Original quantitative research – Factors associated with high health care spending among patients with schizophrenia

HPCDP Journal Home
Published by: The Public Health Agency of Canada
Date published: October 2022
ISSN: 2368-738X
Submit a manuscript
About HPCDP
Browse
Previous | Table of Contents | Next
Andrew J. Stewart, MScAuthor reference footnote 1; Scott B. Patten, PhDAuthor reference footnote 1Author reference footnote 2; Kirsten M. Fiest, PhDAuthor reference footnote 1; Tyler S. Williamson, PhDAuthor reference footnote 1; James P. Wick, MScAuthor reference footnote 2; Paul E. Ronksley, PhDAuthor reference footnote 1
https://doi.org/10.24095/hpcdp.42.10.02
This article has been peer reviewed.
Author references
Correspondence
Paul E. Ronksley, Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Cal Wenzel Precision Health Building, 3280 Hospital Drive NW, Calgary, AB T2N 4Z6; Tel: 403-220-8820; Email: peronksl@ucalgary.ca
Suggested citation
Stewart AJ, Patten SB, Fiest KM, Williamson TS, Wick JP, Ronksley PE. Factors associated with high health care spending among patients with schizophrenia. Health Promot Chronic Dis Prev Can. 2022;42(10):431-9. https://doi.org/10.24095/hpcdp.42.10.02
Abstract
Introduction: Understanding the reasons for the wide variation in health care spending among patients with schizophrenia may benefit the development of interventions aimed at improving patient outcomes and health care spending efficiency. The aim of our study was to determine factors associated with high health care spending in the patient population.
Methods: A serial cross-sectional study used the administrative health records of residents of Alberta, Canada between 1 January 2008 and 31 December 2017 and provincial costing methodologies to calculate total health care spending and sector-specific costs. Factors that modified the odds of being a high cost (i.e. 95th percentile or higher) patient with schizophrenia were estimated using generalized estimating equations.
Results: This study captured 242 818 person-years of observations among 38 177 unique patients with schizophrenia. Increased odds of being a high-cost patient were associated with younger age (18–29 years), male sex, unstable housing status and requiring care from multiple medical specialties. The strongest estimated associations between high cost status and comorbidity were for metastatic cancer (OR = 2.26) and cirrhosis (OR = 2.07). In contrast, polypharmacy was associated with a decreased odds of being high cost compared with untreated patients.
Conclusion: Factors associated with being a high-cost patient are the result of complex interactions between individual, structural and treatment-related factors. Efforts to improve patient outcomes and address rising health care costs must consider the value of allocating resources towards early detection and support of patients with schizophrenia along with the prevention/management of comorbidity.
Keywords: schizophrenia, health services research, comorbidity, health care expenditures
Highlights
- Younger patients with schizophrenia (18–29 years old) were more likely than older patients to be high cost.
- Of the 29 comorbidities assessed, 17 were associated with an increased odds of being a high-cost patient.
- Increasing involvement of medical specialties increases the odds of being a high-cost patient.
- The odds of being a high-cost patient if unstably housed are 2.49 times greater than if stably housed. This suggests that the risk factors driving cost extend beyond clinical or demographic domains.
Introduction
Population-based studies have shown that a small proportion of patients use the majority of health care resources,Footnote 1Footnote 2Footnote 3Footnote 4Footnote 5 and about 5% of the patient population use 65% of the health resources.Footnote 2Footnote 6 The clinical and demographic characteristics of these “high-needs/high-cost” patients vary substantially,Footnote 7 and the presence of underlying mental health conditions has been identified as a key driver of health care use and subsequent spending.Footnote 8Footnote 9
Schizophrenia is a mental health condition known for its association with high levels of disability and health care utilization.Footnote 10Footnote 11Footnote 12 The economic costs of providing mental health services varies widely between patients.Footnote 13 The published literature has linked several factors to increased health spending, including age, sex, number of social contacts, length and frequency of hospitalizations, socioeconomic status, access to community supports, and pharmaceutical management strategies.Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18 Increasing multimorbidity among patients with schizophrenia is expected to further expand health care need and resource use by this population.Footnote 19
The literature describing the characteristics of high-cost patients with schizophrenia continues to grow as the management of this condition evolves. Inpatient costs have long been the largest proportion of direct health-care spending for patients with schizophrenia.Footnote 11 This is changing with prescription costs for second-generation antipsychotic medications outpacing hospital spending for the first time.Footnote 19 Patients with schizophrenia are living longer, and their treatment is increasingly complex as additional chronic conditions develop.Footnote 19 Finally, there remains a need for large studies that characterize the entire spectrum of this patient population.
As the cost of delivering health care in North America continues to increase, it is important to record changing demographic and spending patterns so that discussions on spending efficiency remain relevant. Improving our knowledge of the clinical and sociodemographic factors driving health care spending in this patient population is necessary to determine areas of patient management with the greatest potential effects on health outcomes and quality of life. With this in mind, we conducted a population-based study using administrative health data from the Canadian province of Alberta to determine factors associated with high-cost status among patients with schizophrenia.
Methods
Data sources
We conducted a serial cross-sectional study using administrative and clinical data from the province of Alberta, Canada, collected between 1 January 2008 and 31 December 2017 (i.e. ten 1-year cross-sections). This included information on hospitalizations (Discharge Abstract Database), emergency department visits (National Ambulatory Care Reporting System), practitioner billings, the Pharmaceutical Information Network (to collect outpatient prescription information), the population health registry file, and Alberta Vital Statistics (for date of death information) maintained by Alberta’s Ministry of Health. The choice of datasets used was informed by work previously completed by our research group.Footnote 20
Study population
We identified patients with schizophrenia aged 18 years and older using a validated case-ascertainment algorithm. The presence of schizophrenia was defined as “1 hospitalization or 2 physician billing claims in 2 years or less associated with a F20.X, F21.X, F23.2 or F25.X International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) code or a ‘295.X’ ICD-9 code.”Footnote 21Footnote 22Footnote 23 This algorithm has a reported positive predictive value of 87% and a sensitivity of 87%.Footnote 21 Patients with schizophrenia entered the cohort on the date of their first schizophrenia-specific ICD code and were followed until death, outmigration from the Alberta health system or the end-of-study follow-up (31 December 2017).
Calculating costs
Average cost per patient was estimated for each calendar year from 2008 to 2017. Micro-costing information was not available at the provincial level, so we estimated costs for hospitalizations and emergency department visits using Resource Intensity Weights (RIWs) for each encounter multiplied by the average cost in Alberta of a “typical” encounter. The Canadian Institutes for Health Information (CIHI) calculates annual RIWs by using ICD-10 most responsible diagnosis codes to define case-mix groups from samples of patients.Footnote 24 Resource utilization by these groups is measured and used to determine the average resource utilization for a full course of treatment from admission to discharge for a “typical” case. This value is then weighted to adjust for increased resource utilization associated with additional comorbidity and medical complications. RIW estimates have been found to correlate well with the gold standard of micro-costing.Footnote 25
Outpatient physician visits were costed according to the Alberta Health Schedule of Medical Benefits. Medication costs were estimated by multiplying the Alberta Drug Benefit List cost for dispensed medication by the quantity dispensed.
This costing analysis focussed on direct health-care spending only. Direct nonmedical costs (e.g. transportation and accommodations required to attend medical appointments, informal care provided by unpaid caregivers, formal care provided by professional caregivers, etc.) and indirect nonmedical costs (e.g. incurred by absenteeism from work, forced retirement due to illness, etc.) could not be obtained from the data sources used.
To facilitate comparison with existing literature, a high-cost patient with schizophrenia was defined as one with annual direct health-care costs in the 95th percentile or greater. This was determined independently for each fiscal year between 2008 and 2017.Footnote 1Footnote 6Footnote 9Footnote 26
Gelberg-Andersen Behavioral Model for Vulnerable Populations
Risk factors for high cost status considered in this work were informed by the Gelberg-Andersen Behavioral Model for Vulnerable Populations.Footnote 27 This model uses a holistic approach when describing factors associated with health care utilization.Footnote 27 It includes three domains with special attention to vulnerable exposures: environmental factors (e.g. geography, social environment); population characteristics (e.g. predisposing, enabling and need factors); and health behaviours (e.g. seeking specialist care, other patterns of health care access).Footnote 27 Variables available in the provincial administrative data sources were mapped to these domains (see Table 1).
| Environment | Population characteristics | Health behaviour | ||
|---|---|---|---|---|
| Predisposing characteristics | Enabling resources | Need | ||
| Single-payer health care systemFootnote a |
|
|
|
|
Patient and prescription information
We extracted the demographic and clinical characteristics of all patients from the same administrative datasets. These included age, sex and postal code (to derive urban/rural status). We used census data and postal codes to capture material and social deprivation indices as proxies for socioeconomic status.Footnote 28
Comorbidity profiles were determined using 29 case-ascertainment algorithms defined by Tonelli et al.Footnote 21 A proxy measure for housing stability was generated by searching health care records for a record of homelessness (Z59 ICD-10 code) or a shelter-associated postal code.
Finally, annual prescription information (Anatomical Therapeutic Chemical [ATC] and date of prescription) was obtained for each patient through Alberta’s Pharmaceutical Information Network. We used this information to determine the number of unique pharmaceuticals prescribed to each patient per calendar year. We defined polypharmacy as the prescription of 5 to 9 unique medications and extreme polypharmacy as the prescription of 10 or more unique medications in a single year. This definition is the one used most frequently in the literature.Footnote 29
We used the Drug Identification Number (DIN) of each prescribed antipsychotic medication to determine whether they were administered orally or via injection. Physician claims codes were used to determine the medical specialty associated with care. We generated a proxy measure of treatment for comorbidity by counting the number of unique specialities seen during the study period, that is, beyond care received from general practitioners and psychiatrists.
Models and analysis
Patient demographic and clinical characteristics were summarized using means and standard deviations, medians and interquartile ranges, or proportions as appropriate. The outcome of interest for this work was the odds of being a high-cost patient.
We built models using generalized estimating equations because they provide estimates of population-averaged effects and have been found to offer robust estimates even in cases where uncertainty surrounds covariance structures.Footnote 30 The odds of being a high-cost patient were linked to exposure variables using a logit link. An unstructured covariance matrix was specified given the large number of observations available and the potential for improved model fit by limiting assumptions to do with the covariance structure of relationships.
We developed univariate regression models for each factor and subsequently created a correlation matrix to assess collinearity. After determining an absence of strong correlations between variables, we ran a series of models to estimate associations between exposures and the odds of being a high-cost patient. Models were generated iteratively using a forward building approach to ensure convergence could be attained. To generate the most parsimonious model, we excluded comorbidities associated with a p-value greater than or equal to 0.05. A sensitivity analysis allowed us to understand the implications of choosing different cut-points for high cost (i.e. 90th and 99th percentiles of health care spending).
All analyses were completed using Stata version 16 (StataCorp LLC, College Station, TX, US).Footnote 31 This study follows the REporting of studies Conducted using Observational Routinely-collected Data (RECORD) statement.Footnote 32 The University of Calgary Conjoint Health Research Ethics Board approved this study and granted waiver of patient consent (REB16-1575).
Results
This study captured 242 818 person-years of observations from 38 177 unique adult patients with schizophrenia in the 10-year study time frame. Males contributed 138 285 person-years of observations and females contributed 104 533 (see Table 2). Of the 38 177 unique patients, 2463 were defined as high cost based on the 95% percentile of health spending (12 146 person-years).
| Characteristics | Person-years, n (%) | Total person-years, n (% of cohort person-years) | |
|---|---|---|---|
| High cost | Non-high cost | ||
| Age ranges in years | |||
| 18–29 | 2404 (5.6) | 40 246 (94.4) | 42 650 (17.6) |
| 30–39 | 1905 (4.0) | 45 358 (96.0) | 47 263 (19.5) |
| 40–49 | 1844 (3.8) | 46 869 (96.2) | 48 713 (20.1) |
| 50–59 | 2388 (4.6) | 48 997 (95.4) | 51 385 (21.2) |
| 60–69 | 1818 (6.1) | 27 939 (93.9) | 29 757 (12.3) |
| 70–79 | 1103 (8.0) | 12 713 (92.0) | 13 816 (5.7) |
| 80+ | 672 (7.5) | 8341 (92.5) | 9013 (3.7) |
| Sex | |||
| Male | 6613 (5.0) | 131 672 (95.0) | 138 285 (57.0) |
| Female | 5533 (5.3) | 99 000 (94.7) | 104 533 (43.1) |
| Housing stability | |||
| Unstably housed | 2220 (16.4) | 11 291 (83.6) | 13 511 (5.6) |
| Stably housed | 9926 (4.3) | 219 381 (95.7) | 229 307 (94.4) |
| Missing social deprivation (no address) | 1408 (6.3) | 20 960 (93.7) | 22 368 (10.1) |
| Deprivation index | |||
| 1st quartile (least deprived) | 867 (4.4) | 19 910 (95.6) | 20 777 (9.4) |
| 2nd quartile | 739 (4.1) | 17 157 (95.9) | 17 896 (8.1) |
| 3rd quartile | 1235 (4.3) | 27 478 (95.7) | 28 713 (13.0) |
| 4th quartile | 2324 (5.0) | 44 504 (95.0) | 46 828 (21.2) |
| 5th quartile (most deprived) | 4328 (5.1) | 79 716 (94.9) | 84 044 (38.1) |
| Years since diagnosis | |||
| 0–2 (1st quintile) | 3873 (6.0) | 60 467 (94.0) | 64 340 (26.5) |
| 3–5 (2nd quintile) | 1919 (4.2) | 43 534 (95.8) | 45 453 (18.7) |
| 6–9 (3rd quintile) | 1753 (4.3) | 39 477 (95.7) | 41 230 (17.0) |
| 10–15 (4th quintile) | 2261 (4.7) | 45 962 (95.3) | 48 223 (19.9) |
| 16–23 (5th quintile) | 2340 (5.4) | 41 232 (94.6) | 43 572 (17.9) |
| Number of specialists seenFootnote a | |||
| 0 (none) | 603 (0.6) | 93 945 (99.4) | 94 548 (38.9) |
| 1 | 1547 (3.1) | 49 159 (96.9) | 50 706 (20.9) |
| 2 | 2135 (5.4) | 37 411 (94.6) | 39 546 (16.3) |
| ≥3 | 2340 (4.0) | 41 232 (96.0) | 58 018 (23.9) |
| Access to generalist and psychiatric care | |||
| No GP or psychiatrist | 13 (0.8) | 1644 (99.2) | 1657 (0.7) |
| GP only | 866 (1.0) | 83 561 (99.0) | 84 427 (34.8) |
| Psychiatrist only | 88 (0.6) | 14 057 (99.4) | 14 145 (5.8) |
| Both GP and psychiatrist | 11 179 (7.8) | 131 410 (92.2) | 142 589 (58.7) |
| No. of drugs prescribed | |||
| 0 | 1498 (3.8) | 38 436 (96.2) | 39 934 (16.5) |
| 1–2 | 907 (2.7) | 32 543 (97.3) | 33 450 (13.8) |
| 3–5 (polypharmacy) | 1641 (3.3) | 48 626 (96.7) | 50 267 (20.7) |
| ≥6 (extreme polypharmacy) | 8100 (6.8) | 111 067 (93.2) | 119 167 (49.1) |
| Antipsychotic prescription | |||
| None | 6178 (3.9) | 153 126 (96.1) | 159 304 (65.6) |
| Oral antipsychotic | 5837 (7.0) | 77 008 (93.0) | 82 845 (34.1) |
| Injectable antipsychotic | 40 (16.8) | 198 (83.2) | 238 (0.1) |
| Oral + injectable antipsychotic | 91 (26.8) | 340 (73.2) | 431 (0.2) |
| Comorbidities | |||
| Alcohol misuse | 4893 (8.4) | 58 302 (91.6) | 63 195 (26.0) |
| Asthma | 1658 (8.6) | 17 279 (91.4) | 18 937 (7.8) |
| Atrial fibrillation | 878 (12.0) | 6418 (88.0) | 7296 (3.0) |
| Cancer, lymphoma | 139 (16.0) | 732 (84.0) | 871 (0.4) |
| Cancer, metastatic | 377 (17.5) | 1783 (82.5) | 2160 (0.9) |
| Cancer, non-metastatic | 582 (10.9) | 4771 (89.1) | 5353 (2.2) |
| Chronic heart failure | 1570 (12.8) | 10 722 (87.2) | 12 292 (5.1) |
| Chronic kidney disease | 4612 (6.8) | 62 744 (93.2) | 67 356 (27.7) |
| Chronic pain | 2912 (7.8) | 34 566 (92.2) | 37 478 (15.4) |
| Chronic pulmonary disease | 3595 (8.3) | 39 735 (91.7) | 43 330 (17.8) |
| Chronic viral hepatitis B | 48 (6.6) | 679 (93.4) | 727 (0.3) |
| Cirrhosis | 231 (21.1) | 862 (78.9) | 1093 (0.5) |
| Dementia | 3523 (10.9) | 28 774 (89.1) | 32 297 (13.3) |
| Depression | 9745 (8.4) | 106 090 (91.6) | 115 835 (47.7) |
| Diabetes | 3336 (7.6) | 40 637 (92.4) | 43 973 (18.1) |
| Epilepsy | 1599 (7.9) | 18 601 (92.1) | 20 200 (8.3) |
| Hypertension | 5349 (7.4) | 66551 (92.6) | 71900 (29.6) |
| Hypothyroidism | 2354 (7.4) | 29 461 (92.6) | 31 815 (13.1) |
| Inflammatory bowel disease | 250 (7.7) | 2977 (92.3) | 3227 (1.3) |
| Irritable bowel syndrome | 727 (8.0) | 8323 (92.0) | 9050 (3.7) |
| Multiple sclerosis | 312 (10.1) | 2762 (89.9) | 3074 (1.3) |
| Myocardial infarction | 285 (8.5) | 3074 (91.5) | 3359 (1.4) |
| Parkinson disease | 1028 (10.0) | 9300 (90.0) | 10 328 (4.3) |
| Peptic ulcer disease | 183 (23.6) | 592 (76.4) | 775 (0.3) |
| Peripheral vascular disease | 258 (10.0) | 2310 (90.0) | 2568 (1.1) |
| Psoriasis | 210 (6.7) | 2907 (93.3) | 3117 (1.3) |
| Stroke | 1813 (9.8) | 16 742 (90.2) | 18 555 (7.6) |
| Rheumatoid arthritis | 457 (9.5) | 4377 (90.5) | 4834 (2.0) |
| Severe constipation | 1287 (15.8) | 6879 (84.2) | 8166 (3.4) |
Adjusting for inflation, the 95% percentile cut-off for cost ranged from $62 998 to $74 906 (2017 CAD) over the 10-year period, and the top 5% of patients accounted for between 47.4% and 54.9% of total spending in the cohort in a given year (data not shown).
Person-years of exposure were not distributed equally between high-cost and non-high-cost patients. For example, high-cost patients contributed 16.4% of person-years spent as unstably housed. Older patients also contributed a higher proportion of observations to the high cost group. All 29 comorbidities assessed were over-represented in the high cost group (see Table 2).
Most factors identified in the Gelberg-Andersen Behavioral Model for Vulnerable PopulationsFootnote 27 were found to be significant in our univariate analysis. Notable exceptions were an individual’s rural/urban status, and the number of visits to general practitioners and psychiatrists (data available on request from the authors).
Several trends emerged in the multivariable analysis (see Figure 1). The estimated odds of being high cost were highest for patients aged 18–29 years (OR = 1.63, 95% CI: 1.51–1.77, p < 0.01) compared to those aged 40–49 years. Female patients with schizophrenia had lower odds of being high cost than male patients (OR = 0.87, 95% CI: 0.83–0.92, p < 0.01). The odds of being a high-cost patient if unstably housed were 2.49 times greater than if stably housed (95% CI: 2.34–2.65, p < 0.01).
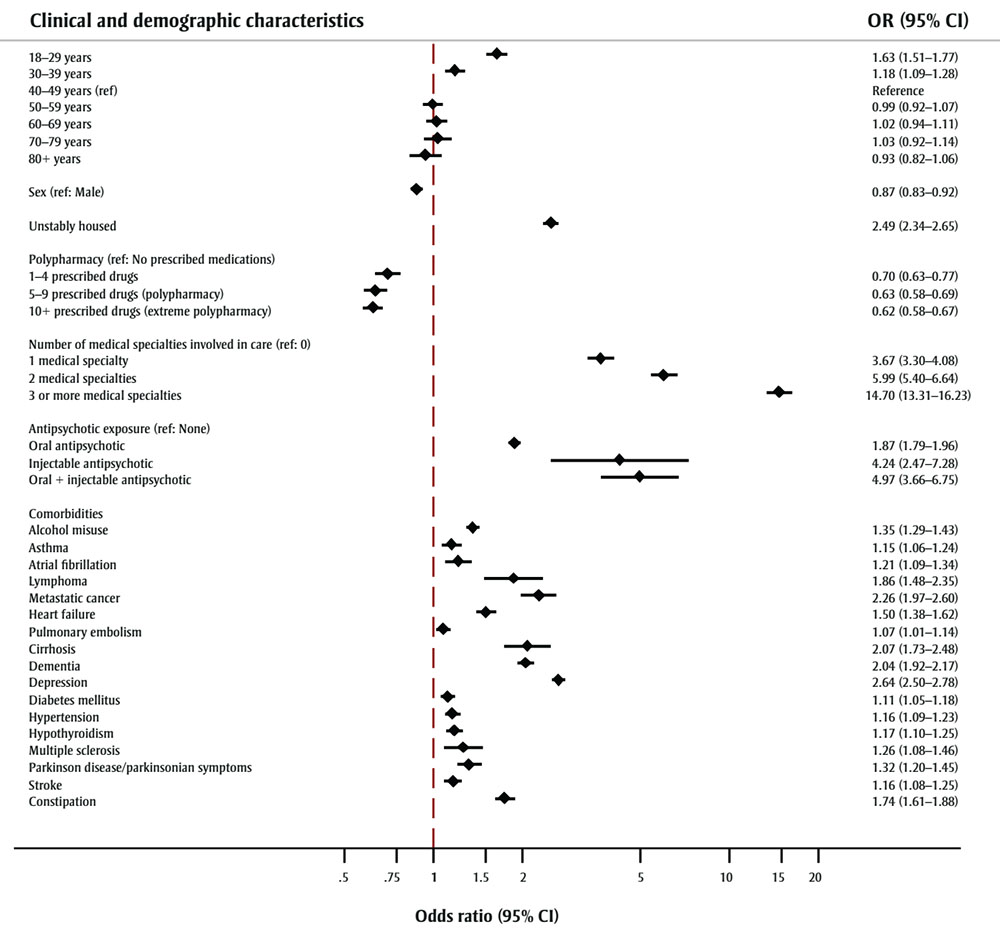
Figure 1 - Text description
| Clinical and demographic characteristics | OR (95% CI) |
|---|---|
| 18–29 years | 1.63 (1.51–1.77) |
| 30–39 years | 1.18 (1.09–1.28) |
| 40–49 years (ref) | Reference |
| 50–59 years | 0.99 (0.92–1.07) |
| 60–69 years | 1.02 (0.94–1.11) |
| 70–79 years | 1.03 (0.92–1.14) |
| 80+ years | 0.93 (0.82–1.06) |
| Sex (ref: Male) | 0.87 (0.83–0.92) |
| Unstably housed | 2.49 (2.34–2.65) |
| Polypharmacy (ref: No prescribed medications) | |
| 1–4 prescribed drugs | 0.70 (0.63–0.77) |
| 5–9 prescribed drugs (polypharmacy) | 0.63 (0.58–0.69) |
| 10+ prescribed drugs (extreme polypharmacy) | 0.62 (0.58–0.67) |
| Number of medical specialties involved in care (ref: 0) | |
| 1 medical specialty | 3.67 (3.30–4.08) |
| 2 medical specialties | 5.99 (5.40–6.64) |
| 3 or more medical specialties | 14.70 (13.31–16.23) |
| Antipsychotic exposure (ref: None) | |
| Oral antipsychotic | 1.87 (1.79–1.96) |
| Injectable antipsychotic | 4.24 (2.47–7.28) |
| Oral + injectable antipsychotic | 4.97 (3.66–6.75) |
| Comorbidities | |
| Alcohol misuse | 1.35 (1.29–1.43) |
| Asthma | 1.15 (1.06–1.24) |
| Atrial fibrillation | 1.21 (1.06–1.34) |
| Lymphoma | 1.86 (1.38–2.35) |
| Metastatic cancer | 2.26 (1.97–2.60) |
| Heart failure | 1.50 (1.38–1.62) |
| Pulmonary embolism | 1.07 (1.01–1.14) |
| Cirrhosis | 2.07 (1.73–2.48) |
| Dementia | 2.04 (1.92–2.17) |
| Depression | 2.64 (2.50–2.78) |
| Diabetes mellitus | 1.11 (1.05–1.18) |
| Hypertension | 1.16 (1.09–1.23) |
| Hypothyroidism | 1.17 (1.10–1.25) |
| Multiple sclerosis | 1.26 (1.08–1.46) |
| Parkinson disease/parkinsonian symptoms | 1.32 (1.20–1.45) |
| Stroke | 1.16 (1.08–1.25) |
| Constipation | 1.74 (1.61–1.88) |
Abbreviations: CI, confidence interval; OR, odds ratio; ref, reference.
Increasing numbers of prescribed medications were associated with decreased odds of being high cost. Patients classified as having extreme polypharmacy (10 or more unique prescribed medications) were about 0.62 times less likely to be high cost than patients who did not receive prescription drugs (95% CI: 0.58–0.67, p < 0.01). Patients receiving both injectable and oral antipsychotic formulations were associated with the highest estimated odds of being high cost (OR = 4.97, 95% CI: 3.66–6.75, p < 0.01).
In the regression model, the greatest difference in odds of being high cost was observed among patients who received care from three or more medical specialties compared with those who had not received specialist care (excluding general practitioners and psychiatrists). Estimated odds ratio was 14.70 (95% CI: 13.31–16.23, p < 0.01). Increasing involvement of medical specialties resulted in increasing odds of being a high-cost patient (see Figure 1).
Of the comorbidities assessed, the highest odds of being a high-cost patient (relative to those without the comorbidity of interest) were associated with metastatic cancer (OR = 2.26, 95% CI: 1.97–2.60, p < 0.01), cirrhosis (OR = 2.07, 95% CI: 1.73–2.48, p < 0.01) and lymphoma (OR = 1.86, 95% CI: 1.48–2.35, p < 0.01). However, other mental health disorders also ranked high among comorbidities. Depression was associated with an odds ratio of 2.63 (95% CI: 2.50–2.78, p < 0.01) and dementia with an odds ratio of 2.04 (95% CI: 1.92–2.17, p < 0.01).
Sensitivity analyses using the 90th and 99th percentile for “high cost” produced similar results (available from the authors on request).
Discussion
Using 10 years of administrative health data collected from patients with schizophrenia in Alberta, Canada, we identified several notable associations between the odds of being a high-cost patient and a patient’s demographic characteristics, their underlying medical complexity and the care they received. Many of these factors are policy relevant and require discussion on the ways current management strategies can be adapted to improve health outcomes while addressing health system sustainability.
We found that younger patients with schizophrenia (aged 18–29 years) were more likely to be high cost. The high level of health care need among younger, newly diagnosed patients with schizophrenia is well established. Nicholl et al.Footnote 33 observed a considerably higher economic burden in the year following treatment for a first psychotic episode than for chronic patients. Similarly, Jin et al.Footnote 34 reported that, compared to older patients, younger patients with schizophrenia had higher rates of service use for five of six service categories assessed (i.e. inpatient, emergency room, crisis house, outpatient and day treatment).
Early detection of schizophrenia, giving patients and clinicians more time to stave off severe outcomes associated with the development of psychosis, could address this association with age. Schizophrenia is often first identified in early adulthood with the onset of psychosis.Footnote 12 A series of risk factors detectable at birth or during childhood allow for the identification of at-risk children. These factors include a family history of schizophrenia, the presence of 22q11.2 deletion syndrome (or several other copy number variants) and the childhood manifestation of transient psychotic symptoms.Footnote 35 Sommer et al.Footnote 35 suggested that health care efficiencies could be realized by applying targeted interventions to children with these risk factors. Promising interventions include those that work to address suboptimal maturation of neuronal pathways during childhood (e.g. treatment with neurotransmitter antagonists and dietary supplementation), the reduction of environmental insults during childhood through the use of social skills training and early interventions to prevent drug use, and the improvement of resilience through cognitive remediation and exercise training.Footnote 35
The association between age and high cost status reinforces the value of the early detection of schizophrenia. First episode/early psychosis programs exist in Alberta, and our finding suggests that patients may benefit from expanding these programs.
The early onset of schizophrenia has far-reaching consequences as the development of symptoms often occurs during critical years. Schizophrenia often impacts a patient’s level of education attainment, career development and personal relationships.Footnote 12 As a result, homelessness is common among patients. This is of concern as a patient’s living situation impacts their ability to access treatment and health care supports. Our work identified a strong association between being unstably housed (our proxy measure for homelessness) and being a high-cost patient.
We previously identified that new antipsychotic formulations may be associated with reduced hospitalization costs.Footnote 19 However, these treatments cannot help an individual who is unable to access them consistently. While this work focusses on direct health-care costs, investment in supportive housing initiatives such as “Housing First” policies that aim to move mental health patients into stable housing as rapidly as possible may represent an important avenue for improving outcomes and health care spending efficiency. In a Canadian pilot study, At Home/Chez Soi participants randomized to receive a Housing First intervention were able to attain stable housing much more rapidly than the treatment-as-usual control group.Footnote 36 This has important implications for patients with schizophrenia who are experiencing homelessness, and we believe that early access to this type of support for younger patients may markedly change disease trajectories and quality of life. Furthermore, housing price to income ratios continue to climb and low-income, older and single Canadians are increasingly being priced out of the housing market.Footnote 37 Efforts must be made to prevent patients with schizophrenia from being left behind.
Patients with schizophrenia are highly reliant on the pharmaceutical management of symptoms. Contrary to our assumptions, we found a negative association between the odds of being high cost and the number of prescribed medications, with the greatest reduction in odds for those with extreme polypharmacy (10 or more unique medications prescribed in a single year). The reduction in odds for those with polypharmacy may be a function of improved management of comorbidity as well as a more active approach in finding efficacious antipsychotic treatment options while minimizing treatment side effects. Alternatively, different polypharmacy profiles may be differentially associated with the odds of being a high-cost patient. Given this uncertainty, further exploration of this trend is necessary.
Underlying comorbidity is also an important factor to consider when discussing high medical costs. Of the 29 comorbidities assessed, 17 were associated with an increased odds of being a high-cost patient. We observed that neurological and mental health conditions such as dementia and depression were highly associated with high cost status. Co-occurring medical conditions are often overlooked during the treatment of patients with schizophrenia.Footnote 38 The strength of these associations highlights the fact that a diagnosis of schizophrenia should not overshadow patient need for the management and prevention of the many comorbid conditions in this population. Like many medical conditions, improving care coordination and connections between medical specialties may pay dividends in the improvement of patient outcomes and result in reductions in total patient spending.
While consideration of single morbidities helps improve our understanding of the conditions that contribute to high health care spending, it does not provide a complete picture. Multimorbidity among patients with schizophrenia is common, with some studies estimating that it occurs 2 to 3 times more often than in the general population.Footnote 39 Although we did not assess multimorbidity directly in our models, we did include information on the number of different medical specialities involved in a patient’s care. Increasing numbers of medical specialties have the strongest association with the odds of being high cost. It may be that level of specialist involvement is a proxy for the level of medical complexity in this population. This would suggest that interventions aimed at addressing medical complexity of patients would result in increases in health care efficiency.
While not specific to schizophrenia, the Lancet Psychiatry Commission on protecting the physical health of people living with mental illness has developed a comprehensive blueprint of interventions for individuals at-risk for developing mental illness, patients undergoing their initial treatment and those undergoing continuing care.Footnote 40 Reductions in medical complexity may be realized by improving the integration between physical and mental health care, training interventions for the reduction of diagnostic overshadowing, and further investigating the attenuation of the long-term effects of antipsychotic medications.Footnote 40
Strengths and limitations
A strength of this study is the use of population-based administrative data that allow for a comprehensive evaluation of the subset of patients with schizophrenia who incur high direct health-care costs. We were able to capture 12 146 person-years of exposures from 2463 patients who were deemed high cost over a 10-year period. This information allowed us to estimate a diverse set of individual, systemic and treatment-associated variables related to health care use and spending.
The use of administrative data for this investigation does have some unavoidable limitations. First, as data were not collected specifically for this investigation, we used several proxy variables. For example, an algorithm for identifying homelessness in administrative data was recently developed in Ontario.Footnote 41 This algorithm relies on data from the Ontario Mental Health Reporting System (OMHRS) that includes detailed information on the use of mental health services not available in Alberta. As a result, we used an adapted algorithm that we believe has high specificity but that may nevertheless underestimate the association between homelessness and high cost status.
Second, community and indirect costs associated with schizophrenia (absenteeism, sick leave, decreased work productivity among patients and caregivers, unemployment, permanent disability and lost productivity due to premature death) were not available in these datasets. With indirect costs associated with schizophrenia estimated as contributing between 50% and 85% of total costs,Footnote 42 the lack of this information limited our ability to quantify the total cost attributable to schizophrenia or the potential role community support programs play on the trends observed.
Despite this, we believe that our description of the association between the various patient- and care-related factors and the odds of being a high-cost patient with schizophrenia captures important dynamics that can help inform evidence-based approaches to improving patient outcomes and cost-savings.
Our findings may not be generalizable to other countries given Canada’s single-payer health care system, but they appear generalizable to other Canadian provinces. For example, de Oliveira et al.Footnote 8 found a similar association between high cost status and age among patients with schizophrenia in Ontario, and Rais et al.Footnote 43 reported a similar cost distribution between high-cost patients and the general population in Ontario.
Finally, our work addresses an important gap by analyzing data that do not depend on sample selection pressures associated with clinics and is therefore more representative of the true patient population that access health services.
Conclusion
The evidence presented here suggests that individual, structural and treatment-related factors all play a role in determining high health spending among patients with schizophrenia. This work confirms several relationships reported in the literature and highlights that underlying medical complexity and subsequent management of multimorbidity play important roles in the subset of patients that drive health care spending. We hope these findings spark further investigation, and inform policy discussions on resource allocation and continued efforts to curb health spending while improving care for patients with schizophrenia.
Acknowledgements
This work is supported by an operating grant from the Canadian Institutes of Health Research (CIHR) and is based in part by data provided by Alberta Health and Alberta Health Services.
Conflicts of interest
None.
Authors’ contributions and statement
Conceptualization – AS, PR and SP. Methodology – AS, PR and SP. Software – AS and JW. Validation – JW. Formal Analysis – AS and PR. Investigator – AS. Resources – PR. Data Curator – JW. Writing – Original Draft: AS. Writing – Review and Editing – AS, PR, SP, TW and KF. Visualization – AS. Supervision – PR and SP. Project Administration – AS. Funding Acquisition – PR.
The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
