Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-6, June 2023: Acute Hepatitis in Children in Canada
Date published: June 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-6, June 2023: Acute Hepatitis in Children in Canada
Surveillance
Surveillance for Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens in Canada, 2020
Christy Wilson1, Salima Gasmi2, Annie-Claude Bourgeois1, Jacqueline Badcock3, Justin Carr4, Navdeep Chahil5, Heather Coatsworth6, Antonia Dibernardo6, Priya Goundar7, Patrick Leighton8, Min-Kuang Lee5, Muhammad Morshed5,9, Marion Ripoche10, Jade Savage11 on behalf of eTick, Hanan Smadi3, Christa Smolarchuk12, Karine Thivierge13,14, Jules Koffi2
Affiliations
1 Centre for Food-borne, Environmental and Zoonotic Infectious Diseases, Public Health Agency of Canada, Ottawa, ON
2 Centre for Food-borne, Environmental and Zoonotic Infectious Diseases, Public Health Agency of Canada, Saint-Hyacinthe, QC
3 Public Health New Brunswick, New Brunswick Department of Health, Fredericton, NB
4 New Brunswick Provincial Veterinary Laboratory, Department of Agriculture, Aquaculture and Fisheries, Fredericton, NB
5 BCCDC Public Health Laboratory, BC Centre for Disease Control, Vancouver, BC
6 National Microbiology Laboratory Branch, Public Health Agency of Canada, Winnipeg, MB
7 Ministry of Health, Regina, SK
8 Epidemiology of Zoonoses and Public Health Research Group (GREZOSP), Faculty of Veterinary Medicine, Université de Montréal, Saint-Hyacinthe, QC
9 Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC
10 Institut national de santé publique du Québec, Montréal, QC
11 Bishop’s University, Sherbrooke, QC
12 Analytics and Performance Reporting Branch, Health Standards, Quality and Performance Division, Alberta Health, Edmonton, AB
13 Laboratoire de santé publique du Québec, Sainte-Anne-de-Bellevue, QC
14 Institute of Parasitology, McGill University, Sainte-Anne-de-Bellevue, QC
Correspondence
Suggested citation
Wilson CH, Gasmi S, Bourgeois A-C, Badcock J, Carr J, Chahil N, Coatsworth H, Dibernardo A, Goundar P, Leighton PA, Lee M-K, Morshed MG, Ripoche M, Savage J on behalf of eTick, Smadi HN, Smolarchuk C, Thivierge K, Koffi JK. Surveillance for Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens in Canada, 2020. Can Commun Dis Rep 2023;49(6):288–98. https://doi.org/10.14745/ccdr.v49i06a06
Keywords: Ixodes scapularis, Ixodes pacificus, surveillance, Borrelia, Anaplasma, Babesia, Powassan virus
Abstract
Background: Ixodes scapularis and Ixodes pacificus ticks are the principal vectors of the agent of Lyme disease and several other tick-borne diseases in Canada. Tick surveillance data can be used to identify local tick-borne disease risk areas and direct public health interventions. The objective of this article is to describe the seasonal and spatial characteristics of the main Lyme disease vectors in Canada, and the tick-borne pathogens they carry, using passive and active surveillance data from 2020.
Methods: Passive and active surveillance data were compiled from the National Microbiology Laboratory Branch (Public Health Agency of Canada), provincial and local public health authorities, and eTick (an online, image-based platform). Seasonal and spatial analyses of ticks and their associated pathogens are presented, including infection prevalence estimates.
Results: In passive surveillance, I. scapularis (n=7,534) were submitted from all provinces except Manitoba and British Columbia, while I. pacificus (n=718) were submitted only from British Columbia. No ticks were submitted from the Territories. The seasonal distribution of I. scapularis submissions was bimodal, but unimodal for I. pacificus. Four tick-borne pathogens were identified in I. scapularis (Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti and Borrelia miyamotoi) and one in I. pacificus (B. miyamotoi). In active surveillance, I. scapularis (n=688) were collected in Ontario, Québec and New Brunswick. Five tick-borne pathogens were identified: B. burgdorferi, A. phagocytophilum, B. microti, B. miyamotoi and Powassan virus.
Conclusion: This article provides a snapshot of the distribution of I. scapularis and I. pacificus and their associated human pathogens in Canada in 2020, which can help assess the risk of exposure to tick-borne pathogens in different provinces.
Introduction
Ixodes scapularis and Ixodes pacificus ticks can transmit several bacterial, viral and protozoan pathogens to humansFootnote 1. The geographic range and population of I. scapularis is increasing in southern central and eastern CanadaFootnote 2Footnote 3, due to climate and environmental changes that have enhanced habitat suitability for ticks in more areasFootnote 4Footnote 5. These changes can further alter tick behaviour and extend their periods of activity, which can increase exposure to tick-borne diseases (TBD)Footnote 1Footnote 6. To reduce the burden from TBD, the continued range expansion of ticks in Canada must be met with increased capacity for and awareness of TBD prevention and surveillance Footnote 1. Tick surveillance data inform the environmental risk of Lyme disease (LD), which can guide public health authorities in targeting prevention and control efforts and support LD diagnostics by healthcare professionalsFootnote 7.
The causative agent of LD, Borrelia burgdorferi, is transmitted by I. scapularis in central and eastern Canada and by I. pacificus in British Columbia. Reported incidence of LD in people has increased more than 10-fold (from 144 to 1,615 cases) from 2009 to 2020Footnote 8. Additional TBD, transmitted by I. scapularis or I. pacificus, are emerging in Canada; including anaplasmosisFootnote 9, babesiosisFootnote 10, hard tick-borne relapsing feverFootnote 11 and Powassan virus diseaseFootnote 12.
Passive tick surveillance has been used since the 1990s to identify I. scapularis and I. pacificus tick populations and the presence of tick-borne pathogensFootnote 13Footnote 14. Active tick surveillance began in the 2000s to detect areas with established tick populations where LD risk may become endemic (LD risk areas)Footnote 15. Efforts to summarize passive and active tick surveillance annually at the national level began in 2019 Footnote 16, providing a baseline for TBD risk that over time will facilitate the identification of current trends and enable the projection of future trends.
The objective of this surveillance report is to summarize the geographic and seasonal characteristics of the main LD vectors in Canada, I. scapularis and I. pacificus, collected through passive and active surveillance in 2020. This article will also summarize the prevalence and spatial distribution of their associated human pathogens.
Methods
Data sources
This report uses two types of surveillance data from ten different providers. Passive tick surveillance data was provided by the National Microbiology Laboratory (NML) Branch of the Public Health Agency of Canada (PHAC), British Columbia Centre for Disease Control (BCCDC), Alberta Health, Saskatchewan Ministry of Health, and eTick. Active tick surveillance data were provided by Thunder Bay District Health Unit, Kingston, Frontenac and Lennox & Addington Public Health, Laboratoire de santé publique du Québec, New Brunswick Department of Health and New Brunswick Provincial Veterinary Laboratory.
Passive tick surveillance
Passive tick surveillance is the voluntary submission by the public of ticks (or their images) to medical or veterinary clinics, regional public health authorities or other institutions (e.g. university laboratory) for species identification and laboratory testingFootnote 13. This analysis was limited to I. scapularis and I. pacificus ticks collected within Canada in 2020, although several other tick species were also identified. Ticks could be submitted at any point during the year. Ticks with a location of acquisition outside of Canada, with a submitter's history of travel to another province, or from within Canada but could not be geocoded were excluded. Ticks were submitted individually (single submission) or in groups of two or more (multiple submission). Provinces with five or fewer ticks submitted for species identification and laboratory testing were excluded from the study to avoid misinterpretation of results. No ticks were submitted from Northwest Territories, Nunavut or Yukon as no passive surveillance programs exist for I. scapularis and I. pacificus.
Since 2009, regional passive tick surveillance programs have been gradually discontinued in several jurisdictions (e.g. Nova Scotia, southwestern Québec and eastern Ontario) dependent on laboratory capacity and as I. scapularis populations have become established. However, ticks (or their images) acquired in these jurisdictions could be submitted by the public directly to NML or to eTick.
eTick is a validated, web-based, community-science passive surveillance system for tick identificationFootnote 17. Individuals submit images of ticks they encounter to the online platform, which are then examined by trained personnel for species identification. The system began in 2017 in Québec, with five additional provinces added by 2020 (Saskatchewan, Ontario, Newfoundland and Labrador, New Brunswick and Nova Scotia). Similar to provincial tick surveillance data sources, eTick collects information on location of acquisition, date of collection, submitter travel history, tick host, tick species and tick instar. All ticks from eTick were classified as single submissions, as users must upload images of each tick individually.
Ticks acquired and submitted in Saskatchewan, Ontario, Québec, Newfoundland and Labrador, New Brunswick, Nova Scotia and Prince Edward Island were tested for A. phagocytophilum, B. burgdorferi, B. miyamotoi and B. microti at NML or University of Saskatchewan using the methods previously describedFootnote 16Footnote 18. Ticks from BCCDC were tested only for B. burgdorferi and B. miyamotoi Footnote 14. Laboratory results for ticks from Alberta Health were not available. Specimens from tick records submitted through eTick were not routinely requested for testing of tick-borne pathogens but could be forwarded onto a laboratory for this purpose at the request of local public health authorities.
Active tick surveillance
In active surveillance, ticks are collected from the environment using drag sampling or by capturing host mammals that are then examined for ticks. This analysis used I. scapularis ticks collected during drag sampling from 7 sites in Ontario, 24 sites in Québec and 14 sites in New Brunswick. Drag sampling takes place in late spring/summer (May through July) and fall (September through November), with some sites visited during both periods.
All ticks were tested at NML for A. phagocytophilum, B. microti, B. burgdorferi, B. miyamotoi and Powassan virus. Ticks were collected and tested using the methods previously describedFootnote 16Footnote 18Footnote 19.
Analysis
Tick characteristics
For passive surveillance, descriptive statistics were calculated for submission type (sample-based or image-based), tick species, province of acquisition, instar (larva, nymph, adult female or adult male), level of engorgement (unfed or engorged), host (human, dog, cat or other) and month of collection. Where date of collection was not available, the date the sample was received was used to ascertain the month of collection. For active surveillance, descriptive statistics were calculated for province of collection and instar (larva, nymph, adult female or adult male). All data were cleaned and analysed in R (version 4.0.2).
Ticks that were acquired in Canada in passive surveillance were mapped using QGIS (version 3.8.1) based on their location of acquisition, except for ticks from Alberta that were mapped to the centroid of the forward sortation area (the first three characters of the postal code) of acquisition. Ticks from submitters with a history of travel in the previous 14 days within the same province as the locality of acquisition were geocoded to the location of exposure during travel. Ticks from submitters with multiple travel locations listed were not mapped. In active surveillance, the location of tick dragging was geocoded and mapped.
Infection prevalence
To account for pooled testing of ticks from some jurisdictions for passive surveillance, maximum likelihood estimates (MLE) of prevalence were calculated in Excel (version 16.0) with 95% confidence intervals (CI) using the PooledInfRate add-in (version 4.0)Footnote 20Footnote 21. This estimates the probability of infection for an individual tick in the population using the results of testing of the pooled samples (i.e. a group of one or more ticks submitted and tested together). Co-infection prevalence was calculated among single submissions only to ascertain true co-infections; that is, two or more pathogens in a single tick. Where ticks were not tested in pools, prevalence was the number of positive ticks divided by the number of ticks tested.
Results
Passive surveillance tick characteristics
In 2020, a total of 8,252 ticks were submitted from nine provinces (Table 1, Figure 1). Ticks from Manitoba were excluded as five or fewer ticks were submitted. No ticks were submitted from Northwest Territories, Nunavut or Yukon. The majority (71.49%) of ticks were sample-based submissions (n=5,899) and the remainder were image-based submissions (n=2,353). Ticks from Ontario and Québec comprised 77.24% of all ticks submitted. The majority (96.80%) of ticks were from single submissions, but there were 109 multiple submissions (range: 2–6 ticks per submission; median: 2).
| Province | Tick species (number of ticks) |
Type of surveillance (number of ticks)Footnote a |
Type of submission (number of submissions)Footnote b |
||||
|---|---|---|---|---|---|---|---|
| Ixodes pacificus | Ixodes scapularis | Total | Sample-based | Image-based | Single submissions | Multiple submissions | |
| British Columbia | 718 | 0 | 718 | 718 | N/A | 670 | 22 |
| Alberta | 0 | 81 | 81 | 81 | N/A | 81 | 0 |
| Saskatchewan | 0 | 12 | 12 | 7 | 5 | 12 | 0 |
| Ontario | 0 | 5,139 | 5,139 | 3,713 | 1,426 | 4,964 | 68 |
| Québec | 0 | 1,235 | 1,235 | 809 | 426 | 1,208 | 12 |
| Newfoundland and Labrador | 0 | 14 | 14 | 4 | 10 | 14 | 0 |
| New Brunswick | 0 | 646 | 646 | 516 | 130 | 634 | 6 |
| Nova Scotia | 0 | 392 | 392 | 36 | 356 | 392 | 0 |
| Prince Edward Island | 0 | 15 | 15 | 15 | N/A | 13 | 1 |
| Total | 718 | 7,534 | 8,252 | 5,899 | 2,353 | 7,988 | 109 |
Figure 1: Ixodes pacificus and Ixodes scapularis ticks submitted through passive tick surveillance, Canada, 2020Footnote a
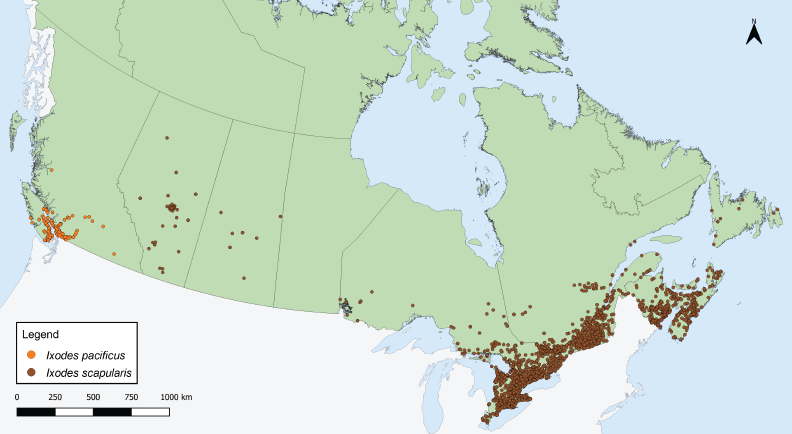
Figure 1 - Text description
This map shows the probable location of acquisition of Ixodes scapularis and Ixodes pacificus ticks submitted through passive surveillance. Ixodes pacificus ticks were present in British Columbia. Ixodes scapularis ticks were present in Alberta, Saskatchewan, Ontario, Québec, Newfoundland and Labrador, New Brunswick, Nova Scotia, and Prince Edward Island to varying extents.
Tick instar, level of engorgement and host were available for 100% of I. pacificus. Tick instar, level of engorgement and host were available for 89.66%, 67.60% and 99.92% of I. scapularis, respectively. The majority of ticks submitted were adult female ticks (I. pacificus: 97.21%; I. scapularis: 92.36%) (Table 2). Adult males, nymphs and larvae were submitted less frequently. Overall, 8.91% of I. pacificus and 41.76% of I. scapularis were engorged. Humans were the most common host among I. pacificus and I. scapularis (90.39% and 82.98%, respectively) followed by dogs (8.91% and 13.34%, respectively).
| Characteristics | Tick species | |||
|---|---|---|---|---|
| Ixodes pacificus | Ixodes scapularis | |||
| n | % | n | % | |
| Instar | ||||
| Larva | 0 | 0 | 9 | 0.13 |
| Nymph | 1 | 0.14 | 284 | 4.20 |
| Adult female | 698 | 97.21 | 6,239 | 92.36 |
| Adult male | 19 | 2.65 | 223 | 3.30 |
| Total | 718 | 100 | 6,755 | 100 |
| Level of engorgement | ||||
| Engorged | 64 | 8.91 | 2,127 | 41.76 |
| Unfed | 654 | 91.09 | 2,966 | 58.24 |
| Total | 718 | 100 | 5,093 | 100 |
| Host | ||||
| Human | 649 | 90.39 | 6,247 | 82.98 |
| Dog | 64 | 8.91 | 1,004 | 13.34 |
| Cat | 3 | 0.42 | 132 | 1.75 |
| OtherFootnote b | 2 | 0.28 | 145 | 1.93 |
| Total | 718 | 100 | 7,528 | 100 |
Month of acquisition and tick instar was available for 100% of I. pacificus and 89.66% of I. scapularis (Figure 2). Adult I. scapularis ticks submitted peaked in May and October through November, while adult I. pacificus submitted peaked only in May. Only 0.14% of I. pacificus submitted were nymphs, while 4.20% of I. scapularis submitted were nymphs, peaking in June. Larvae of I. scapularis (0.13%) were submitted June through September; no I. pacificus larvae were submitted.
Figure 2: Number of Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance, by month and tick instar, Canada, 2020Footnote a
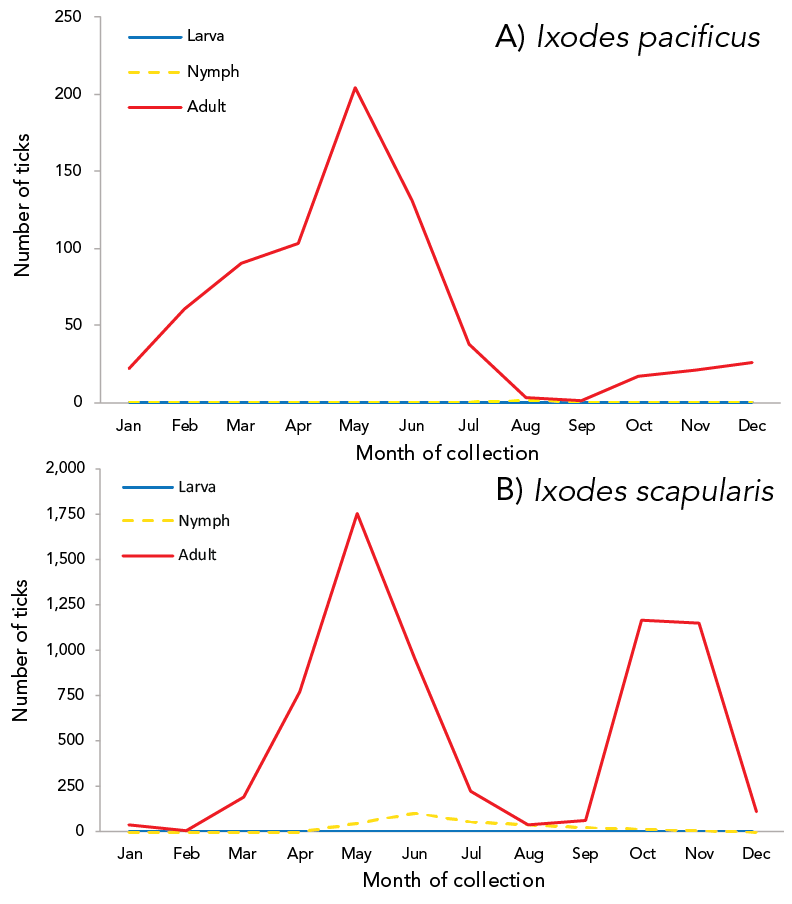
Figure 2 - Text description
| Month | Stage | ||
|---|---|---|---|
| Adult | Nymph | Larva | |
| Jan | 22 | 0 | 0 |
| Feb | 61 | 0 | 0 |
| Mar | 90 | 0 | 0 |
| Apr | 103 | 0 | 0 |
| May | 204 | 0 | 0 |
| Jun | 131 | 0 | 0 |
| Jul | 38 | 0 | 0 |
| Aug | 3 | 1 | 0 |
| Sep | 1 | 0 | 0 |
| Oct | 17 | 0 | 0 |
| Nov | 21 | 0 | 0 |
| Dec | 26 | 0 | 0 |
Month |
Stage | ||
|---|---|---|---|
| Adult | Larva | Nymph | |
| Jan | 35 | 0 | 1 |
| Feb | 2 | 0 | 0 |
| Mar | 195 | 0 | 1 |
| Apr | 775 | 0 | 0 |
| May | 1,753 | 0 | 43 |
| Jun | 957 | 1 | 104 |
| Jul | 221 | 0 | 55 |
| Aug | 38 | 4 | 42 |
| Sep | 63 | 4 | 19 |
| Oct | 1,166 | 0 | 16 |
| Nov | 1,147 | 0 | 3 |
| Dec | 110 | 0 | 0 |
Passive surveillance infection prevalence
Data on laboratory testing were available for 98.27% of I. pacificus and 98.20%–98.40% of I. scapularis from sample-based submissions, depending on pathogen. The most prevalent pathogen was B. burgdorferi, detected in 17.19% of I. scapularis (95% CI: 16.17–18.26) (Table 3). Other tick-borne pathogens (A. phagocytophilum, B. microti and B. miyamotoi) and co-infections were estimated to have a prevalence rate of less than 1%. Among I. pacificus, only B. miyamotoi was identified (0.14%, 95% CI: 0.01–0.68).
Pathogen |
Infection prevalence |
|||
|---|---|---|---|---|
Ixodes pacificus |
Ixodes scapularis |
|||
Single agent |
Maximum likelihood estimate |
|||
% | 95% CI |
% |
95% CI |
|
Anaplasma phagocytophilum |
N/A |
N/A |
0.87 |
0.64–1.15 |
Babesia microti |
N/A |
N/A |
0.02 |
0–0.09 |
Borrelia burgdorferi |
0 |
0–0.54 |
17.19 |
16.17–18.26 |
Borrelia miyamotoi |
0.14 |
0.01–0.68 |
0.49 |
0.33–0.71 |
Total single agent |
0.14 |
0.01–0.68 |
18.21 |
17.16–19.29 |
Co-infection |
Co-infection rate |
|||
% |
Number co-infected ticks/number ticks tested |
% |
Number co-infected ticks/number ticks tested |
|
Anaplasma phagocytophilum + |
N/A |
N/A |
0 |
0/4,874 |
Anaplasma phagocytophilum + |
N/A |
N/A |
0.12 |
6/4,874 |
Anaplasma phagocytophilum + |
N/A |
N/A |
0.02 |
1/4,874 |
Babesia microti + |
N/A |
N/A |
0 |
0/4,882 |
Babesia microti + |
N/A |
N/A |
0 |
0/4,883 |
Borrelia burgdorferi + |
0 |
0/705 |
0.14 |
7/4,882 |
Total co-infected |
0 |
0/705 |
0.29 |
14/4,883 |
Prevalence of B. burgdorferi was higher in multiple submissions of I. scapularis (32.31%, 95% CI: 25.27–40.34) than from single submissions (16.71%, 95% CI: 15.69–17.78). Infection prevalence did not differ significantly by submission type for any other pathogen. Ixodes scapularis submitted from human hosts did not have significantly different infection prevalence compared to I. scapularis submitted from non-human hosts.
Tick-borne pathogens were largely found in southern and eastern Ontario, southern Québec and southern New Brunswick (Figure 3, Figure 4, and Table 4). Borrelia burgdorferi-infected I. scapularis were found in six provinces: Saskatchewan, Ontario, Québec, Newfoundland and Labrador, New Brunswick and Nova Scotia. Three quarters of B. burgdorferi-infected I. scapularis submissions were within previously identified LD risk areas (74.88%; 644/860). Lyme disease risk areas are localities in which there is evidence of reproducing populations of known tick vector species (particularly I. scapularis and I. pacificus) and the likely transmission of B. burgdorferiFootnote 22. Most multiple submissions came from LD risk areas (76.15%; 83/109), of which 51.81% were infected with B. burgdorferi (43/83).
Figure 3: Ixodes scapularis ticks submitted through passive surveillance infected with Borrelia burgdorferi, Canada, 2020Footnote aFootnote b
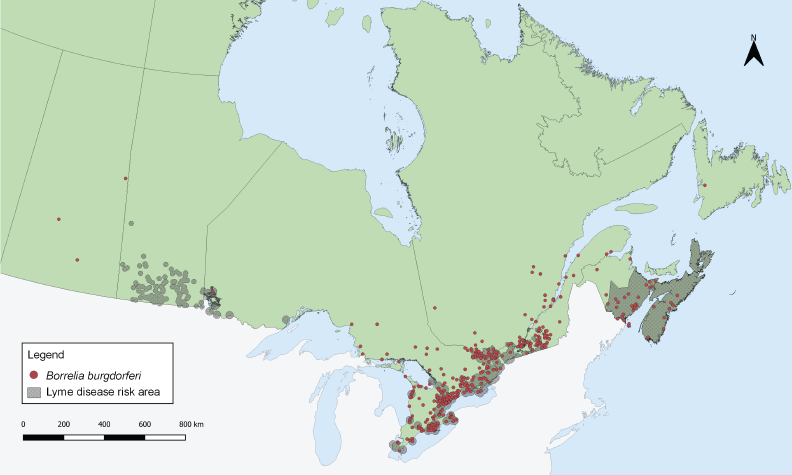
Figure 3 - Text description
This map shows the probable location of acquisition of Ixodes scapularis ticks submitted through passive surveillance that were infected with Borrelia burgdorferi. The map also shows Lyme disease risk areas. Infected ticks are found in Saskatchewan, Ontario, Québec, Newfoundland and Labrador, New Brunswick, and Nova Scotia to varying extents. Most infected ticks are found in southern Ontario and Québec.
Figure 4: Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance infected with Anaplasma phagocytophilum, Babesia microti, Borrelia miyamotoi and co-infections, Canada, 2020Footnote a
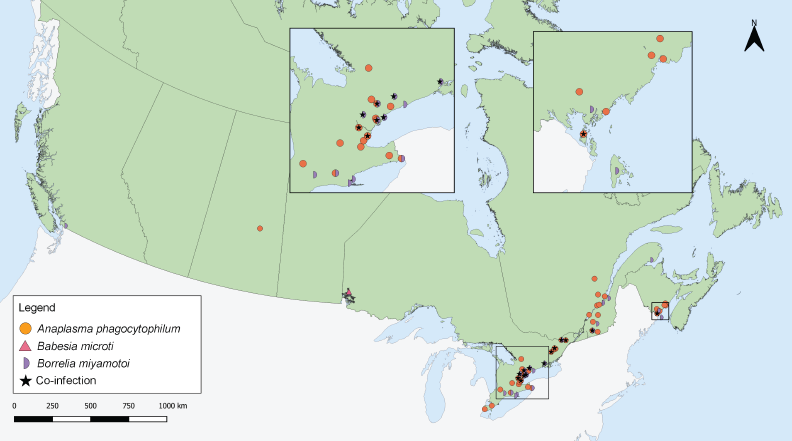
Figure 4 - Text description
This map shows the probable location of acquisition of Ixodes scapularis ticks submitted through passive surveillance that were infected with Anaplasma phagocytophilum, Babesia microti, Borrelia miyamotoi or a co-infection with two of the following: A. phagocytophilum, Borrelia burgdorferi, B. miyamotoi and B. microti. Ticks infected with A. phagocytophilum were found in Saskatchewan, Ontario, Québec, and New Brunswick. Ticks infected with B. microti were found in Ontario. Ticks infected with B. miyamotoi were found in British Columbia, Ontario, Québec, and New Brunswick. Ticks with co-infections were found in Ontario, Québec, and New Brunswick.
Province |
Infection prevalence Maximum likelihood estimate | |||||||
|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Babesia microti | Borrelia burgdorferi | Borrelia miyamotoi | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Ixodes pacificus | ||||||||
| British Columbia | N/A | N/A | N/A | N/A | 0 | 0–0.54 | 0.14 | 0.01–0.68 |
| Ixodes scapularis | ||||||||
| Alberta | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Saskatchewan | 14.29 | 0.85–51.51 | 0 | 0–35.43 | 42.86 | 12.96–77.51 | 0 | 0–35.43 |
| Ontario | 0.73 | 0.49–1.04 | 0.03 | 0–0.13 | 17.78 | 16.56–19.04 | 0.46 | 0.28–0.72 |
| Québec | 1.24 | 0.63–2.19 | 0 | 0–0.47 | 19.50 | 16.87–22.35 | 0.62 | 0.23–1.36 |
| Newfoundland and Labrador | 0 | 0–48.99 | 0 | 0–48.99 | 25.00 | 1.52–73.74 | 0 | 0–48.99 |
| New Brunswick | 1.17 | 0.48–2.40 | 0 | 0–0.74 | 8.97 | 6.72–11.68 | 0.58 | 0.15–1.57 |
| Nova Scotia | 0 | 0–9.64 | 0 | 0–9.64 | 25.00 | 13.03–40.81 | 0 | 0–9.64 |
| Prince Edward Island | 0 | 0–20.15 | 0 | 0–20.15 | 0 | 0–20.15 | 0 | 0–20.15 |
| Total | 0.87 | 0.45–1.15 | 0.02 | 0–0.09 | 17.19 | 16.17–18.26 | 0.49 | 0.33–0.71 |
Anaplasma phagocytophilum was found in I. scapularis (0.87%) in four provinces: Saskatchewan, Ontario, Québec, and New Brunswick (Figure 4, Table 4). Borrelia miyamotoi was found in British Columbia, Ontario, Québec and New Brunswick. Babesia microti was found only in Ontario. Co-infections were found in Ontario, Québec and New Brunswick.
Active surveillance tick characteristics
In 2020, I. scapularis (n=688) were collected in three provinces in active surveillance: New Brunswick (n=445), Ontario (n=128) and Québec (n=115). Adult males (n=264/688; 38.37%) and females (n=214/688; 31.10%) were collected most often, followed by nymphs (n=209/688; 30.38%) and larva (1/688; 0.15%).
Active surveillance infection prevalence
Laboratory testing results were available for 99.27% of I. scapularis. The most prevalent pathogen was B. burgdorferi (29.28%), present in Ontario, Québec and New Brunswick (Table 5). Anaplasma phagocytophilum (4.54%) was found in ticks in Ontario and New Brunswick. The remaining pathogens were found in less than 0.5% of I. scapularis: three B. miyamotoi-positive and one B. microti-positive ticks were found in New Brunswick, and one tick with Powassan virus (deer tick lineage) was found in Québec. The site locations where I. scapularis was collected in active surveillance are shown in Figure 5.
| Province | Infection prevalence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Babesia microti | Borrelia burgdorferi | Borrelia miyamotoi | Powassan virus | ||||||
| Proportion positive tickFootnote a | % | Proportion positive tick | % | Proportion positive tick | % | Proportion positive tick | % | Proportion positive tick | % | |
| Ontario | 2/128 | 1.56 | 0/128 | 0 | 53/128 | 41.41 | 0/128 | 0 | 0/128 | 0 |
| Québec | 0/110 | 0 | 0/110 | 0 | 40/110 | 36.36 | 0/110 | 0 | 1/110 | 0.91 |
| New Brunswick | 29/445 | 6.52 | 1/445 | 0.22 | 107/445 | 24.04 | 3/445 | 0.67 | 0/445 | 0 |
| Total | 31/683 | 4.54 | 1/683 | 0.15 | 200/683 | 29.28 | 3/683 | 0.44 | 1/683 | 0.15 |
Figure 5: Ixodes scapularis ticks with associated pathogens collected through active surveillance, Canada, 2020Footnote aFootnote b
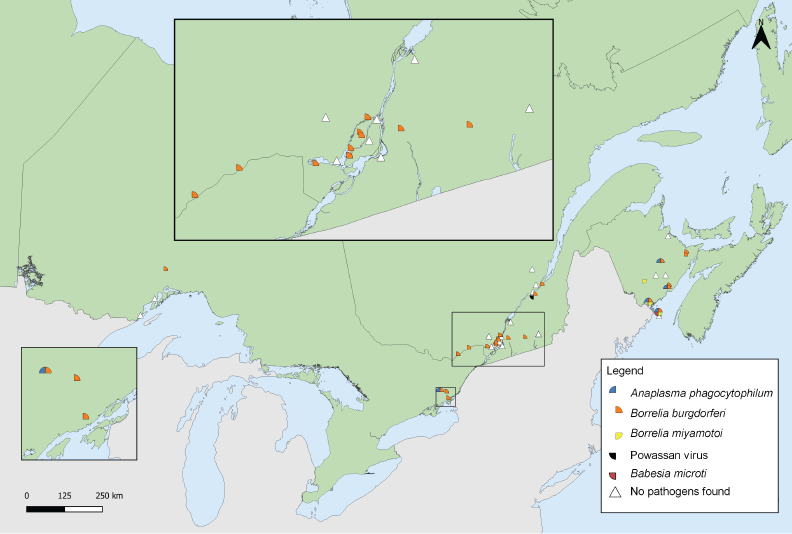
Figure 5: Text description
This map shows the locations where Ixodes scapularis ticks infected with Borrelia burgdorferi, Anaplasma phagocytophilum, Borrelia miyamotoi, Babesia microti or Powassan virus were found in active surveillance. Borrelia burgdorferi was found in ticks in Ontario, Québec, and New Brunswick. Anaplasma phagocytophilum was found in ticks in Ontario and New Brunswick. Babesia microti and Borrelia miyamotoi was found in New Brunswick. Powassan virus was found in a tick in Québec.
Discussion
In 2020, I. scapularis and I. pacificus were submitted in passive surveillance from nine provinces. Only I. pacificus were submitted in British Columbia. The majority of ticks were female adults and obtained from human hosts. Among ticks that were tested, 18.21% of I. scapularis and 0.14% of I. pacificus were infected with at least one tick-borne pathogen, mainly B. burgdorferi. In active surveillance, five tick-borne pathogens (A. phagocytophilum, B. burgdorferi, B. miyamotoi, B. microti and Powassan virus) were identified among the I. scapularis collected in Ontario, Québec and New Brunswick.
From passive surveillance, 5,899 ticks were sample-based submissions, a decrease of 44% from the 10,549 ticks submitted in 2019Footnote 16, which could be due, in part, to impacts from the coronavirus disease 2019 (COVID-19) pandemic. Beginning in spring 2020, COVID-19 pandemic restrictions affected traditional passive surveillance, as health units, medical clinics and veterinary clinics were limited in their ability to accept physical tick specimens at some locations (e.g. Simcoe Muskoka District Health Unit)Footnote 23. The decrease in submissions could also be due to changes to sample-based submission programs and greater emphasis on image-based submission programs in most jurisdictions. Active surveillance was also affected by pandemic restrictions, as in-person activities like field surveillance were limited (e.g. Institut national de santé publique du Québec) Footnote 24. Data from the Canadian Lyme Sentinel Network, which was included in the 2019 reportFootnote 16, was unavailable in 2020 as Canadian Lyme Sentinel Network activities were suspended (personal communication, C. Guillot, 2022).
In passive surveillance, ticks were submitted every month, but submissions followed distinct species-specific patterns influenced by location and weather. Despite fewer ticks submitted to passive surveillance than in 2019 Footnote 16, the same bimodal peaks for I. scapularis adults that have been shown historically in central and eastern CanadaFootnote 13Footnote 25Footnote 26Footnote 27 were observed in 2020. For I. pacificus, a single springtime peak was observed as shown previously in British ColumbiaFootnote 14Footnote 16 and the western United StatesFootnote 28. While risk of exposure to ticks was present year-round, exposure to tick-borne pathogens is dependent on infection prevalence and attachment time.
The proportion of ticks submitted from dogs or cats increased from 8.9% in 2019 to 15.1% in 2020Footnote 16. This increase is likely from including data from eTick: whereas sample-based passive surveillance programs in some localities (e.g. health units, municipalities) are restricted to ticks from human hosts only, image-based passive surveillance has no such restriction, leading to a greater proportion of ticks from animal hosts when eTick data was included in this report.
Compared to 2019Footnote 16, province and pathogen-specific infection prevalence estimates were similar, but geographic distribution was more limited in some cases (e.g. I. scapularis with A. phagocytophilum were limited to only the southernmost parts of New Brunswick compared to 2019). Several factors influence infection prevalence estimates from year-to-year or between provinces, including annual variation in weather, surveillance effort, habitat suitability, presence of established vector and reservoir populations and interactions between humans, ticks and the environment. Because of small sample sizes tested (n=<10), infection prevalence estimates from Saskatchewan and Newfoundland and Labrador should be interpreted with caution.
Ixodes pacificus (found in British Columbia) historically have low rates of B. burgdorferi infectionFootnote 14Footnote 16, while B. burgdorferi infection prevalence in I. scapularis found in central and eastern Canada is typically higherFootnote 18Footnote 25Footnote 29; both trends continued to be observed in 2020. Jacob et al.Footnote 30 report higher infection prevalence among companion animals of several tick-borne pathogens compared to our estimates; however, participating veterinary clinics in that study were skewed towards areas with higher or emerging risk of TBD, likely leading to overestimation of the province-level infection prevalence. The one-year study also concluded in spring 2020, thus not accounting for the effects of pandemic restrictions on tick exposure for the remainder of 2020.
The majority of B. burgdorferi-infected I. scapularis had probable location of acquisition within LD risk areasFootnote 8Footnote 22. The remaining B. burgdorferi-infected I. scapularis may be adventitious ticks carried by migrating birds or mammalsFootnote 15 or collected from areas with emerging LD risk. Provinces routinely review LD risk areas based on new surveillance data according to the 2016 case definitionFootnote 22.
Despite limited opportunities for active field surveillance due to ongoing COVID-19 pandemic restrictions, over 600 I. scapularis were collected in drag sampling from 45 sites across Ontario, Québec and New Brunswick. Five tick-borne pathogens were identified, ranging in prevalence from 0.15% to 29.28%. This was the first detection of Powassan virus (deer tick lineage) in active surveillance in QuébecFootnote 24, which has previously been identified in small numbers of Ixodes spp. in Manitoba, Ontario and New BrunswickFootnote 12Footnote 31.
In addition to single-agent infection with B. burgdorferi and the four other tick-borne pathogens, three distinct types of co-infections were identified. Surveillance beyond LD for other TBD is warranted to monitor the emergence and spread of these pathogens, especially as suitable habitat for Ixodes spp. is predicted to increase due to changes in climate and environmentFootnote 1Footnote 32Footnote 33.
Co-infections have been reported to varying extents in ticks found in CanadaFootnote 16Footnote 18 and the United StatesFootnote 34. Humans who are co-infected may experience a greater number and duration of symptoms compared to single-agent infectionsFootnote 35Footnote 36. Many factors influence the risk of co-infection, including attachment time, but preventing tick bites can help prevent transmission of all TBDs.
Strengths and limitations
This article presents a snapshot of infection prevalence and range estimates for the main LD vectors in Canada. While traditional passive surveillance programs have been discontinued or limited to specific hosts in some regions, incorporating data from eTick allows broader geographic and host representation from these regions in this summary. Combining passive and active surveillance also allows the strengths and weaknesses of the systems to complement each other. For example, while active surveillance is limited in geographic and temporal scope, passive surveillance programs gather data from large areas throughout the year.
There are several limitations to this study. Due to competing public health priorities, passive surveillance programs and the effort of active surveillance vary across Canada. As previously noted, COVID-19 pandemic restrictions affected public health services and surveillance in 2020, resulting in fewer sample-based submissions to passive surveillance and active surveillance that was less geographically representative compared to the previous yearFootnote 16. Shifts in passive tick surveillance programs (e.g. limits on tick host or location of acquisition of tick; discontinuation of regional or provincial programs) have also limited the number of submissions. While digital platforms like eTick offer timely tick identification, tick specimens are not routinely requested for tick-borne pathogen testing from imaging identification platformsFootnote 17. Recall bias in reporting locality of acquisition and travel history in passive surveillance might create uncertainty as to the exact location where ticks were found. Finally, there are likely other active surveillance programs conducted in 2020 not included here in this summary if ticks were not sent for pathogen testing at NML. Furthermore, the number of larvae included in active surveillance is an underestimate, since our dataset only includes ticks sent for testing, for which larvae are rarely sent. These underestimates of the number of ticks may affect the accuracy of infection prevalence of various pathogens.
Conclusion
Ixodes scapularis and I. pacificus were identified across Canada in passive and active surveillance, some of which were infected with B. burgdorferi, the LD pathogen, but also with emerging tick-borne pathogen(s). Healthcare professionals and the public should be aware that there is a risk of exposure to infected ticks outside of known LD risk areas, even if the risk is low in those areas. The identification of new tick-borne pathogens in several jurisdictions in active surveillance may help public health authorities update their prevention strategies, as some of those emerging tick-borne illnesses, like Powassan virus disease, may have infection transmission patterns that differ from LD. As climate change alters the habitat and seasonality of tick vectors, continued surveillance can help in timely identification of new risk areas for LD and other emerging TBD, and directing public health interventions towards these at-risk areas.
Authors’ statement
- CW — Formal analysis, visualization, writing–original draft, writing–review and editing
- SG, AB, JK — Conceptualization, supervision, writing–review and editing
- JB, JC, NC, HC, AD, PG, ML, PL, MM, MR, JS, HS, CS, KT — Writing–review and editing
Competing interests
None.
Acknowledgements
We thank all those involved with tick collection and testing at regional, provincial, and national levels, including Thunder Bay District Health Unit; Kingston, Frontenac and Lennox & Addington Public Health; and members of the public who submitted ticks. We thank M Stefopulos (PHAC) for assistance in creating Figure 3. In addition to co-author J Savage, the composition of the team involved in collection and processing of eTick data included several students as well as the following people: C Jardine (Department of Pathobiology, University of Guelph, Guelph, Ontario [ON]); K Clow (Department of Population Medicine, University of Guelph, Guelph, ON); M Kulkarni (School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON); J Nocera (Faculty of Forestry and Environmental Management, University of New Brunswick, Fredericton, New Brunswick [NB]); S Heard (Department of Biology, University of New Brunswick, Fredericton, NB); E Jenkins, M Voordouw (Department of Veterinary Microbiology, University of Saskatchewan, Saskatoon, Saskatchewan); D Shutler, K Hillier (Department of Biology, Acadia University, Wolfville, Nova Scotia); J Bowden (Natural Resources Canada, Canadian Forest Service, Atlantic Forestry Centre, Corner Brook, Newfoundland and Labrador); P Chuard, J Bouffard (Department of Biology and Biochemistry, Bishop’s University, Sherbrooke, Québec).
Funding
This study was supported by the Public Health Agency of Canada. Passive surveillance from British Columbia was supported by the BC Centre for Disease Control Foundation. Passive surveillance in Saskatchewan is partially funded by the Government of Saskatchewan. Passive and active surveillance from Québec was supported by the Ministère de la Santé et des Services sociaux (MSSS).
