Ministerial Briefing Volume II: COVID-19 overview
Table of Contents
- 1: COVID-19 overview and critical information
- 2: Roles and responsibilities
- 3: FPT and international governance
- 4: Key levers and federal actions
1: COVID-19 overview and critical information
Epidemiology update
The Public Health Agency of Canada provides daily updates on epidemiological data and trends, which are posted online for Canadians: COVID-19 daily epidemiology update – Canada.ca
Additionally, the Chief Public Health Officer provides regular updates on epidemiological trends and modelling, which are also posted online for Canadians: Mathematical modelling and COVID-19 – Canada.ca
Officials will be available to provide early briefings on the latest modelling and expert advice.
Overview of Canada's response to COVID-19
COVID-19 pandemic and federal response to date
The COVID-19 pandemic has caused:
- adverse impacts on health of Canadians and the health care system (with disproportionate impact on vulnerable populations);
- major economic and employment dislocation;
- restrictions on social and family interactions and mobility affecting overall well-being; and
- fundamental changes in how Canadians live and work
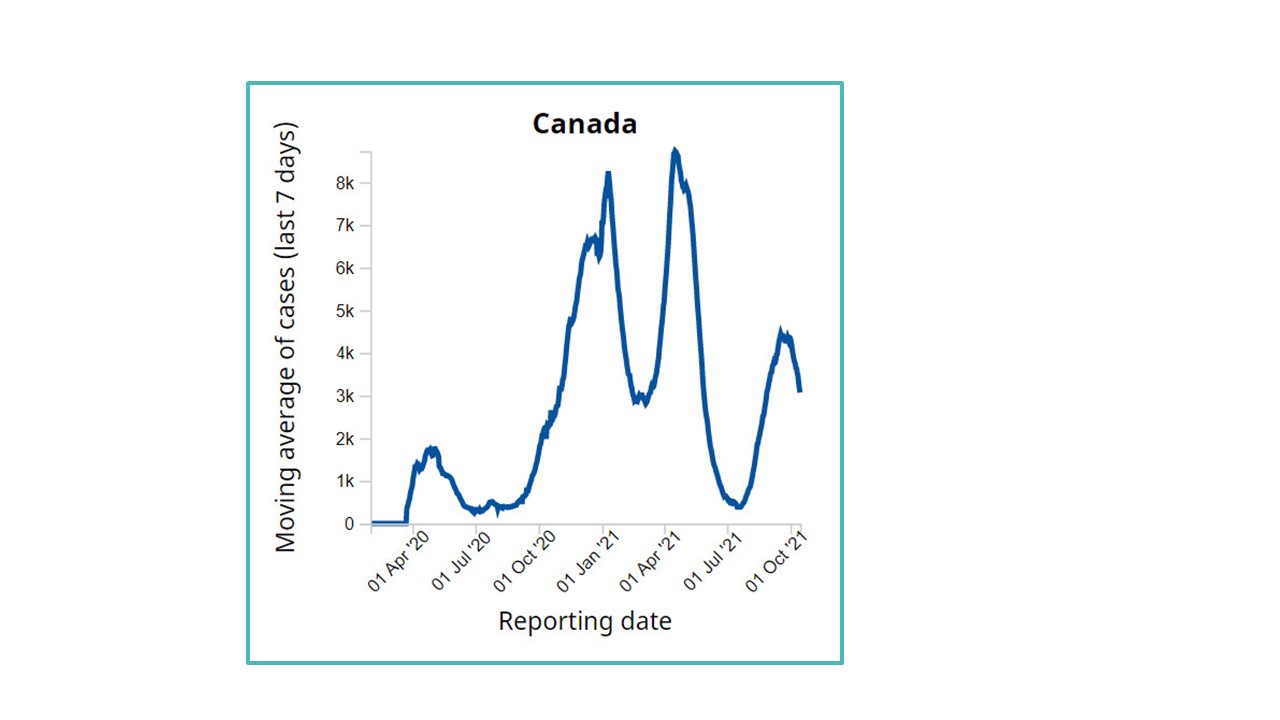
Figure 1: Text description
This line graph shows Canada's moving average of cases and their reporting date. The data is representative of April 2020 to October 2021. The graph shows an initial increase from less than 100 cases up to April 2020 to nearly 2,000 cases in July 2020. The next large increase is from October 2020 to January 2021, reaching a peak of around 8,000 cases. The line goes down briefly for March 2021 at around 3,000 cases, but then increases to the chart's peak at around 9,000 cases in April 2021, which then decreases to only a few hundred cases by July 2021. The line then increases to around 4,000 cases in September 2021 and ends at around 3,000 cases in October 2021.
Overall Goal of COVID-19 Pandemic Response: Minimize serious illness and overall deaths while minimizing societal disruption as a result of the pandemic
A whole-of-government approach is required for pandemic response – from public health and border measures, to new economic and social supports.
To deliver results, new partnerships have been both created and strengthened with:
- provinces and territories,
- Indigenous peoples,
- the private sector,
- international partners,
- health experts,
- the Canadian Red Cross, and,
- other civil society organizations
The Health Portfolio supports federal efforts by:
Response
Providing leadership and decision-making support through the COVID-19 taskforce; implementing innovative regulatory measures to authorize and approve vaccines, treatments, medical devices, and other supplies; scaling up testing and tracing; supporting PTs, including enhancing health care capacity/targeted funding; and supporting notification apps to alert Canadians of COVID exposure.
PHAC is responsible for protecting Canadians by coordinating a national public health response with PTs, providing epidemiological and modelling information, preventing the spread of COVID-19 by financing and rolling out vaccines and supporting a public health focused border posture. It also plays a key role in promoting science-based information through public communications and health guidance.
First wave – Federal response (Winter / Spring 2020)
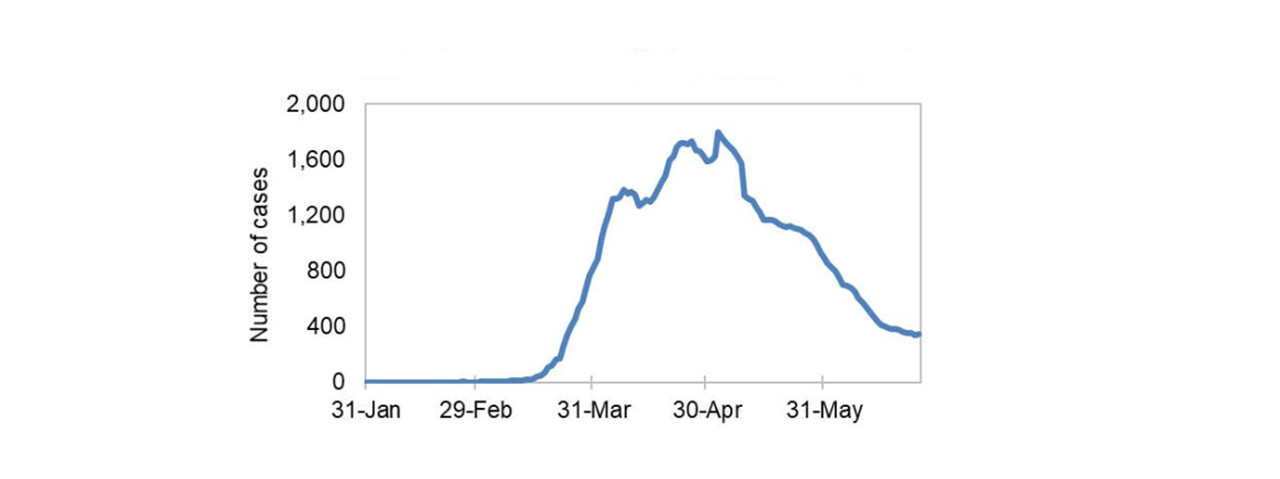
Figure 2: Text description
This line graph shows daily COVID-19 cases from January 31, 2020 through May 31, 2020. The line shows a sharp increase in late March, 2020 to approximately 1,400 cases per day, followed by a slight dip then an increase in April, 2020 peaking around 1,800 cases at the end of the month. Cases then taper off to less than 400 cases past June 2020.
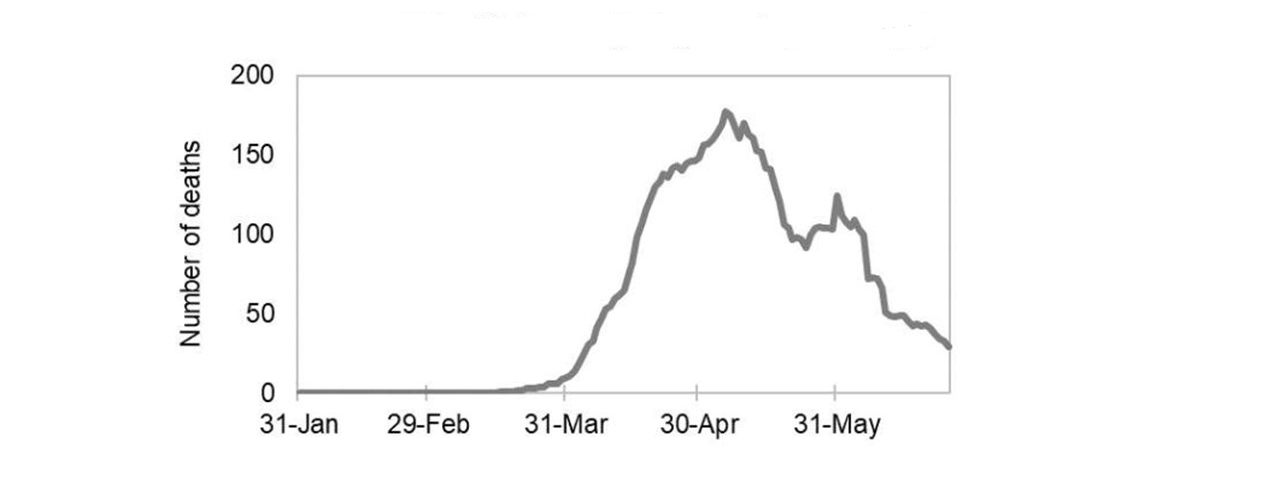
Figure 3: Text description
This line graph shows daily COVID-19 deaths from January 31, 2020 through May 31, 2020. The line begins to rise towards the end of March, 2020 reaching its peak at around 175 deaths in May 2020. The line then decreases, but spikes back up to approximately 125 deaths near the end of May. The line then continues to decrease to less than 30 deaths by the end of June 2020.
December 31, 2019 GPHIN identified pneumonia-like illness of unknown cause originating in Wuhan, China.
January / February 2020 engaged in monitoring and detection systems; activating the FPT Public Health Response Plan and establishing/ convening the Special Advisory Committee and regular Health Ministers' meetings; repatriating, screening, and quarantining returning Canadians, and supporting preparedness.
March 2020 Pandemic declared and stringent public health measures implemented across country; Health Portfolio leadership across various pandemic response areas.
Action taken
- $1 billion federal investment in the COVID-19 Response Fund
- $500M for PTs to support health care system, testing, equipment, surveillance and monitoring
- $275M for large-scale research toward countermeasures to combat COVID-19, including potential vaccines and treatment
- Minister of Health signed an Interim Order to expedite access to key COVID-related supplies including PPE and diagnostic tests
Borders
- On March 18, Canada banned non-essential travel for non-Canadians to limit the spread of COVID-19
- A risk-based framework for easing border restrictions was subsequently implemented, based on epidemiology and public health guidance
First wave – Federal response
The Chief Public Health Officer (CPHO)
The CPHO is the lead public health professional of the Government of Canada. The CPHO has played a key leadership role in the COVID-19 response by providing evidence-based public health advice to the Government and working with PTs to protect Canadians.
Health Canada COVID-19 Task Force
Established to provide strategic leadership and direction, the Task Force supports Health Canada and the Health Portfolio in managing the pandemic.
New governance structures implemented to support coordination across federal departments and agencies, and ensure close cooperation with PTs and stakeholders.
Testing secretariat
Established to oversee COVID-19 testing, screening and tracing. Leads and supports regulatory approvals, FPT leadership, supply strategy and innovative partnerships.
Government appointed advisory bodies provide critical guidance and for collaboration:
- Immunity Task Force
- Vaccine Task Force
- Therapeutics Task Force
Canadian Armed Forces
Supported 7 LTCs in Ontario and 47 in Quebec – Medical and support personnel for planning, liaison, and PPE logistics.
Canadian Red Cross
$100 million in federal funding provided for outbreak response, purchase of equipment and supplies (i.e. mobile field hospitals).
PPE and other medical supplies
In response to the challenges of global demand and supply chain disruption, the Government of Canada secured essential supplies, engaged domestic manufacturers through the 'Call to Action' and supported them via a 'Made-in-Canada' strategy, streamlined and accelerated regulatory approvals, and improved transparency through a new supply model.
Research – Treatment and vaccines
The Government invested in research funding for COVID-19 treatments and vaccines, and established the Canadian COVID Genomics Network (CanCOGeN) to help track the virus and its different strains.
Summer 2020 – End of the first wave
In late Spring, cases had declined as a result of public health measures including lockdowns. With a return to outdoor activities, it was possible to ease some restrictive public health measures.
Health Canada put in place emergency authorization frameworks for vaccine and drug approvals – accelerating regulatory reviews and clinical trials.
PHAC working with PSPC secures agreements with pharmaceutical companies to produce millions of vaccine doses.
A Critical Drug Reserve established with PTs to ensure future drug supply for ventilation, ICU and other procedures needed to support COVID-19 patients.
Contingency planning
The Portfolio worked with Public Safety to put in place plans and tools in the event of resurgence:
- PHAC-led single window to coordinate assistance requests from PTs
- Renewal of health human resource rosters
- Expanded Canadian Red Cross partnership and standing capacity
- Announcement of COVID-19 Testing Assistance Response Teams
The federal government supported PTs and Canadians through public health guidance, financial support for virtual health, procuring rapid tests and for developing response tools, such as COVID Alert.
FPT collaboration
Collaboration on FPT approach to reopening, including First Ministers' Agreement. PT outreach and exchanges to better understand needs and outline support available from federal surge response.
Safe restart agreement
$19 billion provided to Provinces and Territories to help them safely restart their economies and prepare for potential resurgence.
Modelling and scenario analysis
With external experts in fields such as pandemic modelling and genomic analysis, worked to develop scenarios to inform potential responses going forward; regular monthly public briefings on modelling.
Key lessons learned
Continually adjust response to keep pace with a changing pandemic and evolving scientific understanding.
Second wave – Fall 2020
- Cases began increasing in fall, leading to a second wave that peaked in early January 2021
- Timing of regional outbreaks differed across the country beginning in western provinces before moving east.
- Atlantic provinces were largely successful in preventing major resurgence.
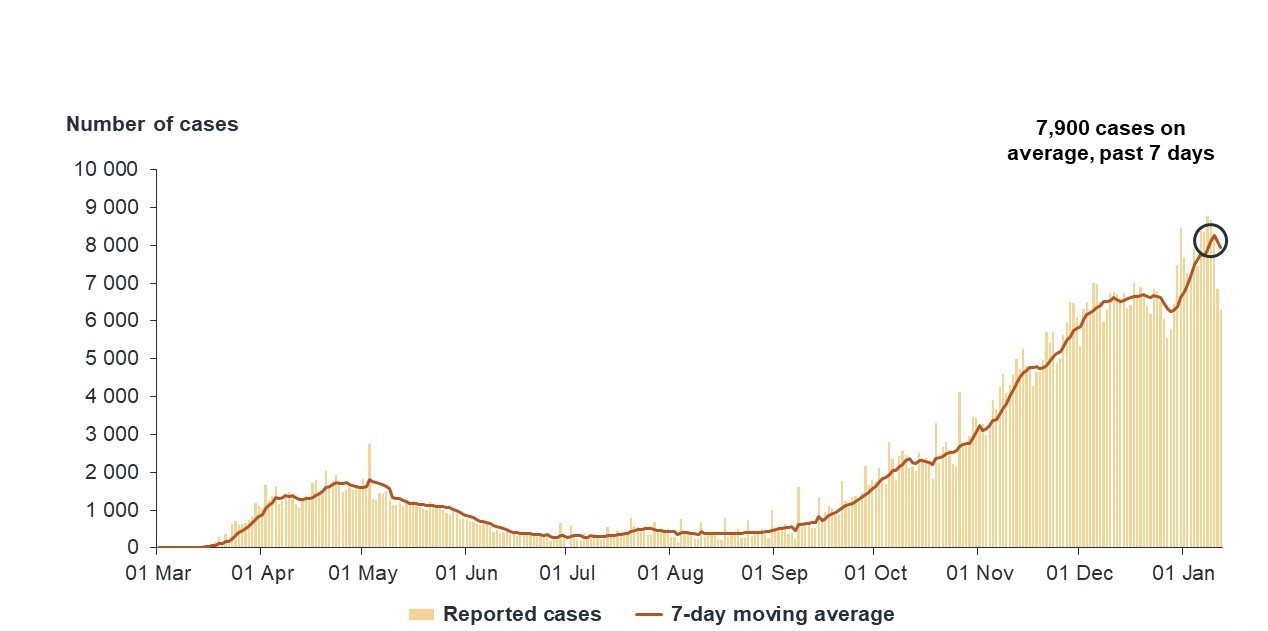
Figure 4: Text description
This graph uses bars to show newly reported cases each day, with the 7-day moving average of cases overlaid as a red line. The data are presented from March 2020 to January 2021. The line shows a slight increase until May 2020, when a peak is reached of around 2,000 cases, then goes down and remains stable below 500 cases until September 2020. The line then displays a steady increase until early January 2021, ending at a peak of approximately 7,900 cases per day on average, calculated over the previous 7 days. The bars follow the same trend throughout.
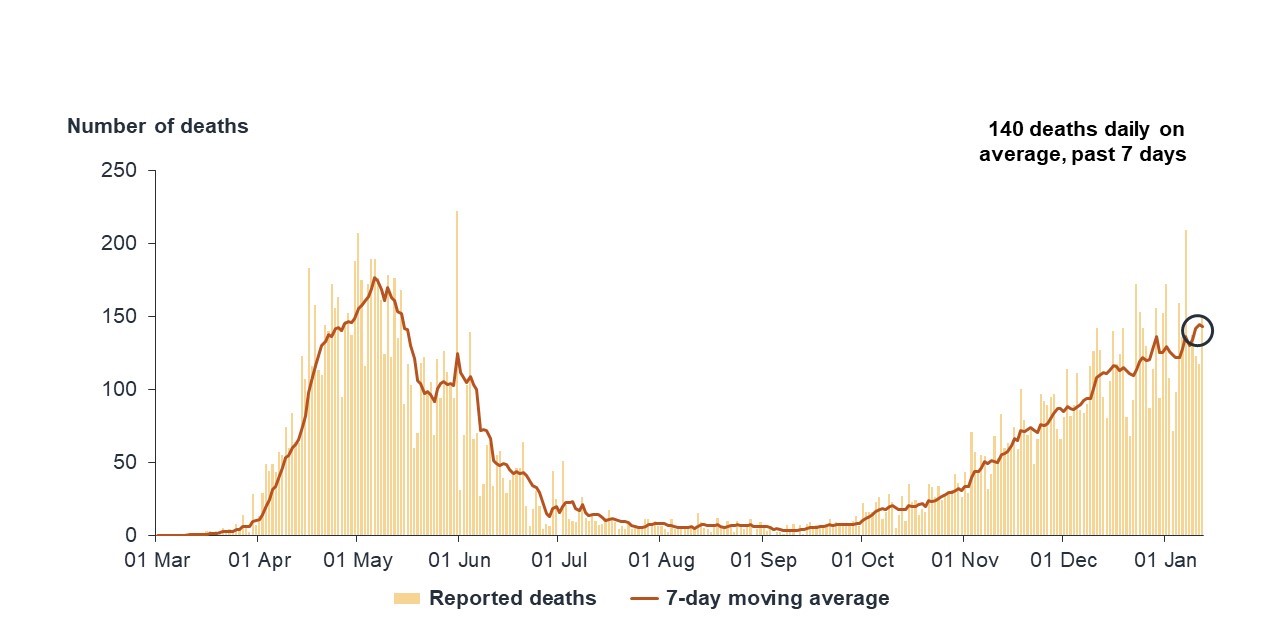
Source: PHAC, January 15th, 2021
Figure 5: Text description
This graph uses bars to show the number of deaths each day due to COVID-19, with the 7-day moving average of daily deaths overlaid as a red line. The data are presented from March 1, 2020 to January 15, 2021. The line shows an increase starting in April 2020 and peaking in May 2020 at around 170 deaths. The line then illustrates a decrease to under 100 deaths in late May 2020. There is then a small increase in June 2020 to around 120 deaths. After this, the line decreases and remains stable over the period July 2020 to October 2020, at less than 25 deaths per day, calculated as a 7-day moving average. The line then then increases steadily from October 2020 to January 15, 2021, ending at 140 deaths daily, calculated as a 7-day average. The bars follow the same trend throughout.
The government worked with partners, and took steps to build up surge capacity:
- Canadian Red Cross deployed teams and resources to deal with acute issues across the country
- Canadian Armed Forces were ready to intervene as a last resort.
- Government of Canada provided surge testing supports to PTs and established a number of distribution channels for rapid testing resources and kits
December 2020: Health Canada authorized use of the Pfizer-BioNTech vaccine, followed by the Moderna vaccine, initiating Canada's vaccination campaign. The campaign, launched on December 14, initially focused on vulnerable populations, including elderly Canadians living in long-term care.
Second wave ended due to restrictive public health measures and individual practices. A targeted approach to the administration of vaccinations protected the most vulnerable first, including long-term care residents.
Key lessons learned
Series of "regional pandemics" required approaches targeted to unique circumstances, with need for agile surge resources
Impact of vaccinations
- The impact of vaccines quickly became apparent as incidence rates dropped among prioritized populations (e.g., elderly in long-term care, healthcare workers, Indigenous peoples)
- Evidence clearly showed vaccines were protective, with a low percentage of cases reported following vaccination.
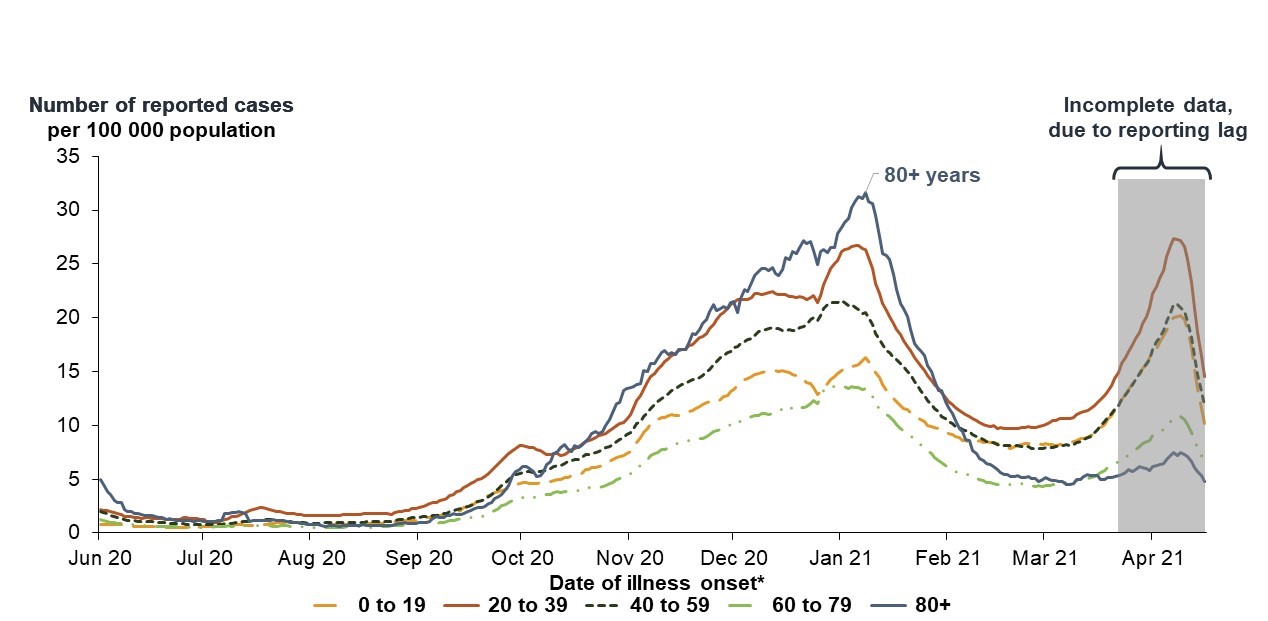
Source: PHAC. April 23rd, 2021
Figure 6: Text description
This line graph shows the number of reported cases of COVID-19 per 100,000 population for the following age groups: 0-19, 20-39, 40-59, 60-79, and 80+. Cases are plotted by the date of the onset of illness.
Each age group is represented by a different coloured line. All age groups follow a similar trend and peak in late 2020/early January 2021. Those 80+ years peak at over 30 cases per 100,000 population, followed by the 20-39 age group with around 25 per 100,000, the 40-59 age group with around 20 per 100,000, the 0-19 age group with 15 per 100,000, and finally the 60-79 age group peak with around 12 per 100,000 in January 2021.
The line for incidence rate goes down for all age groups to at or below 10 cases per 100,000 in March 2021. Although there is incomplete data during April 2021 due to a lag in reporting, the available data show an increase in the number of reported cases per 100,000 population to a level similar or higher than the peaks experienced in January 2021 for certain age groups (e.g., 0-19, 20-39, 40-59 age groups).
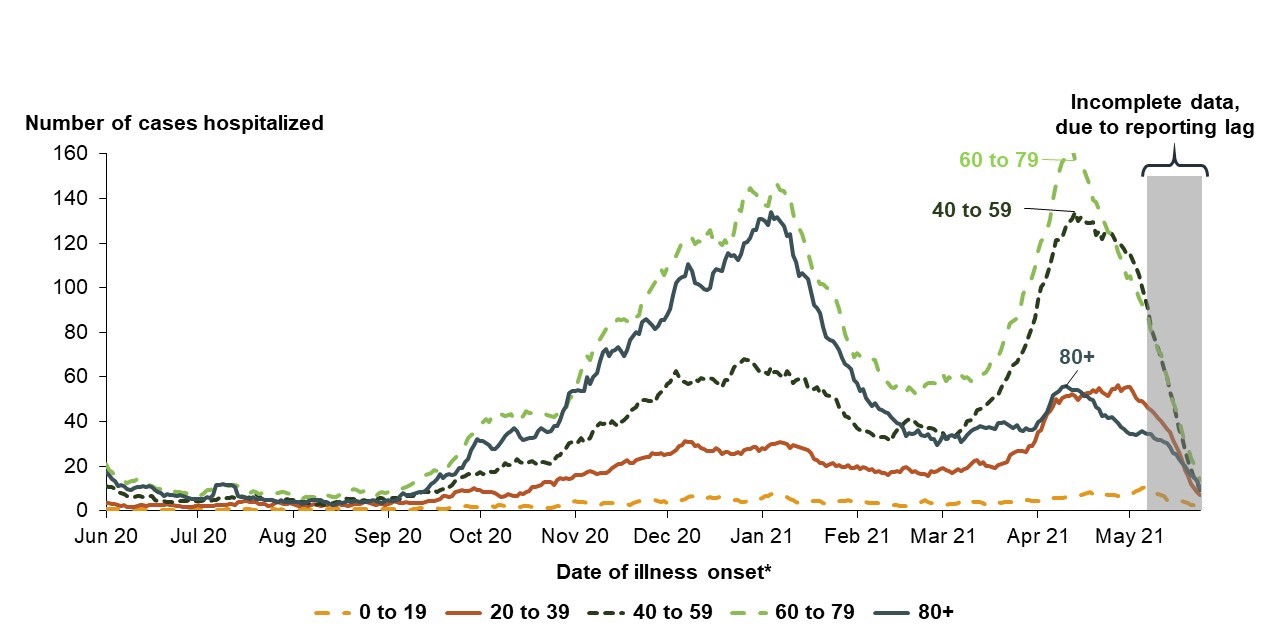
Source: PHAC. May 28th, 2021
Figure 7: Text description
This line graph shows the number of Canadians hospitalized by date, using the date of illness onset. Data is presented for the period June 2020 to May 2021 for the following age groups: 0-19, 20-39, 40-59, 60-79, and 80+.
Each age group is represented by a different coloured line. The lines show two similar peaks in January 2021 and mid-April 2021. These peaks are most pronounced for the age groups 40 and over, with hospitalizations in younger age groups staying relatively stable.
In January 2021, hospitalizations for persons aged 60-79 reached over 140. This number was over 120 for persons aged over 80, over 60 for persons aged 40-59, and over 20 for those aged 20-39. For those aged 0-19, hospitalizations remained steady throughout January at under 10.
For the April 2021 peak, hospitalizations for persons aged 60-79 reached nearly 160. This number was approximately 130 for persons aged 40-59, and approximately 40 for both the 20-39 and over 80 age groups; the 20-39 age group peaked at the end of April 2021. The 0-19 age group again remained steady, with hospitalizations staying below 10.
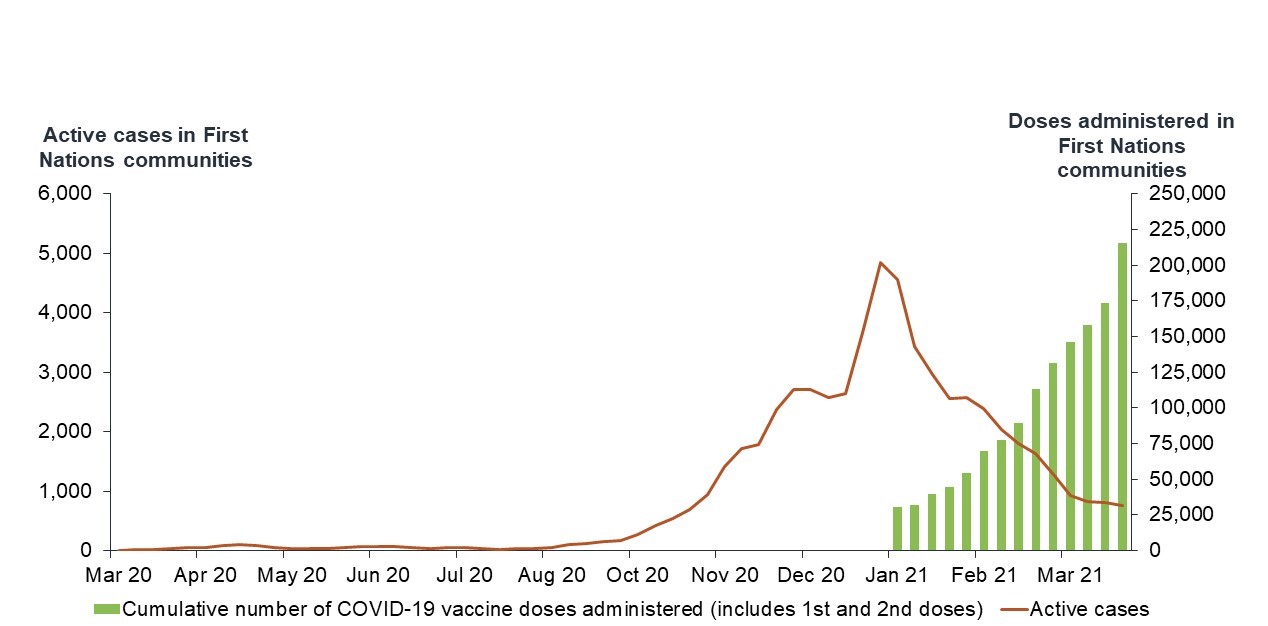
Source: PHAC. April 23rd, 2021
Figure 8: Text description
This graph uses a red line to display active cases in First Nations communities from March 2020 to March 2021. The cumulative number of vaccine doses given is represented by green bars. The data is from March 2020 to March 2021.
Case counts remain nil until late August, 2020, when they begin to rise. The number of active cases rises steadily from October 2020 to the beginning of December 2020, the number of active cases begins to increase more sharply, reaching a peak in January 2021 at around 5,000 active cases. The number of active cases then trends downward over roughly the same period that vaccination increases, beginning in January 2021.
The cumulative number of vaccinations is displayed as green bars, showing a steady increase from January to the end of March 2021. At the end of March 2021, the total number of doses provided in First Nations communities is over 222,500.
Source: PHAC. June 25th, 2021
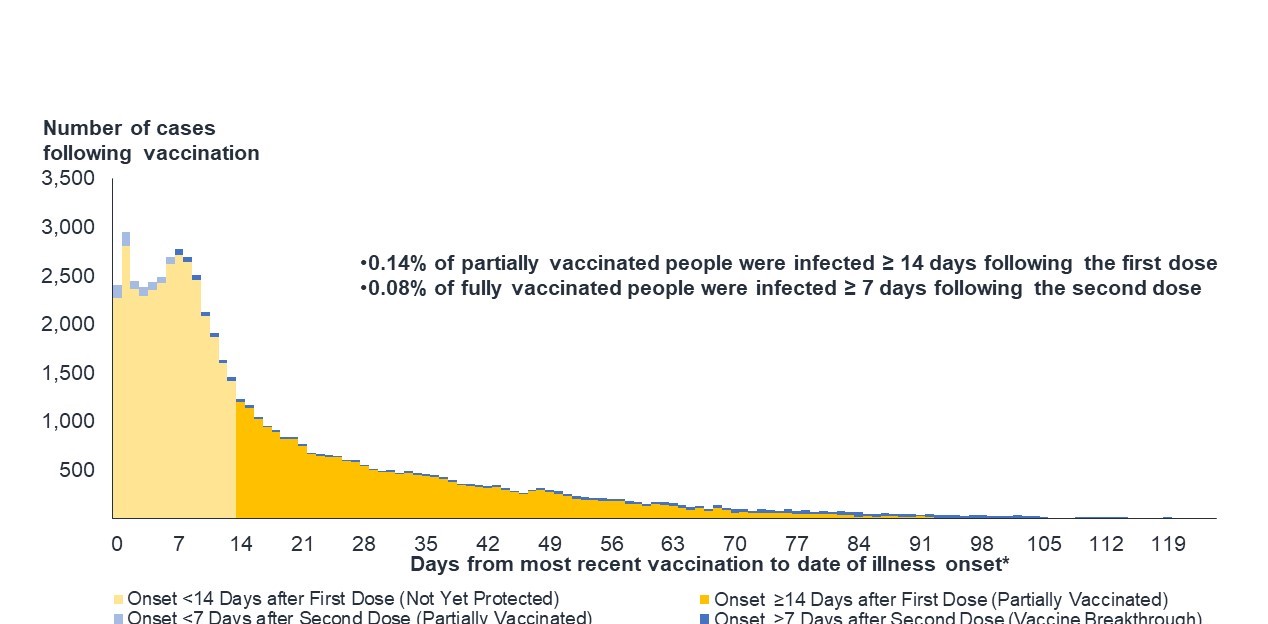
Figure 9: Text description
A low number of cases reported in the fully vaccinated two weeks post vaccination.
This bar graph takes the number of COVID-19 cases reported among people who have received at least one dose of vaccine, and breaks it down by the number of days between when the person received their latest dose of vaccine and the date of onset of illness. The data was collected on June 25, 2021.
The graph is made up of 4 colours. Light yellow shows illness onset less than 14 days after first dose (not yet protected), bright yellow for onset more than 14 days after the first dose (partially vaccinated), light blue for onset less than 7 days after second dose (partially vaccinated), and dark blue for more than 7 days after the second dose. From day 0-14, the majority of cases are made up individuals who were less than 14 days after their first dose (vaccine breakthrough).
The graph illustrates that just below 3,000 cases were reported in people who had received a first dose less than 14 days prior to onset of illness, representing the vast majority of total cases among those at least partially vaccinated.
Cases decline steadily based on two factors: the more days that have passed since a person's last dose, and whether that person has had one or two doses.
Approximately 0.14% of partially vaccinated people were infected 14 days or more after their first dose. Only 0.08% of fully vaccinated people were infected 7 days or more after receiving their second dose.
Third wave – Federal response
Source: PHAC. May 25th, 2021
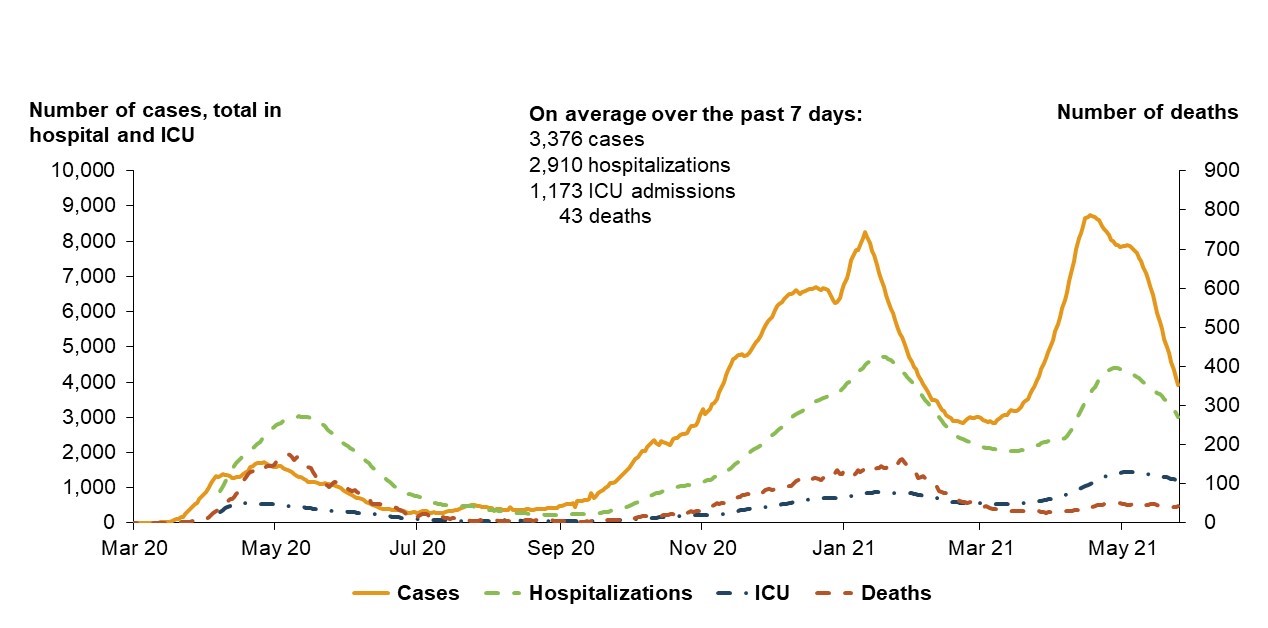
Figure 10: Text description
This graph uses four different coloured lines to present the number of reported COVID-19 cases, how many are in hospital, how many are in ICU, and how many have died during the Third Wave. This graph captures data from March 2020-May 2021. The number of cases, hospitalizations and ICU admissions are depicted on the left side of the graph while the number of deaths is shown on a separate scale on the right side of the graph.
The 4 lines show peaks around May 2020, January 2021, and May 2021. During these peaks, hospitalizations, ICU admissions and deaths peak slightly later than cases, as severe outcomes such as hospitalizations typically occur several days after COVID-19 has been diagnosed.
At the first peak of severe outcomes around May 2020, there are approximately 3,000 hospitalizations, approximately 400 ICU admissions and approximately 200 deaths. There are just under 2,000 cases.
At the second peak of severe outcomes around January 2021, there are approximately 4,300 hospitalizations, approximately 800 ICU admissions and under 200 deaths. There are roughly 8,000 cases.
At the third peak of severe outcomes around May 2021, there are over 4,000 hospitalizations, over 1,000 ICU admissions and approximately 40 deaths. There are roughly just under 9,000 cases.
December 2020 concerns about new, more transmissible variants of concern (VOC).
February 2021 VOC strategy launched to help rapidly scale up surveillance, sequencing and research efforts.
Public health measures eased in some PTs as the 2nd wave declined – VOCs began fueling a 3rd wave larger than previous waves in Spring 2021.
Borders
Implemented risk-based measures including mandatory testing in addition to mandatory quarantine for travellers.
Surge support
Federal response included working with the Canadian Red Cross, the Canadian Armed Forces, the private sector and others to provide surge support to PTs.
Vaccines
Thanks to the "Big Lift" and to strong collaboration with PTs, increasing numbers of Canadians were vaccinated – and incidence rates in the general population started to drop.
A combination of more stringent public health measures including lockdowns and vaccination were used to control the third wave.
Key lessons learned
Governments need to be cautious and measured in lifting public health measures; declining cases are a signal that they work, not that they should be removed.
Managing the pandemic – 4th wave
Source: PHAC. October 13rd, 2021
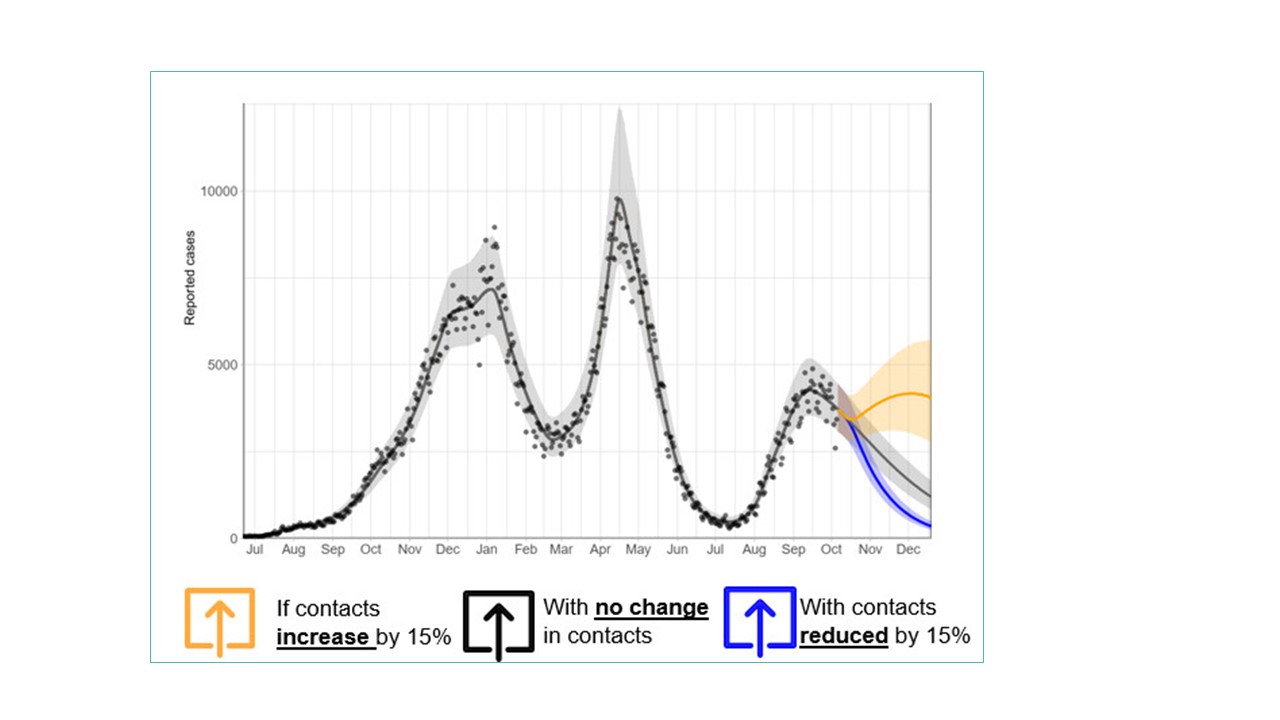
Figure 11: Text description
This graph was created on October 13, 2021.
It is generated using the PHAC-McMaster infectious disease model that is calibrated to the actual reported daily cases over the period July 2020 to October 2021. The calibrated model can then be used to forecast the future trajectory of the pandemic over the next few months. The graph shows the forecasted daily reported cases from mid-October 2021 to December 2021. These forecasts are displayed using three colored lines. The first line shows daily cases increasing from approximately 3000 to approximately 4000 if contacts increase by 15% from what they were in mid-October. The second line shows cases decreasing to approximately 1200 if contact rates remain the same, and the third line shows cases dropping to under 1000 if contact rates are reduced by 15%.
Many PTs implemented re-opening plans over the summer of 2021 based on epidemiological and vaccine coverage indicators.
VOCs, in particular the Delta variant, remain a threat, particularly among the unvaccinated, including children under 12.
The trajectory is towards decline in the coming two months, assuming current contact rates; incidence could be further reduced if contact rates decreased.
Governments need to be cautious when reopening, continue to use public health measures, and delay reopening and reinstitute restrictions if necessary.
Fourth wave action plan
Outlines the government's broad strategy to address this latest surge, including:
- Strategic communication on 4th wave risk and vaccination imperative to drive action;
- Vaccinate all eligible Canadians, supported by ongoing communications efforts and leadership by mandating vaccination for the federal Public Service and in key federal sectors;
- Minimize importation of infection and variants of concern while enabling phased border reopening;
- Mobilize private sector for workplace, self-test distribution, vaccination mandates;
- Support PT and private sector targeted actions in high risk settings (e.g. workplaces, educational institutions); and,
- Proactively identify hot spots and support PTs with surge resources
Borders
Gradual reopening to accommodate non-essential fully vaccinated visitors from the US (August 9) and the rest of the world (September 7), with potential adjustments depending on public health considerations.
Managing the pandemic – 4th wave and beyond
Source: PHAC. September 3rd, 2021
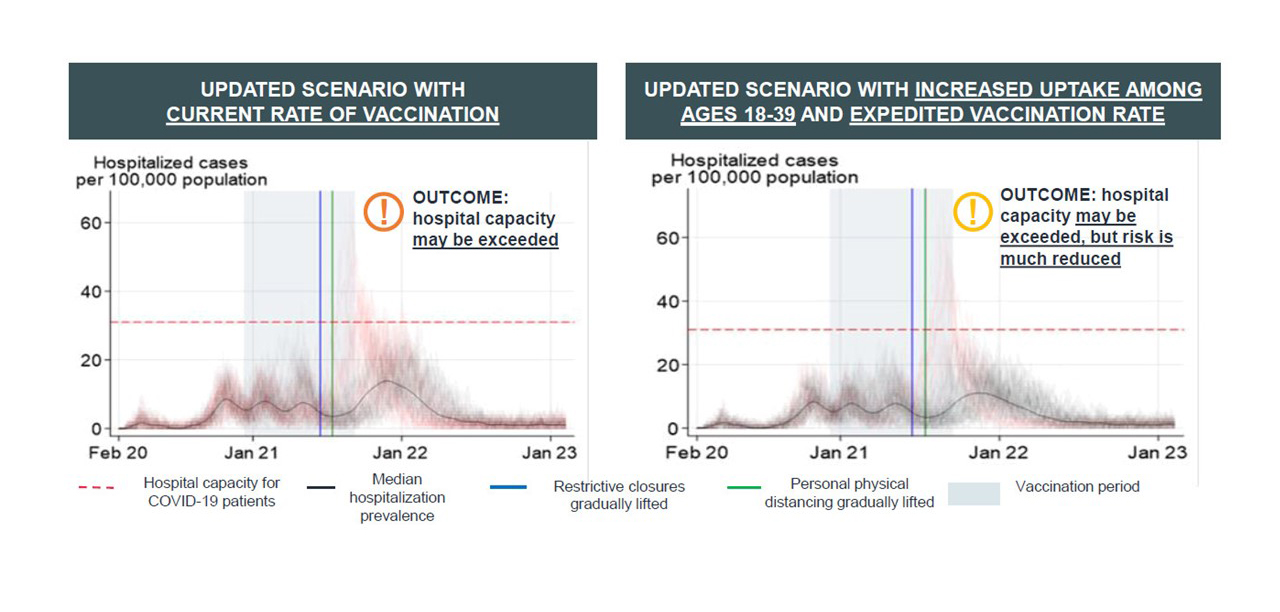
Figure 12: Text description
Updated Scenario With Current rate of vaccination
This graph shows the results from an agent based model for the scenario in which vaccination proceeds at the current rate. One of the outputs is an estimate of the hospitalization prevalence per 100,000 population over the period February 2020 to January 2023. The graph shows that, with public health closures lifted and social distancing measures relaxed, median hospitalization prevalence is expected to increase in January 2022, but remain below 20 hospitalizations per 100,000 population. In some cases hospital capacity may be exceeded but this is not expected.
Updated scenario with increased uptake among ages 18-39 and expedited vaccination rate
This graph shows the results from an agent based model for the scenario in which there is an increased uptake of vaccination in the 18-39 age group as well as a faster vaccination rate. One of the outputs is an estimate of the hospitalization prevalence per 100,000 population over the period February 2020 to January 2023. The graph shows that, with public health closures lifted and social distancing measures relaxed, median hospitalization prevalence is expected to increase in January 2022, but remain below 20 hospitalizations per 100,000 population. It remains lower than in the scenario using current vaccination rates and the risk of exceeding hospital capacity is also lower.
Increased uptake of vaccines, particularly among those ages 18-39, could help reduce hospitalization. Incidence could be lowered if contact rates decreased while vaccination coverage continues to increase.
Key considerations
Vaccination
Higher levels of vaccination are expected to prevent severe outcomes and impacts on hospitals and ICUs; public and private sectors increasingly moving towards requiring vaccinations.
Moving indoors
Fall brings a return to higher risk indoor settings and increased risk of transmission.
PHM adherence
Possible impacts on PHM adherence due to pandemic fatigue, as well as differing views on proof of vaccination.
Adverse events
Additional pressures on health care infrastructure will emerge, such as a return to a normal or higher than expected flu season, and other surge events (floods, wildfires, heat).
Health capacity
Health capacity is limited and exhausted by the ongoing response, and must also turn its attention to addressing backlogs and other health priorities including the wider impacts of COVID-19.
COVID will eventually shift from pandemic to endemic, and our response will shift accordingly.
With effective, widespread vaccines, COVID-19 will likely be controlled and a new normal achieved, but it is unlikely to disappear, and the timeframe is uncertain.
Pandemics burn out when they are unable to spread – when there is enough immunity that they are unable to continue transmission.
Most diseases persist in some corners of the world, becoming endemic, and causing periodic or seasonal smaller outbreaks (i.e. seasonal flu).
Vaccine (including boosters) and surveillance programs, as well as effective therapeutics and treatment capacity, will likely need to be maintained well into the future, which will also enhance our preparedness for future outbreaks.
Globally, there have been many deaths from influenza pandemics.
Spanish Flu
- The Spanish Flu from 1918-1920 caused the most global deaths, with estimates ranging from 24.7 million to over 100 million
- "Ended" due to a combination of herd immunity and public health measures that cut off its ability to spread – but the virus (H1N1) continued to circulate seasonally for 38 years
Asian Flu
- The Asian Flu from 1957-1958 is ranked second with estimates between 1.5 million to 2.4 million global deaths.
COVID-19
- Global COVID-19 deaths are shown in comparison at 4.9 million as of October 15, 2021
Hong Kong Flu
- The Hong Kong Flu from 1968-1969 caused an estimated 1-4 million global deaths
Russian Flu
- The Russian Flu from 1977-1978 caused an estimated 700,000 deaths
Typical Flu
- The typical flu season causes approximately 400,000 global deaths
Areas of post-pandemic planning
Public health emergencies are expected to increase in frequency and severity due to globalization and climate change – action is required in key areas:
Strengthening readiness capacity
- Recruiting and retaining health human resources
- Increasing mental health and substance use resources
- Implement Bio-manufacturing Strategy to ensure domestic research and production capability for vaccines, treatments and diagnostics
Enhancing Government of Canada capacity
- Contingency preparedness and surge capacity
- Review of federal emergency response system to identify gaps, challenges, lessons learned and best practices, to inform and improve future responses
- Enhanced federal science capacity
- Analysis of international partners' pandemic preparedness approaches
- Working with vulnerable communities to better understand health inequities in the social determinants of health
Improving pandemic surveillance and management systems
- Improve surveillance and response, working with experts, PTs and stakeholders – on guidance and action
- Build sustainable approach to managing border measures
- Ensuring clear and consistent public communications as evidence emerges
- Update and regularly test federal and FPT emergency preparedness and response plans
Conclusion
Since the start of the pandemic, the Health Portfolio has played a lead role in responding to COVID-19, working with other federal departments and agencies to tackle issues related to health, social and economic impacts.
Working closely with PTs, Indigenous partners, health experts, academia, civil society, private sector and other stakeholders has proven key to supporting and protecting the health and safety of Canadians.
Work continues to build and maintain public trust through the provision of open and transparent communications – reassuring Canadians that our response is guided by the latest data, science, research and evidence.
COVID-19 has had a disproportionately negative effect on the more vulnerable members of society. Our response requires a focus on understanding and addressing ongoing inequalities.
As COVID-19 transitions from a pandemic to an endemic state, the focus will shift to what is required to maintain a "new normal" and also to what actions are required to ensure Canada is well prepared to face the challenges that future pandemics may present.
About Coronavirus disease – Infographic
Please read, About Coronavirus Disease: Infographic
2: Roles and responsibilities
COVID-19: Key roles and responsibilities
Health Canada (HC)
Key HC responsibilities include providing leadership and decision-making support through the COVID-19 taskforce; implementing innovative regulatory measures to authorize and approve vaccines, treatments, medical devices, and other supplies; scaling up testing and tracing; supporting PTs, including enhancing health care capacity, targeted funding and investments in virtual care and long term care; and supporting notification apps to alert Canadians of COVID exposure.
Public Health Agency of Canada (PHAC)
Key PHAC responsibilities include protecting Canadians by coordinating a national public health response with PTs, providing the epidemiological and modelling information to support good decision making, preventing the spread of COVID-19 by financing and rolling out vaccines and supporting a public health focused border posture. Finally, it plays a key role in promoting science-based information through public communications and public health guidance.
The Chief Public Health Officer (CPHO) is the lead public health professional of the Government of Canada. The CPHO has played a key leadership role in the COVID-19 response by providing evidence-based public health advice to the Government and working with PTs to protect Canadians.
Canadian Institutes of Health Research (CIHR)
CIHR funds COVID-19 research that contributes to the evidence base to support response to the pandemic. It has also established a Centre for Research on Pandemic Preparedness and Health Emergencies to continue to mobilize the research community as the pandemic evolves, and beyond.
Canadian Food Inspection Agency (CFIA)
The CFIA has provided various services during the pandemic to ensure food safety, animal and plant health, and access to food supply and markets. This includes offering industry guidance and information for COVID-19, regulatory flexibilities, inspection and testing services and animal susceptibility studies.
Provincial/Territorial (PT) governments
PTs are responsible for the delivery and administration of health care services, including public health surveillance, screening initiatives, public health measures, vaccine administration, and access to personal protective equipment and medical devices for health care professionals.
Other government departments
Key departments with distinct and important roles include:
- Indigenous Services Canada (ISC)
- Public Services and Procurement Canada (PSPC)
- Innovation Science and Economic Development (ISED)
- Transport Canada (TC)
- Public Safety (PS)
- Canada Border Services Agency (CBSA)
- Department of National Defence (DND)
- Employment and Social Development Canada (ESDC)
- Global Affairs Canada (GAC)
- Justice Canada (JC)
- Finance Canada (FC)
- Immigration, Refugees and Citizenship Canada (IRCC)
- National Research Council (NRC)
External consultative bodies
The National Advisory Committee on Immunization (NACI), Vaccine Task Force, COVID-19 Immunity Task Force, Therapeutics Task Force, COVID-19 Testing and Screening Expert Advisory Panel, and similar bodies play a key role in informing the COVID response.
Other partners
Non Governmental Partners such as the Canadian Red Cross (CRC) also play a key role in providing surge capacity and disseminating information. The World Health Organization and COVAX are important international partners.
Roles and responsibilities: Other government departments
| Key files | Implicated departments |
|---|---|
| Vaccines | Public Services and Procurement; Indigenous Services Canada; Innovation, Science and Economic Development; National Research Council |
| Testing, PPE and Medical Supplies | Public Services and Procurement; Innovation, Science and Economic Development; National Research Council |
| Borders | Canada Border Services Agency; Transport Canada; Immigration, Refugees and Citizenship Canada; Global Affairs Canada |
| Emergency Response | Public Safety; Department of National Defence – Canadian Armed Forces |
| Surge Support | Public Safety; Indigenous Services Canada; Department of National Defence – Canadian Armed Forces |
| International Engagement | Global Affairs Canada |
| Economic and Social Support | Finance Canada; Economic and Social Development Canada |
| Research/Biomanufacturing | Innovation, Science and Economic Development; National Research Council |
3: FPT and international governance
Federal Provincial Territorial governance
Key Federal-Provincial-Territorial (FPT) structures
High-level FPT collaboration is maintained through well-developed formal structures including: FPT Health Ministers' Meetings (HMM), the Conference of Deputy Ministers of Health (CDM), and the pan-Canadian Public Health Network (PHN). There has been unprecedented FPT collaboration since the beginning of the pandemic, with regular CDM and HMM meetings.
The Federal Provincial Territorial Special Advisory Committee (SAC) on COVID-19
In times of an urgent public health event of national concern, the PHN Council may choose to activate a Special Advisory Committee (SAC), as a time-limited mechanism to lead a coordinated FPT response. In January 2020, the PHN Council activated the SAC on COVID-19. SAC advises the CDM on the coordination, public health policy, and technical content related to the COVID-19.
SAC members include Chief Medical Officers of Health and Assistant Deputy Ministers responsible for public health from FPT governments. The Committee is chaired by Canada's CPHO, and the current PT co-chair is New Brunswick's Chief Medical Health Officer.
Since January 2020, SAC and Chief Medical Officers of Health have held more than 200 meetings on COVID-19, touching on:
- coordinated approaches to testing;
- public health measures, outbreak management, and clinical guidance;
- risk communication products and messaging;
- vaccination rollout and education; and,
- border and travel health measures.
Additional FPT tables
A number of additional FPT tables were established to support a coordinated approach to pandemic response efforts across Canada, focusing on issues such as Testing, Contact Tracing, and Data Management, Long-Term Care, Virtual Care, Drug Shortages, COVID Alert, the Vaccine Injury Support Program, and Personal Protective Equipment.
International governance structures
The COVID-19 pandemic has placed a spotlight on key international bodies responsible for pandemic preparedness and response, global health security, and global health governance. The global pandemic, and associated reviews of the response, are expected to drive numerous changes in the global health architecture and impact global health governance.
World Health Organization (WHO)
The WHO has played a critical role in the early and ongoing pandemic response. This includes the global coordination of the International Health Regulations (2005); developing technical guidance; convening experts to accelerate COVID-19 research; developing coordinated COVID-19 supply chain systems for PPE, diagnostics and clinical supplies; and accelerating global vaccine rollout. The WHO has also convened several COVID-19 response reviews with the aim of identifying lessons learned and strengthening global pandemic preparedness and response.
Access to COVID-19 Tools (ACT) Accelerator
The ACT Accelerator, launched by the WHO and partners, is a global collaborative effort to accelerate the development, production, and equitable access to COVID-19 tests, treatments, and vaccines, particularly in low and middle income counties. This initiative comprises of four pillars: Diagnostics; Therapeutic; Vaccines (COVAX); and the Health Systems Connector, which works across the other three. COVAX aims to accelerate the development and manufacturing of COVID-19 vaccines, and to guarantee fair and equitable access for every country in the world.
Pan American Health Organization
The Pan American Health Organization (PAHO) is the specialized international health agency for the Region of the Americas. PAHO's Incident Management Support Team provides technical cooperation and expertise to help guide countries' strategies and policies to address the impacts of COVID-19. This includes the rapid dissemination of information, ongoing surveillance, capacity building, and supporting the deployment of vaccines across the Region.
Global Health Security Initiative
The Global Health Security Initiative (GHSI) is an informal network of countries and organizations that seek to exchange information and coordinate practices for confronting new health threats and risks to global health. Canada serves as the GHSI Secretariat and co-chairs the Senior Officials-level Global Health Security Action Group along with the U.K.
Global Health Security Agenda
The Global Health Security Agenda (GHSA) is a group of 70 countries, international and non-government organizations, and private sector companies. Its objectives are to enhance country capacities to prevent, detect and respond to infectious diseases; galvanize high-level commitments to global health security; promote multi-sectoral engagement and collaboration; and focus on common, measurable targets. Canada is a rotating member of its Steering Group.
G7/G20
The G7 is an informal grouping of seven of the world's advanced economies: Canada, France, Germany, Italy, Japan, the United Kingdom (UK), and the United States (US), as well as the European Union (EU). The G7 provides global leadership and plays a powerful catalyst role by influencing global trends. This year, the UK, as President, has undertaken an agenda reflecting commitments to strengthening global health security and improving pandemic preparedness.
The G20 is an informal group of 19 countries and the EU. This year, Italy, as G20 President, has outlined a health track that includes four pillars: healthy and sustainable recovery; building One Health resilience; coordinated and collaborative response; and accessible control tools.
4: Key levers and federal actions
Vaccines treatments and products for COVID-19
Regulation of COVID-19 health products
Issue
The COVID-19 pandemic placed an unprecedented demand on Canada's health care systems, with an urgent need to access health products, such as test kits, drugs (including COVID-19 treatments and vaccines), sanitizers and disinfectants, and personal protective equipment. Health Canada (HC), in conjunction with the Public Health Agency of Canada (PHAC), played a key role in providing an agile and efficient regulatory framework to support safe and timely pandemic response.
Current status
Health Canada's rapid regulatory response used flexibilities within existing authorities, using interim orders to establish additional authorization pathways. As a top-tier global regulator, HC also collaborates closely with other regulators, such as the U.S. Food and Drug Administration, to share information, coordinate, and align pandemic responses where appropriate. The innovative measures Health Canada adopted successfully expedited regulatory review and authorization without compromising safety, efficacy or quality standards, enabling Canada to be among the first in the world to approve COVID-19 vaccines in December 2020.
Regulatory flexibilities
HC used existing flexibilities within its regulatory frameworks to prioritize COVID-19 related applications and streamline processes, while continuing to protect the health and safety of Canadians. This resulted in the expedited issuance of over 3,300 medical device licenses, and 225 drug establishment licenses to facilitate immediate access to these critically needed tools. Other flexibilities included the issuance of drug establishment licences electronically and the introduction of enhanced flexibilities to allow for virtual/remote inspections and electronic submission of documentation. PHAC also used regulatory flexibilities to facilitate inspections under the Human Pathogens and Toxins Act, while continuing to protect the health and safety of the public.
Interim Orders (IO)
Throughout the pandemic, IOs have been used to create temporary regulatory pathways to allow for the expedited scientific review of COVID-19 clinical trial applications and health products, including vaccines, drugs, medical devices (e.g., test kits and personal protective equipment), and disinfectants. For example, the IO for treatments and vaccines introduced rolling submissions, which allow companies to submit evidence on safety, effectiveness and quality to HC as it becomes available – rather than waiting until the end when a full package is assembled. The IOs also provided the Department with the ability to apply terms and conditions on an authorization, allowing additional oversight measures to be put in place and requiring enhanced post-market monitoring. As of March 2021, these temporary regulatory tools were moved permanently into the Food and Drug Regulations to ensure continued, expedited COVID-19 vaccines and treatments.
IOs were also used to prevent and/or alleviate shortages of key health products by allowing the exceptional importation of certain products not authorized for sale in Canada but that were manufactured elsewhere with comparable standards. In September 2021, these provisions were permanently transitioned into the Food and Drug Regulations to further prevent and alleviate shortages where possible.
Collaborating with partners
HC and PHAC have taken a leadership role in enhanced engagement with domestic and international partners. Since March 2020, Health Canada has worked intensively with provincial and territorial partners to manage 47 national, critical drug shortages, 27 of which were pandemic-related. HC also engaged industry leaders in the health products sector to identify and encourage potential health product submissions to help ensure regulatory reviews are completed in a timely manner.
PHAC has led the Government of Canada's border response under the Quarantine Act, engaging with provinces and territories to coordinate efforts and consulting with various government departments to develop and implement emergency Orders-in-Council to minimize the risk of introduction or spread of COVID-19.
Internationally, HC and PHAC have been working with the International Coalition for Medicines Regulatory Authorities, the World Health Organization, and the Pan American Health Organization on a coordinated and aligned approach to clinical trials, drug and medical device market authorizations, health product risk assessments, and sharing information on potential drug and medical device shortages. For example, while still making independent decisions for Canadians, HC is working closely with other major regulators who are reviewing new data regarding COVID-19 vaccines, which allows for the sharing of scientific evidence and the alignment and streamlining of review processes.
Considerations and next steps
The pandemic has reinforced the need for more agile, innovative, flexible and efficient approaches to regulation. As such, HC will build on the lessons learned from the COVID regulatory response to further inform policy and regulatory development and to ensure the continued access of Canadians to the novel treatments and innovations critical to our preparedness for future health emergencies.
Vaccine approvals, procurement and supply management
Issue
The Government of Canada continues to act proactively to protect Canadians against COVID-19 through the approval, acquisition and distribution of safe and effective vaccines.
Health Canada's role
As the regulator HC is responsible for evaluating and authorizing the use of safe and effective vaccines in Canada. The Department evaluates vaccines for safety, efficacy, and quality, authorizes only those whose benefits outweigh the risks, monitors the quality of vaccines authorized for sale in Canada, and conducts post-market surveillance to manage risks.
PHAC's role
PHAC manages COVID-19 vaccine-related funding, including providing PSPC direction on which vaccines and quantities to procure. As well, PHAC purchases auxiliary supplies (e.g., needles, syringes, ultra-cold freezers) through its National Emergency Strategic Stockpile.
PHAC established an Immunization National Operations Centre (NOC), which acts as the federal logistical authority for managing vaccine distribution and collaborates with PTs on the allocation and delivery of vaccines. The Canadian Armed Forces supported the NOC and assisted with planning and with logistical challenges, such as cold storage and reaching rural and Indigenous communities.
PSPC leads negotiations and finalizes agreements with COVID-19 vaccine suppliers and Global Affairs Canada identifies potential donation recipients.
National Advisory Committee on Immunization's role
The National Advisory Committee on Immunization (NACI) operates as an independent external body to the Government of Canada. NACI has been providing guidance on the use of vaccines in Canada since 1964. They also identify at risk groups for targeted vaccination for vaccine-preventable diseases. NACI recommendations are published in literature reviews, statements and updates. NACI advice provides guidance and information needed for public health decision-makers and healthcare providers.
NACI has published over 20 COVID-19-related statements since May 2020, including statements and updates on the prioritization and use of COVID-19 vaccines, as well as various other stand-alone statements, rapid responses, and guidance documents. As evidence evolves, NACI continually reviews the evidence and updates its recommendations, as required.
Considerations
Regulatory approvals
In response to the urgent need caused by the pandemic, Health Canada (HC) quickly instituted innovative and agile measures to prioritize and expedite the regulatory review of COVID-related vaccines without compromising standards for safety, efficacy, and quality. The effort involved unprecedented collaboration and engagement with international regulatory partners, industry, and health professionals. This included the development and publication of scientific guidance to support the submission and approval of vaccines for COVID-19.
HC also worked with manufacturers of vaccines and treatments to ensure they coordinated their data submissions with those of other major international regulators, enabling the authorization of those products at the same time as other leading jurisdictions. The Department hired additional scientists and established dedicated review teams to ensure timely and thorough vaccine approvals.
HC has authorized five vaccines (Pfizer-BioNTech, Moderna, AstraZeneca, Serum Institute of India, and Janssen [Johnson & Johnson]) to date, with other vaccine submissions currently under review.
Procurement, supply and donations
The Public Health Agency of Canada (PHAC) worked closely with Public Service and Procurement Canada (PSPC) to negotiate and finalize APAs with suppliers to secure early access to a range of vaccine candidates. Canada secured access to up to 404 million vaccine doses under these APAs in 2020.
Canada signed Advanced Purchase Agreements (APAs) with seven suppliers to access a diverse range of vaccine candidates, including mRNA, viral vector, virus-like particle, and protein subunit vaccines. These purchases aligned with recommendations from the COVID-19 Vaccine Task Force.
The VTF is comprised of multidisciplinary experts and industry leaders in the field of vaccines. Established in the spring of 2020, the VTF is mandated to provide advice on vaccines to the Ministers of Health and of Innovation, Science and Industry. Canada's vaccine roll-out started in December 2020 with regulatory approvals and initial delivery of the Pfizer and Moderna vaccines and later expanded in February 2021 to include AstraZeneca.
Based on a two-dose regime, Canada required 66.4M doses, a milestone that was reached on July 27, 2021, to allow it to vaccinate 100% of the eligible population (12 years and older). At this time, vaccines are not authorized for use in children under the age of 12. On October 18, 2021, Health Canada received a submission from Pfizer-BioNTech seeking authorization for use of its vaccine in children 5 to 11 years of age. As Canada has reached a position of surplus, attention has turned to effectively managing the rest of our 2021 supply, including deferring, re-profiling, and donating excess doses.
In addition, Canada joined the COVAX Facility, a global collaboration that aims to ensure equitable access to vaccines around the world, in order to support global COVID-19 vaccination efforts. In support of global vaccine equity, Canada has committed to donate 27.7M surplus doses that it would have received via its APAs. Canada has also contributed $545M to the COVAX facility and has donated back to COVAX almost 13M doses it bought from the facility.
In anticipation of a need for boosters or second-generation vaccines beyond 2021, Canada is advancing a diverse procurement strategy for 2022-2024. Through the Government's Biomanufacturing Strategy, Canada has also recently invested in a range of vaccine platforms and production processes to further build its bio-manufacturing capacity and enhance its readiness for health emergencies.
Vaccine surveillance, uptake and hesitancy
Issue
Monitoring and reporting on vaccine safety, including potential adverse COVID-19 vaccine-related events, as well as understanding vaccine effectiveness, coverage, and the nature of hesitancy among the population, can help with efforts to build public confidence in Canada's immunization programs, encourage informed decision-making by Canadians, and support maximum vaccine uptake.
Vaccine safety
Health Canada (HC) is the national regulator for vaccine quality, safety and efficacy, and conducts post-market surveillance. The Public Health Agency of Canada (PHAC) monitors vaccine safety, coverage, and effectiveness.
Market authorization holders are required to report serious adverse events following immunization (AEFIs) to the Canada Vigilance Program in HC. Manufacturers are also required to report serious adverse reaction reports from Canada as well as international reports.
PHAC manages the Canadian Adverse Events Following Immunization Surveillance System, a post-market vaccine safety monitoring system that receives AEFI reports from PTs and HC. PHAC assesses whether anything about them is unusual, which ultimately supports appropriate regulatory action by HC.
PHAC also funds and leverages external surveillance networks to provide additional safety surveillance support (e.g. Immunization Monitoring Program ACTive (IMPACT) network, Canadian Vaccine Safety (CANVAS) Network).
PHAC posts weekly vaccine safety reports on its website, to provide timely, accessible, and factual information to help Canadians make informed decisions about vaccination.
Vaccine coverage
The purpose of PHAC's vaccine coverage surveillance activities is to monitor COVID-19 vaccination coverage across Canada, by key demographic and priority group, to inform vaccination program delivery and public health recommendations, meet stakeholder information needs, and build public trust through transparent communications. To that end, PHAC integrates data from Federal-Provincial-Territorial partners to produce daily updates on the number of COVID-19 vaccines administered, and weekly updates on COVID-19 vaccination coverage in Canada.
Vaccine effectiveness
Vaccine effectiveness measures how well a vaccine works under real-world conditions and populations. Understanding vaccine effectiveness can inform decisions on which vaccines should be used and when, which sub-populations and/or groups should be vaccinated, and whether there is a need for booster doses. PHAC's vaccine effectiveness monitoring activities focus on funding studies and consolidating evidence on COVID-19 vaccine effectiveness, in collaboration with internal and external partners working in this area. Evidence on the effectiveness of vaccines in Canada helps to instill confidence in the benefits of vaccination.
Vaccine hesitancy and acceptance
Despite the generally high acceptance of COVID-19 vaccines among Canadians, there remains considerable work ahead in addressing vaccine hesitancy. Vaccination rates plateaued this summer, and we are now seeing only incremental increases in first and second doses each day. Public opinion research demonstrates that a minority people are resistant to and/or have challenges accessing COVID-19 vaccination. Young adults (18-39 year olds) have the lowest uptake to date. Polling has consistently indicated that 6 to 9% of Canadian adults say that they will not get vaccinated against COVID-19.
Efforts to support COVID-19 vaccine acceptance
As the lead, PHAC works with other federal government departments and its partners to advance a multi-prong strategy for supporting high COVID-19 vaccine coverage and addressing vaccine hesitancy. This complements efforts taken by PTs and community based organizations. Key federal levers in this strategy include:
- Introducing vaccination requirements in the federal public service, and for employees in the federally regulated air, rail, and marine transportation sectors;
- Carrying out targeted advertising and public education for priority populations;
- The Chief Public Health Officer stakeholder outreach and engagement;
- Supporting communities and innovative leaders in building vaccine confidence and increasing access to COVID-19 vaccines through grants and contributions programs; and,
- Supporting provinces and territories in removing access barriers.
Throughout rollout, federal vaccine confidence efforts have targeted the populations and focus areas of greatest concern, and have evolved in accordance with the dynamic COVID-19 context. For example, in the initial months of vaccine rollout, efforts focused on addressing training needs and boosting confidence among health care providers, who were among the populations prioritized for COVID-19 vaccination and play a critical role in administering vaccination. Examples of present focus areas include addressing complacency among young adults and access barriers and mistrust among equity-deserving groups.
Treatments and other drugs needed for COVID-19
Issue summary
Timely procurement of emerging COVID-19 treatments is critical to treating Canadian patients and protecting Canada's health care system from being overwhelmed. The Government of Canada took immediate action to ensure that Canada had access to potentially promising COVID-19 therapies.
In response, the COVID-19 Therapeutics Task Force (CTTF) was established in July 2020 to provide expert advice to the Government of Canada on possible COVID-19 therapeutics. The CTTF met 33 times, reviewed over 40 proposals submitted to the SIF, and considered over 100 therapeutics initiatives. The CTTF mandate ended in late February 2021.
Current status
Once authorized by Health Canada, the federal government considers procurement of COVID-19 therapeutics in response to PT needs/demand, in consultation with clinical experts and PTs.
The Canadian Agency For Drugs And Technologies In Health (CADTH) and Institut national d'excellence en santé et en services sociaux (INESSS) provide PT health care decision-makers with objective evidence, advice and recommendations to help make informed decisions about the optimal use health technologies (including COVID-19 therapeutics).
While traditionally the responsibility of provinces and territories (PTs), the Public Health Agency of Canada (PHAC), with the support of Public Services and Procurement Canada (PSPC), has procured COVID-19 therapeutics to support timely access to, and equitable distribution of, promising COVID-19 therapies for Canadians. Over the course of the pandemic five purchase agreements have been finalized with suppliers of COVID-19 therapeutics, including, remdesivir (VekluryTM; Gilead), bamlanivimab (Eli Lilly), tocilizumab (ActemraTM; Roche), casirivimab/imdevimab (REGEN-COVTM; Roche), and sotrovimab (GlaxoSmith Kline).
Critical drug reserve
Established in October 2020 the Critical Drug Reserve is a safety net of a 3-month supply of twelve key drugs used in the treatment of COVID-19 symptoms. Critical care experts working with the PTs identified these drugs based on their critical role in treating COVID-19 symptoms.
| Sedatives | Neuroblockers | Analgesics | Vassopressors | Other |
|---|---|---|---|---|
propofol midazolam |
cisatracurium rocuronium |
fentanyl hydromorphone |
vassopressin epinephrine norepinephrine |
salbutamol ceftriaxone dexamethasone |
Planning and preparedness
Contingency planning and surge supports
Health Canada (HC) and the Public Health Agency of Canada (PHAC) continue to work with partners on contingency plans and operational support to protect the health of all Canadians.
While provision of healthcare is under provincial jurisdiction, the extraordinary demands of the COVID-19 pandemic require additional federal support to complement provincial and territorial (PT) capacity. Strong collaboration between federal partners, PTs and non-governmental organizations (NGOs) is key. Notable partners include Public Safety Canada (PS), Statistics Canada, Indigenous Services Canada (ISC), Public Services and Procurement Canada (PSPC), the Canadian Armed Forces (CAF), and the Canadian Red Cross (CRC).
Federal surge capacity
To reduce the impact of COVID-19 on Canadians, the Government of Canada established a Federal Rapid Surge Capacity initiative. Surge resources are available to PTs and Indigenous Communities to support congregate living environments, such as hospitals and long-term care facilities and prevent community transmission. Types of surge supports provided to date include:
- CRC and federal health human resources (HHR) deployments to outbreak settings to assist with immunizations, testing and contact tracing;
- Human health resources deployed to support critical care nursing functions in Intensive Care Units (ICUs);
- CAF and CRC deployments to long-term care homes to support outbreak management and to provide epidemic prevention and control training to facility staff;
- CAF/Canadian Ranger deployments and ISC supports in Indigenous communities to support vaccination, logistics and personal care;
- The distribution of biomedical supplies and personal protective equipment to PTs via the National Emergency Strategic Stockpile and the Critical Drug Reserve;
- The procurement of mobile health units and their deployment to provincial partners, for example with two units mobilized for Toronto and Hamilton during the third wave.
Requests for assistance
PHAC serves as a single window for pandemic response and convenes federal partners to engage with PTs on a full range of Health Portfolio supports as required. Public Safety (PS) is responsible for emergency management coordination through the Government Operations Centre. A request for federal assistance (RFA) can be made in two ways:
- 1) a province or territory can go directly to PS; or
- 2) a province or territory, as well as Indigenous Services Canada (ISC) on behalf of Indigenous communities, can engage PHAC directly for medical-related supports.
Given that federal resources are finite, PTs are responsible for prioritizing local requests within their jurisdiction.
To access federal resources, PTs are expected to: prioritize their own requests across competing jurisdictional needs; agree to indemnification agreements with the CRC or other NGOs if their resources are to be deployed in response; put in place public health measures and other mitigation tools to manage outbreaks and exponential case growth, and commit to developing longer-term strategies to avoid recurring surge needs.
PTs can also request mutual assistance from each other, through the PHAC-managed Operational Framework for Mutual Aid Requests (OFMAR). This approach factors strongly into contingency planning given that some jurisdictions may be impacted less than others. This also ensures that limited federal resources are prioritized for the areas of greatest need.
Humanitarian workforce
As an additional surge resource, the federal government created a Humanitarian Workforce program with an initial investment of up to $150M over 2021-2023. The funding supports capacity building, maintaining a standing capacity and operational deployments by participating NGOs in support of PTs. The CRC is the largest organization in the workforce.
PS administers this program and the contribution agreements associated with it. HC plays a role in advising and contributing to decisions around how workforce supports will be structured and operationalized.
Way forward
Health Canada and other federal organizations are active and willing partners, standing by to provide supports should COVID-19 further strain public health and healthcare capacity in the months ahead. Areas of current action include ongoing monitoring for resurgence, proactive engagement with PTs on emerging needs and the pre-positioning of assets, and maintaining a heightened readiness posture for inter-departmental and inter-organizational deployments.
PPE & medical equipment procurement and distribution
Issue
At the start of the COVID-19 pandemic, Canada and many other countries faced an unprecedented global shortage of personal protective and medical equipment required to support the front-line health care response, including N95 respirators, surgical masks, gloves, face shields and gowns.
Current context
To support the needs of front-line health care, and other essential services in Canada, the Government of Canada (GoC), in coordination with the provinces and territories (PTs), rapidly coordinated bulk procurement and leveraged collective buying power. The federal government provided over $7.5 billion to purchase over 3.5 billion units of PPE, vaccine ancillary supplies, medical equipment and medical supplies. These continue to be distributed to support the front-line health care response, along with support from Public Services and Procurement Canada (PSPC) for other essential services.
This procurement bolstered federal and PT pandemic stockpiles by engaging a diverse number of new suppliers and manufacturers both internationally and domestically, with support for domestic production through the GoC's 'Call to Action' and investments from some provincial governments, resulting in a new, 'Made-in-Canada' capacity to produce key products. Health Canada (HC) ensured that key medical supplies received timely regulatory approval for use in Canada.
Starting in Spring 2020, HC worked with the Public Health Agency of Canada (PHAC), other federal partners and PTs to develop and maintain a supply and demand modelling tool to support procurement decisions for emerging pressures.
Health Canada's role
HC's COVID-19 Task Force works with PHAC, other federal departments and PTs to support efforts related to ensure adequate supplies of PPE and other medical commodities. HC also provides strategic policy analysis, data integration and supply and demand modelling, with support from Statistics Canada. HC continues to regulate medical devices, including PPE, to ensure their safety, quality and effectiveness.
Public Health Agency of Canada's role
PHAC, as the agency responsible for the management of the National Emergency Strategic Stockpile (NESS), plays a central role in all aspects of life cycle materiel management of COVID-19 assets. PHAC supported bulk procurement efforts, expanded its logistics and warehousing infrastructure, established product testing capacities, and developed data systems to support analysis, monitoring and reporting. Through the NESS, PHAC acquired, managed and distributed PPE to jurisdictions according to the Allocation of Scarce Resources COVID-19 Interim Response Strategy, as approved by the FPT Ministers of Health in March 2020.
Other federal government departments
The Health Portfolio works in partnership with other federal departments, including:
- Statistics Canada: providing data collection, management, modelling and analytical support to fill key gaps and economy wide view of PPE supply and demand;
- PSPC: leading procurement efforts, securing emergency bulk procurement authorities, supporting PPE procurement for other federal departments and creating the new Essential Services Contingency Reserve for distributing PPE outside the health sector;
- Innovation, Science and Economic Development Canada: leading the 'Call to Action' process and providing strategic investments to support Canadian manufacturers through its 'Made-in-Canada' initiative and other programs;
- National Research Council of Canada: supporting domestic manufacturers with expertise, organizing challenges for innovations in PPE and providing industrial research assistance funding; and
- Global Affairs Canada: leading efforts on trade diplomacy related to PPE supply and regulation, as well as taking on an expanded role in facilitating procurement.
Next steps
Combined FPT stockpiles and incoming supplies are currently assessed as sufficient to meet the expected demand for the health care sector. Some PTs have reduced reliance on the NESS and there are no immediate identified needs for additional federal bulk procurement for PPE.
Given continued uncertainty about the evolution, duration and impact of the pandemic, continued efforts are required to monitor the overall state of PPE and position Canada for the longer term.
Testing, screening and tracing
Issue
Testing and screening remain important tools for COVID-19 case detection, even in the context of increasing vaccination rates. While vaccines have proven effective at protecting against deaths, hospitalization and symptomatic infection, they do not necessarily break the chain of transmission. As such, testing, contact tracing and screening will continue to be an important part of the overall strategy.
Health Portfolio roles
Diagnostic testing: The Government of Canada has provided funding for provinces and territories that supported diagnostic lab-based molecular test capacity, with surge capacity available when needed, to support regional efforts on testing. The national lab-based PCR testing capacity has exceeded the FPT target of 200,000/day since early 2021 and currently stands at about 240,000/day.
Screening: To support screening, the Government of Canada procures and distributes rapid tests to the Provinces and Territories and to the private sector through a number of distribution channels. Screening is undertaken to identify unknown, often asymptomatic, cases so that measures can be taken to prevent further transmission. The Government has purchased different types of rapid tests. As of October 15, 2021, over 62 million tests have been delivered to Canada, with more than 52 million having been shipped to PTs. In addition, as of October 12, 2021, approximately 4 million tests have been shipped to organizations in the private and public sectors. Five rapid test distribution channels have been established: delivery to Provinces and Territories, direct delivery to large private businesses, small and medium enterprises (SME) via pharmacies and Chambers of Commerce, and to non-profits, charities and Indigenous organizations via the Canadian Red Cross.
Surge capacity available for provinces and territories
Testing Assistance Response Team: In collaboration with the Canadian Red Cross, the Government of Canada offers COVID-19 Testing Assistance Response Teams to be deployed and provides immediate testing support for PTs experiencing surges in COVID-19 cases including in remote and northern communities and at borders.
Federal emergency supply of rapid tests: As part of the procurement strategy, and to ensure the availability of tests in case of sudden increased need, the Government of Canada has a supply of rapid tests for emergency purposes.
Expert advice, guidance on testing and screening
In October 2020, Health Canada (HC) established the Industry Advisory Roundtable on COVID-19 testing, screening, tracing and data management to provide insights and advice to support the safe reopening of the economy. In August 2021, the Roundtable published its latest report, which reviewed some of the achievements of the Government of Canada (GoC) in championing workplace screening and provided new recommendations on the way forward. In July 2021, the Canadian Centre for Occupational Health and Safety (CCOHS) published its Guidance on Rapid Testing in the Workplace, outlining the benefits and limitations of rapid testing as well as practical considerations for implementing rapid testing in the workplace. HC is also pursuing collaboration with CCOHS' industry networks to provide targeted advice to employers to establish screening in their organizations.
In November 2020, the Minister of Health established the Testing and Screening Expert Advisory Panel (Expert Panel) to provide evidence-informed advice on science and policy related to COVID-19 testing. In January 2021, the Expert Panel released their first report which recommended the use of rapid tests for screening purposes. From January to August 2021, the Panel produced reports that provided specific advice for long-term care settings, schools, border measures and self-tests.
In August 2021, HC, in collaboration with the Public Health Agency of Canada, published Testing for COVID-19 in Vaccinated Populations, which helps guide provincial and territorial (PT) testing and screening strategies in the context of vaccinated populations, and serves as a foundational element supporting the updates to the Pan-Canadian Guidance on COVID-19 Testing and Screening.
Next steps
Working with other federal government agencies and partners to encourage voluntary asymptomatic screening. In the public sector, testing and screening also plays a role in the GoC's vaccination policy in the federal and federally regulated workplace.
Supporting the long-term growth of the biomanufacturing sector and life sciences ecosystem
Context
The COVID-19 pandemic exposed gaps in Canada's domestic capacity to research, develop and produce vaccines and therapeutic drugs at sufficient scale and pace. It also reinforced the potential to leverage significant breakthroughs in health science and technology in the future.
On July 28, 2021, Innovation, Science, and Economic Development (ISED) and Health Canada launched a Biomanufacturing and Life Sciences Strategy ("Strategy") to continue growing a strong and competitive sector and ensure Canada is prepared for future pandemics. The implementation of the Strategy is supported by Budget 2021 investments of $2.2 billion for the revitalization of Canada's biomanufacturing and life science sector. This investment includes $250 million over three years for the Canadian Institutes of Health Research (CIHR) to implement the Clinical Trials Fund (CTF) to support the development of new, scientifically proven treatments and improved health outcomes for Canadians.
The objectives in advancing this Strategy are multifold: Canada requires a well-coordinated plan to ensure readiness for future pandemics and other health threats, which includes domestic vaccine and therapeutic development and production capabilities across priority and next-generation platforms; and, Canada stands to benefit from a plan to advance other important health and economic outcomes associated with the biomanufacturing and life sciences sector.
The Strategy consists of five pillars:
- Strong and Coordinated Governance: Develop, align, and implement priorities via centralized priority-setting, with coordinated strategic planning, decision-making, performance monitoring and risk management.
- Laying a Solid Foundation by Strengthening Research Systems and the Talent Pipeline: Invest in research, infrastructure, and talent to spur sector growth, drive innovation, and equip Canada with expertise needed to respond to future health crises.
- Growing Businesses by Doubling Down on Existing and Emerging Areas of Strength: Continue to grow a robust, sustainable domestic life science sector that offers greater self-sufficiency in producing vaccines, therapeutics, critical supply chain inputs and demonstrates emerging technology leadership, supporting Made-in-Canada solutions through ISED's Strategic Innovation Fund.
- Building Public Capacity: Strengthen pandemic and infectious-disease response through increased public sector capacity to develop and manufacture vaccines, train and develop workforce, and support pre-clinical and clinical trial activities through Canada's National Research Council, including its new Biologics Manufacturing Centre,
- Enabling Innovation by Ensuring World Class Regulation: Make Canada more attractive for leading life sciences firms to establish and grow a strong and competitive domestic clinical trail environment and life sciences sector, and ensure Canada's readiness for future pandemics or other health emergencies.
Health Portfolio role
A strengthened domestic biomanufacturing and life sciences sector offers opportunities beyond pandemic preparedness to health innovation for drugs and medical devices. HC and ISED share accountability for the execution and oversight of the Strategy.
Ensuring Best-in-Class regulation
HC will continue to make regulations for clinical trials and market access more agile, and clinical trials more responsive to health innovations from discovery to application. HC's plan for increased regulatory agility builds off lessons learned during the pandemic, the Health and Biosciences Regulatory Review of 2018, and recommendations from the Health and Biosciences Economic Strategy Table.
World-class clinical trials system
CIHR will work in parallel on the science policy context needed for success and to develop and implement three complementary components ($250M over 3 years through the CTF) to carry out the proposed initiative through investments in projects, people, and platforms:
- Operating grants to support the full spectrum of clinical trials research, including dedicated support for domestic and multi-jurisdictional trials with international linkages;
- Training and mentorship opportunities to develop highly qualified personnel in trials research who can direct the conception, design and implementation of controlled trials, using a broad range of supports; and
- A clinical trials network to provide coordination and collaboration infrastructure for clinical trials activities across the country, building on existing initiatives supported by CIHR.
International
International engagement
Issue
The COVID-19 pandemic has highlighted Canada's need to respond to global calls for action, mobilize Canadian health research and expertise, and maximize international engagement to strengthen global public health and health systems.
Role of the Health Portfolio
The Health Portfolio, along with Global Affairs Canada (GAC), provides leadership in global health by engaging with key multilateral fora, as well as bilateral and regional partners to strengthen global governance, protect and advance the health of Canadians, champion health issues where Canada can make a difference, advance best practices and model lessons learned, ensure policy coherence and support broader foreign policy objectives. In addition, the Canadian Institutes for Health Research supports research and provides expertise to the global health research agenda, including for COVID-19.
Current status
Canada's international engagement on global health seeks to advance Canadian health priorities, including health equity, the protection and promotion of the health and safety of Canadians, and enhancing health beyond Canada's border.
Currently, key global health issues include:
- Equitable Access and Vaccine Donations
- Without access to vaccines, many countries are unable to make progress in controlling the pandemic.
- To date Canada has committed to donating over 40 million vaccine doses for those who need them most. The allocation of these doses is being managed by the COVID-19 Vaccine Global Access (COVAX) dose sharing mechanism to help accelerate deliveries to countries in the Caribbean and Latin America, while also helping priority countries in other regions. Canada has also provided $545 million for the COVAX Facility for vaccine procurement, distribution and delivery for 92 low and middle income economies eligible for the COVAX Advance Market Commitment (AMC).
- Canada will continue to prioritize delivery of excess doses through COVAX, explore other sharing options, as appropriate, and take necessary action to increase global supply.
- Vaccine Certification and Border Measures
- Canada, like many countries around the world, has implemented border measures and vaccination requirements in response to COVID-19. As proof of vaccination credentials are implemented at the PT level, work is underway to align them with international travel standards.
- International Recognition of Mixed Vaccine Schedules
- Mixed vaccine schedules have been implemented in different countries without mutual recognition. As such, some fully vaccinated Canadians may not be eligible for vaccination-based exemptions when entering some countries.
- Canada has been advocating for recognition of mixed doses with multilateral partners (e.g. G7 and G20), and with key countries, including the top ten travel destinations for Canadians (e.g., U.S., UK, France, Germany, Italy).
Considerations and next steps
COVID-19 has led many countries to consider how to improve overall preparedness of the global community to prevent and respond to health emergencies. Key considerations include:
Strengthened Global Health Security Governance
- Aligning and strengthening efforts to prepare for, detect and respond to global health threats requires new and innovative approaches. Countries and international partners must align efforts and advance their commitments to global health security.
Strengthening multilateral partnerships
- Canada will continue to work effectively and collaboratively with key multilateral partners on a broad range of global health challenges including:
- World Health Organization (WHO) – COVID-19 and WHO's work in emergency preparedness and response; mental health; non-communicable diseases;
- Pan American Health Organization (PAHO) – One Health: including zoonotic diseases and antimicrobial resistance (AMR), building resilient health systems;
- Global Health Security Initiative – Emergency preparedness and response; Travel health and border measures; Laboratory activities; COVID-19 variants;
- G7 – Global health security, ongoing global COVID-19 review processes; scientific cooperation on therapeutics and vaccines trials;
- G20 – Including monitoring the impact of the pandemic, especially on the achievement of the sustainable development goals; advocating for attention to mental health, gender, sexual and reproductive health and rights; advancing global efforts on AMR, digital health, and universal health coverage; and
- Other multilateral partners, e.g., the Global Health Security Agenda, International Association of National Public Health Institutes, the United Nations System.
Continued bilateral engagement
- Regular outreach with a variety of international partners to compare approaches to COVID response and to collaborate on solutions. This includes regular discussion with the US on bilateral approaches and border management, as well as regulatory cooperation, climate change, antimicrobial resistance (AMR), and global health security.
Border and travel measures
Border measures
Issue
Border measures are central to Canada's COVID-19 pandemic response and the overall protection of public health safety and security. Canada's approach to managing COVID-19 has focused on controlling importation at Ports of Entry (POEs), reducing transmission through testing, quarantine, contact tracing, and gradually and systematically easing measures as domestic and international vaccination rates increase.
Key considerations
Following the introduction and maintenance of strict border measures in 2020, there has been continual reassessment of the global and domestic epidemiological situations in order to evaluate whether measures should be adjusted. A phased approach to re-opening and easing border measures has been taken, in line with the epidemiological context.
There has been a steady increase in travel volumes since the beginning of July 2021, linked to the elimination of a 14-day quarantine period for fully-vaccinated Canadian citizens, permanent residents, and individuals registered under the Indian Act. Volumes continued to grow with the opening of the border to discretionary travel from the US by fully vaccinated US citizens and permanent residents on August 9, 2021 and the reopening to fully vaccinated foreign nationals from anywhere in the world on September 7, 2021. The continued growth of traveller volumes is expected as traveller confidence and vaccination rates increase and with the further easing of border measures abroad.
Roles and responsibilities
In addition to overall responsibility for the border, during a pandemic, the federal government is responsible for managing international aspects of the response, which has included exercising powers under the Quarantine Act and providing the public with information about health risks resulting from international travel. PHAC works with the CBSA to administer the Quarantine Act, which gives the Minister of Health powers related to border measures as they apply to travellers and conveyances entering or exiting Canada. This includes the authority to designate screening, quarantine and review officers; establish and designate any place in Canada as a quarantine facility; designate any point in Canada as a point of entry/departure; and issue interim orders for immediate action on issues addressed through regulation.
To deliver on the border and travel health elements of the Health Portfolio's mandate during the pandemic, Health Canada and PHAC work closely with federal, provincial/territorial, and Indigenous partners as well as other stakeholders, using a variety of authorities to introduce, maintain, and amend border measures in response to the changing domestic and international epidemiological situation. Canada has in place a multi-step COVID-19 testing strategy to prevent infected travellers from entering Canada and to detect and isolate infected travellers upon arrival in Canada. Data from the robust COVID-19 border testing program allows monitoring of imported cases into Canada to support decisions on border measures.
Next steps
Canada's border reopening is guided by domestic and international epidemiological indicators that inform decisions on continuing, enhancing, easing or re-imposing border measures. Provisions are in place to rapidly adjust border measures should the situation warrant.
Important statistics
Border Measures:
- 67 Emergency Order in Councils were put into place from March 2020 to September 21, 2021
Traveller Volumes:
- 19.5 Million individuals crossed the border into Canada from March 21, 2020 to September 26, 2021
Compliance and Enforcement:
- 7.3 million compliance verification calls made
- 480,000 compliance verification visits
- 8,700 enforcement actions
- This data is provided up until October 13, 2021
Quarantine:
- 17 federal designated sites with 14,333 users as of September 13, 2021
- 70 3-day quarantine hotels with 500,000 clients. Please note that this program was discontinued as of August 9, 2021.
Testing:
- 2.1 million post-arrival tests as of September 16, 2021
- Individuals who took these tests had a 0.6% positivity rate as of September 16, 2021
- 6,034 cases variants of concern or variants of interest as of October 7, 2021
Timeline of Border Measures in response to COVID-19 pandemic
- Pre-Phase One (Early 2021): Range of measures applicable to all non-exempted travellers permitted to enter Canada, including: Test prior to arrival, testing Day 1 and Day 8, Government Authorized Accommodations (GAA) and 14 day quarantine.
- Phase One (CIF July 5): Eased some measures for fully vaccinated travelers who are permitted to enter Canada including: removed GAA requirement, test on arrival or Day 1, and no quarantine.
- Phase Two (CIF August 9): Eased measures and expanded scope of those able to enter Canada. This included: removed GAA requirements for all, moved to mandatory random testing for vaccinated travellers, allowed entry for fully vaccinated US citizens and permanent residents for discretionary optional travel and expanded the number of international airports being used.
- Phase three (CIF Sept. 7): All discretionary/optional travel of all fully-vaccinated travellers (non US foreign nationals).
Research and surveillance
COVID-19 research
Context
Since the start of the pandemic, the Government of Canada has invested $259.5 million in COVID-19 research through the Canadian Institutes of Health Research (CIHR). This funding leveraged an additional $20.3 million from partners, for a total of $279.8 million.
These investments support 609 research projects. Canadians have benefited from innovative research on enhanced prevention, detection, and treatment options including, but not limited to, vaccines, therapeutics, and diagnostics, as well as the development of an evidence base on which to inform effective social and public health policy responses to support the government's COVID-19 efforts. These investments:
- Supported clinical trials, trial networks, coordination platforms, and other studies to scale-up, reorient or initiate rapid responses.
- Provided research evidence to decision makers to mitigate the impacts of COVID-19 on the mental health of Canadians through the COVID-19 and Mental Health Initiative.
- Supported priority populations with research on Long-Term Care/retirement homes, the opioid crisis, the health needs of Indigenous Peoples, and children, youth and families.
- Responded to emerging gaps and priorities with investments in a series of competitions to target key priorities including: variants of concern, confidence in science and healthcare providers, long-term effects of COVID-19, surveillance, long-term care, structural inequities including systemic racism in the COVID-19 response and ethics; ongoing impacts on health services; and societal reopening.
In addition to these major investments in COVID-19, the Government of Canada, through CIHR, announced funding in April 2020 to create the Centre for Research on Pandemic Preparedness and Health Emergencies to improve domestic and global pandemic research coordination. Moreover, CIHR led the Government of Canada's participation in the international research response, including by supporting the Canadian Treatments for COVID-19 (CATCO) clinical trial that aims to investigate the safety and efficacy of different COVID-19 medications, and to bring scientists and partners together to lead the way forward.
As part of the Variants of Concern Strategy, PHAC's National Microbiology Laboratory, and Genome Canada, through the Canadian COVID-19 Genomics Network, have invested $20 million, and $8 million respectively, in the improvement of genomic sequencing and data sharing capacity, to detect and address variants of concern in Canada by bringing together expertise in public health, genomic sequencing, epidemiology, immunology, virology, and mathematical modelling. CIHR supported the Government of Canada's Variants of Concern Strategy including the creation of the Coronavirus Variants Rapid Response Network (CoVaRR-Net) that aims to answer questions about the effects of variants on transmissibility, likelihood to cause severe cases, resistance to vaccines, and others.
Government and FPT collaboration
CIHR has taken a leadership role in collaborating with provinces and territories and has promoted knowledge sharing to maximize the impact of its COVID-19 research investments, support coordination, and bring the best available evidence forward to support decision-making and action. Virtual Best Brains Exchanges have brought the most up to date evidence, across five events, to inform policies and practices for federal and provincial partners. CIHR has also coordinated training for policymakers to learn about accessing evidence from the COVID-19 Evidence Network to support Decision-making (COVID-END).
The COVID-19 Immunity Task Force (CITF) contributes scientific evidence to the Health Portfolio to inform policy, guidance and decision-making in response to COVID-19. The CITF collaborated with CIHR to fund 22 studies focused on understanding COVID-19 immunity in Canada and determining the number of people infected with COVID-19, including in healthcare workers, children, people who face homelessness, and the LGBTQ community.
Furthermore, the Government of Canada, through CIHR and PHAC, has provided support to the Canadian Immunization Research Network to evaluate vaccines as they pertain to safety, immunity, effectiveness, and program implementation and evaluation.
Research on modelling COVID-19 at PHAC
Starting in February 2020, PHAC's National Microbiology Laboratory developed mathematical models to explore how the pandemic might affect cases, hospitalizations and deaths, and to understand how public health measures could help manage the epidemic. Recent models include many factors such as vaccination status, and wastewater testing, to produce long-range predictions and are shared with federal, provincial, and territorial partners as well as in published journals.
PHAC funds and collaborates directly with modelling experts at Canadian universities, and has set up an External Expert Modelling Group with members from Canadian universities, federal departments and provincial and territorial public health organizations. This group aims to foster knowledge-sharing, capacity-building for jurisdictions without modelling support, and collaboration amongst university-based modellers and public health organizations.
The Natural Sciences and Engineering Research Council of Canada (NSERC) and PHAC have also provided $10 million in new funding over two years to establish multi-disciplinary networks of specialists in modelling emerging infectious diseases to support public health actions across Canada in the context of pandemics.
Public health surveillance and risk assessment
Issue
Predicting and identifying emerging public health risks, and taking measures to keep Canadians safe and healthy are key responsibilities of the Public Health Agency of Canada. Canadians expect their government to be able to predict, identify and prevent public health risks, and to keep them safe and healthy should risks arise.
To meet this goal, the right data needs to be accessed at the right time (Detect) in order to skillfully assess threats and risks to Canadians (Understand), and share the results of the assessments with appropriate partners for action (Act). The Public Health Agency of Canada (PHAC) uses data collected from approximately 85 surveillance systems along with forecasting, modelling, media analysis and science data, to identify and propose actions to address potential and emerging public health threats and risks domestically and internationally.
Current status
The pandemic has revealed challenges that many countries, including Canada, are facing with respect to surveillance and risk assessment for public health. Action is required to better protect citizens from public health threats, including mitigating against future epidemics and pandemics.
Recent external reviews and audits, including the 2021 Office of the Auditor General (OAG) Report on Pandemic Preparedness and the 2021 independent review of the Global Public Health Intelligence Network (GPHIN), have pointed to three areas that require particular attention:
- Integrating surveillance and risk assessment so the right people have the right intelligence at the right time for action;
- Strengthening data sharing to ensure we have timely access to complete and comprehensive data, particularly in the areas of health equity (e.g.: race/ethnicity, distinction based); and
- Supporting enabling functions (e.g.; technology, HR capacity).
PHAC is taking concrete action to address these recommendations through Management Response Action Plans as well through the Federal-Provincial-Territorial (FPT) co-creation of the COVID surveillance system. This system, which uses existing governance tables, allowed for the early monitoring of COVID Canada-wide. PHAC is strengthening surveillance governance with a view to better integrating surveillance programs, linking surveillance with risk assessment, enhancing data sharing, and translating intelligence into action.
The following are a few key initiatives that have already been undertaken:
- Launched PHAC's COVID-19 Health Data Portal in Fall 2020, a new cloud-based platform designed to improve data flow with PTs, improve analytics, and serve as a foundation portal for further data sharing. One has been created for vaccines, another for COVID.
- Launched the creation of a pan-Canadian Health Data Strategy to address the foundational issues impacting the optimal collection, sharing and use of health data to support the health of Canadians.
- Published PHAC's Data Strategy in Fall 2019 that sets out a phased approach for implementing objectives under key themes, including improving PHAC's data infrastructure.
- Created the Corporate Data and Surveillance Branch in Fall 2020, which provides enterprise-wide leadership for public health surveillance, data innovation and management, and integrated risk assessment.
- Championing the importance of surveillance capacity-building globally and facilitating the sharing of knowledge and best practices with international partners (e.g. via International Zoonoses Community of Experts, One Health Intelligence Collaboration, WHO Hub for Pandemic and Epidemic Intelligence, the European Centre for Disease Control, the Caribbean Public Health Agency, and the United Kingdom).
PHAC's role
The legislative authority over health (including public health) under the constitution is a shared responsibility between Federal and PT jurisdictions. While the Minister of Health has a legislated mandate to monitor diseases, there is no legislative authority to compel or enforce data sharing. Each province and territory decides what information they will share with Canada, which is then governed by formal and informal data sharing agreements.
PHAC has a legislative mandate for surveillance and risk assessment through the Public Health Agency of Canada Act and the Department of Health Act. PHAC also supports the role of the CPHO in providing science-based public health advice to Canadians.
The Agency relies on a number of surveillance systems to meet its mandate to detect, understand and act on a range of public health issues from cancer prevention to addressing the opioid crisis, from mental health promotion to containing food-borne outbreaks.
Next steps
Continue to advance Canada's data, surveillance and risk assessment capacity, addressing commitments in response to recent reviews and audits.
Communications and public health measures
Public communications
Summary/Issue
Communications and risk communications have been an integral part of Health Canada and the Public Health Agency of Canada's (PHAC) pandemic planning for more than a decade. Risk communications – including the provision of evidence-based information in a timely and transparent manner; the integration of communications with the overall pandemic response; and the focus on individuals and stakeholders most at risk – have been core to the COVID-19 communications response.
The Chief Public Health Officer (CPHO) and Deputy CPHO (DCPHO) serve as the central focal point for providing evidence-based information and guidance on the current epidemiological situation in Canada and around the world. Throughout the COVID-19 pandemic, they have provided clear, credible and timely information to keep Canadians, their families and their communities safe.
Identifying creative and multiple ways of reaching at-risk populations will continue to play a prominent role in the ongoing pandemic response, including leveraging tools such as behavioral science and public opinion research.
The CPHO role and communications
PHAC and the position of CPHO were created in 2004 in order to improve public health in Canada and strengthen the country's ability to respond to public health threats, outbreaks and emergencies. The CPHO is the federal government's lead public health professional.
Throughout the COVID-19 pandemic, the CPHO and DCPHO have become familiar, trusted health professionals that Canadians now rely on for clear, credible public health information.
The CPHO's mandate is to provide leadership and guidance on public health issues to Canadians, health professionals, and stakeholders. This includes communicating directly to Canadians through media briefings; timely posting of data, information and public health guidance; infographics; social media; advertising on traditional media and digital platforms; media outreach, and stakeholder and PT engagement. The CPHO and DCPHO also meet regularly with Canada's provincial and territorial chief medical officers of health as part of the Federal-Provincial/Territorial (FPT) Special Advisory Committee on COVID-19, and communicate FPT consensus guidance to Canadians.
Next steps
PHAC will develop communications plans and strategies to support continued adherence to public health measures and work with partners and stakeholders to continue proactive outreach to hard-to-reach populations and those most at risk of COVID-19. A key focus includes Indigenous and racialized communities with customized, in-language material delivered through trusted leaders.
Ongoing proactive communications will update Canadians on the latest public health information related to the COVID-19 pandemic while continuing to build vaccine confidence and counter misinformation through targeted communications, public education and outreach.