Guidance document for the New Substances Notification Regulations (Chemicals and Polymers)
Version 1.0
This Guidance Document provides assistance for complying with the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) of the Canadian Environmental Protection Act, 1999 (the Act). It is meant to help notifiers determine whether a substance is subject to notification under the Regulations and identify the information requirements. It also includes step-by-step instructions for the completion of a New Substances Notification. This Guidance Document replaces the 2005 Guidelines for the notification and testing of new substances – chemicals and polymers.
Although care has been taken to ensure that this Guidance Document accurately reflects requirements prescribed in the Act and the Regulations, notifiers are advised that should any inconsistencies be found, the Act and the Regulations will prevail.
To access a PDF version of the Guidance Document, please visit the Government of Canada Publications website.
Consultation on the Guidance Document
In April 2021, the Guidance Document was published online for a 90-day public comment period. Comments were taken into account in finalizing the updated Guidance Document. A summary of these comments and program responses is accessible on the Consultation webpage.
Abstract
This document (referred to as the Guidance Document) has been prepared to assist notifiers responsible for complying with the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) of the Canadian Environmental Protection Act, 1999 (the Act).
This Guidance Document is meant to help notifiers determine whether a substance is subject to notification under the Regulations and identify the information requirements. In addition, it provides information including but not limited to:
- step-by-step instructions for the completion of a New Substances Notification (NSN)
- user-friendly flowcharts to aid in determining the appropriate Schedule to file (consult Appendix 1)
- technical considerations of the information requirements
- detailed instructions on how to complete the New Substances Notification Form
- identification of appropriate test procedures and practices to use and
- an outline of how confidential information should be submitted
This Guidance Document concludes with an explanation of how the New Substances (NS) program uses and assesses the information submitted in a New Substances Notification and the possible implications of the assessment decisions for notifiers.
Note: Living organisms not on the Domestic Substances List may be subject to the New Substances Notification Regulations (Organisms) and are not addressed in this Guidance Document, which is specific to chemicals, biochemicals, polymers and biopolymers. To determine whether a living organism is subject to notification under the New Substances Notification Regulations (Organisms), please refer to the Guidance document for the notification and testing of new living organisms.
For an overview of the processes applied by the NS program when administrating submissions received in accordance with the Regulations, please refer to the Administration of the New Substances program – Processing of submissions.
How to use the Guidance Document
This Guidance Document has been prepared for the benefit of any person interested in the provisions of the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) made under the Canadian Environmental Protection Act, 1999 (the Act). A review of the sections of this Guidance Document, listed below, will allow the reader to focus on requirements specific to their circumstances.
The key to avoiding unnecessary delays when preparing a New Substances Notification is to thoroughly understand the properties of a substance in question and how to apply the Regulations, which this Guidance Document will help you understand.
This Guidance Document is organized into 10 sections:
- Introduction and overview: explains the purpose, statutory powers and features of the New Substances program
- The inventories: explains the Domestic Substances List and the Non-domestic Substances List, how these are amended and how to locate a substance specified on them
- Substances: helps to determine whether the substance to be manufactured, imported or used must be notified; provides definitions of special categories, substances not subject to notification and substances subject to notification
- Notification information requirements: if the substance is subject to notification, this section helps identify the appropriate Schedule to be provided and determine when the New Substances Notification must be provided to the Minister of the Environment via the New Substances program
- New Substances Notifications: provides instructions for completing the information required for a New Substances Notification
- The New Substances Notification Form: describes the process to complete the New Substances Notification Form and the meaning and intent of each information requirement; also elaborates when data elements are not required
- Confidential information: describes issues pertaining to confidential business information, such as confidentiality claims, masking of substance identities and determining the presence of confidential substances on the Domestic Substances List and the Non-domestic Substances List
- Recommended test protocols and alternative approaches: provides guidance on acceptable test methods and “alternative” information and describes features of subsection 81(8) of the Act, which provides for the waiver of information requirements when one of several criteria are met. The New Substances program provides the opportunity for notifiers to submit a Pre-notification Consultation request (consult section 8.8) to resolve notification issues while the New Substances Notification is being prepared
- Processing a New Substances Notification: explains what happens after a New Substances Notification is received, including how the New Substances Notification is processed and reviewed and the types of correspondence that could be issued by the New Substances program
- Post-notification responsibilities: reviews obligations of notifiers and the New Substances program after a New Substances Notification has been submitted
Further clarification and updates on any topic covered by this Guidance Document can be obtained from the New Substances program website or by contacting the Substances Management Information Line by telephone at 800-567-1999 (within Canada) or 819-938-3232 (outside Canada) or by email: substances@ec.gc.ca.
Section 1. Introduction and overview
1.1 Purpose of this Guidance Document
This Guidance Document provides assistance for complying with the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations). It explains the information that a personFootnote 1 manufacturing or importing a new substanceFootnote 2 into Canada (the notifier) must submit to the Minister of the Environment (the Minister) under subsections 81(1) and 81(4) of the Canadian Environmental Protection Act, 1999 (the Act) before manufacturing or importing a chemical/biochemicalFootnote 3, nanomaterial or polymer/biopolymerFootnote 4 that is not on the Domestic Substances List (DSL).Footnote 5 This information is required so that the Minister or the Minister of Health may determine whether the substance is toxic or capable of becoming toxic within the meaning of section 64 of the Act. When the term "toxic" is used in this Guidance Document, it refers to the interpretation in section 64 of the Act (consult section 9.5.2). This Guidance Document also discusses the obligations of the Minister of the Environment and the Minister of Health (the ministers) to respect assessment periods and those of the Minister to add a chemical or polymer to the DSL under section 87 of the Act.
Note that the Guidance document for the New Substances Notification Regulations (Chemicals and Polymers) does not address the New Substances Notification Regulations (Organisms). Information pertaining to the regulations for living organisms can be found in the Guidance document for the notification and testing of new living organisms.
The New Substances (NS) program consists of officials from both Environment and Climate Change Canada and Health Canada. Each department conducts an assessment of the information provided to the Minister in the New Substances Notification (NSN).
1.2 The Canadian Environmental Protection Act, 1999
The Act is a statute about sustainable development and pollution prevention. These purposes are achieved or furthered through many mechanisms, among them the Substances and Activities New to Canada provisions, requiring the ministers to assess substances not on the DSL in order to determine whether the substances meet the criteria set out in section 64 of the Act and whether they should be subject to action after the assessment.
1.3 Overview of the new substances provisions under the Act
Notification is required if a substance is subject to sections 80 to 89 of the Act. Substances that require notification are the following:
- substances new to Canada (that is, those not on the DSL) that are manufactured in Canada or imported into Canada and
- substances used to undertake a Significant New Activity (SNAc) (consult section 9.6)
The Act features a number of provisions, including criteria for identifying substances requiring notification; notification obligations for manufacturers and importers; a detailed assessment mechanism; and enabling authorities to take action after the assessment.
In the Act, the approach to the management of new substances is both proactive and preventive, employing a pre-manufacture or pre-import notification and assessment process. When this process identifies a new substance that may pose a risk to human health or the environment, the Act empowers the Minister to intervene prior to or during the earliest stages of its introduction to Canada. This ability to act early makes the NS program a unique and essential component of the federal approach to the sound management of chemicals in Canada.
The Regulations specify the information that must be provided to meet the notification obligations. The main regulatory features of the NS program are:
- establishment of categories of substances
- identification of administrative and other information requirements
- specification of conditions, test procedures and laboratory practices to be followed when developing test data
- timing of notification before manufacture or import or beginning of a significant new activity and
- requirements for the NS program to assess information within a set time
To meet the need for evaluating different categories of substances, information requirements are determined by separating substances into categories and notification groups. Substances are first generically categorized by substance type (for example, chemicals and polymers), and then each substance type is further separated into notification groups based on factors such as quantity of manufacture or import or proposed use (for example, research and development).
The assessment process begins when the NS program receives a complete NSN for a new substance proposed to be manufactured or imported, which must contain all required administrative and technical information prescribed in the Regulations, including the appropriate fee (if applicable), and substantiation of confidentiality claims. NSNs must be provided to the NS program at least 5 to 75 calendar days prior to exceeding the applicable trigger quantity according to the notified Schedule.
Significant New Activity Notifications (SNANs) must contain all prescribed information specified in the SNAc Notice or SNAc Order (consult section 9.6.2) and must be provided prior to undertaking a significant new activity according to the timelimes prescribed in the SNAc Notice or SNAc Order (typically 90 days prior to the commencement of the significant new activity).Footnote 6
Sometimes a quantity is specified in the SNAc Notice or SNAc Order (for example, any activity involving more than 10 kilograms per calendar year). In these cases, any person proposing a significant new activity for the substance shall provide the prescribed information at least 90 days before exceeding the specified quantity per calendar year.
When a quantity is not specified in the SNAc Notice or SNAc Order, any person proposing a significant new activity for the substance (0 kilograms per calendar year) shall provide the prescribed information required in the SNAc Notice or SNAc Order 90 days prior to the commencement of the proposed significant new activity.
Environmental and human health risk assessments are conducted based on information provided and any other information that is available to the NS program to determine whether the substance is toxic or capable of becoming toxic (consult section 9.5.2). These assessments are required to be completed within the prescribed assessment period (consult section 1.5.1) and may result in any of the following by the NS Program on behalf of the Minister:
- A determination that the substance is not toxic or capable of becoming toxic
- A determination that the substance is toxic or capable of becoming toxic, which may require
- the establishment of conditions to restrict the manner in which the notifier may manufacture or import of the substance
- prohibition of manufacture or import of the substance or
- prohibition of manufacture or import of the substance to the person to whom the request is directed pending submission and assessment of additional information determined to be required for the purpose of the assessment or
- A suspicion that a significant new activity in relation to the substance may result in the substance becoming toxic. In such instances, a SNAc Notice will be issued for the substance
1.4 Who is required to notify?
Under the Regulations and section 81 of the Act, any person (individual or corporation) manufacturing a new substance in or importing a new substance into Canada (notifier) must provide the NS program with an NSN (consult also section 1.4.2). This NSN must contain all information specified in the Regulations.
The notifier is responsible for complying with the Regulations and must submit the appropriate NSN corresponding to the quantities of the substance being manufactured or imported. The notifier is required to provide the information in the NSN Form (consult section 6).
By signing the certification statement (block A.1.1) on the NSN Form, the notifier accepts all other compliance responsibilities, including filing any subsequent Schedules that may be required and providing the appropriate fee(s), and will be required to keep the information and any supporting data for a period of 5 years, as per section 13 of the Regulations.
1.4.1 Transfer of notification status – Certification Form – Interpretation of Person
Subsection 81(5) of the Act provides a rule of succession in the case of the transfer of certain rights in respect to substances subject to section 81 of the Act.
Successors to which subsection 81(5) applies are requested to sign a Certification Form prior to change of ownership if they wish to take advantage of the current notification status of a substance. This includes companies that are undergoing a company name change. This form indicates the transfer of rights or privileges, in relation to information provided for the substance, from the original notifier to the successor.
The Interpretation of Person Certification Form is available on the NS program website or by contacting the Substances Management Information Line.
The Certification Form must be signed by an officer of the successor and include all NSNs to which the change of ownership applies.
This provision allows successors to continue manufacturing or importing a new substance without having to submit a new notification.
1.4.2 Canadian Agent – subsection 14(3) of the Regulations
If the notifier providing the NSN is not a Canadian resident, this person must identify, under paragraph 14(1)(b) of the Regulations, a Canadian resident who is authorized to act on their behalf as the “Canadian Agent.” All notices and correspondence from the NS program will be sent to the “Canadian Agent,” who will be required to keep the information and any supporting data for a period of 5 years after the end of the year in which the information is provided as per section 13 of the Regulations.
As an example, a notifier who is not a Canadian resident but, for the substance being imported, possesses “Canadian Importer Status” and is listed as the “Importer of Record” on the Commercial Accounting Declaration Form as issued by the Canada Border Services Agency must identify a person residing in Canada who is authorized to act on the notifier’s behalf as the “Canadian Agent.”
The “Canadian Agent” is responsible for ensuring that information in the NSN is accurate and complete.
Please note that the “Canadian Agent” cannot be the importer of the new substance. If the “Canadian Agent” is importing the substance directly and reselling, repackaging, distributing, etc., from their location in Canada, then this person is the Importer of Record and an NSN must be completed identifying this person as the Notifier in block A.2 (consult section 6.2.2) and not as the “Canadian Agent”; in that case, block A.4 would be blank. The Third Party Information Supplier may be identified in block A.5 if this person is supplying proprietary confidential information to complete the NSN (consult section 6.2.5).
1.4.3 Toll manufacturer
Toll manufacturing occurs when a company contracts a manufacturer to process its raw materials and create a new substance. Ownership of the raw materials and resulting substance remains with the contracting company throughout the activity. For new substances that are manufactured on toll, the contracting company is designated as the notifier. If any actions are taken as a result of the assessment, the notifier must inform the toll manufacturer of these actions and the toll manufacturer is responsible for complying with these actions.
1.5 When to submit a New Substances Notification to the New Substances program
The timing of an NSN depends on the notification group (Schedule, which prescribes the assessment period) and when the quantity specified by the Schedule (trigger quantity) is likely to be exceeded.
1.5.1 New Substances Notification assessment periods
Assessment periods range from 5 to 75 calendar days, depending on the type and amount of the substance being manufactured or imported (consult section 4). NSNs must be provided at least the number of calendar days prescribed according to the notified Schedule prior to exceeding the applicable trigger quantity. For example, a Schedule 9 NSN must be provided at least 30 days prior to exceeding 1 000 kg/year. The assessment periods are shown in Table 1-1.
| Schedulea | Class | Assessment period (days) | Annual quantities (kg) |
|---|---|---|---|
| Sch. 1 – Special categoryb (NDSLc and not on NDSL) | Chemicals | 30 | 1 000 |
| Sch. 1 – Special category (update of information) | Chemicals | 30 | 10 000 |
| Sch. 3 – Special category (NDSL and not on NDSL) | Polymers | 30 | 10 000 |
| Sch. 4 – Not on NDSL | Chemicals | 5 | 100 |
| Sch. 4 – NDSL | Chemicals | 30 | 1 000 |
| Sch. 5 – Not on NDSL | Chemicals | 60 | 1 000 |
| Sch. 5 – NDSL | Chemicals | 60 | 10 000 |
| Sch. 5 – NDSL (high release /significant public exposured) | Chemicals | 75 | 50 000 |
| Sch. 6 – Not on NDSL | Chemicals | 75 | 10 000 |
| Sch. 9 – All polymers | Polymers | 30 | 1 000 |
| Sch. 10 – Non-RRR polymerse either on NDSL or all reactants on DSL/NDSL | Polymers | 60 | 10 000 |
| Sch. 10 – Non-RRR polymers either on NDSL or all reactants on DSL/NDSL (high release/significant public exposure) | Polymers | 60 | 50 000 |
| Sch. 11 – Non-RRR polymers not on NDSL and not all reactants on DSL/NDSL | Polymers | 60 | 10 000 |
a Additional information is required from Schedule 2 if the substance is a biochemical or biopolymer for all notified substances (consult sections 4.2 through 4.9).
b Special categories include research and development, contained site-limited intermediate and contained export-only substances (consult section 4.2).
c NDSL means Non-domestic Substances List.
d There may be an additional assessment period for substances that exceed 50 000 kg/year if they meet one of the following criteria: releases anticipated to exceed 3 kg/day into the aquatic environment after wastewater treatment; or significant public exposure (consult section 4.4.3 or 4.9.2). If these criteria are not met, then Schedule 5 or 10 is the final requirement.
e Non-RRR polymers means Non-Reduced Regulatory Requirement Polymers (consult section 3.3.1.6).
1.5.2 New Substances Notification fees
The New Substances Fees Regulations (NSFR) were developed to incorporate service fees; these fees must be provided with most NSNs submitted under the Regulations. The amount of fees required is dependent on the amount of annual sales in Canada for the notifier, the specific Schedule being submitted and other services being requested (for example, confidential search on the DSL or Non-domestic Substances List (NDSL), or masked name request). A fee schedule for different levels of service is provided on the New substances notification fees webpage. Additional information can also be found in the NSFR.
Fee reductions are available for notifiers meeting the criteria for small- or medium-sized enterprises (consult the New substances notification fees webpage) and for matched or consolidated notifications, as described in sections 5.1 and 5.3 of this Guidance Document, respectively.
1.5.3 New substances not subject to notification fees
The NSFR do not apply to biochemicals, biopolymers, research and development substances or to substances that are intended solely for use in products regulated under any other Act of Parliament, including the Food and Drugs Act (F&DA), the Fisheries Act and the Health of Animals Act.
The fees also do not apply to SNANs (consult sections 1.3 and 9.6.2), to the submission of the update of information at 10 000 kg/year required for special category Schedule 1 notification (consult section 4.2.2) and to the submission of additional information at 50 000 kg/year for substances that have high release to the aquatic environment or significant public exposure (consult sections 4.4.3 and 4.9.2).
1.6 Enforcement
For information about the enforcement of the Act and the Regulations, notifiers should consult the Compliance and Enforcement Policy for the Canadian Environmental Protection Act, 1999. This policy was established to ensure that the Act is applied throughout Canada, fairly, predictably and consistently.
Section 2. The inventories
2.1 Role of the Domestic Substances List
The term “Domestic Substances List” (DSL) used in this Guidance Document encompasses substances that are both on the public and confidential parts of this inventory.
For a comprehensive description of each part of the DSL, consult Appendix 12 of this Guidance Document.
2.1.1 The Domestic Substances List – definition of a new substance
The DSL provides an inventory of substances manufactured in, or imported into Canada on a commercial scale. A substance not on the DSL is therefore a new substance in Canada. The DSL is the sole basis for determining whether a substance is new for the purposes of the Canadian Environmental Protection Act, 1999 (the Act) and the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations). Substances are added to the DSL using a unique substance identifier (that is, a Chemical Abstracts Service (CAS)Footnote 7 Registry Number, Confidential Substance Identity NumberFootnote 8 (consult section 2.1.2) or Enzyme Commission number (consult section 6.4.2.4)).
Substances on the DSL are not subject to notification under the Regulations; however, when a substance identifier on the DSL is followed by a regulatory flag (that is S flag, S prime flag, or P flag) this substance is subject to notification under certain circumstances (consult section 2.1.4.1). The DSL includes the original list, published in the Canada Gazette, Part II, on May 4, 1994, and all additions or deletions subsequently published in the Canada Gazette, Part II.
2.1.2 Confidential substances on the Domestic Substances List
A notifier may request that the substance they notify be added confidentially to the DSL using a masked name that complies with the Masked Name Regulations (consult section 2.1.2.1). Procedures for generating masked names are described in section 7.2.2 and Appendix 5 of this Guidance Document. A Third Party Information Supplier who requests that substance information be kept confidential from the notifier may also request that a substance be added confidentially to the DSL. A substance eligible for addition to the DSL under a masked name will be assigned a Confidential Substance Identity Number even when a CAS Registry Number is available. The Confidential Substance Identity Number will be provided to the notifier or the Third Party Information Supplier by the New Substances (NS) program. Once eligible, the Confidential Substance Identity Number and acceptable masked name for the substance will be published in the Canada Gazette, Part II. Substances that are on the confidential portion of the DSL are treated the same as substances that are on the public portion of the DSL.
2.1.2.1 Justification for masking the substance identity
If the identity of the notified substance is claimed as confidential, the notifier will be asked to provide a justification, in addition to a masked name that complies with the Masked Name Regulations, explaining why the information should be treated as confidential. This justification should be selected from the following criteria:
- The substance identity is a trade secret of the submitter
- The substance identity is of a financial, commercial, scientific or technical nature that is treated consistently in a confidential manner by the submitter
- The disclosure of the substance identity could reasonably be expected to result in material financial loss or gain to, or could reasonably be expected to prejudice the competitive position of, the submitter or
- The disclosure of the substance identity could reasonably be expected to interfere with contractual or other negotiations of the submitter
The justification to be provided must also include the information set out in section 7.2.2 of this Guidance Document. If the supporting documentation is deemed to be inadequate in relation to the justification criteria, the notifier will be informed that the NS program intends to list the appropriate CAS Registry Number (consult section 6.2.16). The notifier will have the opportunity to appeal this decision before the information is published.
Subsection 315(1) of the Act states that the Minister of the Environment (the Minister) may, however, disclose information where
- (a) the disclosure is in the interest of public health, public safety or the protection of the environment and
- (b) the public interest in the disclosure clearly outweighs in importance
- (i) any material financial loss or prejudice to the competitive position of the person who provided the information or on whose behalf it was provided, and
- (ii) any damage to the privacy, reputation or human dignity of any individual that may result from the disclosure
2.1.3 Amendments to the Domestic Substances List
As a result of statutory requirements, the DSL is amended from time to time for the following reasons:
- Nomination of a substance to the DSL that was manufactured in or imported into Canada between January 1, 1984, and December 31, 1986 (subsection 66(1) of the Act)
- All prescribed or additional information as well as tests results has been provided to the Minister; the Minister of the Environment and the Minister of Health are satisfied that the substance has been imported or manufactured in Canada by the person who provided the information; an assessment of the substance has been performed under section 83 and no conditions imposed by the Minister under paragraph 84(1)(a) concerning the manufacture or import of the substance remain in effect (subsection 87(1) or 87(5) of the Act) or
- To maintain the list, for example by correcting a typo in a substance identifier (subsection 66(1) of the Act)
Amendments to the DSL are published in the Canada Gazette, Part II, approximately every 6 to 8 weeks.
For additional information about the eligibility requirements for adding substances to the DSL, consult section 10.2.1 of this Guidance Document.
2.1.3.1 Nominating a substance to the Domestic Substances List
Any chemical, biochemical, nanomaterial, polymer or biopolymer that was, between January 1, 1984 and December 31, 1986, manufactured in or imported into Canada in a quantity of not less than 100 kg in any one calendar year, or in Canadian commerce or used for commercial manufacturing purposes in Canada can be nominated for addition to the DSL. To nominate a substance, a Domestic Substances List Nomination Form with applicable documentation demonstrating that substance meets the requirements specified above (that is, records of sale, purchase, production/manufacture and import) must be provided to the NS program. These substances can be added to the DSL publicly or confidentially.
Further instructions for nominating substances to the DSL can be found on the Domestic Substances List Nomination Form available on the NS program website or by contacting the Substances Management Information Line. There are no prescribed deadlines for adding substances that become eligible for addition to the DSL through submittal of a nomination form.
Although there are no fees associated with nominating a substance to the DSL, there is a fee for a masked name request (consult fee table on the New substances notification fees webpage) when substances are added confidentially to the DSL.
2.1.4 Domestic Substances List flags
The DSL contains 5 different flags for substances, and depending on the situation, flags can be combined. Some flags are used for governmental tracking purposes, and others indicate that notification requirements may apply. The onus is on the notifier to identify and comply with obligations resulting from any applicable flags or regulations imposed on a substance.
2.1.4.1 Regulatory flags
The following 3 regulatory flags indicate to notifiers that notification requirements may apply prior to manufacturing, importing or using the substance.
The S flag
The letter S after a substance identifier indicates that the substance is subject to subsection 81(3) of the Act. This flag is used for a new substance that was assessed under section 83 of the Act and the assessment concluded that a significant new activity, in relation to the substance, may result in the substance becoming toxic according to the Act. When the substance was added to the DSL it was added with an S flag.
The S′ (S prime) flag
The letter S′ after a substance identifier indicates that the substance is subject to subsection 81(3) of the Act. This flag is used for a substance that was assessed under sections such as 68 or 74 of the Act where the assessment concluded that a significant new activity, in relation to the substance, may result in the substance becoming toxic according to the Act. The DSL was then updated to include the S′ after a substance identifier.
The purpose of the S and S′ flags is to indicate that information respecting the flagged substance must be submitted if the substance is proposed for a significant new activity. Anyone considering undertaking a significant new activity in relation to the substance must provide the Minister with the prescribed information in the prescribed timeframe prior to the commencement of the proposed significant new activity. This new information will allow the NS program to assess the environmental and human health risks associated with the significant new activity, and modify the SNAc requirements, or to further develop risk management measures, if deemed necessary (consult section 9.6.2).
For a comprehensive listing of substances that are subject to SNAc requirements, consult the Significant New Activity Publications under the Canadian Environment Protection Act, 1999.
The P flag
The letter P after a substance identifier indicates that the substance, which was subject to subsection 81(1) or 81(2) of the Act, was assessed and added to the DSL on the basis that it met the Reduced Regulatory Requirement (RRR) polymer criteria (consult section 3.3.1.5).
The purpose of the P flag is to indicate that any person who intends to manufacture in or import into Canada the flagged polymer in a form that is not considered RRR in a quantity above prescribed thresholds must submit a Non-Reduced Regulatory Requirement (non-RRR) Schedule New Substances Notification (NSN). For greater certainty, this obligation also applies to the original notifier.
In the case where the NS program assesses the re-notified substance and concludes that there is no suspicion of toxicity for the non-RRR polymer and it is again eligible for addition to the DSL, the DSL will be updated accordingly, and the P flag will be removed. In the case where the NS program assesses the non-RRR form of the polymer and concludes that there is a suspicion of toxicity, appropriate actions (consult section 9.6) will be taken post-assessment.
2.1.4.2 Administrative flags
The following 2 administrative flags are used by the NS program to identify substances added to the DSL under specific scenarios.
The T flag
The letter T after a substance identifier indicates that the substance was manufactured or imported during the transitional period (that is between January 1, 1987, and July 1, 1994) and the prescribed information was provided to and assessed by the NS program in accordance with subsection 81(2) and section 83 of the Act, respectively.
The N flag
The letter N after a substance identifier indicates that the substance was added to the DSL based on the substance being manufactured or imported into Canada after July 1, 1994, and the prescribed information was provided to and assessed by the Minister in accordance with subsection 81(1) and section 83 of the Act, respectively.
When there is no flag associated with a substance that is on the DSL, the substance was added to the list via a nomination of the substance under section 66 of the Act (consult section 2.1.3).
2.2 Role of the Non-domestic Substances List
2.2.1 The Non-domestic Substances List
The term Non-domestic Substances List (NDSL) used in this Guidance Document encompasses substances that are both on public and confidential parts of this inventory.
The NDSL is a list of substances not used commercially in Canada above the trigger quantities specified in the Regulations, and known to be in international commerce. Substances on the NDSL are subject to the notification requirements set out in the Regulations; however, they are subject to fewer information requirements in comparison to new substances that are not on the NDSL.
For a comprehensive description of each part of the NDSL, consult Appendix 12 of this Guidance Document.
2.2.2 Confidential substances on the Non-domestic Substances List
The NDSL includes substances with masked identity to protect Confidential Business Information (CBI). These substances are identified by their masked name developed in accordance with requirements set out in the Masked Name Regulations and their Confidential Substance Identity Number assigned by the NS program. A Confidential Substance Identity Number can be assigned whether a CAS Registry Number is available or not. A masked substance on the NDSL is treated the same way as a substance listed publicly. As such, it is subject to fewer information requirements in comparison to new substances not on the NDSL. When known, the Confidential Substance Identity Number should be used to identify the substance for notification purposes.
2.2.3 Amendments to the Non-domestic Substances List
The NDSL is amended on a regular basis for the following reasons:
- As a result of DSL amendments (substances are deleted from the NDSL when they are added to the DSL)
- For annual updates based on the United States Environmental Protection Agency’s (US EPA) Toxic Substances Control Act (TSCA) Chemical Substances Inventory
- Following nomination of a substance through submission of a Nomination Form – NDSL and
- To maintain the list, for example by correcting a typo in a substance identifier
Amendments to the NDSL are published in the Canada Gazette, Part I, approximately every 6 to 8 weeks. There are no statutory timelines for NDSL amendments, including for annual updates or following nomination of a substance.
2.2.3.1 Updates based on the US EPA’s TSCA chemical substances inventory
The NDSL is amended annually to add substances that have been on the public portion of the US EPA’s TSCA Inventory for a minimum period of one year. Substances on the confidential portion of the US EPA’s TSCA Inventory are not automatically added to the NDSL in the annual update process but can be nominated for addition (consult section 2.2.3.2).
Certain substances on the TSCA Inventory are not added to the NDSL. This includes substances subject to risk management controls in Canada or the U.S. and substances subject to the Stockholm Convention on Persistent Organic Pollutants or the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade. When it is determined that a substance on the NDSL is of potential concerns (for example when it is subject to such risk management or controls), this substance may be deleted from the NDSL, following public consultation.
2.2.3.2 Nominating a substance to the Non-domestic Substances List
Any substance that has been on the US EPA’s TSCA Inventory for at least one year can be nominated for addition to the NDSL. This includes substances on the confidential portion of the US EPA’s TSCA Inventory. To nominate a substance, a Non-domestic Substances List Nomination Form and applicable documentation demonstrating that the substance has been on the US EPA’s TSCA Inventory for at least one year must be provided to the NS program. These substances can be added to the NDSL publicly or confidentially. When deemed eligible, nominated substances are bundled with the annual updates to the NDSL.
To nominate a substance for confidential listing, the nomination form must include a proposed masked name and applicable fees for a masked name request (consult the fee table on the New substances notification fees webpage). The person requesting a confidential listing also needs to provide a justification for the confidential claim (consult section 2.1.2.1). If no masked name is provided, the substance is added to the NDSL under its CAS Registry Number.
Further instructions for nominating substances to the NDSL can be found on the Non-domestic Substances List Nomination Form available on the NS program website or by contacting the Substances Management Information Line.
2.3 Determining the presence of substances on inventories
To find out whether a substance is on the DSL or on the NDSL, the substance name, the CAS Registry Number, the Confidential Substance Identity Number (if available) or the Enzyme Commission (International Union of Biochemistry and Molecular Biology (IUBMB)) number can be entered into the Substances search engine.
If the Confidential Substance Identity Number is unknown and the notifier wishes to determine whether the substance is on the confidential portion of either the DSL or NDSL, a confidential search request (consult section 2.3.1) must be filed to the NS program. The CAS Registry Number or Confidential Substance Identity Number can also be provided directly to the CAS, which will, for a fee, search all inventories for that substance (for more information about the CAS, consult Appendix 4).
It is important to note that the search engine does not show any flags. The onus is on the notifier to consult the Canada Gazette publication to ensure that there are no flags associated with a particular substance. (For more information about the flags, consult section 2.1.4).
2.3.1 Confidential search request
Substances on the confidential portion of either the DSL or NDSL are published with Confidential Substance Identity Numbers using masked identities that are named in a manner prescribed by the Masked Name Regulations. Any person who intends to manufacture, import or use a substance may seek confirmation that the substance is on the confidential portion of either of these lists from the NS program by providing a Confidential Search Request Form available on the NS program website. Further instructions can be found on the form or by contacting the Substances Management Information Line.
If a notifier who wants to import a substance is unable to supply all of the required information because a Third Party Information Supplier considers this information confidential, the notifier is required to ensure that the Third Party Information Supplier submits the confidential information directly to the NS program.
After the notifier has provided a confidential search request, the NS program will search substances on the confidential portion of the DSL and NDSL, and will indicate, within 15 days of receipt of the complete documentation, whether the substance is on either of the lists. Note that there are fees associated with a confidential search request (consult the fee table on the New substances notification fees webpage).
2.3.2 Copies of the Domestic Substances List and Non-domestic Substances List
The DSL and the NDSL are available to view online or export to Excel (xlsx) using the Substances search engine. Chemicals, biochemicals, polymers and biopolymers are listed by their respective CAS Registry Number, while biochemicals that are enzymes are listed by CAS Registry Number or Enzyme Commission numbers designated by the IUBMB. Confidential substances are listed under their respective Confidential Substance Identity Numbers and masked identities that are named in a manner prescribed by the Masked Name Regulations (consult section 7.2.2). These lists are amended several times per year and notifiers should consult them regularly (consult sections 2.1.3 and 2.2.3).
Section 3. Substances
3.1 Definition of “substance”
For the purposes of the New Substances Notification (NSN) regime, section 3 of the Canadian Environmental Protection Act, 1999 (the Act) defines a “substance” as:
- any distinguishable kind of organic or inorganic matter, whether animate or inanimate, and includes
- (a) any matter that is capable of being dispersed in the environment or of being transformed in the environment into matter that is capable of being so dispersed or that is capable of causing such transformations in the environment
- (b) any element or free radical
- (c) any combination of elements of a particular molecular identity that occurs in nature or as a result of a chemical reaction and
- (d) complex combinations of different molecules that originate in nature or are the result of chemical reactions but that could not practicably be formed by simply combining individual constituents
In some instances, materials derived from natural sources and complex reactions are considered single substances for notification purposes. These materials are commonly referred to as substances of Unknown or Variable composition, Complex reaction products or Biological materials (UVCBs) (consult section 3.3.1.3).
3.2 Substances not subject to notification
Substances referred to in sections 3.2.1 to 3.2.11 of this Guidance Document do not require notification under the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations).
3.2.1 Mixtures
According to subsection 3(1) of the Act,
any mixture that is a combination of substances and does not itself produce a substance that is different from the substances that were combined
is excluded from the definition of “substance” for the purpose of the new substances and Significant New Activity (SNAc) provisions of the Act and consequently, does not require notification.
Note: If any constituent of a mixture is a new substance, that constituent is subject to notification.
Note: Some mixtures derived from natural sources or complex reactions may be considered a single substance and may be subject to notification (for example UVCBs, consult section 3.3.1.3).
Mixtures such as those described below are examples of mixtures that are not subject to notification.
Hydrates
Hydrates of a substance or hydrated ions formed by association of a substance with water are considered to be a mixture of that substance and water. Therefore, if the anhydrous form is on the Domestic Substances List (DSL), hydrated forms are not notifiable substances. An example of an anhydrous substance that is on the DSL is carbonic acid, magnesium salt (1:1) (Chemical Abstracts Service (CAS) Registry Number 546-93-0); therefore, the hydrated form MgCO3·nH2O is not notifiable.
Note: Metallic hydroxides, often termed metal hydrates, do not contain water of hydration and are not considered hydrates for notification purposes. Such substances must be notified if not on the DSL. An example of a metal hydroxide is copper hydroxide, Cu(OH)2.
Homogeneous and heterogeneous alloys
Homogeneous and heterogeneous alloys are considered mixtures and should not be notified. Alloys that are solid or liquid mixtures of 2 or more metals or are mixtures of one or more metals with certain non-metallic elements (for example, certain carbon steels) are considered mixtures and are not notifiable. An example of a homogeneous alloy is copper, compound with zinc (CuZn); an example of a heterogeneous alloy is copper, compound with cobalt (CuCo).
Note: Intermetallic compounds of well defined stoichiometry are not considered alloys and should be notified. An example of an intermetallic compound is intermetallic tin (In–49Sn).
3.2.2 Manufactured items
According to subsection 3(1) of the Act,
any manufactured item that is formed into a specific physical shape or design during manufacture and has, for its final use, a function or functions dependent in whole or in part on its shape or design
is excluded from the definition of “substance” for the purposes of the new substances and SNAc provisions of the Act and consequently, does not require notification.
Shape describes the macrostructure (for example the physical 3-dimensional structure) of the final item. Examples of items whose end use depends on final manufactured shape are clothing, storage containers, furniture, tiles, electrical wire, etc.
Note: Solid substances formed into a particular shape to meet subsequent processing and manufacturing requirements rather than final use (for example metal ingots and polymer pellets) are not considered to meet this definition of manufactured item and are subject to notification. Fluids (for example gases, liquids, waxes, solutions and suspensions) and particles (for example dusts, powders, dispersions, granules, lumps, flakes and aggregates of unspecified size) are not considered manufactured items even if the usefulness of the product depends on the particle’s shape.
Design refers to the organization or arrangement of the solid components within the macrostructure (for example the weave of fabric and carpeting, layering of plywood or binding of paper fibers) that is not altered in any subsequent processing. For example, fabric retains its final physical design regardless of whether it is a bolt of cloth or an article of clothing, because the manufacture of the clothing does not alter the design (weave) of the cloth.
Substances that are not intentionally released from a manufactured item during normal use are considered an integral part of that item and are thus not subject to notification.
Note: If a substance is intended to be released from a manufactured item, the substance may be subject to notification. The release of a substance is considered to be intended if it occurs during use of the manufactured item and the release contributes to a function of the manufactured item. The transfer of substances from a manufactured item to storage vessels during maintenance is not considered a release that contributes to a function of the item.
| Example | Manufactured item (not subject to notification) | Substance intended to be released from the manufactured item (subject to notification) |
|---|---|---|
| 1 | Electric air freshener diffuser | Substances intended to be emitted from the air freshener diffuser, such as fragrances, solvents, etc. |
| 2 | Personal care wipes | Substances intended to be delivered by the wipes such as surfactants, fragrances, etc. |
| 3 | Deodorant/antiperspirant container/delivery device | Substances intended to be released by the deodorant/antiperspirant container/delivery device such as antimicrobials, chelating agents, propellants, fragrances, etc. |
| 4 | Writing instruments (for example, pens, dry-erase markers) | Substances intended to be released from the writing instrument (components of the ink) such as pigments, dyes, solubilizing agents, solvents, fragrances, etc. |
| 5 | Printer cartridge | Substances intended to be released from the cartridge (components of the ink or toner) such as antistatic agents, pigments, etc. |
| 6 | Dryer sheets | Substances intended to be released during use such as fragrances, antistatic substances, etc. |
| 7 | Pre-loaded syringe | Substances intended to be delivered by the syringe such as pharmaceutically active and non-active ingredients. |
| 8 | Lipstick container or dispenser | Substances intended to be delivered by the lipstick container or dispenser such as pigments, emollients, etc. |
| 9 | Motor vehicle | Substances intended to be released such as substances in windshield washer fluid. |
| Example | Manufactured item (not subject to notification) | Substance not intended to be released from the manufactured item (not subject to notification) |
|---|---|---|
| 1 | Electronic devices (for example, computer) | Substances such as flame retardants that are not intended to be released from the device’s casing (any release of such a substance would not contribute to a function of the item). |
| 2 | Textiles (for example, carpet, towels, clothing) | Substances such as stain repellents and dyes that are not intended to be released from the textile (any release of such a substance would not contribute to a function of the item). |
| 3 | Motor vehicle | Substances such as lubricants, antioxidants, etc. in crankcase oil that are not intended to be released from the motor vehicle (any release of such a substance would not contribute to a function of the item). The transfer of substances from a vehicle to storage vessels during maintenance, for example, oil changes, is not considered a release that contributes to a function of the item. |
3.2.3 Wastes
According to subsection 3(1) of the Act,
any animate matter that is, or any complex mixtures of different molecules that are, contained in effluents, emissions or wastes that result from any work, undertaking or activity
is excluded from the definition of “substance’’ for the purposes of the new substances and SNAc provisions of the Act and consequently, does not require notification.
Note: If a material described above is isolated and commercialized and the resulting substance is not on the DSL, it may be subject to notification under the Regulations.
3.2.4 Other Acts of Parliament
According to paragraph 81(6)(a) of the Act and subsection 3(1) of the Regulations, the Regulations and the SNAc provisions of the Act do not apply in respect of
a substance that is manufactured or imported for a use that is regulated under any other Act of Parliament that provides for notice to be given before the manufacture, import or sale of the substance and for an assessment of whether it is toxic or capable of becoming toxic
and consequently, a substance that is manufactured or imported for a use that is regulated under any act or regulation listed in Schedule 2 of the Act does not require notification.
Note: Substances excluded from the scope of the other Acts of Parliament or regulations listed in Schedule 2 of the Act may be subject to notification under the Regulations. This includes isolated reaction intermediates, feedstocks and other starting materials used in the manufacture of any new substance.
Notifiers of new substances intended for uses regulated under other Acts of Parliament or regulations should monitor federal government websites (Canadian Environmental Protection Act, 1999 Registry) and the Canada Gazette to determine whether the use for the substance remains under the jurisdiction of other Acts of Parliament or Regulations. For example, the Food and Drugs Act (F&DA) is not listed in Schedule 2 of the Canadian Environmental Protection Act, 1999; therefore, new substances intended for use in products regulated under the F&DA may be subject to notification under the Regulations.
Substances for which uses may be subject to more than one Act of Parliament or regulation must be in compliance with the requirements of those Acts of Parliament or regulations. For example, a substance used in a pesticide product that is regulated under the Pest Control Products Act may also have non-pesticidal applications that could be subject to the Act and the Regulations.
3.2.5 Transient reaction intermediates
According to paragraph 81(6)(b) of the Act, the Regulations and the SNAc provisions of the Act do not apply in respect of
transient reaction intermediates that are not isolated and are not likely to be released into the environment
and consequently, these do not require notification.
Transient reaction intermediates are substances produced within a sequence of chemical reactions between the starting materials and the end product and are:
- contained in a reaction vessel or a closed manufacturing system (including process holding tanks) located within a single building or single process area
- intended to be fully consumed in the course of the chemical reaction
- part of an uninterrupted manufacturing process (for example, at any one time, starting materials or intermediates within the reaction sequence are being processed, except in the event of an unscheduled shutdown) and
- not likely to be released into the environment during normal operations, and measures are in place to minimize releases during accidental breaches of the closed manufacturing system
Notifiers are advised to maintain technical data (process and environmental release information) to support claims that a substance is a transient reaction intermediate as described above.
3.2.6 Impurities
According to paragraph 81(6)(c) of the Act, the Regulations and the SNAc provisions of the Act do not apply in respect of
impurities, contaminants and partially unreacted materials, the formation of which is related to the preparation of a substance
and consequently, these do not require notification.
Impurities and contaminants are substances that are usually found in minimal concentrations in the starting materials or are the result of secondary reactions that occur during the manufacturing process. These substances and partially unreacted starting materials that are present in the final product are the direct result of the preparation, are not necessary to the end-use of the product, have not been intentionally added to the substance, and do not enhance the value of the substance.
3.2.7 Incidental reaction products
According to paragraph 81(6)(d) of the Act, the Regulations and the SNAc provisions of the Act do not apply in respect of
substances produced when a substance undergoes a chemical reaction that is incidental to the use to which the substance is put or that results from storage or from environmental factors
and consequently, these do not require notification.
Examples of incidental reaction products include substances formed from chemical reactions during:
- exposure to environmental factors such as air, moisture, microbial organisms and sunlight (substances produced from deliberate reactions with water may be subject to notification, for example, metal hydroxides formed from a metal oxide and water)
- storage (for example, partial polymerization of drying oils)
- the intended use of a substance or mixture containing it (for example, adhesives, paints, cleansers, combustion products from fuels, fuel additives and water softeners) and
- the blending of a formulation when there is no intention to produce new substances and any ensuing chemical reactions do not enhance the value of the formulation (for example, blending monomers to a precise ratio for customer convenience would not result in a notifiable substance even if some reactions occurred; however, intentional manufacture of a pre-polymer to satisfy a customer’s processing specifications would produce a notifiable substance)
3.2.8 Low-quantity exemptions
According to paragraph 81(6)(e) of the Act, the Regulations and the SNAc provisions of the Act do not apply in respect of
a substance that is manufactured, used or imported in a quantity that does not exceed the maximum quantity prescribed
and consequently, such a substance does not require notification.
The Regulations do not apply to substances manufactured or imported in a quantity that does not exceed the prescribed quantity (that is, trigger quantity). The specific quantities that trigger notification requirements under the Regulations can be found in Table 1-1 of this Guidance Document.
3.2.9 Substances carried through Canada
According to subsection 3(2) of the Regulations, these do not apply in respect of
a substance that is loaded on a carrier outside Canada and moved through Canada to a location outside Canada, whether or not there is a change of carrier during transit
and consequently, such a substance does not required notification under the Regulations.
Note: If a substance is brought into Canada and stored for subsequent distribution, the substance may be subject to notification.
3.2.10 Polymers on the Domestic Substances List modified by less than or equal to 2% by weight
A polymer on the DSL that is modified by adding reactants, none of which constitutes more than 2% by weight of the polymer, does not require the explicit substance name to be changed and is therefore not subject to notification. The term “modified” refers to the amount of additional reactant that has been incorporated into the structure of the polymer or the amount charged to the vessel.
Note: The explicit substance name and CAS Registry Number identify a specific substance, and therefore a name or CAS Registry Number change may result in the substance being subject to notification.
For biopolymers, monomer units and reactants are considered to be the repeating units within the polymeric substance, which are produced in situ by the micro-organism or are added to the reaction vessel.
3.2.11 Substances occurring in nature
The New Substances (NS) program considers that substances occurring in nature are not subject to notification. These substances are defined as naturally occurring and must be:
- unprocessed
- processed only by manual, mechanical or gravitational means, by dissolution in water, by flotation, or by heating solely to remove water or
- extracted from air by any means
The criteria for substances occurring in nature limits inclusion only to those substances derived from nature (including the land, water, atmosphere and life forms which naturally inhabit the Earth) by means specified. The interpretation of the criteria is literal and strict. For example, distillation is not considered a mechanical process, and dissolution in solvents other than water does not fall within this definition.
3.3 Substances subject to notification
Notification is required pursuant to section 81 of the Act in relation to the following:
- Substances new to Canada (that is, those not on the DSL) that are manufactured in Canada or imported into Canada and
- Substances used to undertake a significant new activity (consult section 9.6)
3.3.1 Classification of substances
For the purposes of the Regulations, new substances are grouped into 2 major classes, each subject to its own specific information requirements. These classes are non-polymeric substances (referred to in this Guidance Document as chemicals and biochemicals) and polymeric substances (referred to in this Guidance Document as polymers and biopolymers). This document describes the notification requirements and processes for chemicals, biochemicals, polymers and biopolymers (including UVCB substances and nanomaterials).
3.3.1.1 Chemicals and biochemicals
The information requirements for chemicals and biochemicals are prescribed in the Regulations and apply to all substances subject to notification that are neither polymers nor living organisms. The term “biochemical”, means a chemical that is produced by a micro-organism, or means a protein or a nucleic acid derived from a plant or an animal (refer to subsection 1(1) of the Regulations). Note that chemicals derived from a whole plant or animal or from parts of a whole plant or animal are not biochemicals for the purpose of the Regulations. An example of a biochemical is the enzyme subtilisin produced by Bacillus subtilis.
Note: The production organism of a biochemical may be subject to the New Substances Notification Regulations (Organisms) if it meets the definition of a living organism set out in section 104 of the Act and is not on the DSL.
3.3.1.2 Polymers and biopolymers
Polymers are defined in subsection 1(1) of the Regulations as substances that consist of:
- (a) molecules characterized by the sequence of one or more types of monomer units
- (b) greater than 50% by weight of molecules having three or more monomer units that are covalently bound to one or more other monomer units or reactants
- (c) less than 50% by weight of molecules of the same molecular weight and
- (d) molecules distributed over a range of molecular weights whose differences in molecular weights are primarily attributable to differences in the number of monomer units
The term “biopolymer” means a polymer that is produced by a micro-organism, or means a protein or a nucleic acid derived from a plant or an animal (refer to subsection 1(1) of the Regulations). Note that polymers derived from a whole plant or animal or from parts of a whole plant or animal are not biopolymers for the purpose of the Regulations and must be notified as polymers. For biopolymers, monomer units and reactants are considered to be the repeating units within the polymeric substance, which are either produced in situ by the micro-organism or added to the reaction vessel. An example of a biopolymer is the polysaccharide xanthan gum, produced by Xanthomonas camprestris.
Note: The production organism of a biopolymer may be subject to the New Substances Notification Regulations (Organisms) if it meets the definition of a living organism set out in section 104 of the Act and is not on the DSL.
3.3.1.3 Substances of Unknown or Variable composition, Complex reaction products or Biological materials
Generally, UVCBs have the following characteristicsFootnote 9:
- They contain numerous chemicals and cannot be represented by a simple chemical structure or defined by a specific molecular formula
- They are not intentional mixtures of chemicals
- Many are of natural origin (for example, crude oil, coal, plant extracts, reaction products) and cannot be separated into their constituent chemical species
- The concept of “impurities” typically does not apply to complex substances or
- They are often produced according to a performance specification related to their physico-chemical properties
UVCBs are considered single substances for notification purposes (consult section A3.2 for examples). Unless the substance meets the polymer definition, information requirements for chemicals apply.
Note: For greater certainty, when a substance meets the polymer definition, the information requirements for polymers apply.
3.3.1.4 Nano-scale substances (referred to as nanomaterials)
The NS program is currently using the Health Canada Working Definition of Nanomaterials as the basis for identifying nanomaterials. Based on this definition, a substance is considered to be a nanomaterial if it is a manufactured substance and:
- it is at or within the nanoscale in at least one external dimension, or has internal or surface structure at the nanoscale, or
- it is smaller or larger than the nanoscale in all dimensions and exhibits one or more nanoscale properties/phenomena
For the purposes of this definition:
- the term "nanoscale" means 1 to 100 nanometres, inclusive
- the term "nanoscale properties/phenomena" means properties which are attributable to size and their effects; these properties are distinguishable from the chemical or physical properties of individual atoms, individual molecules and bulk material and
- the term "manufactured" includes engineering processes and control of matter that lead to the synthesis, generation, fabrication or isolation of nanomaterials. Health Canada may request information regarding a deliberately or incidentally manufactured nanomaterial for risk assessment purposes. This term also includes natural components that have been deliberately used or engineered to have nanoscale properties/phenomena used in nanoscale encapsulation of bioactive compounds, or used in tissue engineering
More details on these terms can be found in the Health Canada Working Definition.
In order to provide greater regulatory clarity, the NS program will evaluate a substance as a nanomaterial if it meets the criteria described in the Working Definition of Nanomaterials and has 10% or more of its primary particle distribution by number in the nanoscale range (1 to 100 nanometres, inclusive). The 10% by number threshold is consistent with reporting requirements used in a 2015 information-gathering survey for nanomaterials.Footnote 10 Alternatively, if a particle size distribution by number is not available, the NS program will evaluate a substance as a nanomaterial if it meets the criteria described in the Working Definition of Nanomaterials and at least 1% (by mass) of the particles are in the nanoscale range. The use of 1% (by mass) as particle size distribution threshold is in line with the United States Environmental Protection Agency’s (US EPA) final rule for Toxic Substances Control Act (TSCA) reporting and recordkeeping requirements for nanoscale materials.Footnote 11
In order to better account for the presence of smaller nanoscale particles, it is also recommended that the particle size distribution be measured by number count, rather than by mass or volume. For size characterization of nanomaterials, using a single method may not accurately represent the overall size distribution. Therefore, using a combination of measurement methods to determine the overall particle size distribution is recommended (consult Appendix 10).
The DSL is the sole basis for determining whether a substance is new for the purposes of the Act. As such, substances made at the nanoscale that are not on the DSL are considered to be new to Canada and are subject to notification under the Regulations, the same as other non-nanoscale substances not on the DSL.
Substances on the DSL that can be made at the nanoscale are not subject to notification under the Regulations. However, these substances are addressed under the Approach to nanoscale forms of substances on the DSL.
For additional details on nanomaterials, consult Appendix 10.
3.3.1.5 Reduced Regulatory Requirement polymers
Reduced Regulatory Requirement (RRR) polymers are polymers that meet specific criteria concerning molecular weight, oligomer content, elemental composition, stability and relative amounts of cationic and reactive functional groups. These criteria are listed in paragraph 9(a) to (c) as well as in items 1 to 5 of Schedule 7 of the Regulations (consult Appendix 2). RRR polymers are subject to the notification requirements set out in the Regulations; however, they are subject to fewer information requirements in comparison to Non-Reduced Regulatory Requirement (non-RRR) polymers (consult section 4.8). The letter ‘’P’’ after the identifier of a substance on the DSL (consult section 2.1.4.1) indicates that the substance was assessed and added on the basis that it met the RRR polymer criteria. This letter also indicates that a non-RRR form of the P-flagged polymer is subject to the appropriate non-RRR NSN Schedule if it is manufactured or imported into Canada (consult section 2.1.4.1).
Section 9 of the Regulations describes RRR polymers as one of the following:
- (a) a polymer that is not one of the types listed in items 1 to 4 of Schedule 7 of the Regulations (consult section 3.3.1.7) and that has a number average molecular weight greater than 10 000 daltons, with less than 2% of its components having molecular weights less than 500 daltons and less than 5% of its components having molecular weights less than 1 000 daltons
- (b) a polymer that is not one of the types listed in Schedule 7 of the Regulations (consult section 3.3.1.7) and that has a number average molecular weight greater than 1 000 daltons and equal to or less than 10 000 daltons, with less than 10% of its components having molecular weights less than 500 daltons and less than 25% of its components having molecular weights less than 1 000 daltons or
- (c) a polymer that is a polyester manufactured solely from reactants listed in Schedule 8 of the Regulations (consult Appendix 2) or an anhydrous form of those reactants, other than the reactants or their anhydrous forms that include both 1-butanol and fumaric or maleic acid
Note: Paragraph 9(c) of the Regulations does not reference Schedule 7 and does not include restrictions on molecular weight. A polymer meeting the criterion under this paragraph is considered RRR, irrespective of stability and molecular weight.
3.3.1.6 Non-Reduced Regulatory Requirement polymers
Polymers that do not meet the criteria under section 9 of the Regulations are referred to as non-RRR polymers. Non-RRR polymers require additional notification requirements at higher manufacture or import quantities (consult Table 1-1). Non-RRR polymers are added to the DSL once all criteria for addition are met (consult section 4.9).
3.3.1.7 Polymers described in Schedule 7 of the Regulations
Schedule 7 of the Regulations outlines criteria used to determine whether a polymer is considered RRR under paragraphs 9(a) and (b) of the Regulations, based on the amounts of cationic groups, stability, elemental composition and amounts of reactive functional groups. Schedule 7 includes 5 items; items 1 to 4 apply under paragraph 9(a) and items 1 to 5 apply under paragraph 9(b). For the purpose of paragraphs 9(a) and 9(b) of the Regulations, a polymer described in Schedule 7 is considered non-RRR.
According to item 1, a polymer that is reasonably expected to become cationic in a natural aquatic environment is considered non-RRR. However, a polymer that is potentially cationic could be considered RRR if it meets the criterion under either paragraph 1(a) or (b). To determine the applicability of paragraph 1(a), the functional group equivalent weight (FGEW) of cationic groups must be calculated (consult section 3.3.1.8). To determine the applicability of paragraph 1(b), no FGEW calculation is required.
Item 2 describes a polymer that is considered non-RRR based on stability (consult section 3.3.1.9).
Items 3 and 4 describe a polymer that is considered non-RRR based on elemental composition.
Item 5 is only applicable under paragraph 9(b). It describes a polymer that is considered non-RRR based on combined FGEW of specific reactive functional groups:
- A polymer that has a combined FGEW less than 5 000 daltons for reactive functional groups other than those listed in paragraph 5(a) is considered a non-RRR polymer and
- A polymer that has a combined FGEW less than 1 000 daltons for the functional groups listed in paragraph 5(b) is also considered a non-RRR polymer
The higher FGEW threshold in paragraph 5(a) indicates higher concerns associated with the functional groups referred to in this paragraph.
Methods for calculating the FGEW of cationic or reactive functional groups for different functional group distributions are presented in section 3.3.1.8.
Figure 3-1 below presents a decision tree for determining whether a polymer is considered an RRR polymer or a non-RRR polymer.

Long description
Polymers are considered RRR or non-RRR based on criteria listed in Section 9 and Schedule 7 of the Regulations. Figure 3-1 illustrates these criteria in a decision tree with Yes/No questions that are grouped in 3 step-wise categories (Monomer/reactant criteria, Molecular weight criteria, Schedule 7 criteria) to determine whether a polymer is considered RRR or non-RRR:
- Monomer/reactant criteria:
- Is the polymer a polyester manufactured solely from monomers and reactants (including anhydrous forms) listed in Schedule 8 and that does not contain either butanol or fumaric/maleic acid?
- If Yes, polymer is considered RRR.
- If No, proceed to category 2 questions.
- Is the polymer a polyester manufactured solely from monomers and reactants (including anhydrous forms) listed in Schedule 8 and that does not contain either butanol or fumaric/maleic acid?
- Molecular weight criteria:
- Does the polymer have a number average molecular weight (Mn) < 1 000 daltons?
- If Yes, polymer is considered non-RRR.
- If No, proceed to next question 2b.
- Does the polymer have a number average molecular weight 1 000 < Mn ≤ 10 000 daltons, with less than 10% of its components having molecular weights < 500 daltons and less than 25% of its components having molecular weights < 1 000 daltons?
- If Yes, proceed to category 3 question a.
- If No, proceed to next question 2c.
- Does the polymer have a Mn > 10 000 daltons, with less than 2% of its components having molecular weights < 500 daltons and less than 5% of its components having molecular weights < 1 000 daltons?
- If Yes, proceed to category 3 question b.
- If No, polymer is considered non-RRR.
- Does the polymer have a number average molecular weight (Mn) < 1 000 daltons?
- Schedule 7 criteria:
- Is the polymer one of the types listed in item 5 of Schedule 7?
- If Yes, polymer is considered non-RRR.
- If No, proceed to next question 3b.
- Is the polymer one of the types listed in items 1 to 4 of Schedule 7?
- If Yes, polymer is considered non-RRR.
- If No, polymer is considered RRR.
- Is the polymer one of the types listed in item 5 of Schedule 7?
3.3.1.8 The functional group equivalent weight
The FGEW is the theoretical molecular weight of the polymer that contains one equivalent weight (one mole) of a particular functional group. There is an inverse relationship between the FGEW value for a particular functional group and the expected number of occurrences of that functional group in the polymer. For example, a FGEW of 700 daltons means there is on average one functional group in every 700 daltons of the polymer, while a FGEW of 4 500 daltons means there is on average one functional group in every 4 500 daltons of the polymer. Consequently, a higher FGEW value is associated with a lower potential level of concern.
Functional groups can be distributed throughout the polymer or located in terminal positions. To calculate the FGEW for any reactive functional group of concern or cationic group, it is essential to know how the functional groups are distributed within the polymer, and in the case of terminal positioning, whether the polymer is linear or branched.
| Eq. # | Calculation description | Equation |
|---|---|---|
| 1 | FGEW of functional group distributed throughout the polymer | |
| 2 | FGEW of functional group in end-group (=terminal) position, linear polymers | |
| 3 | FGEW of functional group in end-group (=terminal) position, branched polymers | |
| 4 | Combined FGEW calculation | |
| 5 | FGEW derived from amine number | |
| 6 |
# moles of a functional group (# moles of functional group / 100 g polymer) |
List of abbreviations for the equations to calculate the FGEW:
- FGEW: functional group equivalent weight (daltons)
- wt%: weight percent
- nFG: number of available functional groups, where available means functional groups that must be considered in the FGEW calculation
- Mn: number average molecular weight (daltons)
- mw: molecular weight (daltons)
- nEG: number of end-groups
- nRS: number of reactive sites
- xamine: amine number, mg KOH/g polymer and
- mw KOH: molecular weight of KOH = 56.1 g/mol
List of indices for the equations to calculate the FGEW:
- mon: monomer
- BA: branching agent
- FGEG: functional group in end group position
- FGEG_LP: FGEG linear polymer
- FGEG_BP: FGEG branched polymer
- comb.: combined
- FGEWn n=1,2,3…: individual FGEW calculations and
- Amine: cationic amine
Equation 1 takes into account how much reactant is charged to the reactor and assumes that the reactants are completely incorporated into the polymer during the reaction. This equation should be used whenever the incorporation of the reactant would always result in its reactive functional groups being available: that is the availability of the reactive functional groups is not affected by the position of the reactant. This equation can also be used to check the results of other equations (consult examples 1, 2 and 3).
Functional groups that are consumed and incorporated into the polymer backbone are not considered in the FGEW calculation. When a functional group is incorporated into the backbone but still appears in the terminal position of a polymer, the FGEW should be calculated using equation 2 or 3. If equation 1 were used when the functional groups were only available in the terminal positions, the resulting FGEW would be artificially low and would overestimate the concern since this equation does not account for functional groups that become unavailable after being incorporated into the backbone of the polymer. Equations 2 and 3 account for polymer reactants whose functional groups are consumed during polymerization and whose only remaining available functional groups are found at the terminal positions of linear or branched polymers, respectively. Note that this calculation assumes that all terminal positions are occupied by the reactant carrying the functional group of concern. Additional equations accounting for the molar ratios of the other functional groups of the polymer reactants and the order of addition can also improve accuracy of the FGEW calculation, however this exceeds the scope of the materials presented in this guidance.
Example 1
An acrylic polymer has a Mn of 2 745 daltons, 21 wt.% below 1 000 daltons and 7 wt.% below 500 daltons.
The acrylic backbone has randomly distributed pendant aliphatic amines derived solely from its content of 2% by weight 2-propenoic acid, 2-aminoethyl ester (CAS Registry Number 7659-38-3, C5H9NO2, mw = 115 daltons).
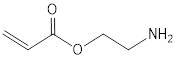 C5H9NO2, mw = 115 daltons
C5H9NO2, mw = 115 daltons
Note: the acrylic functional group of this reactant will react into the backbone of the polymer; only the cationic primary amine needs to be considered.
The FGEWamine is calculated using equation 1: FGEW of functional group distributed throughout the polymer.
The polymer meets the exception criterion under paragraph 1(a) of Schedule 7 (the combined FGEW of cationic groups must be greater than 5 000 daltons), and therefore, this polymer is not one of the types listed in item 1 of Schedule 7. This polymer is considered RRR.
Example 2
A reaction product of 2-propenoic acid (CAS Registry Number 79-10-7) and ethenol, homopolymer (CAS Registry Number 9002-89-5) has a Mn of 8 500 daltons, 17 wt.% below 1 000 daltons and 4.7 wt.% below 500 daltons.
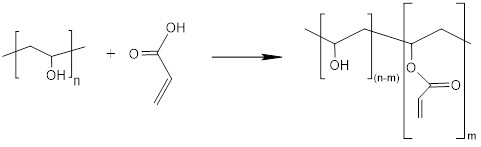
The carboxylic acid group of 2-propenoic acid (mw = 72 daltons) reacts with the pendant alcohol groups of the pre-polymer; the resulting polymer therefore contains randomly distributed pendant acrylates.
Based on the description provided above (Mn and reactants), this polymer does not meet the definition of neither paragraph 9(a) nor (c) of the Regulations. According to paragraph 9(b), it must be determined whether the polymer is described in Schedule 7, including in item 5.
Pendant acrylates are reactive functional groups that are not listed in paragraph 5(a) of Schedule 7; correspondingly the FGEW threshold of 5 000 daltons applies to this functional group.
The polymer contains 5.5 wt.% of the reactant 2-propenoic acid, which has a molecular weight of 72 daltons.
The FGEW of the pendant acrylate functional group is also calculated according to equation 1:
The FGEW for the pendant acrylate group is less than 5 000 daltons; the polymer is therefore described in paragraph 5(a) of Schedule 7. This polymer is considered non-RRR.
Note: A polymer with the same composition and meeting the Mn and oligomer content requirements of paragraph 9(a) would be considered RRR. A FGEW calculation would not be required.
Example 3
Note: The following example illustrates that even when the functional group of concern is located in terminal position only, calculating the FGEW via endgroup analysis might still be inappropriate.
The example polymer has a Mn of 6 800 daltons, 7 wt.% of components of molecular weight below 1 000 daltons and 3 wt.% of components of molecular weight below 500 daltons.
The polymer consists of 91.6 wt.% of a pre-polymer of hexanedioic acid, polymer with 1,6-hexanediol (CAS Registry Number 25212-06-0) that is reacted with 8.4 wt.% pentaerythritol triacrylate (CAS Registry Number 3524-68-3).
CAS Registry Number 25212-06-0 consists of CAS Registry Number 629-11-8 and 124-04-9.
Hexanediol, mw = 118 daltons
CAS Registry Number 629-11-8
Hexanedioic, mw = 146 daltons
CAS Registry Number 124-04-9
Pentaerythritol triacrylate, mw = 298 daltons, CAS Registry Number 3524-68-3
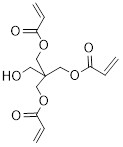
Pentaerythritol triacrylate can only react with the pre-polymer via its alcohol group; the 3 acrylate functional groups will remain pendant.
Based on the description provided above (Mn and reactants), this polymer does not meet the definition of neither paragraph 9(a) nor (c) of the Regulations. According to paragraph 9(b), it must be determined whether the polymer is described in Schedule 7, including in item 5.
Pendant acrylates are reactive functional groups that are not listed in paragraph 5(a) of Schedule 7; correspondingly the FGEW threshold of 5 000 daltons applies to this functional group.
The pre-polymer is linear and its only available functional groups are in terminal positions. The alcohol group of pentaerythritol triacrylate can only react with hexanedioic acid, that is with those end-positions that are terminated by hexanedioic acid.
If equal molar amounts of hexanedioic acid and 1,6-hexanediol are reacted, the end-positions of the resulting polymer will ideally be equally terminated by acid and alcohol groups.
![OC(CCCCC(OCCCCCCO[H])=O)=O polymer](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG6.jpg)
This would be the case if the polymer consisted of 55.3 wt.% hexanedioic acid and 44.7 wt.% of 1,6-hexanediol, thus reacting equal molar amounts of each reactant. Equation 6 is used for the comparison of molar amounts/100g:
In case of sufficient excessFootnote 12 of hexanedioic acid, the resulting polymer will only contain terminal acid groups.
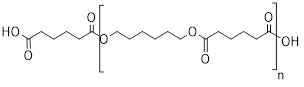
Whether hexanedioic acid is present in one or both end-positions will determine whether pentaerythritol triacrylate can react with one (A) or both (B) end-positions.
(A) ![O=C(OCC(CO)(COC(=O)C=C)COC(=O)C=C)C=C reacting with one end-position of OC(CCCCC(OCCCCCCO[H])=O)=O polymer](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG8.jpg)
(B) ![O=C(OCC(CO)(COC(=O)C=C)COC(=O)C=C)C=C reacting with both end-position of OC(CCCCC(OCCCCCCO[H])=O)=O polymer](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG9.jpg)
The FGEWacrylate could therefore be calculated using equation 2: FGEW of functional group in end-group (=terminal) position, linear polymers.
For (A), the calculation would be
For (B), the calculation would be
Neither of these 2 polymers (A) or (B) would therefore be considered RRR, as the polymer would be considered as one of the polymer types described in paragraph 5(a).
Important
This calculation assumes that the final polymer is intended to have as many terminal acrylate groups as supported by the composition of the pre-polymer, that is enough pentaerythritol triacrylate is added to cover all available end-positions.
To double-check that this is indeed the case, equation 1 has to be used, as this equation takes the amount of reactant into consideration.
Calculating the FGEW via this equation would have provided the answer to whether the polymer is considered RRR or not immediately.
Considering the elaborate calculations above, the FGEWacrylate calculated based on this equation indicates that sufficient acrylate is charged to have all available end-groups of case (B) covered by this reactant. If the polymer was actually version (A), charging this amount of acrylate would be in excess (often noticeable as low molecular weight peak of unreacted monomer in the Gel Permeation Chromatography (GPC)).
Example 4
A linear polyurethane polymer has a Mn of 100 000 daltons and 0% of components of molecular weight below 1 000 daltons. Its isocyanate-containing reactant is aliphatic and therefore considered cationic after hydrolysis. Since the functional group reacts into urethane bonds within the polymer chain and hydrolyses to a cationic amine in terminal position only, the FGEW has to be assessed via the analysis of available endgroups. Assuming all terminal positions of the polymer are occupied by aliphatic isocyanate groups, the FGEWFGEG is therefore calculated according to equation 2:
The FGEW for the cationic amine (that is after hydrolysis of the aliphatic isocyanate) is greater than the FGEW threshold for cationic groups of 5 000 daltons; therefore this polymer meets the exception criterion under paragraph 1(a) of Schedule 7. This polymer is not one of the types listed in item 1 of Schedule 7. This polymer is considered RRR.
Note: An aromatic isocyanate would not have required calculation since aromatic isocyanates do not become cationic and the polymer would have met the requirements under paragraph 9(a) (item 5 of Schedule 7 is only applicable under paragraph 9(b)).
Example 5

A branched polyurethane polymer of 9 600 daltons contains isocyanate groups at chain ends from an aromatic diisocyanate. The branching agent 1,3-propanediol, 2-ethyl-2-(hydroxymethyl) (CAS Registry Number 77-99-6) has a molecular weight of 134 daltons and accounts for 1 wt.% of the polymer; it has 3 reactive sites.
The FGEW for the functional group in end group position of a branched polymer has to be calculated according to equation 3:
With a FGEW for the isocyanates of 3 534, the polymer is described by paragraph 5(a) of Schedule 7 and therefore is not considered RRR.
Example 6
![NCOC(C(C[R])CC(C[R])C(OCN(C)C)=O)=O polymer](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG12.jpg)
An acrylic polymer contains aliphatic amines from 1 wt.% of 2-propenoic acid, 2-aminoethyl ester (CAS Registry Number 7659-38-3, molecular weight = 115 daltons) and 2 wt.% of (dimethylamino)ethyl methacrylate (CAS Registry Number 2867-47-2, molecular weight = 157 daltons). The FGEW for each of the cationic groups is calculated using equation 1:
The combined FGEW is then calculated using equation 4:
With a combined FGEW for cationic groups of less than 5 000 daltons the polymer is described by item 1 of Schedule 7 and is therefore not considered RRR.
Example 7
For many complex polymers (for example polymers containing pre-polymers), it is impossible to calculate the FGEW for a specific functional group of concern without requiring additional information; for example, the pre-polymer’s composition or Mn or both.
In the case of cationic functional groups, an empirically determined amine number provides the most accurate information on the actual cationic charge present. For example for an amine number of 7.5 mg KOH/g polymer, the calculation according to equation 5 would be:
Turning this equation around, any amine number above 11.22 indicates that the polymer is mentioned under item 1 of Schedule 7 and therefore is considered non-RRR:
3.3.1.9 Polymers that substantially degrade, decompose or depolymerize
One of the criteria for determining whether a polymer qualifies as an RRR polymer is whether it is designed or expected to “substantially degrade, decompose or depolymerize.” This criterion is set out in Schedule 7, item 2 of the Regulations. If the substance meets this or other criteria set out in Schedule 7, it would not qualify as an RRR polymer.
In interpreting this criterion, the NS program will consider both the extent of degradation, decomposition or depolymerization and the hazard of the transformation products. If the transformation products of a polymer are of low hazard, it may qualify as an RRR polymer, despite degrading, decomposing or depolymerizing, depending on whether other criteria are met.
When a notifier claims that a polymer is to be considered RRR, they should provide information to support the assessment of whether the polymer is substantially degradable. In the absence of such information, the NS program will use its best judgement in making the determination. To support the claim, notifiers should provide information about whether the polymer is degradable, including whether the polymer is a member of a class of polymers known to be readily transformed (for example, polysaccharides or certain bio-based polyesters). In cases where it is anticipated that the polymer will degrade, or where there is empirical evidence that it will degrade, the notifier should identify the known or anticipated transformation products and provide information concerning their hazard so that the program can assess the RRR claim.
Since experimental information may not be available, predictive programs may be used to address the stability of the polymer, the formation of stable transformation products and their hazard characteristics. Other methods such as obtaining data from the literature or using “read-across” techniques may be acceptable.
As with all information and claims provided in a notification, the NS program will assess their merits in determining whether this criterion is met.
3.4 Special category substances
A special category substance is defined as any substance that is manufactured or imported as:
- (a) a research and development substance
- (b) a contained site-limited intermediate substance or
- (c) a contained export-only substance
3.4.1 Research and development substances
Subsection 1(1) of the Regulations defines a research and development substance as one that is undergoing systematic investigation or research, by means of experimentation or analysis other than test marketing, whose primary objective is any of the following:
- (a) to create or improve a product or process
- (b) to determine the technical viability or performance characteristics of a product or process or
- (c) to evaluate a substance prior to its commercialization, by pilot plant trials, production trials (including scale-up) or customer plant trials, so that technical specifications can be modified in response to the performance requirements of potential customers
This category includes chemicals or polymers manufactured by a toll manufacturer for domestic or foreign customers that are conducting research (consult section 1.4.3).
The Regulations also define “Test marketing,” in respect of a product as referred to above, as “the exploration of its market capability in a competitive situation where the creation or improvement of the product is not the primary objective.”
3.4.2 Contained site-limited intermediates
Subsection 1(1) of the Regulations defines a site-limited intermediate as a substance that is consumed in a chemical reaction used for the manufacture of another substance and that is:
- (a) manufactured and consumed at the site of manufacture
- (b) manufactured at one site and transported to a second site where it is consumed or
- (c) imported and transported directly to the site where it is consumed
The Regulations also define:
- “contained” as “an absolute release limit of 1 kg per day per site of the substance to the aquatic environment after wastewater treatment” and
- “consumed” as “destroyed or completely converted to another substance”
If a substance is classified as a site-limited intermediate, it must, at all times during its existence (manufacture, importation, storage, transport, handling, use and disposal), be contained, as defined above, to prevent any significant environmental release.
A substance that is a direct precursor in the manufacture of an item defined in section 3.2.2 is not considered a site-limited intermediate and would be subject to the notification requirements. However, if the direct precursor of the item meets the criteria of a “transient reaction intermediate” (consult section 3.2.5), it would not be subject to notification.
3.4.3 Contained export-only substances
Contained export-only substances are limited to new substances manufactured in or imported into Canada that are destined solely for foreign markets and that are contained.
“Contained” is defined in subsection 1(1) of the Regulations as an absolute release limit of 1 kg/day per site of the substance to the aquatic environment after wastewater treatment.
Section 4. Notification information requirements
4.1 How to identify the required notification information
The New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) prescribe information requirements tailored to the use and quantity of the chemical or polymer being manufactured or imported. These requirements are listed in the Schedules of the Regulations, which are presented in Appendix 2 of this Guidance Document. To help select the appropriate Schedule, decision flowcharts are provided in this section and also in Appendix 1.
It is important to note that although the Regulations provide a tiered approach to notification, which links information requirements to factors such as quantity, use, intrinsic properties and class, it is not a requirement to follow this tiered notification approach. A notifier may, if they wish, opt to immediately submit the highest notification Schedule required, as long as the lowest prescribed quantities for the lowest Schedule are not exceeded and the New Substances Notification (NSN) is submitted within the timeframe prescribed for the highest Schedule.
As indicated in the decision diagrams presented below and in Appendix 1, there are a number of factors that must be considered when identifying the nature of the information to be submitted and when it should be submitted. These factors include:
- whether the new substance is a chemical or a polymer (consult section 3.3)
- whether the new substance falls within any of the prescribed special categories (that is, research and development, contained site-limited intermediate or contained export-only; consult section 3.4)
- whether the new substance is on the Non-domestic Substances List (NDSL) (consult section 2.2)
- the annual quantities of the new substance that will be manufactured in or imported into Canada (consult Table 1-1 and sections 4.2, 4.4, 4.5, 4.8 and 4.9)
- if the new substance is a polymer, whether it meets the definition of a Reduced Regulatory Requirement (RRR) polymer (consult section 3.3.1.5)
- if the new substance is a polymer, whether it is manufactured solely from monomers and reactants that are on the Domestic Substances List (DSL) or the NDSL (consult section 4.7.1) and
- whether the new substance will have high release to the aquatic environment or significant public exposure (consult sections 4.4.3 and 4.9.2)
4.1.1 Annual quantities
The Regulations prescribe a pre-manufacture/pre-import notification scheme. As such, the notifier must develop an accurate estimate of the annual (calendar year) quantities of the new substance to be manufactured in or imported into Canada and submit an NSN before each of the prescribed quantities is exceeded.
The prescribed quantities relate to the actual amount of new substance manufactured or imported, not to the quantity of the formulation containing the substance. For example, if 10 000 kg of Formulation A are to be imported during a calendar year and this formulation contains 13% of new substance X, then the annual import quantity of substance X would be 1 300 kg.
The following sections will help identify both the Schedule requirements necessary to comply with the Regulations and the date before which NSNs must be submitted to the New Substances (NS) program.
4.2 Notification of special category substances
Substances being manufactured or imported solely for activities defined under the special categories umbrella (consult section 3.4) must be notified as indicated below. If any amount of the substance is to be used for an activity not within the special category umbrella, the substance is subject to notification under the appropriate Schedules based on the quantity to be used in the non-special category activity (consult sections 4.3 and 4.7). These requirements are specified in the Schedules in Appendix 2 of this Guidance Document.
4.2.1 Lower-quantity special category notifications for chemicals (consult Figure 4-1)
Every notifier who manufactures or imports a chemical for research and development purposes, as a contained site-limited intermediate substance or as a contained export-only substance, must provide the Minister of the Environment (the Minister) with the information prescribed in Schedule 1 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
4.2.1.1 Lower-quantity research and development biochemicals
If the substance is a research and development biochemical, the notifier is required to provide, in addition to the Schedule 1 information noted above, the information specified in items 1 and 2 of Schedule 2 of the Regulations.
4.2.1.2 Lower-quantity contained site-limited intermediate biochemicals
If the substance is a contained site-limited intermediate biochemical that is not manufactured and consumed at the site of manufacture, the notifier is required to provide, in addition to the Schedule 1 information noted above, the information specified in items 1–4 of Schedule 2 of the Regulations and:
- (a) if the biochemical is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations and
- (b) if the biochemical possesses enzymatic capability, the information specified in items 7–13 of Schedule 2 of the Regulations
If the substance is a contained site-limited intermediate biochemical that is manufactured and consumed at the site of manufacture, the notifier is required to provide, in addition to the Schedule 1 information noted above, the information specified in items 1, 2 and 4 of Schedule 2 of the Regulations.
4.2.1.3 Lower-quantity contained export-only biochemicals
If the substance is a contained export-only biochemical, the notifier is required to provide, in addition to the Schedule 1 information noted above, the information specified in items 1–4 of Schedule 2 of the Regulations and:
- (a) if the biochemical is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations and
- (b) if the biochemical possesses enzymatic capability, the information specified in items 7–13 of Schedule 2 of the Regulations
4.2.2 Higher-quantity special category notifications for chemicals (consult Figure 4-1)
In addition to the Schedule 1 information (and Schedule 2 where applicable) noted above, the notifier must update all of the information that was previously provided at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year. If there is no change in the information, that must be indicated at this time.

Long description
Notifiers who manufacture or import a new chemical as referred to in section 5 of the Regulations or a new polymer as referred to in section 6 of the Regulation, that is for research and development purposes, as a contained site-limited intermediate substance or as a contained export-only substance, must provide the Minister with the appropriate Schedule of information prior to exceeding the applicable trigger quantity and within the prescribed timeframe for that Schedule. Figure 4-1 illustrates timelines and information to provide for these chemicals and polymers:
- For a Chemical/Biochemical (section 5 of the Regulations):
- Provide information prescribed in Schedule 11 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
- Update all of the information that was previously provided at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
- For a Polymer/Biopolymer (section 6 of the Regulations):
- Provide information prescribed in Schedule 32 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
1 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subsections 5(2), (3) and (4) of the Regulations).
2 Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult subsections 6(2), (3) and (4) of the Regulations).
4.2.3 Higher-quantity special category notifications for polymers (consult Figure 4-1)
Every notifier who manufactures or imports a polymer for research and development purposes, as a contained site-limited intermediate polymer or as a contained export-only polymer must provide the Minister with the information prescribed in Schedule 3 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
4.2.3.1 Higher-quantity research and development biopolymers
If the substance is a research and development biopolymer, the notifier is required to provide, in addition to the Schedule 3 information noted above, the information specified in items 1 and 2 of Schedule 2 of the Regulations.
4.2.3.2 Higher-quantity contained site-limited intermediate biopolymers
If the substance is a contained site-limited intermediate biopolymer that is not manufactured and consumed at the site of manufacture, the notifier is required to provide, in addition to the Schedule 3 information noted above, the information specified in items 1–4 of Schedule 2 of the Regulations and, if the biopolymer is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations.
If the substance is a contained site-limited intermediate biopolymer that is manufactured and consumed at the site of manufacture, the notifier is required to provide, in addition to the Schedule 3 information noted above, the information specified in items 1, 2 and 4 of Schedule 2 of the Regulations.
4.2.3.3 Higher-quantity contained export-only biopolymers
If the substance is a contained export-only biopolymer, the notifier is required to provide, in addition to the Schedule 3 information noted above, the information specified in items 1–4 of Schedule 2 of the Regulations and, if the biopolymer is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations.
4.3 Notification of chemicals
As indicated in section 4.1, the Regulations prescribe information requirements tailored to the use and quantity of the chemical. These requirements are specified in the Schedules in Appendix 2 of this Guidance Document. A decision flowchart is provided below (consult Figure 4-2) and in Appendix 1 of this Guidance Document to assist in the selection of the appropriate notification Schedule.
Before using the flowchart, Table 1-1 and sections 2.2, 3.3, 3.4, 4.2.1, 4.2.2, 4.4 and 4.5 of this Guidance Document should be reviewed to determine:
- whether the new substance meets the definition of a chemical given in the Regulations (consult section 3.3.1.1)
- whether the new chemical falls within any of the prescribed special categories (that is, research and development, contained site-limited intermediate or contained export-only; consult section 3.4)
- whether the new chemical is on the NDSL (consult section 2.2)
- the annual quantities of the new chemical that will be manufactured in or imported into Canada (consult Table 1-1 and sections 4.2 to 4.5) and
- whether the chemical on the NDSL will have high release to the aquatic environment or significant public exposure (consult section 4.4.3)
The following sections apply only to chemicals that are manufactured or imported for a purpose other than as a special category listed in section 3.4 of this Guidance Document.
4.4 Information requirements for chemicals on the Non-domestic Substances List (consult Figure 4-2)
4.4.1 Lower-quantity chemicals
Every notifier who manufactures or imports a chemical that is on the NDSL must provide the Minister with the information prescribed in Schedule 4 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
If the substance is a biochemical, the notifier is required to provide, in addition to the Schedule 4 information, the information specified in items 1–3 of Schedule 2 of the Regulations.
4.4.2 Higher-quantity chemicals
Every notifier who manufactures or imports a chemical that is on the NDSL must provide the Minister with the information prescribed in Schedule 5 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
If the substance is a biochemical, the notifier is required to provide, in addition to the Schedule 5 information, the information specified in items 1–4 of Schedule 2 of the Regulations and:
- (a) if the biochemical is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations and
- (b) if the biochemical possesses enzymatic capability, the information specified in items 7–13 of Schedule 2 of the Regulations
4.4.3 Chemicals on the Non-domestic Substances List with high release to the aquatic environment and/or significant public exposure
Every notifier who manufactures or imports a chemical that is on the NDSL and:
- that is released to the aquatic environment in a quantity exceeding 3 kg/day, per site, averaged monthly and after wastewater treatment and/or
- where the public may be significantly exposed to the chemical in a product
must provide the Minister with additional test information as prescribed in subsections 7(2) and/or 7(3) of the Regulations, respectively, at least 75 days prior to the day on which the quantity of substance manufactured or imported exceeds 50 000 kg in a calendar year. The additional required information is indicated in the following sections.
4.4.3.1 Chemicals released to the aquatic environment
As per paragraph 10(c) of Schedule 10 of the Regulations, it is the notifier’s responsibility to submit evidence in the NSN to support a claim of the substance not being released to the aquatic environment in the quantity indicated above. This information should include any envisioned future use and quantity by other customers and a description of other envisioned applications. To calculate daily release to the aquatic environment, refer to section 6.6.5 of this Guidance Document.
The NS program will assess this information and if it is determined that the substance is released to the aquatic environment in quantities greater than indicated above, the additional information prescribed in subsection 7(2) of the Regulations must be provided. The NS program’s determination of whether the substance is subject to additional information requirements given below will be provided to the notifier. The notifier may submit additional information to support their claim and request a re-evaluation of the decision made by the NS program by contacting the Substances Management Information Line. The NS program will review and consider the information submitted.
The additional information required, as prescribed in subsection 7(2) of the Regulations, must include the following:
- (a) for chemicals having a water solubility of greater than or equal to 200 µg/L:
- (i) adsorption–desorption screening test data and
- (ii) the hydrolysis rate as a function of pH and, if known, an identification of the products of the hydrolysis and
- (b) the data from a repeated-dose mammalian toxicity test of the chemical of at least 28 days duration, using the most significant route of potential public exposure to the chemical, namely, oral, dermal or inhalation, in addition to
- (i) the age, sex, number, species, strain and source of the animals tested
- (ii) the route by which the chemical is administered and the conditions under which the test is conducted and
- (iii) the dose of the chemical, the vehicle by means of which the chemical is administered and its concentration in that vehicle
4.4.3.2 Where the public may be significantly exposed to the chemical in a product
As per paragraph 10(d) of Schedule 10 of the Regulations, it is the notifier’s responsibility to submit information in the NSN to support a claim of the public not being significantly exposed to the substance in a product. The NS program’s determination of whether the substance is subject to the additional information requirements given below will be provided to the notifier. The notifier may submit additional information to support their claim and request a re-evaluation of the decision made by the NS program by contacting the Substances Management Information Line. The NS program will review and consider the information submitted.
Since public exposure is dependent on many factors, a single calculation to determine “significant exposure” cannot be applicable to all circumstances without being extremely conservative. Therefore, the definition of “significantly exposed” will be assessed, by the NS program, on a case-by-case basis. This information should take into consideration such factors as type of use, duration and frequency of use, concentration of the chemical in the product and circumstances of exposure that may limit direct public exposure (for example, whether the chemical is consumed during use or is able to migrate from the product).
Some examples of consumer applications where significant public exposure of a substance may occur include, but are not limited to, dishwashing detergent, laundry products, soaps, toilet paper, cleaning solutions, waxes, polishes, air fresheners, paints, oils, greases, body lotions, and ink.
If it is determined that the public may be significantly exposed to the chemical in a product, the additional information prescribed in subsection 7(3) of the Regulations must be provided and must include the following:
- (a) the data from a repeated-dose mammalian toxicity test of the chemical of at least 28 days duration, using the most significant route of potential public exposure to the chemical, namely, oral, dermal or inhalation, in addition to:
- (i) the age, sex, number, species, strain and source of the animals tested
- (ii) the route by which the chemical is administered and the conditions under which the test is conducted and
- (iii) the dose of the chemical, the vehicle by means of which the chemical is administered and its concentration in that vehicle and
- (b) the data obtained from an in vitro test, with and without metabolic activation, for chromosomal aberrations in mammalian cells or the data from a previously existing in vivo mammalian test for chromosomal aberrations that, together with data substantiating that the tissue investigated was exposed to the chemical or its metabolites, permits an assessment of in vivo clastogenicity
4.5 Information requirements for chemicals not on the Non-domestic Substances List (consult Figure 4-2)
4.5.1 Lower-quantity chemicals
Before exceeding 100 kg per year
Every notifier who manufactures or imports a chemical that is not on the NDSL must provide the Minister with the information prescribed in Schedule 4 of the Regulations at least 5 days prior to the day on which the quantity of substance manufactured or imported exceeds 100 kg in a calendar year.
If the substance is a biochemical, the notifier is required to provide, in addition to the Schedule 4 information, the information specified in items 1–3 of Schedule 2 of the Regulations.
Before exceeding 1 000 kg per year
Every notifier who manufactures or imports a chemical that is not on the NDSL must provide the Minister with the information prescribed in Schedule 5 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
If the substance is a biochemical, the notifier is required to provide, in addition to the Schedule 5 information, the information specified in items 1–4 of Schedule 2 of the Regulations and:
- (a) if the biochemical is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations and
- (b) if the biochemical possesses enzymatic capability, the information specified in items 7–13 of Schedule 2 of the Regulations
4.5.2 Higher-quantity chemicals
Every notifier who manufactures or imports a chemical that is not on the NDSL must provide the Minister with the information prescribed in Schedule 6 of the Regulations at least 75 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
If the substance is a biochemical, the notifier is required to provide, in addition to the Schedule 6 information, the information specified in items 1–4 of Schedule 2 of the Regulations and:
- (a) if the biochemical is a nucleic acid, the information specified in items 5 and 6 of Schedule 2 of the Regulations and
- (b) if the biochemical possesses enzymatic capability, the information specified in items 7–13 of Schedule 2 of the Regulations
4.6 Information requirements for chemicals that are subsequently added to the Non-domestic Substances List
It is important to note that, should a chemical be added to the NDSL after a Schedule 5 NSN has been submitted, the notifier may, as per subsection 8(2) of the Regulations, advise the NS program, in writing, that the information is to be considered as submitted under paragraph 7(1)(b) of the Regulations. For a final NSN assessment to be triggered, the notice must also include the information referred to in item 10 of Schedule 5 of the Regulations if this information was not already provided. Once the appropriate fee has been submitted (consult the fee table on the New substances notification fees webpage), a 60‑day assessment period will commence, to re-assess the substance as a final NSN.
In addition, once a substance is added to the NDSL, it may require the additional data prescribed in subsection 7(2) or 7(3) of the Regulations to be submitted if the substance has high release to the aquatic environment or significant public exposure (consult section 4.4.3).

Long description
Notifiers who manufacture or import a new chemical other than those referred to in section 5 of the Regulations (that is a new chemical other than those described in Figure 4-1) must provide the Minister with the appropriate Schedule of information prior to exceeding the applicable trigger quantity and within the prescribed timeframe for that Schedule. Figure 4-2 illustrates timelines and information to provide for these chemicals:
- For a Chemical/Biochemical specified on the NDSL (section 7 of the Regulations):
- Provide information prescribed in Schedule 41 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
- Provide information prescribed in Schedule 52 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
- If the chemical is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment and/or if the public may be significantly exposed to the chemical in a product, provide additional test information as prescribed in subsections 7(2) and/or 7(3) of the Regulations, respectively, at least 75 days prior to the day on which the quantity of substance manufactured or imported exceeds 50 000 kg in a calendar year.
- For a Chemical/Biochemical not specified on the NDSL (section 8 of the Regulations)3:
- Provide information prescribed in Schedule 44 of the Regulations at least 5 days prior to the day on which the quantity of substance manufactured or imported exceeds 100 kg in a calendar year.
- Provide information prescribed in Schedule 54 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
- Provide information prescribed in Schedule 64 of the Regulations at least 75 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
1 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subparagraph 7(1)(a)(ii) of the Regulations).
2 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subparagraph 7(1)(b)(ii) of the Regulations). No further information will be required unless: (a) the chemical is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment (consult subsection 7(2) of the Regulations) or (b) the public may be significantly exposed to the chemical in a product (consult subsection 7(3) of the Regulations).
3 Notification must be sent to the Minister if: the chemical or biochemical is specified on the NDSL following submission of the information to in subparagraph 8(1)(b)(i) of the Regulations and item 10 of Schedule 5 (consult subsection 8(2) of the Regulations).
4 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subparagraphs 8(1)(a)(ii), (b)(ii) and (c)(ii) of the Regulations).
4.7 Notification of polymers
Similar to the notification of chemicals and biochemicals, the Regulations prescribe information requirements tailored to the use and quantity of the polymer. These requirements are specified in the Schedules in Appendix 2 of this Guidance Document. A decision flowchart is provided below (consult Figure 4-3) and in Appendix 1 of this Guidance Document to assist in selection of the appropriate notification Schedule.
Before using the flowchart, Table 1-1 and sections 2.2, 3.3, 3.4, 4.2.3, and 4.7 through 4.9 of this Guidance Document should be reviewed to determine:
- whether the new substance meets the definition of a polymer given in the Regulations (consult section 3.3.1.2)
- whether the new polymer falls within any of the prescribed special categories (that is, research and development, contained site-limited intermediate or contained export-only; consult section 3.4)
- whether the new polymer is on the NDSL (consult section 2.2)
- the annual quantities of the new polymer that will be manufactured in or imported into Canada (consult Table 1-1 and sections 4.2.3, 4.8 and 4.9)
- whether the new polymer meets the definition of an RRR polymer (consult section 3.3.1.5)
- whether the new polymer is manufactured solely from monomers and reactants that are on the DSL or NDSL (consult section 4.7.1) and
- whether the polymer on the NDSL or the polymer manufactured solely from monomers and reactants that are on the DSL or NDSL will have high release to the aquatic environment or significant public exposure (consult section 4.9.2)
The following sections apply only to polymers that are manufactured or imported for a purpose other than as a special category listed in section 3.4 of this Guidance Document.
4.7.1 Monomers and reactants on the Domestic Substances List or the Non-domestic Substances List
To determine whether a Non-Reduced Regulatory Requirement polymer (non-RRR) notification can be considered under a Schedule with fewer information requirements, that is, under Schedule 10 instead of Schedule 11 for a 10 000 kg trigger quantity, it is necessary to find out whether the monomers and reactants of the substance are on the DSL or NDSL.
To determine the presence of monomers and reactants on the public or confidential portion of the DSL and NDSL, the Chemical Abstracts Service Registry Number can be searched using the Substances search engine.
Alternatively, a confidential search request can be sent to the NS program (consult section 2.3.1).
4.8 Information requirements for polymers (consult Figure 4-3)
4.8.1 Reduced Regulatory Requirement polymers and lower-quantity Non-Reduced Regulatory Requirement polymers
Every notifier who manufactures or imports any polymer must provide the Minister with the information prescribed in Schedule 9 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year. If the substance is considered an RRR polymer (consult section 3.3.1.5), the Schedule 9 NSN is the final notification requirement.
If the substance is a biopolymer, the notifier is required to provide, in addition to the Schedule 9 information, the information specified in items 1–3 of Schedule 2 of the Regulations.
4.9 Information requirements for Non-Reduced Regulatory Requirement polymers (consult Figure 4-3)
The sections 4.9.1 to 4.9.3 do not apply to polymers that meet the RRR polymer criteria.
4.9.1 Higher-quantity non-RRR polymers either on the NDSL or manufactured from reactants on the DSL or NDSL
Every notifier who manufactures or imports a non-RRR polymer (consult section 3.3.1.6) that is either on the NDSL or manufactured solely from monomers or reactants that are on the DSL or NDSL must provide the Minister with the information prescribed in Schedule 10 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
If the substance is a biopolymer, the notifier is required to provide, in addition to the Schedule 10 information, the information specified in items 1–4 of Schedule 2 of the Regulations. If the biopolymer is a nucleic acid, the notifier is also required to provide the information specified in items 5 and 6 of Schedule 2 of the Regulations.
Health toxicity endpoints referred to in item 4 of Schedule 10 of the Regulations are not required if the polymer is a non-RRR polymer solely due to the presence of any of the following functional groups:
- (a) aldehydes whose functional group equivalent weight (FGEW, consult section 3.3.1.8) is less than or equal to 1 000 daltons
- (b) vinyl ethers whose FGEW is less than or equal to 5 000 daltons or
- (c) sulphonic acids whose FGEW is less than or equal to 5 000 daltons
4.9.2 Polymers with high release to the aquatic environment and/or significant public exposure
Every notifier who manufactures or imports a polymer that is either on the NDSL or manufactured solely from monomers or reactants that are on the DSL or NDSL and:
- that is released to the aquatic environment in a quantity exceeding 3 kg/day, per site, averaged monthly and after wastewater treatment and/or
- where the public may be significantly exposed to the polymer in a product
must provide the Minister with additional test information as prescribed in subsections 11(2) and/or 11(3) of the Regulations, respectively, at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 50 000 kg in a calendar year. The additional required information is indicated in the following sections.
This additional information is not required if the polymer is a non-RRR polymer solely due to the presence of any of the following functional groups:
- (a) aldehydes whose FGEW is less than or equal to 1 000 daltons
- (b) vinyl ethers whose FGEW is less than or equal to 5 000 daltons or
- (c) sulphonic acids whose FGEW is less than or equal to 5 000 daltons
4.9.2.1 Polymers released to the aquatic environment
As per paragraph 5(g) of Schedule 10 of the Regulations, it is the notifier’s responsibility to submit evidence in the NSN to support a claim of the substance not being released to the aquatic environment in the quantity indicated above. This information should include any envisioned future use and quantity by other customers and a description of other envisioned applications. To calculate daily release to the aquatic environment, refer to section 6.6.5 of this Guidance Document.
The NS program will assess this information and if it is determined that the substance is released to the aquatic environment in quantities greater than indicated above, the additional information prescribed in subsection 11(2) of the Regulations must be provided. The NS program’s determination of whether the substance is subject to the additional information requirements given below will be provided to the notifier. The notifier may submit additional information to support their claim and request a re‑evaluation of the decision made by the NS program by contacting the Substances Management Information Line. The NS program will review and consider the information submitted.
The additional information required, as prescribed in subsection 11(2) of the Regulations, must include the following:
- (a) the data from a repeated-dose mammalian toxicity test of the polymer of at least 28 days duration, using the most significant route of potential public exposure to the polymer, namely, oral, dermal or inhalation, in addition to
- (i) the age, sex, number, species, strain and source of the animals tested
- (ii) the route by which the polymer is administered and the conditions under which the test is conducted and
- (iii) the dose of the polymer, the vehicle by means of which the polymer is administered and its concentration in that vehicle and
- (b) the mutagenicity data obtained from an in vitro test, with and without metabolic activation, for gene mutations or chromosomal aberrations in mammalian cells
4.9.2.2 Where the public may be significantly exposed to the polymer in a product
As per paragraph 5(h) of Schedule 10 of the Regulations, it is the notifier’s responsibility to submit information in the NSN to support a claim of the public not being significantly exposed to the substance in a product. The NS program’s determination of whether the substance is subject to the additional information requirements given below will be provided to the notifier. The notifier may submit additional information to support their claim and request a re-evaluation of the decision made by the NS program by contacting the Substances Management Information Line. The NS program will review and consider the information submitted.
Since public exposure is dependent on many factors, a single calculation to determine “significant exposure” cannot be applicable to all circumstances without being extremely conservative. Therefore, the definition of “significantly exposed” will be assessed, by the NS program, on a case-by-case basis. This information should take into consideration such factors as type of use, duration and frequency of use, concentration of the substance in the product and circumstances of exposure that may limit direct public exposure (for example, whether the substance is consumed during use or is able to migrate from the product).
Some examples of consumer applications where significant public exposure of a substance may occur include, but are not limited to, dishwashing detergent, laundry products, soaps, toilet paper, cleaning solutions, waxes, polishes, air fresheners, paints, oils, greases, body lotions, and ink.
If it is determined that the public may be significantly exposed to the polymer in a product, the additional information prescribed in subsection 11(3) of the Regulations must be provided. The additional information required, as prescribed in subsection 11(3) of the Regulations, must include the following:
- (a) the data from a repeated-dose mammalian toxicity test of the polymer of at least 28 days duration, using the most significant route of potential public exposure to the polymer, namely, oral, dermal or inhalation, in addition to
- (i) the age, sex, number, species, strain and source of the animals tested
- (ii) the route by which the polymer is administered and the conditions under which the test is conducted and
- (iii) the dose of the polymer, the vehicle by means of which the polymer is administered and its concentration in that vehicle
- (b) the mutagenicity data obtained from an in vitro test, with and without metabolic activation, for gene mutations and
- (c) the data obtained from an in vitro test, with and without metabolic activation, for chromosomal aberrations in mammalian cells or the data from a previously existing in vivo mammalian test for chromosomal aberrations that, together with data substantiating that the tissue investigated was exposed to the polymer or its metabolites, permits an assessment of in vivo clastogenicity
4.9.3 Higher-quantity non-RRR polymers not on the NDSL and not manufactured solely from reactants on the DSL or NDSL
Every notifier who manufactures or imports a non-RRR polymer (consult section 3.3.1.6) that is not on the NDSL and that contains one or more reactants that is not on either the DSL or NDSL must provide the Minister with the information prescribed in Schedule 11 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
If the substance is a biopolymer, the notifier is required to provide, in addition to the Schedule 11 information, the information specified in items 1–4 of Schedule 2 of the Regulations. If the biopolymer is a nucleic acid, the notifier is also required to provide the information specified in items 5 and 6 of Schedule 2 of the Regulations.
Health toxicity endpoints referred to in items 5 to 10 of Schedule 11 of the Regulations are not required if the polymer is a non-RRR polymer solely due to the presence of any of the following functional groups:
- (a) aldehydes whose FGEW is less than or equal to 1 000 daltons
- (b) vinyl ethers whose FGEW is less than or equal to 5 000 daltons or
- (c) sulphonic acids whose FGEW is less than or equal to 5 000 daltons

Long description
Notifiers who manufacture or import a new polymer other than those referred to in section 6 of the Regulations (that is a new polymer other than those described in Figure 4-1) must provide the Minister with the appropriate Schedule of information prior to exceeding the applicable trigger quantity and within the prescribed timeframe for that Schedule. Figure 4-3 illustrates timelines and information to provide for these polymers:
- For a Polymer/Biopolymer considered RRR (section 9 of the Regulations):
- Provide information prescribed in Schedule 92 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year1.
- For a Polymer/Biopolymer not considered RRR and specified on the NDSL or all of whose reactants are specified on the DSL or NDSL (section 11 of the Regulations):
- Provide information prescribed in Schedule 92 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year1.
- Provide information prescribed in Schedule 103 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
- If the polymer is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment and/or if the public may be significantly exposed to the polymer in a product, provide additional test information as prescribed in subsections 11(2) and/or 11(3) of the Regulations, respectively, at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 50 000 kg in a calendar year.
- For a Polymer/Biopolymer not considered RRR and not specified on the NDSL and one or more reactants are not specified on either the DSL or NDSL (section 12 of the Regulations):
- Provide information prescribed in Schedule 92 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year1.
- Provide information prescribed in Schedule 114 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
1 Section 10 of the Regulations.
2 Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult paragraph 10(b) of the Regulations).
3 Not required for Reduced Regulatory Requirement polymers. Also subject to certain exceptions (consult subsection 11(5) of the Regulations). Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult paragraph 11(1)(b) of the Regulations. No further information will be required unless: (a) the polymer is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment (consult subsection 11(2) of the Regulations) or (b) the public may be significantly exposed to the polymer in a product (consult subsection 11(3) of the Regulations).
4 Not required for Reduced Regulatory Requirement polymers. Also subject to certain exceptions (consult subsection 12(3) of the Regulations). Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult paragraph 12(1)(b) of the Regulations).
Section 5. New Substances Notifications (NSNs)
Subsection 81(1) of the Canadian Environmental Protection Act, 1999 (the Act) prohibits the manufacture or import of any substance that is not on the Domestic Substances List (DSL) unless the notifier manufacturing or importing the substance has provided the prescribed information within the prescribed time and with the prescribed fee (consult fee table on the New substances notification fees webpage); and the period for assessing the information has expired or has been terminated early (consult section 9.3.6). The prescribed information specified in the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) (consult Appendix 2) consists of both administrative and technical information described in sections 6.2 through 6.6.
Note: In order for the notifier to avoid generating new test data using animals, information to support a New Substances Notification (NSN) can be obtained from other persons who already possess the required test data, as described in the scenarios below.
5.1 Matched notifications
A Matched Notification takes place when a notifier requests that the New Substances (NS) program use information that was previously provided by another notifier for the same substance. Such information may include test requirements or additional information. The notifier who is providing the information must submit a letter of authorization to the NS program indicating their NSN reference number as well as the name of the notifier whom they are supporting, together with the latter notifier’s NSN reference number, if known. When files are matched, there may be a price reduction in the required fees (consult New substances notification fees webpage). This is different from a Third Party Information Supplier Submission (consult section 5.2).
5.2 Third party information supplier submission (confidential information provided by a third party)
Any information submitted to the NS program may be claimed as confidential (consult section 7). In cases where the notifier is not given access to information that is considered confidential by a Third Party Information Supplier, the information to support the NSN must be supplied directly to the NS program by the Third Party Information Supplier and will be identified as a “Third Party Information Supplier Submission.”
To submit a Third Party Information Supplier Submission, the notifier must initiate the NSN process by providing:
- all the administrative information (blocks A.1 to A.15 of the NSN Form; consult section 6.2)
- all exposure information, including manufacture, importation, use, transport, exposure, release and disposal information requirements (Part E of the NSN Form; consult section 6.6) and
- any other information the notifier has in their possession pertaining to the substance
The NSN must also include a reference to the pending Third Party Information Supplier Submission. Once the notifier has initiated the NSN process and been provided an NSN reference number, the confidential information required to complete the NSN must be submitted directly to the NS program by the Third Party Information Supplier, referencing the appropriate NSN reference number to which the information is being provided.
If several companies are manufacturing or importing the same substance from the same third party supplier, each notifier must submit individual NSNs to the NS program, and each notifier is responsible for tracking their own manufacture or import quantities. Each NSN will be assigned a different NSN reference number.
If the Third Party Information Supplier has already submitted the confidential information about a substance for one notifier, the same information does not need to be resubmitted for other notifiers. However, a letter of authorization from the Third Party Information Supplier must be sent to the NS program allowing the cross-referencing and use of the information within the original Third Party Information Supplier submission to complete each subsequent NSN by other notifiers for the same substance. Fee reductions may be applicable (consult New substances notification fees webpage).
5.3 Consolidated notifications
Consolidated notifications take place when a notifier simultaneously provides 2 to 6 NSNs for substances of the same class and where the technical information provided for one substance is used to address the technical information requirements for the remaining substances. In these cases, a separate NSN reference number is issued for each of the substances captured by the consolidated NSN, but the NSNs are grouped together for the purposes of a common risk assessment. Consolidated notifications are subject to reduced fees (consult New substances notification fees webpage). Although not required, it is recommended that notifiers who wish to notify a number of very similar substances at one time as consolidated notifications do so after consultation with the NS program, through a Pre-notification Consultation (PNC) request (consult section 8.8), to ensure that the technical information is sufficient to address the NSN requirements for all substances in question.
5.4 Reporting test data
The Regulations prescribe technical information which must be addressed by submitting test data or waiver requests. The NS program accepts the use of appropriate alternative approaches (also known as New Approach Methods (NAM)) to meet these technical information requirements.
The conditions to be met and the test procedures to be followed when developing test data must be consistent with the conditions and procedures set out in the Organisation for Economic Co-operation and Development (OECD) Test Guidelines (TGs) that are current at the time the test data are developed (consult section 8.1).
In addition, the development of certain test data must comply with the practices set out in the Principles of Good Laboratory Practice that are current at the time the test data are developed (consult section 8.3.1).
Protocols and laboratory practices that are recommended by the NS program for the generation of experimental data are described in section 8 of this Guidance Document. Alternative approaches which may also be used to meet a technical information requirement are described in section 8.4.
When test data is provided to fulfill a prescribed technical requirement, full test reports must be provided; summaries will not be accepted. It is important to ensure that the name and/or trade name of the substance indicated in the test report provided correspond to the name and/or trade name in the NSN. Although the values for the test data will be included in the test reports, values and conditions should also be provided in sections B.1, B.2 and B.3 of the NSN Form (consult section 6.3).
If literature papers are referenced, a copy of each paper must be provided. If software estimates/models are being used, information about the model (for example, version of software), the input data and model output must be provided to allow for an assessment of the data by the NS program (consult section 8.4.4).
Test data submitted in a previous NSN, PNC request or notice under section 70 of the Act need not be resubmitted; however, the appropriate reference number must be supplied (consult “P” code in section 6.1.2).
Explanations of the conditions under which waivers of prescribed information may be granted are provided in section 8.7 of this Guidance Document, and examples are given in Appendix 6.
5.4.1 Nanomaterials
OECD guidance documents and TGs specific to nanomaterials have been publishedFootnote 13, including guidance such as on sample preparation and dosimetry for manufactured nanomaterials, and adopted TGs. It is expected that new guidance and TGs for nanomaterials will be made available in the coming years as the documentation is developed. If no OECD TG is available for any given endpoint, a PNC (consult section 8.8) is recommended to ensure the suitability of the proposed methods.
5.5 Record-keeping requirements
Pursuant to section 13 of the Regulations, a notifier who is required to provide information to the Minister of the Environment or the NS program under the Regulations must keep a copy of that information and any supporting data at the notifier’s principal place of business in Canada or at the principal place of business in Canada of a representative of that notifier. The information and the supporting data must be kept for a period of 5 years after the year in which the information is provided.
Section 6. The New Substances Notification Form
The New Substances Notification (NSN) Form serves as an aid for complying with the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations). The form is divided into 5 sections:
- Part A: Administrative and substance identity information requirements
- Part B: Technical information requirements
- Part C: Additional information required for biochemicals or biopolymers
- Part D: Additional information requirements and
- Part E: Human and environmental exposure information (known and anticipated)
In addition, 4 Appendices are included in the NSN Form:
- Appendix I: New Substances Fees Payment Form
- Appendix II: Substances Functional Use Codes
- Appendix III: Application Codes and
- Appendix IV: Data Codes, Attachments and Confidential Information
A complete NSN must contain the specific information requirements of Part A, Part B, Part C and Part E, including all test data, laboratory reports, waiver justifications and other attachments necessary to fulfill the requirements set out in the Regulations. Additional information must be listed in Part D. Appendix I is provided as an aid in determining the fee required for each NSN.
If the NSN Form is incomplete or is filled out incorrectly, the submission may be returned to the notifier. The assessment period will not start until the completed information is submitted correctly (consult section 9.2.1).
Information that will not fit in the appropriate block on the NSN Form should be included in an attachment.
Subsection 14(2) of the Regulations states that all information must be provided in English or French. Information can be submitted electronically by electronic mail (email), by storage device (for example, USB thumb drive) or by logging into the Environment and Climate Change Canada Single Window Information Management (SWIM) system, and reporting via the NSN online form. The New Substances (NS) program does not accept .zip files, .rar files or any other types of compression programs within an email or on a storage device (for example, USB thumb drive). Alternatively, a copy of any information provided under the Regulations can be submitted on paper to the Substances Management Information Line.
The NS program will confirm receipt of the NSN and provide an NSN reference number (consult section 9.3.2) that will be used in all further correspondence concerning the NSN.
The NSN Form or sections of the NSN Form may be reproduced as often as required. An electronic version of the New Substances Notification Form can be obtained from the NS program website or by contacting the Substances Management Information Line.
6.1 Data codes, attachments and confidential information
In addition to the list of information requirements, Part B and C of the NSN Form contain additional columns: Required for Schedule; Data code; Value and conditions; Attachment number; and Confidential information. The following explains the use for each of these columns. Explanations also appear in Appendix IV of the NSN Form.
6.1.1 Required for Schedule
This is a quick reference column that allows notifiers to determine, at a glance, which Schedule requires the information to be provided. Footnotes also provide additional guidance for exceptions and conditions associated with certain data elements. It is important to note that if the first notification for a substance is a higher Schedule, all of the information prescribed in the applicable lower Schedules is also required.
6.1.2 Data code
A data code is a reference to indicate whether data are provided; the type of data provided; or whether a request for waiver of information is being submitted. The data codes, with explanatory notes, are as follows:
- D = test data on notified substance, recommended test protocol: this code is used when the data provided were generated on the notified substance using protocols consistent with those listed in Tables 8-1 to 8-4. This code is to be used even if the information is provided under the additional information requirements of the Schedules (consult section 6.5)
- A = alternative procedures: this code is used when the data provided were generated using an alternative test protocol, surrogate data or Quantitative Structure–Activity Relationships (QSARs) (consult section 8.4). This code is to be used even if the information is provided under the additional information requirements of the Schedules (consult section 6.5)
- W = waiver requested: this code is used when it is requested that prescribed information be waived under subsection 81(8) of the Canadian Environmental Protection Act, 1999 (the Act). A request for a waiver of prescribed information must be accompanied by justifications that satisfy any of the waiver criteria listed in the aforementioned subsection of the Act (consult section 8.7)
- N/A = not applicable: this code is used when the Regulations specify that the provision of information is not required under certain conditions. For example, the adsorption–desorption screening test data is not required when water solubility is less than 200 µg/L. This code cannot be used as an abbreviation for “not available”
- NR = not required: this code is used when the information has not been provided and is not required for a specific Schedule of the Regulations or
- P = previous NSN reference number, Pre-notification Consultation (PNC) reference number or notice under section 70 of the Act: this code is used when the notifier has already provided the information to the NS program in a previous NSN; a previous PNC request; and/or a notice under section 70 of the Act. The applicable NSN, PNC or notice under section 70 reference number must be entered in the Attachment number column
6.1.3 Value and conditions
Although complete physico-chemical data must be submitted in test reports (physical state and whether the notified substance is formulated for dispersal in water excepted), the notifier should enter the value and conditions in the appropriate space provided. This information will assist the notifier in organizing data for use in requesting waivers of information; in justifying cases when data are not applicable; and in discussing notifications with NS program officials.
6.1.4 Attachment number
Notifiers must clearly indicate a reference for accompanying documents (for example, Attachment 6) so they may be readily located within the NSN. Attachments include the following: justifications for requesting waivers of information; reports of experimental procedures; reports of test results; rationale for submitting alternative data; results and validation of modelling studies; rationale for why information is considered “not applicable”; and information supplemental to a request for confidentiality.
6.1.5 Confidential information
Notifiers must check the appropriate box to indicate that the information provided is considered confidential (that is, check “Yes” to indicate that the information provided is considered confidential or check “No” to indicate that the information provided is not confidential). The NS program will not consider the information confidential if neither boxes are checked. If the information provided is considered confidential, the notifier should provide, in the NSN, the supplementary information detailed in section 7.2 of this Guidance Document.
6.2 Administrative and substance identity information requirements (part A)
Explanations of the various administrative and substance identity information requirements are provided below. Subsection 14(1) of the Regulations sets out the information required for the administrative requirements of the NSN.
The information requirements listed in blocks A.1 to A.15 of the NSN Form must be provided for all substances that are subject to any Schedule in the Regulations.
6.2.1 Signature page, confidentiality requests and agreements (block A.1)
6.2.1.1 Representative of the resident manufacturer or importer of the substance identified in block A.2 or A.3 (notifier) (block A.1.1)
The person named in block A.1.1 is the resident notifier who is manufacturing the notified substance in Canada or importing it into Canada (identified in block A.2) or the non-resident notifier (identified in block A.3). The notifier must sign and date the Certification Statement in block A.1.1. The signature is a certification that the information provided in the NSN is accurate and complete to the best of the notifier’s knowledge.
6.2.1.2 Agent of the Non-resident importer of the substance identified in block A.4 (Canadian Agent) (block A.1.2)
When the importer is not a Canadian resident, block A.4 must be completed and a person must be authorized to act on behalf of the “Non-resident Importer” as the “Canadian Agent”. This person must sign block A.1.2 (consult section 6.2.4).
6.2.1.3 Toll manufacturer statement of responsibilities identified in block A.7 (block A.1.3)
For notified substances that are manufactured in Canada on toll (meaning that the person who is actually producing the substance is doing so for the benefit of the notifier), the Toll Manufacturer must sign block A.1.3 (consult section 6.2.7). A statement, signed by the Toll Manufacturer, indicates that this person accepts all compliance responsibilities with respect to the manufacture of the notified substance and any accidental release of the notified substance.
6.2.1.4 Fees provided (if applicable) (block A.1.4)
The notifier should indicate the amount of the fee provided as per the New Substances Fees Regulations (NSFR) (consult fee table on the New substances notification fees webpage). Appendix I of the NSN Form should also be completed and accompany the NSN.
In general, substances subject to notification require fees. However, please note that the NSFR do not apply to biochemicals, biopolymers, research and development substances or to substances that are intended solely for use in products regulated under any other Act of Parliament, including the Food and Drugs Act (F&DA), the Fisheries Act and the Health of Animals Act.
6.2.1.5 Confidentiality request and justification (block A.1.5)
Notifiers must also check the appropriate box to indicate whether the information is confidential or not. If the information is considered Confidential Business Information (CBI), the notifier must provide, in the NSN, the supplementary information detailed in section 7.2 of this Guidance Document. A separate justification is required for substance identity.
Checking the box entails the following:
- Name of manufacturer or importer: the link of the substance identity to the corporation or persons in any or all of blocks A.1.1, A.1.2, A.1.3, A.2, A.3, A.4, A.5 and A.6 is confidential
- Activity with the substance (located in block A.12): the fact that the corporation identified in block A.2 manufactures and/or imports the substance at the site identified in A.7, A.8, or at any site indicated on any attachment provided with this NSN Form, is confidential
- Quantity of substance (located in block A.10): the quantity of substance the notifier anticipates exceeding, as indicated in block A.10, as well as the expected date of the exceedance, as indicated in block A.11, are confidential
- Anticipated uses of the substance (located in block A.15.1): The uses of the substance the notifier anticipates to have, as well as the information in any or all of blocks A15.2, A15.3, A15.4, A15.5 and A15.6, are confidential or
- Substance identity (if checked, please complete block A.18): the identity of this substance as indicated in block A.16 and A.17 is confidential, as well as the information in any or all of the blocks A.19, A.20, A.21, A.22, A.23, A.24, A.25, A.26, A.27 and A.28. The supplemental information described in section 7.2.2 and section A5.4 of this Guidance Document must accompany a confidential substance identity claim
In addition, a company requesting confidentiality for the above submitted information must describe the nature of the confidentiality by selecting the following criteria which the company deems applicable in block A.1.5:
- It is a trade secret of the submitter
- It is information of a financial, commercial, scientific or technical nature that is treated consistently in a confidential manner by the submitter
- Its disclosure could reasonably be expected to result in material financial loss or gain to, or could reasonably be expected to prejudice the competitive position of, the submitter or
- Its disclosure could reasonably be expected to interfere with contractual or other negotiations of the submitter
6.2.1.6 Limited disclosure agreement (block A.1.6)
This is not a mandatory block. It allows Environment and Climate Change Canada to disclose information, regarding the substance, including information for which a confidentiality request is made, with the new chemicals regimes of the United States Environmental Protection Agency (US EPA) and/or the European Chemicals Agency (ECHA) and/or the Australian Industrial Chemicals Introduction Scheme (AICIS). To share the information with one of these agencies, check the associated box.
6.2.1.7 Information sharing agreement (block A.1.7)
Instances may occur where a substance has been notified but has not been added to the Domestic Substances List (DSL) for any of the following reasons:
- The notified substance did not meet all of the criteria in section 87 of the Act
- Risk management measures were taken on the notified substance or
- The assessment or processing of the NSN is still in progress
In such cases, any other notifier intending to manufacture or import that substance will be required to provide a complete NSN. To reduce both duplicate testing and the expense of developing information for an NSN, the NS program provides an opportunity for notifiers of a common substance to exchange data through the use of an Information-Sharing Agreement (ISA). There are no additional fees required for using the ISA.
An ISA starts when a notifier provides the NS program with:
- documentation of intent to manufacture or import a particular substance and
- authorization to release the name, address and phone number of the technical contact within the company to any other company that also meets these 2 criteria
Documentation of intent to manufacture or import a substance may be either an NSN or a confidential search request (consult section 2.3.1). After receipt and acceptance of this documentation, the NS program will conduct a search for ISA candidates and, if any exist, will simultaneously provide each notifier with the name of the other company or companies and the name, address and phone number of the technical contact for each company. The NS program’s contribution to the process will end at this point, and the notifiers may then proceed to negotiate an ISA.
If a notifier is willing to enter into an ISA, the ISA Authorization block (Block A.1.7) must be checked.
6.2.2 Corporate headquarters of the resident manufacturer or resident importer (principal place of business in canada) (block A.2)
A notifier who is a Canadian resident and is manufacturing a substance in or importing a substance into Canada must provide the information required in block A.2:
- The contact name and title of the Canadian notifier
- The Canadian Federal Business Numberootnote 14
- The name and address of the manufacturing or importing company
- The telephone number (including area code), as well as the email address, of the manufacturer or importer of the notified substance and
- The preferred language of correspondence
If the importer or manufacturer is not located in Canada, skip to block A.3.
6.2.3 Corporate headquarters of the Non-resident importer (block A.3)
When a foreign company or “Non-resident Importer” is the “Importer of Record,” as shown on the Commercial Accounting Declaration Form as issued by the Canada Border Services Agency, and:
- possesses a “Canadian Importer” status
- has “Importer of Record” status and
- is importing the notified substance into Canada
the foreign company or “Non-resident Importer” must leave block A.2 blank and provide the information required in block A.3:
- The contact name and title of the “Non-resident Importer”
- The civic and postal addresses, telephone number (including area code), and email address of the “Non-resident Importer” of the notified substance and
- The preferred language of correspondence
When the notifier is a “Non-resident Importer,” then the notifier must identify, under paragraph 14(1)(b) of the Regulations, a person resident in Canada who is authorized to act on the notifier’s behalf as the “Canadian Agent” (consult sections 1.4.2 and 6.2.4).
6.2.4 Canadian Agent of the Non-resident importer (needed if block A.3 is applicable) (block A.4)
As previously stated, subsection 14(3) of the Regulations states that if the notifier who provides the information under the Regulations is not a resident of Canada, the notifier must identify, under paragraph 14(1)(b) of the Regulations, a person resident in Canada who is authorized to act on the notifier’s behalf as the “Canadian Agent.”
Therefore, when a “Non-resident Importer” (consult section 6.2.3) is the “Importer of Record” on the Canadian Customs documentation for the notified substance being imported, information about the “Canadian Agent” must be provided.
When a “Canadian Agent” is required, the following information must be provided:
- The signature of the “Canadian Agent” in block A.1.2
- All information of the “Non-resident Importer” in block A.3 (consult section 6.2.3) and
- The name, title, Canadian Federal Business Number, company, address, telephone number (including area code), email address, and the preferred language of correspondence of the “Canadian Agent” in block A.4
If a “Non-resident Importer” provides the information under the Regulations and does not provide the required information about the “Canadian Agent,” the NSN will be considered incomplete and will be returned.
If the “Non-resident Importer” has more than one Canadian customer for the same notified substance, NSNs are not required for each customer as long as the “Non-resident Importer” is recognized as the “Importer of Record” for all shipments going to their customers. Yearly import quantities should be tracked by both the “Canadian Agent” and the “Non-resident Importer” to ensure that subsequent higher-quantity notification obligations are met.
The notifier may request that they be copied on all correspondence; however, the “Canadian Agent” is legally required to receive all notices or correspondence that may be sent in relation to the NSN and to keep a copy of the complete NSN including the confidential information (except in the case where a Third Party Information Supplier is used) and all correspondence and supporting data with respect to the NSN, for a period of 5 years after the end of the year in which the information is provided (consult section 13 of the Regulations).
6.2.5 Third party information supplier (only needed if the information is provided by a third party) (block A.5)
If any, or all, of the confidential technical information of the NSN Form is being provided by a person who is not the notifier (third party), the name and title, the address, the Canadian Federal Business Number (if applicable), the company name, the telephone number (including area code), the email address and the preferred language of correspondence of the Third Party Information Supplier must be provided in block A.5.
6.2.6 Technical contact (block A.6)
The name of a person who is familiar with the content of the NSN and can assist in resolving issues pertaining to ambiguous, incomplete or missing information must be provided. This person must be identified by their name, title, company name and Canadian Federal Business Number (if applicable), address, telephone number (including area code), email address and the preferred language of correspondence. The technical contact need not be a resident of Canada but must be familiar with the nature and content of the NSN.
6.2.7 Proposed site of manufacture in Canada, including toll manufacturing (block A.7)
For notified substances that are manufactured in Canada, the notifier must provide the contact name and title, the Canadian Federal Business Number, the company name and the civic address of the site of manufacture of the notified substance in Canada. If there is more than one site of manufacture, all must be provided in an attachment.
For notified substances that are manufactured in Canada on toll (meaning that the person who is actually producing the substance is doing so for the benefit of the notifier), the notifier must provide the following information:
- The contact name and title, the company name and the Canadian federal Business Number of the toll manufacturer
- The address and telephone number (including area code), as well as the email address, of the toll manufacturer
- The statement of responsabilities signed by the toll manufacturer (consult section 6.2.1.3) and
- All required information about the manufacturing facility as described in section 6.6
6.2.8 Proposed port of entry into Canada (block A.8)
For notified substances that are imported into Canada, the notifier must identify the port of entry into Canada of the notified substance; this identification should include at least the city and province. If there is more than one port of entry, all of them must be identified in an attachment.
Consult the Canada Border Services Agency List of Services for the recognized ports of entry.
6.2.9 Previous New Substances Notification number/Pre-notification Consultation number or other consultative process (block A.9)
All Schedules require the notifier to provide any previous NSN reference numbers, PNC reference numbers or other consultative process reference numbers, if one has been assigned, and the date (YYYY-MM-DD) of the submission of that information.
6.2.10 Quantity (block A.10)
The notifier must indicate the prescribed annual quantity that triggers the requirement to notify.
A notifier may, if they wish, opt to immediately submit the highest notification Schedule required, as long as the lowest prescribed quantities for the lowest Schedule are respected and the NSN is submitted within the timeframe prescribed for the highest Schedule. For instance, should a notifier wish to submit a final Schedule notification for a non-NDSL chemical (Schedule 6) without having previously submitted any lower Schedules, the Schedule 4 prescribed quantity (100 kg/year) cannot be exceeded until the assessment period for the Schedule 6 notification has ended.
6.2.11 Date when the amount in block A.10 is expected to be exceeded (block A.11)
The notifier must provide the date on which the trigger quantity noted in block A.10 is anticipated to be exceeded. This date should be entered in the form of YYYY-MM-DD.
6.2.12 Activity (block A.12)
The notifier must indicate whether the notified substance will be manufactured in and/or imported into Canada.
6.2.13 Substance type (block A.13)
The notifier must check the appropriate boxes to indicate substance type (chemical, biochemical, polymer, biopolymer, special categories, nanomaterial, UVCBFootnote 15, present on the Non-domestic Substance List (NDSL). If the notified substance is a polymer, additional boxes must be checked for information pertaining to reactants and Reduced Regulatory Requirement (RRR) polymer criteria (consult section 3.3.1.5). If the notified substance is on the confidential portion of the NDSL, the notifier must also provide the Confidential Substance Identity Number (if known).
6.2.14 Schedule number (block A.14)
The appropriate Schedule being provided must be selected for the type of substance that is being notified. NSNs for biochemicals or biopolymers must also contain specific items from Schedule 2 of the Regulations. In these cases both the notified Schedule and Schedule 2 should be checked.
6.2.15 Describe anticipated uses of the substance (blocks A.15.1 to A.15.6)
The anticipated uses of the notified substance should be entered in block A.15.1. Additional information is also required for certain Schedules and should be provided in Part E.2 of the NSN Form (consult section 6.6). If known, the functional use code and application code specified in Appendices II and III of the NSN Form should be provided in blocks A.15.2 and A.15.3.
In block A.15.4, if known, the North American Industry Classification System Code (NAICS) for this Substance should be provided.
In block A.15.5, the notifier should select whether the substance is intended to be manufactured or imported:
- solely for use in products regulated by the F&DA
- for an industrial, commercial, and/or consumer use other than for use in products regulated by the F&DA or
- for use in products regulated by the F&DA and in industrial, commercial, and/or consumer products (dual use)
If the notified substance is intended to be manufactured or imported for the last two scenarios above, the notifier must submit the NSN with the appropriate fees (consult the fee table on the New substances notification fees webpage).
For more information regarding the notification of substances used in products regulated by the F&DA, contact the Environmental Assessment Unit of Health Canada by phone at 1-866-996-9913 or (613) 948-3591 or by email at eau-uee@hc-sc.gc.ca.
In block A.15.6, from a green chemistry perspective, the notifier should also indicate whether the new substance is intended to replace another substance or group of substances currently on the market. The chemical name and the Chemical Abstracts Service (CAS) Registry Number of the substituted substance or information on the group of substances as well as the benefit(s) or reason(s) for the substitution (for example, replaces a toxic substance, reduces impact of climate change, replaces ozone-depleting substance) should also be provided.
6.2.16 Chemical Abstracts Service Registry Number (block A.16)
All Schedules of the Regulations require that the CAS Registry Number be provided, if such a number can be assigned to identify the notified substance. Schedule 2 of the Regulations additionally requires an Enzyme Commission number to be provided for biochemicals that possess enzymatic capability, if one is available (consult section 6.4.2.4).
“Can be assigned” refers to the CAS ability to assign a Registry Number to the substance of interest. Where a CAS Registry Number has not been assigned, the notifier must provide a written justification setting out a reason why a CAS Registry Number has not been assigned to the substance. If a CAS Registry Number is not being assigned, a request for confidentiality must be provided (consult sections 6.2.1.5).
If the CAS Registry Number (or a justification) is not contained in the NSN, the NSN will be deemed as missing mandatory prescribed information and a missing information notice will be issued. The assessment period will not start until all the prescribed information has been received and accepted.
The most precise CAS Registry Number available for the notified substance must be provided.
For example, for biochemicals, CAS Registry Numbers for α-Amylase can be differentiated based on the source organism: 9001-19-8 (α-Amylase, Aspergillus oryzae) versus 75496-59-2 (α-Amylase (mouse salivary gland isoenzyme reduced)).
For example, for chemicals, CAS Registry Number 68527-02-6 (chlorinated olefins (C12–C24)) would not be acceptable for (Z)-1-chloro-5-dodecene; the acceptable CAS Registry Number for this substance is 71673-24-0.
Sources of existing CAS Registry Numbers are described in Appendix 4 of this Guidance Document. To obtain information about CAS Registry Numbers, contact
Chemical Abstracts Service
2540 Olentangy River Road
P.O. Box 3012
Columbus, OH 43210
U.S.A.
Telephone:
- 800-753‑4227 (Canada and United States)
- 614-447‑3713
6.2.17 Explicit chemical name of the substance (block A.17)
All Schedules require that the exact name be used to identify substances established in accordance with the nomenclature rules of the International Union of Pure and Applied Chemistry (IUPAC) or the CAS. The name should enable an unambiguous chemical structural diagram to be drawn, unless the notified substance is considered a UVCB substance.
For UVCB substances, the terms “reaction products of,” “compounds with” or other acceptable nomenclature may be used. Examples of UVCB substances are:
- Carbonic acid disodium salt, reaction products with aniline, p-phenylenediamine, sodium sulphide (Na2(Sx)), sulphur and p-toluidine
- Amines, rosin, compounds with 6'-(diethylamino)‑3'-hydroxy‑3-oxo-spiro[isobenzofuran‑1(3H),9'-[9H]xanthene]‑2'-carboxylic acid and sodium bis[2-hydroxy-benzoato(2-)-O1,O2]chromate(1-) and
- Oils, mint, Mentha arvensis var. piperascens, terpene-free
UVCB substance names should include, where applicable, a description of the synthesis (for example, acetylation, alkaline hydrolysis) and, where applicable, the range of possible compositions (for example, paraffins [petroleum], normal C5–20). Information on UVCB composition should be provided in block A.20. Additional information about the naming of well-defined and UVCB chemicals can be found in Appendix 3 of this Guidance Document.
Where polymer and biopolymer nomenclature, including pre-polymers, incorporates the identity of monomers and reactants used in the manufacture of the polymer or biopolymer, the name of the polymer may or may not include monomers or other reactants that are either incorporated into the polymer or charged to the reaction vessel at 2% or less by weight. However, these substances must be included in the description of the polymer composition (consult section 6.2.24).
Examples of polymer nomenclature are:
- Benzene, ethenyl-, polymer with 1,2-ethanediol, butyl 2-propenoate, (chloromethyl)oxirane, 2,5-furanedione and methyl 2-methylpropenoate and
- Formaldehyde, polymer with (chloromethyl)oxirane, 4-(1,1-dimethylethyl)phenol, 4,4'-(1-methylethylidene)bis[phenol], methyloxirane polymer with oxirane ether with 1,2,3-propanetriol [(3:1)] and oxirane
6.2.18 Proposed masked name (block A.18)
If the chemical name of the notified substance is claimed as confidential, a masked name should be provided in accordance with the Masked Name Regulations. If the substance identity is claimed confidential, the box “Substance Identity” in block A.1.5 must be checked. Procedures for generating masked names are described in section 7.2.2 and Appendix 5 of this Guidance Document. These procedures are in place to obtain a balance between protecting CBI while ensuring some degree of transparency.
6.2.19 Known trade name(s) or synonym(s) of the explicit chemical name of the substance, including internal codes and test substance identifiers (block A.19)
The known trade names of the notified substance and synonyms of the chemical name must be provided for all Schedules of the Regulations. The concentration of notified substance (% by weight) must also be included for each known trade name, including internal codes and test substance identifiers, especially when used as a test substance to satisfy technical information requirements (consult Part B of the NSN Form). Additional information must also be entered in Block A.25 for each name or identifier of the test substance.
6.2.20 UVCB composition (immediate precursors and major or key constituents as anticipated) (block A.20)
If the substance is a UVCB substance, the substance name of immediate precursors and major or key constituents as anticipated, the CAS Registry Number and the range of possible composition expressed in percentages (%) must be provided.
6.2.21 The structural formula of the substance, if possible, or a partial structural formula (block A.21)
The structural formula diagram must be provided for chemicals subject to Schedule 1, 5 or 6 of the Regulations.
The structural formula diagram, if possible, or else a partial structural formula must be provided for polymers subject to Schedule 3, 9, 10 or 11 of the Regulations.
In both cases, these diagrams must be made large enough to clearly indicate the identity of all atoms, types of bonds, ionic charges and relevant stereochemistry. If the structure is too large for the space allocated on the reporting form, it should be provided as an attachment.
For polymers, the number or range of repeating units should be indicated and be correct relative to the number average molecular weight (Mn) (for example, x = 7-15, y = 10-50). Where applicable, proportions of isomers or tautomeric forms must be indicated.
Additional information and examples of structural formula are provided in Appendix 3 of this Guidance Document.
For biochemicals and biopolymers that are proteins, a primary structure (amino acid sequence) can be provided as alternative data if a structural formula cannot be provided.
For UVCB substances, if the structural formula of the substance cannot be provided, a partial structural formula that includes immediate precursors should be provided.
Reaction Scheme
In addition, a reaction scheme showing a detailed description of the process for which the notified substance is made is required for polymers that are considered RRR (consult section 3.3.1.5).
It can be difficult to establish the final structure of a polymer without an understanding of the reaction sequence because monomers are multifunctional. The provision of detailed reaction scheme information will help confirm the RRR status of the polymer and better inform the assessment. The reaction scheme should contain the following information:
- The chemical identity of monomers, pre-polymers, and reactants in the polymer
- The details of polymer synthesis, including the sequence of the addition of the monomers and reactants, their percentage by weight, and the relative number of moles
- The nature of the reactions (for example, hydrolysis, epoxidation, or esterification) and
- The polymer structure and any known by-products
The reaction scheme does not need to include an engineering diagram outlining such details as reaction vessels or containers for storage and transport; it is not intended to be a process description. The reaction scheme must include monomer and reactant information, as well as a sequence description. Additional information regarding requirements for reaction schemes can be found in Appendix 8.
Note that a reaction scheme is only required for RRR polymers meeting criteria set out in paragraph 9(a) or (b) of the Regulations. A reaction scheme is not required for RRR polymers meeting the criteria set out in paragraph 9(c) of the Regulations or for Non-Reduced Regulatory Requirement polymers (non-RRR). However, providing a reaction scheme for non-RRR polymers will better inform the assessment of the notified polymer by illustrating the manufacturing process.
6.2.22 Molecular formula (block A.22)
The molecular formula is required for chemicals that are subject to Schedule 1, 5 or 6 of the Regulations and for polymers that are subject to Schedule 3, 9, 10 or 11 of the Regulations. An undefined molecular formula may also be acceptable (for example, consult section A3.2.2 for UVCB). The empirical formula must be provided and should identify each of the monomer units. Examples are:
- Methyl methacrylate, polymer with ethyl acrylate (C5H8O2×C5H8O2)x and
- Polyoxyethylene sorbitol tetraoleate (C2H4O)n(C2H4O)n(C2H4O)n(C2H4O)n(C2H4O)n(C2H4O)nC78H142O10
6.2.23 Gram molecular weight (block A.23)
This information is required for chemicals that are subject to Schedule 1, 5 or 6 of the Regulations. The gram molecular weight should be provided for chemicals with a definite structural formula. For UVCB substances, an estimate or range of molecular weights must be provided, if known.
The number average molecular weight for polymers is discussed in section 6.3.1.14 of this Guidance Document and should be entered into block B.1 of the NSN Form and not in block A.23.
6.2.24 Monomers and reactants (block A.24)
This information is required for polymers that are subject to Schedule 3, 9, 10 or 11 of the Regulations. Reactants include compounds such as free radical initiators, cross-linking agents, chain-terminating agents, neutralizing agents and chain-transfer agents including monomers that become part of the polymer. The name, CAS Registry Number and percentage by weight of each reactant must be provided. Reactants, either incorporated into the polymer or charged to the reaction vessel at 2% or less by weight in the manufacture of the polymer, must also be reported, even if they were not included in the name of the polymer. The percentage by weight of the reactants must add up to 100%.
Pre-polymer not on the DSL or NDSL but whose reactants are on the DSL or NDSL
If a non-RRR polymer contains a pre-polymer that is not on the DSL or NDSL but all of the pre-polymer’s reactants are on the DSL or NDSL, it can be considered for notification under a Schedule with fewer information requirements, that is, under Schedule 10 instead of Schedule 11 for a 10 000 kg trigger quantity.
The term “reactant” is defined in subsection 1(1) of the Regulations as follows:
in respect of a polymer, means a substance that is used in the manufacture of the polymer and becomes part of its chemical composition, and includes a monomer
For the purpose of deciding whether or not a non-RRR polymer will be eligible for fewer information requirements provided in section 11 of the Regulations (consult section 4.7.1), the term “reactant” includes ultimate precursors of pre-polymers.
For example, polymer ABCDE contains reactants A and E which are on the DSL and pre-polymer BCD which is not on the DSL or NDSL. Pre-polymer BCD contains reactants B, C and D; pre-polymer reactants B and D are on the DSL; pre-polymer reactant C is on the NDSL. Therefore, in this case, the notified substance, polymer ABCDE, could be considered for notification under a Schedule with fewer information requirements.
If a pre-polymer is used in the manufacture of the notified substance and the pre-polymer is not on the DSL or NDSL but all of its reactants are on the DSL or NDSL, the composition data for the pre-polymer must be provided and must include the names and CAS Registry Numbers for each of its reactants. This is necessary in order to determine whether fewer information requirements apply to the notified substance.
The percentage by weight of the composition of the pre-polymer is also required if reactive or cationic moieties are present in the pre-polymer (consult section 3.3.1.5). This is necessary to determine whether the notified substance is considered RRR.
6.2.25 Additives, stabilizers and solvents present when the substance is tested for each name or identifier listed in block A.19 (block A.25)
This information is required for chemicals that are subject to Schedule 1, 5 or 6 of the Regulations and polymers that are subject to Schedule 3, 9, 10 or 11 of the Regulations. Additives are substances that are deliberately introduced into a final product containing the notified substance (for example, stabilizers, emulsifiers, solvents and anti-oxidants) and are present when the notified substance is tested.
For each name or identifier of the test substance identified in block A.19 used to satisfy the technical information requirements (consult Part B of the NSN Form), its compositional information must be provided. This includes the substance name, CAS Registry Number and concentration by weight of each component. For each name or identifier of the test substance identified in block A.19, the percentage by weight of the components must add up to 100% (that is the percentage by weight of the additives, stabilizers, solvents and the percentage by weight of the notified substance specified in block A.19 must add up to 100%). Ranges of percentages may be acceptable in certain cases. Should inadequate compositional information be submitted, the NSN will be deemed as missing prescribed information and a missing information notice will be issued.
6.2.26 Degree of purity in its technical grade composition (block A.26)
This information is required for chemicals that are subject to Schedule 1, 5 or 6 of the Regulations.
6.2.27 Impurities and their concentration by weight (block A.27)
This information is required for substances that are subject to Schedule 1, 3, 5, 6, 9, 10 and 11 of the Regulations.
Impurities are substances that are usually present in low concentrations in the final product containing the notified substance, but are not necessary for its intended use (for example, unreacted starting materials, reaction by-products). The name, CAS Registry Number and concentration by weight of each impurity must be provided, if known.
6.2.28 Safety Data Sheet (block A.28)
All Schedules require that a Safety Data Sheet (SDS) be provided if available. An SDS, as defined in section 2 of the Hazardous Products Act must be provided if one has been prepared.
6.3 Technical information requirements (part B)
All prescribed technical information must be addressed by submitting test data, data obtained from alternative approaches or waiver requests. Alternative approaches include the use of surrogate data, alternative test protocols, and calculation or estimation methods. For more information on these approaches and the criteria for determining whether a proposed alternative approach may be acceptable for risk assessment purposes, consult section 8.4. For more information on waiver requests for information requirements and the circumstances under which they may be granted under the Act, consult section 8.7.
Compositional information must also be provided for each test substance used to satisfy the technical information requirements (consult section 6.2.25). The onus is on the notifier to provide acceptable information. Explanations of the information requirements, which appear in the various Schedules of the Regulations, are provided in order to assist with the generation and compilation of the technical data prescribed in the Regulations. These explanatory notes include details such as under which Schedules the information is required; the conditions under which various tests are required; and what constitutes complete and adequate information according to the NS program.
Part B of the NSN Form contains 3 sections:
- B.1 Physical and Chemical Information
- B.2 Ecotoxicity Information and
- B.3 Health Toxicity Information
Explanatory notes for many of the technical information requirements are given in the following sections of this Guidance Document.
6.3.1 Physical and chemical information (block B.1)
Guidance documents specific to nanomaterials and revisions to the Organisation for Economic Co-operation and Development (OECD) Test Guidelines (TGs) have been published in recent years and should be incorporated into any testing strategies.
6.3.1.1 Melting point
This test is required for chemicals that are subject to Schedule 5 or 6 of the Regulations. A melting point between ‑25 °C and 300 °C must be provided as a single value or a range of values. However, if the value is outside this temperature range, the information may be indicated as “< -25 °C” or “> 300 °C.” In cases where the notified substance undergoes a chemical reaction (for example, degradation, decomposition, rearrangement) other than melting, then the temperature at which the reaction occurs must be reported. As alternative data, a pour point, softening point or sublimation point can be provided instead of a melting point, when appropriate. In the case of biochemicals and biopolymers, an isoelectric point can be provided as alternative data.
6.3.1.2 Boiling point
This test is required for chemicals that are subject to Schedule 5 or 6 of the Regulations. A boiling point between ‑50 °C and 300 °C must be provided as a single value or a range of values. However, if the value is outside this temperature range, the information may be indicated as “< -50 °C” or “> 300 °C.” In cases where the notified substance undergoes a chemical reaction (for example, degradation, decomposition, rearrangement) other than boiling, then the temperature at which the reaction occurs must be reported.
6.3.1.3 Water solubility
Water solubility is required for chemicals that are subject to Schedule 5 or 6 of the Regulations.
Water solubility is also a relevant property for nanomaterials, but it is necessary to distinguish between solubility and dispersibility. Information about dispersion of nanomaterials (for example, colloidal dispersion) should be provided. For certain nanomaterials such as metal oxides, the relevant dissolution test is recommended. For relevant information on metal oxides specifically, consult the OECD Guidance Document on Transformation/Dissolution of Metals and Metal compounds in Aqueous Media [PDF].
6.3.1.4 Water extractability
Water extractability is required for polymers that are subject to Schedule 10 or 11 of the Regulations.
Testing should be performed according to OECD TG 120, a modified version of the shake flask method from OECD TG 105. Testing according to the OECD TG 105 column elution method does not address the required endpoint and is therefore not acceptable. The NS program also recommends the OECD TG 120 for testing polymers containing water-reactive functional groups. Additional information is available in Appendix 9.
Depending on the nature of the new polymer, testing must be performed at the pH defined in the Regulations, that is, anionic and neutral polymers at pH 7, cationic polymers at pH 2 and 7, and amphoteric polymers at pH 2, 7 and 9. Results must be reported in % extractable.
6.3.1.5 Vapour pressure
This test is required for chemicals that are subject to Schedule 5 or 6 of the Regulations. However, vapour pressure is not required if the chemical has a standard boiling point below 0 °C.
6.3.1.6 Density
Density is required for chemicals that are subject to Schedule 5 or 6 of the Regulations.
6.3.1.7 Octanol/water partition coefficient
The octanol/water partition coefficient is required for chemicals that have a water solubility of less than or equal to 5 g/L that are subject to Schedule 5 or 6 of the Regulations. However, there is no water extractability cut-off for polymers; therefore, the octanol/water partition coefficient is required for all substances that are subject to Schedule 10 or 11 of the Regulations.
6.3.1.8 Hydrolysis as a function of pH
This test is required for chemicals that have a water solubility of greater than or equal to 200 µg/L that are subject to Schedule 6 of the Regulations.
This test is also required for chemicals that have a water solubility of greater than or equal to 200 µg/L that are subject to Schedule 5 of the Regulations and are on the NDSL, and meet the high release to the aquatic environment criteria (subsection 7(2) of the Regulations) (consult section 4.4.3.1). The identity of any known hydrolysis products must also be provided.
This test is also required for polymers that are subject to Schedule 10 or 11 of the Regulations and have a water extractability determined to be greater than 2%. The identity of any known hydrolysis products must also be provided.
6.3.1.9 Ready biodegradation
A ready biodegradation test is required for chemicals that are subject to Schedule 5 or 6 of the Regulations. The identity of any known products of biodegradation must also be provided.
This test is also required for polymers that are subject to Schedule 11 of the Regulations. The ready biodegradation test is required on the water-soluble portion of the polymer unless the polymer has a water extractability at pH 7 of less than or equal to 2% or is a branched silicone or siloxane polymer.
This test must comply with Good Laboratory Practice (GLP) (consult section 8.3.1).
6.3.1.10 Adsorption–desorption
This test is required for chemicals that have a water solubility of greater than or equal to 200 µg/L and are subject to Schedule 6 of the Regulations.
This test is also required for chemicals that have a water solubility of greater than or equal to 200 µg/L that are subject to Schedule 5 of the Regulations and are on the NDSL, and meet the high release to the aquatic environment criteria (subsection 7(2) of the Regulations) (consult section 4.4.3.1).
6.3.1.11 Spectroscopy
This test is required for chemicals that are subject to Schedule 6 of the Regulations. At least one spectrum suitable for characterization of the chemical is required (for example, Infrared (IR), Ultraviolet (UV), Nuclear Magnetic Resonance (NMR)). Details of the methodology used (for example, solvent, ionization technique, field strength, band width, instrumentation) must also be provided. UV spectra should include the range down to 290 nm.
6.3.1.12 Formulated for dispersal in water
This information is required for polymers subject to Schedule 3, 10 or 11 of the Regulations. The degree of dispersibility need not be determined; however, if the polymer is formulated for dispersal in water, this must be stated. The requirement for this data point will be satisfied by indicating “yes” or “no” in the column of Block B.1 of the NSN Form. This information requirement does not require quantitative determinations.
6.3.1.13 Physical state
The physical state of the polymer is required for polymers subject to Schedule 3, 10 or 11 of the Regulations. The requirement for physical state will be satisfied with an appropriate term (for example, “solid”, “wax” or “liquid”) in the column of Block B.1 of the NSN Form. This information requirement does not require quantitative determinations.
6.3.1.14 Number average molecular weight
This test is required for polymers that are subject to Schedule 3, 9, 10 or 11 of the Regulations. Generally, if the polymer is available in series of different molecular weight compositions, information must be developed using the lowest Mn composition. However, pre-existing information developed on higher molecular weight compositions should also be submitted. The Mn information must include the test procedures used and the chromatogram, calibration curve and slice tables produced during the test. There are different techniques available to determine the Mn, but the one most often used is Gel Permeation Chromatography (GPC). Additional information about what needs to be provided and frequently encountered difficulties with GPC data can be found in Appendix 7 of this Guidance Document.
If the notified substance’s solubility is greater than or equal to 2% in a suitable solvent for the substance, the Mn must be determined on the extractable portion of the notified substance (for example, if only 5% of the polymer is soluble, then the Mn must be determined on this 5% portion).
When the polymer is insoluble (solubility less than 2%) in solvent systems typically used for GPC, then solubility data over a range of different solvents should be provided. For example, insolubility in typical solvents could indicate a highly cross-linked polymer and alternate methods for Mn determination should be employed or a waiver request should be submitted along with the insolubility results. The Mn for a pre-polymer could also be provided as alternate data in this example.
Only a target Mn is required for polymers that are manufactured or imported as research and development substances and that are subject to Schedule 3 of the Regulations.
6.3.1.15 Residual constituents with molecular weights less than 500 daltons and less than 1 000 daltons
This information is required for polymers that are subject to Schedule 3, 9, 10 or 11 of the Regulations, except for Schedule 3 that is for research and development substances.
The percentage of residual constituents must be determined on the composition that has the lowest Mn of any composition intended for manufacture or import.
6.3.2 Ecotoxicity information (block B.2)
The actual number and type of ecotoxicity tests that must be performed on a substance depend on Schedule number and/or the most sensitive species with regard to the substance. Full test reports must be provided; summaries will not be accepted. Compositional information must be provided for each test substance used to satisfy the technical information requirements (consult section 6.2.25).
For all ecological toxicity information requirements for nanomaterials, the OECD Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials [PDF] should be consulted. Guidance documents specific to nanomaterials and revisions to the OECD TGs have been published in recent years and should be incorporated into any testing strategies.
It is recommended that the particle size distribution be measured by number count in order to better account for the presence of smaller nanoscale particles. If information about primary particle size and particle size distribution is not provided, and the NS program believes that the substance could be a nanomaterial, the substance will be treated as a potential nanomaterial for risk assessment and risk management purposes.
6.3.2.1 Acute aquatic toxicity
One or more of these tests are required for chemicals subject to Schedule 5 or 6 of the Regulations and for polymers subject to Schedule 10 or 11 of the Regulations.
For chemicals subject to Schedule 5 of the Regulations, data from one acute fish, daphnia or algae toxicity test are required.
For chemicals subject to Schedule 6 of the Regulations, data from the remaining 2 ecotoxicity tests (that were not completed for the submission of Schedule 5) are required.
For polymers subject to Schedule 10 of the Regulations, unless the polymer has a water extractability at pH 7 of less than or equal to 2%, data from an acute toxicity test for the most sensitive species (fish, daphnia or algae) or, if the sensitivity of these 3 species is unknown, data from an acute algae toxicity test are required.
For polymers subject to Schedule 11 of the Regulations and that have a water extractability at pH 7 of greater than 2%, data from the following tests are required:
- (a) if the sensitivity of the three species is known, an acute toxicity test of the polymer for each of the two most sensitive species: fish, daphnia or algae
- (b) if the sensitivity of only one species is known and that species is not algae, an acute algae toxicity test and either a fish or daphnia acute toxicity test selected on the basis of the most sensitive of these species or
- (c) if the sensitivity of only one species is known and that species is algae or if the sensitivity of the three species is unknown, an acute algae toxicity test and either a fish or daphnia acute toxicity test
These tests must comply with GLP (consult section 8.3.1).
6.3.3 Health toxicity information (block B.3)
For all health toxicity information requirements, the following test information must also be provided:
- (a) the age, sex, number, species, strain and source of the animals tested
- (b) the route by which the substance is administered and the conditions under which the test is conducted and
- (c) the dose of the substance, the vehicle by which the substance is administered and the concentration of the substance in the vehicle
Compositional information must be provided for each test substance used to satisfy the technical information requirements (consult section 6.2.25).
For all health toxicity information requirements for nanomaterials, the OECD Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials should be consulted. Guidance documents specific to nanomaterials and revisions to the OECD TGs have been published in recent years and should be incorporated into any testing strategies.
It is recommended that the particle size distribution be measured by number count in order to better account for the presence of smaller nanoscale particles. If information about primary particle size and particle size distribution is not provided, and the NS program believes that the substance could be a nanomaterial, the substance will be treated as a potential nanomaterial for risk assessment and risk management purposes.
6.3.3.1 Acute mammalian toxicity
This information is required for chemicals subject to Schedule 5 or 6 of the Regulations and for polymers subject to Schedule 10 or 11 of the Regulations. Test animals must be dosed using the same route or routes of exposure that are anticipated to be the most significant route or routes for potential public exposure (for example, oral, dermal and/or inhalation). In the Regulations, the most significant route of potential public exposure means exposure of the general population in Canada. To select the most appropriate route or routes for testing, the expected concentration of the notified substance in the various environmental media and consumer products and the bioavailability of the substance through ingestion, inhalation and dermal absorption must be considered. The most significant route of exposure to a substance for the general population may be different from exposures for workers in an occupational setting. Consequently, data generated for occupational exposures may not meet the requirement for the most significant route of potential public exposure specified in the Regulations. If it is not evident which route or routes would be the most appropriate for testing under the Act, the NS program (consult section 8.8) should be consulted.
For nanomaterials, an acute inhalation toxicity test is generally recommended. A revised OECD Guidance Document on Inhalation Toxicity Studies [PDF] addressing nanomaterial-specifc issues was published in 2018.
Acute toxicity test data generated after December 16, 2002, using OECD TG 401 will not be considered acceptable to fulfill the regulatory requirements for this endpoint.
These tests must comply with GLP (consult section 8.3.1).
6.3.3.2 Skin irritation
Information sufficient to assess skin irritation is required for chemicals subject to Schedule 6 of the Regulations and for polymers subject to Schedule 11 of the Regulations. This information could be obtained from data from validated test methods for the following endpoints:
- Skin irritation (for example, OECD TG 404)
- Dermal sensitization (for example, OECD TG 406), in which the results of adequate grading of dermal responses are provided
- Dermal toxicity (for example, OECD TGs 402, 410, 411), in which the results of adequate grading of dermal responses are provided
- In vitro skin corrosion (positive response only) (for example, OECD TGs 430, 431) or
- In vitro skin irritation with reconstructed human epidermis (for example, OECD TG 439)
The above list is not intended to be exhaustive. As new methods are developed and validated, the NS program will assess whether they provide sufficient information to permit an assessment of skin irritation. To consult test methods, consult section 8.6.
A human repeat insult patch test (positive or negative response) may be an acceptable alternative to animal testing. The concentration of notified substance to which individuals were exposed will be a critical factor in deciding on the acceptability of human patch tests. Human use experience may also be an acceptable alternative (positive response only), provided the human use experience is well described, including quantifying the exposure and dermal response as accurately as possible. Anecdotal information from persons handling or exposed to the substance is not an acceptable alternative.
In addition, information for the assessment of skin irritation may be obtained from QSARs, with adequate scientific justification provided by the notifier regarding the validation and applicability domain of the model (consult section 8.4.4).
These tests must comply with GLP (consult section 8.3.1).
6.3.3.3 Skin sensitization
This information is required for chemicals subject to Schedule 6 of the Regulations and for polymers subject to Schedule 11 of the Regulations. This information could be obtained from data from the following validated test methods:
- Defined approaches to skin sensitization using OECD TGs 442C, 442D and 442E, which includes a combination of in vitro and in chemico methods to reduce the need for data using animals (consult OECD Guideline 497 for more information on how to use this approach)
- Dermal sensitization (for example, OECD TG 406), in which the results of adequate grading of dermal responses are provided or
- Dermal sensitization Local Lymph Node Assay (LLNA) (OECD TGs 429, 442A, 442B)
The above list is not intended to be exhaustive. As new methods are developed and validated, the NS program will assess whether they provide sufficient information to permit an assessment of skin sensitization. To consult test methods, consult section 8.6.
A human repeat insult patch test (positive or negative response) may be an acceptable alternative to animal testing. The concentration of a notified substance to which individuals were exposed will be a critical factor in deciding the acceptability of human patch tests. Human use experience may also be an acceptable alternative (positive response only), provided the human use experience is well described, including quantifying the exposure and dermal response as accurately as possible. Anecdotal information from persons handling or exposed to the substance is not an acceptable alternative.
These tests must comply with GLP (consult section 8.3.1).
6.3.3.4 Repeated-dose mammalian toxicity
This information is required for chemicals subject to Schedule 6 of the Regulations and for polymers subject to Schedule 11 of the Regulations. A test report from a study of at least 28 days duration must be submitted. As described in section 6.3.3.1 of this Guidance document, “Acute Mammalian Toxicity,” test animals must be dosed using the most significant route of potential exposure for the general population in Canada.
The above-mentioned test is also required for a chemical that is on the NDSL and for a polymer that is on the NDSL or all of whose reactants are on the DSL or NDSL and where the substance meet the high release to the aquatic environment criteria; and/or the public may be significantly exposed to the substance in a product (subsections 7(2), 7(3), 11(2) or 11(3) of the Regulations). For additional information about these data points, consult sections 4.4.3 and 4.9.2 of this Guidance Document.
This test must comply with GLP (consult section 8.3.1).
6.3.3.5 In vitro test for gene mutations
An in vitro test, with and without metabolic activation, for gene mutation is required for chemicals subject to Schedule 5 or 6 of the Regulations and for polymers subject to Schedule 11 of the Regulations.
This test is also required for a polymer that is on the NDSL or all of whose reactants are on the DSL or NDSL and where the substance meet the high release to the aquatic environment criteria; and/or the public may be significantly exposed to the polymer in a product (subsections 11(2) and 11(3) of the Regulations). For additional information about these data points, consult section 4.9.2 of this Guidance Document.
When this information is required under subsection 11(2) of the Regulations, the notifier may provide, in lieu of this test, an in vitro test, with and without metabolic activation, for chromosomal aberrations (consult next section).
This test must comply with GLP (consult section 8.3.1).
The OECD Working Party on Manufactured Nanomaterials Workshop on the Genotoxicity of Manufactured NanomaterialsFootnote 16 held in Ottawa in November 2013 concluded that the Bacterial Reverse Mutation Test (OECD TG 471) is not a recommended test method for investigation of the genotoxicity of nanomaterials. Instead, it is recommended that the OECD Guidelines for the Testing of Chemicals program should consider modifying the applicability domain within TG 471 accordingly. Information from a Bacterial Reverse Mutation Test may be relevant only in instances where the nanomaterial is very small (for example, capable of direct penetration of the cellular membrane), soluble, or capable of producing reactive oxygen species. Consequently, in vitro genotoxicity testing of particles in mammalian cells (for example, in vitro mammalian cell gene mutation assays, and in vitro micronucleus assay) is encouraged in most instances. The “In vitro Mammalian Cell Gene Mutation Test” (OECD TG 476) is the assay recommended to fulfill the data requirement for an in vitro mutagenicity test (item 7 of Schedule 5 of the Regulations) for a nanomaterial.
6.3.3.6 In vitro test for chromosomal aberrations
An in vitro test, with and without metabolic activation, for chromosomal aberrations in mammalian cells is required for chemicals subject to Schedule 6 of the Regulations and for polymers subject to Schedule 11 of the Regulations.
This test is also required for a chemical that is on the NDSL and for a polymer that is on the NDSL or all of whose reactants are on the DSL or NDSL and where the public may be significantly exposed to the substance in a product (subsections 7(3) and 11(3) of the Regulations). For additional information about these data points, consult sections 4.4.3.2 and 4.9.2.2 of this Guidance Document.
When this information is required under subsections 7(3) or 11(3) of the Regulations, the notifier may, in lieu of an in vitro test for chromosomal aberrations, submit data from a previously existing in vivo mammalian test for chromosomal aberrations, together with data substantiating that the tissue investigated was exposed to the notified substance or its metabolites.
This test must comply with GLP (consult section 8.3.1).
6.3.3.7 In vivo mammalian mutagenicity test for chromosomal aberration or gene mutation
An in vivo mammalian test for chromosomal aberrations or gene mutations or another indicator of mutagenicity that, together with data substantiating that the tissue investigated was exposed to the substance or its metabolites, generates an assessment of in vivo mutagenicity acceptable to the NS program, is required for chemicals subject to Schedule 6 of the Regulations and for polymers subject to Schedule 11 of the Regulations.
Criteria for “evidence that the tissue investigated was exposed to the substance or its metabolites” and for what constitutes an “indicator of mutagenicity” and an assessment “acceptable to the NS program” are described in Appendix 13 of this Guidance Document.
Some flexibility is allowed in the choice of in vivo test, so that the most appropriate test can be selected for the substance. The choice of in vivo test should be based on results from in vitro genotoxicity tests, the structure and mechanism of action of the substance, and developments in the field of genotoxicity.
This test must comply with GLP (consult section 8.3.1).
6.3.4 Regulatory exemptions: health toxicity tests not required for certain polymers
The information required for polymers with high release to the aquatic environment and significant public exposure (consult section 4.9.2) that is prescribed in subsections 11(2) and 11(3) of the Regulations, as well as the health toxicity tests described in section 6.3.3 of this Guidance Document, are not required for polymers that fall under one of the classes listed in Table 6-1.
| Polymer class | Definition |
|---|---|
| RRR polymersa | As defined in section 3.3.1.5 of this Guidance Document. |
| Aldehyde | Non-RRR polymersb solely due to the presence of aldehydes whose FGEWc is less than or equal to 1 000 daltons. |
| Vinyl ether | Non-RRR polymers solely due to the presence of vinyl ethers whose FGEW is less than or equal to 5 000 daltons. |
| Sulphonic acid | Non-RRR polymers solely due to the presence of sulphonic acids whose FGEW is less than or equal to 5 000 daltons. |
a RRR polymers – Reduced Regulatory Requirement polymers.
b Non-RRR polymers – Non-Reduced Regulatory Requirement polymers.
c FGEW – functional group equivalent weight.
6.3.5 Waivers for health hazard toxicity data for polymers
Table 6-2 lists some examples of polymers for which waivers could be granted and some examples of polymers for which waivers are less likely to be granted for health toxicity tests. This table is subject to change as more information becomes available. These waivers are evaluated on a case-by-case basis; although not required, the NS program provides the opportunity for notifiers to submit a PNC request (consult section 8.8), while the NSN is being prepared, to determine whether the waivers are acceptable.
| Example | Polymer description | Likelihood of waivers being granted for health toxicity data |
|---|---|---|
| 1 | Non-RRR polymersa solely due to the presence of the following cationic or potentially cationic groups: primary, secondary or tertiary amine groups; carbodiimides; or sulphoniums. | Waivers could be granted. |
| 2 | Polymers containing other cationic groups, such as quaternary amines, hindered amines, azides, isocyanates (free and blocked) and phosphoniums (consult section 8.7.2). | Waivers are less likely to be granted. |
| 3 | Polymer for which inhalation is the main route of exposure (aerosol) or the intended uses are in personal care products and/or children’s toys. | Waivers are less likely to be granted. |
| 4 | Cationic polymers with an Mn greater than 10 000 daltons if inhalation is expected to be the most significant route of exposure for the general population based on expected use or if the substance is used in products regulated under the F&DAb. | Waivers will not be granted for acute and repeated-dose toxicity tests. |
a Non-RRR polymers – Non-Reduced Regulatory Requirement polymers.
b F&DA – Food and Drugs Act.
6.4 Additional information required for biochemicals or biopolymers (part C)
Additional information is required for biochemicals and biopolymers manufactured or imported, including substances being manufactured or imported under one of the special categories indicated in section 3.4 of this Guidance Document. The following information is required to address the nature of the production process (for example, living organism) and the potentially unique biological activity of enzymes and nucleic acids.
6.4.1 Information required for the production organism (block C.1)
6.4.1.1 Identification, source and history of the production organism
The identification of the production organism and the organ, if applicable, from which the substance is isolated is information required for biochemicals and biopolymers subject to any Schedules of the Regulations. Taxonomic designations should follow the International Code of Nomenclature and standard taxonomic sources. The organism used to produce the biochemical or biopolymer must be identified at least to the species level and to a level that distinguishes the organism from closely related pathogenic species. The identity of the organism should be substantiated using methods that are consistent with those currently used in microbial taxonomy. Where the organism is genetically modified, the host organism and the sources of exogenous genetic material (donor organisms) should be identified.
In addition, this information must include:
- any synonyms, common and superseded names for the organism, if known, including synonyms and superseded names of the species must be provided. All known internal company codes and culture collection designations must also be provided and
- its original source and history: information about the historical record of the notified micro-organism from its original source of isolation until final product development should be provided. This information includes any strain bank and accession numbers (for example, American Type Culture Collection) and the history of storage and culture conditions. Copies of any published reports of the strain's isolation, characterization, and any previous genetic modifications should be provided
Where the substance’s name is claimed as confidential, an acceptable masked name should be provided in accordance with the Masked Name Regulations. Guidance for masking micro-organism names is given in the Guidance document for the notification and testing of new living organisms.
6.4.1.2 Adverse environmental or human health effects of the production organism
This information is required for biochemicals and biopolymers subject to any Schedule of the Regulations. This information should include a description of any known adverse environmental or human health effects associated with exposure to the production organism. This information requirement should be supported with a thorough literature search.
Documentation submitted based on a literature search should include a copy of the literature search performed, indicating:
- the time period covered by the search
- the date the search was conducted
- the information sources (databases used)
- titles and abstracts of the search results in French or English and
- search strategy and search terms used
A summary of the findings from the literature search that clearly shows how they are relevant to the information requirements should be provided. If most of this information is available in recent reports, literature dating back a number of years may not be necessary. Where recent reports are unavailable, inconclusive, incomplete or contradictory, a more extensive search over a longer time period should be conducted. Full copies of any papers cited, including patents, must be provided in your response in English or French.
When the information provided is based upon a literature review, it must be conducted within 6 months of the submission of the notification and should cover major scientific information sources.
If a literature search is conducted to address a specific information requirement and there are no results for the literature search performed, this must be clearly indicated in the response for that information requirement and include all information regarding the literature search performed. Note that, in this case, the search should be expanded to cover the past 30 years.
6.4.1.3 Concentration of viable production organism (including in end-use products)
This information is required for biochemicals and biopolymers subject to any Schedule of the Regulations. The concentration of the viable production organism in the biochemical or biopolymer and, if known, in end-use products must be provided with specific units of measure (for example, CFU/mL).
Production organisms that are present in the notified substance may be subject to the New Substances Notification Regulations (Organisms), and the level of these organisms should therefore be determined and provided, together with a description of the assay method. In addition, the presence of viable organisms in a substance may result in an exposure to an organism or its metabolic products and could be a potential hazard.
During the research and development stage of manufacturing, the number of persons exposed to a substance is usually limited, and the pilot-scale manufacturing process is not necessarily representative of the conditions that will exist during full-scale production. For these reasons, determination of the level of production organism(s) in the notified substance is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 or 3 of the Regulations.
6.4.1.4 Method of separation of the production organism from the biochemical or biopolymer
This information is required for biochemicals subject to Schedule 1, 5 or 6 of the Regulations and biopolymers subject to Schedule 3, 10 or 11 of the Regulations. This information must include a description of the method(s) used to separate the production organism from the biochemical or biopolymer.
This information is not required for research and development substances subject to Schedule 1 or 3 of the Regulations.
6.4.2 Information required for biochemicals or biopolymers (block C.2)
6.4.2.1 Encoded products
This information is required for biochemicals that are nucleic acids (repeating units of deoxyribonucleotides or ribonucleotides) and are subject to Schedule 1, 5 or 6 of the Regulations and for biopolymers that are nucleic acids and are subject to Schedule 3, 10 or 11 of the Regulations. This information must include the identification of the encoded products, if known.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 or 3 of the Regulations.
6.4.2.2 Biological activity
This information is required for biochemicals that are nucleic acids (repeating units of deoxyribonucleotides or ribonucleotides) and are subject to Schedule 1, 5 or 6 of the Regulations and for biopolymers that are nucleic acids and are subject to Schedule 3, 10 or 11 of the Regulations. This information must include a description of any known biological activity (for example, antibiotic resistance) or adverse environmental or human health effects associated with the nucleic acid or with the encoded products, specified under item 5 of Schedule 2.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 or 3 of the Regulations.
6.4.2.3 Catalytic function
A description of all known catalytic functions is required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
6.4.2.4 Enzyme Commission number and name
The four-digit Enzyme Commission number, if available, and the enzyme’s name are required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
Biochemicals that are enzymes should be named in accordance with the International Union of Biochemistry and Molecular Biology (IUBMB) or CAS nomenclature conventions. Group terms such as protease are not acceptable. The name must uniquely identify a single enzyme (for example, subtilisin produced by Bacillus subtilis).
Enzyme Commission numbers, as designated by the nomenclature committee of the IUBMB, are also commonly referred to as IUBMB numbers. The Enzyme Commission number is the source for internationally accepted enzyme nomenclature and classification systems.
The Enzyme Commission number is a 4-figure set in which the first figure denotes one of the 6 main classes of catalytic substances based on the reaction catalyzed; the second and third figures indicate subclasses; and the fourth figure is the serial number of the catalytic substance in its subclass. The four-digit Enzyme Commission number is a unique number assigned to substances with catalytic activity. When enzymes are being notified, the most precise fourth-level Enzyme Commission number available must be obtained and submitted. For example, Enzyme Commission number 1.1.2 would not be acceptable for Mannitol dehydrogenase (cytochrome); the acceptable Enzyme Commission number for this substance is 1.1.2.2.
Enzyme Commission numbers can be obtained at the IUBMB enzyme nomenclature webpage.
6.4.2.5 Substrate specificity
This information is required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations. This information must include the known substrate specificity for each known catalytic function specified under item 7 of Schedule 2 of the Regulations.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
6.4.2.6 Optimum pH and temperature
This information is required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations. This information must include the optimum pH and temperature for the substrates specified under item 9 of Schedule 2 of the Regulations.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
6.4.2.7 Catalytic constants KM and Kcat
This information is required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations. This information must include the catalytic constants KM and Kcat and the conditions under which they were measured.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
6.4.2.8 Cofactors
This information is required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations. This information must include the known cofactors necessary for enzymatic activity (for example, NADPH, coenzyme Q).
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
6.4.2.9 Enzymatic activity
This information is required for biochemicals that possess enzymatic capability and that are subject to Schedule 1, 5 or 6 of the Regulations. This information must include the activity per unit weight of products and, if known, of end-use products.
This information is not required for research and development substances or contained site-limited intermediates that are manufactured and consumed at the site of manufacture and that are subject to Schedule 1 of the Regulations.
6.5 Additional information requirements (part D)
When using Part D to list all attachments included, notifiers must check the appropriate box to indicate that the document provided is considered confidential (that is, check “Yes” to indicate that the information provided is considered confidential or check “No” to indicate that the information provided is not confidential). The NS program will not consider the information confidential if neither boxes are checked. If the information provided is considered confidential, the notifier must provide, in the NSN, the supplementary information detailed in section 7.2 of this Guidance Document.
6.5.1 Other agencies (block D.1)
This information is required for all substances subject to any Schedules of the Regulations. This information must include:
- any known instances where the manufacture or importation of the substance has been notified to other government agencies, either outside or within Canada, and the purpose of such notification
- if known, the identity of the agency, including the complete name, city and country where the agency is located and
- if known, the agency’s file number, the outcome of the assessment and the risk management measures imposed by the agency
For example, an American supplier may have notified the US EPA under the Pre-Manufacture Notice (PMN) provisions of the Toxic Substances Control Act.
6.5.2 Other requirements (block D.2)
This information is required for substances subject to any Schedule of the Regulations. It must include a summary of all other information and test data in respect of the substance that are in the possession of the manufacturer or importer or to which they may reasonably be expected to have access and that permit the identification of hazards to the environment and human health and the degree of environmental and public exposure to the substance. The NS program considers all available information to inform its risk assessment, including, but not limited to, data from in vitro screening assays, mechanistic endpoints, toxicogenomics and other emerging technologies. Summaries must provide sufficient detail regarding methodology and results to permit the NS program to determine the relevance and quality of the information. The NS program may ask to consult the full report after reviewing the summaries provided.
“In the possession of the manufacturer or importer” means the information in the company’s offices in Canada if the NSN was submitted by a Canadian company or the information in the offices in the country where the notification originated if the NSN was submitted by a foreign company through a “Canadian Agent.” The phrase “to which they may reasonably be expected to have access” means information in any of the company’s offices worldwide or other locations where the notifier can access the information.
6.5.3 Other requirements for nanomaterials (block D.3)
For nanomaterials, information in addition to the required technical information may be needed by the NS program to conduct an assessment. Information such as primary particle size and particle size distribution, agglomeration and/or aggregation state, shape, surface area, surface functionalization, surface coating and surface charge should be submitted. This information is recommended for substances subject to any Schedule of the Regulations.
For water solubility and in vitro test for gene mutation in mammalian cells, alternative test protocols are recommended for nanomaterials. Refer to sections 6.3.1.3 and 6.3.3.5 of this Guidance Document.
6.5.4 Additional information and attachments (block D.4)
In certain cases, information in addition to the required technical information may be needed by the NS program to conduct an assessment. Since these other information elements apply to a small subset of notified substances, they have not been included in the Regulations. For example, when a substance is known to partition to soil and/or sediment, data from one toxicity test on a soil- or sediment-dwelling organism may be needed to conduct an assessment. It is also possible for the soil or sediment toxicity test to replace prescribed information requirements such as data from acute fish, daphnia or algae toxicity tests.
Additional data that may be needed under certain circumstances are described in Table 6-3. These descriptions are intended to alert notifiers to the potential need for generating additional data. If the notified substance meets any of the circumstance(s) described in Table 6-3, notifiers are advised to submit a PNC request (consult section 8.8) prior to generating the additional technical information in order to discuss the validity and relevance of each data element on a case-by-case basis.
| Item | Substance | Additional technical informationa that may be needed |
|---|---|---|
| 1 | Used as a polymer additive (> 10% wt.), and intended for exterior use and/or exposed to weathering (for example, asphalt, epoxy coatings used for pipelines | Leachability potential, soil toxicity, blooming potential, off-gassing potential, degradation/breakdown products, potential to enter water table |
| 2 | Used in saltwater environments | Marine toxicity, relative solubility in salt/fresh water |
| 3 | Poorly soluble or insoluble in water and/or expected to have a large octanol/water partition coefficient | Slow-stir water solubility, bioconcentration factor, bioaccumulation factor, chronic aquatic toxicity, subchronic mammalian toxicity (toxicokinetics) |
| 4 | Predicted to fail ready biodegradation test criteria | Inherent biodegradation, subchronic mammalian toxicity (toxicokinetics) |
| 5 | Surface active | Surface tension, critical micelle concentration, dermal irritation and sensitization, dermal toxicity |
| 6 | Ionizable | Distribution coefficient (log D), dissociation constant (pKa), surface tension |
| 7 | Known to partition to soil and/or sediment | Benthic toxicity, soil toxicity, terrestrial toxicity |
| 8 | Biologically active (for example, pharmaceuticals) | Chronic aquatic toxicity, subchronic mammalian toxicity/carcinogenicity (toxicokinetics), metabolic breakdown products, relative bioavailability (dermal/oral) |
| 9 | Ozone depleting substance (for example, halons, as defined in the Montreal Protocol) | Ozone depletion potential, global warming potential, inhalational mammalian toxicity (toxicokinetics) |
| 10 | Cationic | Mitigation of ecotoxicity to fish by humic acid |
| 11 | Potential endocrine disruptor | Mechanistic in vitro screening assay, amphibian metamorphosis, 2-generation reproduction toxicity with endocrine screening |
| 12 | Confirmed or potential nanomaterial (consult Appendix 10) | Particle size and size distribution, agglomeration/aggregation state, shape, surface area, surface functionalization, surface coating, surface charge, etc; Release potential of the substance from a final product, soil toxicity, inhalational mammalian toxicity (including toxicokinetics), genotoxicity (other than Ames test) |
| 13 | Phthalate or flame retardant or perfluorinated substance | Chronic aquatic toxicity, reproductive/developmental toxicity, subchronic mammalian toxicity (toxicokinetics), mechanistic in vitro screening assay |
| 14 | Metal and metal compound | Transformation/dissolution in aqueous media, subchronic mammalian toxicity (toxicokinetics) by appropriate route of exposure, skin sensitization, carcinogenicity |
| 15 | Enzyme | Amino acid sequences of native and mutated enzyme |
a Refer to the OECD Guidelines for the Testing of Chemicals for internationally accepted standard test methods.
Additional information requirements refer to any information and data relevant to environmental and health hazard identification, such as:
- experimental data (including negative results)
- summaries of literature reviews
- results of searches from databases conducted by the notifier
- structure–activity relationship analyses performed on the substance or structurally related substances
- reports of adverse effects identified as a result of the use of the notified substance in an occupational setting
- results of studies of the risk to employees, customers, the public or the environment (for example, environmental fate modelling) that may result from the use of the substance and
- toxicogenomic data
Information about possible environmental benefits resulting from the manufacture or use of the notified substance may also be provided. If the benefit relates to the substitution for another substance, information in block A.15.6 of the NSN Form should be provided. Examples of such benefits include the following:
- The substance is a “less toxic” substitute for an existing substance or technology
- The substance is recovered from a waste stream
- The manufacture or use of the substance will generate less waste than an existing substance or
- The substance may be recycled
Any information provided as “additional information” may be provided in the language in which the information was originally prepared. The NS program requests that at least a summary of any additional information be provided in English or French.
6.6 Human and environmental exposure information (known and anticipated) (part E)
Part E of the NSN Form identifies all of the manufacture, import, use, and release information that is prescribed by the Regulations. This section also requests certain information that is not required by the Regulations, but that is highly relevant to help predict releases into the environment and potential human exposure to the new substance.
The information provided in this section is used directly in the risk assessment to evaluate potential exposure and release of the new substance throughout its main life cycle stages. This includes, but is not limited to, transportation, storage, manufacture, formulation/processing, equipment cleaning, use, and waste handling and disposal.
The risk assessment takes into consideration the exposure from the anticipated activities by the notifier as well as the potential activities of downstream processors and users of the substance. If specific information is not known by the notifier, such as in the case where the information relates to operations at sites controlled by others (for example, manufacturing, formulation), responses may be provided to the extent known or ascertainable by contacting suppliers or customers. Exposure information provided in a US EPA PMN can also be provided to assist in the evaluation.
All parts of this section should be filled out as completely as possible if the information is known. In the absence of detailed information, the NS program typically adopts conservative estimates and modelling information to estimate potential exposure.
6.6.1 Anticipated annual manufacture, import, and export quantities of the notified substance (block E.1)
Report the amount of pure substance, not including solvents or other components if the substance is in a mixture. For consolidated notifications, report quantities for each substance.
6.6.1.1 Quantity of the substance manufactured, imported and exported (block E.1.1)
This information is required for substances subject to any of the Schedules prescribed in the Regulations.
Complete the table according to the following instructions:
- Quantity manufactured within Canada: indicate the anticipated annual quantity to be manufactured in Canada, if applicable. This information should include the amounts during the first 12 months and, if known, the expected maximum amount to be manufactured during any future 12-month period in kg/year. If there is none expected, indicate so
- Quantity imported into Canada: indicate the anticipated annual quantity to be imported into Canada, if applicable. This information should include the amounts during the first 12 months and, if known, the expected maximum amount to be imported during any future 12-month period in kg/year. If there is none expected, indicate so and
- Quantity for export: indicate the anticipated annual quantities to be manufactured in Canada or imported into Canada for export, if applicable. This information should include the amounts during the first 12 months and, if known, the expected maximum amount to be exported during any future 12-month period in kg/year. If there is none expected, indicate so
6.6.1.2 Canadian sites of greatest quantity (block E.1.2)
This information is required for chemicals subject to Schedule 5 or 6 and non-RRR polymers subject to Schedule 9, 10 or 11 of the Regulations. For contained site-limited intermediate substances subject to Schedule 1 or 3 of the Regulations, the single location of use is required.
Complete the table according to the following instructions:
- Site: if known, identify the 3 sites (company names and site addresses) in Canada where the greatest quantity of the substance, manufactured or imported by the notifier, is anticipated to be used and/or processed and
- Quantity: provide the estimated quantity used and/or processed at the site (in kg/year)
6.6.2 Uses involving the substance (block E.2)
6.6.2.1 Description of activities in Canada (block E.2.1)
This information is required for substances subject to any of the Schedules prescribed in the Regulations. A description of all industrial, commercial, and consumer activities involving the substance in Canada (for example, manufacture, import and distribution; industrial formulation, reformulation of a concentrate, commercial activity) should be provided, to the extent to which it is known or reasonably ascertainable. This should include the activities undertaken by the notifier and by downstream processors or users of the substance in Canada.
If the substance is imported into Canada, a description of the imported product(s) containing the notified substance (for example, pure notified substance, intermediate product, end-use product) should be provided.
Industrial, commercial, and consumer activities can be defined as:
- Industrial: the substance, or products containing the substance, will be used at the site of manufacturers or large-scale processors/users (for example, textile dyeing, paint formulation, use of a curable resin to manufacture a product)
- Commercial: the substance, or products containing the substance, will be used by a commercial enterprise providing a consumer service (for example, use by commercial dry cleaning establishments, use by painting contractors, use by roofers in commercial building construction) or
- Consumer: the substance, or products containing the substance, will be used by private individuals (for example, personal care products, automotive oil, dishwashing detergent)
6.6.2.2 Anticipated end-uses, functions and concentration of the substance (part E.2.2)
The intent of this section is to describe how the substance is imported, and whether it is blended into intermediate products prior to incorporation into final end-use products. It is also intended to obtain information about the function and end-uses of the substance in products and anticipated products that contain it.
Provide the concentration (or range of concentrations) of the notified substance in the product(s) as imported or manufactured in Canada. This information is required for chemicals subject to Schedule 1, 4, 5 or 6 and non-RRR polymers subject to Schedule 3, 9, 10 or 11 of the Regulations.
Identify and describe each anticipated end-use products containing the new substance (for example, architectural paint, hair shampoo, automotive lubricant). Indicate the function of the substance. The function is related to the inherent physical and chemical properties of the substance (for example, degreaser, catalyst, plasticizer, UV absorber, fragrance). Identify if the end-use is an industrial, commercial and/or consumer activity. Indicate the concentration of the substance, if known and the percent of annual quantity. The percent of annual quantity is the percentage of total annual quantity imported or manufactured for each end-use (when adding the percentages for each end-use, it should equal 100%). In some cases, a substance may be used for several different uses and each of these should be reported. For example, an emollient in hand soap may also be used as a surfactant in automobile spray wax. This information is required for substances subject to any of the Schedules prescribed in the Regulations.
The following are some examples of functions and uses:
- Emollient in hand soaps
- Disperse dye carrier for finishing polyester fibers
- Cross-linking agent for epoxy-type coatings for metal surfaces
- Flame retardant for surface application on cotton apparel, textile home furnishings, and exterior canvas products
- Surfactant in automobile spray wax
- Colorant for paper and other cellulosic products
- Fiber-reactive dye for nylon carpeting and upholstery and
- Antioxidant in fuel oils and lubricants
6.6.2.3 Historical and other likely end-uses, functions and concentrations of the substance (block E.2.3)
This information is required for NDSL chemicals subject to Schedule 5, chemicals subject to Schedule 6, and polymers subject to Schedule 10 or 11 of the Regulations.
The purpose of this section is to obtain information about the historical and other likely functions and end-uses for the new substance. Complete the table following the guidance in section 6.6.2.2. These uses and functions are not envisioned to be pursued by the notifier, but are known historically to exist in other jurisdictions or in the patent literature, or understood based on knowledge of the substance properties.
The NS program recommends that this information be provided to the greatest extent known, even if such uses are not expected to be pursued. For example, surfactants intended for use in industrial applications may also be suitable for use in personal care products. Detailed information of this kind will assist the NS program in determining the exposure characterization of the substance. Where only limited information is provided, exposure evaluations will be based on conservative estimates.
6.6.3 Human exposure (block E.3)
The purpose of this section is to obtain information about the potential for direct human exposure to the notified substance, including from the use of consumer products. If the notifier does not have specific information about the potential for human exposure, then descriptions can be based on information provided by downstream processors and users of the substance or on experience with similar substances. The notifier should provide all information requested, to the extent to which it is known or reasonably ascertainable. Where only limited information is provided, exposure evaluations will be based on conservative estimates.
6.6.3.1 Direct human exposure (block E.3.1)
This information is required for chemicals subject to Schedule 5 or 6 and non-RRR polymers subject to Schedule 9, 10 or 11 of the Regulations.
Describe the anticipated circumstances and degree of direct human exposure to the substance, including the concentration of the substance, the duration and frequency of exposure and the route of exposure (dermal, oral, inhalation).
Indicate if the substance is anticipated to be used in products intended for use by or for children. If yes, describe the types of products (for example, shampoo, markers).
Describe any conditions of use or factors that may limit direct human exposure to the substance.
6.6.3.2 Significant public exposure (block E.3.2)
This information is required for substances subject to Schedule 1, 3 or 10 and NDSL chemicals subject to Schedule 5 of the Regulations. Additional test data may be required prior to importing or manufacturing more than 50 000 kg/year depending on the assessment of this information (review sections 4.4.3.2 and 4.9.2.2 of this document).
Indicate whether the public is anticipated to be significantly exposed to the substance in a product, taking into account factors including concentration of the substance, duration, frequency and circumstances of exposure (for example, route of exposure) and factors that may limit direct human exposure. If not, provide information substantiating that the public is not anticipated to be significantly exposed.
6.6.4 Environmental exposure (block E.4)
6.6.4.1 Description of operations (industrial, commercial and consumer) (block E.4.1)
This section focuses on the major life cycle steps where environmental release could occur, including the manufacturing, processing, commercial use, and consumer use operations involving the substance or products containing the substance. In many cases, these life cycle steps may involve multiple users of the substance, including separate manufacturers, blenders, and end-users. For example, for a surfactant used in metal working fluids, there may be surfactant manufacture, processing into metal working fluids, and use in industrial metal cutting operations.
In most cases, specific information relating to operations under the notifier’s control will be available. Where specific information is not available, for example, in the case where operations are controlled by downstream processors or users of the substance descriptions can be based on available information and experience with similar substances. The notifier should provide all information requested, to the extent to which it is known or reasonably ascertainable. Where only limited information is provided, exposure evaluations will be based on conservative estimates.
Complete sections E.4.1A, E.4.1B and E.4.1C for the substance as applicable.
Manufacture and/or processing of the notified substance in Canada (block E.4.1A)
This information is required for manufacture and/or processing of notified substances in Canada that are subject to any of the Schedules prescribed in the Regulations.
Processing the notified substance can include, for example, formulation or blending the substance.
For the description of operation and/or flow diagram, identify the major steps, focusing on waste streams and potential points of release of the substance during the operation and equipment cleaning.
If the same operation occurs at multiple sites and the processes differ significantly, or if there are multiple operations, the information can be reported by replicating the table.
Complete the table according to the following instructions:
- Number of sites: enter the number of sites where the substance is manufactured and/or processed
- Batch operations: all components are loaded into the vessel together or in a pre-defined sequence until the desired product is formed and subsequently discharged in a single batch. Processing of subsequent batches must wait until the current batch is finished. In the table, provide the maximum quantity produced per batch, the maximum number of batches per day, and the maximum number of batches that are expected to be produced per month
- Continuous operations: components are continuously charged into the vessel and the desired product is continuously formed and discharged. In the table, provide the maximum quantity produced per day, and the maximum expected number of days of operation per month
- Description of operation and/or flow diagram: identify the major operational steps in the manufacture of the substance, focusing on waste streams and potential points of release of the substance. This should include a brief description or flow diagram of the main steps of the manufacturing process that identifies:
- features such as process tanks, holding tanks and distillation towers
- the points of entry of all components and
- the points of release of the substance and
- Procedures for cleaning: provide a brief description of the methods used for cleaning the equipment, transportation lines, and vessels (for example, vacuumed, washed with water, washed with organic solvents) and the maximum cleaning frequency (for example, per month, after each batch)
Industrial and commercial uses (block E.4.1B)
This information is required for substances with industrial and/or commercial uses that are subject to any of the Schedules prescribed in the Regulations.
Describe the industrial and/or commercial uses for the substance. Industrial uses include, for example, painting automotive parts, applying interior pipe coatings, lubricating equipment. Commercial uses include, for example, dry cleaning, car washes, automotive servicing.
To complete the remainder of the table, refer to instructions for Block E.4.1A.
Consumer uses (block E.4.1C)
This information is required for substances with consumer uses that are subject to any of the Schedules prescribed in the Regulations.
Describe the consumer uses for the substance. Consumer uses include, for example, dishwashing, do-it-yourself automotive oil changing.
6.6.4.2 Description of the transportation and storage operations (block E.4.2)
This information is required for chemicals subject to Schedule 1, 5 or 6 and polymers subject to Schedule 3, 10 or 11 of the Regulations.
Cleaning of transport and storage vessels is historically associated with releases to the environment. For this reason, the exposure assessment conducted for each new substance pays particular attention to the vessels used for transporting and storing the substance.
In most cases, specific information relating to operations under the notifier’s control will be available. Where specific information is not available, for example, in the case where operations are controlled by downstream processors or users of the substance, descriptions can be based on available information and experience with similar substances. The notifier should provide all information requested to the extent to which it is known or reasonably ascertainable. Where only limited information is provided, exposure evaluations will be based on conservative estimates.
6.6.4.3 Limiting environmental exposure (block E.4.3)
Describe any factors that may limit environmental exposure to the substance (for example, incineration, chemical treatment, pollution prevention practices, recycling, existing regulatory requirements) including on-site treatment. This information is required for NDSL chemicals subject to Schedule 5, chemicals subject to Schedule 6, and polymers subject to Schedule 10 or 11 of the Regulations.
Describe the methods recommended for destruction or disposal of the substance. This information is required for chemicals subject to Schedule 1, 5 or 6, and polymers subject to Schedule 3, 10 or 11 of the Regulations.
Recycling activities include reclamation of useful chemical components from wastes that would otherwise be released as air emissions, water discharges or land releases during manufacture, process or use. All descriptions may be quantitative or qualitative.
For biochemicals and biopolymers that are proteins, a description of the denaturation or degradation process prior to disposal and its residual activity should be provided, if available.
6.6.4.4 Handling waste containing the substance (block E.4.4)
The information requested in this section is provided to describe and quantify potential releases of the substance and waste to the environment. This should include information from each industrial and commercial operation and consumer use in Canada.
In many cases, releases may occur at separate life cycle steps involving different users of the substance, including separate manufacturers, blenders, and end-users. For example, for a surfactant used in metal working fluids, there may be surfactant manufacture, processing into metal working fluids, and use in industrial metal cutting operations.
In most cases, specific information relating to operations under the notifier’s control will be available. Where specific information is not available, for example, in the case where operations are controlled by downstream processors or users of the substance, descriptions can be based on available information and experience with similar substances. The notifier should provide all information requested to the extent to which it is known or reasonably ascertainable. Where only limited information is provided, exposure evaluations will be based on conservative estimates.
Information about the releases of the substance from each industrial and commercial operations and consumer use in Canada should be provided. Releases generated from operational processes and from cleaning equipment, transport and storage vessels should be included. This information is required for chemicals subject to Schedule 1, 5 or 6, and polymers subject to Schedule 3, 10 or 11 of the Regulations.
Amongst the information requested, the component(s) of the environment into which the substance is anticipated to be released (for example, receiving body of water, agricultural land, air) should be provided. This information is required for chemicals subject to Schedule 1, 5 or 6 and polymers subject to Schedule 3 or 11 of the Regulations.
If there are multiple sources of release which differ significantly, the information can be reported by replicating the table.
If there is no production of waste containing the substance from each industrial and commercial operation and commercial use in Canada, an explanation should be provided in the appropriate block to substantiate why there is no production of waste containing the substance.
6.6.4.5 High release to the aquatic environment (block E.4.5)
This information is required for NDSL chemicals subject to Schedule 5 and polymers subject to Schedule 10 of the Regulations. Additional test data may be required prior to importing or manufacturing more than 50 000 kg/year depending on the assessment of this information (review sections 4.4.3.1 and 4.9.2.1 of this document).
Indicate whether the substance is anticipated to be released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment (that is the substance is expected to lead to high release). If the release is less than or equal to 3 kg per day, per site, provide the data substantiating the quantity released. Some detailed guidance on what is required and how to calculate the high release estimate is provided in the next section.
6.6.5 High release to the aquatic environment calculation
In general, to calculate the daily release to the aquatic environment averaged monthly (DRave mo) after wastewater treatment, the following formula can be applied for each site.
Where:
- RDM = number of release days per month
- QR = quantity released on release days
- RE = wastewater treatment removal efficiency
- 30.417 = the average number of days in a month
Release day is typically assumed to involve one release event, but it can involve more. For example, if the notifier or downstream user releases 5 kg of the notified substance to the municipal treatment plant in the morning and another 3 kg in the afternoon, then the release day would involve the sum of those quantities per day, or in this case the quantity released (QR) on the release day would be 8 kg/day.
The average number of days in a month is taken as 30.417 days, which is derived from 365 ÷ 12. This value is used to account for averaged monthly releases.
6.6.5.1 Estimating the number of release days per month
The number of release days per month (RDM) is a function of operations and can vary throughout the year at any site. The following scenarios provide some examples:
- Releases occur 7 days per week, throughout the year: if releases occurred every day all year, then the RDM would equate to the typical number of days in a month, that is, 30.417 days/month
- Releases occur 5 days per week: If releases typically occurred 5 days per week but operations involving the substance only occurred 200 days of the year, to obtain the RDM, take the worst-case month in the year (that is, every week) and multiply this by the average number of weeks per month that is, (4.345 weeks/month) x (5 releases/week) to obtain the RDM of 22 days/month
- note: 30.417 days/month ÷ 7 days/week = 4.345 weeks/month
- Releases occur only during one week in the year: for example, if facility operations release the new substance on only 5 consecutive days in the year, then this would be used to represent the worst-case month and the RDM would be 5 days/month or
- Releases occur once or twice a month throughout the year: if the facility releases the notified substance either once or twice every month throughout the year, then the RDM would reflect the worst-case month and be set at 2 days/month
6.6.5.2 Determining the quantity released on release days
The QR can be determined for continuous or periodic releases. If it is known that a certain quantity of the substance is lost on each release day, then it can be applied directly as the QR. If neither of the scenarios below apply, supporting evidence must be provided to support the QR.
Continuous daily release
If the releases to a wastewater treatment plant are continuous and occur on a daily basis throughout the year, then the QR can be determined based on the annual quantity along with an estimated or measured fraction lost during the operations, for example, from equipment cleaning and/or operational losses. For example, if the annual quantity of the substance is 20 000 kg/year at one site over 250 days and it is known or estimated that 3% is lost during operations, then the average quantity released on release days would be as follows:
Example 1
QR = 20 000 kg/year x 0.03 ÷ 250 days/year = 2.4 kg/day
Therefore, the QR is 2.4 kg/day.
Periodic release
If the release is associated with periodic cleaning of transportation lines and mixing vessels after several batch runs, then one must consider the specific release quantity during that particular process. For example, if the total quantity of the substance in any given batch is 2 000 kg, and the residual level of the substance in the equipment prior to cleaning is 2.5%, and the cleaning operations take place on one day, then the quantity of substance released on the release day is estimated as follows:
Example 2
QR = 2 000 kg/batch x 0.025 ÷ 1 day = 50 kg/day
Therefore, the QR is 50 kg/day.
6.6.5.3 Determining the wastewater treatment removal efficiency
The removal efficiency (RE) of the substance following wastewater treatment is an important part of the equation supporting the high release estimate. There are a variety of ways to determine the RE. For instance, the RE can be determined by monitoring actual influent and effluent from a wastewater treatment facility. In such cases, a description of the monitoring activities must be provided. In most cases, however, it is expected that the RE will be estimated. Estimates can be based on physical and chemical properties and professional judgement or based on computer simulated modelling. In either case, a description of the process or supporting evidence must be provided.
For example, for a particular structure and physical chemical properties, the wastewater treatment RE estimated from the US EPA computer estimation program EPI Suite™ is determined to be 82%. This value can be taken as the RE.
6.6.5.4 Examples of calculations
Based on the above scenarios, the following DRave mo examples are derived.
Example 1
RDM = 22 days/month
QR = 2.4 kg/day
RE = 82%
DRave mo = (22 days/month) x (2.4 kg/day) x (1-0.82)/30.417 days/month
Therefore, the DRave mo = 0.34 kg/day per site, averaged monthly and after wastewater treatment and is not expected to lead to high release to the aquatic environment.
Example 2
RDM = 2 days/month
QR = 50 kg/day
RE = 82%
DRave mo = (2 days/month) x (50 kg/day) x (1-0.82)/30.417 days/month
Therefore, the DRave mo = 0.59 kg/day per site, averaged monthly and after wastewater treatment and is not expected to lead to high release to the aquatic environment.
Section 7. Confidential information
Under section 313 of the Canadian Environmental Protection Act, 1999 (the Act), any notifier who provides information to the government may, at the same time, submit a written request that the information be treated as confidential. This feature ensures that genuine Confidential Business Information (CBI) is protected from public disclosure. The degree of protection given to information claimed to be confidential will be consistent with sections 314–321 of the Act and the provisions of the Access to Information Act.
7.1 Claiming confidentiality
For information to be treated as confidential, the request must be submitted with the New Substances Notification (NSN) and must:
- indicate which particular information is confidential using the appropriate field in the NSN Form (specific information provided in attachments to the NSN Form can also be put in brackets, [ ]) and
- include all supplemental information (detailed in section 7.2)
7.2 Information supporting a confidentiality claim
The New Substances (NS) program aims to obtain a balance between protecting CBI while ensuring some degree of transparency. General confidentiality claims and claims for substance identity confidentiality in an NSN must be accompanied by the supplementary information detailed in sections 7.2.1 and 7.2.2. Notifiers will be advised if their request for confidentiality is incomplete and given an opportunity to review and provide additional substantiation for their claim. Not providing the additional substantiation could lead to the unwanted publication of the notified information. Alternatively, the notifier may choose to withdraw the confidentiality claim.
7.2.1 General confidentiality claims
Claims for confidentiality should only be made when the submitted information is truly confidential, such as when it is a trade secret or where its disclosure could negatively impact the competitive position of the submitter. To reduce the scope of confidentiality requests and focus on what is truly confidential, a request for confidentiality must indicate which specific information or data should be treated as confidential. A justification must be provided to any request for confidentiality describing the nature of the confidentiality. The justification should be selected from the following criteria:
- It is a trade secret of the submitter
- It is information of a financial, commercial, scientific or technical nature that is treated consistently in a confidential manner by the submitter
- Its disclosure could reasonably be expected to result in material financial loss or gain to, or could reasonably be expected to prejudice the competitive position of the submitter or
- Its disclosure could reasonably be expected to interfere with contractual or other negotiations of the submitter
7.2.1.1 Information generally not expected to be confidential
Although submitters may claim any information they submit as confidential, certain types of information of value for risk assessment of substances and for other purposes related to the protection of human health and the environment are generally not expected to be confidential. Release of this information is seen as desirable to promote transparency.
There is consensus within the Organisation for Economic Co-operation and Development (OECD) member states that no restriction needs to be put on the exchange of information described below between governments or on the disclosure of such information to the public.
The following list identifies the kinds of information that would not be expected to be confidential, although it is understood that there will be exceptions. It is not restrictive and is based on the OECD Recommendation of the Council concerning the OECD List of Non-Confidential Data on Chemicals:
- Trade name(s) or name(s) commonly used
- General information about uses (the uses need to be described only broadly: closed or open system, agriculture, domestic use, etc.)
- Safe handling precautions to be observed in the manufacture, storage, transport and use of the substance
- Recommended methods for disposal and elimination
- Safety measures in case of an accident
- Physical and chemical information, with the exception of data revealing the substance identity (for example, spectra). If the physical and chemical information make it possible to deduce the substance identity, non-confidential ranges of values can be identified and
- Summaries of health, safety, and environmental data including precise figures and interpretations. In cases where the study is claimed confidential, the submitter of the health, safety, and environmental study has the option of preparing a non-confidential summary. If no summary is provided, Environment and Climate Change Canada and Health Canada will prepare one following the OECD harmonized template format
7.2.2 Confidential substance identity claims
When the identity of a substance is claimed confidential, the procedures for generating a masked name are prescribed in the Masked Name Regulations. These procedures are further elaborated in Appendix 5 of this Guidance. These procedures are in place to maintain a balance between protecting CBI and ensuring some degree of transparency.
Masking may be accomplished by disguising single distinctive elements of the explicit chemical name of the substance, while retaining the generic identity/molecular structure of the substance. In most cases, masking a single distinctive element of the explicit chemical name of the substance would be sufficient, although masking multiple elements of the substance is also accepted when needed, with supporting justifications.
The explicit chemical name of the substance is the name established in accordance with the current chemical nomenclature rules of the International Union of Pure and Applied Chemistry (IUPAC) or Chemical Abstracts Service (CAS). The explicit name is required when submitting an NSN Form, a Domestic Substances List (DSL) Nomination Form or a Non-domestic Substances List (NDSL) Nomination Form. Please note that a substance will not be eligible for addition to the DSL until an acceptable masked name is received (consult section 10.2 for eligibility requirements).
Masked names will be reviewed upon submission. If the claim for confidentiality of the explicit chemical name is acceptable, the proposed masked name will be evaluated to determine whether or not it is consistent with the Masked Name Regulations. If a masked name is considered unacceptable, the NS program will communicate that decision to the notifier and an alternative name will be requested. If a consensus is not reached, the NS program will publish a masked name that, in its opinion, will respect the confidentiality claim of the company while reflecting the generic molecular structure of the substance. Review of the masked name is separate from the review of the NSN and will not affect the assessment period for the substance. The NS program will indicate within 60 days of receipt of a complete masked name request whether the masked name is acceptable. Note that there are fees associated with a masked name request (consult fee table on the New substances notification fees webpage).
Publication of an acceptable masked name is required under section 88 of the Act if publication of the actual identity of a substance would result in the release of CBI. Therefore, when claiming confidentiality for substance identity, the notifier should, in addition to the justification described in section 7.2.1 above, provide the following information:
- A proposed masked name developed in accordance with the prescribed masking procedures (consult Appendix 5)
- Justification for additional masking, that is disguising more than one distinctive element (consult section A5.4) and
- The following information:
- The detrimental effects on the competitive position of the notifier’s company that would result from the identity of the substance appearing on the DSL or in any other publication
- The manner in which a competitor could use the identity of the substance
- An indication of whether the identity of the substance has been kept confidential to the extent that competitors do not know it is being manufactured, imported or used
- An indication of whether the substance has been patented and, consequently, disclosed through the patent
- An indication of whether it is public knowledge (for example, publications in technical journals or trade publications) that the substance is being manufactured, imported or used
- The measures that have been taken to prevent undesired disclosure of the substance identity and the extent of any disclosures to date
- An indication of whether the substance is, or will be, in an effluent, emission or waste entering the environment
- An indication of whether the substance is, or will be, in a product available to the public, and whether the substance can be identified by analysis of the product
- The purpose for which the substance is being, or will be, manufactured, imported or used and
- An indication, to the best of the notifier’s knowledge, of whether the NS program, another federal agency, a provincial or territorial agency or the agency of a foreign government has ever made a determination that this substance 1) has an immediate or long-term effect on the environment; 2) constitutes, or may constitute, a danger to the environment; or 3) constitutes, or may constitute, a danger to human life or health (if such a determination has been made, details should be provided)
An acceptable masked name disguises the explicit chemical name as described above. As such, replacing components of the explicit chemical name with synonyms and then masking the synonyms will not be accepted.
Duration of confidentiality claims for substance identity
To help increase awareness of the substances in the Canadian market, confidentiality claims for substance identity will be reviewed after a period of 10 years. Before this period expires, the NS program will make reasonable attempts to contact the notifier. A minimum of 30 days’ notice before the expiry date will be provided to the notifier to update their claim if they wish the substance identity to remain confidential for an additional period of 10 years. To update a claim, notifiers need to follow the instructions described above in this section.
7.2.3 Certain purposes for which information may be disclosed
There may be instances where the Government of Canada would wish to release certain confidential information publicly. These would include, but not be limited to, situations where it is in the interest of public health, public safety or the protection of the environment or when it is necessary for the purposes of the administration or enforcement of the Act.
In these situations, a review will be done to determine whether certain information claimed as confidential could be released to promote transparency or because it is in the best interest of Canadians. Reasonable effort will be made to contact the submitter of the information who will be asked to provide additional information to substantiate their original claim.
Under section 316 of the Act, information may be disclosed in any of the following circumstances:
- (a) with the written consent of the person who provided it or on whose behalf it was provided
- (b) as may be necessary for the purposes of the administration or enforcement of this Act
- (c) under an agreement or arrangement between the Government of Canada or any of its institutions and any other government in Canada, the government of a foreign state or an international organization or any of its institutions, or between the Minister and any other minister of the Crown in right of Canada, where
- (i) the purpose of the agreement or arrangement is the administration or enforcement of a law and
- (ii) the government, international organization, institution or other minister undertakes to keep the information confidential
- (d) under an agreement or arrangement between the Government of Canada and the government of a foreign state or an international organization, where the government or organization undertakes to keep the information confidential or
- (e) to a physician or prescribed medical professional who requests the information for the purpose of making a medical diagnosis of, or rendering medical treatment to, a person in an emergency
7.3 Determining presence of confidential substances on lists
Substances on the confidential portion of the DSL or NDSL are published with Confidential Substance Identity Numbers using masked identities that are named in a manner prescribed by the Masked Name Regulations as specified above. Any notifier who intends to manufacture or import a substance that they believe to be on the confidential portion of either of these lists may seek confirmation from the NS program. The NS program will respond to such an inquiry only if the notifier provides the NS program with a confidential search request. For more information about this, consult section 2.3.1.
Section 8. Recommended test protocols and alternative approaches
The New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) prescribe technical information which the notifier must submit using test data or waiver requests. In recognition of tests intended to replace, reduce or refine the use of animals in traditional toxicity tests, the New Substances (NS) program accepts the use of appropriate alternative approaches (also known as New Approach Methods (NAM)) to meet these technical information requirements (consult section 8.4).
8.1 Organisation for Economic Co-operation and Development Test Guidelines
Subsection 15(1) of the Regulations states that the conditions to be met and the test procedures to be followed in developing the required test data for a substance must be consistent with the conditions and procedures set out in the Organisation for Economic Co-operation and Development (OECD) Guidelines for the Testing of Chemicals (the OECD Test Guidelines (TGs)) that are current at the time the test data are developed. The OECD TGs are set out in Annex 1 of the OECD Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted by the OECD on May 12, 1981.
The appropriateness of the OECD TG method for the substance must be determined, and any necessary deviations must be reported and explained. The OECD TGs are not intended to serve as rigid test procedures appropriate for all substances; rather, they allow flexibility for expert judgement and adjustments to new developments.
8.2 Recommended test methods
Examples of test methods, based on the OECD TGs, recommended by the NS program for the generation of physico-chemical, toxicity and ecotoxicity data are provided in Tables 8-1 to 8-4 below. The acceptability of these test methods depends on the applicability of the methods to the substance under investigation. Sources of test methods listed in Tables 8-1 to 8-4 are given in section 8.6 of this Guidance Document.
| Data requirement | Schedules | Test method |
|---|---|---|
| Melting point | 5, 6 | OECD TGa 102 |
| Boiling point | 5, 6 | OECD TG 103 |
| Density | 5, 6 | OECD TG 109 |
| Vapour pressure | 5, 6 | OECD TG 104 |
| Water solubility | 5, 6 | OECD TG 105 |
| Octanol/water partition coefficient | 5, 6 | OECD TG 107 or 117 |
| IRb, UVc, mass or NMRd spectrum | 6 | As appropriate |
| Adsorption–desorption | 6 and high release to the aquatic environment (subsection 7(2) of the Regulations) | OECD TGs 106, 121 as appropriate |
| Hydrolysis rate as a function of pH | 6 and high release to the aquatic environment (subsection 7(2) of the Regulations) | OECD TG 111 |
a OECD TG – Organisation for Economic Co-operation and Development Test Guideline.
b IR – Infrared.
c UV – Ultraviolet.
d NMR – Nuclear Magnetic Resonance.
| Data requirement | Schedules | Test method |
|---|---|---|
| Number average molecular weight | 3, 9, 10, 11 | As appropriate (for example, OECD TGa 118) |
| Residual constituents with molecular weight < 500 daltons and < 1000 daltons | 3, 9, 10, 11 | As appropriate (for example, OECD TG 119) |
| Water extractability | 10, 11 | OECD TG 120 |
| Hydrolysis rate as a function of pH | 10, 11 | OECD TG 111 |
| Octanol/water partition coefficient | 10, 11 | OECD TG 117 |
a OECD TG – Organisation for Economic Co-operation and Development Test Guideline.
| Data requirement | Schedules | Test method |
|---|---|---|
| Acute mammalian toxicity | 5, 6, 10, 11 | OECD TGsa 402, 403, 420, 423, 425, 436 |
| Skin irritation | 6, 11 | OECD TG 404, 430, 431, 439 ; consult also section 6.3.3.2 |
| Skin sensitization | 6, 11 | OECD TGs 406, 429, 442 (A-E)b |
| Repeated-dose toxicity | 6, 11, high release to the aquatic environment and/or significant public exposure (subsections 7(2), 7(3), 11(2) and 11(3) of the Regulations) | OECD TGs 407, 408, 409, 410, 412, 413, 422 |
| Genotoxicity | 5, 6, 11, high release to the aquatic environment and/or significant public exposure (subsections 7(3), 11(2) and 11(3) of the Regulations) | OECD TGs 471, 473, 474, 475, 476,c 487,c 488, 489, 490c |
a OECD TG – Organisation for Economic Co-operation and Development Test Guideline.
b Provide 442A, 442B or 442 (C-E) to address the skin sensitization data requirement.
c Tests recommended for in vitro genotoxicity testing of nanomaterials.
| Data requirement | Schedules | Test method |
|---|---|---|
| Acute fish toxicity | 5, 6, 10, 11 | OECD TGa 203, Environment Canada Biological Test Methods EPS1/RM/9 and EPS1/RM/13 |
| Acute Daphnia toxicity | 5, 6, 10, 11 | OECD TG 202, Environment Canada Biological Test Method EPS1/RM/11 |
| Algae toxicity | 5, 6, 10, 11 | OECD TG 201, Environment Canada Biological Test Method EPS1/RM/25 |
| Ready biodegradability | 5, 6, 11 | OECD TG 301 |
a OECD TG – Organisation for Economic Co-operation and Development Test Guideline.
8.3 Reporting test data
The notifier is obliged to submit a test report with sufficient information to allow the NS program to perform a thorough assessment and evaluation of the quality of these studies and their results. When submitting test data, or data using alternative approaches (consult section 8.4), to fulfill a prescribed information requirement, the full study must be provided, including the following information:
- Identification of the test guideline and methodology employed
- Identification of the test substance (the name of the substance in the test report provided must correspond to the name in block A.19 of New Substances Notification (NSN) Form), and its full composition (consult section 6.2.25)
- Reference methods, standards and controls employed
- Name and address of the test facility and the name of the person responsible for the study
- Dates on which the study was initiated and completed
- Raw data
- Deviations from the test protocol
- Analytical details, including sample preparation and instrument settings and
- A presentation of results, calculations and statistical methods employed
8.3.1 Good Laboratory Practice
Subsection 15(2) of the Regulations states that the laboratory practices to be followed in developing data for the following tests must comply with the practices set out in the Principles of Good Laboratory Practice (GLP) that are current at the time the test data are developed. The principles are set out in Annex 2 of the OECD Decision of the Council Concerning the Mutual Acceptance of Data in the Assessment of Chemicals, adopted by the OECD on May 12, 1981:
- (a) acute mammalian toxicity tests
- (b) repeated-dose mammalian toxicity tests
- (c) genotoxicity tests
- (d) tests to assess skin irritation
- (e) skin sensitization tests
- (f) acute fish, Daphnia or algae toxicity tests and
- (g) biodegradation tests
If any of the tests mentioned above were commenced or completed before the day on which the Regulations came into force (that is, October 31, 2005), the laboratory practices used must be consistent with the practices set out in the Principles of GLP.
The Principles of GLP are intended to promote the quality and validity of test data and to establish a basis for mutual acceptance of data among jurisdictions at the international level. They cover the organizational processes and conditions under which studies are planned, performed, monitored, recorded and reported.
Consult the documents of the OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring.
To be GLP-compliant, the final test report must include the Chemical Abstracts Service (CAS) Registry Number, name or trade name and the purity of the tested substance. The following information must also be provided:
- The name, title and dated signature of the Study Director
- A GLP Compliance Statement from the Study Director
- The name, title and dated signature of the Principal Investigator
- The name, title and dated signature of the Quality Assurance Program
- Quality Assurance Statements from the Quality Assurance Program and
- Dates and explanations of Quality Assurance Audits, including in-life audits
Required studies submitted that are not compliant with GLP or do not contain the above-mentioned items will not be accepted, and the assessment period will not start until the appropriate and acceptable information has been provided.
Note that for studies of physico-chemical properties, GLP compliance is not mandatory.
8.3.2 Accreditation of laboratories
If the test data submitted are from an accredited facility, then the accreditation should be stated and identified.
8.4 Alternative approaches
The NS program accepts the use of appropriate alternative approaches (also known as NAM) to meet technical information requirements. For example, information in support of an NSN may be obtained from alternative test protocols, surrogate substances, or from calculation or estimation methods, instead of generating new test data on the notified substance. These alternative approaches will be acceptable when, in the opinion of the NS program, they are determined to provide a scientifically valid measure of the endpoint under investigation that is deemed sufficient for the purposes of the risk assessment.
Requests for waivers of information are not required when submitting information from an acceptable alternative approach.
Although not required, notifiers are encouraged to submit a Pre-notification Consultation (PNC) request (consult section 8.8), while the NSN is being prepared, in order to seek advice on the acceptability and use of the alternative approaches to meet a technical information requirement.
8.4.1 Alternative test protocols
Alternative protocols include other domestic or internationally recognized protocols, for example, test methods developed or recognized by the NS program, the International Organization for Standardization (ISO), the American Society for Testing and Materials (ASTM), the United States Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the United States Toxic Substances Control Act (TSCA). In addition, protocols developed by individual companies or associations may also be acceptable, including, but not limited to, protocols for in vitro screening assays, mechanistic endpoints, toxicogenomics and other emerging technologies.
The alternative protocol must be clearly referenced and provide the desired data to a degree of accuracy acceptable to the NS program and must be described by the notifier in sufficient detail to allow an evaluation of the procedure and results. The NS program assesses whether the alternative protocol provides sufficient information compared to the relevant OECD guideline (consult section 8.1) and ensures the data was produced with a degree of accuracy acceptable to the program so that proper evaluations can be conducted.
The description of the alternative protocol should include, but not be limited to:
- a detailed description of the test principles and design
- the methodology and controls used
- validation studies of the accuracy and variability of the test method in comparison with the prescribed method and
- any references to the protocol in the scientific or technical literature
The notifier should submit a test report with sufficient information to allow the NS program to perform a thorough assessment and evaluation of the quality of these studies and their results (consult section 8.3).
For example, a human repeat insult patch test (positive or negative response) may be an acceptable alternative to animal testing for skin irritation or skin sensitization. The concentration of substance used in the test will be a critical factor in determining the acceptability of this information. Well-documented human use reports may also be an acceptable alternative to the prescribed test protocols for toxicological endpoints, especially skin irritation or skin sensitization tests (for positive responses only). The human use experience must be well-described and give particular emphasis to quantifying the exposure (concentration, duration, frequency) as accurately as possible. Anecdotal information from persons handling or exposed to the substance is not an acceptable alternative for performing a prescribed test.
Although not required, notifiers are encouraged to submit a PNC request (consult section 8.8), while the NSN is being prepared, in order to seek advice on the acceptability and use of the alternative protocol.
8.4.2 Replacement, reduction or refinement
The NS program supports the principles of the replacement, reduction or refinement approach to the use of alternative test protocols for minimizing unnecessary and avoidable animal use and suffering, where the quality of the information generated to conduct a risk assessment is not compromised. The method must have been satisfactorily validated in terms of scientific rigour, reproducibility and predictability.
A replacement alternative is one that does not involve the use of mammals. These alternatives include the use of validated computer-based models, physico-chemical information (for example, information about pH to assess irritation potential), non-mammalian organisms (for example, zebrafish, amphibians) and in vitro tests on mammalian tissues, embryos, and cell cultures.
An alternative that would reflect the reduction approach is one in which the number of animals needed to assess a particular endpoint can be decreased without compromising the scientific value of the test. Examples of reduction alternatives already accepted by international regulatory agencies include the updated OECD TGs for acute toxicity (OECD TGs 420, 423 and 425) and skin sensitization (OECD TG 429). Another example of a reduction approach is the use of organisms other than mammals (for example, zebrafish embryos) to assess a given endpoint.
Refinement alternatives are aimed at improving the design or efficiency of a test to better predict toxicity (for example, including additional biomarkers such as immunochemistry markers for the analysis of a blood sample). By increasing the number of toxicity endpoints evaluated in a single test, the need for additional whole organism tests is decreased. Refinement can also involve increased measures to reduce distress or discomfort experienced by laboratory animals, during and following testing, such as group housing to decrease isolation anxiety and the elimination of unnecessary handling and restraint of animals.
8.4.3 Use of surrogate data
Read-across is an approach where an experimentally derived endpoint from one substance (called a surrogate or analogue substance) is used to predict the same endpoint for another substance that is considered to be similar with respect to that endpoint. The general principle of read-across is that substances which are similar in one or many aspects should also be similar in other aspects, such as physical, chemical, mammalian toxicological and ecotoxicological properties.
Read-across is acceptable when, in the opinion of the NS program, the data on the surrogate are as suited as, or better suited than, the data on the notified substance for measuring the endpoint under investigation. This approach can be used to fulfill a data requirement prescribed by the Regulations for which experimental data or study information are not available. However, it is important to note that a single surrogate substance may not be appropriate to fulfill all the data endpoints of a notified substance.
Supporting read-across information may be qualitative (for example, the substance is mutagenic) or quantitative (for example, median effective concentration (EC50)).
8.4.3.1 Justification for using surrogate data
Any surrogate data submitted in lieu of experimental data on the notified substance must be supported by a scientific rationale justifying the selection of the surrogate substance and use of the read-across approach. A separate rationale is required for each technical requirement for which surrogate data is used. In addition to the written rationale, a table with a side-by-side comparison of the notified and surrogate substance(s) is recommended (consult section 8.4.3.3). In the justification, the following information should be provided for both notified and surrogate substances:
- Identification information, including but not limited to the structural formula, molecular weight and functional group(s) and
- Physico-chemical and environmental fate data
For ecotoxicological and mammalian toxicological endpoints, in addition to water solubility and octanol/water partition coefficient comparisons, the following information should also be provided, if available:
- Mode of action
- Mechanism of action
- Bioavailability
- Reactivity
- Toxicokinetics
- Metabolic pathways and products and
- Degradation pathways and products
The structural formula should be presented graphically. It should be detailed and the key structural elements or functional groups that are likely to affect a particular endpoint should be identified. Both structural similarities and differences between the substances should be considered and discussed.
For ecotoxicological and mammalian toxicological endpoints, confidence in surrogate data may be strengthened by showing that the notified and surrogate substances fit into a certain chemical group which has a pattern of potency or toxicological similarity across the group.
When surrogate data are being used to fulfill a regulatory requirement, full test reports for the studies and their results must be provided, including elements described in section 8.3 of this Guidance Document. The NS program recommends that full test reports for any other studies used in the comparison be provided. If available, an assessment of the reliability of the study results should also be provided.
If literature papers are referenced, a copy of each paper must be provided. Reviews by other regulatory agencies should be provided, if available.
Quantitative Structure–Activity Relationship (QSAR) estimates may be used to support the comparison of the notified and surrogate substances in a read-across approach, especially if the validity of QSAR estimate can be demonstrated (consult section 8.4.4).
8.4.3.2 Additional justification requirements for certain substance categories
The justification for the substance categories described below should include, but not be limited to, a comparison of the following additional points for both notified and surrogate substances:
- Polymers:
- Molecular weight information (that is, Gel Permeation/Size-exclusion Chromatography (GPC/SEC) chromatograms, polydispersity, number average molecular weight (Mn), weight average molecular weight (Mw), weight percent less than 500 and less than 1 000 daltons)
- Monomer composition and initial starting concentrations of monomers used to synthesize the polymers
- Concentrations or amounts of any residual or excess monomers
- The presence of any reactive functional groups, and any associated functional group equivalent weight (FGEW) calculations (consult section 3.3.1.8) and
- Reaction scheme of the polymer(s)
- Substances of Unknown or Variable composition, Complex reaction products or Biological materials (UVCBs):
- Composition (representative structures and ratios)
- Toxicity of major or key constituents and
- Starting materials and reaction conditions
- Inorganic chemicals:
- Crystal structure or 3-dimensional data
- Metal moiety and dissolution rate
- Ionic state and
- Stability
- Biochemicals and biopolymers (for example, enzymes):
- Primary structure (amino acid sequence) aligned with the notified substance to highlight differences and
- Enzyme-specific properties (for example, catalytic constant KM and Kcat, optimum pH and temperature; consult section 6.4.2)
The factors considered for the acceptance of any potential surrogate for the substance categories described above may be complex; so notifiers are encouraged to submit a PNC request (consult section 8.8).
8.4.3.3 Comparison table
To facilitate the comparison of the notified and surrogate substances, it is recommended that their data be placed in a table. Examples of comparison tables that can be used to structure the comparisons have been provided below for notifiers’ convenience. If multiple surrogates are used, they can be arranged in multiple columns in a suitable order (for example, according to molecular weight) to show a trend or progression for a target endpoint across the group. The cells in the table should also indicate whether the data are unavailable. Where applicable, a qualitative description may also be provided. If possible, reliability of the study results should also be indicated.
These are general tables covering some sample endpoints; they are not meant to be an exhaustive list of all possible endpoints. Since the use of the read-across approach is endpoint-specific, all of the endpoints for which a potential surrogate is being submitted for consideration must be clearly indicated.
| Parameter | Type | Notified substance | Surrogate substance 1 |
|---|---|---|---|
| CAS Registry Numbera | Identification | * | * |
| Chemical name | Identification | * | * |
| Structural formula (image) | Identification | * | * |
| Molecular weight (g/mol) | Identification | * | * |
| Similarity indices (for example, Tanimoto, Dice) | Identification | * | * |
| Relevant functional groups or structural features | Identification | * | * |
| Vapour pressure (Pa or mm Hg) | Physico-chemicalb | * | * |
| Water solubility (mg/L) | Physico-chemicalb | * | * |
| Critical micelle concentration | Physico-chemicalb | * | * |
| Octanol/water partition coefficient | Physico-chemicalb | * | * |
| Hydrolysis as a function of pH | Physico-chemicalb | * | * |
| Other (as needed) | Physico-chemicalb | ** | ** |
| Biodegradationc | Environmental fate | * | * |
| Fish chronic toxicity | Ecotoxicologicald | ** | ** |
| Daphnia acute toxicity | Ecotoxicologicald | ** | ** |
| Other (as needed) | Ecotoxicologicald | ** | ** |
| Acute mammalian toxicity | Toxicologicale | ** | ** |
| Chronic and sub-chronic toxicity | Toxicologicale | ** | ** |
| Sensitization | Toxicologicale | ** | ** |
| Other (as needed) | Toxicologicale | ** | ** |
a CAS – Chemical Abstracts Service.
b SI units are preferred for all endpoints.
c Amount and identity of any stable degradation products.
d Endpoints such as LC50 – median lethal concentration, LOEC – lowest-observed-effect-concentration, NOEC – no-observed-effect concentration, including species and duration.
e Endpoints such as LD50 – median lethal dose, LOEC, NOEC, including species and duration.
* Denotes parameters that should be included in the comparison table. Qualitative description may be provided where applicable. If the data are unavailable, this should be indicated.
** Denotes parameters that are provided here as example. Qualitative description may be provided where applicable.
| Parameter | Type | Notified polymer | Surrogate polymer 1 |
|---|---|---|---|
| CAS Registry Numbera | Identification | * | * |
| Chemical name of the polymer | Identification | * | * |
| Representative structural formula (image) | Identification | * | * |
| Monomer 1 concentration (%)b | Identification | * | * |
| Monomer 2 concentration (%)b | Identification | * | * |
| Monomer 3 concentration (%)b | Identification | * | * |
| Polymer molecular weight distribution and polydispersity for example, Mnc / Mwd, Mw/Mn | Physico-chemical | * | * |
| Weight percent < 1000 daltons | Physico-chemical | * | * |
| Weight percent < 500 daltons | Physico-chemical | * | * |
| Reactive functional groups and FGEWe | Physico-chemical | * | * |
| Other (as needed) | Physico-chemical | ** | ** |
a CAS – Chemical Abstracts Service.
b Include name and CAS Registry Number for all monomers.
c Mn – number average molecular weight.
d Mw – weight average molecular weight.
e FGEW – functional group equivalent weight.
* Denotes parameters that should be included in the comparison table.
** Denotes parameters that are provided here as example. Qualitative description may be provided where applicable.
8.4.4 Quantitative Structure–Activity Relationship estimates
QSAR estimates provide quantitative estimates of particular properties and are often generated by computer programs that use regression analysis or molecular descriptors that mathematically represent the structural components of a molecule. Linear or multiple regression of a particular property against another property (for example, octanol/water partition coefficient versus water solubility, or vapour pressure versus boiling point) can be used to derive an empirical relationship for one or several classes of chemicals.
The validity of the QSAR estimate must be explained in the NSN, in terms of whether the estimate is reasonable in comparison with measured data, taking into account the structural features of the notified substance in comparison with the structural features or chemical classes used to develop the estimate. The appropriateness of the QSAR must be discussed in terms of the ability of the model to correctly predict the targeted endpoint for the notified substance.
Information to support the acceptance of data based on QSARs should include:
- information about the model (for example, version of software), the input data and model output
- a validation of the estimate, including the reporting of types of chemicals and/or structures used to generate the estimate and the experimental data for these chemicals and
- the level of confidence associated with the estimate
A recommended method to support the acceptance of data based on QSARs is presented in the OECD Report from the Expert Group on (Quantitative) Structure–Activity Relationships [(Q)SARs] on the Principles for the Validation of (Q)SARs [PDF].
According to the 5 OECD validation principles, the QSAR model should:
- be associated with a defined endpoint
- be based on an unambiguous algorithm
- have a defined domain of applicability
- be associated with appropriate measures of goodness-of-fit, robustness and predictivity and
- be associated with a mechanistic interpretation, if possible
Any QSAR estimates generated by following these OECD validation principles should be adequately documented using 2 publicly available reporting formats: the QSAR Model Reporting Format (QMRF) and the QSAR Prediction Reporting Format (QPRF). The QMRF provides validation of the QSAR model itself, while the QPRF provides information about the applicability of the model to the chemical under consideration. Both of these documents should be provided, if available. The NS program will consider the adequacy of any QSAR estimate on a case-by-case basis, taking into account the validity and applicability of the model, as provided in the documentation.
The NS program assesses a wide variety of substances, many of which are considered “model difficult” due to the substance falling outside the applicability domain of a model, with features of the molecule not represented in the training set. Consequently, the NS program advocates the judicious use of modelled data. The use of predictive modelling for estimating substance properties should be limited to classes of well-understood chemicals for which there exists robust models developed with strong training sets.
Presently, the NS Program considers QSARs on nanomaterials to be under development, and therefore will require evidence that they are sufficiently reliable in using physico-chemical properties to make the targeted endpoint predictions.
Although not required, notifiers are encouraged to submit a PNC request (consult section 8.8), while the NSN is being prepared, in order to seek advice on the acceptability, use and documentation of estimates obtained from QSAR.
8.4.5 Weight of evidence approach
Where data are available through more than one of the alternative approaches listed in section 8.4, the NS program strongly encourages the use of different lines of evidence in a weight of evidence approach to support the technical information requirement of an NSN. The notifier may make use of any combination of these approaches to address a technical information requirement. The approaches may not on their own be sufficient to provide the required information; however, together with other information, it forms a body of evidence which may be sufficient to indicate the effect of a substance. For example, information may be gathered from the scientific literature on a surrogate substance and QSAR on the notified substance. The weight of evidence approach must be supported by a scientific rationale justifying the use of the different lines of evidence and explaining how they are relevant to characterize the risk of the notified substance (that is, the potential for the substance to be harmful to human health or the environment according to the criteria under section 64 of the Canadian Environmental Protection Act, 1999 (the Act)).
Although not required, notifiers are encouraged to submit a PNC request (consult section 8.8), while the NSN is being prepared, in order to seek advice on the acceptability of using multiple alternative approaches as a weight of evidence.
8.5 Test data on UVCBs and impure substances
UVCB substances are defined as substances of unknown or variable composition complex reaction products or biological materials. These materials are derived from natural sources or complex reactions and are considered single substances for notification purposes and under the New Substances provisions of the Act; therefore, all tests should be performed on the entire UVCB substance. Where a prescribed test is not appropriate (for example, melting point), the use of alternative methods should be considered (for example, softening point). Also, the provision of information about any of the known constituents of the UVCB substance will assist in the interpretation of data generated on the UVCB substance.
Due to the complex nature of this group of substances, the NS program encourages notifiers to submit any available additional information about starting materials, reaction steps and mechanisms related to the UVCB substances which will assist in the risk assessment.
Difficulties may also occur when testing substances that contain high levels of impurities (for example, residual starting materials, solvents and by-products), because impurities can confound the interpretation of test data. Consequently, tests should be performed on a high-purity sample of the substance. However, if further purification of the substance is neither technically feasible nor practical, tests on the crude product may be acceptable. In all cases, the purity of the tested material must be stated and information documenting efforts to isolate the substance provided. Information about the physico-chemical or toxicological properties of any of the impurities will assist in the interpretation of the data generated on the impure substance. In cases where information generated about the mixture would not be meaningful for the assessment of the notified substance (for example, notified substance comprises only a very small proportion of the mixture and further purification is not feasible), a request for a waiver on the grounds of technical infeasibility will be considered (consult section 8.7.1).
8.6 Sources of test methods
Test methods can be accessed through the OECD, Environment and Climate Change Canada or the United States Environmental Protection Agency (US EPA) websites:
- OECD testing of chemicals
- Environment and Climate Change Canada biological test methods publications or
- US EPA National Service Center for Environmental Publications
8.7 Waiver requests for information requirements
8.7.1 Introduction
Under subsection 81(8) of the Act, a request to waive the requirement for any of the prescribed information may be made to the NS program. The decision to grant a waiver will be made on a case-by-case basis and will depend on whether at least one of 3 criteria has been met. The statutory criteria for a waiver of information, identified in subsection 81(8) of the Act, are the following:
- (a) in the opinion of the ministers,Footnote 17 the information is not needed in order to determine whether the substance is toxic or capable of becoming toxic
- (b) the substance is to be used for a prescribed purpose or manufactured at a location where, in the opinion of the ministers, the notifier requesting the waiver is able to contain the substance so as to satisfactorily protect the environment and human health or
- (c) it is not, in the opinion of the ministers, practicable or feasible to obtain the test data necessary to generate the information
Waiver requests may be submitted in writing as part of the NSN and should include a well-documented scientific rationale to support each request as well as an identification of the statutory criterion under which the request is being made. Failure to provide a proper rationale with supporting documentation will result in a delay of the start of the assessment period (consult sections 9.3.3 and 9.3.4). To determine whether waivers are acceptable and to avoid unnecessary delays, the NS program provides the opportunity for and encourages notifiers to submit a PNC request (consult section 8.8) while the NSN is being prepared.
Appendix 6 of this Guidance Document provides examples of conditions under which waivers may be granted. This list is not intended to be exhaustive, but describes some independent conditions that, in most cases, would be considered to be sufficient justification to grant a waiver. Waiver requests may also be based on a combination of factors (for example, physical properties, inherent toxicity and potential for exposure to the substance).
Once the waiver is granted, its particulars will be published in the Canada Gazette, Part I, in accordance with subsection 81(9) of the Act. The published waiver notice will contain only a) the name of the notifier (or company) to whom the waiver is granted; and b) the type of information to which it relates (for example, Company X, Data from a ready biodegradability test). The notice will not specify the substance to which the waiver applies or the NSN reference number.
Generally, the eligibility of a substance for addition to the DSL will not be affected by waivers granted under paragraphs 81(8)(a), 81(8)(b) or 81(8)(c) of the Act.
When waivers have been granted, the notifier must provide any corrections to the information used to justify and assess the waivers as per subsection 81(11) of the Act (consult section 10.1.1). The Minister of the Environment may then, if necessary, request that the notifier provide the information item that was waived or take appropriate control measures.
A waiver should not be requested when information to address the data element is provided about a surrogate substance or using alternative methods.
8.7.1.1 Waivers requested under paragraph 81(8)(a) of the Act
A waiver may be granted if it can be established that the test is unnecessary to determine whether the substance is toxic or capable of becoming toxic. In cases where the requirement for one part of a prescribed test depends on the result of a previous part (for example, mutagenicity test data), it is suggested that the tests be completed based on a self-evaluation of test results or a consultation with the NS program through a PNC request (consult section 8.8). After receipt of the PNC request or the NSN, the NS program will assess the submitted information to determine whether the information provided is acceptable.
8.7.1.2 Waivers requested under paragraph 81(8)(b) of the Act
A waiver may be granted if the substance is to be used for a purpose prescribed by regulations. No regulations have been developed in relation to these waivers.
Waivers may also be granted if the substance is manufactured at a location where, in the opinion of the ministers, the person requesting the waiver is able to contain the substance so as to satisfactorily protect the environment and human health.
8.7.1.3 Waivers requested under paragraph 81(8)(c) of the Act
Many of the potential waivers that can be requested under paragraph 81(8)(c) relate to instances where it is technically arduous or impossible to perform the required tests using conventional technology because of the physical or chemical properties of the substance.
The use of alternative protocols or surrogate data to fulfill the information requirement should be considered before it is judged to be infeasible or impractical to provide certain information. In these cases, a waiver should not be requested. The cost of obtaining data cannot be used as the sole reason for the infeasibility or impracticability of providing the prescribed information.
8.7.2 Class considerations of waivers
As a result of the NS program’s experience in assessing new substances, a body of knowledge now exists on classes of substances that can be applied to newly notified substances in those classes. A systematic review of the properties of a class, corresponding to regulatory requirements, can reveal established trends in its properties. In such cases, information for specified endpoints for notified members of the class will likely not be needed to determine whether the substance is toxic or capable of becoming toxic.
Notifiers who are preparing NSNs for substances that meet the definition of such a class may request waivers in relation to data concerning specified endpoints under paragraph 81(8)(a) of the Act.
Notifiers may contact the NS program through the Substances Management Information Line to discuss the information needed to nominate a new class of substances.
8.7.2.1 Cationic class waivers
Available information is considered sufficient to indicate that a class comprising certain cationic polymers is expected to have low toxicity in the health toxicological tests prescribed in the Regulations. Thus, notifiers may request waivers for all the toxicological test requirements for polymers that meet this class definition.
Currently, this class is defined as polymers that are Non-Reduced Regulatory Requirement polymers solely due to the presence of the following cationic or potentially cationic groups:
- Primary, secondary or tertiary amine groups
- Carbodiimides or
- Sulphoniums
Polymers containing other cationic polymers (such as quaternary amines, hindered amines, azides, isocyanates (free and blocked) and phosphoniums) are not included in the above class definition, either because there is currently insufficient information available regarding their toxicity to warrant their inclusion or because available information indicates that there are adverse effects associated with them. For cationic polymers that do not meet the above definition and therefore are not eligible for a class waiver, notifiers may still request waivers with sufficient rationale for specific tests or submit surrogate information for consideration by the NS program on a case-by-case basis. As well, polymers with a Mn greater than 10 000 daltons will generally not be eligible for waivers for acute and repeated-dose toxicity tests if inhalation is expected to be the most significant route of exposure for the general population based on expected use.
8.8 Pre-notification Consultation
A PNC is an option for notifiers who wish to consult with the NS program during the planning or preparation of their NSN to discuss any questions or concerns regarding information requirements. A PNC request is recommended when assistance is needed in determining the acceptability of waiver requests, test protocols, consolidated notifications, alternative data, other endpoints (consult section 6.5.4), or when clarification is needed regarding substance classification.
PNC requests can be addressed in writing (by mail or email) or through a meeting or conference call.
For meeting and conference call PNC requests, the NS program will make every effort to respond to the proposed queries during the meeting. The NS program requests a minimum of 2 weeks between receiving the preliminary PNC request, which contains sufficient information, and conducting the meeting. This allows time for the NS program to make an informed response to the question(s) at hand during the meeting.
For chemical and polymer PNC requests, the NS program will make every effort to respond in writing to the queries within a period of 30 days. This period will start after sufficient information has been provided for the PNC request to proceed.
The information required to begin a PNC includes:
- substance identity information (substance name, trade name, CAS Registry Number, molecular structure, list of reactants)
- contact information (contact name, title, company, email, and mailing address)
- intended notification Schedule of the Regulations
- intended use(s) of the substance
- the specific questions and/or concerns to be answered by the NS program
- any data and test reports in the notifier’s possession, if applicable
- Confidential Business Information (CBI) claims (corporation, manufacture, import, amount, substance identity, use), if applicable and
- a brief agenda, if a conference call is requested
The NS program will give opinions based on the information received with the PNC request. The professional opinions of the NS program, expressed during the PNC, are not an official commitment, since technical conclusions may differ after a more in-depth assessment has been conducted on the complete NSN.
In addition to PNC requests, the NS program encourages discussions to clarify any other issues related to the NS program.
It is recommended to submit a PNC request with the Pre-notification Consultation Form available on the NS program website. The completed form can be securely submitted through the Environment and Climate Change Canada Single Window Information Management (SWIM) system or emailed to the Substances Management Information Line.
Section 9. Processing a New Substances Notification
This section describes the administrative procedures and responsibilities of the New Substances (NS) program when a New Substances Notification (NSN) is received.
9.1 Overview of the New Substances Notification assessment process
Figure 9-1 gives an overview of the assessment process from the day the NSN is received by the NS program to the day the substance is added to the Domestic Substances List (DSL) or risk management measures are taken on the substance.

Long description
The new substances notification assessment process begins when the NS program receives a complete NSN. Then, the NSN is subject to preliminary content screening before progressing to a joint evaluation by Environment and Climate change Canada for environmental risk assessment and Health Canada for human health risk assessment. The Minister of the Environment and the Minister of Health assess the information and determine if the substance is suspected to be toxic according to the criteria under section 64 of the Act .
- If the substance is suspected to be toxic:
- Risk management measures are developed in consultation with the notifier, including ministerial conditions, ministerial request for addition information or prohibition of manufacture and import.
- If the substance is not suspected to be toxic:
- The notifier is provided with a notice of assessment decision confirming that the manufacture or import may proceed.
- When a significant new activity (SNAc) in relation to the substance could result in the substance being harmful to human health or the environment, a SNAc Notice is issued.
- The substance is added to the DSL when the listing criteria are met.
9.2 Receipt of a New Substances Notification
9.2.1 Assessment period
The assessment period refers to time, in calendar days, allotted for the NS program to assess a NSN. The number of days for an assessment period is indicated in Table 1‑1.
Day 1 of an assessment period is the day following the day on which the complete NSN is received by the NS program. The assessment period may be affected by missing or incomplete information. For example:
- if an NSN is submitted without fees (consult fee table on the New substances notification fees webpage), the assessment period will not begin until the fees are received
- if an NSN is grossly inadequate or missing information prescribed in the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations), the assessment period will not begin until a corrected NSN has been received
- if proprietary information is being sent directly to the NS program by a Third Party Information Supplier, the assessment period will not start until all the required information has been received
- if minor information is found to be missing or erroneous during the assessment, the assessment period would continue, provided the correct information is supplied by a date specified by the program (usually within a few working days) or
- if during the assessment period, the information within the NSN is found to be incomplete or erroneous, it will be deemed incomplete and the assessment period will be restarted on Day 1 once the complete NSN has been submitted
9.3 Correspondence
Official correspondence between the NS program and the notifier or the “Canadian Agent” will occur throughout the assessment process. The NS program will communicate with the notifier via email. Notifiers who still wish to receive correspondence by mail should make the request in their NSN cover letter. lf no such request is made, originals will not be sent by mail. Sections 9.3.1 to 9.3.6 describe types of correspondence a notifier may receive for NSNs and Significant New Activity Notifications (SNANs).
9.3.1 Notice of initiation
When a Third Party Information Supplier (consult section 5.2) is involved in an NSN, the notifier must submit a partial NSN to initiate the process. A notice of initiation is issued to the notifier to acknowledge receipt of this partial information that is required to complete the NSN. The assessment period does not start until all of the prescribed information has been provided by the Third Party Information Supplier. Once the complete package of information has been received, a notice of acknowledgement of the complete NSN is issued (consult section 9.3.2), and the assessment period will start.
9.3.2 Notice of acknowledgement of complete New Substances Notification
After receipt and acceptance of the information provided in the NSN, a notice of acknowledgement will be issued specifying the starting date of the assessment period and the NSN reference number. A notice of acknowledgement indicates that the administrative information is satisfactory and that all prescribed information including the prescribed fees have been received, but the file has not yet been assessed. The notice of acknowledgement also provides the expected end date of the assessment period.
A notifier may, either at the time of filing or after an NSN has been filed, request an early termination of the assessment period (subsection 83(6) of the Canadian Environmental Protection Act, 1999 (the Act)) specified for that specific Schedule in the the Regulations. A notifier may include a target date for early termination and a reason for why early termination is being requested. If such a request is received, the notice of acknowledgement would indicate that the NS program will consider the request during the assessment period. Although the NS program will make every effort to terminate the assessment by the target date, a request for early termination does not guarantee accommodation.
9.3.3 Notice of missing information
A notice of missing information will be issued if the NSN contains omissions or errors in the mandatory prescribed information requirements. This notice will describe all deficiencies in the NSN. Consult section 9.2.1 of this Guidance Document for examples of reasons for this type of notice. The assessment period does not start until all the required information has been received and accepted. If the omissions or errors are identified following the communication of a notice of acknowledgement of complete NSN, the assessment period may be restarted at Day 1 when the additional or corrected information is received.
9.3.4 Notice of rejection
A notice of rejection will be issued if the NSN contains significant omissions or errors in the mandatory information requirements. This notice will describe all deficiencies in the NSN. Original documentation may be returned. Consult section 9.2.1 of this Guidance Document for examples of reasons for this type of notice.
9.3.5 Notice of extension of assessment period
All NSNs and SNANs are eligible to have their assessment periods extended when additional time is required to complete an assessment. The Minister of the Environment (the Minister) may extend the assessment period only once, for a length of time not exceeding the time prescribed for the initial assessment period. Typically, an extension of assessment period will occur when control measures are considered. The notifier will be issued a notice of extension of assessment period at or before the end of the initial assessment period, advising them that the assessment period has been extended.
9.3.6 Notice of assessment decision
The assessment decision will be communicated to the notifier at or before the end of the applicable assessment period.
Note: Pursuant to subsection 83(6) of the Act, the assessment period of an NSN or SNAN may be terminated early. These provisions would be implemented when the evaluation is completed prior to the end of the prescribed assessment period. In such a case, the notice would indicate the day on which the assessment period ends.
Based on the assessment conclusions (consult section 9.6), the notice will indicate that:
- there is no suspicion that the substance is toxic or capable of becoming toxic
- there is no suspicion of toxicity for the current activities associated with the substance, but it is suspected that other activities could result in the substance becoming toxic or
- there is a suspicion of toxicity and risk management measures are imposed
When applicable, the notice will also indicate that manufacture or import may begin either in amounts exceeding the quantity that triggered the notification, or in accordance with the terms of the risk management measures imposed.
The notice may also include any additional information required for the substance to become eligible for addition to the DSL.
9.4 Withdrawing a New Substances Notification
A notifier may request that an NSN be withdrawn if it is determined that:
- the substance has been previously notified by the same company at that threshold
- the substance is already on the DSL or
- the notifier no longer intends to manufacture or import the substance in a quantity at or above the threshold, and the quantity already manufactured or imported does not exceed this threshold
Withdrawal requests for NSNs can be emailed or mailed to the NS program. Withdrawal requests will not be accepted if the notifier has been informed of a proposed decision to take risk management measures or issue a Significant New Activity (SNAc) Notice for the substance. The notifier will be advised in writing as to whether the withdrawal request has been accepted or rejected.
9.5 Assessment of the New Substances Notification
The purpose of the assessment and risk management process is to ensure that, either because of the inherent properties of the substance or because of measures taken to mitigate exposure to the substance, the use of the substance will not pose a risk to human health or the environment.
9.5.1 Information review
Evaluators within the NS program will assess the NSN to determine the acceptability of:
- the substance identity and masked names
- claims for Confidential Business Information
- test protocols and procedures
- test data
- rationales for requests for waivers of information
- rationales for use of alternative test protocols or surrogate information and
- exposure information
Deficiencies in the submitted information that cannot be easily resolved may result in rejection of the NSN and termination of the assessment period (consult section 9.3.4).
9.5.2 Determination of toxicity
The purpose of the NSN assessment process is to determine whether or not the substance is toxic or capable of becoming toxic as per any of the criteria set out in section 64 of the Act and stated below:
64. […] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- (a) have or may have an immediate or long-term harmful effect on the environment or its biological diversity
- (b) constitute or may constitute a danger to the environment on which life depends or
- (c) constitute or may constitute a danger in Canada to human life or health
Consequently, the determination of whether a substance is, or is suspected of being, toxic or capable of becoming toxic involves assessment of the potential for exposure of humans and components of the environment and of the adverse effects of the substance on humans or the environment (including other living organisms, interacting natural systems and the abiotic components of the environment).
The potential for exposure to a substance depends on the quantity, rate, frequency and conditions of release of the substance into the environment at all points in its life cycle, as well as the mobility, environmental compartmentalization and persistence of the substance. The exposure assessment considers the use of the substance identified by the notifier, as well as other possible ways in which the substance might be used if it were on the DSL without restrictions.
The assessment of adverse effects on humans and other living organisms considers endpoints such as lethality, mutagenicity, reproductive and developmental effects and organ toxicity, whereas adverse effects on the abiotic components of the environment include consequences such as depletion of the ozone layer, global warming and production of acid rain.
A substance may be suspected of being toxic if either the adverse effects of a substance or the potential exposure to a substance is of concern. For example, substances with considerable potential for exposure because of continuous release of high quantities or persistence in the environment may be suspected of being toxic, although there may be uncertainty regarding any biological or environmental hazard from the information available for the initial assessment. When an assessment has led to a “suspicion of toxicity,” the Act has a unique provision, under subsection 84(1), which permits the Minister to undertake one of several risk management measures (consult section 9.6).
9.6 Assessment conclusions
There are 3 possible outcomes of an assessment:
- There is no suspicion of toxicity, and no action is taken
- There is no suspicion of toxicity for the current activities associated with the substance, and a SNAc Notice is published for the substance because it is suspected that other activities could result in the substance becoming toxic (consult section 9.6.2) or
- There is a suspicion of toxicity, and risk management measures are imposed (consult section 9.6.3)
The notifier will be advised in writing, before the end of the assessment period, if the NS program suspects that the substance is toxic or capable of becoming toxic, and will be informed as to what action will be taken. The notifier will also be advised in writing, before the end of the assessment period, if the NS program intends to develop a SNAc Notice in relation to the substance (consult section 9.6.2).
9.6.1 No suspicion of toxicity and no action taken
If the completed environmental and human health assessment reports on the notified substance determine that there is no suspicion that the substance is toxic or capable of becoming toxic, no action is taken. If no action is taken prior to the end of the assessment period, the notifier may, after the assessment period has expired, commence manufacturing or importing the substance in amounts exceeding the quantity that triggered the notification.
9.6.2 Significant New Activity Notices
A new substance assessment takes into consideration potential risks concerning the notified activities as well as any other possible activities involving the substance. When there is suspicion that a significant new activity may result in the substance becoming toxic, the SNAc provisions of the Act (consult section 85 of the Act) can be applied to a new substance with the publication of a SNAc Notice in the Canada Gazette, Part I. A SNAc Notice is published within 90 days of the end of the assessment period. Typically, the notifier is informed of the development of a SNAc Notice prior to the end of the assessment period.
A SNAc Notice describes activities that may result in:
- a significantly greater quantity or concentration of the substance in the environment or
- a significantly different manner or circumstances of exposure to the substance
The SNAc Notice includes:
- the identification of the substance (by explicit substance name, or by masked name if claimed confidential)
- a description of the significant new activities
- a description of the information that must be provided to the Minister before the start of the significant new activities
- timelines for the notifier to provide the information and
- the period for the Minister of the Environment and the Minister of Health (the ministers) to assess the information
A SNAc Notice applies to anyone using the substance. Any person wishing to engage in a significant new activity in relation to the substance is required to submit a SNAN to the Minister containing all of the information prescribed in the Notice prior to using the substance for the proposed activity. After the complete notification is received, the ministers assess the information provided and other available information, within the timelines set out in the Notice.
Reporting obligations under the Regulations and the Act apply, whether or not a SNAc Notice has been issued for a new substance. Where applicable, the notifier is required to provide:
- the subsequent Schedules of information under the Regulations, if necessary
- the prescribed additional information in subsection 7(2), 7(3), 11(2) or 11(3) of the Regulations in the case of high release to the aquatic environment or significant public exposure and
- the appropriate notice to fulfill the criteria for DSL addition (consult section 10)
The substance may become eligible for addition to the DSL once the above-mentioned information has been received, accepted and assessed. Until the substance is added to the DSL, other notifiers must continue to notify the manufacture or import of the new substance as specified by the Regulations.
The SNAc provisions of the Act can also be applied to a substance on the DSL with the publication of a SNAc Order in the Canada Gazette, Part II. When a substance subject to a SNAc Notice is added to the DSL, this SNAc Notice no longer applies. To maintain the reporting obligations on the substance, the SNAc requirements are added to the DSL with the publication of a SNAc Order.
After a complete SNAN is received, the NS program will assess the information within the time period specified by the SNAc Notice or SNAc Order. From the assessment of this information, SNAc requirements may be varied or rescinded, or other risk management measures may be imposed, if necessary (consult section 9.6.3).
9.6.3 Risk management measures
When a substance is suspected to be toxic or capable of becoming toxic, risk management measures may be applied to mitigate any risk to human health or the environment. Notifiers will be advised, prior to the end of the assessment period, that there are concerns with the substance. Usually the assessment period is extended (consult section 9.3.5), which provides time to develop the risk management measure and obtain ministerial approval. The notifier will be advised of the extension of the assessment period and of proposed risk management measures prior to the end of the initial assessment period.
Section 84 of the Act states that when the ministers suspect that a substance is toxic or capable of becoming toxic, the Minister may:
- (a) permit any person to manufacture or import the substance subject to specified conditions
- (b) prohibit any person from manufacturing or importing the substance for a period not exceeding two years (this prohibition lapses at the end of the two-year period unless, before the end of this period, a notice of proposed regulations under section 93 of the Act is published in the Canada Gazette) or
- (c) prohibit the manufacture or import of the substance until additional information or test results have been submitted to the Minister (subsection 84(2) of the Act) and assessed (the assessment period for this supplementary information expires 90 days after receipt of the information)
These measures must be taken by the Minister before the expiration of the assessment period. A copy of the ministerial correspondence and notice will be emailed to the notifier. When a condition or prohibition is issued or varied, the notice must be published in the Canada Gazette describing the action and the substance to which it applies. A substance that is subject to conditions imposed pursuant to section 84(1)(a) of the Act cannot be added to the DSL.
9.6.3.1 Conditions under paragraph 84(1)(a) of the Act
When a substance is suspected to be toxic or capable of becoming toxic, conditions may be imposed to mitigate any risk to human health or the environment. Conditions under paragraph 84(1)(a) of the Act allow the manufacture or importation of a substance with restrictions. Types of restrictions on the substance include, but are not limited to:
- the quantity allowed
- the physical form (for example, must be imported as a plastic pellet)
- the use or
- the disposal of the substance or containers that held the substance
The notifier and, if prescribed, the notifier’s customers are obliged to abide by the conditions imposed on the substance by the Minister and keep records as indicated. Ministerial conditions are published in the Canada Gazette, Part I, after they have been issued to the notifier. Substances subject to ministerial conditions are not eligible for addition to the DSL. Therefore, any new notifier who wishes to manufacture or import the same substance must submit an NSN, as prescribed by the Regulations. This may result in the same or similar conditions being imposed.
A notifier may submit additional information and request a re-evaluation of the decision made by the NS program. The NS program will review and consider this additional information and may amend or rescind the conditions. The conditions stand unless a notice is published in the Canada Gazette to amend or rescind the conditions based on the additional information.
9.6.3.2 Prohibitions under paragraph 84(1)(b) of the Act
When a substance is suspected to be toxic or capable of becoming toxic, a prohibition may be imposed to mitigate any risk to human health or the environment. Prohibitions imposed under paragraph 84(1)(b) of the Act prohibit any person from manufacturing or importing the substance in any amounts. Ministerial prohibitions are published in the Canada Gazette, Part I, after they have been issued to the notifier. Subsection 84(4) of the Act states that this prohibition expires 2 years after it is imposed unless, before the expiry of the 2 years, the Governor in Council publishes in the Canada Gazette a notice of proposed regulations under section 93 of the Act in respect of the substance, in which case the prohibition expires on the day the regulations come into force.
The notifier may submit additional information and request a re-evaluation of the decision made by the NS program. The NS program will review and consider this additional information and may amend or rescind the prohibition or take alternative risk management measures. The prohibition stands unless a notice is published in the Canada Gazette to amend or rescind the prohibition, or the prohibition expires as discussed above.
9.6.3.3 Request for additional information under paragraph 84(1)(c) of the Act
When the NS program requires additional information to be provided to determine whether the substance is toxic or capable of becoming toxic, a request for additional information with a prohibition of manufacture or import pending this testing may be imposed to mitigate any risk to human health or the environment. The request for additional information is imposed under paragraph 84(1)(c) of the Act, and the prohibition of manufacture or import is imposed under subsection 84(2) of the Act. Subsection 84(2) of the Act states that the person who is required to submit the information is prohibited from manufacturing or importing the substance unless the information is provided and a period of 90 days after the additional information was provided has expired. Once the required additional information has been submitted it will be assessed to determine whether the substance is toxic or capable of becoming toxic and whether further risk management measures are required.
Section 10. Post-notification responsibilities
10.1 Notifier’s responsibilities
The onus is on the notifier to ensure that all information provided to the New Substances (NS) program is accurate and complete.
10.1.1 Correction of information
Under subsection 81(11) of the Canadian Environmental Protection Act, 1999 (the Act), any notifier who has submitted information in support of a New Substances Notification (NSN) and later finds that the information is erroneous must immediately notify the NS program, via correspondence, of that fact and submit the necessary correction to their NSN.
This requirement relates only to the correction of information that existed at the time the NSN was submitted.
10.1.2 Section 70 of the Act
Information generated after an NSN was submitted which reasonably supports the conclusion that the substance is toxic or is capable of becoming toxic must be provided to the NS program under the provisions of section 70 of the Act. This information must be provided unless the notifier has actual knowledge that the NS program already has the information.
To obtain the procedures for submitting information under section 70 of the Act, contact the Substances Management Information Line.
10.1.3 Notice of Excess Quantity
Under subsection 81(14) of the Act, a notifier who has met the requirements to manufacture or import a substance, other than research and development, contained site-limited intermediate or contained export-only substances, is required to submit a Notice of Excess Quantity (NOEQ) within 30 days of exceeding manufacture or import trigger quantities (consult section 10.1.3.1). The information required in a NOEQ is indicated in section 10.1.5 of this Guidance Document.
10.1.3.1 Trigger quantities
As prescribed in sections 17 and 18 of the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) and subsection 81(14) of the Act, any notifier who did not provide a Notice of Manufacture or Import (NOMI) (consult section 10.1.4) must provide a NOEQ within 30 days of meeting one of the following trigger quantities:
17 (1) […]
- (a) in the case of a chemical or a biochemical that is not on the Non-domestic Substances List (NDSL), a quantity that exceeds 10 000 kg in any calendar year or
- (b) in the case of a chemical or a biochemical that is on the NDSL, a quantity that exceeds in any calendar year:
- (i) 50 000 kg if:
- (A) the chemical or biochemical is released to the aquatic environment in a quantity exceeding 3 kg/day, per site, averaged monthly and after wastewater treatment or
- (B) the public may be significantly exposed to the chemical or biochemical in a product or
- (ii) 10 000 kg, in any other case
18 (1) […]
- (a) in the case of a Reduced Regulatory Requirement (RRR) polymer, a quantity that exceeds 1 000 kg in any calendar year and
- (b) in the case of any other polymer or biopolymer, a quantity that exceeds in any calendar year:
- (i) 50 000 kg if the polymer or biopolymer is on the NDSL or the polymer or biopolymer is not on the NDSL but all of its reactants are on the Domestic Substance Lists (DSL) or the NDSL and:
- (A) that polymer or biopolymer is released to the aquatic environment in a quantity exceeding 3 kg/day, per site, averaged monthly and after wastewater treatment or
- (B) the public may be significantly exposed to that polymer or biopolymer in a product or
- (ii) 10 000 kg, in any other case
10.1.4 Notice of Manufacture or Import
Alternatively, paragraphs 17(2)(a) and 18(2)(a) of the Regulations prescribe the requirement for a NOMI for chemicals and polymers. This type of notice is an alternative to the requirement for the submission of a NOEQ so that a substance may be eligible for addition to the DSL under paragraph 87(5)(a) of the Act without requiring the tracking of manufacture or import quantities. Once a NOMI has been submitted, a NOEQ is no longer required. The information required in a NOMI is indicated below.
10.1.5 Content and submission of the notices
Any notifier who has submitted the full complement of information for a substance and has begun to manufacture or import the substance may submit a NOMI at any time prior to reaching the trigger quantities specified in section 17 or 18 of the Regulations (consult section 10.1.3.1). A notifier who has previously notified a substance for which the full complement of information was not provided and who has begun to manufacture or import the substance in limited quantities may submit the NOMI at the same time as submitting the full complement of information for that substance.
Any notifier who has submitted the full complement of information for a substance and chooses not to submit a NOMI is required to submit a NOEQ within 30 days of meeting the trigger quantities, as indicated in section 10.1.3.1 above.
The NOEQ or NOMI must be signed by the representative of the resident manufacturing or importing the substance as the “Notifier” or the agent of the "Non-resident importer” of the substance as the “Canadian Agent”. The notice should state the following:
- The person’s name and company name
- The name of the substance and its approved masked name (if applicable)
- The NSN reference number of the NSN for which the full complement of information was submitted for that substance and
- A statement indicating that the trigger quantity (consult section 10.1.3.1) was exceeded and the date on which this quantity was exceeded (this information is required only for a NOEQ) or
- A statement indicating that the substance has been manufactured or imported (this information is required only for a NOMI; consult section 10.1.4)
The above information must be submitted to the Substances Management Information Line.
Once either notice is received by the NS program, the substance may be eligible for addition to the DSL if all other requirements described in section 87 of the Act have been met (consult section 10.2.1).
10.2 The New Substances program’s responsibilities
10.2.1 Additions to the Domestic Substances List
Pursuant to section 87 of the Act, a substance must be added to the DSL and, if on the NDSL, deleted from that list within 120 days after the following criteria are met:
- The information prescribed in section 81 or 82 of the Act and any additional information or test results required under subsection 84(1) of the Act have been provided
- The period for assessing the information under section 83 of the Act has expired
- No conditions have been imposed on the substance under paragraph 84(1)(a) of the Act
- Justification has been provided to warrant confidentiality requests, if applicable (consult section 2.1.2) and
- A NOMI has been received which satisfies that the substance has been manufactured or imported as specified in section 10.1.4 or
- A NOEQ has been received within 30 days of exceeding manufacture or import trigger quantities as specified in section 10.1.3.1
Substances that are not anticipated to pose a risk to the environment and human health, regardless of their current use, quantity or any other anticipated activity, will be added to the DSL without restrictions. Substances that may have a significant new activity that may change the outcome of the assessment will be added to the DSL with a flag requiring additional notification requirements (consult sections 2.1.4 and 9.6.2). Polymers that are not anticipated to pose a risk to the environment and human health and life when manufactured or imported as an RRR polymer will be added to the DSL with a flag requiring renotification if they are subsequently manufactured in or imported into Canada in a form that no longer meets the RRR criteria.
When the identity of a DSL-eligible substance is claimed confidential by the notifier and an acceptable masked name has been provided, a Confidential Substance Identity Number is assigned to that substance and is provided to the notifier. This Confidential Substance Identity Number and acceptable masked name are then added to the confidential portion of the DSL.
Appendix 1. Flowcharts
The decision schemes shown in this appendix can be used to determine the required Schedule of information for a substance under the New Substances Notification Regulations (Chemicals and Polymers). The information requirements for each subdivision are cumulative. When consulting the flowcharts, the user should first choose the appropriate flowchart according to the type of substance they wish to notify. The flowcharts will guide the user in identifying what information is to be provided and when it is to be provided, based upon the quantity that triggers the requirement to provide the information.

Long description
Notifiers who manufacture or import a new chemical as referred to in section 5 of the Regulations or a new polymer as referred to in section 6 of the Regulation, that is for research and development purposes, as a contained site-limited intermediate substance or as a contained export-only substance, must provide the Minister with the appropriate Schedule of information prior to exceeding the applicable trigger quantity and within the prescribed timeframe for that Schedule. Figure A1-1 illustrates timelines and information to provide for these chemicals and polymers:
- For a Chemical/Biochemical (section 5 of the Regulations):
- Provide information prescribed in Schedule 11 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
- Update all of the information that was previously provided at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
- For a Polymer/Biopolymer (section 6 of the Regulations):
- Provide information prescribed in Schedule 32 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
1 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subsections 5(2), (3) and (4) of the Regulations).
2 Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult subsections 6(2), (3) and (4) of the Regulations).

Long description
Notifiers who manufacture or import a new chemical other than those referred to in section 5 of the Regulations (that is a new chemical other than those described in Figure A1-1) must provide the Minister with the appropriate Schedule of information prior to exceeding the applicable trigger quantity and within the prescribed timeframe for that Schedule. Figure A1-2 illustrates timelines and information to provide for these chemicals:
- For a Chemical/Biochemical specified on the NDSL (section 7 of the Regulations):
- Provide information prescribed in Schedule 41 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
- Provide information prescribed in Schedule 52 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
- If the chemical is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment and/or if the public may be significantly exposed to the chemical in a product, provide additional test information as prescribed in subsections 7(2) and/or 7(3) of the Regulations, respectively, at least 75 days prior to the day on which the quantity of substance manufactured or imported exceeds 50 000 kg in a calendar year.
- For a Chemical/Biochemical not specified on the NDSL (section 8 of the Regulations)3:
- Provide information prescribed in Schedule 44 of the Regulations at least 5 days prior to the day on which the quantity of substance manufactured or imported exceeds 100 kg in a calendar year.
- Provide information prescribed in Schedule 54 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year.
- Provide information prescribed in Schedule 64 of the Regulations at least 75 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
1 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subparagraph 7(1)(a)(ii) of the Regulations).
2 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subparagraph 7(1)(b)(ii) of the Regulations). No further information will be required unless: (a) the chemical is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment (consult subsection 7(2) of the Regulations) or (b) the public may be significantly exposed to the chemical in a product (consult subsection 7(3) of the Regulations).
3 Notification must be sent to the Minister if: the chemical or biochemical is specified on the NDSL following submission of the information to in subparagraph 8(1)(b)(i) of the Regulations and item 10 of Schedule 5 (consult subsection 8(2) of the Regulations).
4 Additional information specified in Schedule 2 is also required if the chemical is a biochemical (consult subparagraphs 8(1)(a)(ii), b(ii) and c(ii) of the Regulations).

Long description
Notifiers who manufacture or import a new polymer other than those referred to in section 6 of the Regulations (that is a new polymer other than those described in Figure A1-1) must provide the Minister with the appropriate Schedule of information prior to exceeding the applicable trigger quantity and within the prescribed timeframe for that Schedule. Figure A1-3 illustrates timelines and information to provide for these polymers:
- For a Polymer/Biopolymer considered RRR (section 9 of the Regulations):
- Provide information prescribed in Schedule 92 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year1.
- For a Polymer/Biopolymer not considered RRR and specified on the NDSL or all of whose reactants are specified on the DSL or NDSL (section 11 of the Regulations):
- Provide information prescribed in Schedule 92 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year1.
- Provide information prescribed in Schedule 103 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
- If the polymer is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment and/or if the public may be significantly exposed to the polymer in a product, provide additional test information as prescribed in subsections 11(2) and/or 11(3) of the Regulations, respectively, at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 50 000 kg in a calendar year.
- For a Polymer/Biopolymer not considered RRR and not specified on the NDSL and one or more reactants are not specified on either the DSL or NDSL (section 12 of the Regulations):
- Provide information prescribed in Schedule 92 of the Regulations at least 30 days prior to the day on which the quantity of substance manufactured or imported exceeds 1 000 kg in a calendar year1.
- Provide information prescribed in Schedule 114 of the Regulations at least 60 days prior to the day on which the quantity of substance manufactured or imported exceeds 10 000 kg in a calendar year.
1 Section 10 of the Regulations.
2 Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult paragraph 10(b) of the Regulations).
3 Not required for Reduced Regulatory Requirement polymers. Also subject to certain exceptions (consult subsection 11(5) of the Regulations). Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult paragraph 11(1)(b) of the Regulations. No further information will be required unless: (a) the polymer is released to the aquatic environment in a quantity exceeding 3 kg per day, per site, averaged monthly and after wastewater treatment (consult subsection 11(2) of the Regulations) or (b) the public may be significantly exposed to the polymer in a product (consult subsection 11(3) of the Regulations).
4 Not required for Reduced Regulatory Requirement polymers. Also subject to certain exceptions (consult subsection 12(3) of the Regulations). Additional information specified in Schedule 2 is also required if the polymer is a biopolymer (consult paragraph 12(1)(b) of the Regulations).
Appendix 2. Schedules of information under the Regulations
Schedule 1
(Subsections 2(2) and 5(1) to (4))
Information Respecting Chemicals and Biochemicals That Are Research and Development Substances, Contained Site-Limited Intermediate Substances or Contained Export-Only Substances
Schedule 2
(Subsections 2(2), 5(2) to (4) and 6(2) to (4), subparagraphs 7(1)(a)(ii) and (b)(ii) and 8(1)(a)(ii), (b)(ii) and (c)(ii) and paragraphs10(b), 11(1)(b), 12(1)(b), 17(2)(b) and 18(2)(b))
Information Respecting Biochemicals and Biopolymers
Schedule 3
(Subsection 2(2) and section 6)
Information Respecting Polymers and Biopolymers That Are Research and Development Substances, Contained Site-Limited Intermediate Substances or Contained Export-Only Substances
Schedule 4
(Subsection 2(2), subparagraphs 7(1)(a)(i), 8(1)(a)(i) and 17(2)(c)(i) and paragraph 17(2)(d))
Information Respecting Other Chemicals and Biochemicals Not on the NDSL (100 kg) or on the NDSL (1 000 kg)
Schedule 5
(Subsection 2(2), subparagraphs 7(1)(b)(i), 8(1)(b)(i), subsection 16(3), subparagraph 17(2)(c)(i) and paragraph 17(2)(d))
Information Respecting Other Chemicals and Biochemicals Not on the NDSL (1 000 kg) or on the NDSL (10 000 kg)
Schedule 6
(Subsection 2(2), subparagraph 8(1)(c)(i) and paragraph 17(2)(d))
Information Respecting Other Chemicals and Biochemicals Not on the NDSL (10 000 kg)
Schedule 7
(Subsection 2(2) and paragraphs 9(a) and (b))
Types of Polymers
Schedule 8
(Subsection 2(2) and paragraph 9(c))
List Of Reactants and their Chemical Abstracts Service Registry Number
Schedule 9
(Subsection 2(2), paragraphs 10(a) and 18(2)(b), subparagraph 18(2)(d)(i) and paragraph 18(2)(e))
Information Respecting Reduced Regulatory Requirement Polymers and Other Polymers and Biopolymers (1 000 kg)
Schedule 10
(Subsection 2(2), paragraph 11(1)(a), subsection 11(5), subparagraph 18(2)(d)(i) and paragraph 18(2)(e))
Information Respecting Other Polymers and Biopolymers on the NDSL or All of Whose Reactants Are on the DSL or NDSL (10 000 kg)
Schedule 11
(Subsection 2(2), paragraph 12(1)(a), subsection 12(3))
Information Respecting Other Polymers and Biopolymers Not on the NDSL (10 000 kg)
Schedule 12
(Subsection 2(2))
Overview of Information Requirements
Appendix 3. Naming substances
A3.1 Representing substances with well-defined structures
A3.1.1 Chemical name of the substance
A name must be provided that describes the substance using the Chemical Abstracts Service (CAS) or International Union of Pure and Applied Chemistry (IUPAC) nomenclature. Ambiguous or incomplete names are not appropriate for substance identification or for any subsequent addition to the DSL. Abbreviations, acronyms, laboratory designations, trade names, trademarks, or any trivial names that are not chemically descriptive should not be submitted. Further clarification of the level of specificity required is provided in Table A3-1.
Do not assume that an ambiguous name is adequate simply because there is only one isomer used in a particular industry or because the structure diagram has been provided with the notification.
Commercial dye names should not be used unless they correspond to Colour Index Names in Volume 5 of the Colour Index. The Colour Index is a reference publication for manufacturers and users of dyes. It is published by the Society of Dyers and Colourists with assistance from the American Association of Textile Chemists and Colorists.
Inorganic substance names should identify all the elements and specify the element ratios. The use of empirical formulas or Stock Numbers is encouraged. Stock Numbers are Roman numerals added parenthetically to indicate the state or states of oxidation.
A3.1.2 Molecular formula
The molecular formula is a summation of the actual numbers and kinds of atoms present in a molecule of a substance. In the case of salts or addition compounds, the molecular formula may be presented as a single summation formula or in the "dot-disconnect" format used by CAS.
Example: Succinic acid, dilithium salt
LiO2C(CH2)2CO2Li
C4H4Li2O4
(summation)
or
HO2C(CH2)2CO2H · 2Li
C4H6O4 · 2Li
(dot-disconnect)
| Example | Structural representation | Unacceptable name | Acceptable name |
|---|---|---|---|
| 1 |  |
Anisidine |
o-Anisidine or 2-Methoxyaniline |
| 2 | 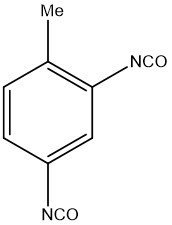 |
Toluene diisocyanate or TDI |
Toluene 2,4-diisocyanate |
| 3 | ![C(=C\C(O)=O)/C(O)=O.[Na]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-1-3.jpg) |
Sodium fumarate or Monosodium butenedioate |
Monosodium fumarate or Monosodium trans-butenedioate or Monosodium E-butenedioate |
| 4 | 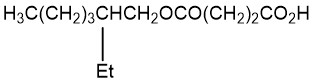 |
Octyl succinate or Ethylhexyl succinate |
Mono(2-ethylhexyl) succinate |
| 5 | 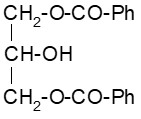 |
Glycerol benzoate or Glycerol dibenzoate |
Glycerol 1,3-dibenzoate |
| 6 | 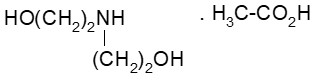 |
Diethanolamine acetate | Diethanolamine acetate salt |
| 7 | 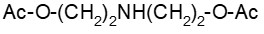 |
Diethanolamine acetate or Diethanolamine acetate ester |
Diethanolamine diacetate ester |
| 8 | 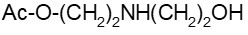 |
Diethanolamine acetate or Diethanolamine acetate ester |
Diethanolamine monoacetate ester |
| 9 | 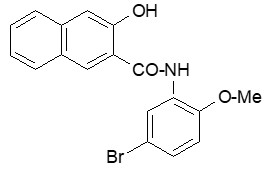 |
Blue APM or EMS 17 |
Brenthol BA or C.I. 37532 or C.I. Azoic Coupling Component 6 or 5'-Bromo-3-hydroxy-2-naphth-o-anisidine or N-(5-Bromo-2-methoxyphenyl)-3-hydroxy-2-naphthalenecarboxamide |
| 10 | ![O=[Ti]O[Ti]=O](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-1-10.jpg) |
Titanium oxide | Titanium oxide (Ti2O3) |
A3.1.3 Structural information
The structure diagram should clearly indicate the identity of the atoms and the nature of the bonds joining them. Guidelines for preparing these diagrams are included in this appendix.
Common abbreviations are acceptable as long as they are unambiguous. Table A3-2 presents examples of abbreviations that may be used.
| Example | Structural representation | Abbreviation |
|---|---|---|
| 1 | ![[CH3]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-1.jpg) |
-Me |
| 2 | ![[CH2]C](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-2.jpg) |
-Et |
| 3 | ![[CH2]CC](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-3.jpg) |
-Pr |
| 4 | C](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-4.jpg) |
-Pr-i or -Pr-iso |
| 5 | ![[CH2]CCC](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-5.jpg) |
-Bu |
| 6 | ![[CH2]C(C)C](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-6.jpg) |
-Bu-i or -Bu-iso |
| 7 | CC](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-7.jpg) |
-Bu-s or -Bu-sec |
| 8 | (C)C](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-8.jpg) |
-Bu-t or -Bu-tert |
| 9 | O](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-9.jpg) |
-CO2H |
| 10 | ](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-10.jpg) |
-CO- |
| 11 | ![[CH]=O](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-11.jpg) |
-CHO |
| 12 | C](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-12.jpg) |
-Ac |
| 13 | (=O)O](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-13.jpg) |
-SO3H |
| 14 | (=O)](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-14.jpg) |
-SO2- |
| 15 | ![[N]=O](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-15.jpg) |
-NO |
| 16 | ![[C]=1C=CC=CC1](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-2-16.jpg) |
-Ph |
Alkyl groups will be assumed to be normal (linear) unless otherwise designated. If a substance has alkyl groups that are not linear, then the nature of the branching should be described as specifically as possible. Table A3-3 illustrates several different representations for nonylphenol.
| Example | Submitted name | Structural representation | CAS Registry Number | CA Index Name |
|---|---|---|---|---|
| 1 | p-Nonylphenol | 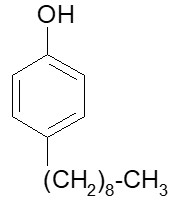 |
104-40-5 | Phenol, 4-nonyl- |
| 2 | p-Isononylphenol | ![OC1=CC=C([C9H19-iso])C=C1](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-3-2.jpg) |
26543-97-5 | Phenol, 4-isononyl- |
| 3 | Branched, 4-nonylphenol | ![OC1=CC=C([C9H19-branched])C=C1](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-3-3.jpg) |
84852-15-3* | Phenol, 4-nonyl-, branched |
| 4 | p-Tripropylene phenol | ![OC1=CC=C([C9H19])C=C1](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-3-4.jpg) |
87247-00-5 | Phenol, 4-tripropylene- |
Carbon atoms in ring systems and their attached hydrogen atoms need not be explicitly shown.
For example:
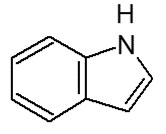
All known stereochemical details should be provided. Indicate whether the stereochemistry is absolute or relative. For example:
CCC1](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG17.jpg)
The ratio of the components of an addition compound or salt should be clearly indicated if more than one form is theoretically possible. It should also be noted if the ratio is unknown.
For example:
![N(CC(O)=O)(CC(O)=O)CCN(CC(O)=O)(CC(O)=O).[Na].[Na]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG18.jpg)
![N(CC(O)=O)(CC(O)=O)CCN(CC(O)=O)(CC(O)=O).[Na].[Na].[Na].[Na]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG19.jpg)
![N(CC(O)=O)(CC(O)=O)CCN(CC(O)=O)(CC(O)=O).[Na] where the number of [Na] salts is x](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-IMG20.jpg)
A3.1.3.1 Examples of well-defined substances
The following examples (1-17) illustrate the information necessary to uniquely identify and represent substances with a well-defined structure.
Example 1
- Chemical name of the substance: N-s-Butoxymethyl)acrylamide
- Molecular formula: C8H15NO2
- Structural information:
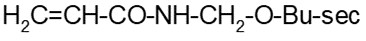
- Comment: Branching of alkyl groups must be indicated or the group will be assumed to be linear. For example, the Bu group on the following diagram would be represented linearly as - CH2CH2CH2CH3.
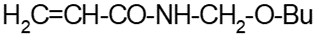
Example 2
- Chemical name of the substance: 1,1-Di-3,4-xylylethane; 1,1-Bis(3,4-dimethylphenyl)ethane
- Molecular formula: C18H22
- Structural information:
- Comment: The semicolon is used to separate the 2 names. Both names cite locants. Dixylylethane would not be an appropriate name for this substance.
Example 3
- Chemical name of the substance: Sodium sebacate; Sodium decanedioate
- Molecular formula: C10H18O4 · x Na
- Structural information:
![O=C(O)CCCCCCCCC(=O)O.[Na] where the number of [Na] salts is x](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example3.jpg)
- Comment: Use of "x" in the molecular formula and structure diagram clearly indicates that the ratio of the salt is unknown.
Example 4
- Chemical name of the substance: Disodium sebacate; Disodium decanedioate
- Molecular formula: C10H18O4 · 2 Na
- Structural information:
![O=C(O)CCCCCCCCC(=O)O.[Na].[Na]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example4-1.jpg)
- Comment: When known, ratios should be cited in the name, formula, and structure. The formula could also be given as C10H16Na2O4. The structure could also be shown as
![O=C(O[Na])CCCCCCCCC(=O)O[Na]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example4-2.jpg)
Example 5
- Chemical name of the substance: 1,3-Pentadiene; Piperylene
- Molecular formula: C5H8
- Structural information:
- Comment: Stereochemistry is not cited in the name or structure. Consult example 6 for a specific stereoisomer.
Example 6
- Chemical name of the substance: cis-1,3-Pentadiene; Z-1,3-Pentadiene; cis-Piperylene
- Molecular formula: C5H8
- Structural information:
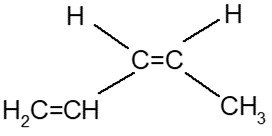
- Comment: Stereochemistry is cited in both the name and structure. Consult example 5 for an example of a non-stereospecific substance.
Example 7
- Chemical name of the substance: Manganese (II) chromate (IV); Manganese chromate (MnCrO4); Chromium manganese oxide (MnCrO4)
- Molecular formula: H2CrO4 · Mn
- Structural information:
(O)O.[Mn]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example7-1.jpg)
- Comment: Stock numbers or empirical formulas should be included in the name when known. The following diagram is also acceptable:
([O-])[O-].[Mn++]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example7-2.jpg)
Example 8
- Chemical name of the substance: PVC; Polyvinyl chloride
- Molecular formula: (C2H3Cl)x
- Structural information:

- Comment: Polymeric substances are to be described in terms of their starting reactants. Starting reactants are defined as those that become part of the polymer composition. If the role of the reactant ABIN is an initiator, it should not be included in the polymer description appearing on the DSL. ABIN, if placed in commerce, must be reported separately.
Example 9
- Chemical name of the substance: Maleic acid-dimethyl phthalate-ethylene glycol copolymer; cis-2-Butenedioic acid-dimethyl phthalate-ethylene glycol polymer
- Molecular formula: (C2H6O2-C4H4O4-C10H10O4)x
- Structural information:
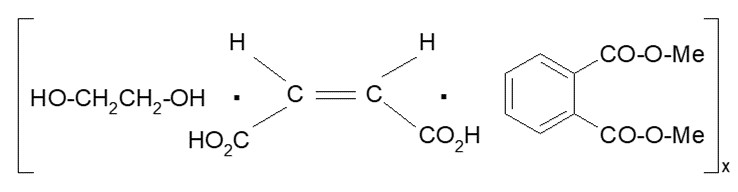
Example 10
- Chemical name of the substance: Styrene-polyethyleneglycol monoallylether
- Molecular formula: ((C2H4O)nC3H6O · C8H8)x
- Structural information:
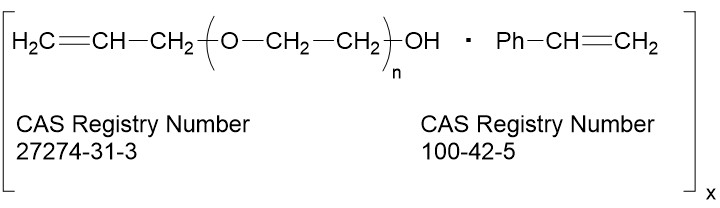
- Comment: Names and CAS Registry Numbers rather than structure diagrams may be used to describe reactants. Polyglycol derivatives should be represented on the basis of their polymeric structure.
Example 11
- Chemical name of the substance: 2,4,4-Trimethyl-2-pentene
- Molecular formula: C8H16
- Structural information:
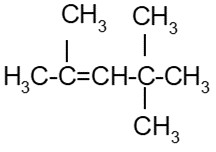
- Comment: Isobutylene dimer would not be an appropriate chemical name of the substance for this structure. Designations such as dimer and trimer are appropriate only when the degree of polymerization is a specific value from 2 through 10 but the specific structure is unknown.
Example 12
- Chemical name of the substance: ar-Nitro-6-hexyl-1-naphthol; ar-Nitro-6-hexyl-1-hydroxynaphthalene
- Molecular formula: C16H19NO3
- Structural information:
=O) where ([N+]([O-])=O) specificity on the first aromatic ring is unknown](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example12.jpg)
- Comment: Compare to examples 13 and 14. The structural representation should represent all known specificity.
Example 13
- Chemical name of the substance: 6-(Nitrohexyl)-1-naphthol; 6-(Nitrohexyl)-1-hydroxynaphthalene
- Molecular formula: C16H19NO3
- Structural information:
CCCCCCC1=CC=C2C(O)=CC=CC2=C1 where O=[N+]([O-]) specificity on the hexyl group is unknown](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example13.jpg)
- Comment: Compare to examples 12 and 14. The structural representation should represent all known specificity.
Example 14
- Chemical name of the substance: 2 or 3-Nitro-6-hexyl-1-naphthol; 2 or 3-Nitro-6-hexyl-1-hydroxynaphthalene
- Molecular formula: C16H19NO3
- Structural information:
=O)C=CC2=C1 or CCCCCCC1=CC=C2C(O)=CC([N+]([O-])=O)=CC2=C1](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example14.jpg)
- Comment: Compare to examples 12 and 13. The structural representation should represent all known specificity.
Example 15
- Chemical name of the substance: Aluminum nickel
- Molecular formula: Ni3Al
- Structural information: Ni3Al
- Comment: Known stoichiometry should be indicated. Ni-Al would be unacceptable.
Example 16
- Chemical name of the substance: Synthetic geikielite
- Molecular formula: Mg-O3Ti
- Structural information: Mg(TiO3)
- Comment: Synthetic minerals should indicate in the chemical name of the substance that they are synthetic.
Example 17
- Chemical name of the substance: Piperazine hexahydrate; Arpezine
- Molecular formula: C4H10N2 · 6 H2O
- Structural information:
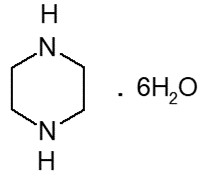
- Comment: Substances that are described as hydrates should be represented as the anhydrous form.
A3.2 Representing substances that are complex and variable
Substances that cannot be represented by a complete structure diagram and specific molecular formula are known as substances of Unknown or Variable composition, Complex reaction products or Biological materials (UVCBs).
A3.2.1 Chemical name of the substance
The guidelines for names for UVCB substances are similar to the instructions given in section A3.1 for Well-Defined Substances and should be reviewed for additional information. Consult Table A3-4 for further clarification of the level of specificity required.
| Example | Structural representation | Unacceptable name | Acceptable name |
|---|---|---|---|
| 1 |
=O)=CC([N+]([O-])=O)=C1C)(O[Na])=O reaction product with sodium polysulfide](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-4-1.jpg)
|
RGP Brown or Sodium dinitrotoluenesulfonic acid polysulfide |
C.I. Sulphur Brown 42 or C.I. 53030 or Thionone Brown R0 or Sodium 3,5-dinitro-o-toluenesulfonic acid reaction product with sodium polysulfide |
| 2 |
![Bromination chlorination of C=C[R] where R = C10-28 Alkyl](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-4-2.jpg)
|
Halogenated C12-30 α-alkenes or Bromo and chloroalkenes |
C12-30 α-alkenes bromo and chloro derivs. or C12-30 α-(alkenes, brominated and chlorinated) or Alkenes, C12-30 α-brominated and chlorinated |
| 3 |
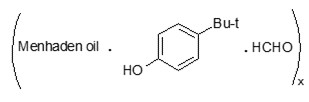
|
Fish oil-butyl phenol-formaldehyde resin or Marine oil, p-tert-butylphenol, formaldehyde resin or Menhaden oil, 4-butylphenol, formaldehyde resin |
Menhaden oil, p-tert-butylphenol, formaldehyde resin |
| 4 | Linseed oil fatty acids · x Na |
Vegetable fatty acids sodium salts or Linseed sodium salts or Linseed oil sodium salts |
Linseed oil fatty acids sodium salts or Fatty acids, linseed-oil, sodium salts |
| 5 |
![O=C(OR)C=1C=CC=CC1C(=O)O where R = [C8-10 branched alkyl]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-4-4.jpg)
|
Nonyl phthalate or Isononyl phthalate or Mono-C8-10-alkyl phthalate |
Mono-C8-10-branched alkyl phthalate or 1,2-Benzenedicarboxylic acid, mono-C8-10-branched alkyl esters |
| 6 |
![O=C(OR)C=1C=CC=CC1C(=O)OR where R = [C8-10 branched alkyl]](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-4-5.jpg)
|
Dinonyl phthalate or Diisononyl phthalate or Di-C8-10-alkyl phthalate |
Di-C8-10-branched alkyl phthalate or 1,2-Benzenedicarboxylic acid, di-C8-10-branched alkyl esters |
| 7 |
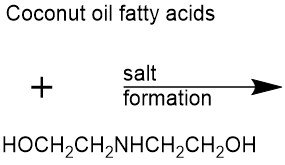
|
Coconut oil fatty acids reaction product with diethanolamine |
Coconut oil fatty acids-diethanolamine salt or Coconut oil fatty acids, compound with diethanolamine or Fatty acids, coco, compds. with diethanolamine |
| 8 |
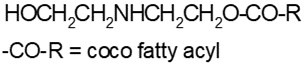
|
Coconut oil fatty acids reaction product with diethanolamine |
Coconut oil fatty acids diethanolamine monoester or Fatty acids, coco, 2-[[(2-hydroxyethyl)amino]ethyl] esters |
| 9 |
![N=1CCN(CCO)C1([nor coco alkyl])](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-TableA3-4-8.jpg)
|
Coconut oil reaction product with aminoethyl ethanolamine or Coco alkylimidazolineethanol |
Coconut oil and N-(2-aminoethyl)ethanolamine cyclization product or 1H-Imidazole-ethanol, 4,5-dihydro-2-norcoco alkyl derivs |
A3.2.2 Molecular formula
Most UVCB substances cannot be represented by a specific molecular formula. However, in some cases, it may be possible to provide a molecular formula that is a summation of the range of numbers and specific kinds of atoms present in a molecule of a substance. Hypothetical or idealized molecular formulas must not be cited.
Molecular formulas for salts and addition compounds, if provided, may be presented as a single summation formula or in the dot-disconnect format used by CAS.
Example: C6-12-alkyldicarboxylic acid, disodium salt
NaO2C-C6-12alkyl-CO2Na
C8-14H12-24Na2O4
or
HO2C-C6-12alkyl-CO2H · 2Na
C8-14H14-26O4 · 2Na
A3.2.3 General guidelines
Because, in most cases, a unique structure diagram cannot be provided, descriptive information for the substance, constituents, or precursors should be given.
If a partial structure diagram can be provided, it should clearly indicate the identity of the atoms and the nature of the bonds joining them. Common abbreviations for substituents and functional groups are acceptable if they are unambiguous. Alkyl groups will be assumed to be normal (linear) unless otherwise designated.
Substance representations should describe all known specificity, such as salt ratios and stereochemical details.
The following examples (18-23) are intended to illustrate the level of specificity that should be provided. It is strongly recommended that the notifier follow the style of the examples.
Example 18
- Chemical name of the substance: N,N-Diisopropyl tall oil fatty amides
- Molecular formula: None possible
- Structural information:
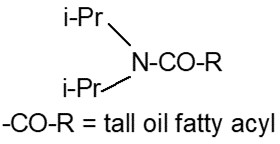
- Comment: A substance can be described using a partial structure diagram.
Example 19
- Chemical name of the substance: 4-(C5-11-alkyl)-1,2-oxathiolane, S,S-dioxide
- Molecular formula: None possible
- Structural information:
![[C5-11 alkyl]C1COS(C1)(=O)=O](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example19.jpg)
- Comment: Carbon ranges of alkyl groups must be defined.
Example 20
- Chemical name of the substance: C8 branched alkylphenol ethoxylate
- Molecular formula: (C2H4O)nC14H23O
- Structural information:
![C=1C(OCC)=CC=C([C8H17-branched])C1 where OCC is a polymeric unit repeated n times and (OCC) and ([C8H17-branched]) specificity is unknown](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example20.jpg)
- Comment: Representations should describe all known specificity, including structural information for alkyl groups.
Example 21
- Chemical name of the substance: Chlorinated 5-norbornene-2,3-dicarboxylic acid; Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid, chloro derivs.
- Molecular formula: C9H(10-x)ClxO4
- Structural information:
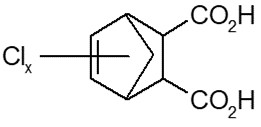
Example 22
- Chemical name of the substance: Safflower oil, polymer with adipic acid, glycerol and phthalic anhydride
- Molecular formula: None possible
- Structural information:
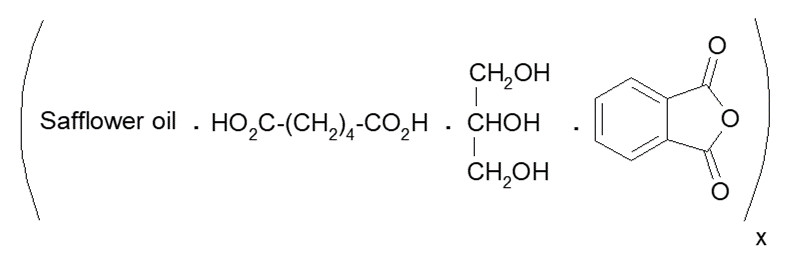
Example 23
- Chemical name of the substance: Phosphoric acid, mono(branched nonyl) phenyl ester, disodium salt
- Molecular formula: C15H25O4P · 2 Na
- Structural information:
![C=1C(OP(=O)(O)O)=CC=C([C9H19-branched])C1.[Na].[Na] where (OP(=O)(O)O) and ([C9H19-branched]) specificity is unknown](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example23.jpg)
A3.2.4 Plant and animal products
Complex and variable substances that are produced by chemical modification of naturally occurring products or are separated from them by physical processingFootnote 18 must be identified by specifying the genus and species as well as other unambiguous common names of the source.
Do not assume that a common name is adequate simply because there is only one source used in a particular industry. Some examples: mint oil should not be used to identify Japanese mint oil, Bergamot oil, Spearmint oil, or Peppermint oil; vegetable oil should not be used to identify corn oil, soybean oil, or linseed oil.
The following examples (24-30) are intended to illustrate the level of specificity that should be provided.
Example 24
- Chemical name of the substance: Soybean fatty acids, diethylenetriamine salt
- Molecular formula: None possible
- Structural information:
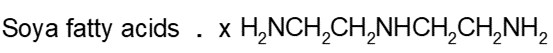
Example 25
- Chemical name of the substance: Mixed vegetable oils fatty acids methyl esters
- Molecular formula: None possible
- Structural information: Methyl esters of mixed vegetable oils fatty acids
- Comment: If a substance is obtained from a manufacturing process that used different types of plants to produce an oil, then the term, "mixed vegetable" should be used in the name.
Example 26
- Chemical name of the substance: Japanese mint oil; Japanese peppermint oil
- Molecular formula: None possible
- Structural information: Oil extracted from Mentha arvensis var. piperascens
- Comment: The genus and species of the plant that was processed to produce the oil must be identified.
Example 27
- Chemical name of the substance: Mentha citrata oil; Bergamot mint oil
- Molecular formula: None possible
- Structural information: Oil extracted from Mentha citrata
- Comment: Bergamot oil would not be an appropriate chemical name of the substance because bergamot oil is also extracted from Citrus bergamia.
Example 28
- Chemical name of the substance: Acetylated lemongrass oils
- Molecular formula: None possible
- Structural information:
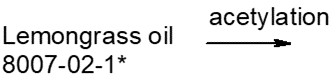
- Comment: The genus and species, Cymbopogon citratus, is associated with the CAS Registry Number 8007-02-1* in the Chemical Definition Section of the Toxic Substances Control Act (TSCA).
Example 29
- Chemical name of the substance: Terpene-free bergamot oil fraction
- Molecular formula: None possible
- Structural information: Terpene-free fraction distilled from oil extracted from Citrus bergamia
Example 30
- Chemical name of the substance: Corn oil deodorizer distillate
- Molecular formula: None possible
- Structural information: A complex mixture of fatty acids, sterols, aldehydes, ketones, and other materials prepared by the steam distillation of corn oil followed by condensation of the steam containing these materials
A3.2.5 Reaction products
The reaction scheme should include the chemical identity of the immediate precursors, the nature of the reaction, and the reactants, whether or not they are implied by the reaction term. Reaction terms should be as specific as possible (for example, acetylation, alkaline hydrolysis, chlorination, diazotization, epoxidation). General reaction terms such as addition, condensation, and reaction should not be used.
Although the substance itself may be a UVCB substance, the precursors or constituents may be well-defined substances. Any descriptions provided for well-defined precursors or constituents should meet the specifications discussed previously.
The following examples (31-36) are intended to illustrate the level of specificity that should be provided.
Example 31
- Chemical name of the substance: Polymer of methyl methacrylate, methacrylic acid, and bromotrichloromethane
- Molecular formula: (C4H6O2 · C5H8O2)x · x CBrCl3
- Structural information:
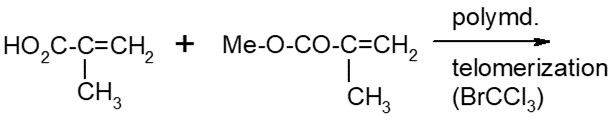
Example 32
- Chemical name of the substance: Chlorinated 5-norbornene-2,3-dicarboxylic acid; Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid, chloroderivs.
- Molecular formula: C9H(10-x)ClxO4
- Structural information:
- Comment: Compare to example 21. Either method is acceptable. Both depict the same degree of specificity.
Example 33
- Chemical name of the substance: Phosphoric acid, mono(branched nonyl) phenyl ester, disodium salt
- Molecular formula: C15H25O4P · 2Na
- Structural information:
![Esterification of OP(=O)(O)O and C=1C(OH)=CC=C([C9H19-branched])C1 where OH and ([C9H19-branched]) specificity is unknown, [Na]O neutralized](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example33.jpg)
- Comment: Compare to example 23. Either method is acceptable. Both depict the same degree of specificity.
Example 34
- Chemical name of the substance: Phthalic anhydride-trimethylolpropane copolymer, pelargonate
- Molecular formula: (C8H4O3 · C6H14O3)x · x C9H17O2
- Structural information:
![Esterification with O=C([O-])CCCCCCCC of copolymer O=C1OC(=O)C2=CC=CC=C12.OCC(CO)(CO)CC](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example34.jpg)
Example 35
- Chemical name of the substance: C.I. Acid Black 47; C.I. 56055; Sulfonine Grey G
- Molecular formula: C16H12N2O2 · x H2SO4
- Structural information:
Example 36
- Chemical name of the substance: Tallow fatty acid ethanolamine amides salt
- Molecular formula: None possible
- Structural information:
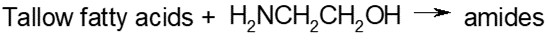
- Comment: Because tallow fatty acids and ethanolamine may react to form a variety of different products (for example, salts, esters, cyclization products), the product description should be as specific as possible and include typical composition.
A3.2.6 Products from industrial processes
Some complex and variable substances are most conveniently described by text rather than structure diagrams or reaction schemes.
The description should include precursors, method of preparation, process terms (low-boiling, catalytic reformed), physical properties (if known), and typical chemical composition. Specifically, the substance information should describe the substance as uniquely as possible and include (if known):
- process description (for example, catalytic cracking, dewaxed, destructive distillation)
- carbon (alkyl) range (for example, C4 through C12)
- physical properties (for example, boiling range, viscosity, solid, slag, and softening point)
- principal chemical composition (for example, hydrocarbons, sulfides, terpenes); and
- source (for example, petroleum, coal)
It is recommended that, whenever appropriate, schematic diagrams (depicting the industrial process and the point where the notified substance is isolated) be provided.
The description should not include process terms that are unqualified or broadly descriptive or undefined trade jargon.
The following examples (37-45) illustrate the level of specificity that should be provided. Additional examples of the type of descriptive information required can be found in the Chemical Substance Definition field of the TSCA inventory (consult Appendix 4).
Example 37
- Chemical name of the substance: C9-13 Alkylbenzene distillation residues
- Molecular formula: None possible
- Structural information:Complex residue from the distillation of C9-13 alkylbenzenes having a boiling point > 600 °F. Composed primarily of diphenylalkanes, diphenylbenzenes, and diphenyldialkanes. The alkyl groups are linear C9-13.
Example 38
- Chemical name of the substance: Ferrous metals blast furnace slag
- Molecular formula: None possible
- Structural information:Fused substance formed by the action of a flux on the gangue of iron-bearing materials charged to the blast furnace and on oxidized impurities of the iron produced. Composed primarily of sulfur and oxides of Al, Ca, Mg, and Si.
Example 39
- Chemical name of the substance: Oxidized black liquor; Spent pulping liquor, oxidized
- Molecular formula: None possible
- Structural information:Substance produced by the oxidation of black liquor with pulping chemicals used in Kraft, sulfite, semichemical, or other pulping processes. Composed primarily of partially oxidized ligosulfonates, sugars and hemicelluloses.
Example 40
- Chemical name of the substance: Quinoline fraction of coal tar alkaline extract residue
- Molecular formula: None possible
- Structural information:
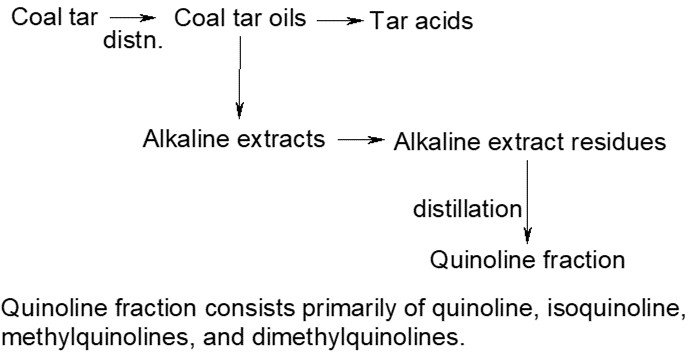
Example 41
- Chemical name of the substance: Coal coke
- Molecular formula: None possible
- Structural information:Carbonaceous residue from the high temperature (> 700 °C) destructive distillation of coal. Composed primarily of carbon but may contain sulfur and ash.
Example 42
- Chemical name of the substance: Petroleum coke
- Molecular formula: None possible
- Structural information:Carbonaceous residue from the high temperature destructive distillation of petroleum fractions. Composed primarily of carbon but may contain some hydrocarbons with high carbon to hydrogen ratios.
Example 43
- Chemical name of the substance: Naphtha, petroleum, hydrodesulfurized full-range
- Molecular formula: None possible
- Structural information:A complex combination of hydrocarbons obtained from a catalytic hydrodesulphurization process. It consists of hydrocarbons having carbon numbers predominantly in the range of C4 through C12 and in the boiling range of approximately 30 to 250 °C.
Example 44
- Chemical name of the substance: Copper smelting slag
- Molecular formula: None possible
- Structural information:Substance resulting from the smelting of copper and previous metals obtained from primary and secondary sources and plant reverts. Composed primarily of iron oxides and SiO2. May contain Cu, Pb, Ni, and other non-ferrous metals and oxides.
Example 45
- Chemical name of the substance: Olivine vanadium blue
- Molecular formula: None possible
- Structural information:An organic pigment formed by the high temperature calcination of vanadium (IV) oxide and silicon oxide in varying amounts. Ionic diffusion occurs to form a crystalline matrix. Alkali or alkaline earth halides may be included as modifiers.
A3.2.7 Combinations of UVCB substances
Because of their complexity, precursors, reactants, reaction scheme, and nominated substance should be described as specifically as possible when notifying substances produced by the combination of UVCB substances. It is strongly recommended that before reporting these types of substances all sections of this appendix be carefully reviewed.
The following examples (46-48) illustrate the level of specificity that should be provided.
Example 46
- Chemical name of the substance: Palm oil and diethylenetriamine cyclization product, compound with distillation residue
- Molecular formula: None possible
- Structural information:
![N=1CCN(CCN)C1([palm oil alkyl])](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example46.jpg) Residue from distillation of C6-18 saturated and unsaturated monobasic acids and C8-15 dibasic acids. Consists of C9-18 saturated dibasic acids salts. May also contain polymers, anhydrides, and polyesters.
Residue from distillation of C6-18 saturated and unsaturated monobasic acids and C8-15 dibasic acids. Consists of C9-18 saturated dibasic acids salts. May also contain polymers, anhydrides, and polyesters.
Example 47
- Chemical name of the substance: Palm oil and diethylenetriamine cyclization product compound with oxidized light petroleum distillates
- Molecular formula: None possible
- Structural information:
![N=1CCN(CCN)C1([palm oil alkyl]) and oxidized light petroleum distillates (64742-98-9*) that react into salts](/content/dam/eccc/images/pded/final-guidance-document-cp/20220317-Example47.jpg)
- Comment: The use of CAS Registry Number 64742-98-9 eliminates the need to include a lengthy description of the starting material.
Example 48
- Chemical name of the substance: Oxidized sesquiterpene fraction of cedarwood oil
- Molecular formula: None possible
- Structural information:

Appendix 4. Locating Chemical Abstracts Service Registry Numbers
This appendix describes sources used to identify Chemical Abstracts Service (CAS) Registry Numbers.
A4.1 Chemical Abstracts Service Registry Services
Using CAS Registry Services, notifiers can obtain CAS Registry Numbers for their substances or Chemical Abstracts Index Names for confidential substances. This service furnishes CAS Registry Numbers to customers either by retrieving existing CAS Registry Numbers and/or assigning new CAS Registry Numbers for substances that meet CAS criteria for registration.
Existing CAS Registry Numbers can be searched in the CAS content collection via the CAS SciFindern online tool. For more information on CAS Registry Number, consult the CAS Registry and CAS Registry Number FAQs.
A4.2 Other inventories and databases
The following inventories and databases can be searched to identify CAS Registry Numbers and other technical information to help with the identification of substances:
- TSCA Inventory: the Toxic Substances Control Act (TSCA) Chemical Substance Inventory includes more than 85 000 chemical substances manufactured, imported, or processed in the United States of America
- EC inventory: the EC Inventory of the European Chemicals Agency (ECHA) includes more than 100 000 chemical substances from the European Inventory of Existing Commercial chemical Substances (EINECS), the European List of Notified Chemical Substances (ELINCS) and the No-Longer Polymers (NLP) list
- PCPC On-line Infobase: the Personal Care Products Council (PCPC) includes more than 27 000 cosmetic ingredients identified by their International Nomenclature Cosmetic Ingredient (INCI) names
- INN Programme and Classification of Medical Products: the International Nonproprietary Names (INN) facilitates the identification of pharmaceutical substances or active pharmaceutical ingredients. These names are unique to each active substance to be marketed as a pharmaceutical and are recognized worldwide
- RTECS database: the Registry of Toxic Effects of Chemical Substances (RTECS) database enhanced by the Canadian Centre for Occupational Health and Safety (CCOHS) contains toxicological information on more than 194 000 chemical substances
- Merck Index Online database: the Merck Index Online of the Royal Society of Chemistry is an online database of chemicals, drugs and biologicals that contains over 11 500 monographs and
- USP Dictionary: this publication by the United States Pharmacopeia (USP) is a dictionary of non-proprietary names for drugs and chemical structures
Appendix 5. Masking of substance names
The procedures presented below provide guidance to notifiers submitting a New Substances Notification (NSN) in which they wish to claim the substance identity as confidential. A masked name should also be submitted for publication purposes to add a substance confidentially to the Domestic Substances List (DSL) or the Non-domestic Substances List (NDSL). The intent of masking is to conceal, only to the extent necessary, the explicit chemical name of the substance. Although this appendix illustrates the masking of only single distinctive elements, additionnal masking is permitted if the notifier can provide justifications (consult section A5.4).
There are inherent differences between explicit chemical names of substances having definite structure diagrams and definite molecular formulas and those that cannot be represented by definite structure diagrams and may or may not be represented by definite molecular formulas. Each of these possibilities is addressed separately in the following subsection.
An acceptable masked name disguises the explicit chemical name. As such, replacing components of the explicit chemical name with synonyms and then masking the synonyms will not be accepted.
A5.1 Substances having definite chemical structure diagrams and molecular formulas
A substance having a definite chemical structure and molecular formula can be represented by a unique structure diagram and unique molecular formula. The explicit chemical name of the substance normally discloses the following structural information:
- The identity of the parent structure (that is, a chain of carbon atoms, a ring system, or a coordinated metal)
- The identity, number, and position of chemical group(s) that are attached to the parent structure(s) or to other chemical groups
- The identity and number of cations and counter ions (for salts) and
- The stereochemical relationships
The masked name may be created by disguising structurally descriptive segments of the explicit chemical name of the substance. Masking may be accomplished by replacing distinct elements of the explicit chemical name with non-descriptive terms and/or removing locants. The number of distinctive elements in an explicit chemical name that can be replaced or removed will be limited to the minimum number necessary to ensure confidentiality (excluding the removal of a stereochemical indicator from an explicit chemical name).
The single distinctive elements of an explicit chemical name that may be masked when creating a proposed masked name are the following:
- A locant that specifies the placement of a chemical group
- The locant and multiplicative prefix (for example, di-, tri-, and tetra-) that together specify the number and placement of a given chemical group
- The name of a given chemical group
- The name of a given parent structure, and locants of chemical groups attached to the parent structure and
- The name and multiplicative prefixes (specifying the number) of a given simple cation or anion of a salt
Table A5-1 lists by name and molecular formula the type of chemical groups that can be masked. The groups of atoms found in Table A5-1 are common structural units; a given group may be listed under more than one name. Each group includes at least one atom other than carbon or hydrogen.
If the substance contains a chemical group that includes a carbon atom having more than a single valence (for example, carbonyl -CO-), the name of that chemical group cannot be masked if the carbon atom is directly attached to an acyclic carbon atom or is included within a ring system. In this circumstance, only the atom or group of atoms attached to the valence carbon atom can be masked.
Certain chemical groups in Table A5-1 include hydrogen atoms that are often additionally substituted, for example, an ethyl group may be substituted for a hydrogen of the sulfamyl group (H2NSO2-) to give C2H5NHSO2-. If additionally substituted, only the chemical group listed in Table A5-1 should be masked, not the substituent.
Table A5-1 lists most of the common chemical functional groups that contain oxygen, for example, H2NCO-. Although not always listed, the Group VIa element (sulfur, selenium, and tellurium) analogs of these functional groups, for example, H2NCSe-, are considered to be included within Table A5-1 and, accordingly, may be used in masking.
| Item | Chemical group | Formula |
|---|---|---|
| 1 | aldo | H(C=O)- |
| 2 | amidino | NH2(C=NH)CH2- |
| 3 | amino | H2N- |
| 4 | (aminoamidino) | H2NC(=NNH2)- or H2NNHC(=NH)- |
| 5 | (aminocarbonyl) | H2NCO- |
| 6 | [(aminocarbonyl)amino] | H2NCONH- |
| 7 | [2-(aminocarbonyl)hydrazino] | H2NCONHNH- |
| 8 | [(aminocarbonyl)hydrazono] | H2NCONHN= |
| 9 | (aminohydrazonomethyl) | H2NC(=NNH2)- |
| 10 | [(aminohydroxymethylene)hydrazino] | H2NC(OH)=NNH- |
| 11 | (aminoiminomethyl) | H2NC(=NH)- |
| 12 | (aminoiminophosphoranyl) | H2NPH(=NH)- |
| 13 | (p-aminophosphinimyl) | H2NPH(=NH)- |
| 14 | (aminosulfinyl) | H2NSO- |
| 15 | (aminosulfonyl) | H2NSO2- |
| 16 | (aminothio) | H2NS- |
| 17 | (aminothioxomethyl) | H2NCS- |
| 18 | ammonio | H3N- |
| 19 | antimono | -Sb=Sb- |
| 20 | arseno | -As=As- |
| 21 | arsenoso | OAs- |
| 22 | arsinico | HOAs(O)≡ |
| 23 | arsinidene | AsH= |
| 24 | arsinidyne | As≡ |
| 25 | arsinimyl | AsH2(=NH)- |
| 26 | arsino | AsH2- |
| 27 | arsinothioyl | AsH2(S)- |
| 28 | arsinyl | AsH2(O)- |
| 29 | arsinylidene | AsH(O)≡ |
| 30 | arso | O2As- |
| 31 | arsono | (HO)2As(O)- |
| 32 | (arsonooxy) | (HO)2As(O)O- |
| 33 | arsononitridyl | AsH(=N)- |
| 34 | arsoranyl | AsH4- |
| 35 | arsoranylidyne | AsH2≡ |
| 36 | arsylene | AsH= |
| 37 | arsylidyne | As≡ |
| 38 | astato | At- |
| 39 | astatoxy | O2At- |
| 40 | astatyl | O2At- |
| 41 | azi | -N=N- |
| 42 | azido | N3- |
| 43 | (azidocarbonyl) | N3CO- |
| 44 | (azidofurmyl) | N3CO- |
| 45 | (azidosulfonyl) | N3SO2- |
| 46 | azino | =NN= |
| 47 | azo | -N=N- |
| 48 | azoxy | -N(O)=N- |
| 49 | bismuthino | BiH2- |
| 50 | bismuthylene | BiH= |
| 51 | bismuthylidyne | Bi≡ |
| 52 | borono | (HO)2B- |
| 53 | (boronooxy) | (HO)2BO- |
| 54 | boryl | BH2- |
| 55 | borylene | BH= |
| 56 | borylidyne | B≡ |
| 57 | bromo | Br- |
| 58 | (bromocarbonyl) | BrCO- |
| 59 | (bromoiminomethyl) | BrC(=NH)- |
| 60 | (bromosulfonyl) | BrSO2- |
| 61 | carbamido | H2NCONH- |
| 62 | carbamoyl | H2NCO- |
| 63 | carbamyl | H2NCO- |
| 64 | carbonimidoyl | -C(=NH)= |
| 65 | (carbonimidoylamino) | H2N=C=N- |
| 66 | carbonothioyl | -CS- |
| 67 | carbonyl | -CO- |
| 68 | (carbonylidiimino) | -NHCONH- |
| 69 | (carbonyldioxy) | -OC(O)O- |
| 70 | carboxy | HO2C- |
| 71 | chloro | Cl- |
| 72 | (chlorocarbonyl) | ClCO- |
| 73 | (chloroformyl) | ClCO- |
| 74 | (chloroiminomethyl) | ClC(=NH)- |
| 75 | (chlorosulfinyl) | ClSO- |
| 76 | (chlorosulfonyl) | ClSO2- |
| 77 | chlorosyl | OCl- |
| 78 | (chlorothio) | ClS- |
| 79 | chloryl | O2Cl- |
| 80 | cyanato | NCO- |
| 81 | cyano | NC- |
| 82 | 1,2-diarsenediyl | -As=As- |
| 83 | diarsenyl | HAs=As- |
| 84 | diarsinetetrayl | =AsAs= |
| 85 | diarsinyl | H2AsAsH- |
| 86 | 1,2-diazenediyl | -N=N- |
| 87 | diazeno | HN=N- |
| 88 | diazo | N2= |
| 89 | diazoamino | -NHN=N- |
| 90 | diazonio | N2+- |
| 91 | 1,2-diborane(4)diylidene | =BB= |
| 92 | diborane(4)tetrayl | =BB= |
| 93 | digermanylene | -GeH2GeH2- |
| 94 | digermathianyl | H3GeSGeH2- |
| 95 | dioxy | -OO- |
| 96 | 1,2-diphosphenediyl | -P=P- |
| 97 | 1,2-diphosphinediyl | -PHPH- |
| 98 | 1,2-diphosphinediylidene | =PP= |
| 99 | diphosphinetetrayl | =PP= |
| 100 | diphosphinyl | H2PPH- |
| 101 | diseleno | -SeSe- |
| 102 | 1,2-disilanediyl | -SiH2SiH2- |
| 103 | disilanoxy | H3SiSiH2O- |
| 104 | disilanyl | H3SiSiH2- |
| 105 | disilanylene | -SiH2SiH2- |
| 106 | (disilanyloxy) | H3SiSiH2O- |
| 107 | (disilathianyloxy) | H3SiSSiH2O- |
| 108 | disilazanoxy | H3SiNHSiH2O- |
| 109 | disilazanyl | H3SiNHSiH2- |
| 110 | 2-disilazanyl | (H3Si)2N- |
| 111 | (disilazanyloxy) | H3SiNHSiH2O- |
| 112 | 1,3-disiloxanediyl | -SiH2OSiH2- |
| 113 | 1,3-disiloxanediylidene | =SiHOSiH= |
| 114 | disiloxanoxy | H3SiOSiH2O- |
| 115 | disiloxanylene | -SiH2OSiH2- |
| 116 | (disiloxanyloxy) | H3SiOSiH2O- |
| 117 | disilthianoxy | H3SiSSiH2O- |
| 118 | 1,2-distannanediyl | SnH2SnH2- |
| 119 | distannanylene | -SnH2SnH2- |
| 120 | 1,3-distannathianediylidene | =SnHSSnH= |
| 121 | 1,2-distibenediyl | -Sb=Sb- |
| 122 | disulfinyl | -S(O)S(O)- |
| 123 | dithio | -SS- |
| 124 | (dithiocarboxy) | HSCS- |
| 125 | (dithiohydroperoxy) | HSS- |
| 126 | epidioxy | -OO- |
| 127 | epidiseleno | -SeSe- |
| 128 | epidithio | -SS- |
| 129 | epioxy | -O- |
| 130 | episeleno | -Se- |
| 131 | epithio | -S- |
| 132 | epoxy | -O- |
| 133 | fluoro | F- |
| 134 | (fluorocarbonyl) | FCO- |
| 135 | fluoryl | O2F- |
| 136 | formamido | HCONH- |
| 137 | 1,5-formazanidyl | -N=NCH=NNH- |
| 138 | 1-formazano | H2NN=CHN=N- |
| 139 | 5-formazano | HN=NCH=NNH- |
| 140 | formazanyl | HN=NC(=NNH2)- |
| 141 | formimidoyl | HC(=NH)- |
| 142 | formyl | HCO- |
| 143 | (formylamino) | HCONH- |
| 144 | germanetetrayl | =Ge= |
| 145 | germyl | H3Ge- |
| 146 | germylene | H2Ge= |
| 147 | germylidyne | HGe≡ |
| 148 | guanyl | H2NC(=NH)- |
| 149 | hydrazi | -NHNH- |
| 150 | 1,2-hydrazinediylidene | =NN= |
| 151 | hydrazino | H2NNH- |
| 152 | (hydrazinocarbonyl) | H2NNHCO- |
| 153 | (hydrazinoiminomethyl) | H2NNHC(=NH)- |
| 154 | (hydrazinosulfinyl) | H2NNHSO- |
| 155 | (hydrazinosulfonyl) | H2NNHSO2- |
| 156 | (hydrazinothioxomethyl) | H2NNHCS- |
| 157 | 1-hydrazinyl-2-ylidene | -NHN= |
| 158 | hydrazo | -NHNH- |
| 159 | hydrazono | H2NN= |
| 160 | hydroperoxy | HOO- |
| 161 | (hydroperoxycarbonyl) | HOOCO- |
| 162 | (hydroperoxyiminomethyl) | HOOC(=NH)- |
| 163 | (hydroperoxysulfinyl) | HOOS(=O)- |
| 164 | (hydroperoxysulfonyl) | HOOS(=O)2- |
| 165 | (hydroperoxythioxomethyl) | HOOCS- |
| 166 | hydroxy | HO- |
| 167 | (hydroxyamino) | HONH- |
| 168 | (hydroxyimino) | HON= |
| 169 | (hydroxyiminomethyl) | HOC(=NH)- |
| 170 | hydroxyl | HO- |
| 171 | (hydroxyphosphinyl) | HOPH(O)- |
| 172 | imidocarbonyl | -C(=NH)- |
| 173 | (imidocarbonylamino) | HN=C=N- |
| 174 | imino | HN= |
| 175 | (iminomercaptomethyl) | HSC(=NH)- |
| 176 | [imino(mercaptooxy)methyl] | HSOC(=NH)- |
| 177 | (iminomethyl) | HN=CH- |
| 178 | (iminonitrilo) | -NHN= |
| 179 | (iminophosphoranyl) | H2P(=NH)- |
| 180 | (iminosulfenomethyl) | HOSC(=NH)- |
| 181 | iodo | I- |
| 182 | (iodocarbonyl) | ICO- |
| 183 | iodosyl | OI- |
| 184 | iodyl | O2I- |
| 185 | isocyanato | OCN- |
| 186 | (isocyanatocarbonyl) | OCNCO- |
| 187 | (isocyanatosulfonyl) | OCNSO2- |
| 188 | isocyano | CN- |
| 189 | (isocyanocarbonyl) | CNCO- |
| 190 | isonitro | HON(O)= |
| 191 | isonitroso | HON= |
| 192 | isosemicarbazido | H2NC(OH)=NNH- |
| 193 | isothiocyanato | SCN- |
| 194 | (isothiocyanatocarbonyl) | SCNCO- |
| 195 | (isothiocyanatosulfonyl) | SCNSO2- |
| 196 | isothiocyano | SCN- |
| 197 | keto | O= |
| 198 | mercapto | HS- |
| 199 | (mercaptoamino) | HSNH- |
| 200 | (mercaptooxy) | HSO- |
| 201 | [(mercaptooxy)carbonyl] | HSOCO- |
| 202 | [(mercaptooxy)sulfinyl] | HSOS(=O)- |
| 203 | [(mercaptooxy)sulfonyl] | HSOS(=O)2- |
| 204 | [(mercaptooxy)thioxomethyl] | HSOCS- |
| 205 | (mercaptotelluro) | HSTe- |
| 206 | nitramino | O2NNH- |
| 207 | aci-nitramino | HON(O)=N- |
| 208 | nitrilio | HN+≡ |
| 209 | nitrilo | N≡ |
| 210 | (nitrilophosphoranyl) | HP(=N)- |
| 211 | nitro | O2N- |
| 212 | acinitro | HON(O)= |
| 213 | (nitroamino) | O2NNH- |
| 214 | (aci-nitroamino) | HON(O)=N- |
| 215 | (nitrooxy) | O2NO- |
| 216 | nitroso | ON- |
| 217 | (nitrosoamino) | ONNH- |
| 218 | (nitrosoimino) | ONN= |
| 219 | (nitrosooxy) | ONO- |
| 220 | (nitrothio) | O2NS- |
| 221 | oximido | HON= |
| 222 | oxo | O= |
| 223 | (oxoboryl) | OB- |
| 224 | oxy | -O- |
| 225 | 1,3-pentazadienyl | H2NN=NN=N- |
| 226 | perchloryl | O3Cl- |
| 227 | perseleno | Se=Se= |
| 228 | perthio | S=S= |
| 229 | phosphinico | HOP(O)= |
| 230 | phosphinidene | HP= |
| 231 | phosphinidyne | P≡ |
| 232 | phosphinimyl | H2P(=NH)- |
| 233 | phosphino | H2P- |
| 234 | phosphinothioyl | H2P(S)- |
| 235 | phosphinothioylidene | HP(S)= |
| 236 | phosphinyl | H2P(O)- |
| 237 | phosphinylidene | HP(O)= |
| 238 | phosphinylidyne | P(O)= |
| 239 | phospho | O2P- |
| 240 | phosphono | (HO)2P(O)- |
| 241 | (phosphonocarbonyl) | (HO)2P(CO)- |
| 242 | phosphononitridyl | HP(=N)- |
| 243 | (phosphonooxy) | (HO)2P(O)O- |
| 244 | phosphoranyl | H4P- |
| 245 | phosphoranylidene | H3P= |
| 246 | phosphoranylidyne | H2P≡ |
| 247 | phosphoro | -P=P- |
| 248 | phosphoroso | OP- |
| 249 | plumbanetetrayl | =Pb= |
| 250 | plumbyl | H3Pb- |
| 251 | plumbylene | H2Pb= |
| 252 | plumbylidyne | HPb= |
| 253 | seleneno | HOSe- |
| 254 | selenino | HOSe(O)- |
| 255 | seleninoselenoyl | Se=Se= |
| 256 | seleninyl | OSe= |
| 257 | seleno | -Se- |
| 258 | selenocyanato | NCSe- |
| 259 | selenono | (HO)SeO2- |
| 260 | selenonyl | O2Se= |
| 261 | selenoxo | Se= |
| 262 | selenyl | HSe- |
| 263 | semicarbazido | H2NCONHNH- |
| 264 | semicarbazono | H2NCONHN= |
| 265 | silanetetrayl | =Si= |
| 266 | silyl | H3Si- |
| 267 | silylene | H2Si= |
| 268 | silylidyne | HSi≡ |
| 269 | (silyloxy) | H3SiO- |
| 270 | stannanetetrayl | =Sn= |
| 271 | stannono | HOSn(O)- |
| 272 | stannyl | H3Sn- |
| 273 | stannylene | H2Sn= |
| 274 | stannylidyne | HSn≡ |
| 275 | stibinico | HOSb(O)= |
| 276 | stibino | H2Sb- |
| 277 | stibo | O2Sb- |
| 278 | stibono | (HO)2Sb(O)- |
| 279 | (stibonooxy) | (HO)2Sb(O)O- |
| 280 | stiboso | OSb- |
| 281 | stibyl | H2Sb- |
| 282 | stibylene | HSb= |
| 283 | stibylidyne | Sb≡ |
| 284 | sulfamino | HOSO2NH- |
| 285 | sulfamoyl | H2NSO2- |
| 286 | sulfamyl | H2NSO2- |
| 287 | sulfeno | HOS- |
| 288 | (sulfenocarbonyl) | HOSCO- |
| 289 | (sulfenosulfinyl) | HOSS(=O)- |
| 290 | (sulfenosulfonyl) | HOSS(=O)2- |
| 291 | (sulfenothioxomethyl) | HOSCS- |
| 292 | sulfhydryl | HS- |
| 293 | sulfinimidoyl | HN=S= |
| 294 | sulfino | HOS(O)- |
| 295 | (sulfinooxy) | HOS(O)O- |
| 296 | sulfinothioyl | S=S= |
| 297 | sulfinyl | OS= |
| 298 | sulfo | HO3S- |
| 299 | (sulfoamino) | HOSO2NH- |
| 300 | sulfonimidoyl | HN=S(O)= |
| 301 | sulfonodiimidoyl | (HN=)2S= |
| 302 | sulfonyl | -SO2- |
| 303 | (sulfooxy) | HO3SO- |
| 304 | sulfuryl | -SO2- |
| 305 | telluro | -Te- |
| 306 | telluroxo | Te= |
| 307 | telluryl | HTe- |
| 308 | 1,4-tetraphosphinediyl | -(PH)4- |
| 309 | 1,7-tetrasiloxanediyl | -SiH2(OSiH2)2OSiH2- |
| 310 | tetrathio | -SSSS- |
| 311 | 1,4-tetrazanediyl | -(NH)4- |
| 312 | 1,4-tetrazanediylidene | =N(NH)2N= |
| 313 | 1-tetrazenyl | H2NNHN=N- |
| 314 | thio | -S- |
| 315 | (thioarsenoso) | S=As- |
| 316 | (thiocarbamoyl) | H2NCS- |
| 317 | thiocarbamyl | H2NCS- |
| 318 | (thiocarbonyl) | -CS- |
| 319 | (thiocarboxy) | HOSC- |
| 320 | thiocyanato | NCS- |
| 321 | thiocyano | NCS- |
| 322 | (thioformyl) | HCS- |
| 323 | thiohydroperoxy | HOS- or HSO- |
| 324 | (thiohydroxy) | HS- |
| 325 | (thionitroso) | SN- |
| 326 | thionyl | -SO- |
| 327 | (thioseleneno) | HSSe- |
| 328 | (thiosulfeno) | HSS- |
| 329 | (thiosulfo) | (HO2S2)- |
| 330 | thioxo | S= |
| 331 | (thioxoarsino) | S=As- |
| 332 | (thioxomethyl) | HCS- |
| 333 | thiuram | H2NCS- |
| 334 | triazanyl | H2NNHNH- |
| 335 | 1-triazene-1,3-diyl | -NHN=N- |
| 336 | 1-triazenyl | H2NN=N- |
| 337 | triseleno | -SeSeSe- |
| 338 | 1,3-trisilanediyl | -(SiH2)3- |
| 339 | 1,3,5-trisiloxanetriyl | -SiH(OSiH2-)2 |
| 340 | trithio | -SSS- |
| 341 | uramino | H2NCONH- |
| 342 | ureido | H2NCONH- |
| 343 | ureylene | -NHCONH- |
A5.1.1 Parent masking
The name of a parent structure of a substance that can be described with a definite structure diagram and definite molecular formula may be masked in the explicit chemical name only by the following non-descriptive terms:
- Alkyl or alkane
- Alkenyl or alkene
- Alkynyl or alkyne
- Carbomonocyclic or carbomonocycle (for example, benzene, cyclopentane)
- Carbopolycyclic or carbopolycycle (for example, naphthalene, spiroundecane)
- Heteromonocyclic or heteromonocycle (for example, pyrrole, p-dioxane) or
- Heteropolycyclic or heteropolycycle (for example, indole, benzothiazole)
In the case of a coordinated metal compound, the identity of the metal atom may be masked by the term “metal” in the explicit chemical name.
Only one such parent group or multiple occurrences of the same parent group should be masked.
The following examples show common explicit chemical names for which a single distinctive element is masked.
Example 1
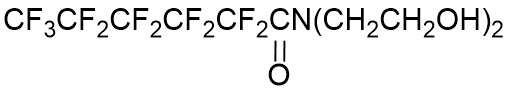
Fully Defined Explicit Chemical Name:
- 2,2,3,3,4,4,5,5,6,6,6-Undecafluoro-N,N-bis(2-hydroxyethyl) hexanamide
Acceptable Single Masking:
- Fluorine atoms masked:
- 2,2,3,3,4,4,5,5,6,6,6-Undecahalo-N,N-bis(2-hydroxyethyl) hexanamide
- Locants and multiplicative prefix of fluorine atoms masked:
- Polyfluoro-N,N-bis(2-hydroxyethyl) hexanamide
- Hydroxyl groups masked:
- 2,2,3,3,4,4,5,5,6,6,6-Undecafluoro-N,N-bis(2-substituted ethyl) hexanamide
- Hexane parent (plus locants of chemical groups) masked:
- Undecafluoro-N,N-bis(2-hydroxyethyl) alkanamide
- Amide group (plus nitrogen locants) masked:
- 2,2,3,3,4,4,5,5,6,6,6-Undecafluoro-bis(2-hydroxyethyl) hexane derivative
Example 2
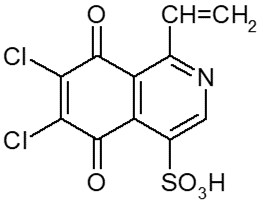
Fully Defined Explicit Chemical Name:
- 6,7-Dichloro-1-ethenyl-5,8-dihydro-5,8-dioxo-4-isoquinolinesulfonic acid
Acceptable Single Masking:
- Chlorine atoms masked:
- 6,7-Dihalo-1-ethenyl-5,8-dihydro-5,8-dioxo-4-isoquinolinesulfonic acid
- Vinyl group masked:
- 6,7-Dichloro-1-alkenyl-5,8-dihydro-5,8-dioxo-4-isoquinolinesulfonic acid
- Oxo group masked:
- 6,7-Dichloro-1-ethenyl-5,8-dihydro-5,8-disubstituted-4-isoquinoline sulfonic acid
- Sulfo group masked:
- 6,7-Dichloro-1-ethenyl-5,8-dihydro-5,8-dioxo-4-substituted isoquinoline
- Isoquinoline ring (plus locants of chemical groups attached to isoquinoline) masked:
- Dichloroethenyldihydrodioxoheteropolycyclic sulfonic acid or
- Dichloroethenyldihydrodioxosulfoheteropolycycle
A5.2 Substances not having definite structure diagrams and molecular formulas
Some substances, such as polymers, cannot be represented by definite structure diagrams and may or may not have definite molecular formulas. In other instances, the composition can be described only in terms of a complex combination of several different known or unknown components such as substances of Unknown or Variable composition, Complex reaction products or Biological materials (UVCBs).
The method of manufacture can also identify a substance. For a substance manufactured by means of a chemical reaction, identification can be stated in terms of the immediate precursor substances and other reactants that participate in the final reaction sequence used to manufacture the substance, and the nature of the reaction (for example, ethoxylation or bromination). For a substance obtained from a source without chemical reaction, processing information identifies the source and method of preparation (for example, distillation, or extraction with methylene chloride).
Although the explicit chemical name of a substance lacking a definite chemical structure or unique structure diagram may be based on variable types of descriptive terms, the procedures of masking are similar to those used for substances with definite structure diagrams and definite molecular formulas (consult section A5.1).
The composition of a substance that can be represented by a partial or incomplete chemical structure diagram can generally be described by a common chemical name that encompasses the variability or incompleteness in the structure. A masked name for such a substance will usually be acceptable if masking the partial structure diagram follows the same procedures used for substances with definite structure diagrams and definite molecular formulas.
In other instances, the explicit chemical name may identify a predominant component or components of its composition, an immediate precursor or precursors, and other reactants by specific chemical name. A proposed masked name will usually be acceptable for such a substance if it is constructed by masking the chemical name of one such component, precursor, or reactant.
Clearly, the masking procedures in this appendix are most useful for masking the identity of substances having a single distinctive element, and will only be useful for some types of substances that cannot be described with a unique structure diagram. In some of these latter cases, the masking procedures provided may have little applicability. For consistency, submitters must base their choice of a masked name on an explicit chemical name of the substance established in accordance with the current chemical nomenclature rules of the International Union of Pure and Applied Chemistry (IUPAC) or the Chemical Abstracts Service (CAS), as provided on the new substances submission form (for example, NSN Form, DSL Nomination Form, NDSL Nomination Form, etc.). The New Substances (NS) program will consider each such proposed masked name on a case-by-case basis.
Changing the order of components of the explicit chemical name of a polymer
Generally, it may be acceptable to change the order of monomers and reactants in the explicit chemical name of a polymer before masking. However, the first element of the name may not be moved. Furthermore, a written statement substantiating the need for moving the monomers and reactants will be required, together with a listing of the new position of each of the moved monomers and reactants with associated chemical names and structure diagrams.
Masking a pre-polymer
A pre-polymer that is conserved as part of the explicit chemical name is considered a single reactant for naming purposes, and thus cannot be broken up into its constituents. As a result, if the name of a pre-polymer is conserved in the explicit name of a polymer, then the pre-polymer chemical name must be equally conserved in the masked name. Structural components of the pre-polymer name can still be masked in accordance with the provisions of the Masked Name Regulations, just as they can be for single distinctive elements of the polymer explicit chemical name as a whole.
Example 3
Substance Description:
- Linseed-oil fatty acids-fumaric acid-glycerol-maleic anhydride polymer
Specific Explicit Chemical Name:
- Fatty acids, linseed-oil, polymers with fumaric acid, glycerol and maleic anhydride
Acceptable Single Masking:
- Linseed-oil masked:
- Fatty acids, plant-based oil, polymers with fumaric acid, glycerol and maleic anhydride
- Fumaric acid masked:
- Fatty acids, linseed-oil, polymers with alkenedioic acid, glycerol and maleic anhydride
Example 4
Substance Description:
- Polyethylene glycol, mono-C12-15-alkyl ethers, phosphates, potassium salts
Specific Explicit Chemical Name:
- Poly(oxy-1,2-ethanediyl), α-hydro-ω-hydroxy-, mono-C12-15-alkyl ethers, phosphates, potassium salts
Acceptable Masked Names:
- Potassium masked:
- Poly(oxy-1,2-ethanediyl), α-hydro-ω-hydroxy-, mono-C12-15-alkyl ethers, phosphates, metal salts
- C12-15-alkyl group masked:
- Poly(oxy-1,2-ethanediyl), α-hydro-ω-hydroxy-, monoalkyl ethers, phosphates, potassium salts
- Ethane masked:
- Poly(oxy-alkanediyl), α-hydro-ω-hydroxy-, mono-C12-15-alkyl ethers, phosphates, potassium salts
A5.3 Masking of biochemicals and biopolymers
Biochemicals and biopolymers that do not have catalytic activity can be masked by disguising descriptive segments of the explicit chemical name. Masking of more than one segment of the explicit chemical name is considered additional masking and would not be permitted without justification. Masking may be accomplished by replacing single distinctive elements of the explicit biological name of a substance with non-descriptive terms and/or removing the locants (consult sections A5.1 and A5.2).
A5.3.1 Enzymatic substances
For enzymes, masked names should be created by disguising the fourth level Enzyme Commission number and using the corresponding description of the selected Enzyme Commission level as the disguising term. Removal of each number of the Enzyme Commission number (a.b.c.d.) is a single masking. For example, removing “d” is single masking; removing “c.d” is double masking and “b.c.d” is triple masking. Note that the first number “a” is not maskable. In instances where a fourth level Enzyme Commission number only consists of one entry, the New Substances program will accept reverting to the second level Enzyme Commission number as a single masking.
Example 5
Substance Description:
- 6-Hydroxynicotinate reductase (Enzyme Commission number 1.3.7.1)
Proposed double masking:
- Substituted-heteromonocycle reductase (Enzyme Commission number 1.3)
Example 6
Substance Description:
CAS Registry Number 9042-64-2:
- Aromatic-L-amino-acid decarboxylase
- Associated Enzyme Commission number: 4.1.1.28
Proposed single masking:
- Decarboxylase
- Associated Enzyme Commission number: 4.1.1
Example 7
Substance Description:
CAS Registry Number 341585-05-5:
- Xylose isomerase (Enzyme Commission number 5.3.1.5) (Lactococcus lactis lactis strain IL1403 gene xylA) (9CI)
Proposed single masking:
- Xylose isomerase (Enzyme Commission number 5.3.1.5) (Lactococcus lactis lactis gene xylA)
Proposed double masking:
- Xylose isomerase (Enzyme Commission number 5.3.1.5) (Lactococcus lactis lactis)
Proposed triple masking:
- Intramolecular oxidoreductase (Enzyme Commission number 5.3) (Lactococcus lactis lactis strain IL1403)
A5.4 Justifying the use of additional masking
If strict application of the masking procedures (for example, the masking of only one single distinctive element) would not adequately mask a specific substance identity, then the notifier may propose a masked name that disguises the substance identity to a greater extent. This proposed masked name must be generated in accordance with the Masked Name Regulations and each additional masking must be accompanied by a separate justification to substantiate the necessity to further disguise the explicit chemical name.
For greater certainty on the use of additional masking:
- Notifiers may propose generally up to 5 masking terms
- The first masking term does not require additional justification, besides those already provided in the confidentiality substantiation of section 6.2.1.5 and section 7.2.2-3 of the Guidance Document and
- Additional justification is required for each additional masking, notifiers must clearly explain why each masking is required, using a stepwise approach
For example, a single masking reveals information about the chemical structure that could adversely affect the intellectual property or market value of the substance, so a second masking is required to protect the information.
Appendix 6. Examples of waiver requests
The requirement to provide test data on a chemical or polymer may be waived if, in the opinion of the Minister of the Environment (the Minister), one of the 3 statutory criteria for a waiver of information is being met (consult section 8.7).
Conditions for accepting a waiver request will be considered on a case-by-case basis. Each waiver request should be accompanied by a well-documented scientific rationale as well as an identification of the statutory criterion under which the request is being made. Failure to provide a proper rationale, with supporting documentation, will result in the rejection of the waiver request.
Examples of conditions under which a waiver may be granted by the Minister are described below. Unless specified otherwise, the examples are in accordance with paragraph 81(8)(c) of the Canadian Environmental Protection Act, 1999 (the Act).
A6.1 Chemicals
The following are examples of circumstances under which the Minister may grant waivers, which may apply to any test:
- The substance is created in situ during the manufacturing process and decomposes during isolation attempts
- The substance coexists with another component or components that cannot be feasibly isolated and that adversely affects test results or
- The substance reacts dangerously during the performance of the test
A6.1.1 Physico-chemical endpoints
A6.1.1.1 Melting point and boiling point
The following is an example of conditions under which a waiver may be granted by the Minister:
- The substance is a salt that is stable only as an aqueous solution
A waiver is not required when:
- the substance has a melting point of less than -25 °C or more than 300 °C. A statement to that effect with substantiating information is sufficient to address the melting point requirement
- the substance has a boiling point of less than -50 °C or or more than 300 °C. A statement to that effect with substantiating information is sufficient to address the boiling point requirement or
- the substance undergoes a chemical reaction other than melting or boiling (for example, degradation, rearrangement). However, the temperature of the chemical reaction must be reported
When applicable and available, alternative data such as a decomposition point, pour point, softening point or sublimation point can be reported as alternative data. In the case of biochemicals and biopolymers, an isoelectric point can be reported as alternative data for the melting point. A waiver request is not required when alternative data is provided.
A6.1.1.2 Density
A waiver is not required when:
- the substance is only stable in solution in a particular solvent and the solution density is similar to that of the solvent. In such cases, an indication of whether the solution density is higher or lower than the solvent density would be sufficient
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(c) of the Act for the determination of density, on the basis that the notified substance cannot be isolated and it is technically infeasible to determine the density
A6.1.1.3 Vapour pressure
The following are examples of conditions under which a waiver may be granted by the Minister:
- The substance has a large molecular weight (more than 1 000 Da)
- The substance is an ionic solid or
- The substance is a solid with a high melting point (more than 300 °C)
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(a) of the Act for the determination of vapour pressure on the basis that the substance is an ionic solid and therefore has negligible vapour pressure
A waiver is not required when:
- the substance has a standard boiling point of less than 0 °C. A statement to that effect with substantiating information is sufficient to address this requirement
A6.1.1.4 Water solubility
The following is an example of conditions under which a waiver may be granted by the Minister:
- The substance forms a stable emulsion in water which cannot be separated by filtration or centrifugation methods (for example, surface active substances)
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(c) of the Act on the basis that the notified substance reacts dangerously during performance of the test.
A waiver is not required when:
- the substance is 100% miscible in water (more than 1 000 g/L, as per the Organisation for Economic Co-operation and Development (OECD) Test Guideline (TG) 105) or
- the substance is produced in an aqueous solution and is not available in an isolated form. A statement to that effect with substantiating information is sufficient to address this requirement
A6.1.1.5 Octanol/water partition coefficient
The following are examples of conditions under which a waiver may be granted by the Minister:
- The substance is inorganic (that is, does not contain any carbon atoms)
- The solubility of the substance in water and/or octanol cannot be measured quantitatively or
- The substance is surface active (surface tension of less than 60 mN/m, as prescribed in OECD TG 115)
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(c) of the Act for the determination of octanol/water partition coefficient on the basis that it was technically infeasible to determine this endpoint using OECD 107 and 117 TGs owing to the surface active (surface tension of less than 60 mN/m) nature of the notified substance
A waiver is not required when:
- the substance is a chemical with a water solubility of more than 5 g/L. A statement to that effect with substantiating information is sufficient to address this requirement
A6.1.1.6 Ready biodegradation
The following is an example of conditions under which a waiver may be granted by the Minister:
- The substance is inorganic (that is, contains no carbon atoms)
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(a) of the Act for biodegradation on the basis that the notified substance contains no carbon atoms and is therefore not susceptible to biodegradation
A6.1.1.7 Adsorption–desorption
The following is an example of conditions under which a waiver may be granted by the Minister:
- The solubility of the substance in water cannot be measured quantitatively
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(c) of the Act for the determination of adsorption-desorption on the basis that the water solubility for the notified substance could not be determined because the substance cannot be measured quantitatively
A waiver is not required when:
- the substance has a water solubility of less than 200 µg/L. A statement to that with substantiating information effect is sufficient to address this requirement
A6.1.1.8 Hydrolysis rate as a function of pH
The following is an example of conditions under which a waiver may be granted by the Minister:
- The substance has no readily hydrolysable groups and therefore is not expected to hydrolyse. The following provides examples of non-hydrolysable groups:
- Alcohols
- Aldehydes
- Alkanes
- Alkenes
- Alkynes
- Aromatic amines
- Aromatic nitro compounds
- Benzenes/Biphenyls
- Carboxylic acids
- Ethers
- Glycols
- Halogenated aromatics
- Heterocyclic polycyclic aromatic hydrocarbons
- Hydrocarbons
- Ketones
- Phenols
- Polycyclic aromatic hydrocarbons or
- Sulphonic acids
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(a) of the Act for the determination of hydrolysis rate as a function of pH on the basis that the substance does not contain any readily hydrolysable groups and is expected to be stable
A waiver is not required when:
- the substance has a water solubility of less than 200 µg/L. A statement to that effect with substantiating information is sufficient to address this requirement
A6.1.2 Toxicological endpoints
A6.1.2.1 Acute mammalian toxicity
The following are examples of conditions under which a waiver may be granted by the Minister:
- The substance is corrosive and is expected to cause severe and enduring pain to the test animal. This waiver must be requested under paragraph 81(8)(a) of the Act or
- It is not technically feasible to administer known doses of the substance because of its chemical or physical properties. This waiver must be requested under paragraph 81(8)(c) of the Act
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested for acute dermal toxicity under paragraph 81(8)(a) of the Act on the basis that the notified substance is corrosive or severely irritating to the skin of laboratory animals as shown in at least one animal test or has a pH less than 2 or greater than 11.5
If the vapour pressure of the notified substance is very high and, as such, oral or dermal exposure is not considered to be a significant route of exposure, an inhalation test should be submitted instead. High vapour pressure alone does not necessarily support a waiver request.
A6.1.2.2 Skin irritation
The following are examples of conditions under which a waiver may be granted by the Minister:
- It is not technically feasible to administer the substance topically. This waiver must be requested under paragraph 81(8)(c) of the Act or
- The substance is expected to be corrosive to the skin of test animals or has demonstrated high acute dermal toxicity. This waiver must be requested under paragraph 81(8)(a) of the Act
A6.1.2.3 Skin sensitization
The following is an example of a condition under which a waiver may be granted by the Minister:
- It is not technically feasible to administer the substance topically. This waiver could be requested under paragraph 81(8)(c) of the Act
A6.1.2.4 Repeated-dose mammalian toxicity
The following is an example of a condition under which a waiver may be granted by the Minister:
- It is not technically feasible to administer known doses of the substance because of its chemical or physical properties. This waiver could be requested under paragraph 81(8)(c) of the Act
If the vapour pressure of the notified substance is very high and, as such, oral or dermal exposure is not considered to be a significant route of exposure, an inhalation exposure test should be submitted instead. High vapour pressure alone does not necessarily support a waiver request.
A6.1.2.5 In vitro test for gene mutations
The following is an example of a condition under which a waiver may be granted by the Minister:
- An in vivo mammalian genotoxicity assay indicates that the substance has mutagenic activity. This waiver could be requested under paragraph 81(8)(a) of the Act
A6.1.2.6 In vitro mammalian test for chromosomal aberrations
The following is an example of a condition under which a waiver may be granted by the Minister:
- An in vivo mammalian genotoxicity test indicates that the substance has clastogenic activity. This waiver could be requested under paragraph 81(8)(a) of the Act
A6.1.2.7 In vivo mammalian test for genotoxicity
A waiver could be requested under paragraph 81(8)(a) of the Act for the determination of in vivo genotoxicity on the basis that the following conditions are met in relation to the substance:
- The intended use of the substance will not involve direct, repeated, or prolonged public exposure
- The results of both the in vitro gene mutation and the in vitro mammalian chromosomal aberration tests indicate that the substance has no genotoxic activity in those tests and
- The chemical structure of the substance, or part thereof, is not related to a known mutagen or carcinogen
Alternatively, a waiver could be requested under paragraph 81(8)(a) of the Act for the determination of in vivo genotoxicity on the basis that the 2 in vitro genotoxicity tests (one gene mutation in bacteria and one mammalian chromosomal aberration study) were positive. This waiver will result in the conclusion that the substance is genotoxic.
A6.2 Polymers
The following are examples of conditions under which the Minister may grant a waiver, which may apply to any test:
- The substance is created in situ during the manufacturing process and decomposes during isolation attempts or
- The substance reacts dangerously during the performance of the test
A6.2.1 Physico-chemical endpoints
A6.2.1.1 Number average molecular weight and concentration/amount of residual/low molecular weight constituents
The following are examples of conditions under which a waiver may be granted by the Minister:
- The substance is a highly cross-linked polymer such that the test is not technically feasible (accompanied with a sound scientific justification) or
- The substance is insoluble in solvents required to perform Gel Permeation Chromatography (GPC) analysis and no other technique is practicable
A6.2.1.2 Octanol/water partition coefficient
In cases of water-reactive polymers, the New Substances (NS) program recognizes there may be issues with self-condensation and the formation of precipitates. Consult Appendix 9 of this Guidance Document for information on the NS program approach to water-reactive polymers.
The following are examples of conditions under which a waiver may be granted by the Minister:
- The solubility of the substance in water and/or octanol cannot be measured analytically or
- The substance is surface active (surface tension of less than 60 mN/m, as prescribed in OECD TG 115)
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(c) of the Act for the determination of the octanol/water partition coefficient on the basis that it was technically infeasible to determine this endpoint using OECD 107 and 117 TGs owing to the surface active (surface tension of less than 60 mN/m) nature of the notified substance.
A6.2.1.3 Hydrolysis rate as a function of pH
In cases of water-reactive polymers, the NS program recognizes there may be issues with self-condensation and the formation of precipitates. Consult Appendix 9 of this Guidance Document for information on the NS program approach to water-reactive polymers.
The following is an example of a condition under which a waiver may be granted by the Minister:
- The polymer has no readily hydrolysable groups and therefore is not expected to hydrolyse. The following provides examples of non-hydrolysable groups:
- Alcohols
- Aldehydes
- Alkanes
- Alkenes
- Alkynes
- Aromatic amines
- Aromatic nitro compounds
- Benzenes/Biphenyls
- Carboxylic acids
- Ethers
- Glycols
- Halogenated aromatics
- Heterocyclic polycyclic aromatic hydrocarbons
- Hydrocarbons
- Ketones
- Phenols
- Polycyclic aromatic hydrocarbons or
- Sulphonic acids
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(a) of the Act for the determination of hydrolysis rate as a function of pH on the basis that the substance does not contain any hydrolysable groups and is expected to be stable
A waiver is not required when:
- the substance has a water extractability less than or equal to 2%. A statement to that effect with substantiating information is sufficient to address this requirement
A6.2.1.4 Ready biodegradation
In cases of water-reactive polymers, the NS program recognizes there may be issues with self-condensation and the formation of precipitates. Consult Appendix 9 of this Guidance Document for information on the NS program approach to water-reactive polymers.
The following is an example of a condition under which a waiver may be granted by the Minister:
- The polymer is inorganic (that is, contains no carbon atoms)
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(a) of the Act for biodegradation on the basis that the notified substance contains no carbon atoms and is therefore not susceptible to biodegradation
A waivers is not required when:
- the substance has a water extractability less than or equal to 2% at pH 7 or is a branched silicone or siloxane polymer. A statement to that effect with substantiating information is sufficient to address this requirement
A6.2.2 Toxicological endpoints
Mammalian toxicity data can potentially be waived for Non-Reduced Regulatory Requirement polymers (non-RRR) solely due to the presence of the following cationic or potentially cationic groups: primary, secondary, tertiary amine groups, carbodiimides or sulphoniums. This will be dependent upon, for example, considerations of use patterns and low anticipated potential exposure to the general population. This waiver must be requested under paragraph 81(8)(a) of the Act.
Polymers intended for use in personal care products, children’s toys or direct food contact materials will generally not be eligible for a waiver of acute and repeated-dose toxicity tests if prolonged dermal contact or oral ingestion is expected to be a significant route of exposure.
Polymers will generally not be eligible for a waiver of acute and repeated-dose toxicity tests if inhalation is expected to be the most significant route of exposure of the general population based on expected type of use.
A typical waiver request may be formulated as follows, accompanied by a sound scientific rationale:
- A waiver is requested under paragraph 81(8)(a) of the Act for 28-day repeated-dose mammalian toxicity on the basis that the notified substance meets the cationic class definition and use patterns of the substance will not involve direct, repeated or prolonged public exposure
Health toxicity endpoints referred to in item 4 of Schedule 10 of the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) and items 5 to 10 of Schedule 11 of the Regulations are not required if the polymer is a non-RRR polymer solely due to the presence of any of the following functional groups:
- (a) aldehydes whose functional group equivalent weight (FGEW, consult section 3.3.1.8) is less than or equal to 1 000 daltons
- (b) vinyl ethers whose FGEW is less than or equal to 5 000 daltons or
- (c) sulphonic acids whose FGEW is less than or equal to 5 000 daltons
A6.2.2.1 Acute mammalian toxicity
The following are examples of conditions under which a waiver may be granted by the Minister:
- The polymer is corrosive to the skin of test animals and is expected to cause severe and enduring pain to the test animal. This waiver could be requested under paragraph 81(8)(a) of the Act or
- It is not technically feasible to administer known doses of the polymer because of its chemical or physical properties. This waiver could be requested under paragraph 81(8)(c) of the Act
A6.2.2.2 Skin irritation
The following are examples of conditions under which a waiver may be granted by the Minister:
- It is not technically feasible to administer the polymer topically. This waiver could be requested under paragraph 81(8)(c) of the Act or
- The polymer is expected to be corrosive the skin of test animals or has demonstrated high acute dermal toxicity. This waiver could be requested under paragraph 81(8)(a) of the Act
A6.2.2.3 Skin sensitization
The following is an example of a condition under which a waiver may be granted by the Minister:
- It is not technically feasible to administer the polymer topically. This waiver could be requested under paragraph 81(8)(c) of the Act
A6.2.2.4 Repeated-dose mammalian toxicity
The following is an example of a condition under which a waiver may be granted by the Minister:
- It is not technically feasible to administer known doses of the polymer because of its chemical or physical properties. This waiver could be requested under paragraph 81(8)(c) of the Act
A6.2.2.5 In vitro test for gene mutations
The following is an example of a condition under which a waiver may be granted by the Minister:
- An in vivo mammalian genotoxicity test indicates that the polymer has mutagenic activity. This waiver could be requested under paragraph 81(8)(a) of the Act
A6.2.2.6 In vitro mammalian test for chromosomal aberrations
The following is an example of a condition under which a waiver may be granted by the Minister:
- An in vivo mammalian genotoxicity test indicates that the polymer has clastogenic activity. This waiver could be requested under paragraph 81(8)(a) of the Act
A6.2.2.7 In vivo mammalian test for genotoxicity
A waiver may be requested under paragraph 81(8)(a) of the Act for the determination of in vivo genotoxicity on the basis that the following conditions are met in relation to the polymer:
- The intended use of the polymer will not involve direct, repeated, or prolonged public exposure
- The results of both the in vitro gene mutation and the in vitro mammalian chromosomal aberration tests indicate that the polymer has no genotoxic activity in those tests and
- The chemical structure of the polymer, or part thereof, is not related to a known mutagen or carcinogen
A waiver may be also requested under paragraph 81(8)(a) of the Act for the determination of in vivo genotoxicity on the basis that the 2 in vitro genotoxicity tests (one gene mutation in bacteria and one mammalian chromosomal aberration study) were positive. This waiver will result in the substance to be concluded as genotoxic.
Appendix 7. Fulfilling number average molecular weight requirements with Gel Permeation Chromatography data
A7.1 Test procedures
The test procedures used to generate the number average molecular weight (Mn) data requirements must be specified. The New Substances (NS) program recommends test protocols of the Organisation for Economic Co-operation and Development (OECD) Test Guideline (TG) 118 for the determination of the Mn using Gel Permeation Chromatography (GPC), and OECD TG 119 for residual constituents with molecular weights less than 500 daltons and less than 1 000 daltons.
A7.2 Gel permeation chromatogram
A chromatogram with the calculated Mn must be provided.
The name of the notified substance must be clearly identified on the GPC test data. The full original GPC curve, with the integration interval clearly identified must be provided.
A blank run should also be provided.
In the case of GPC curves where a portion has been cut off (that is, not integrated over the full peak area) a justification explaining why certain peaks were not included in the calculation of Mn and percentage of low molecular weight components must be provided. This may involve identification of the cut-off peaks as, for example, residual monomer or additives or other solvents found in the sample.
The name of the test and the calibration curve referenced must be clearly identified on the print-out of the chromatogram. A lab-assigned sample identification code must be identified as the new substance in block A.19 of the New Substances Notification (NSN) Form. Figure A7-1 is an example of GPC chromatograph with recommended description.

List of recommended description to include with GPC chromatogram:
- Project name: GPC of Trade Name
- Sample name: Trade Name1, reference should match trade names or substance name provided in block A.19 of the NSN Form
- Injection volume: 100.00
- Instrument: reference to procedure and instrument used
- Mobile phase: reference to procedure and mobile phase information
- Column set: reference to procedure and column settings output from software and
- Date processed: 02/02/2019 11:00:00 AM EDT
| Sample name | MPa | Mnb | Mzc | Mwd | Weight % < 500 daltons | Weight % < 1 000 daltons | Mw/Mn (polydispersity) |
|---|---|---|---|---|---|---|---|
| Trade Name1 | 985 | 1 584 | 4 994 | 3 068 | 3.8 | 17.4 | 1.9 |
a MP – molecular weight of the highest peak.
b Mn – number average molecular weight.
c Mz – z average molecular weight.
d Mw – weight average molecular weight.
A7.3 Calibration
The calibration must identify all running conditions and the chemical identity of standards used. The date of calibration should be within one month of the GPC data for the notified substance. Data for samples acquired over one month from the calibration date should include controls demonstrating that the system is suitable for the measurement (consult section A7.6.4).
All measurements used for constructing the calibration curves have to be documented, preferably in a table (consult Table A7-2). Figure A7-2 is an example of a calibration curve with a recommended description.
Multi Angle Light Scattering (MALS) or Multi Angle Laser Light Scattering (MALLS) produces absolute molecular weight information and therefore does not require calibration with standards.
| Slice | Retention time (minutes) | Molecular weight (daltons) | Calculated weight (daltons) | Percent residual |
|---|---|---|---|---|
| 1 | 15.923 | 1 090 000 | 1 054 689 | 3.348 |
| 2 | 16.456 | 579 000 | 590 573 | -1.960 |
| 3 | 17.259 | 246 000 | 262 182 | -6.172 |
| 4 | 18.035 | 130 000 | 127 883 | 1.656 |
| 5 | 18.866 | 67 000 | 63 238 | 5.949 |
| 6 | 19.630 | 34 800 | 34 981 | -0.516 |
| 7 | 20.595 | 17 800 | 17 732 | 0.381 |
| 8 | 21.751 | 8 400 | 8 577 | -2.062 |
| 9 | 23.382 | 3 420 | 3 519 | -2.825 |
| 10 | 25.073 | 1 620 | 1 578 | 2.632 |
| 11 | 27.094 | 682 | 665 | 2.628 |
| 12 | 27.455 | 578 | 572 | 1.053 |
| 13 | 27.893 | 474 | 477 | -0.623 |
| 14 | 28.445 | 370 | 379 | -2.346 |
| 15 | 29.229 | 266 | 272 | -2.180 |
| 16 | 30.441 | 162 | 159 | 1.700 |

List of recommended description to include with GPC calibration curve:
- Date processed: 02/01/2019 10:00:00 AM EDT
- Instrument: instrument information
- Mobile phase: reference to procedure and mobile phase information
- Column set: reference to procedure and column settings output from software
- Sample: reference to procedure and sample information
- Computer: computer information and
- Calibration standards: the calibration was established with polystyrene standards
A7.4 Slice tables
Slice tables are not explicitly mentioned as a mandatory requirement in the New Substances Notification Regulations (Chemicals and Polymers). However, they may be required to prove the polymer status or the notification status (Reduced Regulatory Requirement or Non-Reduced Regulatory Requirement polymer, consult section 3.3.1.5 and section 3.3.1.6).
Slice information should be acquired at reasonable intervals. Depending on the dispersity of the polymer, 1 to 3 pages of information is sufficient. Retention time or retention volume should be used as slice-defining parameter.
If supplied, slice tables data must match the GPC curve. Molecular weight at a certain time (or volume) must correspond to the curve.
Slice tables must include a minimum of 3 columns: retention time (or volume), molecular weight, and the cumulative percent (or similar). Table A7-3 is an example of slice table with recommended description.
| Slice | Retention time (minutes) | Molecular weight (daltons) | Cumulative percent |
|---|---|---|---|
| 1 | 21.24 | 11 729 | 1 |
| 2 | 21.91 | 7 802 | 5 |
| 3 | 22.31 | 6 197 | 10 |
| 4 | 22.61 | 5 268 | 15 |
| 5 | 22.87 | 4 590 | 20 |
| 6 | 23.09 | 4 096 | 25 |
| 7 | 23.32 | 3 630 | 30 |
| 8 | 23.53 | 3 270 | 35 |
| 9 | 23.70 | 3 008 | 40 |
| 10 | 23.92 | 2 698 | 45 |
| 11 | 24.23 | 2 327 | 50 |
| 12 | 24.42 | 2 129 | 55 |
| 13 | 24.55 | 2 003 | 60 |
| 14 | 24.70 | 1 863 | 65 |
| 15 | 25.13 | 1 541 | 70 |
| 16 | 25.81 | 1 143 | 75 |
| 17 | 26.06 | 1 026 | 80 |
| 18 | 26.09 | 1 015 | 81 |
| 19 | 26.11 | 1 005 | 82 |
| 20 | 26.13 | 996 | 83 |
| 21 | 26.17 | 978 | 85 |
| 22 | 26.30 | 926 | 90 |
| 23 | 26.92 | 714 | 95 |
| 24 | 27.17 | 513 | 96 |
| 25 | 28.13 | 432 | 97 |
| 26 | 28.77 | 330 | 98 |
| 27 | 29.41 | 252 | 99 |
| 28 | 31.59 | 92 | 100 |
List of recommended description to include with slice table:
- Sample name: Trade Name1
- Date acquired: 01/31/2019 11:00:00 AM EDT and
- Date processed: 02/02/2019 11:00:00 AM EDT
A7.5 Reporting
The reporting information specified in OECD TGs 118 and 119 should be submitted for assessment, including:
- available information about the composition of the test substance and its purity (identity, additives, impurities)
- preparation and pre-treatment procedures of the sample before submission to the testing laboratory
- a description of the sample preparation, and any observations or problems encountered during the GPC analysis
- an evaluation of the presence of undissolved particles, if any
- column type and all other technical relevant information about the instrumentation
- solvent and its purity
- information about all extrapolations, assumptions and approximations made during calibration and experimentation and
- any other information and observations relevant for the interpretation of the results
Note: Reporting on the actual test performance (for example, treatment of the sample, observations or problems) is often omitted and should be included to better inform the assessment.
When OECD TG 118 or 119 are not followed, an identification and description of the test guideline and methodology employed should also be provided. Any modifications to the testing procedures must be described in detail.
A7.6 Frequently encountered difficulties
A7.6.1 Limited solubility of the sample
When the polymer is insoluble (solubility less than 2%) in solvent systems typically used for GPC, then solubility data over a range of different solvents should be provided. Dissolution should be attempted in at least 3 different solvents. If it is reasonably successful, select the attempt with the highest solubility and report the amount undissolved. If all attempts to dissolve the substance in GPC-suitable solvents fail, an alternate method may be necessary.
Insolubility in typical solvents could indicate a highly cross-linked polymer and alternate methods for Mn determination should be employed or a waiver request should be submitted along with the insolubility results. The Mn for a pre-polymer could also be provided as alternate data in this example.
A7.6.2 Artifacts in the chromatogram
If these same artifacts can be shown in a blank or test marker run, they can be excluded from integration.
A7.6.3 Alternative information
Alternative information is accepted on a case-by-case basis. For a polymer that is being salted, the GPC for the non-salted version is acceptable. The same applies to polymers that are being end-capped.
A7.6.4 Calibration date differs drastically from the date of the sample run
If general procedures are in place to confirm the accuracy of the GPC column and instrumentation with a few standards before each run, they should be mentioned in the test method description. The positive controls should be polymers of known Mn. The positive controls should demonstrate the suitability of the system over a wide enough range as to include the sample Mn. A system is considered suitable if the positive control Mn is accurately measured within the error of the system.
Appendix 8. Reaction scheme requirements
A reaction scheme showing a detailed description of the process by which the notified substance is made is required for Reduced Regulatory Requirement (RRR) polymers (consult section 3.3.1.5).
The following examples illustrate the level of information that should be provided with the reaction schemes.
A8.1 Reaction scheme examples
These 2 examples illustrate how the regulatory status of a polymer can be affected by changes in the order of the monomers and their molar ratios. The same 3 monomers are for the synthesis of both polymers:
Monomer A: isophorone diisocyanate
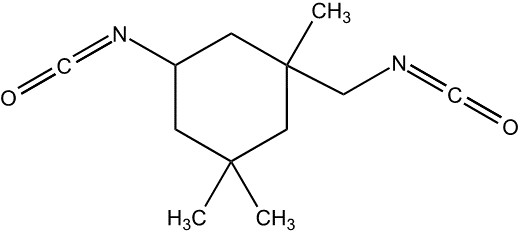
Monomer B: ethylene glycol and
Monomer C: 1,4-butanedioic acid
Example 1: The reaction scheme to obtain Polymer 1 is as follows.
Step 1: Polyurethane formation
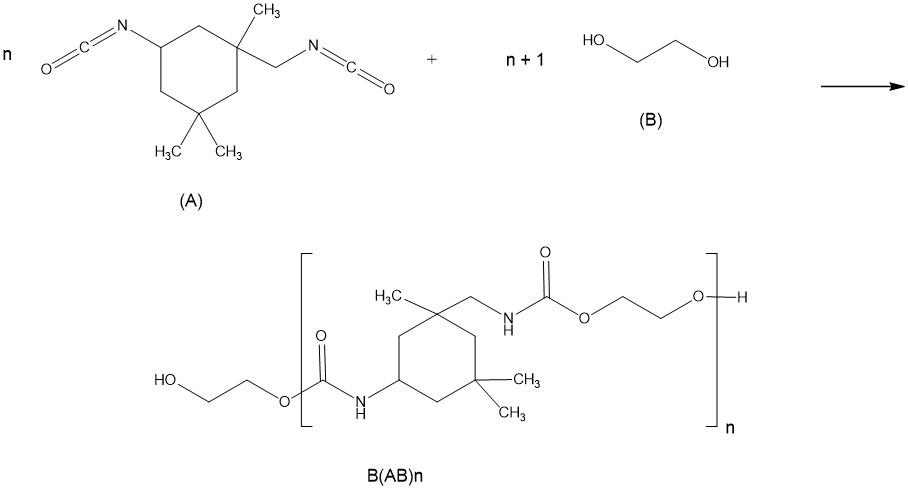
Step 2: Esterification
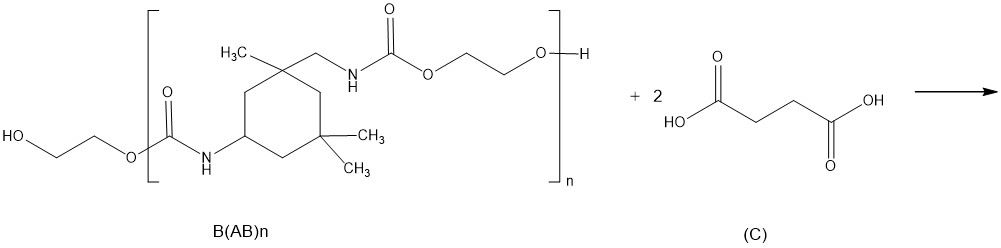
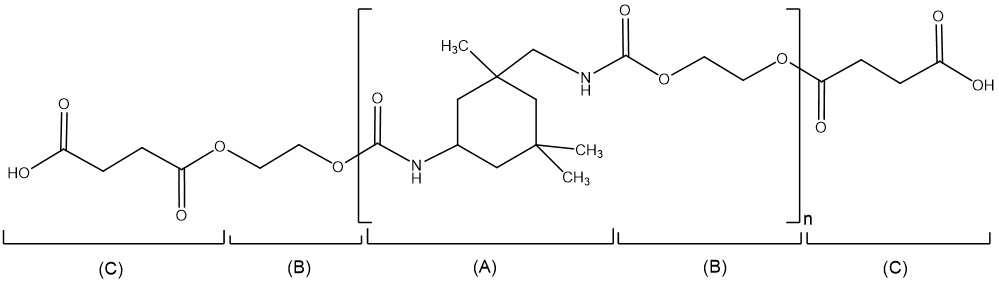
Polymer 1
The final product, Polymer 1, has the structure CB(AB)nC, where component C is derived from a diacid monomer. Because it does not contain reactive groups of concern, it may be considered RRR.
Example 2: The reaction scheme to obtain Polymer 2 from the same 3 monomers is as follows.
Step 1: Esterification
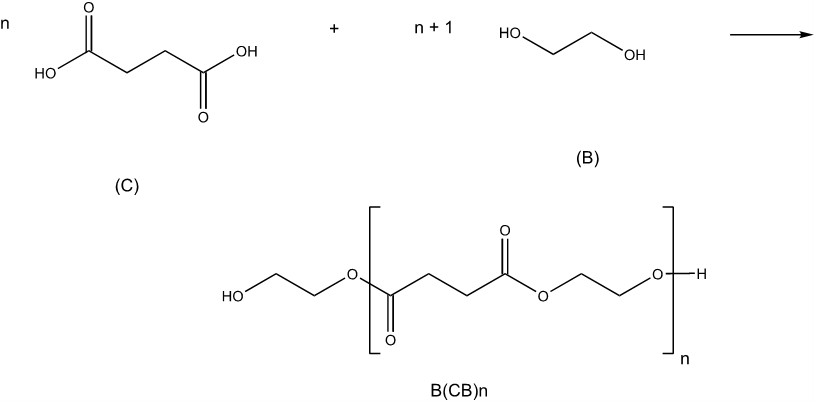
Step 2: Urethane formation
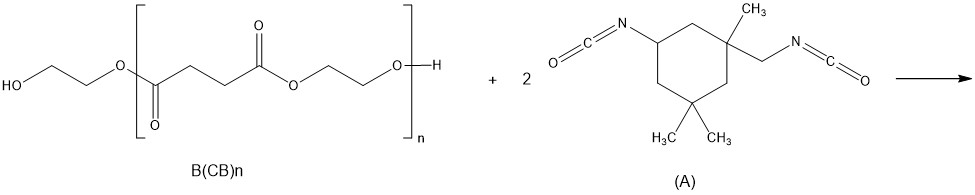
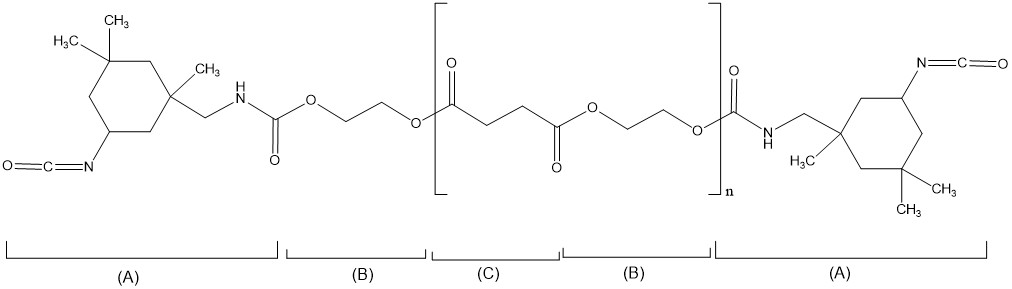
Polymer 2
Polymer 2 has the structure AB(CB)nA and contains unreacted isocyanates, which are reactive groups of concern. Therefore, this polymer may not be considered RRR.
A8.2 Reaction scheme format
The reaction scheme includes both monomer and reactant information and a sequence description.
A8.2.1 Monomer and reactant information
The notifier must provide a table of the chemical identities of all monomers, pre-polymers, and reactants along with their Chemical Abstracts Service (CAS) Registry Number and percent by weight (block A.24 of the New Substances Notification Form). In addition, this table should contain molecular weight and relative number of moles values, which can be provided as an attachment. Each monomer, pre-polymer, and reactant should be assigned an identifier for use in the sequence description.
| ID | Monomers and Reactants | CAS Registry Number | mwa | % by weight | Relatives number of moles |
|---|---|---|---|---|---|
| A |
Cyclohexane, 5-isocyanato- 1-(isocyanatomethyl)-1,3,3- trimethyl- |
4098-71-9 | 222 | 2.52 | 11 |
| B | Poly(oxy-1,2-ethanediyl), α-hydro-ω-hydroxy- | 25322-68-3 | 7 850 | 97.22 | 12 |
| C | Hexane, 1-isocyanato- | 2525-62-4 | 127 | 0.26 | 2 |
a mw – molecular weight
A8.2.2 Sequence description
Either provide the order and description of each step, including the nature of the reactions and the identifiers for all monomers, reactants, and intermediates, for example:
- Step 1: A + B → intermediate AB (polyurethane formation) and
- Step 2: intermediate AB + C → final product (urethane end-cap formation)
or provide a sequencing description that uses structural formulas and includes the order and nature of the reactions and the identifiers for all monomers, reactants, and intermediates, for example:
Step 1: Polyurethane formation
Step 2: Urethane end-cap formation
Appendix 9. Guidance on testing water extractability following the Organisation for Economic Co-operation and Development Test Guideline 120
The water availability of polymers notified under Schedule 10 and Schedule 11 of the New Substances Notification Regulations (Chemicals and Polymers) (the Regulations) is determined through the information requirement for water extractability. To fulfill this information requirement, the New Substances (NS) program recommends following the experimental protocol for determining water extractability outlined in the Organisation for Economic Co-operation and Development (OECD) Test Guideline (TG) 120: Solution/Extraction Behaviour of Polymers in Water.
Polymers are composed of a variety of molecules of differing molecular weights. By virtue of their molecular weight distribution, polymers often form heterogeneous mixtures in water (as opposed to discrete chemicals that can form true thermodynamic solutions). The smaller molecular components of a polymer may dissolve completely, whereas the larger components may form emulsions, dispersions or gels. The water-available fraction of a polymer represents those components that are of greatest interest to human and ecological risk assessments due to their bioavailability.
A9.1 Organisation for Economic Co-operation and Development Test Guideline 120
The OECD TG 120 is an internationally accepted method that can be applied to determine the water extractability of most polymers. The method takes into account that polymers consist of components of different molecular weights and that each component exhibits its own solubility characteristics (that is the fraction that can be extracted into the aqueous medium). The following guidance addresses technical issues related to testing polymers for water extractability using OECD TG 120.
A9.2 Technical guidance for applying the Organisation for Economic Co-operation and Development Test Guideline 120
A9.2.1 Factors affecting water extractability
The water extractability of polymers can be significantly influenced by the test procedures and sample preparation methods. As much as possible, care should be taken to avoid inappropriate test procedures. The NS program may not find studies acceptable where procedures or conditions resulted in interference that affected the study outcomes. Key factors that affect water extractability include the following:
- Sample preparation: methods to separate the polymer from a mixture or isolate it from a solvent must maintain the integrity of the polymeric substance with respect to its molecular weight distribution as well as its water extractability
- Surface area of the sample: because dissolution predominantly occurs at the surface of the polymer bulk, samples with high surface area (that is, composed of small particles) dissolve faster
- Water volume-to-sample mass ratio: using an insufficient amount of water may cause underestimation of extractability results
- Rate of mixing: stirring replenishes the solvent near the surface of the solute
- Temperature: heating the solution imparts a higher kinetic energy to dissolve molecules. Heat may also be generated during water/solute interactions (for example, hydrogen bonding), which in turn, can facilitate dissolution
- Water quality: some polymers that are not readily available in water may dissolve in aqueous salt solutions (“salting-in” effect). In other cases, salts may reduce the hydration of polymer molecules (“salting-out” effect)
- pH: polymers with ionizable moieties, such as carboxylic acid groups or amine groups, can be rendered more water-available in aqueous solutions by the addition of a base or acid, respectively and
- Work-up: the use of high-speed centrifugation or very fine filters may separate extracted material from otherwise stable water dispersion
A9.2.2 Technical guidance
It is recommended that OECD TG 120 be used for investigating the water extractability of all polymer types, despite the method indicating that it is not applicable to liquid polymers, those that appear as liquids due to impurities like solvents, or substances that react with water under the test conditions. The NS program therefore recommends that the water extractability of polymers be determined using OECD TG 120 with the following guidance:
- The water used should be distilled or deionized
- As prescribed under the Regulations, the pH of the aqueous phase should be 2, 7 or 9 respectively, prior to adding the notified polymer. The pH should be adjusted with hydrochloric acid or sodium/potassium hydroxide to avoid the use or formation of a buffer system
- For polymers that are viscous, it is recommended that:
- solvents not be used to dissolve the polymer
- the polymer not be cured or reacted before conducting the test and
- the test vessels be prepared by first spreading the substance on the walls of the vessel. This will maximize the surface area of polymer that is exposed to the water and that may become extractable
- The sample should be agitated for 24 hours at 20°C. Standard laboratory shakers are deemed sufficient to mimic the environmental action of water
- Although some liquid polymers may not be amenable to testing, the formation of stable liquid dispersions (that is, emulsions) should be investigated. Stable emulsions are considered by the NS program to be water-available
- Polymers in solvents should be dried appropriately so that the integrity of the polymer is not affected. For example, heating in an oven to remove residual solvent can result in the loss of oligomers and promote additional polymerization of the substance. Such pre-treatment would be considered by the NS program to invalidate the water extractability results. It is not necessary to attempt to remove all the residual solvent; instead, any remaining solvent can be analyzed along with the polymer sample and that amount can be excluded from the water extractability results and
- Although OECD TG 120 suggests filtration or centrifugation to achieve a clear aqueous phase, the NS program is seeking to quantify the entire bioavailable portion of the polymer, which in certain cases could include a stable dispersion or emulsion in water. Therefore, one of the following techniques may be considered when removing suspended material (consult section A9.2.4):
- Low-speed centrifugation: considered ideal if conducted over a reasonable time frame (typically for 2 hours or less) using speeds below ultra-centrifugation and
- Filtration: use a filter that does not plug or require excessive pressure (filter sizes that are too small may result in separation of larger-molecular-weight fractions and/or polymer degradation by shear forces in the filter). If clogging occurs, the resulting filtrate will not be representative of the polymer’s water availability. Clogging significantly reduces filter pore size, thus invalidating the water extractability result. If the filters clog, centrifugation should be used
A9.2.3 Analysis
OECD TG 120 refers only to a suitable method of analysis for determining the extractable components and suggests different methods for performing this analysis. The NS program recommends aqueous Gel Permeation Chromatography (GPC) as the analytical method, as it allows correlation between molecular weight and water availability, thereby allowing differentiation between extractability of unreacted monomers and additives or impurities.
A9.2.4 Reporting
The test report should contain all information required to theoretically be able to repeat the test, including the following:
- All information available on the test sample (identity, additives, impurities, molecular weight information)
- Detailed descriptions of sample preparation, experimental conditions of test performance and analysis, including verification of the applicability of the analytical methodology used (if required)
- The test result must be reported as a percentage of the nominal concentration (loading rate) of the substance. Any calculations needed to obtain this result must be reported in detail and
- All other calculations and any additional information or observations important for the interpretation of the result must be provided
A9.3 Other considerations
A9.3.1 Completely water-available polymers
Polymers marketed in the form of emulsions or dispersions and those that are capable of forming stable emulsions or dispersions are considered as 100% water-available. Therefore, providing water extractability information is not necessary. However, it must be clearly indicated in block B.1 of the New Substances Notification Form that the polymer is 100% water-available by checking the appropriate box. Ecotoxicity, biodegradation and hydrolysis test information continue to be required, as set out in the Regulations.
A9.3.2 Surface-active and/or water-dispersed polymers
In some cases, surface-active polymers can form colloidal dispersions (solid polymers) or emulsions (liquid polymers).
It is not necessary to provide water extractability data for surface-active polymers and polymers formulated in water and marketed as such, since they will be assumed to be completely water-available. Reporting requirements for this type of polymer are addressed in section A9.3.1.
A9.3.3 Waiver for water extractability
If a polymer is considered completely water-available (consult section A9.3.1), a statement to that effect is sufficient to address the water extractability requirement. A waiver request is not required.
If surrogate or alternate data are provided, a waiver request is not necessary.
If a waiver for water extractability is granted and the percent extractability of the notified polymer remains unknown, ecotoxicity, biodegradation and hydrolysis test information continue to be required, as set out in the Regulations.
A9.3.4 Testing of water-reactive polymers
Polymers containing water-reactive functional groups, such as isocyanates and alkoxysilanes, may be of concern. Consequently, if the presence of these reactive functional groups exceeds the thresholds set out in Schedule 7 of the Regulations, the polymer would not be considered a Reduced Regulatory Requirement polymer (consult section 3.3.1.5). Information about a polymer’s behaviour in water is needed in order to conduct an assessment, and notifiers are required to provide water extractability information under Schedule 10 or Schedule 11 of the Regulations.
It is generally recognized that polymers with water-reactive functional groups, such as isocyanates and alkoxysilanes, undergo hydrolysis. Hydrolysis may be followed by a self-condensation reaction that can lead to an increased molecular weight and the reduced solubility of the polymer. However, the rates of hydrolysis, the potential for self-condensation and the solubility of the resulting substance is dependent on the structural characteristics of the individual polymer.
It is also recognized that the hydrolysis and possible self-condensation reactions may reduce the practicability of conducting water-based testing (for example, ecotoxicity). For these reasons, the Regulations were structured to include an exemption from certain testing requirements (for example, hydrolysis, biodegradation, ecotoxicity) for polymers with limited water extractability.
In order to be eligible for exemptions from certain testing requirements, the Regulations require water extractability information. The NS program recommends that a water extractability test be conducted according to OECD TG 120. While there can be challenges with conducting water extractability testing on water-reactive polymers, the OECD TG 120 test method is considered to be the most relevant method in order to generate information meaningful for the assessment of water-reactive polymers.
Water extractability information will provide direct evidence on the solubility of a polymer and will determine whether or not further testing is required:
- If the water extractability is more than 2%, the polymer is subject to ecotoxicity, biodegradation and hydrolysis testing, as set out in the Regulations or
- If the water extractability is less than or equal to 2%, the notified polymer is not subject to ecotoxicity, biodegradation and hydrolysis testing, as set out in the Regulations
Overall, the testing strategy for water-reactive polymers is best addressed by performing the water extractability test on the notified polymer. Water extractability may also be obtained from alternative approaches (consult section 8.4) or waivers (consult section 8.7). Where waivers are requested, a stand-alone claim that a polymer is highly reactive with moisture converting it into a very high molecular weight cross-linked polymer is not sufficient, given that hydrolysis and self-condensation rates are dependent on the structural characteristics of the individual polymer. A waiver request needs to be accompanied by a well-documented scientific rationale, which includes supporting information (for example, empirical or read-across data) to demonstrate the water-reactivity and condensation behaviour.
If unsure, notifiers should consult with the NS program through a Pre-notification Consultation request (consult section 8.8) in order to determine the most appropriate testing strategy for water-reactive polymers.
Appendix 10. Assessment of nanomaterials under the New Substances program
Nanomaterials typically have larger surface area-to-volume ratios relative to their non-nanoscale forms, which can lead to greater reactivity. They may also exhibit differences in other chemical and physical properties that cannot be predictably extrapolated from their non-nanoscale forms. These differences may affect the potential of a substance to pose a risk to human health or the environment.
In line with the 2013 Organisation for Economic Co-operation and Development (OECD) Council Recommendation,Footnote 19 Canada is using its existing chemical regulatory framework to manage nanomaterials, making adaptations where necessary to take into account the specific properties of nanomaterials.
While there is no internationally aligned regulatory definition, the New Substances (NS) program is using the Health Canada Working Definition of Nanomaterials: “(1) having one or more dimensions (or internal or surface structure) at the nanoscale (1–100 nanometers inclusive); or (2) exhibiting nanoscale-related properties and/or phenomena above or below the nanoscale.”
The NS program may request information about primary particle size and particle size distribution in order to determine whether a notified substance is at the nanoscale. Various methods, based on different physical principles, are available to measure primary particle size and particle size distribution.Footnote 20
If information about primary particle size and particle size distribution is not provided, and the NS program believes that the substance could be a nanomaterial, the substance will be treated as a potential nanomaterial. Providing this information will allow for better identification of new nanomaterials, leading to more informed risk assessment and if necessary, more appropriate control measures. While the New Substances Notification Regulations (Chemicals and Polymers) prescribe the information that must be submitted to the NS program, they also generally require the submission of all other information and test data in the possession of the manufacturer or importer or to which they may reasonably be expected to have access.
The NS program may recommend submitting certain other information to take into account the specific properties of nanomaterials. The information could include, but is not limited to:
- physico-chemical properties specific to each nanomaterial, including agglomeration and/or aggregation state, shape, surface area, surface functionalization, surface coating and surface charge, etc. of the substance
- release potential of the substance from a final product
- ecotoxicity data and test report (for example, soil toxicity) and
- mammalian toxicity test by inhalation route of exposure (including acute toxicity and repeated-dose toxicity) conducted according to revised OECD Test Guidelines (TGs). Revised OECD Guidance Document on Inhalation Toxicity Studies [PDF] and updated inhalation toxicity studies (TGs 412Footnote 21 and 413Footnote 22 ) addressing nanospecific issues are available online
Although not required, notifiers are encouraged to submit a Pre-notification Consultation request (consult section 8.8) while the New Substances Notification is being prepared, in order to seek advice on nanomaterial-specific considerations, such as additional data that could support the risk assessment, and guidance on test methods.
Appendix 11. International arrangements
International arrangements are ongoing and changing. To ensure current information, visit our website New substances: international cooperation on chemical and polymers.
Appendix 12. Parts of the Domestic Substances List and the Non-domestic Substances List
Domestic Substances List
The Domestic Substances List (DSL) (SOR/94-311) provides an inventory of substances in the Canadian marketplace. It was originally published in the Canada Gazette, Part II, in May 1994. The current structure of the DSL was established in 2001 (Order 2001-87-04-01 Amending the Domestic Substances List (SOR/2001-214) [PDF]) and amended in 2012 (Order 2012-87-09-01 Amending the Domestic Substances List (SOR/2012-229)). The DSL is amended, on average, 12 times per year to add, update or delete substances.
The DSL includes 8 parts defined as follows:
- Part 1: Sets out chemicals and polymers, except those referred to in Part 2, 3 or 4 that are identified by their Chemical Abstracts Service (CAS) Registry Numbers, or by their Substance Identity Numbers assigned by the Department of the Environment and the names of the substances
- Part 2: Sets out chemicals and polymers subject to Significant New Activity (SNAc) requirements that are identified by their CAS Registry Numbers
- Part 3: Sets out chemicals and polymers, except those referred to in Part 4, that are identified by their masked names and their Confidential Substance Identity Numbers (also referred to as Confidential Accession Number [CAN]) assigned by the Department of the Environment
- Part 4: Sets out chemicals and polymers subject to SNAc requirements that are identified by their masked names and their Confidential Substance Identity Numbers
- Part 5: Sets out inanimate biotechnology products and living organisms, except those referred to in Part 6, 7 or 8, that are identified by their American Type Culture Collection (ATCC) numbers, International Union of Biochemistry and Molecular Biology (IUBMB) numbers or specific substance names
- Part 6: Sets out inanimate biotechnology products and living organisms subject to SNAc requirements that are identified by their ATCC numbers, IUBMB numbers or specific substance names
- Part 7: Sets out inanimate biotechnology products and living organisms, except those referred to in Part 8, that are identified by their masked names and their Confidential Substance Identity Numbers
- Part 8: Sets out inanimate biotechnology products and living organisms subject to SNAc requirements that are identified by their masked names and their Confidential Substance Identity Numbers
For a complete description of the inventory flags that can appear on the list after a substance identifier, consult section 2.1.4.
Non-domestic Substances List
The Non-domestic Substances List (NDSL) provides an inventory of substances found in international commerce. It was originally published in the Canada Gazette, Part I, in January 1998 (volume 132, no.5) [PDF]. The NDSL is amended, on average, 12 times per year to add, update or delete substances.
The NDSL includes 4 parts defined as follows:
- Part 1: Sets out chemicals and polymers, except those referred to in Part 2, that are identified by their CAS Registry Numbers
- Part 2: Sets out chemicals and polymers that are identified by their masked names in accordance with the Masked Name Regulations, and by their Confidential Substance Identity Numbers assigned by the Department of the Environment
- Part 3: Sets out enzymes, except those referred to in Part 4, identified by their IUBMB numbers
- Part 4: Sets out enzymes identified by their masked names in accordance with the Masked Name Regulations, and by their Confidential Substance Identity Numbers assigned by the Department of the Environment
To find out whether a substance is on the DSL or on the NDSL, the substance name, the CAS Registry Number, the Confidential Substance Identity Number (if available) or the IUBMB number can be entered into the Substances search engine.
Appendix 13. Glossary, abbreviations and acronyms
A13.1 Glossary
Acceptable to the New Substances (NS) program with respect to a test method means a method that enables a sufficient quantity and quality of data to be generated for a meaningful assessment of the endpoint under investigation by the NS program. Important considerations of the method include the use of standards and controls; detection limits; species selected; tissues investigated; doses; adherence to Good Laboratory Practices; validation of the method; and statistical power of the method (consult also indicator of mutagenicity)
Act means the Canadian Environmental Protection Act, 1999
Adequately contained means all precautions and measures necessary to prevent the release of the substance to the environment. With respect to the transportation of a substance, adequate containment requires full compliance with the Transportation of Dangerous Goods Act (consult also contained)
Amphoteric polymer/biopolymer means a polymer that has monomer units that are covalently bound and bear both a negative charge and a positive charge (consult also monomer unit, polymer)
Animal includes a part of an animal, but does not include an animal or part of an animal that exists primarily as a single cell and is without the organization that characterizes tissues or organs
Anionic polymer/biopolymer means a polymer that has one or more monomer units that are covalently bound and bear a net negative charge (consult also monomer unit, polymer)
Assessment period means the number of calendar days that the government has to assess the information submitted by a notifier under the Regulations
Biochemical means a chemical that is produced by a living micro-organism, or means a protein or a nucleic acid derived from a plant or an animal (consult also living organism and micro-organism). Note: dead micro-organisms are considered biochemicals
Biopolymer means a polymer that is produced by a living micro-organism, or means a protein or a nucleic acid derived from a plant or an animal (consult also living organism and micro-organism)
Biotechnology means the application of science and engineering in the direct or indirect use of living organisms or parts or products of living organisms in their natural or modified forms
By-product means a substance produced without separate commercial intent during the manufacture of another substance
Canadian Agent means the agent required when a notifier who provides the information under the Regulations is not a resident in Canada. The notifier must identify, under paragraph 14(1)(b) of the Regulations, a person resident in Canada as a “Canadian Agent” authorized to act on their behalf. The “Canadian Agent” is required to receive any notice or correspondence that may be sent in relation to the New Substances Notfication (NSN) and keep a copy of the NSN and all correspondence and supporting data with respect to the NSN, for the period of 5 years after the end of the year in which the information is provided (consult section 13 of the Regulations). The “Canadian Agent” is responsible for ensuring that information in the NSN is accurate and complete
Chemical Abstracts Service Registry Number means the identification number assigned to a substance by the Chemical Abstracts Service Division of the American Chemical Society
Cationic polymer/biopolymer means a polymer that has one or more monomer units that are covalently bound and bear a net positive charge (consult also monomer unit, polymer)
Chemical means a substance that is not a living organism nor a polymer (consult also substance)
Consumed, in respect of a substance, means destroyed or completely converted to another substance
Contained, in respect of a site-limited intermediate substance or an export-only substance, means an absolute release limit of 1 kg per day per site to the aquatic environment after wastewater treatment
Direct public exposure to a substance results from dermal, inhalation or oral contact with the substance during its end-use (for example in a consumer product), whether knowingly or not. Note that this differs from indirect public exposure to a substance which involves unintended or incidental contact to the substance in environmental media following its release into the environment during one or more stages of its life cycle (manufacture, processing and handling, storage, transportation, disposal) excluding consumer end-uses. With respect to the Regulations, both direct and indirect public exposure refer to exposure of the general population in Canada.
Domestic Substances List means the list maintained by the Minister under subsection 66(1) of the Act, as amended from time to time by the Minister to add, update or delete substances (consult also Non-domestic Substances List)
Evidence that the tissue investigated was exposed to the substance or its metabolites with respect to the in vivo mutagenicity test in Schedule 6 and Schedule 11 of the Regulations, means data that is necessary to determine the appropriateness of the tissue(s) investigated in assessing the in vivo mutagenicity of a substance, and thus the adequacy of the test. This clause indicates the need for sufficient information to support a conclusion that the tissue investigated was exposed to the test substance or its metabolites. The strength of the evidence required will be balanced with the concern of the mutagenic potential of the substance, for example: results from in vitro mutagenicity tests; structure; potential for exposure; tissue investigated; and test method. Examples of what may constitute evidence of tissue exposure include the following:
- A positive result for the test endpoint in the tissue investigated
- Cytotoxicity observed in the tissue investigated, for example, statistically significant reduction in the mitotic index, cell cycle delay, decrease in the ratio of polychromatic to normochromatic erythrocytes
- General organ toxicity in the tissue investigated, for example, significant change in organ weight or hyperplasia and
- Data from a tissue distribution study indicating the presence of the substance or its metabolites in the tissue investigated
Importer or Importer of Record means the person importing the substance as shown on the Commercial Accounting Declaration Form as issued by the Canada Border Services Agency
Impurity means a substance whose presence with another substance is not intentional, is not necessary to the end-use of the product, and does not enhance the commercial value of the product
Indicator of mutagenicity with respect to permitting an assessment of in vitro or in vivo mutagenicity means tests that are “acceptable to the New Substances program” for determining the in vitro or in vivo mutagenic potential of the substance. This wording is intended to permit the selection of the most appropriate test(s) for a substance, and to allow developments in the field of genotoxicity to quickly become part of a testing strategy. It is recommended that the investigator consult with Health Canada officials before testing to determine the acceptability of a test for that specific substance (consult also Acceptable to the New Substances program)
In the possession of the manufacturer or importer means the information in the company’s offices in Canada if the New Substances Notification (NSN) was submitted by a Canadian company or the information in the offices in the country where the notification orginated if the NSN was submitted by a foreign company through a “Canadian Agent” (consult also to which they may reasonably be expected to have access)
Living organism means a substance that is an animate product of biotechnology, and refers to micro-organisms or organisms other than micro-organisms
Masked name means a name based on the Chemical Abstracts Service (CAS), the International Union of Pure and Applied Chemistry (IUPAC) or the International Union of Biochemistry and Molecular Biology (IUBMB) nomenclature, but having one or more of the specific components identified in a manner that prevents the identification of the specific chemical structure. Masking a substance name will only be acceptable to the extent necessary to disguise the full identity of the substance, while retaining the generic molecular structure. Substances published with a masked name are assigned a Confidential Substance Identity Number (also referred to as a Confidential Accession Number by the program)
Micro-organism means a microscopic organism that is:
- (a) classified in the Bacteria, the Archaea, the Protista, which includes protozoa and algae, or the Fungi, which includes yeasts
- (b) a virus, virus-like particle or sub viral particle
- (c) a cultured cell or an organism not referred to in paragraph a) or b), other than a cell used to propagate the organisms or
- (d) any culture other than a pure culture
Minister means the Minister of the Environment; whereas, Ministers means the Minister of the Environment and the Minister of Health
Monomer unit means the reacted form of a monomer in a polymer (consult also polymer)
Most significant route of potential public exposure means exposure of the general population in Canada. To select the most appropriate route or routes for testing, the expected concentration of the notified substance in the various environmental media and consumer products and the bioavailability of the substance through ingestion, inhalation and dermal absorption must be considered. The most significant route of exposure to a substance for the general population may be different from exposures for workers in an occupational setting. Consequently, data generated for occupational exposures may not meet the requirement for the most significant route of potential public exposure specified in the Regulations
New substances mean substances that are not presently on the Domestic Substances List and are considered to be new to Canada. Regulations were created to ensure that no new substances (chemicals, polymers or animate products of biotechnology) are introduced into the Canadian marketplace before an assessment of whether they are potentially toxic has been completed, and any appropriate or required control measures have been taken (consult also substance)
Non-domestic Substances List means the list maintained by the Minister under subsection 66(2) of the Act, as amended from time to time by the Minister to add, update or delete substances (consult also Domestic Substances List)
Person includes legal and natural persons such as corporations or individual residents of Canada
Plant includes a part of a plant, but does not include a plant or part of a plant that exists primarily as a single cell and is without the organization that characterizes tissues or organs
Polymer means a substance that consists of:
- (a) molecules characterized by the sequence of one or more types of monomer units
- (b) greater than 50% by weight of molecules having three of more monomer units that are covalently bound to one or more other monomer units or reactants
- (c) less than 50% by weight of molecules of the same molecular weight and
- (d) molecules distributed over a range of molecular weights whose differences in molecular weights are primarily attributable to differences in the number of monomer units (consult also monomer unit, reactant)
Product development substance means a research and development substance that is evaluated in one program of 2 years or less in length before full commercialization by means of pilot plant trials, production trials, or customer trials to modify technical specifications in response to performance requirements of potential customers but does not include test marketing (consult also research and development substance, test marketing)
Production organism means a living organism that produces a biochemical or biopolymer. A production organism may be subject to the New Substances Notification Regulations (Organisms) if it meets the definition of a living organism set out in section 104 of the Act and is not on the Domestic Substances List. Examples of production organisms include:
- Bacillus subtilis that produces a subtilisin
- Saccharomyces fragilis that produces a lactase and
- Aspergillus flavus that produces a glucosidase
Reactant, in respect of a polymer, means a substance that is used in the manufacture of the polymer and becomes part of its chemical composition, and includes a monomer
Reactive functional group means atoms or an associated group of atoms in a substance that are intended or may reasonably be expected to undergo facile chemical reaction
Read-across estimate means a qualitative estimate of a property of a substance based upon experimental data from one or more compounds having a closely related chemical structure
Reduced Regulatory Requirement polymer means one of the polymers described in section 9 of the Regulations
Regulations means the New Substances Notification Regulations (Chemicals and Polymers) of the Canadian Environmental Protection Act, 1999
Research and development substance means a substance that is undergoing systematic investigation or research, by means of experimentation or analysis other than test marketing, whose primary objective is any of the following:
- (a) to create or improve a product or process
- (b) to determine the technical viability or performance characteristics of a product or process or
- (c) to evaluate the substance prior to its commercialization, by pilot plant trials, production trials, including scale-up, or customer plant trials, so that technical specifications can be modified in response to the performance requirements of potential customers (consult also test marketing)
Safety Data Sheet, in respect of a substance, has the same meaning as in section 2 of the Hazardous Products Act
Site-limited intermediate substance means a substance that is consumed in a chemical reaction used for the manufacture of another substance and that is:
- (a) manufactured and consumed at the site of manufacture
- (b) manufactured at one site and transported to a second site where it is consumed or
- (c) imported and transported directly to the site where it is consumed
Substance is defined in subsection 3(1) and section 80 of the Act as:
- any distinguishable kind of organic and inorganic matter, whether animate or inanimate, and includes
- (a) any matter that is capable of being dispersed in the environment or of being transformed in the environment into matter that is capable of being so dispersed or that is capable of causing such transformations in the environment
- (b) any element or free radical
- (c) any combination of elements of a particular molecular identity that originate in nature or are the result of chemical reactions but could not practicably be formed by simply combining individual constituents and
- (d) complex combinations of different molecules that originate in nature or are the result of chemical reactions but that could not practicably be formed by simply combining individual constituents
- for the purposes of the new substances provisions of the Act (for example, section 66 and sections 80 to 89 concerning chemicals and polymers), does not include:
- (e) any mixture that is a combination of substances and does not itself produce a substance that is different from the substances that were combined
- (f) any manufactured item formed into a specific physical shape or design during manufacture and has, for its final use, a function or functions dependent in whole or in part on its shape or design and
- (g) any animate matter that is, or any complex mixture of different molecules that are, contained in effluents, emissions or wastes that result from any work, undertaking or activity
Substance occurring in nature means a substance that is naturally occurring, and is unprocessed; processed only by manual, gravitational or mechanical means, by dissolution in water, by flotation, or by heating solely to remove water; or extracted from air by any means
Third Party Information Supplier is a term used when the notifier is not given access to information that is considered confidential by the third party. However, that information is used to support the New Substances Notification and will be supplied directly to the New Substances program by the third party and will be identified as a “Third Party Information Supplier Submission.” Third Party Information Supplier include foreign suppliers; a Third Party Information Supplier can be located in Canada or elsewhere
Test marketing, in respect of a product, means the exploration of its market capability in a competitive situation where the creation or improvement of the product is not the primary objective (consult also research and development substance)
Toll Manufacturer means the person who is actually producing the substance on behalf of the notifier, regardless if on toll or otherwise
Transient reaction intermediate means a substance that is formed and consumed in the course of a chemical reaction
To which they may reasonably be expected to have access means information in any of the company’s offices worldwide, or other locations where the person can access the information (consult also in the possession of the manufacturer or importer)
Trigger quantity means the quantity of substance manufactured or imported that, if exceeded, requires the notifier to provide a New Substances Notification. For example, for a chemical/biochemical on the Non-domestic Substances List, the trigger quantity requiring a Schedule 4 notification is 1 000 kg/year
UVCB is an abbreviation for substances of Unknown or Variable composition, Complex reaction products or Biological materials. These materials are derived from natural sources or complex reactions and are considered to be a single substance for notification purposes
A13.2 List of abbreviations and acronyms
- AICIS
Australian Industrial Chemicals Introduction Scheme
- amine
cationic amine
- ASTM
American Society for Testing and Materials
- BA
branching agent
- BP
branched polymer
- CAS
Chemical Abstracts Service
- CBI
Confidential Business Information
- CCOHS
Canadian Centre for Occupational Health and Safety
- comb
combined
- CPI
Consumer Price Index
- DRave mo
daily release to the aquatic environment averaged monthly
- DSL
Domestic Substances List
- EC50
median effective concentration
- ECHA
European Chemicals Agency
- EINECS
European Inventory of Existing Commercial Substances
- ELINCS
European List of Notified Chemical Substances
- F&DA
Food and Drugs Act
- FGEG
functional group in end group position
- FGEW
functional group equivalent weight
- FGEWn
individual functional group equivalent weight calculation (n = 1, 2, 3, …)
- FIFRA
Federal Insecticide, Fungicide, and Rodenticide Act
- GLP
Good Laboratory Practice
- GPC
Gel Permeation Chromatography
- INCI
International Nomenclature Cosmetic Ingredient
- INN
International non-proprietary names
- IR
Infrared
- ISA
Information-Sharing Agreement
- ISO
International Organization for Standardization
- IUBMB
International Union of Biochemistry and Molecular Biology
- IUPAC
International Union of Pure and Applied Chemistry
- LC50
median lethal concentration
- LD50
median lethal dose
- LLNA
Local Lymph Node Assay
- LOEC
lowest-observed-effect concentration
- LP
linear polymer
- MALLS
Multi Angle Laser Light Scattering
- MALS
Multi Angle Light Scattering
- Mn
number average molecular weight
- mon
monomer
- MP
molecular weight of the highest peak
- mw
molecular weight
- Mw
weight average molecular weight
- mw KOH
molecular weight of KOH = 56.1 g/mol
- Mz
z average molecular weight
- NAICS
North American Industry Classification System Code
- NAM
New Approach Methods
- NDSL
Non-domestic Substances List
- nEG
number of end groups
- nFG
number of available functional groups
- NLP
No-Longer Polymers
- NMR
Nuclear Magnetic Resonance
- NOEC
no-observed-effect concentration
- NOEQ
Notice of Excess Quantity
- NOMI
Notice of Manufacture or Import
- non-RRR
Non-Reduced Regulatory Requirement polymers
- nRS
number of reactive sites
- NS program
New Substances program
- NSFR
New Substances Fees Regulations
- NSN
New Substances Notification
- OECD
Organisation for Economic Co-operation and Development
- PCPC
Personal Care Products Council
- PMN
Pre-Manufacture Notice
- PNC
Pre-notification Consultation
- QMRF
QSAR Model Reporting Format
- QPRF
QSAR Prediction Reporting Format
- QR
quantity released
- QSAR
Quantitative Structure-Activity Relationship
- RDM
number of release days per month
- RE
removal efficiency
- RRR
Reduced Regulatory Requirement polymers
- RTECS
Registry of Toxic Effects of Chemical Substances
- SDS
Safety Data Sheet
- SEC
Size-exclusion Chromatography
- SNAc
Significant New Activity
- SNAN
Significant New Activity Notification
- SWIM
Single Window Information Management
- TG
Test Guideline
- the Act
Canadian Environmental Protection Act, 1999
- the Minister
the Minister of the Environment
- the ministers
the Minister of the Environment and the Minister of Health
- the Regulation
New Substances Notification Regulations (Chemicals and Polymers)
- TSCA
Toxic Substances Control Act
- US EPA
United States Environmental Protection Agency
- USP
United States Pharmacopeia
- UV
Ultraviolet
- UVCB
substances of Unknown or Variable composition, Complex reaction products or Biological materials
- wt%
weight percent
- Xamine
amine number, mg KOH/g polymer