Caring for natural history collections
Fiona Graham
Caring for natural history collections is part of CCI's Preventive conservation guidelines for collections online resource. This section presents key aspects of managing the care of natural history specimens in heritage collections based on the principles of preventive conservation and risk management.
Table of contents
- Understanding natural history collections
- Causes of damage to inorganic specimens and preventive conservation strategies
- Causes of damage to organic specimens and preventive conservation strategies
- Examples of preventive conservation practices
- Bibliography
List of abbreviations
- CCI
- Canadian Conservation Institute
- DDT
- dichlorodiphenyltrichloroethane
- HEPA
- high-efficiency particulate air
- portable document format
- RH
- relative humidity
- UV
- ultraviolet
Understanding natural history collections
This resource focuses on natural history specimens that are likely to be found in mixed museum collections or small private collections. It addresses different types of natural history collections as well as health and safety risks associated with pesticide residues in such collections.
Types of natural history collections
Natural history collections consist of materials such as fossils, rocks, minerals, seashells, eggshells, birds' nests, insects (e.g. butterflies) as well as botanical specimens, study skins and taxidermy specimens, which may be mammals (Figure 1), birds, reptiles or amphibians. Wet collections, found in more specialized collections, require the advice of a specialist and will not be addressed here.

Martin Lipman, © Canadian Museum of Nature
Figure 1. Moose specimen in a display diorama at the Canadian Museum of Nature.
In museums with mixed collections, natural history specimens are sometimes neglected when those responsible for them have a limited understanding of the value of these objects. Sometimes the items are used merely as props to provide background atmosphere for displays, and later they are jumbled in a corner of a storage room waiting for someone to take an interest and care for them. Identifying natural history specimens in a collection and recognizing their distinct value, uses and vulnerabilities are the first steps towards their preservation.
Systematic collections, such as those found in natural history museums, universities and some private collections, have additional and sometimes different needs than those of a museum with a mixed collection or those of a small private collection. Typically, specimens in a systematic collection have a high research value and a relatively low aesthetic value compared to specimens in mixed collections (Figure 2). Different methods of preparation (e.g. a study skin versus a trophy head) and different primary values will influence the choice of handling, storage and display techniques. For example, the use of buffered paper storage containers may produce alterations in the chemical composition of plant material. These alterations could be insignificant in some collections but critical in a collection used primarily for scientific research.
As with archaeological collections, natural history specimens in systematic collections will lose most of their value if the associated collection data is lost. Preservation of tags, field notes, etc. is therefore crucial. (Visit Agent of deterioration: dissociation – Vignette 4 for more information on maintaining historical labels.) The specialized management and care of systematic natural history collections is beyond the scope of this resource. For assistance with this type of research collection, contact specialists at natural history museums or use the publications and services of the Society for the Preservation of Natural History Collections.

Figure 2. A study skin of a degu (Octodon degus), a type of rodent, collected in Chile during the U.S. South Seas Exploring Expedition of 1838–1842.
For more information on the wide range of materials represented in natural history collections, consult the following CCI resources:
- Caring for leather, skin and fur – includes information that applies to taxidermy mounts and pelts
- Caring for feathers, quills, horn and other keratinous materials – includes information that applies to birds, taxidermy mounts and trophy heads
- Ivory, horn, bone and antler objects – includes information that applies to skeletons, trophy heads and shell collections
- Caring for basketry and plant materials – includes information that applies to botanical specimens
Health and safety issues
Anyone handling or working around natural history specimens should be aware of potential health and safety hazards associated with these types of collections. Radioactivity in minerals, rocks and fossils is an often overlooked health and safety issue. Samples of uranium ore, for example, have been found in mixed collections. Rocks and fossils from certain areas can have high levels of radon. Identifying these items may require the assistance of a specialist. Special enclosures and handling procedures may be necessary to protect the health of staff, volunteers and visitors. Some mineral specimens, such as arsenic and thallium, may also be toxic.
Many taxidermy specimens, especially those collected prior to the 1970s, were prepared with toxic compounds to prevent bio-deterioration and insect infestations. Arsenic was the most common poison associated with taxidermy, while mercury and a variety of organic pesticides, such as dichlorodiphenyltrichloroethane (DDT), were also used on occasion. Although these products were generally applied to the inside of specimens, residue often migrates to the outer surface. Similarly, mounted botanical specimens, particularly those from the 19th century and earlier, might have been treated with mercury compounds. In some museums, toxic insecticides might have been applied on pelts and taxidermy specimens to eradicate pest infestations.
Residues of these pesticides may be present on the surface of specimens (Figure 3), on storage cabinet walls or in the air inside cabinets. Residues may be visible or invisible. Wear appropriate personal protective equipment when handling all specimens unless it is known for certain, either through testing or from the original preparator, that hazardous materials are not present. Taxidermy mounts destined for educational programs should always be tested for toxic substances prior to use.

Photographer unknown, © Canadian Museum of Nature
Figure 3. Unidentified white powder deposits on a natural history specimen should be treated as pesticide residues.
Causes of damage to inorganic specimens and preventive conservation strategies
Inorganic specimens include minerals, rocks, gems and fossils. Shells and eggs are also categorized here as inorganic, although they may include organic components. When dealing with such wide-ranging materials and object types, all agents of deterioration must be taken into account. However, in particular, the following are specific to inorganic specimens:
- physical forces
- thieves and vandals
- dissociation
- pollutants
- light and ultraviolet (UV)
- incorrect relative humidity (RH)
Physical forces
Physical force is the most common agent of deterioration for inorganic specimens, such as rocks, minerals and shells. These specimens may be more fragile than they appear. They can be damaged by knocking or jostling against hard surfaces, whether due to improper handling or to vibrations in the building while in storage or on display (Figure 4). Damage such as fissures, cracks, breaks, losses or surface abrasion may occur.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0131
Figure 4. Cabinet of exotic-coloured seashells. This is an old-fashioned and visually appealing display, but the shells are vulnerable as they are not restrained or mounted and may chip or break when exposed to shock or vibration. Also, the wood display case can emit organic acids that risk harming the shells.
Projecting structures on some mineral specimens (Figure 5) or on items such as sea urchins (Figures 6a and 6b) are especially prone to damage, e.g. they are at risk of catching and snagging on clothing and packing materials. Even the most careful cleaning procedures can cause damage.
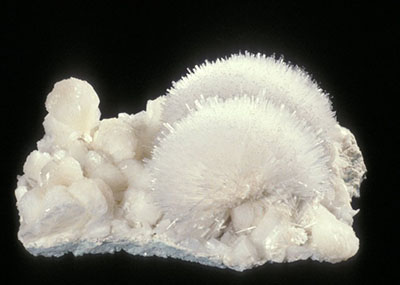
G. Runnels, © Canadian Museum of Nature
Figure 5. A sample of mesolite, a fragile crystalline mineral very susceptible to physical damage.
Eggs are clearly fragile and vulnerable to breakage. Many seashells, such as nautilus shells, are quite thin and also vulnerable.
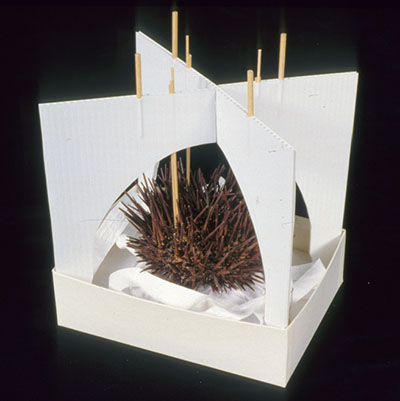
© Government of Canada, Canadian Conservation Institute. CCI 125773-0144
Figure 6a. A mount made for a sea urchin to protect its projecting components. The mount consists of an acid-free matboard box with a carved polyethylene foam (Ethafoam) base and a batting pillow lined with polyethylene non-woven fabric (Tyvek) for the urchin to rest on. Fluted plastic sheets (Coroplast) with wooden dowels were used to create a protective structure over the urchin. (Mount by Carl Schlichting)

© Government of Canada, Canadian Conservation Institute. CCI 125773-0145
Figure 6b. Strips of Tyvek were secured under the sea urchin in order to facilitate lifting. Tyvek is a smooth polyethylene non-woven fabric that does not snag. (Mount by Carl Schlichting)
Fossils may be deceptively fragile, especially when prepared with adhesives that might have deteriorated with age and are prone to failure. Physical damage is also a high risk during transportation (consult Agent of deterioration: physical forces – Vignette 2 for an example).
Recommendations
- Protect projecting parts of specimens at risk of being accidently damaged during handling (Figures 6a and 6b).
- Use non-snagging materials, such as polyethylene non-woven fabric (Tyvek) and smooth foams (Figures 6a, 6b and 7). If snagging materials are present in existing mounts, e.g. cotton batting, replace with non-snagging material.
- Store specimens in drawers, trays and containers, separating objects with spacers or barriers of inert foam or acid-free cardstock (Figure 8). This will prevent the specimens from bumping into one another when a tray is moved or when the drawers are opened and closed.
- Provide individual padded supports or individual padded containers, as necessary, especially for fragile items (Figures 7, 9a and 9b) or for larger or heavier objects (Figures 10a and 10b).
- Tailor foam density and size to the object's needs. For example, use thin sheets of polyethylene foam (Ethafoam) or crosslinked polyethylene foam (Volara) for lightweight objects and denser foam (polyethylene or polystyrene foams) slabs for heavier objects (Figure 11).
- Pad display and storage shelves and work tables with inert foam of appropriate density to protect objects from being damaged by contact with hard surfaces.
- Train staff and volunteers in safe handling procedures. For example, always handle fragile objects with both hands, from below, and preferably with the object in a box or on a tray.
- Take all necessary precautions when shipping and transporting specimens. For further information on the safe shipping of cultural property, consult Agent of deterioration: physical forces.
- For objects on display, provide mounts or retainers to minimize the risk of damage from shock and vibration while maintaining the desired aesthetic standards (visit Secure mounts for a travelling fossil exhibit).
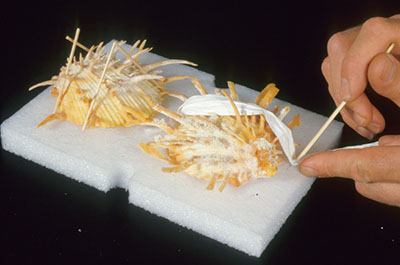
© Government of Canada, Canadian Conservation Institute. CCI 125773-0146
Figure 7. Examples of shells being secured onto a foam pad using sticks and strips of polyethylene non-woven fabric (Tyvek). (Mount by Carl Schlichting)

© Government of Canada, Canadian Conservation Institute. CCI 129976-0001
Figure 8. Example of storage drawers for vertebrae fossil specimens.
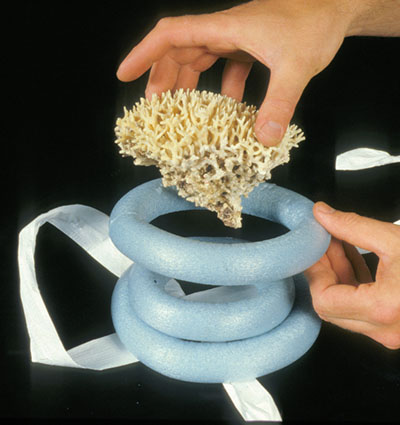
© Government of Canada, Canadian Conservation Institute. CCI 125773-0147
Figure 9a. Three rings of polyethylene foam (i.e. backer rod) are used to make a storage mount for a piece of coral. (Mount by Carl Schlichting)
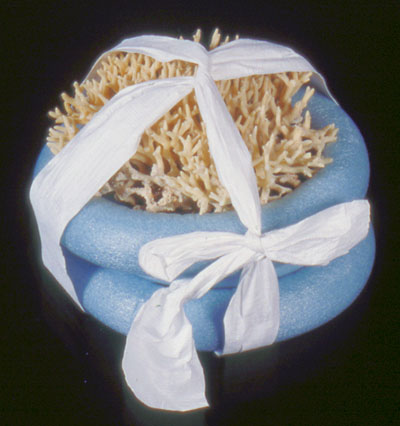
© Government of Canada, Canadian Conservation Institute. CCI 125773-0148
Figure 9b. A strip of polyethylene non-woven fabric (Tyvek) wraps over a piece of coral to secure it to the mount. (Mount by Carl Schlichting)

© Government of Canada, Canadian Conservation Institute. CCI 125773-0137
Figure 10a. Padded storage tray for a heavy mineral specimen. The specimen is wrapped in sheets of polyethylene foam and then in polyethylene non-woven fabric (Tyvek). (Mount by Carl Schlichting)

© Government of Canada, Canadian Conservation Institute. CCI 125773-0138
Figure 10b. A heavy mineral specimen is placed within a custom-fit box made of Hi‑Core, a sturdy fluted polyethylene board. Small blocks of polyethylene foam are inserted between the wrapped specimen and its box to secure it in place. (Mount by Carl Schlichting)

© Government of Canada, Canadian Conservation Institute. CCI 69345-0009
Figure 11. The base of a mount for a large and heavy mammal skull specimen is carved out of a slab of polystyrene foam. Padding will be subsequently added in between the foam base and the object, using a layer of felt or of soft foam sheeting (polyethylene, polypropylene or crosslinked polyethylene).
For further information on handling, consult Handling heritage objects.
Thieves and vandals
Gems and gold nuggets clearly require additional security measures. Some other minerals and some fossils also have high market values and require similar access control. It may be necessary to request assistance from a university or natural history museum in order to identify the specimens and to ascertain their value. Even smaller specimens of low market value, including small detachable bones on large mounted fossils, are vulnerable to opportunistic theft. The chief vulnerability of all such specimens is their general small size and consequently easy concealment.
Dissociation
Dissociation results in the loss of the object, of a part of the object or of valuable object-related data, or a loss in the ability to retrieve or associate objects and data. This is particularly a risk for natural history specimens such as fossils, rocks, minerals, etc. since a significant part of their meaning is associated with the collecting data (where and when they were found, etc.). Specimens which are difficult to label directly on their surface because they are tiny or because the surface is rough and uneven (e.g. the rocks and gems in Figures 13 and 14 below) are particularly at risk. When natural history specimens are neglected and not considered as important as other objects in a mixed collection, this data may never be collected in the first place or may be disposed of due to a lack of understanding of its worth. For more information, consult Agent of deterioration: dissociation.
Pollutants
Alkaline dusts from concrete and organic acids from wood products are two examples of construction-related pollutants that may attack certain mineral ores. Shells are particularly vulnerable to organic acids emitted from wooden drawers and cabinets. Powdery white crystals will appear on affected shells and the smooth, hard surfaces will be destroyed (Figure 12). This is known as Byne's Disease (PDF format).
Dust is a significant issue with specimens like the mesolite shown in Figure 5. Once this kind of surface gets dusty, it is very difficult and time-consuming to clean.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0139
Figure 12. Examples of deterioration to shells when exposed to a high concentration of acetic acid vapour. The two shells on the left were not exposed and show no signs of deterioration. The two shells on the right show deterioration and are covered with powdery white deposits.
Recommendations
- Cover and enclose objects to protect them from dust. Use dust sheets for additional protection.
- During a construction project, protect the collection from construction-related dust by covering with dust sheets, sheets made of polyethylene or non-acidic paper. Ensure that contractors use dust control equipment during construction projects.
- Improve or reorganize collections so that vulnerable items (e.g. shells) are stored or displayed in or on appropriate fittings made of non-emissive materials. Reserve wooden enclosures for collections that are not vulnerable to organic acids.
- Avoid the use of wood products when investing in new storage or display units, particularly if there are shells in the collection.
Light and ultraviolet
Most items in an inorganic collection are not light sensitive; however, there are a few notable exceptions. Minerals such as rose quartz may be faded by exposure to light. Others, like amber (Figure 13) and cinnabar, become discoloured, while minerals like realgar can be transformed into other compounds. In terms of the degree of light sensitivity, amber can be considered to rank in the medium-sensitivity range, while rose quartz, cinnabar and realgar are estimated as ranking in the low to medium range.

© Government of Canada, Canadian Conservation Institute. CCI 123773-0024
Figure 13. Samples of Canadian amber from Medicine Hat. Amber can discolour due to exposure to light.
Mollusks and eggshells may also contain light-sensitive pigments; the pigments are considered highly sensitive to light.
Recommendations
- Identify light-sensitive materials (e.g. certain minerals, mollusks, eggshells).
- Keep light-sensitive materials in opaque enclosures in storage and make them available for viewing (for study, etc.) only upon request.
- Choose not to display light-sensitive minerals, especially the ones of higher sensitivity.
- Where light-sensitive minerals are critical to an exhibition storyline, control light levels and exposure times, avoid natural daylight and eliminate UV from all light sources.
Incorrect relative humidity
Even though most inorganic specimens are not particularly sensitive to RH, it may be surprising to learn that a few are very RH-sensitive. A small fraction of minerals, such as hydrates and pyrites, can crumble and/or weep above or below certain critical RH levels.
Pyrite (FeS2, a mineral also known as fool's gold), which can be found in fossils as well as in discrete mineral specimens (Figure 14), is particularly vulnerable to an RH above 50%: in the presence of oxygen at high RH, it will oxidize and transform into the bulkier iron sulfate. This causes it to powder and eventually disintegrate. Acidic by-products of this reaction (sulfuric acid) are corrosive and will also destroy labels and boxes.

© Government of Canada, Canadian Conservation Institute. CCI 101454-0001
Figure 14. Pyrite appears bright and shiny like gold, as shown on the left. Pyrite suffering from pyrite disease appears dull and gray, covered with a layer of corrosion products, as shown on the right.
For some specimens, incorrect RH may also lead to deliquescence (i.e. absorption of water to the point that the material liquefies) or efflorescence (i.e. the powdering of surfaces). For example, halite (sodium chloride or salt) can begin to liquefy at RH above 75%, and borax may dehydrate to become tincalconite, a change indicated by powdering, at less than 50% RH.
Subfossils are specimens that are not fully fossilized and therefore still contain a portion of organic material (collagen). Ice age specimens, such as mammoth bones and teeth, are common examples of this type of material. These specimens are highly sensitive to fluctuating RH, which can cause cracking and delamination. They should be treated like organic bone or ivory specimens in terms of their vulnerability to incorrect RH.
The organic material that lines eggshells or acts as hinges on mollusk shells is vulnerable to mould under conditions of high RH (above 65% approximately) and to desiccation and weakening at RH below 30%. This material may also be damaged by pests (consult Pests).
Recommendations
- Provide enclosures for specimens vulnerable to high RH. The more airtight the enclosure, the less often the RH in the enclosure will reach a dangerous level for the specimen. For example, use polyethylene containers with gaskets to allow tight sealing (e.g. Rubbermaid or Lock & Lock food storage containers).
- Use a desiccant, such as conditioned silica gel, to provide added protection for the RH-sensitive specimens in their sealed enclosures. Dessicants can also be used to humidify enclosures.
- Establish microclimate enclosures for vulnerable specimens.
Causes of damage to organic specimens and preventive conservation strategies
Organic specimens include taxidermy, non-fossilized bone and skeletons, insects, botanical specimens and birds' nests. When dealing with such wide-ranging materials and object types, all agents of deterioration must be taken into account. Common and effective preservation strategies usually focus on safe handling, pest control and proper measures for storage, display and security. In particular, the following agents are specific to organic natural history specimens:
- Physical forces
- Thieves and vandals
- Dissociation
- Pests
- Pollutants
- Light and ultraviolet (UV)
- Incorrect relative humidity (RH)
Physical forces
The most common forms of damage to organic natural history specimens are breakage and loss due to poor handling and storage practices. Mammal specimens missing ears and tails, or birds with broken legs or damaged wings (Figure 15), are common occurrences. Oversized items, such as large mounted animals, are particularly awkward to move and are easily damaged in the process.

© Government of Canada, Canadian Conservation Institute. CCI 2005306-0001
Figure 15. A stuffed snowy owl exhibiting extensive damage (the straw stuffing is visible at the neck area).
Thin, brittle materials, such as those found in botanical specimens or insect and butterfly collections (Figures 16a to 16c), are highly vulnerable to physical stresses. Breaks and losses that may result will significantly decrease their interpretive, aesthetic and research value (e.g. insect and butterfly specimens with missing legs or antennae, plant samples with fragmented leaves). Birds' nests are also prone to being damaged since they consist of loosely held brittle pieces that are easily detached.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0118
Figure 16a. A bound herbarium from the 19th century showing extremely fragile specimens. Dry and fragile plant specimens were typically pasted onto paper leaves in the bound volume. The paper as well might have become brittle with age. Access to this volume should be controlled; simply turning the pages risks flexing the specimens and causing losses.

© Government of Canada, Canadian Conservation Institute. CCI 98904-0013
Figure 16b. A dried botanical specimen showing damaged leaves.

© Government of Canada, Canadian Conservation Institute. CCI 120500-0004
Figure 16c. Insect specimens (here, mayflies) are particularly fragile.
Recommendations
- Handle specimens as infrequently as possible, especially fragile or brittle ones.
- When handling is necessary, use trays, bases, containers and supports rather than holding specimens directly (Figures 17 and 18). This practice also reduces risks of direct exposure to toxic residues that may be present on specimens.
- Establish safe handling procedures for researchers, visitors and school groups.
- Make customized portable enclosures for specimens that are accessed frequently, such as items used in educational programming (consult Taxidermy mounts for educational programs).
- Identify which specimens in the collection are fragile or brittle, label them and make it a priority to protect them. For example, provide botanical specimens with tailored padded folders, flat mounts and boxes made of corrugated or fluted plastic board (e.g. Cor-X, Coroplast) or acid-free matboard. For pinned insect collections, use standard entomology boxes, adapted with stable plastic window inserts so insects can be viewed without removing lids.
- Store on stable supports and/or in padded boxes, trays and drawers, as appropriate for each object (Figures 17 and 18).
- Provide sufficient space (in terms of volume) for each item on a shelf. Bases should be made as wide as the girth of the object to help delineate the item's shelf space and prevent damage to projecting parts (such as the tail in Figure 19) when the object is placed among others on a storage shelf.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0119
Figure 17. Create nests or padded rings to protect bones and similar materials. For example, wrap skeletal objects in thin polyethylene foam. Store in trays, boxes or containers.
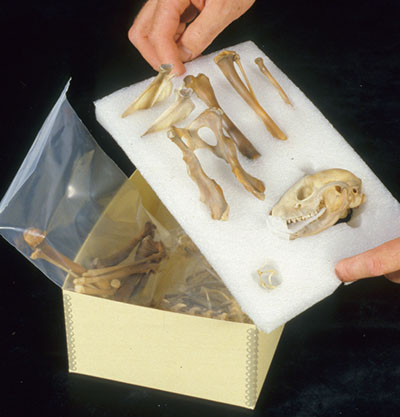
© Government of Canada, Canadian Conservation Institute. CCI 125773-0141
Figure 18. Rather than storing bone items together in bags, where they may knock against one another, keep each item apart, bagged separately or, as shown here, secured within nests carved of polyethylene foam and then placed in a box or on a tray. This also facilitates handling.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0142
Figure 19. A new wider base for this mammalian specimen was made of strong fluted plastic with twill tape ties.
For further information on handling, consult Handling heritage objects.
Thieves and vandals
The display of fur pelts, trophy heads and taxidermy mounts of certain animal species may be controversial in some communities. Vandalism of these items by protesting individuals or groups is a risk.
Recommendations
- Place vulnerable objects in enclosures or at least behind barriers.
- Provide interpretation for these exhibits so as to present the context and defuse potentially aggressive acts.
Dissociation
Dissociation is generally a medium to high risk for organic natural history specimens, for a variety of reasons. One of the principal risk factors is that it is often difficult to inscribe accession numbers directly on items such as plant, insect and taxidermy specimens (Figures 20 and 21), so usually they are only tagged or the number is identified on the support (e.g. the paper onto which a botanical specimen is mounted). Such specimens can easily be separated from their accession numbers.

François Génier, © Canadian Museum of Nature
Figure 20. An insect and label held together on a pin.

Martin Lipman, © Canadian Museum of Nature
Figure 21. Paper tags are attached by string to bird study specimens.
Historical labels can be of high value. Consult Agent of deterioration: dissociation – Vignette 4 for guidance on maintaining and preserving historical labels.
In the case of natural history specimens that are endangered species or consisting of materials that are internationally controlled (ivory, tortoiseshell, certain feathers, etc.), permits must be secured for loans and moves because legal statutes apply to the movement and use of components or materials of objects (e.g. feathers, ivory) and not only to the initial collection of the animal specimens. Omissions of required paperwork during international transit could result in confiscation.
Pests
Pests pose a very high risk to specimens made of fur, feathers and oily skins. Clothes moths and dermestid beetles (Figure 22a) are the most commonly found pests in natural history collections. Unless they have been previously treated with arsenic, mercury or other pesticides, these collections should be considered particularly vulnerable. Identification of frass (i.e. excrement or other residue of insect pest activity) may prove more difficult with taxidermy specimens that are also leaking stuffing materials and shedding fur or feathers. Many pelts and taxidermy specimens show evidence of old infestations, which may further confuse the issue. In addition, fur and feathers may not necessarily appear to be detached; they could be chewed at skin level yet held in place by the surrounding hair and feathers.
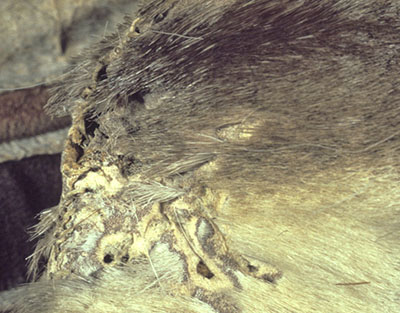
© Government of Canada, Canadian Conservation Institute. CCI 125773-0134
Figure 22a. The localized loss of hair and the holes in the skin of this pelt are examples of insect attack.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0123
Figure 22b. Mounted specimens in storage require periodic examination in order to detect signs of insect activity early on. This storage cabinet with sliding trays protects the specimens on many levels while providing easy access for periodic inspections. Each specimen is secured to the shelf drawer to prevent risk of toppling when accessed. The enclosed cabinet also shelters the specimens from light and dust.
Butterfly and other insect collections are also at very high risk of insect infestation.
Recommendations
- Inspect organic specimens (especially taxidermy and insect specimens) carefully upon acquisition.
- Check delicate structures, such as insect wings and ornamental feathers, as damage may first be visible in these areas.
- Use a fan brush to check the dark, undisturbed areas of specimens that pests prefer, such as under the wings, in armpits or under the feet.
- To distinguish between active and inactive infestations, clean the specimens and then isolate them, or monitor them with sticky traps.
- Line shelves and drawers in storage areas with white acid-free tissue. This makes it easier to quickly identify the presence of frass or other insect activity.
- Regularly inspect specimens in storage and on display in order to catch pest problems before they become widespread (Figure 22b).
- Establish an integrated pest management program that includes monitoring and housekeeping, as well as treatment options, and that takes into account building and equipment features.
- Cold or anoxic storage of pelts and taxidermy specimens are options; consult Caring for leather, skin and fur – Furs: cold storage versus anoxic storage for a discussion on these storage options.
Pollutants
Dust is the chief pollutant to guard against when caring for organic natural history specimens. In particular, it is a major concern for mammalian and bird specimens because dust gets trapped in delicate feather structures or enmeshed within the hair. Removing dust deposited on furs and feather surfaces is a delicate, time-consuming process that requires special equipment and training to prevent damaging the specimen. It may be impossible to remove it completely. Surface colours may also become obscured, and specimens may end up having significantly reduced interpretative and research value (Figure 23).

© Government of Canada, Canadian Conservation Institute. CCI 2005307-0001
Figure 23. A northern bald eagle taxidermy specimen needs to be protected from dust deposition if its feathers at the head and neck are to remain white, as they are in nature.
Oily bone or hide materials are also highly vulnerable because dust deposits will stick to the oils and darken the surface. Dust is typically abrasive and contains compounds that contribute to the oils' degradation, which in turn contributes to surface darkening and causes embrittlement.
Botanical and insect specimens need to be kept free of dust because their sheer material fragility makes them extremely difficult to clean.
Dust also often attracts pests, compounding the problems for hides, furs, feathers and insect collections already quite prone to insect infestation.
Recommendations
- Vacuum rooms regularly to reduce the amount of dust in both storage and display environments, thereby reducing dust accumulation on objects. Use a vacuum cleaner with a HEPA filter to prevent the redistribution of small dust particles and to protect staff and visitors from any toxic dust shed by taxidermy specimens.
- Use closed cabinets or other enclosures in storage, or provide open storage shelves with cotton drapes to reduce dust accumulation on objects.
- Contain oversized organic specimens within a framed structure covered with plastic sheeting or, at a minimum, drape specimens with dust covers.
- Use display cases to reduce or eliminate dust accumulation on exhibited objects.
- Use appropriate filtration through central air handling systems or a positive pressure air system to reduce the level of dust and other pollutants in the museum environment.
Light and ultraviolet
The natural colourants found in organic specimens are typically at medium to high risk of light and UV damage. Visible light and UV-induced fading, discolouration and embrittlement may be observed on bird, animal and plant specimens that have been on display for many years. Variations in pigmentation from species to species make it difficult to predict rates of fading. The colour of most furs and feathers (Figure 24) is particularly vulnerable, ranging from medium to high in sensitivity.

Luci Cipera, © Canadian Museum of Nature
Figure 24. Bright colours on bird specimens are sensitive to light damage; brown tones are moderately sensitive. White feathers can yellow.
Recommendations
- Identify and label sensitive objects.
- Choose not to display valuable light-sensitive objects.
- Keep light-sensitive items in darkened storage, preferably in opaque containers.
- Make light-sensitive items available for study only, upon request.
- Choose less valuable light-sensitive specimens for display with the understanding that they will suffer light damage.
- Control light levels and exposure times for light-sensitive specimens on display.
- Avoid natural daylight and eliminate the unnecessary UV from all light sources.
Incorrect relative humidity
Organic materials may be damaged by incorrect RH in the form of dampness, dryness or significant fluctuations. Above 65% RH, mould can grow. Consult Agent of deterioration: incorrect relative humidity, which provides a chart indicating the rate of mould growth by humidity level. Under dry conditions (RH below 30%), skins of mammals, birds, reptiles, fish, etc. are more brittle and therefore more vulnerable to physical damage during handling. Skins of mounted specimens are usually held taut and are therefore prone to tears due to large drops in RH.
Bone and especially ivory are sensitive to RH fluctuations. Teeth are particularly vulnerable due to their structure, frequently cracking shortly after being collected. In ivory, dust can become trapped in cracks as they open and close, leaving telltale dark lines on the material.
Keratinous materials, such as hooves and claws, are also prone to distortion, cracking and delamination, although they are much less sensitive to RH than bone or ivory.
Recommendations
- Identify and label sensitive objects, such as taxidermy mounts, ivory and teeth, and provide them with the most stable storage and display environments and, if possible, enhanced RH control measures (display cases, etc.).
- Inspect sensitive objects regularly to detect early signs of inadequate RH conditions.
- Wrap sensitive objects in layers of buffering materials and store them in enclosures. (Objects kept enclosed within multiple layers are better sheltered from rapid RH fluctuations that may occur in the environment.)
For more information on RH and its effect on collections, consult Agent of deterioration: incorrect relative humidity.
Examples of preventive conservation practices
Storage for crab specimens
In storage, crab specimens are susceptible to damage when randomly placed on coarse fabric, which has a tendency to snag small projecting parts (Figure 25a). Also, specimens placed closely together could rub or jostle against each other when the drawer is opened and closed.

Barbara Njie, © Canadian Museum of Nature
Figure 25a. Crab specimens stored loose in a large drawer are susceptible to damage.
In order to better protect these specimens in storage, each specimen was placed in a polystyrene box with custom-made dividers cut from stable foam plastic (Figure 25b). This way, the specimens are prevented from sliding into one another as the drawer is pulled open and closed. The boxes can be removed individually for examination purposes.

Barbara Njie, © Canadian Museum of Nature
Figure 25b. Crab specimens stored in boxes and with padding are less susceptible to being damaged when accessed.

Barbara Njie, © Canadian Museum of Nature
Figure 25c. Crab specimens in boxes and with padding as per Figure 25b, now in a higher-quality metal storage cabinet.
Purchasing improved cabinets is expensive and requires long-term planning. In this example, the entire storage cabinet was changed from a wooden construction to powder coated steel (Figure 25c). Metal cabinets are robust, they do not emit any harmful volatiles which may affect parts of the collection, and their drawers open and close quite smoothly, minimizing risks of accidental jerks which could reverberate onto the specimens within and cause physical damage. The addition of a powder coating seals the metal and inhibits the formation of corrosion (rust).
Taxidermy mounts for educational programs
Often, objects that are used for educational purposes take more than their fair share of handling. This is especially the case with specimens used for school groups and similar clients. Such specimens should be securely attached to a mount that can be held instead of handling the object itself (Figure 26).

Martin Lipman, © Canadian Museum of Nature
Figure 26. This mounted specimen of a red fox is secured to the base of a transparent carrying case, so it is protected from all physical forces while in use.
For more details on building mounts and supports, consult Mount-making for Museum Objects (Barclay et al. 1998).
Secure mounts for a travelling fossil exhibit
Figures 27 and 28 show examples of highly effective mounting hardware and techniques that enabled the Canadian Museum of Nature's Ice Age Mammals exhibition to be safely displayed and shipped from Ottawa to Montréal without needing to remove, handle or package the individual specimens.

© Government of Canada, Canadian Conservation Institute. CCI 125773-0135
Figure 27. The mount of a fossil specimen from the Canadian Museum of Nature's Ice Age Mammals travelling exhibition is attached to the base of its travelling display case. This eliminates the need for dismounting and remounting at each venue. (Mount design and fabrication by Ron Seguin – Creative Nature Studio)

© Government of Canada, Canadian Conservation Institute. CCI 125773-0136
Figure 28. The mounts of two fossil specimens are attached directly to their display case's base in the Canadian Museum of Nature's Ice Age Mammals travelling exhibition. (Mount design and fabrication by Ron Seguin – Creative Nature Studio)
Gentle but firm restraint is an important part of preventing shipping damage to fragile objects. The strong, padded, custom-made mounts hold the specimens firmly in place, and the display cases are securely attached to the base units. Complete base unit assemblies such as these were moved by museum staff and art handlers and covered with clean protective blankets inside the art shipper's air ride transport trailer. Selected base units were mounted on protective pads. All base units were securely tied to the trailer. This example is further discussed in Agent of deterioration: physical forces.
Proper documentation for loans
It is important to keep a record of natural history collections that require assembly, as discussed in the case study under Agent of deterioration: dissociation — Vignette 2.
Bibliography
Barclay, R., A. Bergeron and C. Dignard. Mount-making for Museum Objects. Illustrated by C. Schlichting. 2nd ed. Ottawa, ON: Canadian Conservation Institute, 2002.
Carter, D., and A.K. Walker. Care and Conservation of Natural History Collections. Oxford, UK: Butterworth-Heinemann, 1999.
Howie, F.M.P. Care and Conservation of Geological Material: Minerals, Rocks, Meteorites and Lunar Finds. Oxford, UK: Butterworth-Heinemann, 1992.
Kite, M., and R. Thomson, eds. Conservation of Leather and Related Materials. Oxford, UK: Butterworth-Heinemann, 2005.
Rose, C.L., and A.R. de Torres, eds. Storage of Natural History Collections: Ideas and Practical Solutions. Pittsburgh, PA: Society for the Preservation of Natural History Collections, 1992.
Rose, C.L., C.A. Hawks and H.H. Genoways, eds. Storage of Natural History Collections: A Preventive Conservation Approach. Pittsburgh, PA: Society for the Preservation of Natural History Collections, 1995.
Stone, T., and C. Dignard. Care of Mounted Specimens and Pelts, revised. Notes 8/3. Ottawa, ON: Canadian Conservation Institute, 2015.
Waller, R. "Temperature and Humidity-sensitive Mineralogical and Petrological Specimens." In F.M. Howie, ed., The Care and Conservation of Geological Material: Minerals, Rocks, Meteorites, and Lunar Finds. Oxford, UK: Butterworth-Heinemann, 1992, pp. 25–50.
Williams, S., and C. Hawks. "Appendix T: Curatorial Care of Biological Collections." In Museum Handbook, Part 1. Washington, D.C.: National Park Service, 2005, pp. T1‑T134.
© Government of Canada, Canadian Conservation Institute, 2018
Published by:
Canadian Conservation Institute
Department of Canadian Heritage
1030 Innes Road
Ottawa, ON K1B 4S7
Canada
Cat. No.: CH57-4/6-8-2018E-PDF
ISBN 978-0-660-27981-7