Hepatitis C outbreak at a colonoscopy clinic, Ontario

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 47-4: COVID-19: A Year Later
Date published: April 2021
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 47-4: COVID-19: A Year Later
Outbreak
An outbreak of hepatitis C virus attributed to the use of multi-dose vials at a colonoscopy clinic, Waterloo Region, Ontario
Arianne Folkema1, Hsiu-Li Wang1,2, Kristy Wright1, M Mustafa Hirji2,3, Anton Andonov4, Kathryn Bromley1, Chad Ludwig1, Amy MacArthur1
Affiliations
1 Region of Waterloo Public Health and Emergency Services, Waterloo, ON
2 Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON
3 Niagara Region Public Health & Emergency Services, Thorold, ON
4 National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
Correspondence
Suggested citation
Folkema A, Wang HL, Wright K, Hirji MM, Andonov A, Bromley K, Ludwig C, MacArthur A. An outbreak of hepatitis C virus attributed to the use of multi-dose vials at a colonoscopy clinic, Waterloo Region, Ontario. Can Commun Dis Rep 2021;47(4):224–31. https://doi.org/10.14745/ccdr.v47i04a07
Keywords: HCV, infection prevention and control practices, IPAC, contamination, outbreak, out-of-hospital healthcare settings
Abstract
Background: Hepatitis C virus (HCV) transmission has been epidemiologically linked to healthcare settings, particularly out-of-hospital settings such as endoscopy clinics and hemodialysis clinics. These have been largely attributed to lapses in infection prevention and control practices (IPAC).
Objective: To describe the public health response to an outbreak of HCV that was detected among patients of a colonoscopy clinic in Ontario, and to highlight the risks of using multi-dose vials and the need for improved IPAC practices in out-of-hospital settings.
Methods: Screening for HCV was conducted on patients and staff who attended or worked at the clinic within the same timeframe as the index case’s procedure. Blood samples from positive cases underwent viral sequencing. Inspections of the clinic assessed IPAC practices, and a chart review was done to identify plausible mechanisms for transmission.
Outcome: A total of 38% of patients who underwent procedures at the clinic on the same day as the index case tested positive for HCV. Genetic sequencing showed a high degree of similarity in the HCV genetic sequence among the samples positive for HCV. Chart review and clinic inspection identified use of multi-dose vials of anesthesia medication across multiple patients as the plausible mechanism for transmission.
Conclusion: Healthcare workers, especially those in out-of-hospital procedural/surgical premises, should be vigilant in following IPAC best practices, including those related to the use of multi-dose vials, to prevent the transmission of bloodborne infections in healthcare settings.
Introduction
About 246,000 Canadians were living with chronic hepatitis C virus (HCV) infection in 2011Footnote 1.
HCV is a bloodborne virus, and the most common modes of infection are through using drug paraphernalia contaminated with infected blood, receiving body services (e.g. tattooing) that use unsanitary tools or work practices, or sharing personal care itemsFootnote 2.
HCV outbreaks have been epidemiologically linked to healthcare settings in Canada and elsewhere, particularly out-of-hospital surgical/procedural settings such as endoscopy clinics and hemodialysis clinicsFootnote 3Footnote 4Footnote 5Footnote 6Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16. Transmission in these settings has been attributed to syringe reuse; contamination of medication vials used on multiple patients; storage and preparation of medication, intravenous solution and injections in a contaminated environment; and other infection prevention and control (IPAC) lapses that resulted in the contamination of injectable medications or flush solution used for multiple patientsFootnote 3Footnote 4Footnote 5Footnote 6Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16.
The purpose of this report is to:
- Describe the public health response to this outbreak of HCV in a colonoscopy clinic
- Highlight the risk of using multi-dose vials
- Show the need for continued improvement in IPAC practices in out-of-hospital settings
Background
As mandated through the Health Protection and Promotion Act, HCV is a reportable disease and Ontario public health departments must investigate all new diagnoses of HCV that occur within their jurisdictionFootnote 17Footnote 18.
Region of Waterloo Public Health and Emergency Services (hereafter referred to as Public Health), in southern Ontario, coordinates the public health activities for its urban and rural population of approximately 550,000. As part of its reportable disease investigation, Public Health follows up with all HCV cases to identify possible sources of infection and to take measures to prevent further spreadFootnote 18.
Methods
Detection of the outbreak
In October 2014, during a routine follow-up with an individual who had been recently diagnosed with HCV and who had previously tested negative for HCV through repeat blood donation screening, Public Health identified that this individual’s only risk factor was a procedure at a colonoscopy clinic on December 24, 2013. All other HCV cases reported within the jurisdiction since January 1, 2010 (the year the clinic opened) were subsequently reviewed to determine if other cases had identified colonoscopy as a risk factor.
The review identified a second individual who had been reported to Public Health earlier in 2014 and who had also undergone a procedure on the same day at the same clinic. Although this person had been born in an endemic country, they had no other obvious risk factors. Given that the two cases shared a common risk factor and were linked by time and place, an outbreak was suspected and further investigation was conducted. Blood samples from the two identified HCV cases were obtained and sent to the National Microbiology Laboratory of the Public Health Agency of Canada in Winnipeg for genotyping and sequencing.
Investigation
Public Health used the Centers for Disease Control and Prevention (CDC) Viral hepatitis: Healthcare Investigation Guide and documentation from a previous health care–associated investigation as guidance for a systematic approach to the investigation and public health response to this outbreakFootnote 19Footnote 20.
Patient lists for December 23 and 24, 2013 were obtained from the colonoscopy clinic and the provincial reportable diseases database was searched to determine whether any other cases of HCV reported in the province had undergone procedures at the clinic since its inception on October 14, 2010. Patient screening was not conducted for the two-day interval before and after December 23 and 24, 2013 because the clinic was closed on December 21 and 22 (a Saturday and a Sunday), and December 25 and 26 (statutory holidays).
As per the Public Health Ontario Laboratory Protocol for HCV testing, patients were first screened for HCV antibodies using an anti-hepatitis C antibody assay; those with anti-hepatitis C antibodies then had HCV antibody supplemental testing performed for confirmationFootnote 21. Any patients with positive results on the confirmatory testing then had new samples submitted for molecular testing for HCV ribonucleic acid RNA and genotyping.
Public Health conducted a search on the College of Physicians and Surgeons of Ontario’s (CPSO) website to confirm that all physicians working at the clinic on December 23 and 24, 2013 held valid professional licences and to determine if any previous IPAC violations had been reported.
Public Health staff contacted all patients who underwent procedures at the clinic on December 23 and 24, 2013 and recommended HCV screening. Daytime and early evening screening clinics were offered locally. For patients who resided outside of the health department’s jurisdiction, Public Health facilitated follow-up with physicians in their areas. Blood samples were submitted to the National Microbiology Laboratory in Winnipeg for HCV testing and positive samples underwent genetic sequencing.
A case was defined as an individual with a laboratory-confirmed HCV infection (both HCV antibody and RNA testing) that had undergone any procedure performed at the clinic on December 23 or 24 that could be associated with disease transmission (colonoscopy, esophagogastroduodenoscopy/gastroscopy, glucose monitoring and intravenous medication administration).
Between November 17, 2014 and April 21, 2015, Public Health conducted multiple inspections of the clinic to assess and follow-up on IPAC practices. The inspection was guided by a comprehensive assessment tool based on the Ontario Provincial Infectious Diseases Advisory Committee’s (PIDAC) Infection Prevention and Control for Clinical Office Practice documentFootnote 22. Inspections were supplemented by lengthy interviews with staff to understand their processes and IPAC practices. The final inspection was conducted jointly with the CPSO, which has regulatory oversight over community colonoscopy clinics (public health departments in Ontario only investigate out-of-hospital premises in response to suspected infection control lapses)Footnote 23.
The inspections involved observation of high-risk procedures including the preparation and storage of medications; use of multi-dose vials; endoscope reprocessing practices; cleaning and disinfection of surfaces and equipment; use of materials and equipment such as medical gels, intravenous saline flushes and glucometers; as well as a review of the clinic’s IPAC policies and procedures. Several colonoscopy procedures were observed directly to assess the IPAC practices, including anesthesia administration.
An extensive review of all the charts of patients who visited the clinic for procedures on December 23 and 24, 2013 was also conducted to identify patterns that might indicate potential routes of transmission. The chart review included review of the time of the visit; procedure room used; endoscope serial number; glucometer use; anesthetic (propofol) dose received; other medications received; and the attending surgeon, anesthesiologist and nursing staff.
Results
In total, 40 individuals underwent procedures at the clinic on December 23 and 24, 2013. Public Health was successful in screening 39 out of the 40 patients and 11 out of 13 staff (Table 1); one patient and two staff could not be contacted. Of the 26 patients who attended and nine staff who worked at the clinic on December 23, all screened negative for HCV. Of the patients who attended the clinic on December 24, 5 out of 13 (38%) tested positive for HCV (Table 2).
| Clinic date | Total | HCV positive | HCV negative | Not screenedTable 1 footnote a |
|---|---|---|---|---|
| Patients | ||||
| 2013-12-23 | 27 | 0 | 26 | 1 |
| 2013-12-24 | 13 | 5 | 8 | 0 |
| Staff | ||||
| 2013-12-23 only | 8 | 0 | 6 | 2 |
| 2013-12-24 only | 2 | 0 | 2 | 0 |
| Both 2013-12-23 and 2013-12-24 | 3 | 0 | 3 | 0 |
| Total | 53 | 5 | 45 | 3 |
| Characteristics | Number of cases | |
|---|---|---|
| Sex | Male | 4 |
| Female | 1 | |
| Age group, years | 0–19 | 0 |
| 20–49 | 1 | |
| 50–69 | 3 | |
| 70+ | 1 | |
| Risk factorsTable 2 footnote a | Born in endemic country | 1 |
| Previous medical/surgical procedure (>20 years prior) | 2 | |
| Procedure at colonoscopy clinic on Dec. 24, 2020 | 5 | |
None of the staff who worked on December 24 tested positive. The review of all known HCV cases in Ontario since the clinic’s inception in October 2010 did not identify any additional cases of HCV linked to this clinic. The search on the CPSO website indicated that all physicians working at the clinic at the time of the outbreak held valid licences.
Phylogenetic analysis conducted by the National Microbiology Laboratory based on partial sequences from the HCV core (C), envelope (E1) and non-structural (NS5B) genes clearly indicated that the HCV strains from the five outbreak patients were genetically closely related (Figure 1), consistent with a cluster of transmission. All five cases clustered in a monophyletic group based on the E1 gene sequences. A more detailed analysis based on next-generation sequencing (NGS) revealed that the HCV quasispecies population of all five patients clearly clustered together on a single branch with a 99% probability (Figure 2). The average genetic distance within the five cases involved in the outbreak quasispecies was 0.036 while the genetic distance between this group and similar outbreaks as well as unrelated Ontario cases and GenBank HCV strains was significantly higher (p<0.001).
Figure 1: Phylogenetic analysis of Sanger population-based sequencing of HCV E1/NS5B subgenomic regions from five patients in an outbreak investigationFigure 1 footnote a
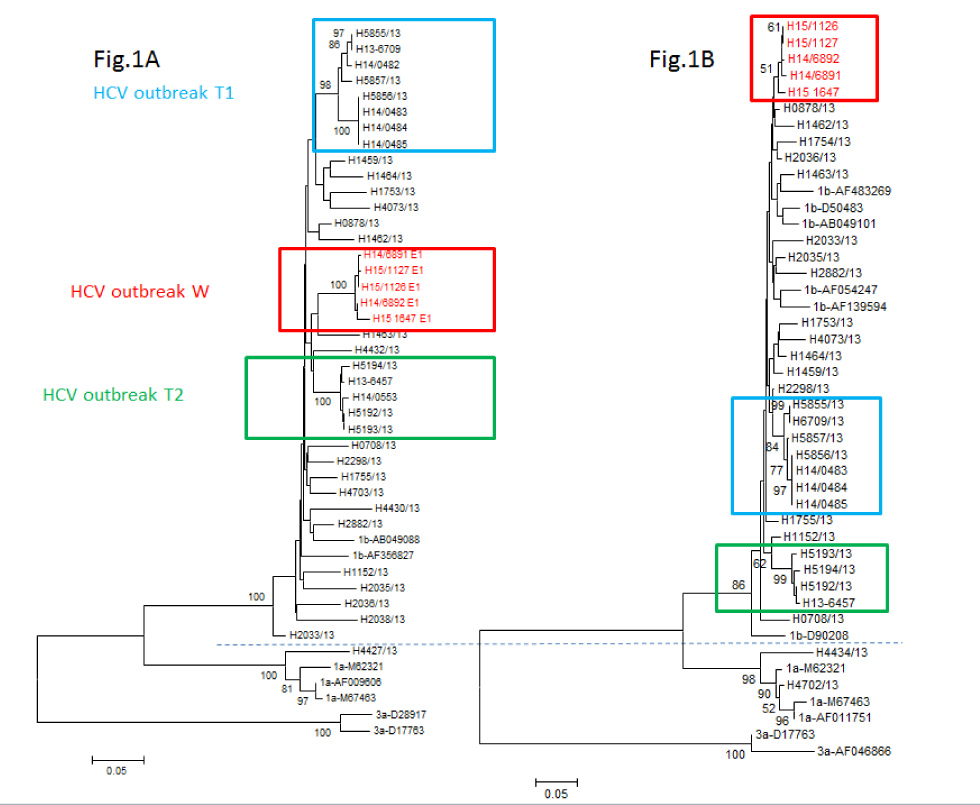
Text description: Figure 1
Phylogenetic analysis of Sanger population-based sequencing of HCV E1/NS5B subgenomic regions from five patients in an outbreak investigation. Neighbour-joining tree was created by using Kimura’s two-parameter model in MEGA v.6. Outbreak sequences from this outbreak (W) and two other similar outbreaks in endoscopy clinics from Toronto (T1 and T2) are in red, green and blue boxes respectively. Epidemiologically unrelated HCV sequences from the province of Ontario collected in the same year (2013) as well as some randomly selected sequences from GenBank are in black. The dendrogram in Fig. 1A is based on E1 region and that in Fig. 1B is based on the NS5B region. Note that phylogenetic analysis based on NS5B region did not have the same strong bootstrap support as that observed for E1 region. In comparison the bootstrap measures for the two other similar outbreaks T1 and T2 remained robust for both the E1 and NS5b although dropped somewhat from 98 to 84 for T1.
Figure 2: Phylogenetic analysis of HCV HVR-1 quasispecies in samples of five patients involved in a transmission event in this outbreak (outbreak W)Figure 2 footnote a
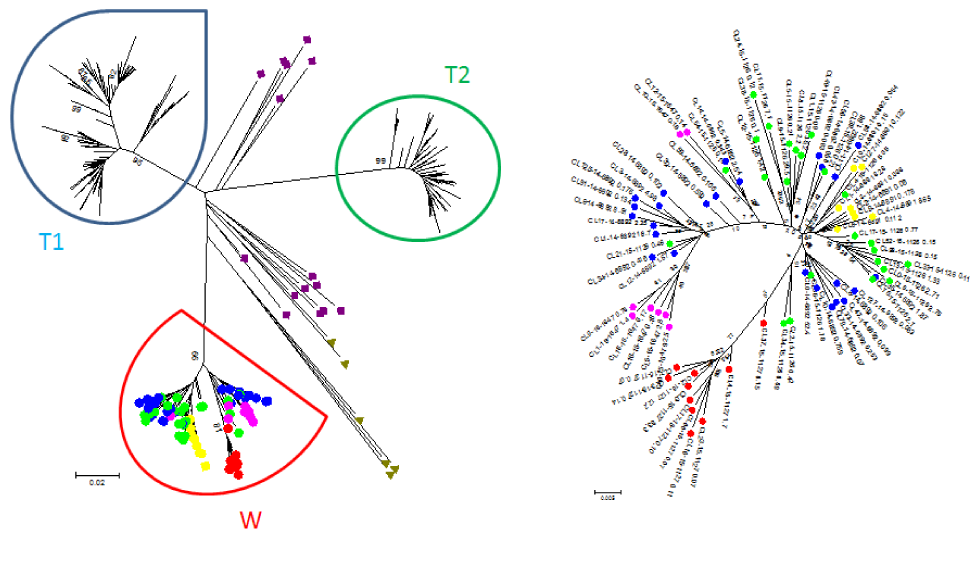
Text description: Figure 2
Phylogenetic analysis of HCV HVR-1 quasispecies in samples of five patients involved in a transmission event in outbreak W. Sequences of HCV genotype 1b quasispecies population derived from three different outbreaks (T1, T2 and W), sequences of unrelated HCV strains from Ontario and GenBank (green triangles) and randomly selected HCV strains from Genbank (purple squares) are presented on the left side of the figure. Bootstrap values are shown at the bottom of the nodes. Sequences belonging to the five cases from outbreak W are colour coded and magnified on the right corner of the figure. Each colour dot (node) represents a single HCV variant. Quasispecies of patient #6 (transmission source) green; patient #7 red; patient #8 pink; patient #10 blue; patient #13 yellow. Note that consensus sequences from the unrelated HCV strains from Ontario and GenBank, as well as the quasispecies from two other HCV outbreaks T1 and T2 occupy entirely different sequence space.
The inspections of the clinic and interviews with staff identified no concerns or deficiencies with respect to the use, cleaning or reprocessing of endoscopes. Glucose monitoring was not performed on all five HCV patients, ruling out the use of a shared glucometer as a potential source of infection. Of the 13 patients who received an intravenous saline flush, only five were HCV positive, which decreased the likelihood that the bag of intravenous saline used for the entire day was a potential source of infection.
The chart review of patients attending the clinic on December 24 showed that only one procedure room was used. All patients who attended the clinic that day had the same surgeon, anesthesiologist and nurses during their procedures. The first 11 patients had the same pre-procedure nurse, while the last two patients had a different pre-procedure nurse. The procedures for the five patients who tested positive for HCV used different endoscopes.
All HCV patients were administered the anesthetic drug, propofol, using multi-dose medication vials across multiple patients, and this was the only plausible mechanism of HCV transmission identified. Based on the amount of propofol administered to each patient according to their medical charts, a patient medication map was developed to present the hypothesized distribution of contaminated 100 mL vials of propofol alongside the distribution of HCV cases at the clinic on December 24, 2013 (Table 3). Since all patients who had a procedure performed also had propofol administered during their procedure, a pattern was identified in the occurrence of HCV positivity and the use of propofol.
| Patient number | Procedure start time | Procedure type | HCV lab result | Medication administered | Total dose administered (mg)Table 3 footnote a | Total volume administered (mL)Table 3 footnote b | Hypothesized vial # | Hypothesized use of propofol vials (100 mL per vial) |
|---|---|---|---|---|---|---|---|---|
| 1 | 08:54 | Colonoscopy | Negative | Propofol | 250 | 25 | Vial #1 | All 100 mL of the first vial of propofol (Vial #1) was used on the first five patients. |
| 2 | 09:16 | Colonoscopy | Negative | Propofol | 150 | 15 | ||
| 3 | 09:54 | Endoscopy | Negative | Propofol | 200 | 20 | ||
| 4 | 10:08 | Colonoscopy | Negative | Propofol | 200 | 20 | ||
| 5 | 10:42 | Colonoscopy | Negative | Propofol | 200 | 20 | ||
| 6 | 11:10 | Endoscopy/colonoscopy | Positive | Propofol | 250 | 25 | Vial #2 | The second vial of propofol (Vial #2) was contaminated with the blood of Patient #6 with HCV and was then used on Patients #7, #8 and #10. |
| 7 | 11:54 | Colonoscopy | Positive | Propofol | 200 | 20 | ||
| 8 | 12:25 | Colonoscopy | Positive | Propofol | 200 | 20 | ||
| 9 | N/A | IV start only | Negative | None | None | None | ||
| 10 | 13:40 | Colonoscopy | Positive | Propofol | 200 | 20 | ||
| 11 | 14:10 | Colonoscopy | Negative | Propofol | 200 | 20 | Vial #3 | The last 15 mL of Vial #2 was mixed with 5 mL of propofol from a third vial (Vial #3) to make 20 mL of propofol for Patient #11, thereby contaminating Vial #3, but diluting the amount of contamination. This dilution could explain why only one of the three patients who received propofol from Vial #3 tested positive for HCV. |
| 12 | 14:35 | Endoscopy/colonoscopy | Negative | Propofol | 250 | 25 | ||
| 13 | 15:11 | Colonoscopy | Positive | Propofol | 200 | 20 | ||
As shown in Table 3, it is hypothesized that all 100 mL of the first vial of propofol (Vial #1) was used up on the first five patients, none of whom tested positive for HCV. Then, propofol Vial #2 was contaminated with the blood of patient #6 who had a pre-existing undiagnosed HCV infection. This same vial was then used for patients #7, #8 and #10. Finally, the remaining 15 mL of propofol in Vial #2 was mixed with 5 mL of propofol from a third vial to make 20 mL of propofol for patient #11, thereby contaminating Vial #3 but diluting the amount of contamination. This dilution could explain why only one of the three patients who received propofol from Vial #3 tested positive for HCV.
Given that the actual propofol vial used for each patient was not identified in the patient charts, this interpretation was hypothesized based on the type of vials used by the clinics, the doses administered to the patients as recorded in their charts and finding out from the inspections and interviews that multi-dose vials were being used across multiple patients.
Discussion
The findings from the outbreak investigation support the hypothesis of HCV transmission from a previously undiagnosed HCV patient to four uninfected persons. Laboratory results demonstrated that all five cases of HCV had genetically related viruses, indicating a high likelihood of transmission from a common source. The investigation identified an association between the administration of propofol from multi-dose vials and the patients who tested positive for HCV.
Contamination of multi-dose vials has been associated with other instances of transmission of bloodborne infections in colonoscopy clinics, among other placesFootnote 4Footnote 8Footnote 12Footnote 24Footnote 25. There is a risk of bloodborne pathogen transmission when devices (e.g. blood glucose monitors) and medications (e.g. multi-dose vials, saline bags) are shared among patients, even in the absence of visible blood on objectsFootnote 6Footnote 25Footnote 26Footnote 27Footnote 28.
Out-of-hospital settings may be more vulnerable to infection control lapses than hospitals because specific IPAC resources and oversight have been less robust in this practice settingFootnote 29Footnote 30. Out-of-hospital settings typically do not have on-site infection control specialists, guidance on policies and procedures tailored to their practice setting, requirements to audit staff practices, nor in many jurisdictions, a clear procedure to report and investigate infection control lapsesFootnote 29.
In addition, non-hospital settings may have physically smaller procedure rooms; this may provide more opportunities for body fluids from patients to contaminate nearby surfaces, supplies and equipmentFootnote 23Footnote 31. In 2014, Canadian hospital operating rooms were a minimum of 400 square feet; in comparison, non-hospital procedure rooms do not have a set minimum size and only require that the space allow for the physician and assisting staff to move around the procedure table with access to the patient without contaminationFootnote 23Footnote 31.
At the time of the outbreak, out-of-hospital premises were inspected only once every five years, which resulted in out-of-date practices, declined adherence to standards of practice over time and/or failure to recognize critical errors in practice for long periods Footnote 23Footnote 32Footnote 33.
It is not routine practice to screen clients for bloodborne pathogens prior to invasive medical procedures, and IPAC procedures have been put in place to prevent transmission of bloodborne pathogens. However, researchers estimate that 44% of Canadians with HCV infection remain undiagnosed due to the asymptomatic nature and slow progression of infectionFootnote 34. Further, for a sub-group of people born between 1945 and 1975 (“baby boomers”), up to 70% of people who have the virus are unaware of their infection statusFootnote 34. In its updated guidelines, the Canadian Association for the Study of the Liver has emphasized that healthcare providers should offer HCV testing to people at risk for infection, including baby boomersFootnote 35. These updated guidelines for screening could help raise awareness of HCV-positive status prior to medical procedures and contribute to the prevention of bloodborne pathogens in all settings.
Outbreak response challenges
The approaches of Public Health and the regulatory bodies were not necessarily consistent and had to be aligned. Further, knowledge of infection control and prevention best practices varied among healthcare practitioner groups. The IPAC investigation also needed to be conducted while allowing the clinic to continue operating and providing procedures for clients during the investigation.
Finally, at the time of the outbreak, although cases were identified through patient screening, not all cases were eligible for treatment. Fortunately, changes have since been made to the Ontario Drug Benefit to cover the cost of medication for all HCV patients.
Conclusion
This outbreak investigation resulted in increased local and provincial awareness of medication injection safetyFootnote 23. On April 30, 2015, Public Health Ontario published updated guidance on the use of multi-dose vials. The CPSO adopted the Public Health Ontario best practices guidance as the standard for IPAC in out-of-hospital premisesFootnote 22. The updated guidelines refer to the overwhelming preference for single-use medication vials and state that multi-dose vials should be restricted to single patients. The guidelines also state that patient safety should be prioritized over cost when choosing between multi-dose and single-use mediation vials.
Since this outbreak in 2013, other outbreaks related to IPAC lapses in out-of-hospital premises have occurred in CanadaFootnote 15Footnote 16. Public health investigations of IPAC complaints are also on the rise in Ontario, including in settings where regulated health professionals workFootnote 36. Given continued pressures on delivery of health care in Canada and around the world, the number of procedures that occur in out-of-hospital premises could increase, leading to an increased risk of outbreaks if IPAC practices are suboptimal. Careful consideration of IPAC resources, supports and regulations is needed as such an expansion of out-of-hospital premises occurs.
Authors’ statement
- AF — Writing–original draft, writing–review & editing
- HW — Supervision of outbreak management and public health response, writing–review & editing
- KW — Supervision of outbreak management and public health response, writing–review & editing
- MH — Investigation, writing–review & editing
- AA — Viral sequencing and interpretation, writing–review & editing
- KB — Writing–review & editing
- CL — Investigation, writing–review & editing
- AM — Writing–review & editing
Competing interests
None.
Acknowledgements
We thank Public Health Ontario and the National Microbiology Laboratory of the Public Health Agency of Canada for their expertise and guidance during the investigation. We would also like to thank BM Hutchison and the Sexual Health and Harm Reduction and Infectious Diseases and Tuberculosis Control teams at Region of Waterloo Public Health for their contributions to the investigation of this outbreak.
Funding
This work was supported by the Ontario Ministry of Health and the Region of Waterloo.
References
- Footnote 1
-
Trubnikov M, Yan P, Archibald C. Estimated prevalence of hepatitis C virus infection in Canada, 2011. Can Commun Dis Rep 2014;40(19):429-36. https://doi.org/10.14745/ccdr.v40i19a02
- Footnote 2
-
Government of Canada. Hepatitis C. Ottawa (ON): Government of Canada; 2016-03-10 (accessed 2021-03-21). http://healthycanadians.gc.ca/diseases-conditions-maladies-affections/disease-maladie/hepatitis-c-hepatite/index-eng.php
- Footnote 3
-
Germain JM, Carbonne A, Thiers V, Gros H, Chastan S, Bouvet E, Astagneau P. Patient-to-patient transmission of hepatitis C virus through the use of multidose vials during general anesthesia. Infect Control Hosp Epidemiol 2005;26(9):789-92. https://doi.org/10.1086/502618
- Footnote 4
-
Fischer GE, Schaefer MK, Labus BJ, Sands L, Rowley P, Azzam IA, Armour P, Khudyakov YE, Lin Y, Xia G, Patel PR, Perz JF, Holmberg SD. Hepatitis C virus infections from unsafe injection practices at an endoscopy clinic in Las Vegas, Nevada, 2007-2008. Clin Infect Dis 2010;51(3):267-73. https://doi.org/10.1086/653937
- Footnote 5
-
Centers for Disease Control and Prevention (CDC). Acute hepatitis C virus infections attributed to unsafe injection practices at an endoscopy clinic--Nevada, 2007. MMWR Morb Mortal Wkly Rep 2008;57(19):513-7.
- Footnote 6
-
Centers for Disease Control and Prevention. Healthcare-associated hepatitis B and C outbreaks (≥ 2 cases) reported to the Centers for Disease Control and Prevention 2008-2019. Atlanta (GA): CDC; 2015 (accessed 2016-03-21). http://www.cdc.gov/hepatitis/outbreaks/healthcarehepoutbreaktable.htm
- Footnote 7
-
Heikens E, Hetem DJ, Jousma-Rutjes JP, Nijhuis W, Boland GJ, Hommes NH, Thang OH, Schuurman R. Hepatitis C virus transmission in a Dutch haemodialysis unit: detailed outbreak investigation using NS5A gene sequencing. J Hosp Infect 2019;101(3):333-8. https://doi.org/10.1016/j.jhin.2018.11.015
- Footnote 8
-
Chung YS, Choi JY, Han MG, Park KR, Park SJ, Lee H, Jee Y, Kang C. A large healthcare-associated outbreak of hepatitis C virus genotype 1a in a clinic in Korea. J Clin Virol 2018;106:53-7. https://doi.org/10.1016/j.jcv.2018.07.006
- Footnote 9
-
Coyle JR, Goerge E, Kacynski K, Rodgers R, Raines P, Vail LS, Lowhim S. Hepatitis C virus infections associated with unsafe injection practices at a pain management clinic, Michigan, 2014-2015. Pain Med 2017;18(2):322-9. https://doi.org/10.1093/pm/pnw157
- Footnote 10
-
Nguyen DB, Gutowski J, Ghiselli M, Cheng T, Bel Hamdounia S, Suryaprasad A, Xu F, Moulton-Meissner H, Hayden T, Forbi JC, Xia GL, Arduino MJ, Patel A, Patel PR. A large outbreak of hepatitis C virus infections in a hemodialysis clinic. Infect Control Hosp Epidemiol 2016;37(2):125-33. https://doi.org/10.1017/ice.2015.247
- Footnote 11
-
Perz JF, Thompson ND, Schaefer MK, Patel PR. US outbreak investigations highlight the need for safe injection practices and basic infection control. Clin Liver Dis 2010;14(1):137-51. https://doi.org/10.1016/j.cld.2009.11.004
- Footnote 12
-
Macedo de Oliveira A, White KL, Leschinsky DP, Beecham BD, Vogt TM, Moolenaar RL, Perz JF, Safranek TJ. An outbreak of hepatitis C virus infections among outpatients at a hematology/oncology clinic. Ann Intern Med 2005;142(11):898-902. https://doi.org/10.7326/0003-4819-142-11-200506070-00007
- Footnote 13
-
Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: united States, 1998-2008. Ann Intern Med 2009;150(1):33-9. https://doi.org/10.7326/0003-4819-150-1-200901060-00007
- Footnote 14
-
Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, Desenclos JC. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol 2005;26(9):752-60. https://doi.org/10.1086/502613
- Footnote 15
-
Toronto Public Health. HCV Outbreak Investigation - North Scarborough Endoscopy Clinic Final Report. Toronto (ON): Toronto Public Health; 2014.
- Footnote 16
-
Toronto Public Health. HCV outbreak investigation - Ontario Endoscopy Clinic Final Report. Toronto (ON): Toronto Public Health; 2015.
- Footnote 17
-
Government of Ontario. Health Protection and Promotion Act, R.S.O. 1990, c. H.7. 2015:1-59. Toronto (ON): Government of Ontario; 2020 (accessed 2019-12-24). https://www.ontario.ca/laws/statute/90h07?search=Public+Health+Act&use_exact=on
- Footnote 18
-
Ontario Ministry of Health and Long-Term Care. Ontario Public Health Standards: Requirements for programs, services, and accountability (Standards). Toronto (ON): Government of Ontario; 2018.
- Footnote 19
-
Centers for Disease Control and Prevention. Viral hepatitis: Healthcare investigation guide. Atlanta (GA): CDC; 2015 (accessed 2019-12-21). http://www.cdc.gov/hepatitis/Outbreaks/HealthcareInvestigationGuide.htm
- Footnote 20
-
Bornschlegel K, Dentinger C, Layton M, Balter S, France AM; Centers for Disease Control and Prevention (CDC). Investigation of viral hepatitis infections possibly associated with health-care delivery--New York City, 2008-2011. MMWR Morb Mortal Wkly Rep 2012;61(19):333-8.
- Footnote 21
-
Ontario Agency for Health Protection and Promotion (Public Health Ontario). Labstract: Hepatitis C virus (HCV) RNA and genotype testing and interpretation. Toronto (ON): Public Health Ontario; 2008 (updated 2019-12; accessed 2020-11-07). https://www.publichealthontario.ca/-/media/documents/lab/lab-sd-033-hep-c-rna-testing-update.pdf?la=en
- Footnote 22
-
Provincial Infectious Diseases Advisory Committee. Infection prevention and control for clinical office practice. Toronto (ON): Public Health Ontario; 2013 (accessed 2019-12-24). https://www.publichealthontario.ca/-/media/documents/b/2013/bp-clinical-office-practice.pdf?la=en
- Footnote 23
-
College of Physicians and Surgeons of Ontario. Out-of-Hospital Premises Inspection Program (OPHPIP) Program Standards. Toronto (ON): CPSO; 2013 (updated 2017-10). https://www.cpso.on.ca/admin/CPSO/media/Documents/physician/your-practice/quality-in-practice/clinic-inspections-special-programs/ohpip-standards.pdf
- Footnote 24
-
Greeley RD, Semple S, Thompson ND, High P, Rudowski E, Handschur E, Guo-Liang X, Ganova-Raeva L, Crawford J, Robertson C, Tan C, Montana B. Hepatitis B outbreak associated with a hematology-oncology office practice in New Jersey, 2009. Am J Infect Control 2011;39(8):663-70. https://doi.org/10.1016/j.ajic.2010.11.011
- Footnote 25
-
County of Los Angeles Public Health. 2010 Pain Clinic Hepatitis Investigation Report - OB 201016. Los Angeles (CA): County of Los Angeles Public Health; 2011. http://publichealth.lacounty.gov/acd/docs/HepInfo/Final Report Hepatitis Investigation at Pain Clinic.pdf.PDF
- Footnote 26
-
Comstock RD, Mallonee S, Fox JL, Moolenaar RL, Vogt TM, Perz JF, Bell BP, Crutcher JM. A large nosocomial outbreak of hepatitis C and hepatitis B among patients receiving pain remediation treatments. Infect Control Hosp Epidemiol 2004;25(7):576-83. https://doi.org/10.1086/502442
- Footnote 27
-
Centers for Disease Control and Prevention (CDC). Notes from the field: deaths from acute hepatitis B virus infection associated with assisted blood glucose monitoring in an assisted-living facility--North Carolina, August-October 2010. MMWR Morb Mortal Wkly Rep 2011;60(6):182.
- Footnote 28
-
Centers for Disease Control and Prevention (CDC). Hepatitis C virus transmission at an outpatient hemodialysis unit--New York, 2001-2008. MMWR Morb Mortal Wkly Rep 2009;58(8):189-94. https://doi.org/10.1097/01.NEP.0000350585.46820.f0
- Footnote 29
-
Williams IT, Perz JF, Bell BP. Viral hepatitis transmission in ambulatory health care settings. Clin Infect Dis 2004;38(11):1592-8. https://doi.org/10.1086/420935
- Footnote 30
-
Schaefer MK, Jhung M, Dahl M, Schillie S, Simpson C, Llata E, Link-Gelles R, Sinkowitz-Cochran R, Patel P, Bolyard E, Sehulster L, Srinivasan A, Perz JF. Infection control assessment of ambulatory surgical centers. JAMA 2010;303(22):2273-9. https://doi.org/10.1001/jama.2010.744
- Footnote 31
-
Facility Guidelines Institute. Guidelines for design and construction of hospitals and outpatient facilities. Chicago (IL): American Hospital Association; 2014.
- Footnote 32
-
Government of Ontario. Excellent Care for All Act, 2010, S.O. 2010, c. 14.; 2014. Toronto (ON): Government of Ontario; 2020 (accessed 2019-12-21). https://www.ontario.ca/laws/statute/10e14
- Footnote 33
-
Government of Ontario. Ontario Regulation 490/09: Designated Substances; 2013:21. http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_090490_e.htm#
- Footnote 34
-
Rotermann M, Langlois K, Andonov A, Trubnikov M. Seroprevalence of hepatitis B and C virus infections: results from the 2007 to 2009 and 2009 to 2011 Canadian Health Measures Survey. Health Rep 2013;24(11):3-13.
- Footnote 35
-
Shah H, Bilodeau M, Burak KW, Cooper C, Klein M, Ramji A, Smyth D, Feld JJ; Canadian Association for the Study of the Liver. The management of chronic hepatitis C: 2018 guideline update from the Canadian Association for the Study of the Liver. CMAJ 2018;190(22):E677-87. https://doi.org/10.1503/cmaj.170453
- Footnote 36
-
Cadieux G, Brown C, Sachdeva H. Public health investigation of infection prevention and control complaints in Ontario, 2015-2018. Can Commun Dis Rep 2019;45(11):289-95. https://doi.org/10.14745/ccdr.v45i11a03
