Transfusion error surveillance system (TESS), 2020 to 2021
Download the alternative format
(PDF format, 270 Kb, 1 page)
- Organization: Health Canada
- Date published: March 2023
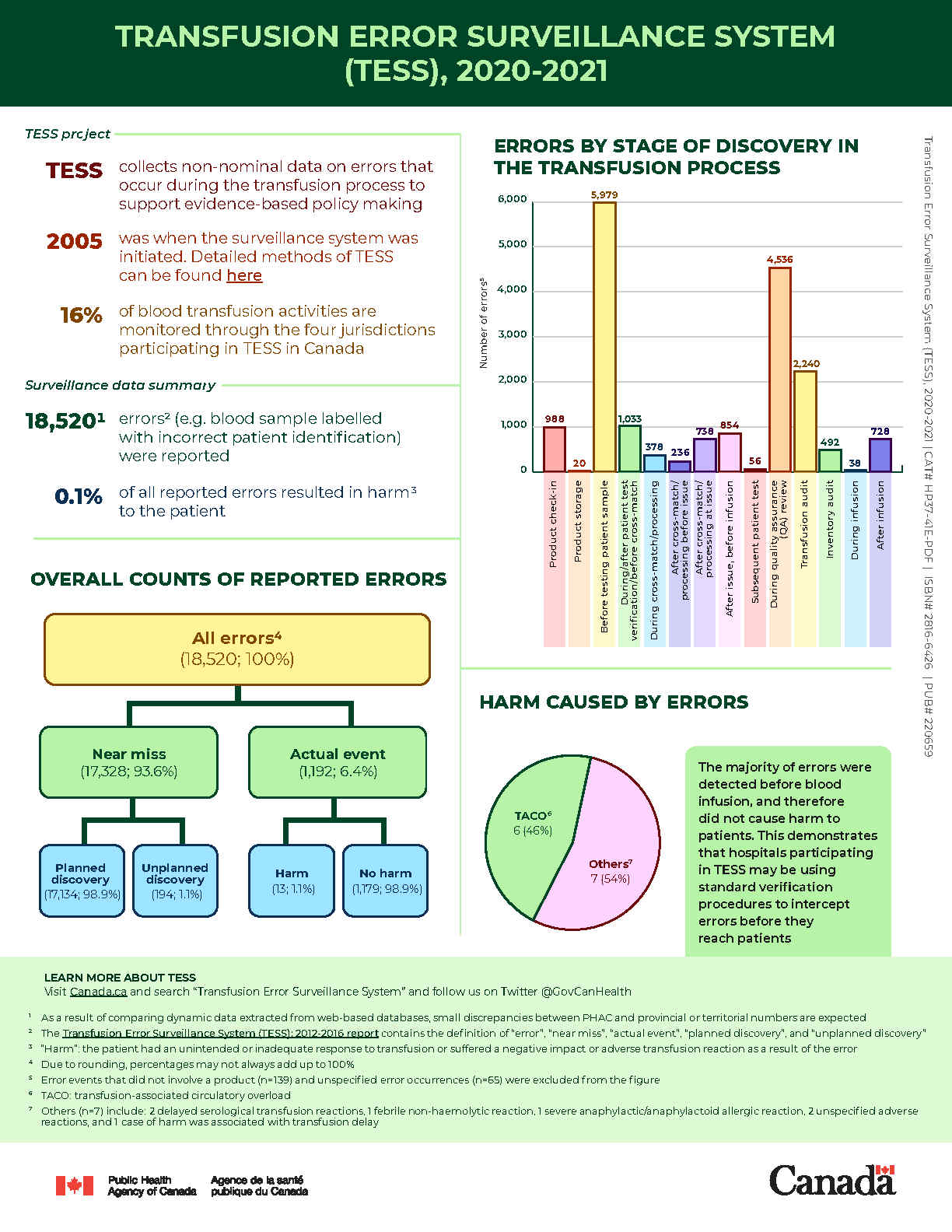
The TESS project
The Transfusion Error Surveillance System (TESS) collects non-nominal data on errors that occur during the transfusion process. The objective of TESS is to support evidence-based decision making by public health authorities and departments. The surveillance system was initiated in 2005. TESS now monitors four provinces and territories and 16% of blood transfusion activities in Canada. Detailed methods of TESS can be found in the Transfusion Error Surveillance System (TESS): 2012-2016 Report.
Surveillance data summary
See below for a summary of the collected data on transfusion errors.
- 18,520Footnote 1 errorsFootnote 2 (such as blood samples labelled with incorrect patient identification) were reported.
- 0.1% of all reported errors resulted in harmFootnote 3 to the patient.
Overall counts of reported errors
See below for a breakdown of the counts and percentages of reported errors according to their categories.
- All errors: 18,520; 100%Footnote 4
- Near miss: 17,328; 93.6%
- Planned discovery: 17,134; 98.9%
- Unplanned discovery: 194; 1.1%
- Actual event: 1,192; 6.4%
- Harm: 13; 1.1%
- No harm: 1,179; 98.9%
Frequency of errors by stages of discovery
See below for the number of errors discovered at various stages in the transfusion processFootnote 5.
- Product check-in: 988
- Product storage: 20
- Before testing patient sample: 5,979
- During/after patient test verification / before crossmatch: 1,033
- During crossmatch / processing before issue: 378
- After crossmatch / processing before issue: 236
- After crossmatch / processing at issue: 738
- After issue, before infusion: 854
- Subsequent patient test: 56
- During quality assurance review: 4,536
- Transfusion audit: 2,240
- Inventory audit: 492
- During infusion: 38
- After infusion: 728
Harm caused by errors
See below for the counts and proportions of adverse transfusion reactions that were caused by transfusion errors.
- Transfusion-associated circulatory overload: 6; 46%
- Others: 7; 54%
- Delayed serological transfusion reaction: 2
- Unspecified adverse transfusion reaction: 2
- Febrile non-haemolytic transfusion reaction: 1
- Severe anaphylactic/anaphylactoid allergic reaction: 1
- Harm associated with transfusion delay: 1
Conclusion
Most of errors were detected before infusion, and therefore did not cause harm to patients. This demonstrates that hospitals participating in TESS may be using standardized verification procedures to intercept errors before they reach patients.
To learn more about TESS, please visit Canada.ca and search “Transfusion Error Surveillance System”, as well as follow us on Twitter @GovCanHealth.
Footnotes
- Footnote 1
-
As a result of using dynamic data extracted from web-based databases, small discrepancies between PHAC and provincial or territorial numbers are expected.
- Footnote 2
-
See the Transfusion Error Surveillance System (TESS): 2012-2016 Report at https://www.canada.ca/en/public-health/services/publications/drugs-health-products/transfusion-error-surveillance-system-tess-2012-2016-report.html for the definitions of “error”, “near miss”, “actual event”, “planned discovery”, and “unplanned discovery”
- Footnote 3
-
“Harm”: the patient had an unintended or inadequate response to transfusion or suffered a negative impact or adverse transfusion reaction because of the error
- Footnote 4
-
Due to rounding, percentages may not always add up to 100%
- Footnote 5
-
Error events that did not involve a product (n=139) and unspecified error occurrences (n=65) were excluded from the figure