Analysis of the SARS-CoV-2 main protease and presence of nirmatrelvir-resistant SARS-CoV-2 Omicron mutants

 Download this article as a PDF (206 KB)
Download this article as a PDF (206 KB)Published by: The Public Health Agency of Canada
Issue: CCDR Volume 50-10, October 2024: COVID-19 after the pandemic
Date published: October 2024
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 50-10, October 2024: COVID-19 after the pandemic
Surveillance
Large scale analysis of the SARS-CoV-2 main protease reveals marginal presence of nirmatrelvir-resistant SARS-CoV-2 Omicron mutants in Ontario, Canada, December 2021–September 2023
Venkata Duvvuri1,2, Fatima Shire1,3, Sandra Isabel1, Thomas Braukmann1, Shawn Clark1, Alex Marchand-Austin1, Alireza Eshaghi1, Hina Bandukwala1, Nobish Varghese1, Ye Li1,3, Karthikeyan Sivaraman1, Hadia Hussain1, Kirby Cronin1, Ashleigh Sullivan1, Aimin Li1, Austin Zygmunt1,4, Karam Ramotar1, Julianne Kus1,2, Maan Hasso1, Antoine Corbeil1, Jonathan Gubbay1, Samir Patel1,2
Affiliations
1 Public Health Ontario, Toronto, ON
2 Department of Laboratory Medicine and Pathobiology, Temerty Faculty of Medicine, University of Toronto, Toronto, ON
3 Division of Biostatistics, Dalla Lana School of Public Health, University of Toronto, Toronto, ON
4 Department of Family Medicine, University of Ottawa, Ottawa, ON
Correspondence
Suggested citation
Duvvuri VR, Shire F, Isabel S, Braukmann T, Clark ST, Marchand-Austin A, Eshaghi A, Bandukwala H, Varghese N, Li Y, Sivaraman K, Hussain H, Cronin K, Sullivan A, Li A, Zygmunt A, Ramotar K, Kus J, Hasso M, Corbeil A, Gubbay JB, Patel SN. Large scale analysis of the SARS-CoV-2 main protease reveals marginal presence of nirmatrelvir-resistant SARS-CoV-2 Omicron mutants in Ontario, Canada, December 2021–September 2023. Can Commun Dis Rep 2024;50(10):365–74. https://doi.org/10.14745/ccdr.v50i10a05
Keywords: SARS-CoV-2, Omicron, Paxlovid, nirmatrelvir-ritonavir, main protease gene (Mpro), in vitro resistant mutations, genomic surveillance, Ontario
Abstract
Background: In response to the COVID-19 pandemic, a new oral antiviral called nirmatrelvir-ritonavir (PaxlovidTM) was authorized for use in Canada in January 2022. In vitro studies have reported mutations in Mpro protein that may be associated with the development of nirmatrelvir resistance.
Objectives: To survey the prevalence, relevance and temporal patterns of Mpro mutations among SARS-CoV-2 Omicron lineages in Ontario, Canada.
Methods: A total of 93,082 Mpro gene sequences from December 2021 to September 2023 were analyzed. Reported in vitro Mpro mutations were screened against our database using in-house data science pipelines to determine the nirmatrelvir resistance. Negative binomial regression was conducted to analyze the temporal trends in Mpro mutation counts over the study time period.
Results: A declining trend was observed in non-synonymous mutations of Mpro sequences, showing a 7.9% reduction (95% CI: 6.5%–9.4%; p<0.001) every 30 days. The P132H was the most prevalent mutation (higher than 95%) in all Omicron lineages. In vitro nirmatrelvir-resistant mutations were found in 3.12% (n=29/929) Omicron lineages with very low counts, ranging from one to 19. Only two mutations, A7T (n=19) and M82I (n=9), showed temporal presence among the BA.1.1 in 2022 and the BQ.1.2.3 in 2022, respectively.
Conclusion: The observations suggest that, as of September 2023, no significant or widespread resistance to nirmatrelvir has developed among SARS-CoV-2 Omicron variants in Ontario. This study highlights the importance of creating automated monitoring systems to track the emergence of nirmatrelvir-resistant mutations within the SARS-CoV-2 virus, utilizing genomic data generated in real-time.
Introduction
Nirmatrelvir-ritonavir (brand name PaxlovidTM, Pfizer Inc.) is an orally administered antiviral therapy. This combination received an Emergency Use Authorization from United States Food and Drug Administration in December 2021 Footnote 1Footnote 2Footnote 3. Nirmatrelvir-ritonavir was subsequently approved by Health Canada for adults with COVID-19 who were at high risk of progressing to severe disease in January 2022 Footnote 4Footnote 5. Nirmatrelvir (PF-07322332), an active component of Paxlovid, is a novel inhibitor of the SARS-CoV-2 3-chymotrypsin-like protease (3CLpro) or main protease (Mpro, also known as non-structural protein, nsp5), which is critical for viral replication and assembly. This inhibitory mechanism prevents the production of new viruses in infected cells Footnote 6. Importantly, nirmatrelvir is highly specific to the viral protease, which reduces the risk of off-target effects on human proteases Footnote 7. Ritonavir inhibits the cytochrome P4503A4 (CYP3A4) enzyme, a major human hepatic drug-metabolizing enzyme, increasing the plasma concentrations of nirmatrelvir in vivo Footnote 8.
Clinical efficacy studies on nirmatrelvir-ritonavir reported fewer visits to the emergency department, lower hospitalizations, and lower all-cause mortality in patients infected with SARS-CoV-2 variants of concern (Delta B.1.617.2 and Omicron B.1.1.529, BA.2, BA2.12.1, BA.4 and BA.5) Footnote 9Footnote 10Footnote 11Footnote 12. A retrospective observational study from Ontario, Canada, reported a significant reduction in hospital admission from COVID-19 and all-cause mortality among outpatients who used nirmatrelvir-ritonavir between April and August 2022, with greater benefits being noted among individuals who were under-vaccinated or unvaccinated and 70 years of age and older Footnote 13. The Canadian Nosocomial Infection Surveillance Program found that 13% (n=490/3,731) of adult patients with COVID-19 received nirmatrelvir-ritonavir, either at admission or during hospitalization in Canada, although the results on treatment efficacy remain unreported Footnote 14.
The therapeutic effectiveness of nirmatrelvir-ritonavir can be influenced by the emergence of resistant variants. Given the continuous evolution of the SARS-CoV-2 virus and selection pressures from the introduction of nirmatrelvir-ritonavir, resistance is likely to emerge Footnote 15. Evidence of in vitro nirmatrelvir-resistant SARS-CoV-2 variants Footnote 16Footnote 17Footnote 18, variable potencies of nirmatrelvir to different human coronaviruses Footnote 19 and resistance of other viruses to protease inhibitors Footnote 20 support the need for continuous monitoring of SARS-CoV-2 Mpro gene sequences to quickly identify mutations that may affect nirmatrelvir's potency. Such genomic surveillance could provide insights into the mechanisms of antiviral evasion that are crucial for policy guidelines and in the development of next-generation Mpro inhibitors Footnote 18.
The purpose of this study was to survey the prevalence, relevance and temporal patterns of Mpro mutations among circulating SARS-CoV-2 lineages in Ontario. First, we conducted a scientific and grey literature review (May 2022 to August 2023) to compile a list of Mpro mutations that have been characterized as conferring in vitro resistance to nirmatrelvir Footnote 21. This complied list was subsequently used to identify the presence of nirmatrelvir-resistant mutations within the dataset. We then analyzed 93,082 Mpro sequences derived from SARS-CoV-2 Omicron-positive clinical specimens sequenced in Ontario between December 2021 and September 2023.
Methods
Clinical specimen selection and SARS-CoV-2 whole genome sequencing
Diagnostic laboratories in Ontario provided a proportion of all SARS-CoV-2 positive clinical specimens to designated whole-genome sequencing (WGS) laboratories as part of the Ontario COVID-19 Genomics Network Footnote 22. The acceptable criteria for WGS sampling included a SARS-CoV-2 polymerase chain reaction (PCR) cycle threshold (Ct) of 30 or fewer and a sufficient sample volume. The sampling proportion ranged from 10% to 100% and was adjusted over time based on projected case counts and Ontario COVID-19 Genomics Network sequencing capacity from December 2021 to September 2023. The diagnostic PCR testing for SARS-CoV-2/COVID-19 was restricted to high-risk populations Footnote 23Footnote 24 and, as such, representative surveillance pertains only to those populations tested at the time of sampling.
SARS-CoV-2 main protease sequences
Raw sequence data from the Illumina platform were analyzed using ARTIC pipeline v1.7 (the Ontario Institute for Cancer Research pipelines) and ARTIC primer scheme version 4.1. Post-analysis quality filtering was performed using ncov-tools version 1.8. Samples were annotated for lineage with Pangolin v4.3 using constellations v.0.1.12 (Pangolin-assignment v1.15.1, Scorpio 0.3.17, and usher 0.5.6). The ARTIC nanopolish v1.3.0-dev (+0.3.1 patch) pipeline and associated ncov-tools version were used for samples sequenced on the nanopore platform. All available Mpro gene sequences of SARS-CoV-2 Omicron (n=93,082 unique sequences) were collected between December 1, 2021, and September 21, 2023, from Public Health Ontario's SARS-CoV-2 WGS database (PHO-SARS-CoV-2 WGS database). These Mpro sequences were screened against the reference SARS-COV-2 genome, Wuhan-Hu-1 (accession no. NC_045512.2), to identify both synonymous and non-synonymous mutations across all Omicron lineages.
Temporal tracking of main protease mutations in Omicron lineages
An in-house data science pipeline was developed in Python v.3.9.16 to track the temporality and prevalence of observed Mpro mutations among Omicron lineages in Ontario. A generalized additive model with restricted cubic spline was fit on the log transformed mutation count. We examined the patterns of the Mpro non-synonymous mutations over the study time-period; based on these patterns, a negative binomial regression (R package mgcv v.1.9-0) was used to model the decline of the number of non-synonymous mutations over time.
Results
A total of 93,082 Mpro gene sequences corresponding to 929 Omicron lineages of SARS-CoV-2 from Ontario were analyzed. Omicron lineages were grouped by their prevalence of total sequences analyzed as low (less than one percent) or high (greater than or equal to one percent). Twelve SARS-CoV-2 lineages were categorized with high prevalence. The five lineages with the highest prevalence during defined period were: BA.1.1 (9.3%), XBB.1.5 (8.3%), BQ.1.1 (7.8%), BA.2 (7.4%) and BA.5.2.1 (6.0%).
We studied the evolution of Mpro nucleotide sequences of SARS-CoV-2; we observed cyclic variations for total counts for both synonymous (no change in protein sequence) and non-synonymous (change in protein sequence) mutations. The negative binomial regression on Mpro non-synonymous mutations showed a 7.9% (95% CI: 6.5%–9.4%; p<0.001) decrease in mutation counts every 30 days (Figure 1). The non-synonymous mutational burden, with sequences carrying at least one mutation across the Mpro protein sequence, accounted for approximately 67.7% (207 AAs/306 AAs of Mpro) (Figure 2). Table 1 presents details of low and high prevalent lineages with Mpro non-synonymous mutations reported in at least 10 sequences of the total sequence data collected for each lineage. For example, the T21I mutation is observed in 31 of 2,801 total BA.2.12.1 sequences during the study period. Only six mutations, L67, L75, K90, A116, P184 and R279, were found to be common in both high and low-prevalent lineages; however, none of these mutations were relevant to the reported in vitro nirmatrelvir-resistant mutations.

Figure 1: Descriptive text
This figure displays the mutational counts observed over time in the main protease protein of SARS-CoV-2 Omicron lineages, categorized by non-synonymous (red line) and synonymous (blue line) mutation types. A total of 93,082 nucleotide sequences were analyzed. The data shows a gradual increase in both mutation types in lineages circulating in early 2022, followed by a noticeable dip in lineages from May 2023. Specifically for non-synonymous mutations, there is a statistically significant decrease in mutational counts every 30 days, as shown by negative binomial regression.
Abbreviation: Mpro, main protease
Footnotes- Footnote a
-
Each solid circle or dot represents one mutation and is colour-coded based on the Mpro protein structural details Footnote 25. The mutational count is the observed absolute value of mutations at each position. Log transformed Y-axis presents mutational counts
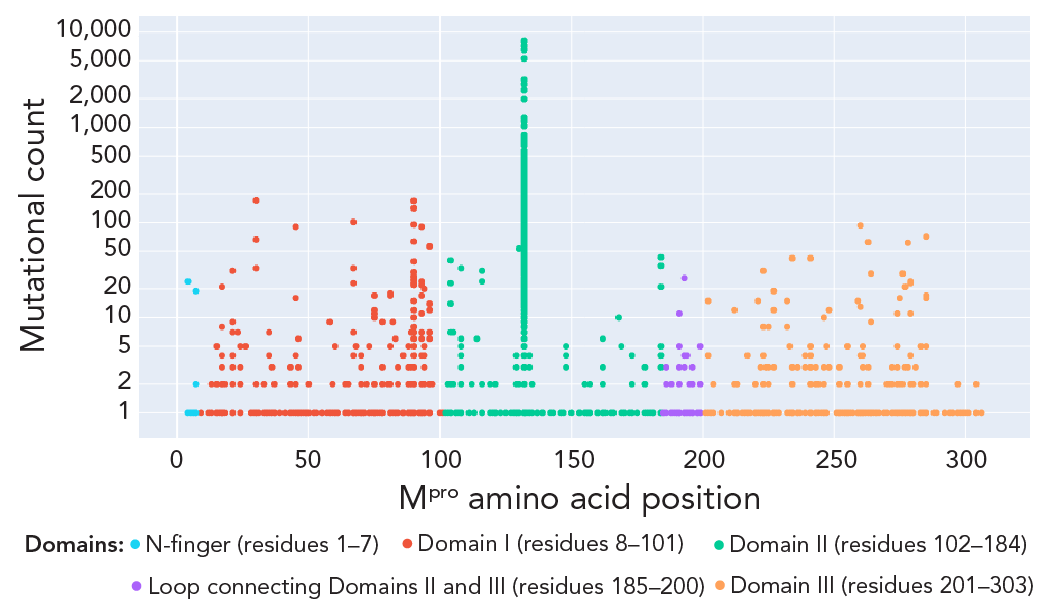
Figure 2: Descriptive text
The figure presents a detailed visual distribution of observed non-synonymous mutations across the different domains of the main protease protein of SARS-CoV-2 Omicron lineages. The x-axis denotes the amino acid positions, and the y-axis represents the mutational count on a logarithmic scale. Consistent with the literature on the most prevalent mutations in Omicron lineages, the 132nd position in Domain II, corresponding to the P132H mutation, showed the highest peak in the mutational count and was present in more than 95% of the Omicron lineages analyzed in the current study.
Abbreviation: Mpro, main protease
Footnotes- Footnote a
-
Each solid circle or dot represents one mutation and is colour-coded based on the Mpro protein structural details Footnote 25. The mutational count is the observed absolute value of mutations at each position. Log transformed Y-axis presents mutational counts
| Mpro structure region | MutationFootnote a | Pango lineage | Lineage prevalenceFootnote b | Total sequences | Count of sequences with mutation | FrequencyFootnote c | Observation from prior literature (reference) |
|---|---|---|---|---|---|---|---|
| N-finger (1 to 7 AA) |
R4K | BQ.1.2 | Low | 267 | 24 | 8.99 | Mutation contributes to Mpro dimerization Footnote 26 |
| A7T | BA.1.1 | High | 8,143 | 19 | 0.23 | Mutation contributes to Mpro dimerization Footnote 27Footnote 28Footnote 29Footnote 30 | |
| Domain I (8 to 101 AA) |
M17V | XBB.1.5 | High | 7,281 | 18 | 0.25 | - |
| T21I | BA.2.12.1 | High | 2,801 | 31 | 1.11 | In vitro study reported as founder or precursor mutation Footnote 18 | |
| L30I | BQ.1.3 | Low | 33 | 33 | 100 | L30F, an in vitro-reported nirmatrelvir-resistant mutation Footnote 9 but L30I has not been tested | |
| BQ.1.3.1 | Low | 170 | 170 | 100 | |||
| BQ.1.3.2 | Low | 66 | 66 | 100 | |||
| T45N | BE.4 | Low | 90 | 90 | 100 | - | |
| BE.4.1 | Low | 16 | 16 | 100 | |||
| CQ.2 | Low | 16 | 16 | 100 | |||
| L67S | BA.5.2.1 | High | 5,312 | 23 | 0.43 | - | |
| L67V | BF.14 | Low | 46 | 32 | 69.57 | - | |
| BQ.1.1.40 | Low | 448 | 101 | 22.54 | |||
| L75F | BA.1.1 | High | 8,143 | 11 | 0.14 | - | |
| BA.2 | High | 6,551 | 12 | 0.18 | |||
| BA.4.6 | High | 1,250 | 17 | 1.36 | |||
| BA.5.5 | Low | 705 | 10 | 1.42 | |||
| S81C | XBB.1.5 | High | 7,281 | 18 | 0.25 | - | |
| K90R | BA.1.1 | High | 8,143 | 95 | 1.17 | Prevalent mutation in Beta (B.1.351) variants Footnote 27 | |
| BA.2 | High | 6,551 | 140 | 2.14 | |||
| BA.2.12.1 | High | 2,801 | 23 | 0.82 | |||
| BA.5.2 | High | 3,083 | 24 | 0.78 | |||
| BA.5.2.1 | High | 5,312 | 25 | 0.47 | |||
| BQ.1 | High | 1,987 | 12 | 0.6 | |||
| BQ.1.1 | High | 6,513 | 39 | 0.6 | |||
| XBB.1.5 | High | 7,281 | 27 | 0.37 | |||
| BA.2.3 | Low | 751 | 166 | 22.1 | |||
| BA.5.9 | Low | 70 | 10 | 14.29 | |||
| BF.14 | Low | 46 | 29 | 63.04 | |||
| BF.21 | Low | 104 | 12 | 11.54 | |||
| BQ.1.1.51 | Low | 133 | 15 | 11.28 | |||
| T93I | BA.2.12.1 | High | 2,801 | 11 | 0.39 | - | |
| XBB.1.5 | High | 7,281 | 6 | 0.08 | |||
| A94V | BU.1 | Low | 20 | 20 | 100 | - | |
| P96S | BQ.1.1 | High | 6,513 | 56 | 0.86 | - | |
| P96L | XBB.1.5 | High | 7,281 | 14 | 0.19 | - | |
| Domain II (102 to 184 AA) |
V104I | BN.1.4 | Low | 15 | 14 | 93.33 | - |
| P108T | BA.5.2.1 | High | 5,312 | 33 | 0.62 | - | |
| A116V | XBB.1.5 | High | 7,281 | 31 | 0.43 | - | |
| A116T | BQ.1.2 | Low | 267 | 24 | 8.99 | - | |
| M130L | BA.5.2.9 | Low | 534 | 53 | 9.93 | - | |
| P168S | BA.5.1 | High | 2,452 | 10 | 0.41 | Prevalent mutation in pre-Omicron lineages Footnote 17 | |
| P184S | BA.1.1 | High | 8,143 | 21 | 0.26 | - | |
| BA.2.3 | Low | 751 | 43 | 5.73 | - | ||
| P184L | BQ.1.14 | Low | 250 | 35 | 14 | - | |
| Loop (185 to 200 AA) |
A193V | XBB.1.16.1 | Low | 227 | 4 | 1.76 | - |
| Domain III (201 to 303 AA) |
V202I | BQ.1.2.3 | Low | 289 | 15 | 5.19 | - |
| V212I | FL.7 | Low | 17 | 12 | 70.59 | - | |
| N221S | BQ.1.22 | Low | 112 | 15 | 13.39 | - | |
| F223L | BN.1.3.1 | Low | 31 | 31 | 100 | - | |
| L227F | BA.5.2.1 | High | 5,312 | 19 | 0.36 | - | |
| BF.7 | High | 999 | 12 | 1.2 | |||
| L232F | BA.5.2 | High | 3,083 | 14 | 0.45 | - | |
| A234V | BQ.1.13 | Low | 435 | 42 | 9.66 | - | |
| P241L | XBB.1.5 | High | 7,281 | 42 | 0.58 | - | |
| H246Y | BA.5.1.15 | Low | 10 | 10 | 100 | - | |
| D248N | BU.1 | Low | 20 | 12 | 60 | - | |
| A260V | BQ.1.1 | High | 6,513 | 92 | 1.41 | No impact shown on the reducing drug potency in biochemical assay Footnote 2Footnote 3 | |
| D263A | BQ.1.1 | High | 6,513 | 62 | 0.95 | - | |
| M264I | XBB.1.5 | High | 7,281 | 29 | 0.4 | - | |
| N274T | BQ.1.1 | High | 6,513 | 11 | 0.17 | - | |
| N274S | BQ.1.14 | Low | 250 | 10 | 4 | - | |
| G275S | BN.1.5.2 | Low | 18 | 16 | 88.89 | - | |
| M276I | BA.5.1.2 | Low | 154 | 28 | 18.18 | - | |
| N277I | BA.5.1.23 | Low | 236 | 21 | 8.9 | - | |
| G278R | BF.7 | High | 999 | 58 | 5.81 | - | |
| R279C | BF.7 | High | 999 | 23 | 2.3 | - | |
| BA.5.5 | Low | 705 | 22 | 3.12 | - | ||
| BF.1 | Low | 95 | 11 | 11.58 | - | ||
| A285T | BA.2 | High | 6,551 | 16 | 0.24 | Mutation contributes to Mpro dimerization Footnote 26 and potential decrease in Mpro catalytic efficiency Footnote 31 | |
| BF.1 | Low | 95 | 11 | 11.58 | - | ||
| Abbreviations: Mpro, main protease; -, not applicable Footnotes
|
|||||||
Pattern of documented highly prevalent mutations in SARS-CoV-2 Omicron lineages, Ontario
Of the nine most prevalent Mpro mutations in SARS-CoV-2 (G15S, T21I, K88R, L89F, K90R, P108S, P132H, L205V and A260V) Footnote 17Footnote 26Footnote 27Footnote 32, albeit with unaltered susceptibility to nirmatrelvir Footnote 2Footnote 3, only P132H accumulated at a noticeable frequency, eventually accounting for more than 95% in the Omicron lineages in Ontario Footnote 27. The K90R mutation was observed in the following Omicron lineages: BA.1.1, BA.2, BA.2.12.1, BA.5.2, BA.5.2.1, BQ.1, BQ.1.1 and XBB.1.15 (within lineage rates ranged from 0.37% to 2.14%). While the A260V substitution was observed in 1.41% (n=92/6,513 sequences) of BQ.1.1 variants circulated in 2022, the T21I mutation accumulated in BA.2.12.1 lineage with 1.1% (n=31/2,801 sequences) mutational frequency.
Low prevalence and no temporality of nirmatrelvir drug resistance in SARS-CoV-2 Omicron lineages, Ontario
Sixteen of 34 in vitro characterized nirmatrelvir-resistant mutations Footnote 2Footnote 3Footnote 32Footnote 33, corresponding to A7T/S/V (Mpro N-finger), G15S, L30F, L50F, M82I (Mpro Domain I), P132S, T135I, E166V, A173S/T/V (Mpro Domain II), Q189K, T196A (loop that connects Mpro Domains II and III), W207S, D248E, A260T, D263E and A266V (Mpro Domain III), were observed with lineage-specificity (3.12%, n=29/929 lineages) (Figure 3). The burden of these mutations ranged from 1 to 19 counts, with A7T being the most frequently observed in BA.1.1 (within lineage rate=0.23%, n=19/8,143 sequences; observed only once in FT.1, XBB.1.22 and XBB.1.5), followed by M82I in nine sequences of BQ.1.2.3. The rest were observed in n=4 sequences of BA.4.6 for the A173T mutation, according to Appendix, Table A1. Only A7T and M82I exhibited some temporality; A7T was notable during weeks three, four and 10 to 15 in 2022 among the BA.1.1 lineage and M82I during weeks 46 to 51 in late 2022 among the BQ.1.2.3 lineage (Figure 3).
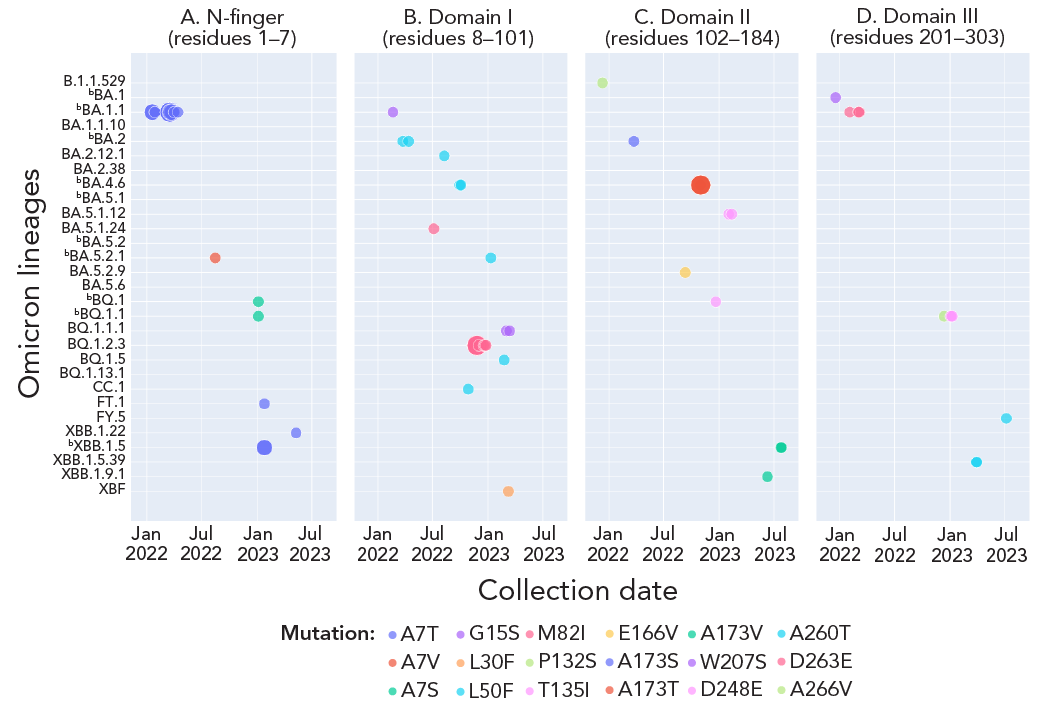
Figure 3 - Text description
This figure shows the prevalence and temporality of Mpro mutations that conferred in vitro resistance to nirmatrelvir within the Omicron lineages collected from December 2021 to September 2023. Among the mutations analyzed, only A7T and M82I exhibited noticeable prevalence in the BA.1.1 and BQ.1.2.3 Omicron lineages, respectively. Additionally, A7T and M82I are also the only mutations that exhibited temporality. The remaining mutations were observed in four or fewer sequences of the lineages analyzed, with no observable temporality.
- Footnote a
-
Each solid circle or dot represents a count of the corresponding colour-coded Mpro mutation and the size of solid circle denotes its count value. The listed Mpro mutations correspond to the Mpro structural regions of the mutation Footnote 25
- Footnote b
-
Denotes highly prevalent lineages. Appendix, Table A1 provides counts of each mutation and associated lineages in time
We also examined our database for double Footnote 18Footnote 21Footnote 33Footnote 34Footnote 35, triple Footnote 2Footnote 3, quadruple and quintuple Footnote 2Footnote 3 mutants, as have been reported in the literature, since these multiple mutations have the potential to confer synergistic resistance to nirmatrelvir. However, none of these mutations were identified within SARS-CoV-2 lineages circulating at the time of sampling in Ontario.
Discussion
A comprehensive analysis of SARS-CoV-2 Omicron lineage Mpro sequences from Ontario revealed that approximately 3% of lineages (n=29/929) exhibited in vitro characterized nirmatrelvir-resistant Mpro mutations, without any discernible temporal pattern.
Consistent with the global literature Footnote 26Footnote 27Footnote 32, the missense mutation P132H in the Mpro structural Domain II region was the most widespread with higher than 95% prevalence in all Ontario Omicron lineages. In addition, K90R, the most prevalent mutation of Beta variants, was observed with modest prevalence in the Ontario Omicron lineages Footnote 27. However, despite their predominance, these two mutations were not reported to reduce nirmatrelvir potency Footnote 2Footnote 3. Structural assessments of Mpro revealed that both mutations (P132H and K90R) are distal to the nirmatrelvir binding site and, thus, do not alter structural conformation at or around the binding site Footnote 34. The A260V substitution, another highly prevalent Mpro mutation observed in BQ.1.1 variants reported as an infrequent natural polymorphism, was flagged in the EPIC-HR clinical trial with impact on nirmatrelvir-resistance pending Footnote 2Footnote 3.
In our dataset, we observed a low frequency of Mpro point mutations, such as T21I, P252L and T304I, which are known to function as “precursor” mutations for the emergence of nirmatrelvir resistance in SARS-CoV-2 Footnote 18. These three mutations may independently limit the replication of the SARS-CoV-2 virus Footnote 32, but no data are available on their potential contribution to resistance. None of the low prevalence mutations observed in our dataset, including A7T and M82I, are implicated in nirmatrelvir-resistance Footnote 35. Notably, A7 is situated within the N-finger region, known to play a role in dimerization which is crucial to Mpro enzyme activity Footnote 28Footnote 29. According to Iketani et al. Footnote 30, variants with mutations of A7 to V/C/S/T have comparable protease activity to wild type. Consistently, structural studies suggest that the alanine substitution by threonine at position 7 only has a modest effect on protease activity of Mpro, a reduction in efficiency by 1.5 times Footnote 29. Altogether, these studies suggest that the A7V/S/T mutations observed in BA.1.1 variants in early 2022 were unlikely to contribute to nirmatrelvir-resistance or protease activity. No known in vitro nirmatrelvir-resistant mutations were found (as of September 17 to 30, 2023) in Ontario's recently circulating variants, EG.5.1.1, FL.1.5.1, HV.1, HK.3 and XBB.1.16.6 Footnote 36.
The declining pattern seen in non-synonymous Mpro mutations (Figure 1) suggests the possibility of either a reduced heterogeneity among Ontario's circulating viral variants or a decreased propensity for the Mpro protein to evolve in response to selective pressure Footnote 27. Alternatively, Schwartz et al. Footnote 13 reported only 5% of patients (n=8,876/177,545) had been treated with nirmatrelvir-ritonavir between April 4, 2022, and August 31, 2022, in Ontario. These data, although specific to a brief study period within the timeframe of our study, suggest limited selection pressure, potentially contributing to the lower prevalence of antiviral-resistant Omicron variants observed in the population studied. Overall, our observations suggests that Omicron variants analyzed at the time of study period have not yet developed significant and widespread resistance to nirmatrelvir Footnote 37.
Strengths and limitations
A major strength of the study is the large scale of the analysis of the Mpro sequences from the Omicron lineages that circulated between December 2021 and September 2023 in Ontario. A comprehensive analysis led to insights related to in vitro mutations relevant to nirmatrelvir resistance (both mutational frequencies and temporality), protease activity and the identification of mutations of unknown function unique to our dataset that may be investigated further in experimental studies. A key limitation of our study is its generalizability, because only a defined sampling proportion was sequenced at given time (i.e., targeted population for COVID-19 diagnostic testing, proportions of specimens sequenced that vary in time, specimens with PCR Ct of fewer than 30). Because of this stringent criteria for sequencing samples, our study dataset may not be directly representative of Mpro sequences of Ontario. Furthermore, a lack of availability of sociodemographic, clinical and treatment data limited the interpretation of our findings in the context of nirmatrelvir-ritonavir treatment.
Conclusion
Overall, we found very low presence of nirmatrelvir-resistant mutant strains with lack of temporality. Our data suggest that the current use of nirmatrelvir-ritonavir targeting specific populations in Ontario may not provide selective pressure for the emergence of resistant mutants Footnote 37. Finally, this study underpins the need for continuous genomic surveillance and also forms the foundation for the creation of an automated monitoring system designed to track the emergence of nirmatrelvir-resistant mutations within the SARS-CoV-2 virus, utilizing real-time genome data. The ability to track, in near real-time, the frequency of mutations associated with antimicrobial resistance can inform the antimicrobial stewardship necessary to maintain drug efficacy over a longer period.
Authors' statement
- VD — Conceptualization, software, formal analysis, writing–original draft, writing–review & editing
- FS — Software, formal analysis, writing–review & editing
- SI — Formal analysis, writing–review & editing
- TB — Writing–review & editing
- SC — Formal analysis, writing–review & editing
- AMA — Writing–review & editing
- AE — Writing–review & editing
- HB — Writing–review & editing
- NV — Writing–review & editing
- YL — Software, formal analysis, writing–review & editing
- KS — Writing–review & editing
- HH — Writing–review & editing
- KC — Writing–review & editing
- AS — Writing–review & editing
- AL — Writing–review & editing
- AZ — Writing–review & editing
- KR — Writing–review & editing
- JK — Writing–review & editing
- MH — Writing–review & editing
- AC — Writing–review & editing
- JG — Writing–review & editing
- SP — Conceptualization, writing–review & editing
The content and view expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
Competing interests
JB Gubbay is a paid consultant scientific editor for GIDEON Informatics, Inc., which is unrelated to the current work. All other authors have no competing interest to declare.
Acknowledgements
We are grateful to the Public Health Ontario Library Service team who supported the literature review and the Public Health Ontario team who contributed to this study.
References
- Footnote 1
-
U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. Washington, DC: FDA; 2021. [Accessed 2023 Aug 21]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
- Footnote 2
-
U.S. Food and Drug Administration. Emergency Use Authorization (EUA) for Paxlovid (nirmatrelvir tablets co-packaged with ritonavir tablets) - Center for Drug Evaluation and Research (CDER) Review. Silver Springs, MD: USDHHS; 2021. [Accessed 2023 Aug 21]. https://www.fda.gov/media/155194/download
- Footnote 3
-
Pfizer Labs. Fact sheet for healthcare providers: Emergency Use Authorization for PaxlovidTM. New York, NY: Pfizer; 2024. [Accessed 2023 Aug 26]. https://www.fda.gov/media/155050/download
- Footnote 4
-
Health Canada. Health Canada authorizes PAXLOVIDTM for patients with mild to moderate COVID-19 at high risk of developing serious disease. Ottawa, ON: HC; 2022. [Accessed 2023 Aug 23]. https://www.canada.ca/en/health-canada/news/2022/01/health-canada-authorizes-paxlovidtm-for-patients-with-mild-to-moderate-covid-19-at-high-risk-of-developing-serious-disease.html
- Footnote 5
-
Ontario Health. Recommendations for Antiviral Therapy for Adults with Mild to Moderate COVID-19. [Accessed 2024 Jul 7]. https://www.ontariohealth.ca/sites/ontariohealth/files/Recommendations-for-Antiviral-Therapy-for-Adults-with-Mild-to-Moderate-COVID-19.pdf
- Footnote 6
-
Hilgenfeld R. From SARS to MERS: Crystallographic Studies on Coronaviral Proteases Enable Antiviral Drug Design. FEBS J 2014;281(18):4085–96. https://doi.org/10.1111/febs.12936
- Footnote 7
-
Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003;300(5626):1763–7. https://doi.org/10.1126/science.1085658
- Footnote 8
-
Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, Dantonio A, Di L, Eng H, Ferre R, Gajiwala KS, Gibson SA, Greasley SE, Hurst BL, Kadar EP, Kalgutkar AS, Lee JC, Lee J, Liu W, Mason SW, Noell S, Novak JJ, Obach RS, Ogilvie K, Patel NC, Pettersson M, Rai DK, Reese MR, Sammons MF, Sathish JG, Singh RSP, Steppan CM, Stewart AE, Tuttle JB, Updyke L, Verhoest PR, Wei L, Yang Q, Zhu Y. An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19. Science 2021;374(6575):1586–93. https://doi.org/10.1126/science.abl4784
- Footnote 9
-
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM; EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022;386(15):1397–408. https://doi.org/10.1056/NEJMoa2118542
- Footnote 10
-
Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-World Effectiveness of Molnupiravir and Nirmatrelvir plus Ritonavir against Mortality, Hospitalisation, and in-Hospital Outcomes among Community-Dwelling, Ambulatory Patients with Confirmed SARS-CoV-2 Infection during the Omicron Wave in Hong Kong: An Observational Study. Lancet 2022;400(10359):1213–22. https://doi.org/10.1016/S0140-6736(22)01586-0
- Footnote 11
-
Aggarwal NR, Molina KC, Beaty LE, Bennett TD, Carlson NE, Mayer DA, Peers JL, Russell S, Wynia MK, Ginde AA. Real-World Use of Nirmatrelvir–Ritonavir in Outpatients with COVID-19 during the Era of Omicron Variants Including BA. 4 and BA. 5 in Colorado, USA: A Retrospective Cohort Study. Lancet Infect Dis 2023;23(6):696–705. https://doi.org/10.1016/S1473-3099(23)00011-7
- Footnote 12
-
Shah MM, Joyce B, Plumb ID, Sahakian S, Feldstein LR, Barkley E, Paccione M, Deckert J, Sandmann D, Gerhart JL, Hagen MB. Paxlovid Associated with Decreased Hospitalization Rate among Adults with COVID-19—United States, April–September 2022. MMWR Morb Mortal Wkly Rep 2022;71(48):1531–7. https://doi.org/10.15585/mmwr.mm7148e2
- Footnote 13
-
Schwartz KL, Wang J, Tadrous M, Langford BJ, Daneman N, Leung V, Gomes T, Friedman L, Daley P, Brown KA. Population-Based Evaluation of the Effectiveness of Nirmatrelvir–Ritonavir for Reducing Hospital Admissions and Mortality from COVID-19. CMAJ 2023;195(6):E220–6. https://doi.org/10.1503/cmaj.221608
- Footnote 14
-
Mitchell R, Lee D, Pelude L, Comeau J, Conly J, Ellis C, Ellison J, Embil J, Evans G, Johnston L, Johnstone J, Katz K, Kibsey P, Lee B, Lefebvre MA, Longtin Y, McGeer A, Mertz D, Minion J, Smith S, Srigley J, Suh K, Tomlinson J, Wong A, Thampi N, Frenette C. Nirmatrelvir-Ritonavir Use among Adults Hospitalized with COVID-19 during the Omicron Phase of the COVID-19 Pandemic, Canadian Nosocomial Infection Surveillance Program. Can Commun Dis Rep 2023;49(7/8):351–7. https://doi.org/10.14745/ccdr.v49i78a07
- Footnote 15
-
Banerjee A, Mossman K, Grandvaux N. Molecular Determinants of SARS-CoV-2 Variants. Trends Microbiol 2021;29(10):871–3. https://doi.org/10.1016/j.tim.2021.07.002
- Footnote 16
-
Hu Y, Lewandowski EM, Tan H, Zhang X, Morgan RT, Zhang X, Jacobs LMC, Butler SG, Gongora MV, Choy J, Deng X, Chen Y, Wang J. Naturally Occurring Mutations of SARS-CoV-2 Main Protease Confer Drug Resistance to Nirmatrelvir. ACS Cent Sci 2023;9(8):1658–69. https://doi.org/10.1021/acscentsci.3c00538
- Footnote 17
-
Moghadasi SA, Heilmann E, Khalil AM, Nnabuife C, Kearns FL, Ye C, Moraes SN, Costacurta F, Esler MA, Aihara H, von Laer D, Martinez-Sobrido L, Palzkill T, Amaro RE, Harris RS. Transmissible SARS-CoV-2 Variants with Resistance to Clinical Protease Inhibitors. Sci Adv 2023;9(13):eade8778. https://doi.org/10.1126/sciadv.ade8778
- Footnote 18
-
Iketani S, Mohri H, Culbertson B, Hong SJ, Duan Y, Luck MI, Annavajhala MK, Guo Y, Sheng Z, Uhlemann AC, Goff SP, Sabo Y, Yang H, Chavez A, Ho DD. Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir. Nature 2023;613(7944):558–64. https://doi.org/10.1038/s41586-022-05514-2
- Footnote 19
-
Li J, Wang Y, Solanki K, Atre R, Lavrijsen M, Pan Q, Baig MS, Li P. Nirmatrelvir Exerts Distinct Antiviral Potency against Different Human Coronaviruses. Antiviral Res 2023;211:105555. https://doi.org/10.1016/j.antiviral.2023.105555
- Footnote 20
-
Clavel F, Hance AJ. HIV Drug Resistance. N Engl J Med 2004;350(10):1023–35. https://doi.org/10.1056/NEJMra025195
- Footnote 21
-
Public Health Ontario. Impact of SARS-CoV-2 Main Protease Mutations on Nirmatrelvir/Ritonavir (Paxlovid) Resistance. Toronto, ON: PHO; 2022. [Accessed 2023 Aug 23]. https://www.publichealthontario.ca/-/media/Documents/nCoV/ipac/2022/06/sars-cov2-protease-mutations-paxlovid-resistance.pdf?sc_lang=en.
- Footnote 22
-
Public Health Ontario. Coronavirus Disease 2019 (COVID-19) - Variant of Concern Screening and Whole Genome Sequencing Surveillance. Toronto, ON: PHO; 2022. [Accessed 2023 Oct 9]. https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/COVID-19-VoC
- Footnote 23
-
Ontario Ministry of Health. COVID-19 Provincial Testing Guidance. Toronto, ON: MOH; 2023. [Accessed 2023 Oct 9]. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/covid-19_provincial_testing_guidance.pdf
- Footnote 24
-
Government of Ontario. Updated Eligibility for PCR Testing and Case and Contact Management Guidance in Ontario. Toronto, ON: Government of Ontario; 2021. [Accessed 2023 Oct 23]. https://news.ontario.ca/en/backgrounder/1001387/updated-eligibility-for-pcr-testing-and-case-and-contact-management-guidance-in-ontario
- Footnote 25
-
Kneller DW, Phillips G, O'Neill HM, Jedrzejczak R, Stols L, Langan P, Joachimiak A, Coates L, Kovalevsky A. Structural Plasticity of SARS-CoV-2 3CL Mpro Active Site Cavity Revealed by Room Temperature X-Ray Crystallography. Nat Commun 2020;11(1):3202. https://doi.org/10.1038/s41467-020-16954-7
- Footnote 26
-
Ullrich S, Ekanayake KB, Otting G, Nitsche C. Main Protease Mutants of SARS-CoV-2 Variants Remain Susceptible to Nirmatrelvir. Bioorg Med Chem Lett 2022;62:128629. https://doi.org/10.1016/j.bmcl.2022.128629
- Footnote 27
-
Lee JT, Yang Q, Gribenko A, Perrin BS Jr, Zhu Y, Cardin R, Liberator PA, Anderson AS, Hao L. Surveillance of SARS-CoV-2 Mpro Reveals High Sequence and Structural Conservation Prior to the Introduction of Protease Inhibitor Paxlovid. mBio 2022;13(4):e0086922. https://doi.org/10.1128/mbio.00869-22
- Footnote 28
-
Arutyunova E, Khan MB, Fischer C, Lu J, Lamer T, Vuong W, van Belkum MJ, McKay RT, Tyrrell DL, Vederas JC, Young HS, Lemieux MJ. N-Terminal Finger Stabilizes the S1 Pocket for the Reversible Feline Drug GC376 in the SARS-CoV-2 Mpro Dimer. J Mol Biol 2021;433(13):167003. https://doi.org/10.1016/j.jmb.2021.167003
- Footnote 29
-
Chen SA, Arutyunova E, Lu J, Khan MB, Rut W, Zmudzinski M, Shahbaz S, Iyyathurai J, Moussa EW, Turner Z, Bai B, Lamer T, Nieman JA, Vederas JC, Julien O, Drag M, Elahi S, Young HS, Lemieux MJ. SARS-CoV-2 Mpro Protease Variants of Concern Display Altered Viral Substrate and Cell Host Target Galectin-8 Processing but Retain Sensitivity toward Antivirals. ACS Cent Sci 2023;9(4):696–708. https://doi.org/10.1021/acscentsci.3c00054
- Footnote 30
-
Iketani S, Hong SJ, Sheng J, Bahari F, Culbertson B, Atanaki FF, Aditham AK, Kratz AF, Luck MI, Tian R, Goff SP, Montazeri H, Sabo Y, Ho DD, Chavez A. Functional Map of SARS-CoV-2 3CL Protease Reveals Tolerant and Immutable Sites. Cell Host Microbe 2022;30(10):1354–62.e6. https://doi.org/10.1016/j.chom.2022.08.003
- Footnote 31
-
Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, Becker S, Rox K, Hilgenfeld R. Crystal Structure of SARS-CoV-2 Main Protease Provides a Basis for Design of Improved α-Ketoamide Inhibitors. Science 2020;368(6489):409–12. https://doi.org/10.1126/science.abb3405
- Footnote 32
-
Ip JD, Wing-Ho Chu A, Chan WM, Cheuk-Ying Leung R, Umer Abdullah SM, Sun Y, Kai-Wang To K. Global Prevalence of SARS-CoV-2 3CL Protease Mutations Associated with Nirmatrelvir or Ensitrelvir Resistance. EBioMedicine 2023;91:104559. https://doi.org/10.1016/j.ebiom.2023.104559
- Footnote 33
-
Zhou Y, Gammeltoft KA, Ryberg LA, Pham LV, Tjørnelund HD, Binderup A, Duarte Hernandez CR, Fernandez-Antunez C, Offersgaard A, Fahnøe U, Peters GHJ, Ramirez S, Bukh J, Gottwein JM. Nirmatrelvir-Resistant SARS-CoV-2 Variants with High Fitness in an Infectious Cell Culture System. Sci Adv 2022;8(51):eadd7197. https://doi.org/10.1126/sciadv.add7197
- Footnote 34
-
Greasley SE, Noell S, Plotnikova O, Ferre R, Liu W, Bolanos B, Fennell K, Nicki J, Craig T, Zhu Y, Stewart AE, Steppan CM. Structural Basis for the in Vitro Efficacy of Nirmatrelvir against SARS-CoV-2 Variants. J Biol Chem 2022;298(6):101972. https://doi.org/10.1016/j.jbc.2022.101972
- Footnote 35
-
Heilmann E, Costacurta F, Moghadasi SA, Ye C, Pavan M, Bassani D, Volland A, Ascher C, Weiss AKH, Bante D, Harris RS, Moro S, Rupp B, Martinez-Sobrido L, von Laer D. SARS-CoV-2 3CLpro Mutations Selected in a VSV-Based System Confer Resistance to Nirmatrelvir, Ensitrelvir, and GC376. Sci Transl Med 2023;15(678):eabq7360. https://doi.org/10.1126/scitranslmed.abq7360
- Footnote 36
-
Public Health Ontario. Weekly Epidemiologic Summary: SARS-CoV-2 Whole Genome Sequencing in Ontario, October 16, 2023. Toronto, ON: PHO; 2023. [Accessed 2023 Oct 22]. https://www.publichealthontario.ca/-/media/Documents/nCoV/epi/covid-19-sars-cov2-whole-genome-sequencing-epi-summary.pdf?rev=66a6cdcde04046b0abb44b0eaf7d648f&sc_lang=en
- Footnote 37
-
Sjaarda CP, Lau L, Simpson JT, Fattouh R, Biondi MJ, Maguire F, Campigotto A, Feng Y, Tozer K, Wong H, Sung WWL, Kim S, Marshall CR, Sheth PM, Kozak R. Prevalence of Low-Frequency, Antiviral Resistance Variants in SARS-CoV-2 Isolates in Ontario, Canada, 2020-2023. JAMA Netw Open 2023;6(7):e2324963. https://doi.org/10.1001/jamanetworkopen.2023.24963
Appendix
| Mpro mutation | Current evidence | Associated lineage | Year of lineage circulation | In vitro reported nirmatrelvir-resistant mutations in each lineage |
|---|---|---|---|---|
| A7T | Post-treatment emergence | BA.1.1 | 2022 | 19 |
| FT.1 | 2023 | 1 | ||
| XBB.1.22 | 2023 | 1 | ||
| XBB.1.5 | 2023 | 2 | ||
| A7V | Post-treatment emergence | BA.5.2.1 | 2022 | 1 |
| A7S | Post-treatment emergence | BQ.1 | 2023 | 1 |
| BQ.1.1 | 2023 | 1 | ||
| G15S | Biochemical assay, resistance selection study | BA.1.1 | 2022 | 1 |
| BQ.1.1.1 | 2023 | 2 | ||
| L30F | Post-treatment emergence | XBF | 2023 | 1 |
| L50F | Resistance selection study | BA.2 | 2022 | 2 |
| BA.2.12.1 | 2022 | 1 | ||
| BA.4.6 | 2022 | 2 | ||
| CC.1 | 2022 | 1 | ||
| BA.5.2.1 | 2023 | 1 | ||
| BQ.1.5 | 2023 | 1 | ||
| M82I | Post-treatment emergence | BA.5.1.24 | 2022 | 1 |
| BQ.1.2.3 | 2022 | 9 | ||
| T135I | Biochemical assay | BQ.1 | 2022 | 1 |
| BA.5.1.12 | 2023 | 2 | ||
| E166V | Biochemical assay, post-treatment emergence | BA.5.2.9 | 2022 | 1 |
| A173S | Biochemical assay, cell culture assay | BA.2 | 2022 | 1 |
| A173T | Biochemical assay, cell culture assay | BA.4.6 | 2022 | 4 |
| A173V | Biochemical assay, cell culture assay | XBB.1.5 | 2023 | 2 |
| XBB.1.9.1 | 2023 | 1 | ||
| Q189K | Biochemical assay | XBB.1.5 | 2023 | 1 |
| T196A | Post-treatment emergence | BA.2 | 2022 | 1 |
| BA.2.3 | 2022 | 1 | ||
| BA.4.1 | 2022 | 1 | ||
| W207S | Post-treatment emergence | BA.1 | 2021 | 1 |
| D248E | Biochemical assay | BQ.1.1 | 2023 | 3 |
| A260T | Post-treatment emergence | FY.5 | 2023 | 1 |
| XBB.1.5.39 | 2023 | 2 | ||
| D263E | Post-treatment emergence | BA.1.1 | 2022 | 3 |
| A266V | Post-treatment emergence | BQ.1.1 | 2022 | 1 |
Abbreviation: Mpro, main protease |
||||
