Enhanced screening for tuberculosis infection among immigrants in southern New Brunswick

 Download this article as a PDF (211 KB)
Download this article as a PDF (211 KB)Published by: The Public Health Agency of Canada
Issue: Volume 51-5, May 2025: Travel Health
Date published: May 2025
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 51-5, May 2025: Travel Health
Epidemiologic Study
Enhanced screening for tuberculosis infection among immigrants in southern New Brunswick: A cross-sectional pilot study
Isdore Chola Shamputa1, Duyen Thi Kim Nguyen2,3, Hope Mackenzie4, Derek J Gaudet5, Alicia Harquail2, Kim Barker2, Duncan Webster6,7,8
Affiliations
1 Department of Nursing and Health Sciences, University of New Brunswick, Saint John, NB
2 Department of Health, Government of New Brunswick, Saint John, NB
3 Faculty of Business, University of New Brunswick, Saint John, NB
4 Microbiology Laboratory, Saint John Regional Hospital, Saint John, NB
5 Department of Psychology, University of New Brunswick, Saint John, NB
6 Dalhousie Medicine New Brunswick, Dalhousie University, Saint John, NB
7 Division of Medical Microbiology, Department of Laboratory Medicine, Saint John Regional Hospital, Saint John, NB
8 Division of Infectious Diseases, Department of Medicine, Saint John Regional Hospital, Saint John, NB
Correspondence
Suggested citation
Shamputa IC, Nguyen DTK, Mackenzie H, Gaudet DJ, Harquail A, Barker K, Webster D. Enhanced screening for tuberculosis infection among immigrants in southern New Brunswick: A cross-sectional pilot study. Can Commun Dis Rep 2025;51(5):167–78. https://doi.org/10.14745/ccdr.v51i05a03
Keywords: tuberculosis infection, screening, prevention, immigrant health, IGRA
Abstract
Background: In 2021, approximately 77% of active tuberculosis (TB) disease (TBD) cases in Canada were among foreign-born individuals. Less than 3% of TBD cases in Canada are detected through pre-arrival Canadian immigration medical examinations (i.e., chest X-rays), and the remaining 97% are likely due to reactivation of undiagnosed latent TB infection (TBI) post-arrival. In New Brunswick, the proportion of TBD cases among foreign-born individuals gradually increased from about 33% (1/3 individuals) in 2013 to 100% (14/14 individuals) in 2023. The objective of this study was to estimate the prevalence of TBI among immigrants in southern New Brunswick, identify potential predictors for positive TBI screening and assess participant experiences with the pilot TBI screening procedure.
Methods: A cross-sectional study was conducted from November 2021 to November 2023 among immigrants ≥19 years old who had no history of TBD and were born in a country with a TB incidence rate of ≥40/100,000 population or were referred by healthcare professionals. Participants were recruited through various channels and underwent TBI screening using the interferon-gamma release assay, followed by a survey on their screening experience.
Results: Of the 264 participants, 49 (18.6%) screened positive for TBI. Factors associated with higher odds of screening TBI-positive included birthplace in a “highly to severely endemic” (≥300/100,000 population) TB-incidence country (OR=3.24; 95% CI: 1.07–9.81) and increased age (OR=1.05; 95% CI: 1.01–1.08). Participants rated the pilot TBI screening procedure positively (mean scores ranged from 4.03–4.55 on a five-point Likert scale).
Conclusion: Results suggest that immigrants born in countries with TB incidences of ≥300/100,000 population should be considered for screening and treatment of TBI. The pilot TBI screening procedure yielded positive feedback. Further research with a larger sample is recommended.
Introduction
Approximately 25% of the global population has latent tuberculosis (TB) infection (TBI) Footnote 1Footnote 2, of which 5%–10% go on to develop active TB disease (TBD) Footnote 3Footnote 4Footnote 5. In 2023, there were 10.8 million TBD cases and 1.25 million TBD-related deaths worldwide Footnote 5. While more than 80% of the TBD cases and deaths occur in low- and middle-income countries Footnote 5, high-income countries also report TBD cases, in part due to immigration and international travel Footnote 6Footnote 7Footnote 8Footnote 9.
In 2021, Canada had a TBD incidence rate of 4.8 cases per 100,000 population and 76.7% of the cases were among individuals born outside Canada Footnote 10. The Atlantic Canadian province of New Brunswick (NB) has experienced a notable rise in immigration in the last decade Footnote 11Footnote 12. Coinciding with this immigration growth is the increase in the proportion of TBD cases among foreign-born individuals. For example, the proportion of foreign-born TBD cases has gradually risen from 33% (n=1) in 2013 to 100% of cases in 2022 (n=17) and 2023 (n=14) (personal communication, Public Health Agency of Canada TB Task Force Meeting, November 26–27, 2024). The rise of TBD in NB provides an opportunity for the provincial healthcare system to explore additional preventative strategies to curb the increase of TBD and protect and promote the health of its population, while continuing to welcome new immigrants to the province.
Current pre-arrival Canadian immigration medical examinations focus on detecting TBD through screening with chest X-ray Footnote 13. The current screening system does not assess for TBI, thus creating a gap where individuals with TBI are at-risk of TB reactivation post-arrival. Research has shown that <3% of TBD cases diagnosed among immigrants to Canada are detected through the immigration post-landing surveillance program Footnote 14. In 2019, Immigration, Refugees and Citizenship Canada (IRCC) broadened screening requirements to include pre-arrival TBI screening for certain high-risk immigration applicants (Box 1) Footnote 13. To our knowledge, this program has not been evaluated and the number of TBD cases averted is unknown. While the TB incidence rate of a person's country of birth has been shown to be a risk factor for TBI Footnote 15Footnote 16Footnote 17Footnote 18, it is noticeably absent from the recent IRCC recommendations Footnote 13. Interestingly, there are currently no widely established avenues for routine TBI screening for immigrants in Canada, despite its potential to curb increasing prevalence of TBD, and the call from experts for enhanced screening efforts Footnote 19.
Box 1: Immigration, Refugees and Citizenship Canada screening criteria for tuberculosis infectionFootnote a
Screening criteria for tuberculosis infection
- Individuals seropositive for human immunodeficiency syndrome
- Individuals who have been in close contact with TB disease in the last five years
- Individuals with a history of certain head and neck cancers within the previous five years
- Individuals undergoing dialysis or suffering from advanced chronic kidney disease
- Individuals who have had solid organ or bone marrow transplants and are receiving immunosuppressive therapy
- Box 1 Footnote a
-
Adapted from Immigration, Refugees and Citizenship Canada. Canadian panel member guide to immigration medical examinations Immigration, Refugees and Citizenship Canada. Ottawa, ON: Government of Canada. https://www.canada.ca/en/immigration-refugees-citizenship/corporate/publications-manuals/panel-members-guide.html
The purpose of this study was to enhance current TBI screening in southern NB, which presently follows the IRCC TB screening protocol, by investigating the potential value of a targeted TBI pilot screening program among immigrants in southern NB. This study seeks to address the gap from IRCC's current TB screening system, by estimating the TBI prevalence among immigrants in southern NB, identifying potential predictors of screening positive for TBI and evaluating the experience of immigrants taking part in the pilot TBI screening procedure. To our knowledge, this is the first Canadian study to offer routine TBI screening to all immigrant streams (e.g., temporary foreign workers, family reunion, permanent residents, international students, refugees), whereas in previous Canadian TBI studies, the primary focus was on the refugee populations Footnote 20Footnote 21Footnote 22Footnote 23.
The interferon-gamma release assay (IGRA) was used for TBI screening in this study instead of the traditional tuberculin skin test (TST). The IGRA offers several advantages, including the use of antigens more specific to Mycobacterium tuberculosis, a single visit for blood draw, screening not influenced by the Bacille Calmette−Guérin (BCG) vaccination or previous exposure to certain non-tuberculous mycobacteria and improved consistency in results with fewer concerns regarding inter-rater reliability Footnote 24Footnote 25.
Methods
Study design
A cross-sectional study was conducted among immigrants to Canada, from November 4, 2021, to November 21, 2023. Participants were eligible if they were ≥19 years old and resided in southern NB, and were either: a) born in a high-TB-incidence country, defined as ≥40 cases per 100,000 population) Footnote 7, b) were referred to Public Health NB by IRCC or c) were at high risk of TBI due to having a TBD-positive partner or having lived in a high-TB-incidence country, or were considered to be at high-risk of TBI following the post-arrival health assessments (PAHAs) of government-assisted refugees (GARs) by NB Primary Health Care. Participants with a history of TBD or TBD treatment were excluded from this study.
Participant recruitment
Participants were recruited using posters, social media channels, snowball sampling, public health channels or PAHAs of GARs. Prospective participants contacted the research team using the phone number or email provided on recruitment materials or met with a research team member in-person after their PAHAs. To help minimize potential recruitment bias, this study included all eligible immigrants at risk of TBI, irrespective of the language they spoke, when they arrived in Canada, or their immigration stream.
Sample size was estimated a priori as previously described Footnote 26Footnote 27. Based on an estimated prevalence of TBI of 25% Footnote 2, our calculation yielded an estimated minimum sample size of 160 individuals. The published study protocol describes a minimum sample size estimate of 240 individuals, but this discrepancy is due to removing two predictors (i.e., comorbidities and immigration stream) Footnote 28. Co-morbidities were removed because they were not feasible to collect due to lack of access to personal medical records, and immigration stream was omitted because it resulted in too many dummy variables, which would have reduced the power of the analysis. Due to the aforementioned reasons, only four of the six original variables were included in the study's regression analyses (i.e., age, sex, gender and incidence rate classification).
Procedure
Potential participants were asked to provide demographic information, including sex, gender, date of birth, country of birth, date of arrival in Canada and type of Canadian entry visa to assist with screening for eligibility.
Pilot tuberculosis infection screening process
Those that met the study's eligibility criteria were provided a consent form to review. Consent forms were translated into the seven most spoken languages among immigrants in southern NB Footnote 29. Following written consent, a research member organized phlebotomy appointments at one of two local hospitals, chosen by the participant. During phlebotomy, hospital staff guided participants through established protocols. For participants recruited at PAHAs, the study was explained by a research team member in their chosen language with the help of a virtual translation service. The YMCA of Greater Saint John staff, which is the local immigrant-serving organization responsible for GAR settlement, arranged phlebotomy appointments and accompanied participants. Other community partners, such as the Saint John Newcomers Centre and PRUDE Inc., also offered close collaboration to support study participants.
Materials
All blood samples were transported to the Saint John Regional Hospital Microbiology Laboratory for TBI screening. Screening was performed using the IGRA (QuantiFERON-TB Gold Plus; QIAGEN, Germantown, Maryland, United States [US]) as per the manufacturer's recommendations. The IGRA results were categorized as either positive (≥0.35 IU/mL), negative (<0.35 IU/mL) or indeterminate Footnote 29. An IGRA was repeated if samples yielded an indeterminate result. If the result remained indeterminate, next steps were determined through review and clinical assessment by an infectious disease specialist. The IGRA results and relevant diagnostic data were accessed and communicated to participants by a healthcare provider. Participants positive for TBI were offered clinical assessment. In cases, where the IGRA was positive, TBD was ruled out and participants were offered TB preventive treatment (TPT). The TPT results will be provided in a follow-up paper.
Following the screening procedure, participants were asked to complete a TBI pilot screening process experience survey using Qualtrics, an online survey platform. The survey was intended to gather information regarding their experience of participating in this study, and included 11 questions on five-point Likert scales, along with open-ended questions. Participants completed the survey virtually through an email containing an attachment or a link to the survey, provided by a research team member. The survey questions focused on 1) equity, diversity, and inclusion; 2) barriers and facilitators to TBI screening, such as language, cultural factors, accessibility of the blood collection facility, interactions with healthcare professionals; and 3) the overall ease or difficulty of participating in the study (see Appendix 1) Footnote 28.
Data analysis and management
The TB incidence rate of a participant's country of birth was obtained through World Health Organization (WHO) data Footnote 30 to create incidence rate categories. Participants were classified as being born in countries with incidence rates considered to be low (<10/100,000 population), lower-moderate (10–49/100,000 population), upper moderate (50–99/100,000 population), endemic (100–299/100,000 population), highly endemic (300–499/100,000 population) or severely endemic (≥500/100,000 population), as per the WHO classification Footnote 31. Due to low counts in certain categories, and to maintain adequate statistical power, incidence categories were combined into three new categories for the analyses: “sub-endemic” (0–99/100,000 population), comprising low, lower-moderate and upper moderate, and “highly to severely endemic” (≥300/100,000 population), comprising highly endemic and severely endemic. The “endemic” category (100–299/100,000 population) remained unchanged Footnote 31.
In terms of data management and analysis, survey data was completed in Qualtrics, exported to Excel and merged with participants' demographic data; thereafter, the data was de-identified and stored on an encrypted OneDrive account. The quantitative de-identified data was imported into IBM SPSS Statistics (version 29; Armonk, New York, US) for analysis. Categorical measures were presented as frequencies and percentages, and continuous measures were presented using means and standard deviations (SD). Associations between predictors and TBI were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
For the sensitivity analysis, literature suggests that recent immigrants are at a higher risk of developing TBD from TBI within the first two to five years of their arrival Footnote 32; thus, binary logistic regression analysis was repeated using data from those that had arrived in the last five years, as this cohort would benefit most from being screened and treated.
Ethical approval
This study was approved by the Horizon Health Network (file #: RS 2021–3046) and University of New Brunswick (file #: 033-2021) Research Ethics Boards.
Results
Participants
Of 292 participants who consented to this study, a total of 28 participants were excluded as they had not submitted blood specimens for TBI screening (n=26) or had a previously undisclosed history of TBD and treatment (n=2). Among 26 participants who did not provide blood samples for TBI screening, 17 were born in “endemic” to “highly to severely endemic” TB incidence countries, of whom 16 (94.1%) relocated to other provinces. After excluding the aforementioned participants, the total sample size was 264 (Figure 1).
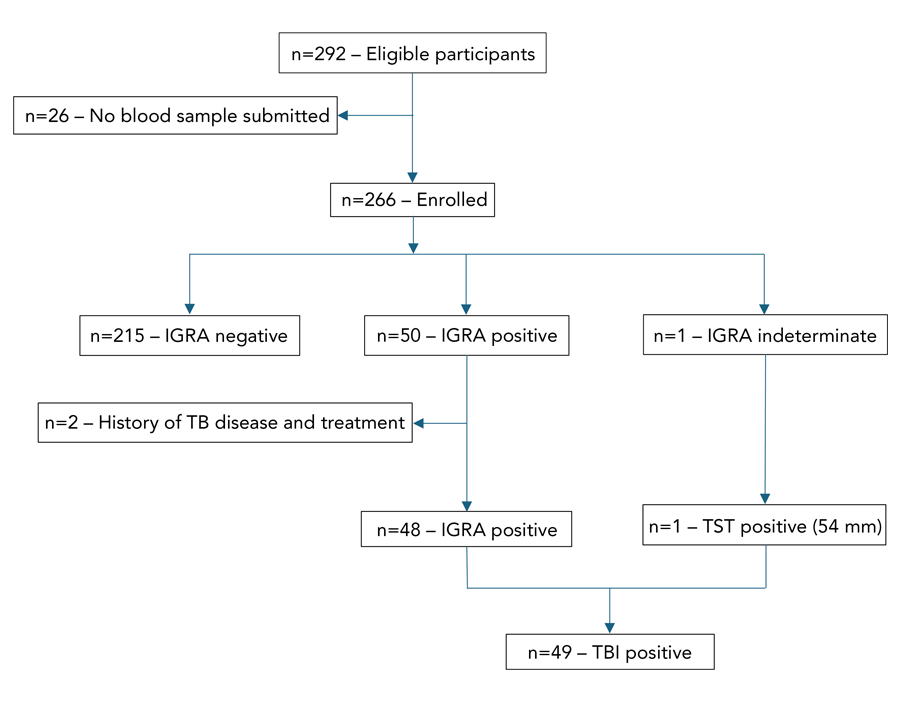
Figure 1: Descriptive text
This figure demonstrates enrollment of eligible participants into the study with flow through the screening stream. A total of 292 eligible participants provided consent and were recruited into the study. However, 26 participants did not provide the required blood specimen for screening to move through study enrollment. As such, 266 participants were enrolled following screening bloodwork, of whom 215 screened negative. Of the remaining 51 participants, 50 screened positive with the interferon-gamma release assay (IGRA) and underwent further assessment. Two of the 50 IGRA-positive participants were identified as having a prior history of tuberculosis (TB) disease with prior treatment and were found not to have a clinical picture consistent with TB infection and were excluded from the study. One participant had indeterminate screening with the IGRA and subsequently had a positive tuberculin skin test and with further clinical assessment was diagnosed with TB infection. As such, a total of 49 individuals were diagnosed with TB infection in this screening study.
Abbreviations: IGRA, interferon-gamma release assay; TB, tuberculosis; TBI, tuberculosis infection; TST, tuberculin skin test
The average age of the 264 participants was 36.8 years (SD=10.27), ranging from 19 to 67 years. More than half (53.8%) identified as female. Self-reported biological sex and gender identity was consistent across participants, except for one participant who identified their biological sex as male and identified their gender as a trans-female. Given the near-identical count for biological sex and gender identity, sex was retained in the regression analyses, while gender was dropped. Additional descriptive characteristics are presented in Table 1.
| Characteristic | Variables | TBI | n | |
|---|---|---|---|---|
| PositiveFootnote a (%) | Negative (%) | |||
Sex |
Male | 21 (17.2%) | 101 (82.8%) | 122 |
| Female | 28 (19.7%) | 114 (80.3%) | 142 | |
| Total | 49 (18.6%) | 215 (81.4%) | 264 | |
Age group (years) |
19‒24 | 3 (8.6%) | 32 (91.4%) | 35 |
| 25‒34 | 11 (14.1%) | 67 (85.9%) | 78 | |
| 35‒44 | 22 (22.0%) | 78 (78.0%) | 100 | |
| 45‒54 | 8 (21.1%) | 30 (78.9%) | 38 | |
| 55‒64 | 4 (36.4%) | 7 (63.6%) | 11 | |
| 65 and older | 1 (50.0%) | 1 (50.0%) | 2 | |
| Total | 19 (7.2%) | 215 (81.4%) | 264 | |
Year of arrival in Canada |
2000 | 0 (0.0%) | 1 (100%) | 1 |
| 2001 | 1 (100%) | 0 (0.0%) | 1 | |
| 2002 | 0 (0.0%) | 1 (100%) | 1 | |
| 2007 | 0 (0.0%) | 3 (100%) | 3 | |
| 2010 | 0 (0.0%) | 4 (100%) | 4 | |
| 2012 | 0 (0.0%) | 2 (100%) | 2 | |
| 2013 | 0 (0.0%) | 2 (100%) | 2 | |
| 2014 | 1 (100%) | 0 (0.0%) | 1 | |
| 2015 | 0 (0.0%) | 1 (100%) | 1 | |
| 2016 | 1 (33.3%) | 2 (66.7%) | 3 | |
| 2017 | 0 (0.0%) | 7 (100%) | 7 | |
| 2018 | 1 (10.0%) | 9 (90.0%) | 10 | |
| 2019 | 3 (16.7%) | 15 (83.3%) | 18 | |
| 2020 | 0 (0.0%) | 6 (100%) | 6 | |
| 2021 | 5 (16.1%) | 26 (83.8%) | 31 | |
| 2022 | 18 (19.4%) | 75 (80.6%) | 93 | |
| 2023 | 19 (23.8%) | 61 (76.2%) | 80 | |
| Total | 49 (18.6%) | 215 (81.4%) | 264 | |
Country of birth by World Health region |
Western Pacific | 7 (20.6%) | 27 (79.4%) | 34 |
| Americas | 4 (16.7%) | 20 (83.3%) | 24 | |
| Africa | 11 (24.4%) | 34 (75.6%) | 45 | |
| South-East Asia | 2 (6.7%) | 28 (93.3%) | 30 | |
| Europe | 0 (0.0%) | 8 (100%) | 8 | |
| Eastern Mediterranean | 25 (20.3%) | 98 (79.7%) | 123 | |
| Total | 49 (18.6%) | 215 (81.4%) | 264 | |
TBI screening results by recruitment category |
Born in high TB incidence countryFootnote b |
44 (20.4%) | 172 (79.6%) | 216 |
Referred by Public HealthFootnote b |
1 (50.0%) | 1 (50.0%) | 2 | |
Recruited via PAHAsFootnote b |
4 (10.3%) | 35 (89.7%) | 39 | |
| At-risk of TBIFootnote c | 0 (0.0%) | 7 (100%) | 7 | |
| Total | 49 (18.6%) | 215 (81.4%) | 264 | |
TB incidence by country of birth (per 100,000 population) |
Sub-endemic (0–99) | 15 (13.8%) | 94 (86.2%) | 109 |
| Endemic (100–299) | 25 (20.0%) | 100 (80.0%) | 125 | |
| Highly to severely endemic (≥300) | 9 (30.0%) | 21 (70.0%) | 30 | |
| Total | 49 (18.6%) | 215 (81.4%) | 264 | |
Primary healthcare provider (doctor/nurse practitioner) in Canada |
No | 49 (21.7%) | 177 (78.3%) | 226 |
| Yes | 0 (0.0%) | 38 (100%) | 38 | |
| Total | 49 (18.6%) | 215 (81.4%) | 264 | |
Abbreviations: PAHA, post-arrival health assessment; TB, tuberculosis; TBI, tuberculosis infection Footnote
|
||||
Tuberculosis infection screen outcome
Of 264 participants, 18.6% (n=49) had a positive TBI screening. One participant had an indeterminate IGRA result on two separate screens due to a reactive negative control. Subsequent TST in the indeterminate case revealed an induration of 54 mm. Based on the participant's demographics, clinical evaluation and radiological studies, this participant was categorized as TBI-positive (Figure 1). Table 1 includes TBI screening results by sex, age range, year of arrival in Canada, country of birth by WHO region, recruitment category, TB incidence of country of birth and primary healthcare provider.
When comparing IGRA screening results by recruitment categories, most of the positive results were found among immigrants born in high-TB-incidence countries. Similarly, when analyzed based on TB incidence rate classifications, a higher proportion of participants born in “highly to severely endemic” TB countries screened positive for TBI (30%), compared to those born in TB-“endemic” (20%) and “sub-endemic” (14%) countries (Table 1).
Binary logistic regression
Binary logistic regression was used to predict TBI screen outcome using age, sex and TB incidence category by country of birth as predictors. Odds ratios were examined to determine the impact of each variable. The binary logistic regression model was statistically significant, χ2 (4)=13.42, p=0.009, with a Nagelkerke R2 value of 0.08. The odds of a positive screen were approximately 3.5 times higher for individuals born in a “highly to severely endemic” country compared to those born in countries classified as “sub-endemic” (OR=3.45; 95% CI: 1.28–9.27). Also, age was found to increase the odds of a positive TBI screen (OR=1.05; 95% CI: 1.02–1.08). Neither sex, nor birth in a TB “endemic” country, was found to be statistically significant (Table 2). Sensitivity analyses results were consistent with the main results (Appendix 2, Table A1).
| Predictor variable | ß | SE | p-value | Odds | 95% CI for odds ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.048 | 0.016 | 0.003 | 1.049 | 1.016 | 1.083 |
| Sex | −0.106 | 0.329 | 0.747 | 0.899 | 0.472 | 1.713 |
| Endemic | 0.516 | 0.364 | 0.156 | 1.675 | 0.821 | 3.421 |
| Highly to severely endemic | 1.237 | 0.505 | 0.014 | 3.446 | 1.280 | 9.272 |
Abbreviations: CI, confidence interval; SE, standard error |
||||||
Participant experience of the tuberculosis infection screen process
To assess the participants' experience in the TBI pilot screening procedure, means and SDs were calculated to assess each survey item response and interpreted with guidance described previously Footnote 33. Surveys regarding the pilot TBI screening procedure were completed by 176 participants (66.7 overall response rate), with more than half (54.1%) being self-reported female respondents. The mean age was 36.78 years (SD=9.75). Participant responses are presented in Table 3. Participants rated the ease of locating the phlebotomy site most favourably (M=4.55, SD=0.68) and wait times for phlebotomy least favourably (M=4.03, SD=1.03). Participants reported positive attitudes towards TB and expressed willingness to recommend TBI screening to others.
| Item | N | Mean | SD |
|---|---|---|---|
| I received information on why the latent tuberculosis test was being done. | 153 | 4.52 | 0.61 |
| The blood collection office was easy to find. | 172 | 4.55 | 0.68 |
| The blood collection office was easy to travel to. | 171 | 4.53 | 0.61 |
| The blood collection process was simple (e.g., registration, blood collection). | 172 | 4.49 | 0.64 |
| The waiting time for blood collection was reasonable. | 166 | 4.03 | 1.03 |
| The healthcare provider (i.e., doctor, nurse) answered all my questions. | 168 | 4.35 | 0.81 |
| I was satisfied with the overall experience with the latent tuberculosis screening process and/or care I received. | 172 | 4.46 | 0.77 |
| I would recommend other people to do a latent tuberculosis screening test. | 169 | 4.52 | 0.65 |
| My knowledge regarding tuberculosis improved by participating in the study. | 169 | 4.12 | 0.96 |
| My attitudes regarding tuberculosis improved by participating in the study. | 167 | 4.25 | 0.79 |
Abbreviation: SD, standard deviation |
|||
Discussion
This pilot study was the first to offer routine TBI screening among immigrants in Atlantic Canada. It differs from previous studies in Canada in several key ways: a) TBI screening was offered to all immigrant groups, rather than focusing on refugee populations; b) it employed the IGRA, in contrast to the traditional TST; and c) the research team collaborated closely with immigrant-serving community partners, which was deemed important for raising awareness and the successful completion of TBI screening and treatment programs in this setting. With respect to the current TB screening practices in NB, our study identified nearly 50 TBI-positive individuals of which only one was referred by IRCC to Public Health NB. These results highlight potential opportunities for increased screening for TBI amongst immigrants in NB.
This study had three key findings. First, a TBI prevalence of 18.6% was found among immigrants in southern NB. Second, increased age and birth in a country with a “highly to severely endemic” TB incidence were identified as factors associated with greater odds of screening positive for TBI. Third, the IGRA worked well in this setting and study participants reported that the pilot TBI screening procedure used in this study was satisfactory.
The prevalence of TBI in this study is comparable to earlier reports of immigrants in other low TB incidence countries Footnote 34Footnote 35, but lower than global and Canadian estimates of 25% Footnote 36Footnote 37. We suspect this difference may be attributed to the local pattern of immigration during the study period and/or the smaller study sample size.
Results regarding the association between birth in a “highly to severely endemic” TB incidence country and TBI are akin to previous research Footnote 35Footnote 37Footnote 38. These findings indicate that TBI screening for immigrants born in “endemic” and “sub-endemic” countries may be of less value than screening immigrants from countries with “highly to severely endemic” TB incidence. Likewise, our results associating older age with positive TBI screening are congruent with earlier reports Footnote 39Footnote 40Footnote 41Footnote 42. One plausible explanation for these associations is that older age and high TB incidence in the immigrant's country of birth increases the participants' vulnerability for TBI due to greater time to the potential exposure to TBD, emphasizing the need to institute mitigating factors, such as TB awareness campaigns, early detection and TPT to protect and enhance overall health.
It is noteworthy that most participants who relocated to other provinces before providing blood samples for TBI screening were born in “endemic” and “highly to severely endemic” countries. This is concerning, as TBI is not reportable in most public health jurisdictions and cases may go unidentified with the potential for development of TBD. Study participants expressed general satisfaction with the TBI screening process, and the use of the IGRA as the screening tool was a novel component of this study. However, participants desired shorter wait times for sample collection. Additionally, there was a recognized need for increased awareness campaigns aimed at improving understanding and attitudes toward TB.
Regarding the year of arrival and TBI screening, it is important to recognize that immigrants to Canada who have lived in the country for an extended time may travel back to their home country. Although they may have tested negative for TBD during their initial immigration screening, these subsequent visits could be associated with new exposures and the potential for subsequent development of TBD Footnote 43. However, this would have had minimal impact on our sample, as over three-quarters of study participants arrived within the previous three years.
Use of the IGRA allowed for several distinct advantages in this pilot screening study. Many study participants had a prior history of BCG, which lowers the specificity of the TST Footnote 25Footnote 44. In addition, many participants were recent immigrants with multiple competing obligations and new to a local health system with many barriers. As such, minimizing the complexity and the number of clinic visits was desirable. Many immigrants were undergoing phlebotomy for other PAHA-associated testing and thus the IGRA could be easily incorporated into this process. No additional follow-up reading was required, as would have been the case with the use of the TST Footnote 25. In a setting of low TB incidence, where proficiency with TST administration and reading may be inadequate, the IGRA provided objective results, avoiding issues of inter-observer variability Footnote 44. Furthermore, in a post-pandemic setting with strained healthcare resources, a shortage of clinical staff, and a lack of quality assessment with TST, the IGRA can promote efficiency in the use of local resources. The cost effectiveness of the IGRA over the TST in this setting continues to be assessed and will benefit from further study.
In March 2024, the Public Health Agency of Canada established a one-year time-limited TB task group, whose membership includes representatives from each province, territory and national Indigenous organizations. The timeliness of these findings is relevant to the anticipated recommendations to be shared with the Communicable and Infectious Disease Subcommittee of the Public Health Agency of Canada in March of 2025.
Limitations
Results from this study should be interpreted with caution. First, the study participation acceptance rate was not calculated, due to various recruitment methods, which made it difficult to track those who declined to participate. Thus, feedback regarding participants' experiences of the pilot TBI screening procedure may be biased towards those who are concerned about TB. Second, this study had a relatively small sample size, and the fluctuating immigration patterns during the study period may limit the generalization and application of the study's results to inform policy. Also, the study produced fewer positive screening results than the expected number that was based on the global TBI incidence estimate. Although statistical power was maintained, this came at the cost of excluding predictors such as immigration stream. Further, while we intended to include all six WHO TB recommended incidence categories into the model, sample size necessitated the collapse of some categories, resulting in the use of only three broader categories. Future research should aim to include all six recommended categories to establish a more granular understanding of the risk associated with WHO-designated incidence rate categories. Third, data on comorbidities were not gathered, due to challenges in obtaining accurate information. Such data could have offered insights into the risk of early progression from TBI to TBD. More stringent inclusion criteria, refining recruitment methods, and expanding the sample size could help address these limitations. Fourth, the nature of the study limited the ability to account for potential confounding factors, as it was not an experimental study with random sampling or stringent controls. While all immigrants were eligible to participate, only those who volunteered during the recruitment period were included, which may introduce self-selection bias. We recognize the potential for other confounding variables, but addressing them was beyond the scope of this study. Although the regression analysis accounts for age and sex, no additional data were collected to control for other potential confounders.
Conclusion
This study provides initial insights into the prevalence and contributing factors associated with screening positive for TBI among immigrants in southern NB. Study results highlight the role for screening immigrants from countries with a TB incidence of ≥300/100,000 population using the IGRA. With further refinement, the TBI screening procedure used in this study could be valuable for broader program application.
Authors' statement
- ICS — Conceptualization, methodology, acquired the financial support, investigation, data curation, software, validation, formal analysis, writing–original draft, writing–review and editing
- DTKN — Conceptualization, methodology, validation, acquired the financial support, writing–original draft, writing–review and editing
- HM — Writing–review and editing, supervision
- DJG — Validation, data curation, software, formal analysis, writing–original draft, writing–review and editing
- AH — Investigation, writing–review and editing
- KB — Conceptualization, supervision, acquired the financial support, writing–review and editing
- DW — Conceptualization, investigation, validation, acquired the financial support, supervision, data development, writing–review and editing
The content and view expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
Competing interests
ICS, KB and DW report receiving in-kind donation of QuantiFERON®-TB Gold Plus test kits used in the study from QIAGEN Inc. QIAGEN Inc. also covered a portion of the article processing charges for the published protocol of this study.
ORCID numbers
- Isdore Chola Shamputa — 0000-0003-1888-8936
- Kim Barker — 0000-0001-5391-8999
- Duncan Webster — 0000-0001-7692-7150
Acknowledgements
We would like to thank the staff of immigrant-serving organizations including the YMCA of Greater Saint John, Saint John Newcomer Centre and PRUDE Inc., as well as laboratory technologists at the Saint John Regional Hospital, primary care nurses, study participants and language translators.
Funding
This work was funded by the Chesley Family Research Award, Research NB, New Brunswick Innovation Fund (Emerging projects ref #: EP_2022_017), the University of New Brunswick (Student Work Program), and QIAGEN Inc. The funders did not play any role in the design of the study, collection and analysis of data, decision to publish or manuscript preparation.
References
- Footnote 1
-
Delogu G, Goletti D. The spectrum of tuberculosis infection: new perspectives in the era of biologics. J Rheumatol Suppl 2014;91:11–6. https://doi.org/10.3899/jrheum.140097
- Footnote 2
-
Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016;13(10):e1002152. https://doi.org/10.1371/journal.pmed.1002152
- Footnote 3
-
Rahlwes KC, Dias BR, Campos PC, Alvarez-Arguedas S, Shiloh MU. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023;14(1):2150449. https://doi.org/10.1080/21505594.2022.2150449
- Footnote 4
-
Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 2009;10(9):943–8. https://doi.org/10.1038/ni.1781
- Footnote 5
-
World Health Organization. Global Tuberculosis Report 2024. Geneva, CH: WHO; 2024. [Accessed 2024 Nov 20]. https://iris.who.int/bitstream/handle/10665/379339/9789240101531-eng.pdf?sequence=1
- Footnote 6
-
Creatore MI, Lam M, Wobeser WL. Patterns of tuberculosis risk over time among recent immigrants to Ontario, Canada. Int J Tuberc Lung Dis 2005;9(6):667–72. https://pubmed.ncbi.nlm.nih.gov/15971395/
- Footnote 7
-
Dobler CC, Fox GJ, Douglas P, Viney KA, Ahmad Khan F, Temesgen Z, Marais BJ. Screening for tuberculosis in migrants and visitors from high-incidence settings: present and future perspectives. Eur Respir J 2018;52(1):1800591. https://doi.org/10.1183/13993003.00591-2018
- Footnote 8
-
Lillebaek T, Andersen AB, Dirksen A, Smith E, Skovgaard LT, Kok-Jensen A. Persistent high incidence of tuberculosis in immigrants in a low-incidence country. Emerg Infect Dis 2002;8(7):679–84. https://doi.org/10.3201/eid0807.010482
- Footnote 9
-
Lönnroth K, Mor Z, Erkens C, Bruchfeld J, Nathavitharana RR, van der Werf MJ, Lange C. Tuberculosis in migrants in low-incidence countries: epidemiology and intervention entry points. Int J Tuberc Lung Dis 2017;21(6):624–36. https://doi.org/10.5588/ijtld.16.0845
- Footnote 10
-
Public Health Agency of Canada. Tuberculosis surveillance in Canada. Summary report 2012–2021. Ottawa, ON: PHAC; 2023. [Accessed 2024 July 31]. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/tuberculosis-surveillance-canada-summary-2012-2021/tuberculosis-surveillance-canada-summary-2012-2021.pdf
- Footnote 11
-
Government of Canada. Atlantic Immigration Program. Ottawa, ON: Government of Canada; 2024. https://www.canada.ca/en/immigration-refugees-citizenship/services/immigrate-canada/atlantic-immigration.html
- Footnote 12
-
Statistics Canada. Immigrants make up the largest share of the population in over 150 years and continue to shape who we are as Canadians. Ottawa, ON: StatCan; 2022. https://www150.statcan.gc.ca/n1/en/daily-quotidien/221026/dq221026a-eng.pdf?st=iy49GRG0
- Footnote 13
-
Immigration, Refugees and Citizenship Canada. Canadian panel member guide to immigration medical examinations 2020. Ottawa, ON: IRCC. [Accessed 2024 July 31]. https://www.canada.ca/en/immigration-refugees-citizenship/corporate/publications-manuals/panel-members-guide.html
- Footnote 14
-
Greenaway C, Diefenbach-Elstob T, Schwartzman K, Cook VJ, Giovinazzo G, Njoo H, Mounchili A, Brooks J. Chapter 13: tuberculosis surveillance and tuberculosis infection testing and treatment in migrants. Can J Respir Crit Care Sleep Med 2022;6(sup1):194–204. https://doi.org/10.1080/24745332.2022.2035544
- Footnote 15
-
Khan K, Hirji MM, Miniota J, Hu W, Wang J, Gardam M, Rawal S, Ellis E, Chan A, Creatore MI, Rea E. Domestic impact of tuberculosis screening among new immigrants to Ontario, Canada. CMAJ 2015;187(16):E473–81. https://doi.org/10.1503/cmaj.150011
- Footnote 16
-
Ronald LA, Campbell JR, Balshaw RF, Romanowski K, Roth DZ, Marra F, Cook VJ, Johnston JC. Demographic predictors of active tuberculosis in people migrating to British Columbia, Canada: a retrospective cohort study. CMAJ 2018;190(8):E209–16. https://doi.org/10.1503/cmaj.170817
- Footnote 17
-
Shea KM, Kammerer JS, Winston CA, Navin TR, Horsburgh CR Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am J Epidemiol 2014;179(2):216–25. https://doi.org/10.1093/aje/kwt246
- Footnote 18
-
White HA, Okhai H, Kirwan P, Rafeeq SH, Dillon H, Hefford P, Wiselka MJ, Pareek M. Tuberculosis incidence in country of origin is a key determinant of the risk of active tuberculosis in people living with HIV: data from a 30-year observational cohort study. HIV Med 2022;23(6):650–60. https://doi.org/10.1111/hiv.13222
- Footnote 19
-
Margineanu I, Rustage K, Noori T, Zenner D, Greenaway C, Pareek M, Akkerman O, Hayward S, Friedland JS, Goletti D, Stienstra Y, Hargreaves S; ESGITM/ESGMYC Study Groups. Country-specific approaches to latent tuberculosis screening targeting migrants in EU/EEA* countries: A survey of national experts, September 2019 to February 2020. Euro Surveill 2022;27(12):2002070. https://doi.org/10.2807/1560-7917.ES.2022.27.12.2002070
- Footnote 20
-
Benjumea-Bedoya D, Becker M, Haworth-Brockman M, Balakumar S, Hiebert K, Lutz JA, Bertram Farough A, Keynan Y, Plourde P. Integrated Care for Latent Tuberculosis Infection (LTBI) at a Primary Health Care Facility for Refugees in Winnipeg, Canada: A Mixed-Methods Evaluation. Front Public Health 2019;7:57. https://doi.org/10.3389/fpubh.2019.00057
- Footnote 21
-
Harwood-Johnson E, Leis KS, Hanson J, Olfert J, Blonde Y, Brindamour M. Community treatment of latent tuberculosis in child and adult refugee populations: outcomes and successes. Front Public Health 2023;11:1225217. https://doi.org/10.3389/fpubh.2023.1225217
- Footnote 22
-
Pépin J, Desjardins F, Carignan A, Lambert M, Vaillancourt I, Labrie C, Mercier D, Bourque R, LeBlanc L. Impact and benefit-cost ratio of a program for the management of latent tuberculosis infection among refugees in a region of Canada. PLoS One 2022;17(5):e0267781. https://doi.org/10.1371/journal.pone.0267781
- Footnote 23
-
Warrington P, Tyrrell G, Choy K, Eisenbeis L, Long R, Cooper R. Prevalence of latent tuberculosis infection in Syrian refugees to Canada. Can J Public Health 2018;109(1):8–14. https://doi.org/10.17269/s41997-018-0028-7
- Footnote 24
-
Nijhawan AE, Iroh PA, Brown LS, Winetsky D, Porsa E. Cost analysis of tuberculin skin test and the QuantiFERON-TB Gold In-tube test for tuberculosis screening in a correctional setting in Dallas, Texas, USA. BMC Infect Dis 2016;16(1):564. https://doi.org/10.1186/s12879-016-1901-8
- Footnote 25
-
Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27(1):3–20. https://doi.org/10.1128/CMR.00034-13
- Footnote 26
-
Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48(12):1503–10. https://doi.org/10.1016/0895-4356(95)00048-8
- Footnote 27
-
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49(12):1373–9. https://doi.org/10.1016/S0895-4356(96)00236-3
- Footnote 28
-
Shamputa IC, Nguyen DT, Higazy D, Abdelhadi A, MacKenzie H, Reddin M, Barker K, Webster D. Optimizing tuberculosis screening for immigrants in southern New Brunswick: A pilot study protocol. PLoS One 2022;17(11):e0277255. https://doi.org/10.1371/journal.pone.0277255
- Footnote 29
-
Qiagen. QuantiFERON-TB Gold Plus(QFT-Plus) Package Insert. [Accessed 2024 Dec 9]. https://www.quantiferon.com/wp-content/uploads/2017/04/English_QFTPlus_ELISA_R04_022016.pdf
- Footnote 30
-
World Health Organization. Global Tuberculosis Report 2020. Geneva, CH: WHO; 2020. [Accessed 2024 July 31]. https://iris.who.int/bitstream/handle/10665/336069/9789240013131-eng.pdf?sequence=1
- Footnote 31
-
World Health Organization. WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025: background document. Geneva, CH: WHO; 2021. [Accessed 2024 July 31]. https://cdn.who.int/media/docs/default-source/hq-tuberculosis/who_globalhbcliststb_2021-2025_backgrounddocument.pdf?sfvrsn=f6b854c2_9
- Footnote 32
-
Mounchili A, Perera R, Lee RS, Njoo H, Brooks J. Chapter 1: Epidemiology of tuberculosis in Canada. Can J Respir Crit Care Sleep Med 2022;6(sup1):8–21. https://doi.org/10.1080/24745332.2022.2033062
- Footnote 33
-
Sözen E, Guven U. The effect of online assessments on students’ attitudes towards undergraduate level geography courses. Int Educ Stud 2019;12(10):1–8. https://doi.org/10.5539/ies.v12n10p1
- Footnote 34
-
Bodenmann P, Vaucher P, Wolff H, Favrat B, de Tribolet F, Masserey E, Zellweger JP. Screening for latent tuberculosis infection among undocumented immigrants in Swiss healthcare centres; a descriptive exploratory study. BMC Infect Dis 2009;9:34. https://doi.org/10.1186/1471-2334-9-34
- Footnote 35
-
Spruijt I, Erkens C, Suurmond J, Huisman E, Koenders M, Kouw P, Toumanian S, Cobelens F, van den Hof S. Implementation of latent tuberculosis infection screening and treatment among newly arriving immigrants in the Netherlands: A mixed methods pilot evaluation. PLoS One 2019;14(7):e0219252. https://doi.org/10.1371/journal.pone.0219252
- Footnote 36
-
Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 2019;54(3):1900655. https://doi.org/10.1183/13993003.00655-2019
- Footnote 37
-
Jordan AE, Nsengiyumva NP, Houben RM, Dodd PJ, Dale KD, Trauer JM, Denholm JT, Johnston JC, Khan FA, Campbell JR, Schwartzman K. The prevalence of tuberculosis infection among foreign-born Canadians: a modelling study. CMAJ 2023;195(48):E1651–9. https://doi.org/10.1503/cmaj.230228
- Footnote 38
-
Pareek M, Watson JP, Ormerod LP, Kon OM, Woltmann G, White PJ, Abubakar I, Lalvani A. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 2011;11(6):435–44. https://doi.org/10.1016/S1473-3099(11)70069-X
- Footnote 39
-
Chen C, Zhu T, Wang Z, Peng H, Kong W, Zhou Y, Shao Y, Zhu L, Lu W. High latent TB infection rate and associated risk factors in the Eastern China of low TB incidence. PLoS One 2015;10(10):e0141511. https://doi.org/10.1371/journal.pone.0141511
- Footnote 40
-
de Jezus SV, do Prado TN, Arcêncio RA, Mascarello KC, Sales CM, Fauth MM, de Faria Marcos Terena N, Amorim RF, Araujo VM, Aragón MA, Maciel EL. Factors associated with latent tuberculosis among international migrants in Brazil: a cross-sectional study (2020). BMC Infect Dis 2021;21(1):512. https://doi.org/10.1186/s12879-021-06227-z
- Footnote 41
-
Yap P, Tan KH, Lim WY, Barkham T, Tan LW, Chen MI, Wang YT, Chee CB. Prevalence of and risk factors associated with latent tuberculosis in Singapore: A cross-sectional survey. Int J Infect Dis 2018;72:55–62. https://doi.org/10.1016/j.ijid.2018.05.004
- Footnote 42
-
Yuan Y, Wang X, Zhou Y, Zhou C, Li S. Prevalence and risk factors of latent tuberculosis infection among college students: a systematic review and meta-analysis. Public Health 2022;213:135–46. https://doi.org/10.1016/j.puhe.2022.10.003
- Footnote 43
-
Public Health Agency of Canada. Canadian Tuberculosis Standards, 7th edition. Ottawa, ON: PHAC; 2014. [Accessed 2024 Nov 20]. https://www.phac-aspc.gc.ca/tbpc-latb/pubs/tb-canada-7/assets/pdf/tb-standards-tb-normes-ch13-eng.pdf
- Footnote 44
-
Campbell JR, Pease C, Daley P, Pai M, Menzies D. Chapter 4: Diagnosis of tuberculosis infection. Can J Respir Crit Care Sleep Med 2022;6(sup1):49–65. https://doi.org/10.1080/24745332.2022.2036503
Appendix 1: Participant Survey
Instructions:
Please circle the response that best describes your agreement with the statement.
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
If you were not satisfied, why?
_____________________________________________________________________________________________________________________________________
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
If you not, please explain:
_____________________________________________________________________________________________________________________________________
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
Please explain:
_____________________________________________________________________________________________________________________________________
- | 1. Strongly disagree |
- 2. Disagree |
- 3. Neutral |
- 4. Agree |
- 5. Strongly agree |
Please explain:
_____________________________________________________________________________________________________________________________________
- | 1. Oui |
- 2. Non |
If yes, please provide your suggestions below:
_____________________________________________________________________________________________________________________________________
Appendix 2: Sensitivity analysis
Analyses were conducted using one additional strategy. As research has shown that recent immigrants are more at risk of developing tuberculosis (TB) disease (TBD) from remotely acquired TB infection (TBI), we decided to run the binary logistic regression using data from immigrants who had arrived in the last five years (i.e., since 2019). There was no significant change in the pattern of results. Two hundred and twenty-eight individuals were included in this alternative analysis, with 183 testing negative and 45 testing positive for TBI. Binary logistic regression was used to predict TBI test outcome using age, sex and TB incidence category of the participants' country of birth as predictors. Odds ratios were examined to determine the impact of each variable. The binary logistic regression model was statistically significant, χ2 (4)=13.98, p=0.007, with a Nagelkerke R2 value of 0.09. The odds of a positive test were approximately 3.6 times higher for individuals born in a country classified as highly to severely endemic as compared to those born in countries classified as sub-endemic (OR=3.64; 95% CI: 1.26–10.55). Age was found to increase the odds of a positive TBI screen (OR=1.05; 95% CI: 1.02–1.09); that is, every additional year of age increased the odds of a positive screen by 1.05 times. In other words, the odds of obtaining a positive screen increased by 5% for every additional year a participant was aged. Neither participant sex, nor being born in a TB endemic country, was found to be statistically significant (Table A1).
| Predictor variable | ß | SE | p-value | Odds | 95% CI for odds ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.051 | 0.018 | 0.004 | 1.053 | 1.017 | 1.090 |
| Sex | −0.258 | 0.347 | 0.458 | 0.773 | 0.391 | 1.526 |
| Endemic | 0.592 | 0.386 | 0.126 | 1.807 | 0.847 | 3.854 |
| Highly to severely endemic | 1.292 | 0.543 | 0.017 | 3.641 | 1.256 | 10.550 |
Abbreviations: CI, confidence interval; SE, standard error |
||||||
