Original quantitative research – The Alberta Congenital Anomalies Surveillance System: a 40-year review with prevalence and trends for selected congenital anomalies, 1997–2019

HPCDP Journal Home
Published by: The Public Health Agency of Canada
Date published: January 2023
ISSN: 2368-738X
Submit a manuscript
About HPCDP
Browse
Previous | Table of Contents | Next
R. Brian Lowry, MDAuthor reference footnote 1Author reference footnote 2Author reference footnote 3; Tanya Bedard, MPHAuthor reference footnote 1; Xin Grevers, MPHAuthor reference footnote 1; Susan Crawford, MScAuthor reference footnote 4; Steven C. Greenway, MDAuthor reference footnote 3Author reference footnote 5Author reference footnote 6Author reference footnote 7; Mary E. Brindle, MDAuthor reference footnote 5Author reference footnote 8; Harvey B. Sarnat, MDAuthor reference footnote 3Author reference footnote 9; A. Robertson Harrop, MDAuthor reference footnote 5Author reference footnote 10Author reference footnote 11; Gerhard N. Kiefer, MDAuthor reference footnote 11Author reference footnote 12; Mary Ann Thomas, MDAuthor reference footnote 1Author reference footnote 2Author reference footnote 3
https://doi.org/10.24095/hpcdp.43.1.04
This article has been peer reviewed.
Author references
Correspondence
Dr. R. Brian Lowry, Alberta Congenital Anomalies Surveillance System, Clinical Genetics, Alberta Health Services, 28 Oki Drive NW, Calgary, AB T3B 6A8; Email: brian.lowry@ahs.ca; Tel: 403-955-7370
Suggested citation
Lowry RB, Bedard T, Grevers X, Crawford S, Greenway SC, Brindle ME, Sarnat HB, Harrop AR, Kiefer GN, Thomas MA. The Alberta Congenital Anomalies Surveillance System: a 40-year review with prevalence and trends for selected congenital anomalies, 1997–2019. Health Promot Chronic Dis Prev Can. 2023;43(1):40-8. https://doi.org/10.24095/hpcdp.43.1.04
Abstract
Introduction: Current published long-term provincial or territorial congenital anomaly data are lacking for Canada. We report on prevalence (per 1000 total births) and trends in 1997–2019, in Alberta, Canada, for selected congenital anomalies. Associated risk factors are also discussed.
Methods: We used data from the Alberta Congenital Anomalies Surveillance System (ACASS) to calculate the prevalence and perform chi-square linear trend analyses.
Results: From 1997 to 2019, the overall prevalence of neural tube defects was stable, at 0.74 per 1000 total births. The same was true for spina bifida (0.38), orofacial clefts (1.99), more severe CHDs (transposition of the great arteries, 0.38; tetralogy of Fallot, 0.33; and hypoplastic left heart syndrome, 0.32); and gastroschisis (0.38). Anencephaly, cleft palate and anorectal malformation significantly decreased with a prevalence of 0.23, 0.75 and 0.54 per 1000 total births, respectively. Significantly increasing trends were reported for anotia/microtia (0.24), limb reduction anomalies (0.73), omphalocele (0.36) and Down syndrome (2.21) and for hypospadias and undescended testes (4.68 and 5.29, respectively, per 1000 male births).
Conclusion: Congenital anomalies are an important public health concern with significant social and societal costs. Surveillance data gathered by ACASS for over 40 years can be used for planning and policy decisions and the evaluation of prevention strategies. Contributing genetic and environmental factors are discussed as is the need for continued surveillance and research.
Keywords: congenital anomalies, surveillance, prevalence, trends, Alberta
Highlights
- The Alberta Congenital Anomalies Surveillance System reports prevalence of anomalies and trends from 1997 to 2019 among live births, stillbirths and terminations of pregnancy at less than 20 weeks gestation.
- Overall prevalence of each of the following was stable, showing no significant trends: neural tube defects, spina bifida, orofacial clefts, cleft lip with or without cleft palate, severe congenital heart defects and gastroschisis.
- Anencephaly, cleft palate and anorectal malformations show significantly decreasing trends.
- Anotia/microtia, ventricular septal defects, hypospadias, undescended testes, limb reductions, omphalocele and Down syndrome show significantly increasing trends.
- Precise risk factors are challenging to address, supporting the need for continued congenital anomalies surveillance and research to be integral to public health.
Introduction
Congenital anomalies surveillance in Alberta started in 1963 in response to malformations caused by thalidomide in the late 1950s. In addition to collecting data on structural congenital anomalies, this surveillance system included all physical and neurodevelopmental disabilities of children and included adults. In 1979, the Alberta government restricted surveillance to only congenital anomalies. In 1982, the government proposed to discontinue congenital anomaly surveillance completely, but agreed to transfer the surveillance system to the Alberta Children’s Hospital, Department of Medical Genetics, without transfer of funds. Funds were secured by grant applications until 1994, when the government resumed funding.
The Alberta Congenital Anomalies Surveillance System (ACASS), a provincial population-based program, has data from 1980. Current, published long-term national, provincial or territorial congenital anomaly data are lacking for Canada.
The objectives of this paper are to report prevalence rates and trends for neural tube defects (NTDs), anotia/microtia, orofacial clefts, anorectal malformations, specific congenital heart defects (CHDs), hypospadias, undescended testes, limb reduction anomalies, gastroschisis, omphalocele and Down syndrome for 1997 to 2019, using data from ACASS. These data allow for other Canadian provinces and territories and the Canadian Congenital Anomalies Surveillance System (CCASS) to compare rates and trends.
ACASS is one of the only surveillance systems in Canada with data on termination of pregnancies at less than 20 weeks gestation with more complete ascertainment to contribute to better prevalence estimates. Although inclusion of ascertainment of termination of pregnancies was recommended for CCASS in 1997,Footnote 1 it has not been sufficiently achieved.
ACASS also provides context to the reported rates and trends, which is necessary for valid interpretation. Currently, CCASS reports numbers without context via their Public Health Infobase,Footnote 2 with their last comprehensive report published in 2013 with data to 2009.Footnote 3
Methods
ACASS is primarily a passive system that relies on health care professionals and administrative data for case ascertainment as opposed to an active system where trained surveillance staff abstract case data. Still, it is best described as a hybrid system because we have both aspects, with legal permission to access patient medical records including supporting documentation (e.g. reports from consultations, operations, cytogenetics, diagnostic imaging and pathology). Thus, we can verify or clarify diagnoses including those that occur after termination of pregnancies at less than 20 weeks gestation. Eligible cases are born in Alberta to mothers who reside in Alberta at the time of delivery. Cases that have structural, syndromic, chromosomal, neoplasm, endocrine and/or metabolic abnormalities are ascertained for up to 1 year after delivery. Anomalies are coded using the Royal College of Paediatrics and Child Health (RCPCH) adaptation of the International Classification of Diseases, 10th Revision (ICD-10). Only selected congenital anomalies are included in this paper; however, data for additional anomalies are available.Footnote 4
Multiple ascertainment sources are used (see Table 1) to include live births and stillbirths (>20 weeks gestation and/or >500 g) born since 1 January 1980. Data since 1 January 1997 also include early fetal deaths and termination of pregnancies (<20 weeks gestation and/or <500 g), which is why this paper focusses on the period 1 January 1997 to 31 December 2019.
| Alberta Vital Statistics | Physicians’ Notice of Birth |
|---|---|
| Medical Certificate of Death | |
| Medical Certificate of Stillbirth | |
| All Alberta hospitals | Case notifications from Alberta Hospital Health Records Department via the Congenital Anomalies Reporting Form (CARF) |
| Alberta Children’s Hospital (Calgary) and Stollery Children’s Hospital (Edmonton) | |
| Specialty data sources | Outpatient Clinics (e.g. genetics, prenatal, metabolics) |
| Alberta Precision Laboratories (e.g. Cytogenetics, Newborn Metabolic Screening) | |
| Calgary and Edmonton Pathology |
The ACASS methodology is described in greater detail by Lowry et al.Footnote 4
Alberta Vital Statistics provided denominators. We calculated prevalence as number of cases divided by total number of live births and stillbirths and, for hypospadias and undescended testes, as number of cases divided by total number of male live births and stillbirths, with 95% confidence intervals. Chi-square linear trend analysis was performed.
As this Registry is a part of public health surveillance in Alberta, which is covered by provincial legislation, no ethics approval is required from Alberta Health or the University of Calgary.
Results
Table 2 shows the case prevalence for selected congenital anomalies per 1000 total births. The overall rate of NTDs is 0.74 and of orofacial clefts is 1.99. The most frequent CHDs are septal defects (ventricular septal defects [VSDs] at 3.10 and atrial septal defects [ASDs] at 2.01). The rates of the more severe CHDs, including hypoplastic left heart syndrome (HLHS; 0.32), transposition of the great arteries (0.38) and tetralogy of Fallot (0.33), are comparable. Gastroschisis and omphalocele rates are similar (0.38 and 0.36, respectively). The rate of Down syndrome is 2.21. The prevalence, per 1000 total male births, of hypospadias is 4.68 and of undescended testes is 5.29.
| Congenital anomaly | ICD-10 RCPCH code | Prevalence per 1000 total birthsFootnote a (95% CI) |
|---|---|---|
| NTDs (all) | Q00…, Q01…, Q05… | 0.74 (0.69–0.79) |
| Anencephaly | Q00.00, Q00.01, Q00.1 | 0.23 (0.20–0.26) |
| Spina bifida | Q05… | 0.38 (0.35–0.42) |
| Anotia/microtia | Q16.0, Q17.2 | 0.24 (0.21–0.27) |
| Orofacial clefts (all) | Q35…, Q36…, Q37… | 1.99 (1.91–2.08) |
| CLP | Q36…, Q37… | 1.23 (1.17–1.30) |
| Cleft palate only | Q35… | 0.75 (0.70–0.81) |
| Anorectal malformations | Q42… | 0.54 (0.50–0.58) |
| CHDs | ||
| Transposition of the great arteries | Q20.11, Q20.3, Q20.5 | 0.38 (0.34–0.42) |
| Tetralogy of Fallot | Q21.3…, Q21.82 | 0.33 (0.30–0.36) |
| VSD | Q21.0 | 3.10 (3.00–3.21) |
| ASDFootnote b | Q21.1… | 2.01 (1.93–2.10) |
| Hypoplastic left heart syndrome | Q23.4 | 0.32 (0.29–0.35) |
| HypospadiasFootnote c | Q54… (exclude Q54.4) | 4.68 (4.51–4.87) |
| Undescended testesFootnote bFootnote c | Q53… | 5.29 (5.10–5.48) |
| Limb reduction | Q71…, Q72… | 0.73 (0.68–0.78) |
| Gastroschisis | Q79.3 | 0.38 (0.35–0.42) |
| Omphalocele | Q79.2 | 0.36 (0.32–0.39) |
| Down syndrome | Q90… | 2.21 (2.12–2.30) |
Results from the chi-square linear trend analyses for 1997–2019 are shown in Table 3. While there are no significant trends for NTDs overall or for spina bifida, anencephaly is significantly decreasing. Rates for cleft palate are also decreasing, while cleft lip with or without cleft palate (CLP) and overall orofacial clefts rates show no significant change. Anorectal malformation rates are significantly decreasing. Although the majority of selected CHD rates show no change, VSD rates are significantly increasing. Rates of both hypospadias and undescended testes show significant increases, as do limb reductions. Gastroschisis has stabilized, while omphalocele is significantly increasing, as is Down syndrome.
| Congenital anomaly | Trend direction | Chi-square analysis (χ2LT) | p value |
|---|---|---|---|
| NTDs (all) | No significant change | 3.58 | 0.0585 |
| Anencephaly | Decreasing | 7.00 | 0.0082 |
| Spina bifida | No significant change | 0.01 | 0.9203 |
| Anotia/microtia | Increasing | 5.67 | 0.0173 |
| Orofacial clefts (all) | No significant change | 0.88 | 0.3482 |
| CLP | No significant change | 0.32 | 0.5716 |
| Cleft palate only | Decreasing | 5.05 | 0.0246 |
| Anorectal malformations | Decreasing | 10.39 | 0.0013 |
| CHDs | |||
| Transposition of the great arteries | No significant change | 1.14 | 0.2857 |
| Tetralogy of Fallot | No significant change | 0.90 | 0.3428 |
| VSD | Increasing | 4.79 | 0.0286 |
| ASDFootnote a | No significant change | 0.08 | 0.7773 |
| Hypoplastic left heart syndrome | No significant change | 2.26 | 0.1328 |
| Hypospadias | Increasing | 55.83 | < 0.0001 |
| Undescended testesFootnote a | Increasing | 14.22 | 0.0002 |
| Limb reduction | Increasing | 4.49 | 0.0341 |
| Gastroschisis | No significant change | 0.07 | 0.7913 |
| Omphalocele | Increasing | 12.07 | 0.0005 |
| Down syndrome | Increasing | 23.54 | < 0.0001 |
Discussion
Neural tube defects
NTDs show no significant change (p = 0.0585). Anencephaly prevalence rates started to decline in 2016 and continued to 2019. In contrast, spina bifida rates have remained stable.
Anencephaly rates are influenced by very early termination of pregnancies and perhaps by the terminology used to describe prenatal findings, for example, “absent calvarium,” which is coded in ICD-10 RCPCH under the musculoskeletal system (Q75.8) and not with anencephaly/exencephaly. As a result, such cases are not classified as NTDs.
Acrania can progress to exencephaly and anencephaly.Footnote 5 The method of termination often precludes an accurate postmortem diagnosis.
The most recent statistics from the Public Health Agency of Canada (PHAC) use data to 2014 and show no trend for NTDs.Footnote 2
Folic acid fortification was introduced in Canada in 1998 and has had a significant impact on the prevalence of NTDs. Nevertheless, a substantial number of such defects remain,Footnote 6 which may be due to red blood cell folate levels being below 906 nmol/L and/or the need to supplement with vitamin B12Footnote 7 or inositol.Footnote 8 Additional risk factors include maternal obesity, diabetes mellitus and the use of anticonvulsants and folic acid antagonists.Footnote 9
Anotia/microtia
Most clinicians and surveillance programs classify anotia/microtia into four categories, with type 4 anotia the most severe and type 1 being a smaller ear with normal structure. Some studies record only types 2 to 4,Footnote 10 which include most ACASS cases. While the rate sharply dropped in 2019, ACASS closely monitors the overall significantly increasing trend, which remains unexplained.
Risk factors include male sex, maternal diabetes and obesity, Hispanic ethnicity, advanced maternal age, high parity, multifetal gestation, cold symptoms and viral infection.Footnote 10 Luquetti et al. summarized the epidemiology and genetics of microtia, including higher risks associated with Asian, Pacific Islander, Native/Alaskan and Indigenous ethnicities.Footnote 11 Living at an altitude greater than 2000 m is a risk factor, but this risk factor does not apply in Alberta (Calgary is at 1048 m and Edmonton at 645 m). Maternal smoking and alcohol are reported as risk factors in nonisolated casesFootnote 10 and alcohol exposure in isolated cases.Footnote 11 Known teratogens include thalidomide, isotretinoin and mycophenolate mofetil.Footnote 11
Orofacial clefts
The overall rates for CLP have remained stable in Alberta for over 40 years and in other jurisdictions for over 30–50 years.Footnote 12 In contrast, cleft palate has shown a significantly declining trend (see Table 3). A decline in California has been reported for CLP, but not for cleft palate (1987–2010), suggesting a possible contribution of folic acid fortification to this decline.Footnote 13 Lowry et al.Footnote 14 compared the period prior to the introduction of folic acid fortification (1993–1997) with two periods after (2000–2004 and 2012–2016), in Alberta, and reported no decline for total CLP cases or for isolated cases over the three timeframes.
A decline was reported in prevalence of orofacial clefts for 1994–2017 in Ontario, especially for cleft palate; however, data from stillbirths and terminations of pregnancy were lacking.Footnote 15 The only national Canadian reported data covers 2005–2014 and show no change in trend for CLP but a possible downward trend for cleft palate.Footnote 2
Risk factors include active and passive smoking; alcohol consumption, particularly binge drinking; and maternal obesity. Gene polymorphisms also play a role.Footnote 16Footnote 17 Meta-analysis of maternal supplementation suggests that periconception intake of folic acid plus multivitamins can reduce occurrence as well as recurrence.Footnote 18 The Hutterite Brethren, whose smoking and alcohol consumption is limited and nutrition probably adequate, had zero cases of cleft lip with cleft palate in 1980–2016.Footnote 19
Anorectal malformations
The overall trend is significantly decreasing (p = 0.0013). A 2007 ACASS study for the years 1990–2004 showed stable ratesFootnote 20 that compared favourably with the results of other studies of that time. The current decline is for both isolated and associated anomaly cases. Khanna et al.Footnote 21 reviewed genetic factors contributing to the etiopathogenesis of isolated cases and concluded that a number of copy number variants and/or single nucleotide variants contributed to the defect. Families with autosomal dominant inheritance are reported to exist.Footnote 21
Risk factors include maternal smoking, maternal body mass index (BMI) greater than 30, assisted reproductive technology, maternal chronic respiratory disease, maternal use of anti-asthmatic medications, hypnotics and benzodiazepine.Footnote 22 Zwink and JenetzkyFootnote 22 report inconsistent results for the protective effects of folic acid supplements.
Zwink and JenetzkyFootnote 22 found that in the majority of studies in their systematic review approximately 60% of cases have an associated anomaly; this compares with 82% of ACASS cases. This difference should be interpreted with caution, as inclusion criteria and case classification differed. Other studies may only include live-born and surgically treated cases.
Congenital heart defects
The more severe anomalies show no significant trends with similar case prevalence rates (per 1000 total births: HLHS, 0.32; tetralogy of Fallot, 0.33; and transposition of the great arteries, 0.38). Öhman et al.Footnote 23 reported a decrease of live births with HLHS in Sweden, and suggest that this decrease was due to increased prenatal detection and termination of pregnancies. This highlights the importance of ascertaining termination of pregnancies to determine more accurate prevalence.
While the prevalence of ASDs remained stable between 1997 and 2019 (p = 0.7773), the prevalence of VSDs has statistically significantly increased (p = 0.0286), likely because small septal defects are better diagnosed as a result of advances in echocardiography and heart ultrasound. However, ACASS does not accept patent foramen ovales, ASDs in premature infants or ASDs that are smaller than 3 mm and spontaneously close; conversely, ACASS does accept VSDs, regardless of their size, the need for intervention or their spontaneous closing.
Although most CHDs are multifactorial, genetic diagnoses have been reported in 15.7% of cases that have a severe CHD requiring surgery or therapeutic intervention in the first year of life.Footnote 24 Cases with a known aneuploidy were excluded.Footnote 24 There is emerging evidence that complex single-gene disorders often present as isolated CHDs prenatally, as complete phenotyping may not be possible.Footnote 25 In the past two decades, genetic variants have been associated with nonsyndromic or isolated CHDs, particularly for highly conserved transcription factors essential for cardiac development (e.g. GATA4 variants associated with tetralogy of Fallot, ASDs, VSDs, atrioventricular septal defects and pulmonary stenosis).Footnote 26
Reported risk factors for CHDs include teratogens (e.g. thalidomide, isotretinoin, anticonvulsants, potassium channel blockers, lithium, alcohol), nutritional deficiencies (e.g. vitamin A, vitamin B3) and maternal conditions (diabetes, obesity, phenylketonuria, viral infections and hyperthermia).Footnote 27 Dolk et al.Footnote 28 reported significant associations with low maternal education, vaginal infections, maternal clotting disorders and prescriptions for the anticlotting medication enoxaparin. With limited evidence to support such an association, more research is needed to confirm this reported increased risk with enoxaparin. Although the data did not support a protective effect of folic acid supplementation, risk was significantly increased for mothers with diets particularly low in fruits and vegetables, emphasizing the need to consider the entire dietary context.Footnote 28
Placental abnormalities (e.g. low placental weight, altered gene expression in placental tissue) have also been reported to be associated with CHDs.Footnote 29 A more comprehensive framework has been proposed to include the environmental complement to the genome, an emerging field of the exposome.Footnote 30 Instead of a siloed approach, the interplay between internal and external prenatal environmental exposures that influence placental vascularization and subsequent fetal growth and development needs to be advanced.Footnote 30
Hypospadias
The prevalence of hypospadias for both isolated and nonisolated cases peaked in 2015 and shows an overall significant increase (p < 0.0001) for 1997–2019. It is difficult to compare prevalence rates because of methodological differences, such as differences in the degree of severity, the inclusion of surgical cases only and whether rates are for total births versus male births. The EUROCAT report showed wide variability per 1000, with Portugal at 0.51 and Mainz (Germany) at 3.68.Footnote 31
George et al.Footnote 32 have described the challenges with associations to determine the etiology of hypospadias and summarized the genetic and environmental factors. Consistent associated risk factors include a positive family history, low birth weight and/or small gestational age, maternal hypertension, preeclampsia, multiple gestations, placental insufficiency, diabetes mellitus and exposures to certain drugs such as progesterone derivatives or valproic acid. Evidence is inconsistent for risk factors such as maternal age and weight, paternal or maternal occupations and agriculture practices.
Genetic variants, such as the diacylglycerol kinase kappa (DGKK) variants, have been shown to be significant risk factors.Footnote 33 In California, cases with the DGKK variants and residential proximity to pesticide application had the highest odds ratios for hypospadias.Footnote 34 In Nova Scotia, the highest prevalence rates of hypospadias were in two counties that were associated with intense farming.Footnote 35 The prevalence of isolated hypospadias in the Hutterite Brethren is approximately double that of the general Alberta population, which may be associated with farming and agricultural practices.Footnote 19
Undescended testes
While there was a sharp drop in rates of undescended testes in 2019, the trend from 1997 to 2019 shows a significant increase (p = 0.0002). These results have to be interpreted with caution, as this condition may resolve spontaneously or may in fact be retractile testes. A more accurate prevalence would be determined by knowing which full-term and normal birth-weight cases came to orchidopexy. Surgical numbers could include preterm and low birth-weight babies. Hence, the difficulty in obtaining a true prevalence rate.
ACASS does not accept cases born before 37 weeks gestation or with a birth weight of less than 2500 g, but considers these to be physiological and caused by immaturity.
Although the etiology is likely multifactorial, there are some familial cases as well as multiple susceptibility genes.Footnote 36 Consistent risk factors are maternal smoking and diabetes, while maternal obesity, alcohol use, use of analgesics and exposure to endocrine-disrupting chemicals, such as agricultural pesticides, are inconsistently reported as risk factors.Footnote 37 No differences were reported in the prevalence of undescended testes in the Hutterite population and the general Alberta population.Footnote 19
Limb reductions
Since 1980, rates have fluctuatedFootnote 38 and we report a significant increase (p = 0.0341) for 1997–2019. As one case may have multiple limb reduction anomalies, we report both anomaly and case rates. Our rate of 0.73/1000 total births is comparable to studies from, for example, northern Netherlands (0.64/1000 for 1981–2017), which did not report a trend.Footnote 39
Results of studies of folic acid, with or without supplements, reducing the risk of limb reductions are equivocal,Footnote 40 but it is clear that folic acid fortification has had no effect in Alberta. In most cases, the precise cause is unknown. Bergman et al.Footnote 39 recently found that an etiological cause was more likely to be identified in a case when more than one limb is affected or in a multiple congenital anomalies case with one affected limb, compared to cases with one limb affected and no other congenital anomalies. Risk factors include maternal smoking, pregestational diabetes, gestational hypertension, maternal age less than 25 years, upper respiratory tract infection in the first trimester, antiepileptic medications and lower educational level of parents.Footnote 41
Gastroschisis
An increase in the prevalence of gastroschisis was noted in the early 1970s in many jurisdictions. In Alberta, the rate rose from 0.15 to 0.57/1000 total births between 1980 and 2011. Rates subsequently declined every year and have now stabilized (see Figure 1), which coincides with a decline in the number of teenage pregnancies (mothers <20 years old) (see Figure 2). Young maternal age is a known risk factor, and the percentage of mothers younger than 20 years in Alberta fell from 7.3% in 2000 to 1.8% in 2019.Footnote 4
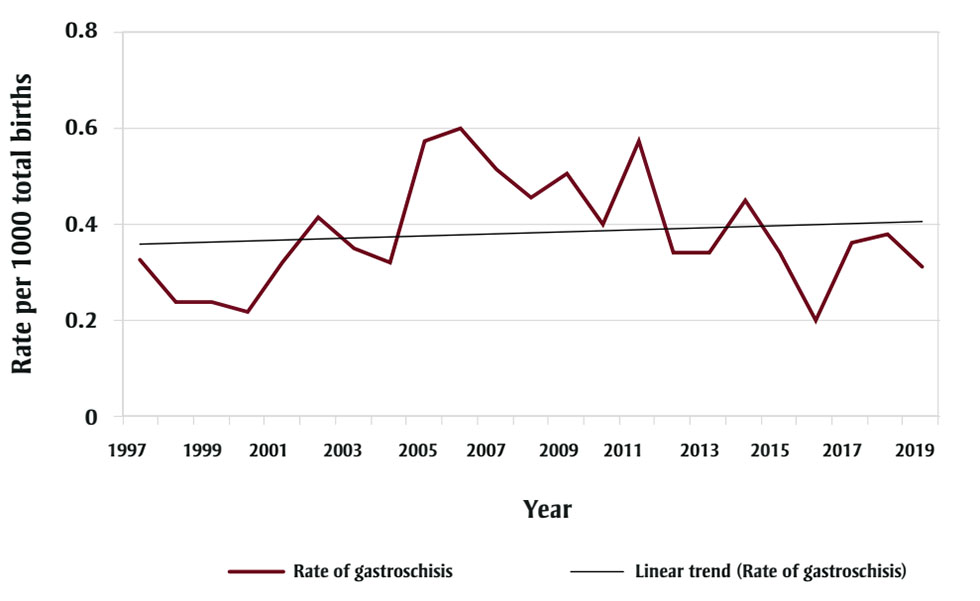
Figure 1 - Text description
| Year | Rate per 1000 total births |
|---|---|
| 1997 | 0.33 |
| 1998 | 0.24 |
| 1999 | 0.24 |
| 2000 | 0.22 |
| 2001 | 0.32 |
| 2002 | 0.42 |
| 2003 | 0.35 |
| 2004 | 0.32 |
| 2005 | 0.57 |
| 2006 | 0.60 |
| 2007 | 0.51 |
| 2008 | 0.46 |
| 2009 | 0.51 |
| 2010 | 0.40 |
| 2011 | 0.57 |
| 2012 | 0.34 |
| 2013 | 0.34 |
| 2014 | 0.45 |
| 2015 | 0.34 |
| 2016 | 0.20 |
| 2017 | 0.36 |
| 2018 | 0.38 |
| 2019 | 0.31 |
* p = 0.7913.
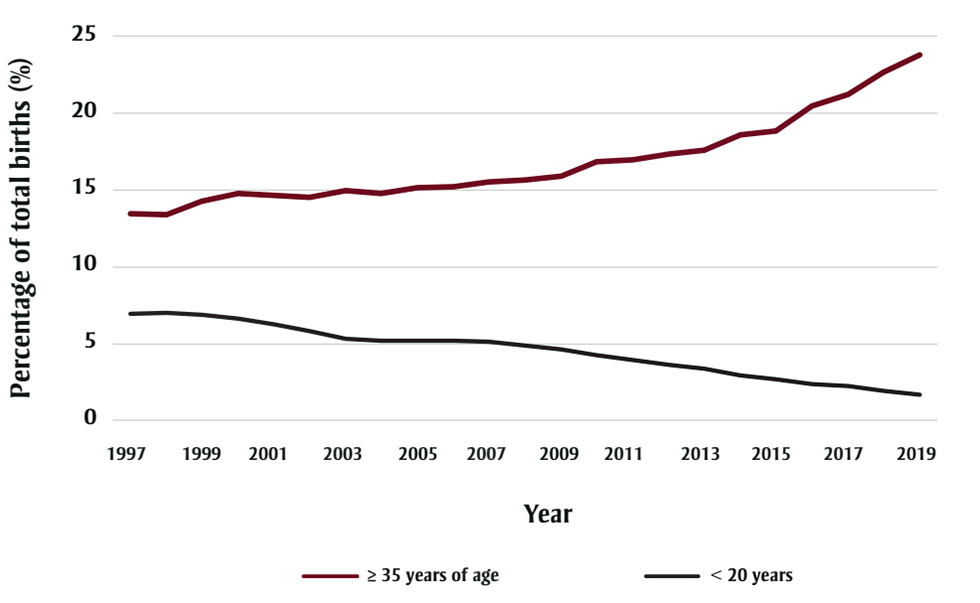
Figure 2 - Text description
| Year | Percentage of total births (%), women 35 years old or older | Percentage of total births (%), women less than 20 years old |
|---|---|---|
| 1997 | 13.48 | 6.97 |
| 1998 | 13.36 | 7.00 |
| 1999 | 14.27 | 6.89 |
| 2000 | 14.74 | 6.66 |
| 2001 | 14.63 | 6.23 |
| 2002 | 14.54 | 5.81 |
| 2003 | 14.94 | 5.35 |
| 2004 | 14.78 | 5.21 |
| 2005 | 15.16 | 5.18 |
| 2006 | 15.23 | 5.17 |
| 2007 | 15.54 | 5.14 |
| 2008 | 15.67 | 4.87 |
| 2009 | 15.87 | 4.63 |
| 2010 | 16.82 | 4.25 |
| 2011 | 16.94 | 3.91 |
| 2012 | 17.33 | 3.62 |
| 2013 | 17.60 | 3.39 |
| 2014 | 18.60 | 2.92 |
| 2015 | 18.86 | 2.67 |
| 2016 | 20.46 | 2.37 |
| 2017 | 21.20 | 2.24 |
| 2018 | 22.69 | 1.93 |
| 2019 | 23.79 | 1.69 |
A recent Canadian study using 2006–2017 data found similar results to those of ACASS for trend and a decrease in mothers younger than 20 years.Footnote 42 However, the North–South classification methodology the authors used and their interpretation of a geographical variation is problematic.Footnote 42 An Ontario study (2012–2018) reported no trend.Footnote 43 Neither study included early fetal deaths or terminations.Footnote 42Footnote 43
Additional social risk factors include maternal smoking, use of marijuana, illicit drugs and alcohol, low BMI, poor nutrition and socioeconomic disadvantage.Footnote 43 There are fewer exposures to many of these risk factors in the Hutterite population, where there were no cases of gastroschisis between 1980 and 2016.Footnote 19 A recently recognized risk factor is exposure to wildfires during pre-pregnancy and the first trimester.Footnote 44
While gastroschisis is usually an isolated anomaly, 28% of ACASS cases (data not shown) had a co-occurring anomaly; this is similar to findings in a study from Sweden,Footnote 45 but was not mentioned by either of the recent Canadian studies.Footnote 42Footnote 43 Although gastroschisis is usually sporadic, there are familial reports of inheritance, including parent to child, full siblings, half siblings and distant relatives.Footnote 46 Geospatial studies have reported some provincial differences and clusters, with urban/rural differences in Ontario.Footnote 43
Omphalocele
Comparable prevalence rates of omphalocele per 1000 total births have been recorded for several jurisdictions despite differing study years: 0.31 for 1997–2016;Footnote 47 0.47 for 1993–2014;Footnote 48 and 0.38 for 2005–2011.Footnote 49 Neither trends for live birthsFootnote 47Footnote 48 nor for total birthsFootnote 49 were reported, but ACASS has a significantly increased trend for 1997–2019 (p = 0.005) (see Figure 3).
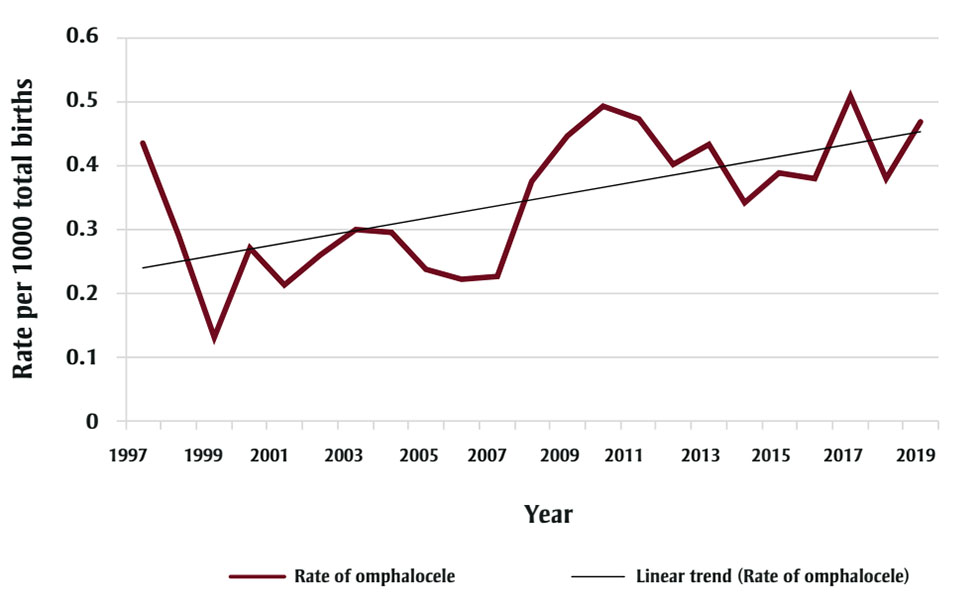
Figure 3 - Text description
| Year | Rate per 1000 total births |
|---|---|
| 1997 | 0.43 |
| 1998 | 0.29 |
| 1999 | 0.13 |
| 2000 | 0.27 |
| 2001 | 0.21 |
| 2002 | 0.26 |
| 2003 | 0.30 |
| 2004 | 0.30 |
| 2005 | 0.24 |
| 2006 | 0.22 |
| 2007 | 0.23 |
| 2008 | 0.38 |
| 2009 | 0.45 |
| 2010 | 0.49 |
| 2011 | 0.47 |
| 2012 | 0.40 |
| 2013 | 0.43 |
| 2014 | 0.34 |
| 2015 | 0.39 |
| 2016 | 0.38 |
| 2017 | 0.51 |
| 2018 | 0.38 |
| 2019 | 0.47 |
* p = 0.0005.
Associated anomalies, which include a malformation in another organ system, chromosomal abnormalities and syndromes, are present in 78% of cases recorded by ACASS. Trisomy 18 is very common, but a wide variety of abnormal karyotypes have been reported.
Risk factors include maternal age greater than 35 years or less than 20 years, maternal obesity and diabetes mellitus. Risks for exposures to smoking and alcohol are inconclusive.Footnote 50 A recent study has linked first trimester broad spectrum penicillin treatment with a reduced risk.Footnote 51
Down syndrome
Down syndrome is significantly increasing (p < 0.0001) and is strongly correlated with increasing maternal age. In 1983, approximately 4% of mothers were 35 years or older; in 2019, 24% were in that age group.Footnote 4
Frequently associated major malformations include CHDs and duodenal atresia. As most live-born infants with trisomy 21 require ongoing health services, ascertaining associated anomalies can help with future health care planning.
Strengths and limitations
The strengths of this study are supported by the principal features of ACASS and include long-term baseline data, which are fundamental for valid descriptive and analytic studies. Additional features include provincial population-based coverage, multiple sources of ascertainment, ability to critically assess notifications and verify diagnoses that are reported to the system, and the expertise of ACASS personnel.
A limitation is that ACASS is technically a “passive” system, although it is augmented by active components, such as access to hospital records and correspondence with attending physicians for verification. ACASS primarily depends on others for case notifications and thus may not have complete ascertainment. The best systems, practised in many US States (e.g. Texas, Utah) and European and South American countries, have “active” ascertainment.
Conclusion
Congenital anomalies occur in approximately 3–5% of live births and 15% of stillbirths. They are an important public health concern and have significant social and societal costs. The majority of congenital anomalies are multifactorial, with established risk factors often requiring a change in behaviour, which can be challenging (e.g. smoking and alcohol cessation, better control of maternal obesity and diabetes, folic acid/multivitamin supplementation and better nutrition).
Congenital anomalies surveillance data can be used for planning and policy decisions and the evaluation of prevention strategies, as exemplified by the success of folic acid fortification in the prevention of NTDs. These data are also required to respond to real and potential emerging threats such as Zika virus and the identification of congenital Zika syndrome. Many congenital anomalies surveillance programs now track outcomes of COVID-19 infection in pregnancy.
While funding is often challenging to obtain and maintain in Canada, PHAC is working with the provinces and territories to enhance CCASS data with more local datasets, which will provide more accurate prevalence rates of congenital anomalies across Canada. The last comprehensive congenital anomaly report published by PHAC used Canadian Institute for Health Information data from 1998–2009.Footnote 3 The British Columbia Health Status Registry was a world-class congenital anomalies surveillance system and after 70 years, the data were archived in 2021. Their last report was in 2005 using data to 2002.
With over 40 years in operation, ACASS has the most published prevalence data in Canada and provides context for more prevention. Congenital anomalies surveillance constitutes an essential data source for further research and to guide public health actions.Footnote 52
Acknowledgements
We would like to recognize that our work takes place on historical and contemporary Indigenous lands, including the territories of Treaties 6, 7 and 8 and the homeland of the Métis. We also acknowledge the many Indigenous communities that have been forged in urban centres across Alberta.
The Alberta Congenital Anomalies Surveillance System (ACASS) receives funding from Alberta Health Services. We are grateful for the support and to be a part of the Clinical and Metabolic Genetics Program. We are thankful for the continued provision of congenital reporting documents and denominator data from the Alberta Ministry of Health and Alberta Vital Statistics. Our success depends upon the interest and activities of hospital health records personnel, paramedical professionals, nurses and physicians.
ACASS would also like to thank Caroline Kokorudz for help with the literature search and Esteban Soriano for administrative support in preparing this article.
Conflicts of interest
The authors declare no conflicts of interest.
Authors’ contributions and statement
RBL: Writing – Original draft. RBL, TB: Conceptualization of the work. RBL, TB, XG, SC, MAT: Data curation and analysis.
All the authors revised the manuscript for relevant and important intellectual content, edited the working manuscript and approved the final version for submission.
The content and views expressed in this article are those of the authors and do not necessarily reflect those of the Government of Canada.
