Information for authors: 2017

 Download this article as a PDF (138 KB - 3 pages)
Download this article as a PDF (138 KB - 3 pages) Published by: The Public Health Agency of Canada
Issue: Volume 43-1: Enteric disease outbreaks
Date published: January 5, 2017
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 43-1, January 5, 2017: Enteric disease outbreaks
Editorial policy
Information for authors: 2017
Introduction
The Canada Communicable Disease Report (CCDR) is a bilingual, peer-reviewed, open-access, online scientific journal published by the Public Health Agency of Canada (the Agency). It will soon be available in full text on PubMed Central. The CCDR provides practical and authoritative information on infectious diseases to clinicians, public health professionals, researchers, teachers, students and others who are interested in infectious diseases. The CCDR is published on the first Thursday of every month. In 2017 there will be joint issues published in March/April and July/August.
The CCDR welcomes submissions from across Canada and elsewhere of manuscripts that include practical, authoritative information on infectious diseases to inform communicable disease policy, program development and practice. The CCDR follows the recommendations of the International Committee of Medical Journal Editors (ICMJE), Canada’s Tri-Council Policy Statement on Ethical Conduct on Research Involving Humans, the Canadian Council of Animal Care Guidelines, the Council of Scientific Editors’ Scientific Style and Format, the Treasury Board of Canada Secretariat’s Policy on Official Languages and Standard on Web Accessibility and the Agency’s Policy for the Publication of Scientific and Research Findings. The CCDR does not contain policy statements, except in summaries of advisory committee statements. Authors retain the responsibility for the content of their articles and opinions expressed are not necessarily those of the Agency.
Types of articles
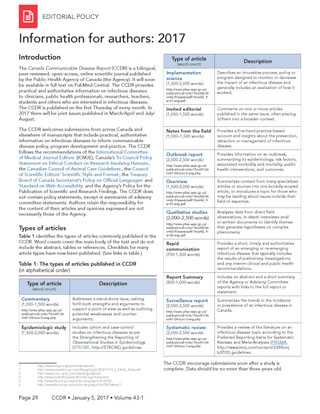
Table 1 identifies the types of articles commonly published in the CCDR. Word counts cover the main body of the text and do not include the abstract, tables or references. Checklists for many article types have now been published. (See links in table.)
| Type of article (word count) |
Description |
|---|---|
| Commentary (1,000−1,500 words) |
Addresses a stand-alone issue, setting forth both strengths and arguments to support a point of view as well as outlining potential weaknesses and counter-arguments. |
| Epidemiologic study (1,500–2,000 words) |
Includes cohort and case-control studies on infectious diseases as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. |
| Implementation science (1,500–2,000 words) |
Describes an innovative process, policy or program designed to monitor or decrease the impact of an infectious disease and generally includes an evaluation of how it worked. |
| Invited editorial (1,000−1,500 words) |
Comments on one or more articles published in the same issue, often placing it/them into broader context. |
| Notes from the field (1,000–1,500 words) |
Provides a first-hand practice-based account and insights about the prevention, detection or management of infectious disease. |
| Outbreak report (2,000–2,500 words) |
Provides information about an outbreak, summarizing its epidemiology, risk factors, associated morbidity and mortality, public health interventions and outcomes. |
| Overview (1,500–2,000 words) |
Summarizes content from many specialized articlesor sources into one broadly‑scoped article or introduces a topic for those who may be reading about issues outside their field of expertise. |
| Qualitative studies (2,000–2,500 words) |
Analyzes data from direct field observations, in-depth interviews and/or written documents to identify themes that generate hypotheses on complex phenomena. |
| Rapid communication (750−1,500 words) |
Provides a short, timely and authoritative report of an emerging or re-emerging infectious disease that typically includes the results of preliminary investigations and any interim clinical and public health recommendations. |
| Report summary (500−1,000 words) |
Includes an abstract and short summary of an Agency or advisory committee report with links to the full report or statement. |
| Surveillance report (2,000−2,500 words) | Summarizes the trends in the incidence or prevalence of an infectious disease in Canada. |
| Systematic review (2,000−2,500 words) | Provides a review of the literature on an infectious disease topic as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. |
The CCDR encourage submissions soon after a study is complete. Data should be no more than three years old.
Other types of manuscripts may be appropriate. To assess potential suitability, consult the Editor-in-Chief prior to submission.
Manuscript preparation and submission
Manuscript preparation
Manuscripts may be submitted in either English or French and prepared with Microsoft Word (.docx). All author(s) and their primary affiliation(s) should be identified as well as the email address of the corresponding author. Research articles should include a 200- to 250-word structured abstract (Background, Objective, Methods, Results and Conclusion). Commentaries and editorials should include a 150- to 200-word text abstract. Tables and figures should be sent as separate files. Figures must be created as editable files, such as Excel or PowerPoint to permit formatting and translation. It is useful to review previous issues of the CCDR to check the formatting of tables and figures. For additional guidance, the ICMJE article “Recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals” provides more detail on general manuscript preparation.
Authorship, contributorship and acknowledgements
All authors need to meet the four criteria for authorship as set out by the ICMJE:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The CCDR encourages the use of the “CRediT taxonomy”. This taxonomy identifies all the contributions that can be made in the development of a manuscript so that the roles of authors and contributors can be identified based on this taxonomy (see Table 2 below).
| Contribution | Definition |
|---|---|
| Conceptualization | Ideas; formulation or evolution of overarching research goals and aims. |
| Methodology | Development or design of methodology; creation of models. |
| Software | Programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components. |
| Validation | Verification, whether as a part of the activity or separate, of the overall replication/ reproducibility of results/experiments and other research outputs. |
| Formal analysis | Application of statistical, mathematical, computational or other formal techniques to analyze or synthesize study data. |
| Investigation | Conducting a research and investigation process, specifically performing the experiments, or data/evidence collection. |
| Resources | Provision of study materials, reagents, patients, laboratory samples, animals, instrumentation, computing resources or other analysis tools. |
| Data collection and curation | Collection of data and management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later reuse. |
| Writing – original draft | Preparation, creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation). |
| Writing – review and editing | Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision. |
| Visualization | Preparation, creation and/or presentation of the published work, specifically visualization/ data presentation. |
| Supervision | Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. |
| Project administration | Management and coordination responsibility for the research activity planning and execution. |
| Funding acquisition | Acquisition of the financial support for the project leading to publication. |
Authors are identified at the end of the manuscript by their initials and contributors are identified by their name. For example:
- Authors: AJ – Conceptualization, investigation, writing-original draft, review and editing; BJ – Methodology, software, validation, writing – review and editing.
- Contributors: John Smith – Supervision, resources, project administration.
Acknowledgements may also be noted. It is the responsibility of the corresponding author to ensure that anyone who is acknowledged has provided permission.
Manuscript submission
Manuscripts should be submitted by email to: ccdr-rmtc@phac-aspc.gc.ca with a copy to the Editor-in-Chief. Authors are invited to identify their ORCID number.
Cover letter
When submitting a manuscript, a cover letter is sent that includes the following:
- A statement that the manuscript has not been published previously. (The CCDR generally considers only previously unpublished work.)
- An assurance that the manuscript has been reviewed and approved by all the authors and the ICMJE criteria for authorship have been met.
- Attachments of a completed ICMJE Conflicts of Interest Form from each author.
Prior to submission, authors employed by a government organization are responsible for obtaining approval or clearance that their manuscript may be submitted. Authors who work for the Agency require director-level approval for submission, in keeping with the Agency’s Policy for the Publication of Scientific and Research Findings. It is an expected courtesy to copy those who have provided clearance in the cover letter.
The editorial and production process
Assessment and revision
Manuscripts that have been correctly submitted are assessed by the editorial team for appropriateness and will soon be routinely screened with antiplagiarism software. Once a manuscript passes the initial evaluation, it undergoes a double-blind peer review process (reviewers do not know who the authors are; authors do not know who the reviewers are). Reviewers assess the manuscript for relevance, content and methodological quality, and identify what improvements might be made.
After analyzing the manuscript and considering the reviewers’ comments, the Editor-in-Chief decides whether to request further revisions or declines the manuscript for publication. If revisions are indicated, an editor sends the reviewers’ comments and any additional editorial comments to the corresponding author and invites them to revise the manuscript and provide a detailed response to each of the reviewer’s comments. When the revised manuscript and response to comments are received, an associate editor and/or the Editor-in-Chief make the final decision whether to accept or decline the manuscript, or request additional revisions. The corresponding author is notified by email of the editorial decision.
The copyright of all papers published in the CCDR belongs to the Government of Canada. Therefore, once a manuscript is accepted for publication, authors are asked to transfer copyright. Authors who are outside the Government of Canada are required to sign a transfer of copyright agreement. For authors who are federal government employees, the copyright remains with the Government of Canada.
Production
All manuscripts accepted for publication are copy-edited, translated, desktop published and web-coded. Corresponding authors are sent a copy-edited version of their article to review for accuracy (the final quality control check) prior to web-coding; authors may also review the translated version upon request.
For further information
Contact the CCDR Editorial Office or the Editor-in-Chief.
Page details
- Date modified: