NACI update on invasive meningococcal disease epidemiology and prevention

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-9, September 2023: Common Infectious Diseases Caused by Bacteria
Date published: September 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-9, September 2023: Common Infectious Diseases Caused by Bacteria
Advisory Committee Statement
A National Advisory Committee on Immunization (NACI) update on invasive meningococcal disease (IMD) epidemiology and program-relevant considerations for preventing IMD in individuals at high risk of exposure
Anne Pham-Huy1, Joseline Zafack2, Courtney Primeau3, Oliver Baclic2, Marina Salvadori3,4, Shelley Deeks5 on behalf of the National Advisory Committee on Immunization
Affiliations
1 Department of Pediatrics, Children’s Hospital of Eastern Ontario, University of Ottawa, Ottawa, ON
2 Centre for Immunization Programs, Public Health Agency of Canada, Ottawa, ON
3 Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada, Ottawa, ON
4 Department of Pediatrics, McGill University, Montréal, QC
5 Nova Scotia Department of Health and Wellness, Halifax, NS
Correspondence
Suggested citation
Pham-Huy A, Zafack J, Deeks S, Zafack J, Primeau C, Baclic O, Salvadori M, Deeks S on behalf of the National Advisory Committee on Immunization. A National Advisory Committee on Immunization (NACI) update on invasive meningococcal disease (IMD) epidemiology and program-relevant considerations for preventing IMD in individuals at high risk of exposure. Can Commun Dis Rep 2023;49(9):358−67. https://doi.org/10.14745/ccdr.v49i09a01
Keywords: National Advisory Committee on Immunization, NACI, invasive meningococcal disease, adolescents and young adults, meningococcal vaccine, vaccination policy, guidance
Abstract
Following recent outbreaks of invasive meningococcal disease (IMD) in Canada and updates to provincial vaccination guidelines, the National Advisory Committee on Immunization (NACI) conducted a targeted review of evidence with a focus on immunization of adolescents and young adults. NACI reviewed national and international immunization recommendations for populations at high-risk of IMD, national IMD epidemiology and program-relevant considerations. Given the varied IMD epidemiology, NACI determined that recommending a pan-Canadian targeted program is currently challenging and that regional programs may be better suited to prevent IMD in population groups considered to be at high-risk of exposure. Further data is needed to ascertain contemporary risk factors for IMD (including activities and settings associated with bacterial acquisition, carriage and transmission) and estimate the true cost of meningococcal vaccine-preventable infections in Canada. To support provinces and territories in their decision-making, an outline of program-relevant elements for provincial and territorial consideration is provided.
Introduction
Invasive meningococcal disease (IMD) is a rare but serious bacterial disease with a relatively high case fatality rate and significant long-term sequelae, including limb amputations and permanent central nervous system injuryFootnote 1. Following the recent cases of IMD on university campuses in the winter of 2022/2023 in Atlantic CanadaFootnote 2 as well as the subsequent recommendations for the immunization of post-secondary students and other young adults living in congregate living settings by some provinces and territories (PTs)Footnote 3Footnote 4, the Public Health Agency of Canada and the National Advisory Committee on Immunization (NACI) were requested by the Council of Chief Medical Officers of Health to review the current national guidance on use of serogroup B meningococcal vaccines and quadrivalent conjugate meningococcal (Men-C-ACYW) vaccine boosters in post-secondary settings. Specifically, the policy question reviewed by NACI was, “Should additional high-risk populations be offered a serogroup B meningococcal vaccine and/or Men-C-ACYW booster vaccine in order to prevent IMD outbreaks in older adolescents and young adults, 15 to 24 years of age?”
Methods
To answer the policy question, NACI conducted a targeted review of evidence, with a focus in adolescents and young adults, that included national and international guidelines for the prevention of IMD in populations at high risk of IMD exposure, national and international definitions of high-risk populations, meningococcal vaccine characteristics, and EEFA (ethics, equity, feasibility, acceptability) programmatic considerations related to immunization of individuals at high risk of IMD exposure. The epidemiological data for IMD cases with disease onset between January 1, 2012 and December 31, 2019, in Canada was obtained from a previous analysisFootnote 5. The Public Health Agency of Canada compiled updated Canadian epidemiological data, including an outbreak analysis for IMD cases occurring between 2020 and 2022, through a data request to PTs participating in the National Enhanced Invasive Meningococcal Disease Surveillance System, through which PTs voluntarily report epidemiologic data on confirmed IMD cases on an annual basis. A request was made to PTs not currently participating in the National Enhanced Invasive Meningococcal Disease Surveillance System to also obtain these data for the same period. The data were validated for 12 of the 13 PTs, while the analyses for the remaining PTs was based on the isolate submissions provided to the National Microbiology Laboratory for confirmation of serogroup and further strain characterization. All age-standardized analyses were done with the direct method using the 2011 Canadian census data. The NACI IMD Working Group met on May 10 and 24, 2023, and the full committee reviewed the evidence presented to the NACI IMD Working Group on June 5, 2023. NACI approved the conclusions on July 14, 2023.
National and international immunization recommendations
Currently, NACI recommends that adolescents and young adults, depending on local epidemiology and programmatic considerations, receive a dose of monovalent conjugate meningococcal C (Men-C-C) or quadrivalent Men-C-ACYW vaccine routinely at the age of 12 (grade six or seven), even if previously vaccinated as infants or toddlersFootnote 6. NACI also recommends the use of protein-based meningococcal vaccines that primarily target serogroup B (serogroup B meningococcal vaccines: Bexsero™, 4CMenB; or Trumenba™, MenB-fHBP) on an individual basis, taking into consideration the individual preferences, regional serogroup B epidemiology and strain susceptibility. For individuals at high risk of IMD due to exposure or underlying medical conditions, NACI recommends immunization with a serogroup B meningococcal vaccine and Men-C-ACYW vaccine, as well as Men-C-ACYW booster immunization for those at ongoing riskFootnote 7.
In Canada, the adolescent dose of Men-C-ACYW is primarily provided through school-based immunization programs in grades four through 12 (children 9–17 years of age)Footnote 8Footnote 9. Eight PTs (Prince Edward Island [PE], British Columbia [BC], Alberta [AB], Nunavut [NU], New Brunswick [NB], Yukon [YT], Northwest Territories [NT], Québec [QC]) currently provide vaccination in grade nine or later, typically less than five years prior to the initiation of post-secondary studies. Based on a generally accepted assumption that protection from vaccination lasts at least five years, immunization offered through late adolescent school-based programs is likely to see protection last into the first years of post-secondary settings. Recently, PE and Nova Scotia [NS] also expanded their IMD programs to include serogroup B meningococcal immunization of adolescents and young adults who are living in group settings while attending post-secondary education (e.g. living in dormitory or other residence) and living for the first time in a youth-based congregate living setting, respectivelyFootnote 3Footnote 4.
In 2021, 89% of 17-year-old adolescents in Canada had received at least one dose of meningococcal vaccine, which is consistent with the national goal of 90% vaccine coverage at this ageFootnote 10Footnote 11. In addition, through its current immunization programs, Canada has also been able to achieve its disease reduction goal of fewer than five cases per year of IMD caused by serogroup C in children younger than 18 years of ageFootnote 10. Most cases of serogroup C IMD currently occur in unvaccinated adults over 40 years of ageFootnote 5.
While the majority of IMD cases in Canada are sporadic, outbreaks have occurred across the country with variable magnitudes. As part of a comprehensive public health responses to these outbreaks, Canadian PTs have previously implemented targeted immunization programsFootnote 12. Most recently, meningococcal vaccines have been used to control hypervirulent serogroup B (ST-269) and W (ST-11) clones at the provincial or regional level in QC, BC and ABFootnote 12Footnote 13.
Internationally, several jurisdictions recommend catch-up or an additional dose of meningococcal vaccine to adolescents and young adults who are attending post-secondary studies or living in close quarters, including university students living in residential colleges and residential accommodation. The United States, United Kingdom, Australia and New Zealand identify post-secondary students, particularly those during the first year of attendance and those residing in close-living situations, as being at increased risk of IMD and have recommended vaccinationFootnote 14Footnote 15Footnote 16Footnote 17. Increased relative risk for serogroup B IMD in these jurisdictions has previously been estimated to be approximately three times higher for students compared to non-students in the same age groupFootnote 18Footnote 19.
Epidemiology of invasive meningococcal disease in Canada, 2012–2022
NACI reviewed the epidemiological risks associated with different serogroups of IMD in Canada by age group and geography. Since the introduction of meningococcal immunization programs in the early 2000s, the epidemiology of IMD in Canada has changed significantly. The incidence of IMD due to serogroup C declined by 93% and the overall IMD incidence declined by 55% from the pre-vaccine era to 2015Footnote 20.
Between 2012 and 2022, there were a total of 1,196 cases of IMD reported in Canada. Overall, the mean incidence of IMD during this period was 0.31 cases per 100,000 population per year (Table 1); however, the distribution according to the number of cases, incidence rates and serogroups varied substantially across age groups and PTs (Figure 1).
| Age group (years) |
2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020Footnote b | 2021Footnote b | 2022Footnote b | 2012–2022 (mean) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Younger than 1 | 3.42 | 3.65 | 4.96 | 2.86 | 1.56 | 3.38 | 4.20 | 4.46 | 2.16 | 1.94 | 1.63 | 3.11 |
| 1–4 | 1.18 | 1.17 | 0.97 | 0.91 | 0.84 | 0.51 | 0.83 | 1.02 | 0.65 | 0.39 | 0.60 | 0.82 |
| 5–9 | 0.44 | 0.27 | 0.10 | 0.05 | 0.20 | 0.20 | 0.05 | 0.15 | 0.20 | 0.10 | 0.15 | 0.17 |
| 10–14 | 0.68 | 0.16 | 0.11 | 0.21 | 0.10 | 0.16 | 0.05 | 0.10 | 0.00 | 0.10 | 0.28 | 0.18 |
| 15–19 | 1.17 | 0.73 | 0.84 | 0.66 | 0.52 | 0.71 | 0.47 | 0.47 | 0.14 | 0.19 | 0.47 | 0.58 |
| 20–24 | 0.51 | 0.25 | 0.17 | 0.33 | 0.25 | 0.79 | 0.37 | 0.53 | 0.36 | 0.24 | 0.28 | 0.37 |
| 25–29 | 0.25 | 0.25 | 0.12 | 0.12 | 0.16 | 0.08 | 0.12 | 0.39 | 0.15 | 0.11 | 0.18 | 0.18 |
| 30–39 | 0.13 | 0.13 | 0.06 | 0.15 | 0.10 | 0.10 | 0.16 | 0.20 | 0.19 | 0.07 | 0.09 | 0.13 |
| 40–59 | 0.31 | 0.16 | 0.18 | 0.20 | 0.13 | 0.15 | 0.31 | 0.20 | 0.19 | 0.09 | 0.15 | 0.19 |
| 60 and older | 0.28 | 0.42 | 0.22 | 0.33 | 0.41 | 0.42 | 0.52 | 0.42 | 0.20 | 0.06 | 0.14 | 0.31 |
| Overall (crude rate) | 0.45 | 0.35 | 0.29 | 0.30 | 0.27 | 0.33 | 0.37 | 0.37 | 0.23 | 0.13 | 0.21 | 0.30 |
|
||||||||||||
Figure 1: Serogroup distribution of invasive meningococcal disease case isolatesFootnote a by province/regions, 2015–2020Footnote b
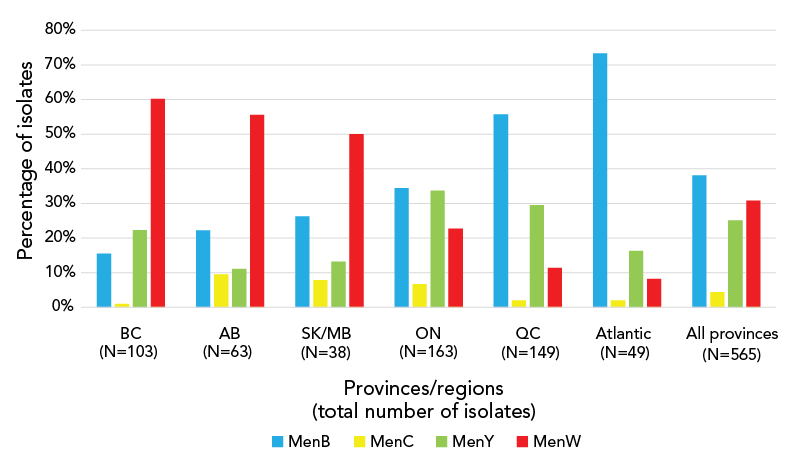
Figure 1 - Text description
Percent of isolates |
Provinces/regions (total number of isolatesFootnote a) | ||||||
|---|---|---|---|---|---|---|---|
| BC (N=103) |
AB (N=63) |
SK/MB (N=38) |
ON (N=163) |
QC (N=149) |
Atlantic provinces (N=49) |
All provinces (N=565) |
|
| B | 16% | 22% | 26% | 34% | 56% | 73% | 38% |
| C | 1% | 10% | 8% | 7% | 2% | 2% | 4% |
| Y | 20% | 11% | 13% | 34% | 30% | 16% | 25% |
| W | 60% | 56% | 50% | 23% | 11% | 8% | 31% |
When considering age, the highest annual incidence rates between 2012 and 2022 were observed for infants younger than one year of age (mean incidence: 3.11 cases per 100,000 population), followed by children 1–4 years of age (0.82 cases per 100,000 population). Adolescents 15–19 years of age and young adults 20–24 years of age had slightly lower mean incidence compared to children 1–4 years of age at 0.58 cases per 100,000 population and 0.37 cases per 100,000 population, respectively. From 2012 to 2022, children younger than five years of age accounted for the largest number of cases (N=265, or 23% of total IMD cases) followed by adolescents 15–19 years of age (N=138, or 12% of total IMD cases) and adults 20–24 years of age (N=98, or 8% of total IMD cases).
Between 2012 and 2022, the highest incidence of IMD was serogroup B (0.14 cases per 100,000 population), followed by serogroup W and serogroup Y (both 0.06 cases per 100,000 population, respectively). Serogroup B incidence was highest in children younger than one year of age and children in the 1–4 years age group (2.03 and 0.59 cases per 100,000 population, respectively), followed by serogroup W in children younger than one year of age (0.48 cases per 100,000 population) and serogroup B in the 15–19 and 20–24 years age groups (0.34 and 0.17 cases per 100,000 population, Table 2). During this period, the highest number of cases were reported for serogroup B in the younger than five (N=189), 15–19 (N=79) and 20–24 (N=46) years age groups. This was followed by cases caused by serogroups W and Y in children younger than five years of age (N=39 serogroup W cases), adolescents 15–19 years of age (N=15 and N=30 serogroup W and Y cases, respectively) and young adults 20–24 years of age (N=18 and N=23 serogroup W and Y cases, respectively).
| Serogroup | Age group (years) | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Younger than 1 | 1–4 | 5–9 | 10–14 | 15–19 | 20–24 | 25–29 | 30–39 | 40–59 | 60 and older | ||
| B | 2.03 | 0.59 | 0.12 | 0.10 | 0.34 | 0.17 | 0.10 | 0.05 | 0.06 | 0.09 | 0.14 |
| C | 0.07 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.00 | 0.02 | 0.02 | 0.02 | 0.02 |
| W | 0.48 | 0.09 | 0.01 | 0.01 | 0.06 | 0.06 | 0.04 | 0.02 | 0.05 | 0.08 | 0.06 |
| Y | 0.17 | 0.02 | 0.01 | 0.03 | 0.13 | 0.07 | 0.03 | 0.02 | 0.05 | 0.09 | 0.06 |
| Non-groupable | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.001 |
| OtherFootnote b | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.003 |
| UnknownFootnote c | 0.24 | 0.06 | 0.01 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
|
|||||||||||
Most PTs had an annual mean IMD incidence rate of less than 0.50 cases per 100,000 population (Table 3). However, the age-standardized incidence rates were highest in NU (1.15 cases per 100,000 population per year, 95% CI: 0.28–2.00), followed by NT (0.49 cases per 100,000 population per year, 95% CI: 0.07–1.10), NS (0.47 cases per 100,000 population per year, 95% CI: 0.27–0.66) and QC (0.44 cases per 100,000 population, 95% CI: 0.29–0.59).
| Provinces and territories | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2012–2022 Mean(95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| British Columbia | 0.36 | 0.24 | 0.29 | 0.22 | 0.18 | 0.54 | 0.52 | 0.52 | 0.25 | 0.13 | 0.08 | 0.30 (0.19–0.41) |
| Alberta | 0.41 | 0.35 | 0.22 | 0.28 | 0.19 | 0.20 | 0.58 | 0.32 | 0.28 | 0.19 | 0.10 | 0.28 (0.20–0.37) |
| Saskatchewan | 0.26 | 0.24 | 0.24 | 0.07 | 0.16 | 0.26 | 0.21 | 0.17 | 0.23 | 0.1 | 0.27 | 0.20 (0.15–0.25) |
| Manitoba | 0.16 | 0.66 | 0.23 | 0.36 | 0.60 | 0.41 | 0.37 | 0.48 | 0.27 | 0.34 | 0.53 | 0.40 (0.30–0.51) |
| Ontario | 0.26 | 0.17 | 0.19 | 0.25 | 0.20 | 0.22 | 0.26 | 0.25 | 0.16 | 0.07 | 0.17 | 0.20 (0.16–0.24) |
| Québec | 0.92 | 0.74 | 0.47 | 0.43 | 0.40 | 0.41 | 0.29 | 0.44 | 0.30 | 0.14 | 0.28 | 0.44 (0.29–0.59) |
| New Brunswick | 0.83 | 0.45 | 0.26 | 0.72 | 0 | 0.16 | 0.95 | 0.58 | 0 | 0.31 | 0.08 | 0.39 (0.17–0.62) |
| Nova Scotia | 0.10 | 0 | 0.35 | 0.82 | 0.44 | 0.66 | 0.70 | 0.60 | 0.34 | 0.26 | 0.85 | 0.47 (0.27–0.66) |
| Newfoundland and Labrador | 0.24 | 0 | 0.41 | 0 | 0.68 | 0.62 | 0 | 0.71 | 0.45 | 0.23 | 0.41 | 0.34 (0.16–0.52) |
| Prince Edward Island | 0.76 | 0 | 0 | 0.70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.13 (0.07–0.33) |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 2.30 | 0 | 0 | 0 | 0 | 0.21 (0.03–0.67) |
| Northwest Territories | 1.79 | 0 | 0 | 0 | 1.87 | 0 | 0 | 1.76 | 0 | 0 | 0 | 0.49 (0.07–1.10) |
| Nunavut | 1.34 | 0 | 3.74 | 0 | 0 | 1.28 | 2.51 | 2.36 | 0 | 1.38 | 0 | 1.15 (0.28–2.00) |
|
||||||||||||
Recently, trends in geographical differences across Canadian jurisdictions have been observed for prevalent serogroups. Between 2015–2020, culture-confirmed IMD due to serogroup W was common in Western Canada, accounting for more cases (60.2% and 55.6% of IMD cases in BC and AB, respectively) than all other serogroups combined. In contrast, IMD due to serogroup B was more common Eastern Canada in QC and Atlantic Canada, accounting for 55.3% and 73.3% of IMD cases, respectively (Figure 1).
Overall, the majority of IMD cases in Canada occurred in the fall and winter months with the peak onset observed in the month of January (N=116, 11.8%), March (N=111, 11.3%) and December (N=97, 9.9%) (Figure 2).
Figure 2: Number of invasive meningococcal disease cases in Canada by month, 2012–2022 (N=979Footnote a)
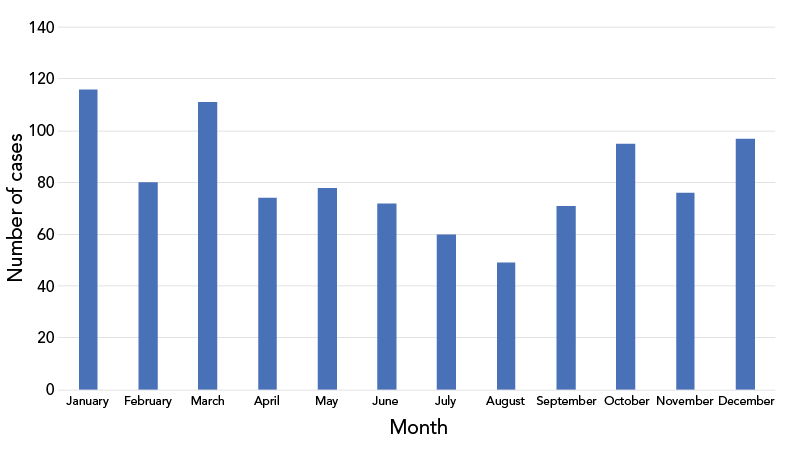
Figure 2 - Text description
| Month | Cases |
|---|---|
| January | 116 |
| February | 80 |
| March | 111 |
| April | 74 |
| May | 78 |
| June | 72 |
| July | 60 |
| August | 49 |
| September | 71 |
| October | 95 |
| November | 76 |
| December | 97 |
Based on the genetic testing of IMD strains from Canadian provinces that was conducted by the National Microbiology Laboratory for the period from 2010 to 2020, over 90% of serogroup B strains have been predicted to have a sufficient antigen expression that would elicit an immune response in individuals vaccinated with either of the two currently authorised serogroup B vaccinesFootnote 21Footnote 22Footnote 23. Similarly, based on the level of bacterial antigen surface expression determined by the meningococcal antigen typing system, 4CMenB vaccine was previously predicted to confer protection against a high proportion of serogroup B IMD isolates collected between 2010 and 2014 from all parts of CanadaFootnote 21.
Vaccine protection against invasive meningococcal disease
High levels of antibody are important for protection against IMD due to the rapid disease progression and because bactericidal activity (the presumed primary immunologic mechanism of protection) is predominantly achieved through antibody-mediated complement activationFootnote 24Footnote 25. Available data suggest that protection against IMD decreases in many adolescents and young adults within five years following immunization with conjugate meningococcal vaccinesFootnote 14Footnote 17Footnote 26Footnote 27Footnote 28. While vaccine effectiveness data are limited for serogroup B vaccines, it is likely that over 50% of vaccine recipients maintain protection up to four years post immunizationFootnote 13Footnote 29Footnote 30Footnote 31Footnote 32Footnote 33Footnote 34. Serogroup B vaccines are also likely to broaden the protection against non-B serogroups expressing the vaccine-contained antigens. While not authorized for this indication, 4CMenB may also provide some cross-protection against Neisseria gonorrhoeae Footnote 29Footnote 35Footnote 36Footnote 37. However, neither of the serogroup B vaccines appear to have an effect on carriage and, consequently, herd immunityFootnote 38Footnote 39Footnote 40.
Invasive meningococcal disease risk assessment and program-relevant considerations
In addition to the assessment of the national disease burden, vaccine characteristics and existing PT immunization programs, NACI also considered EEFA program-relevant elements in the context of the Canadian Immunization Guide definitions and NACI recommendations for high-risk groups due to increased risk of exposure.
Based on the principle of equity and ethics, it was acknowledged that all population groups identified as being at high risk of exposure should be equally considered for, and have access to, vaccination against IMD. However, it was also recognized that, given the very small number of cases, as well as due to high cost of meningococcal vaccines, immunization of all individuals who may be at increased risk of exposure may not be equally feasible across PTs.
In general, NACI concluded that, given the diversity of the Canadian health care system and differences in the individual PT disease burden, it was important to allow flexibility for PTs to make individual decisions about which populations they wish to prioritize for immunization. However, while the level of risk is influenced by local epidemiology and the timing and type of adolescent PT programs, it was recognised that any permissive recommendations should not lead to increased inequity relative to vaccine access (e.g. depending on the place of residence or ability of high-risk individuals to purchase the recommended vaccines).
To support PTs in their decision making, NACI provided an outline of program-relevant elements to be considered when assessing the population-group risk and deciding on whether an IMD program for that population is warranted (Table 4).
| Program-relevant factors | Elements for consideration |
|---|---|
| Epidemiology and risk factors |
|
| Vaccine characteristics |
|
| Ethics and equity |
|
| Feasibility |
|
| Acceptability |
|
| Others |
|
|
|
Conclusion
In Canada, the age groups with the highest incidence of IMD include children younger than five years of age, followed by people 15–24 years of age. Determining particular risk factors (e.g. those associated with a particular activity or setting) beyond age and underlying medical conditions is challenging due to the limitations of currently collected data. Given that IMD epidemiology varies across the country, jurisdictions with higher incidence in specific population groups may therefore consider introducing targeted programs (e.g. offering a serogroup-appropriate meningococcal vaccine to an age group with a higher incidence of IMD), which may also include populations that are believed to be at higher risk of exposure (e.g. students residing in congregate settings or children and adolescents living in regions with circulating hypervirulent clones). When planning targeted programs, consideration should be given to the specific regional circulating strains and epidemiology.
Due to the PT differences in circulating strains and epidemiology, NACI concluded that recommending a single pan-Canadian program targeting additional population groups at high risk of exposure would be challenging and that regional programs may be better suited to address the currently circulating serogroups and prevent IMD in population groups considered to be at high risk of exposure.
While much is known about IMD, further studies are needed to better understand the contemporary risk factors of IMD in high-incidence population groups (including adolescents and young adults) in Canada, including activities and settings associated with bacterial acquisition, carriage and transmission. In addition, further research is needed with regards to estimating the true cost of IMD and meningococcal infections in Canada, including those associated with the absence of immunization programs (e.g. costs associated with contact tracing, school disruptions, outbreak management, etc.). Robust surveillance systems with enhanced data collection are required for the continuous monitoring of vaccine-preventable diseases, program evaluation and timely adjustment of recommendations that are focused on equity.
Authors’ statement
- APH — Review, editing
- JZ — Writing, original draft, review, editing
- CP — Writing, review, editing
- OB — Writing, review, editing
- MS — Review, editing
- SD — Review, editing
The update was prepared by J Zafack, C Primeau, A Pham-Huy and S Deeks, on behalf of the National Advisory Committee on Immunization (NACI) Invasive Meningococcal Disease Working Group and was approved by NACI.
Competing interests
None.
Acknowledgements
The National Advisory Committee on Immunization (NACI) acknowledges and appreciates the contribution of R Tsang, K Franklin, L Coward, S Kelly, M Tunis, and K Young to this update.
NACI IMD Working Group
Members: A Pham-Huy (Chair), J Bettinger, Y Bui, S Crowe, P De Wals, V Dubey, J Embree, M Lavoie, C Muecke, M O’Driscoll, and M Sadarangani.
PHAC participants: O Baclic, H Birdi, G Coleman, L Coward, K Franklin, C Primeau, M Salvadori, F Schwarz, R Tsang, K Young, and J Zafack.
NACI
Members: S Deeks (Chair), R Harrison (Vice Chair), R Harrison (Vice-Chair), M Andrew, J Bettinger, N Brousseau, H Decaluwe, P De Wals, E Dubé, V Dubey, K Hildebrand, K Klein, M O’Driscoll, J Papenburg, A Pham-Huy, B Sander, and S Wilson.
Liaison representatives: L Bill/M Nowgesic (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), S Buchan (Canadian Association for Immunization Research and Evaluation), E Castillo (Society of Obstetricians and Gynaecologists of Canada), J Comeau (Association of Medical Microbiology and Infectious Disease Canada), M Lavoie (Council of Chief Medical Officers of Health), J MacNeil (Centers for Disease Control and Prevention, United States), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), M Osmack (Indigenous Physicians Association of Canada), J Potter (College of Family Physicians of Canada), and A Ung (Canadian Pharmacists Association).
Ex-officio representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (Centre for Immunization and Respiratory Infectious Diseases [CIRID], Public Health Agency of Canada [PHAC]), M Lacroix (Public Health Ethics Consultative Group, PHAC), P Fandja (Marketed Health Products Directorate, Health Canada), M Su (COVID-19 Epidemiology and Surveillance, PHAC), S Ogunnaike-Cooke (CIRID, PHAC), C Pham (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), M Routledge (National Microbiology Laboratory, PHAC) and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
Funding
The work of the National Advisory Committee on Immunization is supported by the Public Health Agency of Canada.
References
- Footnote 1
-
Public Health Agency of Canada. Invasive Meningococcal Disease: For Health Professionals. Ottawa, ON: PHAC; 2014. [Accessed 2023 Jul 12]. https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/invasive-meningococcal-disease/health-professionals.html
- Footnote 2
-
Gorman M. Meningitis outbreak declared at Dalhousie University residence following student death. CBC. 2022, Dec 16. [Accessed 2023 Jul 12]. https://www.cbc.ca/news/canada/nova-scotia/meningitis-outbreak-dalhousie-residence-1.6688166
- Footnote 3
-
Government of Nova Scotia. More Nova Scotians Eligible for Meningococcal B Vaccine. News Releases. Halifax, NS: Government of NS; 2023. [Accessed 2023 Jul 12]. https://novascotia.ca/news/release/?id=20230525003
- Footnote 4
-
Government of Prince Edward Island. Free meningitis vaccine available to post-secondary students living in residence. Charlottetown, PE: Government of PE; 2023. [Accessed 2023 Jul 12]. https://www.princeedwardisland.ca/en/news/free-meningitis-vaccine-available-to-post-secondary-students-living-in-residence
- Footnote 5
-
Saboui M, Tsang RS, MacTavish R, Agarwal A, Li YA, Salvadori MI, Squires SG. Epidemiology of invasive meningococcal disease in Canada, 2012-2019. Can Commun Dis Rep 2022;48(5):228–36. https://doi.org/10.14745/ccdr.v48i05a06
- Footnote 6
-
Henry B; National Advisory Committee on Immunization. Summary of the National Advisory Committee on Immunization’s Update on quadrivalent meningococcal vaccines available in Canada. Can Commun Dis Rep 2015;41 Suppl 3:17–8. https://doi.org/10.14745/ccdr.v41is3a05
- Footnote 7
-
Public Health Agency of Canada. Meningococcal vaccine: Canadian Immunization Guide: For health professionals. Ottawa, ON: PHAC; 2023. [Accessed 2023 Jul 12]. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-13-meningococcal-vaccine.html
- Footnote 8
-
Public Health Agency of Canada. Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada. Ottawa, ON: PHAC; 2023. [Accessed 2023 Jul 12]. https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html
- Footnote 9
-
Ministère de la Santé et des Services Sociaux. Men-C-ACWY: quadrivalent meningococcal Conjugate vaccine. Quebec, QC: MSSS; 2023. [Accessed 2023 Jul 12]. https://www.msss.gouv.qc.ca/professionnels/vaccination/piq-vaccins/men-c-acwy-vaccin-conjugue-quadrivalent-contre-le-meningocoque/
- Footnote 10
-
Public Health Agency of Canada. Vaccination Coverage Goals and Vaccine Preventable Disease Reduction Targets by 2025. Ottawa, ON: PHAC; 2022. [Accessed 2023 Jul 12]. https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/national-immunization-strategy/vaccination-coverage-goals-vaccine-preventable-diseases-reduction-targets-2025.html
- Footnote 11
-
Statistics Canada. Childhood National Immunization Coverage Survey, 2021. Ottawa, ON: StatCan; 2023. [Accessed 2023 Jul 12]. https://www150.statcan.gc.ca/n1/daily-quotidien/230612/dq230612b-eng.htm
- Footnote 12
-
De Wals P. Epidemiology and Control of Meningococcal Disease in Canada: A Long, Complex, and Unfinished Story. Can J Infect Dis Med Microbiol 2019;2019:8901847. https://doi.org/10.1155/2019/8901847
- Footnote 13
-
Deceuninck G, Lefebvre B, Tsang R, Betala-Belinga JF, De Serres G, De Wals P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine 2019;37(31):4243–5. https://doi.org/10.1016/j.vaccine.2019.06.021
- Footnote 14
-
Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep 2020;69(9):1–41. https://doi.org/10.15585/mmwr.rr6909a1
- Footnote 15
-
Australian Government, Department of Health and Aged Care. The Australian Immunisation Handbook. Adolescents and young adults living in close quarters are recommended to receive MenACWY and MenB vaccines. Canberra (AU): Government of AU; 2023. [Accessed 2023 Jul 12]. https://immunisationhandbook.health.gov.au/recommendations/adolescents-and-young-adults-living-in-close-quarters-are-recommended-to-receive-menacwy-and-menb-vaccines
- Footnote 16
-
Ministry of Health New Zealand. Immunization Handbook 2020. Chapter 13. Meningococcal disease. Wellington (NZ): Government of NZ; 2023. [Accessed 2023 Jul 12]. https://www.health.govt.nz/our-work/immunisation-handbook-2020/13-meningococcal-disease
- Footnote 17
-
UK Health Security Agency. The Green Book: information for public health professionals on immunisation. Chapter 22: Meningococcal: Meningococcal meningitis and septicaemia notifiable. London (UK): Government of UK; 2020. [Accessed 2023 Jul 12]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1076053/Meningococcal-greenbook-chapter-22_17May2022.pdf
- Footnote 18
-
Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal Disease Among College-Aged Young Adults: 2014-2016. Pediatrics 2019;143(1):e20182130. https://doi.org/10.1542/peds.2018-2130
- Footnote 19
-
Mandal S, Campbell H, Ribeiro S, Gray S, Carr T, White J, Ladhani SN, Ramsay ME. Risk of invasive meningococcal disease in university students in England and optimal strategies for protection using MenACWY vaccine. Vaccine 2017;35(43):5814–8. https://doi.org/10.1016/j.vaccine.2017.09.024
- Footnote 20
-
Public Health Agency of Canada. Vaccine Preventable Disease Surveillance Report to December 31, 2015. Ottawa, ON: PHAC; 2017. [Accessed 2023 Jul 12]. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/vaccine-preventable-disease-surveillance-report-december-31-2015/vaccine-preventable-disease-eng.pdf
- Footnote 21
-
Tsang RS, Law DK, De Paola R, Giuliani M, Stella M, Zhou J, Deng S, Boccadifuoco G, Giuliani MM, Serino L. Culture-Confirmed Invasive Meningococcal Disease in Canada, 2010 to 2014: Characterization of Serogroup B Neisseria meningitidis Strains and Their Predicted Coverage by the 4CMenB Vaccine. MSphere 2020;5(2):e00883–19. https://doi.org/10.1128/mSphere.00883-19
- Footnote 22
-
Tsang RS, Law DK, Zhou J, Haldane D, Garceau R, Zahariadis G, Mead K, Alexander D. Characterization of invasive meningococcal disease case isolates in Atlantic Canada, 2014 to 2020: spatial-temporal variations of clones and predicted meningococcal B vaccine coverage. J Med Microbiol 2022;71(12). https://doi.org/10.1099/jmm.0.001615
- Footnote 23
-
Bettinger JA, Liberator P, Halperin SA, Vaudry W, Sadarangani M, Hao L, Lambert N, Jansen KU, Anderson AS, Tsang R; members of the Canadian Immunization Monitoring Program, Active (IMPACT). Estimated susceptibility of Canadian meningococcal B isolates to a meningococcal serogroup B vaccine (MenB-FHbp). Vaccine 2020;38(8):2026–33. https://doi.org/10.1016/j.vaccine.2019.12.051
- Footnote 24
-
Lewis LA, Ram S. Complement interactions with the pathogenic Neisseriae: clinical features, deficiency states, and evasion mechanisms. FEBS Lett 2020;594(16):2670–94. https://doi.org/10.1002/1873-3468.13760
- Footnote 25
-
Krüger S, Eichler E, Strobel L, Schubert-Unkmeir A, Johswich KO. Differential influences of complement on neutrophil responses to Neisseria meningitidis infection. Pathog Dis 2018;76(8). https://doi.org/10.1093/femspd/fty086
- Footnote 26
-
Baxter R, Reisinger K, Block SL, Izu A, Odrljin T, Dull P. Antibody persistence and booster response of a quadrivalent meningococcal Conjugate vaccine in adolescents. J Pediatr 2014;164(6):1409–15.e4. https://doi.org/10.1016/j.jpeds.2014.02.025
- Footnote 27
-
Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE; Active Bacterial Core Surveillance (ABCs) Team and MeningNet Surveillance Partners. Effectiveness and Duration of Protection of One Dose of a Meningococcal Conjugate Vaccine. Pediatrics 2017;139(2):e20162193. https://doi.org/10.1542/peds.2016-2193
- Footnote 28
-
Vesikari T, Forsten A, Laudat F, Li P, Van Der Wielen M, Hezareh M, Perez JL, Webber C. Long-term antibody persistence after a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine in healthy 5-year-old children. Vaccine 2020;38(22):3902–8. https://doi.org/10.1016/j.vaccine.2020.02.030
- Footnote 29
-
Wang B, Giles L, Andraweera P, McMillan M, Almond S, Beazley R, Mitchell J, Ahoure M, Denehy E, Flood L, Marshall H. 4CMenB sustained vaccine effectiveness against invasive meningococcal B disease and gonorrhoea at three years post programme implementation. J Infect 2023;87(2):95–102. https://doi.org/10.1016/j.jinf.2023.05.021
- Footnote 30
-
Flacco ME, Manzoli L, Rosso A, Marzuillo C, Bergamini M, Stefanati A, Cultrera R, Villari P, Ricciardi W, Ioannidis JP, Contopoulos-Ioannidis DG. Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: a systematic review and meta-analysis. Lancet Infect Dis 2018;18(4):461–72. https://doi.org/10.1016/S1473-3099(18)30048-3
- Footnote 31
-
Langley JM, Gantt S, Quach C, Bettinger JA, Halperin SA, Mutch J, McNeil SA, Ward BJ, MacKinnon-Cameron D, Ye L, Marty K, Scheifele D, Brown E, Alcantara J; Canadian Immunization Research Network. Randomized Trial of 2 Schedules of Meningococcal B Vaccine in Adolescents and Young Adults, Canada. Emerg Infect Dis 2020;26(3):454–62. https://doi.org/10.3201/eid2603.190160
- Footnote 32
-
Marshall HS, Richmond PC, Beeslaar J, Jiang Q, Jansen KU, Garcés-Sánchez M, Martinón-Torres F, Szenborn L, Wysocki J, Eiden J, Harris SL, Jones TR, Lee SS, Perez JL; 6108A12001 Study Investigators. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2017;17(1):58–67. https://doi.org/10.1016/S1473-3099(16)30314-0
- Footnote 33
-
Lucidarme J, Bai X, Lekshmi A, Clark SA, Willerton L, Ribeiro S, Campbell H, Serino L, De Paola R, Holland A, Louth J, Ramsay ME, Ladhani SN, Borrow R. Invasive serogroup B meningococci in England following three years of 4CMenB vaccination - First real-world data. J Infect 2022;84(2):136–44. https://doi.org/10.1016/j.jinf.2021.11.015
- Footnote 34
-
McMillan M, Chandrakumar A, Wang HL, Clarke M, Sullivan TR, Andrews RM, Ramsay M, Marshall HS. Effectiveness of Meningococcal Vaccines at Reducing Invasive Meningococcal Disease and Pharyngeal Neisseria meningitidis Carriage: A Systematic Review and Meta-analysis. Clin Infect Dis 2021;73(3):e609–19. https://doi.org/10.1093/cid/ciaa1733
- Footnote 35
-
Ruiz García Y, Sohn WY, Seib KL, Taha MK, Vázquez JA, de Lemos AP, Vadivelu K, Pizza M, Rappuoli R, Bekkat-Berkani R. Looking beyond meningococcal B with the 4CMenB vaccine: the Neisseria effect. NPJ Vaccines 2021;6(1):130. https://doi.org/10.1038/s41541-021-00388-3
- Footnote 36
-
Abara WE, Bernstein KT, Lewis FM, Schillinger JA, Feemster K, Pathela P, Hariri S, Islam A, Eberhart M, Cheng I, Ternier A, Slutsker JS, Mbaeyi S, Madera R, Kirkcaldy RD. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: a retrospective observational study. Lancet Infect Dis 2022;22(7):1021–9. https://doi.org/10.1016/S1473-3099(21)00812-4
- Footnote 37
-
Fazio C, Biolchi A, Neri A, Tomei S, Vacca P, Ambrosio L, Palmieri A, Mori E, La Gaetana R, Pizza M, Giuliani MM, Serino L, Stefanelli P. Cross-reactivity of 4CMenB vaccine-induced antibodies against meningococci belonging to non-B serogroups in Italy. Hum Vaccin Immunother 2021;17(7):2225–31. https://doi.org/10.1080/21645515.2020.1855951
- Footnote 38
-
Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, Maiden MC, Ladhani SN, Ramsay ME, Trotter C, Borrow R, Finn A, Kahler CM, Whelan J, Vadivelu K, Richmond P, Meningococcal B. Meningococcal B Vaccine and Meningococcal Carriage in Adolescents in Australia. N Engl J Med 2020;382(4):318–27. https://doi.org/10.1056/NEJMoa1900236
- Footnote 39
-
Perez JL, Absalon J, Beeslaar J, Balmer P, Jansen KU, Jones TR, Harris S, York LJ, Jiang Q, Radley D, Anderson AS, Crowther G, Eiden JJ. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines 2018;17(6):461–77. https://doi.org/10.1080/14760584.2018.1483726
- Footnote 40
-
Sohn WY, Tahrat H, Novy P, Bekkat-Berkani R. Real-world implementation of 4-component meningococcal serogroup B vaccine (4CMenB): implications for clinical practices. Expert Rev Vaccines 2022;21(3):325–35. https://doi.org/10.1080/14760584.2022.2021881
- Footnote 41
-
Marshall GS, Abbing-Karahagopian V, Marshall HS, Cenci S, Conway JH, Occhipinti E, Bekkat-Berkani R, Banzhoff A, Sohn WY. A comprehensive review of clinical and real-world safety data for the four-component serogroup B meningococcal vaccine (4CMenB). Expert Rev Vaccines 2023;22(1):530–44. https://doi.org/10.1080/14760584.2023.2222015
- Footnote 42
-
Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike IO, Oeser C, Kent A, Oliver J, Taylor P, Morales-Aza B, Clarke SC, Casey M, Martins F, Kitchin NR, Anderson AS, Jones H, Jansen KU, Eiden J, Pedneault L, Heath PT, Finn A, Faust SN, Pollard AJ. Meningococcal Carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect 2015;71(1):43–52. https://doi.org/10.1016/j.jinf.2015.02.006
- Footnote 43
-
Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, Mbaeyi SA, Serogroup B; Serogroup B Meningococcal Disease University Outbreak Group. University-Based Outbreaks of Meningococcal Disease Caused by Serogroup B, United States, 2013-2018. Emerg Infect Dis 2019;25(3):434–40. https://doi.org/10.3201/eid2503.181574
- Footnote 44
-
Soumahoro L, Abitbol V, Vicic N, Bekkat-Berkani R, Safadi MA. Meningococcal Disease Outbreaks: A Moving Target and a Case for Routine Preventative Vaccination. Infect Dis Ther 2021;10(4):1949–88. https://doi.org/10.1007/s40121-021-00499-3
- Footnote 45
-
Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal Carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010;10(12):853–61. https://doi.org/10.1016/S1473-3099(10)70251-6
- Footnote 46
-
Asturias EJ, Bai X, Bettinger JA, Borrow R, Castillo DN, Caugant DA, Chacon GC, Dinleyici EC, Echaniz-Aviles G, Garcia L, Glennie L, Harrison LH, Howie RL, Itsko M, Lucidarme J, Marin JE, Marjuki H, McNamara LA, Mustapha MM, Robinson JL, Romeu B, Sadarangani M, Sáez-Llorens X, Sáfadi MA, Stephens DS, Stuart JM, Taha MK, Tsang RS, Vazquez J, De Wals P. Meningococcal disease in North America: Updates from the Global Meningococcal Initiative. J Infect 2022;85(6):611–22. https://doi.org/10.1016/j.jinf.2022.10.022
- Footnote 47
-
World Health Organization. Defeating meningitis by 2030. Geneva (CH): WHO. [Accessed 2023 Jul 12]. https://www.who.int/initiatives/defeating-meningitis-by-2030
