVO) [briefing document from Vaccines and Related Biological Products Advisory Committee Meeting May 18, 2023]. FDA; 2023 May 18. Table 32, Adverse Events by Category Reported After Birth - Infant Participants - Safety Population; p.100. Available from: https://www.fda.gov/media/168186/download.
- Footnote 65
-
Pfizer. Respiratory syncytial virus stabilized bivalent prefusion F subunit vaccine (RSVPREF / ABRYSVO) [briefing document from Vaccines and Related Biological Products Advisory Committee Meeting May 18, 2023]. FDA; 2023 May 18. Available from: https://www.fda.gov/media/168186/download.
- Footnote 66
-
Pfizer. Respiratory syncytial virus vaccine (proposed trade name: Abrysvo) [briefing document from Vaccines and Related Biological Products Advisory Committee Meeting May 18, 2023]. FDA; 2023 May 18. Table 15, Live birth outcomes, infant participants, safety population and Table 23, Birth outcomes, study 1003, maternal participants, safety population; p.35-36 & 46. Available from: https://www.fda.gov/media/168185/download.
- Footnote 67
-
Statistics Canada. Health fact sheets preterm live births in Canada, 2000 to 2013 [Internet]. Statistics Canada; 2016 Oct 26 [cited 2024 Feb 27]. Available from: https://www150.statcan.gc.ca/n1/pub/82-625-x/2016001/article/14675-eng.htm.
- Footnote 68
-
Shah PS, Ye XY, Yang J, Campitelli MA. Preterm birth and stillbirth rates during the COVID-19 pandemic: A population-based cohort study. Canadian Medical Association Journal (CMAJ). 2021 Aug 3;193(30):E1164-72. https://doi.org/10.1503/cmaj.210081.
- Footnote 69
-
Fleming-Dutra K. Evidence to recommendations framework updates Pfizer maternal RSVpreF vaccine [slides presented at ACIP General Meeting on Sep 22, 2023] [Internet]. CDC; 2023 Sep 22. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/06-Mat-Peds-Fleming-Dutra-508.pdf.
- Footnote 70
-
GSK. RSVPreF3 OA [briefing document provided at the Vaccines and Related Biological Products Advisory Committee Meeting on February 28-March 1, 2023]. FDA U.S. Food & Drug Administration; 2023 Feb 28 [cited 2024 Mar 05]. Available from: https://www.fda.gov/media/165621/download.
- Footnote 71
-
Dieussaert I, Kim JH, Luik S, Seidl C, Pu W, Stegmann J, et al. RSV prefusion F protein–based maternal vaccine — preterm birth and other outcomes. The New England Journal of Medicine. 2024 Mar 14;390:1009-21. https://doi.org/10.1056/NEJMoa2305478.
- Footnote 72
-
Moro PL, Gallego R, Scheffey A, Fleming-Dutra KE, Hall E, Zhang B, et al. Administration of the GSK Respiratory Syncytial Virus Vaccine to Pregnant Persons in Error. Obstetrics and Gynecology. 2024 Feb 23. https://doi.org/10.1097/AOG.0000000000005551.
- Footnote 73
-
Public Health Agency of Canada (PHAC). Immunization of immunocompromised persons: Canadian Immunization Guide [Internet]. Ottawa (ON): Government of Canada; 2018 May [cited 2024 Feb 28]. Available from: /en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html.
- Footnote 74
-
Pfizer. Prescribing information ABRYSVO™ (respiratory syncytial virus vaccine) solution [Internet]. FDA; 2023 Aug [cited 2024 Mar 15]. Available from: https://www.fda.gov/media/168889/download.
- Footnote 75
-
Advisory Committee on Immunization Practices (ACIP). Guiding principles for development of ACIP recommendations for vaccination during pregnancy and breastfeeding. Morb Mortal Weekly Rep. 2008;57(21):580.
- Footnote 76
-
Robinson JL, Le Saux N. Preventing hospitalizations for respiratory syncytial virus infection. Paediatrics & Child Health. 2015 Aug 1;20(6):321-6. https://doi.org/10.1093/pch/20.6.321.
- Footnote 77
-
Verbrugghe S, Mahood Q, Tiggelaar S. Cost-Effectiveness of an RSVpreF Vaccine for Prevention of Respiratory Syncytial Virus Outcomes in Infants. Canadian Journal of Health Technologies. 2023 Aug 8;3(8). https://doi.org/10.51731/cjht.2023.711.
- Footnote 78
-
Brown R, Tiggelaar S, Tsoi B, Cromwell I. Cost-effectiveness of nirsevimab for the prevention of respiratory syncytial virus infection in infants. Canadian Journal of Health Technologies. 2023 Oct 19;3(10). https://doi.org/10.51731/cjht.2023.760.
- Footnote 79
-
Kieffer A, Beuvelet M, Sardesai A, Musci R, Milev S, Roiz J, et al. Expected impact of universal immunization with nirsevimab against RSV-related outcomes and costs among all US infants in their first RSV season: A static model. The Journal of Infectious Diseases. 2022 Aug 15;226(Supplement_2):S282-92. https://doi.org/10.1093/infdis/jiac216.
- Footnote 80
-
Regnier SA. Respiratory syncytial virus immunization program for the United States: Impact of performance determinants of a theoretical vaccine. Vaccine. 2013 Sep 13;31(40):4347-54. https://doi.org/10.1016/j.vaccine.2013.07.024.
- Footnote 81
-
Hodgson D, Koltai M, Krauer F, Flasche S, Jit M, Atkins KE. Optimal respiratory syncytial virus intervention programmes using Nirsevimab in England and Wales. Vaccine. 2022 Nov 22;40(49):7151-7. https://doi.org/10.1016/j.vaccine.2022.10.041.
- Footnote 82
-
Joanna Briggs Institute (JBI). Critical appraisal checklist for economic evaluations [Internet]. JBI; 2020 [cited 2024 Feb 1]. Available from: https://jbi.global/critical-appraisal-tools.
- Footnote 83
-
Nourbakhsh S, Shoukat A, Zhang K, Poliquin G, Halperin D, Sheffield H, et al. Effectiveness and cost-effectiveness of RSV infant and maternal immunization programs: A case study of Nunavik, Canada. EClinicalMedicine. 2021 Nov 1;41:101141. https://doi.org/10.1016/j.eclinm.2021.101141.
- Footnote 84
-
84Shoukat A, Abdollahi E, Galvani AP, Halperin SA, Langley JM, Moghadas SM. Cost-effectiveness analysis of nirsevimab and maternal RSVpreF vaccine strategies for prevention of respiratory syncytial virus disease among infants in Canada: a simulation study. Lancet Regional Health - Americas. 2023 Nov 9;28:100629. https://doi.org/10.1016/j.lana.2023.100629.
- Footnote 85
-
Getaneh AM, Li X, Mao Z, Johannesen CK, Barbieri E, van Summeren J, et al. Cost-effectiveness of monoclonal antibody and maternal immunization against respiratory syncytial virus (RSV) in infants: Evaluation for six European countries. Vaccine. 2023 Feb 24;41(9):1623-31. https://doi.org/10.1016/j.vaccine.2023.01.058.
- Footnote 86
-
Li X, Bilcke J, Vázquez Fernández L, Bont L, Willem L, Wisløff T, et al. Cost-effectiveness of respiratory syncytial virus disease prevention strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. The Journal of Infectious Diseases. 2022 Aug 12;226(Supplement_1):S95-S101. https://doi.org/10.1093/infdis/jiac064.
- Footnote 87
-
Hutton DW. Economics of Pfizer maternal RSVpreF vaccine [slides presented at Advisory Committee on Immunization (ACIP) Meeting June 22, 2023]. CDC; 2023 Jun 22. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-RSV-Mat-Ped-Hutton-508.pdf.
- Footnote 88
-
Hutton DW. Economic analysis of nirsevimab in pediatric populations [slides presented at Advisory Committee on Immunization (ACIP) General Meeting February 23, 2023]. CDC; 2023 Feb 23. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Pediatric-02-Hutton-508.pdf.
- Footnote 89
-
Hutton DW. Economic analysis of RSVpreF maternal vaccination [slides presented at Advisory Committee on Immunization (ACIP) Meeting September 22, 2023]. CDC; 2023 Sep 22. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/04-Mat-Peds-Hutton-508.pdf.
- Footnote 90
-
Hutton DW. Economics of combined use of Pfizer maternal RSVpreF vaccine and nirsevimab [slides presented at Advisory Committee on Immunization (ACIP) Meeting June 22, 2023]. CDC; 2023 Jun 22. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/04-RSV-Mat-Ped-Hutton-508.pdf.
- Footnote 91
-
Hodgson D, Wilkins N, van Leeuwen E, Watson CH, Crofts J, Flasche S, et al. Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination. The Lancet Regional Health - Europe. 2024 Jan 8;38:100829. https://doi.org/10.1016/j.lanepe.2023.100829.
- Footnote 92
-
Gebretekle BG, Yeung MW, Ximenes R, Cernat A, Alison ES, Killikelly A, et al. Cost-effectiveness of RSVpreF vaccine and nirsevimab for the prevention of respiratory syncytial virus disease in Canadian infants. medRxiv. 2024 Mar 24. https://doi.org/10.1101/2024.03.21.24304675.
- Footnote 93
-
Nguyen NH, Subhan FB, Williams K, Chan CB. Barriers and mitigating strategies to healthcare access in Indigenous communities of Canada: A narrative review. Healthcare. 2020 Apr 26;8(2):112. https://doi.org/10.3390/healthcare8020112.
- Footnote 94
-
Prendergast C, Robinson J, Caya C, Perez Trejo ME, Guan I, Hébert-Murakami V, et al. Urgent air transfers for acute respiratory infections among children from Northern Canada, 2005–2014. PLOS One. 2022 Jul 28;17(7):e0272154. https://doi.org/10.1371/journal.pone.0272154.
- Footnote 95
-
Lorcy A, Gilca R, Dubé E, Rochette M, De Serres G. Feasibility and ethical issues: experiences and concerns of healthcare workers regarding a new RSV prophylaxis programme in Nunavik, Quebec. International Journal of Circumpolar Health. 2020 Jan 1;79(1):1742564. https://doi.org/10.1080/22423982.2020.1742564.
- Footnote 96
-
Office of the High Commissioner for Human Rights. UN declaration on the rights of Indigenous Peoples [Internet]. United Nations; 2007 Sept 13 [cited 2023 Dec 29]. Available from: https://www.ohchr.org/en/indigenous-peoples/un-declaration-rights-indigenous-peoples.
- Footnote 97
-
Stoll K, Wang JJ, Niles P, Wells L, Vedam S. I felt so much conflict instead of joy: an analysis of open-ended comments from people in British Columbia who declined care recommendations during pregnancy and childbirth. Reproductive Health. 2021 Apr 15;18(1):79. https://doi.org/10.1186/s12978-021-01134-7.
- Footnote 98
-
Statistics Canada. Childhood national immunization coverage survey (CNICS) 2021 [Internet]. Statistics Canada: Statistics Canada; 2023 Jun 12 [cited 2024 Mar 6]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/230612/dq230612b-eng.htm.
- Footnote 99
-
Dugovich AM, Cox TH, Weeda ER, Garner SS. First hepatitis B vaccine uptake in neonates prior to and during the COVID-19 pandemic. Vaccine. 2023 Apr 24;41(17):2824-8. https://doi.org/10.1016/j.vaccine.2023.03.039.
- Footnote 100
-
The COVID-19 snapshot monitoring study (COSMO) in Canada. Data collected: COSMO Wave 2.8, May 19 – June 6, 2023. [Unpublished]. Impact Canada & Behavioural Science Office; 2023 Jun.
- Footnote 101
-
Dirección Xeral de Saúde Pública. Follow-up report on immunization with nirsevimab in Galicia [Internet]. Galicia: Xunta de Galicia; 2024 Jan 31 [cited 2024 Feb 1]. Available from: https://assets-global.website-files.com/65774b0d3a50ee58b24dba82/65ba733ec40156763da2918e_Report_RSV_week4.pdf.
- Footnote 102
-
McCormack S, Thompson C, Chathasaigh CN, Nolan M, Imcha M, Dee A, et al. Maternal awareness, acceptability and willingness towards respiratory syncytial virus (RSV) vaccination during pregnancy. Arch.Dis.Child. 2019;104:A135. https://doi.org/10.1136/archdischild-2019-epa.312.
- Footnote 103
-
Wilcox CR, Calvert A, Metz J, Kilich E, MacLeod R, Beadon K, et al. Attitudes of pregnant women and healthcare professionals toward clinical trials and routine implementation of antenatal vaccination against respiratory syncytial virus: A multicenter questionnaire study. The Pediatric Infectious Disease Journal. 2019 Sep;38(9):944-51. https://doi.org/10.1097/INF.0000000000002384.
- Footnote 104
-
HABIT study: health attitudes, and behavioural insights tracker. Data collected: September 27 to October 9, 2023. [Unpublished]. Impact Canada & Behavioural Science Office; 2023 Nov.
- Footnote 105
-
Public Health Agency of Canada (PHAC). Results of the survey on vaccination during pregnancy 2021 [Internet]. Ottawa (ON): Government of Canada; 2022 Dec 13 [cited 2024 Mar 6]. Available from: /en/public-health/services/publications/vaccines-immunization/survey-vaccination-during-pregnancy-2021.html.
- Footnote 106
-
Centers for Disease Control and Prevention, (CDC). Weekly respiratory syncytial virus (RSV) vaccination dashboard. Data cut-off January 27, 2024 [Internet]. CDC; 2024 Feb 28 [cited 2024 Jan 18]. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/rsvvaxview/index.html.
- Footnote 107
-
Public Health Agency of Canada (PHAC). COVID-19 vaccines: Canadian Immunization Guide [Internet]. Ottawa (ON): Government of Canada; 2023 Dec 15 [cited 2023 Dec 18]. Available from: /en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html#a6.4.