CANVax - Trust in vaccines

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–6: Artificial intelligence in public health
Date published: June 4, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–6, June 4, 2020: Artificial intelligence in public health
Series
Managing immunization stress-related response: A contributor to sustaining trust in vaccines
C Meghan McMurtry1,2,3,4
Affiliations
1 Department of Psychology, University of Guelph, Guelph, ON
2 Pediatric Chronic Pain Program, McMaster University, Hamilton, ON
3 Adjunct Research Professor, Department of Paediatrics, Western University, London, ON
4 Associate Scientist, Children’s Health Research Institute, London, ON
Correspondence
Suggested citation
McMurtry CM. Managing immunization stress-related response: A contributor to sustaining trust in vaccines. Can Commun Dis Rep 2020;46(6):210–8. https://doi.org/10.14745/ccdr.v46i06a10
Keywords: vaccine, immunization, stress, fear, pain, syncope, fainting
Abstract
Adverse events following immunizations (AEFI) are important to identify and manage effectively so as to sustain trust in vaccines and optimize health. The AEFI category related to “anxiety about the immunization” was considered problematic as it did not adequately capture the range of stress responses that can occur. The currently used term for this category, immunization stress-related responses (ISRR), is broader, including the full spectrum of signs and symptoms that can arise in response to stress. ISRR can include vasovagal reactions (fainting), hyperventilation and functional neurological symptoms (e.g. weakness, nonepileptic seizures). It is based on a biopsychosocial framework in which biological (e.g. age, sex), psychological (e.g. preparedness, previous experiences, anxiety) and social factors (e.g. response by others, social media) interact to create an individual’s stress response to the immunization process.
New guidance is available on prevention, early detection and management of ISRRs which is summarized in the article.
Introduction
Vaccines are a clear public health success story, protecting people from a number of diseases. Adverse events following immunizations (AEFI) are important to identify and manage appropriately so as to sustain trust in vaccines and optimize health. In 2015, the Global Advisory Committee on Vaccine Safety of the World Health Organization (WHO) brought together an Expert Working Group to discuss what was previously known as an “AEFI arising from anxiety about the immunization.” Following review by the Global Advisory Committee in 2017 and 2018 and endorsement by the Strategic Advisory Committee for Vaccine Safety in April 2019, a detailed guidance manual for healthcare professionals, Immunization stress-related response: a manual for program managers and health professionals to prevent, identify and respond to stress-related responses following immunization, was publishedFootnote 1 along with a synopsisFootnote 2 and a peer-reviewed publicationFootnote 3.
The guidance manual provides details on understanding, preventing, identifying and managing what are now termed “immunization stress-related responses,” or ISRRFootnote 1.
The objective of this paper is to briefly describe ISRR, direct readers to detailed guidance on the topic and provide an overview of prevention and management.
This is the fifth article produced by the Canadian Vaccination Evidence Resource and Exchange Centre (CANVax) in the CANVax Briefs series. Multidisciplinary professionals at CANVax identify and develop useful resources to foster vaccine uptakeFootnote 4Footnote 5.
ISRR as part of AEFI
The safety of vaccinations is monitored globally, and AEFI, including events that are seen as arising from “anxiety” about the immunization, are grouped into five different categoriesFootnote 6. Naming these “anxiety reactions” is problematic for two reasons: anxiety does not paint an accurate or complete picture of what can be quite complex; and this description is indicative of a biomedical lens that classifies physiological responses as “physical” versus “psychological,” which does not take into account that each individual’s mind and body are intricately connectedFootnote 1.
The term immunization stress-related responses (ISRR) acknowledges the full spectrum of signs and symptoms experienced in response to stress: vasovagal reactions (fainting), hyperventilation, and functional neurological symptoms (e.g. weakness, nonepileptic seizures), among others. The biopsychosocial framework helps to understand that biological (e.g. age, sex), psychological (e.g. preparedness, previous experiences, anxiety) and social (e.g. peer behaviour and experiences, social media, community trust in health care) factors interact to develop an individual’s stress response and ISRRFootnote 1.
ISRR and other AEFI require different prevention and treatment responses. For example, it is important to distinguish ISRR from anaphylaxis, which is life-threatening and requires urgent recognition and a particular pharmacologic response (intramuscular epinephrine) and expert management. ISRR is neither life-threatening nor helped by epinephrine, and requires different management.
The role of the immunization process
Immunizations are typically delivered through injections. The process has several characteristics that can increase distress: pain from the injection, fear, sight of a needle, sight of blood, prolonged standing and responses by others in the environmentFootnote 1Footnote 7. Children and adolescents are particularly at risk as immunizations are common at this age. Pain and fear can go hand in hand: the more scared an individual is about a needle, the more pain they report feelingFootnote 7Footnote 8. Most people who have high levels of needle fear report a previous negative experienceFootnote 7Footnote 9Footnote 10Footnote 11.
There are short and long-term consequences of needle fear. In the short-term, individuals may require longer procedure times, have an increased risk of fainting, try to run away and experience greater distress and painFootnote 7Footnote 8Footnote 10Footnote 12. Fear of the procedure can develop in the long-term along with fear of healthcare professionals, avoidance of medical procedures, vaccine hesitancy and lack of benefit from traditional pain management techniquesFootnote 7Footnote 8Footnote 13Footnote 14Footnote 15.
Identification of ISRR: Timing and manifestation
Understanding and recognizing ISRRs is key to facilitating prevention and appropriate management of this category of AEFIs. While other AEFIs occur only after immunization, an ISRR can occur immediately before, during or after immunizationFootnote 1Footnote 6. The manifestations are acute stress responses, vasovagal reactions or dissociative neurological symptom reactions (DNSRs)Footnote 1. An acute stress response (“fight–flight–freeze” response) can vary in severity, from “butterflies in the stomach” and low to moderate levels of worry to more severe responses including difficulty breathing or rapid breathing/hyperventilation with tingling in the fingers and toes and increased heart rateFootnote 1Footnote 16Footnote 17. A vasovagal reaction is a fainting response that can cause a range of effects, from feeling mildly dizzy to losing consciousness due to insufficient blood flow to the brainFootnote 18.
An acute stress response may be followed by a vasovagal reaction after a sudden decrease in heart rate and a drop in blood pressure. Headache and nausea can also accompany stress reactionsFootnote 1. Symptoms of an acute stress response and vasovagal reaction can present before, during or immediately after immunization, usually within five minutesFootnote 1.
DNSRs are characterized by neurological symptoms with no physical findings, otherwise known as functional neurological symptomsFootnote 19Footnote 20. Symptoms can include difficulty walking or moving a limb, weakness, tingling sensations in the muscles and nonepileptic seizures. These symptoms are considered involuntary. DNSRs have not been well documented or reported in individuals following immunization, but there are reports of “masses” or “clusters” of these reactions in multiple people in close proximityFootnote 21. The current evidence suggests that DNSRs result from complex multifactorial etiologiesFootnote 22. DNSRs most commonly occur independently of immunization; a DNSR that develops after an immunization is best understood using a biopsychosocial framework in which the immunization process is one of a number of contributing factorsFootnote 1.
A variety of postimmunization events, syndromes and disorders have been reported that have no confirmed relationship with immunizationFootnote 1. These include complex regional pain syndrome (CRPS) type 1 with delayed onset; chronic fatigue syndrome; postural orthostatic tachycardia syndrome (POTS); and dissociative neurological symptom disorders (also known as conversion disorders) with delayed onset. They are not considered to be ISRRFootnote 1.
Acute stress response, vasovagal reaction and DNSR in individuals or “clusters” of individuals can occur independent of immunization. They have also been reported after immunization. WHO uses a detailed causality process to determine whether there is any relation between the symptoms and the immunizationFootnote 6; more details can be found in the WHO ISRR guidance manualFootnote 1. ISRR are not caused by the vaccine, a defect in vaccine quality or an error in the immunization program or process.
Each person who comes to be immunized has their own history, psychological strengths and vulnerabilities, and perceptions of the procedure and social context. Experiencing an ISRR is not the person’s faultFootnote 1. Figure 1 illustrates ISRR in individual and group contextsFootnote 1.
Figure 1: ISRR in individual and group contexts
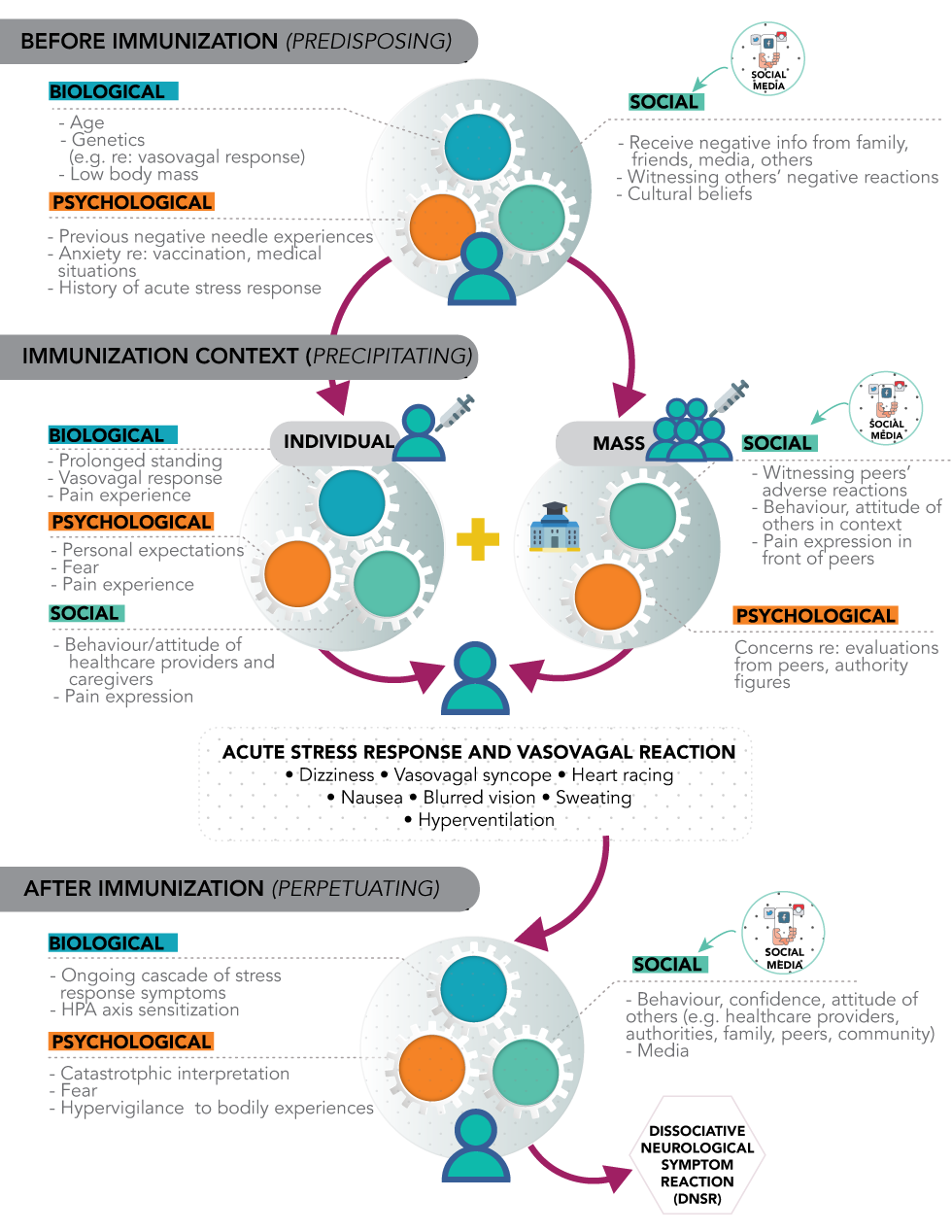
Text description: Figure 1
Figure 1: ISRR in individual and group contexts
The diagram illustrates immunization stress-related responses (ISRR) in individual and group contexts across three broad time points. The first time point is occurs before the immunization and highlights historical, predisposing risk factors for developing an ISRR. The second time point is in the immunization context which represents precipitating factors and the initial response. The third time point is after the immunization in which a delayed response is influenced by perpetuating factors. At each time point, potential biological, psychological, and social risk factors are shown which interact with each other. The potential for social media to provide negative information is highlighted. Although the diagram shows a progression through the three time points, not everyone will progress from one stage to another in a step-by-step fashion.
Facilitating prevention and appropriate intervention of ISRR
Prevention
Prevention relies on targeting predisposing risk factors. Clinicians should be educated on ISRRs, their prevention, screening and managementFootnote 1. Brief reminders/educational materials on display in immunization clinics, for example, a poster describing the difference between anaphylaxis and ISRR, could be helpful. As social media can play a particularly strong negative role in mass immunization contexts, including school immunization programs, communication is important before, during and after the immunization to reduce the risk of ISRRFootnote 23. Planning for mass immunizations should take into account existing rates of ISRR and vulnerability factors, that is, the age and sex of the recipients because adolescents and females are at greater risk of a vasovagal reactionFootnote 24. Therefore, planning for immunization clinics should include familiarizing healthcare providers with how to screen, prevent and manage vasovagal reactionsFootnote 1. Targeted education sessions teaching coping strategies can also be helpfulFootnote 25Footnote 26.
In every clinic, environmental strategies can reduce risk factors for ISRR. The immunization environment should be at a comfortable temperature (rather than overheated) and those at risk of an ISRR should be vaccinated in privateFootnote 1. The flow of individuals through the clinic should be such that the waiting area has only a few people (i.e. should not be crowded) and no one should be waiting for long. Allowing space to sit rather than having to stand for a long time is helpful. To build trust, the healthcare team should be calm, confident and friendly and able to communicate well with the recipients and any caregivers; they will also need to address any caregivers who are nervous and exacerbating fear in the vaccine recipientFootnote 1.
Screening
People at high risk for ISRR should be identified by screening for high levels of needle fear and previous negative experiences with needles, including faintingFootnote 1. For school vaccination campaigns, teachers, school nurses or other staff may be able to flag students at high risk for ISRR ahead of time, or individuals may self-identify. Late school-age and adolescent youth appear to be at higher risk of ISRR than other age groups. Individuals with a history of vasovagal reactions, including syncope and/or a high level of needle fear may be particularly at risk. Individuals with preexisting anxiety disorders and/or developmental disorders (including autism spectrum disorder) may also need extra time and careFootnote 1.
Each vaccine recipient should be asked if they have ever fainted (i.e. lost consciousness) and/or had prodromal symptoms (e.g. felt dizzy, nauseated and/or clammy and/or saw spots) before, during or after a needle procedureFootnote 1. Individuals who have a history of vasovagal reactions should be immunized in a seated or supine position and only move to sitting (from supine) or standing (from sitting) if there have no signs of a vasovagal reaction. Ideally the individual should stay seated for 15 to 30 minutes following the procedure, and the healthcare provider should monitor them for signs of a vasovagal reactionFootnote 1. In addition, the muscle tension technique can be taught to and used by the recipient (see Targeted Interventions for ISRR).
Although no current gold standard exists for screening for high levels of fear, it is recommended that healthcare providers ask vaccine recipients the questions shown in Table 1Footnote 27. Caregivers can be asked similar questions about their younger children.
| Age group, years | Question |
|---|---|
| 5–8 | 1) How afraid of needles are you? Not at all; a little bit; a medium amount; a lot; very, very much/most possible? |
| 2) Do you try hard to miss having a needle because you are so scared? | |
| Older than 8 | 1) How afraid of needles are you? Not afraid; a little bit; a moderate amount; a lot; or the most afraid possible? |
| 2) Do you think this level is higher than it should be (or higher than that of most of your friends)? | |
| 3) Do you avoid getting needles because you are afraid? |
Universal interventions
All recipients should be shown age-appropriate ways to manage pain and low to moderate fearFootnote 1Footnote 27. See Table 2 for physical, psychological, procedural and pharmacological strategies recommended for different age groups. A supportive caregiver could also be present to help with coping strategies. For further details see Reducing pain during vaccine injections: clinical practice guidelineFootnote 27.
| Type of strategy | Newborn | Infant (1–35 months) |
Preschool (3–5 years) |
School-aged (6–12 years) |
Adolescent (13–18 years) |
Adult (19 years+) |
|---|---|---|---|---|---|---|
| Procedural | ||||||
| Inject into anterolateral thigh | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 (1–11 months) |
– | – | – | – |
| No aspiration when injecting | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Give most painful vaccine last | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 (or simultaneous injection 0–1 year) |
Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Physical | ||||||
| Skin-to-skin (kangaroo care) before, during, after | Footnote ✓ of Table 2 | – | – | – | – | – |
| Cradled in parent’s arms | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | – | – | – | – |
| Breastfeeding before, during, after OR sweet-tasting solutions before and/or nonnutritive sucking before, during, after | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | – | – | – | – |
| Seated uprightFootnote a of Table 2 | – | – | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| External vibrating device with cold | – | – | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | – |
| CommunicationFootnote b of Table 2 and psychological | ||||||
| Calm voice, simple language | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Don’t say it won’t hurt | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Use neutral words to signal procedure (e.g. “1, 2, 3, here we go”) | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Avoid repeated excessive reassurance (e.g. “it’s okay, it’s okay, it’s okay”) before, during, after | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Talk about things other than the procedure (verbal distraction) before, during, after | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Distraction (age appropriate) | – | Footnote ✓ of Table 2 (e.g. toy, video with adult coaching to pay attention to distractor) |
Footnote ✓ of Table 2 (e.g. blowing bubbles, toys, video, singing) |
Footnote ✓ of Table 2 (e.g. video game, video, blowing bubbles, toys, music) |
– | – |
| Breathing strategy | – | – | Footnote ✓ of Table 2 (breathing with toy) |
Footnote ✓ of Table 2 (breathing with toy) |
– | Footnote ✓ of Table 2 (cough, breath-hold) |
| Pharmacological | ||||||
| Topical anesthetic applied before (check product instructions for time)Footnote c of Table 2 | – | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 | Footnote ✓ of Table 2 |
| Vapocoolant spray right before | – | – | – | – | – | Footnote ✓ of Table 2 |
Targeted interventions for ISRR
If an individual is at elevated risk for an ISRR, additional measures need to be put in place, such as avoiding having them wait in the general waiting area, immunizing them at the beginning of the clinic and immunizing them in privateFootnote 1. These strategies are designed to reduce contagion of fear and other negative emotions as well as to contain any negative effects of an ISRR, should one occur. These individuals may benefit from having a calm, supportive caregiver or friend with them; as noted above, a fearful caregiver or friend can exacerbate the situation and they need to be addressed immediatelyFootnote 1.
If an individual’s level of fear is high but they are not avoiding the vaccination, two approaches could be used: first, identify what can be done in the immunization clinic to create a positive experience for the individual, for example, taking more time, making further environmental modifications, etc.; and second, determine whether treatment of the needle fear by a mental health professional outside of the immunization context is necessary before future immunizationsFootnote 1Footnote 28. If high levels of needle fear and avoidance are present, consider delaying the needle to address these factors. For extreme fear, pharmacological strategies (e.g. anxiolytics, sedationsFootnote 1) could also be considered if the expertise is available.
If the individual is at risk for a vasovagal reaction, immunizing them in a reclining or supine position while they are using the muscle tension technique can be helpfulFootnote 1. Muscle tension keeps an individual’s blood pressure up and prevents the precipitous drop that can lead to a faint. This technique has been recommended for those aged seven years and older (adolescents are at greater risk for vasovagal syncope)Footnote 27Footnote 28. First, the individual tenses their major muscle groups (e.g. abdomen, legs, contralateral arm to where the injection will be administered) for 15 to 30 seconds until they feel flushed or warm in the face. Next, they release the tension for 15 to 30 seconds, but they do not fully relax. They repeat these steps in cycles before, during and after the procedure until they have no prodromal symptoms.
It is critical to differentiate anaphylaxis from a DNSRFootnote 1. (The WHO ISRR guidance manual contains a table that can help healthcare providers distinguish between anaphylaxis and an ISRRFootnote 29). If an individual loses consciousness following a vaccination, it could be a result of vasovagal syncope or anaphylaxis. Anaphylaxis is potentially life-threatening and requires medicationFootnote 30. While the recipient is in the recovery position (supine, on their side), a healthcare provider should monitor the recipient’s pulse, respiration, blood pressure and peripheral circulationFootnote 1, watch their skin for rash or swelling and listen to their lungs for wheeze or stridor.
If an ISRR has been identified, it is important to communicate that the response is not due to a vaccine product or procedural error; that the response is a known event that staff resolve by following specific guidance; and that the response can resolve spontaneously without medication or hospitalization.
A DNSR that occurs following an immunization does not causally implicate either the immunization or the immunization process. A specific assessment is used to determine causalityFootnote 6; the WHO ISRR guidance manual provides a list that can help to diagnose a DNSR. Examples include symptoms that are inconsistent with known disorders and inconsistent presentation of symptoms (e.g. that disappear inexplicably or do not respond typically to interventions)Footnote 31. Nonepileptic seizures are one example of a DNSR; these resemble epileptic seizures but do not have neural discharges in the way epileptic seizures do (epileptic seizures are differentiated from nonepileptic seizures in the WHO ISRR guidance manualFootnote 32). Nonepileptic seizures are typically a diagnosis of exclusionFootnote 33. Although an electroencephalogram is the gold standard assessment for seizures, conducting one may not be practicable.
A DNSR may resolve spontaneously or may require the involvement of a multidisciplinary team including a mental health professional. The biopsychosocial framework that is used to understand ISRR should also guide treatmentFootnote 1. Medical and psychological expertise is needed for further assessment and management to reduce functional disability. Treatment is specific to the presenting symptoms but may include physiotherapy, cognitive behavioural therapy and/or pharmacological strategiesFootnote 34Footnote 35. In the short term, the healthcare providers at the immunization clinic should attempt to put the affected individual and others present at ease. They should note that anxiety about and fear of immunization are normal and can result in a bodily response that may seem extreme but can resolve spontaneously without any injuryFootnote 1. The affected individual should be kept in a separate, calm, quiet space with only key people present. The healthcare providers can answer the questions raised by the affected individual and/or their caregiver(s). If the recipient and caregiver(s) are relatively calm, they may be able to be distracted by talking about something else or listening to music to further calm them. The goal is to encourage return to normal activityFootnote 1.
Similar or identical symptoms appearing in more than one person with no physiological cause have been the source of attention and curiosity for hundreds of years; the “spread” is thought to be due to shared beliefs and contagion of anxiety and fearFootnote 36Footnote 37Footnote 38Footnote 39. These “clusters” have been known as “mass psychogenic illness” or “mass hysteria” and have been reported inside and outside the immunization contextFootnote 21Footnote 40. These terms can be inflammatory and demeaning to the affected individualsFootnote 1.
Trying to complete a mass vaccination campaign in a short amount of time is a risk factor for the development of ISRRFootnote 1. The biopsychosocial framework is used to understand clusters, with particular attention paid toward understanding and managing social factors. Known clusters in the immunization context have occurred in adolescents and adults but not in infants and young childrenFootnote 1. Anaphylaxis is rare and extremely unlikely to occur in clustersFootnote 1. If an ISRR cluster occurs, the affected individuals should be separated from others and each other to enable containment and appropriate managementFootnote 1. The general strategies outlined above (i.e. training of staff, communication, environmental modifications, screening for people at risk for ISRR and targeted strategies such as privacy) are also critical in mass immunization contexts. Community leaders and healthcare providers who are known to the recipients can help keep them calm and comfortable. Educational materials such as posters differentiating anaphylaxis from ISRR and epileptic seizures from nonepileptic seizures could be designed and posted in the clinicFootnote 1.
Conclusion
In summary, ISRRs are the redefined way to think about, identify and manage what was previously known as AEFI stemming from “anxiety” related to the immunization. ISRRs can occur before, during or after the immunization and are not due to the vaccine product itself or an error in the process. Prevention strategies include proactive communication, managing social media use and in-clinic environmental strategies. Screening can identify those with increased risk of an ISRR, including those with high levels of needle fear or with previous vasovagal reaction. Age-appropriate pain management strategies should be standard for all immunization recipients. Targeted interventions for those experiencing an ISRR include muscle tension for vasovagal reactions, reducing vaccine recipients’ fear, increasing comfort and avoiding the contagion of fear and misinformation.
Understanding the nature of ISRRs and their occurrence provides an opportunity for their prevention and appropriate management, warding off future negative reactions towards immunization and health care in general, and contributing to sustaining trust in vaccines. The ISRR should be reported as part of AEFI surveillance.
See the WHO ISRR guidance manual for further informationFootnote 1 and the CANVax website for updates.
Author’s statement
CMM — Conceptualization, writing – original draft, review, editing. This manuscript is based on Immunization stress-related response: a manual for program managers and health professionals to prevent, identify and respond to stress-related responses following immunization. The content of the World Health Organization (WHO) immunization stress-related response (ISRR) guidance manual was developed by M Gold, NE MacDonald, CM McMurtry, R Pless and U Heininger with coordination and supervision by MR Balakrishnan and P Zuber and support by L Menning and O Benes from WHO.
Conflict of interest
CMM was a member of the immunization stress-related response (ISRR) expert working group. There are no other conflicts of interest.
Acknowledgements
The author is grateful for the efforts of the other members of the immunization stress-related response (ISRR) working group (MR Balakrishnan, O Benes, M Gold, U Heininger, NE MacDonald, L Menning, R Pless and P Zuber). Thank you also to NE MacDonald for reviewing and commenting on an earlier version of this manuscript. Thank you to K Constantin for reviewing the French version of the article for accuracy.
Funding
No external funding was received.
