A PICNIC analysis of Haemophilus influenzae bacteremia in children

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 49-9, September 2023: Common Infectious Diseases Caused by Bacteria
Date published: September 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-9, September 2023: Common Infectious Diseases Caused by Bacteria
Epidemiologic Study
A Pediatric Investigators Collaborative Network on Infections in Children (PICNIC) multi-centre Canadian descriptive analysis of Haemophilus influenzae bacteremia in children: Emerging serotypes
Craig Frankel1, Joan Robinson2, Sarah Khan3, Mohammad Alghounaim4, Jane McDonald4, Alison Lopez5, Sergio Fanella5, John Gunawan2, Jacqueline Wong3, Jeannette Comeau6, Jennifer Bowes7, Robert Slinger7, Angela Kalia8, Ashley Roberts8, Kirk Leifso9, Marina Ulanova10, Michelle Barton1
Affiliations
1 Department of Paediatrics, Children’s Hospital, London Health Sciences Centre, London, ON
2 Department of Pediatrics, Stollery Children’s Hospital, Edmonton, AB
3 Department of Pediatrics, McMaster Children’s Hospital, Hamilton, ON
4 Department of Pediatrics, Montreal Children’s Hospital, Montréal, QC
5 Department of Pediatrics and Child Health, Winnipeg Children’s Hospital, Winnipeg, MB
6 Department of Pediatrics, IWK Health Centre, Halifax, NS
7 Department of Pediatrics, Children’s Hospital of Eastern Ontario, Ottawa, ON
8 Department of Pediatrics, BC Children’s Hospital, Vancouver, BC
9 Department of Pediatrics, Kingston Health Sciences Centre, Kingston, ON
10 Northern Ontario School of Medicine University, Thunder Bay, ON
Correspondence
Suggested citation
Frankel C, Robinson J, Khan S, Alghounaim M, McDonald J, Lopez A, Fanella S, Gunawan J, Wong J, Comeau JL, Bowes J, Slinger R, Kalia A, Roberts A, Leifso K, Ulanova M, Barton M. A Pediatric Investigators Collaborative Network on Infections in Children (PICNIC) multi-centre Canadian descriptive analysis of Haemophilus influenzae bacteremia in children: Emerging serotypes. Can Comm Dis Rep 2023;49(9):368−74. https://doi.org/10.14745/ccdr.v49i09a02
Keywords: Haemophilus influenzae, invasive disease, bacteremia, meningitis, serotype a, serotype b, serotype f, non-typeable, children
Abstract
Background: There has been dramatic reduction in Haemophilus influenzae serotype b (Hib) since introduction of Hib vaccines, but children still experience serious invasive Haemophilus influenzae (Hi) disease caused by various serotype and non-typeable bacteria. The object of this study was to describe the serotype distribution and clinical spectrum of Hi bacteremia in children admitted to Canadian hospitals.
Methods: All children with Hi bacteremia admitted 2013 through 2017 to 10 centres across Canada were included. Demographic, clinical, treatment and outcome data were collected.
Results: Haemophilus influenzae bacteremia occurred in 118 children of median age 12 months (inter-quartile range: 7–48 months). Forty-three (36%) isolates were non-typeable (NTHi) and 8 were not typed. Of the 67 typeable (THi), Hia (H. influenzae serotype a) (n=36, 54%), Hif (serotype f) (n=19, 26%) and Hib (serotype b) (n=9, 13%) dominated. The THi was more likely than NTHi bacteremia to present as meningitis (p<0.001), particularly serotype a (p=0.04) and less likely to present as pneumonia (p<0.001). Complicated disease (defined as intensive care unit admission, need for surgery, long-term sequelae or death) occurred in 31 (26%) cases and were more likely to have meningitis (p<0.001) than were those with uncomplicated disease.
Conclusion: In the era of efficacious conjugate Hib vaccines, NTHi, Hia and Hif have emerged as the leading causes of invasive Hi in Canadian children, with Hia being most likely to result in meningitis and complicated disease. A vaccine for all NTHi and THi would be ideal, but knowledge of the current disease burden from circulating strains will inform prioritization of vaccine targets.
Introduction
Haemophilus influenzae serotype b (Hib) was overwhelmingly the leading cause of invasive Hi disease until the introduction of the Hib vaccines into the routine childhood immunization schedule in the United States (US) and Canada in the late 1980sFootnote 1. This was followed by the emergence of Hi serotype a, particularly in Indigenous children in Canada and in AlaskaFootnote 2Footnote 3Footnote 4. Recent publications from the US reported an increase in the incidence of invasive Hi disease in children, possibly due to an increase in the incidence of cases due to non-typeable Hi (NTHi)Footnote 5Footnote 6. We sought to describe the serotype distribution and clinical spectrum of Hi bacteremia in Canadian children across several provinces and to determine factors associated with complicated disease.
Methods
Study population and design
Ten centres within the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) retrospectively enrolled all hospitalized children younger than 18 years of age with blood culture isolates of Hi from January 1, 2013, through December 31, 2017. For nine centres (London, Ontario; Hamilton, Ontario; Ottawa, Ontario; Kingston, Ontario; Winnipeg, Manitoba; Edmonton, Alberta; Vancouver, British Columbia; Halifax, Nova Scotia and Montréal, Québec), this was a sub-study arising from a retrospective cohort of gram-negative bacteremia, while the tenth centre (Sioux Lookout, Ontario) was added for this sub-study due to their known high incidence of Hi infections. Ethics approval was obtained at each participating centre and the need for parental consent was waived.
Study definitions
The focus of infection was classified as meningitis, pneumonia, epiglottitis, skin and soft tissue infection, osteoarticular infection, other or none (isolated bacteremia with no focus). Multifocal disease was defined as bacteremia with two or more foci.
Disease was defined as complicated if any of the following occurred related to Hi disease: intensive care unit (ICU) admission; organ failure; surgical interventions including amputations for purpura fulminans or drainage of purulent collections (arthrocentesis did not qualify unless performed more than once); complications relating to disease focus including motor deficits, seizures, hydrocephalus, visual or hearing deficits, or necrotizing skin or lung infections; and death.
Serotyping
Serotyping of isolates was completed using monovalent antisera at reference laboratories. Strains were classified by capsular type (a to f) or as non-typeable. When serotyping was not available, the isolates were recorded as not typed.
Data collection
Demographic, clinical, microbiological, treatment, outcome and follow-up data were extracted from medical records and entered into REDCap (Research Electronic Data Capture) tools hosted at the University of Alberta by each participating centreFootnote 7.
Statistical analysis
Descriptive analysis was conducted. Chi-square or Fisher’s exact test was used to compare categorical variables and nonparametric tests were used to compare continuous variables. Univariate analysis was used to explore potential factors associated with Hia disease and complicated disease course. Variables with a univariate p value of ≤0.2 and potential confounding factors (e.g. age and sex) were considered for inclusion in multivariable logistic regression model aimed at determining independent risk factors for complicated disease. The IBM SPSS version 28 was used for statistical analysis.
Results
There were 118 cases of Hi bacteremia of which 74 (63%) were male (Table 1). The median age was 12 months (interquartile range [IQR]: 7–48 months) with 7 cases (6%) being neonates and 25 (21%) being 5 years or older.
FeaturesFootnote a |
Total N=118 |
THi (a–f) n=67 |
Hia n=36 |
Hib n=9 |
Hic n=1 |
Hie n=3 |
Hif n=18 |
NTHi n=43 |
Untyped n=8 |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Median age in months, [IQR]Footnote b | 12, [7–48] |
12, [7–36] |
12, [7–24] |
7, [6–12] |
8 | 48, [12–108] |
18, [12–60] |
24, [5–60] |
30, [7–96] |
| Age 1 month or younger | 7 (6%) | 2 (3%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 1 (6%) | 4 (9%) | 1 (13%) |
| Age younger than 12 months | 43 (36%) | 24 (36%) | 14 (39%) | 5 (56%) | 1 (100%) | 0 (0%) | 4 (22%) | 17 (40%) | 2 (25%) |
| Age younger than 24 months | 69 (58%) | 45 (67%) | 26 (72%) | 8 (89%) | 1 (100%) | 1 (33%) | 9 (50%) | 20 (47%) | 4 (50%) |
| Age younger than 60 months | 93 (79%) | 59 (88%) | 32 (89%) | 9 (100%) | 1 (100%) | 2 (67%) | 13 (72%) | 31 (72%) | 5 (63%) |
| Male sex | 74 (63%) | 38 (57%) | 16 (44%) | 9 (100%) | 1 (100%) | 1 (33%) | 11 (61%) | 30 (70%) | 6 (75%) |
| Clinical fociFootnote c | |||||||||
| Meningitis | 25 (21%) | 21 (31%) | 14 (39%) | 3 (33%) | 1 (100%) | 0 (0%) | 3 (17%) | 3 (7%) | 1 (13%) |
| SSTI | 8 (7%) | 8 (12%) | 4 (11%) | 4 (44%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Osteoarticular infection | 6 (5%) | 6 (9%) | 4 (11%) | 2 (22%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pneumonia | 41 (35%) | 13 (19%) | 5 (14%) | 0 (0%) | 0 (0%) | 1 (33%) | 7 (39%) | 23 (53%) | 5 (63%) |
| Epiglottitis | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6%) | 0 (0%) | 0 (0%) |
| Infective endocarditisFootnote d | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Multifocal diseaseFootnote c | 7 (6%) | 7 (10%) | 5 (14%)
1) CNS/IE |
0 (0%) | 0 (0%) | 0 (0%) | 2 (11%) 1) Men/IE |
0 (0%) | 0 (0%) |
| Isolated bacteremia | 29 (25%) | 11 (16%) | 4 (11%) | 0 (0%) | 0 (0%) | 2 (67%) | 5 (28%) | 16 (37%) | 2 (25%) |
| Microbiology | |||||||||
| Number of isolates with laboratory confirmed viral co-infectionFootnote e | 32 (27%) | 14 (21%) | 10 (28%) | 1 (11%) | 0 (0%) | 0 (0%) | 3 (17%) | 15 (35%) | 1 (13%) |
| Number of isolates with ampicillin resistance | 25/106 (24%) | 6/63 (10%) | 0/34 (0%) | 2/8 (25%) | 0/1 (0%) | 0/3 (0%) | 4/17 (24%) | 18/35 (51%) | 1/8 (13%) |
| Outcome | |||||||||
| Median antibiotic durationFootnote a (days), [IQR] | 13.5, [10–23] |
14, [10–28] |
15, [11–29] |
21, [10–31] |
53 | 11, [11–11] |
13, [9–16] |
13, [10–21] |
10, [10–19] |
| ICU admissionFootnote f | 39 (33%) | 21 (31%) | 13 (36%) | 1 (13%) | 1 (100%) | 0 (0%) | 6 (33%) | 14 (33%) | 4 (50%) |
| Complicated courseFootnote f [% with CNS complication] | 31 (26%) [18/31 (58%)] |
24 (36%) [15/24 (63%)] |
14 (39%) [11/14 (79%)] |
2 (22%) [1/2 (50%)] |
1 (100%) [1/1 (100%)] |
0 (0%) [0 (0%)] |
7 (39%) [2/7 (29%)] |
6 (14%) [2/6 (33%)] |
1 (13%) [1/1 (100%)] |
| Median length of stayFootnote g (days), [IQR] | 10, [5–21] |
9, [4–16.5] |
10, [4–18] |
13, [7–20] |
55 | 1, [0–5] |
7, [2–13] |
12, [7–30] |
12, [8–30] |
| Death | 2 (2%) | 2 (3%) | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Death/sequelaeFootnote h | 17 (14%) | 14 (21%) | 9 (25%) | 2 (22%) | 1 (100%) | 0 (0%) | 2 (11%) | 2 (5%) | 1 (13%) |
|
|||||||||
Typing was available for 110 Hi isolates, of which 67 (61%) were typeable Haemophilus influenzae (THi) and 43 (39%) were NTHi. Serotypes a, f and b were the leading serotypes accounting for 36 (54%), 18 (27%) and 9 (13%) of THi respectively (Table 1). Age distribution across serotypes was not significantly different (Table 1). Case numbers were similar across the years of the study (mean: 23.6±7.33), with a peak in 2016 (Figure 1). Centre-specific contributions are shown in Figure 2. Centres in Winnipeg, Montréal and London contributed most Hi cases, with disease predominantly caused by THi.
Figure 1: Haemophilus influenzae serotype distribution by year in paediatric population, Canada
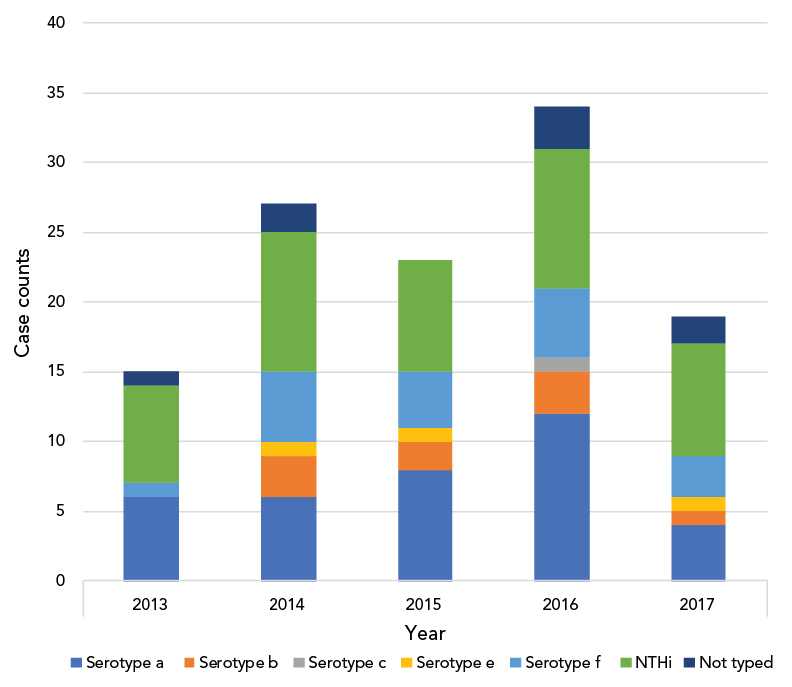
Figure 1 - Text description
| Serotype | Year | ||||
|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | |
| Serotype a | 6 | 6 | 8 | 12 | 4 |
| Serotype b | 0 | 3 | 2 | 3 | 1 |
| Serotype c | 0 | 0 | 0 | 1 | 0 |
| Serotype e | 0 | 1 | 1 | 0 | 1 |
| Serotype f | 1 | 5 | 4 | 5 | 3 |
| NTHi | 7 | 10 | 8 | 10 | 8 |
| Not typed | 1 | 2 | 0 | 3 | 2 |
Figure 2: Serotype-specific distribution of Haemophilus influenzae invasive disease in ten centres across CanadaFootnote a
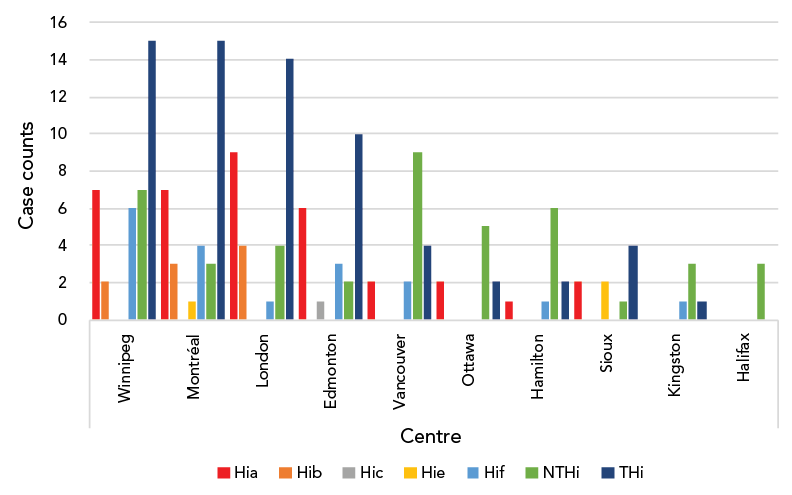
Figure 2 - Text description
| Centre | Hia | Hib | Hic | Hie | Hif | NTHi | THi |
|---|---|---|---|---|---|---|---|
| Winnipeg | 7 | 2 | 0 | 0 | 6 | 7 | 15 |
| Montréal | 7 | 3 | 0 | 1 | 4 | 3 | 15 |
| London | 9 | 4 | 0 | 0 | 1 | 4 | 14 |
| Edmonton | 6 | 0 | 1 | 0 | 3 | 2 | 10 |
| Vancouver | 2 | 0 | 0 | 0 | 2 | 9 | 4 |
| Ottawa | 2 | 0 | 0 | 0 | 0 | 5 | 2 |
| Hamilton | 1 | 0 | 0 | 0 | 1 | 6 | 2 |
| Sioux | 2 | 0 | 0 | 2 | 0 | 1 | 4 |
| Kingston | 0 | 0 | 0 | 0 | 1 | 3 | 1 |
| Halifax | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
Yearly case frequency ranged from 15–34 cases annually with the peak frequency occurring in 2016. Non-typeable H. influenzae followed by serotype a then serotype f disease were the leading causes of Hi disease in 2013, 2014 and 2017. Serotype a followed by NTHi and then serotype f were the leading causes in 2015 and 2016.
Winnipeg, Montréal, London and Edmonton had the most total cases of THi. Serotypes a and f accounted for most cases of THi across all centres, with the exception of London, which identified predominantly cases of serotypes a and b. Frequency of both Hia and Hib were highest in London. Vancouver, Winnipeg, Ottawa and Hamilton had the most cases of NTHi.
Thirty-one (27%) of 116 children with available clinical details (data missing for two cases) had at least one underlying medical condition which included prematurity (n=11; 9%), malignancy (n=11; 9%), immunodeficiency (n=9; 8%) and genetic or metabolic syndrome (n=8; 7%). Of the 108 children with serotyping who had available data, 13/67 (19%) of THi versus 15/41 (35%) with NTHi had an underlying condition (p=0.048). Among six Hib cases with available information on vaccine history, five (83%) had not received any Hib vaccine (n=3) or had inadequate number of doses for age (n=2).
Twenty-five (24%) of 106 isolates with susceptibility reporting available were resistant to ampicillin while none were resistant to ceftriaxone (Table 1). The NTHi isolates were more likely to demonstrate resistance to ampicillin than were THi (n=18/35, 51% vs. n=6/63, 9%; p<0.001).
Among 67 children with THi, 11 (16%) had isolated bacteremia with no focus. Forty-nine had a single focus, including meningitis (n=21), pneumonia (n=13), skin and soft tissue infection (n=8), osteoarticular infection (n=6) and epiglottitis (n=1) (Table 1). Multifocal disease occurred in seven other children (five due to Hia and two due to Hif), with five cases having meningitis as one of the foci.
Among the 43 children with NTHi, 16 (37%) had isolated bacteremia with no focus when compared with 11/67 (16%) of THi (p=0.013). The remaining 27 children had a single focus including pneumonia (n=23), meningitis (n=3) and infective endocarditis (n=1) (Table 1).
Meningitis was more common with THi than NTHi (n=26/67, 39% vs. n=3/43, 7%; p<0.001) and was equally common with Hia and Hib (47% vs. 33%; p>0.05). Pneumonia (as a single focus) was more common with NTHi than THi (n=23/43, 53% vs. n=13/67, 19%; p<0.001).
The median duration of antibiotic therapy in the entire cohort was 13 days [IQR: 10–23] with prolonged duration for osteoarticular infection and meningitis with median duration of 30 days [IQR: 26–41] and 24.5 days [IQR: 12–43], respectively (Table 1). Thirty (26%) of 113 cases with available information who received a median of 14 days (IQR: 11–28) in total were transitioned from parenteral after a median of 7 days (IQR: 4–13) to oral antibiotics to complete remainder as oral therapy.
Complicated disease was more common with THi than with NTHi (n=24/67, 36% vs. n=6/43, 14%; p=0.015). The fatal cases were both caused by Hia and occurred in an infant with meningitis and endocarditis despite no congenital heart disease and in a four-year-old with meningitis complicated by subdural empyema. One infant with Hib meningitis required bilateral limb amputations due to purpura fulminans but survived. The composite outcome of mortality or sequelae at discharge was significantly associated with Hia as compared to non-Hia disease (n=9/36, 25% vs. n=7/74, 9%; p<0.001) (see Appendix).
In the univariate analysis (after adjusting for multiple comparisons), Hi cases with meningitis (p<0.001) were more likely to have a complicated clinical course whereas those with isolated bacteremia were less likely (p<0.001) (Table 2). In the multivariate analysis, meningitis (p<0.001) predicted a complicated disease course after controlling for age (p>0.05) and Hia serotype (p>0.05) (Table 2).
FeaturesFootnote a |
All N=118 |
Complicated disease n=31 |
Uncomplicated disease n=87 |
Significance p<0.006Footnote b |
Multivariate analysisFootnote c | |
|---|---|---|---|---|---|---|
| Odds ratio, [95% CI] | p value | |||||
| Demographics | ||||||
| Males | 74 (63%) | 21 (68%) | 55 (63%) | 0.800 | 1.27, [0.445–3.62] |
0.67 |
| Median age (months), [IQR] | 12, [7–48] |
12, [6–36] |
12, [9–60] |
0.021 | 1.02, [1.00–1.03] |
0.12 |
| Patients younger than 1 year | 43 (36%) | 15 (48%) | 28 (32%) | 0.107 | N/A | N/A |
| Clinical spectrum | ||||||
| Isolated bacteremia | 29 (25%) | 1 (3%) | 30 (34%) | <0.001 | 14.77, [5.22–41.76] |
<0.001 |
| Meningitis | 29 (25%) | 20 (65%) | 9 (10%) | <0.001 | N/A | N/A |
| Pneumonia | 43 (36%) | 11 (35%) | 33 (38%) | 0.809 | N/A | N/A |
| Microbiology | ||||||
| NTHi | 43 (36%) | 6 (19%) | 25 (29%) | 0.029 | N/A | N/A |
| Hia | 36 (31%) | 14 (45%) | 21 (24%) | 0.028 | N/A | N/A |
| Inflammatory markers | ||||||
| Median peak white cell count, [IQR] | 18.1, [12.1–26.3] |
21.3, [16.3–30.3] |
16.7, [11.8–21.3] |
0.072 | N/A | N/A |
| Median C-reactive protein (mg/L), [IQR] | 155, [72–223] |
194, [134–301] |
114, [65–194] |
0.03 | N/A | N/A |
| Treatment | ||||||
| Median duration of antibiotic therapy (days), [IQR] | 14, [10–23] |
26, [11–44] |
13, [10–20] |
0.039 | N/A | N/A |
| Median length of stay (days), [IQR] | 10, [5–21] |
18.5, [9–43.5] |
9, [4–16] |
0.043 | N/A | N/A |
|
||||||
Discussion
This multicentre study from 10 sites across Canada provides insight into the current serotype distribution of Hi bacteremia in Canadian children. Prior to the era of Hib vaccine, invasive Hi disease was very rare in children over four years of age, while approximately one quarter of our cases were older. Invasive and non-invasive Hi disease from 2013 to 2019 has been reviewed from epidemiological data from a single Canadian site with a predominant Indigenous population (Sioux Lookout)Footnote 8. At this centre, invasive Hi disease was identified in 10 children under 4 years, in two aged 5–15 years and in eight aged 16 years and olderFootnote 8, suggesting a bimodal distribution which differs from the pre-Hib vaccine era where younger children were primarily affected. Our data highlight the pediatric centres in Winnipeg, Montréal, London and Edmonton as the highest contributors to THi, and may reflect that these centres are referral centres for large communities of Indigenous children as well as communities with reduced Hib vaccine uptake. The Hib cases still accounted for almost 10% of cases in the current study. As expected, Hib occurred predominantly in unimmunized or under-immunized children.
Serotypes a and f were the most common, accounting for 55% and 25% of THi, respectively, consistent with recent literatureFootnote 4Footnote 6. While Hia meningitis was significantly associated with a complicated course, it otherwise mirrored the Hib experience in terms of age and clinical syndromes. Children with Hif were often older with pneumonia as the focus of infection.
The less virulent NTHi accounted for just over one third of all Hi bacteremic events, where fortunately the incidence of complicated disease was only 4%. The NTHi cases presented as isolated bacteremia or pneumonia and rarely as central nervous system disease, in keeping with recent US dataFootnote 5Footnote 6. Comorbid medical conditions were a risk factor for developing NTHi disease. In a retrospective surveillance study of NTHi invasive disease in children and adults in the Netherlands, comorbid conditions in children, including immunocompromise, malignancy, neurological disease and other conditions, were identified in a large proportion of NTHi cases (n=327/396, 83%) over a seven-year spanFootnote 9.
It is exciting that Phase I trials of a Hia vaccine will begin in Canada in 2023Footnote 10 (personal communication, M. Ulanova, Canadian Immunization Research Network, 2022). A vaccine has been shown to be cost-effective in the Canadian territory of Nunavut, given the high incidence of Hia among Indigenous children and their high risk of diseaseFootnote 11. Post-marketing studies of the multivalent pneumococcal vaccines that employ protein D from NTHi as the carrier protein suggest that they prevent some otitis media due to NHTi diseases, so it is plausible that these vaccines may also prevent Hi bacteremiaFootnote 12.
Limitations
A significant limitation of our study is that it did not capture ethnicity/race data as this variable is not reliably recorded in health records in Canada. This is especially important given the established high rates of Hia in Indigenous populationsFootnote 4. Our study may underestimate the burden and spectrum of Hi disease given that it was limited to bacteremic cases; blood cultures are not always drawn prior to administration of antibiotics and can be falsely negative if an inadequate volume is obtained. As neurodevelopmental and long-term follow-up data were not uniformly available, we reported sequelae documented at or before discharge.
Conclusion
Our study provides detailed clinical comparisons of THi and NTHi, highlighting serotype-specific clinical patterns and outcomes. Although there has been dramatic reduction in Hib since introduction of Hib vaccines, children still experience serious invasive Hi disease caused by both NHTi and by non-b serotypes, especially Hia. Preventive strategies are needed to reduce the morbidity associated with this disease.
Authors’ statement
- CF — Collected data, interpreted data, writing–original draft, writing–revision and editing, final approval
- JR, SK — Data acquisition, writing–original draft, writing–revision and editing, final approval
- MA, JM, AL, SF, JG, JW, JC, JB, RS, AK, AR, KL and MU — Data acquisition, revised manuscript
- MB — Conceptualized, data acquisition, data analysis, data interpretation, writing–revision and editing, final approval
The content and view expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
Competing interests
None.
Acknowledgements
None.
Funding
None.
References
- Footnote 1
-
Gilsdorf JR. Hib Vaccines: Their Impact on Haemophilus influenzae Type b Disease. J Infect Dis 2021;224(12 Suppl 2):S321–30. https://doi.org/10.1093/infdis/jiaa537
- Footnote 2
-
Ulanova M, Tsang RSW. Haemophilus influenzae serotype a as a cause of serious invasive infection. Lancet Infect Dis 2014;14(1)1:70–82. https://doi.org/10.1016/S1473-3099(13)70170-1
- Footnote 3
-
Tsang RSW, Ulanova M. The changing epidemiology of invasive Haemophilus influenzae disease: Emergence and global presence of serotype a strains that may require a new vaccine for control. Vaccine 2017;35(33):4270–5. https://doi.org/10.1016/j.vaccine.2017.06.001
- Footnote 4
-
Zulz T, Huang G, Rudolph K, DeByle C, Tsang R, Desai S, Massey S, Bruce MG. Epidemiology of invasive Haemophilus influenzae serotype a disease in the North American Arctic, 2006–2017. Int J Circumpolar Health 2022;81(1):2150382. https://doi.org/10.1080/22423982.2022.2150382
- Footnote 5
-
Soeters HM, Blain A, Pondo T, Doman B, Farley MM, Harrison LH, Lynfield R, Miller L, Petit S, Reingold A, Schaffner W, Thomas A, Zansky SM, Wang X, Briere EC. Current Epidemiology and Trends in Invasive Haemophilus influenzae Disease-United States, 2009–2015. Clin Infect Dis 2018;67(6):881–9. https://doi.org/10.1093/cid/ciy187
- Footnote 6
-
Vallejo JG, McNeil JC, Hultén KG, Sommer LM, Dunn JJ, Kaplan SL. Invasive Haemophilus influenzae Disease at Texas Children’s Hospital, 2011 to 2018. Pediatr Infect Dis J 2019;38(9):900–5. https://doi.org/10.1097/INF.0000000000002383
- Footnote 7
-
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010
- Footnote 8
-
Ulanova M, Tsang RSW, Nix EB, Kelly L, Shuel M, Lance, B. Canadian Immunization Research Network Investigators. Epidemiology of invasive Haemophilus influenzae disease in northwestern Ontario: comparison of invasive and noninvasive H. influenzae clinical isolates. Can J Microbiol 2023;69(6):219–27. https://doi.org/10.1139/cjm-2022-0208
- Footnote 9
-
van Wessel K, Rodenburg GD, Veenhoven RH, Spanjaard L, van der Ende A, Sanders EA. Nontypeable Haemophilus influenzae Invasive Disease in the Netherlands: A Retrospective Surveillance Study 2001–2008. Clin Infect Dis 2011;53(1):e1–7. https://doi.org/10.1093/cid/cir268
- Footnote 10
-
Barreto L, Cox AD, Ulanova M, Bruce MG, Tsang R. The emerging Haemophilus influenzae serotype a infection and a potential vaccine: Implementation science in action. Can Commun Dis Rep 2017;43(5):85–8. https://doi.org/10.14745/ccdr.v43i05a01
- Footnote 11
-
Shoukat A, Van Exan R, Moghadas SM. Cost-effectiveness of a potential vaccine candidate for Haemophilus influenzae serotype ‘a’. Vaccine 2018;36(12):1681–8. https://doi.org/10.1016/j.vaccine.2018.01.047
- Footnote 12
-
Clarke C, Bakaletz LO, Ruiz-Guiñazú J, Borys D, Mrkvan T. Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage. Expert Rev Vaccines 2017;16(7):1–14. https://doi.org/10.1080/14760584.2017.1333905
Appendix
FeaturesFootnote a |
All N=110 (excluding untyped) |
Hia n=36 |
Non-Hia n=74 |
Univariate analysis significance p value<0.006Footnote b |
|---|---|---|---|---|
| Demographics | ||||
| Females | 42 (38%) | 20 (56%) | 22 (30%) | 0.009 |
| Median age (months), [IQR] | 12, [7–48] | 12, [7–24] | 12, [7–60] | 0.155 |
| Clinical spectrum | ||||
| Meningitis | 29 (26%) | 17 (47%) | 12 (16%) | <0.001 |
| Pneumonia | 38 (35%) | 7 (19%) | 31 (42%) | 0.021 |
| Bacteremia | 27 (25%) | 4 (11%) | 23 (31%) | 0.032 |
| Complicated disease | 30 (27%) | 14 (39%) | 16 (22%) | 0.002 |
| Treatment/outcome | ||||
| Median duration of antibiotic therapy (days), [IQR] | 14, [10–24] | 15, [11–29] | 13, [10–21] | 0.097 |
| Median length of stay (days), [IQR] | 14, [10–24] | 10, [4–18] | 10.5, [5–21] | 0.987 |
| Death/sequelaeFootnote c | 16 (15%) | 9 (25%) | 7 (9%) | <0.001 |
|
||||
