Pre-exposure prophylaxis use in Canada, 2014–2018

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 47-5/6: Responding to New Clusters of COVID-19
Date published: May/June 2021
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 47-5/6: Responding to New Clusters of COVID-19
Surveillance
Trends in HIV pre-exposure prophylaxis use in eight Canadian provinces, 2014–2018
Nashira Popovic1, Qiuying Yang1, Chris Archibald1
Affiliation
1 Centre for Communicable Disease and Infection Control, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Popovic N, Yang Q, Archibald C. Trends in HIV pre-exposure prophylaxis use in eight Canadian provinces, 2014–2018. Can Commun Dis Rep 2021;47(5/6):251–8. https://doi.org/10.14745/ccdr.v47i56a02
Keywords: HIV, Canada, pre-exposure prophylaxis, prevention
Abstract
Introduction: Canada has endorsed the Joint United National Programme on HIV and AIDS global targets to end the acquired immunodeficiency syndrome (AIDS) epidemic, including reducing new human immunodeficiency virus (HIV) infections to zero, by 2030. Given the effectiveness of pre-exposure prophylaxis (PrEP) to prevent new infections, it is important to measure and report on PrEP utilization to help inform planning for HIV prevention programs and policies.
Methods: Annual estimates of persons using PrEP in Canada were generated for 2014–2018 from IQVIA’s geographical prescription monitor dataset. An algorithm was used to distinguish users of tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for PrEP versus treatment or post-exposure prophylaxis. We provide the estimated number of people using PrEP in eight Canadian provinces by sex, age group, prescriber specialty and payment type.
Results: The estimated number of PrEP users increased dramatically over the five-year study period, showing a 21-fold increase from 460 in 2014 to 9,657 in 2018. Estimated PrEP prevalence was 416 users per million persons across the eight provinces in 2018. Almost all PrEP users were male. Use increased in both sexes, but increase was greater for males (23-fold) than females (five-fold). Use increased across all provinces, although there were jurisdictional differences in the prevalence of use, age distribution and prescriber types.
Conclusion: The PrEP use in Canada increased from 2014 to 2018, demonstrating increased awareness and uptake of its use for preventing HIV transmission. However, there was uneven uptake by age, sex and geography. Since new HIV infections continue to occur in Canada, it will be important to further refine the use of PrEP, as populations at higher risk of HIV infection need to be offered PrEP as part of comprehensive sexual healthcare.
Introduction
The government of Canada has endorsed the Joint United National Programme on HIV and AIDS (UNAIDS) global targets to end the AIDS epidemicFootnote 1Footnote 2Footnote 3, including reducing new human immunodeficiency virus (HIV) infections to zero, by 2030. Given the effectiveness of pre-exposure prophylaxis (PrEP) to prevent new infections, and the goal of increasing access to combination prevention for key populations, it is important to measure and report on its uptake in Canada. Increasing our understanding of trends in PrEP utilization will help to inform planning for HIV prevention programs and policies.
The estimated number of new HIV infections in Canada has decreased from about 4,000 per year in the mid-1980s to an estimated 2,165 in 2016Footnote 4. This decrease is likely due, in part, to the introduction of effective antiretroviral treatment, which can suppress viral load and thereby decrease HIV transmissionFootnote 5Footnote 6. The estimated number of new HIV infections in Canada decreased until 2011, but has been stable or has increased slightly since thenFootnote 4, despite the availability of antiretroviral therapy as well as behavioural interventions. Pre-exposure prophylaxis is one of the highly effective strategies to reduce the risk of acquiring an HIV infection, and has the potential to contribute to decreasing HIV incidence in Canada. In 2016, Health Canada approved the drug combination tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for use as PrEP; and in July 2017, lower cost generic versions became available in Canada.
Because PrEP use is not included in national HIV surveillance in Canada, one feasible method to estimate uptake is through the analysis of administrative prescription data. The Public Health Agency of Canada purchased and analysed data from the IQVIA longitudinal prescriptions database to estimate the number of persons prescribed PrEP (“PrEP users”) from eight Canadian provinces, and to describe their basic demographic characteristics.
Methods
Data source
Data on antiretroviral drug prescriptions dispensed between January 1, 2014 and December 31, 2018, were extracted by IQVIA from their geographical prescription monitor dataset. The IQVIA database includes Canadian aggregate dispensed prescription data projected from a sample of approximately 6,000 pharmacies in the eight available provinces, representing close to 60% of all retail pharmacies in Canada. Patient counts are then projected from this sample of pharmacies. While dispensation data provided to IQVIA is de-identified, it is linkable by IQVIA for the same person using anonymous identifiers, allowing for counts of unique individuals. The database includes antiretroviral drugs dispensed and de-identified, individual-level information on patient demographics (sex, age group), the physician specialty and payer type (private insurance, public insurance or out of pocket).
Missing data on PrEP users are possible within this dataset, since only prescriptions that were acquired from a community pharmacy are included. Dispensations from hospital pharmacies, those provided at no cost, and those purchased online are not included.
Algorithm to identify pre-exposure prophylaxis users
Specific diagnostic or procedural codes for PrEP use are not available within the IQVIA database; therefore, an algorithm was used to estimate the annual number of PrEP users (Figure 1). This algorithm discerned whether TDF/FTC was prescribed for PrEP, HIV treatment, hepatitis B treatment or HIV post-exposure prophylaxis (PEP), and was adapted from a validated United States Centers for Disease Control algorithmFootnote 7Footnote 8Footnote 9 and modified to fit the Canadian context. Briefly, in a given year, we selected persons older than two months of age who had one or more TDF-FTC prescription. Since TDF-FTC is also used to treat HIV or hepatitis B infections and as HIV PEP, we applied several exclusion criteria. First, we excluded persons who were prescribed antiretrovirals other than TDF-FTC within ±3 months (persons on HIV treatment). Second, we excluded persons who were prescribed with TDF alone (for hepatitis B treatment). Third, we excluded persons who were prescribed TDF-FTC for fewer than 30 days (PEP users). In any given year, persons prescribed TDF-FTC who were not excluded with our algorithm were considered PrEP users.
Figure 1: Algorithm to assign pre-exposure prophylaxis treatment indication
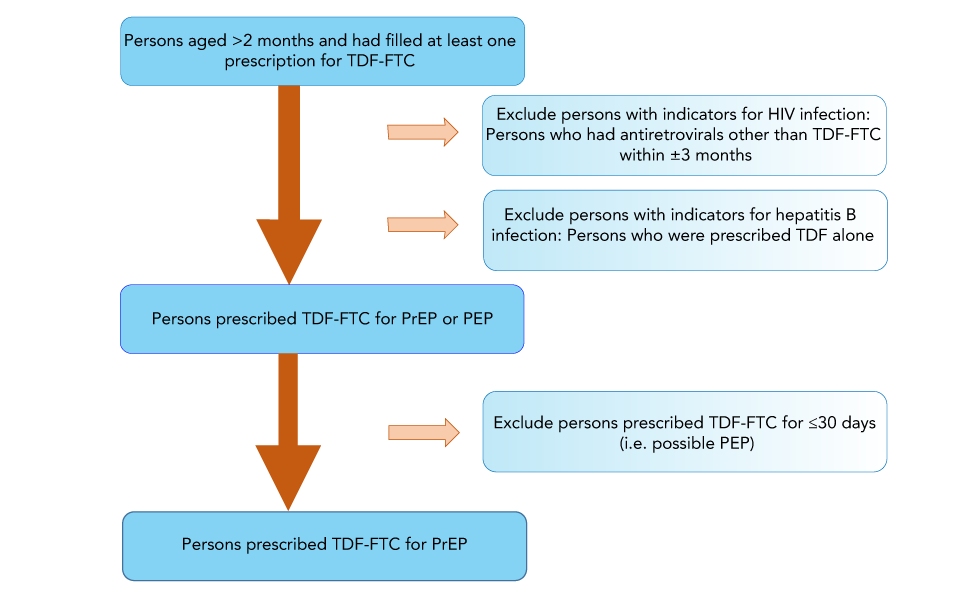
Text description: Figure 1
This diagram shows how we get the cohort for pre-exposure prophylaxis (PrEP) users. All persons aged older than two months old who had had filled at least one prescription for tenofovir disoproxil fumarate/emtricitabine (TDF-FTC) are included in the cohort. First, we excluded persons with indicators for HIV infection (i.e. persons who had antiretrovials other than TDF-FTC within ±3 months). Second, we excluded persons with indicators for hepatitis B infections (i.e. persons who were prescribed TDF alone). The remaining people would be those prescribed TDF-FTC for PrEP or post-exposure prophylaxis (PEP). Then we further excluded persons prescribed TDF-FTC for less than 30 days (i.e. those are likely PEP users). After all of the exclusions, the persons remaining are the cohort prescribed TDF-FTC for PrEP.
For the entire analysis, all ages were taken into account when IQVIA extracted the data and estimated the number of projected patients by indication. However, the results for patients younger than 15 years of age were omitted in the age and sex analysis due to small counts.
Analysis
Pre-exposure prophylaxis use estimates by sex, age group, payer type and physician specialty are descriptive. The prevalence of persons who used PrEP among all persons 15 years of age and older per million for each year were also estimated. Cochran Armitage trend tests were conducted to determine whether the proportion of PrEP uptake changed significantly over time. Analyses were performed using SAS Version 9.4 (SAS Institute).
Results
In 2018, a total of 9,657 people were estimated to be on PrEP in eight Canadian provinces (Saskatchewan (SK), Manitoba (MB), Ontario (ON), Québec (QC), New Brunswick (NB), Nova Scotia (NS), Prince Edward Island (PE) and Newfoundland and Labrador (NL)). The estimated number of PrEP users increased dramatically over the five-year study period (Table 1), showing a 21-fold increase from 460 in 2014 to 9,657 in 2018. Almost all (98%) PrEP users were male during the five-year time period and the number of users increased in both sexes, but increases were greater for males (23-fold) than females (five-fold) (Table 1).
| Estimated PrEP users | Number (%) by year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | |||||||
| Sex | # | % | # | % | # | % | # | % | # | % | |
| Male | 411 | 89.5 | 1,267 | 96.8 | 2,842 | 97.3 | 5,147 | 97.3 | 9,401 | 97.6 | |
| Female | 48 | 10.5 | 42 | 3.2 | 79 | 2.7 | 141 | 2.7 | 235 | 2.4 | |
| Total | 460 | 100.0 | 1,309 | 100.0 | 2,922 | 100.0 | 5,291 | 100.0 | 9,657 | 100.0 | |
| All estimated PrEP users | |||||||||||
| Age group (years) | # | % | # | % | # | % | # | % | # | % | |
| 15–17 | 0 | 0.0 | 3 | 0.2 | 3 | 0.1 | 6 | 0.1 | 19 | 0.2 | |
| 18–24 | 15 | 3.3 | 30 | 2.3 | 91 | 3.1 | 234 | 4.4 | 860 | 8.9 | |
| 25–35 | 99 | 21.5 | 351 | 26.8 | 913 | 31.2 | 1,815 | 34.3 | 3,527 | 36.5 | |
| 36–45 | 136 | 29.6 | 430 | 32.8 | 920 | 31.5 | 1,626 | 30.7 | 2,604 | 27.0 | |
| 46–55 | 120 | 26.1 | 316 | 24.1 | 637 | 21.8 | 985 | 18.6 | 1,683 | 17.4 | |
| 56–64 | 30 | 12.6 | 90 | 9.2 | 196 | 9.2 | 332 | 9.0 | 435 | 7.5 | |
| 65+ | 32 | 7.0 | 53 | 4.0 | 90 | 3.1 | 148 | 2.8 | 237 | 2.5 | |
| Male PrEP users | |||||||||||
| Age group (years) | # | % | # | % | # | % | # | % | # | % | |
| 15–17 | 0 | 0.0 | 3 | 0.2 | 3 | 0.1 | 6 | 0.1 | 17 | 0.2 | |
| 18–24 | 12 | 2.9 | 27 | 2.1 | 88 | 3.1 | 225 | 4.4 | 807 | 8.6 | |
| 25–35 | 81 | 19.7 | 338 | 26.7 | 881 | 31.0 | 1,755 | 34.1 | 3,433 | 36.5 | |
| 36–45 | 124 | 30.2 | 415 | 32.8 | 898 | 31.6 | 1,586 | 30.8 | 2,552 | 27.1 | |
| 46–55 | 112 | 27.3 | 310 | 24.5 | 624 | 22.0 | 966 | 18.8 | 1,650 | 17.6 | |
| 56–64 | 53 | 12.9 | 121 | 9.6 | 259 | 9.1 | 462 | 9.0 | 707 | 7.5 | |
| 65+ | 29 | 7.1 | 53 | 4.2 | 89 | 3.1 | 147 | 2.9 | 235 | 2.5 | |
| Female PrEP users | |||||||||||
| Age group (years) | # | % | # | % | # | % | # | % | # | % | |
| 15–17 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.9 | |
| 18–24 | 3 | 6.3 | 3 | 7.1 | 3 | 3.8 | 9 | 6.4 | 51 | 21.7 | |
| 25–35 | 17 | 35.4 | 13 | 31.0 | 32 | 40.5 | 57 | 40.4 | 82 | 34.9 | |
| 36–45 | 12 | 25.0 | 15 | 35.7 | 21 | 26.6 | 40 | 28.4 | 50 | 21.3 | |
| 46–55 | 8 | 16.7 | 6 | 14.3 | 13 | 16.5 | 19 | 13.5 | 30 | 12.8 | |
| 56–64 | 5 | 10.4 | 5 | 11.9 | 9 | 11.4 | 15 | 10.6 | 18 | 7.7 | |
| 65+ | 3 | 6.3 | 0 | 0.0 | 1 | 1.3 | 1 | 0.7 | 2 | 0.9 | |
The prevalence of persons prescribed PrEP among those 15 years of age or older increased significantly, from 20.7 per million in 2014 to 416.0 per million in 2018 (Ptrend < 0.001) (Figure 2). When stratified by sex, PrEP prevalence among the male population increased significantly over time (Ptrend < 0.001) with a very large increase in 2018 to 821.4 persons prescribed PrEP per million. The PrEP prevalence among the female population also showed an increasing trend, from 4.2 per million in 2014 to 20.0 per million in 2018 (Ptrend < 0.001); however, the overall uptake among females was much lower than that in the male population (Figure 2).
Figure 2: Estimated prevalence (per million) of persons prescribed pre-exposure prophylaxisFootnote a, by sex and overall, in eight provinces in CanadaFootnote b, 2014–2018
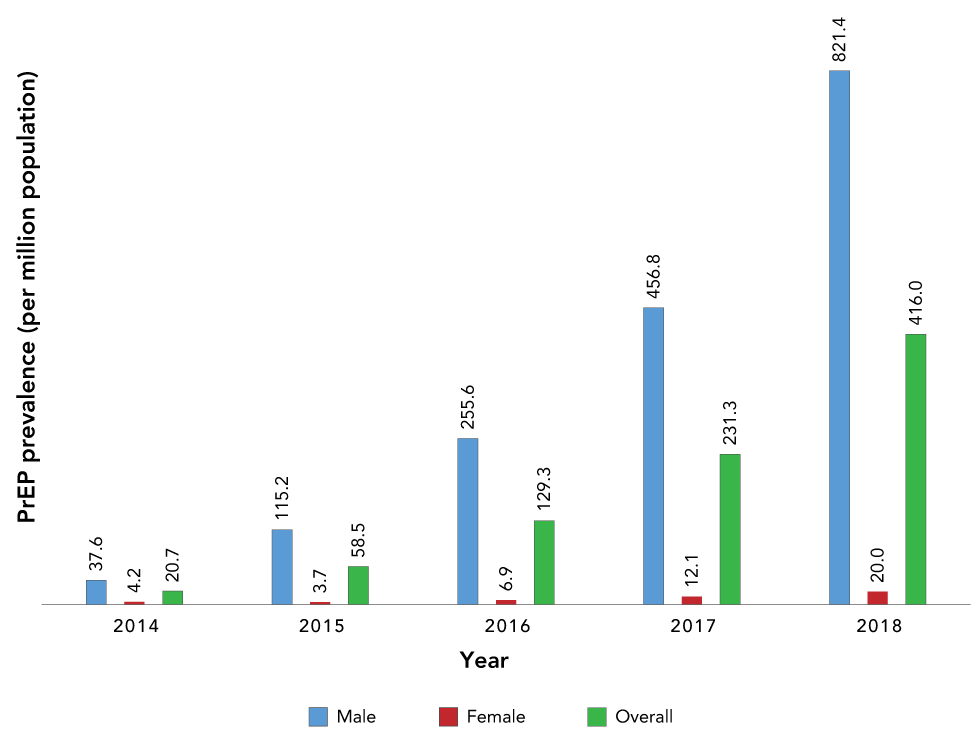
Text description: Figure 2
| Year | PrEP prevalence (per million) | ||
|---|---|---|---|
| Male | Female | Overall | |
| 2014 | 37.6 | 4.2 | 20.7 |
| 2015 | 115.2 | 3.7 | 58.5 |
| 2016 | 255.6 | 6.9 | 129.3 |
| 2017 | 456.8 | 12.1 | 231.3 |
| 2018 | 821.4 | 20.0 | 416.0 |
The estimated number of male PrEP users increased across all age groups between 2014 and 2018, while the relative increase in male PrEP users was greatest in the 18–24 year age category (67-fold) (Table 1). Males aged 36–45 years comprised the greatest proportion of PrEP users from 2014 to 2016; however, in 2017 and 2018, there was a shift to the younger age category with males aged 25–35 years making up the highest proportion of PrEP users (Table 1).
The estimated number of female PrEP users also increased across all age groups between 2015 and 2018, with the exception of the 65+ age group (Table 1). The relative increase in female PrEP users was greatest in the 18–24 years of age category (17-fold increase). The 25–35 years of age category consistently made up the greatest proportion of female PrEP users except for 2015, when females aged 36–45 years accounted for the greatest proportion (Table 1). These percentages were based on relatively small numbers; therefore, these trends should be interpreted with caution.
Pre-exposure prophylaxis was most frequently prescribed by primary care providers (family and general practitioners), and this trend was consistent over the five-year period. In 2018, the majority of the estimated PrEP users were prescribed TDF/FTC by primary care providers (75.5%), followed by infectious disease specialists (11.9%), internal medicine specialists (4.7%) and others (3.8%) (Table 2). From 2014 to 2018, the estimated proportion of users whose PrEP was prescribed by infectious disease and internal medicine physicians decreased by 30% while the estimated proportion prescribed by primary care providers increased by 10 % (Table 2).
| Estimated PrEP users | Number (%) by year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | ||||||
| Prescriber specialty | # | % | # | % | # | % | # | % | # | % |
| Primary care provider | 275 | 68.9 | 896 | 79.6 | 2,037 | 78.9 | 3,616 | 78.8 | 6,107 | 75.5 |
| Infectious diseases | 68 | 17.0 | 125 | 11.1 | 294 | 11.4 | 589 | 12.8 | 965 | 11.9 |
| Internal medicine | 28 | 7.0 | 43 | 3.8 | 73 | 2.8 | 113 | 2.5 | 381 | 4.7 |
| Public health and preventive medicine | 0 | 0.0 | 7 | 0.6 | 40 | 1.5 | 49 | 1.1 | 196 | 2.4 |
| Medical microbiology | 8 | 2.0 | 16 | 1.4 | 59 | 2.3 | 88 | 1.9 | 130 | 1.6 |
| Others | 20 | 5.0 | 39 | 3.5 | 78 | 3.0 | 131 | 2.9 | 308 | 3.8 |
| Payer type | # | % | # | % | # | % | # | % | # | % |
| Out of pocket | 19 | 4.1 | 45 | 3.4 | 89 | 3.0 | 191 | 3.6 | 258 | 2.7 |
| Private insurance | 282 | 61.3 | 899 | 68.8 | 2,068 | 70.6 | 3,874 | 73.2 | 6,612 | 68.4 |
| Public insurance | 159 | 34.6 | 362 | 27.7 | 771 | 26.3 | 1,226 | 23.2 | 2,793 | 28.9 |
| Province | ||||||||||
| Manitoba | 8 | 9 | 16 | 43 | 129 | |||||
| New Brunswick | 0 | 0 | 60 | 100 | 136 | |||||
| Newfoundland and Labrador | 0 | 1 | 4 | 12 | 37 | |||||
| Nova Scotia | 0 | 5 | 98 | 178 | 281 | |||||
| Ontario | 239 | 579 | 1,397 | 2,715 | 5,684 | |||||
| Prince Edward Island | 0 | 0 | 0 | 0 | 12 | |||||
| Québec | 192 | 696 | 1,316 | 2,182 | 3,244 | |||||
| Saskatchewan | 0 | 0 | 11 | 44 | 342 | |||||
On average, more than two-thirds of the estimated PrEP users covered the cost of the prescription through private health insurance, and this trend was consistent over time (Table 2). The estimated number of PrEP prescriptions covered by private and public insurance increased from 2015 to 2018 by 23-fold and 18-fold, respectively (Table 2). Approximately 3%–4% of PrEP prescriptions were paid “out of pocket” by the individual. However, no follow-up was done for these individuals whose expenses could then have been reimbursed by private or public health insurance (Table 2).
Annual PrEP use increased in every province (Table 2); however, there was variation within the increasing trend of people on PrEP between the eight provinces. Annual PrEP prevalence for each province by year, showed that 2018 PrEP prevalence was highest in ON, QC and SK, at 471, 446 and 355 per million persons, respectively (Table 3). Consistently, more than 85% (range 87%–100%) of PrEP users were males across all provinces (data not shown).
Annual estimated PrEP prevalence (by province) |
Number (per million) by year | ||||
|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | |
| Manitoba | 7.7 | 9.5 | 15.0 | 44.4 | 107.5 |
| New Brunswick | 0.0 | 0.0 | 92.0 | 151.0 | 204.7 |
| Newfoundland and Labrador | 0.0 | 2.2 | 8.8 | 26.4 | 81.7 |
| Nova Scotia | 0.0 | 5.0 | 107.6 | 193.7 | 292.5 |
| Ontario | 21.0 | 50.4 | 120.1 | 229.6 | 471.5 |
| Prince Edward Island | 0.0 | 0.0 | 0.0 | 0.0 | 26.5 |
| Québec | 30.4 | 102.2 | 192.0 | 308.6 | 445.9 |
| Saskatchewan | 0.0 | 0.0 | 13.1 | 69.0 | 354.9 |
In five of the provinces (NL, NS, ON, PE and SK), the age group with the highest proportion of PrEP use was 25–35 years, followed by 36–45 years (Figure 3). The age of PrEP users differed for MB, with those aged 46–55 years being the second highest proportion of users. In NB, PrEP users were older, with the highest proportion of PrEP users among those aged 36–45 years, followed by 56–64 years. In QC, the highest proportion was among people aged 36–45 years followed by 25–35 years.
Figure 3: Estimated proportion of individuals prescribed pre-exposure prophylaxisFootnote a by age group, 2014–2018
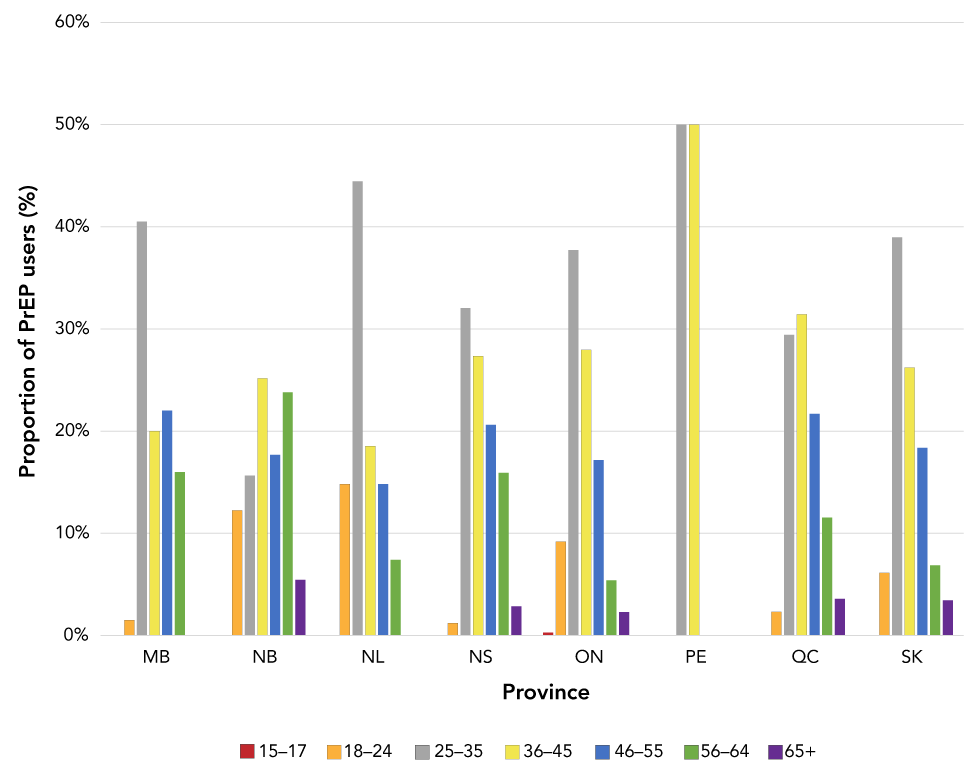
Text description: Figure 3
| Age group | Proportion of individual prescribed PrEP | |||||||
|---|---|---|---|---|---|---|---|---|
| MB | NB | NL | NS | ON | PE | QC | SK | |
| 15–17 | 0.0% | 0.0% | 0.0% | 0.0% | 0.3% | 0.0% | 0.0% | 0.0% |
| 18–24 | 1.5% | 12.2% | 14.8% | 1.2% | 9.2% | 0.0% | 2.3% | 6.1% |
| 25–35 | 40.5% | 15.6% | 44.4% | 32.0% | 37.7% | 50.0% | 29.4% | 39.0% |
| 36–45 | 20.0% | 25.2% | 18.5% | 27.3% | 28.0% | 50.0% | 31.4% | 26.2% |
| 46–55 | 22.0% | 17.7% | 14.8% | 20.6% | 17.2% | 0.0% | 21.7% | 18.4% |
| 56–64 | 16.0% | 23.8% | 7.4% | 15.9% | 5.4% | 0.0% | 11.5% | 6.9% |
| 65+ | 0.0% | 5.4% | 0.0% | 2.9% | 2.3% | 0.0% | 3.6% | 3.4% |
Information on prescription by physician speciality was available for five of the eight provinces (Figure 4). Primary care providers prescribed PrEP most frequently in all provinces, followed by infectious disease specialists in all provinces but NS. In contrast, infectious disease specialists in SK prescribed almost 40% of PrEP prescriptions (Figure 4). For some provinces, there was a large amount of data missing for prescriber specialty; therefore, these data should be interpreted with caution. Over the 5-year period, the most common payer type was private insurance in the majority of provinces, ranging between 58% and 100%, with the exception of SK, where public insurance covered more than 80% of PrEP prescriptions between 2014 and 2018 (data not shown).
Figure 4: Estimated proportion of individuals prescribed pre-exposure prophylaxisFootnote a by physician specialty, 2014–2018
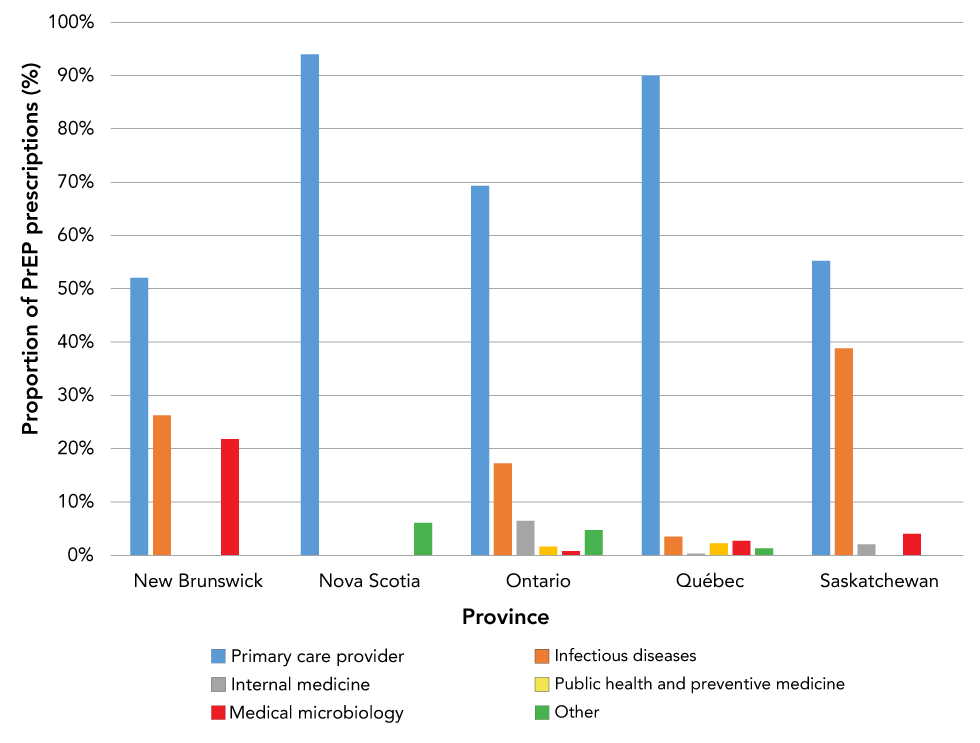
Text description: Figure 4
| Physician specialty | Proportion of individuals prescribed PrEP | ||||
|---|---|---|---|---|---|
| New Brunswick | Nova Scotia | Ontario | Québec | Saskatchewan | |
| Primary care provider | 52.0% | 94.0% | 69.3% | 90.0% | 55.2% |
| Infectious diseases | 26.2% | 0.0% | 17.2% | 3.5% | 38.8% |
| Internal medicine | 0.0% | 0.0% | 6.4% | 0.3% | 2.0% |
| Public health and preventive medicine | 0.0% | 0.0% | 1.6% | 2.2% | 0.0% |
| Medical microbiology | 21.8% | 0.0% | 0.8% | 2.7% | 4.0% |
| Other | 0.0% | 6.0% | 4.7% | 1.3% | 0.0% |
Discussion
In the current analysis, we found an increasing trend in the estimated number of persons prescribed TDF-FTC for PrEP in eight Canadian provinces from 2014 to 2018. During this time period, we found an almost 2,000% increase in PrEP users, with an estimated 9,657 individuals using PrEP in the eight Canadian provinces at the end of 2018. This resulted in an estimated PrEP prevalence of 416 per million persons across the eight provinces in 2018. This increase is likely due to several factors: the approval of TDC/FTC for use as PrEP by Health Canada in February 2016 followed by the availability of lower cost generic versions in July 2017; the publication of a Canadian guideline on HIV pre-exposure prophylaxis and non-occupational post-exposure prophylaxis in 2018Footnote 10; the inclusion of PrEP in an increasing number of provincial drug plans between 2014 and 2018; and increased awareness of PrEP as an effective HIV prevention measure among clinical providers and populations that could benefit from PrEP—notably gay, bisexual and other men who have sex with men (gbMSM).
When all eight provinces (for which data were available) were combined, coverage for PrEP prescriptions was consistent over the five-year period with approximately two-thirds of prescriptions being covered by private insurance. When looking at payer type by province, the payer type was consistently private insurance for all provinces except SK where public insurance covered more than 80% of PrEP prescriptions between 2014 and 2018. A recent summary of PrEP coverage across Canada showed that almost all provinces and territories in Canada had coverage for PrEPFootnote 11; however, there were variations with respect to coverage requirements, ranging from co-payments to requirements for valid provincial health coverage or for approval from senior public health officials. Each of these requirements may have an impact not only on the type of payment used for the PrEP prescription but may also impact the level of uptake of PrEP across Canadian jurisdictions.
In 2014, among 460 estimated PrEP users, 90% were male. We observed an increasing trend in PrEP use among men, with an almost 23-fold increase in the number of male PrEP users from 2014 to 2018. This increase in the estimated number of males taking PrEP during the five-year period is important, given that the largest proportion of estimated new HIV infections and HIV diagnoses in 2018 were among the gbMSM populationFootnote 4Footnote 12. Although the number of females on PrEP was consistently lower than the number of males, female use of PrEP increased five-fold from 2014 to 2018. National surveillance data show that the rate of HIV diagnoses has increased among females in last five years; from 2.5/100,000 population in 2013 to 4.0/100,000 population in 2018, whereas the diagnosis rate for males remained stable at approximately 9/100,000 population during the same time periodFootnote 12. This difference highlights the need to develop or refine strategies for identifying women who have PrEP indications.
By age group, the highest proportion of PrEP use was observed in those 36–45 years of age in 2014 and 2015, and then there was a shift to a younger age group (25–35 years) in 2017–2018. This is important since within national HIV surveillance the 30–39 years age group had the highest number and proportion of reported HIV cases, followed by the 20–29 years age groupFootnote 12. The age group with the lowest proportion of PrEP users was 15–24 years; however, the number of PrEP users among this age group increased by 266% between 2017 and 2018. This increase in PrEP use is encouraging since youth and young adults have been reported to have barriers to PrEP uptakeFootnote 13.
In more recent years, there was a decreasing trend in PrEP prescribed by specialists (infectious diseases and internal medicine), with an increase in PrEP prescribed by primary care providers. These findings are important to consider as increased availability of PrEP for individuals at risk for HIV acquisition continues to be a priority in Canada. Primary care providers can play a key role to increase PrEP uptake as part of a sexual health and disease prevention approach given their large representation in the health care work force Footnote 14Footnote 15.
Pre-exposure prophylaxis use increased across all provinces, although there were jurisdictional differences in the prevalence of PrEP use, age distribution and prescriber types. Several provinces showed PrEP use before 2016, the year when Health Canada approved the drug combination (TDF/FTC) for use as PrEP, and other provinces did not report PrEP use until 2016. Saskatchewan had the highest HIV diagnosis rate in Canada in 2018 at 14.9/100,000 population but had the third highest estimated PrEP prevalence per million—behind only ON and QC. It is important to note that as of April 2018, PrEP became available at no cost to all SK residents; therefore, an increase in PrEP uptake may be observed post-2018 (e.g. 2019–2020 prescription data). The PrEP users tended to be older in some provinces, more frequently prescribed by specialists in some provinces, and commonly covered by private insurance in most provinces: all highlighting the continued need for tailored programs across each jurisdiction in Canada.
Strengths and limitations
This is the first time that estimates of PrEP uptake across Canada have been published, and these data represent a population-based data source for PrEP use; however, there are important limitations to the data. Firstly, the results do not reflect the national picture of PrEP use in Canada, as these data only include eight provinces. British Columbia publishes its own PrEP summary report, which indicated that there were 2,423 PrEP users at the 4th quarter of 2018Footnote 16. The characteristics of PrEP users in British Columbia (BC) were very similar to the eight provinces included in this analysis. For example, 99% of PrEP users in BC were male, the highest proportion of PrEP users was among individuals aged 29–40 years old, and family physicians prescribed 77% of PrEPFootnote 16. The addition of information from BC and Alberta would provide a more representative overview of PrEP uptake in Canada. Second, IQVIA data only included prescriptions that were acquired from a community pharmacy. Dispensations from hospital pharmacies, drugs provided at no cost and drugs purchased online were not included.
Additionally, the dispensation data from IQVIA covered approximately 60% of all retail pharmacies in Canada. Patient counts from participating pharmacies were projected to the whole population of each province by IQVIA, and the algorithm used to project dispensations is proprietary. Sensitivity analysis with other data sources to corroborate the accuracy of the projected patient counts was not completed.
Dispensation data do not include information on medical indication; therefore, an algorithm was used to assign a treatment indication to each dispensation. Although the algorithm for classifying TDF/FTC users as PrEP users has been validated, it is possible that some dispensations were misclassified.
Finally, not all dispensed prescription drugs are consumed, as some people may fill a prescription but not consume the medication. These limitations could result in an under or over-estimate of the number of projected patient counts.
Future directions
The preliminary analysis of this administrative data from 2014 to 2018 showed that there has been substantial growth in the uptake of PrEP across eight Canadian provinces. Nonetheless, the PrEP uptake and its potential prevention of HIV transmission is not distributed equitably, as demographic profiles of new HIV diagnoses by sex and age group do not always align with rates of PrEP use, and the growth of PrEP use has not occurred equally for females. A similar study conducted by the United States Centres for Disease Control also found that annual PrEP use increased faster among males than among females, increased fastest among those aged 25–34 years, and that geographic variations in PrEP uptake existed across the countryFootnote 7Footnote 9.
The analysis of prescription data is helpful to understand where PrEP uptake is greatest, or where there are areas for improvement; however, these data alone cannot distinguish the underlying reasons why PrEP use is lower in specific populations. Results from a four-year longitudinal study of gbMSM in Vancouver, BC, demonstrated that awareness of PrEP increased over time; up to 80% in 2016Footnote 17. Canadian results from the European Men-who have-sex-with-men Internet survey (EMIS) indicated that over 85% of participants had heard of PrEP. Among HIV-negative or untested men, 52% reported that they were likely to use PrEP if it was affordable and available, while only 8.4% of participants were using PrEP at the time of the surveyFootnote 18. Data from the recent Tracks survey of people who inject drugs in Canada (2017–2019) highlighted that only 14% of the participants had heard about PrEPFootnote 19. Recent research showed that HIV treatment adherence information can be used to inform PrEP interventions, and that new strategies are needed to engage vulnerable and marginalized populations in PrEP-related programmingFootnote 20. This research highlights the continued need for complimentary research at the national level, assessing PrEP awareness and willingness to use and factors related to access—focussing on differences between specific populations and across geographic regions. These data, together with data available through population-specific surveys, show an increase in PrEP uptake and awarenessFootnote 17Footnote 19; however, there are gaps in PrEP uptake data for a range of key populations most affected by HIV, including Indigenous people, racialized people (including African, Black and Caribbean communities), transgender and non-binary people, sex workers and people in correctional facilities.
Conclusion
This analysis shows that PrEP use in Canada has increased since 2014, demonstrating increased awareness and uptake of its use for preventing HIV transmission; however, there was uneven uptake of PrEP by different age groups and sex, and across the Canadian provinces. Other Canadian evidence suggests a large unmet need in some population groups (e.g. gbMSM, people who inject drugs), and there is still a need for similar data for other populations.
Presently, the IQVIA prescription database provides the most feasible means to monitor PrEP uptake in Canada; however, sensitivity analysis using provincial prescription databases would help to validate the proprietary IQVIA algorithm for projected patient counts.
Since new HIV infections continue to occur in Canada, the use of PrEP in adult men and women at high risk should continue to be considered in combination with safer sex practices to reduce the risk of sexually acquired HIV infection. In Canada, it will be important to further refine the use of PrEP, as there is progress to be made to ensure that populations at higher risk of HIV infection are offered PrEP as part of comprehensive sexual health care.
Authors’ statement
- NP — Conceptualization, interpretation of data, writing original draft, review, editing, validation, writing final draft, visualization
- QY — Data curation, interpretation of data, contributed to first draft
- CA — Conceptualization, review–revision of the paper, final approval
Competing interests
None.
Acknowledgements
We would like to acknowledge G Tremblay and MA LeBlanc for their contribution. Any analysis of IQVIA data was arrived at independently and IQVIA is not responsible for any reliance by recipients of the data or any analysis thereof. The analyses, conclusions, opinions and statements expressed herein are those of the authors and not necessarily those of IQVIA.
Funding
This work was supported by the Public Health Agency of Canada as part of its core mandate.
References
- Footnote 1
-
Joint United Nations Programme on HIV and AIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva (Switzerland): UNAIDS; 2014. https://www.unaids.org/en/resources/documents/2017/90-90-90#:~:text=Documents-,90%E2%80%9390%E2%80%9390%20%2D%20An%20ambitious%20treatment%20target%20to,help%20end%20the%20AIDS%20epidemic&text=By%202020%2C%2090%25%20of%20all,will%20receive%20sustained%20antiretroviral%20therapy
- Footnote 2
-
Centre for Communicable Diseases and Infection Control1. A summary of the Pan-Canadian framework on sexually-transmitted and blood-borne infections. Can Commun Dis Rep 2018 44(7-8):179-81. https://doi.org/10.14745/ccdr.v44i78a05
- Footnote 3
-
Public Health Agency of Canada. Accelerating our response: Government of Canada five-year action plan on sexually transmitted and blood-borne infections. Ottawa (ON): PHAC; 2019. https://www.canada.ca/en/public-health/services/reports-publications/accelerating-our-response-five-year-action-plan-sexually-transmitted-blood-borne-infections.html
- Footnote 4
-
Public Health Agency of Canada. Estimates of HIV incidence, prevalence and Canada's progress on meeting the 90-90-90 HIV targets, 2016. Ottawa (ON): PHAC; (updated 2019). https://www.canada.ca/en/public-health/services/publications/diseases-conditions/summary-estimates-hiv-incidence-prevalence-canadas-progress-90-90-90.html#t1
- Footnote 5
-
Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD; INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015;373(9):795-807. https://doi.org/10.1056/NEJMoa1506816
- Footnote 6
-
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493-505. https://doi.org/10.1056/NEJMoa1105243
- Footnote 7
-
Wu H, Mendoza MC, Huang YA, Hayes T, Smith DK, Hoover KW. Uptake of HIV Preexposure Prophylaxis Among Commercially Insured Persons-United States, 2010-2014. Clin Infect Dis 2017;64(2):144-9. https://doi.org/10.1093/cid/ciw701
- Footnote 8
-
Mera R, McAllister S, Palmer B, Mayer G, Magnuson D, Rawlings K. Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States (2013-2015) (Abstract TUAX0105LB). 2 Proceedings of the AIDS 2016 Conference; 2016; Durban, South Africa. http://programme.aids2016.org/Abstract/Abstract/10159
- Footnote 9
-
Sullivan PS, Giler RM, Mouhanna F, Pembleton E, Guest J, Jones J, Castel A, Yeung H, Kramer M, McCallister S, Siegler A. Trends in active prescriptions of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infections, United States, 2012-2017. Ann Epidemiol 2018;28(12):833-40. https://doi.org/10.1016/j.annepidem.2018.06.009
- Footnote 10
-
Tan DH, Hull MW, Yoong D, Tremblay C, O'Byrne P, Thomas R, Kille J, Baril JG, Cox J, Giguere P, Harris M, Hughes C, MacPherson P, O'Donnell S, Reimer J, Singh A, Barrett L, Bogoch I, Jollimore J, Lambert G, Lebouche B, Metz G, Rogers T, Shafran S; Biomedical HIV Prevention Working Group of the CIHR Canadian HIV Trials Network. Canadian guideline on HIV pre-exposure prophylaxis and nonoccupational postexposure prophylaxis. CMAJ 2017;189(47):E1448-58. https://doi.org/10.1503/cmaj.170494
- Footnote 11
-
Yoong D. Provincial/Territorial Coverage of ARV drugs for HIV prevention across Canada: Post-exposure prophylaxis (PEP) and Pre-exposure prophylaxis (PrEP). Toronto (ON): St. Michael's Hospital; 2019. https://hivclinic.ca/wp-content/uploads/2019/07/ARV-Coverage_July-2019.pdf
- Footnote 12
-
Haddad N, Robert A, Weeks A, Popovic N, Siu W, Archibald C. HIV in Canada—surveillance Report, 2018. Can Commun Dis Rep 2019;45(12):304-12. https://doi.org/10.14745/ccdr.v45i12a01
- Footnote 13
-
Hosek S, Celum C, Wilson CM, Kapogiannis B, Delany‐Moretlwe S, Bekker L‐G. Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa. JIAS.19: 21107. https://doi.org/10.7448/IAS.19.7.21107
- Footnote 14
-
Silapaswan A, Krakower D, Mayer KH. Pre-exposure prophylaxis: a narrative review of provider behavior and interventions to increase PrEP implementation in primary care. J Gen Intern Med 2017 Feb;32(2):192-8. https://doi.org/10.1007/s11606-016-3899-4
- Footnote 15
-
Hoffman S, Guidry JA, Collier KL, Mantell JE, Boccher-Lattimore D, Kaighobadi F, Sandfort TGM. A Clinical Home for Pre-exposure Prophylaxis: Diverse Health Care Providers' Perspectives on the "Purview Paradox". J Int Assoc Provid AIDS Care. 2016;15(1):59-65. https://doi.org/10.1177/2325957415600798
- Footnote 16
-
British Columbia Ministry of Health. British Columbia Centre for Excellence in HIV/AIDS. HIV Pre-exposure Prophylaxis (PREP) Quarterly Report for British Columbia, Second Quarter 2019. http://bccfe.ca/sites/default/files/uploads/publications/centredocs/prep_indicators_report_220620201.pdf
- Footnote 17
-
Mosley T, Khaketla M, Armstrong HL, Cui Z, Sereda P, Lachowsky NJ, Hull MW, Olarewaju G, Jollimore J, Edward J, Montaner JS, Hogg RS, Roth EA, Moore DM. Trends in Awareness and Use of HIV PrEP Among Gay, Bisexual, and Other Men who have Sex with Men in Vancouver, Canada 2012-2016. AIDS Behav 2018;22(11):3550-65. https://doi.org/10.1007/s10461-018-2026-4
- Footnote 18
-
Brogan N, Paquette DM, Lachowsky NJ, Blais M, Brennan DJ, Hart TA, Adam B. Canadian results from the European Men-who-have-sex-with-men Internet survey (EMIS-2017). Can Commun Dis Rep 2019;45(11):271-82. https://doi.org/10.14745/ccdr.v45i11a01
- Footnote 19
-
Tarasuk J, Zhang J, Lemyre A, Cholette F, Bryson M, Paquette D. National findings from the Tracks survey of people who inject drugs in Canada, Phase 4, 2017-2019. Can Commun Dis Rep 2020;46(5):138-48. https://doi.org/10.14745/ccdr.v46i05a07
- Footnote 0
-
Bazzi AR, Drainoni ML, Biancarelli DL, Hartman JJ, Mimiaga MJ, Mayer KH, Biello KB. Systematic review of HIV treatment adherence research among people who inject drugs in the United States and Canada: evidence to inform pre-exposure prophylaxis (PrEP) adherence interventions. BMC Public Health 2019;19(1):31. https://doi.org/10.1186/s12889-018-6314-8
