Predictive modelling of COVID-19 in Canada

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–6: Artificial intelligence in public health
Date published: June 4, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–6, June 4, 2020: Artificial intelligence in public health
Implementation science
Modelling scenarios of the epidemic of COVID-19 in Canada
Nick H Ogden1, Aamir Fazil1, Julien Arino2, Philippe Berthiaume1, David N Fisman3, Amy L Greer4, Antoinette Ludwig1, Victoria Ng1, Ashleigh R Tuite3, Patricia Turgeon1, Lisa A Waddell1, Jianhong Wu5,6
Affiliations
1 Public Health Risk Sciences Division, National Microbiology Laboratory, Public Health Agency of Canada, St. Hyacinthe, QC and Guelph, ON
2 Department of Mathematics & Data Science NEXUS, University of Manitoba, Winnipeg, MB
3 Dalla Lana School of Public Health, University of Toronto, Toronto, ON
4 Department of Population Medicine, University of Guelph, Guelph, ON
5 Laboratory for Industrial and Applied Mathematics, Department of Mathematics and Statistics, York University, Toronto, ON
6 Fields-CQAM Laboratory of Mathematics for Public Health, York University, Toronto, ON
Correspondence
Suggested citation
Ogden NH, Fazil A, Arino J, Berthiaume P, Fisman DN, Greer AL, Ludwig A, Ng V, Tuite AR, Turgeon P, Waddell LA, Wu J. Modelling scenarios of the epidemic of COVID-19 in Canada. Can Commun Dis Rep 2020;46(6):198–204. https://doi.org/10.14745/ccdr.v46i06a08
Keywords: modelling, COVID-19, Canada, non-pharmaceutical interventions
Abstract
Background: Severe acute respiratory syndrome virus 2 (SARS-CoV-2), likely a bat-origin coronavirus, spilled over from wildlife to humans in China in late 2019, manifesting as a respiratory disease. Coronavirus disease 2019 (COVID-19) spread initially within China and then globally, resulting in a pandemic.
Objective: This article describes predictive modelling of COVID-19 in general, and efforts within the Public Health Agency of Canada to model the effects of non-pharmaceutical interventions (NPIs) on transmission of SARS-CoV-2 in the Canadian population to support public health decisions.
Methods: The broad objectives of two modelling approaches, 1) an agent-based model and 2) a deterministic compartmental model, are described and a synopsis of studies is illustrated using a model developed in Analytica 5.3 software.
Results: Without intervention, more than 70% of the Canadian population may become infected. Non-pharmaceutical interventions, applied with an intensity insufficient to cause the epidemic to die out, reduce the attack rate to 50% or less, and the epidemic is longer with a lower peak. If NPIs are lifted early, the epidemic may rebound, resulting in high percentages (more than 70%) of the population affected. If NPIs are applied with intensity high enough to cause the epidemic to die out, the attack rate can be reduced to between 1% and 25% of the population.
Conclusion: Applying NPIs with intensity high enough to cause the epidemic to die out would seem to be the preferred choice. Lifting disruptive NPIs such as shut-downs must be accompanied by enhancements to other NPIs to prevent new introductions and to identify and control any new transmission chains.
Introduction
In this article, we review efforts within the Public Health Agency of Canada (PHAC) to model transmission of severe acute respiratory syndrome virus 2 (SARS-CoV-2), the agent of coronavirus disease 2019 (COVID-19), to support public health decisions. The COVID-19 pandemic has emerged at remarkable speed. The SARS-CoV-2 is likely a bat-origin coronavirusFootnote 1 that may have “spilled over” into humans from an intermediary animal reservoir not yet identified. The first detected human exposure event was linked to a “wet market” in the city of Wuhan, the capital city of the province of Hubei, China some time during late 2019Footnote 2. The virus was likely already capable of human-to-human transmission, but evolved more efficient transmissibility during late 2019Footnote 3. Human-to-human transmission was officially recognised by the global public health community in mid-January 2020Footnote 4. Shortly after this, spatial spread in China was reported. Intense public health measures (or non-pharmaceutical interventions; NPIs) of case detection, contact tracing and quarantine, and social distancing were implemented within the province of Hubei, and the region was isolated from the rest of China by travel restrictionsFootnote 5. International travel restrictions to and from China were introduced but cases had already escaped outside of ChinaFootnote 6, and the consequent global spread resulted in declaration of a pandemicFootnote 4. The first travel-related cases were identified in Canada in January 2020 and by April 2020, community transmission was occurring in all provinces with the possible exception of Prince Edward Island. Community transmission has yet to be reported from the territories. The majority of cases and deaths have been reported from the four largest provinces (British Columbia, Alberta, Ontario and Quebec). Physical distancing (including school, college, university and “non-essential” business closures) was implemented from mid-March 2020 in Canada and subsequent reductions in disease transmission suggest that these and other NPIs (detailed below) are slowing the epidemicFootnote 7.
Evolution of COVID-19 modelling
At first, modelling studies focused on the epidemic in China, and particularly on the dynamics of the epidemic in the city of Wuhan and throughout the province of Hubei. At this early stage, there was much effort to analyse surveillance data from China to obtain parameter estimates such as the basic reproduction number (R0), case fatality rate and incubation periodFootnote 8. For the first attempts at Susceptible-Exposed-Infectious-Recovered (SEIR) type dynamic models, parameter estimates were “borrowed” from what was known about other coronaviruses (SARS-CoV and MERS-CoV)Footnote 6 and/or obtained by fitting the models to surveillance dataFootnote 9Footnote 10. As more data on SARS-CoV-2 virus transmission and the course of infection in humans have become available, models have become increasingly parameterised using SARS-CoV-2-specific dataFootnote 11. With global spread of the disease, and with a vaccine likely more than a year away, modelling efforts turned to assessing the possible extent of the epidemic in countries outside China, and the impact of different NPIsFootnote 11Footnote 12. Emerging science has revealed that SARS-CoV-2 virus is highly transmissible by respiratory and possibly fecal-oral routes, is transmitted before symptoms appear and some cases may be entirely asymptomaticFootnote 13Footnote 14. The virus can be highly pathogenic for older people and some younger people, particularly those with co-morbiditiesFootnote 14. Presymptomatic transmission, mild symptoms (particularly in younger age groups) and asymptomatic cases all hinder detection of infective cases (in contrast to SARS)Footnote 15, making control difficult. Modelling efforts to date have illuminated the magnitude of the challenge we face: 1) the global population is entirely immunologically naive, 2) the virus is very highly transmissible (R0 values may be greater than five in some settings)Footnote 16 and 3) the level of pathogenicity of SARS-CoV-2 means even the most advanced healthcare systems in the world may be completely overwhelmed if the virus is allowed to spread without introducing NPIs. At the same time, and in contrast to pandemic influenza, there are no known effective antivirals.
COVID-19 modelling in Canada
Predictive modelling of COVID-19 by Canadian scientists is a field of study that was approximately three months old at the time of writing (early May 2020), but there is extensive skill in Canada in modelling the transmission of infectious diseases. Some previously-developed models that investigated interventions to control H1N1 and other influenzasFootnote 17Footnote 18 have been adapted to assess the transmission of SARS-CoV-2 and the impacts of different NPIsFootnote 9Footnote 19Footnote 20. Tang et al.Footnote 9 and Li et al.Footnote 21 have developed SEIR-type models to explore transmission and learn from NPIs implemented in China and South Korea. An Expert Modelling Group, comprising more than 50 federal, provincial, territorial and university-based modellers and epidemiologists, has been assembled by PHAC to develop a Canadian COVID-19 modelling network that supports decision-making. Similar groups have been developed in other countries, with liaison between them facilitated by a World Health Organization modelling group.
COVID-19 modelling at PHAC
In January 2020, a modelling group was convened, and development of two complementary modelling approaches was initiated. The prime objective of this modelling was to assess the impact of different NPIs and levels of efficacy needed to control the epidemic in Canada. In the absence of a vaccine or treatment, the NPIs available to control the epidemic, which were explored in modelling, are 1) physical distancing (closure of schools, colleges and universities, meeting places, gatherings and personal distancing) that reduces the rates of contact between members of society (including those who may be infected), 2) detection of cases by surveillance, and their isolation to prevent them from transmitting infection and 3) tracing and quarantine of people who have had contacts with cases.
The two modelling approaches used will be published as separate, more detailed articles, and are termed “approaches” as the models themselves have evolved with evolving knowledge. What follows is a broad description of these approaches, which are based on 1) an agent-based modelFootnote 22 and 2) a deterministic compartment modelFootnote 23. The agent-based approach was developed de novo using AnyLogic© software, while the deterministic model runs in RFootnote 24. Initial versions of the deterministic model were adapted from Tang et al.Footnote 9. Both are SEIR-type models with elements to model SARS-CoV-2 and impacts of NPIs, with more realism (Figure 1). These elements include compartments for isolated cases and quarantined “exposed” contacts from which onward transmission to susceptible people is limited or absent, compartments for asymptomatic cases that may or may not be detected by surveillance, as well as flows to “isolation” and “quarantine” compartments that allow variation according to different levels of public health effort. Parameters in the models are calibrated according to values obtained by literature searches, which are conducted each day to ensure evolving knowledge is captured by the models.
Figure 1: A schematic of the Analytica 5.3 compartment model showing the flow of populations between compartments
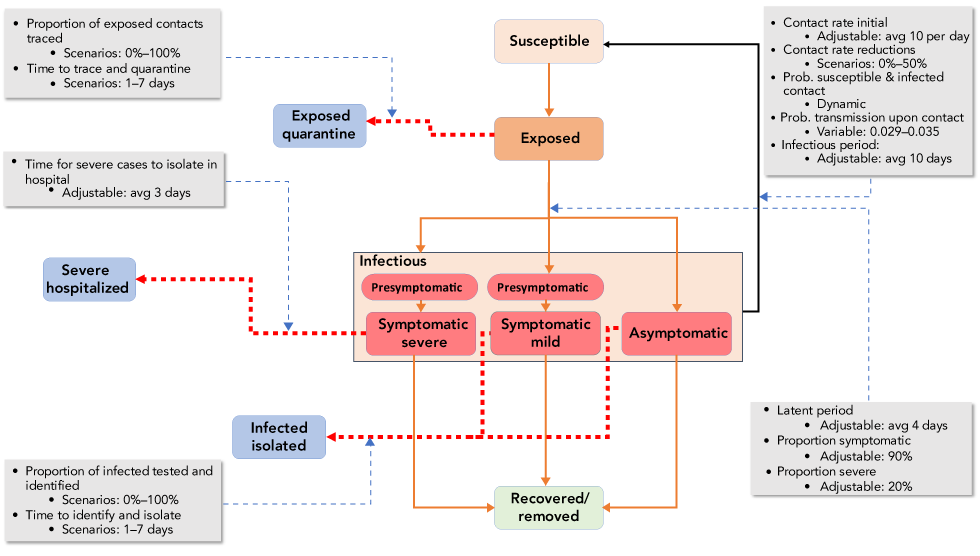
Text description: Figure 1
Figure 1: A schematic of the Analytica 5.3 compartment model showing the flow of populations between compartments
This figure is a schematic of the Analytica 5.3 compartment model showing the flow of populations between compartments. The main compartments are (from top to bottom) susceptible (in pink), exposed (in orange), infectious (red boxes including presymptomtic infectious people, people with mild and severe infection, and asymptomatic infectious people), and recovered/removed (a pale green box). Orange lines show the flow from one of these compartments to the next. Dashed red lines show how people in the exposed or infectious compartments can move to other compartments due to public health or health system actions. These other compartments (coloured blue) are people who have contacted a case and are in quarantine, people isolated by virtue of being severely affected in hospital, and people isolated as a consequence of testing positive. Around these boxes are five gray boxes that detail important parameters that determine rate of flow between the model compartments. These include the contact rate between people (10/day, which may be reduced by up to 50% by public health measures), the probability transmission occurs when a contact takes place (0.029–0.035), the infectious period (average 10 days), the latent period (4 days), the proportion of cases symptomatic (90%), and the proportion severe (20%). Variables more linked to public health action are the proportion of exposed contacts traced (0%–100%), the time to trace and quarantine (1–7 days), the time for severe cases to be isolated in hospital (average 3 days), the proportion of infected people tested and identified (0%–100%), and the time it takes to identify and isolate then (1–7 days).
In the deterministic modelling approach, effects of physical distancing are modelled in a simplistic way by reducing daily per capita contact rates informed by a number of metrics including cellphone dataFootnote 25. The agent-based model approach, which contains stochastic elements, uses a more detailed representation of communities, including homes and communal meeting spaces that may represent workplaces, schools, malls and restaurants etc., so this model is capable of modelling effects of closures in more detail and predicting different realizations of disease spread in the community. The agent-based approach includes contact rates within and between age groups that areFootnote 20 based on the POLYMOD studyFootnote 26. Contact data specific for the United Kingdom (UK) and European countries were used as a surrogate for contact rates in Canada given that similar studies have not been conducted in Canada. The deterministic approach has used homogenous mixing but is also being modified to include contact rates that vary within and between age groups. For illustrative purposes, a deterministic compartment model has been developed in Analytica 5.3 (Lumina Inc.) using the knowledge of COVID-19 transmission elucidated by the two modelling approaches. Code for this model is available upon request with instructions on how to explore the model and generate results.
Key outputs from the models are 1) under what circumstances the NPIs cause the epidemic to die out by reducing the effective reproduction rate (Re) below unity (i.e. one infected person infects fewer than one other person, on average) and 2) the final attack rate (i.e. the total percentage of the population infected) and 3) the approximate duration of the epidemic.
Synopsis of findings of modelling conducted within and outside Public Health Agency of Canada
The following synopsis of results of modelling studies includes those conducted by PHAC with reference to studies conducted by modelling groups outside the Government of Canada. The outputs are illustrated here using graphs generated by the model developed in Analytica 5.3.
1. What happens in the absence of NPIs?
The baseline to compare impacts of NPIs is a scenario where there is no effort to control disease spread. In this case, the attack rate is predicted to be greater than 70%, and the epidemic lasts approximately one year (Figure 2). These findings are consistent with studies estimating impacts on UK and United States populationsFootnote 11.
Figure 2: Impacts of partially-effective NPIs on the epidemic compared to the baseline with no control efforts
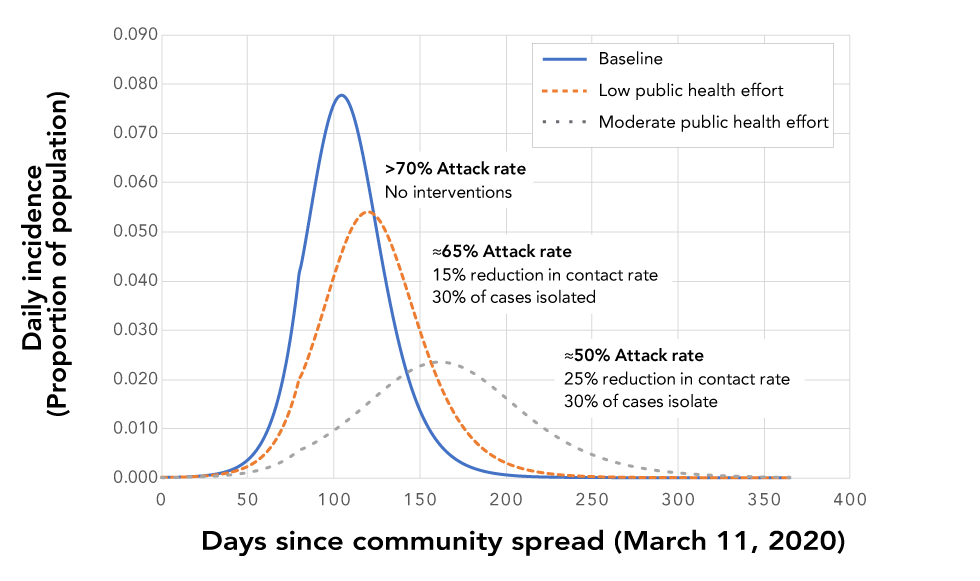
Text description: Figure 2
Figure 2: Impacts of partially-effective NPIs on the epidemic compared to the baseline with no control efforts
This figure shows a graph with three different line graphs that represent possible impacts of partially-effective non-pharmaceutical interventions (NPIs) on the epidemic compared to the baseline with no control efforts. The x-axis is time in days (from 0 when the community transmission starts to 400) and the y-axis is the daily incidence as a proportion of the total population. The three line graphs are i) a blue solid line showing the baseline which has a label identifying that the attack rate is >70% when there are no interventions; ii) a brown dotted line showing the effect of low public health effort which has a label identifying that the attack rate is 65% when there is a 15% reduction in contact rates and 30% of cases are isolated; and iii) a gray dotted line showing the effect of moderate public health effort which has a label identifying that the attack rate is 50% when there is a 25% reduction in contact rates and 30% of cases are isolated.
2. What happens when NPIs are partially effective?
If NPIs, maintained throughout the epidemic, are partially effective (i.e. they have impacts on the epidemic but do not cause it to die out), the main effects are as follows: the epidemic is prolonged, the peak is reduced, the epidemiological curve is flattened and the attack rate is reduced to approximately 50% (to 25% in some models)Footnote 20 (Figure 2). This finding is consistent with equivalent modelling studiesFootnote 11Footnote 20. This scenario has been termed “delay and reduce.”
If the NPIs do not cause the epidemic to die out and are lifted before the epidemic is over, the epidemic is predicted to rebound and the attack rate can be as high as without NPIs because the majority of the population remains naive (Figure 3)Footnote 11Footnote 20.
Figure 3: The effect of initiating partially-effective non-pharmaceutical interventions (in this case physical distancing), and then removing these interventions before the epidemic endsFigure 3 footnote a
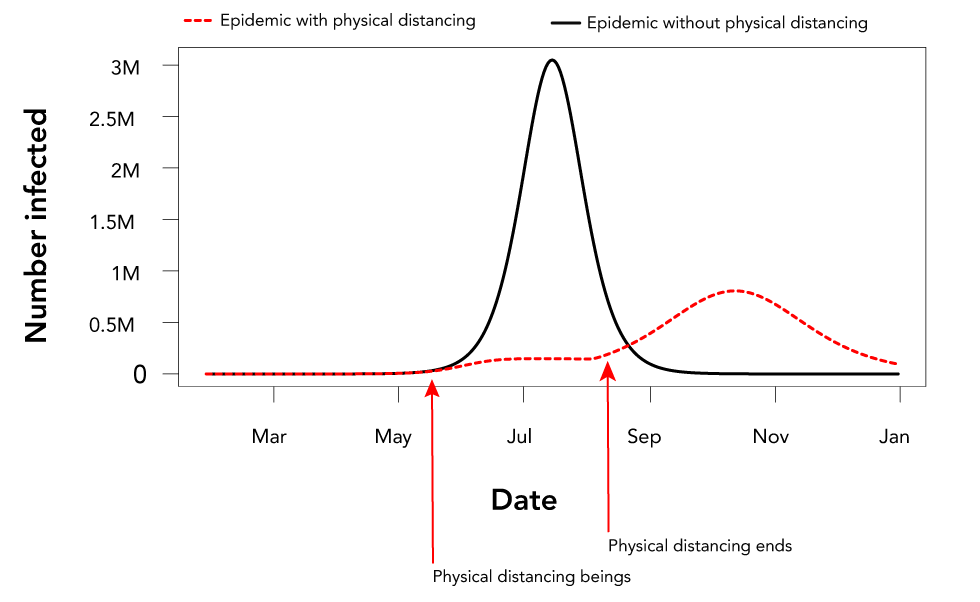
Text description: Figure 3
Figure 3: The effect of initiating partially-effective non-pharmaceutical interventions (in this case physical distancing), and then removing these interventions before the epidemic endsFigure 3 footnote a
This figure is a graph that shows the effect of initiating partially-effective non-pharmaceutical interventions (in this case physical distancing), and then removing these interventions before the epidemic ends. The x-axis is time in months (from March when community transmission starts to January the following year) and the y-axis is the daily incidence as the number of daily cases in the Canadian population. There are two graphs. One, shown by a black line is the baseline with no public health intervention (physical distancing in this case). The other is a red dotted line that shows that the incidence is very low if physical distancing is implemented in early May, but that if the epidemic does not die out, the epidemic rebounds when physical distancing ends in August.
3. What happens when NPIs are highly effective?
When NPIs are highly effective, Re falls below unity, the epidemic dies out and does not rebound if NPIs are lifted (also referred to as epidemic control). How soon that happens, and thus the final attack rate (which may be anywhere between less than 1% and 25%), depends on a number of factors including at what point in the epidemic NPIs are implemented, the intensity with which NPIs are implemented, the duration of implementation and the level of compliance (Figure 4)Footnote 12.
Figure 4: Effects of different levels and combinations of non-pharmaceutical interventions on whether or not, and how quickly, epidemic control is reached
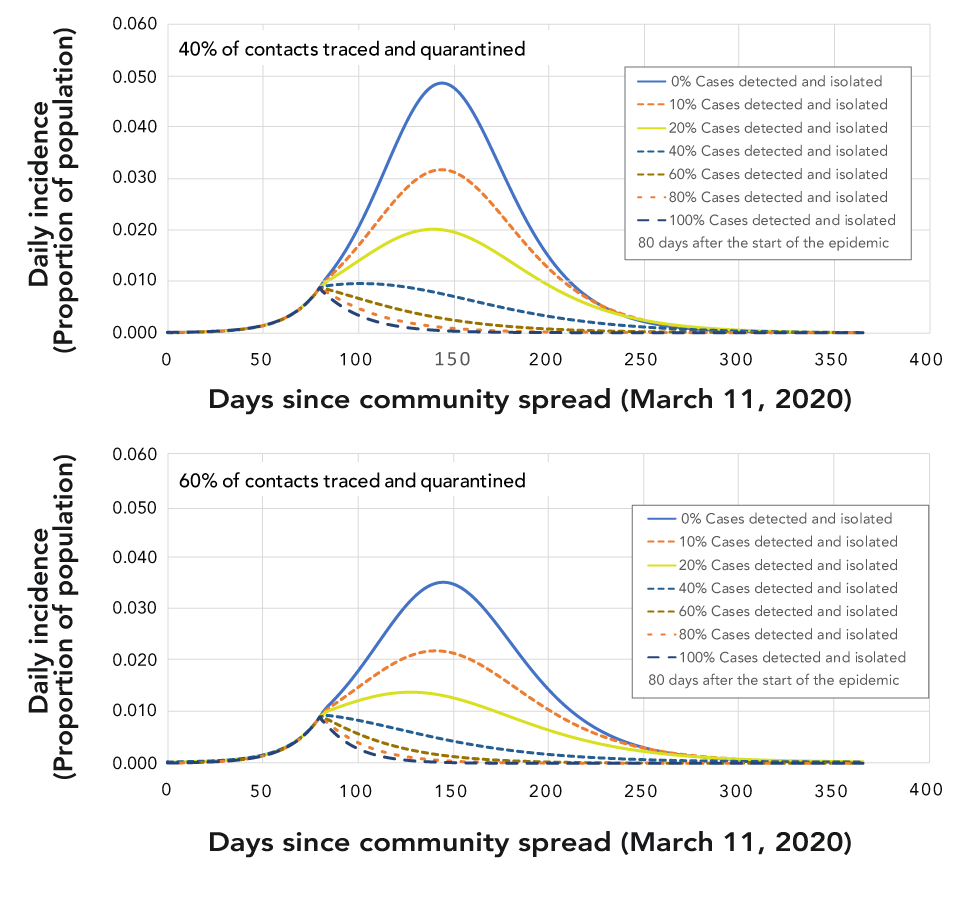
Text description: Figure 4
Figure 4: Effects of different levels and combinations of non-pharmaceutical interventions on whether or not, and how quickly, epidemic control is reached
This figure shows two graphs that have the same format, and axes, as the graph in Figure 2. The graphs show what happens to the epidemic when different levels of non-pharmaceutical interventions (NPIs) are applied 80 days after the start of the epidemic. In each graph there are six line graphs, one for each of the following percentages of cases detected and isolated 0% (blue solid line), 20% (dark orange dotted line), 40% (blue dotted line), 60% (brown dotted line), 80% (orange dotted line), 100% (dark blue dotted line). The upper graphs show the epidemic with these case detection values and when 40% of contacts are traced and quarantined, while the low graphs show the epidemic with these case detection values and when 60% of contacts are traced and quarantined. In all cases, the number of people affected is reduced when 60% rather than 40% of contacts are traced and quarantined. In both graphs only when 60% or more of contacts are traced does the epidemic die out.
Assessing hospitalisation and mortality rates from attack rates
The main objective of the modelling approaches was to compare the impacts of different NPIs. There remains much uncertainty in some model parameters and their distributions, including the duration of the latent period, the proportion of cases that are asymptomatic and the duration of infectivity. The strength of these models is their ability to compare amongst different NPIs using current best estimates of parameter values. However, in order to ensure health care capacity is sufficient to respond to the pandemic, planners need to have a range of estimates for the expected numbers of cases, hospitalizations, cases needing care in intensive care units (ICU) and fatalities. Initial modelling approaches focused on estimating total attack rates, and were not designed to estimate hospitalizations, cases needing ICU care and fatalities. To obtain these estimates from total attack rates, age-specific severity estimates from analysis of international surveillance dataFootnote 27 were used to assess the proportion of cases in Canada that would be mild or asymptomatic, require hospitalization or ICU treatment, and may die, according to the demography of the Canadian population as a wholeFootnote 28. The estimates per one million population are shown in Table 1.
| Level of epidemic control | “Delay and reduce” | Epidemic controlled | ||||
|---|---|---|---|---|---|---|
| Attack rate | 50% | 25% | 10% | 5% | 2.5% | 1% |
| All cases | 500,000 | 250,000 | 100,000 | 50,000 | 25,000 | 10,000 |
| Mild (89.5%) | 450,000 | 225,000 | 90,000 | 45,000 | 22,000 | 9,000 |
| Hospitalised—not ICU (8%) | 39,000 | 19,000 | 7,800 | 3,900 | 2,000 | 800 |
| Hospitalised— ICU (2.5%) | 12,000 | 6,000 | 2,400 | 1,200 | 600 | 200 |
| Fatalities (1.2% all cases) | 6,000 | 3,000 | 1,200 | 600 | 300 | 100 |
These estimates are crude and more precise health care needs estimates should be calculated at the community level (e.g. catchment area for a hospital or group of hospitals) so the specific age structure and co-morbidities of the community under evaluation can be accounted for in the modelFootnote 19.
Observations from Canada and elsewhere in the world
The outputs of modelling studies are theoretical, but their insights and policy implications have been bolstered by real-world evidence. Epidemic control has been realised in Singapore, China and South Korea, with Re falling below unity by application of a prompt and intense level of NPIsFootnote 5Footnote 21Footnote 29. In contrast, in Europe, NPIs to date do not seem to have brought Re below unityFootnote 30. At the time of writing, in Canada the epidemic is geographically heterogeneous, but unpublished estimates suggest that in some jurisdictions Re may be below unity, while in others this state has not been reached (unpublished; Dr. Ashleigh Tuite and Dr. David Champredon). At the time of writing, the observed case fatality rate is higher than that predicted using methods described above because of extensive transmission in long-term care and seniors facilities. In these facilities, contact rates are likely very highFootnote 31 and the population very vulnerable to COVID-19.
Conclusion
The modelling studies described here provide information for planning of public health policies to combat the unprecedented risk of COVID-19 to the health and well-being of Canadians and the rest of the world. These studies underline that without NPIs, the majority of Canadians would acquire infection in a relatively short period of time, and the health care system would most likely be overwhelmed, resulting in a higher case fatality rate, particularly in the most vulnerable age groups. The intensity of the NPIs, and the compliance of the public, will determine whether the epidemic is brought under control, or delayed and reduced. The former would seem the preferred objective as the numbers of Canadians affected would be minimized. However, this will require a very high degree of public health effort and public buy-in and, if successful, will require a high level of vigilance to identify imported cases and any transmission chains that may result, because the Canadian population remains largely infection naive. If transmission in Canada is not completely extinguished, strong NPIs will have to remain in place or the epidemic will rebound. Any lifting of physical distancing, which appears to be bearing fruit at present, will have to be matched by increased efforts to detect cases by surveillance and to trace and quarantine contacts.
Modelling studies are not predictions, they present plausible outcomes with different levels of NPIs, given our current knowledge of the virus and its transmission, and can be used to support planning, particularly in evidence-limited situations, such as emerging infectious disease epidemics. Our knowledge is constantly evolving, and the models and their outcomes will evolve accordingly. The models provide information that is useful for decision-making, but they do not make decisions. Decisions on public health programs to control COVID-19 in Canada will be made accounting for a range of additional factors that include (but are not limited to) economic impacts, ethical and legal concerns, and the negative health impacts of aggressive physical distancing.
Funding
This work was funded by the Public Health Agency of Canada. JA and JW’s work has also been funded by the Canadian Institute of Health Research (CIHR) 2019 Novel Coronavirus (COVID-19) rapid research program.
