Original quantitative research – Strengthening surveillance of consumer products in Canada: the vaping example
Health Promotion and Chronic Disease Prevention in Canada
Minh T. Do, PhDAuthor reference footnote 1Author reference footnote 2Author reference footnote 3; Steven R. McFaull, MScAuthor reference footnote 4; Lauren Guttman, MScAuthor reference footnote 1; Lina Ghandour, MScAuthor reference footnote 1; James Hardy, BScAuthor reference footnote 1
https://doi.org/10.24095/hpcdp.40.10.02
This article has been peer reviewed.
Correspondence: James Hardy, Consumer and Hazardous Products Safety Directorate, Health Canada, 269 Laurier Avenue West, Ottawa, ON K1A 0K9; Email: james.hardy@canada.ca
Abstract
Introduction: The overall objective of this study was to demonstrate how information collected by the Consumer Product Safety Program (“the Program”) can be used to identify emerging hazards. Specifically, this study characterized and quantified trends associated with vaping reports received by the Program over the past five years.
Methods: Data collated by the Program were extracted for the period from 1 January, 2015 to 30 September, 2019. The data were summarized using descriptive statistics and trends were quantified for annual percent change. In order to compare characteristics of vaping reports, the proportionate injury ratios (PIRs) and corresponding 95% CIs were used to compare vaping-related injuries to all other reports received by the Program.
Results: A total of 71 vaping-related reports were received between 1 January, 2015 and 30 September, 2019. During this period, the annual percent change increase in the number of reports received was approximately 73% annually (p < .05). Among the reported injuries, 41% were burn injuries. Proportionally, there were more vaping reports involving males (PIR = 1.89; 95% CI: 1.51–2.36) and individuals between the ages of 15 and 19 years (PIR = 11.53; 95 % CI: 4.95–26.8) as compared to all other reports submitted to the Program.
Conclusion: While the number of reports relating to vaping products is small, the results of this analysis suggest that certain groups, including males and youth, are more likely to be the subject of a vaping-related incident.
Keywords: vaping, consumer, e-cigarettes, injury
Highlights
- Among those who reported gender, the majority were males.
- The number (and proportion per 100 000 reports received) of reports related to vaping products increased significantly between 2015 and 2019.
- Among those who reported age, teens (aged 15–19 years) are significantly more likely to report an incident.
- Compared to the average of all consumer product reports received by the Program, proportionally, those related to vaping involved more visits to emergency departments.
Introduction
Surveillance of consumer product–related incidents plays a key role in identifying risks to health and guiding the response to manage and mitigate those risks. Specifically, through systematic and ongoing monitoring of consumer products, surveillance intelligence informs risk assessment, risk management, compliance, enforcement and other domestic and international activities that aim to protect the health and safety of Canadians. In support of the Canada Consumer Product Safety ActFootnote 1 (CCPSA; “the Act”), the Consumer Product Safety Program (CPSP; “the Program”) of Health Canada collects information on safety-related incidents through its online reporting portal for a wide variety of consumer products, including reports related to vaping products.Footnote 2 Consumer products include appliances, housewares, children’s products, electronics, grooming products (excludes products governed by cosmetics under the Food and Drugs Act), home and automobile maintenance products, textiles, and outdoor living, sports and recreation products.
In Canada, the prevalence of vaping has been increasing in recent years, particularly among youth.Footnote 3 According to the 2018-19 Canadian Student Tobacco, Alcohol and Drugs Survey (CSTADS), e-cigarette prevalence rates have doubled over the past couple of years. In 2016/17, approximately 10% of students in Grades 7 to 12 reported using e-cigarettes over the past 30 days. However, this same statistic increased to 20% in 2018/19.Footnote 3
Since the introduction of vaping products, there have been a number of injuries including poisoning due to ingestion of vaping liquid and burns resulting from malfunctioning vaping devices.Footnote 4Footnote 5 While adverse health effects associated with exposure to some types of tobacco products are well documented, the health implications of vaping are not fully understood.
Vaping devices are relatively new products in Canada. They can place a significant strain on their lithium-ion (Li-ion) batteries by drawing large electrical currents while in operation. Although Li-ion technology is safe in general, straining a battery (particularly one of poor quality) can lead to explosions and fires. Since a vaping device is often in intimate contact with the user, this can result in a unique hazard to consumers.Footnote 4Footnote 5 Rossheim and colleaguesFootnote 4 estimated that between 2015 and 2017, there were 2035 individuals who presented to US hospital emergency departments for burn injuries due to the explosion of vaping devices. Approximately 26% of these patients were treated and then either transferred to another unit, admitted or held for observation.Footnote 4 Many vaping liquids contain nicotine, which has high acute toxicity and has resulted in a number of fatal and non-fatal poisonings after ingestion, including among children. More recently, there has been a focus on vaping-associated lung illness, with over 2000 cases of lung injuries in the United StatesFootnote 6 and 19 cases in Canada.Footnote 7 The risk of injury, poisoning and other adverse health consequences underscores the need for continued monitoring and analysis of surveillance data in order to identify actionable intelligence.
The purpose of this study was to evaluate vaping information reported to the Program. Specifically, the objectives of this study were to
- describe the epidemiology (person, time and exposure) of vaping-related reports; and
- to examine temporal trends and compare reports related to vaping products to those of all other products.
Methods
Data source
The Program receives reports from industry and consumers (including third parties such as health care providers) through its online reporting portal.Footnote 8 On average, the Program received 2500 reports on all types of consumer products annually during the study period. These reports provide related health and safety information about consumer products. Industry (i.e. manufacturers, importers and sellers) is required to report under Section 14 of the Act for consumer products. Although consumers are not required to submit this information, the Program routinely receives voluntary reports from them. Reports may originate from Canada or from abroad. In addition, the Program scans media articles involving consumer products and includes them in its database. Using Google alerts, the Program scans media articles primarily from major news outlets.
The online reporting portal is used to collect information on the affected persons (date received, gender, age range, type of injury incurred and treatment outcome), the narrative (what happened and how), the product involved (brand, model number, serial number, bar code, etc.), and how the product was acquired (date of purchase, business name and address). All of this information is stored in a database called RADAR, a bilingual palindromic acronym that stands for Regulatory Action Depot/Dépôt d’actions réglementaires. Information pertaining to the person (age and gender), time (year, day of the week and time of day) and exposure (e.g. products used) was extracted from RADAR for analysis. Once the reports are received into RADAR, triage analysts code additional information such as product category, injury type, injury severity, treatment and primary hazards, which were then extracted for analysis.
Study period
The study period included all reports collated in RADAR for the period between 1 January, 2015 and 30 September, 2019. The unit of analysis for this study is any report relating to vaping. Vaping-related reports were identified from RADAR using two methods. The first method relied on coding of reports using the injury and hazard coding manuals developed by the Program. The Program coding manual is similar to the National Electronic Injury Surveillance System (NEISS) coding system used by the U.S. Consumer Product Safety Commission (CPSC).Footnote 4 All reports coded as “vaping device” were extracted. In addition, a second method using search strings was applied to incident description narratives to extract syndromic events (e.g. “breath”, “cough”, “chest pain”, “nausea”, “vomit”, “lung”, “pulmonary”, “poumon”, “pulmonaires”, “empoisonner”, “à l’abri”, “respiratoire”, etc.) occurring in either English or French. Furthermore, search strings were also used to identify cases in the product brand and name description field (e.g. “atomiser”, “cartomiser”, “vap*”, “dab”, “cig*”, etc.). A list of search terms is available upon request. All extracted records were reviewed manually to avoid coding errors.
Statistical analysis
Descriptive statistics were generated to examine the distribution of different characteristics of vaping reports identified. Counts and percentages were calculated for all relevant variables. Proportionate injury ratios (PIRs) and 95% confidence intervals (CIs) were computed to compare specific characteristics of vaping cases (N = 71). The PIR was calculated as a ratio of the observed number of cases for a given characteristic (for example, injury type) to the expected number of cases of that characteristic based on all RADAR reports.Footnote 9Footnote 10Footnote 11 A PIR of unity (1.00) indicates that the expected number of injuries attributed to vaping is the same as that of all other RADAR reports. If the PIR is higher than unity and the lower confidence limit excludes 1.00, then the result is interpreted as being significantly higher than expected. The analysis included reports submitted by consumers and industry, and those found in the media. To determine the robustness of the study results, sensitivity analyses were conducted (excluding all reports identified from the media). Annual percent change was computed using Joinpoint regression.Footnote 12 Statistical significance level (alpha) of .05 was determined prior to any analyses. Analyses were conducted using Microsoft Excel, SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and Joinpoint Desktop software version 4.7 (National Cancer Institute, Bethesda, MD, USA).
Results
A total of 71 vaping-related reports were received by the Program between January 2015 and September 2019. Figure 1 shows the distribution of reports over the years. In 2015, four reports were submitted to the Program. In 2019 (up to September only), 28 reports were received. Proportionally, this represents an annual percent increase of 72.6% (p < .05) for the study period.
Figure 1. Number of vaping-related reports, expressed as proportions and annual percent change, reported to RADAR between 1 January, 2015 and 30 September 30, 2019
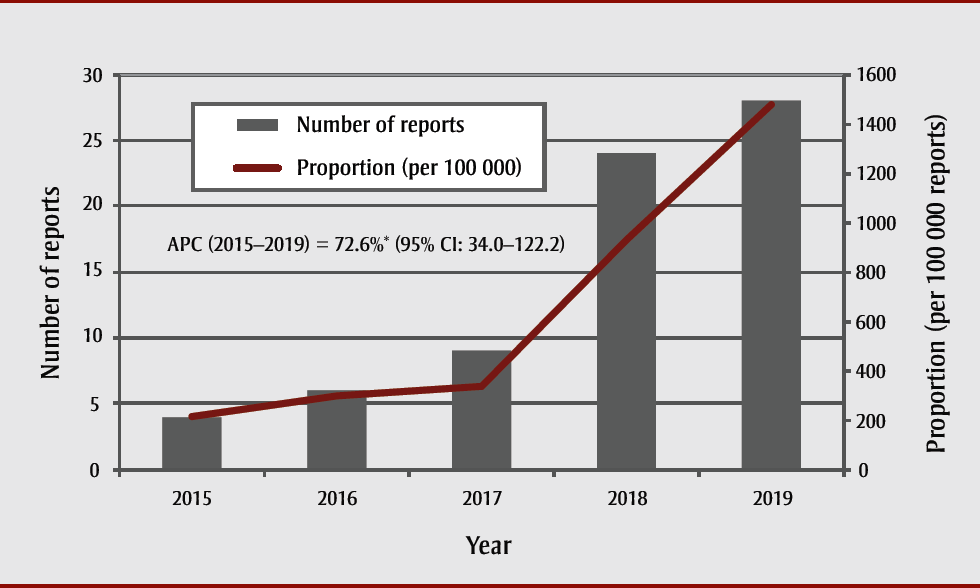
Text description: Figure 1
Figure 1. Number of vaping-related reports, expressed as proportions and annual percent change, reported to RADAR between 1 January, 2015 and 30 September 30, 2019
| Year | Vaping status (number of reports) | Proportion (per 100 000 reports) | |
|---|---|---|---|
| Yes | No | ||
| 2015 | 4 | 1866 | 214 |
| 2016 | 6 | 2001 | 300 |
| 2017 | 9 | 2693 | 334 |
| 2018 | 24 | 2583 | 929 |
| 2019 | 28 | 1895 | 1478 |
Table 1 presents summary demographic and injury characteristics of those described in the reports. Overall, the mean age (in years) was 34.1 (standard deviation [SD] 18.6). Among those who reported age (N = 26; 37%), most were adults aged 35 to 59 (38.5%), whereas children under 14 and seniors aged 60 or older accounted for 11.5% and 7.7% of cases, respectively. Among those reports that included gender, most involved males (69.8%). The most commonly reported hazard was toxicological (e.g. poisonings through ingestion; 53.5%), followed by explosion (23.9%) and other hazards (e.g. mechanical; 22.5%). Overall, 45.1% (N = 32) of the reports did not involve injuries. Among those that reported an injury (N = 39; 54.9%), 41.0% (N = 16/39) were due to burns. Other injury types reported include asphyxia or poisoning (15.4%), irritation or allergic reaction (10.3%) and fractures caused by the exploding battery (5.1%). The largest proportion of injury severity reported was moderate (N = 14; 35.9%); followed by minor (N = 10; 25.6%); severe (N = 8; 20.5%); and fatal, life threatening or disabling (N = 2; 5.1%). Of the injuries involving a treatment, 13 (18.3%) resulted in an emergency room visit, 7 were treated by another medical professional and 3 resulted in hospital admission.
Table 1. Demographic and injury data analyzed from vaping-related reports submitted to Canada’s Consumer Product Safety Program, January 2015 to September 2019
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 37 (52.1) |
| Female | 16 (22.5) |
| Unknown | 18 (25.4) |
| Age group (years) | |
| 0–14 | 3 (4.2) |
| 15–19 | 5 (7.0) |
| 20–34 | 6 (8.5) |
| 35–59 | 10 (14.1) |
| ≥ 60 | 2 (2.8) |
| Unknown | 45 (63.4) |
| Age (years) | |
| Mean (SD) | 34.1 (18.6) |
| Median (IQR) | 34 (18–46.5) |
| Primary hazard | |
| Toxicological | 38 (53.5) |
| Explosion | 17 (23.9) |
| Other | 16 (22.5) |
| Injury type | |
| No injury | 32 (45.1) |
| Injury specified | 39 (54.9) |
| Burn (41.0%) | |
| Asphyxia or poisoningFootnote a of Table 1 (15.4%) | |
| Irritation or allergic reaction (10.3%) | |
| Fracture (5.1%) | |
| Other/unknown (28.2%) | |
| Injury severity | |
| No injury | 32 (45.1) |
| Minor | 10 (14.1) |
| Moderate | 14 (19.7) |
| Severe | 8 (11.3) |
| Fatal, life-threatening or disabling | 2 (2.8) |
| Unknown | 5 (7.0) |
| Treatment | |
| No injury—no treatment | 32 (45.1) |
| Injury—no treatment | 5 (7.0) |
| Other medical professional | 7 (9.9) |
| Emergency department visit | 13 (18.3) |
| Hospital—admission | 3 (4.2) |
| Unknown | 11 (15.5) |
Table 2 provides results of the comparison of vaping-related reports to all other reports received by the Program. Proportionally, males are overrepresented compared to all other reports received by the Program (PIR = 1.89; 95% CI: 1.51–2.36). Vaping-related reports from those between the ages of 15 and 19 years represented 12 times more reports than all others received by the Program (PIR = 11.53; 95% CI: 4.95–26.8). The most common hazards for vaping were toxicological (PIR = 2.79; 95% CI: 2.24–3.47) and explosions (PIR = 6.39; 95% CI: 4.22–9.61). Many of the reports coded as toxicological were due to poisoning attributed to ingestion of vaping substances. Reports coded as poisoning (including breathing difficulties) were overrepresented among vaping reports when compared to all other reports submitted to the Program (PIR = 2.33; 95% CI: 1.08–5.01). Similarly, the explosions caused burns to those reporting this hazard, and lithium-ion batteries were also responsible for the high proportion of vaping reports involving burns (PIR = 3.50; 95% CI: 2.27–5.39) as compared to all other reports received by the Program. Many of the reports involving burn injuries mentioned severe injuries (PIR = 2.21; 95% CI: 1.15–4.24), requiring visits to the emergency department (PIR = 2.00; 95% CI: 1.22–3.27).
Table 2. Proportionate injury ratio by selected demographic and injury characteristics reported to Canada’s Consumer Product Safety Program, January 2015 to September 2019
| Vaping characteristics | Number of incidents | Expected values | PIR (95% CI) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 37 | 19.60 | 1.89 | (1.51–2.36) |
| Female | 16 | 20.35 | 0.79 | (0.51–1.21) |
| Age (years) | ||||
| 0–14 | 3 | 9.08 | 0.33 | (0.11–1.00) |
| 15–19 | 5 | 0.43 | 11.53 | (4.95–26.8) |
| 20–34 | 6 | 2.91 | 2.06 | (0.96–4.94) |
| 35–59 | 10 | 6.89 | 1.45 | (0.81–2.57) |
| 60+ | 2 | 3.63 | 0.55 | (0.14–2.16) |
| Primary hazard | ||||
| Toxicological | 38 | 13.62 | 2.79 | (2.24–3.47) |
| Explosion | 17 | 2.66 | 6.39 | (4.22–9.61) |
| Injury type | ||||
| No injuries | 32 | 43.73 | 0.73 | (0.51–0.95) |
| Burns | 16 | 4.57 | 3.50 | (2.27–5.39) |
| PoisoningFootnote a of Table 2 | 6 | 2.57 | 2.33 | (1.08–5.01) |
| Irritation or allergic reaction | 4 | 7.53 | 0.53 | (0.20–1.38) |
| Injury severity | ||||
| Minor | 10 | 9.93 | 1.01 | (0.57–1.79) |
| Moderate | 14 | 9.31 | 1.50 | (0.94–2.40) |
| Severe | 8 | 3.63 | 2.21 | (1.15–4.24) |
| Treatment | ||||
| No treatment | 32 | 43.73 | 0.73 | (0.56–0.95) |
| Emergency department | 13 | 6.49 | 2.00 | (1.22–3.27) |
| Admitted to hospital | 3 | 3.83 | 0.78 | (0.25–2.37) |
Discussion
Surveillance is an important public health tool for identifying emerging hazards that can potentially cause harm to individuals and populations. The systematic and ongoing monitoring of these hazards allows for early detection of potential health threats and provides opportunities for collating actionable intelligence. In this context, the Program has been monitoring reports on a wide variety of consumer products, including vaping products, and collating them once received through the online reporting portal. In this study, analysis of the reports received by the Program for vaping showed that the majority of the reports for vaping products involved males (N = 37; 69.8% of those who reported gender), and that the number of total reports related to vaping products increased significantly between 1 January, 2015 and 30 September, 2019. The increase in the number of vaping reports received is consistent with the increase in the prevalence of vaping in Canada.Footnote 3
This situation is not unique to Canada. Injuries related to vaping have previously been reported in the United States using data from the National Electronic Injury Surveillance System (NEISS).Footnote 4Footnote 5 Similar to the aforementioned studies, most of the cases seen in this study involved males, and many of the reports described burns due to the malfunction of vaping devices’ lithium-ion batteries, which overheated to the point of catching fire or exploding.Footnote 5 However, these problems could be mitigated as new devices are designed to conform to newly introduced hardware safety standards. In addition to burns, a number of physiological effects were also observed. These include poisoning (including breathing difficulties), irritation and/or allergic reaction and fracture (due to exploding batteries).
Strengths and limitations
The preceding analysis relied on records of consumer and industry reports submitted to the Program. A strength of this data is that reporting of incidents is a mandatory requirement for industry; however, identifying industry’s compliance with that provision of the Canada Consumer Product Safety Act is challenging if industry is not aware of the problems with their products. Furthermore, the reports received almost certainly do not reflect all reports that have taken place in Canada over the study period, particularly since consumer reporting is voluntary. While the study reflects an analysis of the data presently available, there are several key limitations. The number of reports related to vaping products is small and does not capture all incidents in Canada. Additionally, Health Canada does not validate the details of every report it receives. Finally, it is likely that a variety of unquantified factors may lead to over- or underreporting, and this reporting bias may differ by subgroup. Despite these limitations, this study highlights issues that can provide valuable insight into the health risks associated with new and emerging product categories, such as vaping products.
Conclusion
The number of vaping-related reports received by the Program annually has increased significantly since 2015. The available data suggest that certain subgroups (i.e. males, youth) are more likely to be involved in such incidents. Furthermore, reports related to vaping mentioned more visits to emergency departments proportionally than all other reports received by the Program. This study highlights the importance of surveillance systems in the monitoring of potential hazards posed by consumer products.
Conflicts of interest
The authors declare there are no conflicts of interest.
Authors’ contributions and statement
MTD and JH contributed to conceptualizing the study. MTD, L. Guttman and L. Ghandour contributed to the literature review. MTD, SRM, L. Guttman and L. Ghandour analyzed the data. All authors contributed to drafting and revising the article.
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.