Surveillance of laboratory exposures to human pathogens and toxins, Canada, 2023

 Download this article as a PDF (360 KB)
Download this article as a PDF (360 KB)Published by: The Public Health Agency of Canada
Issue: CCDR Volume 51-1, January 2025: Personal Protective Measures
Date published: January 2025
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 51-1, January 2025: Personal Protective Measures
Surveillance
Surveillance of laboratory exposures to human pathogens and toxins, Canada, 2023
Abdulwadud Nafees1, Audrey Gauthier1, Antoinette N Davis1, Emily F Tran1, Christine Abalos1, Christa M Girincuti1, Samuel Bonti-Ankomah1
Affiliation
1 Regulatory, Operations and Emergency Management Branch, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Nafees A, Gauthier A, Davis AN, Tran EF, Abalos C, Girincuti CM, Bonti-Ankomah S. Surveillance of laboratory exposures to human pathogens and toxins, Canada, 2023. Can Commun Dis Rep 2025;51(1):16–25. https://doi.org/10.14745/ccdr.v51i01a03
Keywords: Centre for Biosecurity, human pathogens and toxins, laboratory-acquired infections, laboratory exposures, laboratory incidents, Laboratory Incident Notification Canada, surveillance
Abstract
Background: The Public Health Agency of Canada oversees the Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations, and monitors human pathogen and toxin incidents in licensed facilities to minimize exposure impact at the individual and population level.
Objective: To provide an overview of confirmed laboratory exposure incidents in Canada in 2023.
Methods: Confirmed exposure incident reports in 2023 were analyzed using R 4.2.2, Microsoft Excel and SAS.
Results: In 2023, 207 incident reports were received, including 63 confirmed exposure incidents that affected 85 individuals. The academic sector accounted for 50.8% (n=32) of the reported confirmed exposure incidents. Microbiology (n=33; 52.4%) was the predominant activity being performed, with the most common occurrence types being sharps-related (n=22; 27.2%) and procedure-related (n=16; 19.8%). Human interaction (n=36; 57.1%) and standard operating procedures (n=24; 38.1%) were the most frequent root causes cited, with corrective actions often directly addressing these causes. Most of the 85 affected individuals were technicians/technologists (n=55; 64.7%) and had a median of 11 years of laboratory experience. Sixty-seven human pathogens and toxins (HPTs) were implicated in the confirmed exposure incidents, with bacteria (n=36; 53.7%) being the most common biological agent type. The median time between the incident and the reporting date was six days.
Conclusion: The number of confirmed exposure incidents increased in 2023 compared to 2022. Microbiology was most often the activity being performed at the time of exposure, and occurrence-types, root causes and HPTs implicated in 2023 mirrored those cited in 2022.
Introduction
In the field of biosafety, the management of human pathogens and toxins (HPTs) is a matter of importance due to the potential for laboratory-acquired infections (LAIs) Footnote 1Footnote 2Footnote 3Footnote 4Footnote 5Footnote 6. Recognizing this risk, a rigorous approach to biosafety in facilities where controlled activities are conducted is necessary, including regulated safety practices and incident surveillance.
The backbone of Canada's regulatory framework in laboratory safety is the Human Pathogens and Toxins Act (HPTA) Footnote 7 and the Human Pathogens and Toxins Regulations (HPTR) Footnote 8, which are administered and enforced by the Public Health Agency of Canada's (PHAC's) Centre for Biosecurity. Since the enactment of the HPTA in 2009 and the HPTR in 2015, the HPTA/HPTR have set the standards for working with HPTs in various sectors such as hospitals, academic institutions and public or private institutions in Canada. There are four risk groups that classify HPTs based on their potential to harm individual and community health. For instance, risk group 1 (RG1) HPTs, like non-pathogenic Escherichia coli, are not expected to cause disease in humans, while risk group 4 (RG4) HPTs, such as the Ebola virus, are known for their potential to cause life-threatening diseases that spread rapidly through the community Footnote 9. Also included amongst these regulated HPTs are a class of risk group 3 (RG3) and RG4 HPTs known as security sensitive biological agents (SSBAs) that are specified due to their potential for use as biological weapons and for bioterrorism Footnote 9.
The Centre for Biosecurity established the Laboratory Incident Notification Canada (LINC) surveillance system in late 2015 to oversee HPT incident reporting, identification, monitoring and analysis and ensure appropriate follow-up and support to licensed facilities with the goal of reducing the risk of recurrence Footnote 10 and minimizing the impact of exposures on the health and wellbeing of facility personnel and the general population. Compared to incident surveillance systems in other developed countries, LINC remains the most comprehensive in terms of its scope. For instance, both the Federal Select Agent Program Footnote 11 in the United States and the Security Sensitive Biological Agents Standards Footnote 12 in Australia were established to provide regulatory oversight for only SSBAs, with the former producing an annual report on its inspections, compliance actions, transfer of biological select agents and toxins as well as the theft, loss or release of biological select agents and toxins in order to improve understanding of their mandate Footnote 13. Operating under the HPTA and HPTR, LINC's scope includes a much broader range of HPTs and is not limited to SSBAs Footnote 14.
Under the HPTA, any facility working with risk group 2 (RG2) pathogens and above must obtain a pathogen and toxin licence to conduct controlled activities with HPTs Footnote 7Footnote 15. The licence requires facilities to adhere to the outlined safety protocols and reporting standards. Licensed facilities are mandated to report various types of incidents to LINC without delay, including exposure incidents, which involve potential or actual contact with pathogens, and non-exposure incidents, such as a missing, lost or stolen biological agent, the inadvertent possession, production or release of an HPT and SSBAs not received at the facility within their expected arrival time. Other incidents that must be reported without delay include changes to biocontainment and other biosafety-related occurrences that may not directly involve pathogen exposure but have significant implications for laboratory safety. The reporting of incidents involving agents in their natural environment is voluntary. Pathogens in their natural environment refer to those present in uncultured or unprocessed samples collected directly from humans or animals. Such biological materials may include blood, serum, saliva, milk or urine.
The year 2023 marked the eighth year of the LINC surveillance system. The program's duration has allowed for the meaningful analysis of incident data from more than 361 confirmed exposure reports Footnote 16, which provided insight into laboratory safety measures, highlighted areas of progress and ongoing challenges Footnote 10Footnote 14Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21 and illuminated exposure incident trends such as the most common biological agent types (bacteria and virus) and leading root causes (standard operating procedures [SOPs] and human interaction).
This report summarizes exposure incidents in Canada that were reported to LINC in 2023 with the goal of enhancing awareness of the risks associated with handling HPTs, informing biosafety measures in facilities and comparing the incident data to those from previous years.
Methods
Data sources
Laboratory Incident Notification Canada is the Government of Canada's primary mechanism for collecting and monitoring incidents involving HPTs in licensed facilities across Canada under the HPTA and the HPTR. This system, which is accessible through an online Biosecurity Portal, facilitates the reporting of exposure, non-exposure and other types of incidents by licensed facilities. Once reported, these incidents are viewed and processed by LINC in the Integrated Suite of Tools for Operational Processes (iSTOP) of the Microsoft Customer Relationship Management system.
When a licensed facility reports an exposure incident, they are required to submit one or more follow-up reports in addition to their initial exposure report in order to provide further details and the most updated information regarding the incident.
Incidents reported between January 1, 2023, and December 31, 2023, were extracted from iSTOP on February 6, 2024, and analyzed. The analysis included incidents without a specified occurrence date, provided they were reported within this timeframe. Utilizing only the most recent follow-up reports ensured that the analysis was based on the latest and most accurate information pertaining to each incident. In cases where follow-up reports were not yet submitted to LINC, initial exposure report data were used. The extraction process involved examination for outliers and the removal of any duplicate entries to maintain the integrity of the data. The total number of active licences was extracted from the Customer Relationship Management on February 18, 2024, and additional filters were applied in iSTOP to obtain the number of active licences per sector. Some licences did not have a specified sector.
Report variables
The following variables were used to describe the confirmed exposure reports: the main activity being performed at the time of the exposure incident; sector affected; individuals, pathogens and toxins involved; root causes and corrective actions; occurrence types; and time delay in reporting. The definitions for the main activities are provided in Appendix Table A1. Sector variables include nine categories: academic; hospital; public health; veterinary/animal health; private industry/business; other government; environmental health; not specified; and “do-it-yourself biology,” where “do-it-yourself biology” refers to any individual not working in an institutionalized facility who is conducting their own experiments. Information about affected individuals, such as their role, years of experience and highest level of education, was also collected. Data on other characteristics, such as their age, gender and socioeconomic status, were not collected.
Data analysis
This report focuses on the confirmed exposure incidents reported to LINC in 2023. The classification of incidents into confirmed or ruled-out categories was based on a review of follow-up reports. Data were run in R 4.2.2 software for data wrangling, cleaning and generating descriptive statistics. Microsoft Excel and SAS 9.4 were used for data validation and to generate figures and tables. This dual approach allowed for cross-validation and ensured the quality of data for analysis. This year's analysis also re-examined data from 2016–2022 to account for any updates to previously submitted reports.
The exposure incident rate per 1,000 active licences was calculated by comparing the total number of reported exposure incidents against the total number of active licences during the surveillance period, multiplied by 1,000, to provide a standardized measure to assess trends over time and across different regulatory sectors.
Baseline establishment
An annual and monthly average of exposure incidents from 2016–2022 was calculated along with 95% confidence intervals using Microsoft Excel. To establish an annual baseline incidence, data from 2016–2022 were pooled and the total number of confirmed exposure incidents from 2016–2022 was summed and divided by the total number of active licences from 2016–2022 and multiplied by 1,000 to obtain the annual baseline incidence of exposures per 1,000 active licences.
Results
Figure 1 depicts the 207 laboratory incident reports submitted to LINC from January 1, 2023, to December 31, 2023. Out of these, 93 (44.9%) were exposure reports, 87 (42.0%) were non-exposure reports and 27 (13.0%) were other reports. Thirty exposure reports and 30 non-exposure reports were ruled out, leaving 63 confirmed exposure incidents with 85 affected individuals in 2023. Amongst the confirmed exposure incidents, there was one suspected LAI and three confirmed LAIs.
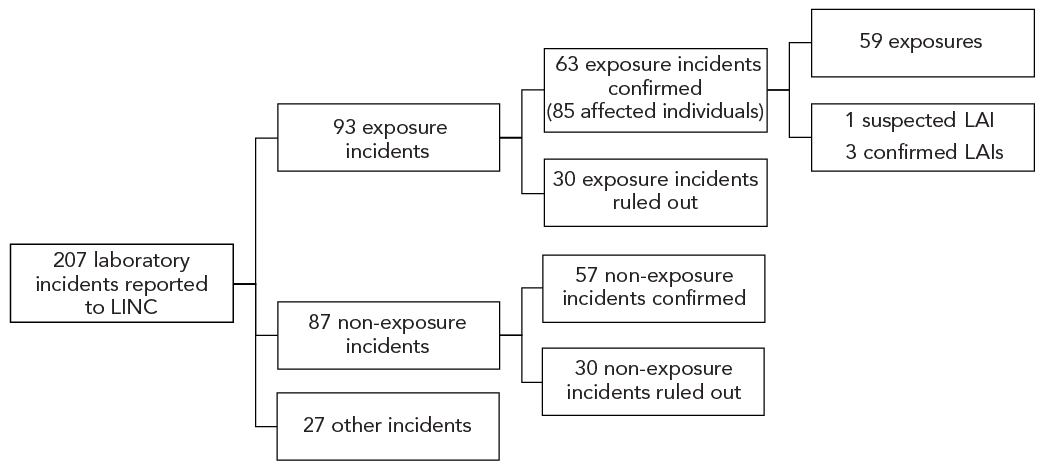
Figure 1: Descriptive text
| Incident | Count |
|---|---|
| All reports | 207 |
| Exposure | 93 |
| Non-exposure | 87 |
| Other | 27 |
| Incident | Confirmed |
| Exposure | 63 |
| Affected individuals | 85 |
| Non-exposure | 57 |
| Incident | Ruled out |
| Exposure | 30 |
| Non-exposure | 30 |
| Incident | Count |
| Exposure | 59 |
| LAI - suspected | 1 |
| LAI - confirmed | 3 |
Abbreviations: LAI, laboratory-acquired infection; LINC, Laboratory Incidence Notification Canada
There was a total of 1,057 active licences (Figure 2) in 2023, including 981 licences for RG2 HPTs, 70 licences for RG3 pathogens, two licences for RG4 pathogens and four licences for SSBAs. The number of confirmed exposure incidents per 1,000 active licences (the exposure incident rate) was 60. From 2016–2022, there was an average of 53.0 (95% CI: 38.7–7.3) exposure incidents per year and a yearly baseline incidence of 54.6 exposure incidents per 1,000 active licences.

Figure 2: Descriptive text
| Year | Number of active licenses | Exposures (Exposure + LAIs) |
Suspected LAI | Confirmed LAI | Exposure incident rate |
|---|---|---|---|---|---|
| 2016 | 835 | 43 | 2 | 1 | 5.1 |
| 2017 | 905 | 42 | 4 | 2 | 4.6 |
| 2018 | 985 | 96 | 4 | 2 | 9.7 |
| 2019 | 996 | 65 | 3 | 2 | 6.5 |
| 2020 | 999 | 40 | 0 | 0 | 4.0 |
| 2021 | 1,027 | 45 | 0 | 3 | 4.4 |
| 2022 | 1,048 | 40 | 2 | 0 | 3.8 |
| 2023 | 1,057 | 63 | 1 | 3 | 6.0 |
Abbreviation: LAI, laboratory-acquired infection
From 2016–2022, there was an average of 4.4 (95% CI: 3.8–5.0) exposure incidents per month. The number of confirmed exposure incidents remained relatively stable in 2023, with five confirmed exposure reports each month for seven of the 12 months (Figure 3). The lowest number of exposure reports occurred in June (n=2; 3.2%) and the highest occurred in October (n=9; 14.3%). In comparison, the baseline incidence per month per 1,000 active licences and the median from 2016–2022 peaked in May and September.
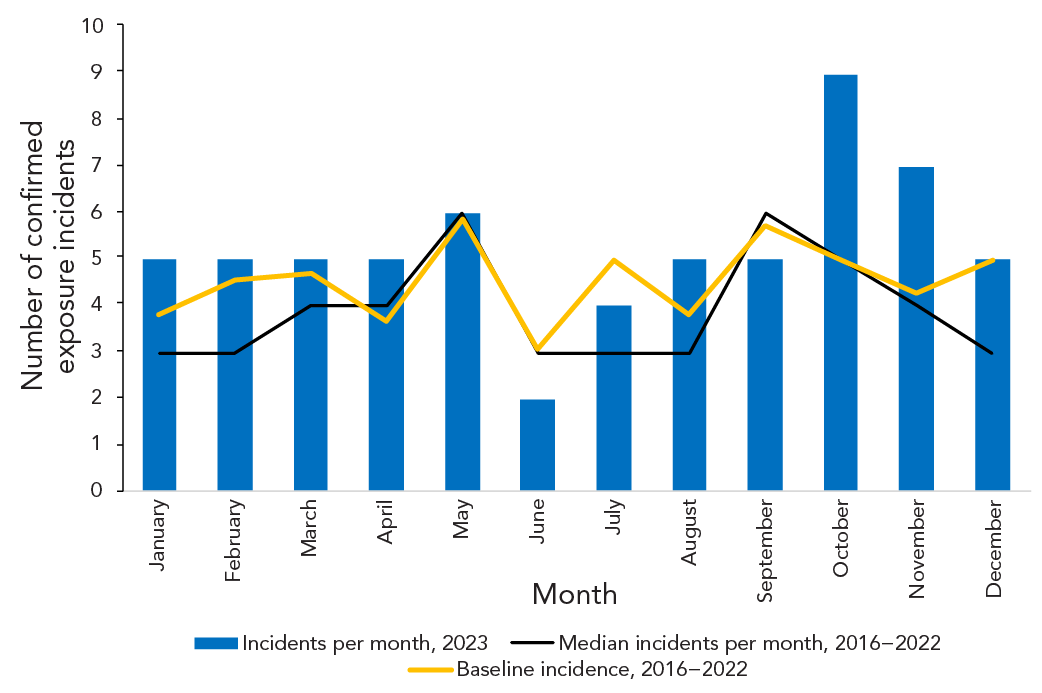
Figure 3: Descriptive text
| Month | Median incidents per month, 2016–2023 |
Incidents per month, 2023 |
Average, 2016–2022 |
Baseline incidence, 2016–2022 |
|---|---|---|---|---|
| January | 3 | 5 | 3.7 | 3.8 |
| February | 3 | 5 | 4.4 | 4.6 |
| March | 4 | 5 | 4.6 | 4.7 |
| April | 4 | 5 | 3.6 | 3.7 |
| May | 6 | 6 | 5.7 | 5.9 |
| June | 3 | 2 | 3.0 | 3.1 |
| July | 3 | 4 | 4.9 | 5.0 |
| August | 3 | 5 | 3.7 | 3.8 |
| September | 6 | 5 | 5.6 | 5.7 |
| October | 5 | 9 | 4.9 | 5.0 |
| November | 4 | 7 | 4.1 | 4.3 |
| December | 3 | 5 | 4.9 | 5.0 |
Exposure incidents by main activity and sector
Microbiology and in vivo animal research were the most common main activities being performed at the time of the confirmed exposure incident (n=33; 52.4% and n=13; 20.6%, respectively) (data not shown). Other activities (n=6; 9.5%), cell culture (n=5; 7.9%), maintenance (n=3; 4.8%), microscopy (n=2; 3.2%) and education or training (n=1; 1.6%) were also mentioned as main activities being performed at the time of exposure.
The largest number of confirmed exposure incidents were reported by the academic (n=32; 50.8%) and hospital (n=20; 31.7%) sectors, as shown on Figure 4. Only four confirmed exposures were reported from the private sector (6.3%). The active licences are distributed among multiple sectors, including academic, hospital, private and public health. Most licences in 2023 were held by private facilities (n=533; 50.7%), academic facilities (n=216; 20.5%) and hospitals (n=177; 16.8%).
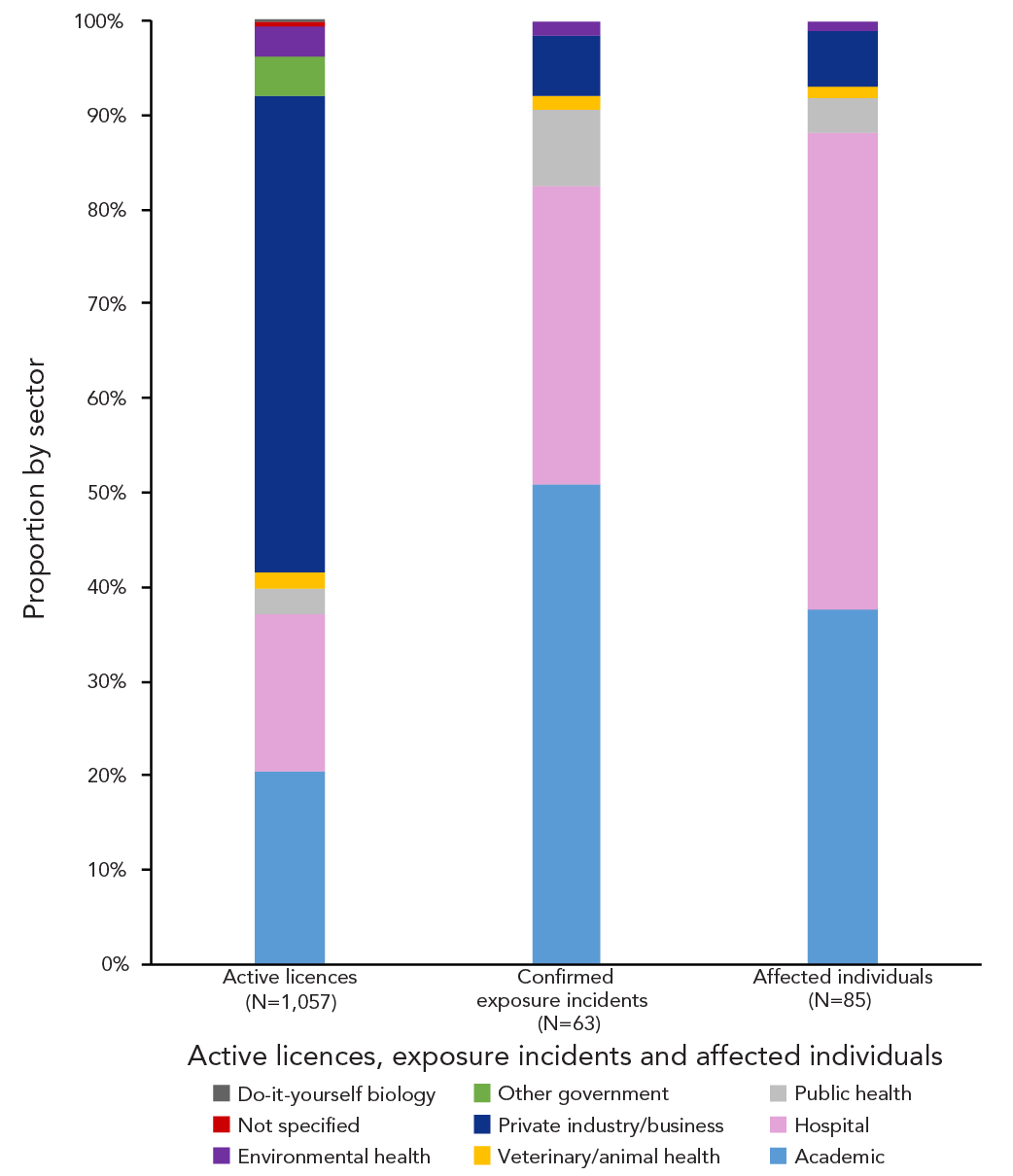
Figure 4: Descriptive text
| Sector | Active licences (N=1,057) |
Confirmed exposure incidents (N=63) |
Affected individuals (N=85) |
|---|---|---|---|
| Academic | 216 | 32 | 32 |
| Hospital | 177 | 20 | 43 |
| Public health | 28 | 5 | 3 |
| Veterinary/animal health | 18 | 1 | 1 |
| Private industry/business | 533 | 4 | 5 |
| Other government | 45 | 0 | 0 |
| Environmental health | 34 | 1 | 1 |
| Not specified | 5 | 0 | 0 |
| Do-it-yourself biology | 1 | 0 | 0 |
| Sum | 1,057 | 63 | 85 |
Affected individuals
An average of 1.57 persons were affected per confirmed exposure incident in 2023, with 85 individuals affected in total. Of these 85 individuals, 43 were affected through confirmed exposure incidents in hospital sector (50.6%), while 32 were affected through confirmed exposure incidents in the academic sector (37.6%), as shown in Figure 4. The veterinary/animal health and environmental health sectors each had one confirmed exposure (1.6%) with one affected individual (1.2%) in each.
The largest number of individuals affected in a single confirmed exposure incident (inhalation of Brucella melitensis caused by an inadvertent possession of the pathogen) was 11 in a hospital laboratory. The majority of individuals affected in confirmed exposure incidents in 2023 were technicians/technologists (n=55; 64.7%) with a median number of 11 years of experience working in a laboratory setting (Figure 5). Among the affected individuals, 20 were students (23.5%) with a median of 2.5 years of experience and seven were researchers (8.2%) with a median of six years of experience. In 2023, only one supervisor/manager was involved in a confirmed exposure incident (1.2%). That individual had 18 years of laboratory experience.
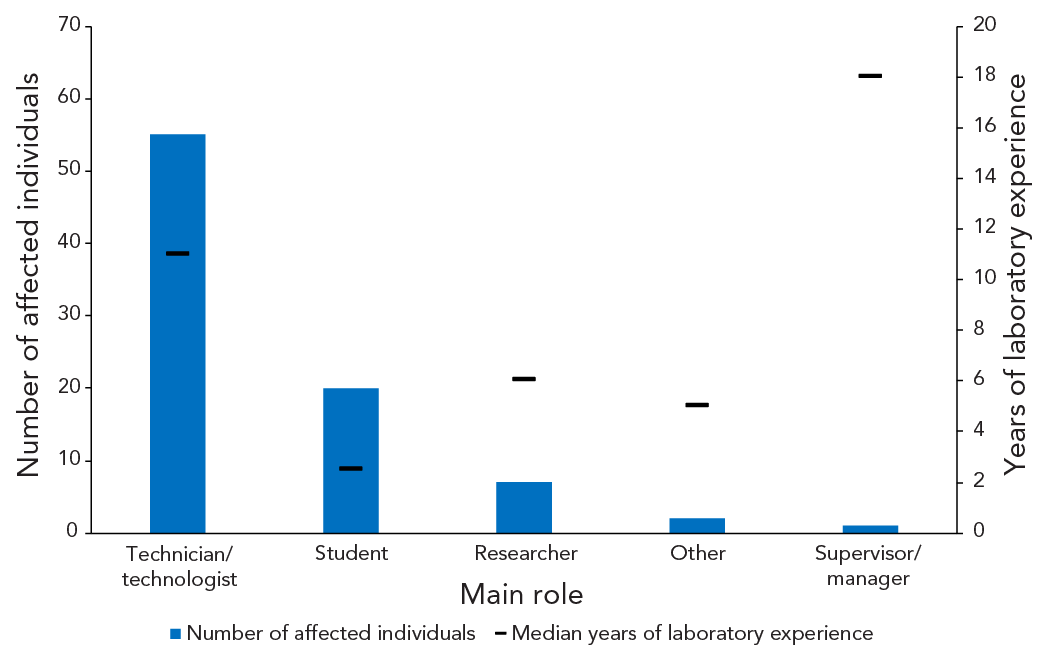
Figure 5: Descriptive text
| Main role | Median years of laboratory experience | Number of affected individuals |
|---|---|---|
| Technician/technologist | 11 | 55 |
| Student | 2.5 | 20 |
| Researcher | 6 | 7 |
| Other | 5 | 2 |
| Supervisor/manager | 18 | 1 |
Implicated human pathogens and toxins
Sixty-seven HPTs were implicated in confirmed exposure incidents in 2023 (Table 1). Exposures were predominantly with non-SSBAs (n=57; 85.1%). Among the RG2 HPTs (n=48; 71.6%), the most common agent types were bacteria (n=30; 44.8%) and viruses (n=14; 20.9%). Other HPT agent types, such as fungus, parasite, prion and cell line, were each implicated in one exposure incident. For exposure incidents involving RG3 HPTs (n=15; 22.4%), the most common agent types were bacteria (n=6; 9.0%), fungus (n=5; 7.5%) and virus (n=3; 4.5%). The RG2 HPTs most frequently implicated in exposure incidents were Neisseria meningitidis (n=8; 16.7%) and Staphylococcus aureus (n=7; 14.6%), while among the RG3 agents, B. melitensis (n=3; 20%) as well as Histoplasma capsulatum and Mycobacterium tuberculosis (n=2; 13.3% each) were most common. Only one exposure incident implicating SARS-CoV-2 was reported in 2023. Enterohemorrhagic E. coli and Salmonella enterica were implicated in two of the three confirmed LAIs, while the HPT implicated in the third confirmed LAI was unknown. Shiga toxin-producing E. coli (STEC) was implicated in the suspected LAI. There were no exposures to RG4 pathogens in 2023.
| Biological agent type by risk group | Non-SSBA | SSBA | Unknown | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| RG2 | 48 | 71.6 | 0 | 0 | 0 | 0 | 48 | 71.6 |
| Bacteria | 30 | 44.8 | 0 | 0 | 0 | 0 | 30 | 44.8 |
| Fungus | 1 | 1.5 | 0 | 0 | 0 | 0 | 1 | 1.5 |
| Parasite | 1 | 1.5 | 0 | 0 | 0 | 0 | 1 | 1.5 |
| Prion | 1 | 1.5 | 0 | 0 | 0 | 0 | 1 | 1.5 |
| Toxin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Virus | 14 | 20.9 | 0 | 0 | 0 | 0 | 14 | 20.9 |
| Cell line | 1 | 1.5 | 0 | 0 | 0 | 0 | 1 | 1.5 |
| RG3 | 9 | 13.4 | 6 | 9.0 | 0 | 0 | 15 | 22.4 |
| Bacteria | 2 | 3.0 | 4 | 6.0 | 0 | 0 | 6 | 9.0 |
| Fungus | 4 | 6.0 | 1 | 1.5 | 0 | 0 | 5 | 7.5 |
| Parasite | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prion | 1 | 1.5 | 0 | 0 | 0 | 0 | 1 | 1.5 |
| Toxin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Virus | 2 | 3.0 | 1 | 1.5 | 0 | 0 | 3 | 4.5 |
| Cell line | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown agents | 0 | 0 | 0 | 0 | 4 | 6.0 | 4 | 6.0 |
| Total | 57 | 85.1 | 6 | 9.0 | 4 | 6.0 | 67 | 100 |
Abbreviations: RG2, risk group 2; RG3, risk group 3; SSBA, security sensitive biological agents |
||||||||
Occurrence types
More than one occurrence type could be selected for each of the 63 confirmed exposure incidents. Eighty-one occurrence types were identified in 2023 (Figure 6). The most frequently cited occurrence type was sharps-related (n=22; 27.2%). There were also 16 (19.8%) procedure-related occurrences, 13 (16.0%) spill-related occurrences and 11 (13.6%) occurrences categorized as “other.” The “other” occurrence type included exposures due to work performed on an open bench and accidental ingestion. There were three (3.7%) unknown occurrence types. Definitions of the occurrence types are provided in Appendix Table A2.

Figure 6: Descriptive text
| Occurrence type | Number of occurrences |
|---|---|
| Insect | 0 |
| Equipment | 1 |
| Loss | 3 |
| Unknown | 3 |
| Animal | 5 |
| PPE | 7 |
| Other | 11 |
| Spill | 13 |
| Procedure | 16 |
| Sharps | 22 |
Abbreviations: animal, animal-related; equipment, equipment-related; loss, loss of containment-related; PPE, personal protective equipment-related; procedure, procedure-related; sharps, sharps-related
Root causes and corrective actions
Many of the confirmed exposure incidents were associated with more than one root cause (Table 2), with a total of 131 root causes identified and an average of 2.08 per exposure incident. Human interaction was the root cause identified in 36 (57.1%) confirmed exposure incidents, while SOPs were identified as the root cause in 24 (38.1%) confirmed exposure incidents.
| Root cause | Examples of areas of concern | Citations | Corrective actions | ||
|---|---|---|---|---|---|
| n | %Footnote a | nFootnote b | %Footnote c | ||
| Human interaction | A violation (cutting a corner, not follow correct procedure, deviating from standard operating procedure) | 36 | 57.1 | 22 | 61.1 |
| An error (a mistake, lapse of concentration or slip of any kind) | |||||
| Standard operating procedure (SOP) | Documents were followed as written but not correct for activity/task | 24 | 38.1 | 20 | 83.3 |
| Procedures that should have been in place were not in place | |||||
| Documents were not followed correctly | |||||
| Training | Training was not in place but should have been in place | 19 | 30.2 | 15 | 78.9 |
| Training was not appropriate for task/activity | |||||
| Staff were not qualified or proficient in performing task | |||||
| Management and oversight | Supervision needed improvement | 17 | 27.0 | 11 | 64.7 |
| Lack of auditing of standards, policies and procedures | |||||
| Risk assessment needed improvement | |||||
| Equipment | Equipment quality control needed improvement | 16 | 25.4 | 8 | 50.0 |
| Equipment failed | |||||
| Equipment was not appropriate for purpose | |||||
| Communication | Communication did not occur but should have | 15 | 23.8 | 12 | 80.0 |
| Communication was unclear, ambiguous, etc. | |||||
| Other | Not applicable | 4 | 6.3 | 0 | 0 |
Footnotes
|
|||||
Corrective actions were compared with the root causes of each confirmed exposure incident (Table 2). The corrective actions that addressed the same root cause were related to SOPs (n=20; 83.3%), communication (n=12; 80.0%) and training (n=15; 78.9%). Only 50.0% of confirmed exposure incidents with an equipment-related root cause were addressed by corrective actions in this same area of concern (n=8).
Reporting delay to Public Health Agency of Canada
The reporting delay refers to the number of days between the date of the confirmed exposure incident's occurrence and the date on which it was first reported to PHAC via LINC. In 2023, the median reporting delay was six days, as was the median reporting delay in 2021 and 2022 (Figure 7). The 25th percentile for reporting delay was two days, consistent with the previous five years, while the 75th percentile was 16.25 days, more than double what it was in 2022 due to retrospective data entry of previously unreported exposure incident reports from 2016–2023 that were discovered during an on-site inspection.

Figure 7: Descriptive text
| Median time | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|---|---|
| MIN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25th percentile | 4.5 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| Median | 16 | 7 | 6 | 5 | 5 | 6 | 6 | 6 |
| 75th percentile | 43.5 | 18.5 | 22 | 11 | 13.5 | 16 | 8 | 16.25 |
| MAX | 456 | 137 | 2,062 | 1,783 | 1,176 | 868 | 552 | 199 |
Discussion
In 2023, an increase in confirmed exposure incidents was observed in comparison with the preceding three years. While there are likely multiple contributing factors to this increase, one of the most significant may be the COVID-19 pandemic. The pandemic, which occurred between 2020 and 2022, significantly altered normal work practices in many fields by limiting the number of workers and changing the volume and type of laboratory activity conducted Footnote 22. The number of confirmed exposure reports in 2023 was similar to the pre-pandemic period. As seen in previous years Footnote 10Footnote 14Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21 the academic and hospital sectors contributed the largest proportion of confirmed exposure incidents. The most common activities being performed during a confirmed exposure event were microbiology and in vivo animal research, and confirmed exposures due to sharps-, procedure- and spill-related occurrences were most often cited, with human interaction and SOPs as the most common root causes. Technicians/technologists made up the majority of affected individuals, while non-SSBAs were implicated most frequently in confirmed exposure incidents. Compared to 2021 and 2022, there was no change in the median reporting delay.
Corrective actions undertaken following a confirmed exposure incident
Understanding the underlying causes of incidents and developing strategies to prevent recurrence, especially for system-level failures rather than individual errors, is important Footnote 23Footnote 24. Part of the exposure incident follow-up process includes the reporting of corrective actions taken by regulated facilities. Corrective actions fall into the same categories as root causes, allowing for an assessment of incidents based on the appropriateness of the applied corrective actions. In 2023, the root cause that was most frequently addressed through corrective actions following a confirmed exposure incident was SOP. Well-designed systems have just as much of an impact on safety as do individual-level capabilities and errors Footnote 23. This understanding drives SOP changes, which generally involve modifications to workflow or communication protocols. The higher rate of corrective action may reflect the tangible nature of these procedural improvements, which can be directly implemented and monitored Footnote 23. Training-related solutions were also frequently observed in 2023, aligning with literature that emphasizes the important role of continuous education in mitigating errors and enhancing safety Footnote 24. Training addresses immediate knowledge gaps and enhances skillsets Footnote 23. Efforts to improve communication through corrective actions, with 80.0% of related incidents addressed in 2023, emphasize the importance of effective communication channels in laboratory settings, which are foundational for error prevention and risk mitigation once an incident has already occurred Footnote 25.
Corrective actions were not reported for some root causes, like “other,” which included unpredictable animal behaviour. This may indicate areas where solutions are more challenging to identify or implement. Corrective actions addressing equipment or ”other” issues may require more resource-intensive solutions or reflect a lower perceived risk Footnote 25.
Non-security sensitive biological agents, risk group 2 and bacteria remain the most reported human pathogen and toxin types
Since the establishment of the LINC program and incident reporting, a large proportion of pathogens implicated in confirmed exposure incidents have consistently been RG2 non-SSBAs and, most commonly, bacterial agents Footnote 10Footnote 14Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21. This trend continued in 2023, with non-SSBAs implicated in 85.1% of confirmed exposure incidents and RG2 HPTs accounting for 71.6% of HPTs identified. Almost 45% of agent types involved in exposure incidents were bacteria, which reflects the findings by Blacksell et al. (2024), where the predominant cause of exposure incidents that resulted in LAIs was a bacterial pathogen Footnote 1. The consistently high percentage of RG2 HPTs involved in confirmed exposure incidents reported to LINC is likely because the majority of active licences (92.8% in 2023) are held by facilities carrying out controlled activities with RG2 HPTs. Similarly, in 2023, the majority of facilities were licensed to work with non-SSBAs, with only 0.4% of active licences granted for SSBAs, thus explaining the higher proportion of non-SSBAs implicated in confirmed exposure incidents compared to SSBAs.
Sharps and procedure-related occurrences and support for licence holders
The leading occurrence-types cited in confirmed exposure incidents in 2023 were sharps (27.2%) and procedures (19.8%). This is consistent with annual report data from previous years Footnote 10Footnote 14Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21. These occurrence types, sharps in particular, frequently occur in laboratories and have often resulted in exposure incidents Footnote 3Footnote 10. For example, a study using data of clinical laboratory workers from private and government health sectors in Al-Madinah, Saudi Arabia also found that sharps-related injuries were commonly experienced among the workers and were associated with a lack of biosafety training Footnote 26. As such, preventing needlestick and sharps-related injuries within laboratories remains crucial due to their potential to transmit pathogens Footnote 27.
To raise awareness of common causes of exposure incidents, mitigate the recurrence and encourage a culture of laboratory biosafety, LINC developed several new resources to support licence holders. These resources, which can be found online in the PHAC Training Portal, facilitate the dissemination of biosafety best practices and clarify reporting procedures using a variety of easily accessible formats, including videos, an e-learning course, webinars, downloadable and fillable forms and a podcast.
Strengths and limitations
A strength of this report is that it involved a comprehensive dataset, encompassing over eight years of data. The standardized reporting forms used as part of the incident reporting process ensured uniform data collection and ensured data reliability for trend analysis and identification of biosafety challenges.
This report has several limitations. Currently, individual-level data of all laboratory workers, such as their age, sex, experience and education background, income and other sociodemographic measures, are not collected. Such data could permit detailed analyses involving inferential statistics and hypothesis-based studies focused on potential variables associated with laboratory exposure incidents. Other limitations include the small sample size and the possibility of underreporting of laboratory exposure incidents, the extent of which remains unknown. It should also be noted that licensed facilities self-identify their sector when creating a user profile in the Biosecurity Portal as part of the licensing process, and they can only select one sector, though overlap with another sector may exist in actuality. For instance, a hospital may select the academic sector as their sector because they are affiliated with a university. This should be kept in mind when interpreting the results. Finally, a lack of comparable national incident reporting surveillance systems outside of Canada made it challenging to compare the findings and trends of this report with those of other countries.
Conclusion
In 2023, the number of confirmed exposure incidents rose and resembled levels seen prior to the COVID-19 pandemic. The most common occurrence-types, main activity being performed, root causes and HPTs implicated in confirmed exposure incidents in 2023 mirrored those cited in 2022. The natural baseline that was calculated will serve as an additional reference point for assessment in future years. Findings from this report can be used to inform biosafety practices and procedures in facilities to reduce the incidence of exposure to HPTs.
Authors' statement
- AN — Methodology, data analysis, writing–original draft, writing–review & editing
- AG — Conceptualization, incident monitoring, methodology, data analysis, data validation, writing–original draft, writing–review & editing
- AND — Conceptualization, writing–original draft, writing–review & editing, supervision
- CA — Incident monitoring, writing–original draft, writing–review & editing
- EFT — Methodology, writing–original draft, writing–review & editing
- CG — Writing–review & editing
- SBA — Writing–review & editing
Competing interests
None.
ORCID numbers
- Antoinette N. Davis — 0009-0002-5720-7742
- Audrey Gauthier — 0009-0005-8729-3743
- Christine Abalos — 0009-0009-5501-549X
- Emily F. Tran — 0009-0005-0414-5025
- Samuel Bonti-Ankomah — 0000-0002-7189-1643
Acknowledgements
Thank you to our licensed facilities for their ongoing compliance with incident notification and reporting.
Funding
This work was supported by the Public Health Agency of Canada as part of its core mandate.
References
- Footnote 1
-
Blacksell SD, Dhawan S, Kusumoto M, Le KK, Summermatter K, O’Keefe J, Kozlovac JP, Almuhairi SS, Sendow I, Scheel CM, Ahumibe A, Masuku ZM, Bennett AM, Kojima K, Harper DR, Hamilton K. Laboratory-acquired infections and pathogen escapes worldwide between 2000 and 2021: a scoping review. Lancet Microbe 2024;5(2):e194–202. https://doi.org/10.1016/S2666-5247(23)00319-1
- Footnote 2
-
Haines CA, Gronvall GK. Improving U.S. Biosafety and Biosecurity: Revisiting Recommendations from the Federal Experts Security Advisory Panel and the Fast Track Action Committee on Select Agent Regulations. Appl Biosaf 2023;28(1):43–54. https://doi.org/10.1089/apb.2022.0025
- Footnote 3
-
El Jaouhari M, Atchessi N, Edjoc R, Striha M, Bonti-Ankomah S. Multivariate analyses of risk factors associated with laboratory exposure incidents. Can Commun Dis Rep 2022;48(7/8):350–5. https://doi.org/10.14745/ccdr.v48i78a06
- Footnote 4
-
El Jaouhari M, Striha M, Edjoc R, Bonti-Ankomah S. Laboratory-acquired infections in Canada from 2016 to 2021. Can Commun Dis Rep 2022;48(7/8):303–7. https://doi.org/10.14745/ccdr.v48i78a02
- Footnote 5
-
Cornish NE, Anderson NL, Arambula DG, Arduino MJ, Bryan A, Burton NC, Chen B, Dickson BA, Giri JG, Griffith NK, Pentella MA, Salerno RM, Sandhu P, Snyder JW, Tormey CA, Wagar EA, Weirich EG, Campbell S. Clinical Laboratory Biosafety Gaps: Lessons Learned from Past Outbreaks Reveal a Path to a Safer Future. Clin Microbiol Rev 2021;34(3):e0012618. https://doi.org/10.1128/CMR.00126-18
- Footnote 6
-
Singh K. Laboratory-acquired infections. Clin Infect Dis 2009;49(1):142–7. https://doi.org/10.1086/599104
- Footnote 7
-
Public Health Agency of Canada. Human Pathogens and Toxins Act (HPTA). Ottawa, ON: PHAC; 2023. [Accessed 2024 Jan 17]. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act.html
- Footnote 8
-
Justice Laws Website. Human Pathogens and Toxins Regulations (SOR/215-44). Ottawa, ON: Justice Laws Website; 2024. [Accessed 2024 Jan 25]. https://laws-lois.justice.gc.ca/eng/regulations/SOR-2015-44/index.html
- Footnote 9
-
Public Health Agency of Canada. Canadian Biosafety Guideline Pathogen Risk Assessment. Ottawa, ON: PHAC; 2018. [Accessed 2024 Sept 25]. https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/pathogen-risk-assessment/document.html
- Footnote 10
-
Abalos C, Gauthier A, Davis A, Ellis C, Balbontin N, Kapur A, Bonti-Ankomah S. Surveillance of laboratory exposures to human pathogens and toxins, Canada, 2022. Can Commun Dis Rep 2023;49(9):398–405. https://doi.org/10.14745/ccdr.v49i09a06
- Footnote 11
-
Centers for Disease Control and Prevention. About Us. Atlanta, GA: CDC; 2023. [Accessed 2024 Jan 17]. https://www.selectagents.gov/overview/
- Footnote 12
-
Department of Health and Aged Care. Security Sensitive Biological Agents (SSBA) Regulatory Scheme. Canberra, AU: DHAC; 2024. [Accessed 2024 Jan 24]. https://www.health.gov.au/our-work/ssba-regulatory-scheme
- Footnote 13
-
Centers for Disease Control and Prevention, Animal and Plant Health Inspection Service. 2022 Annual Report of the Federal Select Agent Program. Atlanta, GA/Riverdale, MD: CDC/APHIS; 2022. [Accessed 2024 Sept 27]. https://www.selectagents.gov/resources/publications/docs/FSAP_Annual_Report_2022_508.pdf
- Footnote 14
-
Pomerleau-Normandin D, Heisz M, Tanguay F. Surveillance of laboratory exposures to human pathogens and toxins: Canada 2017. Can Commun Dis Rep 2018;44(11):297–304. https://doi.org/10.14745/ccdr.v44i11a05
- Footnote 15
-
Public Health Agency of Canada. Licensing program. Ottawa, ON: PHAC; 2024. [Accessed 2024 Jan 17]. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program.html
- Footnote 16
-
Balbontin N, Gauthier A, Abalos C, Davis AN, Lister M. Canadian laboratory incidents with human pathogens and toxins: an overview of reports, 2016−2022. Can Commun Dis Rep 2024;50(5):144–52. https://doi.org/10.14745/ccdr.v50i05a04
- Footnote 17
-
Atchessi N, Striha M, Edjoc R, Thompson E, El Jaouhari M, Heisz M. Surveillance of laboratory exposures to human pathogens and toxins, Canada 2020. Can Commun Dis Rep 2021;47(10):422–9. https://doi.org/10.14745/ccdr.v47i10a04
- Footnote 18
-
Lien A, Abalos C, Atchessi N, Edjoc R, Heisz M. Surveillance of laboratory exposures to human pathogens and toxins, Canada 2019. Can Commun Dis Rep 2020;46(9):292–8. https://doi.org/10.14745/ccdr.v46i09a07
- Footnote 19
-
Choucrallah D, Sarmiento L, Ettles S, Tanguay F, Heisz M, Falardeau E. Surveillance of laboratory exposures to human pathogens and toxins: Canada 2018. Can Commun Dis Rep 2019;45(9):244–51. https://doi.org/10.14745/ccdr.v45i09a04
- Footnote 20
-
Bienek A, Heisz M, Su M. Surveillance of laboratory exposures to human pathogens and toxins: Canada 2016. Can Commun Dis Rep 2017;43(11):228–35. https://doi.org/10.14745/ccdr.v43i11a04
- Footnote 21
-
Thompson ER, El Jaouhari M, Eltayeb N, Abalos C, Striha M, Edjoc R, Ayoo C, Bonti-Ankomah S. Surveillance of laboratory exposures to human pathogens and toxins, Canada, 2021. Can Commun Dis Rep 2022;48(10):484–91.https://doi.org/10.14745/ccdr.v48i10a08
- Footnote 22
-
Stenson MC, Fleming JK, Johnson SL, Caputo JL, Spillios KE, Mel AE. Impact of COVID-19 on access to laboratories and human participants: exercise science faculty perspectives. Adv Physiol Educ 2022;46(2):211–8. https://doi.org/10.1152/advan.00146.2021
- Footnote 23
-
Kellogg KM, Hettinger Z, Shah M, Wears RL, Sellers CR, Squires M, Fairbanks RJ. Our current approach to root cause analysis: is it contributing to our failure to improve patient safety? BMJ Qual Saf 2017;26(5):381–7. https://doi.org/10.1136/bmjqs-2016-005991
- Footnote 24
-
Liu HC, Zhang LJ, Ping YJ, Wang L. Failure mode and effects analysis for proactive healthcare risk evaluation: A systematic literature review. J Eval Clin Pract 2020;26(4):1320–37. https://doi.org/10.1111/jep.13317
- Footnote 25
-
Wood LJ, Wiegmann DA. Beyond the corrective action hierarchy: A systems approach to organizational change. Int J Qual Health Care 2020;32(7):438–44. https://doi.org/10.1093/intqhc/mzaa068
- Footnote 26
-
Khabour OF, Al Ali KH, Mahallawi WH. Occupational infection and needle stick injury among clinical laboratory workers in Al-Madinah city, Saudi Arabia. J Occup Med Toxicol 2018;13:15. https://doi.org/10.1186/s12995-018-0198-5
- Footnote 27
-
Centers for Disease Control and Prevention. Sharps Safety Program Resources. Atlanta, GA: CDEC; 2024. [Accessed 2024 Mar 28]. https://www.cdc.gov/sharpssafety/index.html
Appendices
| Main activity | Definition |
|---|---|
| Animal care | Activities such as attending to the daily care of animals and providing animals with treatment |
| Autopsy or necropsy | Post-mortem surgical examinations for purposes such as determining cause of death or to evaluate disease or injury for research or educational purposes |
| Cell culture | The process of growing cells under controlled conditions. It can also involve the removal of cells from an animal or plant |
| Education or training | Education or training of students and/or personnel on laboratory techniques and procedures |
| In vivo animal research | Experimentation with live, non-human animals |
| Maintenance | The upkeep, repair and/or routine and general cleaning of equipment and facilities |
| Microbiology | Activities involving the manipulation, isolation or analysis of microorganisms in their viable or infectious state |
| Molecular investigations | Activities involving the manipulation of genetic material from microorganisms or other infectious material for further analysis |
| Serology | Diagnostic examination and/or scientific study of immunological reactions and properties of blood serum |
| Hematology | Scientific study of the physiology of blood |
| Occurrence type | Definition |
|---|---|
| Spill | Any unintended release of an agent from its container |
| Loss of containment | Includes malfunction or misuse of containment devices or equipment and other type of failures that results in the agent being spilled outside of, or released from, containment |
| Sharps-related | Includes needle stick, cut with scalpel, blade or other sharps injury (i.e., broken glass) |
| Animal-related | Includes animal bites or scratches, as well as other exposure incidents resulting from animal behavior (i.e., animal movement resulting in a needle stick) |
| Insect-related | Includes insect bites |
| PPE-related | Includes either inadequate PPE for the activity or failure of the PPE in some way |
| Equipment-related | Includes failure of equipment, incorrect equipment for the activity or misuse of equipment |
| Procedure-related | Includes instances when written procedures were not followed, were incorrect for the activity or were inadequate or absent |
Abbreviation: PPE, personal protective equipment |
|
