Quantifying the economic gains associated with COVID-19 vaccination

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-6, June 2023: Acute Hepatitis in Children in Canada
Date published: June 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-6, June 2023: Acute Hepatitis in Children in Canada
Evaluation
Quantifying the economic gains associated with COVID-19 vaccination in the Canadian population: A cost-benefit analysis
Ashleigh R Tuite1,2, Victoria Ng3, Raphael Ximenes1, Alan Diener4, Ellen Rafferty5, Nicholas H Ogden3, Matthew Tunis1
Affiliations
1 Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada, Ottawa, ON
2 Dalla Lana School of Public Health, University of Toronto, Toronto, ON
3 Public Health Risk Sciences Division, National Microbiology Laboratory, Public Health Agency of Canada, Saint-Hyacinthe, QC and Guelph, ON
4 Policy Research, Economics, and Analytics Unit, Strategic Policy Branch, Health Canada, Ottawa, ON
5 Institute of Health Economics, Edmonton, AB
Correspondence
Suggested citation
Tuite AR, Ng V, Ximenes R, Diener A, Rafferty E, Ogden NH, Tunis M. Quantifying the economic gains associated with COVID-19 vaccination in the Canadian population: A cost-benefit analysis. Can Commun Dis Rep 2023;49(6):263–73. https://doi.org/10.14745/ccdr.v49i06a03
Keywords: SARS-CoV-2, COVID-19, vaccination, cost-benefit analysis, health economics, modelling
Abstract
Background: Vaccination has been a key part of Canada’s coronavirus disease 2019 (COVID-19) pandemic response. Although the clinical benefits of vaccination are clear, an understanding of the population-level benefits of vaccination relative to the programmatic costs is of value. The objective of this article is to quantify the economic impact of COVID-19 vaccination in the Canadian population between December 2020 and March 2022.
Methods: We conducted a model-based cost-benefit analysis of Canada’s COVID-19 vaccination program. We used an epidemiological model to estimate the number of COVID-19 symptomatic cases, hospitalizations, post-COVID condition (PCC) cases, and deaths in the presence and absence of vaccination. Median, lower and upper 95% credible interval (95% CrI) outcome values from 100 model simulations were used to estimate the direct and indirect costs of illness, including the value of health. We used a societal perspective and a 1.5% discount rate.
Results: We estimated that the costs of the vaccination program were far outweighed by the savings associated with averted infections and associated downstream consequences. Vaccination increased the net benefit by CAD $298.1 billion (95% CrI: 27.2–494.6) compared to the no vaccination counterfactual. The largest benefits were due to averted premature mortality, resulting in an estimated $222.0 billion (95% CrI: 31.2–379.0) benefit.
Conclusion: Our model-based economic evaluation provides a retrospective assessment of COVID-19 vaccination during the first 16 months of the program in Canada and suggests that it was welfare-improving, considering the decreased hospitalizations and use of healthcare resources, deaths averted and lower morbidity from conditions such as PCC.
Introduction
The availability of coronavirus disease 2019 (COVID-19) vaccines marked a turning point in Canada’s pandemic response, allowing for a reduced reliance on non-pharmaceutical interventions (NPIs) to protect population health. Despite the demonstrated effectiveness of COVID-19 vaccines for preventing severe outcomes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections Footnote 1, quantifying the effect of COVID-19 vaccination programs on Canada’s pandemic trajectory is challenging. Mathematical modelling can be used to compare the Canadian pandemic experience to a counterfactual scenario of how the pandemic might have unfolded in the absence of vaccination. The modelling has shown the substantial clinical benefits of COVID-19 vaccination for preventing SARS-CoV-2 infections, hospitalizations and deathsFootnote 2Footnote 3.
Although the population impacts of COVID-19 vaccination are frequently discussed in terms of health outcomes, undertaking a cost-benefit analysis allows for a more comprehensive evaluation. In a cost-benefit analysis, all outcomes are valued in monetary terms allowing for the inclusion of non-health outcomesFootnote 4. This lens allows for a more complete accounting of the costs of illness, including reduced quality-of-life and labour market effects due to illness-associated disability and mortality, in addition to the direct healthcare costsFootnote 5. This is particularly relevant for post-COVID condition (PCC; also known as long COVID), given emerging data showing the high prevalence of PCC in countries experiencing high rates of SARS-CoV-2 infectionFootnote 6Footnote 7. Additionally, there is measurable negative impact of PCC on workforce productivity, including worker absenteeism and exit from the workforceFootnote 8Footnote 9.
Initial economic evaluations of COVID-19 vaccination in North America have demonstrated that COVID-19 vaccination programs have resulted in substantial economic benefitFootnote 10Footnote 11. An analysis of Canada’s vaccination program estimated a net cost-benefit of −$0.4 billion to $2.1 billion when considering treatment costs and lost productivity due to illness, and a further $27.6 billion benefit due to prevented mortalityFootnote 11. Notably, this study used a statistical model that did not account for the transmissibility of SARS-CoV-2, such that the estimates of COVID-19 cases averted with vaccination are likely to be underestimated.
We used transmission modelling to retrospectively quantify the economic impact of vaccination in the Canadian population due to the prevention of SARS-CoV-2 infections, and associated hospitalizations, deaths and PCC cases. The analysis focuses on a 16-month period, following the first authorization of vaccines in December 2020 until March 2022. Over this time period, approximately 87.5% of Canadians aged five years and older had received at least one vaccine dose, 84% had completed their primary series and 48.8% had received three or more dosesFootnote 12.
Methods
We conducted a cost-benefit analysis of COVID-19 vaccination in the Canadian population. We used an epidemiological model of SARS-CoV-2 transmission to assess the impact of vaccination on COVID-19 burden and evaluate the net benefit associated with vaccination.
Transmission model overview and scenarios
We adapted a previously reported age-structured agent-based model that describes the transmission of SARS-CoV-2 in the Canadian population to estimate COVID-19 cases in the presence and absence of COVID-19 vaccinesFootnote 3Footnote 13. The model simulates transmission in a general community setting and excludes outbreaks in discrete settings such long-term care homes, which experienced high rates of infection. Model outputs were validated by comparison with available administrative dataFootnote 3.
We developed two alternative scenarios using model parameters that were otherwise unchanged from the previously described analysisFootnote 3: a “what happened” baseline scenario and a “no vaccine” counterfactual scenario. The baseline scenario reflected the observed rollout of vaccination programs, in terms of age groups eligible for vaccination and coverage achievedFootnote 14. The model time period included the emergence of the Omicron variant of concern, which triggered an expedited rollout of third doses in the general population in the winter of 2021/2022Footnote 15; additional details about the modelled time period are provided in Ogden et al.Footnote 3. The baseline included observed levels of NPIs over this period since the availability of vaccines did not result in the immediate removal of NPIs.
The counterfactual scenario represented what might have occurred in the absence of vaccination, with continued NPI use to mitigate recurring waves of infection and health system strain. The timing of introduction and lifting of NPIs (“shutdowns”) in the counterfactual scenario was based on intensive care unit (ICU) occupancy, with thresholds based on observed ICU occupancy when NPIs were introduced in the second wave of the pandemic (September 2020 to February 2021). Because the model is stochastic, timing and duration of NPI use varied across model runs for the counterfactual scenario. In both scenarios, lifting of NPIs occurred gradually over a four-week period.
The model population size was 100,000 and outputs were rescaled to represent the size of the Canadian population. Each model scenario was run 100 times. The model was run from February 7, 2020, to March 31, 2022, and outcomes were calculated from December 14, 2020, onwards, to capture the period of divergence between the baseline and counterfactual scenarios following the start of vaccination. Model outputs included COVID-19 clinical cases (all cases experiencing symptoms, regardless of severity), hospitalizations, ICU admissions and deaths in the baseline scenario compared to the counterfactual scenario. We also calculated the number of vaccine doses administered for the baseline scenario, and number and duration of shutdowns. We used the median, lower 95% credible interval (CrI) and upper 95% CrI output values for the economic analysis.
Estimation of post-COVID condition cases averted
Model-projected clinical cases (excluding fatal cases) for the two scenarios were used to estimate the incidence of PCC following SARS-CoV-2 infection in the presence and absence of vaccination. Where possible, we used the World Health Organization case definition of PCCFootnote 16. The probability of developing PCC among clinical cases was derived from a general population cohort with age and sex-matched controlsFootnote 17. We did not apply differential risks of developing PCC by age or infection severity.
Vaccination was assumed to prevent PCC two ways: first, by preventing SARS-CoV-2 infection; and second, by reducing the likelihood of developing PCC if infected. Vaccine effectiveness for preventing infection was assumed to be dependent on the predominant circulating variant of concern at the time of infectionFootnote 3, while vaccine effectiveness for preventing PCC following infection was assumed to be constant (15%), regardless of the infecting variant of concernFootnote 18. Protection against PCC was only assumed among people who had received two or more vaccine doses prior to infection. We did not model a reduction in PCC risk among people vaccinated after SARS-CoV-2 infection and did not include waning of protection from PCC over the model time horizon.
Economic impact of COVID-19 cases averted
We estimated the total costs of illness, including direct and indirect costs and the value of health (morbidity and mortality)Footnote 5 to enumerate the economic impact of COVID-19 cases averted due to vaccination from a societal perspective. We used a lifetime time horizon to enumerate the costs and health consequences associated with COVID-19-attributable mortality. For PCC, we estimated costs and health effects for the first year following onset, given limited data on the longer-term trajectory of PCC. Costs are in 2021 Canadian dollars and where necessary were converted using the Canadian Consumer Price IndexFootnote 19. We used a discount rate of 1.5% per year. Input parameters for the economic model were derived from the published studies, wherever possible, and by assumption and expert opinion otherwise (Table 1).
| Applicable outcome | Parameter | Value | Source |
|---|---|---|---|
| Direct costs | |||
| Clinical case | Net medical cost per outpatient case ($) | 165.2 | Tsui et al.Footnote 20 |
| PCR test ($) | 60.7 | Campbell et al.Footnote 21 | |
| Hospitalization (including ICU) | Healthcare cost per hospitalization ($) | 25,103 | CIHIFootnote 22 |
| PCC case | Cost per case ($, in first year) | 9,683 | Institute for Health Economics, personal communication |
| Vaccination | Vaccine cost per dose ($) | 30 | Office of the Auditor General of Canada Footnote 23 |
| Administration costs per dose ($) | 34 | Office of the Auditor General of OntarioFootnote 24 | |
| Other programmatic costs per dose ($) | 27 | Assumption based on Sah et al.Footnote 10 | |
| Indirect costs | |||
| All | Average employment income, age 16 years and older ($) | 49,095 | Statistics Canada Footnote 25 |
| Average employment income, ages 25–54 years ($) | 58,811 | Statistics CanadaFootnote 25 | |
| Average employment income ($) | Age-specific values | Statistics CanadaFootnote 25 | |
| Productivity loss | |||
| Clinical case | Time off work (days) | 10 | Government of CanadaFootnote 26 |
| Hospitalization (including ICU) | Length of stay in hospital (days) | 13 | CIHI Footnote 22 |
| Time from hospital discharge to return to work (days) | 27 | Chopra et al. Footnote 27 | |
| PCC case | Proportion of PCC cases with ongoing symptoms at one year | 0.15 | Waters and Wernham Footnote 8 |
| Average reduction in earning during first six months of illness (%) | 11 | Wulf Hanson Footnote 28 | |
| Average annual reduction in salary (%) | 8.3 | Extrapolated from Footnote 8 and Footnote 28 | |
| Vaccination | Time off work to receive vaccine (days) | 0.4 | Government of AlbertaFootnote 29 |
| Proportion unable to work one day post-vaccination, dose 1 | 0.05 | Rosenblum et al. Footnote 30 | |
| Proportion unable to work one day post-vaccination, dose 2 | 0.23 | Rosenblum et al. Footnote 30 | |
| Proportion unable to work one day post-vaccination, booster doses | 0.23 | Assumption | |
| All | Labour force participation, age 15 years and older (%) | 64.6 | Statistics Canada Footnote 31 |
| Labour force participation, ages 25–54 years (%) | 87.0 | Statistics Canada Footnote 31 | |
| Labour force participation (%) | Age-specific values | Statistics Canada Footnote 31 | |
| QALY loss | |||
| Clinical case | 0–14 years | 0.0050 | Kirwin et al. Footnote 32 |
| 15–64 years | 0.0077 | Kirwin et al. Footnote 32 | |
| 65 years and older | 0.012 | Kirwin et al.Footnote 32 | |
| Hospitalization (including ICU) | QALY loss (per year) | 0.58 | Kirwin et al. Footnote 32; adjusted for length of hospital stay |
| QALY loss on discharge (per case) | 0.1 | Kirwin et al.Footnote 32 | |
| PCC case | QALY loss (1 year following discharge) | 0.2937 | Weighted decrement for common chronic conditions associated with PCC, Institute for Health Economics, personal communication |
| Death (net present value) | 0–9 years | 41.37 | Kirwin et al. Footnote 32 |
| 10–19 years | 37.19 | Kirwin et al. Footnote 32 | |
| 20–29 years | 33.37 | Kirwin et al.Footnote 32 | |
| 30–39 years | 29.4 | Kirwin et al.Footnote 32 | |
| 40–49 years | 24.9 | Kirwin et al.Footnote 32 | |
| 50–59 years | 20.18 | Kirwin et al.Footnote 32 | |
| 60–69 years | 15.36 | Kirwin et al.Footnote 32 | |
| 70–74 years | 10.35 | Kirwin et al.Footnote 32 | |
| 75 years | 5.17 | Kirwin et al.Footnote 32 | |
| Vaccination | QALY loss if experience adverse event following immunization | 0.00027 | Sandmann et al.Footnote 33 |
| Other | |||
| PCC case | Vaccine effectiveness for preventing PCC following infection | 0.15 | Al-AlyFootnote 18 |
| Clinical case | Percent of clinical cases developing PCC | 12.7 (7.8–17.0) |
BalleringFootnote 17; Thompson Footnote 34 |
| All | Vaccine wastage (%) | 3 | Office of the Auditor General of Ontario Footnote 24; for the period of December 2020 to January 2022 |
| Percent of cases tested by PCR | 20 | Statistics CanadaFootnote 35 and assumption | |
| Discount rate (%) | 1.5 | CADTHFootnote 36 | |
| Cost per QALY threshold ($) | 30,000 (20,000–100,000) |
Ochalek et al.Footnote 37 | |
|
|||
Direct costs included medical costs due to COVID-19 cases, comprising outpatient care and hospitalization for acute COVID-19 and treatment of PCC. Vaccination program costs encompassed the cost of purchasing and administering COVID-19 vaccines, including estimated wastage, as well costs associated with delivery of the program to the population, such as storage and transportation, clinic set up, and advertisement and outreachFootnote 10. The cost of wasted doses excluded vaccine administration costs.
Indirect costs included the value of lost production due to days of employment loss due to illness, disability, death or caregiving responsibilities, as well as production losses associated with time to receive a vaccine and possible adverse events following immunization (AEFI). We did not include out-of-pocket medical costs (e.g. pharmaceutical costs). Productivity loss was quantified using the human capital approachFootnote 4. We used age-specific estimates of labour force participation for the years 2020 and 2021Footnote 31 and average employment income for 2020Footnote 25. Caregiver costs were based on estimates of the average employment income and labour force participation of people aged 25–54 years, adjusted for estimated caregiver productivity lossFootnote 38. We included caregiver costs associated with outpatient infections in children less than 15 years of age, and caregiver costs for hospitalized cases for those age younger than 15 years and 65 years and older. To estimate production loss associated with receiving the vaccine, we used labour force participation rates and average salary in the population aged 16 and older to account for caregiver time off work to accompany children to vaccination appointments.
Health impacts included disutility from symptomatic infection, hospitalization, PCC, death and AEFI. Quality-adjusted life years (QALYs) were monetized using a cost per QALY threshold of $30,000Footnote 37. Net benefit was estimated using the “no vaccination” counterfactual scenario as the baseline. The transmission model was constructed in AnyLogic 8 Professional 8.7.2 and the economic analysis was conducted using RFootnote 39.
Sensitivity analyses
To address uncertainty around vaccine costs, including programmatic costs, we estimated a threshold cost to determine the maximum vaccine cost per dose for which a COVID-19 vaccination would have been cost beneficial. We assumed that administration costs were fixed at the value used in the main analysis.
We explored cost per QALY thresholds values of $20,000, $50,000 and $100,000 in sensitivity analysis. We assessed lower (7.8%) and higher (17.0%) estimates of risk of PCCFootnote 34 to evaluate how these estimates impacted findings.
We re-estimated production losses using the friction cost approach. In contrast with the human capital approach, the friction cost approach assumes that after a “friction period”, workers who have left the workforce will eventually be replaced by currently unemployed workersFootnote 40. We used a three-month friction period for people with PCC or who died of COVID-19Footnote 41.
Results
With vaccination, the average Canadian population experience of the pandemic from December 2020 to March 2022 was represented in the model as a total of three shutdown periods for a total duration of 112 days. In contrast, in the absence of vaccination but with continued implementation of NPIs in the face of healthcare system strain, we would have expected four extended shutdown periods (95% CrI: 3–5) for a total duration of 343 days (95% CrI: 268–399).
Model-estimated health outcomes used for the economic analysis are presented in Table 2 and Figure 1. For the median and upper bound model estimates, incidence of all COVID-19 outcomes was higher in the “no vaccine” counterfactual scenario compared to the baseline. For the lower bound model estimates, although the incidence of symptomatic infections and PCC was higher in the baseline scenario, the occurrence of hospitalizations and deaths was higher for the “no vaccination” counterfactual, due to the effectiveness of vaccination for preventing severe outcomes.
| Health outcome | Scenario | Averted (counterfactual minus baseline) |
|
|---|---|---|---|
| Baseline | Counterfactual (no vaccination)Footnote b |
||
Clinical cases |
13,618,980 |
24,713,530 |
11,094,550 |
Hospitalized cases (excluding ICU) |
86,090 |
1,270,100 |
1,184,010 |
ICU cases |
25,660 |
375,730 |
350,070 |
PCC cases |
1,566,540 |
3,070,700 |
1,504,160 |
Deaths |
10,640 |
534,800 |
524,160 |
|
|||
Figure 1: Age distribution of COVID-19 health outcomes for the two model scenariosFootnote aFootnote b
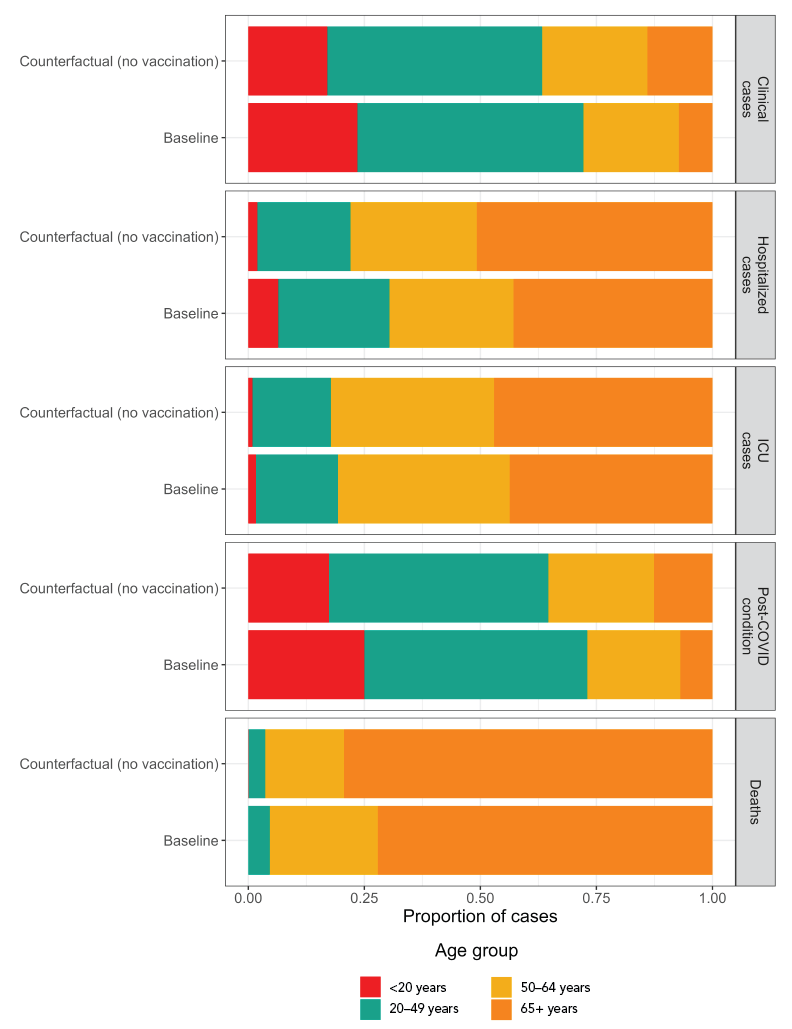
Figure 1 - Text description
| Age group | Outcome | Comparator | Proportion of cases |
|---|---|---|---|
| <20 years | Clinical cases | Baseline | 0.235 |
| 20–49 years | Clinical cases | Baseline | 0.487 |
| 50–64 years | Clinical cases | Baseline | 0.206 |
| 65+ years | Clinical cases | Baseline | 0.072 |
| <20 years | Clinical cases | Counterfactual (no vaccination) | 0.170 |
| 20–49 years | Clinical cases | Counterfactual (no vaccination) | 0.463 |
| 50–64 years | Clinical cases | Counterfactual (no vaccination) | 0.226 |
| 65+ years | Clinical cases | Counterfactual (no vaccination) | 0.140 |
| <20 years | Hospitalized cases | Baseline | 0.065 |
| 20–49 years | Hospitalized cases | Baseline | 0.240 |
| 50–64 years | Hospitalized cases | Baseline | 0.267 |
| 65+ years | Hospitalized cases | Baseline | 0.429 |
| <20 years | Hospitalized cases | Counterfactual (no vaccination) | 0.020 |
| 20–49 years | Hospitalized cases | Counterfactual (no vaccination) | 0.201 |
| 50–64 years | Hospitalized cases | Counterfactual (no vaccination) | 0.272 |
| 65+ years | Hospitalized cases | Counterfactual (no vaccination) | 0.508 |
| <20 years | ICU cases | Baseline | 0.017 |
| 20–49 years | ICU cases | Baseline | 0.176 |
| 50–64 years | ICU cases | Baseline | 0.370 |
| 65+ years | ICU cases | Baseline | 0.437 |
| <20 years | ICU cases | Counterfactual (no vaccination) | 0.009 |
| 20–49 years | ICU cases | Counterfactual (no vaccination) | 0.169 |
| 50–64 years | ICU cases | Counterfactual (no vaccination) | 0.351 |
| 65+ years | ICU cases | Counterfactual (no vaccination) | 0.471 |
| <20 years | Post–COVID condition | Baseline | 0.250 |
| 20–49 years | Post–COVID condition | Baseline | 0.480 |
| 50–64 years | Post–COVID condition | Baseline | 0.200 |
| 65+ years | Post–COVID condition | Baseline | 0.069 |
| <20 years | Post–COVID condition | Counterfactual (no vaccination) | 0.174 |
| 20–49 years | Post–COVID condition | Counterfactual (no vaccination) | 0.472 |
| 50–64 years | Post–COVID condition | Counterfactual (no vaccination) | 0.228 |
| 65+ years | Post–COVID condition | Counterfactual (no vaccination) | 0.126 |
| <20 years | Deaths | Baseline | 0.000 |
| 20–49 years | Deaths | Baseline | 0.047 |
| 50–64 years | Deaths | Baseline | 0.233 |
| 65+ years | Deaths | Baseline | 0.721 |
| <20 years | Deaths | Counterfactual (no vaccination) | 0.001 |
| 20–49 years | Deaths | Counterfactual (no vaccination) | 0.036 |
| 50–64 years | Deaths | Counterfactual (no vaccination) | 0.169 |
| 65+ years | Deaths | Counterfactual (no vaccination) | 0.794 |
Vaccination was associated with 6.61 million (95% CrI: 0.88–10.8) QALYs gained and increased the net benefit by $298.1 billion (95% CrI: 27.2–494.6) compared to the “no vaccination” counterfactual (Table 3). This represents a benefit-cost ratio of 26.7 (3.6–43.3). The largest benefits were due to averted premature mortality, resulting in an estimated $222.0 billion (95% CrI: 31.2–379.0) benefit.
| Health outcome | Incremental benefits ($ billions) | ||
|---|---|---|---|
| Direct | Indirect | Total | |
| Clinical cases | 2 (−0.176–2.77) |
12.8 (−1.1–18) |
14.8 (−1.28–20.8) |
| Hospitalized cases (including ICU) | 29.6 (6.26–45.9) |
10.9 (2.31–17) |
40.6 (8.57–62.9) |
| PCC cases | 14.8 (0.0342–19.8) |
17.5 (0.222–23.7) |
32.3 (0.256–43.5) |
| Deaths | N/A | 222 (31.2–379) |
222 (31.2–379) |
| Vaccination | −7.56 (−7.54–−7.59) |
−4.05 (−4.04–−4.07) |
−11.6 (−11.6–−11.7) |
| Total | 38.83 (−1.426–60.88) |
259.3 (28.59–433.7) |
298.1 (27.16–494.6) |
|
|||
We estimated that if the costs of vaccination were 64 times (95% CrI: 7–104) the assumed baseline value, the vaccination program would still have provided a net benefit, when using a societal perspective that includes both direct and indirect costs. For the lower bound model estimate, this means that for a cost of up to $410 per dose (excluding administration costs), the vaccination program would be considered cost-beneficial; for the median and upper bound estimates, these values are $3,630 and $5,950 per dose, respectively. Considering direct medical costs and monetized QALYs only, which reflects the healthcare payer perspective that is typically used in healthcare decision-making, a cost per dose of up to $390, $2,910 and $4,640 would be cost beneficial for the lower, median and upper bound scenarios, respectively.
The use of higher cost per QALY thresholds increased the welfare gain of vaccination compared to the counterfactual (Figure 2), with a maximum benefit of $1.25 trillion for the upper bound model estimate and a threshold of $100,000 per QALY. Using a lower threshold of $20,000 per QALY and the most conservative model estimates of vaccine impact resulted in an estimated net benefit of $18.3 billion.
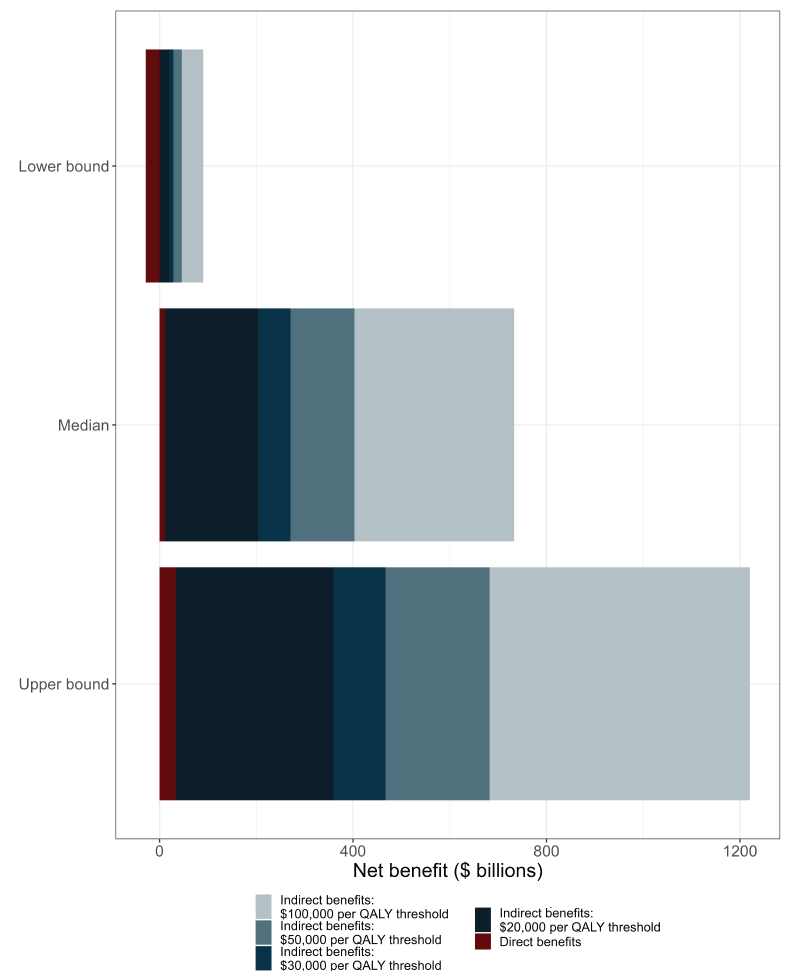
Figure 2 - Text description
| Model outcome measure | Cost component | QALY threshold ($ per QALY gained) |
Incremental benefits ($ billions) |
|---|---|---|---|
| Lower bound | Total | $100,000 | 89.1 |
| Lower bound | Total | $50,000 | 44.9 |
| Lower bound | Total | $30,000 | 27.2 |
| Lower bound | Total | $20,000 | 18.4 |
| Lower bound | Direct only | N/A | -1.4 |
| Median | Total | $100,000 | 760.6 |
| Median | Total | $50,000 | 430.3 |
| Median | Total | $30,000 | 298.1 |
| Median | Total | $20,000 | 232.1 |
| Median | Direct only | N/A | 38.8 |
| Upper bound | Total | $100,000 | 1247.9 |
| Upper bound | Total | $50,000 | 709.8 |
| Upper bound | Total | $30,000 | 494.6 |
| Upper bound | Total | $20,000 | 387 |
| Upper bound | Direct only | N/A | 60.9 |
Lower or higher risk of PCC following infection did not have a substantial impact on estimated benefit of the vaccination program. The net benefit was estimated as $285.7 billion (95% CrI: 27.1–477.8) and $309.1 billion (95% CrI: 27.2–509.3), when PCC occurred in 7.8% or 17% of clinical cases, respectively.
The net benefit of vaccination was reduced when using the friction cost instead of the human capital approach to estimate production losses but remained large at $251.0 billion (95% CrI: 21.6–406.3). Most of the reduced benefit was due to lower estimated indirect costs due to mortality, a reduction of $44.4 billon (95% CrI: 5.4–84.5).
Discussion
We estimate that Canada’s COVID-19 vaccination program resulted in tens to hundreds of billions of dollars in monetary benefit compared to a situation without vaccination and exclusive reliance on NPIs to control transmission. The costs of the vaccination program were far outweighed by the savings associated with averted infections and associated downstream consequences. Although the largest benefit was derived from averted premature mortality, the indirect benefit associated with reduced illness and disability was also substantial.
Our findings are consistent with an analysis of New York City’s COVID-19 vaccination campaignFootnote 10. Despite different epidemiological methods and a different healthcare system, that study also demonstrated substantial cost savings associated with the city’s COVID-19 vaccination programFootnote 10. A recent analysis of Canada’s vaccination program also found the vaccination program to be cost-beneficial, with a net monetary benefit of −$0.4 billion to $2.1 billion, with an additional $27.6 billion in economic benefit associated with averted mortalityFootnote 11; this analysis, which did not use a transmission model to estimate health outcomes averted with vaccination, likely underestimated the benefits of the program. For comparison, the 2022 analysisFootnote 11 estimated that the vaccination program prevented 30,900 deaths from January 2021 to May 2022, while our analysis estimated 524,000 deaths averted over a similar period (December 2020 to March 2022). Another model-based analysisFootnote 2 estimated 314,100 deaths averted in the first year of Canada’s vaccination program (December 2020 to December 2021).
Strengths and limitations
Our estimates of benefit do not include a full accounting of the societal impact of the vaccines for speeding economic recoveryFootnote 42. The counterfactual model showed that without vaccination, the number of days with NPIs in place could have been three times as high as what was observed. A recent analysis estimated that a six-month delay in access to vaccines would have resulted losses of $156 billion in economic activity (or 12.5% of Canada’s gross domestic product)Footnote 11. Relatedly, we did not include the societal costs associated with the prolonged use of NPIs or the downstream effects on the healthcare system resulting from with a higher burden of COVID-19 cases and deferral of care for other health needsFootnote 43Footnote 44; the inclusion of these costs would further increase the economic benefit associated with vaccination.
Due to the confidentiality of COVID-19 vaccine pricing information, we did not use or have access to these data. Instead, we used publicly available estimates of the average cost of the vaccine per dose, which may over or under-estimate the actual cost of vaccines. Similarly, information about other costs associated with the vaccination programs, including storage, transportation, outreach and wastage, were based on public information, assumption and expert opinion. Despite uncertainty in these values, we estimated that the costs of vaccination could have been 10 to 100-fold greater and still been considered a cost-beneficial intervention.
We compared the observed pandemic trajectory to a “no vaccination” counterfactual scenario where implementation of NPIs was tied to ICU capacity. The precise nature of how the pandemic might have been managed in Canada had vaccines not become available is unknowable. Notably, we did not model interventions such as continued used of masking or improvements in ventilation, which might have been more widely adopted had vaccination not become available. Given the uncertainty associated with the counterfactual, we included lower and upper bound model outputs in the economic evaluation. We also noted that vaccination remained a cost-beneficial intervention for the conservative lower bound estimate, where the model predicted higher numbers of symptomatic cases with vaccination, but reduced severe infections, compared to the counterfactual.
The benefit of vaccination for prevention of PCC remains challenging to quantify. We limited our estimates of PCC impacts in the first year following infection, given uncertainty about the longer-term trajectory of illness among cases and thus likely underestimated the total burden associated with PCC. Our model-derived estimates of PCC in the Canadian population of 4.1% (range: 3.5%–4.7%) over the modelled time period are aligned with Canadian survey data indicating that 4.6% of the Canadian population aged 18 years and older reported ongoing symptoms at least three months after SARS-CoV-2 infection, based on data collected between April and August 2022Footnote 35. We assumed that the risk of developing PCC applied equally, regardless of severity of initial infection. The data suggested an increased risk of PCC among more severe casesFootnote 7Footnote 35 and therefore, the estimated impact of vaccination for preventing PCC may be underestimated in our model. Sensitivity analyses revealed that different assumptions about the rate of PCC are unlikely to be very influential on the costs averted by the vaccination program.
We monetized QALYs to estimate the benefits associated with averted COVID-19 morbidity and mortality. The value of statistical life (VSL) approach is an alternative for quantifying the health impacts of an intervention in cost-benefit analysesFootnote 4. The VSL allows for an accounting of the impact of reductions in mortality risk on all aspects of well-being, such as averted medical expenses and the pain and suffering associated with illnessFootnote 4. It has the disadvantage of typically not accounting for the morbidity associated with non-fatal casesFootnote 4. A comparison of VSL and monetized QALY approaches for human papillomavirus vaccination programs showed that VSL was associated with higher estimated benefitFootnote 4. Given the large burden of morbidity associated with COVID-19, we used a monetized QALY approach but note that alternate approaches may result in different estimates of the monetary benefit of COVID-19 vaccination.
Conclusion
Our model-based economic evaluation provides a retrospective assessment of COVID-19 vaccination during the first 16 months of the program in Canada and suggests that it was welfare-improving, considering decreased hospitalizations and use of healthcare resources, deaths averted and lower morbidity from conditions such as PCC. Including the benefits associated with the economic recovery through fewer days in shutdown scenarios would show even greater increases in net benefits. This analysis may help build a foundation for assessment of cost effectiveness and vaccine procurement decisions in future pandemics.
Authors’ statement
- ART — Conceptualization, analysis, manuscript drafting
- VN — Conceptualization, modelling, manuscript review and editing
- RX — Conceptualization, manuscript review and editing
- AD — Conceptualization, manuscript review and editing
- ER — Analysis, manuscript review and editing
- NHO — Conceptualization, manuscript review and editing
- MT — Conceptualization, manuscript review and editing
Competing interests
None.
Acknowledgments
The authors would like to thank H Kim, L Waddell, F Reyes Domingo, R Edjoc, E Kirwin, and A Unsal for helpful feedback and discussion.
Funding
This work was supported by the Public Health Agency of Canada.
References
- Footnote 1
-
Institute for Health Metrics and Evaluation. COVID-19 vaccine efficacy summary. Seattle, WA: IHME; 2022. [Accessed 2022 Dec 8]. https://www.healthdata.org/covid/covid-19-vaccine-efficacy-summary
- Footnote 2
-
Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis 2022;22(9):1293–302. https://doi.org/10.1016/S1473-3099(22)00320-6
- Footnote 3
-
Ogden NH, Turgeon P, Fazil A, Clark J, Gabriele-Rivet V, Tam T, Ng V. Counterfactuals of effects of vaccination and public health measures on COVID-19 cases in Canada: what could have happened? Can Commun Dis Rep 2022;48(7-8):292–302. https://doi.org/10.14745/ccdr.v48i78a01
- Footnote 4
-
Park M, Jit M, Wu JT. Cost-benefit analysis of vaccination: a comparative analysis of eight approaches for valuing changes to mortality and morbidity risks. BMC Med 2018;16(1):139. https://doi.org/10.1186/s12916-018-1130-7
- Footnote 5
-
Public Health Agency of Canada. Economic burden of illness in Canada, 2010. Ottawa, ON: PHAC; 2018. [Accessed 2022 Oct 3]. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/science-research/economic-burden-illness-canada-2010/economic-burden-illness-canada-2010.pdf
- Footnote 6
-
Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, Obeidat M, Obeidat Y, Gerberi D, Taha RM, Kashour Z, Kashour T, Berbari EF, Alkattan K, Tleyjeh IM. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect 2022;28(5):657–66. https://doi.org/10.1016/j.cmi.2022.01.014
- Footnote 7
-
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-Coronavirus Disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis 2022;226(9):1593–607. https://doi.org/10.1093/infdis/jiac136
- Footnote 8
-
Institute for Fiscal Studies. Waters T, Wernham T. Long COVID and the labour market. London (UK): IFS; 2022. [Accessed 2022 Dec 6]. https://ifs.org.uk/publications/long-covid-and-labour-market
- Footnote 9
-
Brookings Institution. Bach K. New data shows long Covid is keeping as many as 4 million people out of work. Washington, DC: Brookings; 2022. [Accessed 2022 Dec 8]. https://www.brookings.edu/research/new-data-shows-long-covid-is-keeping-as-many-as-4-million-people-out-of-work/
- Footnote 10
-
Sah P, Vilches TN, Moghadas SM, Pandey A, Gondi S, Schneider EC, Singer J, Chokshi DA, Galvani AP. Return on investment of the COVID-19 vaccination campaign in New York City. JAMA Netw Open 2022;5(11):e2243127. https://doi.org/10.1001/jamanetworkopen.2022.43127
- Footnote 11
-
C.D. Howe Institute. Wyonch R, Zhang T. Damage Averted: Estimating the Effects of Covid-19 Vaccines on Hospitalizations, Mortality and Costs in Canada. Toronto, ON: CDHowe: 2022. [Accessed 2022 Dec 20]. https://www.cdhowe.org/public-policy-research/damage-averted-estimating-effects-covid-19-vaccines-hospitalizations
- Footnote 12
-
Government of Canada. COVID-19 vaccination in Canada: Vaccination coverage. Ottawa, ON: Government of Canada; 2022. [Accessed 2022 Dec 8]. https://health-infobase.canada.ca/covid-19/vaccination-coverage/
- Footnote 13
-
Ng V, Fazil A, Waddell LA, Bancej C, Turgeon P, Otten A, Atchessi N, Ogden NH. Projected effects of nonpharmaceutical public health interventions to prevent resurgence of SARS-CoV-2 transmission in Canada. CMAJ 2020;192(37):E1053–64. https://doi.org/10.1503/cmaj.200990
- Footnote 14
-
Canadian Institute for Health Information. Canadian COVID-19 Intervention Timeline. CIHI; 2022. [Accessed 2022 Dec 8]. https://www.cihi.ca/en/canadian-covid-19-intervention-timeline
- Footnote 15
-
Government of Canada. NACI updated guidance on booster COVID-19 vaccine doses in Canada [2021-12-03]. Ottawa, ON: Government of Canada; 2021. [Accessed 2022 Dec 8]. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/guidance-booster-covid-19-vaccine-doses.html
- Footnote 16
-
World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. Geneva (CH): WHO; 2021. [Accessed 2022 Sep 26]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- Footnote 17
-
Ballering AV, van Zon SK, Olde Hartman TC, Rosmalen JG; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022;400(10350):452–61. https://doi.org/10.1016/S0140-6736(22)01214-4
- Footnote 18
-
Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022;28(7):1461–7. https://doi.org/10.1038/s41591-022-01840-0
- Footnote 19
-
Statistics Canada. Consumer Price Index, annual average, not seasonally adjusted. Table 18-10-0005-01. Ottawa, ON: StatCan; 2022. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501
- Footnote 20
-
Tsui TC, Zeitouny S, Bremner KE, Cheung DC, Mulder C, Croxford R, Del Giudice L, Lapointe-Shaw L, Mendlowitz A, Wong WW, Perlis N, Sander B, Teckle P, Tomlinson G, Walker JD, Malikov K, McGrail KM, Peacock S, Kulkarni GS, Pataky RE, Krahn MD. Initial health care costs for COVID-19 in British Columbia and Ontario, Canada: an interprovincial population-based cohort study. CMAJ Open 2022;10(3):E818–30. https://doi.org/10.9778/cmajo.20210328
- Footnote 21
-
Campbell JR, Uppal A, Oxlade O, Fregonese F, Bastos ML, Lan Z, Law S, Oh CE, Russell WA, Sulis G, Winters N, Yanes-Lane M, Brisson M, Laszlo S, Evans TG, Menzies D. Active testing of groups at increased risk of acquiring SARS-CoV-2 in Canada: costs and human resource needs. CMAJ 2020;192(40):E1146–E55. https://doi.org/10.1503/cmaj.201128
- Footnote 22
-
Canadian Institute for Health Information. COVID-19 hospitalization and emergency department statistics. CIHI; 2022. [Accessed 2022 Dec 2]. https://www.cihi.ca/en/covid-19-hospitalization-and-emergency-department-statistics
- Footnote 23
-
Office of the Auditor General of Canada. COVID-19 Pandemic. Report 9: COVID-19 vaccines. Ottawa, ON: OAG; 2022. [Accessed 2022 Dec 6]. https://www.oag-bvg.gc.ca/internet/docs/parl_oag_202212_09_e.pdf
- Footnote 24
-
Office of the Auditor General of Ontario. Value-for-Money Audit: COVID-19 Vaccination Program. Toronto, ON: Auditor ON; 2022. [Accessed 2022 Dec 5]. https://www.auditor.on.ca/en/content/annualreports/arreports/en22/AR_COVIDVaccination_en22.pdf
- Footnote 25
-
Statistics Canada. Income of individuals by age group, sex and income source, Canada, provinces and selected census metropolitan areas. Table 11-10-0239-01. Ottawa, ON: StatCan; 2022. https://doi.org/10.25318/1110023901-eng
- Footnote 26
-
Public Health Agency of Canada. Public health management of cases and contacts associated with COVID-19. Ottawa, ON: PHAC; 2021. [Accessed 2022 Dec 2]. http://archive.today/QNixQ
- Footnote 27
-
Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med 2021;174(4):576–8. https://doi.org/10.7326/M20-5661
- Footnote 28
-
Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, Ballouz T, Blyuss O, Bobkova P, Bonsel G, Borzakova S, Buonsenso D, Butnaru D, Carter A, Chu H, De Rose C, Diab MM, Ekbom E, El Tantawi M, Fomin V, Frithiof R, Gamirova A, Glybochko PV, Haagsma JA, Javanmard SH, Hamilton EB, Harris G, Heijenbrok-Kal MH, Helbok R, Hellemons ME, Hillus D, Huijts SM, Hultström M, Jassat W, Kurth F, Larsson IM, Lipcsey M, Liu C, Loflin CD, Malinovschi A, Mao W, Mazankova L, McCulloch D, Menges D, Mohammadifard N, Munblit D, Nekliudov NA, Ogbuoji O, Osmanov IM, Peñalvo JL, Petersen MS, Puhan MA, Rahman M, Rass V, Reinig N, Ribbers GM, Ricchiuto A, Rubertsson S, Samitova E, Sarrafzadegan N, Shikhaleva A, Simpson KE, Sinatti D, Soriano JB, Spiridonova E, Steinbeis F, Svistunov AA, Valentini P, van de Water BJ, van den Berg-Emons R, Wallin E, Witzenrath M, Wu Y, Xu H, Zoller T, Adolph C, Albright J, Amlag JO, Aravkin AY, Bang-Jensen BL, Bisignano C, Castellano R, Castro E, Chakrabarti S, Collins JK, Dai X, Daoud F, Dapper C, Deen A, Duncan BB, Erickson M, Ewald SB, Ferrari AJ, Flaxman AD, Fullman N, Gamkrelidze A, Giles JR, Guo G, Hay SI, He J, Helak M, Hulland EN, Kereselidze M, Krohn KJ, Lazzar-Atwood A, Lindstrom A, Lozano R, Magistro B, Carvalho Malta D, Mansson J, Mantilla Herrera AM, Hokdad AH, Monasta L, Nomura S, Pasovic M, Pigott DM, Reiner RC, Reinke G, Ribeiro ALP, Santomauro DF, Sholokhov A, Spurlock EE, Walcott R, Walker A, Wiysonge CS, Zheng P, Bettger JP, Murray CJI, Vos T. A global systematic analysis of the occurrence, severity, and recovery pattern of long COVID in 2020 and 2021. medRxiv. 2022:2022.05.26.22275532. https://doi.org/10.1101/2022.05.26.22275532
- Footnote 29
-
Government of Alberta. Providing paid COVID-19 vaccination leave. Edmonton, AB; Government of AB; 2022. [Accessed 2022 Dec 8]. https://web.archive.org/web/20221208221344/https://www.alberta.ca/providing-paid-covid-19-vaccination-leave.aspx
- Footnote 30
-
Rosenblum HG, Gee J, Liu R, Marquez PL, Zhang B, Strid P, Abara WE, McNeil MM, Myers TR, Hause AM, Su JR, Markowitz LE, Shimabukuro TT, Shay DK. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis 2022;22(6):802–12. https://doi.org/10.1016/S1473-3099(22)00054-8
- Footnote 31
-
Statistics Canada. Unemployment rate, participate rate and employment rate by sex, annual. Table 14-10-0327-02. Ottawa, ON: StatCan; 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032702
- Footnote 32
-
Kirwin E, Rafferty E, Harback K, Round J, McCabe C. A net benefit approach for the optimal allocation of a COVID-19 vaccine. Pharmacoeconomics 2021;39(9):1059–73. https://doi.org/10.1007/s40273-021-01037-2
- Footnote 33
-
Sandmann FG, Davies NG, Vassall A, Edmunds WJ, Jit M; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis 2021;21(7):962–74. https://doi.org/10.1016/S1473-3099(21)00079-7
- Footnote 34
-
Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, Huggins CF, Kwong AS, Silverwood RJ, Di Gessa G, Bowyer RC, Northstone K, Hou B, Green MJ, Dodgeon B, Doores KJ, Duncan EL, Williams FM, Steptoe A, Porteous DJ, McEachan RR, Tomlinson L, Goldacre B, Patalay P, Ploubidis GB, Katikireddi SV, Tilling K, Rentsch CT, Timpson NJ, Chaturvedi N, Steves CJ; OpenSAFELY Collaborative. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun 2022;13(1):3528. https://doi.org/10.1038/s41467-022-30836-0
- Footnote 35
-
Statistics Canada. Long-term symptoms in Canadian adults who tested positive for COVID-19 or suspected an infection, January 2020 to August 2022. Ottawa, ON: StatCan; 2022. [Accessed 2022 Dec 8]. https://www150.statcan.gc.ca/n1/daily-quotidien/221017/dq221017b-eng.htm
- Footnote 36
-
Canadian Agency for Drugs and Technologies in Health. CADTH Methods and Guidelines: Guidelines for the economic evaluation of health technologies: Canada. Ottawa, ON: CADTH; 2017. [Accessed 2022 Dec 8]. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf
- Footnote 37
-
Patented Medicine Prices Review Board. Ochalek J, Lomas J, Claxton K. Assessing health opportunity costs for the Canadian health care systems. Ottawa, ON: PMPRB; 2018. [Accessed 2022 Dec 5]. http://www.pmprb-cepmb.gc.ca/CMFiles/Consultations/new_guidelines/Canada_report_2018-03-14_Final.pdf
- Footnote 38
-
Reilly M, Mitchell I, Gooch K, Vo P, Virabhak S, Lorimer M, Ruff M, Seidenberg J, Khong H. PRM29 Preliminary validation of the Work Productivity Activity Impairment (WPAI) in Caregivers of Children Hospitalized for Respiratory Illness (WPAI- CHRI) in Germany and Canada. Value Health 2012;15(7):A650. https://doi.org/10.1016/j.jval.2012.08.280
- Footnote 39
-
R Core Team. R: A language and environment for statistical computing. 4.0 ed. Vienna, Austria: R Foundation for Statistical Computing. Copenhagen (DK): GBIF; 2020. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- Footnote 40
-
Pike J, Grosse SD. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with human capital estimates: A review. Appl Health Econ Health Policy 2018;16(6):765–78.https://doi.org/10.1007/s40258-018-0416-4
- Footnote 41
-
Koopmanschap MA, Rutten FF, van Ineveld BM, van Roijen L. The friction cost method for measuring indirect costs of disease. J Health Econ 1995;14(2):171–89. https://doi.org/10.1016/0167-6296(94)00044-5
- Footnote 42
-
Deb P, Furceri D, Jimenez D, Kothari S, Ostry JD, Tawk N. The effects of COVID-19 vaccines on economic activity. Swiss J Econ Stat 2022;158(1):3. https://doi.org/10.1186/s41937-021-00082-0
- Footnote 43
-
Dudevich A, Frood J. Impact of the COVID-19 pandemic on health system use in Canada. Healthc Q 2021;24(2):12–4. https://doi.org/10.12927/hcq.2021.26552
- Footnote 44
-
Malagón T, Yong JH, Tope P, Miller WH Jr, Franco EL; McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer 2022;150(8):1244–54. https://doi.org/10.1002/ijc.33884
