Archived - Enteric infections in British Columbia

 Download this article as a PDF (402 KB - 6 pages)
Download this article as a PDF (402 KB - 6 pages) Published by: The Public Health Agency of Canada
Issue: Volume 42-2: Update on STIs
Date published: February 4, 2016
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 42-2, February 4, 2016: Update on STIs
Surveillance
Are enteric infections sexually transmitted in British Columbia?
Narayan S1*, Galanis E2, BC STEI Group3
Affiliations
1 Simon Fraser University, Burnaby, BC
2 BC Centre for Disease Control, Vancouver, BC
3 BC STEI group membership: Forsting S4, Hoang L2, Jeyes J5, Nowakowski C6, Ritson M4, Stone J7, Tone G8.
4 Vancouver Coastal Health Authority, Vancouver, BC
5 Interior Health Authority, Kelowna, BC
6 Vancouver Island Health Authority, Victoria, BC
7 Fraser Health Authority, Surrey, BC
8 First Nations Health Authority, Prince George, BC
Correspondence
Suggested citation
Nayan S, Galanis E. BC STEI Group. Are enteric infections sexually transmitted in British Columbia? Can Comm Dis Rep 2016; 42-2:24-29. https://doi.org/10.14745/ccdr.v42i02a01
Abstract
Background: Enteric infections may on occasion be sexually transmitted, particularly among people who engage in oral-anal sexual contact. Although outbreaks of enteric infections have been reported among men who have sex with men (MSM) in British Columbia (BC), the epidemiology of sexually transmitted enteric infections has never been assessed.
Objective: To describe the epidemiology of enteric infections in BC in order to determine if sexual transmission may be occurring.
Methods: A descriptive analysis was conducted of all reported cases of shigellosis, amebiasis and giardiasis in BC for the period 2003-2012.
Results: For shigellosis and amebiasis, there was a high male-to-female ratio and a higher rate of infection in males aged 20-59 years as compared to all other age-sex groups. Additionally, for shigellosis, adult males were significantly more likely than females to acquire disease locally (RR 1.9; CI 1.7-2.4).
Conclusion: Analysis suggests that sexual transmission of enteric infections, particularly shigellosis and amebiasis, may be occurring in MSM in BC. Further studies are indicated.
Introduction
Enteric pathogens are most commonly transmitted through consumption of contaminated food or water Footnote 1Footnote 2Footnote 3. However, some enteric pathogens can also be transmitted through sexual practices involving fecal-oral contact, such as oral-anal, oral-genital and anal-genital intercourse Footnote 4Footnote 5Footnote 6. Although these sexually-transmitted enteric infections (STEIs) can occur in heterosexual individuals who engage in unprotected anal sexual contact, they are more common in men who have sex with men (MSM) than any other adult populations Footnote 4Footnote 5Footnote 7Footnote 8Footnote 9.
Pathogens transmitted sexually include Entamoeba histolytica, Giardia lamblia, Shigella Footnote 3Footnote 5Footnote 7Footnote 10Footnote 11Footnote 12 Salmonella Footnote 13 and Campylobacter Footnote 14. However, Entamoeba histolytica, Giardia lambliaand Shigella are most commonly STEIs Footnote 8Footnote 15Footnote 16. Inadvertent ingestion of minute amounts of feces containing as few as 10-100 organisms of Shigella bacteria, Entamoeba histolytica or Giardia lamblia cysts, during oral-anal sexual contacts could deliver a sufficient dose to cause infection. This low infectious dose also explains the tendency of these three pathogens to easily spread from person-to-person Footnote 7Footnote 17Footnote 18.
The incubation period for shigellosis is short; one to two days. It is characterized by diarrhea (which may be bloody and contain pus), fever and tenesmus, and is usually a self-limiting infection Footnote 12. Although 90% of Entamoeba histolytica infections are asymptomatic, fever, diarrhea and abdominal cramps can occur two to four weeks after exposure to the parasites. The infection resolves with treatment in two weeks Footnote 12. Giardiasis is usually asymptomatic in humans but may produce low-grade fever, foul smelling diarrhea and abdominal cramps and bloating, one to two weeks after exposure. Symptoms usually last one to three weeks and people with healthy immune systems normally clear the infection on their own. Treatment may be required for immunocompromised patients Footnote 12Footnote 19.
Shigellosis, amebiasis and giardiasis are reportable communicable disease in British Columbia (BC). While past reports have highlighted outbreaks of shigellosis among the MSM population in BC Footnote 20Footnote 21Footnote 22 the epidemiology of STEIs, including shigellosis, has never been assessed in BC and is therefore not well understood. The objective of this study was to describe the epidemiology of these three infections in BC to determine if sexual transmission may be occurring and to identify the population and regions at risk of STEI.
Methods
A retrospective descriptive analysis was conducted of shigellosis, amebiasis and giardiasis cases reported in BC for the period 2003-2012. All cases were laboratory-confirmed and identified from the Integrated Public Health Information System (iPHIS). Exposure information including sexual activity and travel history was reviewed for all cases. Travel information was obtained from the Primary Access Regional Information Systems (PARIS) for Vancouver Coastal Health Authority cases and from iPHIS for the remaining health authorities. Travel information was classified as either "international travel" (if travel outside of Canada was reported within four days of symptom onset) or "local" (if the case reported no travel, or travel within Canada, within BC, or within the health authority of residence) Footnote 23. Exposure information was available only for shigellosis and amebiasis as giardiasis cases are not routinely followed-up by public health authorities in BC. Information on outbreaks was obtained from the Canadian Network for Public Health Intelligence outbreak summary module, BC Centre for Disease Control outbreak investigation reports and discussion with experts.
Statistical analyses included case counts and incidence rates (IR) by year, geography, sex and age groups. Population estimates for IR calculation were obtained from BC Stats. Cases were grouped into one of four age groups. The 20-59 year age group represented the sexually active population because it was appeared to have an excess number of adult male cases in the preliminary analysis.
Data were analyzed using Microsoft Excel® 2007 and OpenEpi software (version 2.3.1). Chi-square tests were used to compare proportions of shigellosis cases associated with international travel to that of local cases. A p-value of <0.05 was considered to be significant. Relative risk with 95% confidence intervals was calculated to compare the risk of shigellosis in these two groups.
Results
Overall, Vancouver Coastal Health Authority reported the highest average annual IR for all three infections, followed by Fraser Health Authority, Island Health Authority, Interior Health Authority and Northern Health Authority (Table 1). A higher male-to-female ratio was observed for all three infections in all health authorities; however, the majority of shigellosis and amebiasis was reported in those 20-59 years of age (5.9 and 11.2 per 100,000 population) and for giardiasis in children aged 0-9 years (26.9 per 100,000 population).
The average annual IR for shigellosis was 4.6 per 100,000 population (Table 1). The annual IR fluctuated with peak rates seen in 2003, 2005 and 2007. A declining rate was observed, from 6.3 in 2007 to 3.8 per 100,000 population in 2012 (data not shown). Overall, rates were higher in males than females (male-to-female ratio of 1.5:1), with highest IR among males aged 20-59 years (7.3 per 100,000 population) (Figure 1). Vancouver health services delivery area reported the highest average annual rate at 11.2 per 100,000 population (Figure 2).
| Characteristics | Giardiasis (IR [number]) |
Amebiasis (IR [number]) |
Shigellosis (IR [number]) |
|---|---|---|---|
| Provincial average annual IR | 15.2 (6,593) | 7.7 (3,359) | 4.6 (1,986) |
| SexTable 1 - Note 1 | |||
| Female | 12.1 (2,648) | 4.2 (928) | 3.7 (806) |
| Male | 18.3 (3,933) | 11.2 (2,422) | 5.5 (1,176) |
| Male : female | 1.5:1 | 2.6:1 | 1.5:1 |
| Age group (males + females) | |||
| 0 to 9 years | 26.9 (1,184) | 4.1 (159) | 4.8 (200) |
| 10 to 19 years | 10.0 (534) | 3.9 (189) | 2.5 (131) |
| 20 to 59 years | 16.6 (4,177) | 11.2 (2,542) | 5.9 (1,393) |
| 60+ years | 7.9 (698) | 6.1 (469) | 3.2 (262) |
| Health authority | |||
| Fraser Health Authority | 16.4 (2,496) | 7.8 (1,199) | 4.6 (705) |
| Interior Health Authority | 10.7 (757) | 1.2 (89) | 1.8 (127) |
| Northern Health Authority | 9.8 (279) | 0.2 (5) | 1.2 (34) |
| Vancouver Coastal Health Authority | 20.4 (2,225) | 16.2 (1,768) | 8.2 (894) |
| Island Health Authority | 11.4 (836) | 4.0 (298) | 3.1 (226) |
| Age group 20-59 years* | |||
| Female | 12.6 (1,592) | 5.6 (630) | 4.6 (541) |
| Male | 20.6 (2,578) | 16.7 (1,905) | 7.3 (848) |
| Male-to-female ratio | 1.6:1 | 3.0:1 | 1.6:1 |
Figure 1: Distribution of shigellosis cases by age category and sex for BC, 2003-2012
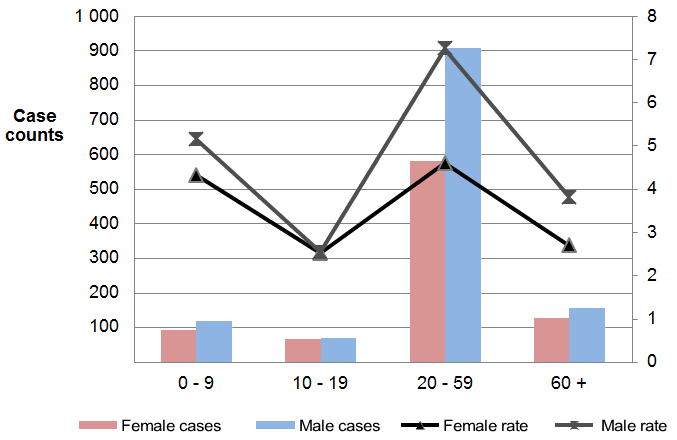
Text description: Figure 1
Figure 1: Distribution of shigellosis cases by age category and sex for BC, 2003-2012
| Age category | Female cases | Male cases | Female rate (%) | Male rate (%) |
|---|---|---|---|---|
| 0 to 9 years | 92 | 117 | 4.33 | 5.16 |
| 10 to 19 years | 65 | 70 | 2.52 | 2.54 |
| 20 to 59 years | 580 | 906 | 4.60 | 7.25 |
| 60+ years | 126 | 156 | 2.70 | 3.82 |
| Grand Total | 863 | 1,249 | 14.15 | 18.77 |
Figure 2: Shigellosis average annual incidence rate by health service delivery area in British Columbia, 2003-2012
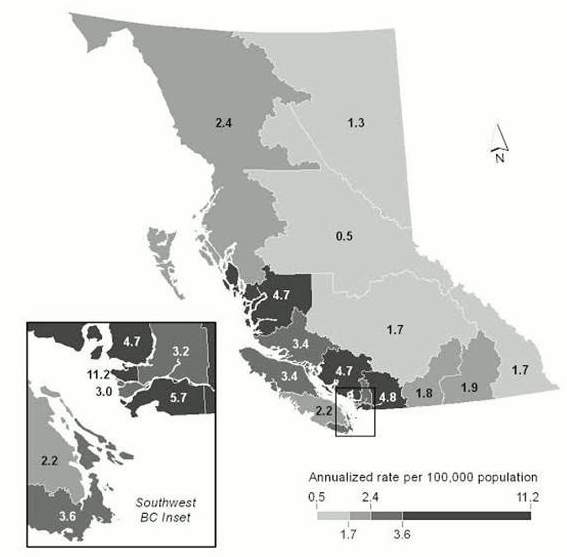
Text description: Figure 2
Figure 2: Shigellosis average annual incidence rate by health service delivery area in British Columbia, 2003-2012
This figure is a map of British Columbia showing the average annual incidence rate of Shigellosis by health service delivery area between 2003-2012. It shows that the greatest rates are in Vancouver and surrounding areas. Annual rates in interior and northern BC ranged between 0.5 to 2.4 whereas annual rates in Vancouver and surrounding areas ranged between 3.0 and 11.2. Vancouver Island ranged from 2.2 to 3.6.
Four shigellosis outbreaks were reported during 2003-2012. The first two outbreaks were caused by Shigella sonnei in 2007 and occurred in the Vancouver health services delivery area and Fraser Health Regions. The first outbreak occurred in the early part of 2007 and affected the MSM population and the second outbreak due to a different Shigella sonnei strain occurred in the latter part of 2007 and affected the homeless population Footnote 22. A third outbreak of Shigella sonnei linked to a local restaurant occurred in 2010 in the Okanagan health service delivery area Footnote 20. The final outbreak, due to Shigella flexneri, occurred during 2008-2012 and affected the MSM population in Vancouver health service delivery area Footnote 20.
Shigella sonnei (56.4%) was the most common strain reported in BC during 2003-2012, followed by Shigella flexneri (35.7%), Shigella boydii (5.1%) and Shigella dysenteriae (2.8%). Analysis demonstrated a shift in the dominating Shigella strain: during 2003-2008, Shigella sonnei was the prevalent infecting species (65.0%) in BC and during 2009-2012, Shigella flexneri was the prevalent infecting species (52.1%).
Limited exposure information was documented. In total, 58.0% of shigellosis cases, 15.1% of giardiasis cases and 8.3% of amebiasis cases had exposure information entered in iPHIS. Of these, less than 1% mentioned sexual activity as an exposure. During 2008-2012, 928 shigellosis cases were reported to PARIS and iPHIS, 654 cases (70.5%) of these had travel information documented, and 461 cases (70.4%) reported international travel. Overall, males were at a greater risk of acquiring shigellosis locally compared to females (RR 1.6; CI 1.4-1.8). Among the 20-59 year age group, males were at greater risk of acquiring shigellosis locally compared to females (RR 1.9; CI 1.7-2.4).
The average annual IR of amebiasis in BC was 7.7 per 100,000 populations (Table 1). Rates were higher in males than females across all age groups (male-to-female ratio of 2.6:1); with highest IR among males aged 20-59 years (16.7 per 100,000 population). Vancouver Coastal Health Authority reported the highest rate (16.2 per 100,000 population). No outbreaks were reported during the study period.
The average annual IR of giardiasis in BC was 15.2 per 100,000 population (Table 1). Vancouver Coastal Health Authority reported the highest average annual IR (20.4 per 100,000 population). The IR was higher in males than females across all age groups (male:female ratio of 1.5:1), with the highest IR observed in males aged 0-9 years (28.9 per 100,000 population) (data not shown). No outbreaks were reported for the study period.
Discussion
The results of this study demonstrate higher rates of all three infections in Vancouver Coastal Health region, with a higher male-to-female ratio. However IR was highest in adult males for shigellosis and amebiasis and in children (0-9 years) for giardiasis. The higher rates in adults (for shigellosis and amebiasis) may be due in part to sexual transmission.
For shigellosis and amebiasis, a high male-to-female ratio was noted, with a higher rate of infection in males aged 20-59 years as compared to all other age-sex groups. Additionally, for shigellosis, adult males were significantly more likely than females to acquire shigellosis locally and an excess of shigellosis cases in adult males was observed in Vancouver health services delivery area (data not shown). Furthermore, two of the four shigellosis outbreaks reported in BC affected the MSM population. In both outbreaks, no other common risk factors such as food, restaurants or travel were noted and sexual practices (oral-anal sex) were thought to be the mode of transmission. This is in contrast to Giardiasis which affected males more than females across all age groups with the highest rate reported in males aged 0-9 years which is less indicative of sexual transmission.
In Canada, other outbreaks of shigellosis where sexual transmission was implicated have been reported. In 1999-2001 an outbreak of S. sonnei and S. flexneri affected the MSM population in Quebec Footnote 24Footnote 25 and in July 2014, a cluster of shigellosis cases was reported among MSM in Toronto. Outbreaks of shigellosis affecting MSM populations are reported by many developed countries Footnote 26Footnote 27Footnote 28Footnote 29Footnote 30Footnote 31Footnote 32Footnote 33. In MSM populations, shigellosis is predominantly a sexually-transmitted infection Footnote 15 with the greatest risk of transmission associated with sexual practices involving direct oral-anal contact Footnote 7Footnote 8Footnote 15Footnote 25Footnote 27Footnote 31. Additionally, having multiple sexual partners could be responsible for widespread dissemination of Shigella in MSM Footnote 34. Human immunodeficiency virus infection has also been identified as an important risk factor for shigellosis in MSM Footnote 7Footnote 15Footnote 27.
Analysis demonstrated a shift in the dominant infecting Shigella species (Shigella sonnei to Shigella flexneri) around 2009. Wilmer et al. Footnote 20 reported similar findings in a study where Shigella flexneri became the dominant circulating strain in the MSM population within Vancouver City Centre after 2008. Others have also reported a change in the dominant Shigella species in the same MSM population Footnote 27Footnote 35Footnote 36. These shifts may reflect some degree of herd immunity towards a given Shigella species Footnote 7.
This study also found that adult males were more likely than adult females to acquire shigellosis locally (RR = 1.9). A similar finding was reported in a shigellosis outbreak in Wales, where locally acquired shigellosis occurred predominantly in males who had reported MSM activity in the week before illness Footnote 33. A higher risk of travel in female cases is indicative of acquiring shigellosis via risk factors considered to be more common during travel including contaminated food and water.
The highest annual average incidence for amebiasis was reported in adult males (16.7 per 100,000 population). The reasons for this are unclear. There was very little sexual exposure information on amebiasis. Entamoeba histolytica is usually transmitted from person-to-person Footnote 10 or via contaminated water Footnote 12. Since most British Columbians have access to safe drinking water Footnote 37 apart from travel to endemic countries, sexual transmission seems likely for this age-sex group. Sexual transmission of E. histolytica has been reported in the MSM population, with oral-anal sexual practices considered to be the mode of transmission Footnote 16Footnote 38Footnote 39.
The rate of giardiasis was higher for males in all age groups, with males aged 0-9 years having the highest rate. This finding is similar to observations reported by other developed countries Footnote 40Footnote 41Footnote 42Footnote 43 where Giardia most commonly infected small children in day-care centres and transmission was associated with poor hand hygiene. Given a similar pattern of giardiasis in BC, sexual transmission of Giardia seems less likely, or may be occurring at low rates, or is being overwhelmed by other transmission routes such as contact with contaminated water, travel to endemic countries and transmission in day-cares centres Footnote 40.
While this study has demonstrated a higher incidence of some enteric infections in males aged 20-59 years, not all adult male excess disease can be attributed to sexual transmission and not all sexual transmission will be observable by an excess in adult males. The combination of adult age and male sex used to identify an at-risk population is not a specific indicator of sexual transmission. Other factors (such as contaminated water, occupational and outdoor recreational exposure) may also account for the higher incidence observed in this group. Additionally, heterosexual adults are also at risk of STEI through oral-anal contact. Missing exposure information due to the lack of case interviews, incomplete assessment of sexual risk factors or incomplete data entry further hampered the ability to attribute cases to a specific transmission route.
Despite growing literature on the risk of STEIs, prevention guidelines and educational information for at-risk populations are not widely available. Public health guidelines on this topic do not appear to be available in Canada. Such guidelines could address the public health investigation required to better assess the risk of STEIs and modes of transmission, the need for contact tracing and educational messaging recommended for cases, at-risk populations and the general population. The findings of this study led to a review of the provincial enteric case follow-up forms to better capture information about sexual practices that increase the risk of STEIs.
A timely diagnosis and treatment of enteric infections will not only decrease the duration of illness but also interrupt its transmission Footnote 4Footnote 29. Currently, the Canadian Guidelines on Sexually Transmitted Infections recommend that health care providers test for enteric pathogens if clients report anorectal sexual activities and/or present with compatible symptoms Footnote 4. Additionally, health care providers should provide safe-sex counseling based on a personalized sexual health risk assessment Footnote 29. Currently, intense efforts to educate the at-risk population have been reported only during STEI outbreaks Footnote 27Footnote 44. However, to increase awareness about STEIs among at-risk populations, sexual health promotion messaging needs to occur on a more routine basis and should include information about STEIs, advice on avoiding unprotected oral-anal contact (especially if the partner is sick), hand hygiene following sexual contact and to seek medical advice for gastroenteritis Footnote 15Footnote 31.
Conclusion
This study suggests that sexual transmission of enteric infections, in particular shigellosis and amebiasis, may be occurring among MSM in Vancouver, BC. This conclusion is supported by outbreak data and limited case exposure (sexual activity, travel) history. To reduce the incidence of STEIs, public health interventions should expand beyond safe food and hand hygiene practices. Continued surveillance especially of case exposure history may also help to guide public health interventions to reduce STEI.
Acknowledgements
The authors thank the regional health authorities and the environmental health officers involved in the surveillance data collection and case follow-ups and the BC laboratories involved in diagnosis of cases included in the manuscript. We also wish to thank Marsha Taylor, Michael Otterstatter, Sophie Li and especially Dr. Pablo Nepomnaschy for their guidance and valuable feedback during this project.
Conflict of interest
None.
Funding
None.