In-depth analysis: Human respiratory disease associated with Middle East Respiratory Syndrome Coronavirus

Download the alternative format
(PDF format, 1.74 MB, 19 pages)
Organization: Public Health Agency of Canada
Published: 2018-08-09
Middle East Respiratory Syndrome Coronavirus (MERS-CoV)
In March 2018, 17 new cases of Middle East Respiratory Syndrome (MERS) were reported. The majority of new cases were reported from Saudi Arabia (n=16). A total of 2183 cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV), including at least 760 deaths, have been reported globally since 2012. Further information can be found in the in depth analysis section below.
Risk to Canada
The Public Health Agency of Canada (PHAC) has conducted a risk assessment for MERS-CoV. To date, no cases have been reported in Canada and the public health risk posed by MERS-CoV to Canadians is considered low based on the available information at this time. Additional cases of MERS-CoV infections are expected to be reported from the Middle East and it is likely that cases will continue to be exported to other countries by individuals who might acquire infection following exposure to an animal (camels) or human source. Human-to-human transmission of the virus has been low and self-limited to only a few generations of infection with occasional amplification in health care settings. Thus, it is important that health care facilities, research laboratories, and public health professionals remain vigilant in the systematic implementation and adherence of infection prevention and control (IPC) measures.
Guidance documents including a public health notice , a travel health notice, IPC guidelines, a biosafety advisory, case and contact management guidelines, and case definitions can be found on the Government of Canada website for MERS-CoV .
Contents
- Background
- Source of exposure
- Virus characteristics
- Clinical manifestations
- Event summary: January to March 2018
- Global epidemiological analysis
- Age and sex distribution
- Temporal distribution
- Geographical distribution
- Vaccines against MERS-CoV
- Limitations
- Conclusion
- References
From Human Emerging Respiratory Pathogens Bulletin Issue 015 (April 2018).
Background
On June 13, 2012, a 60 year old Saudi man was admitted to a private hospital in Jeddah, Saudi Arabia, after reporting respiratory symptoms like cough, expectoration, shortness of breath, and feverFootnote 1Footnote 2. Eleven days after admission, on June 24, 2012, he died of progressive respiratory and renal failureFootnote 1. The investigation into the etiological agent responsible for his condition began at the laboratory attached to the hospital. After two months of testing clinical isolates from the patient for multiple viruses (influenza virus A, influenza virus B, parainfluenza virus, paramyxovirus, hantavirus, enterovirus and adenovirus), the isolate was tested for coronavirus using a pancoronavirus RT-PCRFootnote 3. The test was positive, but negative for SARS specific primers, suggesting the discovery of a novel coronavirusFootnote 3. On September 20th, 2012, after further confirmation by another laboratory (the Erasmus Medical Center), the discovery of a novel coronavirus was announced through ProMEDFootnote 3Footnote 4. This coronavirus, the etiological agent for Middle East Respiratory Syndrome (MERS), was eventually named Middle East Respiratory Syndrome Coronavirus (MERS-CoV), the 6th strain of human coronavirus discoveredFootnote 5.
Since that time, over 2000 cases have been reported globally. Family clusters and hospital outbreaks have occurred both within the Middle East and elsewhere, and the cases have spread from the region to some other countries [Figure 1]Footnote 6. There has also been important progress in laboratory diagnostics, virus characterization, vaccine development, and understanding of the clinical course of illness and the mechanism of transmission [Figure 1]. This in-depth analysis provides a summary of the descriptive epidemiology of human infection with MERS-CoV since its emergence in 2012, with a focus on the demographic, geographic and temporal distribution of reported cases, as well as a review of the clinical symptoms, sources of exposure, and virus characteristics.
Figure 1 . Middle East Respiratory Syndrome (MERS) epidemic: a timeline of major events (2012-2018)
2012

June
A 60-year-old man is admitted to a small private hospital in Saudi Arabia with severe respiratory symptoms.

September
Dr. Ali Mohamed Zaki announces via ProMED the discovery of a novel coronavirus.
September
Development of the first MERS- specific RT-PCR assay.

November
Retrospective identification of the first hospital outbreak of MERS in Jordan. The outbreak occurred in April 2012.

November
Characterization of the full genome sequence of MERS-CoV isolated from the first human case.

November
Phylogenetic analysis of MERS-CoV shows that it is closely related to two bat coronaviruses indicating that it is a lineage C betacoronavirus.

November
First report of a family cluster of MERS from Riyadh Saudi Arabia.
2013

February
First exported case reported from UK. The case had a history of travel to the Middle East.

February
First report of a human cluster of MERS outside the Middle East. This cluster was a result of the first exported case. Evidence of limited human-to-human transmission in the UK family. The index case had history of travel to the Middle East.

March
Dipeptidyl peptidase 4 (DPP4) identified as cellular receptor of MERS-CoV.

July
WHO Director General convenes a meeting of the International Health Regulations Emergency Committee concerning MERS. After reviewing data on the current situation the committee determines that conditions for a Public Health Emergency of International Concern have not yet been met.

August
A comparative serological study finds MERS-CoV neutralising antibodies in camels from Oman and Spain. This is the first study to test animals for antibodies specific to MERS-CoV.

November
Camels associated with a positive human case test positive for MERS-CoV by PCR for the first time.
2014

Spring
Two large nosocomial outbreaks of MERS-CoV occur in Riyadh and Jeddah, Saudi Arabia resulting in more than 150 human infections.
May
First case of MERS reported in North America. The case was a healthcare provider who lived and worked in Saudi Arabia and subsequently traveled back to the US.

June
A phylogenomic study conducted on dromedary camels in Egypt finds that camel MERS-CoV genome is genetically similar (>99% similarity) to the MERS-CoV infecting humans.

December
A MERS-CoV challenge study conducted in dromedary camels demonstrates that camels can show minor clinical signs (rhinorrhea) of disease when infected, and can shed large quantities of the virus in their nasal secretions.
2015

April
A study conducted to determine the risk of MERS-CoV transmission from camels to humans finds no serological evidence of human infection after various levels of exposure to infected camels suggesting that zoonotic transmission of the virus is rare.

April
A large nationwide cross-sectional serological study from Saudi Arabia finds the seroprevalence in the general population to be 0.15%. The study also finds significantly higher seroprevalence in individuals with occupational exposure to camels.

May-June
A large nosocomial outbreak of MERS occurs in South Korea involving four hospitals and resulting in 186 cases. This is the largest nosocomial outbreak of MERS to occur outside the Middle East.
August
Researchers from a Swiss-led international research group report on the first isolation of a MERS-CoV neutralizing antibody (LCA60) from a recovered MERS patient. LCA60 is an anti-MERS monoclonal antibody that potentially neutralizes MERS-CoV infection in vitro and in vivo.

September
The IHR Emergency Committee concerning MERS holds its 10th meeting. The Committee determines that conditions for a PHEIC have not yet been met.

December
The South Korea outbreak is declared over on December 23 2015. It lasted 7 months.
2016

March
A study details the emergence of mutant MERS-CoV during the South Korea outbreak that has reduced affinity to the human DPP4 receptor—impairing its virulence.

March
A study provides the first autopsy, clinicopathologic, immunohistochemical and ultrastructural description of a fatal case of MERS. The autopsy was conducted in 2014. The autopsy revealed extensive lung damage in patient.

June
MERS antibodies are detected in Qatar alpaca herd. This is the first study to find evidence of MERS-CoV in domestic animals other than camels.
August
SAB Biotherapeutics Inc. announce that Phase I clinical trials are underway for their MERS antibody treatment. This is the first potential treatment for MERS to reach this stage.
2017

May
A study evaluating the implementation of infection, prevention and control measures during a large hospital outbreak finds that strict adherence to IPC measures allows for the rapid identification of cases and significant decrease in secondary cases.

June
WHO reports the occurrence of three hospital outbreaks in Riyadh, Saudi Arabia. These outbreaks result in a surge in cases (n>48).
2018

January
A study identified that DPP4, the receptor for MERS-CoV, is upregulated in the lungs of smokers and COPD patients, indicating a potential reason for increased susceptibility in this population.

February
The WHO updated their Blueprint list of priority diseases that require further research and development and retained MERS-CoV on the list.
Note: This timeline is not meant to include all developments regarding the MERS epidemic. Instead, selected items are intended to demonstrate important research advancements and epidemiological milestones in the ongoing epidemicFootnote 4 Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21Footnote 22Footnote 23 Footnote 59Footnote 84.
Source of exposure
Primary cases of MERS are associated with zoonotic transmission from camels. Though research suggests that MERS-CoV emerged from bats, multiple studies on the emergence and transmission of MERS-CoV point to the role of camels as a reservoir and primary source of zoonotic infection due to the high prevalence of MERS-CoV antibodies in camels across the Arabian Peninsula and North and Eastern AfricaFootnote 47Footnote 62Footnote 63Footnote 64Footnote 65. The detection of MERS-CoV by RT-PCR in respiratory secretions, urine, feces and milk of dromedary camels, and demonstration of similarities in the genomic sequence of viruses isolated from infected individuals and camelsFootnote 47Footnote 62Footnote 63Footnote 64Footnote 65 further support the role of camels in zoonotic transmission of MERS-CoV. These findings also highlight the importance of focusing prevention and control measures on camels. Challenge studies conducted in camels suggest that infection of MERS-CoV in dromedary camels results in mild respiratory disease with mild to no signs of illnessFootnote 19. To date, despite the number of reported zoonotic cases, only 14 camel outbreaks of MERS have been reported to the World Organization for Animal Health (OIE) signifying that outbreaks in camel herds are likely to go undetected and allow for an ongoing risk of transmission to humansFootnote 66. Based on sero-epidemiological studies carried out in other livestock, alpacas are the only other domestic livestock in which MERS-CoV-specific antibodies have been identified; however, no human cases with exposure to alpacas have been reportedFootnote 23.
Although the route of camel to human transmission is not fully understood, zoonotic infections of MERS likely occur through direct or indirect contact with camels or camel-related products (e.g. raw milk)Footnote 31. Serological studies that compare the presence of MERS-CoV antibodies in individuals with and without contact to dromedary camels have left a conflicting picture about the role of camel exposure in the zoonotic transmission of MERS. Some studies have found no serological evidence of MERS-CoV infections in humans with various levels of exposure to dromedary camelsFootnote 21Footnote 67Footnote 68. These findings suggest that zoonotic transmission is rareFootnote 21Footnote 67Footnote 68. However, other studies have found a significantly higher seroprevalence of MERS-CoV antibodies in camel-exposed individuals compared to the general population, suggesting a greater risk of MERS-CoV infection among individuals exposed to camelsFootnote 22Footnote 69. Based on the available information to date, further studies are needed to fully understand how human-animal interactions contribute to the transmission of MERS-CoV.
Human-to-human transmission of MERS-CoV has largely been associated with nosocomial outbreaks; however, household transmission has also been reportedFootnote 10Footnote 70. Nosocomial outbreaks of MERS have played an important role in increasing the magnitude of reported cases. To date large scale hospital outbreaks have been reported in Saudi Arabia, United Arab Emirates, and South KoreaFootnote 42Footnote 71Footnote 72. Lack of IPC measures (e.g. overcrowding, delays in isolation of cases, lack of knowledge of MERS case definitions amongst healthcare workers) along with intra- and inter-hospital transmission, super spreaders, and asymptomatic cases are factors that have contributed to these hospital outbreaks in the pastFootnote 73. A study published in 2017 evaluating the implementation of IPC measures during a large hospital outbreak found that strict adherence to IPC measures allowed for rapid identification of cases and a significant decrease in secondary cases, which may explain why recent outbreaks in Saudi Arabia were quickly containedFootnote 27Footnote 28Footnote 74.
Virus characteristics
MERS- CoV is a single stranded RNA virus belonging to the Coronaviridae familyFootnote 2Footnote 75. Though caused by the same genera of coronavirus as SARS, MERS-CoV is distinct in that it belongs to lineage C whereas SARS belongs to lineage B. Entry of MERS-CoV into host cells is mediated by its spike (S) protein binding to the host cell receptor, dipeptidyl peptidase 4 (DPP4)Footnote 76. DDP4 is a surface cell protein that is mainly expressed in the human respiratory tract, but is also extensively expressed on epithelial cells in the kidneys, small intestine, liver, prostate, and activated leukocytesFootnote 55. In the case of SARS, extensive mutations in the virus resulted in its ability to adapt to the human cell receptor angiotensin-converting enzyme 2 (ACE 2) which allowed for increased human-to-human transmissionFootnote 77. Though sequenced viral isolates from camels and humans indicate that MERS-CoV has undergone recombination and genetic changes since its introduction to humans in 2012, none of these viral mutations have allowed more efficient adaptation to the human cell receptor DPP4Footnote 78. In fact, analysis of viral isolates with a mutation in the receptor binding domain from the South Korea outbreak showed reduced affinity to human DPP4Footnote 79. Given the magnitude of South Korea outbreak, these findings further convolute the role of the S protein and DPP4 in the human adaptation of this virus. Overall, the coronaviruses possess the abilities of efficient mutation and recombination, and a propensity to cross host species and adapt to a new environment. These abilities raise concerns that MERS-CoV would gain virulence and an enhanced ability to transmit from human-to-human. However, sustained human-to-human transmission has not occurred in the general population. Potential hotspots of animal-to-human and human-to-human transmission need to be monitored on an on-going basis.
Clinical manifestations
Human infection with MERS-CoV results in a wide range of clinical manifestations, from asymptomatic infection to severe clinical diseaseFootnote 2. Common clinical symptoms include fever, cough and shortness of breath that can rapidly progress to severe acute pneumonia, acute respiratory distress syndrome, septic shock, multi-organ failure and deathFootnote 2Footnote 56. In some instances, cases have experienced gastrointestinal symptoms of abdominal pain, vomiting and diarrheaFootnote 2. In comparison to human illness with other coronaviruses such as Severe Acute Respiratory Syndrome (SARS) (global CFR 14%-15%), MERS has a high case fatality rate of 35% among all cases reported globally and 40% in cases reported from Saudi Arabia Footnote 6Footnote 32Footnote 57. Studies assessing risk factors for disease severity and mortality have found that pre-existing health conditions, older age, sex (being male) and co-infection with other bacterial or viral agents to be significant risk factors for increased odds of mortality and disease severityFootnote 56Footnote 58.
Pediatric cases
Based on case information available to date, pediatric cases of MERS are rareFootnote 34Footnote 60Footnote 61. Thus far, approximately 2% of all cases have occurred in the pediatric population (individual ≤ 18 years of age) and the case fatality rate is 2.5% . In 2016 Al-Tawfiq et al., published a study that aimed to summarize both the epidemiological and clinical characteristics of pediatric cases reported from June 2012 to April 19, 2016 (n=31)Footnote 60. Of the cases for which clinical information was available (n=29), 45% were asymptomatic and 7% presented with mild respiratory symptomsFootnote 60. Of the cases for which information on source of exposure was available (n=15), 68% acquired their infection through household contactFootnote 60. Although the number of pediatric cases is low it is important to remain vigilant in the documentation of the clinical presentation to better understand whether the clinical picture in this population differs from that of the general population.
Event summary: January to March 2018
Between January 1, 2018 and March 31, 2018, 54 cases of MERS including 5 deaths (CFR=36%) of the individuals with known outcomes were reported to the World Health Organization (WHO) and by the Kingdom of Saudi Arabia's Ministry of Health (KSA MOH)Footnote 24. The case count and case fatality rates were comparable to those reported during the same time period in 2016 and 2017, but substantially lower than in 2015 and 2014Footnote 25. The age and sex distribution of cases has remained relatively consistent with previous years: most cases were male (75%), and the median age was 59 years (range 5 years to 89 years). Geographically, cases were less dispersed than previous years with only three countries (Malaysia, Saudi Arabia, and Oman) reporting cases to date. The case reported from Oman was locally acquired, while the case reported from Malaysia was imported from Saudi Arabia – the country with the majority of reported casesFootnote 26. Of the cases for which exposure information was available (n=5), 29% reported exposure to camels while 21% reported exposure to a positive MERS case, giving a similar proportion of secondaryFootnote a cases to primaryFootnote b cases reported to the WHO this year so far. Between January 1 and March 31, 2018, one nosocomial outbreak was reported in a private hospital in the Hafr Albatin region of Saudi Arabia, while a cluster of nosocomial infections was detected in a health facility in the Riyadh region of Saudi ArabiaFootnote 27Footnote 28.
Global epidemiological analysis
Since the emergence of MERS-CoV in 2012, 2183 human infections including at least 760 deaths (CFR =35%) have been reported from 27 countriesFootnote 6Footnote 32. The epidemiological analyses of the MERS-CoV to date reflect sporadic zoonotic infections with amplified human to human transmission in health care settings. Overall, two-thirds of the reported cases (66%) have occurred in men with the median age of 54 years (range of 9 months to 109 years). Though cases of MERS continue to be reported each year, there has been a decline in the number of reported cases and the case fatality rate since 2014 [Table 1]Footnote 29. Given the association between amplified human-to-human transmission and outbreaks in health care settings, strict adherence to infection prevention and control (IPC) measures is likely the main driver for the observed reduction in the incidence of cases according to the WHOFootnote 30.
| Characteristics | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|
| Number of reported cases (CFR %) | 188 (53) | 758 (38) | 672 (33) | 251 (29) | 250 (29) | 54 (36) |
| Median age (range) in years | 51 (2-94) | 48 (4-99) | 54 (9 months-109) | 54 (18-94) | 53 (10-88) | 59 (5-89) |
| % Male | 65 | 65 | 65 | 73 | 72 | 75 |
| Number of countries reporting cases | 10 | 17 | 12 | 7 | 5 | 3 |
| % Healthcare workers | 20 | 26 | 10 | 13 | 17 | 4 |
Note: Data for epidemiological characteristics of MERS cases from 2013-2016 were sourced from the WHO Regional Office for the Eastern Mediterranean (EMRO) January-February 2017 MERS Situation update and compared to data retrieved from WHO Disease Outbreak News (DON) on July 6th on MERS cases reported from Jan-Jun 2017 data. Epidemiological characteristics for cases reported from June 2017-March 2018 were based on data retrieved from WHO DON on March 15th and cases reported by the Kingdom of Saudi Arabia (KSA) Ministry of Health (MOH) up until the end of March 2018. Data reported in this table are subject to change as a result of ongoing investigations and updated analysisFootnote 24Footnote 29Footnote 32. |
||||||
Age and sex distribution
Overall, the age-sex distribution of cases is skewed towards older men [Figure 2]. However, further investigation of exposure type reveals distinctive age-sex patterns between primary and secondary cases [Figure 3]Footnote 31. Exposure and demographic information was available for 109 primary cases and 62 secondary cases (total=171) reported by the WHO Disease Outbreak News (DON) and the KSA MOH between January 1, 2017 and March 31, 2018Footnote 24Footnote 32. Primary cases were skewed towards older men: a significantly higher proportion of primary cases were male (88%) and aged 50 years and older (67%). In contrast, secondary cases had a more balanced age-sex distribution: there was no significant difference between the proportion of males to females or between age groupsFootnote 25. Further, secondary cases had a significantly lower mean age, compared to primary cases (46 years vs. 56 years). The observed age-sex pattern in primary cases – people infected through direct contact with camels – is hypothesized to be due socio-cultural behaviours, such as camel rearing being an activity popular among middle-aged and retired menFootnote 31.
Figure 2 . Comparison of the age distribution of primary to secondary MERS cases reported worldwide between January 1, 2017 and March 31, 2018.
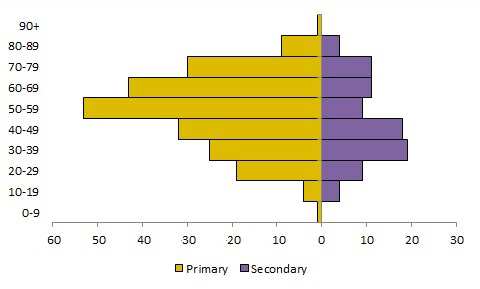
Note: Data displayed on this graph represent a subset of cases reported by the WHO DON and the Kingdom of Saudi Arabia Ministry of Health. Data reported in this figure are subject to change as a result of ongoing investigations and updated analysisFootnote 32.
Text Description
| Primary | Secondary | |
|---|---|---|
| 0-9 | 1 | 0 |
| 10-19 | 4 | 4 |
| 20-29 | 19 | 9 |
| 30-39 | 25 | 19 |
| 40-49 | 32 | 18 |
| 50-59 | 53 | 9 |
| 60-69 | 43 | 11 |
| 70-79 | 30 | 11 |
| 80-89 | 9 | 4 |
| 90+ | 1 | 0 |
Figure 3. Age and sex distribution of primary and secondary MERS cases reported worldwide between January 1, 2017 and March 31, 2018
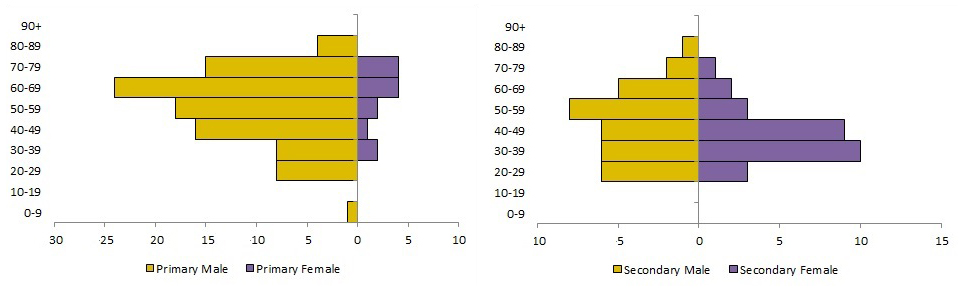
Note: Data displayed on this graph represent a subset of cases reported by the WHO DON and the Kingdom of Saudi Arabia Ministry of Health. Data reported in this figure are subject to change as a result of ongoing investigations and updated analysisFootnote 32.
Text Description
| Primary | Secondary | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 0-9 | 1 | 0 | 0 | 0 |
| 10-19 | 0 | 0 | 0 | 0 |
| 20-29 | 8 | 0 | 6 | 3 |
| 30-39 | 8 | 2 | 6 | 10 |
| 40-49 | 16 | 1 | 6 | 9 |
| 50-59 | 18 | 2 | 8 | 3 |
| 60-69 | 24 | 4 | 5 | 2 |
| 70-79 | 15 | 4 | 2 | 1 |
| 80-89 | 4 | 0 | 1 | 0 |
| 90+ | 0 | 0 | 0 | 0 |
Temporal distribution
The overall temporal distribution of the MERS epidemic can be characterized by multiple intermittent peaks that correspond to large-scale or simultaneously occurring hospital outbreaks. Between 2014 and June 2016, these peaks occurred every 12 to 26 weeks, on average [Figure 4]. However, between 2016 and 2017, there was a 52-week period between peak activity and there has been no peak in activity in 2018 thus far. The recent increased duration between peak activity, resulting in low but ongoing activity, suggests that there have been improvements in the implementation of IPC measures and a decrease in hospital-acquired cases since 2015 as noted by the WHOFootnote 28Footnote 30.
Figure 4 . Global count of human cases of MERS by week of symptom onset or earliest recorded date and location of report, March 2012 to March 31, 2018.
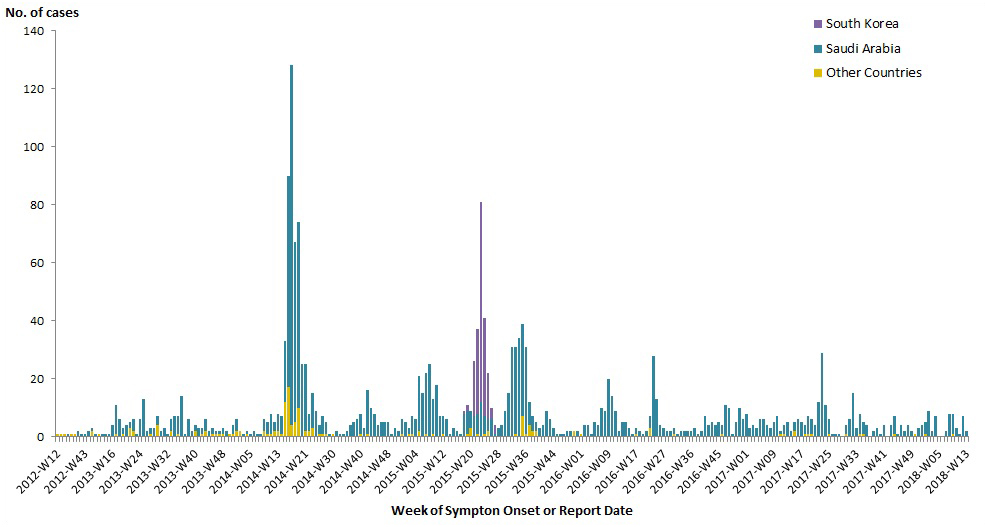
Prepared by the Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada, using data compiled from Global Public Health Intelligence Network (GPHIN), the WHO, and the Kingdom of Saudi Arabia's Ministry of Health.
Text Description
| Weeks | Other Countries | Saudi Arabia | South Korea |
|---|---|---|---|
| 2012-W12 | 1 | - | - |
| 2012-W14 | 1 | - | - |
| 2012-W20 | 1 | - | - |
| 2012-W23 | - | 1 | - |
| 2012-W36 | 1 | - | - |
| 2012-W40 | 1 | - | - |
| 2012-W41 | - | 2 | - |
| 2012-W42 | - | 1 | - |
| 2012-W43 | - | 1 | - |
| 2013-W04 | - | 2 | - |
| 2013-W06 | 2 | 1 | - |
| 2013-W08 | - | 1 | - |
| 2013-W10 | 1 | - | - |
| 2013-W12 | - | 1 | - |
| 2013-W14 | - | 1 | - |
| 2013-W15 | - | 1 | - |
| 2013-W16 | - | 4 | - |
| 2013-W17 | 1 | 10 | - |
| 2013-W18 | - | 6 | - |
| 2013-W19 | 1 | 2 | - |
| 2013-W20 | - | 4 | - |
| 2013-W21 | 3 | 2 | - |
| 2013-W22 | 2 | 4 | - |
| 2013-W23 | - | 1 | - |
| 2013-W24 | - | 6 | - |
| 2013-W25 | - | 13 | - |
| 2013-W26 | - | 2 | - |
| 2013-W27 | 1 | 2 | - |
| 2013-W28 | - | 3 | - |
| 2013-W29 | 4 | 3 | - |
| 2013-W30 | - | 2 | - |
| 2013-W31 | - | 3 | - |
| 2013-W32 | - | 1 | - |
| 2013-W33 | 2 | 4 | - |
| 2013-W34 | - | 7 | - |
| 2013-W35 | 1 | 6 | - |
| 2013-W36 | - | 14 | - |
| 2013-W37 | - | 1 | - |
| 2013-W38 | - | 6 | - |
| 2013-W39 | - | 2 | - |
| 2013-W40 | 2 | 2 | - |
| 2013-W41 | - | 3 | - |
| 2013-W42 | 1 | 2 | - |
| 2013-W43 | 2 | 4 | - |
| 2013-W44 | - | 2 | - |
| 2013-W45 | 1 | 2 | - |
| 2013-W46 | 1 | 1 | - |
| 2013-W47 | 1 | 1 | - |
| 2013-W48 | 1 | 2 | - |
| 2013-W49 | - | 2 | - |
| 2013-W50 | 1 | - | - |
| 2013-W51 | 1 | 3 | - |
| 2013-W52 | 2 | 4 | - |
| 2014-W01 | 2 | - | - |
| 2014-W02 | - | 1 | - |
| 2014-W04 | 1 | 1 | - |
| 2014-W05 | - | 1 | - |
| 2014-W06 | - | 2 | - |
| 2014-W07 | - | 1 | - |
| 2014-W08 | - | 1 | - |
| 2014-W09 | 2 | 4 | - |
| 2014-W10 | 1 | 4 | - |
| 2014-W11 | 1 | 7 | - |
| 2014-W12 | 2 | 3 | - |
| 2014-W13 | 2 | 6 | - |
| 2014-W14 | 1 | 6 | - |
| 2014-W15 | 12 | 21 | - |
| 2014-W16 | 17 | 73 | - |
| 2014-W17 | 4 | 124 | - |
| 2014-W18 | 5 | 62 | - |
| 2014-W19 | 10 | 64 | - |
| 2014-W20 | - | 25 | - |
| 2014-W21 | 2 | 23 | - |
| 2014-W22 | 2 | 6 | - |
| 2014-W23 | 3 | 12 | - |
| 2014-W24 | - | 9 | - |
| 2014-W25 | 1 | 3 | - |
| 2014-W26 | 1 | 6 | - |
| 2014-W27 | 1 | 4 | - |
| 2014-W28 | - | 1 | - |
| 2014-W30 | 1 | - | - |
| 2014-W31 | - | 2 | - |
| 2014-W32 | - | 1 | - |
| 2014-W34 | - | 1 | - |
| 2014-W35 | - | 2 | - |
| 2014-W36 | - | 4 | - |
| 2014-W38 | - | 5 | - |
| 2014-W39 | - | 6 | - |
| 2014-W40 | 1 | 7 | - |
| 2014-W41 | - | 3 | - |
| 2014-W42 | 1 | 15 | - |
| 2014-W43 | - | 10 | - |
| 2014-W44 | - | 8 | - |
| 2014-W45 | - | 4 | - |
| 2014-W46 | - | 5 | - |
| 2014-W47 | - | 5 | - |
| 2014-W48 | - | 5 | - |
| 2014-W49 | - | 1 | - |
| 2014-W50 | - | 3 | - |
| 2014-W51 | - | 2 | - |
| 2014-W52 | 1 | 5 | - |
| 2015-W01 | - | 5 | - |
| 2015-W02 | 1 | 2 | - |
| 2015-W03 | 1 | 6 | - |
| 2015-W04 | - | 6 | - |
| 2015-W05 | 2 | 19 | - |
| 2015-W06 | - | 15 | - |
| 2015-W07 | 1 | 21 | - |
| 2015-W08 | - | 25 | - |
| 2015-W09 | 1 | 12 | - |
| 2015-W10 | - | 18 | - |
| 2015-W11 | - | 7 | - |
| 2015-W12 | 1 | 6 | - |
| 2015-W13 | - | 6 | - |
| 2015-W14 | - | 1 | - |
| 2015-W15 | - | 3 | - |
| 2015-W16 | - | 2 | - |
| 2015-W17 | - | 1 | - |
| 2015-W18 | - | 8 | 1 |
| 2015-W19 | 1 | 8 | 2 |
| 2015-W20 | 3 | 6 | - |
| 2015-W21 | - | 7 | 19 |
| 2015-W22 | 1 | 7 | 29 |
| 2015-W23 | - | 12 | 69 |
| 2015-W24 | 1 | 6 | 34 |
| 2015-W25 | 2 | 4 | 16 |
| 2015-W26 | - | 6 | 4 |
| 2015-W27 | - | 1 | 3 |
| 2015-W28 | - | 3 | - |
| 2015-W29 | - | 4 | - |
| 2015-W30 | - | 9 | - |
| 2015-W31 | - | 15 | - |
| 2015-W32 | - | 31 | - |
| 2015-W33 | 1 | 30 | - |
| 2015-W34 | - | 34 | - |
| 2015-W35 | 7 | 32 | - |
| 2015-W36 | 1 | 30 | - |
| 2015-W37 | 4 | 8 | - |
| 2015-W38 | 2 | 5 | - |
| 2015-W39 | 2 | 3 | - |
| 2015-W40 | - | 3 | - |
| 2015-W41 | - | 4 | - |
| 2015-W42 | - | 9 | - |
| 2015-W43 | - | 6 | - |
| 2015-W44 | - | 3 | - |
| 2015-W45 | - | 1 | - |
| 2015-W47 | - | 1 | - |
| 2015-W48 | - | 1 | - |
| 2015-W50 | - | 2 | - |
| 2015-W51 | - | 2 | - |
| 2015-W52 | 2 | - | - |
| 2015-W53 | - | 2 | - |
| 2016-W01 | 1 | - | - |
| 2016-W02 | - | 4 | - |
| 2016-W03 | - | 4 | - |
| 2016-W04 | - | 1 | - |
| 2016-W05 | - | 5 | - |
| 2016-W06 | - | 4 | - |
| 2016-W07 | - | 10 | - |
| 2016-W08 | - | 9 | - |
| 2016-W09 | - | 20 | - |
| 2016-W10 | - | 14 | - |
| 2016-W11 | - | 9 | - |
| 2016-W12 | - | 2 | - |
| 2016-W13 | - | 5 | - |
| 2016-W14 | - | 5 | - |
| 2016-W15 | - | 3 | - |
| 2016-W16 | - | 1 | - |
| 2016-W17 | 1 | 2 | - |
| 2016-W18 | - | 2 | - |
| 2016-W21 | - | 1 | - |
| 2016-W22 | - | 2 | - |
| 2016-W23 | 3 | 4 | - |
| 2016-W24 | - | 28 | - |
| 2016-W25 | - | 13 | - |
| 2016-W26 | - | 4 | - |
| 2016-W27 | - | 3 | - |
| 2016-W28 | - | 2 | - |
| 2016-W29 | - | 2 | - |
| 2016-W30 | 1 | 2 | - |
| 2016-W31 | - | 1 | - |
| 2016-W32 | - | 2 | - |
| 2016-W33 | - | 2 | - |
| 2016-W35 | - | 2 | - |
| 2016-W36 | - | 2 | - |
| 2016-W37 | - | 3 | - |
| 2016-W38 | - | 1 | - |
| 2016-W40 | - | 2 | - |
| 2016-W41 | - | 7 | - |
| 2016-W42 | - | 4 | - |
| 2016-W43 | - | 5 | - |
| 2016-W44 | - | 4 | - |
| 2016-W45 | - | 5 | - |
| 2016-W46 | 1 | 3 | - |
| 2016-W47 | - | 11 | - |
| 2016-W48 | - | 10 | - |
| 2016-W49 | - | 1 | - |
| 2016-W50 | - | 5 | - |
| 2016-W51 | - | 10 | - |
| 2016-W52 | - | 6 | - |
| 2017-W01 | - | 8 | - |
| 2017-W02 | - | 3 | - |
| 2017-W03 | - | 4 | - |
| 2017-W04 | - | 3 | - |
| 2017-W05 | - | 6 | - |
| 2017-W06 | - | 6 | - |
| 2017-W07 | - | 4 | - |
| 2017-W08 | - | 3 | - |
| 2017-W09 | - | 5 | - |
| 2017-W10 | - | 7 | - |
| 2017-W11 | 1 | 1 | - |
| 2017-W12 | 1 | 3 | - |
| 2017-W13 | - | 5 | - |
| 2017-W14 | - | 2 | - |
| 2017-W15 | 2 | 3 | - |
| 2017-W16 | - | 6 | - |
| 2017-W17 | - | 5 | - |
| 2017-W18 | 1 | 3 | - |
| 2017-W19 | 1 | 6 | - |
| 2017-W20 | 1 | 5 | - |
| 2017-W21 | - | 4 | - |
| 2017-W22 | - | 12 | - |
| 2017-W23 | - | 29 | - |
| 2017-W24 | - | 11 | - |
| 2017-W25 | 1 | 5 | - |
| 2017-W26 | - | 1 | - |
| 2017-W27 | - | 1 | - |
| 2017-W28 | - | 1 | - |
| 2017-W29 | - | 0 | - |
| 2017-W30 | 1 | 3 | - |
| 2017-W31 | - | 6 | - |
| 2017-W32 | - | 15 | - |
| 2017-W33 | - | 3 | - |
| 2017-W34 | 1 | 7 | - |
| 2017-W35 | 1 | 4 | - |
| 2017-W36 | 4 | - | |
| 2017-W37 | - | 0 | - |
| 2017-W38 | - | 2 | - |
| 2017-W39 | - | 3 | - |
| 2017-W40 | - | 1 | - |
| 2017-W41 | - | 4 | - |
| 2017-W42 | - | 0 | - |
| 2017-W43 | - | 4 | - |
| 2017-W44 | 1 | 6 | - |
| 2017-W45 | - | 1 | - |
| 2017-W46 | - | 4 | - |
| 2017-W47 | - | 2 | - |
| 2017-W48 | - | 4 | - |
| 2017-W49 | - | 2 | - |
| 2017-W50 | 1 | - | - |
| 2017-W51 | - | 3 | - |
| 2017-W52 | - | 4 | - |
| 2018-W01 | 1 | 4 | - |
| 2018-W02 | - | 9 | - |
| 2018-W03 | - | 2 | - |
| 2018-W04 | - | 7 | - |
| 2018-W05 | - | 0 | - |
| 2018-W06 | - | 0 | - |
| 2018-W07 | - | 2 | - |
| 2018-W08 | - | 8 | - |
| 2018-W09 | 1 | 7 | - |
| 2018-W10 | - | 3 | - |
| 2018-W11 | - | 1 | - |
| 2018-W12 | - | 7 | - |
| 2018-W13 | - | 2 | - |
Early analysis of the temporal distribution of MERS cases between 2012 and 2014 suggested a seasonal pattern with cases increasing from March to April each year. At the time, this seasonal pattern had been linked to the calving and weaning patterns of camels; however, this pattern has not been observed since 2014Footnote 33. Analysis of the temporal distribution of annual MERS cases reported since 2014 demonstrates that the majority of cases (approximately 61% to 84%) occur during the first 6 months of the year, with peaks corresponding with reported hospital outbreaks that involve more than 20 cases [Figure 5]. Further analysis of the temporal distribution of primary and secondary cases may help determine whether MERS truly exhibits seasonality.
Figure 5 . Temporal distribution of MERS cases, deaths and nosocomial outbreaksFigure 5 Footnote * by month of symptom onset or earliest recorded date, March 2012 to March 31, 2018.
2012
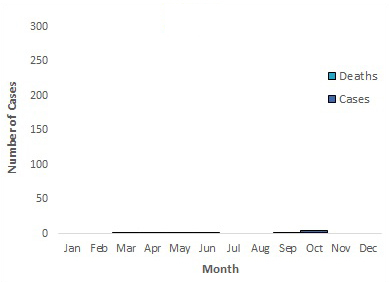
Text Description
| Month | Cases | Deaths | Total |
|---|---|---|---|
| Jan | - | - | 0 |
| Feb | - | - | 0 |
| Mar | - | 1 | 1 |
| Apr | - | 1 | 1 |
| May | 1 | - | 1 |
| Jun | - | 1 | 1 |
| Jul | - | - | 0 |
| Aug | - | - | 0 |
| Sep | - | 1 | 1 |
| Oct | 3 | 2 | 5 |
| Nov | - | - | 0 |
| Dec | - | - | 0 |
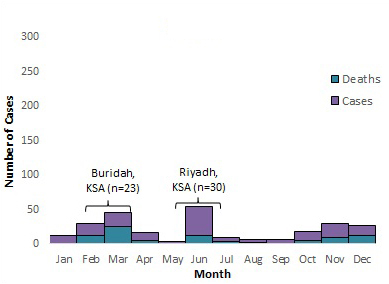
2013
Text Description
| Month | Cases | Deaths | Total |
|---|---|---|---|
| Jan | - | 2 | 2 |
| Feb | 1 | 3 | 4 |
| Mar | 1 | 1 | 2 |
| Apr | 8 | 11 | 19 |
| May | 14 | 8 | 22 |
| Jun | 16 | 6 | 22 |
| Jul | 12 | 3 | 15 |
| Aug | 14 | 7 | 21 |
| Sep | 20 | 7 | 27 |
| Oct | 11 | 5 | 16 |
| Nov | 6 | 5 | 11 |
| Dec | 9 | 6 | 15 |
| - | - | - | 176 |
2014
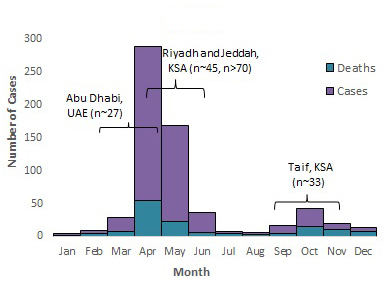
Text Description
| Month | Cases | Deaths | Total |
|---|---|---|---|
| Jan | 3 | 1 | 4 |
| Feb | 5 | 4 | 9 |
| Mar | 20 | 8 | 28 |
| Apr | 234 | 54 | 288 |
| May | 146 | 22 | 168 |
| Jun | 30 | 6 | 36 |
| Jul | 2 | 5 | 7 |
| Aug | 3 | 3 | 6 |
| Sep | 11 | 5 | 16 |
| Oct | 28 | 15 | 43 |
| Nov | 10 | 10 | 20 |
| Dec | 6 | 7 | 13 |
| - | - | - | 638 |
2015
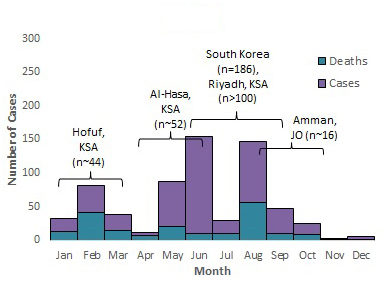
Text Description
| Month | Cases | Deaths | Total |
|---|---|---|---|
| Jan | 20 | 13 | 33 |
| Feb | 40 | 42 | 82 |
| Mar | 25 | 14 | 39 |
| Apr | 4 | 7 | 11 |
| May | 67 | 21 | 88 |
| Jun | 145 | 10 | 155 |
| Jul | 20 | 10 | 30 |
| Aug | 90 | 57 | 147 |
| Sep | 37 | 10 | 47 |
| Oct | 16 | 9 | 25 |
| Nov | 2 | 1 | 3 |
| Dec | 4 | 2 | 6 |
| - | - | - | 666 |
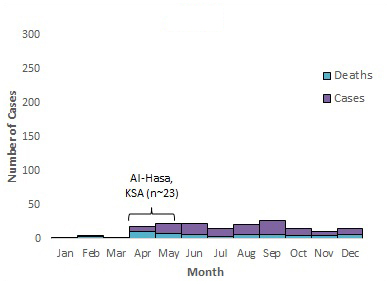
2016
Text Description
| Month | Cases | Deaths | Total |
|---|---|---|---|
| Jan | 11 | 1 | 12 |
| Feb | 17 | 12 | 29 |
| Mar | 20 | 25 | 45 |
| Apr | 11 | 5 | 16 |
| May | 2 | 1 | 3 |
| Jun | 42 | 12 | 54 |
| Jul | 7 | 3 | 10 |
| Aug | 4 | 2 | 6 |
| Sep | 6 | 1 | 7 |
| Oct | 13 | 5 | 18 |
| Nov | 20 | 9 | 29 |
| Dec | 15 | 12 | 27 |
| - | - | - | 256 |
2017
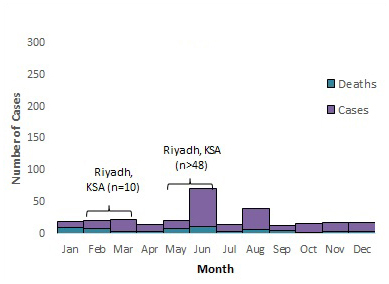
Text Description
| Month | Cases | Deaths | Total |
|---|---|---|---|
| Jan | 10 | 9 | 19 |
| Feb | 12 | 8 | 20 |
| Mar | 20 | 3 | 23 |
| Apr | 11 | 3 | 14 |
| May | 13 | 8 | 21 |
| Jun | 59 | 12 | 71 |
| Jul | 11 | 3 | 14 |
| Aug | 32 | 7 | 39 |
| Sep | 8 | 5 | 13 |
| Oct | 14 | 2 | 16 |
| Nov | 13 | 4 | 17 |
| Dec | 14 | 3 | 17 |
2018
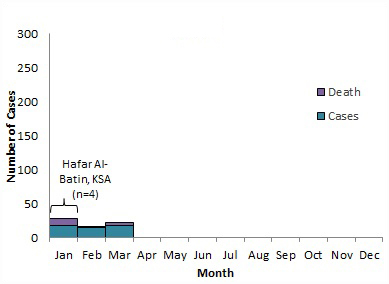
Text Description
| Month | Cases | Death | Total |
|---|---|---|---|
| Jan | 17 | 11 | 28 |
| Feb | 14 | 2 | 16 |
| Mar | 17 | 4 | 21 |
| Apr | - | - | 0 |
| May | - | - | 0 |
| Jun | - | - | 0 |
| Jul | - | - | 0 |
| Aug | - | - | 0 |
| Sep | - | - | 0 |
| Oct | - | - | 0 |
| Nov | - | - | 0 |
| Dec | - | - | 0 |
- Figure 5 Footnote *
Note: The labels on the graph denote nosocomial outbreaks. n represents the approximate number of cases associated with the outbreak. The bracket does not reflect the actual duration of the outbreak but the approximate time period when the majority of cases occurred or were reported. KSA= Kingdom of Saudi Arabia, JO =Jordan and UAE =United Arab Emirates8,27, 34-46.
Geographical distribution
To date, 27 countries located in the Middle East, Northern Africa, Europe, North America and Asia have reported imported and locally-acquired cases of MERS [Figure 6]Footnote 6. The majority of cases (n=1845, 90%), have been reported from countries within the Arabian Peninsula with 92% of those cases reported from Saudi Arabia. Research on the human-animal interactions and the circulation of MERS-CoV in dromedary camels suggest that in addition to the dromedary camel population within the Arabian Peninsula, economic, cultural, and recreational practices specific to the area—such as the movement towards more intensive camel farming clustered around cities—are the cause for the observed concentration of casesFootnote 31Footnote 33 Footnote 47Footnote 48Footnote 49Footnote 50.
Currently, the majority of the world's dromedary camel population (>60%) is located in the Greater Horn of Africa (GHA); a region that regularly exports camels to countries in the Arabian PeninsulaFootnote 48. Though serological studies conducted on dromedary camels from multiple countries in Africa (Egypt, Ethiopia, Nigeria, Somalia, Sudan) have demonstrated that camels in these areas have high seroprevalence of MERS-CoV, no local zoonotic infections with MERS have been reported from any country in Africa; only 6 cases have been reported from countries in Africa and all cases have been imported or linked to an imported case from the Middle EastFootnote 47. Additionally, a study on the circulation of coronavirus species in Saudi Arabia found that local camels had significantly higher carrier rates than imported camelsFootnote 49. Lineage testing identified MERS-CoV found in African camels is genetically different from MERS-CoV found in camels and humans in the Middle EastFootnote 48Footnote 83. This evidence suggests that zoonotic MERS cases seen in Saudi Arabia and other countries in the Arabian Peninsula are connected to factors specific to the Arabian Peninsula and Saudi Arabia.
Given that MERS-CoV has been endemic in Saudi Arabia dromedary camels for over two decades and that the virus is widespread within the Arabian Peninsula, further cases are expected to be reported and exported from these areasFootnote 51. Countries like India and Bangladesh, with a large volume of citizens that travel to Saudi Arabia for religious pilgrimages, have been identified as potential high-risk areas for MERS-CoV importationFootnote 52Footnote 53. To date the largest number of cases to be reported from outside the Middle East occurred in South Korea in 2015: the majority of the 186 reported cases were associated with a large multi-hospital outbreakFootnote 54. Overall, cases were the most geographically dispersed in the 2014 season when 17 countries reported cases [Figure 7].
Figure 6 . Geographical distribution of reported MERS cases between March 2012 and March 31, 2018. (A) Globally, (B) the Arabian Peninsula, (C)Europe, Northern Africa and the Middle East, and (D) Asia.
Figure 6a: Globally
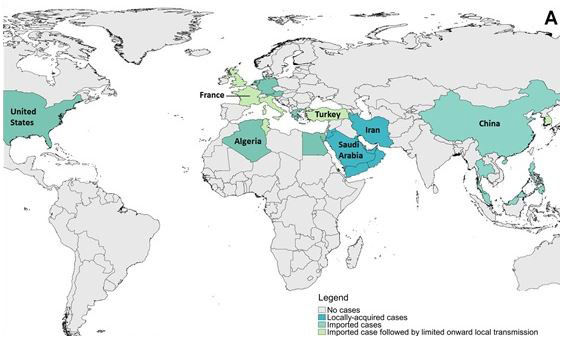
Text Description
This figure shows the cases that have occurred globally.
Figure 6b: Arabian Peninsula
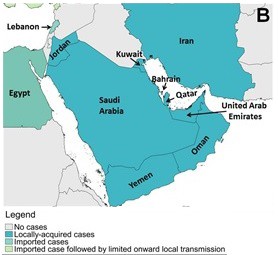
Text Description
This figure shows the cases in the Arabian Peninsula.
Figure 6c: Europe, Northern Africa and the Middle East
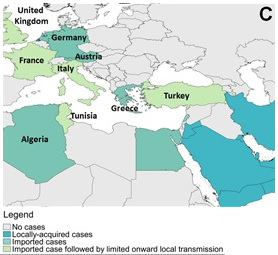
Text Description
This figure shows cases in Europe, Northern Africa and the Middle East.
Figure 6d: Asia
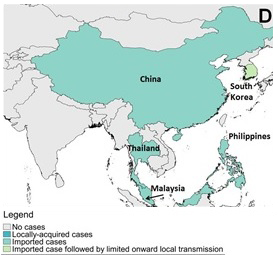
Text Description
This figure shows cases in Asia.
Note: Map was prepared by Centre for Immunization and Respiratory Infectious Diseases (CIRID) using information compiled by the World Health OrganizationFootnote 24.
Figure 7 . Spatial distribution of human cases of MERS between March 2012 and March 31, 2018
Figure 7a: 2012
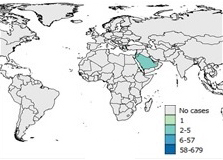
Text Description
Saudi Arabia reported between 2-5 cases.
Figure 7b: 2013
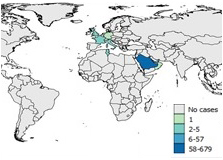
Text Description
Saudi Arabia reported 58-679 cases.
Figure 7c: 2014
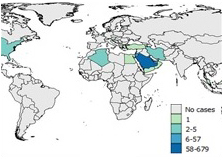
Text Description
Saudi Arabia reported 58-679 cases.
Figure 7d: 2015
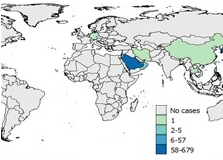
Text Description
Saudi Arabia reported 58-679 cases.
Figure 7e: 2016
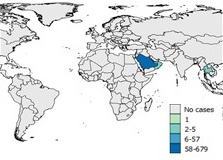
Text Description
Saudi Arabia reported 58-679 cases.
Figure 7f: 2017
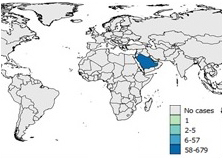
Text Description
Saudi Arabia reported 58-679 cases.
Figure 7g: 2018
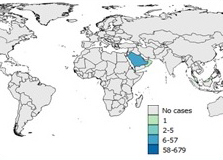
Text Description
Up until March 31, Saudi Arabia has reported 5-67 cases so far.
Note: Map was prepared by Centre for Immunization and Respiratory Infectious Diseases (CIRID) using information compiled by the World Health Organization and the KSA MOHFootnote 24Footnote 32Footnote 39.
Vaccines against MERS-CoV
No vaccine or effective antiviral treatment is currently available for MERS-CoV. Research is underway and many candidate vaccines targeted for humans are under development. These candidates use a wide range of platforms including whole virus vaccine (live attenuated and inactivated), vectored-virus vaccines, DNA vaccine, and protein-based vaccines, with many of the viruses targeting the S (envelope) protein or the DPP4 receptor binding domain (RBD) of the S proteinFootnote 80. Thus far, only one candidate vaccine has advanced to clinical development: a DNA-only vaccine called GLS-5300 that generated protective MERS-CoV antibodies in mice, camels and monkeys, and advanced to Phase 1 human clinical trials in February 2016Footnote 81. This candidate vaccine has also been tested and reduced virus shedding in dromedary camelsFootnote 81. The DNA-based vaccines directed at inducing anti S responses also induce protection in non-human primatesFootnote 80. As the spike protein (specifically S1) is highly divergent among different CoVs, the neutralizing antibodies only provide homotypic protection. In contrast, the amino acid sequence of the spike protein, specifically of S2 domain, is conserved with minimal variability among MERS-CoV strains. Additionally, the circulating MERS-CoV strains also lack significant variation in the serological reactivity. As the S2 domain protein is more conserved among coronaviruses, the adaptive immune response directed against S2 protein can potentially constitute the basis for the development of an vaccine capable of inducing heterotypic protection against circulating MERS-CoV strains. Additionally, a modified vaccinia Ankara based vaccine that reduced virus shedding and generated protective antibodies and mucosal immunity is currently scheduled for a Phase 1 safety trial Footnote 82.
Limitations
This in-depth analysis of MERS-CoV is subject to several limitations. The majority of data in this report represent laboratory-confirmed cases of MERS-CoV reported in the Kingdom of Saudi Arabia. Very limited data are available about cases' clinical manifestation, diagnosis, treatment and comorbidities. There is the possibility of unknown selection biases as the primary cases are only detected following presentation with illness. The asymptomatic primary cases or cases with limited access to health care remain undetected. It remains challenging to interpret risk factors for the acquisition of MERS-CoV particularly in the absence of evidence from extensive case-control epidemiology studies.
Conclusion
In 2015, the WHO created a Blueprint list of priority diseases that pose major public health risks and require further research and development. Both the original list and the update provided in February 2018 deemed MERS-CoV a potential public health emergencyFootnote 59. Since the emergence of MERS in 2012, human cases, predominantly from countries within the Arabian Peninsula, continue to be reported. Transmission patterns of the disease have remained consistent with repeated introductions in the human population occurring through zoonotic transmission followed by amplified human to human transmission in health care settings. A better understanding of the transmission of MERS-CoV in healthcare settings is required, especially of the exposures that result in human-to-human transmission, the potential role of asymptomatic infected health-care workers, and the possible role of environmental contamination, in order to mitigate the risk of nosocomial outbreaks. Though the number of cases reported annually appears to be on the decline, it is clear that continued research and surveillance is needed to further bridge the gaps in knowledge surrounding the virus origin and characteristics, transmission of this disease in the community, the exact mode of zoonotic transmission, and potential environmental sources of disease.
References
- Footnote 1
Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus A DME, Fouchier R AM. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N Engl J Med 2012;367(19):1814-1820.
- Footnote 2
Fehr A.R., Channappanavar R., Perlman S. Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Annu Rev Med 2017 14 Jan 2017;68:387-399.
- Footnote 3
Hussein I. The story of the first MERS patient. 2014; Available at: http://www.natureasia.com/en/nmiddleeast/article/10.1038/nmiddleeast.2014.134. Accessed June 25, 2017.
- Footnote 4
ProMED-mail. Novel coronavirus-Saudi Arabia" human isolate. Archive number 20120920.1302733. 2012; Available at: http://www.eurosurveillance.org/images/dynamic/ES/V18N02/V18N02.pdf. Accessed June 25, 2017.
- Footnote 5
Darling ND, Poss DE, Schoelen MP, Metcalf-Kelly M, Hill SE, Harris S. Retrospective, epidemiological cluster analysis of the Middle East respirator syndrome coronavirus (MERS-CoV) epidemic using open source data. Epidemiol Infect 2017;145:3106-3114.
- Footnote 6
World Health Organization [WHO]. Middle East respiratory syndrome coronavirus (MERS-CoV). 2018; Available at: http://www.who.int/emergencies/mers-cov/en/. Accessed April 1, 2018.
- Footnote 7
Lu X., Whitaker B., Sakthivel S.K.K., Kamili S., Rose L.E., Lowe L., et al. Real-time reverse transcription-pcr assay panel for Middle East Respiratory Syndrome coronavirus. J Clin Microbiol 2014 January 2014;52(1):67-75.
- Footnote 8
Hijawi B, Abdallat M, Sayaydeh A, Alqasrawi S, Haddadin A, Jaarour N, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J 2013;19 Suppl 1:S12-8.
- Footnote 9
van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio 2012;3(6) (pagination):ate of Pubaton: Noember/eember 2012.
- Footnote 10
Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Brief report: Family cluster of Middle East Respiratory Syndrome coronavirus infections. N Engl J Med 2013 2013;369(6):587.
- Footnote 11
Health Protection Agency (HPA) UK Novel Coronavirus Investigation team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro surveillance 2013 March 14 2013;18(11):20427.
- Footnote 12
Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013 Mar 14;495(7440):251-254.
- Footnote 13
World Health Organization [WHO]. First meeting of the IHR emergency committtee concerning MERS-CoV. 2013; Available at: http://www.who.int/ihr/procedures/statements_20130709/en/. Accessed June 20, 2017.
- Footnote 14
Reusken C.B.E.M., Haagmans B.L., Muller M.A., Gutierrez C., Godeke G.J., Meyer B., et al. Middle East Respiratory Syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. The Lancet Infectious Diseases 2013 October 2013;13(10):859-866.
- Footnote 15
ProMed-mail. MERS-CoV Eastern Mediterranean. 2013; Available at: http://www.promedmail.org/direct.php?id=20131112.2051424. Accessed June 12, 2017.
- Footnote 16
Hastings D.L., Tokars J.I., Abdel Aziz I.Z.A.M., Alkhaldi K.Z., Bensadek A.T., Alraddadi B.M., et al. Outbreak of Middle East Respiratory Syndrome at tertiary care hospital, Jeddah, Saudi Arabia, 2014. Emerging Infectious Diseases 2016 May 2016;22(5):794-801.
- Footnote 17
Drosten C., Muth D., Corman V.M., Hussain R., Al Masri M., HajOmar W., et al. An observational, laboratory-based study of outbreaks of Middle East Respiratory Syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clinical Infectious Diseases 2015 01 Feb 2015;60(3):369-377.
- Footnote 18
Centers for Disease Control and Prevention [CDC]. CDC announces first case of Middle East Respiratory Syndrome Coronavirus infection (MERS) in the United States. 2014; Available at: https://www.cdc.gov/media/releases/2014/p0502-us-mers.html. Accessed June 8, 2017.
- Footnote 19
Adney D.R., van Doremalen N., Brown V.R., Bushmaker T., Scott D., de Wit E., et al. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerging Infectious Diseases 2014 01 Dec 2014;20(12):1999-2005.
- Footnote 20
Chu D.K.W., Poon L.L.M., Gomaa M.M., Shehata M.M., Perera R.A.P.M., Zeid D.A., et al. MERS coronaviruses in dromedary camels, Egypt. Emerging Infectious Diseases 2014 June 2014;20(6):1049-1053.
- Footnote 21
Hemida M.G., AlNaeem A., Perera R.A.P.M., Chin A.W.H., Poon L.L.M., Peiris M. Lack of Middle East Respiratory Syndrome coronavirus transmission from infected camels. Emerging Infectious Diseases 2015 2015;21(4):699-701.
- Footnote 22
Muller M.A., Meyer B., Corman V.M., AlMasri M., Turkestani A., Ritz D., et al. Presence of Middle East Respiratory Syndrome coronavirus antibodies in Saudi Arabia: A nationwide, cross-sectional, serological study. The Lancet Infectious Diseases 2015 01 May 2015;15(5):559-564.
- Footnote 23
Adney D.R., BielefeldtOhmann H., Hartwig A.E., Bowen RA. Infection, replication, and transmission of Middle East Respiratory Syndrome coronavirus in alpacas. Emerging Infectious Diseases 2016 June 2016;22(6):1031-1037.
- Footnote 24
World Health Organization [WHO]. Disease outbreak news: Middle East Respiratory Syndrome coronavirus (MERS-CoV) - Oman. 2018; Available at: http://www.who.int/csr/don/15-march-2018-mers-oman/en/. Accessed April 1, 2018.
- Footnote 25
World Health Organization Regional Office for the Eastern Mediterranean [EMRO]. MERS situation update, June 2017. 2017; Available at: http://applications.emro.who.int/docs/EMROPub_2017_EN_19887.pdf. Accessed July 15, 2017.
- Footnote 26
World Health Organization [WHO]. Middle East Respiratory Syndrome coronavirus (MERS-CoV) Lebanon. 2017; Available at: http://www.who.int/csr/don/04-july-2017-mers-lebanon/en/. Accessed July 4, 2017.
- Footnote 27
World Health Organization Regional Office for the Eastern Mediterranean [EMRO]. MERS situation update, January 2018. 2018; Available at: http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2018.html. Accessed April, 2018.
- Footnote 28
World Health Organization Regional Office for the Eastern Mediterranean [EMRO]. MERS situation update, February 2018. 2018; Available at: http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-february-2018.html. Accessed April, 2018.
- Footnote 29
World Health Organization Regional Office for the Eastern Mediterranean [EMRO]. MERS situation update, January-February 2017. 2017; Available at: http://www.emro.who.int/health-topics/mers-cov/news.html. Accessed May 15, 2017.
- Footnote 30
World Health Organization Regional Office for the Eastern Mediterranean [EMRO]. MERS situation update, April 2017. 2017; Available at: http://www.emro.who.int/health-topics/mers-cov/news.html. Accessed May 10, 2017.
- Footnote 31
Gossner C, Danielson N, Gervelmeyer A, Berthe F, Faye B, Kaasik Aaslav K, et al. Human-Dromedary Camel Interactions and the Risk of Acquiring Zoonotic Middle East Respiratory Syndrome Coronavirus Infection. Zoonoses Public Health 2016 Feb;63(1):1-9.
- Footnote 32
Kingdom of Saudi Arabia Ministry of Health [MOH] Command and Control Center. Statistics. 2018; Available at: http://www.moh.gov.sa/en/ccc/pressreleases/pages/default.aspx. Accessed April 1, 2018.
- Footnote 33
Hemida MG, Elmoslemany A, Al-Hizab F, Alnaeem A, Almathen F, Faye B, et al. Dromedary Camels and the Transmission of Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Transbound Emerg Dis 2017 Apr;64(2):344-353.
- Footnote 34
Fagbo S.F., Skakni L., Chu D.K.W., Garbati M.A., Joseph M., Peiris M., et al. Molecular epidemiology of hospital outbreak of Middle East Respiratory Syndrome, Riyadh, Saudi Arabia, 2014. Emerging Infectious Diseases 2015 November 2015;21(11):1981-1988.
- Footnote 35
Abdullah Assiri, Abedi GR, Saeed AA, Abdalla MA, AlMasry M, Choudhry AJ, et al. Multifacility outbreak of Middle East Respiratory Syndrome in Taif, Saudi Arabia. Emerging Infectious Diseases 2016;22(1):32-40. 19 ref.
- Footnote 36
Cho S.Y., Kang J.M., Ha Y.E., Park G.E., Ko J.H., Lee J.Y., et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. The Lancet 2016 03 Sep 2016;388(10048):994-1001.
- Footnote 37
AlDorzi H.M., Aldawood A.S., Khan R., Baharoon S., Alchin J.D., Matroud A.A., et al. The critical care response to a hospital outbreak of Middle East Respiratory Syndrome coronavirus (MERS-CoV) infection: an observational study. Annals of Intensive Care 2016;6(1) (pagination):Arte Number: 101. ate of Pubaton: 01 e 2016.
- Footnote 38
AlAbdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R., et al. Hospital-associated outbreak of Middle East Respiratory Syndrome coronavirus: A serologic, epidemiologic, and clinical description. Clinical Infectious Diseases 2014 01 Nov 2014;59(9):1225-1233.
- Footnote 39
World Health Organization [WHO]. Middle East Respiratory Syndrome coronavirus (MERS-CoV): Summary of current situation, literature update and risk assessment, 7 July 2015. 2015 July 7 2015;15.1.
- Footnote 40
European Centre for Disease Prevention and Control. Middle East Respiratory Syndrome coronavirus (MERS-CoV). 21st update, 21 October 2015. 2015 October 21 2015.
- Footnote 41
World Health Organization [WHO]. Middle East Respiratory Syndrome coronavirus (MERS-CoV): WHO MERS-CoV global summary and risk assessment, 5 December 2016. 2016 5 December 2016;16.1.
- Footnote 42
Hunter J.C., Nguyen D., Aden B., Al Bandar Z., Al Dhaheri W., Abu Elkheir K., et al. Transmission of Middle East Respiratory Syndrome coronavirus infections in healthcare settings, abu dhabi. Emerging Infectious Diseases 2016 April 2016;22(4):647-656.
- Footnote 43
Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med 2015 Feb 26;372(9):846-854.
- Footnote 44
Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DAT, et al. Hospital outbreak of Middle East Respiratory Syndrome coronavirus. N Engl J Med 2013 2013;369(5):407.
- Footnote 45
Balkhy H.H., Alenazi T.H., Alshamrani M.M., BaffoeBonnie H., Arabi Y., Hijazi R., et al. Description of a hospital outbreak of Middle East Respiratory Syndrome in a large tertiary care hospital in Saudi Arabia. Infection Control and Hospital Epidemiology 2016 01 Oct 2016;37(10):1147-1155.
- Footnote 46
El Bushra H.E., Abdalla M.N., Al Arbash H., Alshayeb Z., AlAli S., AlAbdel Latif Z., et al. An outbreak of Middle East Respiratory Syndrome (MERS) due to coronavirus in Al-Ahssa region, Saudi Arabia, 2015. Eastern Mediterranean Health Journal 2016 July 2016;22(7):468-475.
- Footnote 47
Omrani A.S., AlTawfiq J.A., Memish ZA. Middle East Respiratory Syndrome coronavirus (Mers-coV): Animal to human interaction. Pathogens and Global Health 2015 December 2015;109(8):354-362.
- Footnote 48
Younan M., Bornstein S., Gluecks IV. MERS and the dromedary camel trade between Africa and the Middle East. Trop Anim Health Prod 2016 01 Aug 2016;48(6):1277-1282.
- Footnote 49
Sabir JS, Lam TT, Ahmed MM, Li L, Shen Y, Abo-Aba SE, et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science 2016 Jan 1;351(6268):81-84.
- Footnote 50
Wernery U., Lau S.K.P., Woo PCY. Middle East Respiratory Syndrome (MERS) coronavirus and dromedaries. Veterinary Journal 2017 01 Feb 2017;220:75-79.
- Footnote 51
Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., de Wit E., et al. Middle East Respiratory Syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio 2014;5(2) (pagination):Arte Number: e00884-14. ate of Pubaton: 25 Feb 2014.
- Footnote 52
Ma X, Lie F, Lie L, Zhang L, Lu M, Abudukadeer A, Wang L, et al. No MERS-CoV but positive influenza viruses in returning Hajj pilgrims, China, 2013-2015. BMC Infectious Diseases 2017; 17:715
- Footnote 53
Risk of global spread of Middle East respiratory syndrome coronavirus (MERS-CoV) via the air transport network. J Travel Med 2016; 23(6).
- Footnote 54
The Korean Society of Infectious Diseases, Korean Society for Healthcare-associated Infection Control and Prevention. The same Middle East Respiratory Syndrome-coronavirus (MERS-CoV) yet different outbreak patterns and public health impacts on the far east expert opinion from the rapid response team of the Republic of Korea. Infection and Chemotherapy 2015 2015;47(4):247-251.
- Footnote 55
Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018; 23:130-137.
- Footnote 56
Alsahafi A.J., Cheng AC. The epidemiology of Middle East Respiratory Syndrome coronavirus in the Kingdom of Saudi Arabia, 2012-2015. International Journal of Infectious Diseases 2016 April 01, 2016;45:1-4.
- Footnote 57
World Health Organization [WHO]. Update 49 : SARS case fatality ratio, incubation period. 2003; Available at: http://www.who.int/csr/sarsarchive/2003_05_07a/en/. Accessed July 5, 2017.
- Footnote 58
Banik G.R., Alqahtani A.S., Booy R., Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: Analysis of publicly available data from Saudi Arabia. Virol Sin 2016 01 Feb 2016;31(1):81-84.
- Footnote 59
World Health Organization [WHO]. 2018 Annual review of diseases prioritized under the Research and Development Blueprint. February 2018. Available at: http://www.who.int/emergencies/diseases/2018prioritization-report.pdf?ua=1. Accessed April 1, 2018.
- Footnote 60
Al-Tawfiq JA, Kattan RF, Memish ZA. Middle East Respiratory Syndrome coronavirus disease is rare in children: An update from Saudi Arabia. World Journal of Clinical Pediatrics 2016 Nov 08;5(4):391-396.
- Footnote 61
Memish Z.A., AlTawfiq J.A., Assiri A., Alrabiah F.A., Hajjar S.A., Albarrak A., et al. Middle East Respiratory Syndrome coronavirus disease in children. Pediatr Infect Dis J 2014 2014;33(9):904-906.
- Footnote 62
SharifYakan A., Kanj SS. Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives. PLoS Pathogens 2014;10(12) (pagination):ate of Pubaton: 01 e 2014.
- Footnote 63
Ithete NL, Stoffberg S, Corman VM, Cottontail VM, Richards LR, Schoeman MC, et al. Close relative of human Middle East Respiratory Syndrome coronavirus in bat, South Africa. Emerg Infect Dis 2013 Oct;19(10):1697-1699.
- Footnote 64
Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H., et al. Middle East Respiratory Syndrome coronavirus in Bats, Saudi Arabia. Emerging Infectious Diseases 2013 November 2013;19(11):1819-1823.
- Footnote 65
Wang Q., Qi J., Yuan Y., Xuan Y., Han P., Wan Y., et al. Bat origins of MERS-CoV supported by bat Coronavirus HKU4 usage of human receptor CD26. Cell Host and Microbe 2014 10 Sep 2014;16(3):328-337.
- Footnote 66
World Organisation for Animal Health (OIE). World Animal Health Informaton System (WAHIS). 2017; Available at: hhttp://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Countryreports. Accessed July 8, 2017.
- Footnote 67
Memish Z.A., Alsahly A., Masri M.al., Heil G.L., Anderson B.D., Peiris M., et al. Sparse evidence of MERS-CoV infection among animal workers living in Southern Saudi Arabia during 2012. Influenza and other Respiratory Viruses 2015 01 Mar 2015;9(2):64-67.
- Footnote 68
Aburizaiza A.S., Mattes F.M., Azhar E.I., Hassan A.M., Memish Z.A., Muth D., et al. Investigation of anti-Middle East Respiratory Syndrome antibodies in blood donors and slaughterhouse workers in Jeddah and Makkah, Saudi Arabia, Fall 2012. J Infect Dis 2014 15 Jan 2014;209(2):243-246.
- Footnote 69
Reusken C.B.E.M., Farag E.A.B.A., Haagmans B.L., Mohran K.A., Godeke G.J., Raj V.S., et al. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013-2014. Emerging Infectious Diseases 2015 23 Jul 2015;21(8):1422-1425.
- Footnote 70
Abroug F., Slim A., OuanesBesbes L., Kacem M.A.H., Dachraoui F., Ouanes I., et al. Family cluster of Middle East Respiratory Syndrome coronavirus infections, Tunisia, 2013. Emerging Infectious Diseases 2014 September 2014;20(9):1527-1530.
- Footnote 71
Kim KyungMin, Ki MoRan, Cho SungIl, Sung MinKi, Hong JinKwan, Cheong HaeKwan, et al. Epidemiologic features of the first MERS outbreak in Korea: focus on Pyeongtaek St. Mary's Hospital. Epidemiology and Health; 2015.37:e2015041 2015.
- Footnote 72
Balkhy HH, Alenazi TH, Alshamrani MM, Baffoe-Bonnie H, Al-Abdely HM, El-Saed A, et al. Notes from the Field: Nosocomial Outbreak of Middle East Respiratory Syndrome in a Large Tertiary Care Hospital--Riyadh, Saudi Arabia, 2015. MMWR Morb Mortal Wkly Rep 2016 Feb 19;65(6):163-164.
- Footnote 73
Al-Tawfiq JA, Perl TM. Middle East Respiratory Syndrome coronavirus in healthcare settings. Curr Opin Infect Dis 2015 Aug;28(4):392-396.
- Footnote 74
El Bushra HE, Al Arbash HA, Mohammed M, Abdalla O, Abdallah MN, Al-Mayahi ZK, et al. Outcome of strict implementation of infection prevention control measures during an outbreak of Middle East Respiratory Syndrome. Am J Infect Control 2017 May 1;45(5):502-507.
- Footnote 75
Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K., et al. Middle East Respiratory Syndrome. N Engl J Med 2017 09 Feb 2017;376(6):584-594.
- Footnote 76
Fehr AR, Channappanavar R, Perlman S. Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Annu Rev Med 2017 Jan 14;68:387-399.
- Footnote 77
Chinese SARS Molecular Epidemiology Consortium. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 2004 Mar 12;303(5664):1666-1669.
- Footnote 78
Sabir JS, Lam TT, Ahmed MM, Li L, Shen Y, Abo-Aba SE, et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science 2016 Jan 1;351(6268):81-84.
- Footnote 79
Kim Y, Cheon S, Min CK, Sohn KM, Kang YJ, Cha YJ, et al. Spread of Mutant Middle East Respiratory Syndrome Coronavirus with Reduced Affinity to Human CD26 during the South Korean Outbreak. MBio 2016 Mar 1;7(2):e00019-16.
- Footnote 80
Okba NM, Raj VS, Haagmans BL. Middle East Respiratory Syndrome coronavirus vaccines: current status and novel approaches. Curr Opin Virol 2017 Apr 13;23:49-58.
- Footnote 81
Modjarrad K. MERS-CoV vaccine candidates in development: The current landscape. Vaccine 2016 Jun 3;34(26):2982-2987.
- Footnote 82
Alharbi NK. Vaccines against Middle East Respiratory Syndrome coronavirus for humans and camels. Rev Med Virol 2017 Mar;27(2):10.1002/rmv.1917. Epub 2016 Oct 27.
- Footnote 83
Chu DKW, Hui KPY, Perera RAPM, Miguel E, Niemeyer E, et al. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. PNAS 2018 Mar 20; 115(12):3144-3149.
- Footnote 84
Seys LJM, Widagdo W, Verhamme FM, Kleinjan A, Janssens W, et al. DPP4, the Middle East Respiratory Syndrome Coronavirus Receptor, is Upregulated in Lungs of Smokers and Chronic Obstructive Pulmonary Disease Patients. Clinical Infectious Diseases. 2018 Jan; 66(1): 45-63.
Footnotes
- Footnote a
Secondary cases are cases with a history of contact with a positive MERS case
- Footnote b
Primary cases are cases with no history of contact with a positive MERS case